Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
Contributor Disclosures
Please read the Disclaimer at the end of this page.
INTRODUCTION — Chronic kidney disease (CKD) is defined by the presence of kidney damage or decreased glomerular filtration rate (GFR) for three or more months, irrespective of the cause ( table 1 ) [ 1 ]. This three-month duration distinguishes chronic from acute kidney disease. Additional details on the definitions and staging are presented at length elsewhere. (See "Definition and staging of chronic kidney disease in adults" and "Definition and staging criteria of acute kidney injury in adults" .)
For patients being evaluated for elevated serum creatinine or reduced estimated glomerular filtration rate (eGFR), it is important to distinguish those who have relatively stable CKD from those who have acute or subacute kidney injury, which may be ongoing and reversible. Acute kidney injury (AKI) is defined by a rise in the serum creatinine level that has developed within hours to days ( table 2 ). Subacute kidney injury (also called acute kidney disease) informally refers to any decline in kidney function that evolves over more than 48 hours but less than three months [ 2 ]. Diagnostic approach to these patients is presented in detail elsewhere. (See "Diagnostic approach to adult patients with subacute kidney injury in an outpatient setting" and "Evaluation of acute kidney injury among hospitalized adult patients" .)
An overview of the presentation and evaluation of patients with newly identified CKD is presented in this topic ( algorithm 1 ). Specific aspects of the evaluation are presented separately:
● Assessment of kidney function by eGFR. Estimation of the GFR requires that the patient is in steady state. (See "Assessment of kidney function" .)
● Careful examination of the urine by both qualitative chemical tests and microscopic examination. The urinary findings narrow the differential. (See "Urinalysis in the diagnosis of kidney disease" .)
● Radiologic imaging of the kidneys. (See "Radiologic assessment of kidney disease" .)
● Serologic testing and tissue diagnosis with kidney biopsy if noninvasive evaluation is insufficient for diagnosis. (See "Glomerular disease: Evaluation and differential diagnosis in adults" .)
The epidemiology and management of patients with CKD, as well as clinical presentation and evaluation of CKD in children are discussed elsewhere:
● (See "Epidemiology of chronic kidney disease" .)
● (See "Overview of the management of chronic kidney disease in adults" .)
● (See "Chronic kidney disease in children: Clinical manifestations and evaluation" .)
CLINICAL PRESENTATION — Patients with chronic kidney disease (CKD) may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no clinical symptoms, and kidney disease is often detected in these patients when an elevated serum creatinine, reduced estimated glomerular filtration rate (eGFR), or an abnormal urinalysis is discovered incidentally (when such tests are obtained as part of routine evaluation or for a possibly unrelated disorder). In addition, radiographic findings (eg, small and echogenic kidneys [by ultrasound] suggesting chronic damage, multiple bilateral kidney cysts with enlarged kidneys suggestive of polycystic kidney disease) may be observed on imaging performed for some other reason.
Depending upon the duration and severity of CKD, patients may also present with symptoms and/or signs of prolonged kidney failure, including weakness and easy fatigability, anorexia, vomiting, pruritus, and, in very advanced stages, with encephalopathy or seizures.
An abnormally reduced urine output (ie, oliguria or anuria) is seldom observed with CKD alone and always indicates at least some component of acute kidney injury (AKI). Oliguria or anuria may be present among patients with AKI superimposed on CKD, such as may be observed in a patient with chronic obstruction who develops acute urinary retention. Similarly, anuria as a result of severe or prolonged shock, bilateral urinary tract obstruction, pregnancy-related cortical necrosis, or bilateral renal arterial occlusion (eg, due to a dissecting aortic aneurysm) may occur in patients with underlying CKD. (See "Evaluation of acute kidney injury among hospitalized adult patients", section on 'Clinical manifestations' .)
The most common laboratory findings in patients with CKD include increased serum creatinine and blood urea nitrogen. Urine studies may show proteinuria (or albuminuria) and/or abnormal red or white blood cells on urine microscopy. (See "Assessment of kidney function" and "Urinalysis in the diagnosis of kidney disease" .)
Other common laboratory abnormalities that may be part of the clinical picture include anemia, hyperphosphatemia, hyperkalemia, metabolic acidosis, hypocalcemia, and elevated parathyroid hormone (PTH).
The degree to which these abnormalities are present depends upon the severity of CKD. Hyperphosphatemia is uncommon among patients with CKD with eGFR >45 mL/min/1.73 m 2 . PTH, on the other hand, may be mildly elevated even with a mild reduction of eGFR (ie, 50 to 60 mL/min/1.73 m 2 ). (See "Overview of chronic kidney disease-mineral and bone disorder (CKD-MBD)", section on 'Overview' .)
CAUSES OF CHRONIC KIDNEY DISEASE — The most common causes of chronic kidney disease (CKD) are poorly controlled diabetes mellitus and hypertension. These and other possible etiologies are discussed in detail below. (See 'Subsequent Evaluation' below.)
The causes of kidney injury are classically divided into three categories: prerenal; intrinsic renal; or postrenal. However, any cause of kidney injury, if sufficiently severe or long-standing, may lead to persistently abnormal kidney function and therefore CKD. As an example, a patient with severe heart failure may have recurrent or prolonged acute kidney injury (AKI) due to reduced effective arterial blood volume (ie, prerenal disease). Over time, even if cardiac function and kidney perfusion pressure improve, glomerular filtration rate (GFR) may never fully recover to normal.
In addition, whatever the initial cause of kidney disease, a sustained decrease in GFR can produce adaptive hyperfiltration within the remaining functional nephrons, which may lead to further injury and worsening of CKD. (See "Overview of the management of chronic kidney disease in adults", section on 'Natural history of kidney disease' and "Secondary factors and progression of chronic kidney disease", section on 'Intraglomerular hypertension and glomerular hypertrophy' .)
INITIAL ASSESSMENT AND TRIAGE — The initial assessment for all patients who present with suspected chronic kidney disease (CKD) starts with triage of those who may need urgent dialysis based upon symptoms or life-threatening laboratory abnormalities ( algorithm 1 ). In other patients, the time course of their kidney disease should be established to determine the role and timing of consultation with a nephrologist.
Identifying patients needing urgent dialysis — Patients with CKD may have absolute or relative indications for dialysis at the time that their kidney disease is discovered. Those who have refractory pulmonary edema, life-threatening hyperkalemia or metabolic acidosis, encephalopathy, or a pericardial rub should be referred to the emergency department for rapid evaluation and possible initiation of dialysis. These indications are discussed at length elsewhere. (See "Indications for initiation of dialysis in chronic kidney disease" .)
Even among patients not requiring urgent dialysis, most may benefit from early referral to a nephrologist. (See 'Indications for a nephrology evaluation' below.)
Determine the duration of kidney disease — Among patients who do not require dialysis, we start by evaluating the duration of the kidney disease. Typically, this entails assessing serial serum creatinine values (and associated estimated glomerular filtration rates [eGFRs]) over time. If urine tests or radiologic studies of the kidney are abnormal at the time of CKD discovery, temporal changes in these data should also be assessed.
Establishing the duration and trajectory of the disease accurately is fundamentally important and requires that older data be obtained for comparison. In some cases, it may be necessary to acquire this information from the patient's prior caregivers or from other health centers.
Along with the trend of any clinical symptoms, the trajectory of the laboratory abnormalities will determine if and when additional evaluation or nephrology referral is necessary. Recognition of a rapidly progressive process versus stable disease permits early intervention to curtail an active process and to preserve residual kidney function.
The importance of determining the trajectory of kidney disease is illustrated by the following examples:
● Consider a patient with no significant medical history and a current serum creatinine of 4 mg/dL (354 micromol/L) who had a creatinine of 0.6 mg/dL (53 micromol/L) one month earlier. This patient has acute or subacute kidney injury. This patient needs urgent evaluation and management to stop further injury and to optimize kidney recovery. (See "Diagnostic approach to adult patients with subacute kidney injury in an outpatient setting" .)
● By contrast, consider a different patient who has an identical current serum creatinine of 4 mg/dL (354 micromol/L). However, this patient has had long-standing, poorly-controlled diabetes mellitus and had a serum creatinine of 3.5 mg/dL (309 micromol/L) two years earlier, as well as chronically increased albuminuria. This patient likely has slowly progressive CKD. Although this patient will also benefit from nephrology referral, these laboratory data alone, without concurrent significant symptoms, would not justify urgent or extensive evaluation, since the process is less likely reversible. (See 'Clinical presentation' above and 'Indications for a nephrology evaluation' below.)
The determination of disease duration can also help distinguish between CKD and subacute kidney injury (also called acute kidney disease), although this distinction can be arbitrary. The clinical course of gradually progressive CKD is commonly punctuated by transient, small "spikes" in serum creatinine, which often improve to resume a prior long-term trajectory ( figure 1 ). However, the pace of eGFR decline may increase and, if the rate of decline becomes rapid, such patients may be more appropriately evaluated as subacute kidney injury rather than CKD. (See "Diagnostic approach to adult patients with subacute kidney injury in an outpatient setting", section on 'Evaluation' .)
When prior serum creatinine values, urine studies, or radiographic images are unavailable, certain findings from the history and physical examination, or subsequent laboratory or radiographic evaluation, may suggest the duration of disease [ 3 ]. As examples:
● New symptoms or signs, such as sudden onset of anasarca and discolored urine, suggest a more acute process.
● Oliguria (urine output <0.5 mL/kg/hour [often <500 to 600 mL/day]) or anuria in a patient not on maintenance dialysis indicates an acute process. Prolonged oliguria or anuria do not occur in slowly progressive CKD (even if advanced).
● A daily increase in serum creatinine after the initial discovery of an abnormal value indicates at least some component of an ongoing acute process. Conversely, a serum creatinine that does not change, or changes minimally, over weeks to months suggests the presence of CKD. Distinguishing CKD progression from subacute kidney injury may be difficult in the setting of a serum creatinine that is worsening gradually ( figure 1 ). The level of CKD, magnitude of eGFR decline, changes in clinical symptoms, and other factors (such as reliability of patient follow-up) should dictate the frequency of laboratory monitoring to clearly establish a trend. Overall, a rate of decrease of eGFR >5 mL/min/1.73 m 2 per year (or >25 percent decline in eGFR) should prompt early retesting to establish a clear trajectory and to rule out ongoing subacute injury.
● Imaging that reveals small, echogenic kidneys provides definitive evidence of chronicity of disease. However, the presence of normal-sized kidneys does not exclude chronicity, since some causes of CKD (such as diabetic kidney disease) are associated with preserved kidney size. Kidney parenchymal echogenicity (normally kidneys are less echogenic than the liver), if increased, suggests nonspecific diffuse kidney dysfunction [ 4-6 ]. (See "Radiologic assessment of kidney disease" .)
● Radiologic evidence of renal osteodystrophy such as subperiosteal bone resorption or loss of bone density at the distal third of the clavicles suggests CKD. (See "Overview of chronic kidney disease-mineral and bone disorder (CKD-MBD)" .)
Other findings are less helpful. As an example, anemia due to erythropoietin deficiency is a common (although not absolute) finding in CKD, but acute or subacute kidney injury can also be associated with anemia (from either hemolysis or bleeding). Although hyperphosphatemia commonly affects patients with CKD, it may also be seen in AKI or subacute kidney injury and, therefore, does not help distinguish acute from chronic disease. The absence of anemia or hyperphosphatemia does not exclude the presence of CKD.
SUBSEQUENT EVALUATION — Once the initial assessment and triage is complete, we perform an evaluation to identify the cause of chronic kidney disease (CKD) and identify individuals who may benefit from a nephrology consultation ( algorithm 1 ).
Evaluation to identify cause — We obtain a cause-specific history, perform a targeted physical examination, and, if not done recently, we obtain urine studies and an ultrasound to determine the cause as follows:
Targeted history — We specifically determine if there is a history of any of the following:
● Long-standing diabetes and hypertension commonly lead to CKD. The duration of disease (diabetes and/or hypertension) and the degree of control should be determined in such patients. In addition, the presence of diabetic or hypertensive retinopathy should be ascertained because patients with retinopathy have a higher likelihood of having CKD from diabetes and/or hypertension. (See "Clinical features, diagnosis, and treatment of hypertensive nephrosclerosis" and "Diabetic kidney disease: Pathogenesis and epidemiology" and "Overview of hypertension in acute and chronic kidney disease" .)
● Renovascular disease should be suspected among patients who have peripheral arterial disease in other vascular beds or who have multiple vascular risk factors, such as age over 50 years, hyperlipidemia, or cigarette smoking. Renovascular disease can present as CKD. Features that suggest CKD due to renovascular disease include resistant hypertension, recurrent flash pulmonary edema, or a reversible increase in serum creatinine after receiving antihypertensive therapy, particularly angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which improves after withdrawal of the drug. (See "Chronic kidney disease resulting from atherosclerotic renal artery stenosis" .)
● Patients should be asked about a history of prior severe or prolonged acute kidney injury (AKI) to determine if it could have contributed to their CKD. Prior AKI, particularly if dialysis-requiring, may lead to CKD. CKD may have developed even if the patient had sufficient recovery to stop dialysis. (See "Kidney and patient outcomes after acute kidney injury in adults", section on 'Determinants of kidney outcomes' .)
● Histories of obesity, heart failure, liver failure, autoimmune disease, recurrent and complicated urinary tract infections, and reduced kidney mass (eg, nephrectomy, renal agenesis) should be elicited due to their associations with CKD. (See "Overweight and obesity in adults: Health consequences", section on 'Chronic kidney disease' and "Cardiorenal syndrome: Definition, prevalence, diagnosis, and pathophysiology" and "Hepatorenal syndrome" and "Lupus nephritis: Diagnosis and classification" and "Overview of and approach to the vasculitides in adults" .)
● Inherited disorders, such as some cystic, interstitial, and glomerular kidney diseases, are relatively common causes of CKD. Thus, it is important to ask specific questions about kidney disease in family members, including their history of abnormal kidney imaging (eg, large kidneys with many cysts seen in polycystic kidney disease) or abnormal urine studies (eg, hematuria or proteinuria found in glomerular diseases). (See "Autosomal dominant polycystic kidney disease (ADPKD) in adults: Epidemiology, clinical presentation, and diagnosis" and "Autosomal dominant tubulointerstitial kidney disease" and "Focal segmental glomerulosclerosis: Genetic causes" and "Fabry disease: Kidney manifestations" and "C3 glomerulopathies: Dense deposit disease and C3 glomerulonephritis", section on 'Pathogenesis' and "IgA nephropathy: Pathogenesis", section on 'Genetic susceptibility' .)
● Patients who have a history of cancer (eg, myeloma or renal cell carcinoma) and treatment with chemotherapy or radiotherapy may develop CKD from the cancer itself or from its treatment. Many patients with multiple myeloma, for example, have AKI at the time of diagnosis; such patients often develop CKD as a result of incomplete recovery from AKI. Patients with renal cell carcinoma often require a partial or complete nephrectomy, which can lead to CKD from decreased kidney mass. In addition, patients who are treated with chemotherapy using cisplatin , ifosfamide , immune checkpoint inhibitors, or newer molecular-targeted agents may develop CKD as an adverse consequence of their treatment. (See "Overview of kidney disease in patients with cancer" and "Kidney disease in multiple myeloma and other monoclonal gammopathies: Etiology and evaluation" and "Nephrotoxicity of molecularly targeted agents and immunotherapy" and "Cisplatin nephrotoxicity" and "Ifosfamide nephrotoxicity" .)
● Urinary tract obstruction should be suspected among patients who have a history of prior urological surgery, prior pelvic or retroperitoneal surgery, a known or suspected abdominal or retroperitoneal malignancy, neurologic disease involving the bladder, gross hematuria, lower abdominal, pelvic, or flank pain, or in men with lower urinary tract symptoms. (See "Clinical manifestations and diagnosis of urinary tract obstruction (UTO) and hydronephrosis" and "Chronic urinary retention in females" and "Lower urinary tract symptoms in males" .)
● Clinicians should inquire about risk factors for human immunodeficiency, hepatitis C, or hepatitis B virus infections, such as a history of intravenous drug use or sexually transmitted disease. Patients with risk factors should be tested for the presence of these viruses. Because these viruses may cause a variety of kidney diseases, these etiologies should also be considered when the underlying cause of CKD is not clear. (See "Kidney disease in patients with HIV" and "Kidney disease associated with hepatitis B virus infection" and "Overview of kidney disease associated with hepatitis C virus infection" .)
● Medications should be reviewed, including for potentially nephrotoxic medications that the patient used in the past (even if not currently using). As examples, prolonged use of lithium for psychiatric conditions, certain Chinese herbs from "slimming clinics," or analgesic combination agents may each cause chronic interstitial injury that leads to CKD. In addition, drugs that can precipitate allergic interstitial nephritis may lead to CKD since such patients often do not have complete recovery of kidney function. (See "Renal toxicity of lithium" and "Nephropathy induced by aristolochic acid (AA) containing herbs" and "Clinical manifestations and diagnosis of analgesic nephropathy" .)
● Geography may be a clue to the cause of CKD. A history of agricultural work in hot environments or history of exposure to pesticides and other agrochemicals is associated with CKD of unknown cause (ie, "CKDu," also called Mesoamerican nephropathy). Such a history should raise suspicion for this disorder as the cause of CKD. Patients who lived for many years in particular regions of the Balkans may be at risk for a different chronic tubulointerstitial nephropathy that is slowly progressive. In endemic regions, infections such as schistosomiasis and tuberculosis may be common causes of CKD. (See "Mesoamerican nephropathy" and "Balkan endemic nephropathy" and "Schistosomiasis and glomerular disease" and "Urogenital tuberculosis" .)
● A review of toxic environmental or occupational contaminants may reveal exposure to lead, which is associated with CKD. A history of lead mining, plumbing, auto-repair work, or shipbuilding may be associated with significant lead exposure. (See "Lead nephropathy and lead-related nephrotoxicity" .)
Targeted physical examination — We perform a physical exam to elicit the following signs that may suggest a specific etiology of kidney disease:
● Signs of volume overload may indicate the presence of heart failure or cirrhosis, which are associated with CKD.
● Signs of volume depletion, such as may occur with a chronic diarrheal syndrome or a high-output bowel stoma, may suggest a longstanding state of prerenal azotemia with risk of recurrent AKI, thereby leading to CKD.
● The presence of arteriovenous nicking or retinopathy on funduscopic examination can be seen in chronic hypertensive microvascular disease ( image 1 ), which may also involve the kidney.
● An abdominal bruit or abnormal distal pulses may be detected in patients with renal artery stenosis.
● Enlarged kidneys that are palpable on examination may suggest polycystic kidney disease.
● Peripheral neuropathy may be associated with diabetic microvascular disease or another disorder that causes dysautonomia (such as a paraproteinemia).
● Rashes and skin lesions, such as may occur with leukocytoclastic vasculitis or purpura, suggests that small vessel vasculitis may be the cause of CKD ( picture 1 and picture 2 ).
● Skin thickening and hardening as may be seen with systemic sclerosis (scleroderma), an uncommon cause of CKD ( figure 2 and picture 3 ).
Targeted laboratory assessment — Initial testing for all patients should include:
● Basic metabolic panel that includes serum creatinine for calculation of the estimated glomerular filtration rate (eGFR).
● Complete blood count with white cell differential ("CBC with diff").
● Urinalysis using reagent test strips (dipstick) and automated urine microscopy.
● If an experienced operator is available for manual review of urine microscopy, such a detailed examination of the urine may further guide the evaluation. Urine microscopy is especially helpful if there are cellular elements (red blood cells and/or white blood cells), cellular or granular casts, or crystals in the urine. In the absence of an experienced urine microscopist, or if the urinalysis and automated microscopy are normal, manual review may generally be deferred. (See "Urinalysis in the diagnosis of kidney disease" .)
● Quantification of urine protein and albumin by random (or "spot") protein-to-creatinine ratio (UPCR) and albumin-to-creatinine ratio (UACR); each provides slightly different but related information. Our practice is to check both UPCR and UACR concurrently, and, in some cases, a 24-hour urine collection for protein and creatinine at least once early in the evaluation and to follow one of the values over time. A more detailed discussion of proteinuria is presented elsewhere. (See "Assessment of urinary protein excretion and evaluation of isolated non-nephrotic proteinuria in adults" .)
● In addition, in patients older than 40 years of age who have hypercalcemia, severe anemia, bony lesions suggestive of multiple myeloma, or a progressively worsening eGFR without an obvious cause, we also obtain a serum and urine protein electrophoresis with immunofixation and a serum free light chain assay. (See "Kidney disease in multiple myeloma and other monoclonal gammopathies: Etiology and evaluation", section on 'Evaluation' .)
Specific abnormalities in blood and urine studies guide the utility and timing of further evaluation and referral. In addition to a rapidly declining eGFR, other laboratory abnormalities may require directed evaluation and management by a nephrologist. As an example, microscopic hematuria and/or increased proteinuria (as detected by UPCR or UACR) often prompts specific serologic testing to investigate possible glomerular disease. Sterile pyuria, especially if coupled with peripheral eosinophilia, may direct further testing for a hypersensitivity reaction or autoimmune disease or, in endemic regions, renal tuberculosis. The combination of metabolic acidosis with hyper- or hypokalemia may indicate the presence of a renal tubular acidosis, which is a notable feature in some etiologies of CKD. Evidence of a monoclonal gammopathy should prompt a referral to nephrology or hematology for further evaluation of related kidney or plasma cell diseases. (See 'Indications for a nephrology evaluation' below and "Clinical manifestations and diagnosis of acute interstitial nephritis" and "Glomerular disease: Evaluation and differential diagnosis in adults" and "Kidney disease in primary Sjögren's disease" and "Kidney disease in sarcoidosis" and "Urogenital tuberculosis", section on 'Renal and urologic tuberculosis' and "Kidney disease in multiple myeloma and other monoclonal gammopathies: Etiology and evaluation" and "Renal amyloidosis" and "Membranoproliferative glomerulonephritis: Classification, clinical features, and diagnosis", section on 'Monoclonal gammopathies' .)
Depending upon further testing by a nephrologist, a kidney biopsy may be warranted. (See 'Indications for a nephrology evaluation' below.)
In some cases of newly identified CKD with stable, mild laboratory abnormalities, expectant management with watchful waiting, and without nephrology referral, is reasonable. A patient, for example, with stable eGFR >45 mL/min/1.73 m 2 , normal urine microscopy, UPCR <500 mg/g and UACR <300 mg/g may not require extensive serologic testing or kidney biopsy. (See 'Stable patients not in need of a nephrology evaluation' below.)
Targeted radiologic assessment — Unless recent abdominal imaging is available, we obtain a kidney ultrasound in all patients at the time of their initial evaluation for CKD. Abnormalities in kidney imaging may warrant urologic evaluation and urodynamic studies. (See "Radiologic assessment of kidney disease" .)
Patients who have evidence of urinary tract obstruction (ie, hydronephrosis) on ultrasound require further investigation to determine the cause and duration, and to establish reversibility of kidney injury. Early recognition and correction of urinary obstruction can help salvage kidney function. (See "Clinical manifestations and diagnosis of urinary tract obstruction (UTO) and hydronephrosis" .)
Patients who are at a high risk for renovascular disease should have dedicated imaging to evaluate for renal artery stenosis. Vascular duplex ultrasound of the renal arteries is often a first step. Depending upon each institution's radiologic expertise, computed tomography angiography and/or magnetic resonance angiography may be obtained. This imaging is used in conjunction with vascular surgery or interventional radiology evaluation to determine the possible role of revascularization versus medical management. (See "Chronic kidney disease resulting from atherosclerotic renal artery stenosis" .)
Indications for a nephrology evaluation — Based upon the targeted history, physical examination, laboratory testing, and imaging discussed above, further evaluation with additional tests and kidney biopsy may be warranted. (See "Definition and staging of chronic kidney disease in adults", section on 'Referral to a specialist' and "Overview of the management of chronic kidney disease in adults", section on 'Referral to nephrologists' .)
With increasing accessibility of telehealth visits and electronic consults that can often be completed more efficiently than traditional in-office patient visits, a subspecialty referral may now include a spectrum of possible scenarios. In some cases, a nephrologist may review the electronic medical record for the history, medications, and laboratory data and determine that a formal referral for full evaluation is appropriate. In other cases, she or he may simply render education and guidance to a primary care clinician on further evaluation, if necessary, and on issues of surveillance and management of stable disease. Local practice may vary along this spectrum. In an adult with newly identified CKD, indications for consultation with a nephrologist include:
● eGFR <30 mL/min/1.73 m 2
● Persistent UACR ≥300 mg/g (34 mg/mmol)
● Persistent UPCR ≥500 mg/g (56.5 mg/mmol)
● Abnormal urine microscopy (cellular casts, nonurologic hematuria, sterile pyuria)
● Personal history of systemic autoimmune disease
● Large cystic kidneys by kidney imaging or examination
● Known history of multiple myeloma or monoclonal gammopathy
● Evidence of relatively rapid loss of kidney function (reduction in eGFR >5 mL/min/1.73 m 2 per year or decline >25 percent); because there is common physiologic variability, repeat lab testing within one to two months (or sooner in some cases) may be warranted to clearly establish trajectory of eGFR change ( figure 1 )
● Single kidney with eGFR <60 mL/min/1.73 m 2
● Inability to identify a presumed cause of CKD, especially in younger patients
● Difficult to manage laboratory abnormalities such as hyperkalemia, metabolic acidosis, anemia requiring erythropoietin therapy, hyperphosphatemia, or hypocalcemia
● Resistant hypertension
● Recurrent or extensive nephrolithiasis
● Pregnancy
● Confirmed or presumed hereditary kidney disease, such as polycystic kidney disease, Alport syndrome, or autosomal dominant interstitial kidney disease
● Difficult to manage complications of various medications, such as chemotherapeutic agents that may cause kidney injury or increase proteinuria
Other patients do not require nephrology evaluation. As an example, patients who have an eGFR that does not change over sequential measurements, who have minimal or no proteinuria, and who have absence of cellular elements on urine microscopy undergo a limited evaluation. A kidney biopsy is rarely performed in such patients and the cause of CKD may not be identified with certainty. (See 'Stable patients not in need of a nephrology evaluation' below.)
Stable patients not in need of a nephrology evaluation — Many patients will have the cause of their kidney disease become apparent with the evaluation described above. (See 'Evaluation to identify cause' above.)
However, the cause may not be apparent in some despite a thorough evaluation. Among such patients, further evaluation and management depends in part upon whether the kidney disease is progressive or stable. We monitor serum creatinine within six weeks after CKD was initially recognized. In some cases, the test should be repeated even sooner to determine if there is evidence of rapid progression (ie, an eGFR decline of >5 mL/min/1.73 m 2 per year or decline >25 percent). Those with rapid progression need to be evaluated by nephrology. (See 'Indications for a nephrology evaluation' above.)
Patients who have stable, mild to moderate kidney disease (ie, that is not progressive) can be monitored every three to six months (or a longer interval if laboratory studies and clinical status are clearly stable) for the development of the following findings that would benefit from a nephrology evaluation (see 'Indications for a nephrology evaluation' above):
● A decline in the eGFR to <30 mL/min/1.73 m 2 .
● A persistent increase in either the UACR to ≥300 mg/g (34 mg/mmol) or the UPCR to ≥500 mg/g (56.5 mg/mmol).
● Development of new clinical evidence of autoimmune disease or monoclonal gammopathy. This may be detected by new rash, arthritis, bone pain, cytopenias, or other clinical changes that are otherwise unexplained and were not present at initial evaluation.
● A change in the pace of eGFR decline, such that the patient is rapidly losing kidney function (ie, loss of eGFR >5 mL/min/1.73 m 2 per year or >25 percent decline in eGFR).
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Chronic kidney disease in adults" .)
INFORMATION FOR PATIENTS — UpToDate offers two types of patient education materials, "The Basics" and "Beyond the Basics." The Basics patient education pieces are written in plain language, at the 5 th to 6 th grade reading level, and they answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon.
Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients. (You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword(s) of interest.)
● Basics topics (see "Patient education: Chronic kidney disease (The Basics)" and "Patient education: Acute kidney injury (The Basics)" )
● Beyond the Basics topics (see "Patient education: Chronic kidney disease (Beyond the Basics)" and "Patient education: Dialysis or kidney transplantation — which is right for me? (Beyond the Basics)" and "Patient education: Hemodialysis (Beyond the Basics)" and "Patient education: Peritoneal dialysis (Beyond the Basics)" and "Patient education: Protein in the urine (proteinuria) (Beyond the Basics)" and "Patient education: Split urine collection for orthostatic proteinuria (Beyond the Basics)" )
SUMMARY AND RECOMMENDATIONS
● Chronic kidney disease (CKD) versus acute kidney disease or injury – CKD is defined by the presence of kidney damage or reduced glomerular filtration rate (GFR) for three or more months, irrespective of the cause ( table 1 ). Subacute kidney injury (also called acute kidney disease) informally refers to any decline in kidney function that evolves over more than 48 hours but less than three months. Acute kidney injury (AKI) is defined by a rise in the serum creatinine level that has developed within hours to days ( table 2 ). (See 'Introduction' above and "Diagnostic approach to adult patients with subacute kidney injury in an outpatient setting" and "Evaluation of acute kidney injury among hospitalized adult patients" .)
● Clinical presentation – Patients with CKD may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no clinical symptoms, and kidney disease is often detected in these patients when an elevated serum creatinine, reduced estimated GFR (eGFR), or an abnormal urinalysis is discovered incidentally (when such tests are obtained as part of routine evaluation or for a possibly unrelated disorder). In addition, radiographic findings (eg, multiple bilateral kidney cysts with enlarged kidneys suggestive of polycystic kidney disease) may be observed on imaging performed for some other reason. (See 'Clinical presentation' above.)
● Initial assessment and triage – The initial assessment for patients who present with suspected CKD starts with triage of those who may need urgent dialysis based upon symptoms or life-threatening laboratory abnormalities ( algorithm 1 ).
• Identification of patients needing urgent dialysis – Patients who have refractory pulmonary edema, life-threatening hyperkalemia or metabolic acidosis, encephalopathy, or a pericardial rub should be referred to the emergency department for rapid evaluation and possible initiation of dialysis. (See 'Identifying patients needing urgent dialysis' above.)
• Evaluation of disease duration – Among patients who do not require dialysis, we start by evaluating the duration of the kidney disease. Typically, this entails assessing serial serum creatinine values (and associated eGFRs) over time. If urine tests or radiologic studies of the kidney are abnormal at the time of CKD discovery, temporal changes in these data should also be assessed. Establishing the duration and trajectory of the disease accurately is fundamentally important and requires that older data be obtained for comparison. In some cases, it may be necessary to acquire this information from the patient's prior caregivers or from other health centers. When prior serum creatinine values, urine studies, or radiographic images are unavailable, certain findings from the history and physical examination, or subsequent laboratory or radiographic evaluation, may suggest the duration of disease. (See 'Determine the duration of kidney disease' above.)
● Subsequent evaluation – Once the initial assessment and triage is complete, we perform an evaluation to identify the cause of CKD and identify individuals who may benefit from a nephrology consultation ( algorithm 1 ).
• Evaluation to identify cause – We obtain a cause-specific history, perform a targeted physical examination, and, if not done recently, we obtain urine studies and an ultrasound to determine the cause. (See 'Evaluation to identify cause' above.)
• Indications for a nephrology evaluation – Based upon the targeted history, physical examination, laboratory testing, and imaging, further evaluation with additional tests and kidney biopsy may be warranted. Some adults with newly identified CKD should be referred to a nephrologist. (See 'Indications for a nephrology evaluation' above.)
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3:1.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012; 2:1.
- Rose BD. Pathophysiology of Renal Disease, 2nd ed., McGraw-Hill, New York 1987. p.41.
- Moghazi S, Jones E, Schroepple J, et al. Correlation of renal histopathology with sonographic findings. Kidney Int 2005; 67:1515.
- Manley JA, O'Neill WC. How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am J Kidney Dis 2001; 37:706.
- Platt JF, Rubin JM, Bowerman RA, Marn CS. The inability to detect kidney disease on the basis of echogenicity. AJR Am J Roentgenol 1988; 151:317.
- Patient Care & Health Information
- Diseases & Conditions
- End-stage renal disease
End-stage renal disease, also called end-stage kidney disease or kidney failure, occurs when chronic kidney disease — the gradual loss of kidney function — reaches an advanced state. In end-stage renal disease, your kidneys no longer work as they should to meet your body's needs.
Your kidneys filter wastes and excess fluids from your blood, which are then excreted in your urine. When your kidneys lose their filtering abilities, dangerous levels of fluid, electrolytes and wastes can build up in your body.
With end-stage renal disease, you need dialysis or a kidney transplant to stay alive. But you can also choose to opt for conservative care to manage your symptoms — aiming for the best quality of life during your remaining time.
How kidneys work
One of the important jobs of the kidneys is to clean the blood. As blood moves through the body, it picks up extra fluid, chemicals and waste. The kidneys separate this material from the blood. It's carried out of the body in urine. If the kidneys are unable to do this and the condition is untreated, serious health problems result, with eventual loss of life.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Early in chronic kidney disease, you might have no signs or symptoms. As chronic kidney disease progresses to end-stage renal disease, signs and symptoms might include:
- Loss of appetite
- Fatigue and weakness
- Changes in how much you urinate
- Chest pain, if fluid builds up around the lining of the heart
- Shortness of breath, if fluid builds up in the lungs
- Swelling of feet and ankles
- High blood pressure (hypertension) that's difficult to control
- Difficulty sleeping
- Decreased mental sharpness
- Muscle twitches and cramps
- Persistent itching
- Metallic taste
Signs and symptoms of kidney disease are often nonspecific, meaning they can also be caused by other illnesses. Because your kidneys can make up for lost function, signs and symptoms might not appear until irreversible damage has occurred.
When to seek care
Make an appointment with your health care provider if you have signs or symptoms of kidney disease.
If you have a medical condition that increases your risk of kidney disease, your care provider is likely to monitor your kidney function with urine and blood tests and your blood pressure during regular office visits. Ask your provider whether these tests are necessary for you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
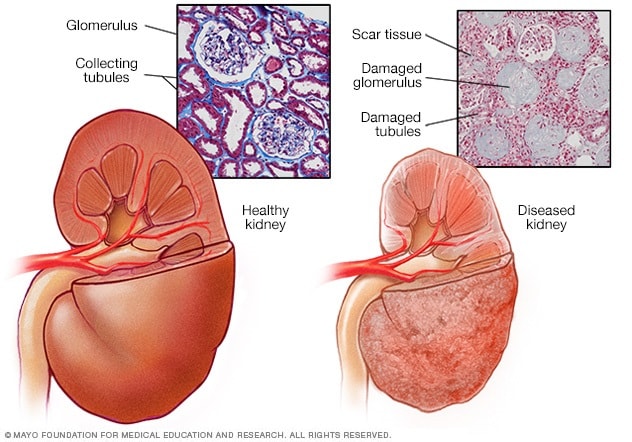
Healthy kidney vs. diseased kidney
A typical kidney has about 1 million filtering units. Each unit, called a glomerulus, joins a tubule. The tubule collects urine. Conditions such as high blood pressure and diabetes harm kidney function by damaging these filtering units and tubules. The damage causes scarring.
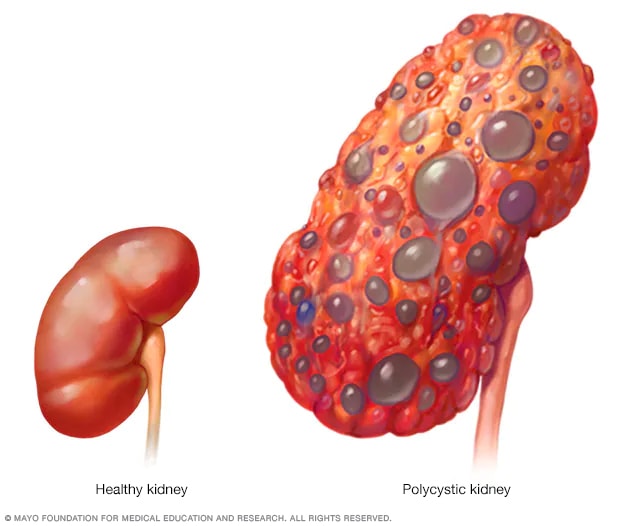
Polycystic kidney
A healthy kidney (left) eliminates waste from the blood and maintains the body's chemical balance. With polycystic kidney disease (right), fluid-filled sacs called cysts develop in the kidneys. The kidneys grow larger and gradually lose the ability to function as they should.
Kidney disease occurs when a disease or condition impairs kidney function, causing kidney damage to worsen over several months or years. For some people, kidney damage can continue to progress even after the underlying condition is resolved.
Diseases and conditions that can lead to kidney disease include:
- Type 1 or type 2 diabetes
- High blood pressure
- Glomerulonephritis (gloe-mer-u-low-nuh-FRY-tis) — an inflammation of the kidney's filtering units (glomeruli)
- Interstitial nephritis (in-tur-STISH-ul nuh-FRY-tis), an inflammation of the kidney's tubules and surrounding structures
- Polycystic kidney disease or other inherited kidney diseases
- Prolonged obstruction of the urinary tract, from conditions such as enlarged prostate, kidney stones and some cancers
- Vesicoureteral (ves-ih-koe-yoo-REE-tur-ul) reflux, a condition that causes urine to back up into your kidneys
- Recurrent kidney infection, also called pyelonephritis (pie-uh-low-nuh-FRY-tis)
Risk factors
Certain factors increase the risk that chronic kidney disease will progress more quickly to end-stage renal disease, including:
- Diabetes with poor blood sugar control
- Kidney disease that affects the glomeruli, the structures in the kidneys that filter wastes from the blood
- Polycystic kidney disease
- Tobacco use
- Black, Hispanic, Asian, Pacific Islander or American Indian heritage
- Family history of kidney failure
- Frequent use of medications that could be damaging to the kidney
Complications
Kidney damage, once it occurs, can't be reversed. Potential complications can affect almost any part of your body and can include:
- Fluid retention, which could lead to swelling in your arms and legs, high blood pressure, or fluid in your lungs (pulmonary edema)
- A sudden rise in potassium levels in your blood (hyperkalemia), which could impair your heart's ability to function and may be life-threatening
- Heart disease
- Weak bones and an increased risk of bone fractures
- Decreased sex drive, erectile dysfunction or reduced fertility
- Damage to your central nervous system, which can cause difficulty concentrating, personality changes or seizures
- Decreased immune response, which makes you more vulnerable to infection
- Pericarditis, an inflammation of the saclike membrane that envelops your heart (pericardium)
- Pregnancy complications that carry risks for the mother and the developing fetus
- Malnutrition
- Irreversible damage to your kidneys (end-stage kidney disease), eventually requiring either dialysis or a kidney transplant for survival
If you have kidney disease, you may be able to slow its progress by making healthy lifestyle choices:
- Achieve and maintain a healthy weight
- Be active most days
- Limit protein and eat a balanced diet of nutritious, low-sodium foods
- Control your blood pressure
- Take your medications as prescribed
- Have your cholesterol levels checked every year
- Control your blood sugar level
- Don't smoke or use tobacco products
- Get regular checkups
End-stage renal disease care at Mayo Clinic
Living with end-stage renal disease?
Connect with others like you for support and answers to your questions in the Transplants support group on Mayo Clinic Connect, a patient community.
Transplants Discussions

