Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 February 2021

The impact of daily caffeine intake on nighttime sleep in young adult men
- Janine Weibel 1 , 2 ,
- Yu-Shiuan Lin 1 , 2 , 3 ,
- Hans-Peter Landolt 4 , 5 ,
- Joshua Kistler 1 , 2 ,
- Sophia Rehm 6 ,
- Katharina M. Rentsch 6 ,
- Helen Slawik 7 ,
- Stefan Borgwardt 3 ,
- Christian Cajochen 1 , 2 na1 &
- Carolin F. Reichert 1 , 2 na1
Scientific Reports volume 11 , Article number: 4668 ( 2021 ) Cite this article
37k Accesses
18 Citations
262 Altmetric
Metrics details
- Slow-wave sleep
Acute caffeine intake can delay sleep initiation and reduce sleep intensity, particularly when consumed in the evening. However, it is not clear whether these sleep disturbances disappear when caffeine is continuously consumed during daytime, which is common for most coffee drinkers. To address this question, we investigated the sleep of twenty male young habitual caffeine consumers during a double-blind, randomized, crossover study including three 10-day conditions: caffeine (3 × 150 mg caffeine daily), withdrawal (3 × 150 mg caffeine for 8 days, then switch to placebo), and placebo (3 × placebo daily). After 9 days of continuous treatment, electroencephalographically (EEG)-derived sleep structure and intensity were recorded during a scheduled 8-h nighttime sleep episode starting 8 (caffeine condition) and 15 h (withdrawal condition) after the last caffeine intake. Upon scheduled wake-up time, subjective sleep quality and caffeine withdrawal symptoms were assessed. Unexpectedly, neither polysomnography-derived total sleep time, sleep latency, sleep architecture nor subjective sleep quality differed among placebo, caffeine, and withdrawal conditions. Nevertheless, EEG power density in the sigma frequencies (12–16 Hz) during non-rapid eye movement sleep was reduced in both caffeine and withdrawal conditions when compared to placebo. These results indicate that daily caffeine intake in the morning and afternoon hours does not strongly impair nighttime sleep structure nor subjective sleep quality in healthy good sleepers who regularly consume caffeine. The reduced EEG power density in the sigma range might represent early signs of overnight withdrawal from the continuous presence of the stimulant during the day.
Similar content being viewed by others

A novel bedtime pulsatile-release caffeine formula ameliorates sleep inertia symptoms immediately upon awakening

Objective sleep quality predicts subjective sleep ratings
Effects of the selective orexin-2 receptor antagonist JNJ-48816274 on sleep initiated in the circadian wake maintenance zone: a randomised trial
Introduction.
Caffeine is the most popular psychoactive substance in the world 1 , consumed daily by around 80% of the population 2 . While caffeine is frequently used to counteract sleepiness and boost performance 3 , its consumption is commonly avoided in the evening 4 , 5 to prevent adverse consequences on nocturnal sleep 3 . The sleep disrupting effects of caffeine are mainly attributed to its influence on the homeostatic component of sleep-wake regulation. Sleep homeostasis describes the increase in sleep pressure during time awake and its dissipation during the following sleep episode 6 , which has been suggested to be related to rising and decreasing concentrations of adenosine 7 . Caffeine is an adenosine receptor antagonist, which blocks the A 1 and A 2A adenosine receptors in the central nervous system 1 . It may, thus, attenuate the increase in sleep pressure during wakefulness 8 and lead to delayed sleep initiation and more superficial sleep 9 .
The effects of caffeine intake on the quality and quantity of sleep depend on the timing of its consumption. More specifically, caffeine consumed in the evening hours prolongs sleep latency 10 , 11 , 12 , 13 , 14 , reduces total sleep time (TST) 10 , 11 , 12 , 14 , 15 , shortens deep sleep 10 , 12 , 13 , 14 , 15 , and decreases electroencephalographically (EEG)-derived slow-wave activity (SWA) 10 , while activity in the sigma range is increased 10 . However, evening caffeine intake only accounts for approximately 10–20% of the total daily caffeine intake in regular consumers 4 , 5 . It needs to be elucidated whether habitual caffeine intake restricted to the morning and afternoon hours similarly affects nighttime sleep.
Furthermore, not only the timing but also the frequency of preceding caffeine intake prior to sleep may be an important factor for the repercussions on sleep. The majority of the worldwide population consumes caffeine on a daily basis 2 , which can lead to tolerance development due to the recurrent supply of the psychostimulant 1 . In line with these results, the sleep-disrupting effects of continuous high-dose caffeine in the morning, afternoon, and evening (3 × 400 mg) intake vanished and only stage 4 sleep remained reduced after 1 week of caffeine intake 12 . However, whether more sensitive markers for sleep intensity such as spectral sleep EEG measures, adapt to the long-term exposure to the stimulant has to our best knowledge not yet been investigated.
Importantly, not only caffeine per se, but also the state of acute abstinence to which regular consumers expose themselves every night, might affect sleep. This so-called overnight abstinence represents the start of a caffeine withdrawal phase 16 . Withdrawal symptoms such as increased tiredness 17 , longer sleep duration, and better sleep quality 18 can be observed at a subjective level starting roughly 12 h after last caffeine intake 17 . However, the influence of caffeine withdrawal on objective EEG-derived sleep variables were not systematically reported up to date and remain to be compared against a placebo-baseline.
Here we aimed at determining whether daily caffeine intake during morning and afternoon hours impairs nighttime sleep structure and sleep intensity after continuous daytime caffeine intake over 9 days. We hypothesized a reduced depth of sleep after caffeine intake, indexed in shortened slow-wave sleep (SWS) duration and a decrease in SWA compared to placebo. Moreover, we hypothesized that the abrupt cessation from the daily intake generates acute subjective withdrawal symptoms, and changes sleep structure and intensity compared to both the daily caffeine intake and the placebo-baseline.
Salivary caffeine levels
Caffeine levels significantly differed between each of the three conditions (main effect of condition: F 2,90.7 = 46.12, p < 0.001) with the highest levels in the caffeine condition and the lowest in the placebo condition (post-hoc comparisons: p all < 0.01). In addition, a significant interaction of the factors condition and time ( F 2,89.6 = 10.65, p < 0.001) confirmed that caffeine levels were modulated by time with levels decreasing during nighttime sleep in the caffeine condition only (post-hoc comparison: p < 0.001), see Fig. 1 .
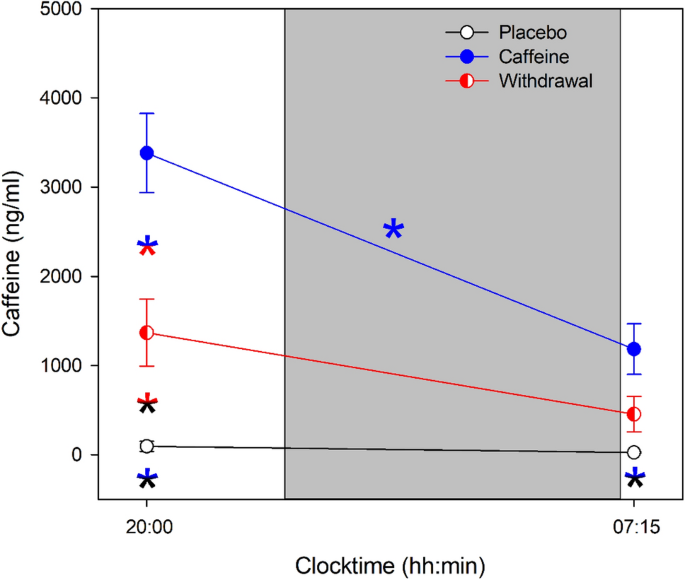
Average caffeine levels collected prior to and after nighttime sleep (grey bar) in the placebo (black open circles), caffeine (blue filled circles), and withdrawal (red semi-filled circles) condition (mean values ± standard errors). The x-axis indicates the mean time of day of sample collection and color-coded asterisks represent significant ( p < 0.05) post-hoc comparisons of the interaction effect condition × time.
Table 1 summarizes the statistical analyses of subjective sleep quality and objective sleep structure assessed during nighttime sleep. Analyses of subjective sleep quality assessed with the Leeds Sleep Evaluation Questionnaire (LSEQ) did not reveal significant differences among the three conditions in any of the four domains of sleep quality ( p all > 0.05).
In line with these results, the analyses of the polysomnography (PSG) did not reveal significant differences in total sleep time (TST), sleep efficiency (SE), sleep latencies, or the relative amount of sleep stages among the three conditions ( p all > 0.05).
In a next step, we analyzed all-night EEG power density in the range of 0.75–32 Hz over the central derivations recorded during non-rapid eye movement (NREM) sleep. In contrast to our assumptions, we did not find any significant differences among the three conditions in the lower frequency bins (0.75–13.25 Hz; p all > 0.05). However, power density was significantly reduced compared to placebo in the sigma range during both withdrawal (frequency bins 13.5–17.25 Hz and 18–18.5 Hz; p all < 0.05) and caffeine (frequency bins 13.5–16 Hz; p all < 0.05).
In a second step, we were interested in the temporal dynamics of both SWA and sigma activity across the night assessed during NREM sleep. As depicted in Fig. 2 (top panel), SWA showed a typical temporal pattern with increased activity during the first NREM cycle followed by a steady decline across the night (main effect of time: F 39,613 = 26.28, p < 0.001). However, differences among the three conditions did not reach significance (main effect of condition: F 2,178 = 1.33, p = 0.27). Also, the interaction of condition and time was not significant ( F 78,1060 = 0.89, p = 0.74).
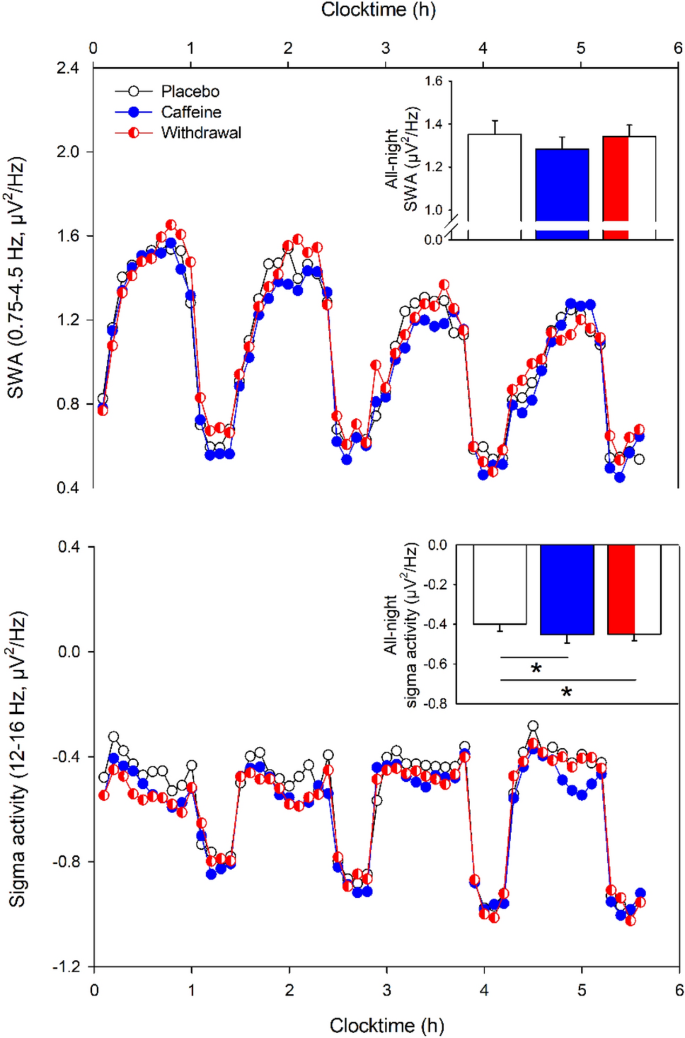
Temporal dynamics of SWA (top) and sigma activity (bottom) during the first four sleep cycles in the placebo (black open circles), caffeine (blue filled circles), and the withdrawal (red semi-filled circles) condition (mean values). The x-axis indicates the mean time of day. While SWA (0.75–4.5 Hz) was not significantly affected by the treatment, sigma activity (12–16 Hz) showed reduced activity during both caffeine and withdrawal compared to the placebo condition ( p all < 0.05). The inset in each right upper corner represents the mean values ± standard errors of the all-night SWA and sigma activity respectively during NREM sleep in the placebo, caffeine, and withdrawal condition. While all-night SWA (0.75–4.5 Hz) did not differ among the conditions, sigma activity (12–16 Hz) was lower in the caffeine and withdrawal condition compared to placebo ( p < 0.05). All analyses are based on log-transformed data.
As illustrated in Fig. 2 (bottom panel), sigma activity was significantly reduced in both the caffeine and withdrawal conditions compared to placebo intake (main effect of condition: F 2,209 = 19.96, p < 0.001; post-hoc comparisons: p < 0.001) and the interaction of condition and time tended to be significant ( F 78,1049 = 1.25, p = 0.08).
Taken together, we could not confirm our assumption of a caffeine-induced reduction of sleep depth, neither in terms of shorter SWS nor in terms of reduced SWA in the caffeine compared to the placebo condition. Based on the discrepancies between the present results and a previous study about the effects of chronic caffeine intake on sleep 12 , we thus explored whether differences in the individual levels of caffeine before sleep could explain the variance within SWS and SWA. However, no significant effects were observed when controlling for dependent observations within subjects ( p > 0.05).
Subjective caffeine withdrawal symptoms
Analyses of the relative withdrawal symptoms yielded a significant main effect of condition ( F 2,20.2 = 11.30, p < 0.01) indicating more withdrawal symptoms during the withdrawal compared to the caffeine condition (post-hoc comparison: p < 0.01), depicted in Fig. 3 . This effect was modulated by time (interaction of condition × time: F 2,37.2 = 3.43, p = 0.04), such that the increase in symptoms during the withdrawal compared to caffeine condition was particularly present during the last measurement ( p < 0.01), i.e. 31 h after the last caffeine intake in the withdrawal condition.

