Registered Nurse RN
Registered Nurse, Free Care Plans, Free NCLEX Review, Nurse Salary, and much more. Join the nursing revolution.

Next Generation NCLEX Case Study Sample Questions
One of the big changes on the Next Generation NCLEX exam is a shift toward case studies. Case studies often require a deeper level of critical thinking, and understanding diseases on a more in-depth level (especially the pathophysiology) will make these types of questions easier to answer.
In this article, you’ll be able to watch a free video to help you prepare for the new Next Generation NCLEX case study format. Nurse Sarah will walk you step-by-step through each scenario and help you understand how to use critical thinking and nursing knowledge to answer these types of questions.
Next Generation NCLEX Case Study Review Questions Video
NGN Case Study Sample Questions and Answers
First, let’s take a look at our case study summary below:
Case Study Summary:
A 68-year-old male is admitted with shortness of breath. He reports difficulty breathing with activity, lying down, or while sleeping. He states that in order to “breathe easier,” he has had to sleep in a recliner for the past week. The patient has a history of hypertension, myocardial infarction (2 years ago), and cholecystectomy (10 years ago). The patient is being transferred to a cardiac progressive care unit for further evaluation and treatment.
Question 1 of 6: The nurse receives the patient admitted with shortness of breath. What findings are significant and require follow-up? The options are listed below. Select all that apply.
To answer this first question in the NGN case study, let’s look at the information provided in the nursing notes and vital signs tabs provided:

This question is asking us to identify findings that are significant and require the nurse to follow-up. In other words, what is presenting that we can’t ignore but need to investigate further.
Therefore, let’s comb through the nursing notes and vital signs to see what is abnormal and requires follow-up.
First, the patient arrived to the room via stretcher. That’s fine and doesn’t necessarily require follow-up.
Next, the patient is alert and oriented x 4 (person, place, time, event). This tells us that the patient’s neuro status is intact so far. Therefore, the shortness of breath isn’t affecting the patient’s mental function yet (we have enough oxygen on board right now for brain activity).
However, the nurse has noticed the shortness of breath with activity and talking, which should not normally happen. This tells us something is wrong and is significant enough to require follow-up. We want to know why is this happening, is it going to get worse, etc.
The patient’s weight and vital signs were collected (this is good). Weight is 155 lbs. and BMI is within a healthy range (doesn’t tell us too much but may be useful later). The patient is also connected to a bedside monitor, so they need to be monitored constantly like on a progressive care unit.
The monitor shows sinus tachycardia . This is significant because it seems the patient’s shortness of breath is causing the heart to compensate by increasing the heart rate to provide more oxygen (hence the lungs may be compromised).
Then we find out that the lungs are indeed compromised because crackles are heard in both lungs , and this may be why our patient is short of breath. This is significant (could the patient have pulmonary edema?)
Then we find out the nurse has noted an S3. This is an extra heart sound noted after S2. And what jumps out to me about this is that it is usually associated with volume overload in the heart like in cases of heart failure . However, S3 may be normal in some people under 40 or during pregnancy, but that’s not the case with our patient based on what we read in the case summary.
Therefore, based on everything I’m reading in this case study, I’m thinking this patient may have heart failure, but we need those test results back (especially the echo and chest x-ray, and hopefully a BNP will be in there too).
We are also told that the patient has an 18 gauge IV inserted (which is good thing to have so we can give medications if required), orders have been received, labs drawn, and testing results are pending.

Now let’s look at the “Vital Signs” tab above, and ask yourself what is normal vs. abnormal for this patient (adult male).
- The heart rate is high at 112 (tachycardia), and should normally be 60-100 bpm (see heart rhythms ).
- Blood pressure is higher than normal (normal is 120/80), which indicates hypertension.
- Oxygen saturation is 94% (this is on the low side as we’d normally want around 95% or higher, and the patient is on 4 L nasal cannula, which tells us the lungs are not okay).
- Respiratory rate is increased (26 breaths per minute)…normal is 12-20 breaths per minute.
Based on the information we were provided, I’ve selected the answers below. These findings are significant and definitely require follow-up by the nurse.

When answering these NGN case study questions, it’s helpful to think of the ABCDE (airway, breathing, circulation, etc.) as all of these fall into that category. If we don’t follow-up on the shortness of breath, crackles, respiratory rate, o2 saturation (94% on 4 L nasal cannula), the respiratory system can further decline.
In addition, the sinus tachycardia, S3 gallop, and hypertension could indicate fluid overload in the heart. This may cause the heart to tire out and lead the lethal rhythm. On the other hand, temperature, pain, weight, and BMI are not abnormal and do not require follow-up.
See the Complete Next Generation NCLEX Case Study Review
Each question in the case study builds on the previous question. To see how these questions evolve based on the patient’s condition and labs, watch the entire Next Generation NCLEX Case Study Review video on our YouTube Channel (RegisteredNurseRN).
NCLEX Practice Quizzes
We’ve developed many free NCLEX review quizzes to test your knowledge on nursing topics and to help you prepare for the Next Generation NCLEX exam.
Nurse Sarah’s Notes and Merch

Just released is “ Fluid and Electrolytes Notes, Mnemonics, and Quizzes by Nurse Sarah “. These notes contain 84 pages of Nurse Sarah’s illustrated, fun notes with mnemonics, worksheets, and 130 test questions with rationales.
You can get an eBook version here or a physical copy of the book here.
Please Share:
- Click to print (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to share on Pocket (Opens in new window)
- Click to share on Telegram (Opens in new window)
Disclosure and Privacy Policy
Important links, follow us on social media.
- Facebook Nursing
- Instagram Nursing
- TikTok Nurse
- Twitter Nursing
- YouTube Nursing
Copyright Notice
- Nursing Career Guide
- Premium Login
- Pass the first time, guaranteed
- Help Center
Your Progress
- 0 Incorrect
- Back to all tests
- Font size Click here to set to default
- 0 Correct
- 0 Incorrect
- 0 Skipped
Next Generation NCLEX: Case Studies 1-2
- 2,500+ NCLEX-Like Questions (including the ones most people fail)
- 80 Realistic Practice Tests & Marathons
- 300+ SATA Questions
- 300+ NCLEX Flashcards
- Unlimited NCLEX Simulations
NCLEX-RN Fact Sheet
Nursing career guide: how to become a nurse.
Expore resources about studying for your NCLEX and becoming a nurse.
Get the NCLEX Genie App
Download our free iOS or Android app to prepare for your NCLEX exam offline or on the go.
More NCLEX-RN Practice Tests
- Basic Care and Comfort 1
- Basic Care and Comfort 2
- Basic Care & Comfort Marathon
- Pharmacological & Parenteral Therapies 1
- Pharmacological & Parenteral Therapies 2
- Pharmacological & Parenteral Therapies 3
- Pharmacological & Parenteral Therapies 4
- Drug Dosage Calculation
- Pharmacological & Parenteral Marathon
- Reduction of Risk Potential 1
- Reduction of Risk Potential 2
- Reduction of Risk Potential 3
- Reduction of Risk Potential 4
- Reduction of Risk Potential Marathon
- Physiological Adaptation 1
- Physiological Adaptation 2
- Physiological Adaptation 3
- Physiological Adaptation 4
- Physiological Adaptation Marathon
- Psychosocial Integrity Test 1
- Psychosocial Integrity Test 2
- Psychosocial Integrity Test 3
- Psychosocial Integrity Test 4
- Psychosocial Integrity Test 5
- Psychosocial Marathon
- Health Promotion and Maintenance 1
- Health Promotion and Maintenance 2
- Health Promotion and Maintenance 3
- Health Promotion and Maintenance 4
- Health Promotion and Maintenance 5
- Health Promotion Marathon
- Management of Care 1
- Management of Care 2
- Management of Care 3
- Management of Care Marathon
- Safety & Infection Control 1
- Safety & Infection Control 2
- Safety & Infection Control 3
- Safety & Infection Control Marathon
- NCLEX Exam Simulator

Breaking Down IV Fluids: The 4 Most Common Types and Their Uses
How Much Do Nurses Make?

How to Pass the NCLEX the First Time
- Skip to content

State Resources
National Resources
Nursing Organizations
- MNWC Initiatives
Maryland Nursing Workforce Center
- NextGen NCLEX
NextGen NCLEX Test Bank
The purpose of this project was to develop a repository of nextgen nclex questions that can be accessed by all faculty and students in maryland..
The questions can be used by faculty to prepare students to understand the new format of Next Generation (NextGen) test items that are like those that will be used by the National Council of State Boards of Nursing (NCSBN) licensing exam beginning in April 2023 to test students’ ability to make clinical judgments.
Disclaimer: The items in the test bank are accessible to all through this nonsecure website. The test questions are not recommended to be used for summative assessments.
The test bank is composed of case studies with six questions each that follow the NCSBN Clinical Judgment Measurement Model steps:
- recognize cues
- analyze cues
- prioritize hypotheses
- generate solutions
- take action
- evaluate outcomes.
In addition, seven questions for reviewing bow-tie or trend items are included. All case studies were subjected to rigorous review both by the project team and subject matter experts.
The names of the case studies are provided with hyperlinks to all items.
- Using the Maryland NextGen Test Bank
- Student Instructions for Using the Test Bank
NCLEX Next Gen Test Bank
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Case study: 60-year-old female presenting with shortness of breath.
Deepa Rawat ; Sandeep Sharma .
Affiliations
Last Update: February 20, 2023 .
- Case Presentation
The patient is a 60-year-old white female presenting to the emergency department with acute onset shortness of breath. Symptoms began approximately 2 days before and had progressively worsened with no associated, aggravating, or relieving factors noted. She had similar symptoms approximately 1 year ago with an acute, chronic obstructive pulmonary disease (COPD) exacerbation requiring hospitalization. She uses BiPAP ventilatory support at night when sleeping and has requested to use this in the emergency department due to shortness of breath and wanting to sleep.
She denies fever, chills, cough, wheezing, sputum production, chest pain, palpitations, pressure, abdominal pain, abdominal distension, nausea, vomiting, and diarrhea.
