An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

BCG vaccination strategies against tuberculosis: updates and perspectives
Xiangmei zhou.
- Author information
- Article notes
- Copyright and License information
CONTACT Hao Li [email protected] College of Veterinary Medicine, China Agricultural University, Beijing, China
Xiangmei Zhou [email protected] College of Veterinary Medicine, China Agricultural University, Beijing, China
Received 2021 Sep 8; Accepted 2021 Nov 15; Collection date 2021.
Bacillus Calmette-Guérin (BCG) is the only licensed vaccine against tuberculosis (TB). However, BCG has variable efficacy and cannot completely prevent TB infection and transmission. Therefore, the worldwide prevalence of TB calls for urgent development of a more effective TB vaccine. In the absence of other approved vaccines, it is also necessary to improve the efficacy of BCG itself. Intravenous (IV) BCG administration and BCG revaccination strategies have recently shown promising results for clinical usage. Therefore, it is necessary for us to revisit the BCG vaccination strategies and summarize the current research updates related to BCG vaccination. This literature review provides an updated overview and perspectives of the immunization strategies against TB using BCG, which may inspire the following research on TB vaccine development.
KEYWORDS: BCG, tuberculosis, vaccine routes, BCG revaccination
1. Introduction
BCG, an attenuated strain of Mycobacterium bovis ( M.bovis ), remains the only approved vaccine against TB for clinical use since 1921. 1 Since 1974, BCG vaccination has been included in the World Health Organization (WHO) Expanded Programme on Immunization (EPI), which was dedicated for infant vaccination worldwide. Different countries have subsequently formulated more favorable BCG vaccination policies according to their own conditions. 1 Countries with high TB incidences continue universal BCG vaccination strategies, while most countries with low to moderate incidence rates consider selective vaccination strategies to target high-risk groups. 2–4 In 2020, 154 countries reported that BCG vaccination is a standard part of childhood immunization programs, of which 53 reported more than 95% coverage. 5 However, previous studies showed that BCG can be only modestly protective, and even completely ineffective against TB in human populations. 6 , 7 The latest WHO report on global TB is still shocking, with an estimated 9.9 million people infected and more than 1.43 million deaths due to the disease in 2020. 5 TB mortality has been more severely impacted by the COVID-19 pandemic in 2020. 5 TB is the leading cause of infectious death worldwide at present, which calls for the development of effective vaccination strategies. 8
The initial development of TB vaccines was mainly focused on devising a vaccine more effective than BCG. Although TB vaccines development have made some progress in the past few years, vaccine evaluation is an extremely long-term, high-risk, and expensive program. 9 On the other hand, BCG has a beneficial heterologous effect, which may prevent diseases other than TB, and modulate immune responses to other vaccines in children. The BCG replacement strategy must take its substantial nonspecific effects into consideration. 10–12 Moreover, strategies of improving existing vaccines by modifying immunization schedules or routes are more cost-effective ways than developing totally new vaccines. Therefore, novel BCG vaccination strategies are being developed. These have shown promising results against Mycobacterium tuberculosis (Mtb) infection.
BCG, which has been used for 100 years as an effective strategy for TB control has protected millions of people from TB. 8 By improving BCG immunization strategies, new and remarkable immune effects have been demonstrated. This has rekindled the hope of BCG to be more effective against TB. 13–17 Under the raging of the global Corona Virus Disease 2019 (COVID-19) pandemic in 2020, BCG has also shown its potential to be protective against COVID-19, which has reignited the research interest in it. 18 , 19 In a retrospective study, among health care workers in a multisite Los Angeles health care organization, BCG vaccination was associated with a reduction in the seroprevalence of anti-SARS-CoV-2 IgG, as well as a decrease in the number of participants who self-reported clinical symptoms associated with COVID-19. 20 Therefore, it is extremely necessary to summarize and update the immunization strategies based on BCG vaccination against TB to provide guidance and inspiration for future research.
2. Why does intradermal BCG vaccination have limited protection against TB?
It is known that BCG is administered via the intradermal (ID) route shortly after birth in TB endemic areas. 6 Although this inoculation method can be easily performed and induce a strong systemic immunity, it can only Mtb provide partial protection in humans and animals models. 21 In addition, this method can produce positive results for the tuberculin skin test (TST), however, it has been shown that the positive conversion rate of TST is irrelevant to the efficacy of BCG immunity. 22 An in-depth discussion on the defects of ID BCG immunization may provide indicative information for the improvement of BCG immune strategy ( Figure 1 ).
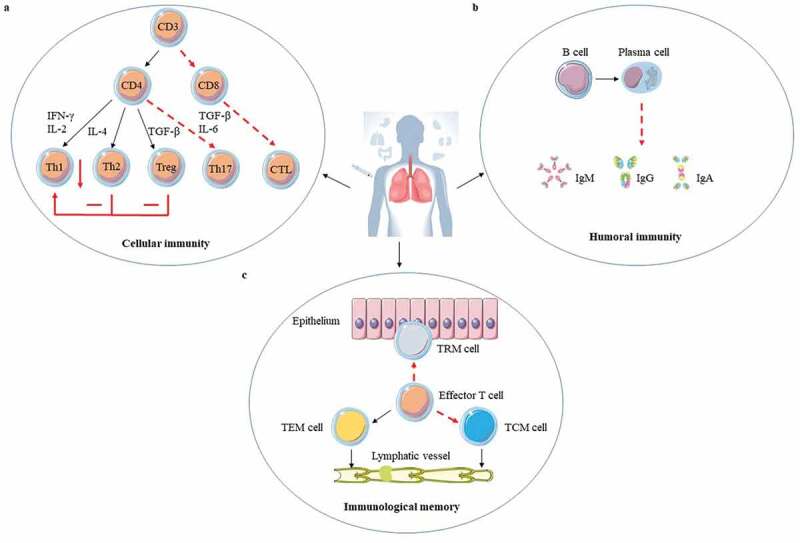
The adaptive immune response to ID BCG vaccination. ID BCG vaccination can arouse a strong adaptive immune response in human body, but these immune responses are not enough to resist Mtb infection for the long term. There are three main reasons: (a). Cellular immunity plays a crucial role in fighting against Mtb infection. Although the T helper 1 (Th1) immune response induced by ID BCG vaccination is relatively robust, it would be inhibited by the Th2 and Treg immune response. In addition, the immune response of Th17 and CD8+ induced by ID BCG vaccination is weak. (b). Recently more and more evidences indicate that humoral immune responses play important roles in protection against Mtb, but the level of antibodies induced by ID BCG immunization is very low, or even almost undetectable. (c). For the memory immune responses, although ID BCG vaccination can induce a large number of effector memory T (TEM) cells, the number of central memory T (TCM) cells and resident memory T (TRM) cells account for small population. Such a composition of memory cells would result in vaccine-induced protection not being sustained for long and make it difficult to respond quickly to the presence of pathogens. The red“ – ” represents a weak immune response, the red“-” represents an adverse immune response, and the red “↓” represents a decrease in the intensity of the immune response.
The performance in inducing T cells immune responses of ID BCG immunization could be an important factor for BCG protection against TB ( Figure 1(a )). First of all, CD4+ and CD8 + T cells cannot be induced efficiently. 23–26 The airway luminal T (ALT) cells are important for the host against Mtb infection, however ID BCG vaccination can only induce a small population of ALT cells and these cells are deficient for at least 10 days after Mtb infection in a TB mouse model. 23 , 24 In a Guinea pig infection model, ID BCG vaccination in the early stage can produce abundant antigen (Ag)-specific lipopeptide-reactive CD4 + T cells in peripheral blood mononuclear cells (PBMCs), but lack functional diversity to prevent granuloma formation. 25 As for CD8 + T cells, BCG can cause significant activation of Ag-specific CD8 + T cells, but its delivery of Ag to the sites of T cell activation is inefficient. 26 Secondly, ID BCG vaccination is not good at inducing T helper 1 (Th1) and Th17 cells. Although ID BCG immunization can induce a robust Th1 immune response, it does not provide sufficient protection and is also negatively regulated by the Th2 and regulatory T cell (Treg) responses. 27 , 28 The same conclusion was reached in a study of neonatal BCG immunization. 29 Human cord blood mononuclear cells selectively produced Th2-type cytokines IL-10 and IL-5 in response to BCG stimulation, and the level of IL-10 was higher than that of unvaccinated infants aged 10 weeks. 29 Furthermore, infants who received BCG at the age of 10 weeks had a stronger lymphoproliferative and Th1 immune response than newborns who received BCG. 29 Th17 cells can trigger the expression of CXCR3 chemokine ligand 9 (CXCL9), CXCL10, and CXCL11 which recruit CD4 + T cells producing interferon (IFN)-γ, and ultimately restrict Mtb growth. 30 Furthermore, interleukin (IL)-17 plays an important role in preventing Mtb infection by inducing CXCL13 to drive neutrophil recruitment to the infection site for pathogens’ control. 31 , 32 However, ID BCG vaccination cannot induce enough Th17 immune responses. 33 Thirdly, as Mtb infection progresses, BCG-induced CD4 + T cells and subsequently CD8 + T cells functionally fade away, gradually resulting in the immune system paralysis. 34 , 35 CD4+ and CD8 + T cells exhaustion after infection is related to mitochondrial dysfunction and the expression of T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), programmed cell death protein 1 (PD1), and other inhibitory receptors. 36 , 37 Therefore, it is necessary to block or offset these complex signals about T cell exhaustion and maintain a reserve of specific self-renewing T cells that can mediate long-term containment in order to improve BCG efficacy.