Relative withdrawal symptoms in the caffeine and withdrawal condition (i.e. withdrawal score of the caffeine and withdrawal condition respectively minus the score of the placebo condition) assessed 35 min, 4 h, and 8 h after wake-up on day ten of treatment. Depicted are mean values and standard errors of the relative values (i.e. difference to placebo). Overall, volunteers reported more withdrawal symptoms in the withdrawal condition compared to the caffeine condition ( p < 0.05). This difference was particularly present 8 h after wake-up during withdrawal compared to caffeine ( p < 0.001).
The aim of the present study was to investigate the influence of daily daytime caffeine intake and its cessation on nighttime sleep in habitual caffeine consumers under strictly controlled laboratory conditions. Strikingly, caffeine consumption did not lead to clear-cut changes in nighttime sleep structure nor in subjective sleep quality when assessed 8 and 15 h after the last intake in the caffeine and withdrawal condition, respectively. The evolution of subjective withdrawal symptoms indicates that withdrawal becomes perceivable at earliest between 27–31 h after intake. However, compared to placebo, EEG power density was reduced in the sigma range during both caffeine and withdrawal conditions. We conclude that daily daytime intake of caffeine does not strongly influence nighttime sleep structure nor subjective sleep quality in healthy men when consumed in the morning, midday, and in the afternoon. In contrast to the reported increases in sigma activity after acute caffeine intake 10 , the observed changes in the sigma frequencies might point to early signs of caffeine withdrawal which occur due to overnight abstinence and presumably derive from preceding caffeine-induced changes in adenosine signaling.
To quantify the influence of caffeine on sleep, the stimulant is commonly administered close to the onset of a sleep episode 10 , 11 , 12 , 13 , 14 , for instance within 1 h prior to bedtime 10 , 11 , 13 , 14 . Taking into account that caffeine plasma levels peak within 30–75 min following caffeine ingestion 19 , consumption within 1 h prior to sleep allows the stimulant to exert its maximum effects at sleep commencement. Indeed, the sleep disrupting effects of caffeine are frequently reported to affect sleep initiation or the first half of the sleep episode 10 , 11 , 12 , 13 , 14 . Moreover, sleep intensity, which is usually strongest at the beginning of the night 20 , was particularly disrupted during the first sleep cycle, as indexed in reduced SWS and SWA 10 . However, caffeine intake in the evening, particularly after 9 pm is rare 5 , presumably to avoid impairment of subsequent sleep 3 . Up to date it remained fairly unclear whether caffeine intake in the morning and afternoon still bears the potential to disrupt nighttime sleep. While we observed a delay of 25 min in sleep episodes during caffeine intake prior to the laboratory part, PSG-derived data after 9 days of regular caffeine intake did not yield a significant change in sleep architecture. Thus, our data provide first evidence that daily daytime caffeine intake does not necessarily alter subsequent sleep structure and SWA when consumed > 8 h prior to sleep. Importantly, our findings do not preclude potential impairments of nighttime sleep after morning caffeine intake, if preceded by several days of abstinence from the stimulant 21 . It rather appears likely that the duration of preceding caffeine consumption drives the discrepancies between acute and chronic effects of caffeine on sleep.
Chronic caffeine intake induces some tolerance development in both physiological measures such as cortisol 22 , blood pressure 23 , heart rate 24 , and also subjective measures such as alertness 18 . Over time, the stimulatory effects of the substance vanish potentially due to changes in adenosine levels 25 and/or adenosine receptors 26 , 27 , 28 . Accordingly, a 1-week treatment of caffeine reduced the sleep disrupting effects, even under conditions of high evening dosages 12 . Thus, the available evidence and the absence of clear-cut changes in the present study point to adaptive processes in sleep initiation, sleep structure, and subjective sleep quality due to the long-term exposure to the stimulant.
However, chronic caffeine consumption bears the risk of withdrawal symptoms when abruptly ceased. These symptoms have been reported to occur as early as 6 h but with peak intensity being reached within 20–51 h after last caffeine intake 17 . While 25 h of caffeine abstinence might not affect nighttime sleep structure 12 , 32 h of abstinence improved subjective sleep quality 18 . Thus, scheduling the start of the sleep episode to 15 h after the last caffeine intake, as in our withdrawal condition, was probably too early to detect changes in sleep structure or subjective sleep quality. In line with this assumption, volunteers subjectively indicated withdrawal symptoms 31 h after caffeine abstinence in the withdrawal condition compared to caffeine. Thus, our findings support the notion that the alterations in sleep structure and subjective sleep quality induced by caffeine abstinence potentially develop at a later stage (> 27 h) of caffeine withdrawal.
Most strikingly and unexpectedly, a reduction in NREM sigma activity during both the withdrawal and caffeine conditions was observed, a phenomenon which is commonly reported under conditions of enhanced sleep pressure 29 , 30 , 31 , 32 . Thus, it seems at first glance in contrast to the reported increases in this frequency range 10 , 21 and the well-known alerting effects after acute caffeine intake 18 . However, during conditions of chronic caffeine intake, mice showed a deeper sleep compared to placebo 33 . Moreover, repeated caffeine intake enhances the sensitivity of adenosine binding 34 presumably due to upregulated adenosine receptors 26 , 27 , 28 or changes in the functions of adenosine receptor heteromers 35 . These neuronal alterations in the adenosinergic system might drive the commonly observed changes in the homeostatic sleep-wake regulation such as increased sleepiness when caffeine intake is suddenly ceased 17 . As reported previously, we also observed in the present study higher subjective sleepiness following caffeine withdrawal when compared to the placebo and caffeine conditions 36 . Thus, the reduction in sigma activity might reflect adenosinergic changes which already emerge 8 and 15 h after the last caffeine intake in the caffeine and withdrawal condition, respectively. This reduction might reflect withdrawal symptoms which chronic consumers reverse daily by the first caffeine dose. Given the high prevalence of daily caffeine consumers in the society, these findings stress the importance to carefully control for prior caffeine intake when assessing sleep in order to exclude potential confounding by induced withdrawal symptoms which are only detectable in the microstructure of sleep.
Our study has some limitations which must be taken into careful consideration when interpreting the present findings. First, age moderates the effects of caffeine on sleep 11 , 14 . Thus, the present results cannot be generalized to other age groups such as to middle-aged consumers which are more vulnerable to the caffeine-induced effects on sleep 11 , 14 . Second, only a limited number of participants were studied. However, a well-controlled study design was employed and power calculation on the basis of an earlier study 12 indicated a sufficient sample size. Third, we do not have any information about the participants’ genetic polymorphisms which have been shown to modulate the metabolism of caffeine 37 . In addition, a genetic variation of the ADORA2A genotype has been linked with caffeine sensitivity to the effects on sleep 38 . Thus, carriers of this genetic variance are more likely to curtail caffeine consumption and are consequently excluded from the present study leading to a selection bias. However, the focus of the present study was to investigate habitual caffeine consumers as they represent the majority of the worldwide population 2 . Fourth, to reduce variance in the data incurred by the influence of the menstrual cycle on sleep 39 and the interaction between caffeine metabolism and the use of oral contraceptives 40 , 41 , only male volunteers were included which clearly reduces the generalizability of the findings.
In conclusion, we report evidence that daily daytime intake of caffeine and its cessation has no strong effect on sleep structure or subjective sleep quality. However, the quantitative EEG analyses revealed reduced activity in the sigma range during both caffeine and withdrawal. These subtle alterations point to early signs of caffeine withdrawal in the homeostatic aspect of sleep-wake regulation which are already present as early as 8 h after the last caffeine intake. Thus, habitual caffeine consumers constantly expose themselves to a continuous change between presence and absence of the stimulant. Around the clock, their organisms dynamically adapt and react to daily presence and nightly abstinence.
Participants
Twenty male volunteers were recruited into the present study through online advertisements and flyers distributed in public areas. Interested individuals aged between 18 and 35 years old (mean age ± SD: 26.4 ± 4 years) and reporting a daily caffeine consumption between 300 and 600 mg (mean intake ± SD: 478.1 ± 102.8 mg) were included. The self-rating assessment for the daily amount of caffeine intake was structured based on Bühler et al. 42 , and the amount of caffeine content was defined according to Snel and Lorist 3 . To ensure good health, volunteers were screened by self-report questionnaires and a medical examination conducted by a physician. Additionally, all volunteers reported good sleep quality assessed with the Pittsburgh Sleep Quality Index (PSQI; score ≤ 5) 43 and showed no signs of sleep disturbances (SE > 70%, periodic leg movements < 15/h, apnea index < 10) in a PSG recorded during an adaptation night in the laboratory scheduled prior to the start of the study. To control for circadian misalignment, volunteers who reported shiftwork within 3 months and transmeridian travels (crossing > 2 time zones) within 1 month prior to study admission were excluded. Further exclusion criteria comprised body mass index (BMI) < 18 or > 26, smoking, drug use, and extreme chronotype assessed by the Morningness-Eveningness Questionnaire (MEQ; score ≤ 30 and ≥ 70) 44 . To reduce variance in the data incurred by the effect of menstrual cycle on sleep 39 and the interaction between caffeine metabolism and the use of oral contraceptives 40 , 41 , only male volunteers were studied. A detailed description of the study sample can be found in Weibel et al. 36 .
All volunteers signed a written informed consent and received financial compensation for study participation. The study was approved by the local Ethics Committee (EKNZ) and conducted according to the Declaration of Helsinki.
Design and protocol
We employed a double-blind, randomized, crossover study including a caffeine, a withdrawal, and a placebo condition. Volunteers were allocated to the order of the three conditions based on pseudo-randomization, for more details see Weibel et al. 36 . As illustrated in Fig. 4 , each condition started with an ambulatory part of 9 days, followed by a laboratory part of 43 h. In each condition, participants took either caffeine (150 mg) or placebo (mannitol) in identical appearing gelatin capsules (Hänseler AG, Herisau, Switzerland) three times daily, scheduled at 45 min, 255 min, and 475 min after awakening, for a duration of 10 days. This regimen was applied based on a previous study investigating tolerance to the effects of caffeine and caffeine cessation 18 . To enhance caffeine withdrawal in the withdrawal condition, treatment was abruptly switched from caffeine to placebo on day nine of the protocol (255 min after wake-up, 15 h before sleep recording).
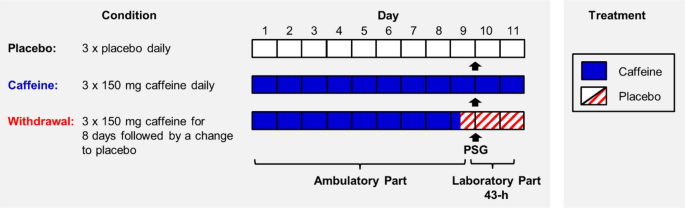
Illustration of the study design. Twenty volunteers participated in a placebo, a caffeine, and a withdrawal condition during which they ingested either caffeine or placebo capsules three times daily (wake-up + 45 min, + 255 min, and + 475 min). Each condition started with an ambulatory part of 9 days and was followed by a laboratory part of 43 h. After 9 days of continuous treatment, we recorded 8 h of polysomnography (PSG), indicated as arrows, during nighttime sleep under controlled laboratory conditions. The sleep episode was scheduled to volunteers’ habitual bedtime.
During the 9 days of the ambulatory part, volunteers were asked to maintain a regular sleep-wake rhythm (± 30 min of self-selected bedtime/wake-up time, 8 h in bed, no daytime napping), verified by wrist actimetry (Actiwatch, Cambridge Neurotechnology Ltd., Cambridge, United Kingdom), and to keep subjective sleep logs. While the participants were compliant, they scheduled sleep episodes differently within the accepted range of ± 30 min. During intake of caffeine (i.e. caffeine and withdrawal condition), the ambulatory sleep episodes were on average around 25 min later as compared to placebo (results see supplements). The duration of the ambulatory part was set for 9 days based on the maximum duration of withdrawal symptoms 17 and thus, to avoid carry-over effects from the previous condition. Furthermore, volunteers were requested to refrain from caffeinated beverages and food (e.g. coffee, tea, soda drinks, and chocolate), alcohol, nicotine, and medications. Caffeine abstinence and compliance to the treatment requirements were checked by caffeine levels from the daily collection of fingertip sweat of which results are reported in the supplemental material of Weibel et al. 36 and which indicate very good adherence to the treatments.
On day nine, volunteers admitted to the laboratory at 5.5 h prior to habitual sleep time. Upon arrival, a urinary drug screen (AccuBioTech Co., Ltd., Beijing, China) was performed to ensure drug abstinence. Electrodes for the PSG were fitted and salivary caffeine levels collected. An 8-h nighttime sleep episode was scheduled at volunteers’ habitual bedtime starting 8 and 15 h after the last caffeine intake in the caffeine and withdrawal condition, respectively. The next day, volunteers rated their subjective sleep quality by the LSEQ 45 and potential withdrawal symptoms by the Caffeine Withdrawal Symptom Questionnaire (CWSQ) 46 .
To reduce potential masking effects on our outcome variables, we standardized food intake, light exposure, and posture changes throughout the laboratory part. Accordingly, volunteers were housed in single apartments under dim-light (< 8 lx) during scheduled wakefulness and 0 lx during sleep. Volunteers were asked to maintain a semi-recumbent position during wakefulness, except for restroom breaks. In addition, volunteers received standardized meals in regular intervals. Social interactions were restricted to team members and no time-of-day cues were provided throughout the in-lab protocol.
Salivary caffeine
To characterize individual caffeine levels during nighttime sleep, we report salivary caffeine levels assessed 3 h prior to the scheduled sleep episode and 5 min after wake-up. Samples were stored at 5 °C following collection, later centrifuged (3000 rpm for 10 min) and subsequently kept at − 28 °C until analyses. Liquid chromatography coupled to tandem mass spectrometry was used to analyze the levels of caffeine. One dataset in the withdrawal condition was lost.
Subjective sleep quality
Subjective sleep quality was assessed 10 min upon scheduled wake-up time with a paper and pencil version of the LSEQ 45 . Volunteers were asked to rate 10 items on visual analogue scales which are grouped into four domains (getting to sleep (GTS), quality of sleep (QOS), awake following sleep (AFS), and behavior following wakening (BFW)).
Polysomnographic recordings
PSG was continuously recorded during 8 h of nighttime sleep using the portable V-Amp device (Brain Products GmbH, Gilching, Germany). Grass gold cup electrodes were applied according to the international 10–20 system including two electrooculographic, two electromyographic, two electrocardiographic, and six electroencephalographic derivations (F3, F4, C3, C4, O1, O2). Channels were referenced online against the linked mastoids (A1, A2). Signals were recorded with a sampling rate of 500 Hz and a notch filter was online applied at 50 Hz.
Each epoch of 30 s of the recorded PSG data was visually scored according to standard criteria 47 by three trained team members blind to the condition. SWS was additionally classified into stage 3 and 4 based on Rechtschaffen and Kales 48 . The scoring agreement between the three scorers was regularly confirmed to reach > 85%.
TST was defined as the sum of the time spent in sleep stages 1–4 and rapid eye movement (REM) sleep. Sleep latency to stage 1 and 2 was calculated as minutes to the first occurrence of the corresponding sleep stage following lights off. REM sleep latency was calculated as minutes to the first occurrence of REM sleep following sleep onset. NREM sleep was calculated as sum of sleep stages 2, 3 and 4. All sleep stages are expressed as relative values (%) of TST.
Spectral analysis was performed by applying fast Fourier transformation (FFT; hamming, 0% overlapped, 0.25 Hz bins) on 4-s time windows. Artifacts were manually removed based on visual inspection, and data were log-transformed prior to spectral analyses. All-night EEG power density during NREM sleep was analyzed for each 0.25 Hz frequency bin in the range of 0.75–32 Hz recorded over the central derivations (C3, C4). SWA was defined as EEG power density between 0.75–4.5 Hz and sigma activity between 12–16 Hz. Sleep cycles were defined based on adapted rules developed by Feinberg and Floyd 49 and divided into 10 NREM and four REM sleep intervals within each cycle. Ten nights were excluded from sleep analyses due to technical problems (placebo: n = 3; caffeine: n = 4; withdrawal: n = 3).
Caffeine withdrawal symptoms
Withdrawal symptoms were first assessed 35 min after wake-up and subsequently prior to each treatment administration with the self-rating CWSQ 46 . Twenty-three items are grouped into seven factors (fatigue/drowsiness, low alertness/difficulty concentrating, mood disturbances, low sociability/motivation to work, nausea/upset stomach, flu-like feelings, headache) and were rated on a 5 point scale by choosing between 1 (not at all) and 5 (extremely). Prior to analyses, eight items have been reversed scored as they were positively worded (e.g. alert or talkative) in the questionnaire. To assess caffeine withdrawal, we first calculated a sum score comprising all 23 items of the caffeine withdrawal questionnaire. Missing responses to single items were replaced by the median response of each condition over all volunteers in the respective time of assessment. In a next step, we calculated relative withdrawal symptoms in the caffeine and withdrawal condition (i.e. the difference of the withdrawal score in the caffeine and withdrawal condition respectively minus the score of the placebo condition). The data of one volunteer was lost due to technical difficulties.
Statistical analyses
Analyses were performed with the statistical package SAS (version 9.4, SAS Institute, Cary, NC, USA) by applying mixed model analyses of variance for repeated measures (PROC MIXED) with the repeated factors ‘condition’ (placebo, caffeine, withdrawal) and/or ‘time’ (levels differ per variable) and the random factor ‘subject’. The LSMEANS statement was used to calculate contrasts and degrees of freedom were based on the approximation by Kenward and Roger 50 . Post-hoc comparisons were adjusted for multiple comparisons by applying the Tukey-Kramer method. A statistical significance was defined as p < 0.05. One dataset has been excluded from all the analyses due to non-compliance with the treatment requirements (caffeine: n = 1).
Data availability
The present data are available upon request from the corresponding author.
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A. & Zvartau, E. E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51 , 83–133 (1999).
CAS PubMed Google Scholar
Heckman, M. A., Weil, J. & GonzalezdeMejia, E. Caffeine (1,3,7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 75 , R77–R87. https://doi.org/10.1111/j.1750-3841.2010.01561.x (2010).
Article CAS PubMed Google Scholar
Snel, J. & Lorist, M. M. Effects of caffeine on sleep and cognition. Prog. Brain Res. 190 , 105–117. https://doi.org/10.1016/B978-0-444-53817-8.00006-2 (2011).
Article PubMed Google Scholar
Martyn, D., Lau, A., Richardson, P. & Roberts, A. Temporal patterns of caffeine intake in the United States. Food Chem. Toxicol. 111 , 71–83. https://doi.org/10.1016/j.fct.2017.10.059 (2018).
Lieberman, H. R., Agarwal, S. & Fulgoni, V. L. 3rd. Daily patterns of caffeine intake and the association of intake with multiple sociodemographic and lifestyle factors in US adults based on the NHANES 2007–2012 surveys. J. Acad. Nutr. Diet 119 , 106–114. https://doi.org/10.1016/j.jand.2018.08.152 (2019).
Borbély, A. A. A two process model of sleep regulation. Hum. Neurobiol. 1 , 195–204 (1982).
PubMed Google Scholar
Porkka-Heiskanen, T. Sleep homeostasis. Curr. Opin. Neurobiol. 23 , 799–805. https://doi.org/10.1016/j.conb.2013.02.010 (2013).
Landolt, H. P. Sleep homeostasis: a role for adenosine in humans?. Biochem. Pharmacol. 75 , 2070–2079. https://doi.org/10.1016/j.bcp.2008.02.024 (2008).
Clark, I. & Landolt, H. P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med. Rev. 31 , 70–78. https://doi.org/10.1016/j.smrv.2016.01.006 (2017).
Landolt, H. P., Dijk, D. J., Gaus, S. E. & Borbély, A. A. Caffeine reduces low-frequency delta-activity in the human sleep EEG. Neuropsychopharmacology 12 , 229–238. https://doi.org/10.1016/0893-133x(94)00079-F (1995).
Drapeau, C. et al. Challenging sleep in aging: the effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. J. Sleep Res. 15 , 133–141. https://doi.org/10.1111/j.1365-2869.2006.00518.x (2006).
Bonnet, M. H. & Arand, D. L. Caffeine use as a model of acute and chronic insomnia. Sleep 15 , 526–536 (1992).
Carrier, J. et al. Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacology 32 , 964–972. https://doi.org/10.1038/sj.npp.1301198 (2007).
Robillard, R., Bouchard, M., Cartier, A., Nicolau, L. & Carrier, J. Sleep is more sensitive to high doses of caffeine in the middle years of life. J. Psychopharmacol. 29 , 688–697. https://doi.org/10.1177/0269881115575535 (2015).
Drake, C., Roehrs, T., Shambroom, J. & Roth, T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J. Clin. Sleep Med. 9 , 1195–1200. https://doi.org/10.5664/jcsm.3170 (2013).
Article PubMed PubMed Central Google Scholar
James, J. E. & Rogers, P. J. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacology 182 , 1–8. https://doi.org/10.1007/s00213-005-0084-6 (2005).
Juliano, L. M. & Griffiths, R. R. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 176 , 1–29. https://doi.org/10.1007/s00213-004-2000-x (2004).
James, J. E. Acute and chronic effects of caffeine on performance, mood, headache, and sleep. Neuropsychobiology 38 , 32–41 (1998).
Article CAS Google Scholar
Mandel, H. G. Update on caffeine consumption, disposition and action. Food Chem. Toxicol. 40 , 1231–1234. https://doi.org/10.1016/s0278-6915(02)00093-5 (2002).
Dijk, D. J. & Czeisler, C. A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15 , 3526–3538 (1995).
Landolt, H. P., Werth, E., Borbély, A. A. & Dijk, D. J. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Res. 675 , 67–74. https://doi.org/10.1016/0006-8993(95)00040-W (1995).
Lovallo, W. R. et al. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom. Med. 67 , 734–739. https://doi.org/10.1097/01.psy.0000181270.20036.06 (2005).
Article CAS PubMed PubMed Central Google Scholar
Lovallo, W. R. et al. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension 43 , 760–765. https://doi.org/10.1161/01.Hyp.0000120965.63962.93 (2004).
Denaro, C. P., Brown, C. R., Jacob, P. 3rd. & Benowitz, N. L. Effects of caffeine with repeated dosing. Eur. J. Clin. Pharmacol. 40 , 273–278. https://doi.org/10.1007/bf00315208 (1991).
Conlay, L. A., Conant, J. A., deBros, F. & Wurtman, R. Caffeine alters plasma adenosine levels. Nature 389 , 136. https://doi.org/10.1038/38160 (1997).
Article ADS CAS PubMed Google Scholar
Johansson, B., Georgiev, V., Lindström, K. & Fredholm, B. B. A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Res. 762 , 153–164 (1997).
Fredholm, B. B. Adenosine actions and adenosine receptors after 1 week treatment with caffeine. Acta Physiol. Scand. 115 , 283–286. https://doi.org/10.1111/j.1748-1716.1982.tb07078.x (1982).
Shi, D., Nikodijevic, O., Jacobson, K. A. & Daly, J. W. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell Mol. Neurobiol. 13 , 247–261 (1993).
Borbély, A. A., Baumann, F., Brandeis, D., Strauch, I. & Lehmann, D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr. Clin. Neurophysiol. 51 , 483–495. https://doi.org/10.1016/0013-4694(81)90225-x (1981).
Dijk, D. J., Hayes, B. & Czeisler, C. A. Dynamics of electroencephalographic sleep spindles and slow-wave activity in men—effect of sleep-deprivation. Brain Res. 626 , 190–199. https://doi.org/10.1016/0006-8993(93)90579-C (1993).
Finelli, L. A., Borbély, A. A. & Achermann, P. Functional topography of the human nonREM sleep electroencephalogram. Eur. J. Neurosci. 13 , 2282–2290. https://doi.org/10.1046/j.0953-816x.2001.01597.x (2001).
Knoblauch, V., Martens, W. L., Wirz-Justice, A. & Cajochen, C. Human sleep spindle characteristics after sleep deprivation. Clin. Neurophysiol. 114 , 2258–2267. https://doi.org/10.1016/s1388-2457(03)00238-4 (2003).
Panagiotou, M., Meijer, M., Meijer, J. H. & Deboer, T. Effects of chronic caffeine consumption on sleep and the sleep electroencephalogram in mice. J. Psychopharmacol. https://doi.org/10.1177/0269881118806300 (2018).
Ferré, S. An update on the mechanisms of the psychostimulant effects of caffeine. J. Neurochem. 105 , 1067–1079. https://doi.org/10.1111/j.1471-4159.2007.05196.x (2008).
Ciruela, F. et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J. Neurosci. 26 , 2080–2087. https://doi.org/10.1523/JNEUROSCI.3574-05.2006 (2006).
Weibel, J. et al. Caffeine-dependent changes of sleep-wake regulation: Evidence for adaptation after repeated intake. Prog. Neuropsychopharmacol. Biol. Psychiatry 99 , 109851. https://doi.org/10.1016/j.pnpbp.2019.109851 (2019).
Nehlig, A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev. 70 , 384–411. https://doi.org/10.1124/pr.117.014407 (2018).
Rétey, J. V. et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 81 , 692–698. https://doi.org/10.1038/sj.clpt.6100102 (2007).
Shechter, A. & Boivin, D. B. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int. J. Endocrinol. 2010 , 259345. https://doi.org/10.1155/2010/259345 (2010).
Balogh, A. et al. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur. J. Clin. Pharmacol. 48 , 161–166. https://doi.org/10.1007/bf00192743 (1995).
Abernethy, D. R. & Todd, E. L. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur. J. Clin. Pharmacol. 28 , 425–428. https://doi.org/10.1007/bf00544361 (1985).
Bühler, E., Lachenmeier, D. W., Schlegel, K. & Winkler, G. Development of a tool to assess the caffeine intake among teenagers and young adults. Ernahrungs Umschau 61 , 58–63 (2013).
Google Scholar
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28 , 193–213 (1989).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol 4 , 97–110 (1976).
Parrott, A. C. & Hindmarch, I. Factor analysis of a sleep evaluation questionnaire. Psychol. Med. 8 , 325–329 (1978).
Juliano, L. M., Huntley, E. D., Harrell, P. T. & Westerman, A. T. Development of the caffeine withdrawal symptom questionnaire: caffeine withdrawal symptoms cluster into 7 factors. Drug Alcohol. Depend. 124 , 229–234. https://doi.org/10.1016/j.drugalcdep.2012.01.009 (2012).
Berry, R. B. et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.0. American Academy of Sleep Medicine: Darien, IL (2012).
Rechtschaffen, A. & Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (US Dept of Health, Education and Welfare, Public Health Service, Bethesda, MD, 1968).
Feinberg, I. & Floyd, T. C. Systematic trends across the night in human sleep cycles. Psychophysiology 16 , 283–291 (1979).
Kenward, M. G. & Roger, J. H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53 , 983–997. https://doi.org/10.2307/2533558 (1997).
Article CAS PubMed MATH Google Scholar
Download references
Acknowledgements
The present work was performed within the framework of a project granted by the Swiss National Science Foundation (320030_163058) and was additionally funded by the Nikolaus und Bertha Burckhardt-Bürgin-Stiftung and the Janggen-Pöhn-Stiftung. Further, we thank our interns Andrea Schumacher, Laura Tincknell, Sven Leach, and all our study helpers for their help in data acquisition and all our volunteers for participating in the study. Moreover, we gratefully acknowledge the help in study organization provided by Dr. Ruta Lasauskaite and the medical screenings conducted by Dr. med. Martin Meyer and Dr. med. Corrado Garbazza.
Author information
These authors contributed equally: Christian Cajochen and Carolin F. Reichert.
Authors and Affiliations
Centre for Chronobiology, Psychiatric Hospital of the University of Basel, Basel, Switzerland
Janine Weibel, Yu-Shiuan Lin, Joshua Kistler, Christian Cajochen & Carolin F. Reichert
Transfaculty Research Platform Molecular and Cognitive Neurosciences, University of Basel, Basel, Switzerland
Neuropsychiatry and Brain Imaging, Psychiatric Hospital of the University of Basel, Basel, Switzerland
Yu-Shiuan Lin & Stefan Borgwardt
Institute of Pharmacology and Toxicology, University of Zürich, Zürich, Switzerland
Hans-Peter Landolt
Sleep & Health Zürich, University Center of Competence, University of Zürich, Zürich, Switzerland
Laboratory Medicine, University Hospital Basel, Basel, Switzerland
Sophia Rehm & Katharina M. Rentsch
Clinical Sleep Laboratory, Psychiatric Hospital of the University of Basel, Basel, Switzerland
Helen Slawik
You can also search for this author in PubMed Google Scholar
Contributions
C.R., C.C. and S.B. designed the study; J.W., Y.S.L. and HS collected the data; J.W., C.R. and C.C. analyzed and interpreted the data; J.W. and C.R. drafted the manuscript; C.C., Y.S.L. and H.P.L. critically revised the manuscript regarding its intellectual content; J.K., S.R. and K.R. provided the resources for the caffeine measurements and performed its analyses; all authors reviewed the present article.
Corresponding author
Correspondence to Christian Cajochen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Weibel, J., Lin, YS., Landolt, HP. et al. The impact of daily caffeine intake on nighttime sleep in young adult men. Sci Rep 11 , 4668 (2021). https://doi.org/10.1038/s41598-021-84088-x
Download citation
Received : 05 August 2020
Accepted : 06 January 2021
Published : 25 February 2021
DOI : https://doi.org/10.1038/s41598-021-84088-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Caffeine and Health
- 1 Associate Editor, JAMA
- Original Investigation Association of Coffee Drinking With Mortality by Genetic Variation in Caffeine Metabolism Erikka Loftfield, PhD; Marilyn C. Cornelis, PhD; Neil Caporaso, MD; Kai Yu, PhD; Rashmi Sinha, PhD; Neal Freedman, PhD JAMA Internal Medicine
- Original Investigation Assessment of Caffeine Consumption and Maternal Cardiometabolic Pregnancy Complications Stefanie N. Hinkle, PhD; Jessica L. Gleason, PhD, MPH; Samrawit F. Yisahak, PhD; Sifang Kathy Zhao, PhD; Sunni L. Mumford, PhD, MSc; Rajeshwari Sundaram, PhD, MS; Jagteshwar Grewal, PhD; Katherine L. Grantz, MD, MS; Cuilin Zhang, MD, PhD, MPH JAMA Network Open
Caffeine is a natural chemical stimulant that can also be created synthetically for consumption.
Natural caffeine is found in coffee beans, tea leaves, cacao beans, guarana berries, and yerba maté leaves. Caffeine preparations can be added to drinks, food, tablets, or powdered supplements. In the US, about 85% of adults consume caffeine daily, and average intake is 135 mg per day (equivalent to 12 oz of coffee). The most common source of caffeine is coffee for adults and soft drinks and tea for teenagers.
How Does the Body Absorb and Metabolize Caffeine?
Caffeine is absorbed into the bloodstream within 45 minutes after ingestion. Metabolism of caffeine varies among individuals, but its duration of action is typically 2.5 to 4.5 hours. Pregnancy and some medications (oral contraceptives, certain antidepressants, cardiovascular medications, and antibiotics) slow caffeine removal from the bloodstream. In contrast, cigarette smoking increases the rate of caffeine removal from the bloodstream.
Beneficial Effects of Caffeine
Caffeine in moderate doses (40-200 mg) acts within the brain to decrease fatigue, increase alertness, and decrease reaction time. Caffeine also may decrease appetite and slightly reduce weight gain. In moderate doses, caffeine has been associated with decreased risk of depression and suicide in some studies.
Medical Uses of Caffeine
Caffeine is used to treat intermittent pauses in breathing (apnea) in premature infants. Addition of caffeine to commonly prescribed pain relievers (such as acetaminophen) can decrease acute pain from certain conditions, such as migraines.
Common Negative Effects of Caffeine
Caffeine leads to temporary increases in blood pressure in individuals with minimal or no prior use. Caffeine, particularly in higher doses, can cause anxiety, as well as difficulty falling asleep if consumed late in the day. Abrupt cessation of caffeine in regular users may result in withdrawal symptoms, which typically peak at 1 to 2 days and include headache, fatigue, and depressed mood. Because higher caffeine intake in pregnancy is associated with lower infant birth weight, caffeine consumption should not exceed 200 mg per day during pregnancy.
Effects of Caffeine in Very High Doses
Ingestion of very high doses of caffeine (1200 mg or more) can cause agitation, severe anxiety, elevated blood pressure, and palpitations. This may occur with overuse of caffeine tablets or supplements in liquid form (energy drinks) or powdered form. Consuming caffeinated energy drinks or energy shots together with alcohol is dangerous and has resulted in deaths.
Possible Health Benefits of Drinking Coffee
Some studies have shown decreased mortality associated with drinking 2 to 5 standard cups of caffeinated or decaffeinated coffee per day. In some reports, regular consumption of both caffeinated and decaffeinated coffee has been associated with a reduced risk of type 2 diabetes and endometrial cancer. In other reports, both caffeinated and decaffeinated coffee consumption was associated with lower risk of liver cancer, gallstones, and gallbladder cancers, but the potential benefit was stronger with caffeinated coffee. Consumption of caffeinated coffee has also been associated with a reduced risk of Parkinson disease and liver cirrhosis.
For More Information
National Library of Medicine medlineplus.gov/caffeine.html
To find this and other JAMA Patient Pages, go to the For Patients collection at jamanetworkpatientpages.com .
Conflict of Interest Disclosures: None reported.
Source: van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med . 2020;383(4):369-378. doi: 10.1056/NEJMra1816604
See More About
Walter K. Caffeine and Health. JAMA. 2022;327(7):693. doi:10.1001/jama.2021.21452
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
News & Publications
New insight into caffeine use disorder.
Johns Hopkins researchers recently conducted the most thorough evaluation to date of the prevalence and clinical significance of caffeine use disorder, as well as the correlates of meeting proposed criteria for the condition.
A new study finds potential for caffeine to cause anxiety, insomnia and other symptoms that interfered with subjects’ lives.
Mary “Maggie” Sweeney wants to make one thing clear: She has no intention of convincing people to give up their coffee or favorite caffeinated beverage. That said, the psychiatry researcher at Johns Hopkins Bayview Medical Center’s Behavioral Pharmacology Research Unit feels compelled to raise awareness about caffeine’s potential to cause distress.
Building on a long-running grant project in collaboration with Roland Griffiths , psychiatry researcher, a recent study on caffeine use disorder revealed responses to questions about caffeine use that Sweeney says were eye-opening and complementary to clinical trials conducted at Johns Hopkins — one in 2016 and one in 2019 . The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) recognized caffeine use disorder as “a condition for further study.”
Caffeine use disorder is a problematic pattern of caffeine consumption characterized by a persistent desire to cut down or control use of the substance along with unsuccessful efforts to do so despite problems caused or worsened by caffeine. Significant withdrawal symptoms or use of the drug to relieve or avoid withdrawal are also characteristics of the condition.
Sweeney, Griffiths and colleagues conducted the online research survey with 1,006 caffeine-consuming adults from across the U.S. Data were collected by an online survey panel aggregator used in other peer-reviewed research studies. The goal was to better understand caffeine use disorder’s prevalence and clinical significance in the general population.
Milligrams of caffeine per serving were calculated using typical milligrams per ounce for brewed/drip coffee (200 mg/12 oz.); brewed tea (40 mg/6 oz.); and soft drinks (40 mg/12 oz.). Total caffeine intake in a typical week from all sources was summed and divided by seven to estimate daily caffeine consumption. To qualify for the study, participants needed to report consuming some caffeine-containing beverage or supplement in a typical week.
The researchers found that 8% of the sample fulfilled DSM- proposed criteria for caffeine use disorder when the structured caffeine use disorder interview questions were adapted to the online survey format.
“What I find fascinating,” says Sweeney, “is how little people think about coffee or other caffeinated drinks as stimulants. Although for many people consumption of caffeine is benign, we learned from our study that there is a small but important subset of caffeine consumers who report that caffeine has interfered with their lives in clinically meaningful ways.”
People who met criteria for caffeine use disorder reported problems such as insomnia, gastrointestinal troubles and anxiety, which were caused by or exacerbated by caffeine. The study also found that participants who met criteria for caffeine use disorder tended to consume more caffeine, and were younger and more likely to be cigarette smokers. A larger sample or sample with greater substance use history may be necessary to detect the association between caffeine use disorder and other substance use.
About 90% of adults in the United States use caffeine regularly, says Griffiths, and their average consumption exceeds 200 milligrams of caffeine per day — more caffeine than is contained in two 6-ounce cups of coffee, or five 12-ounce cans of soft drinks.
This latest research study, notes Sweeney, is the most thorough evaluation to date of the prevalence and clinical significance of caffeine use disorder. These data complement results from their recent clinical trial, which showed that people seeking treatment for caffeine reduction were able to reduce their caffeine consumption and decrease their symptoms following the study intervention.
“In our clinical trial , our hypothesis was that people who have had trouble cutting back on caffeine on their own may be able to reduce their caffeine consumption with our guidelines to cut back over several weeks,” says Sweeney. “We also thought this could help people reduce their caffeine-related distress, such as withdrawal symptoms or consuming more caffeine than they intended.”
In both the online survey study and clinical trial, it was common for participants who met criteria for caffeine use disorder to report withdrawal symptoms from caffeine that reduced their function. Caffeine withdrawal symptoms can include headache, fatigue and irritability, which tend to peak at 24 to 48 hours after stopping caffeine, but can last for as long as 10 days in some individuals.
Prior research has also revealed that caffeine can result in withdrawal symptoms following cessation of much lower doses than previously thought. A 6-ounce cup of regular coffee delivers 100 milligrams of caffeine. Even this small amount of caffeine can cause withdrawal symptoms in some people when they stop using it regularly. Other studies have shown that caffeine doses as low as 10–20 milligrams are psychoactive.
The researchers acknowledge that caffeine can have positive health effects, such as reducing the risk of type 2 diabetes and boosting some aspects of cognition. “I want to be clear that caffeine isn’t all good or bad,” says Sweeney. “We’re not arguing that everyone needs to cut back on their consumption. A moderate amount of caffeine — up to 400 milligrams/day (about two 12-ounce cups of coffee) — is not generally associated with negative health effects. But, caffeine reduction is a good goal if caffeine causes significant impairment through withdrawal symptoms or by worsening an underlying problem, such as insomnia or anxiety.”
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The effects of caffeine intake on weight loss: a systematic review and dos-response meta-analysis of randomized controlled trials
Affiliations.
- 1 Health Policy Research Center, Institute of Health, Student Research Committee, Shiraz University of Medical Sciences , Shiraz , Iran.
- 2 Food Security Research Center, Department of Community Nutrition School of Nutrition and Food Science, Isfahan University of Medical Sciences , Isfahan , Iran.
- 3 Health Policy Research Center, Shiraz University of Medical Sciences , Shiraz , Iran.
- 4 Indigenous and Global Health Research, Department of Medicine, University of Alberta , Edmonton , Canada.
- 5 Department of Community Nutrition School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences , Tehran , Iran.
- 6 Health Information Management Research Center, Kashan University of Medical Sciences , Kashan , Iran.
- 7 Department of Cardiology School of Medicine, Kashan University of Medical Sciences , Kashan , Iran.
- 8 Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences , Kashan , Iran.
- PMID: 30335479
- DOI: 10.1080/10408398.2018.1507996
This systematic review and meta-analysis of randomized controlled trials (RCTs) was performed to summarize the effect of caffeine intake on weight loss. We searched the following databases until November 2017: MEDLINE, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials. The relevant data were extracted and assessed for quality of the studies according to the Cochrane risk of bias tool. We estimated an intake-status regression coefficient (Beta) for each primary study and estimated the overall pooled Beta and SE using random effects meta-analysis on a double-log scale. Heterogeneity between studies was assessed by the Cochran Q statistic and I-squared tests (I 2 ). Thirteen RCTs with 606 participants were included in the meta-analyses. The overall pooled Beta for the effect of caffeine intake was 0.29 (95%CI: 0.19, 0.40; Q = 124.5, I 2 = 91.2%) for weigh, 0.23 (95%CI: 0.09, 0.36; Q = 71.0, I 2 = 93.0%) for BMI, and 0.36 (95% CI: 0.24, 0.48; Q = 167.36, I 2 = 94.0%) for fat mass. For every doubling in caffeine intake, the mean reduction in weight, BMI, and fat mass increased 2 Beta-fold (20.29 = 1.22, 20.23 = 1.17, and 20.36 = 1.28), which corresponding to 22, 17, and 28 percent, respectively. Overall, the current meta-analysis demonstrated that caffeine intake might promote weight, BMI and body fat reduction.
