- Sustainability
- Responsible Business
- Small Medium Business
- Pitch A Story

Image Credit- Wikipedia , Pixabay (Representational)

No Tests, No Homework! Here's How Finland Has Emerged As A Global Example Of Quality, Inclusive Education
Others/world, 15 may 2022 3:40 am gmt, editor : shiva chaudhary | .

Shiva Chaudhary
Digital Editor
A post-graduate in Journalism and Mass Communication with relevant skills, specialising in content editing & writing. I believe in the precise dissemination of information based on facts to the public.
Creatives : Shiva Chaudhary
Student-oriented approach to education in finland has been recognised as the most well-developed educational system in the world and ranks third in education worldwide..
"A quality education grants us the ability to fight the war on ignorance and poverty," - Charles Rangel
The uniqueness of the Finnish education model is encapsulated in its values of neither giving homework to students every day nor conducting regular tests and exams. Instead, it is listening to what the kids want and treating them as independent thinkers of society.
In Finland, the aim is to let students be happy and respect themselves and others.
Goodbye Standardised Exams
There is absolutely no program of nationwide standard testing, such as in India or the U.S, where those exams are the decisive points of one's admission to higher education like Board Examinations or Common Entrance Tests.
In an event organised by Shiksha Sanskriti Utthan Nyas, RSS Chief Mohan Bhagwat remarked, "It is because they teach their children to face life struggles and not score in an examination," reported The Print .
Students in Finland are graded based on individual performance and evaluation criteria decided by their teachers themselves. Overall progress is tracked by their government's Ministry of Education, where they sample groups of students across schools in Finland.
Value-Based Education
They are primarily focused on making school a safe and equal space as children learn from the environment.
All Finland schools have offered since the 1980s free school meals, access to healthcare, a focus on mental health through psychological counselling for everyone and guidance sessions for each student to understand their wants and needs.
Education in Finland is not about marks or ranks but about creating an atmosphere of social equality, harmony and happiness for the students to ease learning experiences.
Most of the students spend half an hour at home after school to work on their studies. They mostly get everything done in the duration of the school timings as they only have a few classes every day. They are given several 15 -20 minutes breaks to eat, do recreational activities, relax, and do other work. There is no regiment in school or a rigid timetable, thus, causing less stress as given in the World Economic Forum .
Everyone Is Equal - Cooperate, Not Compete
The schools do not put pressure on ranking students, schools, or competitions, and they believe that a real winner doesn't compete; they help others come up to their level to make everyone on par.
Even though individualism is promoted during evaluation based on every student's needs, collectivity and fostering cooperation among students and teachers are deemed crucial.
While most schools worldwide believe in Charles Darwin's survival of the fittest, Finland follows the opposite but still comes out at the top.
Student-Oriented Model
The school teachers believe in a simple thumb rule; students are children who need to be happy when they attend school to learn and give their best. Focus is put upon teaching students to be critical thinkers of what they know, engage in society, and decide for themselves what they want.
In various schools, playgrounds are created by children's input as the architect talks to the children about what they want or what they feel like playing before setting up the playground.
Compared To The Indian Education Model
Firstly, Finnish children enrol in schools at the age of six rather than in India, where the school age is usually three or four years old. Their childhood is free from constricting education or forced work, and they are given free rein over how they socialise and participate in society.
Secondly, all schools in Finland are free of tuition fees as there are no private schools. Thus, education is not treated as a business. Even tuition outside schools is not allowed or needed, leaving no scope for commodifying education, unlike in India, where multiple coaching centres and private schools require exorbitant fees.
Thirdly, the school hours in Finland do not start early morning at 6 am, or 7 am as done in India. Finland schools begin from 9.30 am as research in World Economic Forum has indicated that schools starting at an early age is detrimental to their health and maturation. The school ends by mostly 2 pm.
Lastly, there is no homework or surprise test given to students in Finland. Teachers believe that the time wasted on assignments can be used to perform hobbies, art, sports, or cooking. This can teach life lessons and have a therapeutic stress-relieving effect on children. Indian schools tend to give a lot of homework to prove their commitment to studying and constantly revise what they learn in school.
Delhi Govt's Focus On Education
The Delhi model of education transformed under the Aam Aadmi Party's (AAP) tenure in the capital. In line with the Finnish model, Delhi government schools have adopted 'Happiness Classes' to ensure students' mental wellness through courses on mindfulness, problem-solving, social and emotional relationships, etc., from 1st to 8th classes.
Delhi government also introduced 'Entrepreneurial Mindset Classes' in 2019 to instil business and critical thinking skills among students of 9th to 12th classes. The practical approach in this class is indicated in the 'Business Blasters', a competition started by the Delhi government to encourage students to come up with start-up ideas and students were provided with ₹1000. Approximately 51,000 students participated in the first edition of the competition, according to Citizen Matters .
Through these endeavours, India is steadily investing in creating human resources that can get employment and generate employment for themselves.
India is at its demographic dividend stage; more than half of its population is within the working-age group of 14 to 60 years. Education is an essential factor in utilising this considerable advantage to grow economically and socially. Finland's education model is how India can strive closer to its goal and progress as a nation.
Also Read: Connaissance! Delhi Board of School Education Pens MoU To Add French In Government Schools

We are an independent and public-spirited digital media platform for Indian millennials. We report news and issues that matter as well as give you the opportunity to take action.

- The Big Think Interview
- Your Brain on Money
- Explore the Library
- Will true AI turn against us?
- Do we have free will?
- Why are there conspiracy theories?
- Is religion helping or hurting us?
- Are we alone in the universe?
- Should we trust science?
- Michio Kaku
- Neil deGrasse Tyson
- Michelle Thaller
- Steven Pinker
- Ray Kurzweil
- Cornel West
- Helen Fisher
- Smart Skills
- High Culture
- The Present
- Hard Science
- Special Issues
- Starts With A Bang
- Perception Box
- Strange Maps
- The Learning Curve
- Everyday Philosophy
- Free Newsletters
- Memberships
Finland’s education system is failing. Should we look to Asia?

- Finland scored high on the original PISA education assessment, but its scores have slipped in recent years.
- Critics argue that Finland’s success came from earlier education models, not from headline-making features like late start times, lack of homework, and absence of test assessment.
- Asia’s rigorous education system is now eclipsing Finland’s PISA scores. Which approach is the right one? Which is truly shortsighted?
In 2000, the Program for International Student Assessment (PISA) released the results of its first survey of education attainment. Administrated by the Organization for Economic Cooperation and Development, the triennial assessment tested the skills and knowledge of 15-year-olds around the world.
That year , Finland handily came out as a top performer, scoring high in math and science, and number one in reading. The United States’ performance that same year, for comparison’s sake, could best be described as middling . These results led many to claim that Finland had the best education system in the world . Educators and politicians swarmed to the Nordic country in the hopes of discovering the source of their golden touch.
Then things took a turn, and Finland’s standings began to slip. Between 2006 and 2012, its scores in science, reading, and math fell sharply: 18, 23, and 29 points respectively. PISA 2015 saw further drops; meanwhile, other top performers have remained relatively steady.
“Finland was on a downwards slope, not an upwards one,” writes Tim Oates , director of assessment research and development at Cambridge Assessment. “All the assumptions in 2000 seemed to be of Finland at the top and on the rise, not on the way down. And that was mistaking PISA for a longitudinal study, rather than a cross-sectional one.”
While Finland remains a top performer, it has lost its luster in the eyes of many experts, bringing criticisms of Finland’s education system to the debate.
Gabriel Heller Shalgren argues that Finland’s educational successes have their origin with the economic and industrial growth that predates the 2000s.
(Photo: Andrei Niemimaki/Flickr)
The real lesson from Finland
Finland’s meteoric rise certainly had some cause. Looking in, many claimed it to be reforms dedicated to school autonomy and pupil-led education. They pointed to the system’s lack of centralized accountability and features like late start times, lack of homework, absence of test assessment, and a culture that celebrates the teaching profession.
For Gabriel Heller Shalgren, research director at the Center for the Study of Market Reform Education, this view lacks hard evidence. According to him, Finland’s initial successes resulted from educational standards instituted in the 1970s and ’80s, well before the above policies could take root.
In a monograph titled “ Real Finnish Lessons ,” he notes that Finland’s teaching system was centralized and teacher-dominated up until the ’90s, meaning decentralized reform came too late for it to be responsible. Instead, Finland’s late developments in industrialization and economic growth bolstered the country’s educational performance. Late developments, Shalgren points out, that mirror those in East Asia.
Shalgren does agree with some popular explanations, such as Finland’s reverence of teachers. However, he notes this is not a recent phenomenon and stems from the role teachers played in the country’s nation-building process, way back in the 19th century.
“Overall, the strongest policy lesson is the danger of throwing out authority in schools, and especially getting rid of knowledge-based, teacher-dominated instruction,” writes Shalgren. “[T]he story from Finland backs up the increasing amount of evidence, which suggests that pupil-led methods, and less structured school environments in general, are harmful for cognitive achievement.”
For Shalgren, the decline in Finland’s recent test scores results from reality finally catching up to Finnish fantasies.
Asian countries have outdone Finland’s education system in the most recent PISA surveys.
(Photo: Pixabay)
Asian education systems pulling ahead
As Singapore, China, and Japan overcome Finland, especially in math and science, countries like Taiwan are quickly closing the gap. This has led some to wonder if Asian education systems have improved over Finland’s in meaningful ways.
Finnish native and Asia correspondent Hannamiina Tanninen has attended schools in both countries. She agrees that Finland’s education system is one of the world’s finest, especially regarding its quality teachers. However, in her TED talk she argues that Finland must learn lessons from East Asia if it is to stay relevant:
- Students in Asia start their education earlier, work harder, and work longer. Simply put, the more time students put into developing skills and knowledge, the more of both they will acquire.
- Finland’s education system lowers the bar accordingly to match a student’s talent and skill set; East Asian systems require students to work to meet a universal standard and catch up if necessary.
- East Asian systems promote competitiveness and center educational strategies on excelling. In Finnish culture, such open competitiveness is less socially acceptable.
- Finland strives to make learning fun and creative; however, Tanninen argues that this approach may be disadvantageous. It may, for example, sacrifice long-term educational gains if success is always measured on a student’s instant gratification.
“When did [Finland] subscribe to an idea that there is a glass ceiling that says, ‘Good enough’?” Tanninen said. “Where as in Asia, I don’t remember any of my professors saying, ‘Okay, good enough.’ It would be, ‘Okay, Hannah, work hard; you can go further.'”
Girls report that they enjoy reading more than boys the world over, but in Finland’s education system, the gender gap is significantly wider.
The gender gap in Finland’s education system?
Despite Finland’s dedication to equality, its performance gap score continues to languish below the OECD average.
In an analysis titled “ Girls, Boys, and Reading ,” Tom Loveless, director of the Brown Center on Education Policy at the Brookings Institution, notes Finland’s gender gap in reading is twice that of the U.S. While Finnish boys score the average, Finnish girls score nearly double that, meaning the country’s superiority in reading literacy rests solely with one gender.
Interestingly, boys typically score higher on math and science, both in Finland and other OECD countries. However, Finland’s latest PISA scores have girls outperforming the boys in both subjects (though the score differential was significantly less than in reading).
“Finland’s gender gap illustrates the superficiality of much of the commentary on that country’s PISA performance,” writes Loveless. “Have you ever read a warning that even if those policies contribute to Finland’s high PISA scores—which the advocates assume but serious policy scholars know to be unproven—the policies also may be having a negative effect on the 50 percent of Finland’s school population that happens to be male?”
This gap extends beyond PISA scores. In Finland, more women enter higher education and obtain higher levels of education overall.
No doubt many factors are at play, but one pointed out by Pasi Sahlberg, Finnish educator and scholar, is that boys simply don’t read for pleasure. “Finland used to have the best primary school readers in the world until the early 2000s, but not anymore,” he told The Washington Post .
A time frame that matches Shalgren’s point that pupil-led pedagogy may have diminishing effects.
content.jwplatform.com
Finland’s education system the best? Wrong question.
Of course, these criticisms and others are part of an open and ongoing dialogue—not just about Finland’s education system but about efficient pedagogy the world over. They make noteworthy points, but there are counterpoints on the other side, too.
For example, Andreas Schleicher, OECD director of education, disagrees with Shalgren’s analysis. He believes Finland’s recent declines are modest compared to the headway made when the country switched from traditional education.
While Asian education systems may be surpassing Finland’s, their uncompromising schedules and test-driven milieu may be shortchanging their futures for short-term gains. That’s the argument made by journalist and political scientist Fareed Zakaria.
“[We] should be careful before they try to mimic Asian educational systems, which are still oriented around memorization and test taking,” writes Zakaria . “I went through that kind of system and it’s not conductive to thinking, problem solving, or creativity.”
And Finland’s gender gap, though stark, is in keeping with larger trends. Girls outperform boys in all countries , and the debate is ongoing as to how social, biological, and cultural forces perpetuate the gap.
The point isn’t to argue that Finland’s education system isn’t valuable. Rather, it’s that “educational tourists” look to Finland, see what they wanted to see, and don’t bother to ask the questions Finland itself continues to grapple with. As Tim Oates points out, there are important lessons to be gained here. But insights should harmonize with an understanding of Finland’s culture, its history, and a wider range of evidence, not simply be a laundry list of fashionable factoids.
Oates’s conclusion is fitting: “In the case of [Finland’s education system], people have been seriously misled by stories told by people who have looked at Finland through their own, restricted lens. The real story of Finland is more subtle, more challenging, and far, far more interesting.”