Although Mtb is an intracellular pathogen, B cells and antibodies also have important roles in resisting Mtb infection. 38–41 Total immunoglobulin (Ig) isolated from Mtb-exposed healthcare workers in a TB-specialized hospital can offer protection against Mtb infection in the aerosol infection mouse model. 39 Monoclonal antibodies against Mtb phosphate transporter subunit PstS1 isolated from active tuberculosis infection (ATBI) patients could reduce bacterial lung burden by 50% in Mtb infected Balb/c mice. 40 Antibodies may mediate protection against Mtb by Mtb neutralization, phagocytosis enhancement, inflammasome activation, and cytotoxic natural killer (NK) cell activities. 41 Some studies showed that ID BCG immunization not only could produce Mtb-specific antibodies but also antibody levels would increase slightly but significantly with the increase of dose and immunization times, while some studies indicated the opposite results. 42 These results suggest that BCG-mediated humoral immunity is heterogeneous, which may be because of different BCG strains, the health state of the immunized subjects, the number of subjects, and the diagnosis methods. 42 However, it is undeniable that the antibody levels induced by ID BCG vaccination are indeed very low ( Figure 1(b )). Further analysis of the antigenic targets for specific antibodies produced by BCG vaccination revealed that BCG only significantly induced specific antibody against lipoarabinomannan (LAM). 43 , 44 Although the antibody against LAM had been clarified to limit the growth of Mtb, 45 , 46 the antibody produced by ID BCG immunization is obviously not enough to control the invasion of Mtb, probably because of insufficient antibody levels. It was also reported that the inhibitory activity of anti-Mtb antibodies was directly associated with their isotypes. 47 IgA antibodies that target Mtb surface antigens could mediate the blocking effect of Mtb uptake independently of Fc alpha receptors expression, while IgG antibody promoted host cell infection. 47 However, the level of IgA induced by ID BCG vaccination is not adequate to make it effective. 42 Therefore, humoral immunity should be taken into account to improve the immune effects of BCG when develop new BCG vaccine strategies.
For memory immune responses, insufficient induction of the central memory T (TCM) cells and tissue-resident memory T (TRM) cells by ID BCG immunization is another important reason for its immune failure ( Figure 1(c )). The long-term memory response mainly depends on the magnitude of TCM cells, not the T effector memory (TEM) cells. 48 ID BCG immunization induces much fewer TCM cells than TEM cells in the lung. 49 Moreover, TCM cells in the host are gradually depleted due to the long-term exposure to environmental mycobacteria, and this leads to the loss of IL-2 producing CD4 + T cells and the increase of KLRG1+ terminally differentiated T cells. 50 TRM cells, also called the local specialists in immune defense, have the ability to detect infected cells and can respond quickly before host recruitment of circulating memory T cells when exposed to Mtb. 51 CD8+ TRM cells can restrict the entry of Mtb into lung tissue by killing infected macrophages, and trigger protective innate and adaptive immune responses by secreting IFN-γ, TNF-α, and IL-2. When these cytokines are blocked, this protective immune responses disappear completely. 14 , 52 Although ID BCG vaccination can also induce TB-specific lung TRM cells, the frequency of TRM cells is relatively low. 14 Moreover, TRM cells in the lungs are not stable, causing a gradual protection loss. 53 In mouse models, ID BCG vaccination could induce antigen-specific CD4+ TRM cells in lung parenchyma for at least 12 months, but this duration time is still short for vaccination protection. 54 Therefore, the improvement of BCG vaccination strategy should be designed to induce both TRM cells and circulating memory T cells especially TCM cells to obtain a high level of protection against Mtb infection.
3. BCG alternative vaccination routes
The immunogenicity and immuno-protection level of BCG may be improved to some extent by changing the administration route. 21 In recent years, the research of BCG mucosal delivery and intravenous injection ( Figure 2 ) has produced satisfactory results, revealed the importance of immune approaches on the immune response, and also provided a paradigm shift in TB vaccine research. 14–16 , 55
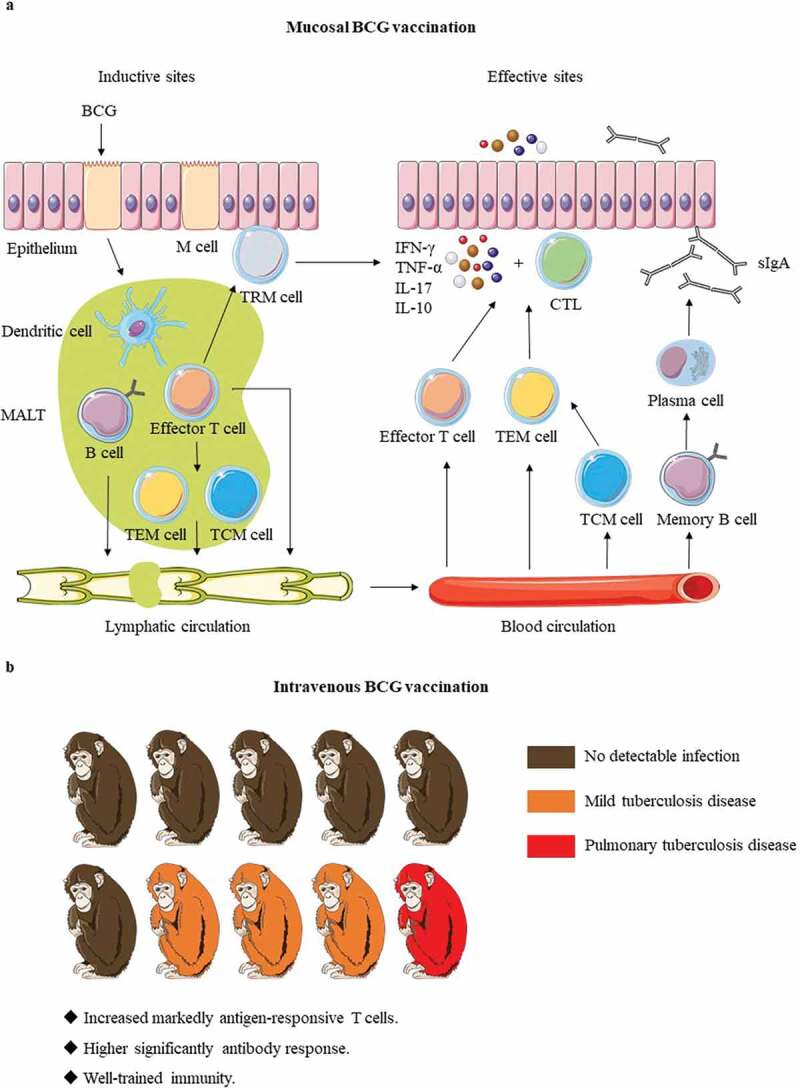
The immune mechanisms of BCG delivered by mucosal and intravenous vaccination. (a). BCG is first taken up by M cells of mucosal epithelium and transported to mucosa-associated lymphoid tissue (MALT). After BCG is processed by dendritic cells, effector T and B lymphocytes are generated, and then differentiated into memory cells. The effector T and B lymphocytes play their protective functions in the effective sites after lymphatic circulation and blood circulation. Except for that tissue-resident memory T (TRM) cells remain constrained within local tissue, central memory T (TCM) cells and effector memory T (TEM) cells migrate to the corresponding lymphatic organs or non-lymphoid tissues. When the body is attacked by Mtb, TRM cells respond quickly, and then the circulating memory cells perform their effector functions. At the same time, memory B cells also rapidly differentiate and secrete IgA (sIgA). (b). Darrah groups 16 showed IV BCG made that 9 out of 10 macaques were highly protected and even 6 showed no signs of infection. The possible protective mechanism of IV BCG vaccination: increased markedly antigen-responsive T cells, higher significantly antibody response, and well-trained immunity. Red shows the presence of bacteria and pulmonary tuberculosis disease, and Orange indicates reduced bacterial burdens and disease, whereas brown remarks no detectable infection.
3.1. Oral immunization
BCG was developed by Calmette and Guerin in 1921 and initially administered orally. 56 And the oral BCG vaccination was used in neonates until 1976 in Brazil, and many data support safety of BCG oral immunization. 13 , 56 Combined with the poor ID BCG immunization, there is a renewed interest in oral BCG. Since Mtb enters the host through infectious aerosols, the mucosa is often the first site to contact with Mtb, and mucosal immunity can trigger a specific protective immune response in the mucosa-associated lymphoid tissue (MALT), which is extremely important for prevention of Mtb infection. 57 The specific immune cells in MALTs are then transported throughout the body generating a systemic immune response ( Figure 2(a )). 58 Besides, B and T cells acquire mucosal homing properties only in the draining lymph nodes from specialized dendritic cells that migrate from the mucosal tissue to these lymph nodes, thus rapidly responding to Mtb. 58 Hence, mucosal delivery can rapidly induce both local and systemic immune responses. 58 Moreover, mucosal immunity can produce specific secretory antibodies in the mucosa to mediate the protective effects against Mtb. 47 , 59 Oral immunization is not only easier to operate but also safer than other mucosal immunization strategies. 60 In this delivery method, BCG can effectively penetrate through the tonsils and intestinal epithelium in newborns and induce specific immunity in the MALTs. 55
Oral BCG can also be used as a booster vaccine. In healthy adults, a combined ID and oral BCG vaccination approach could induce the optimal combination of mucosal and systemic immune responses associated with resistance to TB infection and disease progression. 13 Furthermore, no major safety hazards had been found in the combined ID and oral BCG vaccination approach. 13 ID BCG vaccination-induced systemic Th1 response more powerfully, whereas oral BCG induced a stronger mucosal secretory IgA (sIgA) response and a higher frequency of mucosal cytotoxic T lymphocytes. 60 Therefore, combination of the two vaccination strategies can be considered in clinical practice to enhance the effectiveness of BCG. However, fewer vaccines are using oral immunization methods in clinical practice currently, because this route of administration passes through the body’s first-pass effect, which reduces the drugs’ bioavailability and makes functional burden to the livers and kidneys. 61 In the early days of BCG administration, it was required to take BCG repeatedly to achieve the desired protective effect. 56 However, excessively high doses tend to induce mucosal tolerance, which would avoid triggering an immune response. 62 Hence, it is necessary to use potent adjuvants to make BCG more immunogenic and stable. Nowadays, new materials are often used as transport carriers to wrap BCG, which contribute to mucosal uptake and enhance the BCG protective immunogenicity. 63 , 64
Another promising application of oral BCG is among wild animals living in the nature. 65 , 66 BCG oral vaccination can protect European badgers from virulent M. bovis both experimentally and in the field. 65 , 66 In addition to the general drawbacks of oral vaccines, it is also necessary to consider how to ensure that animals voluntarily consume enough BCG to provide a protective effect. Furthermore, it is required to consider whether BCG could be mixed in the feed avoiding any damage to the vaccine and also preventing environmental pollution.