Keywords: Caffeine; meta-analysis; weight loss.
PubMed Disclaimer
Similar articles
- The effects of resveratrol intake on weight loss: a systematic review and meta-analysis of randomized controlled trials. Tabrizi R, Tamtaji OR, Lankarani KB, Akbari M, Dadgostar E, Dabbaghmanesh MH, Kolahdooz F, Shamshirian A, Momen-Heravi M, Asemi Z. Tabrizi R, et al. Crit Rev Food Sci Nutr. 2020;60(3):375-390. doi: 10.1080/10408398.2018.1529654. Epub 2018 Nov 13. Crit Rev Food Sci Nutr. 2020. PMID: 30421960
- Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet]. LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. LeBlanc EL, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
- Effect of Acute Caffeine Intake on the Fat Oxidation Rate during Exercise: A Systematic Review and Meta-Analysis. Collado-Mateo D, Lavín-Pérez AM, Merellano-Navarro E, Coso JD. Collado-Mateo D, et al. Nutrients. 2020 Nov 24;12(12):3603. doi: 10.3390/nu12123603. Nutrients. 2020. PMID: 33255240 Free PMC article.
- Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies. Cheng M, Hu Z, Lu X, Huang J, Gu D. Cheng M, et al. Can J Cardiol. 2014 Apr;30(4):448-54. doi: 10.1016/j.cjca.2013.12.026. Epub 2014 Jan 2. Can J Cardiol. 2014. PMID: 24680173 Review.
- Effects of caffeine consumption in patients with chronic hepatitis C: A systematic review and meta-analysis. Jaruvongvanich V, Sanguankeo A, Klomjit N, Upala S. Jaruvongvanich V, et al. Clin Res Hepatol Gastroenterol. 2017 Feb;41(1):46-55. doi: 10.1016/j.clinre.2016.05.012. Epub 2016 Jun 24. Clin Res Hepatol Gastroenterol. 2017. PMID: 27350575 Review.
- An intense 60-day weight-loss course leads to an 18 kg body weight reduction and metabolic reprogramming of soldiers with obesity. Albores-Méndez EM, Carrasco-Vargas H, Alaniz Monreal S, Mayen Quinto RD, López García ED, Gutierrez Salmean G, Medina-Quero K, Vargas-Hernández MA, Ferreira Batista CV, López-Hernández Y, Winkler R. Albores-Méndez EM, et al. PeerJ. 2024 Jul 26;12:e17757. doi: 10.7717/peerj.17757. eCollection 2024. PeerJ. 2024. PMID: 39076775 Free PMC article.
- Coffee, tea, and cocoa in obesity prevention: Mechanisms of action and future prospects. Wang Q, Hu GL, Qiu MH, Cao J, Xiong WY. Wang Q, et al. Curr Res Food Sci. 2024 Apr 20;8:100741. doi: 10.1016/j.crfs.2024.100741. eCollection 2024. Curr Res Food Sci. 2024. PMID: 38694556 Free PMC article. Review.
- Common questions and misconceptions about caffeine supplementation: what does the scientific evidence really show? Antonio J, Newmire DE, Stout JR, Antonio B, Gibbons M, Lowery LM, Harper J, Willoughby D, Evans C, Anderson D, Goldstein E, Rojas J, Monsalves-Álvarez M, Forbes SC, Gomez Lopez J, Ziegenfuss T, Moulding BD, Candow D, Sagner M, Arent SM. Antonio J, et al. J Int Soc Sports Nutr. 2024 Dec;21(1):2323919. doi: 10.1080/15502783.2024.2323919. Epub 2024 Mar 11. J Int Soc Sports Nutr. 2024. PMID: 38466174 Free PMC article. Review.
- Associations of urinary caffeine and caffeine metabolites with metabolic syndrome in US adults. Zhou J, Qin L. Zhou J, et al. Front Nutr. 2023 Dec 1;10:1280215. doi: 10.3389/fnut.2023.1280215. eCollection 2023. Front Nutr. 2023. PMID: 38107745 Free PMC article.
- Concomitant medications, functional foods, and supplements: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Tondt J, Bays HE. Tondt J, et al. Obes Pillars. 2022 Apr 6;2:100017. doi: 10.1016/j.obpill.2022.100017. eCollection 2022 Jun. Obes Pillars. 2022. PMID: 37990714 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Taylor & Francis
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 05 March 2018
Effects of caffeine intake on muscle strength and power: a systematic review and meta-analysis
- Jozo Grgic 1 ,
- Eric T. Trexler 2 , 3 ,
- Bruno Lazinica 4 &
- Zeljko Pedisic 1
Journal of the International Society of Sports Nutrition volume 15 , Article number: 11 ( 2018 ) Cite this article
58k Accesses
208 Citations
315 Altmetric
Metrics details
Caffeine is commonly used as an ergogenic aid. Literature about the effects of caffeine ingestion on muscle strength and power is equivocal. The aim of this systematic review and meta-analysis was to summarize results from individual studies on the effects of caffeine intake on muscle strength and power.
A search through eight databases was performed to find studies on the effects of caffeine on: (i) maximal muscle strength measured using 1 repetition maximum tests; and (ii) muscle power assessed by tests of vertical jump. Meta-analyses of standardized mean differences (SMD) between placebo and caffeine trials from individual studies were conducted using the random effects model.
Ten studies on the strength outcome and ten studies on the power outcome met the inclusion criteria for the meta-analyses. Caffeine ingestion improved both strength (SMD = 0.20; 95% confidence interval [CI]: 0.03, 0.36; p = 0.023) and power (SMD = 0.17; 95% CI: 0.00, 0.34; p = 0.047). A subgroup analysis indicated that caffeine significantly improves upper (SMD = 0.21; 95% CI: 0.02, 0.39; p = 0.026) but not lower body strength (SMD = 0.15; 95% CI: -0.05, 0.34; p = 0.147).
The meta-analyses showed significant ergogenic effects of caffeine ingestion on maximal muscle strength of upper body and muscle power. Future studies should more rigorously control the effectiveness of blinding. Due to the paucity of evidence, additional findings are needed in the female population and using different forms of caffeine, such as gum and gel.
Caffeine’s ergogenic potential has been extensively studied in the sports science literature, with research dating back to 1907 [ 1 ]. From investigating caffeine’s effects on aerobic exercise, in recent years the research focus has shifted to anaerobic exercise performance outcomes, such as muscular endurance, muscle strength, and jumping tasks that require muscle power. While caffeine has been found to significantly enhance muscular endurance [ 2 ], the effects of caffeine ingestion on maximal muscle strength (commonly operationalized as one repetition maximum [1RM]) and muscle power (commonly operationalized as vertical jump) remain unclear, and the practical utility of caffeine ingestion for enhancing performance in such physical tasks has not been fully elucidated.
The pioneering work on caffeine’s effects on strength by Astorino et al. [ 3 ] reported no significant strength-enhancing effects with caffeine ingestion in a group of resistance trained men. Recent work by Grgic and Mikulic [ 4 ], however, found a significant 3% increase in lower body strength with caffeine ingestion using the barbell back squat 1RM as a measure of maximal strength. Goldstein et al. [ 5 ] reported a significant increase in upper body strength with caffeine ingestion, while Williams et al. [ 6 ] reported no ergogenic effect. The inconsistent results of individual studies prevent drawing sound conclusions regarding the ergogenic potential of caffeine for maximal strength outcomes.
Equivocal findings have also been presented for the effects of caffeine intake on muscle power. A recent study by Ali et al. [ 7 ] reported no effect on countermovement jump height with caffeine ingestion. However, the findings of Bloms et al. [ 8 ] support conclusions about caffeine as an effective ergogenic aid for achieving acute improvements in countermovement jump height and peak force. Given the importance of jumping abilities for many common sports, it would be of both scientific and practical significance to determine a reasonably precise estimate regarding the potential performance-enhancing impact of caffeine ingestion on muscle power.
Several aspects that vary between studies, including the exercise used, participants’ characteristics (e.g., age, sex, and training experience), and caffeine form, might be responsible for the inconsistency of findings. Most importantly, small sample sizes often limited the statistical power to detect significant effects [ 9 ]. A meta-analysis of individual studies is needed to circumvent these issues and provide in-depth, evidence-based scrutiny of the current body of evidence. The first meta-analytic investigation on the topic of caffeine and strength was performed by Warren et al. [ 10 ], who found a mean increase of approximately 7% in lower body maximal voluntary contraction with caffeine ingestion. A limitation of the meta-analysis is that only two of the included studies tested the effects of caffeine ingestion on 1RM, which significantly restricted the findings to isometric and isokinetic strength outcomes.
The latest meta-analysis on the topic, done by Polito et al. [ 2 ], found no significant effect of caffeine intake on performance in 1RM strength tests. However, only three studies met the inclusion criteria for the meta-analysis. The total number of pooled participants was relatively low ( n = 46), potentially indicating issues with the statistical power of the analysis. Furthermore, the small number of included studies prevented subgroup analyses for possible moderators that may potentially impact the ergogenic potential of caffeine. Since the review by Polito et al. [ 2 ], a number of experimental trials have been published [ 4 , 11 , 12 , 13 , 14 , 15 , 16 ], presenting novel findings for females [ 14 ], trained [ 4 , 16 ] and untrained men [ 11 , 13 ], athletes [ 15 ], and adolescents [ 12 ]; as such, an updated review appears to be warranted.
No previous meta-analyses have pooled the results of individual studies on the effects of caffeine on muscle power. The aim of this systematic review was, therefore, twofold: (a) to perform an updated meta-analysis of the acute effects of caffeine ingestion on maximal muscle strength; and (b) to conduct the first meta-analysis of acute effects of caffeine ingestion on muscle power assessed by vertical jump tests. The results may benefit athletes and practitioners in a variety of sports in which muscle strength and/or power are important determinants of performance.
Search strategy
The systematic literature search was performed following the PRISMA guidelines [ 17 ]. A search of the following databases was performed: PubMed/MEDLINE, Scopus, Cochrane Library, Web of Science (including Science Citation Index Expanded, Social Sciences Citation Index, and Arts & Humanities Citation Index), Google Scholar, Networked Digital Library of Theses and Dissertations, ProQuest Dissertation & Theses and Open Access Theses and Dissertations. The search for the studies on the effects of caffeine on strength was restricted to the documents published from 2015 onwards as the review by Polito et al. [ 2 ], with a search performed in March 2015 was used as a reference point. The review by Polito and colleagues [ 2 ] was assessed for rigor and deemed as of high-quality. Thus, the studies [ 3 , 5 , 6 ] included in the work by Polito et al. [ 2 ] were also included in the present review. The following syntax was used for the primary search: caffeine AND (“muscle strength” OR “ergogenic aid” OR performance OR “resistance exercise” OR “resistance training” OR recovery OR “strength training”).
A separate search was done for the studies on the effects of caffeine on power outcomes. The following syntax with no time restriction was used: caffeine AND (“vertical jump” OR “countermovement jump” OR “squat jump” OR plyometrics OR height OR “drop jump” OR “depth jump” OR “jump training”).
The search results were downloaded and filtered in EndNote software (X8; Clarivate Analytics, New York, USA). A secondary search was performed by screening the reference lists of all selected studies, and by conducting forward citation tracking (using Google Scholar and Scopus) of studies found meeting the inclusion criteria. The search concluded on April 19th, 2017.
Inclusion criteria
To warrant inclusion in the current analysis potential studies were required to meet the following criteria:
an experimental trial published in English in a peer-reviewed journal, or a doctoral or a master’s thesis;
assessed the effects of caffeine ingestion in the form of capsule, liquid, gum or gel on dynamic maximal muscle strength (i.e. the greatest amount of weight lifted in a single repetition – 1RM) using constant external resistance, and/or on muscle power assessed using a vertical jump test (both peak force and vertical jump height were considered);
caffeine was not co-ingested with other drugs/substances or potentially ergogenic compounds;
employed a single or double-blind, randomized crossover design;
used human participants without known chronic disease or injury.
Studies were excluded from the analysis if any of the above criteria were violated. Caffeine ingestion via coffee was not considered as coffee has several other biologically active compounds that might moderate the impact of caffeine.
Study coding and data extraction
For all studies meeting the inclusion criteria, the following information was tabulated on a predefined coding sheet using Microsoft Excel software (Microsoft Corporation, WA, USA):
author(s), title and year of publication;
sample size, participants’ sex, participants’ age (categorized as: adolescents [10–18 years]; young adults [18–39 years]; middle-aged adults [40–64 years];and seniors [≥65 years], and participants’ experience in resistance training (categorized as: untrained [less than 1 year of experience]; and trained [more than 1 year of experience]) for studies assessing strength outcomes, and experience in sport training using the same categories as above for studies assessing muscle power.
caffeine form, dosage, and time of ingestion before the experimental session(s);
the exercises used for assessing muscle strength and power with the accompanying mean ± standard deviation (SD) data for the placebo and caffeine trials;
habitual caffeine intake by the participants;
the number of participants indicating which trial they perceived to be the caffeine trial;
reported side effects;
reported funding for conducting the studies.
Methodological quality
The 11-point PEDro scale was used for the assessment of the methodological quality of studies [ 18 ]. The first item concerns external validity and is not included in the total score; hence, the maximal score on the scale is 10. Studies were classified as in McCrary et al. [ 19 ]. Two authors of the article (JG and BL) performed the search, coding, and appraisal of methodological quality independently, with discussion and consensus over any observed differences. Before correcting for observed differences, the overall agreement between the two independent data extractions was very high (Cohen’s kappa = 0.94).
Statistical analysis
The meta-analysis was performed using the Comprehensive Meta-analysis software, version 2 (Biostat Inc., Englewood, NJ, USA). Standardized mean differences (Hedge’s g [SMD]) and 95% confidence intervals (CI) were calculated between the placebo and caffeine trials based on their means and standard deviations in 1RM (kg) and vertical jump (cm) tests, the correlations between the trials, and the number of participants. An analysis of peak force in the vertical jump test was not performed as only two studies reported such outcomes [ 8 , 16 ]. Since none of the studies reported correlation, a 0.5 correlation was assumed for all trials, as recommended by Follmann et al. [ 20 ]. When a study measured muscle strength and/or power under multiple conditions (e.g. used more than one caffeine dose, tested more than one muscle group), SMDs and variances were averaged across the different conditions. SMDs of ≤0.2, 0.2–0.5, 0.5–0.8, and > 0.8 were considered to represent small, medium, large and very large effects, respectively [ 9 ]. The random effects model was used for analysis of both muscle strength and muscle power outcomes. The statistical significance threshold was set a priori at p < 0.05.
Subgroup analyses for the effects of caffeine on muscle strength were performed for the following study characteristics: (a) upper body strength; (b) lower body strength; (c) the capsule form of caffeine; (d) the liquid form of caffeine; (e) females; (f) males; (g) untrained; and (h) trained. Subgroup analyses for the effects of caffeine on muscle power were performed for the following characteristics: (a) the capsule form of caffeine; (b) the liquid form of caffeine; (c) females; (d) males; (e) athletes; (h) non- athletes; (f) countermovement and squat jump tests; and (g) Sargent jump tests.
The I 2 statistic was used to assess the degree of heterogeneity, with values from ≤50% indicating low heterogeneity, 50–75% moderate heterogeneity and > 75% high level of heterogeneity. Funnel plots were constructed for both muscle strength and muscle power outcomes, plotting standard error against Hedge’s g. Funnel plot asymmetry arising from potential publication bias was assessed using the Trim-and-Fill method [ 21 ].
The literature search yielded a total of 2533 documents. After a preliminary screening of titles and abstracts, 71 full-text studies were scrutinized. In total, ten studies were found meeting the inclusion criteria for strength outcomes [ 3 , 4 , 5 , 6 , 11 , 12 , 13 , 14 , 15 , 16 ] (Table 1 ) with a total of 149 participants (males n = 116, females n = 33). Ten studies were found assessing muscle power outcomes [ 4 , 7 , 8 , 15 , 22 , 23 , 24 , 25 , 26 ] with a total of 145 participants (males n = 116, females n = 29). According to their age, all participants were classified as adolescents or young adults. Three studies [ 4 , 12 , 15 ] assessed both muscle strength and muscle power. The results of the search and study selection process are depicted in Fig. 1 .
Flow diagram of the search and study selection process
Fifteen studies were published in peer-reviewed journals, while two studies were master’s theses [ 14 , 26 ]. The median number of participants per study was 14. Most of the studies used a double-blind design (i.e., 15 studies), with two studies [ 8 , 14 ] using a single-blind design. Caffeine dosage varied from 0.9 mg.kg − 1 to 7 mg.kg − 1 . Only one study administered caffeine in the form of gel [ 16 ], while the rest used capsule or liquid forms. Only nine studies reported habitual caffeine intake, with Astorino et al. [ 3 ] and Goldstein et al. [ 5 ] reporting a large range of habitual caffeine intakes among the participants (0–600 mg.kg − 1 per day). Only three studies [ 3 , 22 , 24 ] reported assessing the effectiveness of the blinding, with 60%, 50% and 33% of the participants correctly differentiating between the placebo and the caffeine trials, respectively. Individual characteristics of the included studies are reported in Table 1 .
Results of the meta-analysis indicated a significant difference ( p = 0.023) between the placebo and caffeine trials on measures of maximal strength (Fig. 2 ). The pooled SMD for the effects of caffeine ingestion on muscle strength was 0.20 (95% CI: 0.03, 0.36). A subgroup analysis indicated that caffeine significantly improves upper (SMD = 0.21; 95% CI: 0.02, 0.39; p = 0.026; Fig. 3 ) but not lower body strength (SMD = 0.15; 95% CI: -0.05, 0.34; p = 0.147; Fig. 4 ). Results from all of the remaining subgroup analysis may be found in Table 2 .
Forest plot showing differences between the effects of placebo and caffeine trials on measures of maximal muscular strength. The size of the plotted squares reflects the relative statistical weight of each study. The numbers on the x -axis denote the standardized mean differences expressed as Hedge’s g. The horizontal lines denote the respective 95% confidence intervals (CI)
Forest plot showing differences between the effects of placebo and caffeine trials on measures of upper-body maximal muscle strength. The size of the plotted squares reflects the relative statistical weight of each study. The numbers on the x -axis denote the standardized mean differences expressed as Hedge’s g. The horizontal lines denote the respective 95% confidence intervals (CI)
Forest plot showing differences between the effects of placebo and caffeine trials on measures of lower-body maximal muscle strength. The size of the plotted squares reflects the relative statistical weight of each study. The numbers on the x -axis denote the standardized mean differences expressed as Hedge’s g. The horizontal lines denote the respective 95% confidence intervals (CI)
The meta-analysis performed for muscle power indicated a significant difference (SMD = 0.17; 95% CI: 0.00, 0.34; p = 0.047) between the placebo and caffeine trials (Fig. 5 ). Results from all of the subgroup analysis can be found in Table 2 .
Forest plot showing differences between the effects of placebo and caffeine trials on measures of muscle power expressed as vertical jump height. The size of the plotted squares reflects the relative statistical weight of each study. The numbers on the x -axis denote the standardized mean differences expressed as Hedge’s g. The horizontal lines denote the respective 95% confidence intervals (CI)
The I 2 statistic showed low heterogeneity for the studies assessing muscle strength and muscle power ( I 2 = 0.0; p = 0.981, and I 2 = 0.0; p = 0.933, respectively). The analysis of funnel plots did not reveal substantial asymmetry for muscle strength or muscle power outcomes. The Trim-and-Fill method changed the pooled SMD for muscle power from 0.17 (95% CI: 0.00, 0.34) to 0.12 (95% CI: -0.01, 0.26). The Trim-and-Fill method did not have an impact on the pooled effect size for muscle strength outcomes.
The mean PEDro methodological quality score was 9.6, with the values for individual studies ranging from 8 to 10. Three studies [ 8 , 14 , 22 ] were categorized as being of “good methodological quality” (PEDro score = 8), while all other studies were classified as being of “excellent quality”.
The results of the meta-analysis show that caffeine may be an effective ergogenic aid for muscle strength and power. The pooled effects of caffeine on performance were small to medium. It is important to note that even small improvements in performance in some sports may translate to meaningful differences in competitive outcomes [ 27 , 28 ]. A previous meta-analysis did not show a significant effect of caffeine supplementation on muscle strength [ 2 ], and the results of individual studies investigating caffeine’s effects on muscle power have not been previously pooled in a meta-analysis. Our novel results showing that caffeine may induce practically meaningful improvements in muscle strength and power can, therefore, be used to inform athletes, coaches, and sports nutritionists, as well as future research endeavors in this area, about the ergogenic potential of caffeine.
Strength outcomes
Upper and lower body strength.
The subgroup analysis indicated a significant increase in upper body, but not lower body strength, with caffeine ingestion. These results are somewhat unexpected, as Warren et al. [ 10 ] suggested that larger muscles, such as those of the lower body, have a greater motor unit recruitment capability with caffeine intake than smaller muscles, such as those of the arm. Motor unit recruitment, in addition to the reduced rate of perceived exertion and the central effects of adenosine on neurotransmission, arousal, and pain perception, are considered to be underlying mechanisms by which caffeine can enhance performance, although the exact mechanisms remain to be fully elucidated [ 29 , 30 ]. Based on the current results, it may be surmised that caffeine is a useful ergogenic aid for achieving acute increases in maximal upper body strength. In the included studies, lower body maximal strength was evaluated using only leg press and squat (machine-based and free weight) tests. Two studies [ 4 , 16 ] used a free weight exercise (barbell back squat), and both reported a significant increase in lower body strength. Warren et al. [ 10 ] concluded that caffeine ingestion might increase lower body isometric strength. Our findings do not indicate a strength increasing effect with caffeine ingestion for lower body dynamic strength. It is worth noting that in general, the included studies did not report on the reliability of their strength assessment, indicating potential reasons for the surprising findings for lower body strength. Further research is needed to examine the effects of caffeine on dynamic strength. Such studies may benefit from using a larger variety of dynamic lower body strength tests, as the current findings are mostly limited to a small selection of primarily machine-based tests.
Training status
The subgroup analysis for training status indicated no significant differences in maximal strength in trained ( p = 0.076) and untrained individuals ( p = 0.144). The meta-analysis of the three studies among untrained individuals was limited by small overall sample size ( n = 32). It may be considered indicative that two of three individual studies reported significant differences in maximal strength with caffeine ingestion, but more individual studies on this topic are needed before drawing firm conclusions. Training status seems to play a significant role in response to caffeine intake in other forms of physical activity, such as swimming, with greater improvements observed in trained athletes [ 31 ]. However, it remains unclear whether the same applies to strength outcomes. More studies are needed before confidently drawing conclusions about the potential differences in effects of caffeine ingestion on muscle strength of trained and untrained individuals.
The subgroup analysis in males showed a significant improvement in strength with caffeine ingestion. The subgroup analysis for females was limited by small sample size, as only three studies [ 5 , 12 , 14 ] were found meeting the inclusion criteria. The landmark study by Goldstein et al. [ 5 ] reported a significant increase in the 1RM bench press in a cohort of resistance trained females. However, the effect size was very small (SMD = 0.07), thereby limiting the practical significance of the finding. Another study among female participants was performed by Sabblah et al. [ 14 ]. The researchers reported an SMD of 0.33 for increases in upper body strength with caffeine ingestion. However, the study employed a single-blind design and hence provided evidence of somewhat lower methodological quality compared to other studies. Additionally, the participants in the study from Sabblah et al. [ 14 ] exhibited lower levels of fitness than the participants in the study from Goldstein et al. [ 5 ], with marked disparities observed for 1RM strength (32 kg and 52 kg, respectively). None of the studies that included female participants controlled for the potential variability attributable to metabolic alterations across the menstrual cycle [ 32 ], which is a limitation of the current body of literature. Additional rigorously controlled studies are needed to provide clarity on the topic.
Caffeine form
The subgroup analysis indicated significant increases in strength after the ingestion of caffeine in the capsule form. The meta-analysis of the effects of the liquid form of caffeine included only three studies and did not report a significant effect. It is likely that the analysis was limited due to the small sample size ( n = 50). Only one study [ 16 ] used caffeine in the form of a gel. Previous studies indicate that there are no practically meaningful pharmacokinetic differences between these routes of caffeine ingestion [ 33 ]; as such, it is unlikely that marked differences exist when comparing ergogenic effects of various forms of caffeine administration. Further investigations are needed for liquid forms of caffeine and others that have rarely or never been studied in this context, such as gum and gel.
Power outcomes
The meta-analysis supports caffeine as an effective ergogenic aid for achieving acute increases in muscle power expressed as vertical jump height. These results may have considerable applicability to many sports, including basketball and volleyball, in which muscle power and jumping ability are highly related to performance outcomes. The magnitude of acute improvement in vertical jump height found in the current analysis for a single caffeine ingestion is roughly equivalent to the effects of ~ 4 weeks of plyometric training [ 34 ]. The current analysis included only studies that used vertical jump as the power outcome; as such, it is possible that caffeine ingestion could produce somewhat different effects on other types of muscle power tests. However, a recent meta-analysis also showed a significant performance-enhancing effect of caffeine on the Wingate test, which is a common test of power [ 35 ]. Furthermore, most of the included studies used countermovement jump for assessing vertical jump; it remains to be explored whether the caffeine ingestion would produce different effects on other forms of vertical jumping. In addition, all of the included studies evaluated these effects in isolated conditions that may not accurately reflect in-game, sport-specific jumping tasks. More evidence may be needed to determine if the performance-enhancing effects of caffeine would transfer in the context of individual sports and/or team-sport matches [ 36 ].
While previous research [ 37 ] has shown an increase in countermovement jump height after ingestion of a caffeine-containing energy drink, it was unclear if the effect was attributable to the caffeine content or the presence of other substances, such as taurine. A recent meta-analysis on caffeinated energy drinks found a significant association between their taurine content and performance, but not between their caffeine content and performance [ 38 ]. As postulated by Bloms et al. [ 8 ], motor schema might play a role when assessing the association between caffeine and muscle power. Bloms et al. [ 8 ] tested the effect of caffeine on muscle power among a cohort of athletes and reported significant increases in jumping height. By contrast, Gauvin [ 26 ] reported no effects of caffeine ingestion on muscle power in a group of untrained men, with no previous experience in the exercise. The subgroup analysis for training status indicated a significant effect for athletes, but not for non-athletes. It may be suggested that future studies should control for this confounding factor by including only participants with or without previous experience in the task, or by performing initial familiarization sessions.
None of the remaining subgroup analysis showed a significant effect of caffeine. These results might be due to the small sample sizes in different subgroup analysis. More studies are needed before reaching conclusions about context-specific effects of caffeine. Furthermore, while the body of evidence evaluating effects of caffeine on muscle power is still limited; the current meta-analysis shows promising findings, but more studies are needed on this topic. Specifically, studies including different forms of vertical jumping and sport-specific jumping tasks, different population groups, larger sample sizes, and different doses and forms of caffeine are required.
The PEDro scale showed good to excellent quality among the included studies, suggesting that the results of the current meta-analysis were not confounded by the inclusion of studies with poor research methodology. Only two studies [ 6 , 25 ] reported receiving funding from parties that may have had commercial interest for conducting the research, so it is improbable that the overall results of the current study were significantly affected by financial bias. To further improve the quality of evidence, future studies should use a double-blind rather than a single-blind design and assess the effectiveness of the blinding. Only three studies [ 3 , 22 , 24 ] reported assessing the effectiveness of the blinding. This information is of importance as participants’ recognition of the caffeine trial may influence outcomes [ 39 ], because psychological effects of ‘expectancy’ and ‘belief’ might have an impact on performance [ 40 ]. In some studies, performance-enhancing responses were found with perceived ‘caffeine’ ingestion, when in fact, a placebo was consumed [ 41 ]. Future studies examining this topic should include a questionnaire of perception of the trials to prevent possible issues associated with such confounding.
While the inclusion of doctoral and master’s theses may be considered as a limitation of this review, their inclusion is supported by their high methodological quality scores. Therefore, the inclusion of such studies may be regarded as a strength rather than a limitation, as it would be inappropriate to omit high-quality contributions to the literature from a comprehensive systematic review. A limitation of the current review is the low number of studies included in the subgroup analysis. Secondly, a limitation is that no studies were found for age groups other than adolescents and young adults. The findings, therefore, pertain mainly to young individuals and cannot be generalized to other age groups. Furthermore, due to the high degree of inter-individual variability of effects [ 42 ], these results should be interpreted with caution when it comes to prescribing caffeine supplementation to individuals. Individuals should also assess their susceptibility to possible side effects as reported in the literature, such as tremor, insomnia, elevated heart rate, headache, abdominal/gut discomfort, muscle soreness, and inability to verbally communicate and stay focused. These side effects may be enhanced in naive caffeine users [ 3 , 5 ], so extra precaution may be warranted in such individuals.
Caffeine appears to provide significant ergogenic effects on muscle strength and power. The expression of strength in the form of 1RM is most specific to the sport of powerlifting but may translate to performance improvements in a variety of other strength-power sports. The effects of caffeine on muscle power may apply to athletes in a variety of sports in which jumping is a predominant activity that affects the sport-specific performance. Subgroup-analyses suggested that the effects of caffeine on strength may be more pronounced in upper body muscles, but further research on this topic is warranted. The results of the present meta-analysis are based on limited evidence, and thus need to be interpreted with caution. Future studies should explore the optimal dosage and form of caffeine for maximizing effects on strength and power. Finally, responses to caffeine ingestion have a high degree of inter-individual variability, and as such, the applicability of the current findings must be assessed on a case-by-case basis, based on the specific characteristics of the individual and the sports activity or other physical tasks.
Rivers WHR, Webber HN. The action of caffeine on the capacity for muscular work. J Physiol. 1907;36:33–47.
Article CAS PubMed PubMed Central Google Scholar
Polito MD, Souza DB, Casonatto J, Farinatti P. Acute effect of caffeine consumption on isotonic muscular strength and endurance: a systematic review and meta-analysis. Sci Sports. 2016;31:119–28.
Article Google Scholar
Astorino TA, Firth K, Rohmann RL. Effect of caffeine ingestion on one-repetition maximum muscular strength. Eur J Appl Physiol. 2008;102:127–32.
Article CAS PubMed Google Scholar
Grgic J, Mikulic P. Caffeine ingestion acutely enhances muscular strength and power but not muscular endurance in resistance trained men. Eur J Sport Sci. 2017;17:1029–36.
Article PubMed Google Scholar
Goldstein E, Jacobs PL, Whitehurst, Penhollow T, Antonio J. Caffeine enhances upper body strength in resistance-trained women. J Int Soc Sports Nutr. 2010;7:18.
Article PubMed PubMed Central Google Scholar
Williams A, Cribb P, Cooke M, Hayes A. The effect of ephedra and caffeine on maximal strength and power in resistance-trained athletes. J Strength Cond Res. 2008;22:464–70.
Ali A, O'Donnell J, Foskett A, Rutherfurd-Markwick K. The influence of caffeine ingestion on strength and power performance in female team-sport players. J Int Soc Sports Nutr. 2016;13:46.
Bloms LP, Fitzgerald JS, Short MW, Whitehead JR. The effects of caffeine on vertical jump height and execution in collegiate athletes. J Strength Cond Res. 2016;30:1855–61.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum; 1988.
Google Scholar
Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML. Effect of caffeine ingestion on muscular strength and endurance: a meta-analysis. Med Sci Sports Exerc. 2010;42:1375–87.
Arazi H, Dehlavinejad N, Gholizadeh R. The acute effect of caffeine supplementation on strength, repetition sustainability and work volume of novice bodybuilders. Turk J Kin. 2016;2:43–8.
Arazi H, Hoseinihaji M, Eghbali E. The effects of different doses of caffeine on performance, rating of perceived exertion and pain perception in teenagers female karate athletes. Braz J Pharm Sci. 2016;52:685–92.
Brooks JH, Wyld K, Chrismas BCR. Acute effects of caffeine on strength performance in trained and untrained individuals. J Athl Enhancement. 2015;4:6.
Sabblah S, Dixon D, Bottoms L. Sex differences on the acute effects of caffeine on maximal strength and muscular endurance. Comp Exerc Physiol. 2015;11:89–94.
Diaz-Lara FJ, Del Coso J, García JM, Portillo LJ, Areces F, Abián-Vicén J. Caffeine improves muscular performance in elite Brazilian Jiu-jitsu athletes. Eur J Sport Sci. 2016;16:1079–86.
Martin J. Does caffeine ingestion prior to high intensity exercise act as an ergogenic aid in sporting performance in male athletes? Cardiff school of sport. 2015 (Master’s thesis).
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21.
PubMed Google Scholar
McCrary JM, Ackermann BJ, Halaki M. A systematic review of the effects of upper body warm-up on performance and injury. Br J Sports Med. 2015;49:935–42.
Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–73.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Andrade-Souza VA, Bertuzzi R, de Araujo GG, Bishop D, Lima-Silva AE. Effects of isolated or combined carbohydrate and caffeine supplementation between 2 daily training sessions on soccer performance. Appl Physiol Nutr Metab. 2015;40:457–63.
Clarke JS, Highton J, Close GL, Twist C. Carbohydrate and caffeine improves high intensity running of elite rugby league interchange players during simulated match play. J Strength Cond Res. 2016; https://doi.org/10.1519/JSC.0000000000001742 .
Foskett A, Ali A, Gant N. Caffeine enhances cognitive function and skill performance during simulated soccer activity. Int J Sport Nutr Exerc Metab. 2009;19:410–23.
Gant N, Ali A, Foskett A. The influence of caffeine and carbohydrate coingestion on simulated soccer performance. Int J Sport Nutr Exerc Metab. 2010;20:191–7.
Gauvin M. The effect of caffeine supplementation on muscular endurance in recreationally active college age males. University of Rhode Island, (Master’s thesis). 2016.
Le Meur Y, Hausswirth C, Mujika I. Tapering for competition: a review. Sci Sports. 2012;27:77–87.
Pyne DB, Mujika I, Reilly T. Peaking for optimal performance: research limitations and future directions. J Sports Sci. 2009;27:195–202.
Davis JK, Green JM. Caffeine and anaerobic performance: ergogenic value and mechanisms of action. Sports Med. 2009;39:813–32.
Graham TE. Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001;31:785–807.
Collomp K, Ahmaidi S, Chatard JC, Audran M, Préfaut C. Benefits of caffeine ingestion on sprint performance in trained and untrained swimmers. Eur J Appl Physiol Occup Physiol. 1992;64:377–80.
Lane JD, Steege JF, Rupp SL, Kuhn CM. Menstrual cycle effects on caffeine elimination in the human female. Eur J Clin Pharmacol. 1992;43:543–6.
Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol Biochem Behav. 1997;58:721–6.
Markovic G. Does plyometric training improve vertical jump height? A meta-analytical review. Br J Sports Med. 2007;41:349–55.
Grgic J. Caffeine ingestion enhances Wingate performance: a meta-analysis. Eur J Sport Sci. 2018;18:219–25.
Bishop D. Dietary supplements and team-sport performance. Sports Med. 2010;40:995–1017.
Abian-Vicen J, Puente C, Salinero JJ, González-Millán C, Areces F, Muñoz G, Muñoz-Guerra J, Del Coso J. A caffeinated energy drink improves jump performance in adolescent basketball players. Amino Acids. 2014;46:1333–41.
Souza DB, Del Coso J, Casonatto J, Polito MD. Acute effects of caffeine-containing energy drinks on physical performance: a systematic review and meta-analysis. Eur J Nutr. 2017;56:13–27.
Saunders B, de Oliveira LF, da Silva RP, de Salles Painelli V, Gonçalves LS, Yamaguchi G, Mutti T, Maciel E, Roschel H, Artioli GG, Gualano B. Placebo in sports nutrition: a proof-of-principle study involving caffeine supplementation. Scand J Med Sci Sports. 2017;27:1240–7.
Tallis J, Muhammad B, Islam M, Duncan MJ. Placebo effects of caffeine on maximal voluntary concentric force of the knee flexors and extensors. Muscle Nerve. 2016;54:479–86.
Pollo A, Carlino E, Benedetti F. The top-down influence of ergogenic placebos on muscle work and fatigue. Eur J Neurosci. 2008;28:379–88.
Pickering C, Kiely J. Are the current guidelines on caffeine use in sport optimal for everyone? Inter-individual variation in caffeine ergogenicity, and a move towards personalised sports nutrition. Sports Med. 2018;48:7–16.
Download references
Acknowledgements
Not applicable
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Author information
Authors and affiliations.
Institute of Sport, Exercise and Active Living (ISEAL), Victoria University, Melbourne, Australia
Jozo Grgic & Zeljko Pedisic
Applied Physiology Laboratory, Department of Exercise and Sport Science, University of North Carolina, Chapel Hill, NC, USA
Eric T. Trexler
Human Movement Science Curriculum, Department of Allied Health Sciences, University of North Carolina, Chapel Hill, NC, USA
Faculty of Education, Department of Kinesiology, J.J. Strossmayer University, Osijek, Croatia
Bruno Lazinica
You can also search for this author in PubMed Google Scholar
Contributions
JG and ZP conceived the idea and conceptualized the review. JG and BL conducted the study selection, data extraction, and methodological quality assessment. JG conducted the meta-analysis. JG drafted the initial manuscript. JG, ET, BL, and ZP contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Zeljko Pedisic .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Grgic, J., Trexler, E.T., Lazinica, B. et al. Effects of caffeine intake on muscle strength and power: a systematic review and meta-analysis. J Int Soc Sports Nutr 15 , 11 (2018). https://doi.org/10.1186/s12970-018-0216-0
Download citation
Received : 19 July 2017
Accepted : 27 February 2018
Published : 05 March 2018
DOI : https://doi.org/10.1186/s12970-018-0216-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ergogenic aid
- Performance
- Data synthesis
Journal of the International Society of Sports Nutrition
ISSN: 1550-2783
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Maintenance work is planned from 21:00 BST on Sunday 18th August 2024 to 21:00 BST on Monday 19th August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Food & Function
The effects of caffeine on pancreatic diseases: the known and possible mechanisms.

