- Privacy Policy
Buy Me a Coffee

Home » Research Summary – Structure, Examples and Writing Guide

Research Summary – Structure, Examples and Writing Guide
Table of Contents

Research Summary
Definition:
A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings. It is often used as a tool to quickly communicate the main findings of a study to other researchers, stakeholders, or decision-makers.
Structure of Research Summary
The Structure of a Research Summary typically include:
- Introduction : This section provides a brief background of the research problem or question, explains the purpose of the study, and outlines the research objectives.
- Methodology : This section explains the research design, methods, and procedures used to conduct the study. It describes the sample size, data collection methods, and data analysis techniques.
- Results : This section presents the main findings of the study, including statistical analysis if applicable. It may include tables, charts, or graphs to visually represent the data.
- Discussion : This section interprets the results and explains their implications. It discusses the significance of the findings, compares them to previous research, and identifies any limitations or future directions for research.
- Conclusion : This section summarizes the main points of the research and provides a conclusion based on the findings. It may also suggest implications for future research or practical applications of the results.
- References : This section lists the sources cited in the research summary, following the appropriate citation style.
How to Write Research Summary
Here are the steps you can follow to write a research summary:
- Read the research article or study thoroughly: To write a summary, you must understand the research article or study you are summarizing. Therefore, read the article or study carefully to understand its purpose, research design, methodology, results, and conclusions.
- Identify the main points : Once you have read the research article or study, identify the main points, key findings, and research question. You can highlight or take notes of the essential points and findings to use as a reference when writing your summary.
- Write the introduction: Start your summary by introducing the research problem, research question, and purpose of the study. Briefly explain why the research is important and its significance.
- Summarize the methodology : In this section, summarize the research design, methods, and procedures used to conduct the study. Explain the sample size, data collection methods, and data analysis techniques.
- Present the results: Summarize the main findings of the study. Use tables, charts, or graphs to visually represent the data if necessary.
- Interpret the results: In this section, interpret the results and explain their implications. Discuss the significance of the findings, compare them to previous research, and identify any limitations or future directions for research.
- Conclude the summary : Summarize the main points of the research and provide a conclusion based on the findings. Suggest implications for future research or practical applications of the results.
- Revise and edit : Once you have written the summary, revise and edit it to ensure that it is clear, concise, and free of errors. Make sure that your summary accurately represents the research article or study.
- Add references: Include a list of references cited in the research summary, following the appropriate citation style.
Example of Research Summary
Here is an example of a research summary:
Title: The Effects of Yoga on Mental Health: A Meta-Analysis
Introduction: This meta-analysis examines the effects of yoga on mental health. The study aimed to investigate whether yoga practice can improve mental health outcomes such as anxiety, depression, stress, and quality of life.
Methodology : The study analyzed data from 14 randomized controlled trials that investigated the effects of yoga on mental health outcomes. The sample included a total of 862 participants. The yoga interventions varied in length and frequency, ranging from four to twelve weeks, with sessions lasting from 45 to 90 minutes.
Results : The meta-analysis found that yoga practice significantly improved mental health outcomes. Participants who practiced yoga showed a significant reduction in anxiety and depression symptoms, as well as stress levels. Quality of life also improved in those who practiced yoga.
Discussion : The findings of this study suggest that yoga can be an effective intervention for improving mental health outcomes. The study supports the growing body of evidence that suggests that yoga can have a positive impact on mental health. Limitations of the study include the variability of the yoga interventions, which may affect the generalizability of the findings.
Conclusion : Overall, the findings of this meta-analysis support the use of yoga as an effective intervention for improving mental health outcomes. Further research is needed to determine the optimal length and frequency of yoga interventions for different populations.
References :
- Cramer, H., Lauche, R., Langhorst, J., Dobos, G., & Berger, B. (2013). Yoga for depression: a systematic review and meta-analysis. Depression and anxiety, 30(11), 1068-1083.
- Khalsa, S. B. (2004). Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian journal of physiology and pharmacology, 48(3), 269-285.
- Ross, A., & Thomas, S. (2010). The health benefits of yoga and exercise: a review of comparison studies. The Journal of Alternative and Complementary Medicine, 16(1), 3-12.
Purpose of Research Summary
The purpose of a research summary is to provide a brief overview of a research project or study, including its main points, findings, and conclusions. The summary allows readers to quickly understand the essential aspects of the research without having to read the entire article or study.
Research summaries serve several purposes, including:
- Facilitating comprehension: A research summary allows readers to quickly understand the main points and findings of a research project or study without having to read the entire article or study. This makes it easier for readers to comprehend the research and its significance.
- Communicating research findings: Research summaries are often used to communicate research findings to a wider audience, such as policymakers, practitioners, or the general public. The summary presents the essential aspects of the research in a clear and concise manner, making it easier for non-experts to understand.
- Supporting decision-making: Research summaries can be used to support decision-making processes by providing a summary of the research evidence on a particular topic. This information can be used by policymakers or practitioners to make informed decisions about interventions, programs, or policies.
- Saving time: Research summaries save time for researchers, practitioners, policymakers, and other stakeholders who need to review multiple research studies. Rather than having to read the entire article or study, they can quickly review the summary to determine whether the research is relevant to their needs.
Characteristics of Research Summary
The following are some of the key characteristics of a research summary:
- Concise : A research summary should be brief and to the point, providing a clear and concise overview of the main points of the research.
- Objective : A research summary should be written in an objective tone, presenting the research findings without bias or personal opinion.
- Comprehensive : A research summary should cover all the essential aspects of the research, including the research question, methodology, results, and conclusions.
- Accurate : A research summary should accurately reflect the key findings and conclusions of the research.
- Clear and well-organized: A research summary should be easy to read and understand, with a clear structure and logical flow.
- Relevant : A research summary should focus on the most important and relevant aspects of the research, highlighting the key findings and their implications.
- Audience-specific: A research summary should be tailored to the intended audience, using language and terminology that is appropriate and accessible to the reader.
- Citations : A research summary should include citations to the original research articles or studies, allowing readers to access the full text of the research if desired.
When to write Research Summary
Here are some situations when it may be appropriate to write a research summary:
- Proposal stage: A research summary can be included in a research proposal to provide a brief overview of the research aims, objectives, methodology, and expected outcomes.
- Conference presentation: A research summary can be prepared for a conference presentation to summarize the main findings of a study or research project.
- Journal submission: Many academic journals require authors to submit a research summary along with their research article or study. The summary provides a brief overview of the study’s main points, findings, and conclusions and helps readers quickly understand the research.
- Funding application: A research summary can be included in a funding application to provide a brief summary of the research aims, objectives, and expected outcomes.
- Policy brief: A research summary can be prepared as a policy brief to communicate research findings to policymakers or stakeholders in a concise and accessible manner.
Advantages of Research Summary
Research summaries offer several advantages, including:
- Time-saving: A research summary saves time for readers who need to understand the key findings and conclusions of a research project quickly. Rather than reading the entire research article or study, readers can quickly review the summary to determine whether the research is relevant to their needs.
- Clarity and accessibility: A research summary provides a clear and accessible overview of the research project’s main points, making it easier for readers to understand the research without having to be experts in the field.
- Improved comprehension: A research summary helps readers comprehend the research by providing a brief and focused overview of the key findings and conclusions, making it easier to understand the research and its significance.
- Enhanced communication: Research summaries can be used to communicate research findings to a wider audience, such as policymakers, practitioners, or the general public, in a concise and accessible manner.
- Facilitated decision-making: Research summaries can support decision-making processes by providing a summary of the research evidence on a particular topic. Policymakers or practitioners can use this information to make informed decisions about interventions, programs, or policies.
- Increased dissemination: Research summaries can be easily shared and disseminated, allowing research findings to reach a wider audience.
Limitations of Research Summary
Limitations of the Research Summary are as follows:
- Limited scope: Research summaries provide a brief overview of the research project’s main points, findings, and conclusions, which can be limiting. They may not include all the details, nuances, and complexities of the research that readers may need to fully understand the study’s implications.
- Risk of oversimplification: Research summaries can be oversimplified, reducing the complexity of the research and potentially distorting the findings or conclusions.
- Lack of context: Research summaries may not provide sufficient context to fully understand the research findings, such as the research background, methodology, or limitations. This may lead to misunderstandings or misinterpretations of the research.
- Possible bias: Research summaries may be biased if they selectively emphasize certain findings or conclusions over others, potentially distorting the overall picture of the research.
- Format limitations: Research summaries may be constrained by the format or length requirements, making it challenging to fully convey the research’s main points, findings, and conclusions.
- Accessibility: Research summaries may not be accessible to all readers, particularly those with limited literacy skills, visual impairments, or language barriers.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
- Resources Home 🏠
- Try SciSpace Copilot
- Search research papers
- Add Copilot Extension
- Try AI Detector
- Try Paraphraser
- Try Citation Generator
- April Papers
- June Papers
- July Papers

How To Write A Research Summary

It’s a common perception that writing a research summary is a quick and easy task. After all, how hard can jotting down 300 words be? But when you consider the weight those 300 words carry, writing a research summary as a part of your dissertation, essay or compelling draft for your paper instantly becomes daunting task.
A research summary requires you to synthesize a complex research paper into an informative, self-explanatory snapshot. It needs to portray what your article contains. Thus, writing it often comes at the end of the task list.
Regardless of when you’re planning to write, it is no less of a challenge, particularly if you’re doing it for the first time. This blog will take you through everything you need to know about research summary so that you have an easier time with it.
What is a Research Summary?
A research summary is the part of your research paper that describes its findings to the audience in a brief yet concise manner. A well-curated research summary represents you and your knowledge about the information written in the research paper.
While writing a quality research summary, you need to discover and identify the significant points in the research and condense it in a more straightforward form. A research summary is like a doorway that provides access to the structure of a research paper's sections.
Since the purpose of a summary is to give an overview of the topic, methodology, and conclusions employed in a paper, it requires an objective approach. No analysis or criticism.
Research summary or Abstract. What’s the Difference?
They’re both brief, concise, and give an overview of an aspect of the research paper. So, it’s easy to understand why many new researchers get the two confused. However, a research summary and abstract are two very different things with individual purpose. To start with, a research summary is written at the end while the abstract comes at the beginning of a research paper.
A research summary captures the essence of the paper at the end of your document. It focuses on your topic, methods, and findings. More like a TL;DR, if you will. An abstract, on the other hand, is a description of what your research paper is about. It tells your reader what your topic or hypothesis is, and sets a context around why you have embarked on your research.
Getting Started with a Research Summary
Before you start writing, you need to get insights into your research’s content, style, and organization. There are three fundamental areas of a research summary that you should focus on.
- While deciding the contents of your research summary, you must include a section on its importance as a whole, the techniques, and the tools that were used to formulate the conclusion. Additionally, there needs to be a short but thorough explanation of how the findings of the research paper have a significance.
- To keep the summary well-organized, try to cover the various sections of the research paper in separate paragraphs. Besides, how the idea of particular factual research came up first must be explained in a separate paragraph.
- As a general practice worldwide, research summaries are restricted to 300-400 words. However, if you have chosen a lengthy research paper, try not to exceed the word limit of 10% of the entire research paper.
How to Structure Your Research Summary
The research summary is nothing but a concise form of the entire research paper. Therefore, the structure of a summary stays the same as the paper. So, include all the section titles and write a little about them. The structural elements that a research summary must consist of are:
It represents the topic of the research. Try to phrase it so that it includes the key findings or conclusion of the task.
The abstract gives a context of the research paper. Unlike the abstract at the beginning of a paper, the abstract here, should be very short since you’ll be working with a limited word count.
Introduction
This is the most crucial section of a research summary as it helps readers get familiarized with the topic. You should include the definition of your topic, the current state of the investigation, and practical relevance in this part. Additionally, you should present the problem statement, investigative measures, and any hypothesis in this section.
Methodology
This section provides details about the methodology and the methods adopted to conduct the study. You should write a brief description of the surveys, sampling, type of experiments, statistical analysis, and the rationality behind choosing those particular methods.
Create a list of evidence obtained from the various experiments with a primary analysis, conclusions, and interpretations made upon that. In the paper research paper, you will find the results section as the most detailed and lengthy part. Therefore, you must pick up the key elements and wisely decide which elements are worth including and which are worth skipping.
This is where you present the interpretation of results in the context of their application. Discussion usually covers results, inferences, and theoretical models explaining the obtained values, key strengths, and limitations. All of these are vital elements that you must include in the summary.
Most research papers merge conclusion with discussions. However, depending upon the instructions, you may have to prepare this as a separate section in your research summary. Usually, conclusion revisits the hypothesis and provides the details about the validation or denial about the arguments made in the research paper, based upon how convincing the results were obtained.
The structure of a research summary closely resembles the anatomy of a scholarly article . Additionally, you should keep your research and references limited to authentic and scholarly sources only.
Tips for Writing a Research Summary
The core concept behind undertaking a research summary is to present a simple and clear understanding of your research paper to the reader. The biggest hurdle while doing that is the number of words you have at your disposal. So, follow the steps below to write a research summary that sticks.
1. Read the parent paper thoroughly
You should go through the research paper thoroughly multiple times to ensure that you have a complete understanding of its contents. A 3-stage reading process helps.
a. Scan: In the first read, go through it to get an understanding of its basic concept and methodologies.
b. Read: For the second step, read the article attentively by going through each section, highlighting the key elements, and subsequently listing the topics that you will include in your research summary.
c. Skim: Flip through the article a few more times to study the interpretation of various experimental results, statistical analysis, and application in different contexts.
Sincerely go through different headings and subheadings as it will allow you to understand the underlying concept of each section. You can try reading the introduction and conclusion simultaneously to understand the motive of the task and how obtained results stay fit to the expected outcome.
2. Identify the key elements in different sections
While exploring different sections of an article, you can try finding answers to simple what, why, and how. Below are a few pointers to give you an idea:
- What is the research question and how is it addressed?
- Is there a hypothesis in the introductory part?
- What type of methods are being adopted?
- What is the sample size for data collection and how is it being analyzed?
- What are the most vital findings?
- Do the results support the hypothesis?
Discussion/Conclusion
- What is the final solution to the problem statement?
- What is the explanation for the obtained results?
- What is the drawn inference?
- What are the various limitations of the study?
3. Prepare the first draft
Now that you’ve listed the key points that the paper tries to demonstrate, you can start writing the summary following the standard structure of a research summary. Just make sure you’re not writing statements from the parent research paper verbatim.
Instead, try writing down each section in your own words. This will not only help in avoiding plagiarism but will also show your complete understanding of the subject. Alternatively, you can use a summarizing tool (AI-based summary generators) to shorten the content or summarize the content without disrupting the actual meaning of the article.
SciSpace Copilot is one such helpful feature! You can easily upload your research paper and ask Copilot to summarize it. You will get an AI-generated, condensed research summary. SciSpace Copilot also enables you to highlight text, clip math and tables, and ask any question relevant to the research paper; it will give you instant answers with deeper context of the article..
4. Include visuals
One of the best ways to summarize and consolidate a research paper is to provide visuals like graphs, charts, pie diagrams, etc.. Visuals make getting across the facts, the past trends, and the probabilistic figures around a concept much more engaging.
5. Double check for plagiarism
It can be very tempting to copy-paste a few statements or the entire paragraphs depending upon the clarity of those sections. But it’s best to stay away from the practice. Even paraphrasing should be done with utmost care and attention.
Also: QuillBot vs SciSpace: Choose the best AI-paraphrasing tool
6. Religiously follow the word count limit
You need to have strict control while writing different sections of a research summary. In many cases, it has been observed that the research summary and the parent research paper become the same length. If that happens, it can lead to discrediting of your efforts and research summary itself. Whatever the standard word limit has been imposed, you must observe that carefully.
7. Proofread your research summary multiple times
The process of writing the research summary can be exhausting and tiring. However, you shouldn’t allow this to become a reason to skip checking your academic writing several times for mistakes like misspellings, grammar, wordiness, and formatting issues. Proofread and edit until you think your research summary can stand out from the others, provided it is drafted perfectly on both technicality and comprehension parameters. You can also seek assistance from editing and proofreading services , and other free tools that help you keep these annoying grammatical errors at bay.
8. Watch while you write
Keep a keen observation of your writing style. You should use the words very precisely, and in any situation, it should not represent your personal opinions on the topic. You should write the entire research summary in utmost impersonal, precise, factually correct, and evidence-based writing.
9. Ask a friend/colleague to help
Once you are done with the final copy of your research summary, you must ask a friend or colleague to read it. You must test whether your friend or colleague could grasp everything without referring to the parent paper. This will help you in ensuring the clarity of the article.
Once you become familiar with the research paper summary concept and understand how to apply the tips discussed above in your current task, summarizing a research summary won’t be that challenging. While traversing the different stages of your academic career, you will face different scenarios where you may have to create several research summaries.
In such cases, you just need to look for answers to simple questions like “Why this study is necessary,” “what were the methods,” “who were the participants,” “what conclusions were drawn from the research,” and “how it is relevant to the wider world.” Once you find out the answers to these questions, you can easily create a good research summary following the standard structure and a precise writing style.
You might also like

Consensus GPT vs. SciSpace GPT: Choose the Best GPT for Research

Literature Review and Theoretical Framework: Understanding the Differences

Types of Essays in Academic Writing - Quick Guide (2024)
How To Write The Results/Findings Chapter
For qualitative studies (dissertations & theses).
By: Jenna Crossley (PhD Cand). Expert Reviewed By: Dr. Eunice Rautenbach | August 2021
So, you’ve collected and analysed your qualitative data, and it’s time to write up your results chapter – exciting! But where do you start? In this post, we’ll guide you through the qualitative results chapter (also called the findings chapter), step by step.
Overview: Qualitative Results Chapter
- What (exactly) the qualitative results chapter is
- What to include in your results chapter
- How to write up your results chapter
- A few tips and tricks to help you along the way
What exactly is the results chapter?
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and discuss its meaning), depending on your university’s preference. We’ll treat the two chapters as separate, as that’s the most common approach.
In contrast to a quantitative results chapter that presents numbers and statistics, a qualitative results chapter presents data primarily in the form of words . But this doesn’t mean that a qualitative study can’t have quantitative elements – you could, for example, present the number of times a theme or topic pops up in your data, depending on the analysis method(s) you adopt.
Adding a quantitative element to your study can add some rigour, which strengthens your results by providing more evidence for your claims. This is particularly common when using qualitative content analysis. Keep in mind though that qualitative research aims to achieve depth, richness and identify nuances , so don’t get tunnel vision by focusing on the numbers. They’re just cream on top in a qualitative analysis.
So, to recap, the results chapter is where you objectively present the findings of your analysis, without interpreting them (you’ll save that for the discussion chapter). With that out the way, let’s take a look at what you should include in your results chapter.