22 Replies Wed, Apr 24, 2024

35 Replies Wed, Apr 24, 2024

16 Replies Tue, Apr 23, 2024
- Goldman L, et al., eds. Chronic kidney disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. http://www.clinicalkey.com. Accessed April 27, 2021.
- Chronic kidney disease (CKD). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd#:~:text=Chronic%20kidney%20disease%20(CKD)%20means,family%20history%20of%20kidney%20failure. Accessed April 26, 2021.
- Rosenberg M. Overview of the management of chronic kidney disease in adults. https://www.uptodate.com/contents/search. Accessed April 26, 2021.
- Chronic kidney disease. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/chronic-kidney-disease/chronic-kidney-disease?query=Chronic%20kidney%20disease. Accessed April 26, 2021.
- Office of Patient Education. Chronic kidney disease treatment options. Mayo Clinic; 2020.
- Are you at increased risk for chronic kidney disease (CKD)? National Kidney Foundation. https://www.kidney.org/atoz/content/atriskckd. Accessed May 25, 2021.
- Warner KJ. Allscripts EPSi. Mayo Clinic. April 12, 2021.
- Hemodialysis
- Kidney transplant
- Peritoneal dialysis
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
End-stage renal disease.
Muhammad F. Hashmi ; Onecia Benjamin ; Sarah L. Lappin .
Affiliations
Last Update: August 28, 2023 .
- Continuing Education Activity
More than 500,000 people in the United States live with end-stage renal disease (ESRD). The development of chronic kidney disease (CKD) and its progression to this terminal disease remains a significant source of reduced quality of life and premature mortality. The Kidney Disease Improving Global Outcomes (KDIGO) define CKD using markers of kidney damage, specifically the ones that determine proteinuria and glomerular filtration rate. Many chronic diseases can cause end-stage renal disease. In many developed and developing countries, diabetes mellitus is the leading cause. This activity explains when this condition should be considered in the differential diagnosis and how to evaluate this condition properly. Furthermore, it highlights the interprofessional team's role in caring for patients with this condition.
- Describe the causes of end-stage renal disease.
- Outline the presentation of a patient with end-stage renal disease.
- Summarize the treatment options for end-stage renal disease.
- Outline interprofessional team strategies for improving care coordination and communication to ensure improvement and best outcomes in end-stage renal disease.
- Introduction
More than 500,000 people in the United States live with end-stage renal disease (ESRD). The development of chronic kidney disease (CKD) and its progression to this terminal disease remains a significant cause of reduced quality of life and premature mortality. [1] Chronic kidney disease (CKD) is a debilitating disease, and standards of medical care involve aggressive monitoring for signs of disease progression and early referral to specialists for dialysis or possible renal transplant. The Kidney Disease Improving Global Outcomes (KDIGO) foundation guidelines define CKD using kidney damage markers, specifically markers that determine proteinuria and glomerular filtration rate. By definition, the presence of both factors (glomerular filtration rate [GFR] less than 60 mL/min and albumin greater than 30 mg per gram of creatinine) along with abnormalities of kidney structure or function for greater than three months signifies chronic kidney disease. End-stage renal disease is defined as a GFR of less than 15 mL/min. [2] [3]
According to KDIGO 2012 clinical practice guideline, CKD is classified into five stages considering the GFR level. [4]
- Stage 1: Kidney damage with normal GFR (greater than 90 ml/min)
- Stage 2: Mild reduction in GFR (60-89 mL/min)
- Stage 3a: Moderate reduction in GFR (45 to 59 mL/min)
- Stage 3b: Moderate reduction in GFR (30 to 44 mL/min)
- Stage 4: Severe reduction in GFR (15 to 29 mL/min)
- Stage 5: Renal failure (GFR less than 15 mL/min)
Many chronic diseases can cause end-stage renal disease. In many developed and developing countries, diabetes mellitus is the leading cause. [5] Other causes include: [6] [7]
- Hypertension
- Vascular disease
- Glomerular disease (primary or secondary)
- Cystic kidney diseases
- Tubulointerstitial disease [8]
- Urinary tract obstruction or dysfunction
- Recurrent kidney stone disease [9]
- Congenital (birth) defects of the kidney or bladder
- Unrecovered acute kidney injury
- Certain medications, including non-steroidal anti-inflammatory drugs (NSAIDs), calcineurin inhibitors, and antiretrovirals [10]
- Renal artery stenosis
- Cytoplasmic pattern antineutrophil cytoplasmic antibody (C-ANCA)–positive and perinuclear pattern antineutrophil cytoplasmic antibody (P-ANCA)–positive vasculitides
- ANCA-negative vasculitides
- Atheroemboli
- Hypertensive nephrosclerosis
- Renal vein thrombosis [11]
- Membranous nephropathy
- Alport syndrome
- Immunoglobulin A (IgA) nephropathy
- Focal and segmental glomerulosclerosis (FSGS)
- Minimal change disease
- Membranoproliferative glomerulonephritis (MPGN)
- Complement-related diseases (atypical hemolytic-uremic syndrome [HUS], dense deposit disease)
- Rapidly progressive (crescentic) glomerulonephritis [12] [13]
- Diabetes mellitus
- Systemic lupus erythematosus
- Rheumatoid arthritis
- Mixed connective tissue disease
- Scleroderma
- Granulomatosis with polyangiitis (formerly known as Wegener granulomatosis)
- Mixed cryoglobulinemia
- Endocarditis
- Hepatitis B and C
- Human immunodeficiency virus (HIV) infection
- Parasitic infection
- Penicillamine
- Amyloidosis
- Light-chain deposition disease
- Thrombotic thrombocytopenic purpura (TTP)
- Shiga-toxin or Streptococcus pneumoniae – related HUS
- Henoch-Schönlein purpura
- Reflux nephropathy [14] [15] [16] [17]
- Drugs (eg, sulfonamides, allopurinol)
- Infection (viral, bacterial, parasitic)
- Sjögren syndrome
- Tubulointerstitial nephritis and uveitis (TINU) syndrome
- Chronic hypokalemia
- Chronic hypercalcemia
- Sarcoidosis
- Multiple myeloma cast nephropathy
- Heavy metals
- Radiation nephritis
- Polycystic kidneys
- Cystinosis and other inherited diseases [18] [19]
- Benign prostatic hypertrophy
- Urolithiasis (kidney stones)
- Urethral stricture
- Neurogenic bladder
- Retroperitoneal fibrosis [20]
- Epidemiology
According to the United States Renal Data System, in 2015, there were 124,411 new ESRD diagnoses, reflecting an increasing burden of kidney failure. The prevalence of the disease has been rising at a stable number of about 20,000 cases per year. [21] [22] Kidney disease is the ninth leading cause of death in the United States.
Race/Ethnicity
The degree of kidney failure varies widely by race in the US. In 2015, the rate of ESRD was three times higher in African Americans compared to Whites (393.5 versus 139.9 per million population). That same year, the ESRD prevalence was about ten times higher in American Indians or Alaska Natives and twice as high in Native Hawaiians or Pacific Islanders. Prevalence rates were 1.3 times higher in Asian Americans, as well. Of note, incidence rates in the African American population have decreased each year since 2006, leading to an overall decrease of 21%. This reduction has been even more pronounced in American Indians/Alaska Natives. [23]
The prevalence of CKD increases with age, with the most rapid growth in people aged 60 years or older. For example, the prevalence is 6.0% at ages 18 to 44 years and 38.1% at ages more than 65 years.
The cumulative incidence of end-stage renal disease is higher in males than females.
- Pathophysiology
Each nephron in a normal kidney contributes to the total glomerular filtration rate (GFR). The decline of kidney function is gradual and may initially present asymptomatically. The natural history of renal failure depends on the etiology of the disease but ultimately involves early homeostatic mechanisms involving hyperfiltration of the nephrons. The kidney maintains GFR, despite the progressive destruction of nephrons because the remaining normal nephrons develop hyperfiltration and compensatory hypertrophy. As a result, the patient with mild renal impairment can show normal creatinine values, and the disease can go undetected for some time. [24]
This nephron adaptability allows for continued normal clearance of plasma solutes. This adaptive mechanism will run its course and eventually cause damage to the glomeruli of the remaining nephrons. At this point, antihypertensives such as ACEs or ARBs may be beneficial in slowing the progress of the disease and preserving renal function. Plasma levels of substances such as urea and creatinine start to show measurable increases only after total GFR has decreased by 50%. For example, a rise in plasma creatinine from 0.6 mg/dL to 1.2 mg/dL in a patient, although within the normal range, actually represents a loss of 50% of functioning nephron mass.
Although hyperfiltration and hypertrophy of residual nephrons are beneficial for maintaining GFR, it is found to be a major cause of progressive renal dysfunction. [25] The increased glomerular capillary pressure may damage the capillaries, leading to focal and segmental glomerulosclerosis (FSGS) and eventually to global glomerulosclerosis.
Factors that may worsen renal injury include:
- Nephrotoxins (NSAIDs)
- Systemic hypertension
- Proteinuria
- Dehydration
- Smoking [26]
- Hyperlipidemia
- Uncontrolled diabetes
- Hyperphosphatemia
- Hyperkalemia
Potassium excretion at near-normal levels is generally maintained in CKD as long as aldosterone secretion and distal flow are maintained. Hyperkalemia develops when GFR falls to less than 20-25 mL/min/1.73 m²; at this point, the kidneys have decreased ability to excrete potassium. [27]
Metabolic Acidosis
Metabolic acidosis in stage 5 CKD is high anion gap metabolic acidosis but with the anion gap generally not higher than 20 mEq/L. In CKD, the kidneys cannot produce enough ammonia in the proximal tubules to excrete endogenous acid into the urine in the form of ammonium. In stage 5 CKD, the accumulation of phosphates, sulfates, and other organic anions is the cause of the increase in the anion gap. [28]
Metabolic acidosis has deleterious effects on protein balance, leading to the following:
- Negative nitrogen balance
- Increased protein degradation
- The increased essential amino acid oxidation
- Reduced albumin synthesis
- Lack of adaptation to a low-protein diet
Metabolic acidosis also plays a role in the development of renal osteodystrophy because bones are buffers for excess acid, with a resultant loss of minerals. Acidosis also interferes with vitamin D metabolism.
Salt and Water Handling Abnormalities
Salt and water handling by the kidney are affected in CKD. Volume overload results from the failure of sodium and free-water excretion and occur when the GFR falls to less than 10-15 mL/min/1.73 m². This leads to peripheral edema, pulmonary edema, and hypertension. Tubulointerstitial renal diseases often cause fluid loss rather than overload. Thus, despite severe reductions in GFR, tubulointerstitial renal diseases may manifest as polyuria and volume depletion, with the inability to concentrate the urine. [29]
Normochromic normocytic anemia develops from the decreased renal synthesis of erythropoietin, the hormone responsible for bone marrow stimulation for red blood cell (RBC) production. [30] Other causes of anemia in CKD include the following:
- Chronic blood loss: Uremia-induced platelet dysfunction enhances the bleeding tendency
- Secondary hyperparathyroidism
- Inflammation
- Nutritional deficiency
Bone Disease
Renal bone disease is a common complication of CKD. Different types of bone disease occur with CKD, as follows:
- High-turnover bone disease from high parathyroid hormone (PTH) levels
- Low-turnover bone disease (adynamic bone disease) [31]
- Defective mineralization (osteomalacia)
- Mixed disease
- Beta-2-microglobulin–associated bone disease
Secondary hyperparathyroidism develops in CKD because of the following factors:
- Hypocalcemia
- Decreased renal synthesis of 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D, or calcitriol)
- Intrinsic alteration in the parathyroid glands gives rise to increased PTH secretion and increased parathyroid growth [32]
- Skeletal resistance to PTH
Hyperphosphatemia develops from the inability of the kidneys to excrete excess phosphate. Hyperphosphatemia suppresses the renal hydroxylation of inactive 25-hydroxyvitamin D to calcitriol. Increased phosphate concentration also affects PTH concentration by directly affecting the parathyroid glands (posttranscriptional effect). Hypocalcemia results from decreased intestinal calcium absorption because of low plasma calcitriol levels.
Hypocalcemia, hyperphosphatemia, and low serum calcitriol levels stimulate PTH synthesis and secretion. With persistent stimulus in advanced CKD, parathyroid glands become hypertrophic and then hyperplastic.
- History and Physical
End-stage renal disease can present with a constellation of signs and symptoms. Some include volume overload refractory to diuretics, hypertension poorly responsive to medication, anemia, mineral and bone disorders, and metabolic derangements including hyperkalemia, hyponatremia, metabolic acidosis, hypo/hypercalcemia, and hyperphosphatemia. [33] Metabolic acidosis in stage 5 CKD presents protein-energy malnutrition, muscle weakness, and loss of lean body mass. Salt and water retention can cause peripheral edema, pulmonary edema, and hypertension. Anemia manifests as fatigue, impaired cognitive function, and reduced quality of life. Anemia can also lead to heart failure.
Other manifestations of uremia in end-stage renal disease (ESRD) are:
- Pericarditis
- Encephalopathy
- Peripheral neuropathy
- Restless leg syndrome
- Anorexia, nausea, vomiting, diarrhea
- Dry skin, pruritus, ecchymosis
- Malnutrition
- Erectile dysfunction, decreased libido, amenorrhea
- Platelet dysfunction
Uremic toxicity is an indication of urgent dialysis. [34] ESRD symptoms generally appear in stages 4 and 5 when the GFR is less than 30 ml/min. Some patients with nephrotic syndrome and cystic renal disease may present earlier. Depression is ubiquitous in patients with ESRD and should be screened for on presentation. [35]
Chronic kidney disease is diagnosed when there is evidence of kidney damage for at least three months or in any patient with a GFR of less than 60 mL/min for that same amount of time. [36] [37]
To calculate GFR, three equations are commonly used (the MDRD [Modification of Diet in Renal Disease Study], CKD-EPI, and Cockcroft-Gault formula). However, the best estimate of GFR is the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, which adjusts for age, race, and gender. However, it is important to note that the formula underestimates the actual GFR at a GFR more significant than 60 mL/min. [38]
Further evaluation of kidney disease can include a renal ultrasound, complete blood count (CBC), basic metabolic panel (BMP), urinalysis, and/or kidney biopsy.
Complete Blood Count
CBC shows normochromic normocytic anemia. [39]
Basic Metabolic Panel (BMP)
The blood urea nitrogen (BUN) and serum creatinine levels are elevated. Hyperkalemia or low bicarbonate levels are usually present. Serum albumin levels are low due to urinary protein loss or malnutrition. Serum phosphate, 25-hydroxyvitamin D, alkaline phosphatase, and intact parathyroid hormone (PTH) levels are obtained to look for evidence of renal bone disease. [40] A lipid profile should be obtained because of the risk of cardiovascular disease.
A spot urine protein/creatinine ratio can be used to quantitate albuminuria. A value higher than 30 mg of albumin per gram of creatinine is considered abnormal, while values greater than 300 mg/g are considered severely impaired renal function. Additionally, a 24-hour urine protein can also be performed. A value greater than 3.5 g is concerning for nephrotic range proteinuria.
Renal Ultrasonography
Renal ultrasonography should be done to look for hydronephrosis or involvement of the retroperitoneum with fibrosis, tumor, or diffuse adenopathy. Small, echogenic kidneys are observed in advanced renal failure. While in diabetic nephropathy, kidneys are normal in size. Structural abnormalities like polycystic kidneys also may be observed on ultrasonograms. An ultrasound can provide data estimating size, obstructions, stones, echogenicity, and cortical thinning. [41]
Plain abdominal radiography can detect radio-opaque stones or nephrocalcinosis, while a voiding cystourethrogram (VCUG) is diagnostic for vesicoureteral reflux. [42]
Computed tomography (CT) scanning can help better describe renal masses and cysts and is also sensitive for identifying renal stones.
Magnetic resonance angiography (MRA) can accurately diagnose renal artery stenosis.
A renal radionuclide scan with captopril administration can diagnose renal artery stenosis, and it also quantitates differential renal contribution to the total glomerular filtration rate (GFR).
Renal Biopsy
Percutaneous ultrasound-guided renal biopsy is indicated when the diagnosis is unclear after an appropriate workup. [43]
Specific Tests
- Serum and urine protein electrophoresis for multiple myeloma
- Antinuclear antibodies (ANA), double-stranded DNA antibody levels for systemic lupus erythematosus
- Serum complement levels
- Cytoplasmic and perinuclear pattern antineutrophil cytoplasmic antibody (C-ANCA and P-ANCA) levels for granulomatosis with polyangiitis (Wegener granulomatosis) and microscopic polyangiitis
- Anti–glomerular basement membrane (anti-GBM) antibodies for Goodpasture syndrome
- Hepatitis B and C, human immunodeficiency virus (HIV), and venereal disease research laboratory (VDRL) serology
- Treatment / Management
Treatment of end-stage renal disease involves correcting parameters at the level of the patient's presentation. [44] Interventions aimed at slowing the rate of kidney disease should be initiated and can include:
- Treating the underlying cause and managing blood pressure and proteinuria. Blood pressure should be targeted to a systolic blood pressure of less than 130 mmHg, and diastolic blood pressure of less than 80 mmHg in adults with or without diabetes mellitus whose urine albumin excretion exceeds 30 mg for 24 hours. For diabetic patients with proteinuria, an angiotensin-converting-enzyme inhibitor (ACEI) or angiotensin 2 receptor blocker (ARB) should be started in cases where urine albumin values range between 30 and 300 mg in 24 hours and greater than 300 mg in 24 hours. These drugs slow the disease progression, particularly when initiated before the GFR decreases to less than 60 mL/min or before plasma creatinine concentration exceeds 1.2 and 1.5 in women and men, respectively. [45]
- Other targets in preventive care and monitoring should include tight glycemic control, cardiovascular risk reduction, and general lifestyle recommendations such as smoking cessation and dietary restriction. Glycemic control is critical. A hemoglobin A1C of less than 7% is generally recommended to prevent or delay microvascular complications in this population. Management with sodium-glucose transporter 2 (SGLT-2) inhibitors may reduce the disease burden in those with type 2 diabetes mellitus. [46]
- Treatment of chronic metabolic acidosis with supplemental renal bicarbonate also may slow the progression of end-stage renal disease. [47]
- Patients with CKD tend to have dyslipidemia, particularly hypertriglyceridemia. Monitoring fasting lipid panels and initiation of cholesterol-lowering agents such as HMG-CoA reductase inhibitors should be done early in the course of the disease. [48]
- Volume overload or pulmonary edema should be treated with loop diuretics or ultrafiltration.
- For uremic manifestations, long-term renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplantation) is needed.
- Anemia is treated with an erythropoiesis-stimulating agent (ESA) such as erythropoietin.
- Hyperphosphatemia is treated with phosphate binders (calcium acetate, sevelamer carbonate, or lanthanum carbonate) and dietary phosphate restriction.
- Lifestyle modification and dietary restrictions are routinely recommended. For example, adhering to a low salt diet (less than 2 g/day), a renal diet (avoiding foods that are high in phosphorus), and restricting daily protein to 0.8 g per kg body weight per day is essential to managing disease burden.
- Hypocalcemia should also be monitored. A 25-OH vitamin D level less than 10 ng/mL warrants initiation of ergocalciferol 50,000 IU weekly for 6 to 8 weeks before switching to cholecalciferol 800 to 1000 IU daily. [49]
- Hyperparathyroidism should be treated with calcitriol, vitamin D analogs, or calcimimetics.
Planning for Long-term Renal Replacement Therapy
Early patient education should be initiated regarding natural disease progression, different modalities for dialysis, and renal transplantation. For patients in whom transplantation is not imminent, a primary arteriovenous fistula should be created in advance of the anticipated date of dialysis. [50] Every patient with end-stage renal disease should be timely referred for renal transplantation.
Indications for renal replacement therapy in patients with CKD include the following:
- Severe metabolic acidosis
- Intractable volume overload
- Failure to thrive and malnutrition
- Intractable gastrointestinal symptoms
- Glomerular filtration rate (GFR) of 5 to 9 mL/min/1.73 m^2, irrespective of the symptoms or the presence or absence of other comorbidities
- Differential Diagnosis
The clinical features of end-stage renal disease mimic many other disorders, and many diseases lead to end-stage renal disease. [51] [52] Therefore the following differentials should be considered whenever assessing a patient with end-stage renal disease.
- Chronic glomerulonephritis
- Chronic pyelonephritis
- Rapidly progressive glomerulonephritis
- Nephropathy of pregnancy/pregnancy toxemia
- Unclassifiable nephritis
- Polycystic kidney disease
- Nephrosclerosis
- Malignant hypertension
- Diabetic nephropathy
- Systemic lupus erythematosus nephritis
- Amyloidal kidney
- Gouty kidney
- Renal failure due to a congenital abnormality of metabolism
- Renal/urinary tract tuberculosis
- Renal/urinary tract calculus
- Renal/urinary tract tumor
- Obstructive urinary tract disease
- Renal hypoplasia