* Corresponding authors
a Shanghai Key Laboratory of Pancreatic Disease, Shanghai JiaoTong University School of Medicine, Shanghai 201600, China
b Department of Gastroenterology, Shanghai General Hospital, Shanghai JiaoTong University School of Medicine, 650 Xinsongjiang Road, Songjiang District, Shanghai 201600, China
c Department of Nutrition, Shanghai General Hospital, Shanghai JiaoTong University School of Medicine, Shanghai 201600, China
d Shanghai JiaoTong University School of Medicine, Shanghai 201600, China
Caffeine, a controversial substance, was once known to be addictive and harmful. In recent years, new effects of caffeine on the human body have been confirmed. Recent research over the past few decades has shown the potential of caffeine in treating pancreas-related diseases. This review aims to analyze the known and possible mechanisms of caffeine on pancreatic diseases and provides an overview of the current research status regarding the correlation between caffeine and pancreatic disease, while enhancing our understanding of their relationship.

- This article is part of the themed collection: Food & Function Review Articles 2024
Article information
Download citation, permissions.
L. Pan, Q. Mei, Q. Gu, M. Duan, C. Yan, Y. Hu, Y. Zeng and J. Fan, Food Funct. , 2024, 15 , 8238 DOI: 10.1039/D4FO02994A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
Coffee and Kidney Disease: Is it Safe?
October 20, 2017

The Amount of Coffee You Drink
First thing to consider is the nutritional content of coffee. An 8 oz. cup of black coffee has 116 mg of potassium 3 . This is considered a low potassium food. However, many people drink more than one cup of coffee each day. Three to four cups of coffee a day is considered high in potassium and could raise your potassium levels. Adding creamers or milk can further raise your coffee’s potassium content. Drinking less than three cups of coffee/day is generally considered safe. Phosphorus, sodium, calories, carbohydrates and protein are minimal in black coffee and not of nutritional consideration.
Your Blood Pressure
Your fluid intake, related kidney topics, which drugs are harmful to your kidneys, what is a plant-based diet, and is it good for your kidneys, foods to avoid after transplantation, related news and stories.

July 23, 2024
Hope and Healing: Counseling Kids with Kidney Disease at Camp ChiMer
July 16, 2024
Fad Diets Versus Prescribed Diets for Kidney Disease

July 09, 2024
5 Summer Picnic Recipes for a Kidney Disease Diet

June 27, 2024
Pride in Practice: Empowering LGBTQIA+ Communities in Kidney Health

October 08, 2019
Top 10 Tips for Reducing Salt in Your Diet

June 27, 2016
10 Common Habits That May Harm Your Kidneys
Related recipes.

Chef Duane: Spicy Roasted Cauliflower Tacos with Cilantro Lime Crema

Chef Duane: Kidney-Friendly Pad Thai

Chef Duane: Mediterranean Chickpea Salad

Apple Bread Loaf

Greek Orzo Salad

Grilled Veggie Kabobs
- UN stoppable care for kids
Children's Emergency Department is now located in Children's Tower: 1001 E. Marshall Street .
- Email this page
- Share via Facebook
- Share via Twitter
- Share via LinkedIn
With enticing new frozen coffees and energy drinks popping up all the time, kids and teens are increasingly interested in trying beverages with caffeine. But, is it harmful for their health?
Recommended caffeine amounts for children and why it’s important
The American Academy of Pediatrics recommends that kids 12 and under have no caffeinated beverages, including soda, energy drinks, coffee or tea, and that adolescents have less than 100 milligrams of caffeine per day. This is about the amount in a standard 8-ounce cup of coffee you’d make at home.
“Caffeine is a stimulant, which means it can briefly help with alertness and attention, but the benefits don’t outweigh the risks,” said pediatrician Dr. Sean McKenna . “It can cause agitation and irritability, which is particularly troublesome for children who may already struggle with these feelings. It can also increase the risk for sudden cardiac arrest in people with known or unknown underlying heart conditions.”
Caffeine consumption can also lead to:
- Dehydration
- Higher blood pressure and heart rate
- Nervousness
- Problems with concentration
- Trouble sleeping
- Upset stomach
Some studies have shown that caffeine can also contribute to increased anxiety, depression and stress in kids, which can be compounded by caffeine-related sleep disruptions. Then there’s the issue of its addictive qualities. Adults who are used to a daily caffeine boost know it can be tough to skip. Quitting cold turkey can lead to headaches, irritability and shakiness. These certainly aren’t situations we want our kids to experience.
Caffeine is marketed to kids and teens
It’s rare to find a child or teen interested in a small cup of black coffee.
Energy drinks come in snazzy, brightly colored packaging to pique kids’ interest, making it easy to miss the tiny print that says they’re not recommended for children. Inside that packaging is more caffeine than found in soda, sometimes triple or quadruple the amount.
Also appealing to the younger crowd are the frozen milkshake-like coffee drinks. The chocolate, caramel, whipped cream and other fun flavors also contain excessive amounts of sugar, calories and fat. Consuming them on a regular basis can contribute to obesity and diabetes.
The bottom line? Caffeinated drinks come with unnecessary health risks and lack nutritional value. It’s best that kids opt for water or milk instead.
Look for more health tips for kids and teens on the CHoR blog .
Subscribe to our blog.
- Health insights

Related articles
From a lone neonatologist to a growing nicu helping thousands of babies thrive.
July 10, 2024
The vaccines your middle schooler needs
June 25, 2024
Meet Dr. Lauren Strauss, headache expert and operations leader at CHoR
June 04, 2024
Donate Find a provider VCU Health MyChart Contact us

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
Your Body's 'Biomolecular' Makeup Shifts in Your 40s and 60s
Key takeaways.
Aging, at least when it comes to your molecules and microbiome, isn't a steady, even process, new research suggests
The body undergoes peak changes in this regard during the mid-40s and the 60s
These changes can affect health and might be due to a mix of genetics and lifestyle
WEDNESDAY, Aug. 14, 2024 (HealthDay News) -- Aging Americans, you're not imagining things: Big shifts in physical well-being do occur at certain points in the life span, new research shows.
A team at Stanford University has found "massive" changes during a person's mid-40s and early 60s in regards to the molecules and microorganisms that help maintain the body.
“We’re not just changing gradually over time; there are some really dramatic changes,” said study senior author Michael Snyder , chair of genetics at Stanford. “It turns out the mid-40s is a time of dramatic change, as is the early 60s. And that’s true no matter what class of molecules you look at.”
As his team explained it, the human body requires many thousands of different types of molecules to function and thrive. It also needs the symbiotic help of a teeming number of microorganisms -- bacteria, fungi and viruses -- that live inside people and on their skin.
However, these molecules and germs aren't static: Their composition changes as people age, according to the new report published Aug. 14 in the journal Nature Aging .
Snyder and the paper's lead author Xiaotao Shen were prompted to conduct their analysis when they noticed that the risk for many illnesses don't rise in a steady, linear fashion over time.
Instead, risks jump sharply at certain time periods: For example, the big jump in risk for Alzheimer's disease that occurs after 60.
Snyder and Shen had already studied the aging of organs, the immune system and metabolism in a group of 108 people. In their new study, the they analyzed blood and other biological samples provided by this group every few months over the span of several years.
The Stanford team focused on changes in crucial molecules -- for example, genetic material called RNA, certain proteins and metabolites -- as well as the participants' microbiome, which is the assorted germs that live within and on a person.
In total, the researchers tracked age-related changes in more than 135,000 different molecules and microbes, for a total of nearly 250 billion distinct data points.
In 81% of cases, changes in molecular or microorganism abundance and composition over time were non-linear, meaning that sharp changes happened at certain periods in life more than others.
The mid-40s and the early 60s were two points where these peaks in changes were most pronounced, Synder and Shen found.
At first, the researchers assumed that menopause -- which many women go through in their late 40s -- was skewing the results, but it turned out that the same changes were occurring for men during this time.
“This suggests that while menopause or perimenopause may contribute to the changes observed in women in their mid-40s, there are likely other, more significant factors influencing these changes in both men and women. Identifying and studying these factors should be a priority for future research,” said Shen, who was a postdoctoral scholar at Stanford when he worked on the study. He's now an an assistant professor at Nanyang Technological University Singapore.
So, how could the molecular and microbial changes he and Synder spotted affect your health?
Many of the shifts could raise a person's odds for heart trouble in the 40s and the 60s, while other changes could dampen the power of the immune system as people enter their 60s, they reasoned.
In a person's 40s, changes occurred among molecules that could influence the health of the skin and muscle, as well as the metabolism of substances such as alcohol, caffeine and fat, according to the study.
During the 60s, changes occurred that further affected skin and muscle, as well as caffeine metabolism. But changes took place that also affected carbohydrate metabolism, as well as the integrity of the immune system, the heart and the kidneys.
According to the researchers, there's a growing consensus that there can be a big difference in a person's chronological age and biological age.
Not all of the molecular or microbiome changes were due to genetics, the team theorized. For example, because a person's 40s can often be a stressful time, people tend to drink more -- and that might influence the molecular changes that occur around alcohol metabolism at that time.
All of that means that individuals can help minimize any deleterious effects of molecular-level change in their 40s and 60s, Snyder and Shen said, simply by living in a healthy way.
“I’m a big believer that we should try to adjust our lifestyles while we’re still healthy,” Snyder said.
More information
Find out more about your microbiome at the National Human Genome Research Institute.
SOURCE: Stanford University, news release, Aug 14, 2024
What This Means For You
Your body undergoes big molecular and microbiome changes during two key decades: Your 40s and your 60s.
Related Stories

- 🔥 Latest News
- 📆 Coffee Events
- ☕️ Coffee Gear
- 📝 Explainers
- ⚡️ Press Releases
- Apple Guides ➚
- ☕️ Brew Guides
- 🛒 Buy Coffee
- 🌇 City Guides
- 🎁 Gift Guides
- 📰 Cafe Spotlights
- 🔍 Find A Cafe
- 📍 Add A Cafe
- 🐱 Sprudge Shop
- Coffee Jobs
- 🚧 Build-Outs Of Coffee
- 🎨 Coffee Design
- 🍹 Coffee Drinks
- 🫘 Roaster Spotlight
- 🥫 Ready-To-Drink Coffee
- 🏆 Sprudge Design Awards
- 🏫 Sprudge Twenty
- 📢 Advertise on Sprudge
- 📔 Book: The New Rules of Coffee
- 📙 Book: But First, Coffee
- 🗞️ Newsletter
- 🏅 Sprudgie Awards
- 📰 Submit a Press Release
- ☕️ Special Projects ➚

How Much Caffeine Is In Your Coffee? Depends On Roast And Brew Method

Build-Outs Of Coffee: Spiller Park Coffee In Atlanta, GA
Build-outs of coffee: victus coffee in hartford, ct, related posts.

I Tried The Pumpkin Spice Slurpee So You Don’t Have To

Chipotle Of Coffee Hires Chipotle CEO

4+ Cups Of Coffee Daily Linked With Higher Levels Of Cognitive Decline In Old Age

Are Blue States More Coffee-Obsessed Than Red States? One Study Says Yes

Now There Is A Pumpkin Spice Slurpee (Or PSLurpee If You Will)

Changes Are Coming To The 2025 US Coffee Championships

Kick Off Coffee Fest Los Angeles With A Party At Califia Farms HQ

Are You Aboard The Specialty Coffee Cruise Right Now? Contact Us Immediately

Howard Schultz-Created Starbucks Drink Uses Howard Schultz-Owned Olive Oil

Roberto Linguanotto, Creator Of The Tiramisu, Has Passed Away

World Coffee Research Planting 10,000 F1 Hybrids In Pre-Commercial Trials

Is This The Most Elaborate In-Flight Pour-Over Ever Made?
Feeling sleepy grabbing a coffee may be shrinking your brain.