What should you include in the results chapter?
As we’ve mentioned, your qualitative results chapter should purely present and describe your results , not interpret them in relation to the existing literature or your research questions . Any speculations or discussion about the implications of your findings should be reserved for your discussion chapter.
In your results chapter, you’ll want to talk about your analysis findings and whether or not they support your hypotheses (if you have any). Naturally, the exact contents of your results chapter will depend on which qualitative analysis method (or methods) you use. For example, if you were to use thematic analysis, you’d detail the themes identified in your analysis, using extracts from the transcripts or text to support your claims.
While you do need to present your analysis findings in some detail, you should avoid dumping large amounts of raw data in this chapter. Instead, focus on presenting the key findings and using a handful of select quotes or text extracts to support each finding . The reams of data and analysis can be relegated to your appendices.
While it’s tempting to include every last detail you found in your qualitative analysis, it is important to make sure that you report only that which is relevant to your research aims, objectives and research questions . Always keep these three components, as well as your hypotheses (if you have any) front of mind when writing the chapter and use them as a filter to decide what’s relevant and what’s not.
Need a helping hand?
How do I write the results chapter?
Now that we’ve covered the basics, it’s time to look at how to structure your chapter. Broadly speaking, the results chapter needs to contain three core components – the introduction, the body and the concluding summary. Let’s take a look at each of these.
Section 1: Introduction
The first step is to craft a brief introduction to the chapter. This intro is vital as it provides some context for your findings. In your introduction, you should begin by reiterating your problem statement and research questions and highlight the purpose of your research . Make sure that you spell this out for the reader so that the rest of your chapter is well contextualised.
The next step is to briefly outline the structure of your results chapter. In other words, explain what’s included in the chapter and what the reader can expect. In the results chapter, you want to tell a story that is coherent, flows logically, and is easy to follow , so make sure that you plan your structure out well and convey that structure (at a high level), so that your reader is well oriented.
The introduction section shouldn’t be lengthy. Two or three short paragraphs should be more than adequate. It is merely an introduction and overview, not a summary of the chapter.
Pro Tip – To help you structure your chapter, it can be useful to set up an initial draft with (sub)section headings so that you’re able to easily (re)arrange parts of your chapter. This will also help your reader to follow your results and give your chapter some coherence. Be sure to use level-based heading styles (e.g. Heading 1, 2, 3 styles) to help the reader differentiate between levels visually. You can find these options in Word (example below).
Section 2: Body
Before we get started on what to include in the body of your chapter, it’s vital to remember that a results section should be completely objective and descriptive, not interpretive . So, be careful not to use words such as, “suggests” or “implies”, as these usually accompany some form of interpretation – that’s reserved for your discussion chapter.
The structure of your body section is very important , so make sure that you plan it out well. When planning out your qualitative results chapter, create sections and subsections so that you can maintain the flow of the story you’re trying to tell. Be sure to systematically and consistently describe each portion of results. Try to adopt a standardised structure for each portion so that you achieve a high level of consistency throughout the chapter.
For qualitative studies, results chapters tend to be structured according to themes , which makes it easier for readers to follow. However, keep in mind that not all results chapters have to be structured in this manner. For example, if you’re conducting a longitudinal study, you may want to structure your chapter chronologically. Similarly, you might structure this chapter based on your theoretical framework . The exact structure of your chapter will depend on the nature of your study , especially your research questions.
As you work through the body of your chapter, make sure that you use quotes to substantiate every one of your claims . You can present these quotes in italics to differentiate them from your own words. A general rule of thumb is to use at least two pieces of evidence per claim, and these should be linked directly to your data. Also, remember that you need to include all relevant results , not just the ones that support your assumptions or initial leanings.
In addition to including quotes, you can also link your claims to the data by using appendices , which you should reference throughout your text. When you reference, make sure that you include both the name/number of the appendix , as well as the line(s) from which you drew your data.
As referencing styles can vary greatly, be sure to look up the appendix referencing conventions of your university’s prescribed style (e.g. APA , Harvard, etc) and keep this consistent throughout your chapter.

Section 3: Concluding summary
The concluding summary is very important because it summarises your key findings and lays the foundation for the discussion chapter . Keep in mind that some readers may skip directly to this section (from the introduction section), so make sure that it can be read and understood well in isolation.
In this section, you need to remind the reader of the key findings. That is, the results that directly relate to your research questions and that you will build upon in your discussion chapter. Remember, your reader has digested a lot of information in this chapter, so you need to use this section to remind them of the most important takeaways.
Importantly, the concluding summary should not present any new information and should only describe what you’ve already presented in your chapter. Keep it concise – you’re not summarising the whole chapter, just the essentials.
Tips and tricks for an A-grade results chapter
Now that you’ve got a clear picture of what the qualitative results chapter is all about, here are some quick tips and reminders to help you craft a high-quality chapter:
- Your results chapter should be written in the past tense . You’ve done the work already, so you want to tell the reader what you found , not what you are currently finding .
- Make sure that you review your work multiple times and check that every claim is adequately backed up by evidence . Aim for at least two examples per claim, and make use of an appendix to reference these.
- When writing up your results, make sure that you stick to only what is relevant . Don’t waste time on data that are not relevant to your research objectives and research questions.
- Use headings and subheadings to create an intuitive, easy to follow piece of writing. Make use of Microsoft Word’s “heading styles” and be sure to use them consistently.
- When referring to numerical data, tables and figures can provide a useful visual aid. When using these, make sure that they can be read and understood independent of your body text (i.e. that they can stand-alone). To this end, use clear, concise labels for each of your tables or figures and make use of colours to code indicate differences or hierarchy.
- Similarly, when you’re writing up your chapter, it can be useful to highlight topics and themes in different colours . This can help you to differentiate between your data if you get a bit overwhelmed and will also help you to ensure that your results flow logically and coherently.
If you have any questions, leave a comment below and we’ll do our best to help. If you’d like 1-on-1 help with your results chapter (or any chapter of your dissertation or thesis), check out our private dissertation coaching service here or book a free initial consultation to discuss how we can help you.

Psst… there’s more (for free)
This post is part of our dissertation mini-course, which covers everything you need to get started with your dissertation, thesis or research project.
You Might Also Like:

20 Comments
This was extremely helpful. Thanks a lot guys
Hi, thanks for the great research support platform created by the gradcoach team!
I wanted to ask- While “suggests” or “implies” are interpretive terms, what terms could we use for the results chapter? Could you share some examples of descriptive terms?
I think that instead of saying, ‘The data suggested, or The data implied,’ you can say, ‘The Data showed or revealed, or illustrated or outlined’…If interview data, you may say Jane Doe illuminated or elaborated, or Jane Doe described… or Jane Doe expressed or stated.
I found this article very useful. Thank you very much for the outstanding work you are doing.
What if i have 3 different interviewees answering the same interview questions? Should i then present the results in form of the table with the division on the 3 perspectives or rather give a results in form of the text and highlight who said what?
I think this tabular representation of results is a great idea. I am doing it too along with the text. Thanks
That was helpful was struggling to separate the discussion from the findings
this was very useful, Thank you.
Very helpful, I am confident to write my results chapter now.
It is so helpful! It is a good job. Thank you very much!
Very useful, well explained. Many thanks.
Hello, I appreciate the way you provided a supportive comments about qualitative results presenting tips
I loved this! It explains everything needed, and it has helped me better organize my thoughts. What words should I not use while writing my results section, other than subjective ones.
Thanks a lot, it is really helpful
Thank you so much dear, i really appropriate your nice explanations about this.
Thank you so much for this! I was wondering if anyone could help with how to prproperly integrate quotations (Excerpts) from interviews in the finding chapter in a qualitative research. Please GradCoach, address this issue and provide examples.
what if I’m not doing any interviews myself and all the information is coming from case studies that have already done the research.
Very helpful thank you.
This was very helpful as I was wondering how to structure this part of my dissertation, to include the quotes… Thanks for this explanation
This is very helpful, thanks! I am required to write up my results chapters with the discussion in each of them – any tips and tricks for this strategy?
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
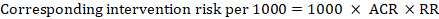
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
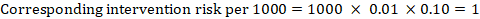
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
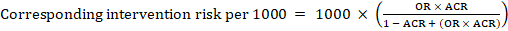
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
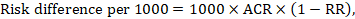
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
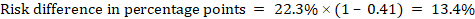
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
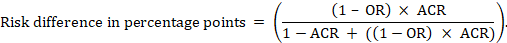
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
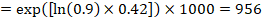
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
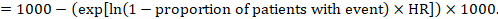
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
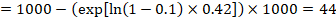
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
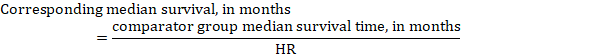
In the example, assuming a comparator group median survival time of 80 months, we obtain:
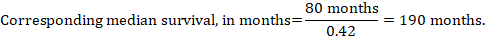
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).
14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).
14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
Intervention:
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
How to Write a Research Paper Summary

One of the most important skills you can imbibe as an academician is to know how to summarize a research paper. During your academic journey, you may need to write a summary of findings in research quite often and for varied reasons – be it to write an introduction for a peer-reviewed publication , to submit a critical review, or to simply create a useful database for future referencing.
It can be quite challenging to effectively write a research paper summary for often complex work, which is where a pre-determined workflow can help you optimize the process. Investing time in developing this skill can also help you improve your scientific acumen, increasing your efficiency and productivity at work. This article illustrates some useful advice on how to write a research summary effectively. But, what is research summary in the first place?
A research paper summary is a crisp, comprehensive overview of a research paper, which encapsulates the purpose, findings, methods, conclusions, and relevance of a study. A well-written research paper summary is an indicator of how well you have understood the author’s work.
Table of Contents
Draft a research paper summary in minutes with paperpal. click here to start writing.
- 2. Invest enough time to understand the topic deeply
Use Paperpal to summarize your research paper. Click here to get started!
- Mistakes to avoid while writing your research paper summary
Let Paperpal do the heavy lifting. Click here to start writing your summary now!
Frequently asked questions (faq), how to write a research paper summary.
Writing a good research paper summary comes with practice and skill. Here is some useful advice on how to write a research paper summary effectively.
1. Determine the focus of your summary
Before you begin to write a summary of research papers, determine the aim of your research paper summary. This will give you more clarity on how to summarize a research paper, including what to highlight and where to find the information you need, which accelerates the entire process. If you are aiming for the summary to be a supporting document or a proof of principle for your current research findings, then you can look for elements that are relevant to your work.
On the other hand, if your research summary is intended to be a critical review of the research article, you may need to use a completely different lens while reading the paper and conduct your own research regarding the accuracy of the data presented. Then again, if the research summary is intended to be a source of information for future referencing, you will likely have a different approach. This makes determining the focus of your summary a key step in the process of writing an effective research paper summary.
2. Invest enough time to understand the topic deeply
In order to author an effective research paper summary, you need to dive into the topic of the research article. Begin by doing a quick scan for relevant information under each section of the paper. The abstract is a great starting point as it helps you to quickly identify the top highlights of the research article, speeding up the process of understanding the key findings in the paper. Be sure to do a careful read of the research paper, preparing notes that describe each section in your own words to put together a summary of research example or a first draft. This will save your time and energy in revisiting the paper to confirm relevant details and ease the entire process of writing a research paper summary.
When reading papers, be sure to acknowledge and ignore any pre-conceived notions that you might have regarding the research topic. This will not only help you understand the topic better but will also help you develop a more balanced perspective, ensuring that your research paper summary is devoid of any personal opinions or biases.
3. Keep the summary crisp, brief and engaging
A research paper summary is usually intended to highlight and explain the key points of any study, saving the time required to read through the entire article. Thus, your primary goal while compiling the summary should be to keep it as brief, crisp and readable as possible. Usually, a short introduction followed by 1-2 paragraphs is adequate for an effective research article summary. Avoid going into too much technical detail while describing the main results and conclusions of the study. Rather focus on connecting the main findings of the study to the hypothesis , which can make the summary more engaging. For example, instead of simply reporting an original finding – “the graph showed a decrease in the mortality rates…”, you can say, “there was a decline in the number of deaths, as predicted by the authors while beginning the study…” or “there was a decline in the number of deaths, which came as a surprise to the authors as this was completely unexpected…”.
Unless you are writing a critical review of the research article, the language used in your research paper summaries should revolve around reporting the findings, not assessing them. On the other hand, if you intend to submit your summary as a critical review, make sure to provide sufficient external evidence to support your final analysis. Invest sufficient time in editing and proofreading your research paper summary thoroughly to ensure you’ve captured the findings accurately. You can also get an external opinion on the preliminary draft of the research paper summary from colleagues or peers who have not worked on the research topic.
Mistakes to avoid while writing your research paper summary
Now that you’ve understood how to summarize a research paper, watch out for these red flags while writing your summary.
- Not paying attention to the word limit and recommended format, especially while submitting a critical review
- Evaluating the findings instead of maintaining an objective , unbiased view while reading the research paper
- Skipping the essential editing step , which can help eliminate avoidable errors and ensure that the language does not misrepresent the findings
- Plagiarism, it is critical to write in your own words or paraphrase appropriately when reporting the findings in your scientific article summary
We hope the recommendations listed above will help answer the question of how to summarize a research paper and enable you to tackle the process effectively.
Summarize your research paper with Paperpal
Paperpal, an AI academic writing assistant, is designed to support academics at every step of the academic writing process. Built on over two decades of experience helping researchers get published and trained on millions of published research articles, Paperpal offers human precision at machine speed. Paperpal Copilot, with advanced generative AI features, can help academics achieve 2x the writing in half the time, while transforming how they research and write.
How to summarize a research paper with Paperpal?
To generate your research paper summary, simply login to the platform and use the Paperpal Copilot Summary feature to create a flawless summary of your work. Here’s a step-by-step process to help you craft a summary in minutes:
- Paste relevant research articles to be summarized into Paperpal; the AI will scan each section and extract key information.
- In minutes, Paperpal will generate a comprehensive summary that showcases the main paper highlights while adhering to academic writing conventions.
- Check the content to polish and refine the language, ensure your own voice, and add citations or references as needed.
The abstract and research paper summary serve similar purposes but differ in scope, length, and placement. The abstract is a concise yet detailed overview of the research, placed at the beginning of a paper, with the aim of providing readers with a quick understanding of the paper’s content and to help them decide whether to read the full article. Usually limited to a few hundred words, it highlights the main objectives, methods, results, and conclusions of the study. On the other hand, a research paper summary provides a crisp account of the entire research paper. Its purpose is to provide a brief recap for readers who may want to quickly grasp the main points of the research without reading the entire paper in detail.
The structure of a research summary can vary depending on the specific requirements or guidelines provided by the target publication or institution. A typical research summary includes the following key sections: introduction (including the research question or objective), methodology (briefly describing the research design and methods), results (summarizing the key findings), discussion (highlighting the implications and significance of the findings), and conclusion (providing a summary of the main points and potential future directions).
The summary of a research paper is important because it provides a condensed overview of the study’s purpose, methods, results, and conclusions. It allows you to quickly grasp the main points and relevance of the research without having to read the entire paper. Research summaries can also be an invaluable way to communicate research findings to a broader audience, such as policymakers or the general public.
When writing a research paper summary, it is crucial to avoid plagiarism by properly attributing the original authors’ work. To learn how to summarize a research paper while avoiding plagiarism, follow these critical guidelines: (1) Read the paper thoroughly to understand the main points and key findings. (2) Use your own words and sentence structures to restate the information, ensuring that the research paper summary reflects your understanding of the paper. (3) Clearly indicate when you are paraphrasing or quoting directly from the original paper by using appropriate citation styles. (4) Cite the original source for any specific ideas, concepts, or data that you include in your summary. (5) Review your summary to ensure it accurately represents the research paper while giving credit to the original authors.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- 5 Reasons for Rejection After Peer Review
- Ethical Research Practices For Research with Human Subjects
- How to Write a Conclusion for Research Papers (with Examples)
- Publish or Perish – Understanding the Importance of Scholarly Publications in Academia
PhD Dissertation Outline: Creating a Roadmap to Success
How ai can improve the academic writing experience, you may also like, is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., should you use ai tools like chatgpt for..., publish research papers: 9 steps for successful publications , how to make translating academic papers less challenging, self-plagiarism in research: what it is and how..., 6 tips for post-doc researchers to take their..., presenting research data effectively through tables and figures, 8 most effective ways to increase motivation for..., publish or perish – understanding the importance of....
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write the Results/Findings Section in Research
What is the research paper Results section and what does it do?
The Results section of a scientific research paper represents the core findings of a study derived from the methods applied to gather and analyze information. It presents these findings in a logical sequence without bias or interpretation from the author, setting up the reader for later interpretation and evaluation in the Discussion section. A major purpose of the Results section is to break down the data into sentences that show its significance to the research question(s).
The Results section appears third in the section sequence in most scientific papers. It follows the presentation of the Methods and Materials and is presented before the Discussion section —although the Results and Discussion are presented together in many journals. This section answers the basic question “What did you find in your research?”
What is included in the Results section?
The Results section should include the findings of your study and ONLY the findings of your study. The findings include:
- Data presented in tables, charts, graphs, and other figures (may be placed into the text or on separate pages at the end of the manuscript)
- A contextual analysis of this data explaining its meaning in sentence form
- All data that corresponds to the central research question(s)
- All secondary findings (secondary outcomes, subgroup analyses, etc.)
If the scope of the study is broad, or if you studied a variety of variables, or if the methodology used yields a wide range of different results, the author should present only those results that are most relevant to the research question stated in the Introduction section .
As a general rule, any information that does not present the direct findings or outcome of the study should be left out of this section. Unless the journal requests that authors combine the Results and Discussion sections, explanations and interpretations should be omitted from the Results.
How are the results organized?
The best way to organize your Results section is “logically.” One logical and clear method of organizing research results is to provide them alongside the research questions—within each research question, present the type of data that addresses that research question.
Let’s look at an example. Your research question is based on a survey among patients who were treated at a hospital and received postoperative care. Let’s say your first research question is:
“What do hospital patients over age 55 think about postoperative care?”
This can actually be represented as a heading within your Results section, though it might be presented as a statement rather than a question:
Attitudes towards postoperative care in patients over the age of 55
Now present the results that address this specific research question first. In this case, perhaps a table illustrating data from a survey. Likert items can be included in this example. Tables can also present standard deviations, probabilities, correlation matrices, etc.
Following this, present a content analysis, in words, of one end of the spectrum of the survey or data table. In our example case, start with the POSITIVE survey responses regarding postoperative care, using descriptive phrases. For example:
“Sixty-five percent of patients over 55 responded positively to the question “ Are you satisfied with your hospital’s postoperative care ?” (Fig. 2)
Include other results such as subcategory analyses. The amount of textual description used will depend on how much interpretation of tables and figures is necessary and how many examples the reader needs in order to understand the significance of your research findings.
Next, present a content analysis of another part of the spectrum of the same research question, perhaps the NEGATIVE or NEUTRAL responses to the survey. For instance:
“As Figure 1 shows, 15 out of 60 patients in Group A responded negatively to Question 2.”
After you have assessed the data in one figure and explained it sufficiently, move on to your next research question. For example:
“How does patient satisfaction correspond to in-hospital improvements made to postoperative care?”
This kind of data may be presented through a figure or set of figures (for instance, a paired T-test table).
Explain the data you present, here in a table, with a concise content analysis:
“The p-value for the comparison between the before and after groups of patients was .03% (Fig. 2), indicating that the greater the dissatisfaction among patients, the more frequent the improvements that were made to postoperative care.”
Let’s examine another example of a Results section from a study on plant tolerance to heavy metal stress . In the Introduction section, the aims of the study are presented as “determining the physiological and morphological responses of Allium cepa L. towards increased cadmium toxicity” and “evaluating its potential to accumulate the metal and its associated environmental consequences.” The Results section presents data showing how these aims are achieved in tables alongside a content analysis, beginning with an overview of the findings:
“Cadmium caused inhibition of root and leave elongation, with increasing effects at higher exposure doses (Fig. 1a-c).”
The figure containing this data is cited in parentheses. Note that this author has combined three graphs into one single figure. Separating the data into separate graphs focusing on specific aspects makes it easier for the reader to assess the findings, and consolidating this information into one figure saves space and makes it easy to locate the most relevant results.
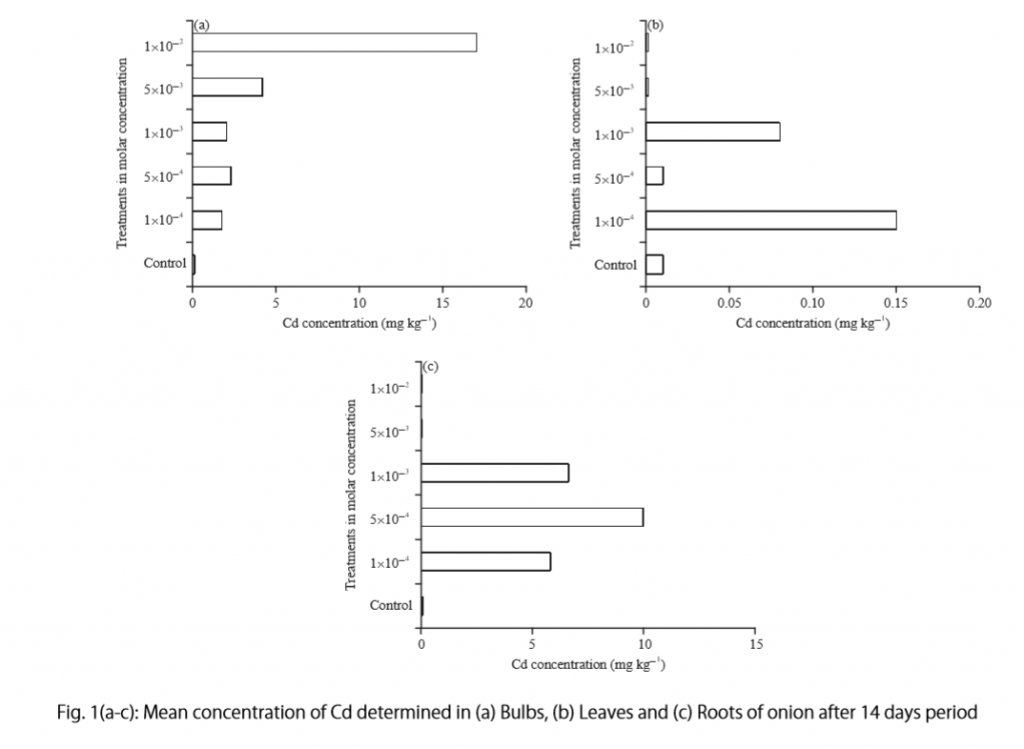
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section.
- “Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M.”
Captioning and Referencing Tables and Figures
Tables and figures are central components of your Results section and you need to carefully think about the most effective way to use graphs and tables to present your findings . Therefore, it is crucial to know how to write strong figure captions and to refer to them within the text of the Results section.
The most important advice one can give here as well as throughout the paper is to check the requirements and standards of the journal to which you are submitting your work. Every journal has its own design and layout standards, which you can find in the author instructions on the target journal’s website. Perusing a journal’s published articles will also give you an idea of the proper number, size, and complexity of your figures.
Regardless of which format you use, the figures should be placed in the order they are referenced in the Results section and be as clear and easy to understand as possible. If there are multiple variables being considered (within one or more research questions), it can be a good idea to split these up into separate figures. Subsequently, these can be referenced and analyzed under separate headings and paragraphs in the text.
To create a caption, consider the research question being asked and change it into a phrase. For instance, if one question is “Which color did participants choose?”, the caption might be “Color choice by participant group.” Or in our last research paper example, where the question was “What is the concentration of cadmium in different parts of the onion after 14 days?” the caption reads:
“Fig. 1(a-c): Mean concentration of Cd determined in (a) bulbs, (b) leaves, and (c) roots of onions after a 14-day period.”
Steps for Composing the Results Section
Because each study is unique, there is no one-size-fits-all approach when it comes to designing a strategy for structuring and writing the section of a research paper where findings are presented. The content and layout of this section will be determined by the specific area of research, the design of the study and its particular methodologies, and the guidelines of the target journal and its editors. However, the following steps can be used to compose the results of most scientific research studies and are essential for researchers who are new to preparing a manuscript for publication or who need a reminder of how to construct the Results section.
Step 1 : Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study.
- The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches.
- Note length limitations on restrictions on content. For instance, while many journals require the Results and Discussion sections to be separate, others do not—qualitative research papers often include results and interpretations in the same section (“Results and Discussion”).
- Reading the aims and scope in the journal’s “ guide for authors ” section and understanding the interests of its readers will be invaluable in preparing to write the Results section.