She reports difficulty breathing at rest, forgetfulness, mild fatigue, feeling chilled, requiring blankets, increased urinary frequency, incontinence, and swelling in her bilateral lower extremities that are new-onset and worsening. Subsequently, she has not ambulated from bed for several days except to use the restroom due to feeling weak, fatigued, and short of breath.
There are no known ill contacts at home. Her family history includes significant heart disease and prostate malignancy in her father. Social history is positive for smoking tobacco use at 30 pack years. She quit smoking 2 years ago due to increasing shortness of breath. She denies all alcohol and illegal drug use. There are no known foods, drugs, or environmental allergies.
Past medical history is significant for coronary artery disease, myocardial infarction, COPD, hypertension, hyperlipidemia, hypothyroidism, diabetes mellitus, peripheral vascular disease, tobacco usage, and obesity. Past surgical history is significant for an appendectomy, cardiac catheterization with stent placement, hysterectomy, and nephrectomy.
Her current medications include fluticasone-vilanterol 100-25 mcg inhaled daily, hydralazine 50 mg by mouth, 3 times per day, hydrochlorothiazide 25 mg by mouth daily, albuterol-ipratropium inhaled every 4 hours PRN, levothyroxine 175 mcg by mouth daily, metformin 500 mg by mouth twice per day, nebivolol 5 mg by mouth daily, aspirin 81 mg by mouth daily, vitamin D3 1000 units by mouth daily, clopidogrel 75 mg by mouth daily, isosorbide mononitrate 60 mg by mouth daily, and rosuvastatin 40 mg by mouth daily.
Physical Exam
Initial physical exam reveals temperature 97.3 F, heart rate 74 bpm, respiratory rate 24, BP 104/54, HT 160 cm, WT 100 kg, BMI 39.1, and O2 saturation 90% on room air.
Constitutional: Extremely obese, acutely ill-appearing female. Well-developed and well-nourished with BiPAP in place. Lying on a hospital stretcher under 3 blankets.
HEENT:
- Head: Normocephalic and atraumatic
- Mouth: Moist mucous membranes
- Macroglossia
- Eyes: Conjunctiva and EOM are normal. Pupils are equal, round, and reactive to light. No scleral icterus. Bilateral periorbital edema present.
- Neck: Neck supple. No JVD present. No masses or surgical scarring.
- Throat: Patent and moist
Cardiovascular: Normal rate, regular rhythm, and normal heart sound with no murmur. 2+ pitting edema bilateral lower extremities and strong pulses in all four extremities.
Pulmonary/Chest: No respiratory status distress at this time, tachypnea present, (+) wheezing noted, bilateral rhonchi, decreased air movement bilaterally. The patient was barely able to finish a full sentence due to shortness of breath.
Abdominal: Soft. Obese. Bowel sounds are normal. No distension and no tenderness
Skin: Skin is very dry
Neurologic: Alert, awake, able to protect her airway. Moving all extremities. No sensation losses
- Initial Evaluation
Initial evaluation to elucidate the source of dyspnea was performed and included CBC to establish if an infectious or anemic source was present, CMP to review electrolyte balance and review renal function, and arterial blood gas to determine the PO2 for hypoxia and any major acid-base derangement, creatinine kinase and troponin I to evaluate the presence of myocardial infarct or rhabdomyolysis, brain natriuretic peptide, ECG, and chest x-ray. Considering that it is winter and influenza is endemic in the community, a rapid influenza assay was obtained as well.
Largely unremarkable and non-contributory to establish a diagnosis.
Showed creatinine elevation above baseline from 1.08 base to 1.81, indicating possible acute injury. EGFR at 28 is consistent with chronic renal disease. Calcium was elevated to 10.2. However, when corrected for albumin, this corrected to 9.8 mg/dL. Mild transaminitis is present as seen in alkaline phosphatase, AST, and ALT measurements which could be due to liver congestion from volume overload.
Initial arterial blood gas with pH 7.491, PCO2 27.6, PO2 53.6, HCO3 20.6, and oxygen saturation 90% on room air, indicating respiratory alkalosis with hypoxic respiratory features.
Creatinine kinase was elevated along with serial elevated troponin I studies. In the setting of her known chronic renal failure and acute injury indicated by the above creatinine value, a differential of rhabdomyolysis is determined.
Influenza A and B: Negative
Normal sinus rhythm with non-specific ST changes in inferior leads. Decreased voltage in leads I, III, aVR, aVL, aVF.
Chest X-ray
Findings: Bibasilar airspace disease that may represent alveolar edema. Cardiomegaly noted. Prominent interstitial markings were noted. Small bilateral pleural effusions
Radiologist Impression: Radiographic changes of congestive failure with bilateral pleural effusions greater on the left compared to the right
- Differential Diagnosis
- Acute on chronic COPD exacerbation
- Acute on chronic renal failure
- Bacterial pneumonia
- Congestive heart failure
- Pericardial effusion
- Hypothyroidism
- Influenza pneumonia
- Pulmonary edema
- Pulmonary embolism
- Confirmatory Evaluation
On the second day of the admission patient’s shortness of breath was not improved, and she was more confused with difficulty arousing on conversation and examination. To further elucidate the etiology of her shortness of breath and confusion, the patient's husband provided further history. He revealed that she is poorly compliant with taking her medications. He reports that she “doesn’t see the need to take so many pills.”
Testing was performed to include TSH, free T4, BNP, repeated arterial blood gas, CT scan of the chest, and echocardiogram. TSH and free T4 evaluate hypothyroidism. BNP evaluates fluid load status and possible congestive heart failure. CT scan of the chest will look for anatomical abnormalities. An echocardiogram is used to evaluate left ventricular ejection fraction, right ventricular function, pulmonary artery pressure, valvular function, pericardial effusion, and any hypokinetic area.
- TSH: 112.717 (H)
- Free T4: 0.56 (L)
- TSH and Free T4 values indicate severe primary hypothyroidism.
BNP can be falsely low in obese patients due to the increased surface area. Additionally, adipose tissue has BNP receptors which augment the true BNP value. Also, African American patients with more excretion may have falsely low values secondary to greater excretion of BNP. This test is not that helpful in renal failure due to the chronic nature of fluid overload. This allows for desensitization of the cardiac tissues with a subsequent decrease in BNP release.
Repeat arterial blood gas on BiPAP ventilation shows pH 7.397, PCO2 35.3, PO2 72.4, HCO3 21.2, and oxygen saturation 90% on 2 L supplemental oxygen.
CT chest without contrast was primarily obtained to evaluate the left hemithorax, especially the retrocardiac area.
Radiologist Impression: Tiny bilateral pleural effusions. Pericardial effusion. Coronary artery calcification. Some left lung base atelectasis with minimal airspace disease.
Echocardiogram
The left ventricular systolic function is normal. The left ventricular cavity is borderline dilated.
The pericardial fluid is collected primarily posteriorly, laterally but not apically. There appeared to be a subtle, early hemodynamic effect of the pericardial fluid on the right-sided chambers by way of an early diastolic collapse of the RA/RV and delayed RV expansion until late diastole. A dedicated tamponade study was not performed.
The estimated ejection fraction appears to be in the range of 66% to 70%. The left ventricular cavity is borderline dilated.
The aortic valve is abnormal in structure and exhibits sclerosis.
The mitral valve is abnormal in structure. Mild mitral annular calcification is present. There is bilateral thickening present. Trace mitral valve regurgitation is present.
- Myxedema coma or severe hypothyroidism
- Pericardial effusion secondary to myxedema coma
- COPD exacerbation
- Acute on chronic hypoxic respiratory failure
- Acute respiratory alkalosis
- Bilateral community-acquired pneumonia
- Small bilateral pleural effusions
- Acute mild rhabdomyolysis
- Acute chronic, stage IV, renal failure
- Elevated troponin I levels, likely secondary to Renal failure
- Diabetes mellitus type 2, non-insulin-dependent
- Extreme obesity
- Hepatic dysfunction
The patient was extremely ill and rapidly decompensating with multisystem organ failure, including respiratory failure, altered mental status, acute on chronic renal failure, and cardiac dysfunction. The primary concerns for the stability of the patient revolved around respiratory failure coupled with altered mental status. In the intensive care unit (ICU), she rapidly began to fail BiPAP therapy. Subsequently, the patient was emergently intubated in the ICU. A systemic review of therapies and hospital course is as follows:
Considering the primary diagnosis of myxedema coma, early supplementation with thyroid hormone is essential. Healthcare providers followed the American Thyroid Association recommendations, which recommend giving combined T3 and T4 supplementation; however, T4 alone may also be used. T3 therapy is given as a bolus of 5 to 20 micrograms intravenously and continued at 2.5 to 10 micrograms every 8 hours. An intravenous loading dose of 300 to 600 micrograms of T4 is followed by a daily intravenous dose of 50 to 100 micrograms. Repeated monitoring of TSH and T4 should be performed every 1 to 2 days to evaluate the effect and to titrate the dose of medication. The goal is to improve mental function. Until coexistent adrenal insufficiency is ruled out using a random serum cortisol measurement, 50 to 100 mg every 8 hours of hydrocortisone should be administered. In this case, clinicians used hydrocortisone 100 mg IV every 8 hours. Dexamethasone 2 to 4 mg every 12 hours is an alternative therapy.
The patient’s mental status rapidly worsened despite therapy. In the setting of her hypothyroidism history, this may be myxedema coma or due to the involvement of another organ system. The thyroid supplementation medications and hydrocortisone were continued. A CT head without contrast was normal.
Respiratory
For worsening metabolic acidosis and airway protection, the patient was emergently intubated. Her airway was deemed high risk due to having a large tongue, short neck, and extreme obesity. As the patient’s heart was preload dependent secondary to pericardial effusion, a 1-liter normal saline bolus was started. Norepinephrine was started at a low dose for vasopressor support, and ketamine with low dose Propofol was used for sedation. Ketamine is a sympathomimetic medication and usually does not cause hypotension as all other sedatives do. The patient was ventilated with AC mode of ventilation, tidal volume of 6 ml/kg ideal body weight, flow 70, initial fio2 100 %, rate 26 per minute (to compensate for metabolic acidosis), PEEP of 8.
