If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Physics archive
Course: physics archive > unit 5.
- What are energy and work?
- What is kinetic energy?
- What is gravitational potential energy?
- What is conservation of energy?
- Work and the work-energy principle
- Work as the transfer of energy
- Work example problems
- Work as area under curve
Thermal energy from friction
What is thermal energy.
- Conservative forces
- What is power?
Thermal energy from drag
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Thermal Energy
Claimed by Erin Yoon (Spring 2023)
- 1.1.1 Temperature
- 1.1.2 Specific Heat Capacity
- 1.1.3 The Kinetic Molecular Theory of Matter
- 1.1.4 Ways to Transfer Thermal Energy
- 1.1.5 Thermal Equilibrium
- 1.2 Computational Model
- 2.2 Middling
- 2.3 Difficult
- 3 Connectedness
- 5.1 Further reading
- 5.2 External links
- 6 References
All objects are made up of atoms and molecules-- so many that it would take longer than the Universe's estimated lifetime to count. The constant and random motion of an object's atoms or molecules is what determines its Thermal Energy. Thermal Energy is a component of internal energy, but is unrelated to the vibrational and rotational energy of a solid's atoms. Instead, Thermal Energy occurs from atoms' translational motion.
When we say "change of thermal energy," we mean that it is the part of the internal energy that is associated with a Temperature change. Thermal Energy is quantified using temperature. This quantification describes the approximate average Thermal Kinetic Energy present in all of the atoms or molecules in the object/sample/system. In the real world, it is often impossible to accurately state how much of an object's internal energy is Thermal. However, if the Heat Capacity and mass of am object is known, we can measure its temperature using a thermometer and calculate its Thermal Energy.
A Mathematical Model
A change in Thermal Energy can be calculated by using the following equation:
This equation states:
Temperature
In order to quantify Thermal Energy, a measurement of the average translational energy of the atoms or molecules in a system is measured. This value is referred to as temperature . In order to measure temperature, a thermometer of some sort must be utilized. The most well known representation of a thermometer is a thin glass column filled with either mercury or an alcohol. When a thermometer is placed into a system and time passes, the average kinetic energies of the system and the thermometer become the same. The change in thermal energy causes the alcohol or mercury to expand or compress. The temperature is then measured on a scale of Celsius, Kelvin, or Fahrenheit.
Specific Heat Capacity
Specific heat capacity is a value unique to each object or material that quantifies the amount of thermal energy it takes to raise the temperature of said object by one degree Celsius. For example, water's specific heat capacity is 4.186 [math]\displaystyle{ \frac{J}{g \ °C} }[/math] , meaning that it takes 4.186 Joules of Thermal Energy to raise the temperature of water by one degree Celsius. Materials with higher specific heat capacity, thus, require more Thermal Energy to increase in temperature than materials with lower specific heat capacity. This principle also applies when materials are cooling down in the absence of heat-- materials with higher specific heat capacity will lose heat energy (and decrease in temperature) more slowly than materials with lower specific heat capacity.
The Kinetic Molecular Theory of Matter
The Idea of Thermal Energy is derived from the Kinetic Molecular Theory of Matter (KMToM). This theory explains why matter can and does exist in different phases. It also provides a description of the interactions and properties of atoms via ideas generally applied to macroscopic systems. According to the KMToM, all matter is comprised of lots of smaller molecules or atoms that are constantly moving. The type of motion of these particles is a result of the Thermal Energy present, and it determines whether the substance is in a gaseous, liquid, solid, or plasma state. When energy is introduced or lost from a material, the resulting change in motion of the individual particles can cause a phase change to occur for the substance, by increasing or decreasing the spacing between atoms and molecules. If enough energy is added to the substance, intermolecular forces can be overcome, leading to a plasmic state.
Ways to Transfer Thermal Energy
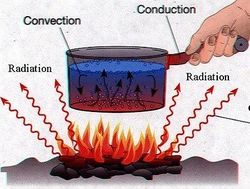
- Convection : The movement in fluids (gases and liquids) caused by gravity, in which hotter fluids (less dense) rise, and cooler fluids (denser) sink, causing an increased transfer of Heat or Thermal Energy. For example, clouds are created by rising, warmed water (evaporation). These clouds move up and down in the atmosphere based on how dense they are, until they become too dense and rain on us (precipitation).
- Conduction : The transfer of Heat or Thermal Energy caused by the direct touching of two separate objects. For example, when you sit down in a seat and feel it is warm, this is due to the seat being warmer than your pants, and thus transferring its Heat to you by conduction.
- Radiation : The transfer of heat over a distance, between two objects that are not touching. Electromagnetic Radiation warms an object by transferring some of its energy to the object. For example, how does the Sun warm the Earth? The Earth and Sun are not touching, so it can't be conduction. Furthermore, the Earth and Sun are not a part of the same fluid that transfers Heat between them, so it is not Convection. Therefore, the Sun warms the Earth through seemingly endless amounts of Radiation bombarding the Earth constantly.
Thermal Equilibrium
Like all systems in nature, Thermal Energy works to reach Thermal Equilibrium. When two substances of differing temperatures come into "contact," the substance of greater Thermal Energy will do microscopic work on the other substance until the two are at the same temperature. Atoms of the higher energy substance will collide with those of the lower energy substance at the boundary. Kinetic energy will be transferred and distribute evenly throughout. Given enough time, this process of propagation of kinetic energy will continue until Thermal Equilibrium is reached. However, do not think that because two substances have reached Thermal Equilibrium that they are not transferring Thermal Energy. Instead, they are continuously transferring Thermal Energy with each other, but at an equal rate, so that neither's Thermal Energy/Temperature changes. The transfer of Thermal Energy is referred to as Heat.
Computational Model
PhET by the University of Colorado Boulder allows free public usage of their simulations in an educational context. Their simulation Energy Forms and Changes is a useful visual companion to practice calculating specific heat capacities and thermal energies of materials.
Simulation by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0 ( https://phet.colorado.edu ).
[math]\displaystyle{ 500 g }[/math] of water is heated from an initial temperature [math]\displaystyle{ T_0 = 20°C }[/math] to a final temperature [math]\displaystyle{ T_f = 50°C }[/math] .
[math]\displaystyle{ 400 g }[/math] of water, with an initial temperature of [math]\displaystyle{ T_{0_{w}} = 90°C }[/math] [math]\displaystyle{ \left(C = 4.2 \ \frac{J}{g°C}\right) }[/math] is poured into an aluminum pan, whose mass is [math]\displaystyle{ 800 g }[/math] with an initial temperature of [math]\displaystyle{ T_{0_{a}} = 20°C }[/math] [math]\displaystyle{ \left(C = 0.9 \ \frac{J}{g°C}\right) }[/math] .
[math]\displaystyle{ 500g }[/math] of water with an initial temperature of [math]\displaystyle{ T_{w_{0}} = 87 \ °C \left(C = 4.2 \ \frac{J}{g°C}\right) }[/math] is poured into an aluminum pan whose mass is [math]\displaystyle{ 800g }[/math] with an initial temperature of [math]\displaystyle{ T_{a_{0}} = 22 \ °C \left(C = 0.9 \ \frac{J}{g°C}\right) }[/math] . The aluminum pan and water are allowed to reach Thermal Equilibrium. Then, you place the pan on a hot electric stove. While the stove is heating the pan, you stir the water doing [math]\displaystyle{ 26,000 \ J }[/math] of work, raising the temperature of the system to [math]\displaystyle{ 82.5 \ °C }[/math] .