Step 2 : Consider your research results in relation to the journal’s requirements and catalogue your results.
- Focus on experimental results and other findings that are especially relevant to your research questions and objectives and include them even if they are unexpected or do not support your ideas and hypotheses.
- Catalogue your findings—use subheadings to streamline and clarify your report. This will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Create appendices that might interest specialists but prove too long or distracting for other readers.
- Decide how you will structure of your results. You might match the order of the research questions and hypotheses to your results, or you could arrange them according to the order presented in the Methods section. A chronological order or even a hierarchy of importance or meaningful grouping of main themes or categories might prove effective. Consider your audience, evidence, and most importantly, the objectives of your research when choosing a structure for presenting your findings.
Step 3 : Design figures and tables to present and illustrate your data.
- Tables and figures should be numbered according to the order in which they are mentioned in the main text of the paper.
- Information in figures should be relatively self-explanatory (with the aid of captions), and their design should include all definitions and other information necessary for readers to understand the findings without reading all of the text.
- Use tables and figures as a focal point to tell a clear and informative story about your research and avoid repeating information. But remember that while figures clarify and enhance the text, they cannot replace it.
Step 4 : Draft your Results section using the findings and figures you have organized.
- The goal is to communicate this complex information as clearly and precisely as possible; precise and compact phrases and sentences are most effective.
- In the opening paragraph of this section, restate your research questions or aims to focus the reader’s attention to what the results are trying to show. It is also a good idea to summarize key findings at the end of this section to create a logical transition to the interpretation and discussion that follows.
- Try to write in the past tense and the active voice to relay the findings since the research has already been done and the agent is usually clear. This will ensure that your explanations are also clear and logical.
- Make sure that any specialized terminology or abbreviation you have used here has been defined and clarified in the Introduction section .
Step 5 : Review your draft; edit and revise until it reports results exactly as you would like to have them reported to your readers.
- Double-check the accuracy and consistency of all the data, as well as all of the visual elements included.
- Read your draft aloud to catch language errors (grammar, spelling, and mechanics), awkward phrases, and missing transitions.
- Ensure that your results are presented in the best order to focus on objectives and prepare readers for interpretations, valuations, and recommendations in the Discussion section . Look back over the paper’s Introduction and background while anticipating the Discussion and Conclusion sections to ensure that the presentation of your results is consistent and effective.
- Consider seeking additional guidance on your paper. Find additional readers to look over your Results section and see if it can be improved in any way. Peers, professors, or qualified experts can provide valuable insights.
One excellent option is to use a professional English proofreading and editing service such as Wordvice, including our paper editing service . With hundreds of qualified editors from dozens of scientific fields, Wordvice has helped thousands of authors revise their manuscripts and get accepted into their target journals. Read more about the proofreading and editing process before proceeding with getting academic editing services and manuscript editing services for your manuscript.
As the representation of your study’s data output, the Results section presents the core information in your research paper. By writing with clarity and conciseness and by highlighting and explaining the crucial findings of their study, authors increase the impact and effectiveness of their research manuscripts.
For more articles and videos on writing your research manuscript, visit Wordvice’s Resources page.
Wordvice Resources
- How to Write a Research Paper Introduction
- Which Verb Tenses to Use in a Research Paper
- How to Write an Abstract for a Research Paper
- How to Write a Research Paper Title
- Useful Phrases for Academic Writing
- Common Transition Terms in Academic Papers
- Active and Passive Voice in Research Papers
- 100+ Verbs That Will Make Your Research Writing Amazing
- Tips for Paraphrasing in Research Papers
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Working with sources
- How to Write a Summary | Guide & Examples
How to Write a Summary | Guide & Examples
Published on November 23, 2020 by Shona McCombes . Revised on May 31, 2023.
Summarizing , or writing a summary, means giving a concise overview of a text’s main points in your own words. A summary is always much shorter than the original text.
There are five key steps that can help you to write a summary:
- Read the text
- Break it down into sections
- Identify the key points in each section
- Write the summary
- Check the summary against the article
Writing a summary does not involve critiquing or evaluating the source . You should simply provide an accurate account of the most important information and ideas (without copying any text from the original).
Table of contents
When to write a summary, step 1: read the text, step 2: break the text down into sections, step 3: identify the key points in each section, step 4: write the summary, step 5: check the summary against the article, other interesting articles, frequently asked questions about summarizing.
There are many situations in which you might have to summarize an article or other source:
- As a stand-alone assignment to show you’ve understood the material
- To keep notes that will help you remember what you’ve read
- To give an overview of other researchers’ work in a literature review
When you’re writing an academic text like an essay , research paper , or dissertation , you’ll integrate sources in a variety of ways. You might use a brief quote to support your point, or paraphrase a few sentences or paragraphs.
But it’s often appropriate to summarize a whole article or chapter if it is especially relevant to your own research, or to provide an overview of a source before you analyze or critique it.
In any case, the goal of summarizing is to give your reader a clear understanding of the original source. Follow the five steps outlined below to write a good summary.
Scribbr Citation Checker New
The AI-powered Citation Checker helps you avoid common mistakes such as:
- Missing commas and periods
- Incorrect usage of “et al.”
- Ampersands (&) in narrative citations
- Missing reference entries
You should read the article more than once to make sure you’ve thoroughly understood it. It’s often effective to read in three stages:
- Scan the article quickly to get a sense of its topic and overall shape.
- Read the article carefully, highlighting important points and taking notes as you read.
- Skim the article again to confirm you’ve understood the key points, and reread any particularly important or difficult passages.
There are some tricks you can use to identify the key points as you read:
- Start by reading the abstract . This already contains the author’s own summary of their work, and it tells you what to expect from the article.
- Pay attention to headings and subheadings . These should give you a good sense of what each part is about.
- Read the introduction and the conclusion together and compare them: What did the author set out to do, and what was the outcome?
To make the text more manageable and understand its sub-points, break it down into smaller sections.
If the text is a scientific paper that follows a standard empirical structure, it is probably already organized into clearly marked sections, usually including an introduction , methods , results , and discussion .
Other types of articles may not be explicitly divided into sections. But most articles and essays will be structured around a series of sub-points or themes.
Now it’s time go through each section and pick out its most important points. What does your reader need to know to understand the overall argument or conclusion of the article?
Keep in mind that a summary does not involve paraphrasing every single paragraph of the article. Your goal is to extract the essential points, leaving out anything that can be considered background information or supplementary detail.
In a scientific article, there are some easy questions you can ask to identify the key points in each part.
If the article takes a different form, you might have to think more carefully about what points are most important for the reader to understand its argument.
In that case, pay particular attention to the thesis statement —the central claim that the author wants us to accept, which usually appears in the introduction—and the topic sentences that signal the main idea of each paragraph.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
Now that you know the key points that the article aims to communicate, you need to put them in your own words.
To avoid plagiarism and show you’ve understood the article, it’s essential to properly paraphrase the author’s ideas. Do not copy and paste parts of the article, not even just a sentence or two.
The best way to do this is to put the article aside and write out your own understanding of the author’s key points.
Examples of article summaries
Let’s take a look at an example. Below, we summarize this article , which scientifically investigates the old saying “an apple a day keeps the doctor away.”
Davis et al. (2015) set out to empirically test the popular saying “an apple a day keeps the doctor away.” Apples are often used to represent a healthy lifestyle, and research has shown their nutritional properties could be beneficial for various aspects of health. The authors’ unique approach is to take the saying literally and ask: do people who eat apples use healthcare services less frequently? If there is indeed such a relationship, they suggest, promoting apple consumption could help reduce healthcare costs.
The study used publicly available cross-sectional data from the National Health and Nutrition Examination Survey. Participants were categorized as either apple eaters or non-apple eaters based on their self-reported apple consumption in an average 24-hour period. They were also categorized as either avoiding or not avoiding the use of healthcare services in the past year. The data was statistically analyzed to test whether there was an association between apple consumption and several dependent variables: physician visits, hospital stays, use of mental health services, and use of prescription medication.
Although apple eaters were slightly more likely to have avoided physician visits, this relationship was not statistically significant after adjusting for various relevant factors. No association was found between apple consumption and hospital stays or mental health service use. However, apple eaters were found to be slightly more likely to have avoided using prescription medication. Based on these results, the authors conclude that an apple a day does not keep the doctor away, but it may keep the pharmacist away. They suggest that this finding could have implications for reducing healthcare costs, considering the high annual costs of prescription medication and the inexpensiveness of apples.
However, the authors also note several limitations of the study: most importantly, that apple eaters are likely to differ from non-apple eaters in ways that may have confounded the results (for example, apple eaters may be more likely to be health-conscious). To establish any causal relationship between apple consumption and avoidance of medication, they recommend experimental research.
An article summary like the above would be appropriate for a stand-alone summary assignment. However, you’ll often want to give an even more concise summary of an article.
For example, in a literature review or meta analysis you may want to briefly summarize this study as part of a wider discussion of various sources. In this case, we can boil our summary down even further to include only the most relevant information.
Using national survey data, Davis et al. (2015) tested the assertion that “an apple a day keeps the doctor away” and did not find statistically significant evidence to support this hypothesis. While people who consumed apples were slightly less likely to use prescription medications, the study was unable to demonstrate a causal relationship between these variables.
Citing the source you’re summarizing
When including a summary as part of a larger text, it’s essential to properly cite the source you’re summarizing. The exact format depends on your citation style , but it usually includes an in-text citation and a full reference at the end of your paper.
You can easily create your citations and references in APA or MLA using our free citation generators.
APA Citation Generator MLA Citation Generator
Finally, read through the article once more to ensure that:
- You’ve accurately represented the author’s work
- You haven’t missed any essential information
- The phrasing is not too similar to any sentences in the original.
If you’re summarizing many articles as part of your own work, it may be a good idea to use a plagiarism checker to double-check that your text is completely original and properly cited. Just be sure to use one that’s safe and reliable.
If you want to know more about ChatGPT, AI tools , citation , and plagiarism , make sure to check out some of our other articles with explanations and examples.
- ChatGPT vs human editor
- ChatGPT citations
- Is ChatGPT trustworthy?
- Using ChatGPT for your studies
- What is ChatGPT?
- Chicago style
- Paraphrasing
Plagiarism
- Types of plagiarism
- Self-plagiarism
- Avoiding plagiarism
- Academic integrity
- Consequences of plagiarism
- Common knowledge
A summary is a short overview of the main points of an article or other source, written entirely in your own words. Want to make your life super easy? Try our free text summarizer today!
A summary is always much shorter than the original text. The length of a summary can range from just a few sentences to several paragraphs; it depends on the length of the article you’re summarizing, and on the purpose of the summary.
You might have to write a summary of a source:
- As a stand-alone assignment to prove you understand the material
- For your own use, to keep notes on your reading
- To provide an overview of other researchers’ work in a literature review
- In a paper , to summarize or introduce a relevant study
To avoid plagiarism when summarizing an article or other source, follow these two rules:
- Write the summary entirely in your own words by paraphrasing the author’s ideas.
- Cite the source with an in-text citation and a full reference so your reader can easily find the original text.
An abstract concisely explains all the key points of an academic text such as a thesis , dissertation or journal article. It should summarize the whole text, not just introduce it.
An abstract is a type of summary , but summaries are also written elsewhere in academic writing . For example, you might summarize a source in a paper , in a literature review , or as a standalone assignment.
All can be done within seconds with our free text summarizer .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, May 31). How to Write a Summary | Guide & Examples. Scribbr. Retrieved April 3, 2024, from https://www.scribbr.com/working-with-sources/how-to-summarize/
Is this article helpful?
Shona McCombes
Other students also liked, how to paraphrase | step-by-step guide & examples, how to quote | citing quotes in apa, mla & chicago, the basics of in-text citation | apa & mla examples, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
- How it works
Writing a Summary – Explanation & Examples
Published by Alvin Nicolas at October 17th, 2023 , Revised On October 17, 2023
In a world bombarded with vast amounts of information, condensing and presenting data in a digestible format becomes invaluable. Enter summaries.
A summary is a brief and concise account of the main points of a larger body of work. It distils complex ideas, narratives, or data into a version that is quicker to read and easier to understand yet still retains the essence of the original content.
Importance of Summaries
The importance of summarising extends far beyond just making reading more manageable. In academic settings, summaries aid students in understanding and retaining complex materials, from textbook chapters to research articles. They also serve as tools to showcase one’s grasp of the subject in essays and reports.
In professional arenas, summaries are pivotal in business reports, executive briefings, and even emails where key points need to be conveyed quickly to decision-makers. Meanwhile, summarising skills come into play in our personal lives when we relay news stories to friends, recap a movie plot, or even scroll through condensed news or app notifications on our smartphones.
Why Do We Write Summaries?
In our modern information age, the sheer volume of content available can be overwhelming. From detailed research papers to comprehensive news articles, the quest for knowledge is often met with lengthy and complex resources. This is where the power of a well-crafted summary comes into play. But what drives us to create or seek out summaries? Let’s discuss.
Makes Important Things Easy to Remember
At the heart of summarisation is the goal to understand. A well-written summary aids in digesting complex material. By distilling larger works into their core points, we reinforce the primary messages, making them easier to remember. This is especially crucial for students who need to retain knowledge for exams or professionals prepping for a meeting based on a lengthy report.
Simplification of Complex Topics
Not everyone is an expert in every field. Often, topics come laden with jargon, intricate details, and nuanced arguments. Summaries act as a bridge, translating this complexity into accessible and straightforward content. This is especially beneficial for individuals new to a topic or those who need just the highlights without the intricacies.
Aid in Researching and Understanding Diverse Sources
Researchers, writers, and academics often wade through many sources when working on a project. This involves finding sources of different types, such as primary or secondary sources , and then understanding their content. Sifting through each source in its entirety can be time-consuming. Summaries offer a streamlined way to understand each source’s main arguments or findings, making synthesising information from diverse materials more efficient.
Condensing Information for Presentation or Sharing
In professional settings, there is often a need to present findings, updates, or recommendations to stakeholders. An executive might not have the time to go through a 50-page report, but they would certainly appreciate a concise summary highlighting the key points. Similarly, in our personal lives, we often summarise movie plots, book stories, or news events when sharing with friends or family.
Characteristics of a Good Summary
Crafting an effective summary is an art. It’s more than just shortening a piece of content; it is about capturing the essence of the original work in a manner that is both accessible and true to its intent. Let’s explore the primary characteristics that distinguish a good summary from a mediocre one:
Conciseness
At the core of a summary is the concept of brevity. But being concise doesn’t mean leaving out vital information. A good summary will:
- Eliminate superfluous details or repetitive points.
- Focus on the primary arguments, events, or findings.
- Use succinct language without compromising the message.
Objectivity
Summarising is not about infusing personal opinions or interpretations. A quality summary will:
- Stick to the facts as presented in the original content.
- Avoid introducing personal biases or perspectives.
- Represent the original author’s intent faithfully.
A summary is meant to simplify and make content accessible. This is only possible if the summary itself is easy to understand. Ensuring clarity involves:
- Avoiding jargon or technical terms unless they are essential to the content. If they are used, they should be clearly defined.
- Structuring sentences in a straightforward manner.
- Making sure ideas are presented in a way that even someone unfamiliar with the topic can grasp the primary points.
A jumble of ideas, no matter how concise, will not make for a good summary. Coherence ensures that there’s a logical flow to the summarised content. A coherent summary will:
- Maintain a logical sequence, often following the structure of the original content.
- Use transition words or phrases to connect ideas and ensure smooth progression.
- Group related ideas together to provide structure and avoid confusion.
Steps of Writing a Summary
The process of creating a compelling summary is not merely about cutting down content. It involves understanding, discerning, and crafting. Here is a step-by-step guide to writing a summary that encapsulates the essence of the original work:
Reading Actively
Engage deeply with the content to ensure a thorough understanding.
- Read the entire document or work first to grasp its overall intent and structure.
- On the second read, underline or highlight the standout points or pivotal moments.
- Make brief notes in the margins or on a separate sheet, capturing the core ideas in your own words.
Identifying the Main Idea
Determine the backbone of the content, around which all other details revolve.
- Ask yourself: “What is the primary message or theme the author wants to convey?”
- This can often be found in the title, introduction, or conclusion of a piece.
- Frame the main idea in a clear and concise statement to guide your summary.
List Key Supporting Points
Understand the pillars that uphold the main idea, providing evidence or depth to the primary message.
- Refer back to the points you underlined or highlighted during your active reading.
- Note major arguments, evidence, or examples that the author uses to back up the main idea.
- Prioritise these points based on their significance to the main idea.
Draft the Summary
Convert your understanding into a condensed, coherent version of the original.
- Start with a statement of the main idea.
- Follow with the key supporting points, maintaining logical order.
- Avoid including trivial details or examples unless they’re crucial to the primary message.
- Use your own words, ensuring you are not plagiarising the original content.
Fine-tune your draft to ensure clarity, accuracy, and brevity.
- Read your draft aloud to check for flow and coherence.
- Ensure that your summary remains objective, avoiding any personal interpretations or biases.
- Check the length. See if any non-essential details can be removed without sacrificing understanding if it is too lengthy.
- Ensure clarity by ensuring the language is straightforward, and the main ideas are easily grasped.
The research done by our experts have:
- Precision and Clarity
- Zero Plagiarism
- Authentic Sources