Why Are Finland’s Schools Successful?
The country’s achievements in education have other nations, especially the United States, doing their homework
LynNell Hancock
Photographs by Stuart Conway
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/cd/ee/cdee1c82-f8e3-4de4-983e-8599d4485745/finland-smiles-wr.jpg)
It was the end of term at Kirkkojarvi Comprehensive School in Espoo, a sprawling suburb west of Helsinki, when Kari Louhivuori, a veteran teacher and the school’s principal, decided to try something extreme—by Finnish standards. One of his sixth-grade students, a Kosovo-Albanian boy, had drifted far off the learning grid, resisting his teacher’s best efforts. The school’s team of special educators—including a social worker, a nurse and a psychologist—convinced Louhivuori that laziness was not to blame. So he decided to hold the boy back a year, a measure so rare in Finland it’s practically obsolete.
Finland has vastly improved in reading, math and science literacy over the past decade in large part because its teachers are trusted to do whatever it takes to turn young lives around. This 13-year-old, Besart Kabashi, received something akin to royal tutoring.
“I took Besart on that year as my private student,” Louhivuori told me in his office, which boasted a Beatles “Yellow Submarine” poster on the wall and an electric guitar in the closet. When Besart was not studying science, geography and math, he was parked next to Louhivuori’s desk at the front of his class of 9- and 10-year- olds, cracking open books from a tall stack, slowly reading one, then another, then devouring them by the dozens. By the end of the year, the son of Kosovo war refugees had conquered his adopted country’s vowel-rich language and arrived at the realization that he could, in fact, learn .
Years later, a 20-year-old Besart showed up at Kirkkojarvi’s Christmas party with a bottle of Cognac and a big grin. “You helped me,” he told his former teacher. Besart had opened his own car repair firm and a cleaning company. “No big fuss,” Louhivuori told me. “This is what we do every day, prepare kids for life.”
This tale of a single rescued child hints at some of the reasons for the tiny Nordic nation’s staggering record of education success, a phenomenon that has inspired, baffled and even irked many of America’s parents and educators. Finnish schooling became an unlikely hot topic after the 2010 documentary film Waiting for “Superman” contrasted it with America’s troubled public schools.
“Whatever it takes” is an attitude that drives not just Kirkkojarvi’s 30 teachers, but most of Finland’s 62,000 educators in 3,500 schools from Lapland to Turku—professionals selected from the top 10 percent of the nation’s graduates to earn a required master’s degree in education. Many schools are small enough so that teachers know every student. If one method fails, teachers consult with colleagues to try something else. They seem to relish the challenges. Nearly 30 percent of Finland’s children receive some kind of special help during their first nine years of school. The school where Louhivuori teaches served 240 first through ninth graders last year; and in contrast with Finland’s reputation for ethnic homogeneity, more than half of its 150 elementary-level students are immigrants—from Somalia, Iraq, Russia, Bangladesh, Estonia and Ethiopia, among other nations. “Children from wealthy families with lots of education can be taught by stupid teachers,” Louhivuori said, smiling. “We try to catch the weak students. It’s deep in our thinking.”
The transformation of the Finns’ education system began some 40 years ago as the key propellent of the country’s economic recovery plan. Educators had little idea it was so successful until 2000, when the first results from the Programme for International Student Assessment (PISA), a standardized test given to 15-year-olds in more than 40 global venues, revealed Finnish youth to be the best young readers in the world. Three years later, they led in math. By 2006, Finland was first out of 57 countries (and a few cities) in science. In the 2009 PISA scores released last year, the nation came in second in science, third in reading and sixth in math among nearly half a million students worldwide. “I’m still surprised,” said Arjariita Heikkinen, principal of a Helsinki comprehensive school. “I didn’t realize we were that good.”
In the United States, which has muddled along in the middle for the past decade, government officials have attempted to introduce marketplace competition into public schools. In recent years, a group of Wall Street financiers and philanthropists such as Bill Gates have put money behind private-sector ideas, such as vouchers, data-driven curriculum and charter schools, which have doubled in number in the past decade. President Obama, too, has apparently bet on competition. His Race to the Top initiative invites states to compete for federal dollars using tests and other methods to measure teachers, a philosophy that would not fly in Finland. “I think, in fact, teachers would tear off their shirts,” said Timo Heikkinen, a Helsinki principal with 24 years of teaching experience. “If you only measure the statistics, you miss the human aspect.”
There are no mandated standardized tests in Finland, apart from one exam at the end of students’ senior year in high school. There are no rankings, no comparisons or competition between students, schools or regions. Finland’s schools are publicly funded. The people in the government agencies running them, from national officials to local authorities, are educators, not business people, military leaders or career politicians. Every school has the same national goals and draws from the same pool of university-trained educators. The result is that a Finnish child has a good shot at getting the same quality education no matter whether he or she lives in a rural village or a university town. The differences between weakest and strongest students are the smallest in the world, according to the most recent survey by the Organization for Economic Co-operation and Development (OECD). “Equality is the most important word in Finnish education. All political parties on the right and left agree on this,” said Olli Luukkainen, president of Finland’s powerful teachers union.
Ninety-three percent of Finns graduate from academic or vocational high schools, 17.5 percentage points higher than the United States, and 66 percent go on to higher education, the highest rate in the European Union. Yet Finland spends about 30 percent less per student than the United States.
Still, there is a distinct absence of chest-thumping among the famously reticent Finns. They are eager to celebrate their recent world hockey championship, but PISA scores, not so much. “We prepare children to learn how to learn, not how to take a test,” said Pasi Sahlberg, a former math and physics teacher who is now in Finland’s Ministry of Education and Culture. “We are not much interested in PISA. It’s not what we are about.”
Maija Rintola stood before her chattering class of twenty-three 7- and 8-year-olds one late April day in Kirkkojarven Koulu. A tangle of multicolored threads topped her copper hair like a painted wig. The 20-year teacher was trying out her look for Vappu, the day teachers and children come to school in riotous costumes to celebrate May Day. The morning sun poured through the slate and lemon linen shades onto containers of Easter grass growing on the wooden sills. Rintola smiled and held up her open hand at a slant—her time-tested “silent giraffe,” which signaled the kids to be quiet. Little hats, coats, shoes stowed in their cubbies, the children wiggled next to their desks in their stocking feet, waiting for a turn to tell their tale from the playground. They had just returned from their regular 15 minutes of playtime outdoors between lessons. “Play is important at this age,” Rintola would later say. “We value play.”
With their wiggles unwound, the students took from their desks little bags of buttons, beans and laminated cards numbered 1 through 20. A teacher’s aide passed around yellow strips representing units of ten. At a smart board at the front of the room, Rintola ushered the class through the principles of base ten. One girl wore cat ears on her head, for no apparent reason. Another kept a stuffed mouse on her desk to remind her of home. Rintola roamed the room helping each child grasp the concepts. Those who finished early played an advanced “nut puzzle” game. After 40 minutes it was time for a hot lunch in the cathedral-like cafeteria.
Teachers in Finland spend fewer hours at school each day and spend less time in classrooms than American teachers. Teachers use the extra time to build curriculums and assess their students. Children spend far more time playing outside, even in the depths of winter. Homework is minimal. Compulsory schooling does not begin until age 7. “We have no hurry,” said Louhivuori. “Children learn better when they are ready. Why stress them out?”
It’s almost unheard of for a child to show up hungry or homeless. Finland provides three years of maternity leave and subsidized day care to parents, and preschool for all 5-year-olds, where the emphasis is on play and socializing. In addition, the state subsidizes parents, paying them around 150 euros per month for every child until he or she turns 17. Ninety-seven percent of 6-year-olds attend public preschool, where children begin some academics. Schools provide food, medical care, counseling and taxi service if needed. Student health care is free.
Even so, Rintola said her children arrived last August miles apart in reading and language levels. By April, nearly every child in the class was reading, and most were writing. Boys had been coaxed into literature with books like Kapteeni Kalsarin (“Captain Underpants”). The school’s special education teacher teamed up with Rintola to teach five children with a variety of behavioral and learning problems. The national goal for the past five years has been to mainstream all children. The only time Rintola’s children are pulled out is for Finnish as a Second Language classes, taught by a teacher with 30 years’ experience and graduate school training.
There are exceptions, though, however rare. One first-grade girl was not in Rintola’s class. The wispy 7-year-old had recently arrived from Thailand speaking not a word of Finnish. She was studying math down the hall in a special “preparing class” taught by an expert in multicultural learning. It is designed to help children keep up with their subjects while they conquer the language. Kirkkojarvi’s teachers have learned to deal with their unusually large number of immigrant students. The city of Espoo helps them out with an extra 82,000 euros a year in “positive discrimination” funds to pay for things like special resource teachers, counselors and six special needs classes.
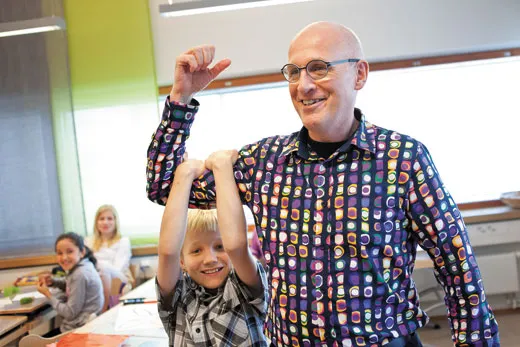
Rintola will teach the same children next year and possibly the next five years, depending on the needs of the school. “It’s a good system. I can make strong connections with the children,” said Rintola, who was handpicked by Louhivuori 20 years ago. “I understand who they are.” Besides Finnish, math and science, the first graders take music, art, sports, religion and textile handcrafts. English begins in third grade, Swedish in fourth. By fifth grade the children have added biology, geography, history, physics and chemistry.