End-stage renal disease is a progressive disorder, and timely renal replacement therapy is necessary to prevent death. The disorder is associated with numerous hospitalizations, increased healthcare costs, and metabolic changes. The mortality rates for patients with end-stage renal disease are significantly higher than those without the disease. Even with timely dialysis, the death rates vary from 20% to 50% over 24 months. The most common cause of death is hyperkalemia, followed by adverse cardiac events. [53] Mortality rates are higher for men than women; similarly, Blacks are more prone to death due to ESRD than Whites. The highest mortality rate is within the first six months of starting dialysis. The 5-year survival rate for a patient undergoing long-term dialysis in the United States is approximately 35% and about 25% in patients with diabetes.
In children, puberty is delayed in both genders, and low vitamin D levels are common, an independent risk factor for death. [54]
- Complications
Complications of end-stage renal disease are divided into two groups—complications due to ESRD and complications due to vascular access or dialysis.
Complications due to ESRD
- Coronary heart disease is a significant complication of chronic kidney disease and is the most common cause of death in this population. Patients on dialysis have a 10 to 30 times higher cardiovascular mortality risk than the general population. [55]
- Peripheral vascular disease is also commonly seen [56]
- Mineral and bone disorders (secondary to hyperparathyroidism, vitamin D deficiency)
- Hyperuricemia
- Metabolic acidosis
- Hypoalbuminemia
- Decreased libido, erectile dysfunction
Complications due to Vascular Access/Dialysis
- Local or disseminated intravascular infection
- Graft occlusion
- Electrolyte abnormalities after dialysis
- Dialysis dementia
- Dialysis disequilibrium syndrome
- Consultations
The management of end-stage renal disease requires a dedicated interprofessional healthcare team comprised of the following:
- Nephrologist
- Intensivist
- Renal transplant surgeon
- Nurse educator
- Nutritionist
- Deterrence and Patient Education
The U.S. Preventive Services Task Force (USPSTF) does not recommend screening asymptomatic individuals for CKD. [57] However, for those at higher risk for the disease, such as those with diabetes or hypertension, USPSTF recommends ongoing screening for CKD with proteinuria testing. However, it is essential to note that screening for proteinuria is not necessary for a patient who is already on ACEI or ARB therapy.
Patients with end-stage renal disease should be educated about the following:
- Avoidance of nephrotoxic drugs like non-steroidal anti-inflammatory drugs
- Advanced counseling for renal replacement modalities, including peritoneal dialysis, hemodialysis, and transplantation
- Timely placement of vascular access for hemodialysis
- Pregnancy could be fatal in ESRD
- Avoid phosphate-rich foods [58]
- Potassium restriction in diet
- Sodium and water restriction to avoid volume overload
- Protein restriction to delay the onset of uremic symptoms [59]
- Reduction in salt intake may slow the progression of diabetic CKD
- Pearls and Other Issues
- End-stage renal disease is a terminal illness with a glomerular filtration rate of less than 15 mL/min.
- The most common cause of ESRD in the US is diabetic nephropathy, followed by hypertension.
- Other etiologies can include glomerulonephritis, cystic kidney disease, recurrent kidney infection, chronic obstruction, etc.
- The disease can present with nausea, vomiting, metabolic, hematologic, electrolyte derangements, seizures, coma, bleeding diathesis, refractory fluid overload, hypertension unresponsive to pharmacotherapy, uremic pericarditis, etc.
- Vigilant monitoring of GFR and proteinuria in diabetics and non-diabetics is essential for managing disease progression in patients with chronic kidney disease.
- Early referral to specialists is necessary for timely dialysis or renal transplant planning.
- Enhancing Healthcare Team Outcomes
Once a patient has been diagnosed with end-stage renal disease, a significant number of patients will require dialysis, and the lucky few may be eligible for a renal transplant. Unfortunately, end-stage renal failure significantly increases morbidity and mortality; it also leads to enormous costs to the healthcare system. Thus, the disorder is best managed by an interprofessional team dedicated to adequate disease control and improving outcomes for these patients.
There is no cure for end-stage renal disease, and all the available treatments are short-term. Thus, the key to improving long-term outcomes is preventing the disease's progression.
A dedicated interprofessional healthcare team should comprise a nurse educator, a specialized pharmacist, a nutritionist, a social worker, and a couple of clinical providers, including a primary care provider and a trained nephrologist.
The specialized nurse educator plays a vital role in educating the patient about lifestyle modifications necessary to prevent the progression of CKD. In patients with advanced CKD, the dedicated nurse's role become crucial in protecting an arm for future fistula placement. During hospitalizations, the clinical nurse should place limb restrictions on that arm to ensure venipunctures and blood pressure readings are not taken on that arm.
The pharmacist should identify those patients who carry a diagnosis of CKD and provide specialized instructions to these patients, particularly concerning avoiding nephrotoxic agents and medications. In addition, the pharmacist plays a crucial role in communicating and guiding the providers about the patient's medications to limit those that can adversely affect the kidneys.
A trained nutritionist should also be involved in the care of these patients to guide an appropriate diet plan specific to their needs. [60]
A social worker should be involved in the care to ensure that the patient has a support system and financial resources to continue therapy.
To improve outcomes, each interprofessional team member should maintain accurate and updated patient records, communicate with the other team members, and act collaboratively to ensure that the patient receives optimal care resulting in the best outcomes. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Onecia Benjamin declares no relevant financial relationships with ineligible companies.
Disclosure: Sarah Lappin declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Hashmi MF, Benjamin O, Lappin SL. End-Stage Renal Disease. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Chronic kidney disease in adults: assessment and management [ 2015] Review Chronic kidney disease in adults: assessment and management . 2015 Jan
- Review Kidney Disease in Diabetes. [Diabetes in America. 2018] Review Kidney Disease in Diabetes. Pavkov ME, Collins AJ, Coresh J, Nelson RG. Diabetes in America. 2018 Aug
- Review Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. [Am J Kidney Dis. 2015] Review Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Akbari A, Clase CM, Acott P, Battistella M, Bello A, Feltmate P, Grill A, Karsanji M, Komenda P, Madore F, et al. Am J Kidney Dis. 2015 Feb; 65(2):177-205. Epub 2014 Nov 4.
- Chronic Kidney Disease. [StatPearls. 2024] Chronic Kidney Disease. Vaidya SR, Aeddula NR. StatPearls. 2024 Jan
- Review Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. [Health Technol Assess. 2010] Review Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, Fluck N, MacLeod A, McNamee P, Prescott G, et al. Health Technol Assess. 2010 Apr; 14(21):1-184.
Recent Activity
- End-Stage Renal Disease - StatPearls End-Stage Renal Disease - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Why Is Chronic Kidney Disease on the Rise? 6 Things to Know
BY KATHY KATELLA April 24, 2024
Chronic kidney disease (CKD) is a condition that affects an estimated 37 million American adults—or one in seven. And yet, many people don’t even know they have it.
That’s because CKD is a silent disease, progressing without symptoms as the kidneys gradually—and permanently—lose function over months or years. When people finally start to experience symptoms, such as itchy skin, an impaired ability to urinate, and unexplained weight loss, among others, it means the disease has reached an irreversible stage. At that point, they may have lost so much kidney function that they will need dialysis or a kidney transplant.
CKD is also on the rise. "Diabetes is the number one cause of kidney disease; so, because more patients have diabetes, we're seeing more kidney disease,” says Randy Luciano, MD , a Yale Medicine nephrologist. High blood pressure is the second leading cause, and that’s on the rise as well. (There are other causes, including obesity and, less commonly, polycystic kidney disease, a genetic condition marked by multiple cysts in the kidneys.)
Once you have CKD, it and the accompanying kidney damage cannot be reversed. However, diagnosing and treating CKD early may help stop it from advancing. There are new treatments in the past few years that have been described as “game-changing” for their ability to slow the progression of CKD for years and possibly even decades, Dr. Luciano says.
Below, Dr. Luciano answers common questions about chronic kidney disease.
1. What, exactly, is chronic kidney disease?
Chronic kidney disease is the medical term used to describe the gradual loss of kidney function over a period of at least three months. Because of this, excess fluid and waste from the blood remain in the body and may cause other health problems, such as heart disease and stroke , in addition to kidney failure.
CKD can occur for a variety of reasons, but diabetes and high blood pressure are the two most common ones. If someone has diabetes, high blood sugar can clog and narrow the kidney’s tiny blood vessels, leading to kidney damage. In people with high blood pressure, there is an increase in the force of blood pushing against vessel walls throughout the body, including in the kidneys, affecting their ability to eliminate waste and extra fluid from the body—key kidney functions. Other issues that can lead to CKD include glomerulonephritis, an inflammation of the tiny filters in the kidneys; lupus nephritis , an autoimmune disease; polycystic kidney disease ; and kidney cancer .
2. How do you know if you’re at risk for CKD?
While anyone can develop CKD, some people are at higher risk.
Risk increases with age, with people over 65 most likely to be diagnosed with CKD. In addition to having diabetes (type 1 or 2) and high blood pressure , people are also at higher risk if they have heart disease, obesity, or past damage to the kidneys from an infection or surgery.
Family history matters, too. "If you have a family history of CKD, kidney failure, or inherited kidney disorders, you should talk to your provider about kidney health when you’re in your 20s,” Dr. Luciano says. “People with first-degree relatives affected by CKD are at higher risk, and those with other relatives who have the condition have an elevated risk as well. A young patient may not have CKD that will impact their life for the next 10 or 15 years, but we can take steps to lower their risk as they age.”
Race, ethnicity, and socioeconomic issues may play a role as well. As many as 20% of non-Hispanic Black adults in the U.S. are estimated to have CKD compared to 11.7% for non-Hispanic white adults, according to the CDC. “This is probably multifactorial,” says Dr. Luciano, adding that access to health care and affordable medications can be a factor in some cases. And minority populations are more likely to have diabetes, heart disease, high blood pressure, and obesity, all conditions that raise the risk for CKD, he says.
“We also know that some Black patients have certain genetic risks that may increase their chances of developing CKD,” Dr. Luciano says. A nephrologist can arrange genetic testing, which involves a tissue swab of the cheek or a blood draw the patient can perform at home. If a patient tests positive for a genetic mutation linked to CKD, “the goal is to be preemptive and manage diabetes or blood pressure using the latest medicines aggressively to get that disease under control,” he says.
3. How is CKD diagnosed?
CKD can be detected (and monitored) using two simple tests: a blood test and a urine test. The first is often done as part of a regular physical examination with routine bloodwork that includes a test called glomerular filtration rate (GFR)—the GFR score is based on the level of creatinine in a person’s blood, combined with their age and sex. (Creatinine is a waste product, resulting from the normal breakdown of muscle tissue and digestion of protein from food; too much of it in the blood is a sign that the kidneys are not cleaning out waste efficiently.)
The second test is a urinalysis to evaluate the urine for albumin, the main protein found in blood. Albumin in the urine is called albuminuria, and its presence is a sign that the kidneys are damaged.
There are five stages of kidney disease, which are determined by GFR scores that remain consistent for at least three months:
- Stage 1. GFR: 90 or higher. Stage 1 CKD means you have a normal GFR, but there is protein in your urine. The presence of protein alone means you are in Stage 1 CKD, even if there is no kidney damage.
- Stage 2. GFR: 60-89. This suggests mild kidney damage, but the kidneys still work well. Because most people don’t notice symptoms until Stage 3, this stage of CKD often goes unnoticed; however, if caught, it may still be possible to slow down the loss of kidney function with lifestyle changes.
- Stage 3. GFR: 30-59. This stage is divided into two subcategories: 3a, which is mild-to-moderate damage, with a GFR between 45 and 59, and 3b, which is moderate-to-severe damage, with a GFR between 30 and 44. At this stage, CKD can cause complications, such as anemia and bone disease. Although some people still may not have symptoms, many experience one or more of a range of symptoms, including feeling weak and tired, lower back pain, dry or itchy skin, urinating more or less than usual, or having foamy or darker-colored urine. Although at this point the kidneys are irreversibly damaged and don’t work as well as they should, with treatment and healthy life changes, many people in this stage do not move to Stage 4 or Stage 5.
- Stage 4. GFR: 15-29. This is the last stage before kidney failure, when damage is severe. The kidneys have a difficult time filtering out waste, which then builds up in the body where it can cause a variety of health problems, including heart disease and stroke. Patients and their providers should begin to plan for future dialysis or a kidney transplant.
- Stage 5. GFR: Less than 15. The kidneys are close to failure or have already failed, the latter of which is called end-stage CKD (ESKD). Symptoms of kidney failure may include some of the issues that some people start to experience in Stage 3, along with some new ones, such as rashes; foamy, frothy, or bubbly urine; feeling less hungry than normal; and feeling sick in the stomach and throwing up. This stage calls for dialysis or a kidney transplant to keep patients alive.
4. How can you stop CKD from progressing?
Treatment usually depends on the underlying cause of kidney disease. “Not all chronic kidney disease progresses in the same way,” Dr. Luciano says. For instance, “if we know a patient’s kidney disease is due to diabetes, we focus on treating that,” he says, because improving diabetes will slow further damage. Likewise, patients with high blood pressure must get their blood pressure under control, whether that includes making lifestyle changes or taking medication.
In the past few years, several relatively new CKD medications have become available. Some of these are also used to treat diabetes and other CKD-related conditions, including obesity and heart disease. These drugs include:
- SGLT-2 inhibitors (also called flozins). These include several medicines, available as pills, that are used in diabetes treatment. Several, including empagliflozin (Jardiance®), are FDA-approved for CKD. One way these medicines work is by preventing the kidneys from reabsorbing blood sugar; the glucose instead goes to the urine and is eliminated from the body, thereby protecting the kidneys.
- Finerenone (Kerendia®) . This is an FDA-approved pill aimed at reducing the risk of kidney function decline and failure, as well as serious heart conditions and events associated with type 2 diabetes . It works by blocking proteins called MRs that can become overactivated in type 2 diabetes, leading to kidney inflammation and scarring.
- GLP-1 receptor agonists (glucagon-like peptide-1 receptor agonists). These include drugs that are FDA-approved for type 2 diabetes and may help people lose weight. One is semaglutide (Ozempic®), which is approved for weight loss under the brand name Wegovy®. These drugs have shown promise in reducing the risk of kidney failure, although they are not FDA-approved for that purpose at this time.
High blood pressure can be both a cause and a result of CKD, so blood pressure medications are often prescribed—and were used to treat CKD for decades before the newer drugs became available. These include ACE inhibitors (angiotensin receptor blockers) and ARBs (angiotensin-converting enzymes). They help blood vessels relax, so the blood can flow smoothly, preserving kidney function.
A series of other medications may also help. For instance, because poor kidney function can weaken bones, calcium and vitamin D supplements may be prescribed to keep them strong. Diuretics, which can help kidneys eliminate salt and water and facilitate urination, may be used to reduce swelling. And iron supplements can help address anemia.
It may also be important to stop taking certain medicines that can worsen kidney damage, such as NSAIDs (nonsteroidal anti-inflammatory drugs) and some arthritis medicines.
5. If you have CKD, will you need dialysis or a kidney transplant?
It depends. Having CKD doesn’t always mean the disease will progress to ESKD and require dialysis or a kidney transplant . “As kidney specialists, we try to establish the cause of the disease, treat it, and determine if a patient will require dialysis in the future,” Dr. Luciano says. “Sometimes, the last part is difficult because we don't know how they're going to respond to treatment, how their kidney disease is going to progress over time, and what other circumstances can develop in their life that can impact the disease.”
If, however, you progress to ESKD and your kidneys fail, you will need either dialysis or a kidney transplant to replace the work of the kidneys.
Out of almost 808,000 people in the U.S. who have ESKD, 69% are on dialysis, which was once done only in a health care setting. Now, many people choose types of dialysis that can be performed at home.
There are two types of dialysis treatment, both of which involve several treatments a week.
- Peritoneal dialysis: In a surgical procedure, a catheter (or plastic tube) is placed in the patient's belly. The patient hooks up a plastic bag of cleansing fluid to the tube, which transports the fluid to the abdomen, and is then used to drain it. Treatment can be continuous, as the person goes about their normal activities, or automated, in which the cleansing fluid is delivered and drained while the person sleeps.
- Hemodialysis: Blood is pumped out of the body through a tube and into an artificial kidney machine, which removes the waste and extra fluid before it’s sent back to the body. This is done three or four times a week, often in a health care setting, but it can sometimes be done at home.
“For those who can do it, we often recommend home dialysis,” Dr. Luciano says. “It tends to be easier for people, gives them more flexibility with their schedule, and is gentler on the body.”
Still, a kidney transplant is often the best solution—preferably before a patient reaches the point of requiring dialysis, Dr. Luciano says. That’s because while dialysis can take over the function of cleaning waste and excess water from the body, treatments can be time-consuming and cause such side effects as skin infections, low blood pressure, muscle cramps, weakness, and fatigue. It can also have a major impact on a person’s life, both emotionally and physically.
“The hope is that some of the newer medications will allow patients to stay on the waiting list longer without needing dialysis,” Dr. Luciano says.
In 2020, the remaining 31% of the almost 808,000 people in the U.S. with ESKD had kidney transplants, according to the CDC. People wait three to five years, more or less, for an organ from a deceased donor, depending on such factors as geographic location and blood type. “But if you have a living donor—either someone you know or a stranger willing to donate a kidney—and everything works out, you may get a kidney within three to six months,” Dr. Luciano says.
There are also kidney registries that can help match people with a living donor who is not a relative.
6. How can you avoid CKD?
The best thing to protect your kidneys is to regularly see your primary care provider and, if relevant, share any family history of kidney disease or dialysis, Dr. Luciano says.
“If you have a yearly physical and bloodwork every two or three years, you’ll be much less likely to miss CKD,” he says. If there is an abnormality, such as an elevated level of creatinine, he recommends repeating the bloodwork once a year. “Your doctor may notice over time that your creatinine is a little off, which will trigger the follow-up appointment with a kidney specialist who can look into it more carefully.”
For anyone with diabetes, high blood pressure, or heart disease, and/or who is 65 or older, it’s especially important to talk to a doctor about CKD risk. People who are young and healthy but have a first-degree relative with a history of kidney disease or dialysis should consider being screened, Dr. Luciano says. “Chances are they're not going to have a serious case of CKD at an early age. But it's always good to make sure that we're not missing anything that would put them at risk as they age.”
As with many diseases, preventing a serious condition from developing also comes down to good general health, he says. This includes eating a diet rich in fruits and vegetables and low in salt, fat, and sugar; drinking enough water; being active for at least 30 minutes most days; sleeping seven to eight hours each night; avoiding smoking; and minimizing alcohol intake.
“There are some people who are going to develop kidney disease no matter what they do, just as there are people who will develop heart disease,” Dr. Luciano says. “But good general care can mean early diagnosis and treatment, which can keep people healthy.”
More news from Yale Medicine