Dos and Don’ts of Summarising Key Points
Summarising, while seemingly straightforward, comes with its nuances. Properly condensing content demands a balance between brevity and fidelity to the original work. To aid in crafting exemplary summaries, here is a guide on the essential dos and don’ts:
Use your Own Words
This ensures that you have truly understood the content and are not merely parroting it. It also prevents issues of plagiarism.
Tip: After reading the original content, take a moment to reflect on it. Then, without looking at the source, write down the main points in your own words.
Attribute Sources Properly
Giving credit is both ethical and provides context to readers, helping them trace back to the original work if needed. How to cite sources correctly is a skill every writer should master.
Tip: Use signal phrases like “According to [Author/Source]…” or “As [Author/Source] points out…” to seamlessly incorporate attributions.
Ensure Accuracy of the Summarised Content
A summary should be a reliable reflection of the original content. Distorting or misrepresenting the original ideas compromises the integrity of the summary.
Tip: After drafting your summary, cross-check with the original content to ensure all key points are represented accurately and ensure you are referencing credible sources .
Avoid Copy-Pasting Chunks of Original Content
This not only raises plagiarism concerns but also shows a lack of genuine engagement with the material.
Tip: If a particular phrase or sentence from the original is pivotal and cannot be reworded without losing its essence, use block quotes , quotation marks, and attribute the source.
Do not Inject your Personal Opinion
A summary should be an objective reflection of the source material. Introducing personal biases or interpretations can mislead readers.
Tip: Stick to the facts and arguments presented in the original content. If you find yourself writing “I think” or “In my opinion,” reevaluate the sentence.
Do not Omit Crucial Information
While a summary is meant to be concise, it shouldn’t be at the expense of vital details that are essential to understanding the original content’s core message.
Tip: Prioritise information. Always include the main idea and its primary supports. If you are unsure whether a detail is crucial, consider its impact on the overall message.
Examples of Summaries
Here are a few examples that will help you get a clearer view of how to write a summary.
Example 1: Summary of a News Article
Original Article: The article reports on the recent discovery of a rare species of frog in the Amazon rainforest. The frog, named the “Emerald Whisperer” due to its unique green hue and the soft chirping sounds it makes, was found by a team of researchers from the University of Texas. The discovery is significant as it offers insights into the biodiversity of the region, and the Emerald Whisperer might also play a pivotal role in understanding the ecosystem balance.
Summary: Researchers from the University of Texas have discovered a unique frog, termed the “Emerald Whisperer,” in the Amazon rainforest. This finding sheds light on the region’s biodiversity and underscores the importance of the frog in ecological studies.
Example 2: Summary of a Research Paper
Original Paper: In a study titled “The Impact of Urbanisation on Bee Populations,” researchers conducted a year-long observation on bee colonies in three urban areas and three rural areas. Using specific metrics like colony health, bee productivity, and population size, the study found that urban environments saw a 30% decline in bee populations compared to rural settings. The research attributes this decline to factors like pollution, reduced green spaces, and increased temperatures in urban areas.
Summary: A study analysing the effects of urbanisation on bee colonies found a significant 30% decrease in bee populations in urban settings compared to rural areas. The decline is linked to urban factors such as pollution, diminished greenery, and elevated temperatures.
Example 3: Summary of a Novel
Original Story: In the novel “Winds of Fate,” protagonist Clara is trapped in a timeless city where memories dictate reality. Throughout her journey, she encounters characters from her past, present, and imagined future. Battling her own perceptions and a menacing shadow figure, Clara seeks an elusive gateway to return to her real world. In the climax, she confronts the shadow, which turns out to be her own fear, and upon overcoming it, she finds her way back, realising that reality is subjective.
Summary: “Winds of Fate” follows Clara’s adventures in a surreal city shaped by memories. Confronting figures from various phases of her life and battling a symbolic shadow of her own fear, Clara eventually discovers that reality’s perception is malleable and subjective.
Frequently Asked Questions
How long is a summary.
A summary condenses a larger piece of content, capturing its main points and essence. It is usually one-fourth of the original content.
What is a summary?
A summary is a concise representation of a larger text or content, highlighting its main ideas and points. It distils complex information into a shorter form, allowing readers to quickly grasp the essence of the original material without delving into extensive details. Summaries prioritise clarity, brevity, and accuracy.
When should I write a summary?
Write a summary when you need to condense lengthy content for easier comprehension and recall. It’s useful in academic settings, professional reports, presentations, and research to highlight key points. Summaries aid in comparing multiple sources, preparing for discussions, and sharing essential details of extensive materials efficiently with others.
How can I summarise a source without plagiarising?
To summarise without plagiarising: Read the source thoroughly, understand its main ideas, and then write the summary in your own words. Avoid copying phrases verbatim. Attribute the source properly. Use paraphrasing techniques and cross-check your summary against the original to ensure distinctiveness while retaining accuracy. Always prioritise understanding over direct replication.
What is the difference between a summary and an abstract?
A summary condenses a text, capturing its main points from various content types like books, articles, or movies. An abstract, typically found in research papers and scientific articles, provides a brief overview of the study’s purpose, methodology, results, and conclusions. Both offer concise versions, but abstracts are more structured and specific.
You May Also Like
From academic research to personal blogs, the bedrock of trust and credibility is often established by one simple act: source citing. Whether we are constructing a thesis for a graduate program or debunking a myth on a personal blog, providing the origins of our information bolsters our arguments and pays homage to the original creators of that knowledge.
When researching or exploring a new topic, the distinction between primary and secondary sources is paramount. The validity, reliability, and relevance of the information you gather will heavily depend on the type of source you consult.
The ability to effectively incorporate multiple sources into one’s work is not just a skill, but a necessity. Whether we are talking about research papers, articles, or even simple blog posts, synthesising sources can elevate our content to a more nuanced, comprehensive, and insightful level.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works

Research Summary

A research paper analyzes a perspective or argues a point. It is an expanded essay based on your interpretation, evaluation or argument about a certain topic.
According to Sunny Empire State College , “When you write a research paper you build upon what you know about the subject and make a deliberate attempt to find out what experts know. A research paper involves surveying a field of knowledge in order to find the best possible information in that field.” Whatever type of research paper you choose to write, it should present your own ideas backed with others’ (especially experts on the field) information and data.
Every research paper has a research summary. A research summary is a brief overview of what the whole research is about. It is a professional piece of writing that describes your research to the readers. It concisely yet perfectly captures the essence of the research as a whole. You may also see What Should Be in an Executive Summary of a Report?

Fundamentals of a Research Summary
Having a good template for a research summary is nothing if you don’t know its importance and basic function. Before you start writing your research summary, you should first know its fundamentals on the areas you need to pay attention to such as its content, style and organization.
- The content of your research summary must briefly discuss the techniques and tools used in the research and the importance of the research as a whole. Explain how the research can be of benefit for the people.
- To organize your research summary, each topic must be discussed in separate paragraphs. How you came up with a factual research must be briefly explained in a separate paragraph.
- If you have a lengthy research paper, try not to write not more than 10% of the entire paper. If it’s not as lengthy, you should not write more than 300 words in your summary.
However, rules may vary according to your research professor’s standards. This is just the basic fundamentals on how to write your research summary. Also see Thesis Outline Examples
How to Write a Research Summary
It is apparent that a research summary is a condensed version of the main idea of your research paper. Because of this, it is advised that the summary of your paper is written after you are done with your entire research. This is to ensure that all the added information in your research can be written in your summary as well and all of those that removed can be edited out. Here are a few steps on how to write a research summary:
Read your paper
It should be a fact you should know beforehand; the importance of reading your entire research paper thoroughly to write an effective research summary. Along the way, take notes of the important details and key findings that you want to highlight in your paper. This will help you organize your summary better. Remember that your research summary is a mini-paper of your study and it should contain the main ideas of your entire research.
Write a draft
For your first draft, focus on the content rather than the length of your summary. Your draft is your first outline on what to include in the final summary. Writing a draft ensures you write a clear, thorough and coherent summary of your research paper. Also see How to Write a Rough Outline
Identify main points
Within your research paper, you must identify the major points that will encourage prospective readers to go through your research paper. These major points must thoroughly and completely explain what the paper is trying to convey.
Separate sections
Identify the differences of the main section in your paper. Write a few sentences describing the main ideas of each section. In short, you should be able to present and thoroughly describe what each main section is focused on. It should have these basic sections:
- Introduction, brief opening statement
- Purpose of the study
- Data gathering method
- Summary of findings
- Description of recommendations with actual justification.
Combine Information
All the information you have gathered must be then used to make your summary. Remember that your summary is just an overview of your research paper as a whole. It should be not be more than 10% of your whole paper. Also see 5 Summary Writing Examples and Samples
Making The First Draft
After establishing the basic way of writing a research summary, it is a must to write a first draft. It should follow the flow of the original paper. Here’s a few steps on how to make a first draft:
First, state the research question in the introduction of your summary. This holds the ground as to the summary’s direction. Provide an explanation why your research is interesting and how it can help your target recipients.
Second, state the hypothesis you wish to prove. This will help you and your readers stay grounded on the topic at hand.
Third, briefly discuss the methodology used in your research. Discuss and describe the procedure, materials, participants, design, etc. The analysis of your data must also be included. You may also see How to Write a Successful Thesis Proposal
Fourth, describe the results and significance of your research. And lastly, briefly discuss the key implications of your research. The results and its interpretation should directly coincide with your hypothesis.

Editing your Research Summary
A research paper is a formal piece of writing. Your summary should be tailored to your expected readers. Say for example the prospective readers are your classmates, so the style of your paper should be clearly understood by them.
Eliminate wordiness. Avoid using unnecessary adjectives and adverbs. Write in a way it would be easier for your readers to understand. It is common for research papers to establish a word count. Avoid elongating your sentences when it has shorter versions.
Being vague in describing and explaining the points of your paper might lead to confusion in your readers part. Use specific, concrete language when presenting results. Use reliable and specific examples and references as well. You should also use scientifically accurate language to help support your claims. Avoid informal words and adjectives to describe the results of your research.
Paraphrase the information you want to include in your research paper. Direct quoting the information you have read from a different source is not oftenly used in formal writings. To give the exact credit for the information you paraphrased, follow the citation format required by your professor.
Reread your paper and let others read it as well. This way minor errors you were not able to notice can be quickly pointed out and corrected.
Research Summary Writing Tips
Your research summary should not be more than 10 pages long or not more than 10% of your original document. This keeps your research summary concise and compact. It should be short enough for your readers to read through but long enough for you to clearly explain your study.
Copy and paste
Avoid simply copy and pasting different parts of your paper into your summary. You should paraphrase parts that you want to include. As most research advisers read through all of your paper, it can easily be identified if you have copy-pasted parts from your research and might give you a bad grade.
Consider the readers
Although not a requirement from your professor, catering your summary to what the readers need is sometimes required. As some studies are given out to different influential people in the field, writing a summary that caters to the readers’ necessities might be required.
Research Article Summary Template

- Google Docs
Size: 158 KB
Research Report Executive Summary Template

Size: 120 KB
Research Summary Example
Size: 130 KB
Research Summary Sample

Size: 486 KB
Research Writing Summary Tips (continuation)
Clarity and organization.
One of the common mistakes in writing a research is publishing an unclear and unpolished summary. Bear in mind that your readers are likely reading about the topic of your research for the first time, avoid unclear and uncertain explanations and a disorganized summary.
Use strong and positive language
Use precise and strong words to help strengthen the foundation of your summary. Your summary should be able to stand alone despite it being a part of the research paper. Once you have convinced your readers with the recommendations regarding the topic of your paper, the readers should be able to find concrete evidence and explanations within your summary. Avoid pleas and biased statements in your summary, but make sure you are able to relay the sense of urgency for the recommendations you have given.
Divide into parts
To make things easier for you, divide your paper into different sections and headings, much like creating an outline. With this in mind, every point should be explained limited to its essence. In this way, you avoid writing too much information about your paper in your summary.
AI Generator
Text prompt
- Instructive
- Professional
10 Examples of Public speaking
20 Examples of Gas lighting