3.2. Intratracheal and intranasal vaccination
Intratracheal (IT) and intranasal (IN) vaccination are also representative routes of mucosal immunity to deliver BCG. This method of immunization does not require large doses compared to oral administration, and vaccine delivery via aerosol spray is more convenient and attractive due to the upgrading of delivery equipment. 67 Furthermore, Mtb usually enters the host through the respiratory tract, suggesting that IT and IN BCG vaccination are highly effective for the induction of protective immunity. 68 These vaccination methods generate a large number of effector T cells and TRM cells in the lung airway, which are the main components of the BCG efficacy. 14 The airway-resident CD8 + T cells exhibit typical TRM characteristics, in addition to expressing IFN-γ and TNF-α, two cytokines that are not only primary mediators of protective immunity against TB but also recruit CD4 + T cells and B cells to the Mtb infected site to enhance local immunity. 14 In contrast, the airway-resident CD4 + T cells contain a mixture of T-bet + effectors and Foxp3 + -expressing regulatory T cells. 14 Furthermore, CD4 + T cells induced by BCG in this manner also exhibit a specific cellular phenotype compared to those induced by intradermal delivery of BCG. 69 Ag-specific CD4 + T cells expressing a PD-1+ KLRG1- phenotype are present in lung parenchyma and bronchoalveolar lavage fluid (BALF), and these cells can enhance the local immune effect at the infection site by improving the homing effect. Such phenotype determines that these cells can be purified from the lung parenchyma rather than the pulmonary vasculature. 69 CD4 + T cells from the lung parenchyma have greater control over Mtb infection because of the homing effect than the ones from the pulmonary vascular system. 70 , 71 Besides these immune cells, the human alveolar lining fluid contains hydrolytic enzymes which can help BCG improve Mtb control in the mouse infection model. 72
In TB animal models, rhesus monkeys, which share the greatest anatomical and physiological similarities with humans, are the most important “gateways” into human performance testing. 73 The IT BCG vaccination route also shows excellent immune protection effects in rhesus monkeys. Dijkman et al . 15 showed the differences between IT and ID BCG vaccination in rhesus macaques by repeatedly infecting the test population with very low doses of Mtb (1 CFU Mtb) to simulate human natural infection. Surprisingly, infection of rhesus macaques immunized by endobronchial instillation was significantly delayed, or even completely absent. In contrast, all unvaccinated animals and animals that received BCG through the skin got infected and subsequently developed TB. 15 The Th1/Th17 response and the expression of IL-10 in lung cells may be significantly associated with the enhanced immune protection of BCG. 15 IL-17-mediated specific mucosal immune responses triggered by BCG mucosal immunity also offer robust protection against Mtb infection. 74 On the contrary, IL-10 is related to Mtb ability to evade immune responses and mediate long-term lung infections. 75 However, a role for IL-10 in protective immunity cannot be excluded as well. A balance between pro- and anti-inflammatory cytokines was associated with clearance of Mtb at the granulomas level. 76 The balance between activities of IL-17 and IL-10 induced by IT BCG immunization constitute the host defense mechanism in overcoming chronic infection established by Mtb. 15 Interestingly, there is no correlation between sIgA and immune protection in this study, possibly due to the limited number of experimental animals. 15
Mimicking the natural infection route of Mtb has been suggested as a possible means to improve the protective efficacy of the vaccine. 69 In conclusion, both oral BCG and IT or IN BCG are effective. Moreover, treatment of BCG with petroleum either removes inflammatory lipids on the surface of BCG while maintaining the vitality of bacteria, thereby reducing the inflammation caused by lung inoculation with BCG. 77 The technical aspects of BCG mucosal immunization need further research including oral or aerosol delivery, immune dosage, and immune adjuvant mechanism, etc. Overall, it is believed that BCG mucosal immunization has a bright application prospect.
3.3. Intravenous vaccination
Intravenously (IV) BCG immunization can effectively prevent Mtb infection. 16 , 21 , 78 , 79 As early as the 1970s, IV BCG in rhesus monkeys had been shown to provide more protection compared with other conventional BCG inoculation methods. 78 Of 7 IV BCG-immunized rhesus monkeys to mimic natural infection with Mtb, 4 had no gross lesion and the other 3 had the only mild disease. 79 A study published in 2016, further confirmed that IV immunization could induce the highest IFN spot-forming units and multifunctional CD4 + T cell frequency to reduce disease pathology caused by TB. 21 The latest research has shown that IV administration of BCG could achieve unprecedented levels of protection to resist Mtb infections and diseases in non-human primates (NHP) ( Figure 2(b )). 16 In the Mtb challenge experiment after BCG vaccination 6 months, 9 out of 10 macaques given BCG intravenously were highly protected, and of which 6 showed no signs of infection. 16 Compared to aerosol and intradermal delivery, IV BCG immunization resulted in large and sustained recruitment of T cells into the airway and parenchyma. Moreover, IV injection-induced more intense antigen-specific CD4+ and CD8 + T cell responses in BALF and PBMCs, which helped rapid elimination of Mtb. 16 In addition, the antibody response aroused by IV BCG vaccination in BALF and plasma was also significantly higher than other routes. IV BCG vaccination in mice models could induce trained immunity to enhance innate immunity, thereby generating better protection against Mtb infection by means of producing epigenetically modified macrophages. 80 However, neither BCG was detected in bone marrow after one month of IV BCG vaccination, nor was there increase in the innate activation of PBMCs against non-Mtb antigens in NHP models. 16 Nevertheless, it was undeniable that trained immunity played a role in this process. The immune correlation of high protection of IV BCG vaccination still need to be further studied.
It is hard to imagine that BCG as a 100 years old vaccine has remarkable high protection level against TB after changing vaccination route. 16 Importantly, the limited set of clinical safety parameters measured suggested that IV BCG might be well tolerated in NHP, which indicates that this immunization strategy may have good prospects in human applications. 16 It is known that IV injection is currently used for drug therapy and is rarely used for vaccination because of difficulties to implement it in mass vaccination. However, IV immunization has shown excellent immune effects in prevention of many diseases. In a recent study, it was reported that IV vaccination induced a higher proportion of TCF1+ PD-1+ CD8 + T cells and produced a higher anti-tumor response as compared to subcutaneous immunization. 81 Another study of the malaria preventive vaccine PfSPZ showed that IV immunization had produced superior immunogenicity and protective effects in humans compared with subcutaneous and ID administration. 82 Similarly, a series of clinical trials have begun in Africa, Europe, the United States and other regions, in anticipation of applying this immunization method to a small number of high-risk groups. 82 However, the PfSPZ is a non-replicating sporeworm vaccine. 82 A recent study showed that intravenous administration of COVID-19 mRNA vaccine might cause acute myopericarditis in mouse model. 83 As for BCG, although such delivery had previously been used in humans to treat cancer, 84 , 85 further in-depth research is still required to study the safety and effectiveness of injecting pathogenic bacteria with replication ability into human blood.
4. Prime-boost vaccination strategy to enhance BCG efficacy
Except for the immunization routes, various booster vaccines are developed to “repair” the immunogenicity and enhance immune memory persistence of BCG. 86–96 The current BCG booster vaccine research strategy is mainly based on the several dominant antigens of Mtb with the help of live virus expression vectors or adjuvants. 33 WHO has made this approach to improving BCG a priority for the research and development of a new TB vaccine. 97 A total of 9 BCG booster candidate vaccines are currently under active evaluation in clinical trials ( Figure 3 ) and only “best-in-class” candidates to late-stage clinical trials. A really excellent BCG booster vaccine can prevent not only the primary Mtb infection but also the progression of the disease in those latently infected individuals. In the recently completed final analysis of the clinical phase IIb trial (ClinicalTrials.gov Identifier: NCT01755598 ), M72/AS01 E could provide 49.7% protection against active pulmonary TB for latent Mtb-infected adults for at least 3 years, excluding differences in age or gender for vaccine efficacy, which was a milestone in the development of a new tuberculosis vaccine. 93 , 98 Notably, the vaccine had a clinically acceptable safety profile and immunogenicity in HIV-infected people, no matter in TB endemic areas or in low-risk areas, regardless of their antiretroviral therapy status. 99 , 100 Although TB is highly prevalent among HIV-positive people, WHO does not recommend BCG vaccination in infants infected with HIV. 101 Hence, there is an urgent need for an effective TB vaccine that can be safely vaccinated to HIV-infected people. M72/AS01 E is expected to fill the gap.
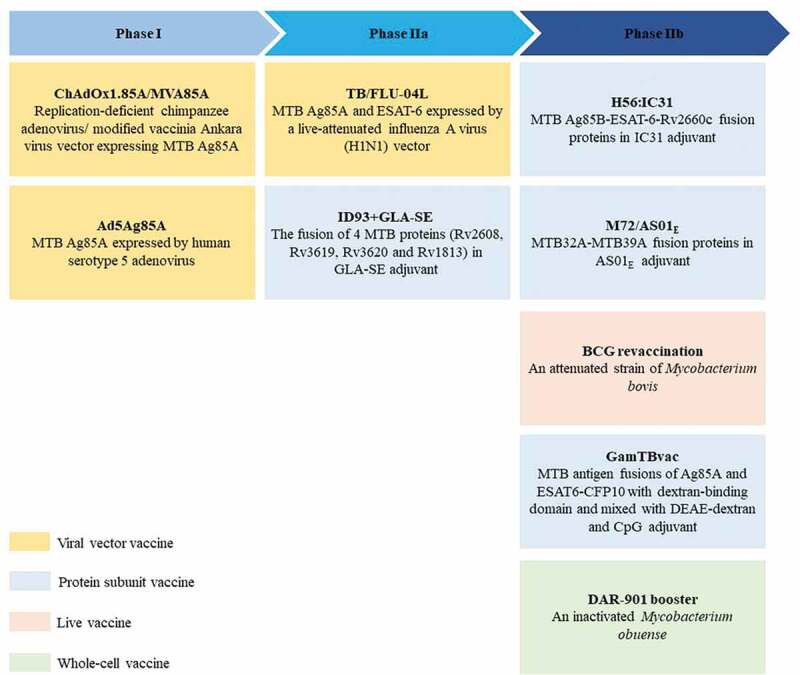
BCG booster vaccine candidates in clinical development. There are currently 9 BCG booster candidates in clinical development, including viral vector vaccines, protein subunit vaccines, live attenuated vaccines, and whole cell vaccines 69–77. The stage of clinical development of vaccine candidates is inferred from data available at ClinicalTrials.gov. Abbreviation: TLR = toll-like receptor.
The easiest and most convenient way to apply the prime-boost strategy is a second BCG vaccination. Previous large randomized clinical trials had shown that BCG revaccination do not contribute to TB prevention. 102 , 103 In 2018, the WHO also announced the same conclusion and did not recommend BCG revaccination. 104 However, recent clinical trials make us rethink about this strategy. BCG revaccination was safe in QuantiFERON-TB Gold In-tube assay (QFT)-negative adolescents and can significantly improve BCG-specific CD4 + T cell response. But not the specific CD8 + T cell response. 17 , 105 Remarkably, BCG revaccination did not prevent the initial conversion of QFT in a context of TB high-transmission, but reached 45.4% efficacy against persistent QFT conversion, while the efficacy of clinical TB vaccine candidate H4:IC31 (Ag85B-TB10.4 fusion proteins in IC31 adjuvant) was only 30.5%. 17 The sustained QFT conversion might reflect sustained Mtb infection and progression to disease. This study reflected BCG revaccination could help prevent sustained Mtb infection, which was of great public health significance.