Cardiovascular
She was determined to be hemodynamically stable with a pericardial effusion. This patient’s cardiac dysfunction was diastolic in nature, as suggested by an ejection fraction of 66% to 70%. The finding of posterior pericardial effusion further supported this conclusion. The posterior nature of this effusion was not amenable to pericardiocentesis. As such, this patient was preload dependent and showed signs of hypotension. The need for crystalloid fluid resuscitation was balanced against the impact increased intravascular volume would have on congestive heart failure and fluid overload status. Thyroid hormone replacement as above should improve hypotension. However, vasopressor agents may be used to maintain vital organ perfusion targeting a mean arterial pressure of greater than 65 mm Hg as needed. BP improved after fluid bolus, and eventually, the norepinephrine was stopped. Serial echocardiograms were obtained to ensure that the patient did not develop tamponade physiology. Total CK was elevated, which was likely due to Hypothyroidism compounded with chronic renal disease.
Infectious Disease
Blood cultures, urine analysis, and sputum cultures were obtained. The patient's white blood cell count was normal. This is likely secondary to her being immunocompromised due to hypothyroidism and diabetes. In part, the pulmonary findings of diffuse edema and bilateral pleural effusions can be explained by cardiac dysfunction. Thoracentesis of pleural fluid was attempted, and the fluid was analyzed for cytology and gram staining to rule out infectious or malignant causes as both a therapeutic and diagnostic measure. Until these results return, broad-spectrum antibiotics are indicated and may be discontinued once the infection is ruled out completely.
Gastrointestinal
Nasogastric tube feedings were started on the patient after intubation. She tolerated feedings well. AST and ALT were mildly elevated, which was thought to be due to hypothyroidism, and as the TSH and free T4 improved, her AST and ALT improved. Eventually, these values became normal once her TSH level was close to 50.
Her baseline creatinine was found to be close to 1.08 in prior medical records. She presented with a creatinine of 1.8 in the emergency department. Since hypothyroidism causes fluid retention in part because thyroid hormone encourages excretion of free water and partly due to decreased lymphatic function in returning fluid to vascular circulation. Aggressive diuresis was attempted. As a result, her creatinine increased initially but improved on repeated evaluation, and the patient had a new baseline creatinine of 1.6. Overall she had a net change in the fluid status of 10 liters negative by her ten days of admission in the ICU.
Mildly anemic otherwise, WBC and platelet counts were normal. Electrolyte balance should be monitored closely, paying attention to sodium, potassium, chloride, and calcium specifically as these are worsened in both renal failure and myxedema.
Daily sedation vacations were enacted, and the patient's mental status improved and was much better when TSH was around 20. The bilateral pleural effusions improved with aggressive diuresis. Breathing trials were initiated when the patient's fio2 requirements decreased to 60% and a PEEP of 8. She was eventually extubated onto BiPAP and then high-flow nasal cannula while off of BiPAP. Pericardial fluid remained stable, and no cardiac tamponade pathology developed. As a result, it was determined that a pericardial window was unnecessary. Furthermore, she was not a candidate for pericardiocentesis as the pericardial effusion was located posterior to the heart. Her renal failure improved with improved cardiac function, diuretics, and thyroid hormone replacement.
After extubation patient had speech and swallow evaluations and was able to resume an oral diet. The patient was eventually transferred out of the ICU to the general medical floor and eventually to a rehabilitation unit.
Despite the name myxedema coma, most patients will not present in a coma status. This illness is at its core a severe hypothyroidism crisis that leads to systemic multiorgan failure. Thyroid hormones T3, and to a lesser extent, T4 act directly on a cellular level to upregulate all metabolic processes in the body. Therefore, deficiency of this hormone is characterized by systemic decreased metabolism and decreased glucose utilization along with increased production and storage of osmotically active mucopolysaccharide protein complexes into peripheral tissues resulting in diffuse edema and swelling of tissue. [1]
Myxedema coma is an illness that occurs primarily in females at a rate of 4:1 compared to men. It typically impacts the elderly at the age of greater than 60 years old, and approximately 90% of cases occur during the winter months. Myxedema coma is the product of longstanding unidentified or undertreated hypothyroidism of any etiology. Thyroid hormone is necessary throughout the body and acts as a regulatory hormone that affects many organ systems. [2] In cardiac tissues, myxedema coma manifests as decreased contractility with subsequent reduction in stroke volume and overall cardiac output. Bradycardia and hypotension are typically present also. Pericardial effusions occur due to the accumulation of mucopolysaccharides in the pericardial sac, which leads to worsened cardiac function and congestive heart failure from diastolic dysfunction. Capillary permeability is also increased throughout the body leading to worsened edema. Electrocardiogram findings may include bradycardia and low-voltage, non-specific ST waveform changes with possible inverted T waves.
Neurologic tissues are impacted in myxedema coma leading to the pathognomonic altered mental status resulting from hypoxia and decreased cerebral blood flow secondary to cardiac dysfunction as above. Additionally, hypothyroidism leads to decreased glucose uptake and utilization in neurological tissue, thus worsening cognitive function.
The pulmonary system typically manifests this disease process through hypoventilation secondary to the central nervous system (CNS) depression of the respiratory drive with blunting of the response to hypoxia and hypercapnia. Additionally, metabolic dysfunction in the muscles of respiration leads to respiratory fatigue and failure, macroglossia from mucopolysaccharide driven edema of the tongue leads to mechanical obstruction of the airway, and obesity hypoventilation syndrome with the decreased respiratory drive as most hypothyroid patients suffer from obesity.
Renal manifestations include decreased glomerular filtration rate from the reduced cardiac output and increased systemic vascular resistance coupled with acute rhabdomyolysis lead to acute kidney injury. In the case of our patient above who has a pre-existing renal disease status post-nephrectomy, this is further worsened. The net effect is worsened fluid overload status compounding the cardiac dysfunction and edema. [3]
The gastrointestinal tract is marked by mucopolysaccharide-driven edema as well leading to malabsorption of nutrients, gastric ileus, and decreased peristalsis. Ascites is common because of increased capillary permeability in the intestines coupled with coexistent congestive heart failure and congestive hepatic failure. Coagulopathies are common to occur as a result of this hepatic dysfunction.
Evaluation: The diagnosis of myxedema coma, as with all other diseases, is heavily reliant on the history and physical exam. A past medical history including hypothyroidism is highly significant whenever decreased mental status or coma is identified. In the absence of identified hypothyroidism, myxedema coma is a diagnosis of exclusion when all other sources of coma have been ruled out. If myxedema coma is suspected, evaluation of thyroid-stimulating hormone (TSH), free thyroxine (T4), and serum cortisol is warranted. T4 will be extremely low. TSH is variable depending on the etiology of hypothyroidism, with a high TSH indicating primary hypothyroidism and a low or normal TSH indicating secondary etiologies. Cortisol may be low indicating adrenal insufficiency because of hypothyroidism. [4]
Prognosis: Myxedema coma is a medical emergency. With proper and rapid diagnosis and initiation of therapy, the mortality rate is still as high as 25% to 50%. The most common cause of death is due to respiratory failure. The factors which suggest a poorer prognosis include increased age, persistent hypothermia, bradycardia, low score Glasgow Coma Scale, or multi-organ impairment indicated by high APACHE (Acute Physiology and Chronic Health Evaluation) II score. For these reasons, placement in an intensive care unit with a low threshold for intubation and mechanical ventilation can improve mortality outcomes. [3] [5]
- Pearls of Wisdom
- Not every case of shortness of breath is COPD or congestive heart failure (CHF). While less likely, a history of hypothyroidism should raise suspicion of myxedema coma in a patient with any cognitive changes.
- Myxedema is the great imitator illness that impacts all organ systems. It can easily be mistaken for congestive heart failure, COPD exacerbation, pneumonia, renal injury or failure, or neurological insult.
- Initial steps in therapy include aggressive airway management, thyroid hormone replacement, glucocorticoid therapy, and supportive measures.
- These patients should be monitored in an intensive care environment with continuous telemetry. [6]
- Enhancing Healthcare Team Outcomes
This case demonstrates how all interprofessional healthcare team members need to be involved in arriving at a correct diagnosis, particularly in more challenging cases such as this one. Clinicians, specialists, nurses, pharmacists, laboratory technicians all bear responsibility for carrying out the duties pertaining to their particular discipline and sharing any findings with all team members. An incorrect diagnosis will almost inevitably lead to incorrect treatment, so coordinated activity, open communication, and empowerment to voice concerns are all part of the dynamic that needs to drive such cases so patients will attain the best possible outcomes.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Case Study of 60 year old female presenting with Shortness of Breath Contributed by Sandeep Sharma, MD
Disclosure: Deepa Rawat declares no relevant financial relationships with ineligible companies.
Disclosure: Sandeep Sharma declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Rawat D, Sharma S. Case Study: 60-Year-Old Female Presenting With Shortness of Breath. [Updated 2023 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PubMed Links to PubMed
Similar articles in PubMed
- Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough. [StatPearls. 2024] Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough. Sharma S, Hashmi MF, Rawat D. StatPearls. 2024 Jan
- Acute Exacerbation of COPD. [J Educ Teach Emerg Med. 2023] Acute Exacerbation of COPD. Pappas D, Vempati A. J Educ Teach Emerg Med. 2023 Apr; 8(2):S35-S61. Epub 2023 Apr 30.
- Review Breathlessness with pulmonary metastases: a multimodal approach. [J Adv Pract Oncol. 2013] Review Breathlessness with pulmonary metastases: a multimodal approach. Brant JM. J Adv Pract Oncol. 2013 Nov; 4(6):415-22.
- Stress Cardiomyopathy in the Setting of COPD Exacerbation. [J Investig Med High Impact Cas...] Stress Cardiomyopathy in the Setting of COPD Exacerbation. Landefeld K, Saleh Q, Sander GE. J Investig Med High Impact Case Rep. 2015 Oct-Dec; 3(4):2324709615612847. Epub 2015 Oct 14.
- Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. [J Adv Pract Oncol. 2017] Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. Doverspike L, Kurtz S, Selvaggi K. J Adv Pract Oncol. 2017 May-Jun; 8(4):382-386. Epub 2017 May 1.
Recent Activity
- Case Study: 60-Year-Old Female Presenting With Shortness of Breath - StatPearls Case Study: 60-Year-Old Female Presenting With Shortness of Breath - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Open access
- Published: 10 April 2024
Phenotypes and outcome of diffuse pulmonary non-amyloid light chain deposition disease
- François Lestelle 1 ,
- Catherine Beigelman 2 ,
- David Rotzinger 2 ,
- Salim Si-Mohamed 3 ,
- Mouhamad Nasser 1 ,
- Lidwine Wemeau 4 ,
- Sandrine Hirschi 5 ,
- Grégoire Prevot 6 ,
- Antoine Roux 7 ,
- Vincent Bunel 8 ,
- Emmanuel Gomez 9 ,
- Laurent Sohier 10 ,
- Helene Morisse Pradier 11 ,
- Martine Reynaud Gaubert 12 ,
- Anne Gondouin 13 ,
- Romain Lazor 14 ,
- Jean-Charles Glerant 15 ,
- Françoise Thivolet Bejui 16 ,
- Magali Colombat 17 ,
- Vincent Cottin 1 , 18 &
the OrphaLung network
Respiratory Research volume 25 , Article number: 159 ( 2024 ) Cite this article
238 Accesses
18 Altmetric
Metrics details
Light chain deposition disease (LCDD) is a very rare entity. Clinical manifestations of LCDD vary according to the organs involved. Data on pulmonary LCDD are scarce and limited to small series or case reports. This study aimed to describe the characteristics and outcome of diffuse pulmonary non-amyloid LCDD localized to the lungs.
Study design and methods
A multicenter retrospective cohort study was conducted. Clinical characteristics were collected, and chest CTs were centrally reviewed. The diagnosis of pulmonary non-amyloid LCDD was confirmed by immunohistochemistry.
Thirty-one cases were identified (68% female), with a median age at diagnosis of 50 years (IQR 20). Baseline FEV1/FVC was < 0.70 in 45% of patients. Mean ( ± SD) FEV1 and DLCO were 86% ± 26.2 and 52% ± 23.9, respectively. CT revealed peculiar patterns of thin-walled cysts (58%) and thin-walled cystic bronchiectases (27%). Increased serum kappa light chain was found in 87% of patients. Histological analysis showed kappa light chain deposits in all patients, except one with lambda chain deposits. Median annual FEV1 decline was 127 ml (IQR 178) and median DLCO decline was 4.3% (IQR 4.3). Sixteen patients received immunomodulatory treatment or chemotherapy; serum light chain levels decreased in 9 cases (75%), without significant improvement in FEV1 ( p = 0.173). Overall, 48% of patients underwent bilateral lung transplantation. Transplant-free survival at 5 and 10 years were 70% and 30%, respectively. An annual FEV1 decline greater than 127 ml/year was associated with increased risk of death or transplantation ( p = 0.005).
Conclusions
Diffuse pulmonary LCDD is characterised by female predominance, a peculiar imaging pattern with bronchiectasis and/or cysts, progressive airway obstruction and severe DLCO impairment, and poor outcome. Lung transplantation is a treatment of choice.

Take-home message
Diffuse pulmonary light chain deposition disease is characterised by female predominance, a peculiar imaging pattern with bronchiectasis and/or cysts, progressive airway obstruction and diffusion capacity impairment, and poor outcome.
Plain language summary
Diffuse pulmonary light chain deposition disease is an exceedingly rare disease of the lungs, whereby parts of abnormal antibodies (proteins involved in host defense especially against microorganisms) deposit in the lungs. This disease is characterised by a peculiar pattern on chest CT, with dilation of bronchi and formation of air-filled cysts (holes), and progresses in several years to chronic respiratory insufficiency.
Immunoglobulins can cause specific forms of lung involvement. Their physiochemical properties and size are important pathogenetic determinants. Two forms of immunoglobulin light chains (LC) can be deposited in tissues: amyloid [ 1 ] and non-amyloid. Light chain deposition disease (LCDD) is a term restricted to the non-amyloid forms of LC deposition.
LCDD is a rare multisystemic entity described by Randall in 1976 [ 2 ] as the deposition of a nonfibrillary, amorphous material that does not have a β-pleated sheet configuration and consequently does not bind Congo red nor have apple-green birefringence with Congo red stain. Contrary to LC amyloidosis [ 3 ], LCDD is mostly composed of kappa LC. Moreover, electronic microscopy does not show a fibrillary pattern but electron-dense granular deposits along basement membranes [ 4 ]. The diagnosis of LCDD is established by immunohistological analysis of affected organs. It requires a formalin-fixed paraffin-embedded sample for microscopic examination and a frozen sample for immunofluorescence analysis with anti-kappa and anti-lambda antibodies. When a frozen tissue sample is not available, mass spectrometry on formalin-fixed paraffin-embedded tissue can be used [ 5 ].
Clinical manifestations of LCDD vary according to the organs involved. Lung involvement appears to be very uncommon but may be underrecognised especially when the deposition of LCs is limited to the lung. Since its first report in 1988 [ 6 ], pulmonary LCDD has been described as either nodular or diffuse [ 7 , 8 ]. The nodular form is generally seen in patients who have no evidence of plasma cell dyscrasia. Diffuse LCDD is characterised by parenchymal cysts, or airway involvement [ 9 , 10 ] including bronchiectasis [ 11 ]. Pathologically, diffuse LCDD is characterised by LC deposits along the basement membranes of the alveolar, bronchial, and vascular walls. The putative pathophysiology of cyst formation involves the degradation of elastic fibers by matrix metalloproteinases [ 12 ].
The clinical symptoms reported in previous studies are chest discomfort, haemoptysis, and progressive dyspnea leading to chronic respiratory failure [ 13 , 14 , 15 ]. Although no treatment is validated, chemotherapy is often prescribed to control monoclonal LC secretion in the serum [ 16 ]. Lung transplantation may be performed [ 17 ].
Data on pulmonary LCDD are scarce and limited to small series or case reports. The main objectives of this study were to: 1) describe the clinical, functional and radiological characteristics at presentation, 2) determine lung function during follow-up and estimate time to transplantation or death.
Patient selection and data collection
This retrospective multicentre study was conducted in the French OrphaLung network, a cooperative group of lung specialists. Patient data regarding clinical, laboratory, functional, radiological characteristics, and outcome were collected using a case report form. Patients were considered eligible if the diagnosis of pulmonary LCDD was confirmed by immunohistochemistry. The exclusion criteria were uncertain diagnosis, solitary pulmonary nodules, and predominance of amyloid deposits.
Ethical consideration
This study was conducted with respect to the Declaration of Helsinki. It was approved by the ethics committee of the Hospices Civils de Lyon and was registered with the national data protection agency (Commission Nationale de l’Informatique et des Libertés, number 20–075). According to the legislation in place at the time of the study, informed consent signature was waived, but each patient was informed by a written letter and could object to the use of their personal data. Several patients were reported in previous publications [ 10 , 11 , 12 , 14 , 17 , 18 ].
Pulmonary function tests
Lung volumes were measured by plethysmography, and forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) by flow–volume curve, using GLI reference equations. Carbon monoxide transfer factor (DLco) was assessed with the single-breath method. Hypoxemia was defined as a partial pressure of oxygen in arterial blood (PaO2) < 80 mmHg.
Chest computed tomography (CT)
Two expert chest radiologists (SSM, DR) blinded to the clinical data reviewed the baseline and latest available computed tomography (CT) images during follow-up and before lung transplantation. If consensus was not obtained, a third expert radiologist (CB) settled the description. A CT grading system was used to document cyst number, size of the largest cyst, shape (round, oval, irregular), internal septation, and distribution. Bronchiectases were classified by their appearance (cylindric, varicose, cystic), their proximal or distal distribution, and the presence of bronchial wall thickening. Then, based on imaging, the predominant imaging pattern was classified as bronchiectatic, cystic, or mixed. To evaluate temporal changes in imaging findings, the last available follow-up CT was compared to the baseline CT.
Pathological assessment
Histology reports of lung biopsies were collected and centrally reviewed by an expert pathologist in the field (MC) to confirm the diagnosis of LCDD, using previously described criteria [ 5 ], i.e. biopsies had to stain negative with Congo red and demonstrate LC deposits on frozen tissue under immunofluorescence microscopy. When frozen tissue was unavailable, mass spectrometry was used for diagnostic confirmation [ 19 ].
Statistical analysis
Although normal distribution could not be assumed due to the small sample size and the expected heterogeneity in disease behavior, Shapiro–Wilk test was used to check for normal and skewed distributions. Non-parametric tests were used in the absence of normality. Continuous variables are presented as means (percentages) ± standard deviation (SD) or as median (range).
The Wilcoxon signed-rank test was applied to compare pre- and post-treatment lung function decline, and the Mann–Whitney U test was used to compare measures between groups. Chi-squared test, Fisher’s exact test and Student’s t test were used where appropriate. For each patient, the estimated annual decline rate in DLCO and FEV1 expressed in %/year and in mL/year, respectively, were calculated by linear regression. The average ∆FEV1 and ΔDLCO were calculated in patients who had completed a minimum of 24 months of follow-up and a minimum of four measures. Event-free survival, defined as the time from the first consultation to transplantation or death from any cause, was estimated using the Kaplan–Meier survival method, and univariate Cox regression was performed to assess hazard ratios (HRs) with 95% confidence intervals.
Statistical significance was set at p < 0.05 (two-tailed). IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp, Armonk, NY) and RStudio (v1.3.959) were used for statistical analyses.
Baseline characteristics
Out of 61 patients identified between 1998 and 2020 in 12 French centres and one Swiss centre, 31 met the eligibility criteria and were included in the analysis (Figure S 1 ). Overall, 68% of patients were women and the mean ± SD age at diagnosis was 50 ± 10.7 years. The median (range) interval between the onset of symptoms and diagnosis was 4 (1–30) years. Twenty (67%) patients were current or former smokers with a median of 23.8 (2.5–120) pack-years. All patients except two had dyspnea (Table 1 ).