Improved Surface Drainage of Pavements: Final Report (1998)
Chapter: chapter 5 summary, findings, and recommendations.
Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
CHAPIER 5 SI~MARY, FINDINGS, AND RECOMMENDATIONS SUGARY The primary objective of this research was to identify unproved methods for draining rainwater from the surface of multi-lane pavements and to develop guidelines for their use. The guidelines, along with details on the rationale for their development, are presented in a separate document' "Proposed Design Guidelines for Improving Pavement Surface Drainage" (2J. The guidelines support an interactive computer program, PAVDRN, that can be used by practicing engineers In the process of designing new pavements or rehabilitating old pavements' is outlined In figure 39. The intended audience for the guidelines is practicing highway design engineers that work for transportation agencies or consulting firms. Improved pavement surface drainage is needed for two reasons: (~) to minimize splash and spray and (2) to control the tendency for hydroplaning. Both issues are primary safety concerns. At the request of the advisory panel for the project, the main focus of this study was on ~mprov~g surface drainage to mammae the tendency for hydroplaning. In terms of reducing the tendency for hydroplaTuT g, the needed level of drainage is defined in terms of the thickness of the film of water on the pavement. Therefore, the guidelines were developed within the context of reducing the thickness of the water film on pavement surfaces to the extent that hydroplaning is unlikely at highway design speeds. Since hydroplaning is ~7
DESIGN CRITERIA Pavement Geometry Number of lanes Section type - Tangent - Horizontal curve - Transition - Vertical crest curve - Vertical sag curve Enviromnental oramaters Rainfall intensity ~ Temperature Pavement Tvpe Dense-graded asphalt Porous asphalt Portland cement concrete ~ Grooved Portland cement concrete Desion Soeed Allowable speed for onset of hydroplaning Recommend Desion Changes Alter geometry Alter pavement surface Add appurtenances Groove (Portland cement concrete) CALCULATIONS Lenoth of flow path Calculate on basis of pavement geometry IT Hydraulic Analvses . No? Water film thickness Equation No. 10 Equation No.'s. 16-19 1 Hvdroolanino Analvsis Hydroplaning speed Equation No.'s 21-24 Rainfall Intensity Equation No. 25 -A I / Meet Design ~ \ Cntena? / \<es? Accent Desinn | Figure 39. Flow diagram representing PAVI)RN design process In "Proposed Guidelines for Improving Pavement Surface DrmT~age" (2). 118
controlled primarily by the thickness of the water film on the pavement surface, the design guidelines focus on the prediction and control of ache depth of water flowing across the pavement surface as a result of rainfall, often referred to as sheet flow. Water film thickness on highway pavements can be controlled In three fundamental ways, by: I. Minimizing the length of the longest flow path of the water over We pavement and thereby the distance over which the flow can develop; 2. Increasing the texture of the pavement surface; and 3. Removing water from the pavement's surface. In the process of using PAVDRN to implement the design guidelines, the designer is guided to (~) minimize the longest drainage path length of the section under design by altering the pavement geometry and (2) reduce the resultant water film thickness that will develop along that drainage path length by increasing the mean texture depth, choosing a surface that maximizes texture, or using permeable pavements, grooving, and appurtenances to remove water from the surface. Through the course of a typical design project, four key areas need to be considered in order to analyze and eventually reduce the potential for hydroplaning. These areas are: ~9
I. Environmental conditions: 2. Geometry of the roadway surface; 3. Pavement surface (texture) properties; and 4. Appurtenances. Each of these areas and their influence on the resulting hydroplaning speed of the designed section are discussed In detail In the guidelines (21. The environmental conditions considered are rainfall ~ntensibr and water temperature, which determines the kinematic viscosity of the water. The designer has no real control over these environmental factors but needs to select appropriate values when analyzing the effect of flow over the pavement surface and hydroplaning potential. Five section types, one for each of the basic geometric configurations used In highway design, are examined. These section are: 1. TaIlgent; 2. Superelevated curve; 3. Transition; 4. Vertical crest curve; and 5. Vertical sag curve. 120
Pavement properties that affect the water fihn thickness mclude surface characteristics, such as mean texture depth and grooving of Portland cement concrete surfaces, are considered In the process of applying PAVDRN. Porous asphalt pavement surfaces can also reduce He water film thickness and thereby contribute to the reduction of hydroplaning tendency and their presence can also be accounted for when using PAVDRN. Finally, PAVDRN also allows the design engineer to consider the effect of drainage appurtenances, such as slotted drain inlets. A complete description of the various elements that are considered In the PAVDRN program is illustrated In figure 40. A more complete description of the design process, the parameters used in the design process, and typical values for the parameters is presented In the "Proposed Design Guidelines for Improving Pavement Surface Drainage" (2) alla in Appendix A. fIN1)INGS The following findings are based on the research accomplished during the project, a survey of the literature, and a state-of-the-art survey of current practice. I. Model. The one~unensional mode} is adequate as a design tool. The simplicity and stability of the one~imensional mode} offsets any increased accuracy afforded by a two-d~mensional model. The one~mensional model as a predictor of water fiDn thickness and How path length was verified by using data from a previous study (11). 121
No. of Planes Length of Plane Grade Step Increment Wdth of Plane Cross Slope Section T,rne 1) Tangent 2) Honzontal Curare 3) Transition 4) Vertical Crest 5) Vertical Sag U=tS 1)U.S. 2) S. I. Rainfall Intenstity ~ , \ |Kinematic Viscosity |Design Speed Note: PC = Point of Curvature PI. = Point of Tangency PCC = Portland cement concrete WAC = Dense graded asphalt concrete 0GAC = 0pcn~raded asphalt concrete where OGAC includes all types of intentally draining asphalt surfaces GPCC = Grooved Ponland cement concrete Taneent Pavement Type Mean Texture Depth 1) PCC 2) DGAC 3) OGAC 4) GPCC Horizontal Cun~c Grade Cross Slope Radius of Cunran~re Wdth Pavement Type _ 2) DGAC 3) OGAC 4) GPCC Mean Texture Depth Step Increment _ Transition Length of Plane Super Elevation Tangent Cross Slope Tangent Grade width of Curve Transition Width Pavement Type_ 1) PCC 3) OGAC 4) GPCC Mean Texture Depth Step Increment Horizontal Length Cross slope width PC Grade PI' Grade Elevation: Pr-PC Vertical Crest Flow Direction Step Increment Pavement Type 1) PC Side I 2) PI. Side | 1)PCC 2) DGAC 3) OGAC 4) GPCC Mean Tex~rc Depth _ _ ~ Figure 40. Factors considered in PAVDRN program. 122 ~1 r - . , Vertical Sad | Horizontal Length | Cross slope Wldth PC Grade PI Grade Elevation: PIE Flow Direction Step Increment / Stored :_ ~ cats ~ 1) PC Side | 2) PI Side | . Pavement Typed 1) PCC 3) OGAC 14) GPCC Mean Texture Depth I I
~ Stored data V ~ 3 L IN1T For use with a second nut using data from the first run.) , 1 EPRINT (Echos input to output ) 1 CONVERT (Converts units to and from SI and English.) ~ , ADVP (Advances Page of output.) KINW (Calculates Minning's n, Water Film Thickness (WEIR), and Hydroplaning Speed UPS).) , EDGE (Determines if flow has reached the edge of the pavement.) out roar Figure 40. Factors considered in PAVDRN program (continued). 123
2. Occurrence of Hydropl~r g. In general, based on the PAVDRN mode! and the assumptions inherent in its development, hydroplaning can be expected at speeds below roadway design speeds if the length of the flow path exceeds two lane widths. 3. Water Film Thickness. Hydroplaning is initiated primarily by the depth of the water film thickness. Therefore, the primary design objective when controlling hydroplaning must be to limit the depth of the water film. 4. Reducing Water Film Thickness. There are no simple means for controlling water John thickness, but a number of methods can effectively reduce water film thickness and consequently hydroplaning potential. These include: Optimizing pavement geometry, especially cross-slope. Providing some means of additional drainage, such as use of grooved surfaces (PCC) or porous mixtures (HMA). Including slotted drains within the roadway. 5. Tests Needed for Design. The design guidelines require an estimate of the surface texture (MTD) and the coefficient of permeability Porous asphalt only). The sand patch is an acceptable test method for measuring surface texture, except for the more open (20-percent air voids) porous asphalt mixes. In these cases, an estimate of the surface texture, based on tabulated data, is sufficient. As an alternative, 124
sand patch measurements can be made on cast replicas of the surface. For the open mixes, the glass beads flow into the voids within the mixture, giving an inaccurate measure of surface texture. Based on the measurements obtained In the laboratory, the coefficient of permeability for the open-graded asphalt concrete does not exhibit a wide range of values, and values of k may be selected for design purposes from tabulated design data (k versus air voids). Given the uncertainty of this property resulting from compaction under traffic and clogging from contaminants and anti-skid material, a direct measurement (e.g., drainage lag permeameter) of k is not warranted. Based on the previous discussion, no new test procedures are needed to adopt the design guidelines developed during this project. 6. Grooving. Grooving of PCC pavements provides a reservoir for surface water and can facilitate the removal of water if the grooves are placed parallel to the flow oath. Parallel orientation is generally not practical because the flow on highway pavements is typically not transverse to the pavement. Thus, the primary contribution offered by grooving is to provide a surface reservoir unless the grooves comlect with drainage at the edge of the pavement. Once the grooves are filled with water, the tops of the grooves are the datum for the Why and do not contribute to the reduction in the hydroplaning potential. 125
7. Porous Pavements. These mixtures can enhance the water removal and Hereby reduce water film tHch~ess. They merit more consideration by highway agencies In the United States, but they are not a panacea for eliminating hydroplaning. As with grooved PCC pavements, the internal voids do not contribute to the reduction of hydroplaning; based on the field tests done In this study. hv~ronImiina can be if, , , ~ expected on these mixtures given sufficient water fiLn thickness. Other than their ability to conduct water through internal flow, the large MTD offered by porous asphalt is the main contribution offered by the mixtures to the reduction of hydroplaning potential. The high-void ~ > 20 percent), modified binder mixes used In Europe merit further evaluation in the United States. They should be used In areas where damage from freezing water and the problems of black ice are not likely. 8. Slotted Drains. These fixtures, when installed between travel lanes, offer perhaps the most effective means of controlling water film thickness from a hydraulics standpoint. They have not been used extensively In the traveled lanes and questions remain unanswered with respect to their installation (especially in rehabilitation situations) and maintenance. The ability to support traffic loads and still maintain surface smoothness has not been demonstrated and they may be susceptible to clogging from roadway debris, ice, or snow. 126
RECOMMENDATIONS AND CONCLUSIONS The following recommendations are offered based on the work accomplished during this project and on the conclusions given previously: I. Implementation. The PAVDRN program and associated guidelines need to be field tested and revised as needed. The program and the guidelines are sufficiently complete so that they can be used in a design office. Some of the parameters and algorithms will I~ely need to be modified as experience is gained with the program. 2. Database of Material Properties. A database of material properties should be gathered to supplement the information contained in PAVDRN. This information should Include typical values for the permeability of porous asphalt and topical values for the surface texture (MTD) for different pavement surfaces to include toned Portland cement concrete surfaces. A series of photographs of typical pavement sections and their associated texture depths should be considered as an addition to the design guide (21. 3. Pavement Geometry. The AASHTO design guidelines (~) should be re-evaluated In terms of current design criteria to determine if they can be modified to enhance drainage without adversely affecting vehicle handling or safety. ~27
4. Use of appurtenances. Slotted drams should be evaluated In the field to determine if they are practical when Installed In the traveled way. Manufacturers should reconsider the design of slotted drains and their Installation recommendations currently In force to maximize them for use In multi-lane pavements and to determine if slotted drains are suitable for installations In the traveled right of way. 5. Porous Asphalt Mixtures. More use should be made of these mixtures, especially the modified high a~r-void mixtures as used In France. Field trials should be conducted to monitor HPS and the long-term effectiveness of these mixtures and to validate the MPS and WDT predicted by PAVDRN. 6. Two-D~mensional Model. Further work should be done with two~mensional models to determine if they improve accuracy of PAVDRN and to determine if they are practical from a computational standpoint. ADDITIONAL STUDIES On the basis of the work done during this study, a number of additional items warrant furler study. These Include: 1. Full-scale skid resistance studies to validate PAVDRN in general and the relationship between water film thickness and hydroplaning potential in particular are needed in light of the unexpectedly low hvdronlanin~ speeds predicted during 128 , . ~. , ~
this study. The effect of water infiltration into pavement cracks and loss of water by splash and spray need to be accounted for In the prediction of water fihn Sickness. Surface Irregularities, especially rutting, need to be considered in the prediction models. 2. Field trials are needed to confirm the effectiveness of alternative asphalt and Portland cement concrete surfaces. These include porous Portland cement concrete surfaces, porous asphalt concrete, and various asphalt m~cro-surfaces. 3. The permeability of porous surface mixtures needs to be confirmed with samples removed from the field, and the practicality of a simplified method for measuring in-situ permeability must be investigated and compared to alternative measurements, such as the outflow meter. 4. For measuring pavement texture, alternatives to the sand patch method should be investigated, especially for use with porous asphalt mixtures. 129
THIS PAGE INTENTIONALLY LEFT BLANK
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
- Open access
- Published: 16 January 2023
Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity
- Stephanie Cowan ORCID: orcid.org/0000-0001-6731-4221 1 ,
- Siew Lim 2 ,
- Chelsea Alycia 1 ,
- Stephanie Pirotta 3 ,
- Rebecca Thomson 4 ,
- Melanie Gibson-Helm 1 , 5 ,
- Rebecca Blackmore 6 ,
- Negar Naderpoor 1 ,
- Christie Bennett 7 ,
- Carolyn Ee 8 ,
- Vibhuti Rao 8 ,
- Aya Mousa 1 ,
- Simon Alesi 1 &
- Lisa Moran 1
BMC Endocrine Disorders volume 23 , Article number: 14 ( 2023 ) Cite this article
30k Accesses
29 Citations
29 Altmetric
Metrics details
Polycystic ovary syndrome (PCOS) is a common condition affecting reproductive-aged women with reproductive, metabolic and psychological consequences. Weight and lifestyle (diet, physical activity and behavioural) management are first-line therapy in international evidence-based guidelines for PCOS. While these recommend following population-level diet and physical activity guidelines, there is ongoing interest and research in the potential benefit of including psychological and sleep interventions, as well as a range of traditional, complimentary and integrative medicine (TCIM) approaches, for optimal management of PCOS. There is limited evidence to recommend a specific diet composition for PCOS with approaches including modifying protein, carbohydrate or fat quality or quantity generally having similar effects on the presentations of PCOS. With regards to physical activity, promising evidence supports the provision of vigorous aerobic exercise, which has been shown to improve body composition, cardiorespiratory fitness and insulin resistance. Psychological and sleep interventions are also important considerations, with women displaying poor emotional wellbeing and higher rates of clinical and subclinical sleep disturbance, potentially limiting their ability to make positive lifestyle change. While optimising sleep and emotional wellbeing may aid symptom management in PCOS, research exploring the efficacy of clinical interventions is lacking. Uptake of TCIM approaches, in particular supplement and herbal medicine use, by women with PCOS is growing. However, there is currently insufficient evidence to support integration into routine clinical practice. Research investigating inositol supplementation have produced the most promising findings, showing improved metabolic profiles and reduced hyperandrogenism. Findings for other supplements, herbal medicines, acupuncture and yoga is so far inconsistent, and to reduce heterogeneity more research in specific PCOS populations, (e.g. defined age and BMI ranges) and consistent approaches to intervention delivery, duration and comparators are needed. While there are a range of lifestyle components in addition to population-recommendations for diet and physical activity of potential benefit in PCOS, robust clinical trials are warranted to expand the relatively limited evidence-base regarding holistic lifestyle management. With consumer interest in holistic healthcare rising, healthcare providers will be required to broaden their knowledge pertaining to how these therapies can be safely and appropriately utilised as adjuncts to conventional medical management.
Peer Review reports
Introduction
Polycystic ovary syndrome (PCOS) is a common condition affecting up to 13% of reproductive-aged women [ 1 ]. It is diagnosed through the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESRHE/ASRM) criteria, requiring two of the following features: polycystic ovaries on ultrasound, oligoovulatory or anovulatory cycles and biochemical or clinical hyperandrogenism [ 2 ]. Women with PCOS experience a combination of reproductive (infertility, pregnancy complications) [ 3 ], metabolic (risk factors for and conditions of type 2 diabetes (T2DM) and cardiovascular disease (CVD)) [ 4 , 5 ] and psychological (conditions including anxiety, depression, poor quality of life (QoL), disordered eating) comorbidities [ 6 , 7 ].
Insulin resistance (IR) is defined as a key pathophysiological feature in PCOS, contributing to hyperandrogenism and worsening the clinical presentation of PCOS. While lean women present with IR in a form that is mechanistically different from IR caused by excess weight, overweight and obesity further exacerbate IR and consequent hyperinsulinaemia [ 8 ]. Women with PCOS also display a higher rate of weight gain over time [ 9 ] and a greater prevalence of overweight and obesity [ 10 ], which can further contribute to this worsening of IR and hence worsening of the presentation of PCOS [ 11 ]. The reason for this is unclear, but may be related to differences in intrinsic psychological and biological mechanisms [ 12 , 13 , 14 , 15 ], or extrinsic lifestyle factors such as diet and physical activity [ 16 , 17 ]. Improving IR and excess adiposity are therefore key targets in PCOS management.
The International Evidence-Based Guideline for the Assessment and Management of PCOS [ 18 ], highlights lifestyle intervention as the primary early management strategy. Lifestyle interventions are traditionally defined as those designed to improve dietary intake or physical activity through appropriate behavioural support. In the 2018 PCOS guideline, lifestyle management is recommended for general health benefits [ 18 ]. Given that excess weight is associated with increased IR in PCOS [ 8 ], the guideline additionally promotes weight management, defined as: 1) weight gain prevention in all women with PCOS, and 2) achieving and maintaining modest weight loss in women with excess weight [ 18 ].
Lifestyle interventions in PCOS management can also be viewed as a broader construct beyond physical health. Since the emergence of the biopsychosocial model of healthcare in 1977, health disciplines have seen a gradual shift away from the classical biomedical model (where health is defined as the ‘absence of disease’) towards whole person or holistic care [ 19 ]. This is an approach that reflects many facets of the patient context, via integrating care that addresses biological, psychological, social, spiritual and ecological aspects [ 20 ]. It therefore requires a range of different treatment strategies to improve health. Provision of whole person or holistic care has been identified as a core objective of healthcare reforms internationally [ 21 , 22 , 23 ]. In line with these reforms the PCOS guideline recognises the importance of emotional wellbeing to overall health and QoL in women living with PCOS [ 18 ]. It also highlights evidence which suggests that the psychological impact associated with PCOS is under-appreciated in clinical care [ 4 , 5 ], and that few women are satisfied with the mental health support they receive [ 6 , 7 ]. Recommendations for appropriate screening, assessment and treatment strategies for anxiety, depression, psychosexual dysfunction, eating disorders and poor body image are provided [ 18 ]. These specific areas of emotional wellbeing are of particular concern, with research showing a higher prevalence and severity of depression and anxiety [ 24 , 25 ], lower scores for satisfaction with sex life and feeling sexually attractive [ 26 ] and a higher prevalence of disordered eating and eating disorders [ 7 ] in women with PCOS. Features of PCOS, in particular hirsutism and increased weight, have also been shown to negatively affect body image [ 27 , 28 ], with poor body image being strongly related to depression in women with PCOS [ 29 , 30 ].
While the current PCOS guideline is comprehensive, considering all available evidence at the time of development and providing best-practice recommendations for necessary screening, risk assessment and management, it could not possibly cover all aspects of PCOS care. An International Delphi process was used to prioritise clinical questions, with consensus reached through extensive consultation with both consumers and multidisciplinary clinicians with expertise in PCOS care. Therapies, such as traditional, complementary and integrative medicine (TCIM), supplement use, sleep and meditation interventions are either briefly considered or not at all included in the 2018 PCOS guideline. Many of these therapies are novel and there is a paucity of evidence to support intervention efficacy on PCOS outcomes. However, as patient interest in these types of non-pharmacological interventions are growing [ 31 , 32 , 33 , 34 , 35 ], it is prudent to provide more guidance to healthcare providers in this area on their potential efficacy in PCOS. Whole person or holistic care recognises that the doctor-patient relationship should be one of open dialogue, where healthcare providers involve the patient in negotiating their care and recognises patient’s autonomy to guide treatment (Figure 1 ) [ 36 ].
Viewing lifestyle modifications through a whole person or holistic care lens. The key features of whole person or holistic care listed in the centre of the figure have been adapted from Thomas et al. [ 20 ]. ‘Recognises individual personhood’ relates to focusing on the unique needs of the person rather than the disease. ‘Importance of therapeutic relationship’ emphasises patient autonomy and responsibility. ‘Acknowledges humanity of the doctor’ considers the doctors’ ability to self-reflect on how they engage in the care of the patient. ‘Health as more than absence of disease’ incorporates the mental, emotional, physical, environmental and social needs of the patient. ‘Employs a range of treatment modalities’ promotes continuity of care across health disciplines, and while it may include traditional, complementary and integrative medicine (TCIM), TCIM is not holistic if used in isolation and without adequate integration into conventional healthcare
This review provides an extensive overview of evidence to date on lifestyle strategies used to optimise management of PCOS. Using a holistic definition of patient care, this review considers the traditional components of lifestyle change (diet, physical activity and behavioural change), psychological and sleep interventions, as well as TCIM approaches (supplements, herbal medicine, acupuncture and yoga). To improve translation of findings, evidence summaries are accompanied by an overview of relevant recommendations from the existing PCOS guideline. This highlights where emerging evidence supports current recommendations or provides new insights for research. As this is a narrative review, while evidence summaries include peer-reviewed journal articles identified from databases including Medline OVID, this is supplemented by expert opinion of the authors.