The immune effect of BCG will be affected by the infection status. This is one of the reasons for the variable immune effect of BCG. 106 Therefore, it is very important to see whether BCG revaccination will be affected by the Mtb infection status, since approximately just under a quarter of the global population are latent tuberculosis infection (LTBI) patients in 2014. 107 The above clinical trials of BCG revaccination were carried out in QFT-negative adolescents. 17 , 105 Similarly, BCG revaccination in healthy adults infected with Mtb, whether or not they were treated with isoniazid before vaccination, had the same robust immunogenicity. 108 , 109 BCG revaccination could transiently promote BCG-specific CD4+, CD8+ and γδ T cell responses, it could particularly boost highly specific natural killer T (NKT) cell and NK cell responses persistently (at least for 1 year) to improve trained immunity, 109 , 110 which might indicate that BCG revaccination could also produce an additional immune effect. In addition, BCG vaccination could significantly enhance Mtb-specific Th17 responses, especially regulatory IL-10+ Th17 responses. 108 The protectiveness of BCG revaccination against Mtb infection in LTBI patients still needs further research.
5. Perspectives
Mtb is an extremely “robust” and “tricky” intracellular pathogen that has highly efficient mechanisms for immune evasion and can coexist with infected hosts for a lifetime. 57 TB vaccines should have the ability to modulate moderately the complex regulatory signals induced by Mtb, create a delicate balance between inflammation and regulatory immune responses, and maintain strong memory immune responses for a long time. The BCG immunization strategy must be continuously improved to ensure the efficacy of the Mtb control strategy worldwide.
Either changing the vaccination route or relying on the prime-boost immune strategy, is a good way to improve the immune effect of BCG. However, there are significant challenges in conducting the process of clinical trials, one of the biggest obstacles in this process is the lack of accurate and reliable immune markers. It is not feasible to overemphasize the Th1 immune response before the classical and reliable immune markers are determined, which may ignore the truly effective immune response and enable Mtb to perform immune evasion. This might suggest that the sample size should be as large as possible and the scope of immunization evaluation should be as wide as possible when conducting TB vaccine research.
Another major challenge is the often glaring difference between the immune assessment of clinical trials and those based on animal models for TB vaccines. To minimize discrepancies, animal models that reflect human infection, such as NHPs, should be selected when evaluating TB vaccines in animal models. Secondly, the number of experimental animals can be increased as much as possible to reduce the randomness of experimental results and the differences caused by the heterogeneity of experimental animals. Besides, TB vaccines need to be evaluated in the context of ongoing chronic infections to reflect people’s lifelong exposure to pathogens and their antigens in many cases. 15 It has been reported that the combination of Monte-Carlo methods and compartmental models can reduce the uncertainty in impact evaluations to a certain extent, improve the evaluation of vaccine candidates and help the decision-making processes of funding agencies. 111
An obvious limitation in the development of BCG booster vaccines is that the type of vaccine function is extremely limited. For most TB vaccine candidates entering preclinical trials and clinical trials, their functional profiles are extremely limited. In most cases, they can be differentiated mostly by the magnitude of antigen-specific T-cell responses. 33 So the studies should focus more on finding promising protective antigens that are not confined only in inducing cellular immunity. The role of antibodies in TB has been initially elucidated and should be taken into account in the design of TB vaccines. 39–41 Additionally, adjuvants are usually required for vaccines to exert enough protective immune responses against pathogens, which can increase the vaccine efficacy significantly. 112 Therefore, new adjuvants technologies should be studied in parallel with vaccines research and development. Moreover, TB vaccine design cannot be limited to its small field and should learn from the experiences of other successful vaccines such as Hib and meningococcal conjugate vaccines. 113 Over last century, BCG vaccine has saved countless lives around world. With the rapid development of science and technologies, we believe that BCG vaccination strategies development will be a crucial and important research direction and will exert its positive roles in public health.
Funding Statement
This research was funded by National Natural Science Foundation of China to Hao Li (No. 32070937), 2015 Talent Development Program of China Agricultural University to Hao Li (No. 00109029) and National Natural Science Foundation of China to Xiangmei Zhou (No.31873005).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Hao Li: Conceptualization, Writing-Review & Editing, Supervision, Project administration and Funding acquisition. Xiangmei Zhou: Writing-Review & Editing, Supervision, Project administration and Funding acquisition. Mengjin Qu: Writing- Original draft preparation.
- 1. Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M.. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012. doi: 10.1371/journal.pmed.1001012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Manissero D, Lopalco PL, Levy-Bruhl D, Ciofi Degli Atti ML, Giesecke J. Assessing the impact of different BCG vaccination strategies on severe childhood TB in low-intermediate prevalence settings. Vaccine. 2008;26(18):2253–59. doi: 10.1016/j.vaccine.2008.02.038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Faust L, Schreiber Y, Bocking N. A systematic review of BCG vaccination policies among high-risk groups in low TB-burden countries: implications for vaccination strategy in Canadian indigenous communities. BMC Public Health. 2019;19(1):1504. doi: 10.1186/s12889-019-7868-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Kobayashi S, Yoshiyama T, Uchimura K, Hamaguchi Y, Kato S. Epidemiology of childhood tuberculosis after ceasing universal Bacillus Calmette-Guérin vaccination. Sci Rep. 2021;11:15902. doi: 10.1038/s41598-021-95294-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. WHO . Global tuberculosis Report 2021. 2021.
- 6. Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3(8):656–62. doi: 10.1038/nrmicro1211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacille Calmette et Guérin (BCG) vaccine diversity and delivery: why does BCG fail to protect against tuberculosis? Vaccine. 2015;33(39):5035–41. doi: 10.1016/j.vaccine.2015.08.033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Kuan R, Muskat K, Peters B, Lindestam Arlehamn CS. Is mapping the BCG vaccine-induced immune responses the key to improving the efficacy against tuberculosis? J Intern Med. 2020;288(6):651–60. doi: 10.1111/joim.13191. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Sable SB, Posey JE, Scriba TJ. Tuberculosis vaccine development: progress in clinical evaluation. Clin Microbiol Rev. 2019;33(1). doi: 10.1128/cmr.00100-19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35(2):109–14. doi: 10.1016/j.clinthera.2013.01.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Kowalewicz-Kulbat M, Locht C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev Vaccines. 2017;16(7):1–10. doi: 10.1080/14760584.2017.1333906. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Butkeviciute E, Jones CE, Smith SG. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13(10):1193–208. doi: 10.2217/fmb-2018-0026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Hoft DF, Xia M, Zhang GL, Blazevic A, Tennant J, Kaplan C, Matuschak G, Dube TJ, Hill H, Schlesinger LS, et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4+ T cell transcriptomal molecular signatures. Mucosal Immunol. 2018;11(2):486–95. doi: 10.1038/mi.2017.67. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Perdomo C, Zedler U, Kühl AA, Lozza L, Saikali P, Sander LE, Vogelzang A, Kaufmann SHE, Kupz A. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. mBio. 2016;7(6). doi: 10.1128/mBio.01686-16 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, Kocken CHM, Ottenhoff THM, Kondova I, Khayum MA, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019;25(2):255–62. doi: 10.1038/s41591-018-0319-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, Pokkali S, Swanson PA, Grant NL, Rodgers MA, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102. doi: 10.1038/s41586-019-1817-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–49. doi: 10.1056/NEJMoa1714021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet (London, England). 2020;395(10236):1545–46. doi: 10.1016/s0140-6736(20)31025-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, Meeks JJ, Bivalacqua TJ, Gontero P, Steinberg GD, et al. 100 years of Bacillus Calmette-Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18:611–22. doi: 10.1038/s41585-021-00481-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, Van Eyk JE, Cheng S, Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131(2). doi: 10.1172/jci145157 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, Basaraba R, Clark S, Hall G, Rayner E, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinburgh, Scotland). 2016;101:174–90. doi: 10.1016/j.tube.2016.09.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Ho MM, Southern J, Kang HN, Knezevic I. WHO informal consultation on standardization and evaluation of BCG vaccines Geneva, Switzerland 22-23 September 2009. Vaccine. 2010;28:6945–50. doi: 10.1016/j.vaccine.2010.07.086. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Horvath CN, Shaler CR, Jeyanathan M, Zganiacz A, Xing Z. Mechanisms of delayed anti-tuberculosis protection in the lung of parenteral BCG-vaccinated hosts: a critical role of airway luminal T cells. Mucosal Immunol. 2012;5(4):420–31. doi: 10.1038/mi.2012.19. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Jeyanathan M, Heriazon A, Xing Z. Airway luminal T cells: a newcomer on the stage of TB vaccination strategies. Trends Immunol. 2010;31(7):247–52. doi: 10.1016/j.it.2010.05.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Kaufmann E, Spohr C, Battenfeld S, De Paepe D, Holzhauser T, Balks E, Homolka S, Reiling N, Gilleron M, Bastian M, et al. BCG vaccination induces robust CD4+ T cell responses to mycobacterium tuberculosis complex–specific lipopeptides in Guinea Pigs. J Immunol (Baltimore, Md: 1950). 2016;196(6):2723–32. doi: 10.4049/jimmunol.1502307. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Ryan AA, Nambiar JK, Wozniak TM, Roediger B, Shklovskaya E, Britton WJ, Groth BF, Triccas JA. Antigen load governs the differential priming of CD8 T cells in response to the bacille Calmette Guerin vaccine or Mycobacterium tuberculosis infection. J Immunol (Baltimore, Md: 1950). 2009;182:7172–77. doi: 10.4049/jimmunol.0801694. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Orr MT, Windish HP, Beebe EA, Argilla D, Huang PWD, Reese VA, Reed SG, Coler RN. Interferon γ and tumor necrosis factor are not essential parameters of CD4+T-cell responses for vaccine control of tuberculosis. J Infect Dis. 2015;212(3):495–504. doi: 10.1093/infdis/jiv055. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Bhattacharya D, Dwivedi VP, Kumar S, Reddy MC, Van Kaer L, Moodley P, Das G. Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances bacillus Calmette-Guerin vaccine efficacy against tuberculosis. J Biol Chem. 2014;289(48):33404–11. doi: 10.1074/jbc.M114.600452. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Hussey GD, Watkins MLV, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105(3):314–24. doi: 10.1046/j.1365-2567.2002.01366.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Junecko BF, Reinhart TA, Kolls J, Randall TD, Connell TD, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–84. doi: 10.1038/mi.2012.135. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko B, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10(5):e1004099. doi: 10.1371/journal.ppat.1004099. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Rodo MJ, Rozot V, Nemes E, Dintwe O, Hatherill M, Little F, Scriba TJ. A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog. 2019;15(3):e1007643. doi: 10.1371/journal.ppat.1007643. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha S-J, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–87. doi: 10.1073/pnas.1118450109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Chodisetti SB, Gowthaman U, Rai PK, Vidyarthi A, Khan N, Agrewala JN. Triggering through Toll-like receptor 2 limits chronically stimulated T-helper type 1 cells from undergoing exhaustion. J Infect Dis. 2015;211:486–96. doi: 10.1093/infdis/jiu472. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Crawford A, Angelosanto J, Kao C, Doering T, Odorizzi P, Barnett B, Wherry E. Molecular and transcriptional basis of CD4⁺ T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, Anderson AC, Kuchroo VK, Behar SM. TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 2016;12:e1005490. doi: 10.1371/journal.ppat.1005490. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–443.e414. doi: 10.1016/j.cell.2016.08.072. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Li H, Wang XX, Wang B, Fu L, Liu G, Lu Y, Cao M, Huang H, Javid B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2017;114:5023–28. doi: 10.1073/pnas.1611776114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Watson A, Li H, Ma B, Weiss R, Bendayan D, Abramovitz L, Ben-Shalom N, Mor M, Pinko E, Bar Oz M, et al . Human antibodies targeting a Mycobacterium transporter protein mediate protection against tuberculosis. Nat Commun. 2021;12(1):602. doi: 10.1038/s41467-021-20930-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Li H, Javid B. Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol. 2018;18(9):591–96. doi: 10.1038/s41577-018-0028-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Tanner R, Villarreal-Ramos B, Vordermeier HM, McShane H. The humoral immune response to BCG vaccination. Front Immunol. 2019;10:1317. doi: 10.3389/fimmu.2019.01317. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Brown RM, Cruz O, Brennan M, Gennaro M, Schlesinger L, Skeiky Y, Hoft D. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J Infect Dis. 2003;187:513–17. doi: 10.1086/368096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Watanabe Y, Watari E, Matsunaga I, Hiromatsu K, Dascher CC, Kawashima T, Norose Y, Shimizu K, Takahashi H, Yano I, et al. BCG vaccine elicits both T-cell mediated and humoral immune responses directed against mycobacterial lipid components. Vaccine. 2006;24(29–30):5700–07. doi: 10.1016/j.vaccine.2006.04.049. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Chen T, Blanc C, Liu Y, Ishida E, Singer S, Xu J, Joe M, Jenny-Avital ER, Chan J, Lowary TL, et al. Capsular glycan recognition provides antibody-mediated immunity against tuberculosis. J Clin Invest. 2020;130(4):1808–22. doi: 10.1172/jci128459. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, Glatman-Freedman A, Joe M, Bai Y, et al. Association of human antibodies to arabinomannan with enhanced Mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis. 2016;214:300–10. doi: 10.1093/infdis/jiw141. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Zimmermann N, Thormann V, Hu B, Köhler A-B, Imai‐Matsushima A, Locht C, Arnett E, Schlesinger LS, Zoller T, Schürmann M, et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–39. doi: 10.15252/emmm.201606330. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin vaccine immunol. 2010;17:618–25. doi: 10.1128/cvi.00368-09. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Lindenstrøm T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol (Baltimore, Md: 1950). 2013;190:6311–19. doi: 10.4049/jimmunol.1300248. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. doi: 10.1038/nri.2015.3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol. 2017:2. doi: 10.1126/sciimmunol.aag2031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Bull NC, Kaveh DA, Garcia-Pelayo MC, Stylianou E, McShane H, Hogarth PJ. Induction and maintenance of a phenotypically heterogeneous lung tissue-resident CD4+ T cell population following BCG immunisation. Vaccine. 2018;36(37):5625–35. doi: 10.1016/j.vaccine.2018.07.035. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Monteiro-Maia R, Pinho RT. Oral Bacillus Calmette-Guérin vaccine against tuberculosis: why not? Mem Inst Oswaldo Cruz. 2014;109(6):838–45. doi: 10.1590/0074-0276140091. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Benévolo-de-andrade TC, Monteiro-Maia R, Cosgrove C, Castello-Branco LR, Moreau BCG. Rio de Janeiro: an oral vaccine against tuberculosis–review. Mem Inst Oswaldo Cruz. 2005;100(5):459–65. doi: 10.1590/s0074-02762005000500002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Chai Q, Lu Z, Liu CH. Host defense mechanisms against Mycobacterium tuberculosis. Cell Mol Life Sci. 2020;77(10):1859–78. doi: 10.1007/s00018-019-03353-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Boyaka PN. Inducing mucosal IgA: a challenge for vaccine adjuvants and delivery systems. J Immunol (Baltimore, Md: 1950). 2017;199:9–16. doi: 10.4049/jimmunol.1601775. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Creighton RL, Woodrow KA. Microneedle-mediated vaccine delivery to the oral mucosa. Adv Healthc Mater. 2019;8:e1801180. doi: 10.1002/adhm.201801180. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116–31. doi: 10.1016/j.addr.2017.04.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi: 10.1016/j.addr.2015.03.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Saleem I, Coombes AGA, and Chambers MA. In vitro evaluation of eudragit matrices for oral delivery of BCG vaccine to animals. Pharmaceutics . 2019;11. doi: 10.3390/pharmaceutics11060270. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Hosseini M, Dobakhti F, Pakzad SR, Ajdary S. Immunization with single oral dose of alginate-encapsulated BCG elicits effective and long-lasting mucosal immune responses. Scand J Immunol. 2015;82(6):489–97. doi: 10.1111/sji.12351. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Chambers MA, Aldwell F, Williams GA, Palmer S, Gowtage S, Ashford R, Dalley DJ, Davé D, Weyer U, Salguero FJ, et al. The effect of oral vaccination with Mycobacterium bovis BCG on the development of tuberculosis in captive European badgers (meles meles). Front Cell Infect Microbiol. 2017;7:6. doi: 10.3389/fcimb.2017.00006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Lesellier S, Birch CPD, Davé D, Dalley D, Gowtage S, Palmer S, McKenna C, Williams GA, Ashford R, and Weyer U. Bioreactor-grown bacillus of Calmette and Guerin (BCG) vaccine protects badgers against virulent mycobacterium bovis when administered orally: identifying limitations in baited vaccine delivery. Pharmaceutics . 2020;12. doi: 10.3390/pharmaceutics12080782. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Lobaina Mato Y. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813. doi: 10.1016/j.ijpharm.2019.118813. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Li W, Deng G, Li M, Liu X, Wang Y. Roles of mucosal immunity against Mycobacterium tuberculosis infection. Tuberc Res Treat. 2012;2012:791728. doi: 10.1155/2012/791728. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Bull NC, Stylianou E, Kaveh DA, Pinpathomrat N, Pasricha J, Harrington-Kandt R, Garcia-Pelayo MC, Hogarth PJ, McShane H. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1(+) KLRG1(-) CD4(+) T cells. Mucosal Immunol. 2019;12:555–64. doi: 10.1038/s41385-018-0109-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL. Cutting edge: control of mycobacterium tuberculosis infection by a subset of lung parenchyma–homing CD4 T cells. J Immunol (Baltimore, Md: 1950). 2014;192:2965–69. doi: 10.4049/jimmunol.1400019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Barber DL. Vaccination for Mycobacterium tuberculosis infection: reprogramming CD4 T-cell homing into the lung. Mucosal Immunol. 2017;10(2):318–21. doi: 10.1038/mi.2016.110. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Moliva JI, Hossfeld AP, Canan CH, Dwivedi V, Wewers MD, Beamer G, Turner J, Torrelles JB. Exposure to human alveolar lining fluid enhances Mycobacterium bovis BCG vaccine efficacy against Mycobacterium tuberculosis infection in a CD8(+) T-cell-dependent manner. Mucosal Immunol. 2018;11:968–78. doi: 10.1038/mi.2017.80. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Peña JC, Ho WZ, Jacobs Jr. WR, McShane H, Mizrahi V, Orme IM. Non-human primate models of tuberculosis. Microbiol Spectr. 2016:4. doi: 10.1128/microbiolspec.TBTB2-0007-2016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Aguilo N, Alvarez-Arguedas S, Uranga S, Marinova D, Monzón M, Badiola J, Martin C. Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-dependent mechanism. J Infect Dis. 2016;213:831–39. doi: 10.1093/infdis/jiv503. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4(3):261–70. doi: 10.1038/mi.2011.7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11:e1004603. doi: 10.1371/journal.ppat.1004603. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Moliva JI, Hossfeld AP, Sidiki S, Canan CH, Dwivedi V, Beamer G, Turner J, Torrelles JB. Selective delipidation of Mycobacterium bovis BCG enables direct pulmonary vaccination and enhances protection against Mycobacterium tuberculosis. Mucosal Immunol. 2019;12(3):805–15. doi: 10.1038/s41385-019-0148-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Anacker RL, Brehmer W, Barclay WR, Leif WR, Ribi E, Simmons JH, Smith AW. Superiority of intravenously administered BCG and BCG cell walls in protecting rhesus monkeys (Macaca mulatta) against airborne tuberculosis. Z Immunitatsforsch Exp Klin Immunol. 1972;143:363–76. [ PubMed ] [ Google Scholar ]