At baseline, FEV1/FVC was < 0.70 in 45% of patients. FEV1 was lower than 80% of the predicted value in 43% of patients. DLCO was 52 ± 24% (Table 1 ). Hypoxemia was present in 68% of patients.
Haematological characteristics and biology
Bone marrow biopsy or aspiration performed in 21 cases showed > 10% of plasma cells in three patients. A bone marrow B-cell clone search was performed in seven cases and was found in four. Immunofixation identified a circulating monoclonal component as IgM in 52% of cases. Kappa/lambda ratio of serum LC was increased in 21 patients. Free monoclonal kappa LC was increased in the serum of 21/23 patients with a mean level of 236.2 ( ± 344.4) mg/L (Table 1 ). An auto immune workup, including antinuclear and antineutrophilic cytoplasmic antibodies, performed in 22 patients, was negative in all but one patient with anti-SSA and anti-SSB antibodies.
Histology and immunohistochemistry
The most frequently used method to obtain tissue was videothoracoscopic lung biopsy (36%) (Figure S 2 A). The pathological examination of lung biopsies demonstrated cystic destruction and bronchiolar dilatation in all cases (Figure S 2 B). In all patients, biopsy specimens showed Congo red negative eosinophilic deposits infiltrating alveolar walls, small airways, and/or vessels. Immunofluorescence assay of frozen tissues was performed in 25 cases, showing LC deposition stained using anti-kappa antibodies in 19 patients, as illustrated in Figure S 2 C. In contrast, anti-lambda antibody staining confirmed the diagnosis in only one patient. Electron microscopy, performed in five patients, revealed granular dense electron deposits in all cases. Mass spectrometry-based analysis of biopsy, performed in 19 cases, showed that the main constituent of the deposits in all cases was the presence of peptides belonging to the constant region of the immunoglobulin kappa chain. A lymphoplasmocytic infiltrate was found in 13 cases. A search for B-cell clone in the lung was performed by PCR in 9 cases and revealed a clone in all patients.
Fiberoptic bronchoscopy
Fiberoptic bronchoscopy was performed in 24 patients. The macroscopic appearance of the bronchial mucosa was inflammatory in 29% of patients, normal in 42%, showed bronchial distorsions in 12.5%, and bronchomalacia in 8%. Bronchial biopsies were performed in 11 cases, including 8 with immunohistochemical analysis (positive in 5), and 3 with proteomic analysis (positive in all 3 cases).
Baseline CT characteristics
The most frequent abnormalities were plurifocal and sometimes extensive lung cysts (100%) and cystic bronchiectasis (77%). Cysts were bilateral and of regular shape and were characterised by internal septation (73%), bronchovascular topography (77%), and thin walls (Supplement Figure S 3 ). There was a wide variation in cyst size; the largest cysts were found abutting the pleura (Table 2 ). The patient population was then split according to whether patients presented with a cystic, bronchiectasis, or mixed CT pattern. FEV1 was significantly lower, and airflow obstruction was significantly more severe in the cystic pattern compared to the bronchiectasis pattern (Table 3 ).
Extrapulmonary manifestations
In four cases, LC deposits were found in salivary glands, one case being associated with primary Sjögren syndrome. Kidney tissue was available in three cases and no deposit was found. Interventricular septum thickening on cardiac ultrasonography was found in none of the cases. No patient had congestive heart failure. Left ventricular ejection fraction was preserved in all cases, and diastolic dysfunction was absent. Low voltage was not present on electrocardiograms. Right heart catheterisation was performed in 11 patients (Supplement Table S 1 ).
Lung function and CT follow-up
Follow-up data were available for FEV1 in 26 patients and for DLCO in 17 patients. The median duration of lung function follow-up was 75 (2–180) months. The median annual overall FEV1 decline was 127 ml/year (2.2 – 877), and the median DLCO annual decline was 4.3%/year (1.9 – 8). Imaging follow-up was available for 16 patients with a median interval between the first and last chest CT of 62 (7–139) months. Cysts increased in size in 13 patients and in number in ten patients (Fig. 1 ). The number of bronchiectases increased in nine patients.
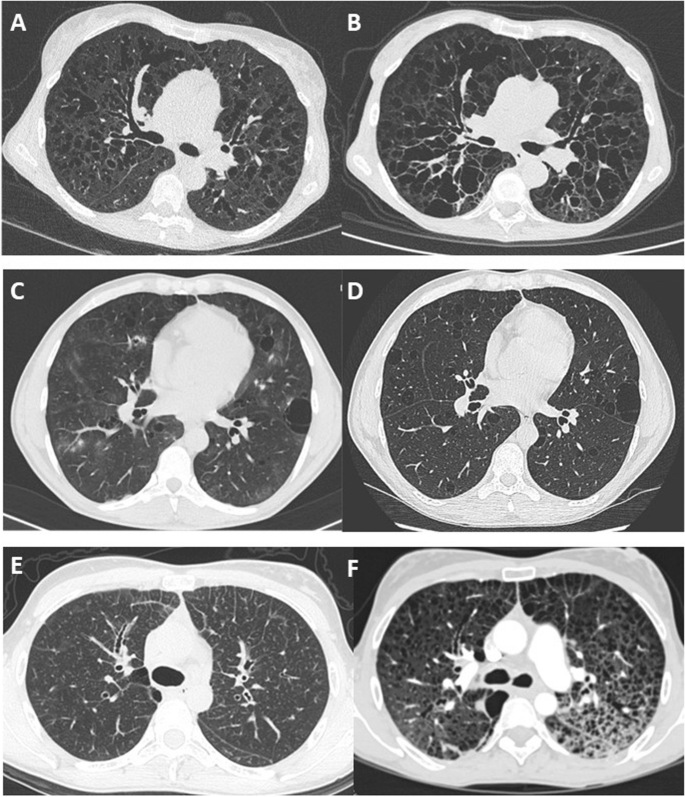
Long-term change in chest CT features in 3 patients showing 3 patterns. A and B CT scan at baseline and after 8 years in a patient with diffuse cystic bronchiectasis. C and D CT scan at baseline and after 1 year in a patient with regular round cysts of various sizes. E and F CT scan at baseline in a patient with micronodules and interlobular reticulation, and 7 years later with multiple cysts and ground glass attenuation
Nonpecific treatment left to the discretion of each investigator included inhaled glucocorticoids ( n = 16), long-acting beta-agonists ( n = 15), long-acting muscarinic antagonist ( n = 6), long-term macrolide treatment ( n = 9). In 16 cases, a treatment was prescribed to attempt treating the disease by targeting the underlying production of LC. In all cases except 4, the first line of chemotherapy was different for each patient, varying according to centres (Supplementary figure S 4 ).
Among the 16 patients treated, ten had evaluable measurements of serum-free LC before and after systemic treatment. A normalisation or reduction > 90% of serum-free LC kappa was obtained only in three patients. Only eight patients had evaluable annual FEV1 before and after systemic treatment. The annual decline in FEV1 did not differ significantly between the pre-and post-treatment periods ( p = 0.73; Supplementary Table S 2 ).
Long-term supplemental nasal oxygen was used in 19 patients (66%). Fifteen patients underwent double lung transplantation, with a median interval between diagnosis and lung transplantation of 5 years (1–11). In 4/15 cases, the diagnosis of LCDD was made before the transplantation. No patient requiring a lung transplant was excluded from receiving it due to contraindications and no patient died on the lung transplant waiting list.
Survival and prognostic factors
When comparing patients deceased or transplanted with those alive or not transplanted, there were significant differences in age at onset of symptoms, baseline FEV1% predicted, FEV1/FVC, and ∆FEV1 (Table 4 ).
Three patients died at the age of 45, 55, and 68 years, all of them due to progressive respiratory failure. The median transplant-free survival was 9 years (Fig. 2 ). There was no significant difference in transplant-free survival between men and women, between smokers and non-smokers, between treated and non-treated, or between cystic and bronchiectatic patterns. A rapid decline in FEV1, defined as ∆FEV1 > 127 ml/year (median value), was associated with an increased risk of death or transplantation ( p = 0.005) (Fig. 2 ).