Coffee For A Cause: The New Friends Blend From Sisters Coffee Company

Members Of *NSYNC And The Backstreet Boys Team Up To Sing About Iced Coffee

Onyx Coffee Lab Teams Up With New Belgium For A Coffee Sour Beer

Nestlé Creates New Higher Yielding, More Resilient Arabica Variety Star 4

Tesla Factory Is Missing 65,000 Coffee Mugs
- Search Please fill out this field.
- Manage Your Subscription
- Give a Gift Subscription
- Newsletters
- Sweepstakes
- Nonalcoholic Drinks
You Might Be Consuming Too Much Caffeine — Here's How Much Is Actually in Your Favorite Drinks
There's caffeine hiding in more places than you'd think.
Korin Miller has spent nearly two decades covering food, health, and nutrition for digital, print, and TV platforms. Her work has appeared in Women's Health, SELF, Prevention, The Washington Post, and more.
:max_bytes(150000):strip_icc():format(webp)/korin-miller-headshot-FW24-6779d1e2ea344172a1c5b0e723ae1f29.jpg)
How does caffeine impact the body?
Do all caffeine sources impact your body the same way, how much caffeine is too much, signs you’ve had too much caffeine.
Food & Wine / Getty Images
Caffeine is having a moment, and not for the best reasons. The drug has been linked to two deaths after it showed up in large quantities in a since-discontinued lemonade offering from chain bakery Panera Bread, and has even come under fire from lawmakers for appearing in energy drinks marketed towards by children.
But despite all the negative attention, caffeine is still a popular element in plenty of products Americans consume on a regular basis. Case in point: Nearly 70% of U.S. adults said in a recent survey that they had a cup of coffee within the previous 24 hours, which is the highest number of daily coffee drinkers reported in the past two decades. Americans also eat about 10 pounds of chocolate a year, and there are plenty of other less obvious sources of caffeine we’re tossing back.
But doctors say it’s important to be aware of what exactly it is you’re consuming when you have caffeinated products, as well as why you don’t want to go overboard. Here’s how caffeine impacts your body, along with exactly how much is in a range of popular products, from a shot of espresso to a can of Red Bull.
Caffeine stimulates your central nervous system, causing you to feel more awake and energetic than you would feel otherwise. “Caffeine reduces the effects of adenosine, a signal that makes you feel sleepy, by blocking the adenosine receptors,” explains Rob M. van Dam, Ph.D. , nutrition researcher and professor in the departments of Exercise and Nutrition Sciences and Epidemiology, Milken School of Public Health, at The George Washington University.
But caffeine is also a diuretic (meaning, it can cause you to pee more), increases the release of acid in your stomach, can interfere with the absorption of calcium in your body, and increase your blood pressure, per MedlinePlus . Caffeine reaches its peak level in your blood after an hour of consuming it, although you can feel the effects for up to six hours.
Caffeine doesn’t impact everyone the same way. “If someone has ADHD, they might feel sleepy after caffeine,” says Jamie Alan, Ph.D., Pharm.D ., an associate professor of pharmacology and toxicology at Michigan State University. She also points out that some people may also be more sensitive to the effects of caffeine than others.
The opposite can also be true, too, especially if you continue to use caffeine over time. The adenosine receptors in your body become less sensitive to caffeine as you continuously expose them to the drug, says Alan. As a result, you can build up a tolerance for caffeine over time.
It’s generally assumed by doctors that all caffeine sources impact your body the same way. “It doesn’t matter how you consume caffeine — eating or drinking — the effect is the same,” says Alan.
However, van Dam notes that emerging research shows some components in coffee may partly inhibit the effects of caffeine, although more studies are needed to explore these relationships further. “Still, it is helpful to keep track of the total amount of caffeine consumed during the day from all sources to avoid consuming too much,” he says.
These are some of the most popular caffeine sources that you may come across on a regular basis, and how much caffeine each contains:
| Popular caffeine sources: | |
|---|---|
| 8-ounce cup of drip coffee | 95–200 milligrams (robusta coffee beans contain about caffeine as arabica) |
| 1-ounce | 60–65 milligrams |
| 12-ounce can of Coke | 34 milligrams |
| 12-ounce can of Pepsi | 38 milligrams |
| 12-ounce can of Diet Coke | 46 milligrams |
| 12-ounce can of Mountain Dew | 54 milligrams |
| 8-ounce cup of black tea | 47 milligrams |
| 8-ounce cup of green tea | 28 milligrams |
| 8-ounce cup of matcha tea | 70 milligrams |
But there are also super-charged products that have hit the market. Those can include:
| Caffeine in super-charged beverages: | |
|---|---|
| , 12-ounce can | 200 milligrams |
| , 20 ounces | 260 milligrams |
| , 16-ounce can | 300 milligrams |
| , 8.4-ounce can | 80 milligrams |
| , 12-ounce can | 200 milligrams |
Of course, caffeine shows up in some foods, too. Dark chocolate (70% to 85%), for example, contains more than 22 milligrams of caffeine per ounce, according to the U.S. Department of Agriculture (USDA). Given that many people eat more than an ounce of chocolate at a time, you can have a significant amount of caffeine from dark chocolate without realizing it.
This is a surprisingly tricky question to answer. Most adults can safely have up to 400 milligrams of caffeine a day, according to the Food and Drug Administration (FDA). “That is similar to four to five 8-fluid ounce cups of coffee,” says van Dam. “However, the speed with which caffeine is metabolized in the body differs substantially from person to person.”
Caffeine recommendations are significantly lower for pregnant people, with the American College of Obstetricians and Gynecologists (ACOG) suggesting having no more than 200 milligrams per day for expectant moms. People with certain heart and cardiovascular conditions may also have lower caffeine thresholds, says Alan.
You’ll usually develop uncomfortable symptoms if you have too much caffeine. Those can include, per the FDA:
- Trouble sleeping
- Anxiousness
- Elevated heart rate
- Upset stomach
- Feeling unhappy
“Very large doses can cause seizures, hallucinations, and agitation,” says Alan.
If you feel like your heart rate is too fast or it seems like it has an abnormal rhythm, Alan recommends seeking medical attention. But if you feel mostly OK after having too much caffeine, van Dam says you should be fine to ride it out. “Typically, waiting it out and reducing caffeine intake in the future is sufficient,” he says.
“You can also take an antacid to settle your stomach or eat something bland, like oatmeal or a banana,” says Alan. Taking a walk or doing light exercise may also help reduce the effects of caffeine, she points out. And, of course, doing your best in the future to have less caffeine.
Related Articles
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Newsletters
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My watchlist
- Stock market
- Biden economy
- Personal finance
- Stocks: most active
- Stocks: gainers
- Stocks: losers
- Trending tickers
- World indices
- US Treasury bonds
- Top mutual funds
- Highest open interest
- Highest implied volatility
- Currency converter
- Basic materials
- Communication services
- Consumer cyclical
- Consumer defensive
- Financial services
- Industrials
- Real estate
- Mutual funds
- Credit cards
- Balance transfer cards
- Cash back cards
- Rewards cards
- Travel cards
- Online checking
- High-yield savings
- Money market
- Home equity loan
- Personal loans
- Student loans
- Options pit
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
New on Yahoo
- Privacy Dashboard
Why does everything hurt? Research shows that our bodies go through rapid changes at these ages
For many people, reaching their mid-40s may bring unpleasant signs the body isn’t working as well as it once did. Injuries seem to happen more frequently. Muscles may feel weaker.
A new study, published Wednesday in Nature Aging, shows what may be causing the physical decline. Researchers have found that molecules and microorganisms both inside and outside our bodies are going through dramatic changes, first at about age 44 and then again when we hit 60. Those alterations may be causing significant differences in cardiovascular health and immune function.
The findings come from Stanford scientists who analyzed blood and other biological samples of 108 volunteers ages 25 to 75, who continued to donate samples for several years.
“While it’s obvious that you’re aging throughout your entire life, there are two big periods where things really shift,” said the study’s senior author, Michael Snyder, a professor of genetics and director of the Center for Genomics and Personalized Medicine at Stanford Medicine. For example, “there’s a big shift in the metabolism of lipids when people are in their 40s and in the metabolism of carbohydrates when people are in their 60s.”
Lipids are fatty substances, including LDL, HDL and triglycerides, that perform a host of functions in the body, but they can be harmful if they build up in the blood.
The scientists tracked many kinds of molecules in the samples, including RNA and proteins, as well as the participants’ microbiomes.
The metabolic changes the researchers discovered indicate not that people in their 40s are burning calories more slowly but rather that the body is breaking food down differently. The scientists aren’t sure exactly what impact those changes have on health.
Previous research showed that resting energy use, or metabolic rate , didn’t change from ages 20 to 60. The new study’s findings don't contradict that.
The changes in metabolism affect how the body reacts to alcohol or caffeine, although the health consequences aren’t yet clear. In the case of caffeine, it may result in higher sensitivity.
It’s also not known yet whether the shifts could be linked to lifestyle or behavioral factors. For example, the changes in alcohol metabolism might be because people are drinking more in their mid-40s, Snyder said.
For now, Snyder suggests people in their 40s keep a close eye on their lipids, especially LDL cholesterol.
“If they start going up, people might want to think about taking statins if that’s what their doctor recommends,” he said. Moreover, “knowing there’s a shift in the molecules that affect muscles and skin, you might want to warm up more before exercising so you don’t hurt yourself.”
Until we know better what those changes mean, the best way to deal with them would be to eat healthy foods and to exercise regularly, Snyder said.
Dr. Josef Coresh, founding director of the Optimal Aging Institute at the NYU Grossman School of Medicine, compared the new findings to the invention of the microscope.
“The beauty of this type of paper is the level of detail we can see in molecular changes,” said Coresh, a professor of medicine at the school. “But it will take time to sort out what individual changes mean and how we can tailor medications to those changes. We do know that the origins of many diseases happen in midlife when people are in their 40s, though the disease may occur decades later.”
The new study “is an important step forward,” said Dr. Lori Zeltser, a professor of pathology and cell biology at the Columbia University Vagelos College of Physicians and Surgeons. While we don’t know what the consequences of those metabolic changes are yet, “right now, we have to acknowledge that we metabolize food differently in our 40s, and that is something really new.”
The shifts the researchers found might help explain numerous age-related health changes, such as muscle loss, because “your body is breaking down food differently,” Zeltser said.
This article was originally published on NBCNews.com
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceuticals (Basel)
- PMC10459237

Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies
Sofia m. saraiva.
1 CPIRN-UDI/IPG, Center of Potential and Innovation of Natural Resources, Research Unit for Inland Development (UDI), Polytechnic Institute of Guarda, 6300-559 Guarda, Portugal; tp.gpi@aviarasaifos (S.M.S.); tp.gpi@otnicajamlet (T.A.J.)
Telma A. Jacinto
Ana c. gonçalves.
2 CICS-UBI—Health Sciences Research Centre, University of Beira Interior, 6201-001 Covilhã, Portugal; tp.opas@sevlacnoganiloracana
Dário Gaspar
3 Department of Sport Sciences, University of Beira Interior, 6201-001 Covilhã, Portugal; moc.liamg@7rapsagoirad
Luís R. Silva
4 Department of Chemical Engineering, University of Coimbra, CIEPQPF, Rua Sílvio Lima, Pólo II—Pinhal de Marrocos, 3030-790 Coimbra, Portugal
Associated Data
Data sharing not applicable.
Caffeine is a naturally occurring alkaloid found in various plants. It acts as a stimulant, antioxidant, anti-inflammatory, and even an aid in pain management, and is found in several over-the-counter medications. This naturally derived bioactive compound is the best-known ingredient in coffee and other beverages, such as tea, soft drinks, and energy drinks, and is widely consumed worldwide. Therefore, it is extremely important to research the effects of this substance on the human body. With this in mind, caffeine and its derivatives have been extensively studied to evaluate its ability to prevent diseases and exert anti-aging and neuroprotective effects. This review is intended to provide an overview of caffeine’s effects on cancer and cardiovascular, immunological, inflammatory, and neurological diseases, among others. The heavily researched area of caffeine in sports will also be discussed. Finally, recent advances in the development of novel nanocarrier-based formulations, to enhance the bioavailability of caffeine and its beneficial effects will be discussed.
1. Introduction
Currently, special attention is being paid to natural molecules and their putative therapeutic effects to delay, or even prevent, the occurrence of many diseases and improve the health status of the population [ 1 ]. Indeed, their ingestion is widely believed to have fewer or no adverse effects on humans than most synthetic molecules, and they are also cheaper and easier to obtain [ 2 , 3 , 4 ]. Caffeine, in particular, has been the subject of intense and in-depth research on the human organism regarding its health-promoting effects and possible beneficial effects on the performance of athletes, especially through its ability to improve anaerobic and aerobic performance, muscle efficiency, and speed, and to reduce fatigue [ 5 , 6 , 7 , 8 , 9 ]. Caffeine is probably the most commonly ingested psychoactive substance in the world, found mainly in coffee, soft drinks, tea, cocoa and chocolate-like products, yerba matte leaves, guarana berries, and some pharmaceuticals [ 10 ]. It is rapidly absorbed and distributed in all human tissues, reaching maximum plasma concentrations 30–120 min after oral intake [ 9 ].
As far as we know, in vivo studies have already reported that caffeine stimulates the central nervous system by acting as an antagonist of A1 and A2 adenosine receptors, promotes adrenaline release, increases dopamine, noradrenalin, and glutamate levels, blood circulation and respiratory rate, mobilizes intracellular calcium stores, and alters fat and carbohydrates metabolism in the human body by stimulating lipolysis, thanks to its ability to inhibit phosphodiesterase enzymes [ 11 , 12 , 13 , 14 , 15 , 16 ]. In addition, caffeine also increases energy, alertness, excitement, and mood [ 17 , 18 ]. According to the European Food Safety Authority (EFSA), a habitual daily consumption of caffeine up to 400 mg (5.7 mg/kg bw per day for a 70 kg adult) by adults, and 200 mg by pregnant or breastfeeding women is considered to be safe [ 19 ]. Exceeding this dose or the sudden cessation of caffeine intake may cause anxiety, insomnia, hallucinations, hypertension and headache, gastrointestinal and sleep disturbances, diuresis, dehydration, tremors, palpitations, and cardiac arrhythmias given the stimulant effects of caffeine [ 20 , 21 , 22 , 23 ]. Regarding children and adolescents, the EFSA considers that there is insufficient information [ 19 ]. Considering caffeine’s high consumption, as well as its increasing commercial availability in pure form, and presence at high concentrations in products such as dietary supplements, the US Food and Drug Administration (FDA) and EFSA warn about the risks of its consumption at high doses [ 24 , 25 ].
Nonetheless, considering the different beneficial biological and physiological effects of caffeine, extensive studies have been conducted to determine its full health potential. In this line, caffeine encapsulation, alone or combined with other molecules, has been performed to increase its biological activities [ 26 , 27 , 28 , 29 , 30 ]. Therefore, the main objective of this review is to discuss the biological potential of caffeine for human health, highlighting its anticancer, immunological, anti-inflammatory, cardiovascular, and neurological protective effects, as well as its effects on the performance of athletes.
2. Chemical Structure and Main Natural Sources of Caffeine
Caffeine (C 8 H 10 N 4 O 2 ; Figure 1 A), also known as 1,3,7-trimethylxanthine, belongs to the group of methylxanthines, which are alkaloids [ 11 , 31 ]. Together with theobromine, its precursor ( Figure 1 B), both are synthetized by the fruits, leaves, and seeds of many plants and trees to protect them from diseases and predators [ 22 , 32 , 33 ].