A Medical Information Site. Great Information. Great Health.
Clinical presentation of kidney disease.
The clinical presentation of kidney disease.
Renal disease may present in many ways, including: (1) the screening of asymptomatic individuals; (2) with symptoms and signs resulting from renal dysfunction; and (3) with symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction.
History and clinical signs—in many cases these are nonspecific or not apparent, and detection of renal disease relies on a combination of clinical suspicion and simple investigations, including urinalysis (by dipstick for proteinuria and haematuria, with quantification of proteinuria most conveniently performed by estimation of the albumin:creatinine ratio, ACR, or protein:creatinine ratio, PCR) and estimation of renal function (by measurement of serum creatinine, expressed as estimated glomerular filtration rate, eGFR).
Asymptomatic renal disease—this is common and most often detected as chronic depression of glomerular filtration rate (known as chronic kidney disease, CKD), proteinuria, or haematuria, either as isolated features or in combination.
Symptomatic renal disease—may present in many ways, including: (1) with features of severe chronic depression of glomerular filtration rate—‘uraemia’, manifesting with some or all of anorexia, nausea, vomiting, fatigue, weakness, pruritus, breathlessness, bleeding tendency, apathy and loss of mental concentration, and muscle twitching and cramps; (2) acute kidney injury—also known as acute renal failure; (3) with urinary symptoms—frequency, polyuria, nocturia, oliguria, anuria, and macroscopic haematuria; and (4) loin pain.
Specific renal syndromes—these include: (1) nephrotic syndrome—comprising oedema, proteinuria, and hypoalbuminaemia—caused by primary or secondary glomerular disease; and (2) rapidly progressive glomerulonephritis with acute renal failure.
Other conditions—renal disease may be associated with and present in the context of many underlying conditions, including: (1) diabetes mellitus; (2) renovascular disease; (3) myeloma and other malignancies; (4) infectious diseases, either as a nonspecific manifestation of the sepsis syndrome or as a specific complication of the particular infection, e.g. haemolytic uraemic syndrome, poststreptococcal glomerulonephritis, hantavirus infection, leptospirosis, HIV nephropathy; (5) systemic inflammatory diseases, e.g. systemic vasculitides, rheumatological disorders, sarcoidosis, amyloidosis; (6) drug-induced renal disease; and (7) pregnancy.
Clinical Presentation of Kidney Disease in detail - technical
Introduction.
Renal disease may present in a multitude of ways. In practice it is usually detected as a result of:
- ◆ screening of asymptomatic individuals
- ◆ symptoms and signs resulting from renal dysfunction
- ◆ symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction
Symptoms and signs of renal pathology are often absent or subtle, even in the presence of significant disease, hence the detection of renal problems requires careful evaluation of the history and clinical findings to assess the potential risk of underlying renal disease. This evaluation should focus on features of systemic and inflammatory disease as well as those relating directly to the renal tract, and similarly a drug and obstetric history may help elucidate the cause of renal disease. However, in many cases the history and clinical signs are nonspecific or not apparent, with detection of renal disease relying on a combination of clinical suspicion and simple investigations, including urinalysis and estimation of renal function.
Presentation of aymptomatic renal disease
Asymptomatic renal disease is common and may often remain stable and undetected. However, some patients with asymptomatic disease are at increased risk of developing renal failure with the passage of time or in the event of intercurrent illnesses. Active screening for subclinical disease is thus carried out in certain subpopulations with the result that patients may be identified with abnormal renal function or with abnormalities on urinalysis that may indicate significant renal pathology. Examples of such screening include:
- ◆ screening patients in primary care—general population screening via eGFR reporting (see below); monitoring of patients at ‘high risk’ of developing renal disease (e.g. hypertension, diabetes, multisystem disease); occupational and insurance medicals
- ◆ screening patients admitted to hospital with acute illnesses—as an incidental finding; as part of renal and electrolyte surveillance in patients at risk (e.g. in the presence of sepsis, hypovolaemia, and usage of nephrotoxic drugs)
- ◆ incidental finding on abdominal imaging—stones, cysts and tumours, reduced renal size
- ◆ screening of the family members of patients with inherited renal disease
Asymptomatic renal dysfunction and screening for chronic kidney disease
Traditionally, the basic means of assessing renal function has been estimation of the serum creatinine, and this can be used—with or without estimation of urinary creatinine excretion—to estimate the GFR. The Cockcoft–Gault equation, which estimates creatinine clearance from serum creatinine, weight, age, and sex, has largely given way as a screening tool to the Modification of Diet in Renal Disease (MDRD) formula, which in its simplest form generates an estimated glomerular filtration rate (eGFR) normalized to body surface area from serum creatinine, age, sex, and race. Using this method, population studies in the Western world have estimated that between 4 and 6% of the adult population have moderate to severe renal failure, with an eGFR of less than 60 ml/min per 1.73 m 2 body surface area (stage 3 to 5 chronic kidney disease (CKD). Most of these are elderly, and only very few of them (1–4%) progress to endstage renal failure when followed over a 5-year period, while over the same time up to 69% die owing to cardiovascular disease.
Population and ‘risk group’ screening for renal dysfunction
Population data suggest that most renal disease identified by screening is not progressive, but there are subpopulations in which progressive renal disease is more likely and in whom early intervention and optimal management may delay or prevent the need for dialysis. Hence in the United States of America and the United Kingdom guidelines have been drafted in which ‘risk groups’ are screened for renal dysfunction/CKD (Bullet list 1). It is not yet known whether screening for renal dysfunction has any effect on outcome, but in the United Kingdom, data on the prevalence of CKD and associated information such as blood pressure measurement, its control, and the use of angiotensin converting enzyme (ACE) inhibitors in the CKD population, are now incorporated into the Primary Care Quality and Outcomes Framework, by which means funding is related to achievement of targets. As a consequence, there has been a substantial rise in the number of patients identified with asymptomatic renal dysfunction, and an increased rate of referral to secondary care, especially of elderly patients. There is still considerable doubt about the validity and value of labelling many very elderly people as having moderate to severe renal failure, especially since in many patients an eGFR in this range seems to confer a very much higher risk of cardiovascular demise than of endstage renal failure.
Bullet list 1 Summary of United Kingdom guidelines for serum creatinine measurement and estimation of GFR
Serum creatinine concentration should be measured at initial assessment and then at least annually in all adult patients with the following conditions:
- • Persistent proteinuria
- • Unexplained haematuria
- • Identified renal pathology
- • Bladder voiding dysfunction (outflow obstruction, neurogenic bladder)
- • Urinary diversion surgery
- • Urinary stones
- • Hypertension
- • Cardiovascular disease—ischaemic heart disease, chronic heart failure, peripheral vascular disease and cerebrovascular disease.
- • ACE inhibitors, angiotensin receptor blockers, NSAIDs, lithium, mesalazine, ciclosporin, tacrolimus
- • Systemic lupus erythematosus
- • Systemic vasculitides
- • Rheumatoid arthritis
- • Individuals with a family history of stage 5 CKD or hereditary renal disease
Employment or insurance health screening
As well as targeted screening of ‘at-risk’ populations, asymptomatic renal disease may also be identified as a result of employment or insurance health screening. Common abnormalities identified are hypertension or abnormal urinalysis, such as proteinuria and microscopic haematuria. Patients identified in this way will often be referred for subsequent investigation.
Screening for renal dysfunction in secondary care
In the secondary care setting, patients in specialist clinics who are at risk of renal disease, such as patients with diabetes, are periodically screened for the development of hypertension, proteinuria, and renal dysfunction. Renal disease also often presents in acute medical and surgical patients. Up to 5% of acute patients have some acute deterioration in renal function during a hospital admission, mostly owing to hypotension, sepsis, or the use of nephrotoxic drugs. Monitoring renal function in such patients may help in the acute management of their illness and may also identify those with underlying chronic renal impairment who require long-term management.
Screening for drug-induced renal disease
Renal disease resulting from the use of nephrotoxic drugs is often asymptomatic, and CKD may develop as a result of long-term use of agents such as nonsteroidal anti-inflammatory drugs (NSAIDs), lithium, and calcineurin inhibitors. Often the only evidence for this is a progressive rise in serum creatinine and fall in eGFR, which may be progressive and—if not detected by routine screening—may present with advanced renal failure. Other drugs such as ACE inhibitors may cause an acute deterioration in renal function, screening for which is required, especially in high-risk groups.
Other incidental findings of renal disease
Subclinical renal disease may also present as an incidental finding on biochemical testing, e.g. abnormalities of potassium and acid–base homeostasis identified on a ‘routine’ sample may indicate a renal tubular acidosis and prompt further investigation for an underlying cause.
Renal disease identified incidentally with imaging
Advances in imaging technology combined with their widespread use have increased the number of incidental renal abnormalities identified. Many of these are anatomical abnormalities which are of little consequence, such as duplex ureters and isolated renal cysts, but significant pathology is sometimes found incidentally, such as polycystic kidneys, renal tumours, and asymmetrical kidneys.
Family screening for renal disease
Patients with a family history of inherited renal disease may also be identified with early, asymptomatic renal disease as a result of screening. The most common example is autosomal dominant polycystic kidney disease, which may be reliably identified by ultrasonography from the third decade onwards. The identification of disease genes for inherited renal diseases such as autosomal dominant polycystic kidney disease, tuberous sclerosis, von Hippel–Lindau disease, Alport’s syndrome, and congenital nephrotic syndrome raises the possibility of future antenatal screening and earlydetection of these diseases long before they become clinically manifest.
Screening and management of asymptomatic proteinuria
The availability of reliable and cheap urine dipstick reagent strips has led to their widespread use to screen for and monitor renal disease in primary and secondary health care. Within the general population, up to 5% of apparently healthy adults and 16% of those aged over 80 years have either a ‘trace’ or ‘+’ of protein, but most of these do not have significant treatable disease, making routine population screening uneconomic and unnecessary. Guidelines aimed at identifying subclinical renal disease therefore suggest proteinuria screening only for patients at increased risk of renal disease. The 2005 United Kingdom guidelines are summarized in Bullet list 2.
Bullet list 2 United Kingdom guidelines for proteinuria screening
Dipstick urinalysis for protein should be undertaken:
- • Newly discovered hypertension, haematuria, or reduced GFR
- • Unexplained oedema or suspected heart failure
- • Suspected multisystem disease, e.g. lupus, vasculitis, and myeloma
- • Diabetes mellitus
- • Urologically unexplained haematuria or persistent proteinuria
- ◆ As part of routine monitoring for patients receiving nephrotoxic drugs, e.g. gold and penicillamine
Detection of proteinuria
Most multireagent strips are sensitive to 100 to 200 mg/litre of protein, giving either a ‘trace’ or ‘+’, although some designed to screen for microalbuminuria are more sensitive. They do not detect low-molecular-weight proteins such as immunoglobulin light chains, and thus assay of light chains using urine immunoelectrophoresis is essential as part of the investigations for myeloma, primary amyloidosis, and light-chain glomerulopathy.
The kidney normally excretes less than 150 mg of protein in 24 h, mainly owing to failed tubular reabsorption of albumin. Urinary protein excretion also reduces overnight while recumbent, but increases during the day owing to posture and exercise. Urinary protein concentration also depends on urine flow rate. To overcome the diurnal variation, proteinuria has been traditionally evaluated from a 24-h urine collection, but these have been largely superseded by measuring the ratio of albumin or protein to creatinine in the urine (albumin:creatinine ratio, ACR; protein:creatinine ratio, PCR). This method has been validated against 24-h urinary collections and—as a rule of thumb—a urinary ACR of 70 mg/mmol or PCR of 100 mg/mmol equates approximately to a 24-h protein excretion of 1 g/24 h.
Management of asymptomatic proteinuria without haematuria
Proteinuria may be an early presentation of renal disease, but transient proteinuria is not associated with significant renal disease. A finding of proteinuria should lead the physician to take a history focusing on risk factors for renal disease (e.g. diabetes, drugs, multisystem disease, and family history), measure the blood pressure, and examine for oedema (Bullet list 3
Bullet list 3 Approach to the patient with dipstick-positive proteinuria
- ◆ Is there any evidence of diabetes or urinary infection?
- ◆ Are there any risk factors for or signs of renal disease?
- ◆ Is proteinuria transient or persistent?
Causes include:
- ◆ Urinary tract infection
- ◆ Orthostatic proteinuria
- ◆ Send urine for spot ACR (or PCR)
- • Diabetes, hypertension, systemic inflammatory disease, myeloma, family history of renal disease
- • Are there any features of nephrotic syndrome (heavy proteinuria with oedema and low serum albumin)?
In the absence of risk factors or signs of renal disease, transient proteinuria is not likely to indicate underlying renal disorder, hence an initial finding of proteinuria on dipstick testing should be repeated a week or so later, and any positive result confirmed and quantitated by estimation of ACR (or PCR). If postural or orthostatic proteinuria is suspected, an early-morning urine specimen should be sent for ACR (or PCR), in which case the diagnosis is substantiated by the finding of normal urinary protein excretion in this specimen.
Persistent proteinuria (ACR >70 mg/mmol, PCR >100 mg/mmol) on two or more occasions requires further investigation with:
- ◆ renal function (eGFR)
- ◆ serum albumin, for diagnosis of nephrotic syndrome
- ◆ serum paraprotein electrophoresis and urinary Bence Jones protein for myeloma
- ◆ immunological screen (antinuclear antibodies, complement, antineutrophil cytoplasmic antibodies (ANCA))
- ◆ renal ultrasonography
- ◆ consideration of renal biopsy if heavy proteinuria (ACR >150–200 mg/mmol, PCR >200–300 mg/mmol) or renal dysfunction
Management of asymptomatic proteinuria with microscopic haematuria
Proteinuria with haematuria on urinalysis indicates intrinsic renal disease. It may be the first sign of a severe glomerulonephritis and acute renal failure, hence this presentation must be considered seriously (see later). Patients with abnormal renal function, haematuria, and proteinuria require urgent referral to a nephrologist for investigation.
Apparently asymptomatic patients with normal renal function but persistent proteinuria and haematuria may describe subtle symptoms of multisystem disease on close questioning (e.g. myalgia, arthralgia, ‘sinusitis’, rash, or fever). These may be clues to an underlying disease, hence patients with such symptoms require screening for multisystem disease with urine microscopy for red cell casts, serum ANCA, antiglomerular basement membrane antibodies, antinuclear and anti-double-stranded DNA antibodies and complement levels, and referral to a nephrologist for further evaluation and consideration of renal biopsy.
Asymptomatic microscopic (non visible) haematuria
Microscopic haematuria may potentially arise from anywhere in the urinary tract. As with renal dysfunction and proteinuria, isolated microscopic haematuria is common. Population studies indicate a prevalence between 0.2 and 16%, with a higher prevalence of 18% in men aged over 50 years. Studies of male army recruits screened and followed up for 12 years showed that 39% had microscopic haematuria on one occasion, and 16% had microscopic haematuria on two or more occasions. Although isolated microscopic haematuria may be associated with benign glomerular disease, in practice the main concern is the possibility of renal and urinary tract malignancy.
Urothelial and bladder carcinomas account for approximately 5% of microscopic haematuria. This risk increases with age, particularly in men over the age of 65 years. In contrast, underlying malignancy in those younger than 40 years is very rare, particularly in the absence of risk factors such as smoking and exposure to azo dyes.
Causes of microscopic (non visible) haematuria
The causes of microscopic haematuria are summarized in Bullet list 4. The true prevalence of intrinsic renal disease is unknown because renal biopsies are not routinely performed in the absence of proteinuria or abnormal renal function. However, small biopsy studies of patients with no other cause for haematuria identified a glomerular cause in 16 to 30%. Within this group, IgA nephropathy and thin basement membrane disease are most common.
Bullet list 4 Causes of microscopic haematuria without proteinuria
- ◆ IgA nephropathy
- ◆ Thin basement membrane disease
- ◆ Hereditary nephritis (Alport’s syndrome)
- ◆ Other glomerulonephritides (mesangiocapillary glomerulonephritis, vasculitis, lupus, etc.)
- ◆ Nephrolithiasis
- ◆ Pyelonephritis
- ◆ Renal cell carcinoma
- ◆ Cystic kidney disease (polycystic and medullary sponge)
- ◆ Papillary necrosis
- ◆ Ureteric strictures
- ◆ Hydronephrosis
- ◆ Sickle cell disease
- ◆ Renal infarcts and arteriovenous malformations
- ◆ Renal tuberculosis
- ◆ Cystitis, prostatitis
- ◆ Bladder carcinoma
- ◆ Benign bladder and ureteral tumours and polyps
- ◆ Urethral strictures
- ◆ Overanticoagulation
- ◆ Factitious
Management strategy for microscopic (non visible) haematuria
The key to managing patients with asymptomatic microscopic haematuria is identifying risk factors for malignancy. In routine practice, patients older than 50 years, smokers, or those with an occupational history of dye exposure should be investigated for malignancy and referred to a urologist for cystoscopy.
Numerous different approaches to the management of patients with microscopic haematuria have been published, reflecting a lack of consensus and an insufficient evidence base. There are no indications for screening for microscopic haematuria as the positive predictive value for malignancy is as low as 5% in an elderly population, and early detection of disease has not been shown to improve prognosis.
Following the detection of microscopic haematuria without proteinuria on urine dipstick, menstruation, recent exercise, or sexual activity should be excluded, and the urine sent for microscopy and culture. Urinalysis should then be repeated after 7 days: if this remains positive, urinary tract infection must be excluded, and blood pressure, urine protein-creatinine ratio (PCR) or albumin creatinine ratio (ACR), and serum creatinine/eGFR measured.
Patients should be referred to urological services to exclude urinary tract malignancy and disease if they are over 40 years of age with:
- (1) persistent asymptomatic haematuria (defined as 2 out of 3 positive dipsticks), or
- (2) symptomatic non-visible (microscopic) haematuria, or
- (3) visible (macroscopic) haematuria.
Patients should be referred to a nephrologist if a urological cause has been excluded, or the criteria for urological assessment are not met, and the patient has:
- (1) declining GFR, or
- (2) CKD stage 4 or 5, or
- (3) proteinuria with urinary PCR >50mg/mmol or ACR >30mg/mmol, or
- (4) age <40 years and hypertension
Patients should be referred to a nephrologist if:
Patients not meeting criteria for referral to urological or renal services, or who have had negative urological or nephrological investigations, need long term monitoring in primary care due to the uncertainty of the underlying diagnosis, with appropriate referral should they develop:
- (1) voiding lower urinary tract symptoms (LUTS), or
- (2) visible haematuria, or
- (3) significant or increasing proteinuria, or
- (4) progressive renal impairment (falling eGFR), or
- (5) hypertension.
Symptomatic renal disease
Many patients with renal disease remain asymptomatic, but others develop symptoms that may be nonspecific, e.g. due to the gradual onset of uraemia in patients with progressive CKD, renal-specific, e.g. loin pain or polyuria, or unrelated to the kidney and manifest as isolated ‘nonrenal’ symptoms or as a constellation of symptoms suggestive of a particular systemic condition. Key features to establish are the duration of symptoms, the presence of nonspecific symptoms possibly related to uraemia, the presence of specific renal symptoms, and the presence of symptoms possibly indicative of systemic disease.
Chronic kidney disease
The symptoms of CKD are attributed to the gradual onset of uraemia, anaemia, and salt and water retention. Patients often develop these slowly and may not report them until renal function is severely impaired, perhaps even an eGFR as low as lesss than 10 ml/min per 1.73 m 2 body surface area. The number of symptoms and their severity tend to increase as renal function declines, forming a spectrum from asymptomatic to overtly symptomatic uraemia. Symptoms and the level of eGFR may not correlate well: some patients with an eGFR of 15 to 20 ml/min per 1.73 m 2 may be symptomatic, whereas a few with an eGFR of less than 5 ml/min per 1.73 m 2 may be remarkably symptom free.
Most patients have some symptoms by the time that they require dialysis (CKD stage 5, eGFR <15 ml/min per 1.73 m 2 ) (Bullet list 5). These include anorexia, nausea, and vomiting (in 76% of patients), fatigue and weakness (72%), pruritus (40%), breathlessness and orthopnoea (26%), bleeding tendency (14%), apathy and loss of mental concentration (12%), and muscle twitching and cramps (11%).
Bullet list 5 Features of uraemia and an eGFR less than 15 ml/min per 1.73 m 2 body surface area
- ◆ Anorexia and malnutrition
- ◆ Nausea and vomiting
- ◆ Tiredness
- ◆ Fluid overload with oedema, breathlessness, and orthopnoea
- ◆ Mental apathy and depression
- ◆ Muscle twitching, restless legs, and cramps
- ◆ Bleeding tendency—haematemesis, epistaxis
- ◆ Sexual dysfunction—loss of libido and impotence
- ◆ Cardiac—pericarditis
Factors contributing to the development of ‘uraemia’ and other symptoms include small-molecule nitrogenous substances, endproducts of protein metabolism, metabolic acidosis, salt and water retention, electrolyte disturbances (e.g. phosphate retention), malnutrition, and anaemia.
Some of these symptoms may be improved by treatment with agents such as erythropoietin, diuretics, and oral sodium bicarbonate, and dietary advice to improve malnutrition and phosphate control. Others respond to the initiation of dialysis. Some symptoms may persist in spite of all these measures.
It is unfortunately not uncommon for patients to present for the first time very late in the course of progressive CKD, with profound and symptomatic uraemia. This is the initial mode of presentation in 20 to 40% of patients entering dialysis programmes in the United Kingdom, who tend to be older, more dependent, and with greater comorbidities than those presenting earlier. Late presentation presents major problems: it is not possible to plan dialysis initiation, and patient choice of modality is limited, with haemodialysis being the default mode. Furthermore, it is often not possible to create definitive vascular access, hence patients often need to begin dialysis with temporary or semipermanent central venous lines. These and other features increase morbidity and mortality after late presentation.
It can be difficult and sometimes impossible to distinguish patients presenting late with advanced chronic renal failure (‘crash-landers’) from those with acute renal failure due to potentially reversible disease. Failure to become dialysis independent by 90 days after initiation is often taken as proof that the acute presentation was with endstage rather than acute renal failure. Patients who ‘crash-land’ are often extremely unwell and may be obtunded with uraemic encephalopathy. Fluid overload is common, with pulmonary and peripheral oedema. Metabolic acidosis is often present and if severe may cause Kussmaul’s respiration as well as cerebral and cardiac depression. Patients may also show signs of muscle twitching, which may be a sign of hyperkalaemia or hypocalcaemia. A pericardial friction rub indicates uraemic pericarditis, which if unrecognized may lead to pericardial tamponade and occasionally to fatal pericardial haemorrhage.
Acute renal failure (acute kidney injury)
Acute kidney injury (in brief) causes can be classified as being prerenal, renal (due to intrinsic renal disease) or postrenal (obstruction). In the general hospital setting, most cases are prerenal and occur as the result of reduced renal perfusion due to volume depletion (30%), cardiac failure (12%), and sepsis (12%). Drug-induced kidney injury accounts for 30%, urinary tract obstruction 10%, and acute glomerular disease and interstitial nephritis cause 5 to 10%.
Key features to establish sequentially when managing a patient with acute renal failure are as follows:
- 1 How ill are they? The condition of patients with similar biochemical abnormalities can range from the asymptomatic to the moribund: those with cardiorespiratory compromise need critical care support.
- 2 Does the patient need emergency haemodialysis or haemofiltration? The major indications are severe hyperkalaemia, pulmonary oedema, profound acidosis, and severe uraemia—the latter being defined more on clinical than biochemical grounds.
- 3 Is there a prerenal element that may respond to volume repletion or inotopic support? Clinical examination, perhaps supplemented by central venous pressure measurement, facilitates this decision.
- 4 Is the patient obstructed? Clinical features can be helpful, and a urinary tract ultrasound is usually diagnostic.
- 5 Is this acute or chronic renal failure? Sometimes this is difficult or impossible to determine on clinical grounds, but small kidneys on ultrasound signify chronic disease.
- 6 Is this intrinsic renal disease? Clinical features of systemic disease and relevant immunological tests (including ANCA and antiglomerular basement membrane antibody) must be pursued, and renal biopsy will usually be required to establish the diagnosis.
As with chronic kidney disease, many of the symptoms and signs attributed to loss of renal function are nonspecific and occur with advanced acute renal failure (GFR <15 ml/min per 1.73 m 2 ). However, in contrast to CKD, the acute metabolic changes are often less well tolerated. The greatest danger is hyperkalaemia, which may develop quickly and is almost always asymptomatic until the onset of cardiac arrhythmias and cardiac arrest. Other potentially life-threatening features include pulmonary oedema, metabolic acidosis, and uraemic pericarditis.
The clinical context and history are of overriding importance in establishing the likely aetiology of acute renal failure. A patient developing acute renal failure postsurgery is likely to have prerenal and acute tubular injury due to a combination of hypovolaemia, sepsis, and analgesia with an NSAID. A patient presenting acutely after a prolonged period of unconsciousness following a drug overdose is likely to have rhabdomyolysis. A patient with a past history of lupus presenting with a recent fever, myalgia, and rash is likely to have rapidly progressive lupus nephritis. A patient with a history of lower urinary tract symptoms or of urinary stones is likely to have obstruction.
It is always important to consider the possibility of urinary tract obstruction as it may be readily reversible. Complete anuria is highly suggestive of total obstruction, although it may also occur in patients with rapidly progressive glomerulonephritis and those with acute obstruction of the renal arterial supply. However, urinary output is generally a poor guide to the presence of urinary tract obstruction, and a normal or even increased output does not exclude the diagnosis. All patients with unexplained acute renal failure should undergo ultrasound imaging of the kidneys and urinary tract. This permits the diagnosis or exclusion of obstruction in most cases, and also allows renal size to be assessed: small kidneys indicate chronic renal failure.