Connectedness
Thermal Energy is a phenomenon present in all aspects of life. Everything from the Sun to an ice cube contains, emits, and absorbs thermal energy. In order to convert thermal energy to other, more useful forms an engine must be used. For example, steam carries a large amount of thermal energy, which can be used mechanically via steam engines. Similarly, Thermal Energy is integral in determining which phase of matter a material exists in. For example, at left is a phase diagram of iron-- it can be seen from the axes that what phase iron is in is determined by the surrounding pressure and the iron's temperature.
One of the interesting industrial application of thermal energy is industrial thermal energy storage. Thermal energy storage is a technology that stocks thermal energy by heating or cooling a storage medium so that the stored energy can be used at a later time for heating and cooling applications and power generation.
Thermal Energy was discovered by a man named James Joule in the 1840's. Careful experiments showed that the temperature increase of an object and its surroundings due to friction is directly related to the amount of mechanical energy lost. Joule carried out one of the most famous experiments, demonstrating this fact. Joule hung some weights from pulleys, so that as they fell, they turned a paddle-wheel apparatus immersed in a bucket of water. The friction between the paddles and the water raised the water’s temperature by an amount that was directly proportional to the distance that the weights fell. In our modern system of units, Joule found that raising the temperature of a kilogram of water by one degree Celsius required a loss in mechanical (gravitational) energy of approximately 4,200 Joules. Joule therefore proposed that this mechanical energy is not actually lost, but converted into a new type of energy: Thermal Energy, which manifests itself as an increase or decrease in temperature.
Further reading
External links, navigation menu.

Importance of the heat (thermal energy) and light energy in our daily life
by Heba Soffar · Published February 11, 2015 · Updated April 9, 2024
Heat is a form of energy that transfers from the higher temperature object to the lower temperature object, and is transferred through conduction, convection and radiation. Anything that produces warmth is referred to as a source of heat. Sources of heat such as the sun , fire, electricity , and gas.
Heat energy
The heat is thermal energy that flows from the warmer areas to the cooler areas, and the thermal energy is the total of all kinetic energies within a given system. The temperature is the degree of hotness or coldness of a body, It is measured by three scales of measurement which are Fahrenheit, Celsius, and Kelvin and it is the average kinetic energy within a given object.
Thermal energy is a part of the total energy of any object, Its measuring unit is the joule, and it is related to the temperature of an object, the heat energy can be transferred in three ways which are convection, conduction, and radiation. Heat and temperature are not the same things because the temperature is related to how hot or cold something is, The measuring devices of the temperature are the thermometers .
Heat is the form of energy crossing the boundary of the thermodynamic system by a temperature difference across the boundary in thermodynamics, It transfers to or from the thermodynamic system, by the mechanism that involves the microscopic atomic modes of motion or the corresponding macroscopic properties.
The measurement of energy transferred as heat is called calorimetry, performed by measuring its effect on the states of interacting bodies, Heat energy has the unit joule (J) in the International System of Units (SI), and many applied branches of engineering use other, traditional units, such as the British thermal unit (BTU) and the calorie.
Importance of the heat
Heat is vital in our daily life in warming the house, heating the water , and drying the washed clothes. Heat has many usages in the industry as making and processing food and manufacturing of glass, paper, textiles, ………etc. Heat can be used for Ironing clothes, and p roviding power to move cars, ships, and hot air balloons, Heat is used in welding and cutting metals.
The steam has a high specific heat (more than the water ), It is used to carry a lot of heat energy at high pressures to run the rail engines or the rotors in AC generators . The water in the swimming pool remains cool even in summer and the people enjoy a lot staying inside the pool because the water has high specific heat. We use heat in cooking food, We use utensils for making tea or coffee, or cooking vegetables or rice. Heat is used to warm our bodies in winter.
Heat is used in Engines: Various engines, such as rail engines, work on heat , All electrical appliances need heat , Heat energy is used to generate electricity . Heat is used as a simple yet effective way to manage pain and joint or muscular stiffness, The deep and penetrating heat not only relieves the pain but also enhances the recovery process.
Heat is a form of energy that exists naturally, It quickly changes into different forms of energy like light, electricity , etc., Life on this earth depends on heat energy for survival, Heat energy, unlike other forms, can be felt by the sense of touch. L ight energy from the sun converts to heat energy that helps plants to make the photosynthesis process .
Heat is used in enzyme reactions , Heat plays a vital role in health care, The heat can counter inflammation and reduce pain, so, heat is used in the treatment of inflammatory and pain-related problems. Heat is used in the water cycle, It is used in chemical reactions, the electrons and atoms in the substances which are in the stable state are set into vibration.
All the vehicles which run on petrol and coal operate due to the generation of heat energy within, The heat energy helps the pistons to move, which is conveyed into cyclic motion, and thus, the wheels rotate. Sterilization is a process to kill any microbes in drugs and other healthcare materials, heat is used for Incubation to grow birds in eggs.
Heat is crucial in our world, playing a vital role in many aspects of our lives and the environment. Heat is essential for most living organisms. It drives biological processes in plants and animals, like enzyme activity and metabolism, which are necessary for growth, reproduction, and survival.
Heat causes changes in the state of matter. We use heat for cooking, boiling water, and melting metals for various purposes. Heat is a fundamental form of energy. We can convert heat from various sources like sunlight, fossil fuels, or geothermal energy to generate electricity, powering our homes and industries.
Heat is essential for most living organisms. It provides the energy for biological processes in plants and animals Plants rely on sunlight, a form of heat energy , for photosynthesis, the process by which they produce food. Warmth is necessary for many animals to maintain internal body temperature, crucial for survival.
Heat causes changes in the state of matter, it can melt solids into liquids and vaporize liquids into gases. This property is used in various applications, like cooking food (boiling water, frying), shaping metals (melting them for manufacturing), and generating electricity in power plants .
Heat is undeniably woven into our daily lives. We use it for essential tasks like cooking, keeping warm (heating systems), and drying clothes. Many industrial processes also rely on heat for various purposes. Heat energy is a fundamental source of power generation. We use heat from burning fossil fuels or nuclear reactions to create steam, which drives turbines to generate electricity in power plants .
Heat is a key player in many natural phenomena. It influences weather patterns, ocean currents, and the formation of clouds. The uneven distribution of heat around the globe drives wind patterns and contributes to the diversity of climates on Earth.
Heat is essential for most living organisms. It provides the energy for biological processes in plants and animals, allowing them to function and survive. Heat causes changes in the state of matter. We use heat for cooking, boiling water, and melting metals for various purposes.
Heat is used in countless ways in our daily lives. From warming our homes in cold weather to drying clothes and running various appliances, heat plays a significant role in our comfort and daily routines. Heat is a key driver of many natural phenomena like weather patterns and ocean currents. It plays a role in geological processes like the formation of rocks and the movement of tectonic plates.
Heat is a fundamental form of energy. It’s important for both natural processes and human civilization. It sustains life, drives physical changes, and has numerous applications in our daily lives and the natural world. Heat is vital in our world, playing a crucial role in many aspects of our lives and the natural world around us.
You can follow Science Online on Youtube from this link: Science online
You can download Science Online application on Google Play from this link: Science online Apps on Google Play
Solar energy (Sun), Ways of heat transfer (conduction, convection & radiation)
Heat therapy (Thermotherapy) & What are the clinical uses of heat?