Chemical structure and functional groups of caffeine ( A ) and theobromine ( B ).
Regarding their structure, both compounds are carbon- and nitrogen-based molecules composed of two purine rings, a pyrimidine ring (C5-ring), and an imidazole ring (C6-ring), both of which have two nitrogen atoms [ 34 , 35 ]. Their functional groups are an amide (a carbonyl group bonded to carbon and nitrogen atoms), an amine (at least one hydrocarbon group bonded to a nitrogen atom), and an alkene (an unsaturated hydrocarbon with a double bond between two carbon atoms) [ 34 ]. Caffeine serves as a hydrogen bond acceptor because three of its four nitrogen atoms are methylated [ 36 ].
Although caffeine and theobromine share similarities at the physical and chemical levels, caffeine has an additional methylene group and exerts stronger central nervous system effects than theobromine [ 37 , 38 ].
Regarding caffeine consumption, recent statistical data show that more than 85% of American adults consume caffeine daily (135 mg per day) [ 39 , 40 ]. In Europe, a higher caffeine consumption is observed (values ranging from 37 to 319 mg per day), especially in the Netherlands (411 mg per day), Denmark (390 mg per day), Finland (329 mg per day), Austria (300 mg per day), and Switzerland (288 mg per day) [ 19 , 41 ]. Compared to other non-European countries, Brazil and Argentina also have a high consumption of caffeine (mean values of 40 and 100 mg per day, respectively), as well as Australia (232 mg per day) and Japan (169 mg per day) [ 41 ]. In contrast, lower amounts of caffeine are consumed in China (16 mg per day), Angola (4 mg per day), and Kenya (50 mg per day) [ 41 ].
The main sources of caffeine are coffee and tea, energy beverages, sodas and soft drinks, and dark chocolate (see Table 1 ) [ 31 , 41 , 42 , 43 , 44 ]. As expected, coffee and chocolate are the most popular sources of caffeine worldwide [ 31 , 42 ]. Among the different types of coffee (beverage), American coffee is the most caffeinated (91.7–213.3 mg per 100.0 mL) [ 42 ], followed by Scotland espresso (66.0–276.0 mg per 13.0–90.0 mL) [ 31 ]. As for tea, black tea and Yerba Mate contain considerable amounts of this molecule (both around 40.0 mg per 236.0 mL) [ 41 ]. Among soft drinks, Mountain Dew Rise and Diet Coke have considerable amounts of caffeine (180.0 mg per 473.0 mL and 46.0 mg for 354.0 mL, respectively) [ 41 ]. Among energy drinks, Java Monster 300 and Rockstar XDurance present the highest caffeine contents (amounts around 300.0 mg per 443.0 and 473.0 mL, respectively), followed by Full Throttle (160.0 mg per 473.0 mL) [ 41 ]. In addition, as expected, energy shots, such as Spike Energy Double Shot and Bang Shot, are also rich in caffeine (levels of 350.0 and 300.0 mg per 125.0 and 88.0 mL, respectively) [ 41 ]. Finally, Cran Energy juice and Water Joe also have significant amounts of caffeine (70.0 mg per 295.0 and 591.0 mL, respectively) [ 41 , 45 ]. The presence of caffeine is also reported in dark chocolate (8.0 mg per 1.0 g) [ 41 ].
Major sources of caffeine and respective levels, according to their usual commercialization volumes/recommend preparations.
| Source | Volume (mL) | Caffeine Range (mg) | References |
|---|---|---|---|
| Americano coffee | 100.0 | 91.7–213.3 | [ ] |
| Decaffeinated coffee | 500.0 | 0.0–13.9 | [ ] |
| Instant coffee | 125.0 | 8.7–120.0 | [ , , , ] |
| Plain coffee | 200.0 | 68.4–136.9 | [ ] |
| Scotland espresso | 13.0–90.0 | 66.0–276.0 | [ ] |
| Italy espresso | 13.0–31.0 | 54.0–150.0 | [ ] |
| Spain espresso | 34.0–104.0 | 82.0–139.0 | [ ] |
| Black tea | 236.0 | 42.0 | [ ] |
| Green tea | 236.0 | 18.0 | [ ] |
| Yerba Mate | 236.0 | 40.0 | [ ] |
| Coca-Cola classic | 354.0 | 34.0 | [ ] |
| Coca-Cola Energy | 354.0 | 38.0 | [ ] |
| Diet Coke | 354.0 | 46.0 | [ ] |
| Pepsi | 354.0 | 38.0 | [ ] |
| Mountain Dew | 354.0 | 54.0 | [ ] |
| Mountain Dew Rise | 473.0 | 180.0 | [ ] |
| Ski | 354.0 | 69.0 | [ ] |
| Sunkist | 354.0 | 19.0 | [ ] |
| Mountain Dew Amp | 473.0 | 142.0 | [ ] |
| Full Throttle | 473.0 | 160.0 | [ ] |
| Monster Dragon Tea | 680.0 | 60.0 | [ ] |
| Java Monster 300 | 443.0 | 300.0 | [ ] |
| Red Bull | 250.0 | 80.0 | [ ] |
| Rockstar Boom | 473.0 | 160.0 | [ ] |
| Rockstar XDurance | 473.0 | 300.0 | [ ] |
| Cran Energy | 295.0 | 70.0 | [ ] |
| Bang Shot | 88.0 | 300.0 | [ ] |
| 5-Hour Energy | 57.0 | 200.0 | [ ] |
| TruBrain Extra | 29.0 | 100.0 | [ ] |
| Spike Energy Double Shot | 125.0 | 350.0 | [ ] |
| Water Joe | 591.0 | 70.0 | [ , ] |
| Dark chocolate | 10.0 * | 8.0 | [ ] |
| Guarana | 1.0 * | 47.0 | [ ] |
* Values in grams.
3. Benefits of Caffeine on Health
For our search, we used Web of Science. The search restrictions were based on language (English), year of publication from 2018 to present, and type of publication set to journal. The keywords used for the search were “caffeine” in combination with any of these other keywords: “cancer”, “anticancer”, “antitumor”, “anti-tumor”, anti-cancer”, “neurodegenerative diseases”, “autoimmune diseases”, “immunological”, “immunomodulatory”, “immune system”, “anti-inflammatory”, and “cardiovascular”. In the following subsections, we provide an overview of the latest research regarding the impact of caffeine in different illnesses such as cancer, autoimmune diseases, immunomodulation, and ocular, respiratory, neurodegenerative, and cardiovascular diseases.
3.1. Cancer
Cancer is one of the leading causes of death worldwide. It was estimated that in 2020, there were 19.3 million cancer cases, which resulted in 10.0 million cancer deaths [ 46 , 47 ]. By 2030, it is estimated that over 22 million people will develop cancer [ 47 , 48 ]. In addition, cancer is responsible for a significant economic burden on both the health care system and patients [ 48 ].
As early as 2000, Hanahan and Weinberg defined the key features (i.e., “hallmarks of cancer”) that describe the characteristics necessary to promote cancer growth and metastasis. These hallmarks are self-sufficiency in growth signals, insensitivity to antiproliferative signals, resistance to apoptosis, limitless replicative potential, the induction of angiogenesis, and the activation of tissue invasion and metastasis [ 49 ]. In 2011, the authors revised the original hallmarks and added two more cancer-promoting features (genomic instability and tumor-promoting inflammation) and two more hallmarks (deregulation of cellular energetics and avoidance of immune destruction) [ 50 ]. As the understanding of cancer underlying mechanisms of progression has grown, as have the available experimental and computational tools; early in 2022, Hanahan reviewed the previously discussed features and included new additional features of cancer, namely, (i) phenotypic plasticity, (ii) non mutational epigenic reprogramming, (iii) polymorphic microbiomes, and (iv) senescent cells [ 51 ].
The role of coffee components in suppressing some of the cancer hallmarks defined by Hanahan and Weinberg [ 52 , 53 ] has been reviewed by Gaascht et al. and Cadóna et al., while other authors have fully elucidated the effect of caffeine on the cell cycle [ 54 ]. Caffeine anticancer activity has been widely studied [ 55 ], and the below-stated findings demonstrate the capacity of caffeine to overcome some of the cancer-promoting hallmarks, such as resistance to cell death and cellular senescence, that play an important role in cancer progression [ 51 ]. Further, several works state that caffeine may induce apoptosis through numerous pathways, such as p-53-dependent and -independent, phosphatase and tensin homolog, PI3K/protein kinase B (AKT), and mammalian target of rapamycin (mTOR) pathways [ 56 ].
El Far et al. studied the effect of caffeine and other natural substances on the senescent cells of colon and breast cancers. After inducing senescence with doxorubicin, the cells were treated with various doses of caffeine (0, 5, 10, 15, 20, 30, 40, 50, and 60 mM). The IC 50 of caffeine against doxorubicin-treated HCT116 and MCF7 cells was 13.36 ± 2.29 mM and 17.67 ± 3.98 mM, respectively. The authors also examined caffeine-induced apoptosis in both senescent and proliferative cells. At concentrations of 10 and 15 mM, caffeine induced a significant increase in apoptosis in senescent HTC116 cells, and at concentrations of 5, 10, and 15 mM in senescent MCF7 cells compared with proliferative cells [ 56 ]. In another study, Machado et al. evaluated the effect of caffeine on two breast cancer cell lines (MCF-7 and MDA-MB-231). The results showed that caffeine at a concentration of 2.5 mM and 5 mM for MCF-7 and MDA-MB-231, respectively, reduced cell viability and induced apoptosis [ 57 ]. The antitumoral effects of caffeine were studied in diverse cancer in vitro models, such as glioblastoma, melanoma, and pancreatic and lung cancers [ 58 , 59 , 60 ].
The antitumoral effects of caffeine have also been evaluated in in vivo tumor models. Venkata Charan Tej and collaborators investigated the effect of caffeine on the carcinogen-induced tumor model of fibrosarcoma. After 250 days of 3-MCA inoculation, there was a dose-dependent decrease in the tumor incidence and growth rate in the groups treated with caffeine (1.030, 2.060, and 4.120 mM) [ 61 ]. The anti-tumoral effect of caffeine was related to its action on cytotoxic T lymphocytes. On one hand, caffeine led to a higher percentage of cytotoxic T cells in the tumor, and on the other hand, it decreased the expression of programmed cell death protein 1 (PD-1) on these cells. In addition, it also increased the levels of pro-inflammatory cytokines such as TNF-α and IFN-γ. These results are in line with the previously known inhibitory effect of caffeine on the adenosine-A2a receptor pathway [ 62 ], which is one of the immunosuppressive pathways involved in cancer [ 63 , 64 ]. This capacity of caffeine to modulate the immune system in the tumor surroundings alters another important hallmark (i.e., the ability to avoid immune destruction). The modulation of the PD-1, an important immune checkpoint, and consequent enhancement of the T cell responses can exert an antitumor effect. In fact, the inhibitors of this protein are one of the immunotherapies approved by the FDA [ 65 ].
The therapeutic effect of caffeine was also demonstrated for renal carcinoma. Xu et al. showed, through in silico studies, that caffeine is able to bind to glucose-6-phosphate dehydrogenase (G6PDH), which is considered a biomarker and potential therapeutic target for this type of cancer. Consistent with the above results, in this study, the use of caffeine at concentrations of up to 0.016 mM for in vitro studies and 60 and 120 mg/kg/day for in vivo studies decreased the viability and proliferation of ACHN and 786-O cancer cells both in vitro and in vivo [ 64 ]. G6PDH is an important target in cancer given that is normally upregulated in different cancers and its dysregulation can provide valuable conditions for cancer progression [ 66 ]. Further, it also has an important role in maintaining the redox balance and biosynthesis of nucleotides and lipids, which is part of another cancer hallmark (i.e., reprogramming cellular metabolism) [ 67 ].
As previously mentioned, caffeine has also been tested in combination with other drugs in order to potentiate the antitumoral effect [ 68 , 69 , 70 , 71 ]. Higuchi et al. evaluated the efficacy of oral recombinant methioninase (o-rMETase) in combination with caffeine and doxorubicin in an orthotopic xenograft mouse model of synovial sarcoma. After two weeks of treatment, the group treated with the combinatorial treatment was able to induce tumor regression. According to the authors, this can be explained by the ability of caffeine to induce mitotic catastrophe [ 72 ]. Other examples of caffeine combination with different drugs are depicted in Table 2 .
Overview of the latest research regarding caffeine anti-cancer activity.
| Target Cancer | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Breast | In vitro | MCF-7 and MDA-MB-231cells | 1–10 mM | Caffeine reduced the cell viability in concentrations greater than 2.5 mM for MCF7 and for 5 and 10 mM for MDA-MB-231 cell lines. At the latter concentrations, caffeine induces apoptosis and necrosis in both cell lines. | [ ] |
| Breast | In vitro | MDA-MB-231, MCF7 and MCF10A cells | 0.000125 mM | After MDA-MB-231 and MCF7 cells’ treatment with caffeine, there was a change in metabolism towards respiratory-chain phosphorylation with low ratio of free to bound NADH. In combination with cisplatin, there was a decrease in viability and preference of cancer cells over normal breast cells. | [ ] |
| Breast and colon | In vitro | HCT116 and MCF7 cells | 0–60 mM | Apoptosis increased in both proliferative and senescent cells after treatment with caffeine at a concentration of 15 mM. | [ ] |
| Carcinoma squamous cells | In vitro | HN5 and KYSE30 cells | 0.5–70 mM | Caffeine at concentrations of 20, 50, and 70 mM presented an inhibitory effect and decreased the proliferation rate of both cell lines. | [ ] |
| Endometrial | In vitro | RL95-2, HEC-1-A and KLE cells | 0–40 mM | Therapeutic concentration of cisplatin decreased from 4.1 to 1.1 µM and from 163 to 6.6 µM, with caffeine concentrations of 1.1 and 5.3 mM, respectively. | [ ] |
| Glioblastoma multiforme | In vitro | Human GBM and U87-MG cells | 1 mM | Pre-treatment of cells with caffeine followed by combined treatment of temozolomide and caffeine significantly decreased cell viability compared to the other groups. | [ ] |
| Glioblastoma multiforme | In vitro | Human GBM, U87MG and T98G 101 cells | 0.5–10 mM | In both cell lines, caffeine at 2.5 mM was able to reduce cellular viability, which was more pronounced under hypoxia. | [ ] |
| Lung | In vitro | NCI-H23 and MLC15 cells | 0–0.5 mM | After of NCl-H23 cells’ treatment with 0.25 and 0.50 mM caffeine, the size of colonies decreased by 78.1% and 63.9%, respectively. In addition, caffeine induced cell arrest in the G0/G1 phase, reduced the S phase of the cell cycle, and suppressed cell invasion. | [ ] |
| Melanoma | In vitro | Normal human melanocytes COLO829 and C32 cells | 100–1000 mM | The results showed the ability of caffeine to reduce the viability of COLO829 and C32 cells by 5–35% and 1–16%, respectively. In addition, it also led to a decrease in thiol degradation and pro-apoptotic effects and did not affect normal melanocytes cells. | [ ] |
| Melanoma | In vitro | B16F10 cells | 0.001–0.04 mM | Cells’ pre-treatment with caffeine enhanced the cytotoxic effects induced by dacarbazine. In addition, caffeine increased oxidative stress in a dose-dependent manner. | [ ] |
| Pancreatic ductal adenocarcinoma | In vitro | AsPC-1, BxPC-3, Capan-1, COLO-357, MiaPaCa-2, SU.86.86, PANC-1, and T3M4 pancreatic cancer cells | 0.1, 0.2 mM | Caffeine enhanced cell death induced by 5-fluorouracil and gemcitabine, and also decreased the IC of both chemotherapeutic agents. | [ ] |
| Prostate | In vitro | PC-3 cells | 0.5 mM | Caffeine affected cell viability in a dose-dependent manner. Cell migration and invasion ability was more affected by the combination of atorvastatin and caffeine than by caffeine alone. The same was observed for the formation of tumor spheres. | [ ] |
| Glioma | In vitro and in vivo | RT2 cells-induced glioma in male Fischer 344 inbred rat | 100 mg/kg/day orally (2 weeks) plus temozolomide given once daily (5 days) | The combination of caffeine with temozolomide inhibited tumor growth compared to the control group. | [ ] |
| Hepatocellular carcinoma | In vitro and in vivo | SMMC-7721 and Hep3 cell lines and Male BALB/c nude mice | 0–32 mM (in vitro) 20 mg/kg/day injected IP every other day for (2 weeks) | Caffeine decreased the viability of both cell lines and had a synergistic effect with 5-fluorouracil. In addition, tumor growth was suppressed, and tumor weight was reduced in mice treated with caffeine alone or in combination with 5-fluorouracil. | [ ] |
| Osteosarcoma, fibrosarcoma | In vitro and in vivo | HOS, HT1080 and LM8 cells and athymic nude mice | 0.5 mM (in vitro) 100 mg/kg injected IP on days 2 to 4 to the treatment (1 week). The treatment was performed two times. | The combination of cisplatin and caffeine decreased cell viability compared with cisplatin alone. In vivo, after implantation of LM8 and HT1080 cells, the combination of cisplatin + caffeine decreased tumor volume and weight. | [ ] |
| Pleomorphic rhabdomyosarcoma | In vitro and in vivo | RMS cells, Athymic nu/nu nude mice | 0.5 and 1 mM (in vitro) 100 mg/kg/day injected IP daily (3 weeks) | Caffeine showed the ability to enhance the antiproliferative effects of valproic acid. In vivo, the group treated with caffeine and valproic acid showed a reduction in tumor volume compared to the control group. This was also confirmed in the group treated with A1 receptor in combination with caffeine and valproic acid. | [ ] |
| Renal cell carcinoma | In silico, in vitro, and in vivo | ACHN and 786-O cells, and BALB/c nude mice | 0–0.016 mM intragastrically administered for 34 consecutive days | The molecular docking studies demonstrated that caffeine was able to bind to G6PDH at the NADP+ binding site, which is a biomarker and potential therapeutic target for renal cell carcinoma. In addition, caffeine was able to decrease the viability and proliferation of both cell lines and in the in vivo studies. | [ ] |
| Colorectal | In vivo and in silico | Swiss Webster mice | 50 mg/kg/day, intragastrically 5 times a week (10 weeks) | Mice treated with caffeine alone or in combination with chlorogenic acid decreased the expression of IL-6, IL-17, and TNF-α. | [ ] |
| Fibrosarcoma | In vivo | Adult albino mice | 1.030, 2.060 and 4.120 mM in drinking water administered daily (8 weeks) | In caffeine-treated mice, tumor incidence, size, and growth rate decreased with the increase in caffeine concentration. In addition, caffeine-treated mice had a higher percentage of cytotoxic T cells and higher TNF-α and IFN-γ levels. | [ ] |
| Fibrosarcoma | In vivo | Adult Syrian golden hamsters | 100 mg/kg/day, intragastrical administration; treatment started 3 days before inoculation with sarcoma cells and continued for 14 days | Administration of metformin and caffeine resulted in inhibition of fibrosarcoma growth. | [ ] |
| Melanoma | In vivo | Albino mice and C57BL/6J mice | 4.120 mM daily in drinking water (3 or 6 weeks) | In the carcinogen-induced tumor model, the groups treated with caffeine alone decreased the tumor growth rate from 5.3 mm /day to 2.6 mm /day. The combination with anti-PD1 led to a more pronounced decrease (0.9 mm /day). | [ ] |
| Osteosarcoma | In vivo | Athymic nu/nu nude mice | 100 kg/kg/day, orally administered for 14 consecutive days | The osteosarcoma mice model (patient-derived orthotopic xenograft) treated with cisplatinum + oral recombinant methioninase + caffeine, showed the most marked decrease in comparison to the other groups. | [ ] |
| Synovial sarcoma | In vivo | Athymic nu/nu nude mice | 100 mg/kg/day, orally administered for 14 consecutive days | The combination of oral recombinant methioninase and caffeine reduced tumor volume. | [ ] |
IC 50 , half-maximal inhibitory concentration; NADH, nicotinamide adenine dinucleotide; MTT assay, (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay; Anti-PD1, anti-Programmed Cell Death Protein 1; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; G6PDH, glucose-6-phosphate dehydrogenase; NADP+, nicotinamide adenine dinucleotide phosphate; IP, intraperitoneally.
Understanding the effects of caffeine on cancer and the mechanisms underlying this effect is of extreme importance. Table 2 summarizes the most recent (from 2018) works on this topic. These studies also contribute to determining the necessary caffeine quantities to achieve a therapeutic effect and to ensure the safe use of caffeine.
3.2. Anti-Inflammatory and Immunomodulation
3.2.1. autoimmune diseases and immunomodulation.
Inflammation is usually caused by infection or damage to a tissue [ 84 ]. Caffeine has the ability to exert modulation on the immune system. The immune response can be divided into two types: (i) innate and (ii) adaptive immunity [ 85 ]. Acute inflammation is a mechanism of innate immunity, whereas chronic inflammation usually contributes to the development of various diseases, such as metabolic disorders, neurodegenerative diseases, and even cancers [ 86 , 87 ]. The effect of caffeine on the innate immune system is related to the reduction in macrophage, neutrophil, and monocyte chemotaxis [ 88 , 89 ]. As for adaptive immunity, the effect of caffeine is due to the inhibition of Th1 and Th2 cell proliferation, as well as to the alteration of B cell function and the consequent reduction in antibody production [ 89 , 90 , 91 , 92 ]. Several authors, such as Horrigan et al., Açıkalın et al., and Al Reef et al., already reviewed, in depth, the impact of caffeine on the immune system and its capacity to alleviate autoimmune diseases [ 88 , 93 , 94 ].
Considering the immunomodulatory effects of caffeine, Wang et al. evaluated its effects on multiple sclerosis. Experimental autoimmune encephalomyelitis is the standard animal model for multiple sclerosis. After inducing the disease in C57BL/6 mice, these were treated with caffeine (10, 20, or 30 mg/kg/day) in drinking water. The results showed that caffeine could reduce inflammatory cell infiltration, the degree of demyelination, and microglial in vivo. It also reduced NLRP3 and p62 protein levels. In vitro assays indicated that caffeine promoted autophagy [ 95 ]. In another study, Ghaffary et al. evaluated the potential of mesenchymal stem cells to reduce the severity of rheumatoid arthritis. Wistar rats were treated with mesenchymal stem cells that had previously been incubated with various concentrations of caffeine. The results showed that the rats treated with mesenchymal stem cells, previously treated with 0.5 mM of caffeine, presented decreased disease severity and serum levels of C-reactive protein, nitric oxide, myeloperoxidase, and TNF-α. In addition, the IL-10 serum levels and the weight of the treated rats increased [ 96 ].
3.2.2. Ocular Diseases
Adenosine receptors are also expressed by retinal endothelial and retinal pigment epithelial (RPE) cells, as well as choroid and choroidal cells [ 97 ]. Therefore, caffeine may also have beneficial effects in ocular diseases, such as choroidal neovascularization and retinal inflammation.
Retinal inflammation is involved in ocular diseases as age-related macular degeneration (AMD) and diabetic retinopathy (DR), among others. For example, AMD is characterized by elevated vitreous levels of IL-1 β [ 98 ] and plasmatic tumor necrosis receptor 2 (TNF-R2) and low levels of brain-derived neurotrophic factor (BDNF) in the aqueous humor, which negatively affect photoreceptor and retinal ganglion cells’ survival [ 99 ]. Conti et al. demonstrated that caffeine has an anti-inflammatory effect in RPE cells, decreasing the expression of IL-1 β , IL-6, and TNF- α , as well as the nuclear translocation of nuclear factor kappa B (NF- κ B). In addition, the topical instillation of caffeine in an ischemia-reperfusion injury mice model was shown to restore physiological BDNF levels and reduce the mRNA levels of IL-6 in the retina, demonstrating its potential for the treatment of retinal inflammation and degeneration [ 100 ]. The effect of caffeine on choroidal adenosine receptors, the reduction in cell migration to the injured area, and angiogenesis demonstrate the importance of caffeine in attenuating choroidal neovascularization [ 97 ]. Despite the potential of caffeine in the management of such ocular conditions, the available studies are still scarce.
3.2.3. Respiratory Diseases
Currently, there are respiratory diseases for which caffeine is used as a clinical treatment, namely, premature infant diseases such as apnea and bronchopulmonary dysplasia (BPD). BPD is a common neonatal pulmonary complication with a prevalence of 45% in preterm infants [ 101 ]. BPD is associated with a nonspecific inflammatory response involving the activation of Toll-like receptors (TLRs), NOD-like receptors (NLRs), and increased levels of pro-inflammatory cytokines (IL-1 β , IL-6, IL-8, IL-18, TNF α ) [ 102 ]. In addition, NLR3 (NOD-, LRR-, and pyrin domain-containing protein 3), a key player in the pathogenesis of BPD, is responsible for the release of pro-inflammatory cytokines (IL-1 β and IL-18) and alveolar cell death through various mechanisms [ 103 , 104 ]. Caffeine is the most commonly used medication for extreme prematurity (less than 28 weeks) and is also very commonly prescribed for very early preterm birth (28 to 32 weeks) [ 105 ]. As clinically shown, the early initiation of caffeine treatment (5 and 10 mg/kg/day) is important to achieve a successful outcome. Early treatment significantly reduced BPD incidence and mortality in low-birth-weight neonates [ 106 ]. Despite the use of caffeine and its clear benefits, the mechanisms behind the clinical benefits in these diseases are not fully understood.
In vitro studies showed that the treatment of lipopolysaccharide (LPS)-induced macrophages with caffeine caused a reduction in caspase-1 expression and the inhibition of the NLRP3 inflammasome, demonstrating its potential effect on this important target. Moreover, in vivo, the treatment of newborn mice with hypoxia-induced lung injury with caffeine was shown to significantly increase A2a receptor expression and inhibit the NLRP3 inflammasome protein and NF- κ B pathway in the lung. The effect of caffeine on these key regulators attenuated inflammatory infiltration, reduced oxidative stress, decreased alveolar cell death, and promoted alveolar development [ 107 ]. Similar results were also observed in another study; specifically, caffeine caused a decrease in NF- κ B and pro-inflammatory factor levels, increased the expression of A1, A2a, and A2b receptors, and decreased cell death in the lung [ 108 ].
Table 3 summarizes recent research findings on the anti-inflammatory effects of caffeine and its effects on autoimmune diseases.
Overview of the latest research regarding caffeine anti-inflammatory activity and impact on the immune system.
| Target/Disease | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Anti-inflammatory effect and immunomodulation | In vitro | Human peripheral blood mononuclear cells | 1.16 mM | Caffeine reduced the levels of several cytokines (IL-8, MIP-1 , IL-6, IFN- , GM-CSF, TNF- , IL-2, IL-4, MCP-1, and IL-10. It also inhibited STAT1 signaling. | [ ] |
| Bronchopulmonary dysplasia | In vitro | THP-1-derived macrophages | 100–800 μM | There was a decrease in NLRP3 inflammasome activation, ASC speck formation, and caspase 1 cleavage. In addition, IL-1β and IL-18 secretion decreased, as well as the phosphorylation of MAPK and NF-kB pathway members. | [ ] |
| Immunomodulation | In vitro | Monocytes and macrophage | 300–1000 µM | Caffeine suppressed TNF-α and Akt signaling in both LPS-activated macrophage subtypes, inhibited STAT/IL-10 signaling in macrophage colony-stimulating factor, and significantly increased the expression of A2a and downregulated mTOR phosphorylation in M-macrophages. | [ ] |
| Immunomodulation | In vitro | Mesenchymal stem cells and neutrophiles | 0.1–1 mM | Caffeine-treated mesenchymal stem cells produced fewer reactive oxygen species and increased phagocytosis of neutrophils co-cultured with mesenchymal stem cells. | [ ] |
| Immunomodulation | In vitro | Mesenchymal stem cells and neutrophiles | 0.1–1 mM | Caffeine treatment increased the viability of co-cultured neutrophils. | [ ] |
| Melanoma | In vitro and in silico | Mel1 and Mel3 cells | 1 and 2 mM | After caffeine treatment, there was a decrease in the levels of IL-1β, IP-10, macrophage inflammatory protein 1-α, and CCL4. On the other hand, the expression of regulated and normal T cells decreased in the Mel3 cell line. | [ ] |
| Autoimmune encephalomyelitis | In vitro and in vivo | Primary microglia and BV2 cells C57BL/6 mice were immunized to induce autoimmune encephalomyelitis | 2 mM (in vitro) 10, 20 and 30 mg/kg/day in drinking water (30 days) after immunization with MOG | Caffeine decreased clinical score, inflammatory cell infiltration degree of the demyelination, and microglia stimulation in mice. In addition, it increased LC3-II/LC3-I levels and decreased NLRP3 and P62 levels. | [ ] |
| Choroidal neovascularization | In vitro and in vivo | Laser photocoagulation C57BL/6j mice model | 200, 400 µM (in vitro); before laser photocoagulation (day 9): 20 mg/kg at day 0 and 10 mg/kg at day 1–4 and day 7 to 8; after laser photocoagulation: 10 mg/kg for 2 weeks (excluding weekends) | Significantly reduced the migration of retinal and choroidal endothelial cells (in vitro). Decreased choroidal neovascularization and inflammatory (mononuclear phagocytes) cells recruitment to the lesion area. | [ ] |
| Depression | In vitro and in vivo | CBA × C57BL/6 F1 mice and syngeneic splenocytes | Transplantation (IV injection) with 15 × 10 splenocytes previously treated with 100 µg of caffeine for 25 min | Immune cells treated with caffeine and transplanted into depressive-like mice resulted in an increase in neuronal density and anti-inflammatory cytokines (IL-10 and IL-4) and a decrease in proinflammatory cytokines (IL-1β, INF-γ, and TNF-α). | [ ] |
| Infection | In vitro and in vivo | Peritoneal macrophages and Swiss mice infected with | 0.0257–25.7 μM (in vitro) 0.05, 0.5, 5 mg/Kg of caffeine IV injected 30 min after mice infection | In mice, the leucocyte infiltration in the peritoneal cavity decreased after caffeine treatment. In addition, mRNA expression of IL-1β, IL-6, and the enzyme inducible nitric oxide synthase were decreased, whereas IL-10 was increased. | [ ] |
| Immunological and metabolic anomalies in obesity | In vitro and in vivo | Male Sprague-Dawley rat, RAW 264.7 macrophage and HepG2 cells | 50, 100, 150 mΜ (in vitro High-fat-diet (6 weeks) induced hepatic steatosis mice were treated with 20 mg/kg/day by oral gavage (6 weeks) | In caffeine-treated mice, the profiles of TNF−α, MCP-1, IL-6, intercellular adhesion molecule, and nitrite were suppressed. In addition, live white adipose tissue and muscle macrophages and their cytokine levels also decreased. | [ ] |
| Retinal inflammation | In vitro and in vivo | Ischemia reperfusion (I/R) injury mice model | 1–100 µM (in vitro); 10 µL at 97.8 mM instilled 60 min before and after I/R reperfusion, twice a day for 72 h | Caffeine reduced the secretion of IL-1β, IL-6, and TNF-α and restored the integrity of retinal cell monolayer (in vitro). Instilled caffeine reduced IL-6 mRNA levels and maintained BDNF physiological levels in the retina. | [ ] |
| Rheumatoid arthritis | In vitro and in vivo | Mesenchymal stem cells and Wistar rats | 0–1 mM (in vitro); 14 days after rheumatoid arthritis induction, mice were injected IP with 2 × 10 cells previously treated with 0.5 mM caffeine for 48 h | Caffeine at a concentration of 0.5 mM promoted lower levels of cytokines, such as IFN-γ, IL-6, and IL-1β, and higher levels of IDO and TGF-β. In addition, cells treated with caffeine diminished the severity of rheumatoid arthritis in vivo and caused a decrease in serum levels of C-reactive protein, nitric oxide, myeloperoxidase, and TNF-α. | [ ] |
| Cognitive impairment | In vivo | BALB/c mice | 0.025, 0.05, 0.1 mg of caffeine intranasally administered (10 µL) 1 day before ischemia-induced cognitive impairment in mice, and the next 7 consecutive days | Caffeine improved the behavior outcomes of ischemic mice and reduced the expression of proinflammatory biomarkers (TNF-α, IL-6) and increased the levels of anti-inflammatory cytokines (IL-10). | [ ] |
| Hepatic fibrosis—antioxidant and anti-inflammatory | In vivo | Hepatic fibrosis Sprague Dawley rats | 50 mg/kg/day orally administered (8 weeks) | Decreased fibrosis and necro-inflammation; decreased LPAR1, TGF-β1, CTGF, α-SMA, and LPAR1 expression; improved liver function. | [ ] |
| Hydrocephalus | In vivo | Kaolin-induced hydrocephalus mice neonates | 50 mg/kg/day of caffeine were administered to dams by gavage or water (21 days) and lactated the neonates | Administration of caffeine to dams reduced cell death and increased the neurons dendritic arborization in the sensorimotor cortex and striatum of the mice neonates and improved hydrocephalic deficits and behavioral development. | [ ] |
| Immunomodulation and anti-inflammatory effect | In vivo | Nile tilapia | Diet containing 5 and 8% / (21 days) | Caffeine supplemented diet prevented alterations caused by hypoxia, such as ATP hydrolysis and consequent accumulation in the extracellular environment. | [ ] |
| Inflammation and adenosinergic system in cerebellum | In vivo | Ethanol-induced inflammation in Wistar and UChB rats | 15.4 mM/day in 10% ethanol solution (55 days) | Caffeine reduced gene expression of A1 and A2a receptors and increased and reduced A1 and A2a protein levels, respectively, in the cerebellum. Caffeine also attenuated the inflammation, demonstrating a neuroprotective role. | [ ] |
| Neuroinflammation | In vivo | Sprague Dawley rats | 60 mg/kg/day administered orally by gavage (2 days) | Caffeine/modafinil increased the levels of anti-inflammatory (IL-4 and IL-10) and decreased proinflammatory (TNF-α, IL-1β) cytokines in the hippocampus. Treatment decreased microglial immunoreactivity and improved inflammatory response and anxious behavior. | [ ] |
| Neurotoxicity | In vivo | Tramadol-induced damage in cerebellum rat model | 37.5 mg/kg/day administered orally by gavage (21 days) | Caffeine upregulated autophagy-related genes and reduced the expression of inflammatory and apoptosis markers, demonstrating neuroprotective effects in the cerebellum. | [ ] |
| Neurotoxicity—antioxidant and anti-inflammatory | In vivo | Albino rats | 20 mg/kg/day IP injected (30 days) | Caffeine reduced oxidative stress and restored TNF-α levels in cerebral tissues. | [ ] |
| Oxygen-induced inflammatory lung injury | In vivo | Neonatal rats | 10 mg/kg IP injected every 48h (15 days) | Under hyperoxia, caffeine decreased pro-inflammatory mediators (TNF-α, IL-1α, IL-1β, IFN-γ) and NF-kB, and decreased infiltrating cells in the lung. Opposite effects were observed in normoxiaconditions. | [ ] |
| Dental pain | Clinical Trial | Patients with acute postoperative dental pain | 100 mg (single dose) | Caffeine improved the effect of ibuprofen in the treatment of moderate postoperative dental pain. | [ ] |
IL, interleukin; TNF- α , tumor necrosis factor alpha; IFN- γ , interferon gamma; MCP-1 (monocyte chemoattractant protein-1); STAT1, Signal Transducer and Activator of Transcription 1; Akt, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian target of rapamycin; NLRP3, NLR family pyrin-domain-containing 3; NF- k B, nuclear factor- κ B MAPK, mitogen-activated protein kinase; IP-10, interferon gamma-induced protein 10; CCL4, CC motif chemokine ligand 4; TGF- β , transforming growth factor beta; CTGF, connective tissue growth factor; α -SMA, alpha smooth muscle actin; LPAR1, lysophosphatidic acid receptor 1; LPS, lipopolysaccharide; M-MFs, inflammation-resolving macrophages; GM-MFs, inflammation-promoting macrophages; NF κ B1, nuclear factor kappa B subunit 1; HMGB1, high mobility group box 1 protein; BDNF, brain-derived growth factor.
3.3. Neurodegenerative Diseases
By 2050, the number of dementia cases worldwide is estimated to be 36.5 million [ 127 ]. There are several neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and multiple sclerosis [ 128 , 129 ]. For example, Parkinson’s disease is triggered by the loss of neurons, which leads to a decrease in dopamine levels. In Alzheimer’s disease, there is a deposition of extracellular deposits of amyloid-beta peptides and neurofibrillary tangles [ 130 , 131 ].
Caffeine is considered the most widely consumed psychoactive stimulant in the world. This natural compound is able to cross the blood–brain barrier [ 132 , 133 ] and, according to the literature, may exert a stimulant effect on the central nervous system by modulating several molecular targets, such as the (i) antagonism of adenosine receptors, (ii) promotion of intracellular calcium mobilization, (iii) inhibition of phosphodiesterase, and (iv) inhibition of GABA A receptors. However, except for the blockade of adenosine receptors and consequent inhibition of neurotransmitter-induced signaling pathways, the other mechanisms only exert their effects at toxic concentrations of caffeine [ 132 , 134 , 135 , 136 ]. Recently, Ruggiero et al. reviewed the available literature on the protective effects of caffeine in various neurodegenerative diseases [ 137 ]. Among these studies, some emphasized the neuroprotective role of caffeine. For example, Manolo et al. showed that caffeine, at a concentration of 10 mM, is able to protect 96% of the dopaminergic neurons. The co-administration of olanzapine and caffeine did not result in neuroprotection, implying that both dopamine D2-like and A2a receptors are required for neuroprotection [ 138 ]. In an in silico study of Parkinson’s disease, the authors demonstrated that caffeine has the ability to bind to both wild-type and mutant parkin protein [ 139 ]. The mutation of parkin protein is the most common cause of Parkinson’s disease, as is the abnormal secretion and accumulation of α-synuclein [ 140 , 141 ]. This last part was detected in the following in vivo studies. Luan et al. investigated whether caffeine could protect against mutant α-synuclein-induced toxicity. Exposing mice to 1 g/L of caffeine in drinking water attenuated apoptotic neuronal cell death as well as microglia and astroglia reactivation, culminating in synucleinopathy [ 142 ]. In a similar study, Yan et al. investigated synergetic neuroprotection between caffeine and eicosanoyl-5-hydroxytryptamide. Both compounds are present in coffee and showed no effect at subtherapeutic doses, whereas their combination reduced the accumulation of phosphorylated α-synuclein, and maintained neuronal integrity and function [ 143 ]. Table 4 summarizes the most recent research on the neuroprotective effects of caffeine in neurodegenerative diseases and other conditions.
Overview of the latest research regarding caffeine in neurodegenerative diseases.
| Disease | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Parkinson’s | In silico | Molecular docking simulations | N/A | Caffeine was able to bind at position 28 in both wild-type and mutant parkin proteins. | [ ] |
| Alzheimer’s | In silico | Molecular docking simulations | N/A | In the presence of caffeine, the distances between the inter-residual increased, leading to the breakdown of hydrophobic contacts, ultimately destabilizing the Aβ protofibrils. | [ ] |
| Parkinson’s | In vitro | Transgenic | 10 mM | Caffeine was able to prevent neuronal cell loss in 96% of dopaminergic neurons. | [ ] |
| Alzheimer’s | In vitro | SHSY5Y cells | 0.6 and 1 mM | Both concentrations were able to reduce beta-amyloid neurotoxicity. | [ ] |
| Alzheimer’s | In vitro | SH-SY5Y wild-type and N2a cells | 100 µM | In the presence of caffeine, the level of ADAM10 protein increased to 138.5 ± 9.2%, and the levels of APP protein level and ROS decreased to 85.4 ± 3.6% and 48.8 ± 3.2%, respectively. | [ ] |
| Alzheimer’s | In vitro | HEK293 cells | 0.1–10 mM | Caffeine induces conformational changes in muscle nicotinic acetylcholine receptors, which are molecular targets of Alzheimer’s disease. | [ ] |
| Synaptic transmission and plasticity | In vitro | Dorsal hippocampus slices of C57bl\6j mice and A2aR knockout mice | 50 μM | Caffeine increased synaptic transmission by 40%, decreased facilitation of paired pulse, and decreased the amplitude of long-term potentiation by 35%. | [ ] |
| Cd-induced neurodegeneration | In vitro and in vivo | HT-22 and BV-2 cells and wild-type C57BL/6N male mice | 30 mg/kg/day IP injected (2 weeks) | Caffeine reduced ROS, lipid peroxidation and 8-dihydro-8-oxoguanine levels. It also attenuated neuronal loss, synaptic dysfunction, and learning and cognitive deficits. | [ ] |
| Parkinson’s | In vivo | Swiss mice and Wistar rats | 31.2 mg/kg given orally by gavage | Caffeine administration reduced the catalepsy index and increased the number of ipsilateral rotations. | [ ] |
| Hypoxic ischemia | In vivo | Sprague Dawley mice | 1.5 mM in drinking water until 16 postnatal days | Pre-treatment with caffeine reduced brain infarct after hypoxia ischemia and also restored brain activity. | [ ] |
| Acetaminophen-induced neurotoxicity | In vivo | Swiss albino mice | 20 mg/kg IP injected 30 min after treatment with acetaminophen | Treatment with caffeine and acetaminophen reduced the formation of ROS compared with the acetaminophen group. In addition, the survival time of caffeine-treated mice increased by 33%. | [ ] |
| Parkinson’s | In vivo | C57BL/6 mice with motor behavioral deficit induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine | 20 mg/kg/day, 7 days before MPTP-induced neurodegeneration and 7 days after | Caffeine improved behavioral and neurotransmitter recovery against the induced toxicity. It was also able to restore antioxidant levels and suppress neuroinflammation. | [ ] |
| Hypoxic ischemia | In vivo | Wild-type C57/bl6 specific pathogen-free mice | 5 mg/kg IP injected (120 days) | Caffeine administration after hypoxic ischemic brain injury reduced lesions in the gray and white matter and the number of amoeboid microglia and apoptotic cells. The expression of pro-inflammatory cytokines also decreased. | [ ] |
| Apnea of prematurity | In vivo | Infection-free pregnant Sprague Dawley rats | 20 mg/kg 1 day followed by 5 mg/kg/day over 14 days or 80 mg/kg 1 day followed by 20 mg/kg/day over 14 days, IP injected | Caffeine administration in normoxia reduced oxidative stress and hypermyelination, and increased Golgi bodies. Caffeine at standard and high doses could provide neuroprotective effects. | [ ] |
| Parkinson’s | In vivo | C57BL/6 male mice | 5.1 mM in drinking water | Caffeine protected against synucleinopathy by modulating α-syn-induced apoptosis, microglial, and astrocytic activation in the striatum. | [ ] |
| Neuroprotection | In vivo | Male Swiss mice | 1.5 mM in drinking water (4 weeks) | The number of A2a receptors was decreased in the hippocampus of mice that consumed caffeine. The aged mice treated with caffeine presented more pyknotic neurons in the hippocampus and reduced damage. | [ ] |
| LPS-induced oxidative stress and neuroinflammation | In vivo | C57BL/6N male mice | 3 mg/kg/day IP injected (6 weeks) | The LPS-injected group had enhanced expression of Bax and caspase-3. On the other hand, these markers were reduced in the group treated with caffeine, and this treatment also caused a restoration of the synaptic markers. | [ ] |
| Diabetes | In vivo | Male GK and Wistar–Hannover–Galas rats | 5.1 mM in drinking water (4 months) | Caffeine prevented the GFAP, vimentin, and SNAP25 alterations caused by diabetes, and also improved memory deficits. | [ ] |
| Alzheimer’s | In vivo | Wild-type N2 and CL2006 worms | Worms were cultured in 200 and 400 μM caffeine-treated plates | The treatment prevented amyloid beta-peptide paralysis, decreased acetylcholinesterase activity, and decreased amyloid beta-peptide mRNA levels. | [ ] |
| Parkinson’s | In vivo | C57BL/6J mice | 50 mg/kg/day in drinking water | The co-administration of caffeine and eicosanoyl-5-hydroxytryptamide resulted in decreased accumulation of phosphorylated α-synuclein, maintenance of neuronal integrity and function, reduction in neuroinflammation, and improvement in behavioral performance. | [ ] |
| Parkinson’s | Clinical trial | Parkinson’s disease patients | 100 mg (single dose) | Caffeine treatment reduced the number of errors in patients and controls on the Stroop and Choice reaction time and enhanced dual item accuracy on the rapid visual serial presentation task. | [ ] |
ADAM10, A disintegrin and metalloproteinase domain-containing protein 10; APP, amyloid-beta precursor protein; ROS, reactive oxygen species; LPS, lipopolysaccharides; GFAP, glial fibrillary acidic protein; SNAP25, synaptosomal-associated protein, 25kDa; N/D, non-disclosed; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; N/A, not applicable.
3.4. Cardiovascular Diseases
Cardiovascular disease (CVD), the leading cause of mortality, accounted for 17.8 million deaths worldwide between 1980 and 2017 [ 160 ]. By 2030, an estimated 23.6 million people per year will die due to CVD. Caffeine intake, particularly through the consumption of coffee, tea, and other products, has shown various cardiovascular effects. Turnbull et al. reviewed more than 300 studies regarding the effects of caffeine on cardiovascular health, published from the late 1980s to 2017. Overall, the results suggest that caffeine consumption does not increase the risk of CVD and may have a protective effect against this group of diseases [ 161 ]. However, recent studies on this topic have shown that high caffeine consumption may have the opposite effect.
A study of 347,077 people (UK Biobank) concluded that coffee consumption may modestly increase the risk of cardiovascular disease. A nonlinear association was found between long-term coffee consumption and cardiovascular disease. Individuals who consumed coffee in high doses (>6 cups/day, >450 mg caffeine/day) were more likely to develop cardiovascular disease (22%) than those who consumed less coffee (1–2 cups/day or 75–150 mg caffeine/day) [ 162 ]. In addition, the authors examined the association between coffee consumption, plasma lipids, and CVD risk in 362,571 individuals (UK Biobank). The results showed that high coffee consumption (>6 cups/day) may increase CVD risk by increasing the levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (total-C), and apolipoprotein B (ApoB) [ 163 ].
However, other studies have reported the potential beneficial effects of moderate coffee consumption, in line with Turnbull et al.’s literature review [ 161 ]. For instance, a study involving 20,487 Italian participants concluded that moderate coffee consumption (3–4 cups/day) was associated with a low risk of CVD-related mortality. In addition, an inverse correlation was found between NT-proBNP levels (N-terminal fragment of the B-type natriuretic peptide, which is associated with higher stroke risk) and coffee consumption [ 164 ]. Similarly, a study of more than 500,000 participants in England reported that a caffeine intake of 121–182 mg/day from coffee (2–3 cups/day) or tea (4–6 cups/day) was associated with a low risk of coronary artery disease [ 165 ]. In addition, a US follow-up study of 23,878 participants over 16 years found that the daily caffeine consumption of about 100–200 mg or >200 mg is associated with a lower risk of CVD mortality [ 166 ]. An inverse association between coffee consumption and CVD risk factors (blood pressure and arterial stiffness) was also observed in another study, showing the beneficial effect of moderate coffee consumption [ 167 ]. A similar association was observed concerning coffee consumption and hypertension risk [ 168 ].
Therefore, despite some studies linking high coffee or caffeine consumption to CVD risk, most studies have reported that its moderate consumption has potentially beneficial and even protective effects on CVD. Table 5 summarizes the recent research on the effects of caffeine on CVD.
Overview of the latest research regarding caffeine’s impact on cardiovascular diseases.
| Study Type | Model | Result | Reference |
|---|---|---|---|
| Systematic review | Review of prospective studies | Regular and moderate coffee consumption (1–2 cups/day) is not associated with hypertension risk. Higher coffee consumption has a protective effect. | [ ] |
| Prospective | 347,077 volunteers (37–73 years old, UK Biobank) | Coffee consumption may lead to a slight increase in CVD risk. | [ ] |
| Prospective | 2278 volunteers (18–80 years old) | Caffeine metabolites are responsible for lowering the risk of hypertension. | [ ] |
| Prospective | 20,487 (35–94 years old) | Moderate coffee consumption (3–4 cups/day) has been associated with lower CVD mortality. | [ ] |
| Prospective | >500,000 individuals (40–69 years old) | The consumption of 2–3 cups of coffee per day (121–182 mg caffeine/day) was associated with a low risk of coronary artery disease. | [ ] |
| Prospective | 23,878 individuals (>20 years old) | Higher caffeine intake (>100 mg/day) was associated with lower CVD mortality. | [ ] |
| Prospective | 362,571 individuals (37–73 years old, UK Biobank) | High coffee consumption (>6 cups/day) increases levels of low-density lipoprotein cholesterol, total cholesterol, and apolipoprotein B, thereby increasing the risk for CVD. | [ ] |
| Prospective | 1095 individuals (mean age 53 ± 14 years old) | Moderate coffee consumption (>3 cups/day) reduces CVD risk factors such as arterial stiffness and high blood pressure | [ ] |
| Randomized Controlled Trial | 12 volunteers (19–39 years old) | Administration of caffeine (200 mg, 12 h intervals) during sleep deprivation reduced HR and increased HF-HRV. The concentration effect was nonlinear. No significant interaction between sleep deprivation and caffeine intake | [ ] |
| In vitro in vivo | Primary human and mouse aortic VSMCs, immortalized mouse aortic VSMCs; restenosis mice model (apoe−/−C57BL/6 J) | In vitro, caffeine (2 mM) induced autophagy by inhibiting mTOR signaling and decreased proliferation of VMCs by inhibiting WNT signaling. In vivo, caffeine at 2.57 mM (in drinking water, 2 weeks before and after injury) decreased vascular restenosis. | [ ] |
| In vivo | Zebrafish | Caffeine (128 and 334 µM in zebrafish culture water) caused a similar decrease in HR. | [ ] |
HR, heart rate; HF-HRV, heart rate variability; mTOR, mammalian target of rapamycin; VSMCs, vascular smooth muscle cells.
4. Caffeine Impact on Sports Performance
Coffee’s best-known constituent, caffeine, is the most widely consumed psychotropic drug in the world, with an estimated daily intake of up to 4 mg/kg body weight in American adults [ 173 , 174 , 175 , 176 ]. It is a psychostimulant that can lead to physical dependence [ 177 ]. Caffeine intake is widespread among inactive individuals and high-performance athletes, especially since 2004, when it was removed from the World Anti-Doping Agency’s list of banned substances for competition [ 178 ]. It is also readily available in various forms such as capsules, powders, caffeinated beverages, and energy drinks [ 173 ].
However, while there is evidence that caffeine improves athletic performance [ 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 ], due to particular protocols and study designs, some research seems conflicting. Some studies show ergogenic effects on aerobic endurance (>90 min), high-intensity efforts (20–60 min), muscular endurance, sprint performance and maximal strength (0 to 5 min), and ultra-endurance (>240 min) and endurance races with prolonged intermittent sprints (team sports), while others report no evidence for its administration [ 180 , 181 , 182 ]. We assume that an ergogenic substance is a substance used with the aim of improving athletic performance and promoting recovery after exercise by delaying fatigue. The word is of Greek origin: ergo (work) and gen (generation). As a result, it is commonly consumed by athletes, and research suggests that 75 to 90% of athletes consume caffeine before or during athletic competition [ 181 ]. In an analysis of 20,686 urine samples from elite athletes, 73.8% of the samples contained caffeine at concentrations greater than 0.1 µg/mL, suggesting that three out of four athletes consume caffeine before or during competition [ 175 ].
It should be recalled that the consumption of caffeine is not prohibited for athletes, with the maximum allowable concentration being 12 mg/L of urine (International Olympic Committee). The fact that caffeine affects the nervous system, adipose tissue, and skeletal muscle originally led to the hypothesis that caffeine might affect athletic performance. For example, caffeine may increase skeletal muscle contractile force at submaximal contraction and increase the athlete’s pain threshold or perceived exertion, which could lead to longer training sessions [ 180 , 181 ].
However, it should be remembered that caffeine intake has several side effects. Blood pressure increases both at rest and during exercise and heart rate increases, and it may impair recovery and sleep patterns, most likely in athletes who do not regularly consume caffeine [ 180 ]. In addition, Martins et al. demonstrated that high doses of caffeine have side effects. In a recent study using a caffeine dose of 12 mg/kg, almost all participants reported side effects such as tachycardia and palpitations, anxiety or nervousness, headache, and insomnia [ 175 ].
However, according to our research, it seems important to us to better evaluate certain aspects to achieve better scientific clarification with implications for practice, such as the ideal dosage, time of intake, abstinence, training time vs. caffeine consumption, physiological factors, gender, and caffeine users or not.
4.1. Optimal Dosage
Higher-than-ideal caffeine doses, 3–6 mg/kg, before exercise do not further improve athletic performance. Additional and higher doses of caffeine may lead to side effects in athletes [ 180 ].
Low doses of caffeine (~200 mg) have also been shown to improve attention, alertness, and mood, and cognitive processes during and after strenuous exercise. Thus, the ergogenic effects of low doses of caffeine appear to be due to changes in the central nervous system [ 180 ].
The generally accepted dosage of caffeine for performance enhancement is between 3 and 6 mg/kg, 60 min before exercise [ 175 ].
Although a meta-analysis reported that caffeine intake can be ergogenic in a variety of physical activities, the “optimal” caffeine dose remains difficult to determine [ 178 ].
4.2. Timing of Intake
The early ingestion of caffeine prior to physical activity has been shown to enhance performance. For example, caffeine can improve performance during high-intensity sprints when taken 45–60 min before exercise [ 176 ].
Because caffeine has so many positive effects on exercise performance, it can—and perhaps should—be taken before or during exercises. For most sports, it is recommended that caffeine be taken about 60 min before the start of the first set of the training session if used before exercise. This period varies depending on the individual, the type of event, and the type of caffeine ingested, with caffeinated mouthwashes and chewing gums generally requiring much less time. For longer training sessions, there is evidence that ingesting caffeine later in the day, and at lower doses, may be effective [ 181 ]. Other interesting data refer to the concentration peak that occurs in the first 15 min [ 183 ].
The isolated consumption of anhydrous caffeine results in maximal plasma peaks of the substance between 30 and 90 min after the consumption of low (2–3 mg/kg), moderate (3–6 mg/kg), or high doses (6–9 mg/kg) [ 175 , 184 ].
4.3. Abstinence
It appears that, short-term, caffeine withdrawal before competitions does not enhance the ergogenic effects of caffeine in habitual users. Withdrawal is associated with numerous negative consequences, including headache, fatigue, irritability, muscle pain, sleep disturbance, and nausea. However, these acute withdrawal symptoms, shortly before important competitions, may have a negative impact on the subjective self-confidence and well-being of the athlete [ 181 ].
4.4. Training Time vs. Caffeine Consumption
Increases in physical performance as a function of training time have been demonstrated in various sports. Studies suggest that anaerobic and aerobic activities may be more powerful due to the diurnal fluctuations of the circadian cycle between 4 and 8 pm. Morning caffeine consumption had a more beneficial effect than afternoon consumption [ 175 ].
4.5. Physiological Factors
Hypothetically, the potential performance enhancement from caffeine ingestion may be greater in trained individuals than in untrained individuals because trained individuals have an enhanced neuromuscular action potential. Trained individuals have a higher concentration of adenosine A2a receptors than untrained individuals [ 175 , 185 ].
The main finding of this review is that very low doses of caffeine (>1–2 mg/kg, generally taken 60 min before exercise) improve resistance training performance in terms of muscle strength, muscle endurance, and average speed [ 174 ].
Aerobic endurance appears to be the sport in which caffeine consumption most consistently produces moderate to significant benefits, although the magnitude of the effect varies among individuals [ 185 ].
4.6. Gender
Caffeine ingestion positively affects resistance exercise performance in women, and the magnitude of these effects appears to be comparable to those observed in men [ 184 ]. Even considering the woman’s menstrual cycle, a study showed that caffeine increased peak aerobic cycling power in the early follicular, preovulatory, and mid-luteal phases. Thus, the ingestion of 3 mg of caffeine per kg of body mass might be considered an ergogenic aid for eumenorrheic women during all three phases of the menstrual cycle [ 186 ].
4.7. Caffeine Consumers or Not
For the first study using a performance test, 17 moderately trained men were recruited, 8 of whom did not routinely consume caffeine (<25 mg/day) and 9 of whom regularly consumed caffeine (>300 mg/day). It was found that there were no differences between the groups in time to exhaustion at any of the doses, suggesting that habitual caffeine consumption does not attenuate the ergogenic effects of caffeine [ 181 ]. In another study, on cycling, habitual caffeine intake was found to have no effect on athletic performance, suggesting that habituation to caffeine has no negative effect on caffeine ergogenesis [ 187 ].
5. Future Directions: Nanotechnology-Based Delivery Strategies
Caffeine is usually consumed through the ingestion of beverages, especially coffee, tea, and pharmaceuticals, which allows for rapid absorption and distribution in all tissues [ 9 ]. However, caffeine has a short half-life (3–5 h) [ 188 ]. In addition, the oral intake of high concentrations of caffeine may cause gastrointestinal problems [ 189 ] and its wide distribution may lead to undesirable side effects, such as the stimulation of the nervous system.
Nanotechnology is a multidisciplinary field that enables the manipulation of matters at the nanoscale (1 to 100 nm) and the creation of novel devices with unique properties [ 190 ]. Nanotechnology is frequently explored for drug delivery to a target tissue. Drug delivery systems (DDS) or nanocarriers offer important advantages for caffeine delivery, namely, a high loading capacity, the co-encapsulation of different drugs, controlled and sustained release, a high surface area allowing greater interaction with tissue, and a high ability to permeate through tissues [ 191 ]. In addition, other routes of administration besides oral can be used, such as intranasal [ 192 ] and dermal [ 188 ] ( Figure 2 ) .