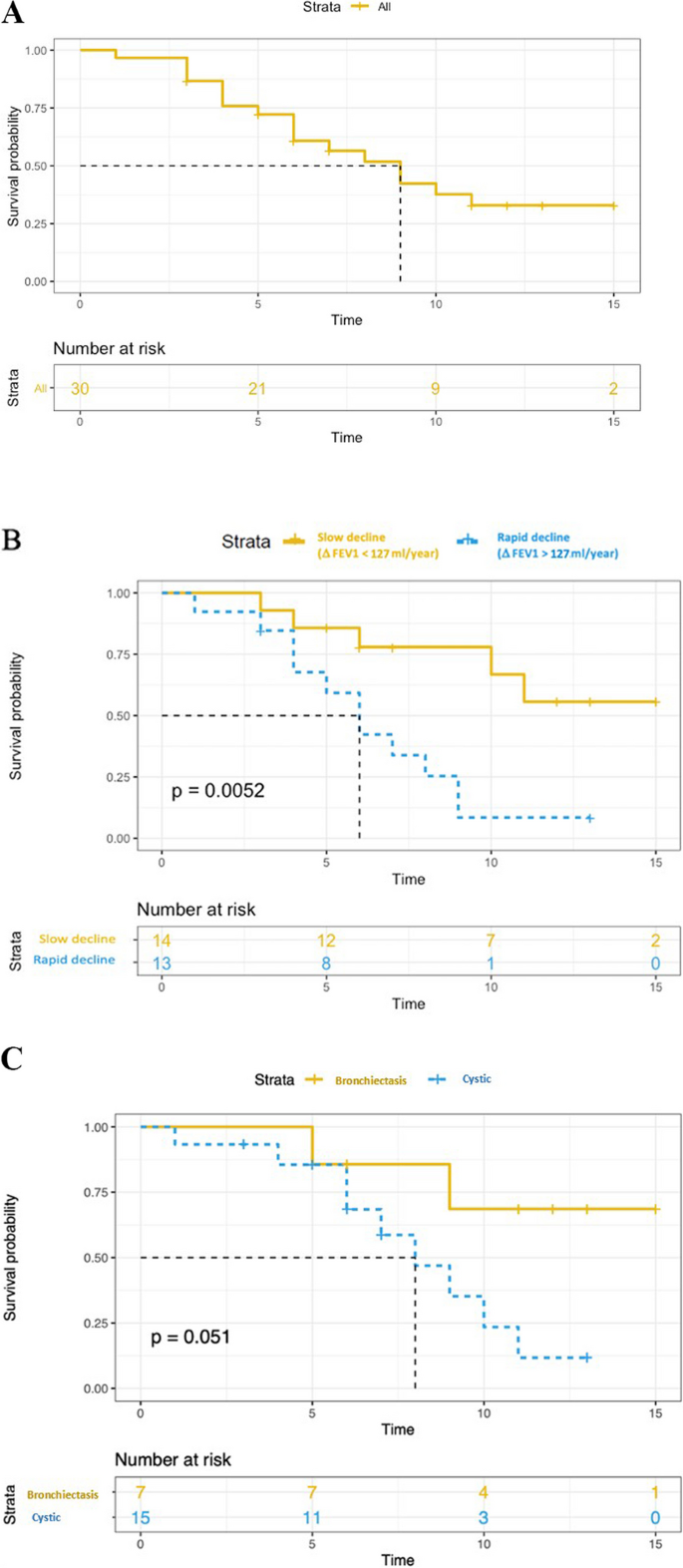
Kaplan–Meier estimates of transplant-free survival. A transplant-free survival in all patients. B Yellow and blue curves correspond to Kaplan Meier estimates of the transplant-free survival for patients with FEV1 decline greater or less than 127 ml/year ( P value for log rank test). C Yellow and blue curves correspond to Kaplan Meier estimates of the transplant-free survival for patients with “bronchiectasis pattern” and “cystic pattern”, respectively ( P value for log rank test)
The present cohort of diffuse pulmonary LCDD, which is the largest to date, shows a female predominance, an increase in serum free monoclonal kappa LC at diagnosis, and a predominance of thin-walled lung cysts and bronchectases on CT. Moreover, pulmonary LCDD appears as a progressive airflow obstruction associated with a poor prognosis and limited response to chemotherapy. Double lung transplantation should be considered as a curative option in this population, and patients should be referred early for transplantation.
Diffuse pulmonary LCDD localized to the lungs is less well described than other cystic lung diseases such as lymphangioleiomyomatosis or pulmonary Langerhans cell histiocytosis. In the patients’ files, the disease was often reported as “unclassifiable diffuse cystic lung disease”, “idiopathic diffuse bronchiectasis”, or “atypical emphysema” before LCDD was considered. The diagnosis is probably often missed since IF is rarely applied to lung biopsies by pulmonary pathologists and frozen material is rarely available, although it is considered the diagnostic method of choice. In these situations, mass spectrometry may confirm the diagnosis, but is not widely available. Paraffin IF was not performed in the present cohort but a prior study suggested that this technique is feasible and could rescue some difficult diagnoses [ 20 ]. The pathological diagnosis was based on the findings of videothoracoscopic lung biopsy, bronchial biopsy, and/or lung explant. Interestingly, bronchial biopsy tissue analysis was sufficient to confirm the diagnosis in a large proportion of the patients in whom the diagnosis was suspected, and in the cases where IF could be performed on frozen bronchial biopsies. Hence, physicians should be alerted to use adequate tissue fixation methods (i.e. frozen tissue) when a diagnosis of LCDD is considered.
Previous studies clearly demonstrated that diffuse pulmonary LCDD differs clinically from the systemic multivisceral form and from the pulmonary nodular form of the disease [ 21 ]. Unlike patients in two recent reports [ 22 , 23 ], the patients herein rarely had underlying autoimmune conditions, in particular primary Sjögren syndrome. Nodular pulmonary LCDD may be associated with Sjögren syndrome more often than the cystic presentation [ 24 ].
The present results indicate that young females more frequently present with respiratory manifestations of LCDD. The most common lung function abnormality at baseline was decreased DLCO, probably related to the damage induced by LC deposits along the alveolar-capillary membrane, as observed in lung amyloidosis [ 25 ]. Airflow obstruction defined by FEV1/FVC < 70% was also common.
The CT findings of the present study were relatively heterogeneous. In contrast to the findings of Sheard et al. [ 26 ], pulmonary involvement primarily consisted of varicose bronchiectasis without nodular infiltrates, except in one case. The coexistence (and possible communication [ 12 ]) of thin-walled bronchiectasis and cystic changes is somewhat characteristic of diffuse pulmonary LCDD when compared to other cystic lung diseases, in particular lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome. Cysts in pulmonary LCDD are regular in shape with a predominance of lower distribution [ 27 ]. During follow-up, the size and the number of bronchiectases and pulmonary cysts increased remarkably. However, in some cases, distinguishing these two patterns was not possible, with extensive emphysema-like changes in severe forms, and it is conceivable that both bronchiectases and cyst result from a common mechanisme of elastolysis. Noteworthy, the majority of the patients herein were current or ex-smokers, and it cannot be excluded that tobacco smoking may have played a role in pulmonary lesion formation.
Monoclonal gammopathy is the presence in the serum of a monoclonal immunoglobulin produced by a B-cell clone. This biological anomaly fits with the concept of monoclonal gammopathy of clinical significance, in which the clone induces severe organ damage [ 28 ]. Herein, the free LC ratio was abnormal in the vast majority of patients, making it a plausible screening tool. Nevertheless, the serum level of kappa LC at baseline was not associated with a greater probability of lung transplant or death. As shown by the plasmacytosis greater than 10% found in certain patients who underwent bone marrow biopsy or aspiration, although the association of LCDD with haematological malignancy is possible [ 13 , 29 ], the absence of bone marrow abnormality upon immunohistology should not exclude pulmonary LCDD. In such cases, LC may be produced within the lungs [ 17 ]. No evidence of extrapulmonary LC deposition was observed in the present cohort, except in accessory salivary glands. As a result, pulmonary LCDD may be considered in the appropriate context, even in the absence of kidney dysfunction or proteinuria.
During follow-up, inter-patient variability in FEV1 decline was observed, however, the decline in lung function was generally faster than that seen in chronic obstructive pulmonary disease [ 30 ] or in other multiple cystic lung diseases [ 31 , 32 , 33 ], and diffusion capacity was severely altered. Patients who required a lung transplantation had a higher rate of FEV1 decline than non-transplanted patients. Consequently, lung function surveillance at 6–12 months intervals is of paramount importance to assess disease progression and identify possible transplantation candidates, especially when ΔFEV1 > 127 ml/year.
There is no validated therapeutic strategy for LCDD localized to the lung. The main treatment approach is to target the synthesis of monoclonal proteins, using the same treatments as those employed in multiple myeloma. In the present cohort, various medications were used (rituximab, alkylating agents, proteasome inhibitor, dexamethasone, antimetabolite) targeting the underlying production of light chains, as well as autologous blood stem cell transplantation. Using an exploratory analysis, no change in the annual rate of FEV1 decline before and after treatment was observed, whereas improved renal function was reported in patients treated with melphalan and prednisone [ 34 ] or autologous stem cell transplantation for renal LCDD [ 35 ]. The fact that tissue damage induced by LC deposits is irreversible may explain the absence of clinical improvement despite serum LC titers that tended to decrease upon treatment. It remains to be determined whether treatments allowing LC serum levels to return to normal may slow disease progression. Lung volume reduction surgery might be considered as an alternative to chemotherapy in selected cases [ 18 ].
Lung transplantation is currently the main treatment option in patients with respiratory failure resulting from LCDD, with no reported recurrence in the transplanted lungs [ 17 ]. This is in line with the relatively long median lung transplant-free survival found herein. However, the present analysis showed that there was a significant difference in lung-transplant free survival according to FEV1 decline, underlying the possible need for some patients to undergo transplantation as early as possible. In contrast, kidney transplantation is not considered an option in end-stage renal disease, as further kidney damage cannot be prevented in the transplanted organ [ 36 ]. In case of cardiac LCDD, LC deposits were identified as early as 3 months after heart transplantation [ 37 ].
This study has several limitations, including the relatively small sample size and the retrospective design. Second, its multicentric nature represents a source of variability in pulmonary function test measurements. Third, the lack of standardised therapy may have contributed to the lack of significant treatment benefit observed on lung function decline.
In conclusion, in this cohort of patients with diffuse pulmonary LCDD, women were more commonly affected than men. Low DLCO was the most commonly observed lung function abnormality. Rapid annual FEV1 decline was associated with a greater risk of death or transplantation. Systemic haematologic treatment did not reduce the annual lung function decline. Prospective larger studies are eagerly awaited.
Availability of data and materials
Data are available upon request to the corresponding author.
Abbreviations
Computed tomography
Diffusing capacity for carbon monoxide
Transfer coefficient for the lung for carbon monoxide
Forced vital capacity
Forced expiratory volume in one second
Immunofluorescence
Light chain
- Light chain deposition disease
Arterial oxygen partial pressure
Total lung capacity
Buxbaum JN, Chuba JV, Hellman GC, Solomon A, Gallo GR. Monoclonal immunoglobulin deposition disease: light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology and molecular analysis. Ann Intern Med. 1990;112:455–64.
Article CAS PubMed Google Scholar
Randall RE, Williamson WC, Mullinax F, Tung MY, Still WJ. Manifestations of systemic light chain deposition. Am J Med. 1976;60:293–9.
Buxbaum J, Gallo G. Nonamyloidotic monoclonal immunoglobulin deposition disease. Light-chain, heavy-chain, and light-and heavy-chain deposition diseases. Hematol Oncol Clin North Am. 1999;13:1235–48.
Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12:1482–92.