- 79. Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect Immun. 1970;2(5):574–82. doi: 10.1128/iai.2.5.574-582.1970. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier J-C, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2):176–190.e119. doi: 10.1016/j.cell.2017.12.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Baharom F, Ramirez-Valdez RA, Tobin KK, Yamane H, Dutertre CA, Khalilnezhad A, Reynoso GV, Coble VL, Lynn GM, and Mulè MP, et al. Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat Immunol . 2021;22:41-52. doi: 10.1038/s41590-020-00810-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Seder RA, Chang L-J, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–65. doi: 10.1126/science.1241800. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Li C, Chen Y, Zhao Y, Lung DC, Ye Z, Song W, Liu FF, Cai JP, Wong WM, Yip CC, et al. Intravenous injection of COVID-19 mRNA vaccine can induce acute myopericarditis in mouse model. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab707. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Lamm DL. Optimal BCG treatment of superficial bladder cancer as defined by American trials. Eur Urol. 1992;21 Suppl 2:12–16. doi: 10.1159/000474915. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Whittaker JA, Slater AJ. The immunotherapy of acute myelogenous leukaemia using intravenous BCG. Br J Haematol. 1977;35(2):263–73. doi: 10.1111/j.1365-2141.1977.tb00583.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–28. doi: 10.1016/S0140-6736(13)60177-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Stylianou E, Griffiths KL, Poyntz HC, Harrington-Kandt R, Dicks MD, Stockdale L, Betts G, McShane H. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine. 2015;33(48):6800–08. doi: 10.1016/j.vaccine.2015.10.017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Darrah PA, DiFazio RM, Maiello P, Gideon HP, Myers AJ, Rodgers MA, Hackney JA, Lindenstrom T, Evans T, Scanga CA, et al. Boosting BCG with proteins or rAd5 does not enhance protection against tuberculosis in rhesus macaques. NPJ Vaccines. 2019;4(1):21. doi: 10.1038/s41541-019-0113-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2(53):53ra74. doi: 10.1126/scitranslmed.3001094. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, Vergara J, Mabwe S, Bilek N, Geldenhuys H, et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med. 2018;6(4):287–98. doi: 10.1016/s2213-2600(18)30077-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Tkachuk AP, Bykonia EN, Popova LI, Kleymenov DA, Semashko MA, Chulanov VP, Fitilev SB, Maksimov SL, Smolyarchuk EA, et al. Safety and immunogenicity of the GamTBvac, the recombinant subunit tuberculosis vaccine candidate: a phase II, multi-center, double-blind, randomized, placebo-controlled study. Vaccines (Basel). 2020;8. doi: 10.3390/vaccines8040652. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Suliman S, Luabeya AKK, Geldenhuys H, Tameris M, Hoff ST, Shi Z, Tait D, Kromann I, Ruhwald M, and Rutkowski KT, et al. Dose optimization of H56: IC31Vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am J Respir Crit Care Med . 2019;199:220–31. doi: 10.1164/rccm.201802-0366OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, Scriba TJ, Akite EJ, Ayles HM, Bollaerts A. Final analysis of a trial of M72/AS01(E) M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381:2429–39. doi: 10.1056/NEJMoa1909953. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Masonou T, Hokey DA, Lahey T, Halliday A, Berrocal-Almanza LC, Wieland-Alter WF, Arbeit RD, Lalvani A, Von Reyn CF. CD4+ T cell cytokine responses to the DAR-901 booster vaccine in BCG-primed adults: a randomized, placebo-controlled trial. PLoS One. 2019;14(5):e0217091. doi: 10.1371/journal.pone.0217091. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Day TA, Penn-Nicholson A, Luabeya AK, Fiore-Gartland A, Du Plessis N, Loxton AG, Vergara J, Rolf TA, Reid TD, Toefy A, et al. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir Med. 2020. doi: 10.1016/s2213-2600(20)30319-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines. 2018;3(1):34. doi: 10.1038/s41541-018-0057-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. WHO . WHO informal consultation on standardization and evaluation of BCG vaccines; 2009. Accessed 12 Oct 2021. https://www.who.int/publications/m/item/BCG-meeting-report-2009v7 [ DOI ] [ PubMed ]
- 98. Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, Ayles HM, Henostroza G, Thienemann F, Scriba TJ. Phase 2b controlled trial of M72/AS01(E) vaccine to prevent tuberculosis. N Engl J Med. 2018;379(17):1621–34. doi: 10.1056/NEJMoa1803484. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Kumarasamy N, Poongulali S, Bollaerts A, Moris P, Beulah FE, Ayuk LN, Demoitié MA, Jongert E, Ofori-Anyinam O. A. A randomized, controlled safety, and immunogenicity trial of the M72/AS01 candidate tuberculosis vaccine in HIV-positive Indian adults. Medicine (Baltimore). 2016;95:e2459. doi: 10.1097/md.0000000000002459. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Thacher EG, Cavassini M, Audran R, Thierry A-C, Bollaerts A, Cohen J, Demoitié M-A, Ejigu D, Mettens P, Moris P. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine in HIV-infected adults on combination antiretroviral therapy: a phase I/II, randomized trial. Aids. 2014;28(12):1769–81. doi: 10.1097/qad.0000000000000343. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Hesseling AC, Cotton MF, Fordham Von Reyn C, Graham SM, Gie RP, Hussey GD. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int J Tuberculosis Lung Dis. 2008;12:1376–79. [ PubMed ] [ Google Scholar ]
- 102. Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet (London, England). 1996;348(9019):17–24. doi: 10.1016/S0140-6736(96)02166-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, Hijjar MA, Cruz AA, Sant’Anna C, Bierrenbach AL, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet (London, England). 2005;366(9493):1290–95. doi: 10.1016/s0140-6736(05)67145-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. WHO . Global Tuberculosis Report 2018. 2018.
- 105. Bekker LG, Dintwe O, Fiore-Gartland A, Middelkoop K, Hutter J, Williams A, Randhawa AK, Ruhwald M, Kromann I, Andersen PL, et al. A phase 1b randomized study of the safety and immunological responses to vaccination with H4: IC31,H56: IC31,and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClin Med. 2020;21:100313. doi: 10.1016/j.eclinm.2020.100313. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Ahmed A, Rakshit S, Adiga V, Dias M, Dwarkanath P, D’Souza G, Vyakarnam A. A century of BCG: impact on tuberculosis control and beyond. Immunol Rev. 2021;301(1):98–121. doi: 10.1111/imr.12968. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Houben RM, Dodd PJ, Metcalfe JZ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Rakshit S, Ahmed A, Adiga V, Sundararaj BK, Sahoo PN, Kenneth J, D’Souza G, Bonam W, Johnson C, Franken KLMC, et al. BCG revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA- Indian adults. JCI Insight. 2019;4(24). doi: 10.1172/jci.insight.130540. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, Toefy A, Lerumo L, Hopley C, Pienaar B, et al. Bacillus Calmette-Guérin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol (Baltimore, Md: 1950). 2016;197:1100–10. doi: 10.4049/jimmunol.1501996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98:347–56. doi: 10.1189/jlb.5RI0315-096R. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Tovar M, Arregui S, Marinova D, Martín C, Sanz J, Moreno Y. Bridging the gap between efficacy trials and model-based impact evaluation for new tuberculosis vaccines. Nat Commun. 2019;10:5457. doi: 10.1038/s41467-019-13387-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Agger EM. Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv Drug Deliv Rev. 2016;102:73–82. doi: 10.1016/j.addr.2015.11.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Mascola JR, Fauci AS. Novel vaccine technologies for the 21st century. Nat Rev Immunol. 2020;20(2):87–88. doi: 10.1038/s41577-019-0243-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (4.8 MB)
- Collections

Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Tuberculosis vaccines update: Is an RNA-based vaccine feasible for tuberculosis?
Sasha e larsen, susan l baldwin, rhea n coler.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2023 Feb 2; Revised 2023 Mar 16; Accepted 2023 Mar 16; Issue date 2023 May.
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
Despite concerted efforts, Mycobacterium tuberculosis (M.tb), the pathogen that causes tuberculosis (TB), continues to be a burden on global health, regaining its dubious distinction in 2022 as the world's biggest infectious killer with global COVID-19 deaths steadily declining. The complex nature of M.tb, coupled with different pathogenic stages, has highlighted the need for the development of novel immunization approaches to combat this ancient infectious agent. Intensive efforts over the last couple of decades have identified alternative approaches to improve upon traditional vaccines that are based on killed pathogens, live attenuated agents, or subunit recombinant antigens formulated with adjuvants. Massive funding and rapid advances in RNA-based vaccines for immunization have recently transformed the possibility of protecting global populations from viral pathogens, such as SARS-CoV-2. Similar efforts to combat bacterial pathogens such as M.tb have been significantly slower to implement.
In this review, we discuss the application of a novel replicating RNA (repRNA)-based vaccine formulated and delivered in nanostructured lipids.
Our preclinical data are the first to report that RNA platforms are a viable system for TB vaccines and should be pursued with high-priority M.tb antigens containing cluster of differentiation (CD4+) and CD8+ T-cell epitopes.
This RNA vaccine shows promise for use against intracellular bacteria such as M.tb as demonstrated by the feasibility of construction, enhanced induction of cell-mediated and humoral immune responses, and improved bacterial burden outcomes in in vivo aerosol-challenged preclinical TB models.
Keywords: RNA vaccine, Tuberculosis, Immunity
Introduction
Since the emergence of SARS-CoV-2 in China in late 2019, over 671 million confirmed cases have been reported. While this statistic is staggering, equally threatening on a global scale is tuberculosis (TB). TB is an airborne disease that can be spread through coughing and sneezing [1] . The World Health Organization estimates that 1.8 billion people—close to one-quarter of the world's population—are infected with Mycobacterium tuberculosis (M.tb). In 2022, TB regained its position as the leading infectious cause of death worldwide, ranking above COVID-19 and HIV/AIDS. Access to TB diagnosis and treatment have been impacted by the COVID-19 pandemic, caused by the SARS-CoV-2, thus slowing and indeed reversing the progress that had been made in previous years leading up to 2019 [1] . Based on the annual death rate, it is estimated that TB kills about 4000 people a day, while recent reports suggest that 1,552 people a day die due to COVID-19 [2] . This global pandemic is responsible for economic devastation and the most vulnerable are women, children, and those living with HIV/AIDS. Given TB burden continues to affect low- and middle-income countries disproportionately, effective vaccines would inherently also help to advance global health equity.