It is important to emphasize that, after stabilization, patients in whom the clinical features and initial investigations do not give sufficient clues to allow a diagnosis to be established will require a renal biopsy to avoid missing potentially reversible intrinsic renal disease.
Urinary symptoms
Micturition.
Most symptoms related to micturition relate to problems arising in the lower urinary tract. Bladder outflow obstruction is commonly associated with symptoms such as urgency, hesitancy, poor urinary stream, nocturia, dysuria, and dribbling. Recognition of these symptoms is important as outflow obstruction may result in complete obstruction with acute renal failure or chronic obstructive uropathy with CKD.
Patients may also describe discomfort or pain on micturition. This symptom of dysuria may also be associated with burning within the urethra or suprapubic pain during or after micturition. When associated with urinary frequency or fevers in young women, dysuria is likely to be caused by a urinary tract infection. However, dysuria occurring in isolation in men of any age suggests structural lesions within the prostate or bladder and warrants further investigation. Perineal or rectal pain associated with micturition suggests prostatic inflammation, such as prostatitis or malignancy.
Patients may present with symptoms of increased frequency of micturition. In this situation, it is important to distinguish between frequent voiding of small volumes of urine and an overall increase in urinary volume with more frequent emptying of a full bladder. Charting urinary frequency and voided volumes over a number of days can allow these to be distinguished. The frequent passage of small volumes of urine suggests bladder irritation (from inflammation, stone, or tumour) or reduced volume from extrinsic compression or contraction (e.g. following radiotherapy). Increased frequency of emptying a full bladder is suggestive of polyuria, especially if the volume and frequency is unaffected during the night.
Polyuria (defined as a urinary output>3 litre/24 h) may result from solute diuresis, water diuresis, or a combination of both. Solute diuresis occurs in conditions such as hyperglycaemia and salt-losing states, e.g. overuse of diuretics and salt-losing nephropathies. Water diuresis may result from primary polydipsia, failure to synthesize or secrete ADH normally (congenital and acquired cranial diabetes insipidus), or failure of cortical and medullary collecting ducts to respond to ADH (congenital and acquired nephrogenic diabetes insipidus).
There are numerous causes of acquired nephrogenic diabetes insipidus, including chronic kidney disease (especially associated with ureteric obstruction, postobstructive states, and chronic interstitial nephritis), electrolyte abnormalities (hypercalcaemia and hyperkalaemia), nephrotoxic drugs (such as lithium and amphotericin), and many other miscellaneous conditions including sickle cell disease, Sjögren’s syndrome, and sarcoidosis. Most patients with polyuria have associated thirst, polydipsia, and nocturia. Polyuria needs confirmation by 24-h urinary collection as most patients are unclear as to their true daily urine output. Once it is established that the patient is polyuric, common causes such as hyperglycaemia and excessive diuretic use need to be excluded, after which investigations should focus on excluding primary polydipsia and distinguishing between cranial and nephrogenic diabetes insipidus.
Nocturia is defined as the need to get up once or more times for nocturnal voids. It may have a considerable negative impact on quality of life and in older people predisposes to falls. Three types of nocturia have been identified: low voided volume, nocturnal polyuria, and mixed origin. Nocturia due to low voided volumes occurs in patients with bladder outflow obstruction and those with hyperactive bladders from any cause. Nocturnal polyuria occurs when there is a reversal of the normal circadian pattern of voiding such that there is an increased nocturnal urine output. These types of nocturia are distinguishable by the use of voiding diaries. Elderly patients who void in excess of 33% of their total 24-h output between 11 p.m. and 7 a.m. are said to have nocturnal polyuria, the corresponding fraction in young adults being 20%. Factors predisposing to nocturnal polyuria include renal impairment, diabetes mellitus, congestive cardiac failure, sleep apnoea, and the mobilization of peripheral oedema due to any cause. In patients without predisposing causes, usually elderly, low nocturnal levels of ADH have been described.
Oliguria and anuria
Oliguria is arbitrarily defined as a urinary output of less than 400 ml/24 h or 0.5 ml/kg body weight per hour. Oliguria is the normal renal physiological response to reduced renal perfusion from any cause and is common in hospital inpatients, particularly those with acute illnesses associated with hypotension and reduced effective circulating volume. Monitoring of fluid balance and urinary output in such patients allows its early detection and treatment, which may help prevent progression to established acute renal failure. The recognition of oliguria should prompt an evaluation of the patient with attention to volume status, blood pressure and the detection/exclusion of sepsis, followed by appropriate management to optimize blood pressure and circulating volume.
Oliguria may also be a feature of intrinsic renal failure due to nephrotoxic drugs, acute glomerulonephritis, or interstitial nephritis, but it is a poor marker of intrinsic renal disease as urinary output often remains normal despite significantly impaired renal function.
The development of anuria, meaning the total absence of urine, is strongly suggestive of urinary tract obstruction, which may occur at any level in the urinary tract. A careful history, examination for an enlarged bladder and digital rectal examination for a prostatic or pelvic mass, should be followed by an urgent ultrasound of the kidneys and bladder. Very occasionally, anuria may be a manifestation of severe intrinsic renal disease, such as a rapidly progressive glomerulonephritis, cortical necrosis, or renal infarction.
Urine appearance and macroscopic haematuria
Macroscopic haematuria is the most common abnormality of the urine noted by patients. As little as 5 ml of blood in a litre of urine will lead to a visible change in urinary colour. Haematuria may arise from anywhere within the urinary tract, but bright red haematuria (with or without clots) is suggestive of lower urinary tract bleeding, whereas dark, smoky brown–black urine is more suggestive of renal pathology. Haematuria at the beginning of micturition, which then clears, suggests urethral pathology, whereas endstream haematuria is consistent with bladder pathology. Although the causes of haematuria are numerous (Bullet list 6), infection, stones, and malignancy are the most common. Macroscopic haematuria warrants investigation in all patients.
Bullet list 6 Causes of macroscopic haematuria
- • Cystitis and pyelonephritis
- • Prostatitis
- • Urethritis
- • Schistosomiasis
- ◆ Urinary stones
- • Renal cell
- • Transitional cell
- • Prostatic
- • IgA nephropathy
- • Alport’s syndrome
- • Crescentic glomerulonephritis
- • Polycystic kidneys
- • Interstitial nephritis
- • Papillary necrosis
- • Tuberculosis
- • Release of urinary obstruction
- • Loin pain–haematuria syndrome
- • Arteriovenous malformations
- • Anticoagulation
- • Factitious
Frank haematuria is uncommon in glomerular disease, with the notable exception of IgA nephropathy in which macroscopic haematuria classically occurs immediately following mucosal inflammation, typically an upper respiratory tract infection. In patients with polycystic disease, cysts may haemorrhage to cause loin pain and haematuria. This may be associated with infection of the cysts and usually resolves with conservative management, with antibiotics if there are signs of infection.
Red–brown–black urine is occasionally caused by haemoglobinuria due to haemolysis or myoglobinuria precipitated by rhabdomyolysis. Beetroot and food colouring may turn the urine pink, whereas drugs such as rifampicin may discolour the urine orange–red. Rarely, urine is found to darken following exposure to light, suggesting a diagnosis of porphyria or alkaptonuria.
The presence of pain in the renal angle (loin pain) is consistent with inflammation, obstruction, or stretching of the renal capsule by a mass lesion. Pain arising from acute obstruction is common and typically colicky in nature, with radiation into the groin and scrotum. The pain may be exacerbated by oral fluids, which increase urinary volume and pressure within the renal pelvis. Pyelonephritis typically causes renal angle pain on the affected side and is often associated with pyrexia and leucocytes in the urine. Similarly, a renal abscess extending into the renal capsule may present with loin pain or with isolated symptoms of diaphragmatic irritation or involvement of the psoas muscle, with pain on leg extension. Patients with polycystic kidneys may also develop loin pain as a result of infection or haemorrhage of single or multiple cysts.
Renal pain is an uncommon feature of glomerulonephritis and other intrinsic renal diseases: IgA nephropathy is very occasionally associated with renal pain, but active destructive glomerulonephritis and interstitial nephritis are invariably pain free.
Loin pain–haematuria syndrome
Rarely, patients may present with recurrent intermittent loin pain, haematuria (microscopic or macroscopic), and normal renal function, with no relevant structural abnormality of the renal tract. The cause of this condition, termed the loin pain–haematuria syndrome, is unknown: it is a diagnosis of exclusion which is most often seen in young women.
The pain—often described as ‘deep’, ‘burning’, or ‘throbbing’—is usually felt in the loin, but can radiate in a typical renal pattern to the groin, genital area, and medial thigh. Some will describe a psychologically traumatic event before the onset of pain. The pain can sometimes be induced or exacerbated by exercise and affected by posture, e.g. sitting for a prolonged length of time can be uncomfortable, and in some cases there is associated nausea and vomiting. Some patients report continuous pain that never goes away, whereas others describe episodic pain that lasts more or less continuously for days or (more typically) weeks, interspersed with periods of remission. The pain is usually unilateral at presentation, but many patients eventually develop pain bilaterally. Many patients are taking large quantities of opioids and other analgaesics (e.g. amitriptyline, gabapentin) by the time they are referred to specialist services.
Urological investigation is unremarkable, or shows incidental abnormalities only. If renal biopsy is performed, the appearances may be normal, but thinning or thickening of the glomerular basement membrane has been reported in about 60% of cases in some series, and appearances of IgA nephropathy are sometime seem, but the relationship—if any—between these findings and symptomatology remains obscure.
Aside from loin pain, many patients will have other medically unexplained somatic symptoms, raising the possibility that this symptom is also a somatoform disorder. They may request nephrectomy and/or renal autotransplantation, which the wise physician will not accede to, preferring to help the patient by sympathetic discussion and referral to pain management services.
Specific renal syndromes
Nephrotic syndrome.
See this link for very detailed technical article about nephrotic syndrome: Nephrotic Syndrome
Nephrotic syndrome is the triad of oedema, proteinuria, and hypoalbuminaemia (see Bullet list 7 for an example). Proteinuria is usually greater than 3.5 g in 24 h, which equates approximately to an ACR of 250 mg/mmol or PCR of 350 mg/mmol. When patients have clinically apparent oedema, serum albumin is usually less than 25 g/litre. However, in practice the definition is somewhat arbitrary, and the correlation between the degree of proteinuria, serum albumin, and presence of oedema is poor. Some patients (particularly older people) may develop oedema with proteinuria less than 3.5 g, whereas others remain free of oedema despite having a serum albumin considerably less than 25 g/litre. Other patients may have heavy proteinuria but maintain a normal serum albumin and remain free of oedema.
Bullet list 7 Case illustration—proteinuria and oedema
A 54-year-old woman presents with worsening peripheral oedema. She had been diagnosed with type 2 diabetes 6 months earlier, but remained well until 4 weeks ago, when she suddenly noted frothy urine and mild peripheral oedema. Over the following weeks the oedema had worsened and she noted some abdominal distension. Her only regular medication is gliclazide.
- ◆ Pitting oedema to her lumbar spine, with bilateral small pleural effusions
- ◆ Jugular venous pressure not elevated and heart sounds normal
- ◆ Mild erythema over right ankle and lower leg
- ◆ Urine dipstick test: protein 4+, no haematuria
- ◆ Urine albumin:creatinine ratio (ACR): 4520 mg/mmol
- ◆ 24-h urinary collection: 6.8 g proteinuria
- ◆ Serum albumin: 13 g/litre
- ◆ Serum creatinine: 82 µmol/litre
- ◆ Autoimmune and hepatitis serology: negative
- ◆ Renal ultrasonography and venous Doppler: normal
- ◆ Doppler ultra sonography of right leg: normal
- ◆ Renal biopsy: membranous nephropathy with subepithelial spikes on silver stain
- ◆ Membranous nephropathy with nephrotic syndrome
Frothy urine, oedema, and hypoalbuminaemia indicate the onset of heavy proteinuria and nephrotic syndrome. The rapid onset of symptoms suggests a primary glomerular lesion rather than long-standing diabetic nephropathy. The presence of leg erythema may be due to infection or deep venous thrombosis, hence a Doppler ultrasound scan was requested. To make the diagnosis, a renal biopsy was performed, which showed membranous nephropathy. The patient was initially managed conservatively with diuretics and low-molecular-weight heparin as thromboembolic prophylaxis.
Nephrotic syndrome indicates the presence of glomerular disease. Causes can usefully be divided into primary glomerular diseases and those arising secondary to systemic disease (Bullet list 8), with the geographical context important in determining the most likely cause in any particular case. The most common cause of nephrotic syndrome in Western countries is diabetes mellitus, whereas in developing countries it is most commonly associated with infection. Nephrotic syndrome due to malaria and hepatitis are particularly common in sub-Saharan Africa, and poststreptococcal glomerulonephritis is also an important cause.
Bullet list 8 Causes of nephrotic syndrome
- • Minimal change
- • Focal segmental glomerulosclerosis (FSGS)
- • Membranous
- • Mesangiocapillary glomerulonephritis (MCGN)
- • Gold, penicillamine, NSAIDs, captopril, heroin
- • Poststreptococcal glomerulonephritis
- • Hepatitis B and C
- ◆ Pre-eclampsia
- • Nail–patella syndrome
Clinical features
One of the earliest symptoms patients may report is that of frothy urine. This often occurs before the onset of oedema and may be a useful indicator of the onset of heavy proteinuria. As proteinuria develops and serum albumin falls, patients gradually develop oedema. This may be noticed first as periorbital swelling and ‘puffiness’ in the morning, or as ankle swelling in the evening due to the effects of gravity. Worsening leg oedema develops as salt and water retention increases, followed by abdominal distension from ascites. In men, scrotal oedema may be marked and very uncomfortable. Further fluid retention leads to pleural effusions, which are often bilateral but may be unilateral. Patients often feel lethargic, with a loss of appetite and nausea due to associated gut oedema.
Clinical examination of the patient’s volume status may reveal a normal or low jugular venous pressure despite marked oedema. Although rare in untreated adult patients, it is important to identify intravascular volume depletion because the use of high-dose diuretic therapy in this setting may provoke circulatory collapse from hypovolaemia, or less dramatically may further reduce renal perfusion and exacerbate renal dysfunction. Conversely, a raised jugular venous pressure with a low blood pressure may suggest a significant pericardial effusion or underlying amyloid with cardiac involvement.
Patients may also present with complications associated with nephrotic syndrome. Thromboembolism may be difficult to detect clinically. Patients with marked peripheral oedema often have swollen legs of unequal size and associated erythema due to an increased susceptibility to cellulitis. These may mask the signs of deep venous thrombosis. Similarly, subtle symptoms of breathlessness, perhaps suggesting pulmonary embolism, or headache, perhaps suggesting cerebral venous sinus thrombosis, may be overlooked. In practice, a low threshold is required for investigation and treatment of suspected thromboembolism.
The combination of severe peripheral oedema and susceptibility to infection following skin breakdown often leads to cellulitis. Long-standing hypoalbuminaemia may lead to leuconychia. Severe hyperlipidaemia, which is a feature of nephrotic syndrome, may lead to cutaneous xanthomas.
Establishing a clinical diagnosis of nephrotic syndrome is often straightforward. The clinical history and examination may also provide clues to an underlying cause, which may be clear, such as in a patient with long-standing diabetes and progressive diabetic nephropathy. Alternatively, the immediate cause may only become apparent after a detailed history revealing long-standing use of drugs that may precipitate the condition (e.g. ACE inhibitors, NSAIDs, gold, or penicillamine). A history of chronic infections (such as hepatitis) may suggest an underlying membranous or mesangiocapillary glomerulonephritis, whereas a rash and arthralgia may lead to a diagnosis of an autoimmune condition such as systemic lupus erythematosus or cryoglobulinaemia. The presence of other long-standing inflammatory conditions, such as rheumatoid arthritis, raises the possibility of systemic amyloidosis. In older patients, an associated malignancy remains a possibility and should be sought in the history and examination, but does not warrant further investigation apart from a chest radiograph in the absence of clinical clues, e.g. disturbance of bowel habit would merit imaging of the colon. Very occasionally, a family history may reveal an inherited nephrotic syndrome such as familial focal segmental glomerulosclerosis.
Rapidly progressive glomerulonephritis with acute renal failure
Around 5% of cases of acute renal failure are caused by a rapidly progressive glomerulonephritis (RPGN). Recognizing this relatively small group of patients is important because many of the causes respond well to treatment, provided the diagnosis is made early and treatment started promptly. The key to making a diagnosis is having a high index of clinical suspicion such that important features of the syndrome are identified (see Bullet list 9for an example).
Bullet list .9 Case illustration—ANCA-associated vasculitis
An 80-year-old woman presents with a 2-week history of increasing malaise and lethargy. On close questioning she also reported arthralgia in the small joints of her hands, and numbness in her hands and feet in the last few months.
Examination
- ◆ Subtle purpuric rash on both legs
- ◆ Bibasal crepitations
- ◆ Reduced pinprick sensation in a glove and stocking distribution
- ◆ Creatinine: 854 µmol/litre (56 µmol/litre 10 months before)
- ◆ Urea: 45 mmol/litre
- ◆ Hb: 8.3 g/dl
- ◆ Urine dipstick test: blood 3+, protein 2+
- ◆ Urine microscopy: red cell casts
- ◆ Serological testing: p-ANCA positive, with myeloperoxidase titre 78%
- ◆ Renal biopsy: focal necrotizing glomerulonephritis
- ◆ Acute renal failure due to microscopic polyangiitis (an ANCA-associated vasculitis) with associated peripheral neuropathy
The history is nonspecific, except that the onset of symptoms is recent and suggestive of a systemic disorder. The presence of a purpuric rash makes the diagnosis of vasculitis a possibility. Dipstick testing of the urine and checking the renal function are critical in making the diagnosis of acute renal failure due to an inflammatory condition. Confirmation of a systemic vasculitis is made with a positive p-ANCA and renal biopsy.
The hallmarks of an RPGN are rapidly declining renal function, haematuria and proteinuria on urine dipstick testing, dysmorphic red cells or red cell casts on urine microscopy, and crescentic and focal necrotizing glomerulonephritis on renal biopsy.
An RPGN may present either de novo in a previously well patient or as a complication in a patient known to have a systemic disease (Bullet list 10). Their clinical features may be diverse. Occasionally, patients may present with very few symptoms and signs, except for proteinuria and haematuria with a recent decline in renal function, and at the other end of the spectrum patients may present with severe acute renal failure associated with features of uraemia. Importantly, patients may also present with clinical features of systemic inflammation that indicate an underlying cause for glomerulonephritis. These range from the subtle, such as arthralgia or myalgia, to the florid, such as a purpuric rash, haemoptysis and peripheral neuropathy. Clinical features of specific inflammatory diseases associated with an RPGN are detailed in Table 1 below.
Bullet list 10 Causes of a rapidly progressive glomerulonephritis
- • Wegener’s granulomatosis
- • Microscopic polyangiitis
- • Churg–Strauss syndrome
- ◆ Other primary systemic vasculitides (ANCA-negative)
- • Cryoglobulinaemia
- • Henoch–Schönlein purpura
- • Postinfectious glomerulonephritis
- • Infective endocarditis
- ◆ Antiglomerular basement membrane disease (Goodpasture’s syndrome)
- • Mesangiocapillary glomerulonephritis
In practice, specific features to elicit in patients presenting with an acute decline in renal function include arthralgia and arthritis, myalgia and muscle tenderness, rashes, eye symptoms (pain and redness), ear, nose, and throat symptoms (epistaxis, nasal crusting, and new deafness), haemoptysis (important, as may be life-threatening if severe), and neuropathic symptoms and signs. Conversely, the clinician should have a high index of suspicion for an RPGN in patients presenting with any of the above features, and in this context suspicions are heightened by the presence of dysmorphic red cells and red cell casts on urinary microscopy.
If an RPGN is suspected, then investigations should include ANCA, antiglomerular basement membrane (anti-GBM) antibodies, antinuclear and anti-double-stranded DNA antibodies, serum complement, antistreptolysin-O titre, and immunoglobulins and serum electrophoresis (including tests for cryoglobulins). It is almost certain that a patient with an RPGN will require a renal biopsy to confirm the diagnosis and to guide management, and thus all patients with suspected RPGN should be referred urgently to a nephrologist.
Presentation of renal disease associated with other underlying diseases
Renal disease is capable of presenting in many complex and diverse ways, and many renal problems arise as either direct or indirect complications of other disease. Examples include acute renal failure caused by sepsis, and progressive chronic kidney disease due to diabetes (Bullet list 11. This section illustrates some common and important presentations of renal disease.
Bullet list 11 Important and common presentations of renal disease
- ◆ Diabetic nephropathy with progressive chronic kidney disease
- • Renal atheroemboli
- • Renal artery stenosis
- • Urinary tract obstruction
- • Hypercalcaemia
- • Acute presentation with renal failure: (1) general syndromes—sepsis, rhabdomyolysis, haemolytic uraemic syndrome, postinfectious glomerulonephritis, tubulointerstitial nephritis; (2) specific syndromes—hantavirus, leptospirosis, malaria
- • Chronic infections associated with renal disease: hepatitis B, hepatitis C, filaria, schistosomiasis, HIV
- • Sarcoidosis
- ◆ Drug-induced renal disease
- ◆ Pregnancy
Diabetic nephropathy
In the Western world, diabetes is the most common cause of renal disease, accounting for 43% of endstage renal failure in the United States of America and 19% in the United Kingdom (Bullet list 12). Diabetic nephropathy develops over the course of years and is preceded by a clinically silent phase of microalbuminuria, which is often detected as a result of diabetic screening programmes, enabling a targeted approach to management in which tight glycaemic control and blood pressure control with the use of agents to block the renin–angiotensin system aim to reduce the rate of progression of the nephropathy. As with other causes of progressive CKD, patients with diabetic nephropathy often only develop symptoms of kidney disease late in the course of their disease, but there is a tendency for those with this condition to become symptomatic, particularly in relation to anaemia and fluid retention, with lesser impairment of renal function than their nondiabetic counterparts. This leads to an earlier requirement for initiation of dialysis in patients with diabetic nephropathy.
Bullet list 12 Case illustration—progressive chronic kidney disease due to diabetic nephropathy
A 66-year-old Asian man presents with nausea, anorexia, ankle swelling and breathlessness. He has a 25-year history of type 2 diabetes mellitus, a 14-year history of hypertension, and had coronary artery bypass grafts 3 years ago. Insulin, furosemide, and ramipril are his only regular medications.
- ◆ Cardiovascular—blood pressure 167/88 mmHg, jugular venous pressure +3 cm, cardiomegaly, bibasal crepitations, and peripheral oedema to sacrum
- ◆ Fundi—treated diabetic retinopathy
- ◆ Neurological—reduced pinprick sensation in stocking distribution to knees, with absent ankle reflexes, proprioception, and vibration sensation
- ◆ Urine albumin:creatinine ratio (ACR): 1720 mg/mmol
- ◆ Serum creatinine: 568 µmol/litre (eGFR 9 ml/min per 1.73 m 2 body surface area)
- ◆ Serum bicarbonate: 15 mmol/litre
- ◆ Full blood count: Hb 9.8 g/dl
- ◆ Hb A1C : 10.2%
Five years previously his blood pressure was 189/92 mmHg with creatinine of 154 µmol/litre and protein+ on urine dipstick. At the time of his coronary surgery, blood pressure was 165/86 mmHg with creatinine 210 µmol/litre. Over the last year he had felt well until the last 2 months, since when he had developed increasing lethargy, anorexia, and breathlessness on exertion, and noted increasing ankle swelling.
This man with long-standing diabetes presents with nonspecific symptoms and oedema. He also has evidence of end-organ damage, with cardiovascular disease, retinopathy, and neuropathy. Five years ago he had evidence of nephropathy with proteinuria and an eGFR of 43 ml/min (CKD stage 3). Since then his blood pressure and glycaemic control have been poor, which contributed to the progression of nephropathy to eGFR of 30 ml/min (CKD stage 3/4) 3 years ago, and now to CKD stage 5 with symptoms of uraemia.
Screening patients with diabetes for microalbuminuria and hypertension enables early diagnosis of complications and intensive management of glucose and blood pressure. As eGFR falls to 30 ml/min per 1.73 m 2 body surface area, patients should be referred to a nephrologist to plan for renal replacement therapy.
Patients with diabetes are also subject to develop other microvascular and macrovascular complications, which may lead to superimposed renal atheroembolic disease and renal artery stenosis. These may present as an abrupt decline in renal function following the introduction of an ACE inhibitor or angiotensin receptor antagonist. Patients with diabetic nephropathy are also at increased risk of acute or chronic renal failure, with common causes for this including use of radiocontrast media for investigations such as coronary or peripheral angiography, surgery (especially cardiac surgery), and, in the context of diabetic emergencies, particularly diabetic ketoacidosis.
See here for a detailed article about diabetic renal disease .
Many patients with diffuse atherosclerosis have evidence of renovascular disease, and a history of cerebrovascular, coronary, or peripheral vascular disease makes a diagnosis of renovascular disease likely. Up to 24% of patients presenting with peripheral vascular disease have stenoses in both renal arteries, and up to 50% have more than 50% stenosis in at least one renal artery. The absence of peripheral pulses and the presence of a femoral bruit make the diagnosis of renovascular disease extremely likely, although most of these patients remain asymptomatic from the renal point of view. Common presentations of renovascular disease are outlined in Bullet list 13
Bullet list 13 Common presentations of renovascular disease
- ◆ As part of the investigation for acute, severe, or refractory hypertension
- ◆ An acute rise (>20%) in creatinine following introduction of an ACE inhibitor or angiotensin receptor antagonist
- ◆ Incidental finding of asymmetric kidney size on renal ultrasound
- ◆ As part of the investigation for progressive CKD.
- ◆ Symptomatically as acute (‘flash’) pulmonary oedema in the absence of cardiac failure or fluid overload
- ◆ Postoperative acute renal failure, especially following coronary artery bypass or aortic aneurysm surgery