Article PubMed Google Scholar
Colombat M, Holifanjaniaina S, Guillonneau F, Mal H, Hirschi S, Reynaud-Gaubert M, Stern M. Mass spectrometry-based proteomic analysis: a good diagnostic tool for cystic lung light chain deposition disease. Am J Respir Crit Care Med. 2013;188:404–5.
Kijner CH, Yousem SA. Systemic light chain deposition disease presenting as multiple pulmonary nodules. A case report and review of the literature. Am J Surg Pathol. 1988;12:405–13.
Khoor A, Myers JL, Tazelaar HD, Kurtin PJ. Amyloid-like pulmonary nodules, including localized light-chain deposition: clinicopathologic analysis of three cases. Am J Clin Pathol. 2004;121:200–4.
Mezzanotte JN, Gibbons-Fideler IS, Shilo K, Lustberg M, Devarakonda S. Nodular pulmonary deposition disease in a patient with the acquired immunodeficiency syndrome: a case report. J Med Case Rep. 2020;14:64.
Article PubMed PubMed Central Google Scholar
Miró O, Fernández-Solá J, Gómez-Angelats E, Andreu MV, Solé M. [Tracheobronchomegaly associated with light chain deposition disease]. Arch Bronconeumol. 1994;30:508–10.
Colombat M, Gounant V, Mal H, Callard P, Milleron B. Light chain deposition disease involving the airways: diagnosis by fibreoptic bonchoscopy. Eur Respir J. 2007;29:1057–60.
Girard N, Vasiljevic A, Cottin V, Falchero L, Meyronet D, Thivolet-Bejui F, Cordier JF. Respiratory failure with diffuse bronchiectases and cryoglobulinaemia. Eur Respir J. 2008;31:1374–8.
Colombat M, Caudroy S, Lagonotte E, Mal H, Danel C, Stern M, Fournier M, Birembaut P. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J. 2008;32:1399–403.
Kato T, Muto H, Hishima T, Kawashima M, Nagai H, Matsui H, Shimada M, Hebisawa A, Doki N, Miyawaki S, Ohashi K. A 56-Year-old woman with multiple pulmonary cysts and severe chest Pain. Chest. 2018;153:e105–12.
Colombat M, Stern M, Groussard O, Droz D, Brauner M, Valeyre D, Mal H, Taille C, Monnet I, Fournier M, Herson S, Danel C. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med. 2006;173:777–80.
Gorospe Sarasúa L, Pacios-Blanco RE, Arrieta P, Chinea-Rodríguez A. Intracystic hemorrhage in a patient with pulmonary cystic disorder related to light-chain deposition disease. Arch Bronconeumol. 2017;53:285–7.
Borgne AL, Prévot G, Rouquette I, Huynh A, Têtu L, Projetti F, Carreiro M, Borie R, Jaccard A, Recher C, Didier A. Blood stem cell transplantation to treat cystic lung light chain deposition disease. Eur Respir J. 2015;46:1199–202.
Hirschi S, Colombat M, Kessler R, Reynaud-Gaubert M, Stern M, Chenard MP, Métivier AC, Jeung MY, Mal H. Lung transplantation for advanced cystic lung disease due to nonamyloid kappa light chain deposits. Ann Am Thorac Soc. 2014;11:1025–31.
Delaey P, Plawny L, Nchimi A, Hirschi S, Weingertner N, Santelmo N, Wirtz G. [Effect of surgery of pulmonary cysts related to immunoglobulin light chain deposits]. Rev Mal Respir. 2020;37:180–6.
Camus M, Hirschi S, Prevot G, Chenard MP, Mal H, Stern M, Reynaud-Gaubert M, Gilhodes J, Burlet-Schiltz O, Brousset P, Colombat M. Proteomic evidence of specific IGKV1-8 association with cystic lung light chain deposition disease. Blood. 2019;133:2741–4.
Gibier JB, Perbet R, Lopez B, Colombat M, Dubois R, Humez S, Terriou L, Copin MC, Gnemmi V. Paraffin immunofluorescence increases light-chain detection in Extra-renal Light Chain Amyloidosis and other Light-Chain-Associated diseases. Arch Pathol Lab Med. 2021;145:352–8.
Colombat M, Mal H, Copie-Bergman C, Diebold J, Damotte D, Callard P, Fournier M, Farcet JP, Stern M, fau-Larue MH. Primary cystic lung light chain deposition disease: a clinicopathologic entity derived from unmutated B cells with a stereotyped IGHV4-34/IGKV1 receptor. Blood. 2008;112:2004–12.
Baqir M, Moua T, White D, Yi ES, Ryu JH. Pulmonary nodular and cystic light chain deposition disease: a retrospective review of 10 cases. Respir Med. 2020;164:105896.
Wei P, Tao R, Liu Y, Xie H, Jiang S, Yu D, Lu H, Cao W. Pulmonary light chain deposition disease: a case series and literature review. Annals Transl Med. 2020;8:588.
Article CAS Google Scholar
Arrossi AV, Merzianu M, Farver C, Yuan C, Wang SH, Nakashima MO, Cotta CV. Nodular pulmonary light chain deposition disease: an entity associated with Sjögren syndrome or marginal zone lymphoma. J Clin Pathol. 2016;69:490–6.
Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in amyloidosis. Eur Respir Rev. 2017;26:170046.
Sheard S, Nicholson AG, Edmunds L, Wotherspoon AC, Hansell DM. Pulmonary light-chain deposition disease: CT and pathology findings in nine patients. Clin Radiol. 2015;70:515–22.
Park HJ, Chae EJ, Do KH, Lee SM, Song JW. Differentiation between Lymphangioleiomyomatosis and Birt-Hogg-Dubé Syndrome: analysis of pulmonary cysts on CT images. AJR Am J Roentgenol. 2019;212:766–72.
Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132:1478–85.
Clayden RC, Macdonald D, Oikonomou A, Cheung MC. Cystic lung disease with kappa light chain deposition in newly diagnosed multiple myeloma. Br J Haematol. 2020;188:201.
Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–92.
Daccord C, Cottin V, Prévot G, Uzunhan Y, Mornex JF, Bonniaud P, Borie R, Briault A, Collonge-Rame MA, Crestani B, Devouassoux G, Freynet O, Gondouin A, Hauss PA, Khouatra C, Leroy S, Marchand-Adam S, Marquette C, Montani D, Naccache JM, Nadeau G, Poulalhon N, Reynaud-Gaubert M, Salaun M, Wallaert B, Cordier JF, Faouzi M, Lazor R. Lung function in Birt-Hogg-Dubé syndrome: a retrospective analysis of 96 patients. Orphanet J Rare Dis. 2020;15:120.
Article CAS PubMed PubMed Central Google Scholar
Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–74.
Tazi A, de Margerie C, Naccache JM, Fry S, Dominique S, Jouneau S, Lorillon G, Bugnet E, Chiron R, Wallaert B, Valeyre D, Chevret S. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J Rare Dis. 2015;10:30.
Heilman RL, Velosa JA, Holley KE, Offord KP, Kyle RA. Long-term follow-up and response to chemotherapy in patients with light-chain deposition disease. Am J Kidney Dis. 1992;20:34–41.
Royer B, Arnulf B, Martinez F, Roy L, Flageul B, Etienne I, Ronco P, Brouet JC, Fermand JP. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65:642–8.
Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, Fervenza FC. Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. 2004;43:147–53.
Aimo A, Vergaro G, Pucci A, Bernazzali S, Maccherini M, Buda G, Passino C, Merlini G, Emdin M. Cardiac light-chain deposition disease relapsing in the transplanted heart. Amyloid. 2017;24:135–7. OrphaLung network (collaborators) 19 Yurdagül UZUNHAN, Avicenne hospital, APHP, Paris Nord university, Paris, France 20 Stéphane JOUNEAU, Pontchaillou Hospital, Rennes, France.
Download references
Acknowledgements
We thank the patients for agreeing to participate in this study. The authors would like to thank R. LAVRUT (Lyon, France), M. RABANT (Paris, France) and R. DUBOIS (Lille, France) for histopathological contribution and pathology slides; V. LANDEL for manuscript preparation;
OrphaLung network (collaborators)
19 Yurdagül UZUNHAN, Avicenne hospital, APHP, Paris Nord university, Paris, France.
20 Stéphane JOUNEAU, Pontchaillou Hospital, Rennes, France.
Author information
Authors and affiliations.