A novel prevention of infection vaccine, or vaccine regimen aimed to reduce disease could save countless lives globally but also significantly reduce morbidity, associated costs of treatment, and likely reduce emergence of drug resistance [3] , [4] , [5] . Mathematical modeling predicts that an effective vaccine deployed for adults and adolescents with partial efficacy would save up to 3 billion United States (US) dollars and reduce the need for at least 22 million drug treatment courses [6] . In addition to new infections or disease progression from latency, the emergence of drug resistance is a major problem for those suffering from this bacterial pathogen. The World Health Organization (WHO) estimates that in 2021, nearly half a million M.tb cases were rifampicin- or multidrug-resistant [1] . Excitingly, TB vaccines are among the most promising candidates for reducing antimicrobial resistance [7] . Given this exceptional promise, TB vaccines should receive heavy financial investments and be an international priority. According to the WHO COVID-19 Vaccine Tracker [8] , in just 3 years there were at least 50 approved vaccines against SARS-CoV-2, and a further 242 candidates were being evaluated across 821 vaccine trials [9] . Comparatively, there are a meager 13 TB vaccine candidates considered to be under active clinical development [10] . The speed and promise of RNA-based vaccines, however, may help to reenergize this lagging pipeline. One important consideration is the selection of TB antigens to include for RNA-based vaccines in development. Several vaccines in clinical trials utilize similar immunogenic antigens which have provided efficacy in preclinical studies and are immunogenic in humans [10] , [11] , [12] , [13] , [14] , [15] , [16] , [17] , [18] , [19] , [20] . For the ID93 polyprotein fusion vaccine antigen developed by our group, we assessed the ability of over 100 different M.tb antigens to induce interferon-γ from peripheral blood mononuclear cells of healthy, purified protein derivative (PPD) positive (antigens that have been used in tuberculin skin tests as a diagnostic for TB), or of healthy PPD negative people, as an initial screen for antigen discovery [ 21 , 22 ]. One key aspect of an RNA approach for vaccine delivery is the ability to induce cluster of differentiation (CD8+) T cells, which is often difficult using subunit vaccine approaches [23] . Some TB antigens have known CD8 epitopes that could be considered [24] . In addition, antigen selection may be different based on whether one is designing a prophylactic or a therapeutic vaccine. If the vaccine is geared toward a therapeutic vaccine, the inclusion of latency antigens in addition to other key antigens expressed as latency antigens may be of importance.
Self-replicating RNA vaccines (RNA replicons) derived from alphavirus vectors, such as Sindbis (SIN) virus, Semliki Forest virus, or Venezuelan equine encephalitis (VEE) virus vectors, emerged as an effective strategy for nucleic acid vaccine development more than 3 decades ago [25] . A good review of the mechanisms of alphavirus-based expression vectors can be found in the review by Frolov et al . [26] . The first report of the successful use of messenger RNA (mRNA) in animals was published in 1990 when mRNAs were injected into mice and protein production was detected [27] . These early promising results did not immediately lead to a considerable investment in developing mRNA vaccines and therapeutics, largely owing to concerns about mRNA instability, high engagement of innate immunogenicity, and inefficient in vivo delivery [28] . RNA vaccines are self-replicating and self-limiting and after adminsitration (or in transfected cells), they are then transcribed into RNA replicons allowing the antigen of interest to be expressed at high levels. RNA replicons can eventually cause lysis of transfected cells and lacking the enzymatic power does not raise the concern of integration into the host genome, unlike predecessor nucleic acid naked DNA vaccines. RNA vaccines were initially designed for cancer applications, targeted toward tumor antigens. Soon thereafter, an RNA construct expressing the M.tb MPT83 antigen was designed for use against M.tb, and was reported to elicit specific humoral and T-cell immune responses and induced modest but significant protection against M.tb challenge in mice. These investigators observed antigen-specific cytotoxicity to the M.tb MPT83 antigen in vitro and that SIN virus encoding M.tb MPT83 reduced M.tb H37Rv pulmonary burden at 4 weeks post-challenge in BALB/c mice [29] . In nearly 2 decades since, there is no literature suggesting the TB vaccine research field followed these findings and developed promising RNA-based vaccine candidates.
Like DNA vaccines, mRNA vaccines are simpler to construct, manufacture, and purify than protein-based subunit vaccines. Subunit vaccines require large-scale cell culture and complex purification schemes that are often protein-specific, while nucleic acid-based vaccines can be synthesized in a single cell-free reaction, and the purification scheme is often the same regardless of antigen sequence, allowing rapid, scalable, and cost-effective production. For example, a 5 l bioreactor can produce close to a million mRNA vaccine doses in a single reaction [30] . In addition, because the protein antigen of interest is eventually produced within host cells, it is more likely to be properly folded and have the requisite post-translational modifications than if it were manufactured in vitro as is the case of protein-based vaccines. An additional advantage is that a single mRNA vaccine can encode multiple antigens, thus inducing the immune response against multiple pathogenic stages of a single microbe or a variety of pathogens and enabling the targeting of multiple microbes with a single formulation. However, RNA vaccines have lagged behind DNA vaccines, given the hurdles to increasing the stability, improving efficacy, excessive immunostimulation, and potency of RNA-based technology [31] . Over the past decade, technological innovation and research investment have enabled mRNA to become a promising therapeutic tool in the fields of vaccine development and protein replacement therapy. Such innovations and optimizations included adding natural nucleoside modifications to mRNA-based vaccines to reduce direct immunogenicity, change coding sequences, and untranslated regions, modify nucleoside profiles, and increase translation of the target antigenic protein [ 28 , 32 ]. Formulations improving the stability of highly RNase-sensitive RNA-based vaccines have pushed this now viable platform to the forefront of development across pathogens. Even so, much remains to be discovered on the outcomes of mRNA vaccination, including efficacy, safety profiles in children versus adults or immunocompromised individuals, and how these recently implemented vaccine strategies compare with vector-based vaccines or more traditional protein-based vaccine strategies [33] , [34] , [35] .
RNA vaccines for tuberculosis
To our knowledge, RNA vaccine platforms have been minimally leveraged for TB vaccine development. Yet, the evidence for targeting multifaceted immune responses against M.tb for improved vaccine efficacy provides a good rationale for evaluating the RNA platform for TB [33] and other infectious diseases. Indeed, BioNTech (Mainz, Germany) recently announced plans to initiate a phase I clinical trial for the safety and immunogenicity testing of an mRNA TB vaccine candidate in collaboration with the Bill and Melinda Gates Foundation (ClinicalTrials.gov Identifier: NCT05547464 ). Building off of the technology in place for SARS-CoV-2, we applied the development of a VEE virus-based replicating RNA (repRNA) delivered in a highly stable nanostructured lipid carrier to target vaccines for Zika, nontuberculous mycobacteria, and TB [ 23 , 36 , 37 ]. This technology has since transitioned through current good manufacturing practices-compliant production of both the repRNA and an improved lipid inorganic nanoparticles (LION) carrier [38] ( Figure 1 ).
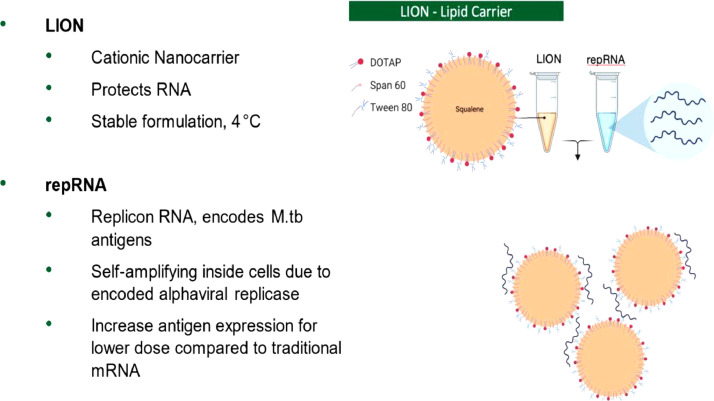
repRNA product description. Development of Venezuelan equine encephalitis virus-based repRNA vaccine encoding M.tb antigens of interest delivered by a LION.
LION, lipid inorganic nanoparticle; M.tb, Mycobacterium tuberculosis ; repRNA, replicating RNA.
In recent studies, we leveraged two delivery platforms as prophylactic vaccines to assess immunity and subsequent efficacy against low-dose and ultra-low-dose aerosol challenges with M.tb H37Rv in C57BL/6 mice. One of our second-generation TB vaccine candidates, ID91, was produced as a fusion protein formulated with a synthetic TLR4 agonist adjuvant (glucopyranosyl lipid adjuvant in a stable emulsion) or as a novel replicon RNA (repRNA) formulated in nanostructured lipid carrier [38] . Both protein subunit- and RNA-based vaccines were evaluated using a homologous TB vaccine prime/boost strategy as well as a heterologous strategy to complement the effects of a protein-adjuvant prophylactic vaccine candidate against an M.tb challenge in mice.
We found that both methods preferentially elicit cellular immune responses to different ID91 epitopes [23] . In prophylactic immunization evaluations, both platforms were found to reduce pulmonary bacterial burden compared to controls. In prime-boost strategies, groups that received heterologous RNA-prime, protein-boost, or combined RNA and protein immunizations demonstrated the greatest reduction in bacterial lung burden and a unique humoral and cellular immune response profile. These data are the first to report that repRNA platforms are a viable option for TB vaccines and should be pursued with high-priority M.tb antigens containing CD4+ and CD8+ T-cell epitopes.
Safety considerations for messenger RNA and a replicon RNA platform for tuberculosis vaccines
Increasing numbers of manuscripts are being published regarding the safety of the mRNA vaccines targeting SARS-CoV-2. Many of these studies suggest that the mRNA vaccines are safe; the most common side effects include local pain, fatigue, and muscle pain [39] . However, there are rare side effects due to mRNA vaccination in certain people, including those with comorbidities such as type 2 diabetes mellitus, where the frequency of adverse effects such as venous thromboembolism, Bell's palsy, pulmonary embolism, and thrombocytopenia can occur [40] . Rare myocarditis cases have also been documented in young men following SARS-CoV-2 mRNA vaccines [ 41 , 42 ]. Therefore, as with any TB candidate vaccine, carefully designed safety studies are needed to test this RNA vaccine platform, if it is to be used against M.tb, in animals that have been Bacille Calmette-Guerin vaccinated or previously infected with M.tb.
Advantages of repRNA vaccines versus mRNA learned from vaccines targeting SARS-CoV-2 are that repRNA typically require a 1000-fold less quantity than mRNA vaccines, can induce immune responses in non-human primates (NHPs) after a single immunization, induces T helper 1 (Th1)-biased T cells as well as antibodies, is effective in young and aged NHP, and has enhanced stability at 4˚C compared to mRNA vaccines [38] . In hamsters, next-generation SARS-CoV-2 repRNA vaccines can decrease the viral load in the lungs as well as protect against lung pathology following infection with SARS-CoV-2 compared to mock-treated hamsters [43] . A repRNA SARS-CoV-2 vaccine is now active in a phase I trial in the US (HDT-301; NCT05132907 ). This repRNA-CoV2S vaccine (HDT/Gennova COVID-19; HGC019) has also completed phase II/III in India and is now approved for emergency use there. This is the first repRNA approved for use in humans [44] . The key to next-generation RNA vaccines for other pathogens, such as the repRNA vaccines, will be the safe delivery and elicitation of both cellular and humoral immunity against intracellular pathogens, such as M.tb. In our own studies, we observed no adverse events in mice receiving an intramuscular repRNA TB candidate [ 23 , 45 ].