Myeloma and other malignancies
Myeloma can cause acute renal failure in several ways. Features suggestive of underlying myeloma in a patient presenting with unexplained renal failure are older age, bone pain (often nonspecific), hypercalcaemia (sometimes mild, and sometimes ‘relative’ considering the degree of renal impairment), anaemia (often inappropriately severe for the degree of renal impairment), abrupt decline in renal function after relatively minor prerenal ‘insult’, and unremarkable urine dipstick.
Up to 50% of patients presenting with myeloma have impaired renal function at the time of diagnosis. This may be reversible and due to hypercalcaemia, dehydration, hyperuricaemia, or infection. Cast nephropathy accounts for 10% of all renal dysfunction in patients with myeloma and is characterized by the formation of tubular casts of excreted light chains and Tamm–Horsfall protein: these are thought to cause renal failure by obstructing the tubule and by direct tubular toxicity.
The key to the diagnosis is to maintain a high index of suspicion, particularly in elderly patients presenting with renal failure and hypercalcaemia. Serum electrophoresis and urinary Bence Jones proteins are the required investigations, followed—if either is positive—by bone marrow examination.
Other malignancies may present with renal involvement due to a number of mechanisms, including acute renal failure due to urinary tract obstruction by pelvic or retroperitoneal tumour. Other possible causes are outlined in Bullet list 14
Bullet list 14 Renal presentations associated with malignancy
- • Tumour lysis with urate nephropathy
- • Chemotherapy (e.g. cisplatin, ifosfamide)
- • Leukaemic infiltration
- • Microangiopathy
Renal presentation of infectious diseases
A wide range of systemic infections, resulting in either acute renal failure or chronic kidney disease, can affect the kidney. The presentation of infection-related kidney disease varies worldwide, and in the developing world—in contrast to the developed world—infectious diseases are the leading cause of acute and chronic kidney disease.
Acute renal failure may occur as part of a general systemic syndrome induced by infection, such as sepsis and septic shock, haemolytic uraemic syndrome (HUS), rhabdomyolysis, postinfectious glomerulonephritis, and tubulointerstitial nephritis. Alternatively, an infectious agent may cause specific nephrotoxicity, e.g. hantavirus, leptospirosis, or malaria.
General systemic syndromes caused by infection
In Western countries, the most common infectious cause for renal disease is sepsis, which accounts for 10% of all hospital-acquired renal failure and, if severe, may lead to acute renal failure in the context of multiorgan failure.
Other general syndromes that may be induced by infection include HUS and rhabdomyolysis. For example, the verotoxin of Escherichia coli O157:H7 causes (D+) HUS, which is a thrombotic microangiopathy characterized by diarrhoea, acute renal failure, and thrombocytopenia. Patients with influenza, legionella, or streptococcal infection may present with fever, severe myalgia, and dark urine in the context of acute renal failure due to rhabdomyolysis.
Poststreptocccal glomerulonephritis is still one of the most common causes of acute renal failure in the developing world, although now seen rarely in the United Kingdom and developed countries. Typical presentation is 10 days to a few weeks following a streptococcal infection of the throat or skin with a ‘nephritic’ syndrome characterized by hypertension, oedema, haematuria and proteinuria, and acute renal failure.
Specific nephrotoxicity caused by infection
Hantavirus and leptospirosis.
Hantaviruses are endemic in specific rodent reservoirs and are transmitted to man by inhalation of infectious aerosols or rodent excreta. In Europe, the main pattern of disease is haemorrhagic fever with renal syndrome (HFRS). The disease presents in four stages: (1) an abrupt febrile stage characterized by fever, loin or abdominal pain, nausea, vomiting, and periorbital oedema, lasting for 3 to 7 days; (2) a hypotensive phase associated with haemorrhages and ecchymoses, lasting hours to 2 days; (3) an oliguric phase for 3 to 14 days, with worsening acute renal failure due to a tubulointerstitial nephritis and haemorrhage; and (4) a polyuric phase as renal function returns to normal.
Leptospirosis may present with similar features to hantavirus. However, leptospirosis is endemic worldwide and is typically associated with jaundice and hepatomegaly. Acute renal failure occurs in 20 to 85% of patients owing to tubulointerstitial nephritis.
Severe infection with Plasmodium falciparum occurs in nonimmune adults. Acute renal failure may occur either in the acute phase of the disease or in the recovery phase. Sequestration of parasitized erythrocytes in the renal vasculature and proinflammatory cytokine release cause tubular cell ischaemia and injury. Rarely, patients with falciparum malaria present with ‘blackwater fever’ due to massive intravascular haemolysis, which often occurs following quinine administration in association with glucose-6-phosphate dehydrogenase deficiency.
Infections and chronic kidney disease
In the developing world, CKD is commonly secondary to infectious disease, with the underlying infection often remaining subclinical until the presentation with renal manifestations. Examples of CKD secondary to infective agents include hepatitis B, hepatitis C, Plasmodium malariae , filaria, schistosomiasis, and HIV.
Hepatitis B is classically associated with nephrotic syndrome due to membranous nephropathy, but occasionally it may result in a mesangiocapillary glomerulonephritis. Hepatitis B virus infection is associated with the development of polyarteritis nodosa, although the reported frequency of this appears to be falling. Patients who develop such complications usually have chronic hepatitis, having contracted the virus in childhood.
Hepatitis C is increasingly recognized as a common cause for cryoglobulinaemia, but this remains asymptomatic in most patients, with only a few developing clinical evidence of vasculitis. The associated mesangiocapillary glomerulonephritis can present as nephrotic syndrome or chronic kidney disease.
Many infectious agents endemic in sub-Saharan Africa may also cause a mesangiocapillary glomerulonephritis presenting as nephrotic syndrome: most common is P. malariae , but filaria and schistosomiasis remain in the differential diagnosis.
HIV-associated nephropathy (HIVAN) is an increasingly recognized complication of HIV infection, and it now accounts for 1% of the dialysis population in the United States of America. Patients usually present with heavy proteinuria and nephrotic syndrome due a collapsing form of focal segmental glomerulosclerosis. HIVAN predominates in young African-American men and is rare in areas endemic for HIV.
Systemic inflammatory diseases
Patients with systemic inflammatory disease are at risk of developing renal disease. Sometimes this may be the presenting feature of the condition, such as systemic vasculitis or systemic lupus, and on other occasions renal disease may develop as a complication later in the course of disease. Examples of systemic inflammatory diseases associated with renal involvement are detailed in Bullet list 15. The presentation of an acute glomerulonephritis and progressive renal failure due to systemic inflammatory diseases such as the vasculitides and systemic lupus erythematosus has been discussed earlier in the chapter, and further details can be found in Chapters 21.10.2 (vasculitides) and 21.10.3 (renal involvement in rheumatological disorders). Other inflammatory diseases may present in different ways.
Bullet list 15 Systemic inflammatory diseases associated with renal involvement
- • Systemic sclerosis
- • Relapsing polychondritis
- • Ankylosing spondylitis
- • Behçet’s disease
- • Amyloidosis (of AA type)
Sarcoidosis
Renal disease is common in sarcoidosis and characterized histologically by granulomatous tubulointerstitial nephritis. The mean prevalence from biopsy studies is 35%, but this is likely to be an overestimate. Most renal disease is subclinical, but may be identified by the presence of proteinuria or tubular dysfunction with a renal tubular acidosis. However, sarcoidosis may present with acute renal failure, which may be caused by an acute tubulointerstitial nephritis associated with an eosinophilia and eosinophiluria, or be precipitated by hypercalcaemia, which may be more common in summer months owing to ultraviolet light exposure. The presence of extrarenal features of sarcoidosis (including bilateral hilar lymphadenopathy and erythema nodosum) helps to establish the diagnosis, but sometimes the diagnosis may only become apparent following a renal biopsy for unexplained renal impairment and measurement of serum ACE.
Systemic sclerosis
Systemic sclerosis may present with an acute crisis characterized by an abrupt rise in blood pressure (>160/90 mmHg) with hypertensive encephalopathy, acute renal failure, and a microangiopathic haemolytic anaemia. This may occur before the onset of the cutaneous features of the disease. Patients are typically tachycardic, with evidence of heart failure and a high systemic vascular resistance. This diagnosis should be suspected in any patient presenting with malignant-phase hypertension and acute renal failure.
Systemic amyloidosis
Systemic amyloidosis is characterized by extracellular deposition of insoluble fibrillar proteins that lead to organ dysfunction. In AL amyloidosis this arises from light chains produced by a malignant plasma cell clone. AA amyloidosis occurs in the setting of long-standing inflammation, the amyloidogenic protein being an N-terminal fragment of serum amyloid A (SAA), an acute phase reactant. Patients may present with heavy proteinuria or nephrotic syndrome. Renal involvement can be demonstrated by serum amyloid P (SAP) scanning or by renal biopsy.
Drug-induced renal disease
Numerous drugs have the potential for causing both acute renal failure and chronic kidney disease (Bullet list 16). As well as prescribed and over-the-counter medication, renal disease may also arise from herbal and traditional Chinese medicines or illicit drugs. Mechanisms by which drugs cause renal disease include salt and water depletion, effects on renal perfusion, direct nephrotoxicity, and intrarenal obstruction.
Bullet list 16 Adverse effects of drugs on the kidney
- • Aminoglycosides
- • Amphotericin
- • Cisplatin
- • Radiocontrast media
- • Paracetamol poisoning
- • Statins (by inducing rhabdomyolysis)
- • β-Lactam antibiotics (penicillins, cephalosporins)
- • Furosemide
- • Allopurinol
- • Azathioprine
- • Sulphonamides
- • Aristolochic acid (‘Chinese herb nephropathy’)
- • High-dose captopril
- • Penicillamine
- • Phenytoin
- • Pamidronate
- • Acetazolamide
- • Demeclocycline
- • Aspirin with phenacetin
- • Aciclovir
- • Indinavir
- • Methotrexate
Salt and water depletion
Acute renal failure may follow hypotension and reduced renal perfusion due to sodium and water depletion. This may be caused by excess diuretic use or diarrhoea and vomiting as a drug side effect.
Effect on renal perfusion and the regulation of intrarenal haemodynamics
Overdose of any hypotensive agent may compromise renal perfusion and interfere with renal function. Agents which block the renin–angiotensin system, the ACE inhibitors and angiotensin receptor blockers, require special consideration. These agents abrogate the effect of angiotensin II on efferent arteriole constriction, which is the normal adaptive response to any reduction in renal perfusion. A small and acceptable deterioration in renal function (up to a 20% increase in serum creatinine) often occurs following the introduction of an ACE inhibitor. A greater increase in serum creatinine may indicate underlying renal artery stenosis, for which further investigation may need to be carefully considered, and these agents are implicated in a significant proportion of cases of acute renal failure in patients with sepsis and dehydration. This is because renal perfusion is frequently compromised in these settings and the kidney is unable to autoregulate its blood flow in the presence of renin–angiotensin system blockade.
NSAIDs have numerous renal effects, including disturbances of autoregulation of intrinsic renal haemodynamics. These effects are mediated by inhibition of prostaglandin synthesis from arachidonic acid by nonspecific blocking of the enzyme cyclooxygenase. This may lead to vasoconstriction and reversible renal impairment in volume-contracted states. Long-term use of NSAIDs may cause chronic renal impairment, with selective COX-2 inhibitors seeming to confer no renal advantage.
Direct nephrotoxicity
The mechanisms of drug toxicity on the kidney include direct tubulotoxicity, drug-induced tubulointerstitial nephritis, tubular dysfunction, and glomerular disease. Common nephrotoxic drugs and mechanism are outlined in Bullet list 16.
Tubulointerstitial nephritis is classically caused by penicillins and NSAIDs, but most drugs have the potential to cause the condition. Drug-induced tubulointerstitial nephritis usually occurs days to weeks after starting the drug. Symptoms may be nonspecific, with malaise, fatigue, and anorexia. A low-grade fever, fleeting rash, and arthralgia may also be reported. Investigations show a variable degree of renal dysfunction along with proteinuria and microscopic haematuria. Urine microscopy may show white and red cell casts, and there may be a blood eosinophilia. However, in practice the key is to suspect the diagnosis, stop the potentially offending drug (or drugs), and proceed with a renal biopsy if renal function does not improve.
Obstruction
Specific drugs, such as aciclovir and the protease inhibitor indinavir, may precipitate as crystals within the tubule, causing obstruction and sometimes renal failure. This is more likely to occur if the patient is dehydrated, hence adequate fluid input to achieve a high urinary volume is advised before these drugs are taken.
Pregnancy provides a unique set of circumstances in which renal disease may present. Renal disease may be present before pregnancy and be detected as part of screening for proteinuria and hypertension during the first trimester. Alternatively, de novo renal disease may be precipitated by pregnancy and present with specific syndromes, such as nephrotic syndrome or acute renal failure due to pre-eclampsia. A summary of the presentation of renal disease in pregnancy is detailed in Bullet list 17.
The clinical presentation of renal disease during pregnancy is often varied and nonspecific, but certain features may guide the diagnosis. Key points to note are as follows:
- ◆ Is there evidence of pre-existing renal disease?
- ◆ Were previous pregnancies complicated by hypertension or pre-eclampsia (PET)?
- ◆ What was the time of onset of renal disease during pregnancy? Onset in late pregnancy implies that the renal disease is likely to be pregnancy induced.
- ◆ Hypertension with proteinuria and oedema suggest PET or underlying renal disease (e.g. systemic lupus erythematosus).
- ◆ Hypotension and hypovolaemia suggest sepsis or haemorrhage.
Bullet list 17 Renal disease in pregnancy
- • Hyperemesis
- • Haemorrhage
- • Abruption
- • Pyelonephritis
- • Septic abortion
- • Puerperal sepsis
- • Gravid uterus
- • Pre-eclampsia
- • Acute fatty liver of pregnancy
- • Syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP)
- • Haemolytic uraemic syndrome (HUS)
- ◆ Any chronic kidney disease with deterioration of function, proteinuria, and pre-eclampsia
- ◆ Flare of systemic and renal disease (systemic lupus erythematosus and systemic sclerosis)
- ◆ Systemic lupus erythematosus
- ◆ Minimal change disease
Pre-eclampsia and HELLP
Pre-eclampsia classically presents with hypertension, oedema, and proteinuria. Other recognized features include elevation of serum urate, liver transaminases, and haematocrit, along with thrombocytopenia. However, patients may not be hypertensive or demonstrate other features, hence distinguishing pre-eclampsia from pre-existing renal disease may be difficult, and the condition may occasionally present with heavy proteinuria and nephrotic syndrome. The HELLP syndrome (haemolysis, elevated liver enzymes, and low platelets) is a severe variant of pre-eclampsia that is commonly associated with renal failure, severe haemolysis, and coagulopathy, and may progress to multiorgan failure. See Chapters 14.4 (hypertension in pregnancy) and 14.9 (liver and gastrointestinal disease in pregnancy) for further discussion.
Haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura (HUS/TTP) are related disorders that are occasionally associated with pregnancy and can both cause acute renal failure. HUS usually occurs 2 days to 10 weeks postpartum and may cause severe renal failure. By contrast, TTP usually presents in the first or second trimester with predominant neurological features and only mild proteinuria and haematuria.
Conditions and Diseases
Highlighted article.
Preparing for pregnancy
Why is being ready for pregnancy so important?
Conception occurs about 2 weeks before your period is due. That means you may not even know you're pregnant until you're more than 3 weeks pregnant. Yet your baby is most sensitive to harm 2 to 8 weeks after conception. This is when your baby's organs (such as the heart) begin to form. Anything you eat, drink, smoke or are exposed to can affect your baby. That's why it's best to start acting as if you're pregnant before you actually are.
Articles about various types of diet:
- A balanced diet
- Acid reflux diet
- Anti-aging diet
- Anti-cancer diet
- Anti-candida diet
- Diet for constipation
- Diet for stress
- A blood pressure diet
- Diet to reduce cancer risk
- Diets: a general overview
- GERD diet - in detail
- GERD diet - top ten tips
- GERD diet plan and table
- Gluten-free diet -labelling and shopping - with tables
- Gluten-free diet - eating and dining
- Gluten-free diet - eating healthily - about nutrition and diet
- Nutrition in pregnancy
- Peptic ulcer diet in brief
- Table of anti-cancer foods
- The eatwell plate
- Tips and guidelines for healthy eating
- Weight loss and heartburn
- Keeping salt out of your diet
Herbal Treatments
Complementary medicine - commonly used herbal therapies and herbal substances - links to article:
- St Johns Wort
- St Johns Wort for Depression and Anxiety
- Treat anxiety and insomnia with Californian poppy, hops and passion flower
Miscellaneous Detailed Articles
- Atherosclerosis biology and pathology
- Bioterrorism
- Cardiac involvement in genetic disease (e.g. Marfan's Syndrome)
- Coronary heart disease - influences acting in utero and early childhood
- Iron metabolism
- Chest pain, breathlessness and fatigue
- Hormones and the gastrointestinal tract
- Vitamins and trace elements
Alzheimer's Disease
- Alzheimer's Disease - main section and non-technical article
- Detailed technical article about dementia - includes Alzheimer's disease technical section
- Alzheimer's disease in detail -technical
- Alzheimer's detected decades before symptoms
- Acute Abdominal Pain
- Chest Pain, Breathlessness and Fatigue
- Chronic pain
- Dysmenorrhoea
- Fibroid Pain
- Pelvic Pain
- Vaginal Discharge
Section about medical treatments - links to articles:
- Coronary artery bypass surgery
- Lipid lowering drugs
- Pain management
- Percutaneous interventional cardiac procedures (PCI and others)
- Psychopharmacology
- Robotic surgery
- Stable angina management and treatment
- Tinnitus treatment
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