Importance of heating in Agriculture, Horticulture and the greenhouse
Tags: 5 importance of heat energy Applications of heat energy in Life conduction Convection educational Energy Topics heat Heat energy Heat energy advantages Heat energy benefits Heat energy definition Heat energy pros Heat importance Heat in houses Heat in industry Heat Transfer Heat uses Importance of heat energy in mining Importance of heat energy to living things Importance of heat energy to our existence Importance of heat in daily life Importance of heat in Earth importance of heat transfer importance of thermal energy Just Energy list 10 uses of heat energy Radiation Sources of Heat The temperature The thermometers Thermal energy Thermodynamics uses of heat energy uses of heat in daily life What are 10 uses of heat? What are useful examples of heat? What is heat used for? what is the importance of heat and light energy in our daily lives Where is heat? why is heat important why is heat important to living things
You may also like...
Energy transformation inside the cars and What is the process of energy transformation?
May 21, 2015
by Heba Soffar · Published May 21, 2015 · Last modified May 11, 2024
Potential energy, Kinetic energy and Law of conservation of mechanical energy
November 3, 2019
by Heba Soffar · Published November 3, 2019 · Last modified February 2, 2024

Nuclear submarines (Nuclear Powered Ships) advantages and disadvantages
July 3, 2016
by Heba Soffar · Published July 3, 2016 · Last modified October 9, 2019
8 Responses
- Pingbacks 0
why heating is important to horticulture
You can read this article about Importance of heating in Agriculture, Horticulture and the greenhouse
what are the clinical uses of heat
You can read this article about Heat therapy ( Thermotherapy ) & What are the clinical uses of heat ?
what are the benefits of heat in day to day life
You can read this articles about Heat therapy ( Thermotherapy ) & What are the clinical uses of heat ? heat in agriculture
hey, thank you, good post
You are welcome
Leave a Reply Cancel reply
You must be logged in to post a comment.

In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation.
Thermal Energy Essay
The motion of the fluid may be natural or forced. If a liquid or gas is heated, its mass per unit volume generally decreases. If the liquid or gas is in a gravitational field, the hotter, lighter fluid rises while the colder, heavier fluid sinks. For example, when water in a pan is heated from below on my stove, the liquid closest to the bottom expands and its density decreases. The hot water as a result rises to the top and some of the cooler fluid descends toward the bottom, thus setting up a circulatory motion.
Related posts:
Leave a comment cancel reply.

Home energy essays thermal energy essay
thermal energy essay
Lab #7: Cold weather Energy
Check the price for your assignment. Get a 100% Plagiarism-Free Essay
Temperature is heat energy getting transferred in one place to an additional, because of heat changes. This may take place simply by three techniques. These three processes will be known as bail, convection, and radiation.
Whenever we place two objects based on a temperatures talking to each other, heat from the hotter object is going to immediately and automatically movement to the colder object. This is certainly known as bail. Some items make good conductors of warmth while others make poor conductors of heat or excellent insulators. Silver, copper, and gold make excellent conductors of heat. Foams and plastics generate good insulators of heat although make poor conductors. Last night for dinner, My spouse and i made myself a grilled cheese meal and a bowl of tomato soup. We heated the soup faster than I cooked the sandwich therefore i poured the hot soup into a bowl and finished cooking the sandwich. Once I had been done preparing food, I gabbed the soups bowl and burned me. The heat from your soup manufactured the bowl hot. This can be an example of conduction.
The process of conduction between an excellent surface and a shifting liquid or gas is known as convection. The motion of the fluid could possibly be natural or forced. If a liquid or gas is definitely heated, its mass every unit quantity generally lessens. If the liquefied or gas is in a gravitational field, the hotter, less heavy fluid rises while the frigid, heavier substance sinks. For example , when drinking water in a griddle is warmed from under on my range, the water closest to the bottom extends and its thickness decreases. The hot water because of this rises to the top plus some of the cooler fluid descends toward the underside, thus setting up a circulatory motion. This is also so why the warming of a place by a rad depends less on radiation than about natural convection currents, the air growing upward along the wall and cooler atmosphere coming back to the radiator in the side of the bottom. Because of the tendencies of hot air to increase and of fresha ir to drain, radiators are positioned near the flooring and a/c outlets nearby the ceiling pertaining to maximum effectiveness.
Radiation is fundamentally not the same as both louage and convection in that the substances exchanging heat does not need to be in contact together. All chemicals emit glowing energy basically by virtue of creating a positive absolute temperature. The bigger the heat, the greater the amount of energy released. In addition to emitting, all substances are equipped for absorbing rays. Thus, although an ice cubes cube is usually continuously emitting radiant energy, it will burn if a tungsten halogen lamp is targeted on it since it will be absorbing a greater quantity of heat than it is emitting. In addition , surfaces can absorb or reveal incident the radiation. Generally, dull, rough surfaces absorb even more heat than bright, refined surfaces, and bright floors reflect more radiant strength than boring surfaces. In addition , good absorbers are also great emitters, great reflectors, or perhaps poor absorbers, are poor emitters. That is why cooking utensils generally possess dull bottoms for good ingestion and finished sides pertaining to minimum release to maximize the heat transfer in the contents of the pot.
Heat and is usually being transported from place to place. Anything on Earth provides a temperature above Zero Kelvin so almost everything on earth consists of heat. Devoid of some kind of warmth and strength, there may not be existence.

No related posts.
NEED AN ESSAY WRITING HELP?
Your Email (required)
Please leave this field empty.
Your Name (required)
Your Message

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
What Is Energy? Energy Definition and Examples (Science)

The concept of energy is key to science and engineering. Here is the definition, examples of energy, and a look at the way it is classified.
Energy Definition
In science, energy is the ability to do work or heat objects. It is a scalar physical quantity, which means it has magnitude, but no direction. Energy is conserved, which means it can change from one form to another, but isn’t created or destroyed. There are many different types of energy, such as kinetic energy, potential energy , light, sound, and nuclear energy.
Word Origin and Units
The term “energy” comes from the Greek word energeia or from the French words en meaning in and ergon which means work. The SI unit of energy is the joule (J), where 1 J = 1kg⋅m 2 ⋅s −2 . Other units include the kilowatt-hour (kW-h), British thermal unit (BTU), calorie (c), kilocalorie (C), electron-volt (EV), erg, and foot-pound (ft-lb).
What Losing Energy Means
One form of energy may be converted into another without violating a law of thermodynamics . Not all of these forms of energy are equally useful for practical applications. When energy is “lost”, it means the energy can’t be recaptured for use. This usually occurs when heat is produced. Losing energy doesn’t mean there is less of it, only that it has changed forms.
Energy may be either renewable or nonrenewable. Photosynthesis is an example of a process the produces renewable energy. Burning coal is an example of nonrenewable energy. The plant continues to produce chemical energy in the form of sugar, by converting solar energy. Once coal is burned, the ash can’t be used to continue the reaction.
Kinetic Energy and Potential Energy
The various forms of energy are classified as kinetic energy, potential energy, or a mixture of them. Kinetic energy is energy of motion, while potential energy is stored energy or energy of position. The total of the sum of the kinetic and potential energy of a system is constant, but energy changes from one form to another.
For example, when you hold an apple motionless above the ground, it has potential energy, but no kinetic energy. When you drop the apple, it has both kinetic and potential energy as it falls. Just before it strikes the ground, it has maximum kinetic energy, but no potential energy.
Renewable and Non-Renewable Energy
Another broad way of classifying energy is as renewable or non-renewable . Renewable energy is energy that replenishes within a human lifetime. Examples include solar energy, wind energy, and biomass. Non-renewable energy either does not regenerate or else takes longer than a human lifespan to do so. Fossil fuels are an example of non-renewable energy.