Traditional lifestyle and weight management
The PCOS guideline recommends the promotion of healthy lifestyle behaviours in all women with PCOS, to achieve and/or maintain a healthy weight and to optimise general health [ 18 ]. In women with excess weight, a weight loss of 5-10% is advised, aiming for an energy deficit of 30% or 500-750 kcal/day (1200-1500 kcal/day). While weight management is seen as a core component of lifestyle interventions, the guideline recognises that a healthy lifestyle provides benefits that occur independent of weight change.
A recent Cochrane review of 15 randomised controlled trials (RCT) and 498 participants, reported that lifestyle interventions compared with minimal intervention or usual care, significantly reduces weight (kg) and body mass index (BMI) and improves secondary reproductive outcomes such as free androgen index (FAI), testosterone (T), sex hormone-binding globulin (SHBG) and hirsutism (Ferriman-Gallwey score) [ 37 ]. In terms of metabolic outcomes, lifestyle intervention resulted in significant reductions in total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and fasting insulin (FINS). These findings are largely similar to that of other systematic reviews [ 38 , 40 , 41 , 41 ]. While no studies in the Cochrane review assessed clinical reproductive outcomes [ 37 ], individual trials that were not included in the review have reported that lifestyle interventions resulting in modest weight loss (2-5% total body weight) improve ovulation and menstrual regularity [ 42 , 44 , 45 , 45 ]. Losing >5% of weight is additionally associated with being able to conceive, having live births, reduction of ovarian volume and reduction in the number of follicles [ 46 , 48 , 49 , 50 , 51 , 52 , 52 ].
Although weight loss has shown clear benefits to PCOS outcomes, including not only on reproductive function, but also glucoregulatory status, androgen status and lipid profiles [ 42 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 52 ], there are varying degrees of responsiveness to weight loss in terms of improvement of PCOS symptoms. One study by Pasquali et al. [ 53 ] found that when women achieved similar levels of weight loss (>5% weight) only one-third displayed a full recovery from PCOS, with the remainder showing only partial or no recovery. Higher waist circumference (WC), waist-hip-ratio (WHR) and androstenedione at baseline were associated with a poorer chance of successful outcomes [ 53 ], suggesting that central adiposity and more severe hyperandrogenism may predict responsiveness to weight loss interventions in PCOS. Huber-Bucholz et al. [ 45 ] also reported women who achieve greater reductions in central fat and insulin sensitivity show greater symptom improvement with weight loss. This suggests that lifestyle interventions which simultaneously reduce IR and improve body composition (namely fat distribution), may help to optimise outcomes in PCOS management independent of changes in weight status.
The 2018 PCOS guideline recognises there is insufficient evidence to suggest that any specific dietary approaches provide greater benefits on health outcomes [ 18 ]. Dietary recommendations may take on a variety of balanced dietary strategies according to the individual’s lifestyle needs and preferences, as per general population recommendations [ 18 ]. This advice is based on a systematic review comparing different dietary compositions (e.g. low carbohydrate, low glycaemic index (GI) and glycaemic load (GL), high protein, monounsaturated fatty acid (MUFA) enriched and fat counting diets) to best manage PCOS, identifying minimal differences between diets on anthropometric outcomes, concluding weight loss improves the presentation of PCOS regardless of dietary composition [ 16 , 54 ]. There is now an emerging body of evidence that suggests a range of dietary strategies may produce favourable effects on PCOS features that occur independent of weight loss. It is important that the emerging findings from these studies are thoroughly considered to support consumer and health professional interests. To summarise current evidence this review has grouped diets in terms of those that modify carbohydrates, protein and fat, as well as specific dietary patterns.
Carbohydrates
The use of altered carbohydrate composition remains the most researched dietary approach for PCOS management. Two systematic reviews published after guideline inception support altered carbohydrate intake to improve intermediate markers of PCOS [ 55 , 56 ], finding that altering carbohydrate type, as opposed to content, is preferable to better manage PCOS [ 55 ]. RCTs [ 57 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 72 ] and pre-post intervention studies [ 73 , 75 , 76 , 77 , 78 , 79 , 80 , 80 ] demonstrate that following a low GI/GL diet for at least eight weeks significantly reduces WC [ 55 , 73 , 74 ] and BMI when compared to high GI/GL [ 56 ] or a regular diet [ 73 , 75 , 76 , 76 ], although levels of weight loss are generally comparable to other dietary compositions [ 59 , 60 , 72 , 74 ]. These reductions are proposed to be a result of decreased hunger, which may reduce energy intake and make it easier to follow dietary recommendations in the long-term [ 78 , 81 , 83 , 84 , 84 ]. Low GI/GL diets also improve insulin sensitivity and reproductive hormones (T, SHBG, FAI) compared to high carbohydrate [ 16 , 55 , 57 , 79 , 85 ] or control diets [ 56 , 59 , 73 , 75 , 76 , 76 ], contributing to improvements in reproductive function, specifically menstrual regularly [ 60 , 79 ]. Lastly, low GI/GL diets can improve risk factors for T2DM and CVD, including glucose [ 86 , 87 ], TC [ 55 , 56 , 59 , 75 , 77 ], LDL-C [ 55 , 59 , 75 , 85 ], TAG [ 55 , 59 , 73 ] and HDL-C [ 75 ], when compared to a regular or high GI/GL diet. It must be noted that beneficial effects of low GI/GL diets may also be attributed to proportional increases in protein and/or fat loads.
In women with PCOS higher protein intakes may be superior at supressing androgen levels when compared to high carbohydrate diets. Postprandial research has shown that high protein meals can reduce insulin and dehydroepidiandrosteone stimulation compared to meals rich in glucose [ 88 ]. Research in the general population has also shown that reduced appetite and energy intakes from low GI/GL diets are related to increased protein intakes [ 89 , 90 ]. RCTs and pre-post intervention studies found that high protein diets (defined here as protein constituting ≥25% energy [ 91 ]) consumed for at least four weeks reduce weight [ 12 , 73 , 74 , 92 , 94 , 95 , 96 , 96 ], BMI [ 73 , 74 , 92 , 95 ], WC [ 73 , 74 , 92 , 97 ], WHR [ 73 ] and fat mass [ 74 , 92 , 97 ]. These reductions in anthropometric measures are accompanied by improved FINS [ 12 , 74 , 95 , 98 ] and HOMA-IR [ 12 , 73 , 95 , 98 ], blood lipids [ 12 , 96 ], T [ 73 , 92 , 94 ] and hirsutism (Ferriman-Gallwey score) [ 73 ]. However, only three of these studies were able to show significant improvements in anthropometric measures [ 97 ], insulin sensitivity [ 98 ] and blood lipids [ 12 ] when compared to low/standard protein [ 12 , 97 ] or control diets [ 98 ]. Only one study investigated effects on mental health outcomes and found that a high protein diet reduced depression and improved self-esteem [ 99 ].
Fatty acid composition is also an important consideration as metabolic disorders associated with PCOS can benefit from increased MUFA and polyunsaturated fatty acid (PUFA) intakes [ 63 , 65 , 65 ]. Postprandial research in PCOS reported prolonged reductions in T for high fat compared to low fat meals, which likely results from delayed nutrient absorption [ 86 ]. Two acute meal studies in lean and obese women with and without PCOS reported that proatherogenic inflammatory markers [ 100 ] and oxidative stress [ 101 ] were elevated, independent of but augmented by obesity, following saturated fat ingestion with this associated with worsened IR and androgens. Two experimental studies in PCOS investigated the effects of habitual walnut (PUFA rich diet) [ 102 , 103 ] and almond (MUFA rich diet) [ 102 ] intake for at least six weeks and reported no differences in glucoregulatory status, lipids or androgens with the exception of HbA1c significantly decreasing in the walnut relative to the almond group. Kasim-Karakas et al. [ 103 ] reported increased fasting and postprandial glucose (oral glucose tolerance test (OGTT)) for increased walnut intake compared to habitual (control), which they postulated may be related to the control diet being rich in oleic acid. Together these findings suggest minimal benefit for improving dietary PUFA compared to MUFA content. Two RCTs in women with PCOS investigated the effects of diets rich in olive [ 104 , 105 ], canola [ 105 ] and sunflower [ 105 ] oil. Yahay et al. [ 105 ] reported 25g/day canola oil caused reductions in TAG, TC/HDL-C, LDL-C/HDL-C, TAG/HDL-C and HOMA, but not androgens, compared to 25 g/day olive and sunflower oils [ 105 ]. This may be related to the more favourable fatty acid composition of canola oil, with comparable MUFA content to olive oil, higher alpha-linolenic acid, lower omega-6/omega-3 ratio and saturated fat than both olive and sunflower oils. Douglas et al. [ 104 ] reported weight and the acute insulin response (OGTT) were lower following a eucaloric low carbohydrate compared to a eucaloric MUFA-enriched olive oil diet, suggesting that reduced carbohydrate intake may have grater glucoregulatory benefits than increased MUFA intake [ 104 ]. Lastly, two RCTs compared hypocaloric low-fat diets to a low carbohydrate [ 106 ] or low GI [ 107 ] diets, with reductions in weight [ 106 ], WC [ 106 ], body fat [ 106 , 107 ], FINS [ 106 ] and FAI [ 106 ] in both groups but no difference between groups.
Dietary and eating patterns
In addition to diets that focus on specific macronutrient manipulations, there are a range of dietary patterns which have been explored in PCOS management. A systematic review (including 19 studies and 1,193 participants) published after guideline development (2020) found that the Dietary Approaches to Stop Hypertension (DASH) diet (rich in fruit, vegetables, wholegrains, nuts, legumes and low-fat dairy and with a predominantly low-GI carbohydrate profile) was the optimal choice for reducing IR [ 85 ]. RCTs in PCOS also report beneficial effects on weight [ 63 , 64 ], BMI [ 62 , 63 ], IR [ 62 ] and hormonal profile, including SHBG [ 64 ], androstenedione [ 64 ] and FAI [ 62 ] for a DASH compared to a control diet after 8-12 weeks. A vegetarian diet also reduced inflammatory markers (CRP, resistin and adiponectin) compared to a meat inclusive diet [ 80 ]. A vegan diet improved weight loss at three, but not six months [ 68 ], and a pulse-based diet led to similar reductions in weight, insulin sensitivity and reproductive hormones compared to a healthy control diet [ 72 ]. All of these dietary patterns are high in fibre and plant proteins, producing favourable effects on microbial diversity and encouraging production of short-chain fatty acids that possess potential anti-inflammatory actions [ 108 , 109 ]. With mechanistic animal studies suggesting a possible pathophysiological role of gut microbiota in IR and ovarian dysfunction, it is possible that metabolic and hormonal benefits associated with plant-based dietary patterns in PCOS are related to increased intakes of dietary prebiotics [ 110 ]. However, further mechanistic studies exploring the role of gut microbiota in PCOS and RCTs investigating effects of dietary prebiotics on PCOS outcomes are required.
Lastly, particular eating patterns, such as eating smaller more frequent meals across the day [ 111 ] and eating a larger breakfast and smaller dinner [ 66 ], have also been found to be beneficial for insulin sensitivity [ 66 , 111 ] and androgen reductions [ 66 ]. This is an important finding, as women with PCOS are more likely to either skip breakfast or consume their breakfast and lunch later in the day [ 112 ].
Studies examining specific food items in relation to PCOS outcomes, including raw onions [ 65 ], concentrated pomegranate juice [ 69 , 113 , 115 , 115 ] and flaxseed powder [ 70 , 116 ] have yielded largely inconsistent results. A core limitation of these single food studies is that foods are never consumed alone within the diet, omitting the influence of the dietary matrix and the interactions that occur amongst dietary constituents within meals. These studies provide limited applicability in the context of formulating practical dietary recommendations [ 117 ]. Please see Table 1 for a summary of available evidence from reviews and experimental studies investigating the effects of different types of diets on PCOS outcomes.
Physical activity
The 2018 PCOS guideline recommends ≥150 minutes per week of moderate or ≥75 minutes per week of vigorous intensity exercise for weight gain prevention, and ≥250 minutes per week of moderate or ≥150 minutes per week of vigorous intensity exercise for weight loss and weight regain prevention [ 18 ]. Minimising sedentary time and the inclusion of strength training exercise for two days per week is also recommended [ 18 ].
To date the most comprehensive review in PCOS (including 27 papers from 18 trials up until June 2017) reported that exercise improved FINS, HOMA-IR, TC, LDL-C, TAG, body composition (body fat percentage and WC) and aerobic fitness (VO 2max ) [ 119 ] compared with usual care or control groups. In regards to exercise type, subgroup analysis reported aerobic exercise improved BMI, WC, body fat percentage, FINS, HOMA-IR, TC, TAG and VO 2max . In contrast, while resistance training produced unfavourable effects on HDL-C (decrease) and BMI (increase), it improved other measures of anthropometry, including WC. Combined interventions (using both aerobic and resistance training) had no effect on any of the measured markers. Subgroup analysis also found that more outcomes improved when interventions were supervised, of a shorter duration (≤ 12 weeks) and were conducted in women who were above a healthy weight [ 119 ].
Three more recent systematic reviews have looked at the effects of specific types of exercise on PCOS outcomes [ 120 , 122 , 122 ]. These reviews found that vigorous aerobic exercise can improve measures of insulin responsiveness and resistance, including HOMA-IR [ 121 ] and the insulin sensitivity index [ 120 ]; body composition, including WC [ 121 ] and BMI [ 122 ]; and cardiorespiratory fitness (VO 2max ) [ 121 ]. High intensity interval training (HIIT) alone may be effective for improving IR and BMI [ 123 ], however this has not been consistently shown [ 124 ]. Interventions involving a combination of aerobic and resistance exercise [ 122 ] or resistance training only [ 120 ] did not result in improvements in BMI [ 122 ] or weight status [ 120 ]. Exercise involving resistance training did result in other beneficial improvements to body composition (reduced body fat, WC and increased lean mass) and strength. This is important, as the degree of central adiposity predicts responsiveness to weight loss interventions in PCOS [ 53 ], and women who achieve greater reductions in central fat show greater symptom improvement with weight loss [ 45 ]. Resistance training may also improve androgen levels, though findings are inconsistent and more research is needed to draw definite conclusions [ 120 ]. There was insufficient evidence from available data to assess the effects of exercise type on reproductive function [ 122 ]. Please see Table 2 for a summary of available evidence from meta-analyses investigating the effects of different types of exercise on PCOS outcomes.
When comparing the effects of exercise and diet combined with diet alone, a systematic review and meta-analysis (three studies) found no differences for any measured outcomes (glucose, insulin HOMA-IR, weight, BMI, WC, body fat, fat free mass, T, SHBG and FAI) [ 119 ]. In regards to exercise and diet combined compared to exercise alone, subgroup analysis (including 17 studies) from a large systematic review found that the addition of diet to exercise, particularly vigorous intensity aerobic exercise, resulted in greater reduction to BMI, WC, FAI and HOMA-IR than exercise only [ 121 ]. In regards to exercise (aerobic) alone versus diet alone, one intervention study found that exercise induced weight loss produced greater improvements in menstrual frequency and ovulation rates [ 125 ], with no differences in pregnancy rates [ 125 ]. However, this study was not randomised and treatments were self-selected, which may have biased the results and precludes firm conclusions [ 125 ].
Behavioural
The 2018 PCOS guideline promotes the use of behavioural interventions that foster self-efficacy [ 18 ]. These include the use of SMART (specific, measurement, achievable, realistic and timely) goals, self-monitoring, stimulus control, problem solving and relapse prevention [ 18 ].
Behavioural and cognitive interventions are required to improve sustainability of lifestyle changes, through considering not only the specific behaviour, but also their antecedents, consequences and cognition [ 126 , 127 ]. Given that women with PCOS show higher rates of weight gain over time [ 9 ] and high attrition rates in clinical weight management research [ 37 ], there is a clear need to improve adherence to diet and physical activity interventions. However, the majority of research investigating lifestyle change in PCOS involve short-term dietary interventions with/without an exercise element, and there is a paucity of research on behavioural change strategies. As such, guideline development relied heavily on evidence taken from the general population. Only three RCTs in women with PCOS included a ‘behavioural intervention’ [ 128 , 130 , 130 ]. While these studies showed enhanced weight loss [ 128 , 130 ] and improved androgen and lipid profiles [ 129 ] when compared with placebo, the interventions were not well defined, with negligible context provided regarding the theoretic framework or behavioural strategies utilised.
More recently, a cross-sectional study in 501 women with PCOS [ 131 ] and two RCTs [ 44 , 132 ] explored the use of self-management strategies [ 131 ] and behavioural modification interventions [ 44 , 132 ] in PCOS. In the cross-sectional study, implementation of physical activity self-management strategies improved the likelihood of meeting physical activity recommendations, but had no association with BMI. Dietary self-management strategies were associated with reductions in BMI, though were not related to weight or nutritional intake [ 131 ]. In the RCTs, only the behavioural modification programme and not the control (general healthy lifestyle recommendations) produced significant weight loss after four months. A significantly greater proportion of women in the intervention group also improved menstrual regularity [ 44 ] and psychological well-being (lower anxiety and depressive symptoms) [ 132 ] when compared to the control group. The women who achieved greater weight loss reported higher social desirability and lower embitterment scores on a personality trait assessment measure [ 132 ]. These findings are particularly novel, as they provide insight into the influence of personality traits and their contribution to success in following behavioural modifications [ 132 ].
Alcohol and smoking
In the clinical setting, smoking and alcohol consumption are often addressed alongside dietary and physical activity changes, employing the same behavioural and cognitive interventions to promote adherence. Hence, alcohol and cigarette use are considered here under traditional lifestyle strategies. The PCOS international guideline highlights the importance of assessing alcohol consumption and cigarette smoking when improving fertility and reproductive outcomes in women with PCOS [ 18 ]. Assessment of cigarette use is also recommended when evaluating CVD risk factors and thromboembolism risk associated with oral contraceptive pills [ 18 ]. These recommendations are based on existing practice guidelines used for the general population.
There is a paucity of observational research characterising alcohol consumption in women with PCOS. One Swedish study comparing women with PCOS ( n =72) to healthy controls ( n =30), demonstrated a lower alcohol intake in the PCOS group [ 133 ]. A larger study in Australia comparing women with ( n =409) and without ( n =7,057) PCOS, reported no significant difference in alcohol intake [ 134 ]. Similarly, a Spanish study ( n =22 PCOS and n =59 controls) and a Chinese study ( n =2,217 PCOS and n =279 controls), found no significant difference in alcohol intake between PCOS and non-PCOS groups [ 135 , 136 ].
Current evidence on the impact of alcohol intake on anovulatory infertility (a common feature of PCOS) is controversial, with some studies showing adverse effects and others reporting no significant correlation [ 136 , 137 ]. One prospective study including 18,555 married women from The Nurses’ Health Study II, who had no history of infertility, found no clinically significant impact of alcohol intake on anovulatory infertility, after adjusting for parity and other factors [ 138 ]. Similarly, a Danish study ( n =6,120 women aged 21 to 45 years) found no fertility effect with alcohol consumption of less than 14 standard drinks per week [ 137 ]. In contrast, a study on 3,833 women who recently gave birth and 1,050 women with infertility, reported an increased risk of anovulatory infertility and endometriosis with increasing alcohol intake [ 139 ].
Current observational evidence does not reveal any significant difference in smoking between women with and without PCOS [ 135 , 136 , 140 ], with the exception of one study in pregnant women which showed a lower smoking rate in women with PCOS ( n =354) compared to women without PCOS at 15 weeks gestation [ 3 ]. However, a significantly higher rate of smoking (including passive and active) is reported in women with PCOS and oligo-anovulation and/or reduced fertility compared to women with PCOS and normal menstruations or healthy controls [ 141 , 142 ]. Smoking is also associated with PCOS risk independent of BMI and age [ 142 ]. A Mendelian randomisation study supports these findings, demonstrating a 38% higher risk of PCOS development in genetically predicted smokers (based on single-nucleotide polymorphisms associated with smoking initiation) compared with those who never smoked [ 143 ]. In PCOS, smoking is associated with increased levels of T, DHEAS, TC, LDL-C and FINS [ 141 , 144 , 145 ]. However, the underlying mechanisms are not fully understood and there are inconsistencies in findings from different studies. Furthermore, smoking is associated with lower conception and live birth rates and less favourable ART outcomes in women with PCOS [ 141 , 146 ].
Psychological
The current guideline highlights the need for awareness, and appropriate assessment (such as stepwise screening) and management, of QoL, depression and anxiety, psychosexual dysfunction, negative body image and disordered eating [ 18 ]. The guideline emphasises the importance of clinicians and women working in partnership to address women’s individual priorities; understanding that the impact of PCOS on an individual’s QoL is key to delivering meaningful outcomes [ 147 , 148 ]. To assist women to communicate with clinicians about what is important to them, the PCOS Question Prompt List [ 149 ] was developed and is consistent with the 2018 guideline. The 2018 guideline recommends screening for risk factors and symptoms of depression and anxiety at time of diagnosis. Women with positive screening results should be supported with further assessment and treatment by appropriately qualified clinicians. To screen for psychosexual dysfunction tools such as the Female Sexual Function Index [ 150 ] should be utilised. If negative body image, disordered eating or eating disorders are suspected, the PCOS guideline outlines a stepped approach for screening, and where appropriate promotes the use of psychological therapy offered by trained health professionals, which should be guided by regional clinical practice guidelines [ 18 ].
While the PCOS guideline provides justification and summarises evidence for mental health screening and diagnostic assessment, there is also a need for consideration of additional aspects, such as the efficacy of different types of psychological interventions and how psychological interventions influence engagement with lifestyle change. This is important, as poorer mental health outcomes at baseline are positively associated with higher rates of attrition in lifestyle interventions [ 13 ]. Cognitive behavioural interventions could be considered to improve engagement and adherence to healthy lifestyle in women with PCOS. Research has shown support for a range of different psychological interventions, such as counselling [ 151 ], cognitive behavioural therapy (CBT) [ 152 , 154 , 154 ] and mindfulness meditation [ 155 , 156 ], helping to change the way clinicians’ approach and deliver optimal PCOS management.
CBT is one of the most widely-researched psychological interventions, and is well-recognised as the most effective psychological treatment for depression and anxiety [ 157 ]. One RCT showed that eight weekly group CBT sessions were effective in improving QoL ratings and reducing psychological fatigue in women with PCOS [ 152 ]. Another more recent RCT investigated the outcome of a 1 year three-component intervention focusing on CBT, diet and exercise [ 154 ] and reported improvements in self-esteem and depressive symptoms as compared to usual care [ 154 ]. Similarly, an RCT by Cooney et al. [ 153 ], comparing the effects of CBT and lifestyle modification versus lifestyle modification alone, reported the CBT/lifestyle modification group lost more than twice as much weight per week and had greater improvements in QoL compared to lifestyle only. Depression scores decreased in the overall group and there was no difference between the two groups [ 153 ]. Lastly, a pilot intervention study of adolescents with PCOS has shown promising results for the use of CBT in the reduction of weight and improvement in depressive symptoms [ 158 ].