teacher appreciation
11 templates

tropical rainforest
29 templates

46 templates

pediatrician
27 templates

spring season
34 templates

23 templates
Kidney Disease
Kidney disease presentation, free google slides theme and powerpoint template.
Kidneys are some of the most important organs, as they take part in various important processes. If you are a physician or urologist, professor or a med student and want to show some details about nephritic conditions and their treatments, use this creative template.
The structure of this template is typical of a disease presentation. It’s conspicuous for the use of different hues of pink for backgrounds and other elements. We have added linear and filled illustrations related to health. There are drawings performed with dashed lines, which gives a very modern air. The condensed heavy titles are rounded, elongated and elegant; this makes them perfect for a theme like this one.
Features of this template
- A creative design with linear and filled illustrations
- 100% editable and easy to modify
- 26 different slides to impress your audience
- Contains easy-to-edit graphics, maps and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

Register for free and start editing online

Diseases and Disorders of the Kidney
Jul 21, 2014
220 likes | 473 Views
Diseases and Disorders of the Kidney. Section 7.7 Page 357. Importance of the kidney. Kidneys help maintain homeostasis Diseases and disorders of the kidneys can have effects on other systems Many kidney disorders are detected by urinalysis. Outline. Diseases : Diabetes mellitus
Share Presentation
- various solutes
- many kidney disorders
- kidney transplant
- filtrate sugar levels
- normal filtrate

Presentation Transcript
Diseases and Disorders of the Kidney Section 7.7 Page 357
Importance of the kidney • Kidneys help maintain homeostasis • Diseases and disorders of the kidneys can have effects on other systems • Many kidney disorders are detected by urinalysis
Outline Diseases: • Diabetes mellitus • Diabetes insipidus • Bright’s disease • Kidney stones Treatments: • Dialysis • Transplant
Diabetes mellitus • The one with which you are probably familiar Cause: • Inadequate secretion of insulin by pancreas Results in: • Cells can’t take up sugar • Blood sugar levels rise Glomerular filtrate sugar levels rise
Diabetes mellitus • More sugar in filtrate than can be reabsorbed • Excess sugar remains in nephron; creates osmotic pressure that keeps water in nephron. • Large volumes of urine are produced.
Diabetes mellitus Detection: • Glucose in urine • Not usual Treatment: • Insulin
Diabetes insipidus Cause: • No ADH to regulate water reabsorption. • Due to: destruction of ADH-producing cells in hypothalamus, or of neural pathway between hypothalamus and pituitary.
Diabetes insipidus Results in: • Increased urine output Treatment: • Synthetic ADH, or drugs stimulating ADH production
Bright’s disease, aka “nephritis” Cause & Result: • A class of diseases, characterized by inflammation of nephrons • Permeability of nephron membrane is altered: • Proteins can pass into nephron • The body has no reabsorption mechanism for proteins • Water remains in nephron due to osmotic pressure • Increased urine output
Bright’s disease Detection: • Protein in urine; waste products in blood Treatment: • Anti-inflammatories, antibiotics, dialysis
Kidney stones Cause: • Precipitation of mineral solutes from blood • Normal filtrate contains chemicals that prevent precipitation • Not enough in an affected individual Result: • Can lodge in renal pelvis, or in ureter • Sharp sides tear tissues of ureter and urethra → Pain
Kidney stones Treatment: • Surgical • Nonsurgical – High-energy shock waves are used to break kidney stones into smaller fragments, which can be excreted in urine Prevention: • Increase fluid consumption in order to produce dilute urine
Dialysis • Malfunctioning kidneys cannot effectively process wastes • Dialysis technology uses the process of diffusion to remove wastes from patients' blood • Two types of dialysis: • Haemodialysis • Peritoneal dialysis
Haemodialysis • Machine is connected to a patient`s vein • Blood is pumped through a series of dialysis tubes • Tubes act like a semi-permeable membrane • Allow diffusion of some substances
Urea: Higher concentration in blood • Moves from blood into solution Glucose: Same concentration in blood and solution • No movement
Tubes are submerged in a bath of various solutes (glucose and salts). This solution is called the dialysate. • Each solute establishes a concentration gradient: • Less solute in the dialysate: Solute will move out of blood • Equal concentrations: No movement either way • No wastes exist in the dialysis solution, so wastes (like urea) are pulled from blood • Dialysis solution is constantly replaced
Peritoneal dialysis • Operates on same principle • Catheter is surgically inserted into the patient`s abdominal cavity • Dialysate is fed into cavity via the catheter
As in haemodialysis, diffusion of substances occurs between dialysate and blood • Fluid is drained via the catheter, and replaced • Patient has greater independence than with haemodialysis
The last resort: Kidney transplant • Advantages: Transplanted kidney can produce and respond to hormones in the body • Disadvantages (same as with all transplants): Immune response, opportunistic infections
Homework • Page 362 #2,3 • #6, 8, 10 to be handed in CHAPTER 7 REVIEW • Pg 368 #1-5, 8-13, 18
- More by User

KIDNEY DISEASES Developmental disorders Glomerular diseases Tubulo-interstitial diseases Urinary stones Obstructive urop
KIDNEY DISEASES Developmental disorders Glomerular diseases Tubulo-interstitial diseases Urinary stones Obstructive uropathy Tumors. LUPUS GLOMERULONEPHRITIS GN-Common feature of SLE Immune complex-deposition -in situ formation Associated with deposits in -blood vessels
1.43k views • 56 slides

DISEASES DISORDERS OF THE LIVER
Primary Liver Cancer . Liver malignancy may arise from hepatocytesBiliary epithelial cells. Primary Liver Cancer. uncommon in the USA, but its incidence is increasing.In Asia and Africa > primary liver cancer is extremely commonover age 50, but a few are found in children, mainly under 2 yea
931 views • 61 slides

Diseases and Disorders of Camellias
Diseases and Disorders of Camellias. David M. Ingram Extension/Research Professor Central MS Research & Extension Center Raymond, Mississippi. Nicely Landscaped Home. Plant Disease.
649 views • 28 slides

Diseases of the Kidney
685 views • 49 slides

Disorders OF KIDNEY AND URINARY TRACT
Disorders OF KIDNEY AND URINARY TRACT. Dr. Shreedhar Paudel 12 April, 2009. Disorders OF KIDNEY AND URINARY TRACT. INTRODUCTION REVIEW OF ANATOMY PHYSIOLOGY OF KIDNEY APPROACH TO KIDNEY DISEASES AGE AT EVALUATION: NEONATE- MULTICYSTIC RENAL DYSPLASIA
514 views • 18 slides