Forms of Energy
There are many different forms energy can take . Here are some examples:
- nuclear energy – energy released by changes in the atomic nucleus, such as fission or fusion
- electrical energy – energy based on the attraction, repulsion, and movement of electrical charge, such as electrons, protons , or ions
- chemical energy – energy based on the difference between the amount required to form chemical bonds versus how much is needed to break them
- mechanical energy – the sum of the translational and rotational kinetic and potential energies of a system
- gravitational energy – energy stored in gravitational fields
- ionization energy – energy that binds an electron to its atom or molecule
- magnetic energy – energy stored within magnetic fields
- elastic energy – energy of a material that causes it to return to its original shape if it’s deformed
- radiant energy – electromagnetic radiation, such as light from the sun or heat from a stove
- thermal energy – kinetic energy due to the motion of subatomic particles, atoms, and molecules
Examples of Energy
Here are some everyday examples of energy and a look at the types of energy:
- Throwing a ball : Throwing a ball is an example of kinetic energy, potential energy, and mechanical energy
- Fire : Fire is thermal energy, chemical energy, and radiant energy. Its source may be either renewable (wood) or non-renewable (coal).
- Charging a phone battery : Charging a phone involves electrical energy, chemical energy (for the battery), and both kinetic and potential energy. The stored electrical charge is potential energy, while moving charge is kinetic energy.
- Harper, Douglas. “Energy”. Online Etymology Dictionary .
- Smith, Crosbie (1998). The Science of Energy – a Cultural History of Energy Physics in Victorian Britain . The University of Chicago Press. ISBN 978-0-226-76420-7.
Related Posts
Solar Energy Essay
500 words essay on solar energy.
Solar energy is the energy which the earth receives from the sun which converts into thermal or electrical energy. Moreover, solar energy influences the climate of the earth and weather to sustain life. It has great potential which we must use to our advantage fully. Through the solar energy essay, we will look at this in detail and know more about it carefully.

Importance of Solar Energy
Solar energy is very important as it is a clean and renewable source of energy. Thus, this means it will not damage the earth in any way. In addition, it is available on a daily basis. Similarly, it does not cause any kind of pollution.
As it is environment-friendly, it is very important in today’s world. It is so much better than other pollution sources of energies like fossil fuels and more. Further, it has low maintenance costs.
Solar panel systems do not require a lot of solar power energy. Moreover, they come with 5-10 years of warranty which is very beneficial. Most importantly, it reduces the cost of electricity bills.
In other words, we use it mostly for cooking and heating up our homes. Thus, it drops the utility bills cost and helps us save some extra money. Further, solar energy also has many possible applications.
A lot of communities and villages make use of solar energy to power their homes, offices and more. Further, we can use it in areas where there is no access to a power grid. For instance, distilling the water is Africa and powering the satellites in space.
Get the huge list of more than 500 Essay Topics and Ideas
Uses of Solar Energy
In today’s world, we use solar energy for a lot of things. Firstly, we use solar power for many things as small as calculators to as big as power plants which power the entire city. We use the most common solar power for small things.
For instance, many calculators use solar cells to operate, thus they never run out of batteries. Moreover, we also have some watches which run on solar cells. Similarly, there are also radios which run on solar cells.
Thus, you see so many things run on solar power. All satellites run on solar power otherwise they won’t be able to function. Moreover, large desalinization plants make use of solar power if there is little or no freshwater.
In addition, many countries have solar furnaces. We also use solar power commercially and residentially. You will find its uses in transportation service too. In fact, soon, solar powers will also be out on the streets.

Conclusion of Solar Energy Essay
To sum it up, solar energy is a cost-effective means of energy which is quite useful for people that have huge families. When we install solar panels, we can get solar energy which will reduce electricity costs and allow us to lead a sustainable lifestyle. Thus, we must all try to use it well to our advantage.
FAQ of Solar Energy Essay
Question 1: What is solar energy in simple words?
Answer 1: Solar energy is basically the transformation of heat, the energy which is derived from the sun. We have been using it for thousands of years in numerous different ways all over the world. The oldest uses of solar energy are for heating, cooking, and drying.
Question 2: What are the advantages of solar energy?
Answer 2: There are many advantages of solar energy. Firstly, it is a renewable source of energy which makes it healthy. Moreover, it also reduces the electricity bills of ours. After that, we can also use it for diverse applications. Further, it also has low maintenance costs.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Geography Notes
Essay on geothermal energy: top 11 essays | energy management.
ADVERTISEMENTS:
Here is a compilation of essays on ‘Geothermal Energy’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Geothermal Energy’ especially written for school and college students.
Essay on Geothermal Energy
Essay Contents:
- Essay on the Effect of Geothermal Energy on Environment
Essay # 1. Introduction to Geothermal Energy:
Geothermal energy is the earth’s natural heat available inside the earth. This thermal energy contained in the rock and fluid that filled up fractures and pores in the earth’s crust can profitably be used for various purposes. Heat from the Earth, or geothermal — Geo (Earth) + thermal (heat) — energy can be and is accessed by drilling water or steam wells in a process similar to drilling for oil.
Geothermal resources range from shallow ground to hot water and rock several miles below the Earth’s surface, and even farther down to the extremely hot molten rock called magma. Mile-or-more-deep wells can be drilled into underground reservoirs to tap steam and very hot water that can be brought to the surface for use in a variety of applications.
This geothermal energy originates from the original formation of the planet, from radioactive decay of minerals, from volcanic activity and from solar energy absorbed at the surface. It has been used for bathing since Paleolithic times and for space heating since ancient Roman times, but is now better known for generating electricity.
Worldwide, about 10,715 megawatts (MW) of geothermal power is online in 24 countries. An additional 28 gigawatts of direct geothermal heating capacity is installed for district heating, space heating, spas, industrial processes, desalination and agricultural applications.
India has reasonably good potential for geothermal; the potential geothermal provinces can produce approximately 10,600 MW of power.
Geothermal power is cost effective, reliable, sustainable, and environmentally friendly, but has historically been limited to areas near tectonic plate boundaries. Recent technological advances have dramatically expanded the range and size of viable resources, especially for applications such as home heating, opening a potential for widespread exploitation.
Geothermal wells release greenhouse gases trapped deep within the earth, but these emissions are much lower per energy unit than those of fossil fuels. As a result, geothermal power has the potential to help mitigate global warming if widely deployed in place of fossil fuels.
The earth’s geothermal resources are theoretically more than adequate to supply humanity’s energy needs, but only a very small fraction may be profitably exploited. Drilling and exploration for deep resources is very expensive. Forecasts for the future of geothermal power depend on assumptions about technology, energy prices, subsidies, and interest rates.
Essay # 2. History of Geothermal Energy Worldwide:
The oldest known pool fed by a hot spring, built in the Qin dynasty in the 3rd century BC.
Hot springs have been used for bathing at least since Paleolithic times. The oldest known spa is a stone pool on China’s Lisan mountain built in the Qin dynasty in the 3rd century BC, at the same site where the Huaqing Chi palace was later built. In the first century AD, Romans conquered Aquae Sulis, now Bath, Somerset, England, and used the hot springs there to feed public baths and underfloor heating.
The admission fees for these baths probably represent the first commercial use of geothermal power. The world’s oldest geothermal district heating system in Chaudes-Aigues, France, has been operating since the 14th century. The earliest industrial exploitation began in 1827 with the use of geyser steam to extract boric acid from volcanic mud in Larderello, Italy.