Possible administration routes for caffein-loaded nanosystems and their main outcomes.
Nanocarriers’ compositions are tailored depending on the drug(s), route of administration, and target tissue. Therefore, different nanocarrier compositions based on lipids, polymers, or metals have been proposed for caffeine delivery, as reported in this section.
Lipidic nanocarriers have been widely explored for topical drug delivery through the skin for cosmetic and pharmaceutical applications [ 193 ]. The composition of lipid carriers is an important factor to be considered to improve skin permeation and therapeutic effects. For example, among the various phospholipids (1,2-distearoyl-snglycero-3-phosphocholine (DSPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol, sodium salt (DPPG), 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)) tested for liposome preparation and the topical delivery of caffeine, DPPG was the most promising. Ex vivo studies showed that DPPG was able to enhance the permeation of encapsulated and free caffeine through hairless rat skin by disrupting the lipid barrier of the stratum corneum (SC) [ 194 ]. A similar effect was observed for lipid nanocapsules (NCs) in porcine skin. The ability of lipid NCs to increase skin permeation of free caffeine has been attributed to the combination of several factors, namely, the occlusion effect of nanoparticles on the skin surface, accumulation in hair follicles, and the effect on barrier function of SC [ 195 ]. On the other hand, the incorporation of propylene glycol into phosphatidyl liposomes has been shown to enhance the permeation of caffeine through the skin, as demonstrated ex vivo in human full-thickness skin [ 196 ]. In this sense, the researchers proposed the combination of the lipolytic activity of caffeine with the increased permeation capacity of propylene glycol liposomes as a noninvasive treatment for cellulitis [ 196 ]. Amasya et al. also proposed semisolid lipid nanoparticles as a promising treatment for cellulitis because they can penetrate the skin and reach the adipose tissue [ 197 ].
Flexible liposomes composed of phosphatidylcholine and higher surfactant content (polysorbate 80 and polysorbate 20) were also proposed for the treatment of alopecia by topical application [ 198 ]. The therapeutic potential of caffeine in alopecia is due to its ability to inhibit 5-α-reductase and phosphodiesterase and increase vasodilatation and blood supply to hair follicles [ 199 ]. The nanocarriers co-encapsulating minoxidil and caffeine resulted in an increase in hair length comparable to the aqueous solution of the drugs and the commercial alcoholic solution. Nevertheless, liposomes loaded with caffeine and minoxidil led to a significant increase in hair weight, an indicator of healthy and strong hair, demonstrating the potential of liposomes for the treatment of hair loss [ 198 ]. Other types of nanosystems, namely, nanoemulsions containing eucalyptol and oleic acid, have been shown to accumulate in hair follicles and increase caffeine retention in these structures, demonstrating the potential of these nanosystems for the treatment of alopecia [ 200 ]. Considering that hair follicles are nourished by blood vessels, targeted accumulation in these structures may enhance the permeation of caffeine. Therefore, these approaches can also be used to develop novel therapeutics for diseases of other tissues to avoid systemic or oral delivery.
In this sense, proniosomes have been proposed for the treatment of migraine by the topical application of caffeine. As expected, caffeine-loaded proniosomes, applied topically to Swiss albino mice, were able to penetrate the skin. Moreover, the treatment resulted in a significantly higher caffeine concentration in the blood and brain, as well as prolonged and sustained effects, compared with orally administered caffeine solution [ 188 ]. Recently, the co-delivery of caffeine and ergotamine to the brain by intranasal administration (olfactory route) has also been proposed. Hybrid lipid–polymer nanoparticles of lecithin, poly(lactic-co-glycolic acid) (PLGA), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine functionalized with polyethylene glycol (PEGylated DPPC) showed a high encapsulation efficiency (87%) and controlled release over a period of 48 h. In addition, the results showed that the nanoparticles had high targeting accuracy in the brain without causing toxic effects. Furthermore, the synergistic effects of the drugs enhanced the anti-migraine effect [ 192 ].
The anti-cancer effect of caffeine has also been enhanced by the use of nanocarriers. Liu et al. prepared lipid-based nanosystems for the co-delivery of caffeine and imiquimod. Caffeine enhanced the therapeutic effect of the immunomodulator imiquimod and radiotherapy in an orthotopic breast cancer model. The authors suggested that this may be due to the modulation of the tumor microenvironment [ 201 ]. On the other hand, polymer-based nanocarriers, i.e., gelatin nanoparticles loaded with caffeine, showed the ability to decrease the viability and proliferative capacity of murine melanoma cells (B16F10) without causing significant cytotoxic effects in normal fibroblast cells (L929) [ 202 ]. Other studies have reported the combination of caffeine with metallic nanocarriers. For example, silver–caffeine complexes anchored to magnetic nanoparticles were proposed for the treatment of hepatocellular carcinoma [ 203 ]. This type of cancer is known to be resistant to radiotherapy and chemotherapy and can be caused by hepatitis-related infections. The most promising nanoparticles showed higher cytotoxicity against the cancer cells (hepatocellular carcinoma cells, HCC) than against the normal cells (normal hepatic cells, WRL-68). On the other hand, the targeted hyperthermia effect of the magnetic nanoparticles can improve the anti-tumor effect of the formulation and avoid the side effects of the commonly used therapeutics. In addition, these silver–caffeine magnetic nanoparticles also showed antibacterial activity against Escherichia coli , Staphylococcus aureus , and Bacillus cereus [ 203 ]. Other caffeine–metal nanoparticles have been developed for antibacterial applications. Khan et al. [ 204 ] demonstrated the ability of caffeine–gold nanoparticles to inhibit biofilm formation and eliminate mature biofilms. In addition, the nanoparticles showed antibacterial activity against resistant pathogenic bacteria ( Escherichia coli , Pseudomonas aeruginosa , Staphylococcus aureus , Listeria monocytogenes ), demonstrating their potential for treating chronic infections.
Overall, the different types of nanocarriers have shown the potential to improve the therapeutic effect of caffeine. Table 6 provides an overview of the recent research on the development of lipid-, polymer-, and metal-based nanocarriers loading caffeine for biomedical applications.
Overview of the latest research regarding nanocarriers loading caffeine for biomedical applications.
| Nanosystem | Method | Composition | Application | Model | Result | Reference |
|---|---|---|---|---|---|---|
| Liposomes | Thin-film hydration | Lecithin, polysorbate 80, polysorbate 20 | Alopecia | Wistar rats | Improves skin delivery, weight, and hair length. | [ ] |
| Liposomes | Thin film hydration | Phospholipid, cholesterol | Skin drug delivery | Abdominal skin of WBN/ILA-Ht hairless rats | DPPG liposomes enhanced skin penetration by disrupting the lipidic barrier of stratum corneum. | [ ] |
| Liposomes | High-pressure homogenization | Phosphatidylcholine, propylene glycol | Skin drug delivery | Full-thickness abdominal human skin | Propylene glycol increased liposome deformability and improved skin permeation of caffeine. | [ ] |
| Lipidic nanosystems | High-pressure homogenization | Trilaurin, oleic acid, pluronic F68, imiquimod | Cancer | Orthotopic breast cancer mice model | Caffeine slightly improved antitumor activity. | [ ] |
| Lipid nanocapsules | Phase inversion temperature | Miglyol 812 N, Kolliphor HS 15, Phospholipon 90G | Skin drug delivery | Porcine skin | Caffeine was not successfully encapsulated. Nanocapsules improved the transdermal permeation of caffeine. | [ ] |
| Semi-solid nanostructured lipid carriers | Two-stage homogenization method, high shear homogenization, ultrasonication | Compritol 888 ATO and Precirol ATO 5, argan oil, Poloxamer 407 | Cosmetics, skin drug delivery | Wistar rat full-thickness dorsal skin | NLCs exhibited a high capacity for deposition and permeation through the skin. | [ ] |
| Proniosomes | Coacervation phase separation | Cholesterol, span 60, lecithin | Brain delivery—migraine | Swiss albino mouse abdominal skin and albino rabbit ear | Increased caffeine permeation through the skin and caffeine levels in blood and brain compared to orally administered caffeine. No evidence of skin irritation. | [ ] |
| Nanoemulsions | Low energy emulsification | Dicaprylyl ether, ethylhexyl isononanoate, potassium lauroyl wheat amino acids, palm glycerides and capryloyl glycine | Cosmetics, skin drug delivery | Abdominal human epidermis | Did not improve skin permeation of caffeine compared to emulsion. | [ ] |
| Nanoemulsions | Low energy emulsification | Volpo-N10, oleic acid or eucalyptol | Skin drug delivery | Human full-thickness skin | Increased permeation and retention of caffeine in hair follicles and skin. | [ ] |
| Pickering emulsions stabilized by magnesium oxide NPs | High shear homogenization | Wheat germ oil, magnesium oxide NPs | Oral drug delivery—hepatoprotective | Wistar rats intoxicated with CCl4 | Decreased proliferation of cancer cells, moderate reduction in oxidative stress and inflammatory markers, similar to caffeine solution. Increased catalase levels compared to caffeine. | [ ] |
| Polymeric nanoparticles | Emulsion polymerization | Methyl methacrylate, CTAB or sodium dodecyl sulfate | Antifungal | CTAB–caffeine nanoparticles inhibited the growth of . | [ ] | |
| Polymeric nanoparticles | Desolvation | Gelatin | Cancer | B16F10, L929 cell lines | Inhibited the proliferation of murine melanoma cells (B16F10) and induced apoptosis without causing cytotoxic effects on normal fibroblast cells (L929). | [ ] |
| Silver complexes anchored to magnetic NPs | Covalent conjugation and complexation | Chloro-functionalized Fe3O4 magnetic NPs, caffeine N-heterocyclic carbene-silver complex | Cancer | HepG2, WRL-68 cell lines; , , , | Enhanced cytotoxic effects against HepG2 cells and antibacterial activity against , and . Hyperthermia studies showed that the nanosystems reached a temperature of 47 °C, which is suitable for anticancer applications | [ ] |
| Silver nanoparticles | Chemical reduction | Silver nitrate, gallic acid, (-)-epicatechin-3-gallate or caffeine | Cancer | B16-F0, COLO 679 cell lines | EGCG- and caffeine-stabilized AgNPs were the most and less effective against the tested cancer cell lines. | [ ] |
| Gold nanoparticles | Chemical reduction | Gold (III) chloride trihydrate | Antibacterial | , , , | Inhibition of biofilm formation and removal of mature biofilms. Antibacterial activity against resistant pathogenic bacteria. | [ ] |
| Nanocrystals | Pearl-milling | Carbopol 981, propylene glycol | Skin drug delivery | Human volunteers, arm skin | Nanocrystals with a size of 694 nm showed a delayed, but higher and longer delivery of caffeine, being detected in serum for at least 5 days. | [ ] |
NLCs, nanostructured lipid carriers; AgNPs, silver nanoparticles; DPPG, 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol, sodium salt; CTAB, cetyltrimethylammonium bromide; EGCG, (-)-epicatechin-3-gallate.
6. Conclusions
Coffee is the most consumed caffeinated beverage, while caffeine can also be found in tea, soft drinks, and energy beverages. Studies on the associations between coffee consumption and a range of health outcomes have been completed. Epidemiological studies reveal that, for the majority of people, coffee consumption is advantageous and adversely connected with risk for a number of diseases. Numerous researchers have recently conducted studies on the effects of caffeine on diseases such as cancer, cardiovascular, immunological, inflammatory, and neurological disorders, among others, as well as in sports, suggesting that this field of study is expanding quickly. To clarify the link between caffeine consumption and specific diseases and to examine consumption patterns in relation to health outcomes, randomized controlled studies are required because association does not imply causality. Because most studies have focused on adults, little is known about the negative consequences of children and adolescents consuming items with caffeine. On the other hand, several advancements in innovative DDS have been made in order to lessen the adverse effects and boost bioavailability for the treatment of various diseases. Thus, DDS have potential importance for clinical applications in several diseases, potentiating the effect of caffeine. However, the growing volume of articles, meta-analyses, and scientific evidence is not yet sufficient to confirm the quality and quantity of caffeine in the treatment of several disorders and in sports, being an avenue to explore in the future.
Funding Statement
This work was funded by the Programa Operacional Regional do Centro (CENTRO-04-3559-FSE-000162) within the European Social Fund (ESF), CICS-UBI (UIDP/00709/2020) financed by National Funds from Fundação para a Ciência e a Tecnologia (FCT), Community Funds (UIDB/00709/2020), by Fundação La Caixa and Fundação para a Ciência e Tecnologia (FCT) under the Programa Promove Project PD21-00023, and project PRR-C05-i03-I-000143 (RedFruit4Health). The authors are also grateful to the Foundation for Science and Technology (FCT), the Ministry of Science, Technology and Higher Education (MCTES), the European Social Fund (EFS), and the Europe Union (EU) for the PhD fellowships of Ana C. Gonçalves (2020.04947.BD).
Author Contributions
Conceptualization, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; methodology, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; software, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; validation, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; formal analysis, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; investigation, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; resources, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; data curation, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; writing—original draft preparation, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; writing—review and editing, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; visualization, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; supervision, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; project administration, S.M.S., T.A.J., A.C.G., D.G. and L.R.S.; funding acquisition, S.M.S., T.A.J., A.C.G., D.G. and L.R.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Search Please fill out this field.
- Newsletters
- Sweepstakes
- Raising Kids
Is It OK To Let Your Kid Drink Coffee?
Is all coffee bad for kids? Here, we spill the beans on whether children and adolescents should consume the occasional cup of joe.
Is Coffee Bad for Kids?
- Coffee Side Effects
- How Much Coffee Can Kids Have?
Caffeine Content in Popular Coffee Shop Drinks
Safer ways for kids to drink coffee.
You'd be hard-pressed to walk by a group of teens without spotting an oversized drink from Starbucks or Dunkin' in their hands. It seems coffee shops are the new hangout for high schoolers, and the trend is quickly extending to middle schoolers too. Whether it's an iced coffee while hanging out at the mall or a post-practice pick-me-up mocha, kids are consuming caffeinated beverages at an alarming rate.
But should kids drink coffee? What are the possible long-term and short-term side effects? In this article, we'll take a look at the effects of caffeine on children and how much coffee kids can safely drink.
ByLorena / Stocksy
In small amounts, coffee is not particularly bad for kids, but there are a couple of things you should consider before allowing them to consume any amount of coffee.
Caffeine content
The American Academy of Pediatrics (AAP) recommends that kids under the age of 12 consume no caffeine, and the American Academy of Child and Adolescent Psychiatry (AACAP) advises that kids aged 12 to 18 years consume no more than 100 milligrams (mg) of caffeine per day.
Yet a study published in the journal Pediatrics revealed that a staggering 73% of children and adolescents drink some amount of caffeine daily—and most of it comes from coffee, soda, or energy drinks.
Fat and added sugar
Most of the coffee beverages kids and teens order at coffee shops contain a lot of added sugar and fat in the form of ingredients like sweetened syrups and whipped cream. This increases the amount of fat and sugar they're consuming and likely reduces their intake of healthier beverages like water .
Side Effects of Coffee in Kids
The caffeine content in coffee can affect kids differently from adults, since their bodies are generally smaller and still developing, and they have different needs overall. Steve Theunissen, a registered dietitian nutritionist and ISSA /IFPA certified personal trainer, says adverse effects from coffee and caffeinated beverages can include:
- Frequent urination and increased risk of dehydration : Coffee is a diuretic, which means that high amounts can possibly dehydrate children.
- Altered mental state : One study shows that caffeine intake in kids is associated with increased feelings of stress, anxiety, and depression. Caffeine consumption is also associated with jitteriness and nervousness.
- Poor sleep patterns : Caffeine can alter kids' sleep cycles, which can interrupt growth in the brain and body.
- Caffeine dependency : Regularly drinking coffee as a child can become a habit, and children can experience the effects of caffeine withdrawal just like adults do. These might include tiredness, mood changes, difficulty concentrating, and headaches.
- Upset stomach or nausea : The caffeine and acid in coffee can cause gastrointestinal symptoms like stomachaches and nausea.
What's more, excessive amounts of caffeine can lead to caffeine overdose, which may require emergency treatment.
"Symptoms of caffeine overdose can include vomiting, high blood pressure, racing heart, heart rhythm problems, and, less commonly, disorientation and hallucinations," according to the AACAP. "Youth with certain health conditions such as heart problems, seizures, or migraines may be more at risk for caffeine-related problems than others."
How Much Coffee Is OK for Kids?
The AAP doesn't recommend that kids or teens drink ever drink coffee, but if you're considering allowing your teen to have it in small amounts, you can use the AACAP's recommended limit for caffeine to guide you (no more than 100 mg for tweens and teens aged 12 to 18).
How Much Coffee Is 100 mg of Caffeine?
The caffeine content in coffee drinks can vary considerably, but one 8-ounce cup of brewed coffee typically contains about 95 mg of caffeine while one shot of espresso contains about 75 mg. Alternatively, one 8-ounce cup of decaf coffee contains between 2 and 15 mg of caffeine. It's important to note, however, that at most coffee shops, the sizes typically start at 12 ounces and some roasts can contain significantly more caffeine than others.
Of course, the best option is to speak to your child's doctor before offering them a caffeinated beverage. If your child or adolescent has a history of anxiety, stomach issues, or heart complications, for example, it may be best to skip coffee and other caffeinated beverages entirely.
If you're considering allowing your child to drink coffee it's a good idea to know the caffeine content of the beverage your child wants. A quick glance at the caffeine content proves that these types of drinks can vastly exceed the recommended guidelines for caffeine consumption in kids. Note that all beverages included are size tall (12 fl oz) for Starbucks and size small (10 fl oz) for Dunkin', unless otherwise noted.
| Beverage | Caffeine Content |
|---|---|
| Starbucks Brewed Coffee (Pike Place) | 235 mg |
| Starbucks Nitro Cold Brew | 215 mg |
| Starbucks Peppermint Mocha | 75 mg |
| Starbucks Hot Chocolate | 20 mg |
| Starbucks Chai Tea Latte | 70 mg |
| Starbucks Vanilla Frappuccino | 65 mg |
| Starbucks Brewed Coffee (Decaf Pike Place) | 20 mg |
| Starbucks Iced Latte or Cappuccino | 75 mg |
| Starbucks Pink Drink | 35 mg |
| Dunkin' Brewed Coffee | 150 mg |
| Dunkin' Dunkaccino | 58 mg |
| Dunkin' Cold Brew | 174 mg |
| Dunkin' Iced Coffee (16 fl oz) | 198 mg |
| Dunkin' Hot Chocolate | 9 mg |
What can parents do if they have coffee shop-loving kids? The best option is to stick to no-caffeine or low-caffeine options. "There are some beverages, even at popular coffee shops, that tend to have less caffeine, and are thus more appropriate for younger kids," says Theunissen.
At Starbucks, for example, you can choose caffeine-free options like a "babyccino" (essentially a cappuccino minus the espresso), herbal teas, Vanilla Crème, Caramel Apple Spice, or White Hot Chocolate.
Another option for kids set on true coffee drinks is to stick with decaf coffee (which can contain up to 15 mg of caffeine in an 8-ounce serving size) or decaf espresso—or to pour only a small amount of coffee and add lots of the milk of your choice to make it lighter and less caffeinated overall.
Caffeine and Children . American Academy of Child and Adolescent Psychiatry . 2020.
Trends in Caffeine Intake Among US Children and Adolescents . Pediatrics. 2014.
Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children . J Psychopharmacol . 2015.
Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update . Nutrients . 2022.
Beverages, coffee, brewed, prepared with tap water . U.S. Department of Agriculture . 2019.
Spilling the Beans: How Much Caffeine is Too Much? . Federal Drug Administration . 2023.
Related Articles
- Weird But True
- Sex & Relationships
- Viral Trends
- Human Interest
- Fashion & Beauty
- Food & Drink
- Health Care
- Men’s Health
- Women’s Health
- Mental Health
- Health & Wellness Products
- Personal Care Products
trending now in Lifestyle