Diseases and Disorders of the Endocrine System
Diseases and Disorders of the Endocrine System. Abnormalities of Growth Hormone Secretion. Panhypopituitarism – refers to decrease secretion of all the anterior pituitary hormones Causes – congenital pituitary tumor (that destroys the pit)
525 views • 21 slides
CYSTIC DISEASES OF THE KIDNEY
CYSTIC DISEASES OF THE KIDNEY . Types 1-Simple Cysts 2-Autosomal Dominant (Adult) Polycystic Kidney Disease 3-Autosomal Recessive (Childhood) Polycystic Kidney Disease 4-Medullary Cystic Disease 1-Simple Cysts
1.1k views • 22 slides

Inherited Kidney Diseases
Inherited Kidney Diseases. Zehra Eren M.D. Nephrology Department. LEARNING OBJECTIVES. Recognize Renal cystic disorders - Autosomal dominant polycystic kidney disease - Autosomal recessive polycystic kidney disease - M edullary sponge kidney - Medullary c ystic kidney d isease
2.07k views • 67 slides

Diseases and Disorders of the Endocrine System. Raul Campusano-Flores Mackenzie Sullivan 7 th block. Hypothalmus. Neurogenic Diabetes Insipidus. Tertiary Hypothyroidism. Deficiency of TRH. Occurs due to low levels of ADH production from the hypothalamus. Kidneys . Nephrotic syndrome .
1.05k views • 11 slides

Nutrition for Diseases of the Kidney
Nutrition for Diseases of the Kidney. Functions of the Kidney. Excretion of metabolic waste through urine Water and Electrolyte Homeostasis Acid/base balance Maintenance of bone health Activation of vitamin D Calcium/phosphorus homeostasis Blood Pressure Regulation
759 views • 50 slides

Disorders and Diseases of the Cardiovascular System
Disorders and Diseases of the Cardiovascular System. Pericarditis:. Inflammation of the pericardium due to viral or bacterial infection Produces adhesions that attach the layers of the pericardium Very painful Interferes with heart movements. Mitral Valve Prolapse:.
532 views • 20 slides

Gender and kidney diseases
Gender and kidney diseases. Dr. Wagner László 1 – Dr. Fekete Andrea 2 1 Transzplantációs és Sebészeti Klinika 2 I. sz. Gyermekklinika. Korenchevsky and Ross London, 1940. Sex difference in …. Anatomy Development Physiology Associated renal diseases
707 views • 53 slides

Cystic Diseases of the Kidney
Cystic Diseases of the Kidney. Dr. Raid Jastania. Objectives. By the end of this session the students should be able to: List the common causes of renal cysts List the types of polycystic kidney disease List the types of renal stones and their implications
1.11k views • 70 slides

Genetics of Kidney Diseases
Genetics of Kidney Diseases. 张咸宁 [email protected] Tel: 13105819271; 88208367 Office: A705, Research Building 2012/03. Learning Objectives. 了解泌尿系统疾病的遗传学研究现状。 掌握相关的疾病基因组学研究技术新进展。. Autosomal dominant polycystic kidney disease.
539 views • 32 slides

Genetics of Kidney Diseases. 张咸宁 [email protected] Tel: 13105819271; 88208367 Office: A705, Research Building 2014/03. Learning Objectives. 了解泌尿系统疾病的遗传学研究现状。 掌握相关的疾病基因组学研究技术新进展。. Autosomal dominant polycystic kidney disease.
606 views • 43 slides

Diseases and Disorders
Unit 4. Diseases and Disorders. Chapter 13. Preventing infectious diseases. What Are Infectious Diseases?. What Causes Infectious Diseases? Infectious diseases are diseases caused by agents invading the body.
725 views • 44 slides

Tubulointerstitial Diseases of the Kidney
Tubulointerstitial Diseases of the Kidney. Pelvis, Cortex, medulla, calyces Renal Artery. 1700 L of blood filtered 180 L of G lomerul filtrate 1.5 L of urine / day. Diseases affecting tubules & interstitium:.
1.11k views • 62 slides

DISEASES OF KIDNEY AND URINARY TRACT
DISEASES OF KIDNEY AND URINARY TRACT. Lecture on pathomorphology for the 3-rd year students by T. Filonenko. Syndromes of renal diseases. Acute nephritic syndrome The nephrotic syndrome Asymptomatic hematuria or proteinuria Acute renal failure Chronic renal failure
1.07k views • 64 slides

Genetics of Kidney Diseases. 张咸宁 [email protected] Tel: 13105819271; 88208367 Office: A705, Research Building 2013/04. Learning Objectives. 了解泌尿系统疾病的遗传学研究现状。 掌握相关的疾病基因组学研究技术新进展。. Autosomal dominant polycystic kidney disease.
479 views • 31 slides

Diseases of the Kidney. Kidney Physiology Kidney Functions: • activate vitamin D (renal 1-alpha hydroxylase) • produces erythropoietin which stimulates RBC formation • helps regulate blood pressure • ELIMINATES METABOLIC WASTE PRODUCTS • HELPS MAINTAIN FLUID, ELECTROLYTE,
199 views • 16 slides

447 views • 43 slides

670 views • 62 slides
10 Signs You May Have Kidney Disease
December 17, 2020, 9:44am EST

More than 37 million American adults are living with kidney disease and most don’t know it. “There are a number of physical signs of kidney disease, but sometimes people attribute them to other conditions. Also, those with kidney disease tend not to experience symptoms until the very late stages, when the kidneys are failing or when there are large amounts of protein in the urine. This is one of the reasons why only 10% of people with chronic kidney disease know that they have it,” says Dr. Joseph Vassalotti, Chief Medical Officer at the National Kidney Foundation.
While the only way to know for sure if you have kidney disease is to get tested, Dr. Vassalotti shares 10 possible signs you may have kidney disease. If you’re at risk for kidney disease due to high blood pressure , diabetes , a family history of kidney failure or if you’re older than age 60, it’s important to get tested annually for kidney disease. Be sure to mention any symptoms you’re experiencing to your healthcare practitioner.
Signs of Kidney Disease
- You're more tired, have less energy or are having trouble concentrating. A severe decrease in kidney function can lead to a buildup of toxins and impurities in the blood. This can cause people to feel tired, weak and can make it hard to concentrate. Another complication of kidney disease is anemia , which can cause weakness and fatigue.
- You're having trouble sleeping. When the kidneys aren't filtering properly, toxins stay in the blood rather than leaving the body through the urine. This can make it difficult to sleep. There is also a link between obesity and chronic kidney disease, and sleep apnea is more common in those with chronic kidney disease, compared with the general population.
- You have dry and itchy skin. Healthy kidneys do many important jobs. They remove wastes and extra fluid from your body, help make red blood cells, help keep bones strong and work to maintain the right amount of minerals in your blood. Dry and itchy skin can be a sign of the mineral and bone disease that often accompanies advanced kidney disease, when the kidneys are no longer able to keep the right balance of minerals and nutrients in your blood.
- You feel the need to urinate more often. If you feel the need to urinate more often, especially at night, this can be a sign of kidney disease. When the kidneys filters are damaged, it can cause an increase in the urge to urinate. Sometimes this can also be a sign of a urinary infection or enlarged prostate in men.
- You see blood in your urine. Healthy kidneys typically keep the blood cells in the body when filtering wastes from the blood to create urine, but when the kidney's filters have been damaged, these blood cells can start to "leak" out into the urine. In addition to signaling kidney disease, blood in the urine can be indicative of tumors, kidney stones or an infection.
- Your urine is foamy. Excessive bubbles in the urine – especially those that require you to flush several times before they go away—indicate protein in the urine. This foam may look like the foam you see when scrambling eggs, as the common protein found in urine, albumin, is the same protein that is found in eggs.
- You're experiencing persistent puffiness around your eyes. Protein in the urine is an early sign that the kidneys’ filters have been damaged, allowing protein to leak into the urine. This puffiness around your eyes can be due to the fact that your kidneys are leaking a large amount of protein in the urine, rather than keeping it in the body.
- Your ankles and feet are swollen. Decreased kidney function can lead to sodium retention, causing swelling in your feet and ankles. Swelling in the lower extremities can also be a sign of heart disease, liver disease and chronic leg vein problems.
- You have a poor appetite. This is a very general symptom, but a buildup of toxins resulting from reduced kidney function can be one of the causes.
- Your muscles are cramping. Electrolyte imbalances can result from impaired kidney function. For example, low calcium levels and poorly controlled phosphorus may contribute to muscle cramping.
Kidney disease & COVID-19
Find answers about kidney disease during the COVID-19 outbreak here.

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
Chronic Kidney Disease (CKD)
Published by Kennedi Warman Modified over 9 years ago
Similar presentations
Presentation on theme: "Chronic Kidney Disease (CKD)"— Presentation transcript:

ABC’s of Nephrology Sobha Malla RD,CSR 9/17/11

William Vega-Ocasio MD. Internal Medicine - Nephrology

+ Understanding Kidney Disease and Renal Dialysis Brooke Grussing Concordia College.

Chronic Kidney Disease Manju Sood GPST3. What is CKD? Chronic renal failure is the progressive loss of nephrons resulting in permanent compromise of renal.

Kidney Physiology Kidney Functions: activate vitamin D (renal 1-alpha hydroxylase)activate vitamin D (renal 1-alpha hydroxylase) produces erythropoietin.

Dinkar Kaw, M.D., Division of Nephrology

Prepared by D. Chaplin Chronic Renal Failure. Prepared by D. Chaplin Chronic Renal Failure Progressive, irreversible damage to the nephrons and glomeruli.

Protein-, Mineral- & Fluid-Modified Diets for Kidney Diseases

Nutrition & Renal Diseases

Uncontrolled secondary hyperparathyroidism in a haemodialysis patient Jordi Bover, MD, PhD Fundació Puigvert Barcelona, Spain © Springer Healthcare, a.

End Stage Renal Disease in Children. End stage kidney disease occurs when the kidneys are no longer able to function at a level that is necessary for.

Chronic Renal Failure (End Stage Renal Disease “ESRD”) Dr. Belal Hijji, RN, PhD April 18 & 23, 2012.

Recent Advances in Management of CRF Yousef Boobess, M.D. Head, Nephrology Division Tawam Hospital.
Chronic Kidney Disease

SLOW- COOKING THE BEANS “OR, HOW TO STOP WORRYING AND APPLY SOME LOVE TO THE KIDNEYS” AN APPROACH TO CKD SARA KATE LEVIN, MD JANUARY 2014.

Calcium & phosphor disturbance CKD- MBD Dr. Atapour.

Kidney Function Tests Contents: Kidney functions Functional units Renal diseases Routine kidney function tests Serum creatinine Creatinine clearance.

This lecture was conducted during the Nephrology Unit Grand Ground by Medical Student rotated under Nephrology Division under the supervision and administration.

Finishing Renal Disease Aging and death. Chronic Renal Failure Results from irreversible, progressive injury to the kidney. Characterized by increased.
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
UCLA Health urologists will showcase groundbreaking research at the 2024 American Urological Association Annual Meeting
The ucla department of urology will deliver over 70 presentations showcasing new discoveries and innovations in urologic research.

Newswise — Faculty, scientists, and urologists from UCLA Health Urology will present research data on the latest discoveries and innovations in urology at the 2024 American Urological Association’s (AUA) Annual Meeting in San Antonio, Texas, May 3-6.
The conference will feature over 70 presentations from UCLA Health physicians and scientists, including abstracts and podium presentations, highlighting topics impacting the field of urology.
These studies review cutting-edge topics, including novel evaluation techniques for Peyronie’s disease, using AI to help plan focal prostate cancer therapy, and evaluating the role of endourology innovations in low-resource communities.
According to the AUA, the annual meeting is the largest gathering of urologists globally – offering researchers in the field access to groundbreaking research, new guidelines, and the latest advances in urologic medicine.
“The AUA Meeting is always an exciting opportunity for our entire department, as it allows us to share our research with professionals across the world, as well as to learn from presentations from other leading academic centers,” said Christopher Saigal, MD, MPH, Professor and Executive Vice Chair of Urology at the UCLA David Geffen School of Medicine . “Collectively as a department, we take pride in the fact that over 70 presentations from UCLA Urology researchers will be featured, demonstrating our commitment to providing new solutions to the most challenging dilemmas faced by patients with urologic diseases.”
Abstracts from UCLA Health researchers and urologists will include:
Bacteria Trigger Calcium Oxalate Crystal Formation
Kymora Scotland, MD, PhD , will present a poster on kidney stone pathogenesis. Her research reveals that approximately 80% of kidney stones are composed of calcium oxalate (CaOx), and recent studies suggest the potential involvement of bacteria in CaOx nephrolithiasis. The study aims to identify evidence of bacteria within kidney stones and investigate biofilm-forming bacteria's impact on the formation and growth of CaOx crystals.
Endourology in Resource Limited Areas
Additionally, Dr. Kymora Scotland will also deliver multiple invited talks, including one on endourology in low-resource communities.
Effect of 3-dimensional, virtual reality models for surgical planning of robotic prostatectomy on trifecta outcomes: A randomized clinical trial
Joseph Shirk, MD , will present research on utilizing virtual three-dimensional (3D) models in planning robotic-assisted laparoscopic radical prostatectomy (RALP). Traditionally, surgeons review two-dimensional Magnetic Resonance Imaging (MRI) images and pathology results to understand the three-dimensional anatomy. The study aims to evaluate the impact of virtual 3D models on the trifecta of cancer control, continence, and erectile function in patients undergoing RALP.
Novel Evaluation Techniques for Peyronie’s Disease
Sriram Eleswarapu , MD, PhD , will discuss the current state of diagnosis for Peyronie’s disease by evaluating newer technologies.
Metrics of Treatment Outcome Following Partial Gland Ablation for Prostate Cancer
Wayne Brisbane, MD , will present an evaluation of the outcomes of treating prostate cancer in men who do not fully meet the standard inclusion criteria recommended by the focal therapy community.
Software to Determine Extent of Tumor Margins in Focal Therapy of Prostate Cancer
Wayne Brisbane, MD , will also present on the challenge of poorly defined margins of prostate cancer by MRI imaging, emphasizing the importance of treating these margins for complete ablation. The objective of the study is to assess the efficacy of Unfold-AI™, a recently FDA-cleared software, in enhancing the definition of tumor margins and guiding focal therapy.
“In presenting these diverse scientific findings, our goal is to illuminate what is happening at the forefront of urologic research and that research’s significant implications for patient care,” said Dr. Saigal. “From novel evaluation techniques for Peyronie’s disease to AI-based approaches that are revolutionizing prostate cancer therapy, each presentation reflects our dedication to pushing boundaries, improving outcomes, and enhancing the lives of our patients.”
MEDIA CONTACT
Type of article.
- Experts keyboard_arrow_right Expert Pitch Expert Query Expert Directory
- Journalists
- About keyboard_arrow_right Member Services Accessibility Statement Newswise Live Invoice Lookup Services for Journalists Archived Wires Participating Institutions Media Subscribers Sample Effectiveness Reports Terms of Service Privacy Policy Our Staff Contact Newswise
- Blog FAQ Help

IMAGES
VIDEO
COMMENTS
Chronic kidney disease can progress to end-stage kidney failure, which is fatal without artificial filtering (dialysis) or a kidney transplant. How kidneys work. Show transcript for video How kidneys work. One of the important jobs of the kidneys is to clean the blood. As blood moves through the body, it picks up extra fluid, chemicals and waste.
Chronic kidney disease (CKD) versus acute kidney disease or injury - CKD is defined by the presence of kidney damage or reduced glomerular filtration rate ... Clinical presentation - Patients with CKD may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no ...
Kidney disease, also known as chronic kidney disease or CKD, causes more deaths than breast cancer or prostate cancer (NVS 2021 report of 2018 data).1 It is the under-recognized public health crisis. • Kidney disease affects an estimated 37 million people in the U.S. (15% of the adult population; more than 1 in 7 adults).2,3,4
What is chronic kidney disease (CKD)? •CKD refers to a decrease in the function of your kidneys based on blood or urine tests for ≥ 3 months. •Direct measurement of glomerular filtration rate (GFR): isotope tests. o Iothalamate, iohexol. •Measurement of surrogate markers of GFR in blood.
Keeping your bones healthy. Chronic kidney disease (CKD) is when the kidneys have become damaged over time (for at least 3 months) and have a hard time doing all their important jobs. CKD also increases the risk of other health problems like heart disease and stroke. Developing CKD is usually a very slow process with very few symptoms at first.
Chronic kidney disease (CKD) is defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 mt2, persisting for 3 months or more, irrespective of the cause.[1] It is a state of progressive loss of kidney function, ultimately resulting in the need for renal replacement therapy (dialysis or transplantation).
Next: Physical Examination. Chronic kidney disease (CKD)—or chronic renal failure (CRF), as it was historically termed—is a term that encompasses all degrees of decreased renal function, from damaged-at risk through mild, moderate, and severe chronic kidney failure. CKD is a worldwide public health problem.
As chronic kidney disease progresses to end-stage renal disease, signs and symptoms might include: Nausea. Vomiting. Loss of appetite. Fatigue and weakness. Changes in how much you urinate. Chest pain, if fluid builds up around the lining of the heart. Shortness of breath, if fluid builds up in the lungs. Swelling of feet and ankles.
Clinical Presentation. Chronic kidney disease is typically identified through routine screening with serum chemistry profile and urine studies or as an incidental finding. Less commonly, patients may present with symptoms such as gross hematuria, "foamy urine" (a sign of albuminuria), nocturia, flank pain, or decreased urine output. ...
The two main causes of kidney failure are diabetes and high blood pressure, which make up about two-thirds of cases. Other diseases can also lead to kidney failure, including IgA nephropathy, lupus nephritis, polycystic kidney disease, Fabry disease, and many others. Social and environmental factors also play a part in kidney disease.
More than 500,000 people in the United States live with end-stage renal disease (ESRD). The development of chronic kidney disease (CKD) and its progression to this terminal disease remains a significant cause of reduced quality of life and premature mortality.[1] Chronic kidney disease (CKD) is a debilitating disease, and standards of medical care involve aggressive monitoring for signs of ...
Introduction. Chronic kidney disease (CKD) is defined as abnormal kidney function or structure present for greater than three months, with subsequent implications for health. 1. CKD is a common condition estimated to affect about nine to thirteen per cent of the adult population worldwide. 2.
Chronic kidney disease is the medical term used to describe the gradual loss of kidney function over a period of at least three months. Because of this, excess fluid and waste from the blood remain in the body and may cause other health problems, such as heart disease and stroke, in addition to kidney failure.. CKD can occur for a variety of reasons, but diabetes and high blood pressure are ...
The clinical presentation of kidney disease. Essentials. Renal disease may present in many ways, including: (1) the screening of asymptomatic individuals; (2) with symptoms and signs resulting from renal dysfunction; and (3) with symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction.
Neuro SIG. Business Health & Medicine. 1 of 51. Download now. Common Kidney Diseases - Download as a PDF or view online for free.
ÐÏ à¡± á> þÿ -Ö Ù þÿÿÿÞ ß à á â ã ä å æ ç è é ê ë ì í î ï ð ñ ò ó ô õ ö ÷ ø ù ú û ü ý þ ÿ × Ø ÿÿÿÿÿÿÿn
Whether for high-profile medical conferences or academic symposiums, equip yourself with this Google Slides and PowerPoint template: It's simple yet informative, helping you present the facts about chronic kidney disease convincingly. It is fully customizable, enabling you to tailor your presentation to different audiences with ease. Moreover ...
Kidney Disease Find out why 1 in 9 American adults has kidney disease — and most don't know it. See if you are at risk for kidney disease. Learn about the 2 simple tests you can have to determine the health of your kidneys. Get tips for keeping your kidneys as healthy as they can be. Help fight kidney disease. Learn more at www.kidney.org
Common presentation and investigation of Kidney diseases. Dec 26, 2011 •. 40 likes • 14,078 views. Ezmeer Emiral. Education Health & Medicine. 1 of 47. Download now. Download to read offline. Common presentation and investigation of Kidney diseases - Download as a PDF or view online for free.
54 likes • 28,822 views. Abhay Rajpoot. Chronic kidney disease (CKD) means your kidneys are damaged and can't filter blood the way they should. The disease is called "chronic" because the damage to your kidneys happens slowly over a long period of time. Read more. Education. 1 of 24. Chronic Kidney Disease (CKD) - Download as a PDF or ...
Kidney Disease Presentation . Medical . Free Google Slides theme and PowerPoint template . Kidneys are some of the most important organs, as they take part in various important processes. If you are a physician or urologist, professor or a med student and want to show some details about nephritic conditions and their treatments, use this ...
Presentation Transcript. Diseases and Disorders of the Kidney Section 7.7 Page 357. Importance of the kidney • Kidneys help maintain homeostasis • Diseases and disorders of the kidneys can have effects on other systems • Many kidney disorders are detected by urinalysis.
Signs of Kidney Disease. You're more tired, have less energy or are having trouble concentrating. A severe decrease in kidney function can lead to a buildup of toxins and impurities in the blood. This can cause people to feel tired, weak and can make it hard to concentrate.
Diseases of the kidney. Aug 20, 2017 • Download as PPTX, PDF •. 47 likes • 15,985 views. Dr. Varughese George. Diseases of the kidney. Health & Medicine. 1 of 55. Download now. Diseases of the kidney - Download as a PDF or view online for free.
Key Concepts Chronic kidney disease (CKD) is classified based on: * the cause of kidney disease, assessment of glomerular filtration rate, and extent of proteinuria. Frequent complications of advanced CKD include altered sodium and water balance, hyperkalemia, metabolic acidosis, anemia, CKD-related mineral and bone disorder (CKD-MBD), and cardiovascular disease. 3. Key mechanisms responsible ...
The conference will feature over 70 presentations from UCLA Health physicians and scientists, including abstracts and podium presentations, highlighting topics impacting the field of urology.