In 1892, America’s first district heating system in Boise, Idaho was powered directly by geothermal energy, and was copied in Klamath Falls, Oregon in 1900. A deep geothermal well was used to heat greenhouses in Boise in 1926, and geysers were used to heat greenhouses in Iceland and Tuscany at about the same time. Charlie Lieb developed the first down-hole heat exchanger in 1930 to heat his house. Steam and hot water from geysers began heating homes in Iceland starting in 1943.
Global geothermal electric capacity. Upper red line is installed capacity; lower green line is realized production.
In the 20th century, demand for electricity led to the consideration of geothermal power as a generating source. Prince Piero Ginori Conti tested the first geothermal power generator on 4 July 1904, at the same Larderello dry steam field where geothermal acid extraction began.
It successfully lit four light bulbs. Later, in 1911, the world’s first commercial geothermal power plant was built there. It was the world’s only industrial producer of geothermal electricity until New Zealand built a plant in 1958.
By this time, Lord Kelvin had already invented the heat pump in 1852, and Heinrich Zoelly had patented the idea of using it to draw heat from the ground in 1912. But it was not until the late 1940s that the geothermal heat pump was successfully implemented. The earliest one was probably Robert C. Webber’s home-made 2.2 kW direct-exchange system, but sources disagree as to the exact timeline of his invention.
J. Donald Kroeker designed the first commercial geothermal heat pump to heat the Commonwealth Building (Portland, Oregon) and demonstrated it in 1946. Professor Carl Nielsen of Ohio State University built the first residential open loop version in his home in 1948. The technology became popular in Sweden as a result of the 1973 oil crisis, and has been growing slowly in worldwide acceptance since then.
In 1960, Pacific Gas and Electric began operation of the first successful geothermal electric power plant in the United States at The Geysers in California. The original turbine lasted for more than 30 years and produced 11 MW net power.
The binary cycle power plant was first demonstrated in 1967 in the U.S.S.R. and later introduced to the U.S. in 1981. This technology allows the generation of electricity from much lower temperature resources than previously. In 2006, a binary cycle plant in Chena Hot Springs, Alaska, came on-line, producing electricity from a record low fluid temperature of 57°C (135°F).
Installed geothermal electric capacity as of 2007 is around 10000 MW. The main countries having major electric generation installed capacities (as of 2007) are USA (3000MW), Philippines(2000MW), Indonesia (1000MW), Mexico (1000MW), Italy (900 MW), Japan(600MW), New Zealand (500MW), Iceland (450MW). The other region includes the Latin American countries, African countries and Russia.
Essay # 3. Formation of Geothermal Resources:
Geothermal energy is made up of heat from the earth. Underneath the earth’s relatively, thin crust, temperature range from 1000-4000°C and in some areas, pressures exceed 20,000 psi. Geothermal energy is most likely generated from radioactive, thorium, potassium and uranium dispersed evenly through the earth’s interior which produce heat as part of the decaying process. This process generates enough heat to keep the lose of the earth at temperature approaching 4000°C.
Composed primarily of molten Ni and Fe the core is surrounded by a layer of molten rock, the mantle at approx. 1000°C. Nine major crystal plates float on the mantle, and currents in the mantle cause the plates to drift, colliding in some areas and diverging in others.
When two continental plates coverage, a complex series of chemical reactions involving water and other substances combine to generate large bodies of molten rock called magna chamber that rise through the crust often resulting in volcanic activity. Molten rock also rises in the earth’s crust where the plates are moving away from each other and in other areas where the crust is thin.
Volcanoes, hot springs, geysers and fumaroles are natural clues as to the presence of geothermal resources near the surface and where economic drilling operations can tap their heat and pressure. Additional heat can be generated by friction as two plates converge and one moves on top of other.
Essay # 4. Types of Geothermal Resources:
There are following types of geothermal resources:
(i) Hydrothermal.
(ii) Geopressured.
(iii) Hot Dry Rock.
(iv) Active Volcanic Vents and Magna.
(i) Hydrothermal:
Hydrothermal resources contain superheated rock trapped by a layer of impermeable rock. The highest quality reserves with temperature over 240°C contain steam with little or no condensate (vapour dominated resources).
Some hydrothermal reserves are very hot ranging from 150-200°C, but roughly 2/3rd are of moderate temperature (100-180°C). Only two sizeable high quality dry steam reserves have been located to date on in the US and one in Italy. The geysers in northern California is perhaps the world’s largest dry steam field and could provide 2000 MWe capacity for upto 30 years.
(ii) Geopressured:
It contains moderate-temperature brines containing dissolved methane. They are trapped under high pressure in deep sedimentary formations sealed between impermeable layers of clay and shale. Pressures vary from 5000 to over 20,000 psi at depths of 1500 to 15000 metres. Temperature range from 90 to over 200°C, although they seldom exceed 150°C, each barrel of fluid at 10,000 psi and 150°C could contain between 20 and 50 standard cubic feed (SCF) of methane.
(iii) Hot Dry Rock:
It contains high temperature rocks, ranging from 90-650°C that may be fractured and contain little or no water. The rocks must be artificially fractured and heat transfer fluid circulated to extract their energy. Hot dry rock resources are much more extensive than hydrothermal or geo-pressured, but extracting their energy is more difficult.
(iv) Active Volcanic Vents and Magma:
It occurs in many parts of the world. Magma is molten rock at temperature ranging from 700°C to 1600°C, lying under the earth crust, the molten rock is part of the mantle and in approx. 24 to 28 km thick. Magma chambers represent a huge energy source, the largest of all geothermal resources but they rarely occur near the surface of the earth and extracting their energy is difficult.
Essay # 5. Geothermal Electricity:
As per the International Geothermal Association (IGA) sources, about 10,715 MW of geothermal power in 24 countries is online. In 2010, the United States led the world in geothermal electricity production with 3,086 MW of installed capacity from 77 power plants.
The largest group of geothermal power plants in the world is located at the Geysers, a geothermal field in California. The Philippines is the second highest producer, with 1,904 MW of capacity online. Geothermal power makes up approximately 18% of the country’s electricity generation.
Geothermal electric plants were traditionally built exclusively on the edges of tectonic plates where high temperature geothermal resources are available near the surface. The development of binary cycle power plants and improvements in drilling and extraction technology enable enhanced geothermal systems over a much greater geographical range.
Demonstration projects are operational in Landau-Pfalz, Germany, and Soultz-sous-Forest, France, while an earlier effort in Basel, Switzerland was shut down after it triggered earthquakes. Other demonstration projects are under construction in Australia, the United Kingdom, and the United States of America.
The thermal efficiency of geothermal electric plants is low, around 10-23%, because geothermal fluids do not reach the high temperatures of steam from boilers. The laws of thermodynamics limits the efficiency of heat engines in extracting useful energy. Exhaust heat is wasted, unless it can be used directly and locally, for example in greenhouses, timber mills, and district heating.
System efficiency does not materially affect operational costs as it would for plants that use fuel, but it does affect return on the capital used to build the plant. In order to produce more energy than the pumps consume, electricity generation requires relatively hot fields and specialized heat cycles. Because geothermal power does not rely on variable sources of energy, unlike, for example, wind or solar, its capacity factor can be quite large – up to 96% has been demonstrated. The global average was 73% in 2005.
Essay # 6. Geothermal Power Plants Technology:
To convert geothermal energy into electrical energy, heat must be extracted first to convert it into useable form. Mile-or-more-deep wells can be drilled into underground reservoirs to tap steam and very hot water that drive turbines that drive electricity generators.
There are basically four types of geothermal power plants which are operating today. The description of these power plants is as follows:
(i) Flashed Steam Plant:
The extremely hot water from drill holes when released from the deep reservoirs high pressure steam (termed as flashed steam) is released. This force of steam is used to rotate turbines. The steam gets condensed and is converted into water again, which is returned to the reservoir. Flashed steam plants are widely distributed throughout the world.
(ii) Dry Steam Plant:
Usually geysers are the main source of dry steam. Those geothermal reservoirs which mostly produce steam and little water are used in electricity production systems. As steam from the reservoir shoots out, it is used to rotate a turbine, after sending the steam through a rock-catcher. The rock-catcher protects the turbine from rocks which come along with the steam.
(iii) Binary Power Plant:
In this type of power plant, the geothermal water is passed through a heat exchanger where its heat is transferred to a secondary liquid, namely isobutene, isopentane or ammonia-water mixture present in an adjacent, separate pipe. Due to this double-liquid heat exchanger system, it is called a binary power plant.
The secondary liquid which is also called as working fluid should have lower boiling point than water. It turns into vapour on getting required heat from the hot water. The vapour from the working fluid is used to rotate turbines.
The binary system is therefore useful in geothermal reservoirs which are relatively low in temperature gradient. Since the system is a completely closed one, there is minimum chance of heat loss. Hot water is immediately recycled back into the reservoir. The working fluid is also condensed back to the liquid and used over and over again.
(iv) Hybrid Power Plant:
Some geothermal fields produce boiling water as well as steam, which are also used in power generation. In this system of power generation, the flashed and binary systems are combined to make use of both steam and hot water. Efficiency of hybrid power plants is however less than that of the dry steam plants.
Enhanced Geothermal System:
The term enhanced geothermal systems (EGS), also known as engineered geothermal systems (formerly hot dry rock geothermal), refers to a variety of engineering techniques used to artificially create hydrothermal resources (underground steam and hot water) that can be used to generate electricity.
Traditional geothermal plants exploit naturally occurring hydrothermal reservoirs and are limited by the size and location of such natural reservoirs. EGS reduces these constraints by allowing for the creation of hydrothermal reservoirs in deep, hot but naturally dry geological formations. EGS techniques can also extend the lifespan of naturally occurring hydrothermal resources.
Given the costs and limited full-scale system research to date, EGS remains in its infancy, with only a few research and pilot projects existing around the world and no commercial-scale EGS plants to date. The technology is so promising, however, that a number of studies have found that EGS could quickly become widespread.
Essay # 7. Other Applications of Geothermal Energy:
In the geothermal industry, low temperature means temperatures of 300°F (149°C) or less. Low-temperature geothermal resources are typically used in direct-use applications, such as district heating, greenhouses, fisheries, mineral recovery, and industrial process heating. However, some low-temperature resources can generate electricity using binary cycle electricity generating technology.
Direct heating is far more efficient than electricity generation and places less demanding temperature requirements on the heat resource. Heat may come from co-generation via., a geothermal electrical plant or from smaller wells or heat exchangers buried in shallow ground.
As a result, geothermal heating is economic at many more sites than geothermal electricity generation. Where natural hot springs are available, the heated water can be piped directly into radiators. If the ground is hot but dry, earth tubes or down-hole heat exchangers can collect the heat.
But even in areas where the ground is colder than room temperature, heat can still be extracted with a geothermal heat pump more cost-effectively and cleanly than by conventional furnaces.
These devices draw on much shallower and colder resources than traditional geothermal techniques, and they frequently combine a variety of functions, including air conditioning, seasonal energy storage, solar energy collection, and electric heating. Geothermal heat pumps can be used for space heating essentially anywhere.
Geothermal heat supports many applications. District heating applications use networks of piped hot water to heat many buildings across entire communities. In Reykjavik, Iceland, spent water from the district heating system is piped below pavement and sidewalks to melt snow.
Essay # 8. Economics Related to Geothermal Energy Harnessing :
Geothermal power requires no fuel (except for pumps), and is therefore immune to fuel cost fluctuations, but capital costs are significant. Drilling accounts for over half the costs, and exploration of deep resources entails significant risks.
Unlike traditional power plants that run on fuel that must be purchased over the life of the plant, geothermal power plants use a renewable resource that is not susceptible to price fluctuations. The price of geothermal is within range of other electricity choices available today when the costs of the lifetime of the plant are considered.
Most of the costs related to geothermal power plants are related to resource exploration and plant construction. Like oil and gas exploration, it is expensive and because only one in five wells yield a reservoir suitable for development. Geothermal developers must prove that they have reliable resource before they can secure millions of dollar required to develop geothermal resources.
Although the cost of generating geothermal has decreased during the last two decades, exploration and drilling remain expensive and risky. Drilling Costs alone account for as much as one-third to one-half to the total cost of a geothermal project. Locating the best resources can be difficult; and developers may drill many dry wells before they discover a viable resource.
Because rocks in geothermal areas are usually extremely hard and hot, developers must frequently replace drilling equipment. Individual productive geothermal wells generally yield between 2 MW and 5 MW of electricity; each may cost from $1 million to $5 million to drill. A few highly productive wells are capable of producing 25 MW or more of electricity.
Transmission:
Geothermal power plants must be located near specific areas near a reservoir because it is not practical to transport steam or hot water over distances greater than two miles. Since many of the best geothermal resources are located in rural areas, developers may be limited by their ability to supply electricity to the grid. New power lines are expensive to construct and difficult to site.
Many existing transmission lines are operating near capacity and may not be able to transmit electricity without significant upgrades. Consequently, any significant increase in the number of geothermal power plants will be limited by those plants ability to connect, upgrade or build new lines to access to the power grid and whether the grid is able to deliver additional power to the market.
Direct heating applications can use much shallower wells with lower temperatures, so smaller systems with lower costs and risks are feasible. Residential geothermal heat pumps with a capacity of 10 kilowatt (kW) are routinely installed.
District heating (Cities etc.) systems may benefit from economies of scale if demand is geographically dense, as in cities, but otherwise piping installation dominates capital costs. Direct systems of any size are much simpler than electric generators and have lower maintenance costs per kW.h, but they must consume electricity to run pumps and compressors.
Essay # 9. Barriers in the Way of Geothermal Energy:
i. Finding a suitable build location.
ii. Energy source such as wind, solar and hydro are more popular and better established; these factors could make developers decided against geothermal.
iii. Main disadvantages of building a geothermal energy plant mainly lie in the exploration stage, which can be extremely capital intensive and high-risk; many companies who commission surveys are often disappointed, as quite often, the land they were interested in, cannot support a geothermal energy plant.
iv. Some areas of land may have the sufficient hot rocks to supply hot water to a power station, but many of these areas are located in harsh areas of the world (near the poles), or high up in mountains.
v. Harmful gases can escape from deep within the earth, through the holes drilled by the constructors. The plant must be able to contain any leaked gases, but disposing of the gas can be very tricky to do safely.
Essay # 10. Sustainability of Geothermal Energy:
Geothermal power is considered to be sustainable because any projected heat extraction is small compared to the Earth’s heat content. The Earth has an internal heat content of 10 31 joules (3. 10 15 TW.hr). About 20% of this is residual heat from planetary accretion, and the remainder is attributed to higher radioactive decay rates that existed in the past.
Natural heat flows are not in equilibrium, and the planet is slowly cooling down on geologic timescales. Human extraction taps a minute fraction of the natural outflow, often without accelerating it.
Even though geothermal power is globally sustainable, extraction must still be monitored to avoid local depletion. Over the course of decades, individual wells draw down local temperatures and water levels until a new equilibrium is reached with natural flows. The three oldest sites, at Larderello, Wairakei, and the Geysers have experienced reduced output because of local depletion.
Heat and water, in uncertain proportions, were extracted faster than they were replenished. If production is reduced and water is re injected, these wells could theoretically recover their full potential. Such mitigation strategies have already been implemented at some sites. The extinction of several geyser fields has also been attributed to geothermal power development.
Essay # 11. Effect of Geothermal Energy on Environment :
Fluids drawn from the deep earth carry a mixture of gases, notably carbon dioxide (CO 2 ), hydrogen sulphide (H 2 S), methane (CH 4 ) and ammonia (NH 3 ). These pollutants contribute to global warming, acid rain, and noxious smells if released.
Existing geothermal electric plants emit an average of 122 kilograms (269 lb) of CO 2 per megawatt-hour (MW-h) of electricity, a small fraction of the emission intensity of conventional fossil fuel plants. Plants that experience high levels of acids and volatile chemicals are usually equipped with emission-control systems to reduce the exhaust.
In addition to dissolved gases, hot water from geothermal sources may hold in solution trace amounts of toxic chemicals such as mercury, arsenic, boron, and antimony. These chemicals precipitate as the water cools, and can cause environmental damage if released. The modern practice of injecting cooled geothermal fluids back into the Earth to stimulate production has the side benefit of reducing this environmental risk.
Direct geothermal heating systems contain pumps and compressors, which may consume energy from a polluting source. This parasitic load is normally a fraction of the heat output, so it is always less polluting than electric heating. However, if the electricity is produced by burning fossil fuels, then the net emissions of geothermal heating may be comparable to directly burning the fuel for heat.
For example, a geothermal heat pump powered by electricity from a combined cycle natural gas plant would produce about as much pollution as a natural gas condensing furnace of the same size. Therefore the environmental value of direct geothermal heating applications is highly dependent on the emissions intensity of the neighbouring electric grid.
Plant construction can adversely affect land stability Enhanced geothermal systems can trigger earthquakes as part of hydraulic fracturing.
Geothermal has minimal land and freshwater requirements. Geothermal plants use 3.5 square kilometres (1.4 sq mi) per gigawatt of electrical production (not capacity) versus 32 and 12 square kilometres (4.6 sq mi) for coal facilities and wind farms respectively. They use 20 litres (5.3 US gal) of freshwater per MW-h versus over 1,000 litres (260 US gal) per MW-h for nuclear, coal, or oil.
Related Articles:
- Essay on the Renewable Sources of Energy (The Best One) | Energy Management
- Essay on Solar Energy: Top 6 Essays | India | Energy Management
- Essay on Ocean Thermal Energy: Top 6 Essays | Energy Management
- Essay on Biomass: Top 7 Essays | India | Bio Energy | Energy Management
Energy Management , Essay , Essay on Geothermal Energy , Geothermal Energy
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
- DOI: 10.1016/j.energy.2024.132134
- Corpus ID: 270630032
The effect of active and passive battery thermal management systems on energy consumption, battery degradation, and carbon emissions of an electric vehicle
- M. A. Bamdezh , G. Molaeimanesh
- Published in Energy 1 June 2024
- Environmental Science, Engineering
45 References
Analysis of ternary hybrid nanofluid in microchannel-cooled cylindrical li-ion battery pack using multi-scale multi-domain framework, a numerical study of thermal management of lithium-ion battery with nanofluid, the electro-thermal equalization behaviors of battery modules with immersion cooling, multi-objective optimization of multi-channel cold plate under intermittent pulsating flow by rsm and nsga-ⅱ for thermal management of electric vehicle lithium-ion battery pack, the performance investigation and optimization of reciprocating flow applied for liquid-cooling-based battery thermal management system, heat dissipation performance research between drop contact and immersion contact of lithium-ion battery cooling, thermal performance assessment for an array of cylindrical lithium-ion battery cells using an air-cooling system, multi-objective optimization of a sandwich rectangular-channel liquid cooling plate battery thermal management system: a deep-learning approach, hybrid single-phase immersion cooling structure for battery thermal management under fast-charging conditions, estimate long-term impact on battery degradation by considering electric vehicle real-world end-use factors, related papers.
Showing 1 through 3 of 0 Related Papers
Laird Performance Materials
EMI Shielding and Thermal Management Solutions Brochure

Laird's new booklet helps design engineers explore innovations in the material sciences which can overcome complex EMI mitigation and growing heat transfer issues. Laird™ brand solutions are used in more than one billion products. Each is engineered to tame unwanted waste energy, curb excessive thermal loads, or both. Resolving these hurdles leads to faster compliance, despite encountering design roadblocks exacerbated by faster, higher power, and more densely packed components.
- Share full article
Advertisement
Supported by
Guest Essay
The Heat Wave Scenario That Keeps Climate Scientists Up at Night

By Jeff Goodell
Mr. Goodell is the author of “The Heat Will Kill You First: Life and Death on a Scorched Planet.”
On a recent Thursday evening, a freakish windstorm called a derecho (Spanish for “straight ahead”) hit Houston, a city of more than two million people that also happens to be the epicenter of the fossil fuel industry in America.
In a matter of minutes, winds of up to 100 miles per hour blew out office building windows, uprooted trees and toppled electric poles and transmission towers. Nearly a million households lost power. Which meant that not only was there no light; there was no air-conditioning. The damage from the storm was so extensive that, five days later, more than 100,000 homes and businesses were still marooned in the heat and darkness.
Luckily, the day the derecho blew in, the temperature in Houston, a city infamous for its swampy summers, was in the low to mid-80s. Hot, to be sure, but for most healthy people, not life-threatening. Of the at least eight deaths reported as a result of the storm, none were from heat exposure.
But if this storm had arrived several days later, perhaps over the Memorial Day weekend, when the temperature in Houston hit 96 degrees, with a heat index as high as 115, it might have been a very different story. “The Hurricane Katrina of extreme heat” is how Mikhail Chester, director of the Metis Center for Infrastructure and Sustainable Engineering at Arizona State University, once put it to me, echoing the memory of the catastrophic 2005 hurricane that struck Louisiana, devastated New Orleans and killed more than 1,300 people.
Most people who died in Louisiana during Katrina died from drownings, injuries or heart conditions. But Dr. Chester was using Katrina as a metaphor for what can happen to a city unprepared for an extreme climate catastrophe. In New Orleans, the levee system was overwhelmed by torrential rains ; eventually, 80 percent of the city was underwater.
What if, instead, the electricity goes out for several days during a blistering summer heat wave in a city that depends on air-conditioning?
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Journal of Materials Chemistry A
In-situ switchable nanofiber films based on photoselective asymmetric assembly towards year-round energy saving.
Buildings thermal management consumes 51% of the world’s energy use. Optimization of the energy use can be potentially achieved via daylight harvesting and radiative cooling approaches, yet their simultaneous utilization under static conditions is challenging due to opposite operation principles. Here, an in-situ switchable photoselective polymer (PSP) material was prepared by sequential electrospinning of light-reflecting and light-absorbing layers made of contrasting polymer nanofibers. As-prepared PSP material exhibited high solar light reflectance of 97.7% and high broadband emissivity of 94.9% resulting in radiative cooling power 111.1 W·m-2. Such “cooling” state of the PSP film can be easily switched to a “heating” one via impregnation of the index matching liquid that suppresses scattering at the film-air interface and reduces solar band reflectivity of the film. Thanks to the highly porous structure of the designed PSP film, its switching takes less than 5 min and allows to achieve an integrated solar absorbance of ~95.6% resulting in the estimated heating power of 781.6 W·m-2. Performed numerical calculations further supported high potential of the developed PSP film for thermal management of the buildings located at high latitudes with the energy savings up to 89.74 GJ m-2·y-1 and reduced CO2 emissions down to 21.69 t.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers
Supplementary files
- Supplementary movie MP4 (2864K)
- Supplementary movie MP4 (2170K)
- Supplementary information PDF (1723K)
Article information
Download citation, permissions.
A. Liuqian, J. Ma, P. Wang, J. Yao, A. Kuchmizhak, H. Xu and W. Wang, J. Mater. Chem. A , 2024, Accepted Manuscript , DOI: 10.1039/D4TA03558E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

IMAGES
VIDEO
COMMENTS
Heat is the flow of thermal energy. A whole branch of physics, thermodynamics, deals with how heat is transferred between different systems and how work is done in the process (see the 1ˢᵗ law of thermodynamics ). In the context of mechanics problems, we are usually interested in the role thermal energy plays in ensuring conservation of energy.
Gas Constant. Solar Car. Thermal Energy. Alpha Decay. Scintillation Counter. Special Theory Of Relativity. Shearing Stress. Thermal energy is a kind of energy and it is generated when the temperature rises. Thermal energy is directly proportional to the change in temperature of the object.
Thermal energy storage is a technology that stocks thermal energy by heating or cooling a storage medium so that the stored energy can be used at a later time for heating and cooling applications and power generation. History. Thermal Energy was discovered by a man named James Joule in the 1840's. Careful experiments showed that the temperature ...
The heat is thermal energy that flows from the warmer areas to the cooler areas, and the thermal energy is the total of all kinetic energies within a given system. The temperature is the degree of hotness or coldness of a body, It is measured by three scales of measurement which are Fahrenheit, Celsius, and Kelvin and it is the average kinetic energy within a given object.
Thermal energy is the energy in an object or system due to the movement of its molecules and atoms. When the particles are moving quickly, the object or system has a lot of thermal energy. When ...
Example 3.5.1 3.5. 1. A 1.2kg block of lead at a temperature of 80oC 80 o C is placed within an insulated container containing 0.6kg of water at a temperature of 20oC 20 o C. The block warms the water as the water cools the lead, eventually bringing them both to a common temperature. Find this equilibrium temperature.
1.5: Heat Transfer, Specific Heat, and Calorimetry Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. As we learned earlier in this chapter, heat transfer is the movement of energy from one place or material to another as a result of a difference in temperature.
Thermal Energy Essay. Heat is thermal energy being transferred from one place to another, because of temperature changes. This can take place by three processes. These three processes are known as conduction, convection, and radiation. When we place two objects with different temperatures in contact with each other, the heat from the hotter ...
thermal energy essay. Lab #7: Cold weather Energy. Check the price for your assignment. Get a 100% Plagiarism-Free Essay. Temperature is heat energy getting transferred in one place to an additional, because of heat changes. This may take place simply by three techniques. These three processes will be known as bail, convection, and radiation ...
Energy Definition. In science, energy is the ability to do work or heat objects. It is a scalar physical quantity, which means it has magnitude, but no direction. Energy is conserved, which means it can change from one form to another, but isn't created or destroyed. There are many different types of energy, such as kinetic energy, potential ...
Block 1. January 5, 2015. Thermal Energy. Thermal energy is the energy a substance or system has related to its temperature. This means the energy of moving or vibrating molecules. Atoms and molecules are always in motion. Generally the motion of thermal energy cannot be seen, but instead the effects it has on the substance can be seen or felt.
Thermal energy is the energy a substance or system has related to its temperature. This means the energy of moving or vibrating molecules. Atoms and molecules are always in motion. ... The Effect Of Heat On The Heat Essay. Heat is a form of energy that is transferred between two substances at different temperatures. The flow of the energy is ...
Thermal energy storage deals with the storage of energy by cooling, heating, melting, solidifying a material; the thermal energy becomes available when the process is reversed [5]. Thermal energy storage using phase change materials have been a main topic in research since 2000, but although the data is quantitatively enormous.
Thermal energy storage: An overview of papers published in Applied Energy 2009-2018. March 2021. Applied Energy 285:116397. DOI: 10.1016/j.apenergy.2020.116397. Authors: J. Yan. Xiaohu Yang. Xi ...
This essay shows that heat is very important to nearly everything in life and how it is used nearly everywhere. 12/12/11. "Heat energy (or just heat) is a form of energy which transfers among particles in a substance (or system) by means of kinetic energy of those particles. In other words, under kinetic theory, the heat is transferred by ...
Thermal Energy Essay Questions (Test!!!!) Explain why putting a dented ping pong ball in a pot of boiling water will help remove the dent? Click the card to flip 👆. as air inside the ball is heated, it expands and pushes outward on the dented ball. often a dented ping pong ball can be put back in a rounded shape this way.
Vol. 55, Issue IV, 2012. THERMAL ENERGY STORAGE: AN OVERVIEW. Lavinia Gabriela SOCACIU. Abstract: Nowadays, as global warming is becoming one of the most urgent problems in the world, we. need to ...
Essay # 1. Introduction to Ocean Thermal Energy: The ocean can produce two types of energy thermal energy from the sun's heat and mechanical energy from the tides and waves. Oceans cover more than 70% of Earth's surface, making them the world's largest solar collectors. The sun's heat warms the surface water a lot more than the deep ...
Energy is the ability to do work, it is the "power that comes from the use of physical or chemical resources.". In your home energy can come in many forms such as heat, light, and even electricity. The problem is some of us, meaning people, in general, know little to anything about the word energy itself. When we think about energy, we ...
Essay On Kinetic Energy 921 Words | 4 Pages. In thermal energy or heat energy is how fast the particles of something are moving. The more temperature you have, the more thermal energy you have which means the particles will be much faster. A hot cup of coffee has lots of thermal energy unlike a cold beverage.
500 Words Essay On Solar Energy. Solar energy is the energy which the earth receives from the sun which converts into thermal or electrical energy. Moreover, solar energy influences the climate of the earth and weather to sustain life. It has great potential which we must use to our advantage fully.
Essay # 5. Geothermal Electricity: As per the International Geothermal Association (IGA) sources, about 10,715 MW of geothermal power in 24 countries is online. In 2010, the United States led the world in geothermal electricity production with 3,086 MW of installed capacity from 77 power plants.
Semantic Scholar extracted view of "The effect of active and passive battery thermal management systems on energy consumption, battery degradation, and carbon emissions of an electric vehicle" by M. A. Bamdezh et al. ... Search 219,228,986 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.1016/j.energy.2024.132134;
Thermal Energy Conversion Essay. The demand for an efficient renewable energy source is a driving force in ongoing research. Thermal energy conversion is one such potential source that is under constant investigation and has endless avenues of possibility. The two requirements of energy production are efficiency and renewability.
Laird's new booklet helps design engineers explore innovations in the material sciences which can overcome complex EMI mitigation and growing heat transfer issues. Laird™ brand solutions are used in more than one billion products. Each is engineered to tame unwanted waste energy, curb excessive thermal loads, or both.
In June 2021, a heat wave led to in nearly 900 excess deaths in the Pacific Northwest. And in 2010, an estimated 56,000 Russians died during a record summer heat wave.
Buildings thermal management consumes 51% of the world's energy use. Optimization of the energy use can be potentially achieved via daylight harvesting and radiative cooling approaches, yet their simultaneous utilization under static conditions is challenging due to opposite operation principles. Here, an in Journal of Materials Chemistry A HOT Papers