Not until sixth grade will kids have the option to sit for a district-wide exam, and then only if the classroom teacher agrees to participate. Most do, out of curiosity. Results are not publicized. Finnish educators have a hard time understanding the United States’ fascination with standardized tests. “Americans like all these bars and graphs and colored charts,” Louhivuori teased, as he rummaged through his closet looking for past years’ results. “Looks like we did better than average two years ago,” he said after he found the reports. “It’s nonsense. We know much more about the children than these tests can tell us.”
I had come to Kirkkojarvi to see how the Finnish approach works with students who are not stereotypically blond, blue-eyed and Lutheran. But I wondered if Kirkkojarvi’s success against the odds might be a fluke. Some of the more vocal conservative reformers in America have grown weary of the “We-Love-Finland crowd” or so-called Finnish Envy. They argue that the United States has little to learn from a country of only 5.4 million people—4 percent of them foreign born. Yet the Finns seem to be onto something. Neighboring Norway, a country of similar size, embraces education policies similar to those in the United States. It employs standardized exams and teachers without master’s degrees. And like America, Norway’s PISA scores have been stalled in the middle ranges for the better part of a decade.
To get a second sampling, I headed east from Espoo to Helsinki and a rough neighborhood called Siilitie, Finnish for “Hedgehog Road” and known for having the oldest low-income housing project in Finland. The 50-year-old boxy school building sat in a wooded area, around the corner from a subway stop flanked by gas stations and convenience stores. Half of its 200 first- through ninth-grade students have learning disabilities. All but the most severely impaired are mixed with the general education children, in keeping with Finnish policies.
A class of first graders scampered among nearby pine and birch trees, each holding a stack of the teacher’s homemade laminated “outdoor math” cards. “Find a stick as big as your foot,” one read. “Gather 50 rocks and acorns and lay them out in groups of ten,” read another. Working in teams, the 7- and 8-year-olds raced to see how quickly they could carry out their tasks. Aleksi Gustafsson, whose master’s degree is from Helsinki University, developed the exercise after attending one of the many workshops available free to teachers. “I did research on how useful this is for kids,” he said. “It’s fun for the children to work outside. They really learn with it.”
Gustafsson’s sister, Nana Germeroth, teaches a class of mostly learning-impaired children; Gustafsson’s students have no learning or behavioral issues. The two combined most of their classes this year to mix their ideas and abilities along with the children’s varying levels. “We know each other really well,” said Germeroth, who is ten years older. “I know what Aleksi is thinking.”
The school receives 47,000 euros a year in positive discrimination money to hire aides and special education teachers, who are paid slightly higher salaries than classroom teachers because of their required sixth year of university training and the demands of their jobs. There is one teacher (or assistant) in Siilitie for every seven students.
In another classroom, two special education teachers had come up with a different kind of team teaching. Last year, Kaisa Summa, a teacher with five years’ experience, was having trouble keeping a gaggle of first-grade boys under control. She had looked longingly into Paivi Kangasvieri’s quiet second-grade room next door, wondering what secrets the 25-year-veteran colleague could share. Each had students of wide-ranging abilities and special needs. Summa asked Kangasvieri if they might combine gymnastics classes in hopes good behavior might be contagious. It worked. This year, the two decided to merge for 16 hours a week. “We complement each other,” said Kangasvieri, who describes herself as a calm and firm “father” to Summa’s warm mothering. “It is cooperative teaching at its best,” she says.
Every so often, principal Arjariita Heikkinen told me, the Helsinki district tries to close the school because the surrounding area has fewer and fewer children, only to have people in the community rise up to save it. After all, nearly 100 percent of the school’s ninth graders go on to high schools. Even many of the most severely disabled will find a place in Finland’s expanded system of vocational high schools, which are attended by 43 percent of Finnish high-school students, who prepare to work in restaurants, hospitals, construction sites and offices. “We help situate them in the right high school,” said then deputy principal Anne Roselius. “We are interested in what will become of them in life.”
Finland’s schools were not always a wonder. Until the late 1960s, Finns were still emerging from the cocoon of Soviet influence. Most children left public school after six years. (The rest went to private schools, academic grammar schools or folk schools, which tended to be less rigorous.) Only the privileged or lucky got a quality education.
The landscape changed when Finland began trying to remold its bloody, fractured past into a unified future. For hundreds of years, these fiercely independent people had been wedged between two rival powers—the Swedish monarchy to the west and the Russian czar to the east. Neither Scandinavian nor Baltic, Finns were proud of their Nordic roots and a unique language only they could love (or pronounce). In 1809, Finland was ceded to Russia by the Swedes, who had ruled its people some 600 years. The czar created the Grand Duchy of Finland, a quasi-state with constitutional ties to the empire. He moved the capital from Turku, near Stockholm, to Helsinki, closer to St. Petersburg. After the czar fell to the Bolsheviks in 1917, Finland declared its independence, pitching the country into civil war. Three more wars between 1939 and 1945—two with the Soviets, one with Germany—left the country scarred by bitter divisions and a punishing debt owed to the Russians. “Still we managed to keep our freedom,” said Pasi Sahlberg, a director general in the Ministry of Education and Culture.
In 1963, the Finnish Parliament made the bold decision to choose public education as its best shot at economic recovery. “I call this the Big Dream of Finnish education,” said Sahlberg, whose upcoming book, Finnish Lessons , is scheduled for release in October. “It was simply the idea that every child would have a very good public school. If we want to be competitive, we need to educate everybody. It all came out of a need to survive."
Practically speaking—and Finns are nothing if not practical—the decision meant that goal would not be allowed to dissipate into rhetoric. Lawmakers landed on a deceptively simple plan that formed the foundation for everything to come. Public schools would be organized into one system of comprehensive schools, or peruskoulu , for ages 7 through 16. Teachers from all over the nation contributed to a national curriculum that provided guidelines, not prescriptions. Besides Finnish and Swedish (the country’s second official language), children would learn a third language (English is a favorite) usually beginning at age 9. Resources were distributed equally. As the comprehensive schools improved, so did the upper secondary schools (grades 10 through 12). The second critical decision came in 1979, when reformers required that every teacher earn a fifth-year master’s degree in theory and practice at one of eight state universities—at state expense. From then on, teachers were effectively granted equal status with doctors and lawyers. Applicants began flooding teaching programs, not because the salaries were so high but because autonomy and respect made the job attractive. In 2010, some 6,600 applicants vied for 660 primary school training slots, according to Sahlberg. By the mid-1980s, a final set of initiatives shook the classrooms free from the last vestiges of top-down regulation. Control over policies shifted to town councils. The national curriculum was distilled into broad guidelines. National math goals for grades one through nine, for example, were reduced to a neat ten pages. Sifting and sorting children into so-called ability groupings was eliminated. All children—clever or less so—were to be taught in the same classrooms, with lots of special teacher help available to make sure no child really would be left behind. The inspectorate closed its doors in the early ’90s, turning accountability and inspection over to teachers and principals. “We have our own motivation to succeed because we love the work,” said Louhivuori. “Our incentives come from inside.”
To be sure, it was only in the past decade that Finland’s international science scores rose. In fact, the country’s earliest efforts could be called somewhat Stalinistic. The first national curriculum, developed in the early ’70s, weighed in at 700 stultifying pages. Timo Heikkinen, who began teaching in Finland’s public schools in 1980 and is now principal of Kallahti Comprehensive School in eastern Helsinki, remembers when most of his high-school teachers sat at their desks dictating to the open notebooks of compliant children.
And there are still challenges. Finland’s crippling financial collapse in the early ’90s brought fresh economic challenges to this “confident and assertive Eurostate,” as David Kirby calls it in A Concise History of Finland . At the same time, immigrants poured into the country, clustering in low-income housing projects and placing added strain on schools. A recent report by the Academy of Finland warned that some schools in the country’s large cities were becoming more skewed by race and class as affluent, white Finns choose schools with fewer poor, immigrant populations.
A few years ago, Kallahti principal Timo Heikkinen began noticing that, increasingly, affluent Finnish parents, perhaps worried about the rising number of Somali children at Kallahti, began sending their children to one of two other schools nearby. In response, Heikkinen and his teachers designed new environmental science courses that take advantage of the school’s proximity to the forest. And a new biology lab with 3-D technology allows older students to observe blood flowing inside the human body.
It has yet to catch on, Heikkinen admits. Then he added: “But we are always looking for ways to improve.”
In other words, whatever it takes.
Get the latest stories in your inbox every weekday.
LynNell Hancock | READ MORE
LynNell Hancock writes about education and teaches at the Columbia Graduate School of Journalism.
Stuart Conway | READ MORE
Stuart Conway is a photographer based in southeast England.
The Hechinger Report
Covering Innovation & Inequality in Education
OPINION: How Finland broke every rule — and created a top school system

Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to email a link to a friend (Opens in new window)
The Hechinger Report is a national nonprofit newsroom that reports on one topic: education. Sign up for our weekly newsletters to get stories like this delivered directly to your inbox. Consider supporting our stories and becoming a member today.
Get important education news and analysis delivered straight to your inbox
- Weekly Update
- Future of Learning
- Higher Education
- Early Childhood
- Proof Points

Spend five minutes in Jussi Hietava’s fourth-grade math class in remote, rural Finland, and you may learn all you need to know about education reform – if you want results, try doing the opposite of what American “education reformers” think we should do in classrooms.
Instead of control, competition, stress, standardized testing, screen-based schools and loosened teacher qualifications, try warmth, collaboration, and highly professionalized, teacher-led encouragement and assessment.
At the University of Eastern Finland’s Normaalikoulu teacher training school in Joensuu, Finland, you can see Hietava’s students enjoying the cutting-edge concept of “personalized learning.”
Related: Everyone aspires to be Finland, but this country beats them in two out of three subjects
But this is not a tale of classroom computers. While the school has the latest technology, there isn’t a tablet or smartphone in sight, just a smart board and a teacher’s desktop.
Screens can only deliver simulations of personalized learning, this is the real thing, pushed to the absolute limit.
This is the story of the quiet, daily, flesh-and-blood miracles that are achieved by Hietava and teachers the world over, in countless face-to-face and over-the-shoulder interactions with schoolchildren.
Related: Ranking countries by worst students
Often, Hietava does two things simultaneously: both mentoring young student teachers and teaching his fourth grade class.
“Finland’s historic achievements in delivering educational excellence and equity to its children are the result of a national love of childhood, a profound respect for teachers as trusted professionals, and a deep understanding of how children learn best.”
Hieteva sets the classroom atmosphere. Children are allowed to slouch, wiggle and giggle from time to time if they want to, since that’s what children are biologically engineered to do, in Finland, America, Asia and everywhere else.
This is a flagship in the “ultimate charter school network” – Finland’s public schools.
Related: Why Americans should not be coming up with their own solutions to teacher preparation issues
Here, as in any other Finnish school, teachers are not strait-jacketed by bureaucrats, scripts or excessive regulations, but have the freedom to innovate and experiment as teams of trusted professionals.
Here, in contrast to the atmosphere in American public schools, Hietava and his colleagues are encouraged to constantly experiment with new approaches to improve learning.
Hietava’s latest innovations are with pilot-testing “self-assessments,” where his students write daily narratives on their learning and progress; and with “peer assessments,” a striking concept where children are carefully guided to offer positive feedback and constructive suggestions to each other.
Related: In Singapore, training teachers for the classroom of the future
The 37 year-old Hietava, a school dad and Finnish champion golfer in his spare time, has trained scores of teachers, Unlike in America, where thousands of teacher positions in inner cities are filled by candidates with five or six weeks of summer training, no teacher in Finland is allowed to lead a primary school class without a master’s degree in education, with specialization in research and classroom practice, from one of this small nation’s eleven elite graduate schools of education.
As a boy, Hietava dreamed of becoming a fighter pilot, but he grew so tall that he couldn’t safely eject from an aircraft without injuring his legs. So he entered an even more respected profession, teaching, which is the most admired job in Finland next to medical doctors.
I am “embedded” at this university as a Fulbright Scholar and university lecturer, as a classroom observer, and as the father of a second grader who attends this school.
Related: Schools exacerbate the growing achievement gap between rich and poor, a 33-country study finds
How did I wind up here in Europe’s biggest national forest, on the edge of the Western world in Joensuu, Finland, the last, farthest-east sizable town in the EU before you hit the guard towers of the Russian border?
In 2012, while helping civil rights hero James Meredith write his memoir “ A Mission From God ,” we interviewed a panel of America’s greatest education experts and asked them for their ideas on improving America’s public schools.
One of the experts, the famed Professor Howard Gardner of the Harvard University Graduate School of Education, told us, “Learn from Finland, which has the most effective schools and which does just about the opposite of what we are doing in the United States. You can read about what Finland has accomplished in ‘Finnish Lessons’ by Pasi Sahlberg.”
Related: While the U.S. struggles, Sweden pushes older students back to college
I read the book and met with Sahlberg, a former Finnish math teacher who is now also at Harvard’s education school as Visiting Professor.
After speaking with him I decided I had to give my own now-eight-year old child a public school experience in what seemed to be the most child-centered, most evidence-based, and most effective primary school system in the world.
Now, after watching Jussi Hietava and other Finnish educators in action for five months, I have come to realize that Finland’s historic achievements in delivering educational excellence and equity to its children are the result of a national love of childhood, a profound respect for teachers as trusted professionals, and a deep understanding of how children learn best.
Related: In Norway, where college is free, children of uneducated parents still don’t go
Children at this and other Finnish public schools are given not only basic subject instruction in math, language and science, but learning-through-play-based preschools and kindergartens, training in second languages, arts, crafts, music, physical education, ethics, and, amazingly, as many as four outdoor free-play breaks per day, each lasting 15 minutes between classes, no matter how cold or wet the weather is. Educators and parents here believe that these breaks are a powerful engine of learning that improves almost all the “metrics” that matter most for children in school – executive function, concentration and cognitive focus, behavior, well-being, attendance, physical health, and yes, test scores, too.
The homework load for children in Finland varies by teacher, but is lighter overall than most other developed countries. This insight is supported by research, which has found little academic benefit in childhood for any more than brief sessions of homework until around high school.
Related: Demark pushes to make students graduate on time
There are some who argue that since Finland has less socio-economic diversity than, for example, the United States, there’s little to learn here. But Finland’s success is not a “Nordic thing,” since Finland significantly out-achieves its “cultural control group” countries like Norway and Sweden on international benchmarks. And Finland’s size, immigration and income levels are roughly similar to those of a number of American states, where the bulk of education policy is implemented.
There are also those who would argue that this kind of approach wouldn’t work in America’s inner city schools, which instead need “no excuses,” boot-camp drilling-and-discipline, relentless standardized test prep, Stakhanovian workloads and stress-and-fear-based “rigor.”
But what if the opposite is true?
What if many of Finland’s educational practices are not cultural quirks or non-replicable national idiosyncrasies — but are instead bare-minimum global best practices that all our children urgently need, especially those children in high-poverty schools?
Related: China downturn, increased competition could affect supply of foreign students
Finland has, like any other nation, a unique culture. But it has identified, often by studying historical educational research and practices that originated in the United States, many fundamental childhood education insights that can inspire, and be tested and adapted by, any other nation.
As Pasi Sahlberg has pointed out, “If you come to Finland, you’ll see how great American schools could be.”
Finland’s education system is hardly perfect, and its schools and society are entering a period of huge budget and social pressures. Reading levels among children have dropped off. Some advanced learners feel bored in school. Finland has launched an expensive, high-risk national push toward universal digitalization and tabletization of childhood education that has little basis in evidence and flies in the face of a recent major OECD study that found very little academic benefit for school children from most classroom technology.
Related: In Brazil, fast-growing universities mirror the U.S. wealth divide
But as a parent or prospective parent, I have spent time in many of the most prestigious private schools in New York City and toured many of the city’s public school classrooms, in the largest public school system in the world. And I am convinced that the primary school education my child is getting in the Normaalikoulu in Joensuu is on a par with, or far surpasses, that available at any other school I’ve seen.
I have a suggestion for every philanthropist, parent, educator and policymaker in the world who wants to improve children’s education.
Start by coming to Finland. Spend some time sitting in the back of Jussi Hietava’s classroom, or any other Finnish classroom.
If you look closely and open your mind, you may see the School of Tomorrow.
William Doyle is a 2015-2016 Fulbright Scholar and New York Times bestselling author from New York City on the faculty of the University of Eastern Finland, and father of an eight year old who attends a Finnish public school.
Related articles
The Hechinger Report provides in-depth, fact-based, unbiased reporting on education that is free to all readers. But that doesn't mean it's free to produce. Our work keeps educators and the public informed about pressing issues at schools and on campuses throughout the country. We tell the whole story, even when the details are inconvenient. Help us keep doing that.
Join us today.
William Doyle
William... More by William Doyle
Letters to the Editor
At The Hechinger Report, we publish thoughtful letters from readers that contribute to the ongoing discussion about the education topics we cover. Please read our guidelines for more information. We will not consider letters that do not contain a full name and valid email address. You may submit news tips or ideas here without a full name, but not letters.
By submitting your name, you grant us permission to publish it with your letter. We will never publish your email address. You must fill out all fields to submit a letter.
Enjoyed reading William Doyle’s piece on school education in Finland. Am independently developing a flexible, interdisciplinary, interactive, and affordable learning model for K-12 education in India that integrates concept learning, hands- on activities, and life skills. Look forward to read more on new thinking in learning and education!
> But it has identified, often by studying historical educational research and practices that originated in the United States, many fundamental childhood education insights that can inspire, and be tested and adapted by, any other nation.
Can you elaborate on this? Did Finland learn from specific research that originated from the USA or studies from the USA? If so, which ones?
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Sign me up for the newsletter!
Submit a letter

Why is Finnish education still at the top of the world?
No tests, no homework, no punishment, here’s why finnish education is still at the top of the world..
Finland has always been ranked first in math, reading and science in the PISA (Programme for International Student Assessment) rankings of the Organization for Economic Cooperation and Development.
Although, Finland has no standardized test. The only “exam” applied nationally is the national entrance exam. At this exam, students are assessed and graded by teachers who have followed them for a long time. Even if students do not want this assessment, students can completely refuse.
Besides, Finnish education does not accept the act of punishing students for mistakes or grades.
As for the homework of students in this country is very little, even none and often very interesting. For example, for a history lesson, students might be asked to ask their grandparents how life in the 1950s was different from today. Children are encouraged to rest and spend time with their families instead of doing homework.
No pressure for grades or homework, so why is Finnish education always admired by the world?

1. Teachers must meet high standards
The standards set for teachers in Finland are very high. All teachers must have a master’s degree to teach. To teach students in grades 1-6, teachers must have a master’s degree in education or higher. In order to teach students in grades 7-9, in addition to a degree in education, teachers must have a master’s degree in the discipline they teach.
The curricula have high requirements and it is not easy to enter the pedagogical schools. In 2014, only 9% of candidates who took the entrance exam to the faculty of teachers of the University of Helsinki were admitted.
In a school, if a teacher is not up to standard or does a bad job, it is the principal’s responsibility to take care of the problem.
2. Students are the center of education
In Finland, the Education Law of 1998 allows student ownership. This educational model puts the student at the center, giving the way to own and take responsibility for all his or her decisions. They can request shorter school hours, less homework, and more nutritious lunches…
In Finnish classrooms, students can choose their seats according to their preferences, freely express their opinions and are encouraged to play to their strengths.
3. Teacher and student “familiar”
Finnish schools usually have very few teachers and students. Each teacher usually teaches a group of students continuously for 6 years. During this time, teachers act as mentors, guides, and even family members. This trust and attachment helps teachers and students understand and respect each other.
No tests, no homework, no penalties, Finnish education is still at the top of the world, why is that? - Photo 1. Each student’s needs and learning style are different, and teachers who understand those things over the years will help them map out the correct orientation for each student, creating conditions for them to achieve their goals.
4. Pressure at school is minimized
According to the Organization for Economic Co-operation and Development (OECD), Finnish students enter school from 9:45 a.m. and finish at 2:30 p.m. with the least amount of work and homework in the world, even none. Finnish students also do not have tutors, but excel in knowledge and culture thanks to a low-stress education.
Students here also attend only a few classes per day. A school day has 5 lessons (for grades 1-2) and a maximum of 7 periods for older classes. After every 45 minutes of study, the children will have a 15-minute break to exercise, entertain or relax together.
Teachers, too, have a time and place to relax, prepare for lessons, or simply socialize with colleagues.

5. Students are taught knowledge close to life
During swimming lessons, students will be taught how to spot a drowning person. When learning about house management, children are equipped with cooking, knitting and sewing skills. Nature is also an area of content that is of interest to educators.
It is important in Finnish education to prepare students with the knowledge to be able to adapt in any situation.
No tests, no homework, no penalties, Finnish education is still at the top of the world, why is that? - Photo 2. With a highly autonomous education, schools in this country are required to each develop at least one ‘multi-disciplinary’ semester, focusing on the phenomenon or topic that students are interested in. heart.
Children participating in the above semesters will be taught about the ability to study together with many teachers at the same time, background knowledge, helping to understand the essence of the problem being discussed. This concept is also known as phenomenological learning. Teachers also assess students through interdisciplinary topics.
6. Prioritize the basics
Schools in many countries are so concerned with grades and understanding math and other natural sciences that they forget what constitutes an appropriate, equitable learning environment that keeps students happy and healthy.
Since the 80s of the last century, Finnish educators have focused on prioritizing the following core things:
Education is a tool to balance social injustice.
- All students get free meals at school.
- Ease of access to medical services and health care
- Psychological counseling
- Instructions, personalized guidance, tailored for each student
Source: CafeF
Recent Posts


Is Finland Still an Education Superstar?
Halsey rogers, alethea dopart.

Get updates from Education for Global Development
Thank you for choosing to be part of the Education for Global Development community!
Your subscription is now active. The latest blog posts and blog-related announcements will be delivered directly to your email inbox. You may unsubscribe at any time.

Lead Economist, Education Global Practice
Consultant, Education Sector, World Bank
Join the Conversation
- Share on mail
- comments added
9 reasons Finland's schools are so much better than America's
by Libby Nelson

If there's any consensus on education in the US, it could be this: other countries are doing it better. And in the doing-education-better sweepstakes, Finland has long been the cold and snowy standout.
In 2001, the world was stunned when Finland ended up at the top of international rankings after a standardized test administered to students in developed countries . Finland's dominance continued unabated for a decade (although it slipped in 2012 ). Endless articles, and some books, all have the same basic gist: what can the United States learn from Finland?
Finland might be a popular example because, no matter your general beliefs on education policy, you can find something to back them up. The result turns into a policy wonk buffet — nearly everybody can a policy lesson to learn from Finland's success, or a factor that explains why it isn't replicable in the US. Even if some of those lessons directly contradict each other.
Here are 9 reasons that have been cited to explain Finland's success.
1) Finland's teachers have high status, professional support, and good pay

Becoming a teacher in Finland is hard, but they enjoy more autonomy and professional development. ( Shutterstock )
Teachers in Finland with 15 years' experience make about as much as the typical college graduate with a bachelor's degree; in the US, they make less than that . And the workload is also less demanding. Teachers in Finland teach about four hours a day, with another two hours of professional development , and they develop their own curriculum based on a set of national guidelines. The leadership ranks of education are also drawn from former teachers. The result, writes Pasi Sahlberg , a Finnish teacher and researcher who has become a one-man promotional machine for the country's schools, is "an inspiring and respectful environment in which teachers work… Parents and authorities regard teachers with the same confidence they do medical doctors. Indeed, Finns trust public schools more than any other public institution, except the police."
American teachers unions point to the high status and professional flexibility for Finnish teachers as something they'd like to have themselves. They also often note that nearly all Finnish teachers are unionized and the unions are relatively powerful . They argue that to improve schools, the US should focus on treating teachers the way Finland does — with more professional support and greater respect — rather than using students' standardized test scores to reward and grade teachers, a trend the Obama administration has encouraged.
2) Finland has more selective and rigorous schools of education
One reason teaching in Finland is prestigious is becoming a teacher isn't easy. Finland, like the US, used to have a large number of teachers' colleges. But in the 1970s, Finland dramatically changed how teachers were trained. Teacher education became the responsibility of the country's eight universities , and teachers are required to earn masters' degrees. It takes five years of teacher education to become a teacher, and only about one in 10 applicants to teacher education programs is accepted. Secondary teachers get a master's degree in the content area they're going to be teaching, and all master's degree recipients have to write a research-based dissertation.
This is the other side of the argument about teaching: education reformers in the US argue that Finnish teachers get more respect because they earn it through a rigorous, selective entry process. The policy lesson they draw isn't that teachers should be treated like they are in Finland — it's that the teacher corps in the US needs to be more like Finland's. Groups like the National Council on Teacher Quality argue that teachers' colleges aren't selective or rigorous enough . About half of all new teachers come from the bottom third of college graduates, as measured by SAT or ACT scores, according to a 2010 McKinsey report.
On the other hand, the fact that Finnish teachers are so intensely trained also appeals to opponents of programs like Teach for America . A popular saying among opponents of the two-year program is that there is no "Teach for Finland," because in Finland, teaching is a lifelong career with a long and rigorous training program.
3) Finland doesn't give standardized tests

( Shutterstock )
The most common praise for Finland (pushed by Sahlberg and others) goes something like this: Finland has no national standardized tests and no rewards or punishments for schools that pass or fail them — and yet they still outperform American students on international exams. Students in Finland take one standardized test at the end of high school. The rest of the time, teachers are responsible for setting expectations and evaluating whether students can meet them. The nation doesn't monitor the quality of schools in any way.
Some people argue that the success of Finnish schools without standardized testing means that testing shouldn't be necessary in the United States, either – and that it's possible for the US to improve its educational performance in other ways.
4) Finland emphasizes subjects other than reading and math
Finnish kids get plenty of recess , more than an hour a day; US kids get less than half an hour. Oh, and students do less than an hour of homework per night all the way through the equivalent of American middle school. Arts and crafts are required — both boys and girls learn needlework, embroidery, and metalwork .
It's not clear how much this has to do with the success of the Finnish school system, but Samuel Abrams, a visiting scholar at Columbia University, argued in the New Republic that these subjects allow students to apply science and math skills in the real world. They're also an example of what some parents fear has been lost in the US as teachers spend more time preparing students for standardized tests.
5) Finland has a history of tight oversight for schools
Finland doesn't have a national curriculum now, and Finnish education experts brag about how much autonomy teachers get in the classroom. But that wasn't always the case. In the 1960s and 1970s, Finland totally overhauled its education system . The change to teacher training was part of this, but the country also worked with teachers to develop a mandatory national curriculum and there were national inspections to check on student learning. That tight national control remained in place for two decades, until it was eased up in the 1990s — the national curriculum is now described as being more like guidelines than a tight prescription for what teachers should teach in the classroom.
Unlike the lack of testing, this is a Finnish tradition that American supporters of education reform, particularly standards like the Common Core, embrace. They argue that Finland can only give teachers the autonomy they have now because of the generation of tight oversight that preceded it.
6) It's easier to learn to spell in Finland

(Shutterstock)
This is the most unusual explanation for Finland's success yet. At The Atlantic, Luba Vangelova argues that the difficulties of learning to read and write English are holding American students back because other languages — including Finnish — are more straightforward.
Masha Bell, the vice chair of the English Spelling Society, says that Finnish is phonetically much simpler than English because there aren't dozens of arcane spelling rules and exceptions ( i before e except after c, for example) to memorize. Once you know the alphabet and how letter sounds correspond with the written word, learning to read is fairly simple. A study found that in Finnish and other European languages, children can read a list of familiar words after about a year of reading instruction; in English, it took nearly three years.
In other words, Finnish children have an advantage: even though they don't start school until age 7, and even though the Finnish language is very complex for English-speakers to learn, it's relatively easy for native speakers to learn to read and write.
7) Finland has low child poverty and state support for parents

Finland doesn't spend as much on education as the United States. But that overlooks a vast social safety net for families, particularly low-income families, that doesn't exist here, either. Baby Finns start their life with a " baby box " of supplies from the Finnish government. Child care is heavily subsidized, and most children attend some kind of early childhood education before mandatory schooling starts at age 7.
Finland also has one of the lowest child poverty rates in the world, around 5 percent (it's over 20 percent in the US). Parents get a monthly allowance to help them care for their children — 100 euros for the first child and more for additional children. Matt Bruenig at Demos has an overview of Finland's extensive child welfare programs .
Perhaps as a result, Finland has very small gaps between rich and poor students' test scores; in the US, those divides are much bigger. Schools in Finland also offer other services, like dentistry and psychological counseling, according to the OECD. These "wraparound services" are something that teachers' unions argue the United States needs more of — although the attempt to create Finland-style community schools generally hasn't resulted in higher test scores.
8) Finland's schools aren't better — they're just homogenous
Some people argue that Finland's schools aren't actually better — they're just serving a much smaller, much more homogenous population. Finland is tiny — the entire country has just 5.4 million people, fewer than New York City. About 5 percent of its residents are immigrants, much lower than the United States.
Schools in the US where most children aren't poor are actually better than low-poverty school systems in Finland. But high-poverty schools in the US struggle in part because of a toxic legacy of segregation, unequal funding, and unequal opportunity. "For a lot of kids who don't score well on these tests, you go back six generations and you have people in bondage," Jack Schneider, a historian of education, told Vox in October.
Finland, which essentially reinvented its school system from scratch in the second half of the 20th century, has none of that baggage. In some ways, the United States has two school systems — well-funded, high-performing suburban schools serving the middle class, and struggling urban school systems where students are overwhelmingly poor and from disadvantaged backgrounds. While Finland has a few schools educating low-income immigrants with an excellent track record of success ( this Smithsonian article features a visit to one ), those schools are fewer and farther between than they are in the US.
9) Finland is culturally different

Another thing Finland has a lot of: saunas. (Shutterstock)
This is another version of the "Finland does better because it's Finland" argument — that Finnish society is just different than American society, and that as a result lessons are harder to translate. Finnish adults are among the most literate in the world, and the country's libraries are treasured institutions . That, as much as an easier language to spell, could explain stellar reading results. Finland also doesn't have a school sports culture like the US's. And children are more independent; helicopter parenting is just not really a thing .
There are other differences that influence social policy, but aren't limited to them. Rick Hess, a scholar at the conservative American Enterprise Institute, is skeptical that Finland can teach the US anything because its society is so different. His list of differences (most tongue-in-cheek) include long winter nights that leave plenty of time for studying and more children in two-parent families.
Finnish test scores dropped in 2012, but the fascination with its education system hasn't faded. The bottom line seems to be that something in Finland is working — but it might be impossible to ever figure out what.
Most Popular
America is not ready for what comes next, project 2025: the myths and the facts, the ugly process of turning beautiful women into dallas cowboys cheerleaders, what we know about the attempt to assassinate trump, stop setting your thermostat at 72, today, explained.
Understand the world with a daily explainer plus the most compelling stories of the day.
More in Politics

3 theories for America’s anti-immigrant shift

American allies have gone from denial to bargaining on Trump’s return


Are betting markets the best way to predict an election?

What Biden’s news conference did, and didn’t, clear up

The controversy over Biden and Parkinson’s disease, explained

Iran’s new president can only change the country so much

Who shot Trump? What we know about the assassination attempt

How Alec Baldwin’s Rust trial went spectacularly off the rails

Kamala Harris’s strengths — and vulnerabilities — explained

- Scandal and Gossip
- Pop Culture
- Performing Arts
- Visual Arts

Countries with Less Homework and Why More Countries Should Follow Them

Countries with less homework and why more countries should follow them: It may sound counter intuitive but studies are showing that less homework might be the right way to go in better learning.
In an ideal world, students are entitled to an evening of some revision, rest and entertainment after a whole day of study. In many school systems, however, kids are assigned tons of assignments to handle in their free time in a bid to improve their grasp of themes and keep them occupied in books.
As much as the intentions are good, more homework only keeps children drowned in books and does little in achieving the latter. A testament to this, countries with fewer homework policies have better statistics of students that join campus and even lesser dropouts.
A testament to the benefits of fewer time commitment to homework, educational systems in powerhouses like Finland and USA have adopted the policies championing for least homework with the US recommending at most 10 minutes of assignment in any unit per night.
For proper insight, here is a list of countries that embrace the motion for least homework and reasons for other countries to emulate this move. For assistance on homework and clarity on concepts, engage experts on myHomework done , thus earning your student spurs and conceptualizing various classes better.
1. Finland
On top of the list of countries giving less assignment is Finland. Apart from boasting of short school terms and extended holidays, the country limits the homework load to 2.8 hours total of homework per week.
Despite their educational system, Finland manages to rank among the top countries in math and science innovations and also with a smaller drop-out rate. Due to their approach on education, students feel a lesser burden imposed on them thus embracing learning.
Even better, Finland educational system discourages cramming of concepts and trains teachers to impart lessons to students in a matter that they all understand the information equally.
2. South Korea
Like the former, South Korea limits its homework duration per week to a maximum of 2.9 hours. By reducing the burden on students, the country boasts of more educated persons per level of education and even lesser dropout rates.
Unlike other countries, South Korea majors on continuous assessments which excel at testing the understanding of students as opposed to daily homework.
3. Japan
Among the leading countries in technology and science is Japan . Although it has the highest amount of hours for homework per week than its counterparts at 3.8 hours, the numbers are way low than the average.
Even better, the Japanese system of study trains students to gather information from social media platforms thus honing their research and creativity skills. By limiting the amounts of homework, students get to spend quality time with parents thus giving them a platform to instill morals and gain perspective for the upcoming classes.
Reasons why more countries should reduce the homework load on students
1. By assigning more homework to students, the level of anxiety increases thus leading to low motivation in school work. As such, the productivity and attitude of kids towards education is lowered which in turn leads to more dropout rates and lesser grades.
2. With alarming rates of obesity and immorality in kids, less homework creates more parent-kid time and allows kids to engage in more co-curricular activities. As such, parents get a chance to instill moral character in kids and also involve kids in sports and exercise.
3. Time off books allows kids to relax their mind thus increasing the ability to grasp more concepts hence getting the most from every session.
Apart from denying students a change for co-curricular activities, students are also deprived of social time which in turn leads to less time for parents to instill morals in children and also spikes anxiety levels in kids.
Whether more homework is helpful or not is a debatable issue. However, the burden on students leads to daunting effects. Given that academic frontiers assign lesser homework; it shows the need for change in lesser ranking countries.
Recent Posts

Trump sniper suspect motive explored as FBI say no obvious clues

Donald Trump sniper assassin suspect id as registered Republican

Donald Trump survives assassination attempt after shot in ear at Butler...
Popular posts.

5 year old boy dies after left in hot car for...

Teen kills mom & younger boyfriend days after ‘transitioning’ argument

Mom tries to BBQ daughter’s cat on grill as animal mewed...
Popular categories.
- Scandal and Gossip 18108
- Pop Culture 2818
- Fashion 960
- Nightlife 550
- Visual Arts 220
- Performing Arts 213
- Eating Out 137
- Contact & Corrections:
- About S&V & Author Bio:
- Privacy Policy
- Editorial Construct:
What Americans Keep Ignoring About Finland's School Success
The Scandinavian country is an education superpower because it values equality more than excellence.

Everyone agrees the United States needs to improve its education system dramatically, but how? One of the hottest trends in education reform lately is looking at the stunning success of the West's reigning education superpower, Finland. Trouble is, when it comes to the lessons that Finnish schools have to offer, most of the discussion seems to be missing the point.
The small Nordic country of Finland used to be known -- if it was known for anything at all -- as the home of Nokia, the mobile phone giant. But lately Finland has been attracting attention on global surveys of quality of life -- Newsweek ranked it number one last year -- and Finland's national education system has been receiving particular praise, because in recent years Finnish students have been turning in some of the highest test scores in the world.
Finland's schools owe their newfound fame primarily to one study: the PISA survey , conducted every three years by the Organization for Economic Co-operation and Development (OECD). The survey compares 15-year-olds in different countries in reading, math, and science. Finland has ranked at or near the top in all three competencies on every survey since 2000, neck and neck with superachievers such as South Korea and Singapore. In the most recent survey in 2009 Finland slipped slightly, with students in Shanghai, China, taking the best scores, but the Finns are still near the very top. Throughout the same period, the PISA performance of the United States has been middling, at best.
Compared with the stereotype of the East Asian model -- long hours of exhaustive cramming and rote memorization -- Finland's success is especially intriguing because Finnish schools assign less homework and engage children in more creative play. All this has led to a continuous stream of foreign delegations making the pilgrimage to Finland to visit schools and talk with the nation's education experts, and constant coverage in the worldwide media marveling at the Finnish miracle.
So there was considerable interest in a recent visit to the U.S. by one of the leading Finnish authorities on education reform, Pasi Sahlberg, director of the Finnish Ministry of Education's Center for International Mobility and author of the new book Finnish Lessons: What Can the World Learn from Educational Change in Finland? Earlier this month, Sahlberg stopped by the Dwight School in New York City to speak with educators and students, and his visit received national media attention and generated much discussion.
And yet it wasn't clear that Sahlberg's message was actually getting through. As Sahlberg put it to me later, there are certain things nobody in America really wants to talk about.
During the afternoon that Sahlberg spent at the Dwight School, a photographer from the New York Times jockeyed for position with Dan Rather's TV crew as Sahlberg participated in a roundtable chat with students. The subsequent article in the Times about the event would focus on Finland as an "intriguing school-reform model."
Yet one of the most significant things Sahlberg said passed practically unnoticed. "Oh," he mentioned at one point, "and there are no private schools in Finland."
This notion may seem difficult for an American to digest, but it's true. Only a small number of independent schools exist in Finland, and even they are all publicly financed. None is allowed to charge tuition fees. There are no private universities, either. This means that practically every person in Finland attends public school, whether for pre-K or a Ph.D.
The irony of Sahlberg's making this comment during a talk at the Dwight School seemed obvious. Like many of America's best schools, Dwight is a private institution that costs high-school students upward of $35,000 a year to attend -- not to mention that Dwight, in particular, is run for profit, an increasing trend in the U.S. Yet no one in the room commented on Sahlberg's statement. I found this surprising. Sahlberg himself did not.
Sahlberg knows what Americans like to talk about when it comes to education, because he's become their go-to guy in Finland. The son of two teachers, he grew up in a Finnish school. He taught mathematics and physics in a junior high school in Helsinki, worked his way through a variety of positions in the Finnish Ministry of Education, and spent years as an education expert at the OECD, the World Bank, and other international organizations.
Now, in addition to his other duties, Sahlberg hosts about a hundred visits a year by foreign educators, including many Americans, who want to know the secret of Finland's success. Sahlberg's new book is partly an attempt to help answer the questions he always gets asked.
From his point of view, Americans are consistently obsessed with certain questions: How can you keep track of students' performance if you don't test them constantly? How can you improve teaching if you have no accountability for bad teachers or merit pay for good teachers? How do you foster competition and engage the private sector? How do you provide school choice?
The answers Finland provides seem to run counter to just about everything America's school reformers are trying to do.
For starters, Finland has no standardized tests. The only exception is what's called the National Matriculation Exam, which everyone takes at the end of a voluntary upper-secondary school, roughly the equivalent of American high school.
Instead, the public school system's teachers are trained to assess children in classrooms using independent tests they create themselves. All children receive a report card at the end of each semester, but these reports are based on individualized grading by each teacher. Periodically, the Ministry of Education tracks national progress by testing a few sample groups across a range of different schools.
As for accountability of teachers and administrators, Sahlberg shrugs. "There's no word for accountability in Finnish," he later told an audience at the Teachers College of Columbia University. "Accountability is something that is left when responsibility has been subtracted."
For Sahlberg what matters is that in Finland all teachers and administrators are given prestige, decent pay, and a lot of responsibility. A master's degree is required to enter the profession, and teacher training programs are among the most selective professional schools in the country. If a teacher is bad, it is the principal's responsibility to notice and deal with it.
And while Americans love to talk about competition, Sahlberg points out that nothing makes Finns more uncomfortable. In his book Sahlberg quotes a line from Finnish writer named Samuli Paronen: "Real winners do not compete." It's hard to think of a more un-American idea, but when it comes to education, Finland's success shows that the Finnish attitude might have merits. There are no lists of best schools or teachers in Finland. The main driver of education policy is not competition between teachers and between schools, but cooperation.
Finally, in Finland, school choice is noticeably not a priority, nor is engaging the private sector at all. Which brings us back to the silence after Sahlberg's comment at the Dwight School that schools like Dwight don't exist in Finland.
"Here in America," Sahlberg said at the Teachers College, "parents can choose to take their kids to private schools. It's the same idea of a marketplace that applies to, say, shops. Schools are a shop and parents can buy what ever they want. In Finland parents can also choose. But the options are all the same."
Herein lay the real shocker. As Sahlberg continued, his core message emerged, whether or not anyone in his American audience heard it.
Decades ago, when the Finnish school system was badly in need of reform, the goal of the program that Finland instituted, resulting in so much success today, was never excellence. It was equity.
Since the 1980s, the main driver of Finnish education policy has been the idea that every child should have exactly the same opportunity to learn, regardless of family background, income, or geographic location. Education has been seen first and foremost not as a way to produce star performers, but as an instrument to even out social inequality.
In the Finnish view, as Sahlberg describes it, this means that schools should be healthy, safe environments for children. This starts with the basics. Finland offers all pupils free school meals, easy access to health care, psychological counseling, and individualized student guidance.
In fact, since academic excellence wasn't a particular priority on the Finnish to-do list, when Finland's students scored so high on the first PISA survey in 2001, many Finns thought the results must be a mistake. But subsequent PISA tests confirmed that Finland -- unlike, say, very similar countries such as Norway -- was producing academic excellence through its particular policy focus on equity.
That this point is almost always ignored or brushed aside in the U.S. seems especially poignant at the moment, after the financial crisis and Occupy Wall Street movement have brought the problems of inequality in America into such sharp focus. The chasm between those who can afford $35,000 in tuition per child per year -- or even just the price of a house in a good public school district -- and the other "99 percent" is painfully plain to see.
Pasi Sahlberg goes out of his way to emphasize that his book Finnish Lessons is not meant as a how-to guide for fixing the education systems of other countries. All countries are different, and as many Americans point out, Finland is a small nation with a much more homogeneous population than the United States.
Yet Sahlberg doesn't think that questions of size or homogeneity should give Americans reason to dismiss the Finnish example. Finland is a relatively homogeneous country -- as of 2010, just 4.6 percent of Finnish residents had been born in another country, compared with 12.7 percent in the United States. But the number of foreign-born residents in Finland doubled during the decade leading up to 2010, and the country didn't lose its edge in education. Immigrants tended to concentrate in certain areas, causing some schools to become much more mixed than others, yet there has not been much change in the remarkable lack of variation between Finnish schools in the PISA surveys across the same period.
Samuel Abrams, a visiting scholar at Columbia University's Teachers College, has addressed the effects of size and homogeneity on a nation's education performance by comparing Finland with another Nordic country: Norway. Like Finland, Norway is small and not especially diverse overall, but unlike Finland it has taken an approach to education that is more American than Finnish. The result? Mediocre performance in the PISA survey. Educational policy, Abrams suggests, is probably more important to the success of a country's school system than the nation's size or ethnic makeup.
Indeed, Finland's population of 5.4 million can be compared to many an American state -- after all, most American education is managed at the state level. According to the Migration Policy Institute , a research organization in Washington, there were 18 states in the U.S. in 2010 with an identical or significantly smaller percentage of foreign-born residents than Finland.
What's more, despite their many differences, Finland and the U.S. have an educational goal in common. When Finnish policymakers decided to reform the country's education system in the 1970s, they did so because they realized that to be competitive, Finland couldn't rely on manufacturing or its scant natural resources and instead had to invest in a knowledge-based economy.
With America's manufacturing industries now in decline, the goal of educational policy in the U.S. -- as articulated by most everyone from President Obama on down -- is to preserve American competitiveness by doing the same thing. Finland's experience suggests that to win at that game, a country has to prepare not just some of its population well, but all of its population well, for the new economy. To possess some of the best schools in the world might still not be good enough if there are children being left behind.
Is that an impossible goal? Sahlberg says that while his book isn't meant to be a how-to manual, it is meant to be a "pamphlet of hope."
"When President Kennedy was making his appeal for advancing American science and technology by putting a man on the moon by the end of the 1960's, many said it couldn't be done," Sahlberg said during his visit to New York. "But he had a dream. Just like Martin Luther King a few years later had a dream. Those dreams came true. Finland's dream was that we want to have a good public education for every child regardless of where they go to school or what kind of families they come from, and many even in Finland said it couldn't be done."
Clearly, many were wrong. It is possible to create equality. And perhaps even more important -- as a challenge to the American way of thinking about education reform -- Finland's experience shows that it is possible to achieve excellence by focusing not on competition, but on cooperation, and not on choice, but on equity.
The problem facing education in America isn't the ethnic diversity of the population but the economic inequality of society, and this is precisely the problem that Finnish education reform addressed. More equity at home might just be what America needs to be more competitive abroad.

Unlocking Finland’s Secret: A Revolutionary Approach to Homework and Testing

- June 26, 2023
Did you know in recent years Finland has been hailed as a global leader in education? Well, yes Finland is consistently achieving top rankings in international assessments such as the Programme for International Student Assessment (PISA). One of the key aspects that sets Finland apart from other countries is its unique approach to homework and testing. Unlike traditional systems that emphasize heavy workloads and high-stakes examinations, Finland’s educational philosophy promotes a balanced and holistic learning experience. In this blog post, we will explore Finland’s innovative strategies regarding homework and testing, and discuss how these approaches contribute to the remarkable success of the Finland education system .
1. The Role of Homework in Finland:
Finland takes a remarkably different approach to homework compared to many other countries. In Finnish schools, the emphasis is not on the quantity but on the quality of homework. Instead of assigning excessive amounts of homework, Finnish educators focus on promoting meaningful and purposeful assignments that reinforce classroom learning. Homework is viewed as a tool for self-reflection, consolidation of knowledge, and promoting independent thinking. Additionally, the Finnish system recognizes the importance of free time for children to engage in recreational activities, develop social skills, and maintain a healthy work-life balance. Consequently, Finnish students have significantly less homework compared to their peers in other nations, allowing them ample time for rest, relaxation, and pursuing extracurricular interests.
Also read: What is Finnish education System?
2. Assessments in Finland:
Moving Beyond Standardized Testing: Unlike many countries that heavily rely on standardized testing as a measure of student performance, Finland adopts a more comprehensive and holistic approach to assessments. The Finnish education system prioritizes continuous evaluation and formative assessments over high-stakes exams. Teachers regularly assess students’ progress through a combination of observation, classroom discussions, project work, and practical assignments. This student-centered approach allows teachers to understand each student’s unique learning style and adapt instruction accordingly. By focusing on individual growth and providing constructive feedback, Finnish educators foster a supportive and nurturing learning environment, free from the stress and pressure associated with high-stakes testing.
3. The Benefits of Finland’s Approach:
Finland’s approach to homework and testing has several notable benefits. Firstly, by reducing the emphasis on homework, Finnish students experience less academic stress and have more time for relaxation and extracurricular activities. This balanced approach promotes overall well-being and fosters the development of well-rounded individuals. Secondly, the shift away from standardized testing allows for a more comprehensive evaluation of students’ abilities, including their critical thinking, problem-solving, and collaborative skills. This holistic assessment aligns with the needs of the 21st-century workforce, which values creativity, adaptability, and teamwork. Additionally, the focus on formative assessments provides students with regular feedback, allowing them to understand their strengths and areas for improvement, and promoting a growth mindset.
Conclusion:
Finland has revolutionized the conventional notions of education through its unique approach to homework and testing. By emphasizing purposeful homework and prioritizing holistic assessments, Finland has cultivated an educational system that nurtures well-rounded individuals, fosters critical thinking, and instills a genuine passion for learning. While every educational system faces its own challenges, Finland’s remarkable success serves as an inspiration for other nations to reassess their approaches to homework and testing. By adopting a more balanced and student-centered methodology, countries can establish educational environments that prioritize well-being, stimulate creativity, and effectively prepare students for the demands of the future. Finland’s educational paradigm shift stands as a testament to the transformative power of reimagining traditional education systems, emphasizing the vital importance of continually questioning and improving our approaches to teaching and learning. If you are interested in providing your child with a Finnish education curriculum, look no further than finlandeducationhub.com – your comprehensive resource for all your needs.
Leave A Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.

27 Surprising Finnish Education System Facts and Statistics
There has been a lot of press recently about how the education system in Finland is one of the best in the world and how they are using radical (compared with the UK and the US) ideas to help achieve their status as one of the best.
Anywhere you look the proof doesn’t seem to lie, yet how exactly is the Finnish Education system achieving such greatness? Their students outperform students in the US and the UK in most, if not all areas and their teachers enjoy a much better work life balance. Let’s take a dive into some of the things the Finnish are doing.
The Program for International Student Assessment (PISA) , a survey taken every three years by the Organization for Economic Cooperation and Development (OECD) routinely releases data which shows that Americans and British students are seriously lagging behind in many educational performance assessments.
The Finnish Education System
#1 Finnish children enter education at a later age than in many countries. They start school at age 7 and believe that “starting children in school before they’re naturally developmentally ready has no scientifically proven long-term advantage”.
#2 Prior to age 7, Finnish school children can attend day care/nursery school but they do not have formal education whilst there, Instead, they focus on creative play . “They need time to play and be physically active. It’s a time for creativity”. says Tiina Marjoniemi, head of Franzenia daycare center in Helsinki. The Guardian
#3 For every 45 minutes of learning, students enjoy 15 minutes of play.
#4 School is only compulsory for 11 years, meaning students can leave education at age 18. Everything after that is optional. This idea is thought to prepare Finnish students for the real world.
#5 Finish students are not measured at all for the first six years of their education.
#6 Students in Finland only have to sit for a centralized exam (National Matriculation Exam) at the age of 18-19 years old (after 12 years of school).
Finland School Hours
#7 Finnish students do the least number of class hours per week in the developed world, yet get the best results in the long term. The school day starts between 8-9am and is finished by 2pm.
Finland Education Ranking
#8 The schools in Finland are not ranked in any way, there are no comparisons made between schools, regions, teachers or even students. They believe that cooperation is the key to success, not competition.
#9 Finnish Teachers are some of the most qualified in the world. The requirements for becoming a teacher in Finland are set very high, only around the top 10% of applicants are successful and all of those have a masters degree (which incidentally is fully subsidised!).
#10 Finnish teachers have the same status as doctors and lawyers. ( I wish that was the case in the UK! )
#11 Finnish Teachers are not graded. This is probably a direct result of their rigorous selection process and because of this, in Finland, they don’t feel the need to constantly assess and grade their teachers. If a teacher isn’t performing satisfactorily, it is up to the schools principal/head to deal with it. Pasi Sahlberg, director of the Finnish Ministry of Education and writer of Finnish Lessons, said this about teachers’ accountability:
“There’s no word for accountability in Finnish… Accountability is something that is left when responsibility has been subtracted.”
#12 Schools are not inspected. School inspections were actually abolished in Finland in the early 1990s. They have the ideology that they can help direct and assist through support and funding. Again, they trust the professionalism of teachers and school leaders. Schools are encouraged to self-evaluate along.
#13 There are no selective schools or private schools. One of the reasons why there is no competition between Finnish schools is that all schools are funded through public funds. No competition = level playing field.
#14 All Finnish school children receive free school meals, all of them, all the way through school!. There has been a healthy hot lunch served to all students been since 1943 for the whole 9 years at school. ( Huffingtonpost.com )
#15 Finnish students all have access to support that is individually based on their specific needs from the start of their school career. They believe that every child has some special needs and therefore special education is for everyone.
#16 The Basics are the priority. Rather than focus on increasing test scores and dominating in math, science and English, the Finnish education system focus on creating a healthy and harmonious environment for students and learning. The ideology of the Finnish education system is that education should be an “ instrument to balance out social inequality “.
#17 Finnish students have the same teacher for up to 6 years of their school career. This is one of the pillars of their harmonious education environment ideology. It allows student/teacher relationships to grow year on year, allowing a much deeper level of trust and respect than only having one year.
Finland Education Curriculum
#18 Finnish Students have less homework than any other student on the planet. Even with fewer school hours, they are still getting everything they need to be done whilst at school. This, in turn, builds on a Finnish child’s ability to grow and learn into a happy and responsible adult.
#19 All classes are mixed ability. This is unpopular in a lot of education systems in the UK and the US (I know, my own school recently adopted this policy (Personally, I love it) and there can be a lot of teachers that don’t like it. However, some of the most successful education systems have mixed ability classes, so it does work!
#20 Finnish Students learn more languages. They learn Finnish from their first day at school. At age 9 they start learning their second language (which is usually English). By age 11 they start learning Swedish, which is Finland’s second language. Many students even start learning a fourth language when they are 13. They are only tested on their first two languages in the final exam at the end of high school.
#21 Teachers only generally spend 4 hours a day in the classroom and have 2 hours every week for professional development , thus reducing teacher stress.
#22 The Finnish national curriculum is a broadly based guideline, allowing teachers to use their own style and ideas in the classroom. This builds on the trust that the Finnish education system has in its teachers.
Finland Education Statistics
#23 93% of students graduate from high school. More than in the US.
#24 66% of high school students go on to further education (college or vocational courses).
#25 Finland spends about 30% less per student than the US, the UK, Japan and Germany. ( OECD Indicators )
#26 Just under 100% of 9th-grade students in Finland go on to high school. This figure includes most of the severely disabled children ( smithsonian.com )
#27 43% of those students in further education (16+) attend vocational school.
So there we have it, Finnish students and teachers are part of a great system. Having worked with several Finnish teachers, I can tell you that their ideology and these strategies work, very well!
Similar Posts:
- 35 of the BEST Educational Apps for Teachers (Updated 2024)
- Discover Your Learning Style – Comprehensive Guide on Different Learning Styles
- 20 Huge Benefits of Using Technology in the Classroom
2 thoughts on “27 Surprising Finnish Education System Facts and Statistics”
As a student in Finland I realized some of this information is outdated. #1 – It states that children start their education at the age of 7. This is no longer correct because they can start is at the ages of 5, 6 or 7. Typically they do at ages 6 or 7. #3 – It is very school based. some schools do not follow this and it depends a lot. A school can have 45 minute lessons and a 5 min break. #4 – Over resent years it has changed into 9 years of compulsory education (basic education) 2-4 years of upper secondary studies/vocational application. #6 – the matriculation examination is at the age of 18 (typically the last two years of upper secondary studies). Not all students do this because they choose to go to vocational school. #7 – It is again very school based because some schools follow periods (certain subjects for 6 weeks and then the timetable changes). Most schools and students most likely have days from 8am-3pm. It depends a lot what day it is. #16 – To apply to upper secondary school and vocational schools they calculate the average of math, English, Finnish, Swedish, history, civics, religion/ethics, physics, chemistry, biology, geography, health education. #17 – Pert of this is true. Best case scenario they do have the same teacher for six years, but most of the time teachers are only qualified to teach certain grade levels. #18 – The amount of homework totally depends on the teacher. It depends how much the teacher wants them to do. Most times homework is tasks that they did not get done on lessons or ones that deepen the meaning of the subject. #20 – there are a lot of confusing things about this. In most schools the child starts learning Finnish from first grade onwards. From grade 3 onwards they start learning English. From grade 5 onwards they can decide if the want another language (typically French, German or Spanish). From grade 6 onwards they start learning Swedish. In the matriculation examinations the test Finnish and a second home language so either Swedish or English. #21 – Subject teachers can have as many hours a day as the pupils. This all depends how many subjects they are qualified to teach.
According to the Bildung Review the Finnish educational system is failing. Not testing and focusing only on cooperation seems to have failed. I hope Finland will shift in the proper educational focus.
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.
- Education System
- Fins and Fun

Homework in Finland School

How many parents are bracing themselves for nightly battles to get their kids to finish their homework every year with the beginning of a school year? Thousands and thousands of them. Though not in Finland. The truth is that there is nearly no homework in the country with one of the top education systems in the world. Finnish people believe that besides homework, there are many more things that can improve child’s performance in school, such as having dinner with their families, exercising or getting a good night’s sleep.
Do We Need Homework?
There are different homework policies around the world. The Organization for Economic Cooperation and Development (OECD) keeps track of such policies and compares the amount of homework of students from different countries. For example, an average high school student in the US has to spend about 6 hours a day doing homework, while in Finland, the amount of time spent on after school learning is about 3 hours a day. Nevertheless, these are exactly Finnish students who lead the world in global scores for math and science. It means that despite the belief that homework increases student performance, OECD graph shows the opposite. Though there are some exceptions such as education system in Japan, South Korea, and some other Asian countries. In fact, according to OECD, the more time students spend on homework, the worse they perform in school.
Finnish education approach shows the world that when it comes to homework, less is more. It is worth to mention that the world has caught onto this idea and, according to the latest OECD report, the average number of hours spent by students doing their homework decreased in nearly all countries around the world.
So what Finland knows about homework that the rest of the world does not? There is no simple answer, as the success of education system in Finland is provided by many factors, starting from poverty rates in the country to parental leave policies to the availability of preschools. Nevertheless, one of the greatest secrets of the success of education system in Finland is the way Finns teach their children.
How to Teach Like The Finns?
There are three main points that have to be mentioned when it comes to the success of education system in Finland.
First of all, Finns teach their children in a “playful” manner and allow them to enjoy their childhood. For example, did you know that in average, students in Finland only have three to four classes a day? Furthermore, there are several breaks and recesses (15-20 minutes) during a school day when children can play outside whatever the weather. According to statistics, children need physical activity in order to learn better. Also, less time in the classroom allows Finnish teachers to think, plan and create more effective lessons.
Secondly, Finns pay high respect to teachers. That is why one of the most sought after positions in Finland is the position of a primary school teacher. Only 10% of applicants to the teaching programs are accepted. In addition to a high competition, each primary school teacher in Finland must earn a Master’s degree that provides Finnish teachers with the same status as doctors or lawyers.
High standards applied to applicants for the university teaching programs assure parents of a high quality of teaching and allow teachers to innovate without bureaucracy or excessive regulation.
Thirdly, there is a lot of individual attention for each student. Classes in Finland are smaller than in the most of other countries and for the first six years of study, teachers get to know their students, their individual needs, and learning styles. If there are some weaker students, they are provided by extra assistance. Overall, Finnish education system promotes warmth, collaboration, encouragement, and assessment which means that teachers in this country are ready to do their best to help students but not to gain more control over them.
The combination of these three fundamentals is the key to success of any education system in the world and Finns are exactly those people who proved by way of example that less is more, especially when it comes to the amount of homework.

System of education in Finland

School System in Finland

Finland Education Reform

Fins and Fun: Distinctive Features of Education in Finland

10 Facts About Education in Finland
- Privacy policy

Harnessing AI in Education: How Finnish Students Outperform Their European Peers

Don’t Let Faulty Plumbing Ruin your Child’s Lesson

There's no Homework in Finland
THERE'S No Homework In Finland Finland's school system accomplishes some impressive feats: 93% THEIR HIGH SCHOOL 78% GRADUATION RATE IS AT 93%. 75% COMPARED TO 78% IN CANADA. AND 75% IN THE US. %! ABOUT 2 IN 3 STUDENTS IN FINLAND WILL GO ON TO COLLEGE. That's the highest rate in all of Europe. AND THEIR TEST SCORES DOMINATE EVERYONE ELSE. Mean scores for PISA test (Program for International Student Assessment) 2006. 520 530 540 550 560 570 Finland Hong Kong Canada Taiwan Estonia Japan New Zealand Australia Netherlands Liechtenstein So what makes Finnish students so successful? STUDENTS GET PLENTY OF TEACHER INTERACTION. Finland and New York City have the same number of teachers. But Finland has nearly half the number of students. FINLAND NYC Students: Students: 600,000 ALMOST 1.1 MILLION Student to teacher ratio: Student to teacher ratio: 1 TO 12 1 TO 24 THOUGH 1 IN 3 FINNISH STUDENTS RECEIVES SOME SORT OF SPECIAL HELP IN SCHOOL... There are no separate classrooms for accelerated learning or special education. STANDARDIZED TESTING IS KEPT TO A MINIMUM. BEFORE A NEW YORK STUDENT REACHES HIGH SCHOOL, HE OR SHE WILL HAVE TAKEN 10 STANDARDIZED TESTS. Collectively, US students take 100 million standardized tests a year. FINLAND'S ONLY STANDARDIZED TEST IS TAKEN WHEN STUDENTS ARE 16 YEARS OLD. KIDS HAVE MORE TIME TO BE KIDS. AN AVERAGE US 5TH Finnish students GRADER HAS 50 MIN. rarely do homework OF HOME WORK until their teens. PER DAY. 10-11 YRS. ÖLD 13-14 YRS. ÖLD And while US elementary students average 27 minutes of recess... ... STUDENTS IN FINLAND GET 27 MIN 75 MIN ABOUT 75 MINUTES DAY. Most importantly? Finland knows good teachers are essential. TEACHERS IN FINLAND ARE ALL REQUIRED TO HAVE A MASTER'S DEGREE. (Which is fully subsidized by the state.) ONLY THE TOP 10% OF GRADUATES ARE ACCEPTED INTO TEACHING PROGRAMS. AND FINLAND'S TEACHERS ARE AS ESTEEMED AS THEIR DOCTORS OR LAWYERS. The rest of the world could learn a lot from you, Finland. Sources: [1] http://www.scribd.com/doc/82204617/Global-Graduation-Rates-By-Country-Source-OECD [2] http://today.msnbc.msn.com/id/48343652/ns/today-back_to_school/t/home work-overload-gets-f-experts/ [3] http://www.businessinsider.com/finland-education-school-2011-12?op=1 [4] http://seattletimes.com/html/localnews/2019676789_finland14m.html [5] http://edpolicy.stanford.edu/sites/default/files/publications/secret-finland%E2%80%99s-success- [6] http://www.time4learning.com/testprep/index.php/new-york-state-standardized-test-prep/educating-teachers.pdf This work is under a Creative Commons license. Brought to you by: Online Classes.org BY NO ND OnlineClasses.org
You may also like...
For hosted site:
For wordpress.com:
Get the Reddit app
Ask away! Disclaimer: This is an anonymous forum so answers may not be correct
if other countries can see that Finland have better education system by lessening the student's homework why don't the other countries also lessen it?

COMMENTS
Student-oriented approach to education in Finland has been recognised as the most well-developed educational system in the world and ranks third in education worldwide.
The truth about Finland's great schools: Yes, kids do get homework, and no, they didn't stop teaching individual subjects. - The Washington Post Advertisement
Finland's recent decline in international test scores has led many to question whether its education system is truly the best.
From tests to teachers, a number of simple changes have transformed Finland's education system into one of the world's most successful. Read to know them.
Finland has vastly improved in reading, math and science literacy over the past decade in large part because its teachers are trusted to do whatever it takes to turn young lives around. This 13 ...
The homework load for children in Finland varies by teacher, but is lighter overall than most other developed countries. This insight is supported by research, which has found little academic benefit in childhood for any more than brief sessions of homework until around high school.
No tests, no homework, no punishment, here's why Finnish education is still at the top of the world. Finland has always been ranked first in math, reading and science in the PISA (Programme for International Student Assessment) rankings of the Organization for Economic Cooperation and Development.
Before we answer that, some background: Ever since international student assessments first flagged Finland as an apparent "education superpower," the country's experience has been mined for lessons. What did Finland do and achieve in education, and how relevant is its experience for other countries?
Finland doesn't have a national curriculum now, and Finnish education experts brag about how much autonomy teachers get in the classroom. But that wasn't always the case.
1. Finland. On top of the list of countries giving less assignment is Finland. Apart from boasting of short school terms and extended holidays, the country limits the homework load to 2.8 hours total of homework per week. Despite their educational system, Finland manages to rank among the top countries in math and science innovations and also ...
What Americans Keep Ignoring About Finland's School Success The Scandinavian country is an education superpower because it values equality more than excellence.
Finland's approach to homework and testing has several notable benefits. Firstly, by reducing the emphasis on homework, Finnish students experience less academic stress and have more time for relaxation and extracurricular activities. This balanced approach promotes overall well-being and fosters the development of well-rounded individuals.
Why is the Finnish education system one of the best in the world? These facts and statistics explain how they've managed it.
Moore discusses Finland's education policy (almost no homework, no standardized testing), speaking with Krista Kiuru, the Finnish Minister of Education. Moor...
How many parents are bracing themselves for nightly battles to get their kids to finish their homework every year with the beginning of a school year? Thousands and thousands of them. Though not in Finland. The truth is that there is nearly no homework in the country with one of the top education systems in the world. Finnish people believe that besides homework, there are many more things ...
Here's a quick reminder: China has a population of 1.3 BILLION people. Dirty math puts that at quadruple the United States and two hundred and sixty times the population of Finland.
There's no Homework in Finland. THERE'S No Homework In Finland Finland's school system accomplishes some impressive feats: 93% THEIR HIGH SCHOOL 78% GRADUATION RATE IS AT 93%. 75% COMPARED TO 78% IN CANADA. AND 75% IN THE US. %! ABOUT 2 IN 3 STUDENTS IN FINLAND WILL GO ON TO COLLEGE. That's the highest rate in all of Europe.
Misconceptions About Homework in Finland I've been seeing all over the internet that there is no homework in Finland. Even if you google it the answer is that there is no homework in Finland. But I live in Finland and I defidently have homework. Where is this misconception coming from? Like we don't have that much homework but its still ...
As you may know Finland has the highest educational achievement rankings in the world. In the youtube comments though Finnish people say they do have homework, but it is not pushed and they don't have to do it. They also contest the claims about time at school. One comment says first grade is 5 hrs a day, 6-8 hrs in 2nd to 6th grade, and 10 hrs ...
if other countries can see that Finland have better education system by lessening the student's homework why don't the other countries also lessen it?
President Joe Biden on Thursday participated in the most high-stakes news conference of his political career on the sidelines of the NATO summit, aiming to convince his detractors and supporters ...