Hospices Civils de Lyon, Centre de Référence Coordinateur Des Maladies Pulmonaires Rares (OrphaLung), Hôpital Louis Pradel, Service de Pneumologie, 69677, Lyon, France
François Lestelle, Mouhamad Nasser & Vincent Cottin
Service de Radiologie Et de Radiologie Interventionnelle, Hôpital Universitaire de Lausanne, Université de Lausanne, Lausanne, Suisse
Catherine Beigelman & David Rotzinger
Hospices Civils de Lyon, Hôpital Louis Pradel, Service de Radiologie, Lyon 69677U1206, Université de Lyon, INSA-Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS, UMR 5220, F‐69621, 7 Avenue Jean Capelle O, 69100, Villeurbanne, France
Salim Si-Mohamed
Centre de Référence Constitutif Des Maladies Pulmonaires Rares (OrphaLung), CHU Lille, Service de Pneumologie, Lille, France
Lidwine Wemeau
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Strasbourg, Service de Pneumologie, Strasbourg, France
Sandrine Hirschi
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Toulouse, Hôpital LarreyUniversité Paul Sabatier, Toulouse, France
Grégoire Prevot
Service de Pneumologie Et de Transplantation Pulmonaire, Hopital Foch, Suresnes, France
Antoine Roux
Service de Pneumologie B Et de Transplantation Pulmonaire, AP-HP, Hôpital Bichat Claude-Bernard, Inserm U1152, Paris, France
Vincent Bunel
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Nancy, Service de Pneumologie, Nancy, France
Emmanuel Gomez
Centre Hospitalier Bretagne Sud, Service de Pneumologie, Lorient, France
Laurent Sohier
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Rouen, Service de Pneumologie, Rouen, France
Helene Morisse Pradier
Service de Pneumologie Et Transplantation Pulmonaire, CHU Marseille Nord, Aix-Marseille Université Marseille, Assistance Publique-Hôpitaux de Marseille, Marseille, France
Martine Reynaud Gaubert
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Besançon, Service de Pneumologie, Besançon, France
Anne Gondouin
Service de Pneumologie, Centre Hospitalier Universitaire Vaudois, Lausanne, CH, Suisse
Romain Lazor
Hospices Civils de Lyon, Hôpital Louis Pradel, Service d’explorations Fonctionnelles Respiratoires, 69677, Lyon, France
Jean-Charles Glerant
Hospices Civils de Lyon, Hôpital Louis Pradel, Service d’Anatomopathologie, 69677, Lyon, France
Françoise Thivolet Bejui
CHU Toulouse, Institut Universitaire du Cancer de Toulouse, Service d’anatomie Et Cytologie Pathologiques, Toulouse, France
Magali Colombat
UMR754, INRAE; Member of RespiFil and ERN-LUNG, Université, Claude Bernard Lyon 1, Lyon, France
Vincent Cottin
You can also search for this author in PubMed Google Scholar
- Yurdagül Uzunhan
- & Stéphane Jouneau
Contributions
François Lestelle: conception of the work; acquisition, analysis, and interpretation of data for the work; AND Drafting the work; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work. Catherine Beigelman: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. David Rotzinger: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Salim Si-Mohamed: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Mouhamad Nasser: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Lidwine Wemeau: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Sandrine Hirschi: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Grégoire Prevot: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Antoine Roux: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Vincent Bunel: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Emmanuel Gomez: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Laurent Sohier: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Helene Morisse Pradier: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Martine Reynaud Gaubert: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Anne Gondouin: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Romain Lazor: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Jean-Charles Glerant: data interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Françoise Thivolet Bejui: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Magali Colombat: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Vincent Cottin (guarantor): conception and design of the work; analysis, and interpretation of data for the work; AND Drafting and revising the work; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work.
Corresponding author
Correspondence to Vincent Cottin .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted with respect to the Declaration of Helsinki. It was approved by the ethics committee of the Hospices Civils de Lyon and was registered with the national data protection agency (Commission Nationale de l’Informatique et des Libertés, number 20–075). According to the legislation in place at the time of the study, informed consent signature was waived, but each patient was informed by a written letter and could object to the use of their personal data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lestelle, F., Beigelman, C., Rotzinger, D. et al. Phenotypes and outcome of diffuse pulmonary non-amyloid light chain deposition disease. Respir Res 25 , 159 (2024). https://doi.org/10.1186/s12931-024-02798-y
Download citation
Received : 18 October 2023
Accepted : 01 April 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s12931-024-02798-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bronchiectasis
- Lung transplantation
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Alspeakstruth. Top creator on Quizlet. ·. Created 2 years ago. A 55-year-old client is admitted to the medical-surgical unit at the hospital after 1 week of experiencing fever, chills, coughing, chest pain upon coughing, and shortness of breath. She had what she believed to be a "bad cold" but never seemed to get better.
Respiratory - Patient 1! So close! Rationale: Chronic obstructive pulmonary disease (COPD) is a chronic, progressive, mostly irreversible condition that is characterized by restrictions in airflow and inflammation. Symptoms include chronic cough, sputum production, and dyspnea. It is most commonly caused by cigarette smoking.
NGN: Respiratory EAQ Case Study. 5.0 (1 review) Respiratory - Patient 1. H&P. 0630 (admission): 52-year-old client presents to ED after an acute onset of sharp chest pain and SOB occurring shortly after awakening this morning. Reports feeling well until this morning. Experienced dizziness and "feeling terrible" shortly after getting out of bed.
Sherpath - Respiratory Case Study. Get a hint. Pediatric Respiratory Dysfunction - Patient 1. Patient Data. Click the card to flip 👆. Nurses notes: A 4-year-old child is brought to the emergency department after developing fever and irritability overnight. The parent is worried that the child seems to be getting worse and is refusing to take ...
This, over time, becomes exhaustive to a patient, and even more so for a patient who is in respiratory distress. The lack of oxygenation or tissue. perfusion will cause a patient to ultimately experience alteration of mentation. They may become delirious and, lastly, experience loss of consciousness. ... Nursing Situation: Respiratory Case Study.
SPRING 2020 · 2 What could it mean? Naturally, it is not sufficient that a nurse simply identifies important information. For that reason, the second question in the NGN Case Study asks candidates to interpret the information presented. For example, in the question below, candidates are asked to relate specific findings to possible disease ...
Study all the case studies uploaded and you will be prepared to pass! respiratory patient aa haa. Skip to document. University; High School. Books; ... NGN Respiratory 2 - HESI Med-Surg Case studies from evolve the developers of HESI. Study all the ... NGN Respiratory 2 - HESI Med-Surg Case studies from evolve the developers of HESI. Study all the.
NGN Case Study Sample Questions and Answers. First, let's take a look at our case study summary below: Case Study Summary: A 68-year-old male is admitted with shortness of breath. He reports difficulty breathing with activity, lying down, or while sleeping. He states that in order to "breathe easier," he has had to sleep in a recliner for ...
Next Generation NCLEX: Case Studies 1-2. Nurse Plus Academy is excited to offer a new Next-Generation NCLEX (NGN) practice test to help NCLEX applicants prepare for the upcoming changes to the exam. The NGN is designed to better measure a candidate's clinical judgment and will include innovative item types such as extended multiple response ...
Unit VI: Interprofessional Collaboration for Patients with Problems of the Respiratory System. Chapter 24 Assessment of the Respiratory System ... NGN Unfolding Case Study #2: Venous Thromboembolism (Concepts of Clotting and Perfusion) NGN Unfolding Case Study #3: Chronic Obstructive Pulmonary Disease (Concepts of Gas Exchange and Infection) ...
Next-Generation NCLEX ® Examination (NGN)-style case studies and question types help you review and practice for the NGN. Contents. Section 1 ... Patients Section 2 Problems Related to Comfort and Coping 7 Stress Management 8 Sleep and Sleep Disorders ... Respiratory System 28 Supporting Ventilation 29 Upper Respiratory Problems
Study with Quizlet and memorize flashcards containing terms like Patient is admitted with a diagnosis of acute respiratory distress secondary to pneumonia. The patient is complaining of acute pain in the right lower lobe of the lung, which is where radiology says the pneumonia is located. As the nurse listens to that area, what should she expect to hear?, The nurse also hears a rather loud ...
The test bank is composed of case studies with six questions each that follow the NCSBN Clinical Judgment Measurement Model steps: recognize cues. analyze cues. prioritize hypotheses. generate solutions. take action. evaluate outcomes. In addition, seven questions for reviewing bow-tie or trend items are included.
Before you attempt writing a case study, you need to understand how to design it. To do that, you need to be familiar with Layer 3 of the NCSBN's Clinical Judgment Measurement Model. Layer 3 illustrates the 6-step process that a nurse goes through in each client encounter. (See the numbered graphic below that depicts the 6 steps.)
The patient is a 60-year-old white female presenting to the emergency department with acute onset shortness of breath. Symptoms began approximately 2 days before and had progressively worsened with no associated, aggravating, or relieving factors noted. She had similar symptoms approximately 1 year ago with an acute, chronic obstructive pulmonary disease (COPD) exacerbation requiring ...
View NGN Case Study Respiratory 2 (2).png from NUR 310 at San Jose City College. Respiratory — Patient 2 The primary healthcare provider diagnoses the client with asthma and provides the client with
NGN CASE STUDY RESPIRATORY.docx - NGN CASE STUDY RESPIRATORY. Doc Preview. Pages 3. Total views 100+ University of Phoenix. BSN. BSN 443. EarlDiscovery10136. 1/30/2023. 42% (19) View full document. Students also studied. TRMA 810 QUIZ 3 STUDY.docx. Liberty University Online Academy. COUNSELING 810. APEA Health Promotion.docx.
The client's serum creatinine level is within normal limits and is not expected to change with pseudoephedrine therapy. NGN case study- Respiratory (patient 3) Client side effect paramater of blood pressure readings. Click the card to flip 👆. Response to/adverse effect of pseudoephedrine. Click the card to flip 👆.
White Paper Using Case Studies to Develop Clinical Judgment and Ensure Next eneration NCLEX ® NGN) Success 2 Background In 2010, the results of a large study of nursing education in the United States were summarized in Educating Nurses: A Call for Radical Transformation (Benner, et al., 2010).One of the key findings was that nurses entering practice do not
NGN News - Spring 2020. Topic: The NGN Case Study. Download Publication. Français. The National Council of State Boards of Nursing (NCSBN) is a not-for-profit organization whose purpose is to provide an organization through which boards of nursing act and counsel together on matters of common interest and concern affecting the public health ...
The patient is assessed and a blood glucose level and vital signs are obtained upon arrival on the unit: BG: 239 HR: ... NGN: Respiratory EAQ Case Study. 9 terms. MngrDwight_K_Schrute. Preview. Respiratory Case Studies. 41 terms. agorm9355. Preview. MED SURGE 2 EXAM 2. 287 terms. karen_duarte33. Preview.
Give your students vital charting exposure with the most trusted educational EHR in nursing education. Designed and refined by nurse educators, Lippincott DocuCare enhances clinical learning by contextualizing realistic patient care scenarios with hands-on documentation, all in an easy-to-use program. Its intuitive educational experience lets ...
Background Light chain deposition disease (LCDD) is a very rare entity. Clinical manifestations of LCDD vary according to the organs involved. Data on pulmonary LCDD are scarce and limited to small series or case reports. This study aimed to describe the characteristics and outcome of diffuse pulmonary non-amyloid LCDD localized to the lungs. Study design and methods A multicenter ...
Study with Quizlet and memorize flashcards containing terms like 1. An 82-year-old woman drove herself to the emergency department (ED) with reports of chest pain, shortness of breath, headache, and extreme fatigue. Client states she has been coughing up a lot of "stuff that looks like egg whites with red string in it." Her chest and abdomen are sore from all the coughing. She has been unable ...