Look for 'really dramatic changes' in your body at these two...

I'm a Playboy model who's traveled to nearly every country —...

Even light drinking is harmful to older adults, study warns:...

I'm a neurologist — this is the scariest thing people do to...

Chick-fil-A fan favorite is coming back for the first time in 13...

If this is how you're disciplining your kids, you're parenting...

Want to prevent diabetes? Stop eating so much of this one food,...

Dear Abby: My boyfriend is addicted to partying with his son
Look for ‘really dramatic changes’ in your body at these two ages: stanford study.
We know the body changes over time, but new research suggests those shifts may be more sudden and staggering than previously thought.
A new study from Stanford Medicine reveals that many of the body’s molecules and microorganisms starkly rise or fall in numbers at two specific times — ages 44 and 60.
Researchers drew this conclusion after assessing thousands of molecules — including RNA, proteins and metabolites — and their microbiomes, the collection of bacteria, viruses and fungi that live on and inside us, in people 25 to 75 years old.

Researchers found that 81% of the molecules studied displayed non-linear fluctuations, meaning they underwent more change at certain times than others. The findings were published Wednesday in the journal Nature Aging .
Michael Snyder , chair of genetics and the study’s senior author, imparts, “We’re not just changing gradually over time; there are some really dramatic changes. It turns out the mid-40s is a time of dramatic change, as is the early 60s. And that’s true no matter what class of molecules you look at.”
Researchers believe these dramatic changes are reflected in significant transformations within the body.
The research team was inspired to study the effects of molecular and microbial shifts after observing that the risk of developing age-related conditions like Alzheimer’s disease and cardiovascular disease is a sharp, rather than a steady, rise.
For those in their 40s, molecular changes were seen in the number of molecules related to alcohol, caffeine, lipid metabolization, cardiovascular disease and skin and muscle.
For those in their 60s, changes were related to carbohydrate and caffeine metabolism, immune regulation, kidney function, cardiovascular disease and skin and muscle.

Among the 108 study participants, researchers identified four “ageotypes,” indicating that the kidney, liver, metabolism and immune systems age at differing rates in different people.
When researchers searched for clusters of molecules with the largest fluctuation in amount, they found these changes occurred the most at two intervals: when people reached their mid-40s and early 60s.

The mid-40s cluster surprised scientists who initially assumed that menopause or perimenopause was directing these changes in women, thereby skewing the group. However, when they divided the study group by sex, they discovered that the cluster shift affected men equally.
Xiaotao Shen, a former Stanford Medicine postdoctoral scholar and the study’s first author, expounds, “This suggests that while menopause or perimenopause may contribute to the changes observed in women in their mid-40s, there are likely other, more significant factors influencing these changes in both men and women. Identifying and studying these factors should be a priority for future research.”
Get the latest breakthroughs in medicine, diet & nutrition tips and more.
Subscribe to our weekly Post Care newsletter!
Thanks for signing up!
Please provide a valid email address.
By clicking above you agree to the Terms of Use and Privacy Policy .
Never miss a story.

As Shen suggests, more research is needed to explore the driving force of these sudden changes, whether the results are the product of behavioral or biological factors.
Regardless of causation, researchers recommend paying particular attention to your health in your 40s and 60s, perhaps increasing exercise and decreasing alcohol consumption to live in better accordance with these biomolecular shifts.
As Snyder maintains, “I’m a big believer that we should try to adjust our lifestyles while we’re still healthy.”

Advertisement
What is matcha? What to know about the green drink taking over coffeeshops.

If you've stepped into a coffeeshop in the last few years, you've probably seen some form of matcha on the menu.
Interest in matcha has been steadily on the rise over the last few years — experts credit rising interest in healthier nutrition swaps as well as the fact that the drink is aesthetically appealing and fun to share pictures of online.
"Matcha tea has become popular in the western world with photogenic social media pictures of this bright green drink popping up everywhere," Virginia-based registered dietitian and diabetes educator Caroline Thomason tells USA TODAY.
Here's what nutrition experts want you to know about drinking matcha.
What is matcha?
Matcha is a type of green tea made from finely grounding green tea leaves into a powder. It has a slightly earthy taste.
The beverage originated in China, but the matcha consumed today was largely influenced by Japan.
Does matcha have caffeine?
Matcha does contain some caffeine , but many enjoy it as an alternative to coffee because it doesn't contain quite as much.
A cup of matcha has about 70 mg of caffeine, which Thomason notes is equal to a shot of espresso and a bit less than a cup of coffee.
"Matcha tea also contains compounds that slow down the absorption of caffeine so that we don’t get such a spike and crash in energy — a benefit most people report enjoying about this green drink," Thomason says.
But, she notes, those who get overly anxious or jittery from caffeine may still want to avoid matcha.
"You may not enjoy drinking caffeinated beverages like matcha despite the fact that they are lower in caffeine and have different effects on energy levels compared to coffee," she says.
What is the healthiest tea? We're breaking down the health benefits of black, herbal, more
Is matcha good for you?
Research has shown that green tea offers a whole host of health benefits including anti-inflammatory properties and possible aids in disease prevention.
Is decaf coffee bad for you? What to know about calls to ban a chemical found in decaf.
As a type of green tea, matcha has many of those benefits, too. Some studies have shown that matcha may boost liver, brain and heart health.
"All types of green tea are also high in antioxidants and contain a compound called ECGC which has been shown to improve metabolism and may impact fat loss when taken consistently," Thomason adds.

IMAGES
COMMENTS
Current research suggests that if caffeine does have an effect on mood, the most significant changes may be anxiety. Studies did not support caffeine as having any significant effect on attention, but that it did play a role in enhancing processing speed. The majority of the studies reviewed suggest caffeine as having a significant positive ...
Coffee is one of the most widely consumed beverages in the world and is also a major source of caffeine for most populations [].This special issue of Nutrients, "The Impact of Caffeine and Coffee on Human Health" contains nine reviews and 10 original publications of timely human research investigating coffee and caffeine habits and the impact of coffee and caffeine intake on various ...
1.1. Caffeine—General Information. Caffeine (1,3,7-trimethylcanthine or 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione), a well-known purine alkaloid, was described by Gennaro [] as a white, odorless powder with a slightly bitter taste.Its chemical formula is C 8 H 10 N 4 O 2.Caffeine occurs in more than 60 plant species globally [].This substance is produced by extraction from green coffee ...
This review examines the effects caffeine has on cognitive and physical function, since most real-world activities require complex decision making, motor processing and movement. Caffeine exerts its effects by blocking adenosine receptors. Following low (∼40 mg or ∼0.5 mg kg −1) to moderate (∼300 mg or 4 mg kg −1) caffeine doses ...
Published July 22, 2020. N Engl J Med 2020;383: 369 - 378. DOI: 10.1056/NEJMra1816604. VOL. 383 NO. 4. Coffee and tea are among the most popular beverages worldwide and contain substantial amounts ...
370 n engl j med 383;4 nejm.org July 23, 2020 The new england journal of medicine levels peaking after 15 minutes to 2 hours.14 Caffeine spreads throughout the body and cross - es the blood ...
Coffee is the most widely consumed source of caffeine worldwide, partly due to the psychoactive effects of this methylxanthine. ... CMRR EPI 2D (R2016A, Center for Magnetic Resonance Research ...
Metrics. Moderate coffee consumption (2-5 cups per day) has been consistently associated with a lower risk of cardiovascular disease in epidemiological studies. For most individuals, a caffeine ...
Salivary caffeine levels. Caffeine levels significantly differed between each of the three conditions (main effect of condition: F 2,90.7 = 46.12, p < 0.001) with the highest levels in the ...
Despite the extensive research on caffeine, certain effects are still unclear, and different studies have produced contradictory findings. For more than a century, the safety of caffeine has been debatable [14].Given the ubiquitous use of caffeine, it is crucial for us to comprehend the pharmacology of the substance and its action mechanisms.
Caffeine in moderate doses (40-200 mg) acts within the brain to decrease fatigue, increase alertness, and decrease reaction time. Caffeine also may decrease appetite and slightly reduce weight gain. In moderate doses, caffeine has been associated with decreased risk of depression and suicide in some studies. Medical Uses of Caffeine.
The results of this systematic review support a shift in caffeine research to focus on characterizing effects in sensitive populations and establishing better quantitative characterization of interindividual variability (e.g., epigenetic trends), subpopulations (e.g., unhealthy populations, individuals with preexisting conditions), conditions ...
Coffee is one of the most commonly consumed beverages in the world, yet its acute health effects remain largely unknown. 1 Despite the common admonition that coffee should be avoided owing to ...
About 90% of adults in the United States use caffeine regularly, says Griffiths, and their average consumption exceeds 200 milligrams of caffeine per day — more caffeine than is contained in two 6-ounce cups of coffee, or five 12-ounce cans of soft drinks. This latest research study, notes Sweeney, is the most thorough evaluation to date of ...
This systematic review and meta-analysis of randomized controlled trials (RCTs) was performed to summarize the effect of caffeine intake on weight loss. We searched the following databases until November 2017: MEDLINE, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials. The r …
Caffeine's ergogenic potential has been extensively studied in the sports science literature, with research dating back to 1907 [].From investigating caffeine's effects on aerobic exercise, in recent years the research focus has shifted to anaerobic exercise performance outcomes, such as muscular endurance, muscle strength, and jumping tasks that require muscle power.
Caffeine, a controversial substance, was once known to be addictive and harmful. In recent years, new effects of caffeine on the human body have been confirmed. Recent research over the past few decades has shown the potential of caffeine in treating pancreas-related diseases. This review aims to analyze the known Food &; Function Review Articles 2024
To provide an overview on the results on caffeine intake reported in these articles and reports, ... (SIP), a marketing research program monitoring the consumption beverages. Data from 10,712 caffeinated beverage consumers, collected in 1999, revealed a daily caffeine intake from beverages ranging from 106 to 170 mg/day (P90: 227-382 mg/day ...
Estimates of Caffeine Consumption. Recent estimates in adults suggest that more than 85% of adults in the U.S. regularly consume caffeine, with an average daily intake of about 180 mg/day, about the amount of caffeine in up to two cups of coffee (6, 26).Among children and adolescents, caffeine use appears to be either stable or slightly decreasing over time, despite the influx of new caffeine ...
Caffeine causes a short but sudden increase in blood pressure. Research has not shown that drinking 3-4 cups of coffee a day increases the risk of kidney disease or increases rate of decline of kidney function. However, moderating how much coffee you drink is a good idea. Those struggling with blood pressure control should especially drink less ...
Recommended caffeine amounts for children and why it's important. The American Academy of Pediatrics recommends that kids 12 and under have no caffeinated beverages, including soda, energy drinks, coffee or tea, and that adolescents have less than 100 milligrams of caffeine per day.
WEDNESDAY, Aug. 14, 2024 (HealthDay News) -- Aging Americans, you're not imagining things: Big shifts in physical well-being do occur at certain points in the life span, new research shows. A team at Stanford University has found "massive" changes during a person's mid-40s and early 60s in regards to the molecules and microorganisms that help ...
According to the research, caffeine content varies wildly by brew method. While the dark roasted versions of each method tended to have more caffeine than their light- and medium-roasted counterparts, the biggest discrepancies came from the brewers. Espresso, for instance ranged between 120mg and 174mg caffeine per 30ml serving.
But despite all the negative attention, caffeine is still a popular element in plenty of products Americans consume on a regular basis. Case in point: Nearly 70% of U.S. adults said in a recent ...
A good caffeinated beverage is a favorite of many folks—especially in the morning. While coffee holds the top spot for the nation's preferred source of caffeine—with an estimated 67 percent ...
Previous research showed that resting energy use, or metabolic rate, didn't change from ages 20 to 60. The new study's findings don't contradict that. ... In the case of caffeine, it may ...
Caffeine, in particular, has been the subject of intense and in-depth research on the human organism regarding its health-promoting effects and possible beneficial effects on the performance of athletes, especially through its ability to improve anaerobic and aerobic performance, muscle efficiency, and speed, and to reduce fatigue [5,6,7,8,9].
The caffeine content in coffee drinks can vary considerably, but one 8-ounce cup of brewed coffee typically contains about 95 mg of caffeine while one shot of espresso contains about 75 mg ...
A new study from Stanford Medicine reveals that many of the body's molecules and microorganisms starkly rise or fall in numbers at two specific times — ages 44 and 60.
A cup of matcha has about 70 mg of caffeine, which Thomason notes is equal to a shot of espresso and a bit less than a cup of coffee. ... Research has shown that green tea offers a whole host of ...