Vaccinations are responsible for saving approximately 9 million lives each year worldwide and eradicating at least one disease, smallpox, from the globe [46] . Tackling the issues faced by the vaccine field in controlling other complex infectious diseases such as TB, malaria, respiratory syncytial virus, and influenza will require significant investments in new technologies like RNA. Additionally, a more comprehensive understanding of the immune system is now beginning to be realized with advances in bioinformatics, systems biology which incorporate omics-level data (transcriptomics, metabolomics, epigenetics, cytokine profiles, and high-dimensional analysis of immune cell populations), machine learning, and genomics. As we unravel host complexities and design novel technologies there is a promise for less suffering from pathogens we have dealt with for centuries.
The global response to the COVID-19 pandemic highlights how billions of research dollars, innovation, and collaboration resulted, with stunning speed, in tremendous successes in the development of novel solutions to counter the SARS-CoV-2 virus, including antivirals, mRNA, and subunit vaccines, in addition to therapeutics. These technologies, if applied to TB, may lead to successful outcomes in our search not only for a better TB vaccine but in countermeasures targeting other intracellular bacterial pathogens of clinical importance.
Declaration of competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
Research reported here was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers R01AI125160, 75N93019C00072, and AI169207. This work was also supported by the Global Challenges Research Fund (GCRF) Networks in Vaccines Research and Development VALIDATE Network which was co-funded by the Medical Research Council (MRC) and Biotechnology and Biological Sciences Research Council (BBSRC) (ref MR/R005850/1). This UK-funded award is part of the European and Developing Countries Clinical Trails Partnership 2 (EDCTP2) program supported by the European Union. Sasha E. Larsen was supported through the University of Washington, Diseases of Public Health Importance training grant number T32A1075090. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, MRC, or BBSRC.
The authors would like to express their gratitude to Seattle Children's Research Institute Leadership for their guidance, compassion, and mentorship. Schematic images created with Biorender.com. The authors would also like to thank Dr. Jesse Erasmus (HDT Bio) for his help with Figure 1 .
Author contributions
SEL, SLB, and RNC designed experiments; RNC prepared figure 1; SEL, SLB and RNC drafted the manuscript; all authors reviewed the manuscript and contributed to edits.
Transparency declaration
This article is part of a supplement entitled Commemorating World Tuberculosis Day, March 24th, 2023: “Yes! We Can End TB” published with support from an unrestricted educational grant from QIAGEN Sciences Inc.
- 1. World Health Organization. Global TB report 2022. Geneva: World Health Organization, 2022.
- 2. Our World in Data. Daily confirmed COVID-19 deaths by world region, https://ourworldindata.org/grapher/daily-covid-deaths-region ; 2023 [accessed 19 January 2023].
- 3. Harris RC, Sumner T, Knight GM, White RG. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum Vaccin Immunother. 2016;12:2813–2832. doi: 10.1080/21645515.2016.1205769. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. White RG, Hanekom WA, Vekemans J, Harris RC. The way forward for tuberculosis vaccines. Lancet Respir Med. 2019;7:204–206. doi: 10.1016/S2213-2600(19)30040-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Harris RC, Sumner T, Knight GM, Zhang H, White RG. Potential impact of tuberculosis vaccines in China, South Africa, and India. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aax4607. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. World Health Organization An investment case for new tuberculosis vaccines. 2022 [ Google Scholar ]
- 7. Frost I, Sati H, Garcia-Vello P, Hasso-Agopsowicz M, Lienhardt C, Gigante V, Beyer P. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe. 2023;4:e113–e125. doi: 10.1016/S2666-5247(22)00303-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. World Health Organization . World Health Organization; Geneva: 2023. COVID-19 vaccine tracker and landscape. [ Google Scholar ]
- 9. COVID-19 vaccine tracker. Vaccines Candidates in Clinical Trials, https://covid19.trackvaccines.org/vaccines/ ; 2023 [accessed 13 March 2023].
- 10. Scriba TJ, Netea MG, Ginsberg AM. Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin Immunol. 2020;50 doi: 10.1016/j.smim.2020.101431. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Day TA, Penn-Nicholson A, Luabeya AKK, Fiore-Gartland A, Du Plessis N, Loxton AG, et al. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir Med. 2021;9:373–386. doi: 10.1016/S2213-2600(20)30319-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. npj Vaccines. 2018;3:34. doi: 10.1038/s41541-018-0057-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med. 2018;6:287–298. doi: 10.1016/S2213-2600(18)30077-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitie MA, Mettens P, et al. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013;31:2196–2206. doi: 10.1016/j.vaccine.2012.05.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, et al. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine. 2015;33:4025–4034. doi: 10.1016/j.vaccine.2015.05.088. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2018;379:1621–1634. doi: 10.1056/NEJMoa1803484. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72:6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A. 2009;106:2301–2306. doi: 10.1073/pnas.0712077106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24:130–143. doi: 10.1038/nm.4473. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Chin KL, Anibarro L, Sarmiento ME, Acosta A. Challenges and the way forward in diagnosis and treatment of tuberculosis infection. Trop Med Infect Dis. 2023;8:89. doi: 10.3390/tropicalmed8020089. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Larsen SE, Erasmus JH, Reese VA, Pecor T, Archer J, Kandahar A, et al. An RNA-based vaccine platform for use against Mycobacterium tuberculosis. Vaccines (Basel) 2023;11:130. doi: 10.3390/vaccines11010130. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Lewinsohn DM, Swarbrick GM, Cansler ME, Null MD, Rajaraman V, Frieder MM, et al. Human Mycobacterium tuberculosis CD8 T cell antigens/epitopes identified by a proteomic peptide library. PLoS One. 2013;8:e67016. doi: 10.1371/journal.pone.0067016. PE9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Rice CM, et al. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene. J Immunol. 2001;166:6218–6226. doi: 10.4049/jimmunol.166.10.6218. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Frolov I, Hoffman TA, Prágai BM, Dryga SA, Huang HV, Schlesinger S, et al. Alphavirus-based expression vectors: strategies and applications. Proc Natl Acad Sci U S A. 1996;93:11371–11377. doi: 10.1073/pnas.93.21.11371. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Xue T, Stavropoulos E, Yang M, Ragno S, Vordermeier M, Chambers M, et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun. 2004;72:6324–6329. doi: 10.1128/IAI.72.11.6324-6329.2004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Kis Z, Kontoravdi C, Dey AK, Shattock R, Shah N. Rapid development and deployment of high-volume vaccines for pandemic response. J Adv Manuf Process. 2020;2:e10060. doi: 10.1002/amp2.10060. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Cable J, Srikantiah P, Crowe JE, Jr, Pulendran B, Hill A, Ginsberg A, et al. Vaccine innovations for emerging infectious diseases-a symposium report. Ann N Y Acad Sci. 2020;1462:14–26. doi: 10.1111/nyas.14235. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Larsen SE, Williams BD, Rais M, Coler RN, Baldwin SL. It takes a village: the multifaceted immune response to Mycobacterium tuberculosis infection and vaccine-induced immunity. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.840225. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Tian Y, Chen L, Shi Y. Safety, efficacy, and immunogenicity of varying types of COVID-19 vaccines in children younger than 18 years: an update of systematic review and meta-analysis. Vaccines (Basel) 2022;11:87. doi: 10.3390/vaccines11010087. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Durand J, Dogné JM, Cohet C, Browne K, Gordillo-Marañón M, Piccolo L, et al. Safety monitoring of COVID-19 vaccines: perspective from the European Medicines Agency. Clin Pharmacol Ther. 2022 doi: 10.1002/cpt.2828. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Erasmus JH, Khandhar AP, Guderian J, Granger B, Archer J, Archer M, et al. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol Ther. 2018;26:2507–2522. doi: 10.1016/j.ymthe.2018.07.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Larsen SE, Reese VA, Pecor T, Berube BJ, Cooper SK, Brewer G, et al. Subunit vaccine protects against a clinical isolate of Mycobacterium avium in wild type and immunocompromised mouse models. Sci Rep. 2021;11:9040. doi: 10.1038/s41598-021-88291-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Erasmus JH, Khandhar AP, O'Connor MA, Walls AC, Hemann EA, Murapa P, et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abc9396. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Rahmani A, Dini G, Montecucco A, Orsi A, Sticchi L, Domnich A, et al. Adverse reactions after the third dose of the BNT162b2 mRNA COVID-19 vaccine among medical school residents in a regional reference university hospital in Italy. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10111779. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Kim HJ, Lee SJ, Sa S, Bae JH, Song G, Lee CW, et al. Safety of COVID-19 vaccines among patients with type 2 diabetes mellitus: real-world data analysis. Diabetes Metab J. 2023 doi: 10.4093/dmj.2022.0129. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Marschner CA, Shaw KE, Tijmes FS, Fronza M, Khullar S, Seidman MA, et al. Myocarditis following COVID-19 vaccination. Heart Fail Clin. 2023;19:251–264. doi: 10.1016/j.hfc.2022.08.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Hawman DW, Meade-White K, Archer J, Leventhal SS, Wilson D, Shaia C, et al. SARS-CoV-2 variant-specific replicating RNA vaccines protect from disease following challenge with heterologous variants of concern. eLife. 2022;11:e75537. doi: 10.7554/eLife.75537. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Oâ Connor MA, Hawman DW, Meade-White K, Leventhal S, Song W, Randall S, et al. A replicon RNA vaccine induces durable protective immunity from SARS-CoV-2 in nonhuman primates after neutralizing antibodies have waned. bioRxiv, https://www.biorxiv.org/content/10.1101/2022.08.08.503239v1 ; 09 August 2022 [accessed 13 March 2023]. [ DOI ] [ PMC free article ] [ PubMed ]
- 45. Rais M, Abdelaal H, Reese VA, Ferede D, Larsen SE, Pecor T, et al. Immunogenicity and protection against Mycobacterium avium with a heterologous RNA prime and protein boost vaccine regimen. Tuberculosis (Edinb) 2023;138 doi: 10.1016/j.tube.2022.102302. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. United Nations Children's Fund (UNICEF). Vaccines bring 7 diseases under control, https://www.unicef.org/pon96/hevaccin.htm ; 2019 [accessed 24 October 2019].
- View on publisher site
- PDF (599.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO