An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A case report of lung metastasis in a cervical cancer presenting as a consolidation
Saeed abughazaleh , md, mohammad tarawneh , md, hamza alzghoul , md, saqr alsakarneh , md, othman saleh, wasey ali yadullahi mir , md.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected] [email protected]
Received 2023 Sep 16; Revised 2023 Nov 15; Accepted 2023 Nov 22; Collection date 2024 Mar.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Cervical cancer is a preventable cancer in the United States. We discuss a case of a 43-year-old woman who presented with signs and symptoms of Cerebrovascular accident (CVA) as well as shortness of breath and chest tightness. Upon investigation, it was concluded that she had developed multiple brain infarcts, pulmonary embolism, and deep venous thrombosis in both lower extremities. However, after her pulmonary symptoms worsened, further investigations revealed an uncommon occurrence of infiltrative lung metastasis. This finding was particularly surprising as she had recently been diagnosed with squamous cell carcinoma of the cervix. It is important to note that patients who have not undergone regular cervical cancer screening can remain without symptoms until the disease has reached an advanced stage, as is the case with this patient. Various screening methods, such as Pap smear cytology, human papillomavirus (HPV) DNA testing, and visual inspection tests, are available to detect and prevent cervical cancer.
Keywords: Cervical cancer, Lung consolidation, CT chest, Metastatic, Pneumonia
Introduction
In 2020, cervical carcinoma was responsible for 4272 cancer-related deaths in women, making it a significant cause of mortality according to the CDC [1] . The main risk factors for cervical neoplasia include specific types of human papillomavirus (HPV), such as types 16, 18, 31, 33, and 45 [2] . However, due to age-appropriate screening measures, the incidence and mortality rates of cervical cancer in the United States have decreased by 70% since the 1950s [3] . Primary prevention through HPV vaccination and effective treatment of preinvasive lesions have also contributed to this decline [4] . Nevertheless, cervical cancer remains a major health issue in underdeveloped countries.
While early-stage or locally advanced cervical cancer has a better prognosis compared to advanced stages, where the median survival is 8-13 months, distant metastasis can occur. The lungs are the most common site of metastasis in cervical cancer, with an incidence ranging from 4.16% to 7.7% [5] . In this case report, we present the case of a patient with cervical cancer who initially exhibited signs and symptoms resembling a cerebral vascular accident. Further examination revealed lung metastasis characterized by patchy opacities and consolidations in both lung bases. The patient was ultimately diagnosed with squamous cell carcinoma of cervical cancer with lung metastasis.
Case presentation
A 43-year-old woman with no notable medical history, who had not undergone age-appropriate cancer screening, arrived at the Emergency Department complaining of symptoms consistent with a cerebral vascular accident, such as visual loss, dysarthria, and weakness in the right face and arm. Additionally, she experienced a gradual increase in shortness of breath during physical activity, accompanied by chest tightness. The patient was also hypoxic (low oxygen levels) and had an elevated heart rate. She denied experiencing any vaginal bleeding or discharge.
The physical examination shows bilateral crackles, tachycardia, and breathlessness at rest. The blood work showed a slightly elevated white blood cell count (13.3 × 10 3 /uL), along with hypoxemia and low hemoglobin (8.7 g/dL). Moreover, the ABG was as the following: PH = 7.47, pCO2 = 35.6 mm Hg, HCO3 = 24 mmol/L, and pO2= 80.0 mm Hg ( Table 1 ). A brain CT scan revealed the presence of multiple acute and subacute infarcts, indicating an embolic phenomenon. A chest computed tomography angiography (CTA) showed an extensive sub-massive pulmonary embolism on the right side, affecting the main, lobar, segmental, and subsegmental arteries ( Fig. 1 ). A duplex venous ultrasound (US) examination identified deep venous thrombosis in both lower extremities, and an echocardiogram indicated right ventricular dilation with wall hypokinesia, consistent with right ventricular strain. Additionally, a patent foramen ovale was detected. The patient was initiated on therapeutic anticoagulation and broad-spectrum antibiotics. Following the administration of anticoagulant medication, the patient began experiencing vaginal bleeding. To further investigate this complication, a CT scan of the abdomen and pelvis was performed. The scan revealed an enlarged uterus accompanied by bilateral pelvic lymphadenopathy. A transvaginal ultrasound showed the presence of a 6.4 cm cervical mass, with a potential invasion of the bladder wall measuring approximately 2.2 cm. A subsequent biopsy confirmed the diagnosis of cervical squamous cell carcinoma. Due to the patient's significant burden of venous thrombosis and limited pulmonary circulation reserve, an inferior vena cava filter was inserted. After nearly 2 weeks of hospitalization, the patient was discharged and prescribed Apixaban along with home oxygen therapy of 2L via a nasal cannula. The patient was advised to follow up with imaging studies in 1 month.
Biochemical tests.
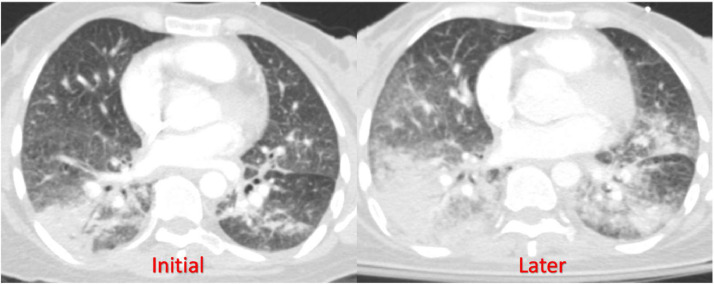
CT Chest axial view showing bilateral lung infiltrate progression over 1 month.
After a 2-week period following discharge, the patient was readmitted to the hospital due to a deterioration in her shortness of breath and an increased need for oxygen support. Initially, she required a high-flow nasal cannula to maintain her oxygen saturation above 94%. A chest CTA was performed, which revealed a worsening of bilateral multifocal infiltrates in her lungs. Moreover, new findings of nodules measuring up to 4 mm were observed in the right upper lobe, indicating a resolution of her prior pulmonary embolism ( Fig. 2 ).
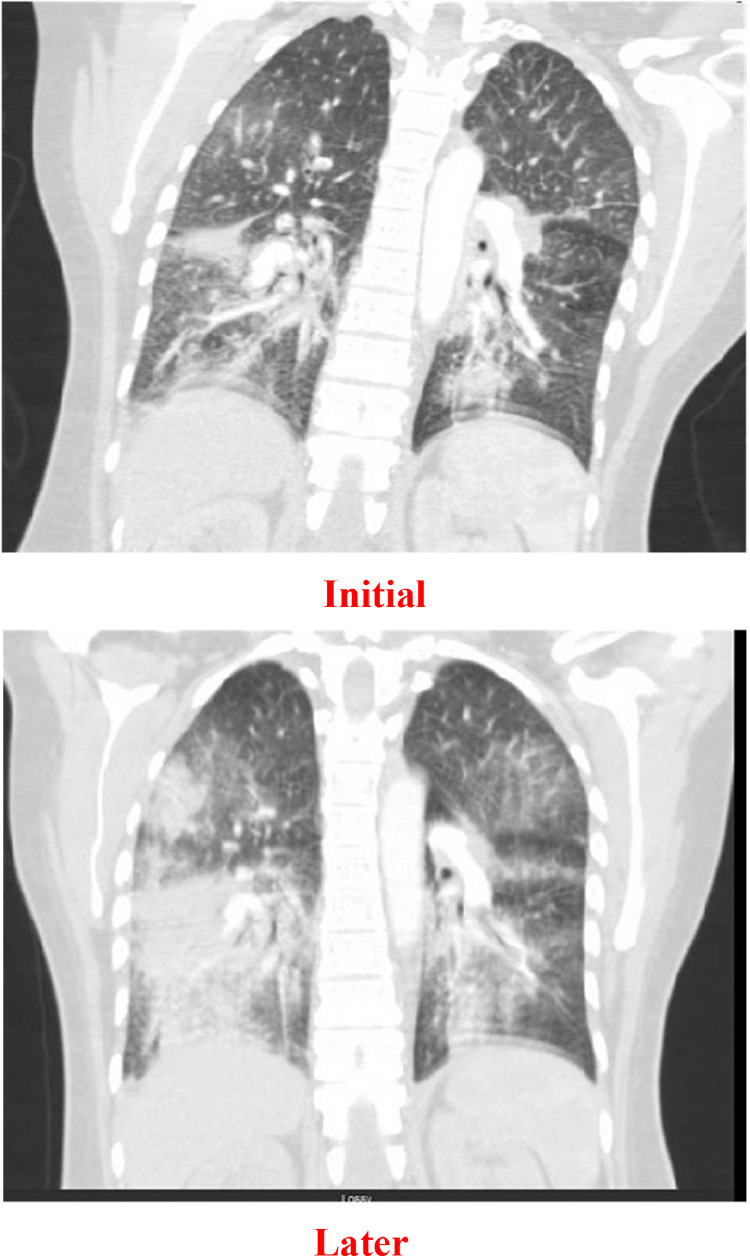
CT Chest coronal view showing bilateral lung infiltrate progression over 1 month.
The patient was transferred to our center's intensive care unit and immediately started on broad-spectrum antibiotics to address potential infections caused by various types of bacteria. Tests for COVID-19, RSV, and influenza A/B were performed, all of which returned negative results. Blood cultures consistently showed no growth of microorganisms. However, her respiratory distress worsened, necessitating intubation 1 day after her transfer. To further investigate the cause, a bronchoalveolar lavage was conducted, which confirmed the presence of malignancy consistent with squamous cell carcinoma. The lavage tested positive for markers CK4 and CK5/6, which establishes the diagnosis. Despite all interventions, the patient's blood pressure continued to decrease, requiring the administration of 3 vasopressors to maintain a mean arterial pressure above 65 mm Hg. The hypotension was likely due to right ventricular failure, considering her known patent foramen ovale. Given the worsening condition and in accordance with the decision made by her healthcare proxy, the patient's care was shifted to comfort measures only, and she was voluntarily removed from mechanical ventilation (extubated). Fig. 3
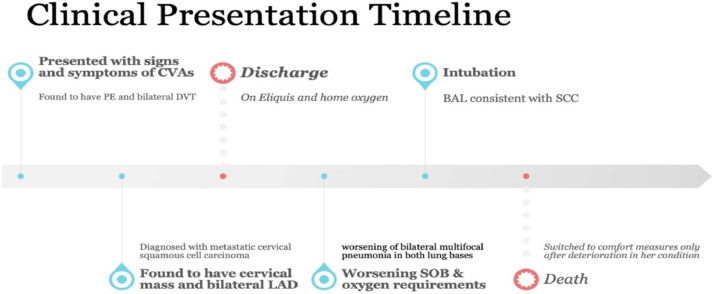
Timeline of the clinical presentation.
Cervical cancer is the growth of abnormal cells in the lining of the cervix. It is typically spreads either locally to nearby tissues such as the vagina, uterus, and pelvic cavity, or distant areas like the lungs, para-aortic lymph nodes, and bones [6] , [7] , [8] . Lung metastasis is considered less common in squamous cell carcinoma of the cervix, accounting for less than 5% of cases. Also, the incidence of lung metastasis varies depending on the stage of the cancer, with higher rates observed in advanced stages. For instance, it occurs in approximately 3.2% of stage I cases, 5.0% of stage II cases, 9.4% of stage III cases, and 20.9% of stage IV cases [9] .
Lung metastasis can present in different ways, including solitary or multiple nodules dispersed across both lungs, lymphangitic carcinomatosis, tumor emboli, endobronchial metastasis, and pleural effusion [10] . In this case, we observed rapidly progressing bilateral lung infiltrates, presenting as a consolidative pattern resembling pneumonia. Despite antibiotic therapy, further investigation using bronchoalveolar lavage (BAL) has confirmed the presence of squamous cell carcinoma (stage 4 cervical cancer with metastases in both lungs). However, it is important to differentiate between metastatic cervical squamous cell carcinoma and primary pulmonary squamous cell carcinoma. The use of the p16 marker can aid in this distinction, as p16 overexpression is typically seen in HPV-related cervical cancer [11] . Unfortunately, this specific test was not conducted for our patient. Moreover, one of the pathological patterns of cell proliferation that resemble infectious pneumonia on imaging is the lepidic growth pattern. It refers to the development of tumor cells along intact alveolar walls [12] . Although we did not have pathological confirmation of lepidic growth in the lung, the rapid progression of the metastases over a month in a pneumonia-like manner and the presence of cancer cells in the lavage fluid strongly indicate lepidic growth. It is worth noting that lepidic growth has not been previously described in cervical cancer.
Many cervical cancer patients lack sufficient screening, with over half not having had a Pap smear in the last 5 years [13] . Detection methods include colposcopy, fluorescence spectroscopy, HPV DNA testing, and nuclear aneuploidy detection [14] . Advanced cases may require pelvic MRI and PET-CT [14] . The 2018 International Federation of Gynecology and Obstetrics staging system emphasizes precise tumor size and lymph node details for accurate clinical staging [15] . Enhancing screening rates is crucial, achieved through proactive patient invitations and utilizing urgent care visits for screening, particularly for those less likely to adhere to recommended guidelines. This proactive approach increases the likelihood of early-stage cervical cancer detection [ 16 , 17 ].
Regarding treatments, patients diagnosed with locally advanced cervical cancer could be suitable candidates for primary chemoradiation, which commonly includes cisplatin. However, for those with stage IIA1 disease, surgical therapy with lymphadenectomy may be a possible option instead of primary chemoradiation. Surgery alone is unlikely to result in a cure for people with stage IIB through IVA disease [18] . In stage IVB disease of cervical cancer, the cancer extends via the lymphatic system to the pelvic and para-aortic lymph nodes, or it travels through the bloodstream to distant organs such as the lungs, liver, bones, and other remote sites [19] . In cases where experiencing oligometastatic illness that may be adequately treated locally, definitive radiation or surgical excision of metastases, in conjunction with systemic therapy, could be considered [20] . In circumstances when patients have recurrent or chronic illness localized to the pelvic region after earlier radiation treatments, a surgery known as pelvic exenteration may still be used for treatment.
Currently, there is ongoing research into a new treatment approach that involves the addition of pembrolizumab to a first-line platinum-based regimen, with or without bevacizumab [21] . While immunotherapy is typically well tolerated, it can cause toxicities in a variety of organ systems, potentially resulting in treatment breaks or termination [22] . Furthermore, the US Food and Drug Administration (FDA) authorized Tisotumab for release in September 2021. The FDA approval was based on the findings of a single-arm experiment with 101 participants. Tisotumab studies are still ongoing, with the goal of gathering more information and knowledge of its efficacy and safety [23] . However, in our case, the cancer has been diagnosed in an advanced stage, and the patient's chances of survival are quite low even with current available treatments.
In summary, our case report underscores the diagnostic challenges in identifying advanced cervical cancer with lung metastasis, initially mistaken for pneumonia. The rarity of this metastatic pattern highlights the need for a comprehensive diagnostic approach. Limited therapeutic options for advanced cervical cancer emphasize the importance of palliative care, prompting ongoing research for improved outcomes. This case underscores the critical importance of effective screening strategies for early detection in such cases.
Patient consent
Informed consent was obtained from the patient’s family.
Acknowledgments: No internal or external sources of funding were obtained for this case report.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- 1. USCS Data Visualizations - CDC [Internet]. [cited 2023 August 2]. Available from: https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/value,1,1,73,1,3,1 . [accessed 15.08.23].
- 2. Meir H, Kenter G, Burggraaf J, Kroep J, Welters M, Melief C, et al. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anticancer Agents Med Chem. 2014;14(2):190–203. doi: 10.2174/18715206113136660372. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Heal. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. http://www.thelancet.com/article/S2214109X19304826/fulltext [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. https://pubmed.ncbi.nlm.nih.gov/17494925/ [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43. doi: 10.3802/jgo.2016.27.e43. https://pmc/articles/PMC4864519/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Sawin SW, Aikins JK, Van Hoeuen KH, Prioleau Y, Morgan MA, Mikuta JJ. Recurrent squamous cell carcinoma of the cervix with pulmonary lymphangitic metastasis. Int J Gynecol Obstet. 1995;48(1):85–90. doi: 10.1016/0020-7292(94)02250-x. https://pubmed.ncbi.nlm.nih.gov/7698389/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Panek G, Gawrychowski K, Sobiczewski P, Derlatka P, Danska-Bidzinska A, Gmyrek L, et al. Results of chemotherapy for pulmonary metastases of carcinoma of the cervix in patients after primary surgical and radiotherapeutic management. Int J Gynecol Cancer. 2007;17(5):1056–1061. doi: 10.1111/j.1525-1438.2007.00879.x. https://pubmed.ncbi.nlm.nih.gov/17466044/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Shin MS, Shingleton HM, Partridge EE, Nicolson VM, Ho KJ. Squamous cell carcinoma of the uterine cervix. Patterns of thoracic metastases. Invest Radiol. 1995;30(12):724–729. doi: 10.1097/00004424-199512000-00006. https://pubmed.ncbi.nlm.nih.gov/8748186/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998-2002. Obstet Gynecol. 2007;109(2 PART 1):360–370. doi: 10.1097/01.AOG.0000254165.92653.e8. https://journals.lww.com/greenjournal/Fulltext/2007/02000/Cervical_Cancer_Incidence_in_a_Prevaccine_Era_in.20.aspx [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Avdalovic M, Chan A. Thoracic manifestations of common nonpulmonary malignancies of women. Clin Chest Med. 2004;25(2):379–390. doi: 10.1016/j.ccm.2004.01.009. https://pubmed.ncbi.nlm.nih.gov/15099897/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Wang CW, Wu TI, Yu CT, Wu YC, Teng YH, Chin SY, et al. Usefulness of p16 for differentiating primary pulmonary squamous cell carcinoma from cervical squamous cell carcinoma metastatic to the lung. Am J Clin Pathol. 2009;131(5):715–722. doi: 10.1309/AJCPTPBC6V5KUITM. https://pubmed.ncbi.nlm.nih.gov/19369633/ [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Young TJ, Salehi-Rad R, Ronaghi R, Yanagawa J, Shahrouki P, Villegas BE, et al. Predictors of invasiveness in adenocarcinoma of lung with lepidic growth pattern. Med Sci. 2022;10(3):34. doi: 10.3390/medsci10030034. https://pmc/articles/PMC9326548/ [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Coleman DV, Poznansky JJR. Review of cervical smears from 76 women with invasive cervical cancer: cytological findings and medicolegal implications. Cytopathology. 2006;17(3):127–136. doi: 10.1111/j.1365-2303.2006.00310.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2303.2006.00310.x [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21139 [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Grigsby PW, Massad LS, Mutch DG, Powell MA, Thaker PH, McCourt C, et al. FIGO 2018 staging criteria for cervical cancer: impact on stage migration and survival. Gynecol Oncol. 2020;157(3):639–643. doi: 10.1016/j.ygyno.2020.03.027. https://pubmed.ncbi.nlm.nih.gov/32248993/ [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Batal H, Biggerstaff S, Dunn T, Mehler PS. Cervical cancer screening in the urgent care setting. J Gen Intern Med. 2000;15(6):389–394. doi: 10.1046/j.1525-1497.2000.08001.x. https://link.springer.com/article/10.1046/j.1525-1497.2000.08001.x [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Forbes CA, Jepson RG, Martin-Hirsch PP. Interventions targeted at women to encourage the uptake of cervical screening. Cochrane Database Syst Rev. 2002;(3) doi: 10.1002/14651858.CD002834. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002834/full [Internet] [cited 2023 July 9] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Yamashita H, Okuma K, Kawana K, Nakagawa S, Oda K, Yano T, et al. Comparison between conventional surgery plus postoperative adjuvant radiotherapy and concurrent chemoradiation for FIGO stage IIB cervical carcinoma: a retrospective study. Am J Clin Oncol. 2010;33(6):583–586. doi: 10.1097/COC.0b013e3181cae5b7. https://pubmed.ncbi.nlm.nih.gov/20065848/ [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Gallup DG. The spread and staging of cervical cancer. Glob Libr Women’s Med. 2008:117–131. http://www.glowm.com/section-view/heading/The Spread and Staging of Cervical Cancer/item/231 [Internet] [cited 2023 July 22] Available from: [ Google Scholar ]
- 20. Chopra S, Mangaj A, Sharma A, Tan LT, Sturdza A, Jürgenliemk-Schulz I, et al. Management of oligo-metastatic and oligo-recurrent cervical cancer: a pattern of care survey within the EMBRACE research network. Radiother Oncol. 2021;155:151–159. doi: 10.1016/j.radonc.2020.10.037. https://pubmed.ncbi.nlm.nih.gov/33144247/ [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. https://www.nejm.org/doi/full/10.1056/NEJMoa2112435 [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(4):387–405. doi: 10.6004/jnccn.2022.0020. https://pubmed.ncbi.nlm.nih.gov/35390769/ [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Coleman RL, Lorusso D, Gennigens C, González-Martín A, Randall L, Cibula D, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–619. doi: 10.1016/S1470-2045(21)00056-5. https://pubmed.ncbi.nlm.nih.gov/33845034/ [Internet] [cited 2023 July 22] Available from: [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (779.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Survival Analysis of Cervical Cancer Patients: A Case Study of Bhutan
Affiliations.
- 1 Department of Mathematics, Faculty of Science, Mahidol University, Rama 6 Rd, Bangkok, Thailand.
- 2 Centre of Excellence in Mathematics, Perdo, Thailand.
- PMID: 34582671
- PMCID: PMC8850885
- DOI: 10.31557/APJCP.2021.22.9.2987
Objective: The purpose of the study is to identify the risk factors such as age, the stage of patients based on the International Federation of Gynecology and Obstetrics system (FIGO stage) and treatment type, and their effect on the survival of cervical cancer patients receiving treatments at Jigme Dorji Wangchuck National Referral Hospital (JDWNRH) in Bhutan between January 2014 and December 2019.
Methods: In this retrospective study, all 357 women diagnosed with cervical cancer were included. Kaplan-Meier model was applied to estimate survival, and the log-rank test was performed to compare survival distributions between subgroups stratified by each of the risk factors. Baseline demographics, cervical cancer stages, and treatment options were analyzed as factors and predictors of survival by Cox proportional hazards model.
Results: The overall estimated 1- to 5-year survival rates are 82.1% (95% CI: 77.8-86.7), 75.6% (70.4-81.1), 65.2% (58.2-73.0), 62.3% (54.7-70.9) and 55.4% (44.9-68.3). The results reveal that age group, FIGO stage, treatment, and frequency of hospital visits are significant factors affecting the survival of cervical cancer patients in Bhutan. Patients aged >45 years increases the risk of dying (HR: 2.1, 95% CI: 1.2-3.9) compared to the young age group (≤45 years). Treatment types other than surgery only are significantly associated with an increased risk of mortality in patients with cervical cancers. The more frequency of hospital visits also reduces the risk of dying (HR: 0.1, 95% CI: 0-0.3). FIGO stage IV is the most significant risk factor for mortality with a hazard ratio of 6 (95% CI: 2.1-17.6).
Conclusion: The five-year survival rate of cervical cancer patients in this study was low. Late diagnosis of cervical cancer appears to be mainly associated with a higher risk of dying. The results provide valuable information for further research and policymaking in the prevention and management of cervical cancer. <br />.
Keywords: Cervical cancer; Cox proportional hazards model; Kaplan-Meier model; Survival Analysis.
- Age Distribution
- Bhutan / epidemiology
- Middle Aged
- Neoplasm Staging
- Proportional Hazards Models
- Retrospective Studies
- Risk Factors
- Survival Analysis
- Survival Rate
- Uterine Cervical Neoplasms / mortality*
- Uterine Cervical Neoplasms / pathology
- Uterine Cervical Neoplasms / therapy
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 10, Issue 2
- Eradicating tumor in a recurrent cervical cancer patient with autologous tumor-infiltrating lymphocytes and a modified lymphodepleting regimen
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Jing Guo 1 ,
- Ning Luo 1 ,
- Guihai Ai 1 ,
- Weihong Yang 1 ,
- Jihui Zhu 1 ,
- Caixia Li 1 ,
- Rong Chen 1 ,
- Changbao Zhang 2 ,
- Shupeng Liu 1 , 3 ,
- Huajun Jin 4 and
- Zhongping Cheng 1 , 3
- 1 Department of Obstetrics and Gynecology , Shanghai Tenth People's Hospital, School of Medicine, Tongji University , Shanghai , People's Republic of China
- 2 Department of Radiology , Shanghai Tenth People's Hospital, School of Medicine, Tongji University , Shanghai , People's Republic of China
- 3 Gynecologic Minimally Invasive Surgery Research Center , School of Medicine, Tongji University , Shanghai , People's Republic of China
- 4 Gencells Therapeutics , Shanghai , People's Republic of China
- Correspondence to Dr Zhongping Cheng; mdcheng18{at}263.net ; Dr Huajun Jin; hj-jin{at}hotmail.com ; Dr Shupeng Liu; lshup{at}tongji.edu.cn
Tumor-infiltrating lymphocyte (TIL) therapy has shown promising results against several cancers. However, traditional lymphodepleting regimens are severe and represent a major limitation for a more widespread use of TIL. The modified pretreatment strategies may alleviate side effects and demonstrate the persistence of tumor-reactive T cells in the blood. Here, we report a case who was diagnosed recurrent cervical cancer with bladder metastasis. Omitting high dose of IL-2, she received intravenous dose of cyclophosphamide (20 mg/kg) for 3 days, approximately 48 hours before receiving the intravenous infusion of TILs. Half dosage (100 mg) of PD1 antibody was administered with purpose of neutralizing PD1 expressed on T cells surface. She achieved complete response 10 weeks after one-time TILs infusion. Adverse reactions were negligible and safely manageable in a general ward without the need for intervention from intensive care units. Time-course peripheral blood counts and TCR repertoire sequencing demonstrated a robust expansion and long-term persistence of the infused TILs. These results illustrated the potential value of modified lymphodepletion, followed by TILs for the treatment of patients with cervical cancer with local recurrence. Trial registration number, NCT04766320 .
- lymphocytes
- tumor-infiltrating
- case reports
- clinical trials as topic
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/jitc-2021-003887
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Cervical cancer is the fourth most common malignancy in women worldwide. 1 Although cervical cancer may be preventable by human papilloma virus (HPV) vaccine and early detection, 2 the incidence and deaths will increase, if no action is taken. 3 Chemotherapy as well as immunotherapy such as antiprogrammed death 1 receptor (anti-PD1) therapy has shown clinical efficacy. 4 The KEYNOTE-158 trial has contributed to the approval of pembrolizumab in recurrent programmed cell death ligand 1 (PD-L1) positive cervical cancer. 4 Seventy-two trials are recruiting with anti-PD1 antibody in cervical cancer. 5 However, the progress remains poor and better treatments are needed. 6
Tumor-infiltrating lymphocyte (TIL) therapy, first reported in the 1980s, is an emerging cellular therapy with limited application to cervical cancer. TILs are mainly composed of CD3 positive T lymphocytes with a minor subset of natural killer cells, and target tumor cells by their relatively enriched tumor epitope-specific T cell receptors (TCRs). 6 7 The studies, HPV-TIL cell products and LN-145 (Iovance Biotherapeutics), are evaluating two autologous cell therapies, respectively. 8 9 Among 27 patients with metastatic cervical carcinoma who had all relapsed on the standard of treatments-chemotherapy, VEGF-targeted agents, and radiation, the objective response rate with LN-145 was 44.4%. 8 Traditional TIL therapy relies on high-intensity lymphodepleting pretreatment and repeated supplementary injection of high-dose IL-2 to promote TIL expansion in vivo by removing inhibitory cells. 10 11 Such combination of preinfusion and postinfusion treatment, however, widely causes adverse events ranging from grade 1 to grade 5, and sometimes proves to be life-threatening for subjects demonstrating low performance status before the infusion. 11 Hence an adjusted pretreatment regimen for TIL infusion is rational to alleviate its side effects.
Here, we report the clinical findings in a single patient having recurrent cervical cancer with bladder metastasis in a phase I clinical trial using autologous TIL infusion pretreated with a modified lymphodepleting regimen. The characteristic of resected tissue indicated limited lymphocytes infiltrating within tumor area. Notably, postinfusion IL-2 injection was removed from the therapy as well. The patient achieved complete response 10 weeks after TIL infusion with less adverse reactions during the therapy. Time-course peripheral blood counts and TCR repertoire sequencing demonstrated a robust expansion and long-term persistence of the infused TILs in vivo. Our results provide a novel clinical therapeutic option for solid tumors with the use of TIL infusion.
Case presentation
The medical history.
A 52-year-old woman diagnosed with recurrent cervical cancer was referred to our hospital in January, 2021 because of irregular bleeding. She was diagnosed with cervical cancer of stage IB3r based on imaging and underwent radical hysterectomy with pelvic lymphadenectomy in May, 2019. The disease stage was restratified as stage IIIC1p, which was confirmed by pelvic lymphnode involvement via pathological examination. She received four cycles of paclitaxel liposome (175 mg/m 2 on day 1 of each 21-day cycle) and oxaliplatin (130 mg/m 2 on day 1 of each 21-day cycle) from June 13, 2019 to August 15, 2019 and was free from disease. Seventeen months later, the disease recurred in her bladder based on magnetic resonance imaging (MRI). She underwent partial cystectomy followed by one cycle of adjuvant chemotherapy and requested tumor resection to be preserved for cellular therapy ( figure 1A ). All clinical parameters for this case were presented in table 1 . She requested to enroll in the present trial when disease progressed and signed the informed consent form. A timeline of interventions was depicted in figure 1B . Briefly, the patient received baseline assessment when TILs were prepared well. Then she performed lymphodepleting regimens followed by TIL infusion and PD1 antibody administration, and the first assessment happened 42 days after infusion. She did not show any symptoms of fever, or weight loss. Neither did she smoke nor use alcohol. On physical examination, she had no palpable bilateral inguinal nodes or palpable supraclavicular or axillary nodes.
- Download figure
- Open in new tab
- Download powerpoint
Clinical response. (A) Pretreatment course of this patient reported. (B) Scheme of study interventions. (C) Change of the metastatic lesions (yellow areas) before and after TIL infusion in the left bladder. TIL, tumor-infiltrating lymphocyte. PFS, progression-free survival; ICF, informed consent form.
- View inline
Characteristics of the patient
Multiplexed immunofluorescence
Pathological examination of the resected tumor tissue revealed squamous-cell carcinoma, which was consistent with her primary cervical tumor. To evaluate the perspective of tumor immune contexture, we did multiplexed immunofluorescence (mIF) analysis of the resected tumor tissue. Briefly, mIF was performed by staining 4 μm thick formalin-fixed, paraffin-embedded whole tissue sections with standard using a TSA 7-color iplexed immunofluokit (D110071-50T, WiSee Bio), as previously described in other studies. 12 For example, deparaffinized slides were incubated with pan-CK (AE1/AE3) primary antibody (GM351507, Gene Tech) for 30 min, followed by the application of polymeric Horseradish peroxidase-conjugated secondary antibodies (A10011-60, Yuanxibio). IF labeling was developed for a strictly observed 10 min with Alexa Flour 488. The slides were rinsed with washing buffer after each step. These steps were repeated for the following antibodies/fluorescent dyes, in order: anti-CD68 (GM087607, Gene Tech)/TSA520, anti-CD11b (ab133357, Abcam)/TSA520, anti-CD56 (3576, Cell Signaling Technology)/TSA570, anti-CD8 (BX50036, Biolynx)/TSA670, and anti-CD3 (BX50022-C3, Biolynx)/TSA440 for panel 1; and anti-Ki-67 (BX50040, Biolynx)/TSA520, anti-fibronectin (26836, Cell Signaling Technology)/TSA620, anti-FAP (ab207178, Abcam)/TSA570, anti-PD-L1 (13684, Cell Signaling Technology)/TSA670, and anti-CD3 (BX50022-C3, Biolynx)/TSA440 for panel 2. Each slide was then treated with 2 drops of DAPI, washed in distilled water, and manually coverslipped. Slides were air dried, mounted with anti-fade mounting medium, and taken pictures with Aperio Versa 8 tissue imaging system (Leica). The fluorescence-stained slides were scanned using a digital microscopy scanner Pannoramic MIDI tissue imaging system (3DHISTECH, Hungary). Images was analyzed using Indica Halo software. The findings showed fewer CD3 and CD8 positive cells intermingled with pan-CK positive tumor cells ( figure 2A , online supplemental figure S1A , online supplemental table S1 ), while most CD3 positive signals colocalized with the fibroblast activation protein (FAP) positive cells. And the rate of PD-L1 in this tissue was 0.3% ( figure 2B , online supplemental figure S1B , online supplemental table S2 ).
Supplemental material
Multiplex immunofluorescent images of tumor tissue sections. (A) The technique labels seven channels within tumor regions as follows: pan-CK (orange), CD68 (green), CD11b (red), CD56 (yellow), CD8 (purple), CD3 (light blue), DAPI (blue). (B) Seven color demonstrates the spatial distribution of different immune lineages and markers within the stroma: pan-CK (orange), Ki67 (green), FN1 (red), FAP (yellow), PD-L1 (purple), CD3 (deep purple), DAPI (blue). CD, cluster of differentiation; FN1, fibronectin; FAP, fibroblast activation protein; PD-L1, programmed death ligand 1.
Autologous TIL therapy
TILs used in this study were provided by Shanghai Gencells Therapeutics, and manufactured according to standard Good Manufacturing Practice of Medical Products (GMP) procedure. Briefly, the tumor specimen was transferred to a GMP manufacturing facility where it was fragmented mechanically. The tumor fragments were subjected to culture in a pre-Rapid Expansion Protocol (pre-REP) medium comprizing X-VIVO 15 plus rhIL-2 (201-GMP-01M, R&D) (2000 IU/mL), rhIL-7 (207-GMP-01M, R&D) (10 ng/mL) and rhIL-15 (247-GMP-01M, R&D) (10 ng/mL). TILs obtained from the pre-REP culture were incubated for 2 days in a plate precoated with anti-CD3 antibody (317302, Biolegend) and anti-CD28 (MAB342-500, R&D) to become reactivated before they were transferred to G-REX100 harboring a REP culture medium containing X-VIVO 15 plus rhIL-2 (300 IU/mL) for a further linear expansion. The final TIL product for autologous adoptive transfer was prepared as cryopreserved drug product (CDP) containing 1×10 10 cells aliquoted in 100 mL. Before it was released, we characterized cell subtypes of the TIL product by staining cell surface markers on TILs through flow cytometry aiming to detect invigorated T cells ( table 2 , online supplemental figure S2 ). Additional details are provided in online supplemental files 1; 2 .
The characterization of TILs
TCR sequencing+
Total RNA was isolated from the peripheral blood of the recipients using the RNeasy Plus Mini Kit (Qiagen). RNA samples were analyzed by NGS for TRBs using the TCR profiling system. Briefly, a 5’ rapid amplification of cDNA ends (RACE) unbiased amplification protocol was used. 13 One common forward adaptor primer and one reverse primer corresponding to the constant (C) regions of each of the TCR β were designed to facilitate PCR amplification of cDNA sequences in a less biased manner. This protocol uses unique molecular identifiers (UMIs) introduced in the course of cDNA synthesis to eliminate PCR and sequencing errors. Sequencing was performed on an Illumina NovaSeq system with PE150 mode.
The UMI attached to each raw sequence reads were applied to correct PCR and sequencing errors correction and PCR duplicates removing. Map V, D, J and C segments with IMGT and then extract CDR3 regions and assemble clonotype for all clones. The resulting nucleotide and amino acid sequences of CDR3 of TCRβ were determined and those with out-of-frame and stop codon sequences were removed from the identified TCRβ repertoire. We further defined amounts of each TCRβ clonotype by adding numbers of TCRβ clones sharing the same nucleotide sequence of CDR3.
Clinical findings
The patient was the first one enrolled in this study in April, 2021 meeting with inclusion criteria for recurrence of gynecological cancers and measurable lesions. Evaluations using MRI based on the enhanced-T1-weighted (T1WI) sequence showed no other disease except a mass that was 3 cm in diameter in her left bladder (day 10). Before TIL infusion, the patient received intravenous dosing of cyclophosphamide (20 mg/kg of body weight) for 3 days (day 5 –3). Forty-eight hours after the last dosing of cyclophosphamide, the intravenous infusion of TILs of 2×10 10 cells was carried out in a general ward over 60 min (day 0). A PD1 antibody drug (Sintilimab, Innovent) reduced by half dosage (100 mg) as compared with the normal dosage was administered intravenously on the same day (d ay 0) before TIL infusion with purpose of neutralizing cell-surface expressed PD1. A second intravenous injection of 100 mg of sintilimab was given 24 days after the infusion (day 24), as observable PD1 expression increased in CD3 +T cell population, to contain the rising immunosuppressive condition. Six weeks after TIL infusion, T1WI showed remarkable shrinkage of the lesion (yellow arrowheads). According to the Response Evaluation Criteria in Solid Tumors v1.1 (RECIST), 14 a complete response was confirmed 10 weeks after infusion ( figure 1C ).
To evaluate the effect of cyclophosphamide and the side effects of TIL infusion, counts of immune cells and cytokines were assessed using peripheral venous blood samples collected at multiple time points during the course of TIL therapy. Significant decrease of total white blood cell count (WBC), neutrophil count and lymphocyte count were observed after cyclophosphamide administration. Fourteen days after lymphodepleting pretreatment, the total WBC and neutrophil count gradually dropped to minima of 0.99×10 9 /L and 0.18×10 9 /L from 5.29×10 9 /L, and 3.79×10 9 /L, respectively. Both total WBC and neutrophil counts remained at a relatively low level for 16 days ( figure 3A ). The lymphocyte count decreased to minina of 0.28×10 9 /L 6 days after lymphodepletion and increased noticeably from day 1 to day 11. The total WBC, absolute neutrophil and lymphocyte level remained stable from 15 days after infusion ( figure 3B ). Interestingly, the mutually complemented change in CD4+ and CD8+ cell frequencies permanently increased the proportion of the systemic CD8+ T cells of the following blood time points ( figure 4A ), reflecting the activation and long-term persistent effects of TIL infusion.
Change of peripheral blood cells and cytokines during treatment. (A–B) Changes of absolute count of cells in blood after lymphodepletion and TIL infusion. Dots represent white cell count; squares mean neutrophils and triangles indicate lymphocytes. (C) Cytokine dynamics after TIL infusions (from day 0 to day 42). Concentrations of IL-2, 4, 6, 8, 10, TNF and IFN-γ were measured by flow cytometry from day 0 to day 42. Values ≤1 pg/mL were marked as 1 pg/mL. (D) Fluctuations of hemoglobin level and platelet count during treatment. TNF, tumor necrosis factor; IFN-γ, interferon γ.
The change of CD4+ and CD8+ cell and PD1 expression. (A) The mutually complemented change in CD4+ and CD8+ cell frequencies increased the proportion of the systemic CD8+ T cells. (B) PD1 expression on TILs increased on 24 days after infusion.
Levels of cytokines, including IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α and IFN-γ were monitored with flow cytometry using a CBA cytokine detection kit (BD Biosciences). It showed that most of these cytokines excluding IL-6 and IL-8 maintained stable base level in peripheral blood. IL-6 level was increased on Day 11 and dropped to base level after then, while IL-8 increased dramatically on day 11, peaked on day 26 and dropped to base level almost on day 30 ( figure 3C ). Additionally, hemoglobin level and platelet count were also measured. Stable with minor to moderate fluctuations were observed in both hemoglobin level and platelet count ( figure 3D ). No other adverse effects were observed in this patient, which confirmed that this new lymphodepletion therapy is safe enough to be performed in a ward.
To estimate the expansion of infused TILs, we used 5’ RACE approach to quantify the expected frequency of TCRβ clonotype sharing the same nucleotide sequence of CDR3. 13 A high frequency of CDR3 sharing had been observed on 31 days after infusion (4.45%), nearly four-fold increase compared with the frequency of CDR3 sharing on the first day of infusion (1.28%), suggesting that the infused TILs had profoundly changed the systemic TCR clonotype diversity ( figure 5A ). We further quantified similarity of the frequency distribution of CDR3 length from two samples of different time points after infusion using the Morisita-Horn (MH) similarity index, which revealed a higher MH value for the two samples of two closer time points ( figure 5B ). A time-course MH value curve calculated using different time-point samples showed a gradual decrease of similarity to nearing the preinfusion similarity on 59 days after infusion ( figure 5C ), while the time-course curve of frequency of CDR3 sharing between infusion product and TCR different time point samples after infusion showed a completely different pattern with multiple downs and falls ( figure 5D ). The two highest frequencies of CDR3 sharing were 27.51% and 28.11% on 2 days and 31 days after infusion, respectively. To determine the relationship between frequency of clonotype sharing and absolute count of lymphocytes, we compared the two datasets which demonstrated frequency of clonotype sharing was highly coincident with absolute count of lymphocytes from peripheral blood ( figure 5D ). These findings suggested a robust expansion of infused TILs and a permanent change to the systemic peripheral blood T lymphocyte profile.
Shared clonotypes from samples of different time points after infusion. (A) Venn diagram of shared clonotype frequency. (B) Clustered distance matrix of subjects, using pairwise Morisita-Horn (MH) similarity of CDR3 as the distance measure. (C) A time course MH value curve using different time point samples. (D) The relationship between frequency of clonotype sharing and absolute count of lymphocytes from peripheral blood. TCR, T cell receptor.
Discussion and conclusion
TIL therapy using in vitro expanded naturally occurring tumor-residing lymphocytes has produced durable, complete regression in patients with cervical cancer. 9 For clinic use, large numbers of antitumor lymphocytes (up to 10 11 ) can be readily grown in vitro and selected for high-avidity tumor antigens and mediation of cancer regression. 11 15
Lymphodepletion using high dose chemotherapeutic drugs is the currently established pretreatment for most TIL therapies, which is believed to be able to improve the efficacy of TIL infusion by creating a favorable environment in vivo. The underlying mechanisms of lymphodepletion may include the clearance of homeostatic cellular cytokine sinks and the removal of Tregs to enhance the efficacy of transferred TILs. 16 The most frequently used pretreatment regimen consists of cyclophosphamide (60 mg/kg) given for 2 days and fludarabine (25 mg/m 2 ) administered over 5 days, followed by TIL infusion and IL-2 administration. 17 Traditional pretreatment and post-TIL infusion regimen lead to numerous adverse events such as constitutional symptoms like fever and chills, as well as side effects of cardiovascular, pulmonary, renal, gastrointestinal, neurologic, dermatological, and endocrine systems. 18–20 We modified the lymphodepleting regimen with half of the reported dosage of cyclophosphamide and excluded fludarabine and IL-2. As expected, on the patient treated with this modified lymphodepleting regimen in the present case showed negligible adverse reactions and achieved complete response. This finding supported the feasibility of our novel pretreatment, at least in this cervical cancer patient.
Following TILs infiltration, the killing ability of T cells might be impaired during a prolonged T cell-tumor interaction referred as T cell exhaustion. 21 T cell exhaustion is typically characterized by reduced effector cytokines, coupled with the upregulation of multiple inhibitory receptors such as PD1, LAG-3 and TIM-3, as well as loss an effective attack on the target cells. 10 22 23 Without high dose lymphodepleting pretreatment coupled with high dose IL-2 utilization after infusion, TILs are more prone to be suppressed by immunosuppressive tumor microenvironment. Blockading immune checkpoints such as PD1 and CTLA-4, has proven to rescue T lymphocytes from immunosuppressive condition. 10 The exhaustion of TIL was assessed of the following blood time points by PD-1 level. Based on PD1 expression on TILs ( figure 4B ), an approved anti-PD1 antibody, sintilimab, was used in a half-reduced dosage as compared with the label-recommended dose with purpose of neutralizing PD1 expression on TILs, and no adverse effects related to the PD1 mAb drug was observed in this case. Additionally, most CD3 positive signals were observed in the extracellular matrix of the tumor, while only a minor proportion residing in the pan-CK positive area ( figure 2 ), indicating the tumor may result from a failure of immune recognition or region-specific barriers to infiltration. 24 The tissue architecture is preserved to improve biomarker discovery and assessment. TCR clone frequency of the infused TILs was monitored by TCR repertoire sequencing, indicating the repertoires from different time point samples were altered by new immunological encounters. 13 It showed that the TCR clone frequency increased along with time after infusion ( figure 5D ) and that the enlarged proportion of CD8+ T cells lasted as long as 100 days after therapy ( online supplemental figure S2A ). These findings suggested a robust expansion of infused TILs and a permanent change to the systemic peripheral blood T lymphocyte profile even though IL-2 was not used after infusion. It is possible that IL-2 secreted by infused TILs involved in the expansion. Moreover, a similarity comparison between TCR samples from various postinfusion time points and the infusion product showed that the similarity descended continuously from 2 days post infusion. Study has demonstrated that clones expandation after infusion mainly originated from infused clusters with higher expression of cytotoxicity and proliferation genes. 25 Taken together, we hypothesized that the infused TILs underwent tumor neoantigen response screening in vivo and as a result, relatively fewer numbers of TCRs, possibly tumor responsive, became enriched. Still, there is a need for more comprehensive immune biomarker analysis in the patient to identify important phenotypic and functional TIL markers as well as the sustained monitoring of persisting TIL subsets in the blood after infusion to further delineate the differential role of CD4+vs CD8+ T cells.
In conclusion, our study provides a promising therapeutic approach for TIL therapy in which mild chemotherapeutic pretreatment is required and post-infusion IL-2 administration is removed completely. This case represented another option for patients receiving TIL infusion. Notably, more patients and other solid tumors are needed to validate the therapeutic efficacy achieved in this case.
Ethics statements
Patient consent for publication.
Consent obtained directly from patient(s)
Ethics approval
This study was approved by the institutional review board of Shanghai Tenth People’s Hospital (IRB No. SHSY-IEC-4.0/20-238/02).
Acknowledgments
The authors would like to thank the patient who participated in the trial. The authors would also like to thank the physicians, nurses, research coordinators and other staff at the hospital and Binghui Zhao from Department of Radiology who assisted with the study. The authors would like to acknowledge Shanghai Gencells Therapeutics team for their contributions.
- Jhingran A ,
- Oaknin A , et al
- Weiderpass E ,
- Bruni L , et al
- Delord J-P , et al
- ↵ Clinicaltrials.gov . Available: https://clinicaltrials.gov/ct2/results?term=PD1&cond=Cervical+Cancer&Search=Apply&recrs=a&age_v=&gndr=&type=&rslt=
- Wendel Naumann R ,
- Sarnaik AA ,
- Khushalani NI , et al
- XAmir Jazaeri A A ,
- Stevanović S ,
- Helman SR ,
- Wunderlich JR , et al
- Restifo NP ,
- Dudley ME ,
- Rosenberg SA
- Creelan BC ,
- Teer JK , et al
- Liu S-P , et al
- Inderbitzin A ,
- Joyce C , et al
- Schwartz LH ,
- Litière S ,
- de Vries E , et al
- Gattinoni L ,
- Finkelstein SE ,
- Klebanoff CA , et al
- Chandran SS ,
- Somerville RPT ,
- Yang JC , et al
- Marabondo S ,
- Starbuck K ,
- Besser MJ ,
- Shapira-Frommer R ,
- Treves AJ , et al
- O'Donnell JS ,
- Parkhurst MR ,
- Robbins PF ,
- Tran E , et al
- McPherson A ,
- Milne K , et al
- Voillet V ,
- Hanafi L-A , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
JG and NL contributed equally.
Contributors ZC, HJ and SL designed and led the clinical trial; JG, NL, GA, WY, CL and RC provided patient care; JG analyzed data and wrote the manuscript; ZC, HJ and SL designed the TIL product and in vitro experiments; JG, NL and SL interpreted data and edited the manuscript; JZ provided clinical assistance; CZ provided MR images.
Funding This work was supported by grant from the National Natural Sciences Foundation of China (82103337; to JG) and Shanghai Biomedical Science and Technology Project (21S11906600; to HJ).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Impact of screening on cervical cancer incidence: A population‐based case–control study in the United States
Rebecca landy, peter d sasieni, christopher mathews, charles l wiggins, michael robertson, yolanda j mcdonald, daniel w goldberg, isabel c scarinci, jack cuzick, cosette m wheeler.
- Author information
- Article notes
- Copyright and License information
Correspondence to: Rebecca Landy, E‐mail: [email protected]
The New Mexico HPV Pap Registry (NMHPVPR) Steering Committee Members are listed in Appendix
Corresponding author.
Received 2019 Sep 5; Revised 2019 Nov 14; Accepted 2019 Nov 25; Issue date 2020 Aug 1.
This is an open access article under the terms of the http://creativecommons.org/licenses/by/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Cervical cancer is widely preventable through screening, but little is known about the duration of protection offered by a negative screen in North America. A case–control study was conducted with records from population‐based registries in New Mexico. Cases were women diagnosed with cervical cancer in 2006–2016, obtained from the Tumor Registry. Five controls per case from the New Mexico HPV Pap Registry were matched to cases by sex, age and place of residence. Dates and results of all cervical screening and diagnostic tests since 2006 were identified from the pap registry. We estimated the odds ratio of nonlocalized (Stage II+) and localized (Stage I) cervical cancer associated with attending screening in the 3 years prior to case‐diagnosis compared to women not screened in 5 years. Of 876 cases, 527 were aged 25–64 years with ≥3 years of potential screening data. Only 38% of cases and 61% of controls attended screening in a 3‐year period. Women screened in the 3 years prior to diagnosis had 83% lower risk of nonlocalized cancer (odds ratio [OR] = 0.17, 95% CI: 0.12–0.24) and 48% lower odds of localized cancer (OR = 0.52, 95% CI: 0.38–0.72), compared to women not screened in the 5 years prior to diagnosis. Women remained at low risk of nonlocalized cancer for 3.5–5 years after a negative screen compared to women with no negative screens in the 5 years prior to diagnosis. Routine cervical screening is effective at preventing localized and nonlocalized cervical cancers; 3 yearly screening prevents 83% of nonlocalized cancers, with no additional benefit of more frequent screening. Increasing screening coverage remains essential to further reduce cervical cancer incidence.
Keywords: cervical screening, cervical cancer, cytology, pap smear, HPV, case–control, cancer screening, cancer registry
Short abstract
What's new?
Screening is an effective means of preventing cervical cancer in women. However, while a negative screening result affords protection against cervical cancer, little is known about how long this protection lasts or when a woman should be screened again. Here, the authors estimate the impact of cervical screening at a state‐wide level, linking a U.S. population‐based screening registry with a SEER cancer registry. The results show that screening once every three years prevents 83 percent of stage 2+ cervical cancers, with no additional benefit from more frequent screening. The findings may help improve adherence to U.S. screening guidelines.
Introduction
Cervical cancer is largely preventable, yet an estimated 13,170 women in the United States (US) will be diagnosed with invasive cervical cancer in 2019, an age‐standardized rate of 7.6 per 100,000 women in 2011–2016. 1 Cervical screening and human papillomavirus (HPV) vaccination are two methods of preventing cervical cancer. In 2012, consensus guidelines were issued for cervical screening in US populations, recommending screening begin at age 21 years; 3 yearly cytology for women aged 21–29 years, and either 3 yearly cytology or 5 yearly cotesting (co‐occurring HPV and cytology testing) for women 30–64 years. 2 , 3 In 2018, the US Preventive Services Task Force (USPSTF) released updated guidelines, adding 5 yearly primary HPV testing as an option for women aged 30–65 years. 4 Most women aged >65 years can cease cervical screening. 2 , 4 The first HPV vaccine was licensed in the US in 2006 5 and the Centers for Disease Control and Prevention first recommended routine HPV vaccination for girls aged 11–12 years in 2007. 6
Screening has been shown to be effective at preventing cervical cancer on a population level since the 1960s. 7 Although the effectiveness of screening has been evaluated in numerous European populations, 7 , 8 , 9 , 10 , 11 , 12 , 13 the sensitivity of cytology varies between screening settings. 14 Previous research on the effectiveness of cervical screening within the US has focused on women enrolled in health plans or integrated health systems, 15 , 16 and/or has focused on women of specific ages. 17 In 2006, HPV was added to the list of reportable conditions for individuals residing in New Mexico. All cervical screening test results (HPV, Pap cytology and cotesting) and all pathology for the cervix, vagina and vulva are reported to the New Mexico HPV Pap Registry (NMHPVPR). 18 The NMHPVPR has previously been described in detail. 19 New Mexico is the only State in the US with a complete record of all cervical screening, diagnosis and treatment, providing appropriate high‐quality data to evaluate the effectiveness of cervical screening on a population basis, across a variety of diverse healthcare delivery settings and populations. The population of New Mexico is diverse; according to 2018 population estimates, 49.1% of the population were of Hispanic or Latino origin, 10.9% were American Indian or Alaska natives and 2.6% were African American. 20
We assessed the effectiveness of cervical screening in New Mexico using a case–control study design. We addressed three questions (outlined in the Methods) which together provide insights into the effectiveness of screening on a state‐wide basis. Our study was approved by the University of New Mexico Human Research Review Committee.
Cervical cancer cases
We collected data on all cervical cancer diagnoses in the population‐based New Mexico Tumor Registry (NMTR) during 2006–2016. For each case, the NMTR provided information on the month/year of birth, month/year of diagnosis, morphology and stage at diagnosis (using the derived AJCC‐6 stage classification system). NMTR records were linked with the NMHPVPR to provide information on each case's history of cervical screening, diagnostic and treatment results within New Mexico since January 2006. The reason why each test was performed was not available; see Supporting Information S1 for details on how we determined which tests were likely due to symptoms. Only colposcopy procedures resulting in a biopsy were captured. With few exceptions, information was available for each woman's census tract of residence at cancer diagnosis and at each screening or diagnostic test.
Since cancers histologically diagnosed within 5 months of an abnormal screening result were almost certainly present at the time of the screen, and in most cases will have been screen‐detected, we took the date of the first abnormal cytology or positive HPV test within 5 months of histological diagnosis as the “date of index diagnosis”. The date of index diagnosis for cases with no such abnormal test result was the date of diagnosis. We note that this definition primarily affects results when considering “time since last screen” since this definition does not count a positive test less than 5 months before histological diagnosis as a prediagnostic test.
Controls were selected from the NMHPVPR. Five women were selected per case, matched on date of birth and census tract of residence at diagnosis. To be eligible as a control, women had to be alive without a known hysterectomy or diagnosis of cervical cancer recorded at the date of the case's diagnosis. Since women were only in the NMHPVPR if they had attended screening from January 2006–December 2016, we added a fractional number of unscreened “virtual‐controls” for each case, to represent women who had not attended screening between January 2006–December 2016, and were therefore not in the NMHPVPR. The number of virtual controls was determined by comparing numbers of women in NMHPVPR with numbers from the census. Details on how the weights were calculated to determine the fractional number of unscreened women are available in Supporting Information S2 , and additional details on matching in Supporting Information S3 . All controls were assigned their matched case's date of diagnosis as a date of pseudodiagnosis.
Measures to evaluate the effectiveness of cervical screening
We address the following primary questions:
What is the risk of ( i ) Stage I (localized) and ( ii ) Stage II+ (nonlocalized) cervical cancer within 3 years of attending screening compared to the risk in women who did not attend screening within the previous 5 years?
For how long do women remain at lower risk of nonlocalized cancer after a negative screen?
How does the risk among women who attend screening frequently (at least once every 2.5 years), regardless of the screening result, compare with the risk among women who do not attend screening or who attend infrequently?
We examined the effect of attending screening on the risk of cervical cancer using the following measures to answer each question. ( i ) Existence of a satisfactory screen in the 3 years prior ( vs . none in the 5 years prior) to the case's date of index diagnosis. This analysis was restricted to women with ≥3 years of potential prediagnosis screening history. ( ii ) Time between the last negative screening test and the case's date of index diagnosis, among women with ≥5 years of screening history available. A screening test was defined to be negative if there was a negative cytology or HPV test which was not taken as part of a positive cotest, nor was it the first negative cytology/HPV test within 12 months of an abnormal screening test. We used the following categories: ≤1.5, 1.5–2.5, 2.5–3.5 and 3.5–5 years, compared to women with no recorded negative screening tests within 5 years of the case's date of index diagnosis. ( iii ) We defined a woman to have been frequently screened if she had at least two screens a minimum of 10 months apart, with no interval >30 months between screens, in the 5 years prior to the date of index diagnosis/pseudodiagnosis. Women with some screening in the 5 years prior to the date of index diagnosis who did not meet the criteria of frequent screening were considered to have attended screening infrequently. This analysis was restricted to women with at least 5 years of screening history, to allow us to distinguish unscreened from infrequently screened women.
Since women are only recommended to attend routine screening until age 65 years, we restrict the main analyses to women aged 25–64 years. Except where explicitly stated otherwise, when analyses considered screening in a 5‐year period, we excluded cases and their matched controls diagnosed before January 1, 2011. All analyses were carried out for all stages combined and separately by stage at diagnosis.
We carried out seven sensitivity analyses (SA) on the first question addressed (What is the risk of ( i ) Stage I (localized) and ( ii ) Stage II+ (nonlocalized) cervical cancer within 3 years of attending screening compared to women who did not attend screening within the previous 5 years?). The first sensitivity analyses (SA1) adjusted for the census‐tract level sociodemographic variables shown in Supporting Information Table S1 , since the controls were matched to the cases on census tract, and we do not have individual‐level sociodemographic data. SA2 excluded women whose address was a P.O. Box or zip code (Supporting Information S2 ). SA3 used an alternative set of weights, where control women from the NMTR who were diagnosed with potentially screen‐detected cancers (breast or colorectal) were excluded when calculating the weights. SA4 excluded the virtual (unscreened) controls from the analysis, to examine the impact of merely selecting controls from the NMHPVPR, without allowing for the fact that it is not a population register, and that women who did not attend screening from January 2006 to December 2016 could not be selected as a control. SA5 included women of all ages, regardless of whether they were recommended to attend screening, and SA6 included women aged 25–69 years, since 65 years was only introduced as the upper age limit of screening in 2012. 3 Finally, SA7 used a reference category of women who had not attended screening in a 3‐year period, rather than a 5‐year period.
Statistical methods
We present results from unadjusted weighted logistic regression analyses (having broken the matching, to allow for the weights) as the primary results.
Reporting under state regulations (New Mexico Administrative Code) specified by the list of Notifiable Diseases and Conditions is exempted from informed consent.
Data availability: Primary data supporting the investigation reported in this article can be made available in de‐identified form subject to establishing a data use agreement with the University of New Mexico Health Sciences Center.
A total of 876 women were diagnosed with cervical cancer in New Mexico between January 1, 2006 and December 31, 2016. Of these 876 cancers, 70% were squamous, 19% adenocarcinoma, 2% adenosquamous and 8% other morphologies. A total of 646 women were diagnosed from January 2009–December 2016, with ≥3 years of potential screening history recorded. Of these, 47.9% were diagnosed at ages 35–54 years, with only 2.3% ( n = 15) diagnosed before age 25 years, and 15.8% ( n = 102) diagnosed aged ≥65 years (Fig. 1 , Table 1 ). The stage at diagnosis was strongly related to age at diagnosis; in women <35 years, 75.0% with a known stage were Stage I, compared to 41.1% among women aged ≥65 years.
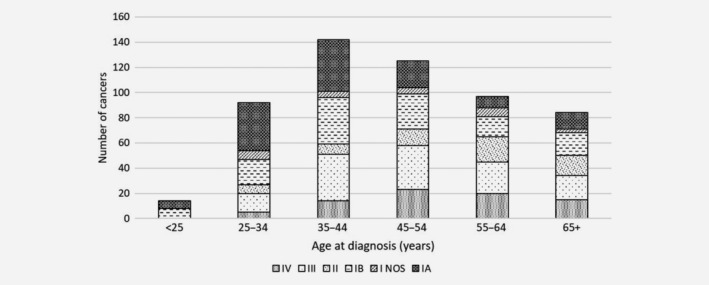
Stage distribution by age of the 646 cervical cancers diagnosed in New Mexico 2009–2016 among women with ≥3 years of screening history.
Stage distribution by age of the 646 cervical cancers diagnosed in New Mexico 2009–2016 among women with ≥3 years of screening history
NOS: not otherwise specified.
Approximately 40% (38.0%) of cases diagnosed aged 25–64 years attended screening in the 3 years prior to the date of index diagnosis (Table 2 ), compared to 61.2% of controls (weighted for women without a record of screening in the NMHPVPR). Women aged 25–64 years who attended screening in a 3‐year period had a lower risk of diagnosis for each cancer stage compared to women not screened in the last 5 years (Table 2 , Supporting Information Table S2 ). Only one‐fifth (22.5%) of women with Stage III+ cancer had been screened in the 3 years prior to the date of index diagnosis, compared to 59.3% of women with Stage IA cancer (Supporting Information Table S2 ). The effect of attending screening in the last 3 years increased with increasing cancer stage, from no effect on the odds of Stage IA cancer (odds ratio (OR) = 0.78, 95% CI:0.48–1.28) to strong effects on Stage III+ cancer (OR = 0.16, 95% CI:0.10–0.23) compared to women who did not attend screening in the last 5 years. Figure 2 shows there were statistically significant effects of screening on nonlocalized cancers for all ages, but only for ages 35–49 years and 50–64 years for Stage I cancers.
Odds ratios and 95% confidence intervals of cervical cancer by screening attendance and stage at diagnosis, among women aged 25–64 years with at least 3 years of potential screening history
NMHPVPR and virtual controls were used in this analysis.
or not screened in the last 3 years with <5 years of potential screening data.
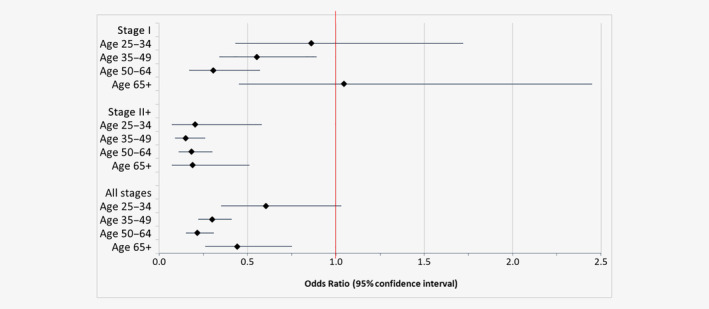
Odds ratios and 95% confidence intervals for risk of cervical cancer by stage for women screened within the last 3 years compared to women not screened in the last 5 years, restricted to women with ≥3 years of screening history. [Color figure can be viewed at wileyonlinelibrary.com ]
The results from SA are presented in Supporting Information Figure S1 . Most of the SA provided extremely similar results, more details are provided in Supporting Information S4 . When we assumed that the population at risk of cervical cancer excluded women with a hysterectomy (who guidelines have recommended against screening since 2012 2 ), and that all hysterectomized women had not attended screening, the proportion of unscreened women was 0 for women aged 20–69. This is equivalent to SA4, when the virtual‐controls were excluded from the analyses; this SA showed a larger effect of screening (SA4).
For time since the last negative screen (Table 3 ), when restricted to women with ≥5 years of potential screening history, women aged 25–64 years with a negative screen remained at lower risk of both Stage I (OR = 0.20, 95% CI: 0.14–0.28) and nonlocalized cancer (OR = 0.11, 95% CI: 0.07–0.17) for at least 3.5 years compared to women with no negative screening in the last 5 years (a mix of women with no screening and those with only abnormal screening results). The risk for Stage II+ cancers remained constant over the first 3.5 years. Results were similar in the SA, adjusting for census‐level socioeconomic variables, and using alternative weights (Supporting Information Table S3 ). There was a significant reduction in risk of nonlocalized cervical cancer for at least 3.5 years following a negative test relative to women with no negative tests in 5 years for women in each age group considered (25–34, 35–49, 50–64 and ≥65 years), except Stage I for women aged ≥65 years (Supporting Information Fig. S2 ). In SA, when the analysis was extended to women with ≥3 years potential screening history rather than 5 years, the results were very similar (Supporting Information Table S4 ).
Odds ratios and 95% confidence intervals of cervical cancer by time since last negative screen and stage at diagnosis, among women aged 25–64 years with at least 5 years of potential screening history
Women who attended screening frequently (at least two screens a minimum of 10 months apart, with no interval >30 months between screens) were at significantly lower risk of both nonlocalized (OR = 0.10, 95% CI: 0.05–0.19) and Stage I cancer (OR = 0.43, 95% CI:0.28–0.65) than women who did not attend screening in a 5‐year period (Table 4 ). Women who attended screening in the previous 5 years, but did not meet the criteria for frequent screening (“infrequently” screened) were at significantly reduced risk of both nonlocalized (OR = 0.26, 95% CI: 0.18–0.37) and Stage I cancer (OR = 0.58, 95%CI: 0.40–0.82) compared to women not screened in 5 years, but at significantly greater risk of nonlocalized cancer compared to those screened frequently (OR = 2.54, 95% CI: 1.33–4.84). SA produced very similar results (Supporting Information Table S5 ). When restricted to women who attended screening in the 2.5 years prior to the date of index diagnosis or who had not attended in 5 years, the results were also very similar (Supporting Information Table S6 ).
Odds ratios and 95% confidence intervals of cervical cancer for women who were frequently and infrequently screened by stage at diagnosis, among women aged 25–64 years with at least 5 years of potential screening history
Women were considered regularly screened if they had at least two screens a minimum of 10 months apart, with no interval >30 months between screens, in the 5 years prior to diagnosis/pseudodiagnosis. NMHPVPR and virtual controls were used in this analysis.
When restricted to women who had only cytology screening (i.e., no HPV tests prior to diagnosis), the results of the three main analyses were very similar (Supporting Information Tables S7–S9 ).
Our study addressed three key relevant questions related to the performance of cervical screening. First, attending screening within a 3‐year period reduced the odds of nonlocalized cancer by 83%, and Stage I cancer by 48% compared to women not screened in 5 years. Second, women who had a negative screening test were at much lower risk of both nonlocalized and Stage I cancer for up to 5 years compared to women without a negative screen in the last 5 years, with a larger benefit in the first 3.5 years. Third, frequently attending cervical screening (at least two screens a minimum of 10 months apart, with no interval >30 months between screens) was associated with a 90% reduction in the odds of nonlocalized cervical cancer, and a 57% reduction in the odds of Stage I cervical cancer, compared to women who did not attend screening for 5 years. Notably, we found similar relative benefits of screening at ages 25–34, 35–49, 50–64 and aged ≥65 years for nonlocalized cancer.
It is important to acknowledge that cancers diagnosed before symptoms developed should be considered a success of cervical screening; 23% of cancers diagnosed at a known stage in New Mexico 2006–2016 were diagnosed at Stage IA. The stage distributions of cervical cancers diagnosed in New Mexico over the study time period including Stage IA were very similar to that computed for SEER18 registries overall (SEER*Stat November 2018; data not shown).
Women who were screened at least once every 2.5 years (frequently) had a relative risk of nonlocalized cancer of 0.39 compared to women screened infrequently. This was also the case when restricted to women who were screened within the 2.5 years prior to the date of index diagnosis, indicating that this is not purely due to the presence of a recent test, but to having had multiple tests in the 5‐year period. This was largely a study of cytology, with little co‐testing. The sensitivity of cytology for CIN2+ is around 71–75%; 21 therefore, there is an advantage to having more frequent screenings, due to the high level of false negatives for a single cytology test. However, this does not mean that annual testing is an improvement, as demonstrated by the very similar risk of nonlocalized cancer 0–1.5 years after a negative screen compared to 2.5–3.5 years after a negative screen. On the contrary, while our study was not designed to assess the disadvantages of screening more frequently than current guidelines recommend, there are many reasons to dissuade this practice. First, more frequent screening increases the probability of having a false‐positive test (when either no precancerous lesion is present, or the precancerous lesion would regress without requiring intervention). Second, false‐positive tests have the potential to increase stress and anxiety if further diagnostic testing is required, in addition to the discomfort from a colposcopy. Additionally, there is the time and expense associated with unnecessary testing; in New Mexico, 28% of women who reside in rural areas must travel more than 30 minutes each‐way to seek diagnostic services. 22
Recent guidelines recommend routine HPV cotesting in women aged 30–65 years. 4 The majority of screening records in New Mexico in 2006–2016 were cytology tests taken alone, though the proportion of HPV tests or cotests increased with time (from 4.2% in 2006 to 54.7% in 2016), and when restricted to women aged 30–65 years, where cotests are routinely recommended, 67.8% were observed in 2016. Cotesting will increase the sensitivity of a single round of screening, and potentially support longer screening intervals versus intervals when screening by cytology alone. 23 Whether longer screening intervals can be successfully adopted by the US in the absence of organized call‐recall systems should be given careful consideration. As cervical screening intervals lengthen for primary HPV testing and cotesting over time, it will be critical to monitor the proportion of women who fail to rescreen at 5‐year intervals. Although HPV‐based technologies are directed at improving screening efficiencies and reducing potential harms from screening, lengthening cervical cancer screening intervals in the US may not be readily implemented due to the lack of organized screening programs. Furthermore, the continuously changing landscape of cervical screening could result in an increase in cervical cancer incidence if women fail to return for screening or return beyond the duration of protection afforded.
While we have shown that cervical screening in New Mexico is effective at preventing cervical cancer, only 61% of controls aged 25–64 years had attended cervical screening in a 3‐year period. Therefore, initiatives which increase screening coverage are likely the best investment for improving the prevention of cervical cancer, especially among women from birth cohorts which did not benefit from HPV vaccination prior to sexual initiation. Since not all attendees return for their next screen, it is important to use the most sensitive screening test available.
Similar methods have been used to explore the effectiveness of cervical screening in Europe 8 , 9 , 10 , 11 , 24 , 25 and Australia. 26 Andrae et al . 8 found a slightly lower effect of screening in women aged 30–65 in Sweden for all stages (OR = 2.52) and Stage II+ (OR = 4.82), when considering women who were not screened compared to women who were screened in the recommended interval (3 yearly for women aged 30–50 and 5 yearly for women ages 50–60). Yang et al . 26 found that even infrequent screening in Australia, defined as a pap test in only 1 year of a 4‐year period, was associated with an 85% reduction in risk of all stages of cervical cancer, and frequent screening (a pap in at least 2 years in a 4‐year period) was associated with around a 95% reduction in risk. These effects are slightly larger than those found for infrequently and frequent screening in our study, though our definition of frequent screening differs slightly.
New Mexico is the only state within the US where cervical screening data of this quality exist on a population basis, enabling the evaluation of cervical screening as practiced across a wide range of healthcare delivery settings. Screening recommendations and implementation approaches vary widely between countries, 27 so results from one setting may not apply to another; for example, in the US, the vast majority of screening is opportunistic whereas in Sweden there is a national program where women are invited for screening. 28 The importance of comprehensive audits of screening programs including the full target population is widely recognized. 29 , 30 Previous research on the effectiveness of cervical screening in the US has relied on data from women enrolled in health plans or integrated health systems 15 , 16 who may be at different risk of cervical cancer than the general population. Screening guidelines for the US have been almost exclusively based on the analysis of cervical screening data which are not representative of women and/or providers in the general population. 2 , 31 Furthermore, studies of cervical screening effectiveness in the US have been conducted in settings where screening is implemented by system‐specific screening guidelines. For example, Kaiser Permanente Northern California introduced HPV as part of a cotest in 2003, 32 whereas HPV cotesting did not even begin utilization in mainstream clinical practice in New Mexico until 2013, following national cervical screening guidelines issued in 2012. 3
It was not possible to select controls from a population register and link to their screening history. Only women who have attended screening at least once could be identified from the NMHPVPR; it was therefore important to augment this with virtual‐controls (who had not been screened since January 2006) based on the census. Had we not included virtual‐controls, we would have overestimated the impact of screening. We weighted the controls selected from the NMHPVPR by identifying the age‐specific proportion of matched women in the NMTR who had a screening record in the NMHPVPR. However, women who develop noncervical cancer may have different screening behaviors compared to the general population; we therefore reweighted the controls excluding women diagnosed with cancers which could have been screen‐detected (breast and colorectal), and the results were extremely similar (Supporting Information Fig. S1 , Tables S3 and S5 ). Our results estimated 75% of controls aged 25–65 years had been screened in the past 5 years; this is consistent with previous investigations which estimated the 5‐year screening coverage for women aged 21–65 years in New Mexico to be around 80%. 19
While we have not included any woman who we know to have had a hysterectomy, we only have incomplete information on hysterectomies (particularly prior to 2006). The situation is further complicated in that prior to 2012, the majority of women with a hysterectomy were still offered screening. If we add together the number of women in the screening registry with the number of women in New Mexico who have had a hysterectomy, the sum, in most age‐groups, is greater than the number in the census. Analyzing the data in this way would be equivalent to not allowing for unscreened (virtual) controls—it makes screening appear better than it is.
We have used the date of the first abnormal cytology or positive HPV within 5 months of diagnosis as the date of index diagnosis rather than the definitive date of diagnosis used by the NMTR, 33 and considered screening in a 3‐ or 5‐year period prior to this date. We only have records of screening tests performed on women where addresses were recorded as a resident of New Mexico or which were taken from a New Mexico provider; whereas some women may have attended screening in other States which would have been missed. Some of the women selected as NMHPVPR controls may only have been resident in New Mexico for a limited period, for example, due to migration, therefore our data may not represent their full screening history since 2006. When limiting the analyses to women diagnosed with cervical cancer at age 25–64 years who had at least 5 years of screening history data, our sample was reduced to 410 cases. Screening guidelines varied both between and within organizations across the period of our study, so we could not evaluate the effect of screening among women who complied with screening guidelines. We have not linked HPV vaccination status to screening histories, but this is likely to have minimal impact on our results due to the long natural history from HPV infection to cervical cancer versus the introduction of HPV vaccination. We do not have sufficient women who were only screened using HPV testing to compare the effect of screening using cytology alone to those with HPV testing, nor sufficient numbers of women with adenocarcinomas who have at least 3 years of screening data when broken down by stage and screening history in order to investigate the effect of screening by histologic subtype.
In conclusion, our study demonstrates that routine screening at a population level has had a beneficial effect in preventing cervical cancer. However, only 61% of controls in our study had attended screening in a 3‐year period. Thus, increasing screening coverage will have the greatest impact in achieving further reductions in cervical cancer rates.
Conflict of interest
J.C. and C.M.W. have received funds from grants, cooperative agreements or subcontracts related to cervical screening and triage through their institutions. J.C. reports grants to his institution and personal fees from Qiagen, Becton Dickinson (BD), Genera Biosystems (GB) and grants to his institution from Hologic, Gene First and Trovagene, all outside the submitted work. CMW reports receiving reagents and equipment for HPV genotyping, from Roche and GB through her institution and personal fees from BD all outside of the submitted work. R.L., P.D.S., C.M., C.L.W., M.R., Y.J.M., D.W.G. and I.C.S. have no interests to report.
Supporting information
Data S1 : Supporting Information
Acknowledgements
This work was supported by the US National Cancer Institute (NCI) U54CA164336 to CMW (CMW, CLW, MR) with subcontracts to Texas A & M University, College Station, Texas (YJM, DWG) and to University of Alabama at Birmingham (ICS) and by the US National Institute of Allergy and Infectious Diseases U19AI113187 to CMW with subcontract to Queen Mary University of London (QMUL) (JC, PDS). This project was also supported by Contract HHSN261201800014I, Task Order HHSN26100001 from the National Cancer Institute (CLW). In addition, support was received from Cancer Research UK programme grants C8161/A1689 to PDS (RL, CM) and C569/A16891 to JC, from NCI P30CA118100 (to CL Willman) (YJM) and the Ford Foundation (YJM).
Appendix 1.
New Mexico HPV Pap Registry (NMHPVPR) Steering Committee Members:
Members of the New Mexico HPV Pap Registry (NMHPVPR) Steering Committee reviewed and gave input to the manuscript and supported the concept and directions of the NMHPVPR including the evaluations presented in this manuscript. The NMHPVPR Steering members participating in this effort are as follows: Nancy E. Joste, MD, University of New Mexico Health Sciences Center and Tricore Reference Laboratories, Albuquerque, New Mexico; Walter Kinney, MD, retired Kaiser Permanente Northern California; Cosette M. Wheeler, PhD, University of New Mexico Health Sciences Center; Ruth M. McDonald, MS, University of New Mexico Health Sciences Center; Michael Robertson, BS, University of New Mexico Health Sciences Center, Alan Waxman, MD MPH, University of New Mexico Health Sciences Center; Steven Jenison, MD, Community Member; Julia C. Gage, PhD, MPH, US National Cancer Institute; Philip E. Castle, PhD MPH, Albert Einstein School of Medicine; Vicki Benard, PhD, US Centers for Disease Control and Prevention; Debbie Saslow, PhD, American Cancer Society; Jane J. Kim PhD, Harvard TH Chan School of Public Health; Mark H. Stoler MD, University of Virginia; Jack Cuzick, PhD, Wolfson Institute of Preventive Medicine, London; and Giovanna Rossi Pressley, MSc, Collective Action Strategies; Kevin English, DrPh MPH, Albuquerque Area Southwest Tribal Epidemiology Center (AASTEC); No compensation was received for contributions to this manuscript by any named authors or by the NMHPVPR Steering Committee members.
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62:147–72. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Moyer VA. Screening for cervical cancer: US preventive services task force recommendation statement. Ann Intern Med 2012;156:880–91. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. US Preventive Services Task Force . Screening for cervical cancer US preventive services task force recommendation statement. JAMA 2018;320:674–86. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. U.S. Food & Drug Administration . Gardasil Vaccine Safety. Silver Spring, MD: US FDA, 2018. 2009. [ Google Scholar ]
- 6. Markowitz L, Dunne E, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:1–24. [ PubMed ] [ Google Scholar ]
- 7. International Agency for Research on Cancer . IARC Handbooks of Cancer Prevention Volume 10: Cervix Cancer Screening ed. Lyon: IARC Press, 2005. [ Google Scholar ]
- 8. Andrae B, Kemetli L, Sparén P, et al. Screening‐preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst 2008;100:622–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Castanon A, Landy R, Cuzick J, et al. Cervical screening at age 50–64 years and the risk of cervical cancer at age 65 years and older: population‐based case control study. PLoS Med 2014;11:e1001585. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer 2016;139:1040–5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Olesen F. A case‐control study of cervical cytology before diagnosis of cervical cancer in Denmark. Int J Epidemiol 1988;17:501–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. IARC Working Group on Evaluation of Cervical Cancer Screening Programmes . Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J 1986;293:659–64. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Hakama M. Effect of population screening for carcinoma of the uterine cervix in Finland. Maturitas 1985;7:3–10. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and north American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095–101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Kamineni A, Weinmann S, Shy KK, et al. Efficacy of screening in preventing cervical cancer among older women. Cancer Causes Control 2013;24:1653–60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst 2014;106:dju153. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Rustagi AS, Kamineni A, Weinmann S, et al. Cervical screening and cervical cancer death among older women: a population‐based, case‐control study. Cancer Epidemiol Biomarkers Prev 2014;179:1107–14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. New Mexico Department of Health . Notifiable diseases or conditions in New Mexico. Santa Fe, NM: New Mexico Department of Health, 2016. [ Google Scholar ]
- 19. Cuzick J, Myers O, Hunt WC, et al. A population‐based evaluation of cervical screening in the United States: 2008–2011. Cancer Epidemiol Biomarkers Prev 2014;23:765–73. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. United States Census Bureau . QuickFacts: New Mexico, population estimates base, April 1, 2010, (V2018). Suitland, MD: United States Census Bureau, 2018. [ Google Scholar ]
- 21. Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening inthe general population. Cochrane Database Syst Rev 2017;8:CD008587. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. McDonald YJ, Goldberg DW, Scarinci IC, et al. Health service accessibility and risk in cervical cancer prevention: comparing rural versus nonrural residence in New Mexico. J Rural Health 2017;33:382–92. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. Br Med J 2008;337:a1754. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case‐control study of prospectively recorded data. BMJ 2009;339:b2968. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Sasieni P, Cuzick J, Lynch‐Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br J Cancer 1996;73:1001–5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Yang B, Morrell S, Zuo Y, et al. A case–control study of the protective benefit of cervical screening against invasive cervical cancer in NSW women. J Cancer Causes Control 2008;19:569–76. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Williams JH, Carter SM, Rychetnik L. ‘Organised’ cervical screening 45 years on: how consistent are organised screening practices? Eur J Cancer 2014;50:3029–38. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Hortlund M, Elfström KM, Sparén P, et al. Cervical cancer screening in Sweden 2014‐2016. PLoS One 2018;13:e0209003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Sasieni P, Cuzick J. Routine audit is an ethical requirement of screening. BMJ 2001;322:1179. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Slater D, Milner P, Radley H. Audit of deaths from cervical cancer: proposal for an essential component of the National Screening Programme. J Clin Pathol 1994;47:27–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Smith RA, Manassaram‐Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin 2015;65:30–54. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Castle PE, de Sanjosé S, Qiao Y‐L, et al. Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine 2012;30:F117–F22. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Tate AR, Martin AG, Murray‐Thomas T, et al. Determining the date of diagnosis—is it a simple matter? The impact of different approaches to dating diagnosis on estimates of delayed care for ovarian cancer in UK primary care. BMC Med Res Methodol 2009;9:42. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.

Supplementary Materials
- View on publisher site
- PDF (483.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2022
Cervical cancer prognosis and related risk factors for patients with cervical cancer: a long-term retrospective cohort study
- Jina Li 1 na1 ,
- Gaoming Liu 2 na1 ,
- Jiayou Luo 1 ,
- Shipeng Yan 2 ,
- Ping Ye 1 ,
- Jie Wang 1 &
- Miyang Luo 1
Scientific Reports volume 12 , Article number: 13994 ( 2022 ) Cite this article
7948 Accesses
10 Citations
10 Altmetric
Metrics details
- Cancer epidemiology
- Cancer prevention
This study aims to explore the recurrence rate and overall survival for patients with cervical cancer after the first treatment and the related risk factors. A retrospective cohort study was conducted on cervical cancer patients enrolled in a cancer specialist hospital in Hunan Province, China from January 1992 to December 2005 and followed up until December 2010. Kaplan–Meier survival analysis was used to estimate the cumulative recurrence rate, and Cox proportional hazards model was utilized to identify risk factors associated with prognosis. A total of 4358 patients were enrolled with a median follow-up of 7.4 years (range 5–19 years), and 372 (8.5%) patients had cancer recurrence. The cumulative recurrence rate showed a rapid increase from 3.8% in the first year after discharge to 8.0% in the fifth year, and the recurrence rate remained relatively stable afterward reaching 9.7% and 10.8% in the 10th and the 15th year, respectively. The median time to recurrence was 15.5 months with an IQR of 5.5–40.0 months. The Cox regression showed that miscarriage, clinical stage, and treatment received were significantly associated with cervical cancer recurrence after adjustment for confounders. Patients with recurrence showed a significantly higher risk for mortality than those without recurrence (HR 2.79, 95% CI 2.42–3.22). This study depicted the long-term recurrence rate and survival after recurrence for patients with cervical cancer after the first treatment, and reported time to recurrence and risk factors related to recurrence. These findings may provide important evidence for designing targeted interventions for the treatment of cervical cancer.
Similar content being viewed by others
Clinicopathological characteristics and prognostic factors of cervical adenocarcinoma
Nomogram for prognosis of elderly patients with cervical cancer who receive combined radiotherapy
Comparing survival outcomes for cervical cancer based on the 2014 and 2018 International Federation of Gynecology and Obstetrics staging systems
Introduction.
Cervical cancer is the most common malignant tumor in the female reproductive system, and it is one of the leading causes of death in women globally 1 . According to the 2020 Global Cancer Statistics Report, there were about 604,127 new cases of cervical cancer and 341,831 deaths caused by cervical cancer worldwide in 2020, accounting for 6.5% and 7.7% of the total number of cancer incidences and deaths in women, respectively 2 . Although there are standardized treatment methods for cervical cancer, patients after treatment often face the dilemma of recurrence 3 . Once the recurrence happens, the patient is left with very limited treatment options and a poor prognosis 4 , 5 . Thus, the prevention of cervical cancer recurrence has become a huge challenge in clinical practice.
Reducing the recurrence of cervical cancer requires an improved understanding of the actual recurrence rate, time to recurrence, and its related risk factors. Previous studies reported that the recurrence rate of cervical cancer varied in different populations (approximately 6.4–21.1%) 6 , 7 , 8 , 9 , 10 . To our knowledge, only a few studies focused on the pattern of recurrence rate and survival after recurrence in China 11 , 12 , 13 . However, most of these studies had a small sample size and a short period of observation, or were based on data from clinical trials which may limit their generalizability. In addition, most of the studies lacked in-depth research on the time to recurrence 14 , 15 , such as the analysis of the time to recurrence for patients with different age groups, parity, clinical stages, and treatment received. Moreover, despite that previous studies reported several risk factors of cervical cancer recurrence and prognosis, including patient's age, ethnicity, pathological type, clinical stage, lymph node metastasis, tumor size, and treatment received 16 , 17 , there is still controversy about certain risk factors, for instance, some studies reported inconsistent results for age, pathological type, and differentiation 6 , 18 , 19 .
This study aims to explore the long-term recurrence rate, time to recurrence, and survival after recurrence for patients with cervical cancer after the first treatment and identify the potential risk factors, using a large retrospective cohort, which may provide a scientific basis for the effective prevention and treatment of cervical cancer recurrence.
Characteristics of the study population
From January 1992 to December 2005, a total of 4374 subjects diagnosed with cervical cancer and completed the initial treatment in the study hospital were identified. After excluding 16 subjects who were lost to follow-up, we included 4358 subjects with a mean age of 46.5 years in the final analyses. The mean follow-up duration was 7.4 years with a range from 5 to 19 years. In this study, 46.6% of subjects were in clinical stage I, 40.8% of subjects were in clinical stage II, and 12.6% of subjects were in clinical stage III and IV (Table 1 ). In addition, 3864 (92.2%) subjects had squamous cell carcinoma, 261 (6.2%) subjects had adenocarcinoma, and 68 (1.6%) subjects had other types of cervical cancer. We found that 479 (12.0%) subjects were well-differentiated, 3332 (83.7%) subjects were moderately differentiated, 172 (4.3%) subjects were poorly differentiated/ undifferentiated.
Around half of the subjects received surgical treatment, with 6% receiving surgery only and 45.4% receiving surgery plus adjuvant therapy; 35.5% of subjects received radiotherapy only; 12.1% of them received chemotherapy plus radiotherapy; only 1% of subjects received chemotherapy only. When stratified by clinical stage, we observed that 8.1% and 84.2% of subjects in clinical stage I received surgery only and surgery plus adjuvant therapy, respectively. 17.3% of subjects in clinical stage II received surgery plus adjuvant therapy, 62.9% of them received radiotherapy only, and 18.3% of them received chemotherapy plus radiotherapy. Among subjects in clinical stage III/IV, 65.1% of subjects received radiotherapy only, and 28.8% of them received chemotherapy plus radiotherapy (Supplemental Table 1 ).
A total of 372 subjects had a cancer relapse during the follow-up period. Subjects with recurrence had significantly younger age, lower parity, and a greater number of miscarriages, and they were more likely to have lymph node metastasis and received chemotherapy ( P < 0.05).
Cumulative rate and time to recurrence
The overall cumulative recurrence rate was 3.8% in the first year after discharge and increased to 5.1% in the second year, and 8.0% in the fifth year. The increasing trend slowed down afterward and remained relatively stable at around 10% after ten years (Fig. 1 ). The median time to recurrence for the 372 subjects during follow-up was 15.5 months (IQR 5.5, 40.0) (Table 2 ). There were significant differences in the recurrence rate when stratified by parity, miscarriage, clinical stage, treatment received, lymph node metastasis, and chemotherapy received ( P < 0.05, Fig. 2 ), and the average time to recurrence was significantly shorter for subjects with lower parity, a greater number of miscarriages, higher clinical stage, lymph node metastasis, and received chemotherapy, especially for those with initial chemotherapy.
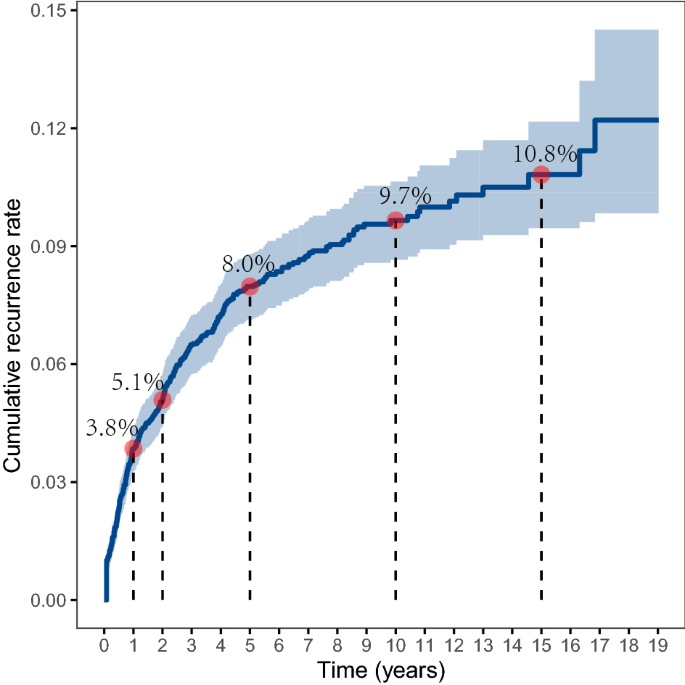
The overall cumulative recurrence rate.
Cumulative recurrence rate by ( A ) parity; ( B ) miscarriage; ( C ) clinical stage; ( D ) treatment received; ( E ) lymph node metastasis; ( F ) chemotherapy.
Risk factors for recurrence
We further analyzed the risk factors for cervical cancer recurrence by constructing Cox proportional hazards regression models (Table 3 ). We observed that miscarriage, clinical stage, and treatment received remained significant after adjustment for confounders. More specifically, the recurrence risk for subjects with three or more times of miscarriages was increased by 65% compared to those without a history of miscarriages (95% CI 1.23–2.22); the HR for subjects with clinical stage II was 1.73 (95% CI 1.24–2.41) and clinical stage III or IV was 2.04 (95% CI 1.32–3.16) compared to those with stage I; subjects received chemotherapy only were 3.62 times more likely to had recurrence compared to those received surgery only (95% CI 1.13–11.62). The sensitivity analysis that excluded subjects with recurrence within 3 months after discharge identified similar risk factors (Supplemental Table 3 ). When only including subjects with clinical stage I and II, we found that age greater than 60 years old was associated with a lower risk of recurrence (HR 0.53, 95% CI 0.30–0.94) than those with age younger than 40 years old; subjects with more than two times of miscarriages (HR 1.74, 95% CI 1.26–2.39) and received chemotherapy only (HR 5.61, 95% CI 1.58–19.95) had a higher risk of recurrence (Supplemental Table 4 ).
Survival after recurrence
The total survival time was plotted for subjects with and without cancer recurrence (Fig. 3 ). The 1-year survival probability for those without cancer recurrence was 96.3%, the 5-year survival probability was 79.5%, and the survival probability dropped to 50.7% after 15 years of follow-up. The survival probability was significantly lower for those with cancer recurrence, which was 90.6% in the first year, 54.0% in the fifth year, 28.7% in the 10th year, and only 15.4% in the 15th year. The Cox regression showed that the HR of mortality was 2.79 (95% CI 2.42–3.22) for subjects with recurrence compared to those without recurrence after adjustment for confounders (Table 4 ). We also found that pathological type and year of admission were significantly associated with mortality in the adjusted model, and subjects with adenocarcinoma showed a higher risk for mortality compared to subjects with squamous cell carcinoma (HR 1.50, 95% CI 1.23–1.83). The sensitivity analysis including subjects with clinical stage I and II also found that recurrence, pathological type, and year of admission was associated with mortality (Supplemental Table 5 ).
The overall survival probability by recurrence.
In this study, we reported the longitudinal pattern of cumulative recurrence rate over 15 years for patients with cervical cancer after the first treatment. We found that the overall crude recurrence rate was 8.5%, and the median time to recurrence was 15.5 months. Also, we found that the cumulative recurrence rate increased rapidly from 3.8% in the first year to 8.0% in the fifth year, and the increasing trend slowed down afterward where the rate was 9.7% in the 10th year and 10.8% in the 15th year. The recurrence rate reported in this study was consistent with a systematic review in 2009 which found that the recurrence rate for patients who completed primary therapy for cervical cancer ranged from 8.0 to 26%, and the median time to recurrence ranged from 7 to 36 months 10 . A more recent cohort study from Denmark that enrolled a population of 1523 women with clinical stage I cervical cancer in 2005–2013 showed a recurrence rate of 6.4% after 5 years of follow-up 6 . A study in China reported that rates of recurrence were 16.9% and 12.4% for 148 stage IIb patients with radical hysterectomy and 290 stage IIb patients with radical radiotherapy, respectively 12 . The variation of recurrence rate reported in different studies may be explained by the composition of patients with different disease severity in each study and different treatments received. Note that the major strength of this study was the analysis of the long-term recurrence rate pattern for a large population over 15 years of follow-up, and we included subjects ranging from FIGO stage Ia to stage IVb, based on medical records of patients admitted to the hospital during 1992 to 2005. These findings may provide evidence for designing treatment and follow-up guidelines for patients with cervical cancer.
Our study reported several risk factors for cervical cancer recurrence, including younger age, lower parity, a greater number of miscarriages, higher clinical stage, lymph node metastasis, and received chemotherapy, and we found that miscarriage, clinical stage, and treatment received remained significant in the adjusted Cox regression model. These risk factors identified have also been reported by previous studies 4 , 20 . Clinical stage was the most commonly reported risk factor for cancer recurrence 19 , 21 , 22 , our study results showed that patients with clinical stage II had 1.73 times the risk of recurrence, and patients with stage III or IV had 2.04 times the risk compared to those with clinical stage I. Patients with advanced clinical stages had a greater range of cancer lesions, and a higher probability of peripheral invasion and lymph node metastasis, thus even if the patient was systematically treated, the possibility of recurrence for these patients was relatively higher 19 , 23 . In this study, we observed that patients with younger age had a higher risk of recurrence in the unadjusted model, while an insignificant association was observed in the adjusted model. It is still controversial whether age is related to recurrence with some studies reported positive associations 6 , 24 and some reported negative associations 14 , 25 . It’s possible that patients with younger age had better adherence to the follow-up schedule, thus they were more likely to have a recorded recurrence. Unfortunately, we did not have detailed records on the adherence to follow-up, and it is important to further explore the reason between age and cancer recurrence. In addition, we did not include some risk factors that have been reported in previous studies, such as smoking, age of first intercourse, HPV infection, parametrial invasion, and vascular invasion 26 , 27 , 28 , and future investigations should take a more comprehensive considerations on related risk factors.
In this study, we observed that the treatment received varied by clinical stages, which jointly influenced the cancer recurrence rate. We found that surgery with adjuvant therapy was the most common treatment received for patients with clinical stage I, and more than half of the patients in clinical stage II and above were mainly treated with radiotherapy alone or chemotherapy plus radiotherapy. The treatment received in this study was generally consistent with the treatment guidelines indicating that surgery was the main treatment option for patients with early-stage cervical cancer 29 , and supplemental adjuvant therapy was added based on whether the patient has a risk factor for recurrence 30 . It’s also important to note that this study included a heterogeneous population with large variations in clinical stages and treatment plans, and we observed that the treatment plan was not consistent with the standard guidelines for a very small number of patients. For example, a few patients in Clinical stage I and II received chemotherapy only without surgery or radiotherapy. One possible explanation was that these patients were not able to receive surgery due to other complications or economic reasons. It is also possible that some patients were transferred to other local hospitals of whom the treatment regimen was not recorded in great detail. In addition, as this study collected data over a long period, we showed that the recurrence rate and survival rate increased during the follow-up period. The increase in recurrence rate may be due to the improved patient management system so that an increased number of subjects were followed up routinely and recurrence was more likely to be recorded. The improved survival rate may result from the improved standard of care over the years 31 , 32 , 33 , 34 . Note that in recent years, cisplatin-based chemotherapy and radiotherapy for advanced cervical cancer and inoperable early cervical cancer have been widely used in clinical practice, which has been shown to improve patient prognosis 35 , 36 .
In this study, we also demonstrated the overall survival rate for subjects with and without cancer recurrence separately and showed that cancer recurrence significantly reduced the overall survival rate, especially over the long term. The 5-year survival probability for patients with and without recurrence was 54.0% and 79.5%, and the 10-year survival probability was 28.7% and 63.6%, respectively. Such differences in survival between patients with and without recurrence were observed in many previous studies 6 , 37 , and these findings re-emphasized the importance of prevention of cancer recurrence in the management of cervical cancer.
Our study had several limitations. First, it was performed on patients in one hospital in South China, which may limit the ability to extrapolate these findings to the different settings. Also, the database is only available until 2010, and it would be necessary to conduct follow-up studies to update the recurrence rate data using more recent and comprehensive data records. Despite that, this study had a relatively large sample size with 5–19 years of follow-up, which provided a rare opportunity to study the overall trend of long-term recurrence rate for cervical cancer patients. Second, a small number of patients were lost to follow-up, which may lead to loss to follow-up bias, although the number has been kept to minimal as we have conducted several rounds of follow-up with multiple tracing methods. Third, despite the large overall sample size, we were not able to conduct multiple stratification analyses due to limited sample size after stratification, and we combined patients in clinical stages III and IV due to the small number of subjects in Stage IV. Fourth, we found that a very small proportion of subjects had a short disease-free time of fewer than 3 months after completion of the primary treatment, and these patients may have a disease progression rather than cancer relapse. However, we conducted a sensitivity analysis that excluded these subjects and reported consistent findings in the Cox regression.
In this study, we reported the pattern of cumulative recurrence rate using a study with 5–19 years of follow-up. The overall cumulative recurrence rate was 3.8% in the first year and increased to 8.0% in the fifth year, then the recurrence rate stayed at 9.7% in the 10th year and 10.8% in the 15th year. The median time to recurrence in this study was 15.5 months. Also, we reported that younger age, lower parity, a greater number of miscarriages, higher clinical stage, lymph node metastasis, and received chemotherapy were significantly associated with cancer recurrence. Patients with recurrence have a much poorer prognosis in terms of survival probability compared to those without recurrence. This study may provide important evidence for designing targeted interventions for cervical cancer treatment.
Material and methods
Study population.
A retrospective cohort study was conducted for patients with cervical cancer that received cancer treatment from a cancer specialist hospital in Hunan Province, China from January 1992 to December 2005. The inclusion criteria were as follows: (1) subject was diagnosed with cervical cancer for the first time and had a clear clinicopathological diagnosis report; (2) subject had completed initial treatment in the study hospital and had a complete medical record; (3) subject was able to record the condition independently or with help to complete the follow-up activities; (4) age of the subject was greater than 18 years old. The exclusion criteria included: (1) subject had cognitive or communication dysfunction, or was unable to cooperate with the investigation; (2) subject was pregnant or had other severe diseases, including severe heart failure, renal failure, deep vein thrombosis, severe peripheral neuropathy, severe arterial insufficiency, other malignancies, etc.; (3) subject and family members did not cooperate with the recruitment process.
Informed written consent was obtained from all participants. Ethical approval was obtained from the Ethics Committee of Xiangya School of Public Health, Central South University, and all research was performed in accordance with the Declaration of Helsinki.
Data collection and follow-up
Baseline information was collected by trained doctors through face-to-face interviews, phone calls, and the extraction of medical records. The trained doctor was responsible for filling in a questionnaire that included baseline characteristics (i.e. age, gravidity, parity, and history of miscarriage), clinicopathological characteristics (i.e. duration of discomfort before diagnosis, clinical stage, pathological type, and level of differentiation), and treatment plan (i.e. surgical method, radiotherapy method, and chemotherapy method). Clinical stage was classified according to the 2009 International Federation of Gynecology and Obstetrics (FIGO 2009) staging criteria, and lymph node metastasis was classified into Yes or No based on postoperative pathological biopsy results. The pathological type and degree of differentiation were obtained according to the pathological examination report. Treatment received referred to the treatment received after the first diagnosis, which was classified into the following categories: surgery only, surgery plus adjuvant therapy, radiotherapy only, concurrent chemotherapy and radiotherapy, and chemotherapy only. The treatment plan was decided by the physician-in-charge according to the standard treatment guidelines based on the patient’s condition and willingness for treatment. The post-treatment follow-up for subjects was planned for every three to 6 months during the first 5 years, and once a year afterward according to treatment guidelines in China 38 . Follow-up for subjects’ recurrence in this study was conducted every year through telephone, mail, and extraction of medical records by trained researchers after the patient was discharged from the hospital until December 31, 2010. Subjects lost to follow-up were censored, and subjects completely lost to follow-up were excluded from the data analysis. Cervical cancer recurrence was defined based on clinical–pathological diagnosis results, and this study was focused on the first recurrence of cervical cancer after the first treatment.
Statistical analysis
Descriptive statistics were used for patient characteristics and summarized as mean \(\mp\) SD or percentages. Differences in baseline characteristics and clinicopathological characteristics and treatment plan of the study participants with and without recurrent cervical cancer during follow-up were examined using student t-tests or chi-square tests, as appropriate. Average time to recurrence was presented with median and interquartile range (IQR) and a Log-rank test was conducted. Kaplan-Meier survival curves were used to plot the recurrence rate and survival rate after recurrence, stratified by different risk factors. Cox proportional hazards regression was used to calculate the hazard ratio (HR) and 95% confidence intervals (95% CIs) for cervical cancer recurrence and mortality. Proportional hazards assumptions were tested using Schoenfeld tests (Supplemental Table 2 ). Univariate analyses were conducted to evaluate patient characteristics associated with cervical cancer recurrence and mortality. The final multivariate model was selected based on the univariate analysis and biological significance, and variance inflation factors were used to test the collinearity between variables. Age, clinical stage, and treatment received were excluded from Cox regression for mortality due to violation of proportional hazards assumptions. A sensitivity analysis was conducted using Cox regression on cancer recurrence by excluding subjects with recurrence within 3 months as these subjects may have a disease progression rather than cancer relapse. We also conducted sensitivity analyses among subjects with clinical stage I and II using Cox regressions to explore factors associated with cancer recurrence and overall mortality. All statistical analyses were conducted using R version 4.0.5.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Peirson, L., Fitzpatrick-Lewis, D., Ciliska, D. & Warren, R. Screening for cervical cancer: A systematic review and meta-analysis. Syst. Rev. 2 , 35. https://doi.org/10.1186/2046-4053-2-35 (2013).
Article PubMed PubMed Central Google Scholar
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 , 209–249. https://doi.org/10.3322/caac.21660 (2021).
Article PubMed Google Scholar
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63 , 11–30. https://doi.org/10.3322/caac.21166 (2013).
Peiretti, M. et al. Management of recurrent cervical cancer: A review of the literature. Surg. Oncol. 21 , e59-66. https://doi.org/10.1016/j.suronc.2011.12.008 (2012).
Article CAS PubMed Google Scholar
Waggoner, S. E. Cervical cancer. Lancet 361 , 2217–2225. https://doi.org/10.1016/S0140-6736(03)13778-6 (2003).
Taarnhøj, G. A. et al. Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005–2013: A national cohort study. Cancer 124 , 943–951. https://doi.org/10.1002/cncr.31165 (2018).
Colturato, L. F., Signorini Filho, R. C., Fernandes, R. C., Gebrim, L. H. & Oliani, A. H. Lymph node micrometastases in initial stage cervical cancer and tumoral recurrence. Int. J. Gynaecol. Obstet. 133 , 69–75. https://doi.org/10.1016/j.ijgo.2015.08.019 (2016).
Lee, W. M. et al. Clinicopathologic factors for central recurrence in patients with locally advanced bulky cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 161 , 219–223. https://doi.org/10.1016/j.ejogrb.2011.12.029 (2012).
Chen, Y. Y., Zhu, Y. M. & Wu, J. C. Prognosis of early stage cervical cancer according to patterns of recurrence. Cancer Manage. Res. 13 , 8131–8136. https://doi.org/10.2147/Cmar.S314384 (2021).
Article ADS CAS Google Scholar
Elit, L. et al. Follow-up for women after treatment for cervical cancer: A systematic review. Gynecol. Oncol. 114 , 528–535. https://doi.org/10.1016/j.ygyno.2009.06.001 (2009).
Qian, Q. et al. Analysis of treatment modalities and prognosis on microinvasive cervical cancer: A 10-year cohort study in China. J. Gynecol. Oncol. 25 , 293–300. https://doi.org/10.3802/jgo.2014.25.4.293 (2014).
Article CAS PubMed PubMed Central Google Scholar
Chai, Y. et al. Radical hysterectomy with adjuvant radiotherapy versus radical radiotherapy for FIGO stage IIB cervical cancer. BMC Cancer 14 , 63. https://doi.org/10.1186/1471-2407-14-63 (2014).
Cao, D. Y. et al. Comparisons of vaginal and abdominal radical trachelectomy for early-stage cervical cancer: Preliminary results of a multi-center research in China. Br. J. Cancer 109 , 2778–2782. https://doi.org/10.1038/bjc.2013.656 (2013).
Wang, J. et al. Patient age, tumor appearance and tumor size are risk factors for early recurrence of cervical cancer. Mol. Clin. Oncol. 3 , 363–366. https://doi.org/10.3892/mco.2014.465 (2015).
Shu, T. et al. Prognostic evaluation of postoperative adjuvant therapy for operable cervical cancer: 10 years’ experience of National Cancer Center in China. Chin. J. Cancer Res. 29 , 510–520. https://doi.org/10.21147/j.issn.1000-9604.2017.06.05 (2017).
Cohen, P. A., Jhingran, A., Oaknin, A. & Denny, L. Cervical cancer. Lancet 393 , 169–182. https://doi.org/10.1016/S0140-6736(18)32470-X (2019).
Lin, H. H. et al. Risk factors for recurrence in patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Obstet. Gynecol. 88 , 274–279. https://doi.org/10.1016/0029-7844(96)00145-7 (1996).
Lim, S., Lee, S. H., Lee, K. B. & Park, C. Y. The influence of number of high risk factors on clinical outcomes in patients with early-stage cervical cancer after radical hysterectomy and adjuvant chemoradiation. Obstet. Gynecol. Sci. 59 , 184–191. https://doi.org/10.5468/ogs.2016.59.3.184 (2016).
Wang, H. et al. Clinicopathological risk factors for recurrence after neoadjuvant chemotherapy and radical hysterectomy in cervical cancer. World J. Surg. Oncol. 11 , 301. https://doi.org/10.1186/1477-7819-11-301 (2013).
Murakami, I. et al. Analysis of pathological and clinical characteristics of cervical conization according to age group in Japan. Heliyon 6 , e05193. https://doi.org/10.1016/j.heliyon.2020.e05193 (2020).
Chan, J. K., Loizzi, V., Burger, R. A., Rutgers, J. & Monk, B. J. Prognostic factors in neuroendocrine small cell cervical carcinoma: A multivariate analysis. Cancer 97 , 568–574. https://doi.org/10.1002/cncr.11086 (2003).
Twu, N. F. et al. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: A Taiwanese Gynecologic Oncology Group (TGOG) study. Surg. Oncol. 25 , 229–235. https://doi.org/10.1016/j.suronc.2016.05.028 (2016).
Rotman, M. et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 65 , 169–176. https://doi.org/10.1016/j.ijrobp.2005.10.019 (2006).
Yang, Y. C. et al. Cervical cancer in young women in Taiwan: Prognosis is independent of papillomavirus or tumor cell type. Gynecol. Oncol. 64 , 59–63. https://doi.org/10.1006/gyno.1996.4543 (1997).
Li, Y. et al. Clinical outcomes observation in stage IIB-IIIB cervical cancer treated by adjuvant surgery following concurrent chemoradiotherapy. BMC Cancer 21 , 442. https://doi.org/10.1186/s12885-021-08146-3 (2021).
Chen, L. et al. Analysis of prognostic factors in patients with cervical squamous cell carcinoma of stage Ib and IIa. Zhonghua Fu Chan Ke Za Zhi 40 , 239–242 (2005).
ADS PubMed Google Scholar
Ruiz, A. M. et al. Proximity of first sexual intercourse to menarche and risk of high-grade cervical disease. J. Infect. Dis. 206 , 1887–1896. https://doi.org/10.1093/infdis/jis612 (2012).
Roset Bahmanyar, E. et al. Prevalence and risk factors for cervical HPV infection and abnormalities in young adult women at enrolment in the multinational PATRICIA trial. Gynecol. Oncol. 127 , 440–450. https://doi.org/10.1016/j.ygyno.2012.08.033 (2012).
Peters, W. A. et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 18 , 1606–1613. https://doi.org/10.1200/Jco.2000.18.8.1606 (2000).
Abu-Rustum, N. R. et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 18 , 660–666. https://doi.org/10.6004/jnccn.2020.0027 (2020).
Article Google Scholar
Tsikouras, P. et al. Cervical cancer: Screening, diagnosis and staging. J. BUON 21 , 320–325 (2016).
PubMed Google Scholar
Leath, C. A. & Monk, B. J. Twenty-first century cervical cancer management: A historical perspective of the gynecologic oncology group/NRG oncology over the past twenty years. Gynecol. Oncol. 150 , 391–397. https://doi.org/10.1016/j.ygyno.2018.06.023 (2018).
Tewari, K. S. & Monk, B. J. Recent achievements and future developments in advanced and recurrent cervical cancer: Trials of the Gynecologic Oncology Group. Semin. Oncol. 36 , 170–180. https://doi.org/10.1053/j.seminoncol.2008.12.008 (2009).
Leath, C. A. 3rd. & Straughn, J. M. Jr. Chemotherapy for advanced and recurrent cervical carcinoma: Results from cooperative group trials. Gynecol. Oncol. 129 , 251–257. https://doi.org/10.1016/j.ygyno.2012.12.035 (2013).
Rose, P. G. Combined-modality therapy of locally advanced cervical cancer. J. Clin. Oncol. 21 , 211s–217s. https://doi.org/10.1200/JCO.2003.01.222 (2003).
Lukka, H. & Johnston, M. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer: A meta-analysis. Clin. Oncol. (R Coll. Radiol.) 16 , 160–161. https://doi.org/10.1016/j.clon.2004.01.002 (2004).
Article CAS Google Scholar
Qiu, J. T. et al. Outcomes and prognosis of patients with recurrent cervical cancer after radical hysterectomy. Gynecol. Oncol. 127 , 472–477. https://doi.org/10.1016/j.ygyno.2012.08.008 (2012).
Tong, J. et al. Short-term recurrence and distant metastasis following robotic-assisted radical hysterectomy with pelvic lymphadenectomy and chemoradiotherapy for a stage IB1 cervical adenocarcinoma: A case report and literature review. Medicine (Baltimore) 98 , e15387. https://doi.org/10.1097/MD.0000000000015387 (2019).
Download references
Acknowledgements
We thank all the participants and researchers who collaborated on the execution of this project. The study was supported by National Natural Science Foundation of China (Grant number: 82003313).
Author information
These authors contributed equally: Jina Li and Gaoming Liu.
Authors and Affiliations
Xiangya School of Public Health, Central South University, No. 238 Shang Ma Yuan Ling Road, Changsha, 410008, Hunan, China
Jina Li, Jiayou Luo, Ping Ye, Jie Wang & Miyang Luo
Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, 410013, Hunan, China
Gaoming Liu & Shipeng Yan
You can also search for this author in PubMed Google Scholar
Contributions
J.Y.L., G.M.L., J.N.L. and S.P.Y. conceived and designed the study; J.Y.L., G.M.L. and S.P.Y. acquired the data; J.N.L., M.Y.L., J.Y.L., P.Y. and J.W. analyzed and interpreted the data; J.N.L. and M.Y.L wrote the manuscript; all authors provided revisions for the manuscript and approved it for submission.
Corresponding author
Correspondence to Miyang Luo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, J., Liu, G., Luo, J. et al. Cervical cancer prognosis and related risk factors for patients with cervical cancer: a long-term retrospective cohort study. Sci Rep 12 , 13994 (2022). https://doi.org/10.1038/s41598-022-17733-8
Download citation
Received : 21 February 2022
Accepted : 29 July 2022
Published : 17 August 2022
DOI : https://doi.org/10.1038/s41598-022-17733-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Time to death from cervical cancer and its predictors in hospitalized patients: a survival approach study in mato grosso, brazil.
- Sancho Pedro Xavier
- Kátia Moreira da Silva
- Ageo Mario Cândido da Silva
World Journal of Surgical Oncology (2024)
Whole-tumor histogram analysis of multiple non-Gaussian diffusion models at high b values for assessing cervical cancer
- Xiaohui Duan
Abdominal Radiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Zoom Journal Club
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 34, Issue Suppl 1
- 775 Prevalence and determinants of anemia in cervical cancer patients: a one-year retrospective study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Shoon Mya Aye
- Karuna Compassionate Care Center, Yangon, Myanmar
Introduction/Background Anemia is a prevalent condition among cancer patients, particularly those with gynecologic malignancies. Its causes are multifaceted, including tumor-specific and treatment-related factors, chronic inflammation, acute blood loss, and malnutrition. This study aimed to assess the prevalence and factors associated with pretreatment anemia among cervical cancer patients.
Methodology The study retrospectively analyzed the clinical data of 50 cervical cancer patients from Karuna Oncology Clinic between March 2022 and March 2023. The inclusion criteria were all patients with cervical cancer who gave consent. Those who had already received any cancer treatment and cases complicated by other primary tumors were excluded. A binary logistic regression model was used to identify covariates affecting the outcome variable
Results The study found that 48% of the cervical cancer patients had anemia before treatment, with 14% having severe anemia. The majority of the patients were Buddhists, urban dwellers, and married, with a mean age of 52.46 years. About 30% of the patients had a history of substance use, and 80% were in the advanced stages (IIB, III and IV). The occurrence of anemia was significantly associated with the advanced stages of cervical cancer [OR= 2.500, 95% CI (1.710–3.654)] [p < 0.001].
Conclusion The study concluded that anemia is a common condition among cervical cancer patients, with a significant association with the advanced stages of the disease. This finding underscores the importance of early detection and treatment of both cervical cancer and anemia, as they can significantly affect the patient’s quality of life. Further research is needed to explore strategies for managing anemia in patients with advanced cervical cancer. However, the findings should be interpreted in light of the study’s limitations, including its retrospective design and the relatively small sample size.
Disclosures I have no potential conflict of interest to report.
- View inline
- Download powerpoint
https://doi.org/10.1136/ijgc-2024-ESGO.201
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:

COMMENTS
In this case, the transverse maximum diameter of the primary tumor was 10.5 cm with bilateral parametrial infiltration, and in recent study, disease-free survival rate of the patients who have stage IIIB cervical cancer more than 8 cm was 16.7%. Another previous study revealed that hypoxia may contribute to lower local control of bulky tumor.
Introduction. In 2020, cervical carcinoma was responsible for 4272 cancer-related deaths in women, making it a significant cause of mortality according to the CDC .The main risk factors for cervical neoplasia include specific types of human papillomavirus (HPV), such as types 16, 18, 31, 33, and 45 .However, due to age-appropriate screening measures, the incidence and mortality rates of ...
Objective: The purpose of the study is to identify the risk factors such as age, the stage of patients based on the International Federation of Gynecology and Obstetrics system (FIGO stage) and treatment type, and their effect on the survival of cervical cancer patients receiving treatments at Jigme Dorji Wangchuck National Referral Hospital (JDWNRH) in Bhutan between January 2014 and December ...
Matalliotakis et al presented that among 647 patients with adenomyosis, 1.1% had cervical intraepithelial neoplasia and 0.8% had CC [Citation 9]. Li et al. reported that patients with CC are less likely to have a history of adenomyomas than those without CC in a nested case-control study [Citation 10]. In this study, we reviewed the medical ...
Tumor-infiltrating lymphocyte (TIL) therapy has shown promising results against several cancers. However, traditional lymphodepleting regimens are severe and represent a major limitation for a more widespread use of TIL. The modified pretreatment strategies may alleviate side effects and demonstrate the persistence of tumor-reactive T cells in the blood. Here, we report a case who was ...
Cervical cancer is widely preventable through screening, but little is known about the duration of protection offered by a negative screen in North America. ... Kamineni A, Weinmann S, et al. Cervical screening and cervical cancer death among older women: a population‐based, case‐control study. Cancer Epidemiol Biomarkers Prev 2014;179:1107 ...
In a double-blind, phase 3 trial, we randomly assigned patients with persistent, recurrent, or metastatic cervical cancer in a 1:1 ratio to receive pembrolizumab (200 mg) or placebo every 3 weeks ...
This study aims to explore the recurrence rate and overall survival for patients with cervical cancer after the first treatment and the related risk factors. A retrospective cohort study was ...
In this study, outcomes of patients with tumor size ≤ 2 cm were similar between the two groups (aHR, 1.15; 95% CI, 0.28 to 4.72; P = .85). However, only 89 patients had a tumor size of ≤ 2 cm, compared with our current study, which has 264 patients with tumors ≤ 2 cm on final pathology (excluding those with no tumor on final pathology).
Results The study found that 48% of the cervical cancer patients had anemia before treatment, with 14% having severe anemia. The majority of the patients were Buddhists, urban dwellers, and married, with a mean age of 52.46 years. About 30% of the patients had a history of substance use, and 80% were in the advanced stages (IIB, III and IV).