Mindfulness meditation programs have gained increasing popularity over the past few decades, and are being included as part of clinical trials to reduce stress and improve psychological wellbeing across a range of medical conditions [ 159 ]. Mindfulness meditation can be used to reduce the production of adrenal androgens, activated via the adrenal glands as a direct result of psychological distress [ 156 ]. Despite the proposed benefits, there are very few studies investigating the use of mindfulness meditation as a treatment for psychological symptoms associated with PCOS. One RCT ( n =86) compared the provision of an eight week mindfulness-based stress reduction (MBSR) program, and found that when compared to the control group (health education), the MBSR group produced greater reductions in perceived stress, depressive symptoms and fasting blood glucose [ 160 ]. Similarly, another RCT investigating the impact of mindfulness meditation for eight weeks in PCOS showed reduced stress, depression and anxiety symptoms, and increased life satisfaction and QoL in the intervention group compared to no treatment [ 156 ]. In adolescents with PCOS ( n =37), a pilot RCT reported higher levels of nutrition and physical activity self-efficacy following a mindfulness and self-management program [ 161 ]. Mindfulness-based cognitive therapy (MBCT) combines both elements of MBSR and CBT, but as yet there are no trials investigating this intervention in PCOS.
In addition to CBT and mindfulness meditation, there is some evidence to support group counselling sessions as beneficial in conjunction with exercise programs to increase and support weight loss [ 151 ]. In one RCT ( n =17) participants followed a high-intensity aerobic exercise program for eight weeks, followed by eight weeks of group counselling [ 151 ]. Qualitative analysis of data taken from the group counselling and physical exercise sessions revealed that development of supportive relationships was important for successful behavioural change. By fostering the exchange of narratives relating to their illness (e.g. effects of PCOS on aspects of everyday life), and generating feedback between group members, counselling sessions helped to reduce social isolation and improve adherence to the exercise intervention [ 151 ]. Please see Table 3 for a summary of experimental studies investigating effects of psychological interventions on PCOS outcomes.
Women with PCOS have an increased risk of both clinical sleep disorders and non-clinical sleep disturbance, which is mediated by hormone derangement, in particular reduced oestrogen, progesterone and melatonin levels [ 164 ]. Oestrogen is required for the metabolism of neurotransmitters (norepinephrine and serotonin) involved in regulating sleep patterns, and plays an important role in maintaining a low body temperature at night [ 165 ]. Progesterone has sedative and anxiolytic actions that can support sleep quality, and acts as a respiratory stimulant that lessens airway resistance in obstructive sleep apnoea (OSA) [ 166 ]. Melatonin is a neuroendocrine hormone that is widely recognised as crucial in maintaining circadian rhythm regulation. However, melatonin is also involved in ovarian function, with actions including delaying ovarian senescence, promoting follicle formation and improving oocyte quality [ 167 , 169 , 170 , 171 , 172 , 173 , 173 ].
The current PCOS guideline recognises that OSA is 6.5-8.3 times more likely in women with PCOS [ 164 , 174 , 176 , 177 , 177 ], and promotes routine screening to identify and treat associated symptoms, such as snoring, excessive sleepiness and the potential for fatigue to worsen mood disorders [ 18 ]. Screening should include a simple questionnaire, such as the Berlin tool [ 178 ], and where appropriate women should be referred onto a specialist for further assessment and treatment [ 18 ]. The guidelines also highlight that treatment of OSA in PCOS should not be used to improve metabolic features. Since guideline inception evidence has emerged reporting weight, PCOS and sleep are interrelated factors that can each contribute to the worsening presentation of one another, whereby sleep disorders and disturbance may worsen the presentation of PCOS related metabolic outcomes and vice versa [ 179 ].
Hypersomnia and insomnia are also common clinical sleep disorders in PCOS [ 164 , 177 , 180 ], with prevalence estimated at 11% versus 1% in women with versus those without PCOS [ 180 ]. Even in the absence of clinically diagnosed sleep disorders, women with PCOS have a higher prevalence of sleep disturbances, including poor sleep quality [ 181 ], issues with sleep initiation [ 182 ], severe fatigue [ 140 ], restless sleep [ 140 ] and difficulty sleeping overnight [ 140 ]. The prevalence of sleep disturbances may be up to 20% higher in women with PCOS compared to women without PCOS [ 183 ]. Emerging research also suggests that social restrictions arising from the COVID-19 pandemic have worsened sleep disturbances in women with PCOS [ 177 ]. Findings from key studies of non-clinical sleep disturbance can be found in Table 4 .
In the general population short and disturbed sleep is consistently associated with excess weight [ 184 ], IR [ 185 ], T2DM [ 185 ] and CVD [ 186 ]. Similar relationships are observed in PCOS, where OSA and sleep disordered breathing exacerbates risk of IR and metabolic consequences of abnormal glucose tolerance [ 187 , 188 ]. A cross-sectional study in adolescents with PCOS ( n =103) reported those with sleep disordered breathing had significantly higher BMI Z-scores, and a higher prevalence of metabolic syndrome (METS) [ 188 ]. Similar metabolic consequences are seen in women with PCOS who suffer from non-clinical sleep disturbance [ 164 ]. Underlying mechanisms linking sleep disorders and disturbance with worsened metabolic outcomes include amplified sympathetic tone and oxidative stress [ 164 ], reduced adipose tissue lipolysis, and an increase in energy intake stemming from heightened hedonic and endocrine appetite signals [ 189 ].
Unfavourable effects on energy metabolism and appetite regulation, may explain why women with PCOS who display sleep disturbance have a reduced capacity to maintain dietary interventions [ 183 ]. Moreover, depression and anxiety share a bidirectional relationship with disrupted and reduced sleep [ 190 ], and as stated previously, interventions that improve mental health can help to increase engagement with dietary and physical activity recommendations [ 131 ]. Optimising sleep may therefore be an important consideration when promoting healthy lifestyle change in women with PCOS [ 183 ].
Traditional, complementary and integrative medicine
The 2018 PCOS guideline includes recommendations on inositol supplementation, though do not include evidence regarding the use of other supplements, herbal medicine or other TCIM approaches, including acupuncture and yoga [ 18 ].
Vitamins, vitamin-like supplements, minerals and other supplements
The 2018 guideline highlights that inositol (including myo-inositol (MI) and di-chiro inositol) is a nutritional supplement that may be involved in insulin signalling transduction [ 191 ]. MI in particular is a key endocrine regulator that displays impaired metabolism in PCOS [ 191 ]. MI supplementation has been explored in a meta-analysis of nine RCTs ( n =496), which showed improved metabolic profiles and reduced hyperandrogenism [ 191 ]. These findings are supported by two earlier meta-analyses, reporting improved ovulation, menstrual cyclicity, and hormonal profiles following MI supplementation [ 192 , 193 ]. The 2018 PCOS guideline recommends that inositol (in any form) should be considered as an experimental therapy in PCOS management. The guideline also recognises that women participating in any form of TCIM should be encouraged to advise their health professional. However, it does not consider emerging evidence for the use of other types of TCIM in PCOS treatment as this was outside of the scope of the 2018 guideline.
B-group vitamins (B 1 , B 6 and B 12 ), folic acid (B 9 ) and vitamins D, E, and K are critical for several biological processes that can affect metabolic and reproductive features of PCOS. B-group vitamins work alongside folic acid (the synthetic form of folate) to regulate homocysteine (Hcy) via re-methylation of Hcy to methionine [ 194 ]. Hcy is an amino acid that confers an increased risk of CVD at high levels, and which is often deranged in women with PCOS [ 195 ], likely related to a higher prevalence of folate deficiency [ 196 , 197 , 198 ]. One RCT explored the use of B-group vitamins combined with folic acid in 60 women with PCOS, and reported a reduction in the Hcy increasing effect of metformin [ 198 ]. Folic acid alone has also been examined in two RCTs of women with PCOS ( n =69 [ 199 ] and n =81 [ 200 ]), improving FINS, HOMA-IR, C-reactive protein, total antioxidant capacity (TAC) and glutathione with doses ≥ 5 mg/day when compared with placebo [ 199 , 200 ]. Regarding vitamin D supplementation, three large-scale meta-analyses reported improvements in measures of IR (HOMA-IR [ 201 , 202 ], FINS [ 201 ]), fasting glucose [ 201 ]), lipid profiles (LDL-C [ 201 , 202 , 203 ], TC [ 203 ] and TAG [ 203 ]) and androgens (T) [ 202 ], when compared with placebo. While vitamin E (or tocopherol) has various reported benefits on fertility outcomes in other populations [ 204 ], and has improved androgen profiles when co-supplemented with coenzyme Q10 (CoQ10) in women with PCOS [ 205 ], to date no RCTs have examined the use of vitamin E supplements alone in PCOS. Vitamin K also has limited available literature in PCOS, with only one RCT ( n =84) demonstrating improvements in anthropometry, insulin and androgen profiles following supplementation (90 μg/day Menaquinone-7 for eight weeks), compared with placebo [ 206 ].
Vitamin-like supplements
Vitamin-like supplements including bioflavonoids, carnitine and alpha-lipoic acid (α-LA) have well-recognised antioxidant properties and play a role in fatty acid and glucose metabolism, providing possible metabolic benefits in PCOS [ 207 ]. Bioflavonoids consist of plant-derived polyphenolic compounds, some of which have been inversely associated with METS in women with PCOS [ 207 ]. In a pilot prospective study of 12 women with PCOS, 36 mg/day of the soy isoflavone genistein for six months improved lipid profiles but not anthropometry, IR, hormonal profiles or menstrual cyclicity [ 208 ]. Carnitine, particularly the active form L-carnitine, is reported to be lower in women with PCOS and linked with hyperandrogenism, hyperinsulinaemia and reduced oocyte quality [ 209 , 210 ]. One RCT explored L-carnitine use in PCOS and found beneficial effects on mental health parameters and markers of oxidative stress [ 211 ], although the integrity of these have come under scrutiny and hence should be interpreted with caution [ 122 212 ]. Regarding α-LA, a small pre-post study ( n =6) administered 1200 mg/day for 16 weeks, and reported improved IR, LDL-C and TAG, though no effects on TAC or plasma oxidation metabolites [ 213 ]. Another RCT reported improved anthropometric (BMI), metabolic (FINS and HDL-C) and reproductive (menstrual cyclicity) features in 46 women with PCOS receiving α-LA supplementation (600 mg/day for 180 days) compared with controls [ 214 ]. However, as these women were co-supplemented with 1000 mg/day D-chiro-inostiol, findings are not isolated to the effects of α-LA alone [ 214 ].
Minerals such as calcium, zinc, selenium, magnesium and chromium picolinate (CrP) have been explored in PCOS due to their reported insulin sensitising, antioxidant and anti-inflammatory properties [ 215 , 216 , 217 ]. A small number of studies have also reported women with PCOS are at higher risk of being deficient in calcium [ 218 ], zinc [ 215 , 217 ] and selenium [ 195 ]. A recent systematic review (six RCTs) reported that vitamin D and calcium co-supplementation in women with PCOS improved lipid and androgen profiles, follicular health and menstrual cyclicity [ 219 ]. While these findings are promising, it is difficult to attribute benefits to calcium alone, given calcium is often co-supplemented with vitamin D due to their complementary mechanisms of action. One systematic review (five RCTs) in PCOS reported zinc (often co-supplemented with other nutrients such as calcium, vitamin D and magnesium), improved HOMA-IR, lipids, T, FSH and DHEAS [ 220 ] compared to placebo. Another systematic review (five RCTs) examining selenium supplementation reported reduced IR, oxidative stress and inflammation, while results for anthropometry, lipids, androgens and hirsutism were inconsistent [ 221 ]. Regarding magnesium (an intracellular cation involved in insulin metabolism), while supplementation in PCOS has been associated with reduced IR in observational research [ 222 ], these findings are not supported by data from RCTs, with considerable inconsistencies between studies [ 222 ]. Two meta-analyses examined CrP in women with PCOS [ 223 , 224 ]. While one reported that CrP supplementation reduced BMI, FINS and free testosterone [ 223 ], the other reported decreased IR, but not BMI, and increased levels of T [ 224 ].
Other supplements
Other supplements purported to provide a range of antioxidant and anti-inflammatory benefits, including omega-3 fatty acids, N-acetyl-cysteine (NAC), CoQ10, probiotics, quercetin, resveratrol and melatonin have been explored in PCOS. A meta-analysis (nine RCTs) of women with PCOS ( n =591) receiving omega-3 supplementation reported reductions in HOMA-IR, TC, TAG and LDL-C, though showed no effect on other metabolic parameters or T [ 225 ]. In a meta-analysis of eight RCTs ( n =910) examining NAC supplementation (the acylated form of L-cysteine), researchers reported improved glucose regulation and a greater likelihood of conception and livebirths in women with PCOS compared with placebo [ 226 ]. In a single RCT ( n =60) CoQ10 supplementation (100 mg/day for 12 weeks) improved fasting glucose and insulin, HOMA-IR, insulin sensitivity index and TC, compared with the placebo group [ 227 ]. Two meta-analyses reported probiotics improved FAI, SHBG, IR and blood lipids, with no differences in weight or hirsutism between intervention and placebo groups [ 228 , 229 ]. These findings may be linked to lower microbial diversity and increased intestinal permeability in women with PCOS [ 230 , 231 ]. In regards to quercetin and resveratrol, which are both food derived polyphenols with a strong antioxidant capacity, one systematic review (three experimental studies, n =246 women with PCOS) reported quercetin supplementation improved measures of IR and testosterone levels, but not anthropometry compared with placebo [ 232 ]. Similarly, one RCT in women with PCOS ( n =61) reported resveratrol (800-1500 mg/day for four days) improved androgen and metabolic profiles and oocyte and embryo quality compared with placebo [ 233 ]. Finally, a systematic review (two RCTs and one cell-culture study) investigating the effects of melatonin supplementation in women with PCOS using assisted reproductive technologies reported melatonin significantly increased clinical pregnancy rates but not live birth rates [ 172 ]. A more recent RCT ( n =56) reported improved levels of T, hirsutism, inflammatory and oxidative stress profiles in women receiving 10 g melatonin/day for 12 weeks, compared with placebo [ 234 ].
Herbal medicine
To date the most recent and comprehensive review (Cochrane review including five RCTs and n =414 women with PCOS) investigating the effects of herbal medicine on reproductive outcomes, reported no difference between the use of Chinese herbal medicine (CHM) and clomiphene for pregnancy rates, and limited evidence of increased pregnancy rate for CHM with clomiphene compared with clomiphene alone [ 235 ]. This review concluded that there was inadequate evidence to promote the use of CHM for the treatment of subfertility in women with PCOS [ 235 ]. Similarly, a smaller systematic review (five studies) investigating the effects of four herbal medicines (green tea, cinnamon, spearmint and black cohosh) on menstrual regularity in PCOS, found limited high-quality evidence from RCTs to support their clinical use and concluded that evidence for safety was lacking [ 236 ].
More recently, a number of small RCTs investigating metabolic and reproductive effects of a range of herbal medicines have been published. Curcumin, an active compound in turmeric ( Curcuma longa), may exert hypoglycemic effects via a number of mechanisms, including attenuation of circulating levels of tumor necrosis factor-α [ 237 ]. One RCT ( n =67) reported decreased levels of fasting glucose following supplementation compared with placebo [ 238 ], while another ( n =51) which used a lower dose (1000 mg/day versus 1500 mg/day) and shorter duration (six weeks versus 12 weeks), reported no between group differences for fasting glucose, HOMA-IR or lipids [ 239 ]. Salvia officinalis or sage contains multiple active compounds that display antioxidant effects and therefore effects on glucose metabolism and insulin sensitivity [ 240 ]. One RCT ( n =72) reported consuming sage extract for eight weeks improved IR and reduced BMI, with no effects on WHR or blood pressure [ 241 ] Foeniculum vulgare or fennel may provide protective effects on hormonal abnormalities in PCOS via its actions as a phytoestrogen [ 242 ]. One RCT ( n =55) reported that six months of fennel tea and dry cupping was as effective as metformin for reducing BMI and menstrual cycle length [ 243 ]. Glycyrrhiza glabra or licorice contains active phytochemicals including isoflavane and glabridin, which have been shown to have antiandrogenic effects [ 244 ]. Two experimental studies in healthy women ( n =9) [ 245 ] and women with PCOS ( n =32) [ 246 ] reported that 3.5 g/day of licorice extract decreased T [ 245 ] and reduced side effects of spironolactone [ 246 ]. Mentha spicata (spearmint), Zingiber offinale Roscoe (ginger), Cinnamomum cassia (cinnamon) and Citrus sinensis (citrus) have been shown to exert anti-inflammatory and hypoglycemic effects [ 247 , 248 , 249 , 250 ]. One RCT in infertile women with PCOS ( n =60) comparing the effects of a herbal mixture (citrus, ginger, cinnamon and spearmint) with clomiphene citrate (CC), herbal mixture alone, or CC alone reported that the herbal mixture, with or without CC, improved circulating antioxidant levels, IR and fasting blood glucose, but not menstrual regularity when compared to CC alone [ 251 ]. While observations from emerging research are promising, to support the safe translation of findings into the clinical setting there is a clear need for larger clinical trials investigating the efficacy and safety of herbal medicine use in PCOS.
Other traditional, complimentary and integrative medicine approaches
Acupuncture may provide beneficial impacts on sympathetic function [ 252 ] and ovarian blood flow [ 253 ] in women with PCOS. A recent meta-analysis of 22 RCTs ( n =2315 women with PCOS) reported recovery of the menstrual period in the acupuncture group when compared with placebo, but no evidence for differences between groups in terms of live birth, pregnancy and ovulation [ 254 ]. While an earlier meta-analysis reported a significant reduction in BMI following acupuncture use, this was mainly due to one RCT ( n =80) which compared acupuncture and the oral contraceptive pill to the oral contraceptive pill alone [ 255 ]. When this study was removed, the pooled analysis was no longer significant [ 255 ].
Yoga gymnastics have been recommended as an example of moderate physical activity in the 2018 evidence-based PCOS guideline [ 18 ]. However, as yoga is considered a mind-body therapy that incorporates aspects of meditation, it may provide additional benefits beyond those gained through other forms of exercise [ 256 ]. While one systematic review (16 observational and experimental studies, n =365 women with PCOS) reported yoga may provide a range of psychological, reproductive and metabolic benefits, no meta-analysis was performed and a limited summary of included studies made it difficult to confirm findings [ 257 ]. A more recent systematic review (11 experimental studies) included a meta-analysis of two RCTs and found that yoga significantly decreased clinical hyperandrogenism, menstrual irregularity and fasting glucose and insulin [ 258 ]. Lastly, findings from a recent RCT ( n =67 women with PCOS) suggests that 90 minutes of yoga per day for six weeks can significantly reduce hirsutism, waist and hip circumference when compared to controls [ 259 ]. Please see Table 5 for a summary of available evidence from meta-analyses and experimental studies investigating the effects of TCIM on PCOS outcomes.
Summary of findings and research gaps
The 2018 International Evidence-Based Guideline for the Assessment and Management of PCOS highlights lifestyle (diet, physical activity and/or behavioural) management as the primary initial treatment strategy [ 18 ]. It is important to consider that the definition of lifestyle management may warrant expansion consistent with the whole person model of healthcare provision, which may include care addressing psychological and sleep interventions, as well as a range of TCIM approaches [ 20 ]. In line with patient interest [ 31 , 32 , 33 , 34 , 35 ], and to assist women and healthcare providers in understanding the evidence to aid safe implementation of adjunct therapies, rigorous assessment of the evidence for these alternative lifestyle strategies in PCOS management in warranted. Using a holistic definition of patient care, this review has summarised evidence to date on the traditional components of lifestyle change (diet, physical activity and behavioural change), psychological interventions and non-pharmacological strategies (sleep, supplements, herbal medicine and other TCIM approaches). Table 6 provides a overview of current guideline recommendations alongside the key findings from this review, summarising the identified research gaps that need to be addressed before evidence-based recommendations for clinical practice can be updated.
With regards to traditional lifestyle treatment, the majority of studies focussed on weight loss as a primary treatment goal. This indicates more research is warranted to understand the role of diet and exercise in lean women and/or in weight gain prevention. RCTs using lifestyle interventions under isocaloric conditions that investigate effects on IR, body composition and androgens independent of weight loss are needed. Given the high risk of failure with long-term weight management [ 9 , 37 , 40 , 261 ] and high attrition in weight loss trials in PCOS [ 13 ], exploring interventions that focus on weight neural messaging around dietary quality and physical activity may also aid in optimising engagement, adherence and sustainability of lifestyle interventions. Future research should also identify subgroups who respond more favourably to weight loss [ 45 , 53 ], to aid provision of a more targeted and personalised treatment approach.
With regards to diet strategies, there is a need for more research understanding the impact of low GI/GL diets on androgen status, as well as the biological mechanisms by which low GI/GL diets may impact reproductive and cardiometabolic outcomes associated with PCOS. With regards to physical activity, additional longer-term studies are required to guide exercise prescription in PCOS, although promising evidence supports the provision of vigorous aerobic exercise performed under supervised conditions (i.e. through referral to an exercise physiologist). While behavioural interventions are essential for long term sustainability of dietary and physical activity change, research in PCOS is scarce and interventions are not well defined. Future research should incorporate appropriate theoretical frameworks and clearly outline behavioural components utilised. This will aid intervention duplication and tailoring of active elements to ensure relevance in women with PCOS.
There is currently a lack of research investigating whether women with PCOS are at a higher risk of alcohol and smoking-related complications. This is particularly relevant given the well-established relationship between higher alcohol and cigarette use and rates of depression and anxiety in the general population [ 262 , 263 , 264 , 265 ]. There is also a need to better understand the relationship between alcohol intake and reproductive outcomes (particularly anovulatory infertility) [ 139 ], as safe alcohol limits in PCOS is currently unknown [ 139 ].
With regards to psychological interventions, the current evidence base for prevalence of mental health concerns in PCOS relies heavily on symptom prevalence. More adequately powered, gold standard prevalence studies using structured diagnostic interviews administered by appropriately qualified professionals are needed. While QoL has recently been highlighted as a core outcome in PCOS research [ 266 ], the application of QoL tools in clinical care is still unclear, with research yet to validate QoL tools longitudinally or identify clinically meaningful differences in QoL scores. The emerging evidence showing support for the use of CBT in PCOS [ 152 , 154 , 154 ] highlights an opportunity for tailoring of this psychological intervention to meet the specific mental health needs of women with PCOS, with a focus on how management of mental health symptoms affect lifestyle modifications. CBT that incorporates elements of mindfulness-based stress reduction also warrants further investigation.
Future research in PCOS and sleep disorders should include more high-quality research in subclinical disorders using objective sleep measures (polysomnography and actigraphy). Future work should also consider emerging evidence showing that disturbed sleep can detrimentally effect energy expenditure, which may increase adipose tissue deposition and exacerbate IR [ 164 , 184 , 186 , 267 , 268 , 269 , 270 , 271 ], thereby worsening the presentation of PCOS. Further, a consideration of how sleep disturbance can reduce engagement with positive lifestyle changes, for example through the disruption of appetite regulation [ 272 , 273 ] or via contributing to poor mental health outcomes [ 190 , 274 ], is warranted. CBT interventions including elements of stimulus control and psychoeducation are effective non-pharmacological treatments for both clinical sleep disorders and sleep disturbances in the general population [ 275 , 276 , 277 ]. RCTs in women with PCOS that investigate effects of CBT on dietary intake, energy metabolism, appetite regulation, anthropometry, adherence to lifestyle changes and PCOS features are required.
With regards to TCIM, there is a vast array of literature suggesting some beneficial effects of vitamins (B-group vitamins, folate, vitamins D, E and K), vitamin-like nutrients (bioflavonoids, carnitine and α-LA), minerals (calcium, zinc, selenium, and CrP) and other formulations (such as melatonin, omega-3 fatty acids, probiotics, NAC and cinnamon) in PCOS [ 278 ]. However, the quality of evidence across studies ranges from meta-analyses of RCTs (vitamin D, omega-3 fatty acids and NAC) to single retrospective observational studies (vitamin K and carnitine). In addition, heterogeneity in results related to factors including variable PCOS presentation and study methodology make it difficult to draw definite conclusions. Future research should focus on specific populations within PCOS, for example age, BMI or phenotype (factors which substantially affect nutrient sufficiency), and outline more consistent approaches to supplement formulation, dosage, intervention duration and type of comparator used. Mechanistic studies are also needed to investigate herb- or nutrient-drug interactions (with common pharmacological treatments used in PCOS) and other possible interactions with the biological processes underpinning PCOS. In regards to acupuncture and yoga, more sufficiently powered RCTs are needed to determine clinical relevance and integration into PCOS management is not yet warranted.
While current research is not sufficiently robust to support integration of TCIM into routine clinical practice, healthcare providers should broaden their knowledge pertaining to how these therapies can be safely and appropriately utilised as adjuncts to conventional medical management [ 279 , 280 , 281 ]. TCIM is frequently used by women, with uptake of TCIM approaches increasing steadily over the past 10 years [ 31 , 32 , 33 , 34 , 35 ]. In women with PCOS, one cross-sectional study ( n =493) found that 70% reported use of TCIM, namely nutritional and herbal supplements [ 282 ]. The most common reasons for use were to treat PCOS symptoms, improve general wellbeing and reduce depression. Of the women using TCIM, 77% had consulted with a complementary practitioner (acupuncturists, chiropractors, naturopaths and massage therapists) [ 282 ]. While the study did not report participants engagement with medical physicians, research in the general population has shown that patients are resistant to discuss TCIM use with their consulting physician [ 283 , 284 , 285 , 286 , 287 , 288 ]. Qualified healthcare providers should be involved in TCIM discussions to help ensure appropriate use, maximise possible benefits and minimize potential harm [ 289 ]. For example, to sustain patient engagement in women who express the desire to experiment with supplementation, healthcare providers could consider inositol supplementation, using a nuanced and case-specific approach that encapsulates the variety of pathologies in PCOS.
When considering all of the research summarised here, across traditional lifestyle, psychological, sleep and TCIM interventions, there is a clear need for more real-world PCOS research. This involves the translation of findings from clinical trials (where highly selected populations, intensive treatment protocols and expert multidisciplinary teams provide an ideal research setting), into the heterogenous situations that face clinicians [ 290 , 291 , 292 ]. Health professionals provide care to women from diverse social contexts, are often restrained by finite resources and are required to juggle many competing demands for their time [ 290 , 291 , 292 ]. While some barriers to implementation, including time, resource and access issues are considered in the current PCOS guideline, they were generated by the guideline development groups and research is needed to validate and clarify their proposed concerns. Real-world research is required to: a) fully understand whether lifestyle recommendations can be practically integrated into current healthcare settings; b) tailor interventions to meet the unique needs of women with PCOS; and c) generate evidence on clinical outcomes that are of great relevance to patients and clinicians, such as live birth, miscarriage and menstrual regularity, which can be collected through routine care.
It is also important to highlight that while lifestyle management is a first-line treatment for PCOS, the addition of pharmacological therapies to further improve clinical features of hyperandrogenism, menstrual irregularity and infertility are often indicated [ 293 ]. In these instances, prescribing physicians should consider how medical management and lifestyle change can be used in adjunct to optimise treatment. For example, the use of combined oral contraceptive pills may have detrimental effects on weight gain [ 294 ] and mental health [ 295 ], which can be mitigated by appropriate lifestyle intervention. Further, the combination of lifestyle modification and metformin has been shown to lower BMI, subcutaneous adipose tissue and improve menstruation compared with lifestyle modification alone, and hence may have an additive effect on improving cardio-metabolic outcomes in high risk groups [ 296 ].
Using the whole person or holistic definition of health, this review has highlighted emerging areas of research that could be considered for integration into future classifications of lifestyle management in PCOS. When developing lifestyle recommendations for PCOS management, interpreting and communicating evidence not only for diet, physical activity and behavioural interventions, but also psychological, sleep and TCIM approaches, will aid clinicians to deliver patient-centred care by affording women more choice and therefore autonomy over their treatment options. This sentiment aligns with the core objectives underpinning the 2018 PCOS guideline, which sought to understand the unmet needs of women with PCOS through continuing to engage consumers in co-design of guideline development, implementation, translation and dissemination.
Availability of data and materials
Not applicable.
Abbreviations
Alpha-lipoic acid
Body mass index
Cardiovascular disease
Chinese herbal medicine
Chromium picolinate
Coenzyme Q10
Cognitive behavioural therapy
Dietary Approaches to Stop Hypertension
Fasting insulin level
Follicle stimulating hormone
Free androgen index
Glycaemic index
Glycaemic load
High density lipoprotein cholesterol
Homocysteine
Insulin resistance
Low density lipoprotein cholesterol
Luteinizing hormone
Maximal rate of oxygen
Metabolic syndrome
Monounsaturated fatty acid
Myo-inositol
N-acetyl-cysteine
Oral glucose tolerance test
- Polycystic ovary syndrome
Polyunsaturated fatty acid
Randomised controlled trial
Sex hormone-binding globulin
Total antioxidant capacity
Total cholesterol
Testosterone
Triglycerides
Type 2 diabetes
Waist circumference
Waist-hip-ratio
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–55.
Article Google Scholar
ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Bahri Khomami M, Moran LJ, Kenny L, Grieger JA, Myers J, Poston L, et al. Lifestyle and pregnancy complications in polycystic ovary syndrome: The SCOPE cohort study. Clin Endocrinol. 2019;90(6):814–21.
Kakoly N, Khomami M, Joham A, Cooray S, Misso M, Norman R, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–67.
Article CAS Google Scholar
Wekker V, Van Dammen L, Koning A, Heida K, Painter R, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942–60.
Barry JA. Exploration of biological causes of psychological problems in polycystic ovary syndrome (PCOS). 2011. City Univerity London (thesis) doi: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.544446
Pirotta S, Barillaro M, Brennan L, Grassi A, Jeanes YM, Joham AE, et al. Disordered eating behaviours and eating disorders in women in Australia with and without polycystic ovary syndrome: a cross-sectional study. J Clin Med. 2019;8(10):1682.
Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–84.
Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity. 2013;21(8):1526–32.
Lim SS, Davies M, Norman RJ, Moran L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–37.
Lim S, Norman RJ, Davies M, Moran L. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109.
Moran LJ, Noakes M, Clifton PM, Tomlinson L, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(2):812–9.
Moran LJ, Noakes M, Clifton P, Buckley J, Brinkworth G, Thomson R, et al. Predictors of Lifestyle Intervention Attrition or Weight Loss Success in Women with Polycystic Ovary Syndrome Who Are Overweight or Obese. Nutrients. 2019;11(3):492.
Farid NR, Diamanti-Kandarakis E. Diagnosis and Management of Polycystic Ovary Syndrome: New York. United States: Springer; 2009.
Book Google Scholar
Moran C, Garcia-Hernandez E, Barahona E, Gonzalez S, Bermudez JA. Relationship between insulin resistance and gonadotropin dissociation in obese and nonobese women with polycystic ovary syndrome. Fertil Steril. 2003;80(6):1466–72.
Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J Acad Nutr Diet. 2013;113(4):520–45.
Tay CT, Moran LJ, Harrison CL, Brown WJ, Joham AE. Physical activity and sedentary behaviour in women with and without polycystic ovary syndrome: An Australian population-based cross-sectional study. Clin Endocrinol. 2020;93(2):154–62.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–18.
Checkland K, Harrison S, McDonald R, Grant S, Campbell S, Guthrie B. Biomedicine, holism and general medical practice: responses to the 2004 General Practitioner contract. Sociol Health Illness. 2008;30(5):788–803.
Thomas H, Mitchell G, Rich J, Best M. Definition of whole person care in general practice in the English language literature: a systematic review. BMJ Open. 2018;8(12):e023758.
Collins A, de Iongh A. Independent Commission on Whole Person Care for the UK Labour Party: British Medical Journal Publishing Group; 2014.
Primary Health Care Advisory Group, Department of Health. Better Outcomes for People with Chronic and Complex Health Conditions. Canberra: Commonwealth of Australia; 2015.
Google Scholar
Ferrante JM, Balasubramanian BA, Hudson SV, Crabtree BF. Principles of the patient-centered medical home and preventive services delivery. Ann Fam Med. 2010;8(2):108–16.
Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–91.
Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73:196–203.
Pastoor H, Timman R, de Klerk C, W MB, Laan ET, Laven JS. Sexual function in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod BioMed Online. 2018;37(6):750–60.
Dawber RP. Guidance for the management of hirsutism. Curr Med Res Opin. 2005;21(8):1227–34.
Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5(2):107–11.
Pastore LM, Patrie JT, Morris WL, Dalal P, Bray MJ. Depression symptoms and body dissatisfaction association among polycystic ovary syndrome women. J Psychosom Res. 2011;71(4):270–6.
Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol. 2006;11(4):613–25.
Smith CA, Bateson DJ, Weisberg E. A survey describing the use of complementary therapies and medicines by women attending a family planning clinic. BMC Complement Altern Med. 2013;13(1):1–8.
Sibbritt DW, Adams J, Young AF. A longitudinal analysis of mid-age women's use of complementary and alternative medicine (CAM) in Australia, 1996–1998. Women Health. 2005;40(4):41–56.
Gollschewski S, Anderson D, Skerman H, Lyons-Wall P. Associations between the use of complementary and alternative medications and demographic, health and lifestyle factors in mid-life Australian women. Climacteric. 2005;8(3):271–8.
Barnes LAJ, Barclay L, McCaffery K, Aslani P. Complementary medicine products used in pregnancy and lactation and an examination of the information sources accessed pertaining to maternal health literacy: a systematic review of qualitative studies. BMC Complement Altern Med. 2018;18(1):229.
Adams J, Lui CW, Sibbritt D, Broom A, Wardle J, Homer C, et al. Women's use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth. 2009;36(3):237–45.
van Velden D. The bio-psycho-social approach to health and disease. SADJ. 2003;58(5):191–6.
Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;3:CD007506.
Haqq L, McFarlane J, Dieberg G, Smart N. The Effect of Lifestyle Intervention on Body Composition, Glycemic Control, and Cardiorespiratory Fitness in Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Int J Sport Nutr Exerc Metab. 2015;25(6):533–40.
Abdolahian S, Tehrani FR, Amiri M, Ghodsi D, Yarandi RB, Jafari M, et al. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):71.
Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4655–63.
Haqq L, McFarlane J, Dieberg G, Smart N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Endocrine Connect. 2014;3(1):36–46.
Ujvari D, Hulchiy M, Calaby A, Nybacka Å, Byström B, Hirschberg AL. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29(7):1526–35.
Soares NP, Santos AC, Costa EC, Azevedo GD, Damasceno DC, Fayh AP, et al. Diet-Induced Weight Loss Reduces DNA Damage and Cardiometabolic Risk Factors in Overweight/Obese Women with Polycystic Ovary Syndrome. Ann Nutr Metab. 2016;68(3):220–7.
Oberg E, Gidlöf S, Jakson I, Mitsell M, Tollet Egnell P, Hirschberg AL. Improved menstrual function in obese women with polycystic ovary syndrome after behavioural modification intervention-A randomized controlled trial. Clin Endocrinol. 2019;90(3):468–78.
Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84(4):1470–4.
CAS Google Scholar
Ornstein RM, Copperman NM, Jacobson MS. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2011;24(3):161–5.
Marzouk TM, Sayed Ahmed WA. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol. 2015;28(6):457–61.
Kuchenbecker WK, Groen H, van Asselt SJ, Bolster JH, Zwerver J, Slart RH, et al. In women with polycystic ovary syndrome and obesity, loss of intra-abdominal fat is associated with resumption of ovulation. Hum Reprod. 2011;26(9):2505–12.
Tolino A, Gambardella V, Caccavale C, D'Ettore A, Giannotti F, D'Antò V, et al. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2005;119(1):87–93.
Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96(11):3533–40.
Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18(9):1928–32.
Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502–5.
Pasquali R, Gambineri A, Cavazza C, Ibarra Gasparini D, Ciampaglia W, Cognigni GE, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164(1):53–60.
Moran LJ, Tassone EC, Boyle J, Brennan L, Harrison CL, Hirschberg AL, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Lifestyle management. Obes Rev. 2020;21(10):e13046.
Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv Nutr. 2020;12(1):161–78.
Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2019;2019:4386401.
Perelman D, Coghlan N, Lamendola C, Carter S, Abbasi F, McLaughlin T. Substituting poly- and mono-unsaturated fat for dietary carbohydrate reduces hyperinsulinemia in women with polycystic ovary syndrome. Gynecol Endocrinol. 2017;33(4):324–7.
Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism. 2014;63(10):1257–64.
Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol. 2013;79(4):550–7.
Atiomo W, Read A, Golding M, Silcocks P, Razali N, Sarkar S, et al. Local recruitment experience in a study comparing the effectiveness of a low glycaemic index diet with a low calorie healthy eating approach at achieving weight loss and reducing the risk of endometrial cancer in women with polycystic ovary syndrome (PCOS). Contemp Clin Trials. 2009;30(5):451–6.
Barr S, Reeves S, Sharp K, Jeanes YM. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J Acad Nutr Diet. 2013;113(11):1523–31.
Foroozanfard F, Rafiei H, Samimi M, Gilasi HR, Gorjizadeh R, Heidar Z, et al. The effects of dietary approaches to stop hypertension diet on weight loss, anti-Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: A randomized clinical trial. Clin Endocrinol. 2017;87(1):51–8.
Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res. 2015;47(3):232–8.
Azadi-Yazdi M, Karimi-Zarchi M, Salehi-Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. J Hum Nutr Diet. 2017;30(3):275–83.
Ebrahimi-Mamaghani M, Saghafi-Asl M, Pirouzpanah S, Asghari-Jafarabadi M. Effects of raw red onion consumption on metabolic features in overweight or obese women with polycystic ovary syndrome: A randomized controlled clinical trial. J Obstet Gynaecol Res. 2014;40(4):1067–76.
Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504–12.
Karamali M, Kashanian M, Alaeinasab S, Asemi Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomised clinical trial. J Hum Nutr Diet. 2018;31(4):533–43.
Turner-McGrievy GM, Davidson CR, Wingard EE, Billings DL. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr Res. 2014;34(6):552–8.
Abedini M, Ghasemi-Tehrani H, Tarrahi M, Amani R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: A randomized controlled trial. Phytother Res. 2020;35:442–51.
Haidari F, Banaei-Jahromi N, Zakerkish M, Ahmadi K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr J. 2020;19(1):8.
Johnson LK, Holven KB, Nordstrand N, Mellembakken JR, Tanbo T, Hjelmesæth J. Fructose content of low calorie diets: effect on cardiometabolic risk factors in obese women with polycystic ovarian syndrome: a randomized controlled trial. Endocrine Connect. 2015;4(3):144–54.
Kazemi M, McBreairty LE, Zello GA, Pierson RA, Gordon JJ, Serrao SB, et al. A pulse-based diet and the Therapeutic Lifestyle Changes diet in combination with health counseling and exercise improve health-related quality of life in women with polycystic ovary syndrome: secondary analysis of a randomized controlled trial. J Psychosom Obstet Gynecol. 2020;41(2):144–53.
Phy JL, Pohlmeier AM, Cooper JA, Watkins P, Spallholz J, Harris KS, et al. Low Starch/Low Dairy Diet Results in Successful Treatment of Obesity and Co-Morbidities Linked to Polycystic Ovary Syndrome (PCOS). J Obes Weight Loss Ther. 2015;5(2):259.
Pohlmeier AM, Phy JL, Watkins P, Boylan M, Spallholz J, Harris KS, et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Applied physiology, nutrition, and metabolism =. Physiol Appl Nutr Metab. 2014;39(11):1237–44.
Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104.
Shishehgar F, Mirmiran P, Rahmati M, Tohidi M, Ramezani TF. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr Disord. 2019;19(1):93.
Szczuko M, Zapałowska-Chwyć M, Drozd A, Maciejewska D, Starczewski A, Wysokiński P, et al. Changes in the IGF-1 and TNF-α synthesis pathways before and after three-month reduction diet with low glicemic index in women with PCOS. Ginekol Pol. 2018;89(6):295–303.
Hoover SE, Gower BA, Cedillo YE, Chandler-Laney PC, Deemer SE, Goss AM. Changes in Ghrelin and Glucagon following a Low Glycemic Load Diet in Women with PCOS. J Clin Endocrinol Metab. 2021;106(5):e2151–e61.
Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83–92.
Ganie MA, Sahar T, Rashid A, Wani IA, Nisar S, Sathyapalan T, et al. Comparative Evaluation of Biomarkers of Inflammation Among Indian Women With Polycystic Ovary Syndrome (PCOS) Consuming Vegetarian vs. Non-vegetarian Diet. Front Endocrinol. 2019;10:699.
McBreairty LE, Kazemi M, Chilibeck PD, Gordon JJ, Chizen DR, Zello GA. Effect of a pulse-based diet and aerobic exercise on bone measures and body composition in women with polycystic ovary syndrome: A randomized controlled trial. Bone Rep. 2020;12:100248.
Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol. 2010;36(5):712–8.
Kazemi M, Pierson RA, McBreairty LE, Chilibeck PD, Zello GA, Chizen DR. A randomized controlled trial of a lifestyle intervention with longitudinal follow-up on ovarian dysmorphology in women with polycystic ovary syndrome. Clin Endocrinol. 2020;92(6):525–35.
Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16(6):1255–60.
Shang Y, Zhou H, Hu M, Feng H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2020;105(10):3346–60.
Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, et al. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril. 2009;91(4):1175–82.
McBreairty LE, Chilibeck PD, Gordon JJ, Chizen DR, Zello GA. Polycystic ovary syndrome is a risk factor for sarcopenic obesity: a case control study. BMC Endocr Disord. 2019;19(1):70.
Kasim-Karakas SE, Cunningham WM, Tsodikov A. Relation of nutrients and hormones in polycystic ovary syndrome. Am J Clin Nutr. 2007;85(3):688–94.
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73.
Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–13.
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98.
Toscani MK, Mario FM, Radavelli-Bagatini S, Wiltgen D, Cristina Matos M, Spritzer PM. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: a randomized study. Gynecol Endocrinol. 2011;27(11):925–30.
Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81(3):630–7.
Nadjarzadeh A, Ghadiri-Anari A, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A, Namayande SM, et al. Effect of hypocaloric high-protein, low-carbohydrate diet supplemented with fennel on androgenic and anthropometric indices in overweight and obese women with polycystic ovary syndrome: A randomized placebo-controlled trial. Complement Ther Med. 2021;56:102633.
Moran LJ, Noakes M, Clifton PM, Wittert G, Tomlinson L, Galletly C, et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab. 2004;89(7):3337–44.
Moran LJ, Noakes M, Clifton PM, Norman RJ. The effect of modifying dietary protein and carbohydrate in weight loss on arterial compliance and postprandial lipidemia in overweight women with polycystic ovary syndrome. Fertil Steril. 2010;94(6):2451–4.
Sørensen LB, Søe M, Halkier KH, Stigsby B, Astrup A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am J Clin Nutr. 2012;95(1):39–48.
Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117–25.
Galletly C, Moran L, Noakes M, Clifton P, Tomlinson L, Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome—a pilot study. Appetite. 2007;49(3):590–3.
González F, Considine RV, Abdelhadi OA, Xue J, Acton AJ. Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2021;321(5):E689–701.
González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(11):5360–71.
Kalgaonkar S, Almario R, Gurusinghe D, Garamendi E, Buchan W, Kim K, et al. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386–93.
Kasim-Karakas SE, Almario RU, Gregory L, Wong R, Todd H, Lasley BL. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(2):615–20.
Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85(3):679–88.
Yahay M, Heidari Z, Allameh Z, Amani R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: a randomized controlled trial. Lipids Health Dis. 2021;20(1):1–12.
Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77–87.
Wong JM, Gallagher M, Gooding H, Feldman HA, Gordon CM, Ludwig DS, et al. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr Obes. 2016;11(3):210–20.
Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):1–17.
Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, et al. Diet and the human gut microbiome: an international review. Dig Dis Sci. 2020;65(3):723–40.
Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, et al. Microbiome and PCOS: state-of-art and future aspects. Int J Mol Sci. 2021;22(4):2048.
Papakonstantinou E, Kechribari I, Mitrou P, Trakakis E, Vassiliadi D, Georgousopoulou E, et al. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial. Eur J Clin Nutr. 2016;70(5):588–94.
Kulshreshtha B, Sharma N, Pant S, Sharma L, Pahuja B, Singh P. PCOS patients differ in meal timings rather than total caloric or macronutrient intake in comparison to weight matched controls. Eur J Obstet Gynecol Reprod Biol. 2022;270:11–6.
Hou C, Zhang W, Li J, Du L, Lv O, Zhao S, et al. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol Nutr Food Res. 2019;63(16):e1800773.
Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari MH, Amooee S, Barati-Boldaji R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutr Metab Cardiovasc Dis. 2019;29(2):201–8.
Esmaeilinezhad Z, Barati-Boldaji R, Brett NR, de Zepetnek JOT, Bellissimo N, Babajafari S, et al. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J Endocrinol Investig. 2020;43(4):539–48.
Mani UV, Mani I, Biswas M, Kumar SN. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J Diet Suppl. 2011;8(3):257–65.
Jacobs DR, Tapsell LC. Food synergy: the key to a healthy diet. Proc Nutr Soc. 2013;72(2):200–6.
Szczuko M, Zapałowska-Chwyć M, Maciejewska D, Drozd A, Starczewski A, Stachowska E. Significant improvement selected mediators of inflammation in phenotypes of women with PCOS after reduction and low GI diet. Mediat Inflamm. 2017;2017:5489523.
Kite C, Lahart IM, Afzal I, Broom DR, Randeva H, Kyrou I, et al. Exercise, or exercise and diet for the management of polycystic ovary syndrome: a systematic review and meta-analysis. Syst Rev. 2019;8(1):51.
Shele G, Genkil J, Speelman D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J Funct Morphol Kinesiol. 2020;5(2):35.
Patten RK, Boyle RA, Moholdt T, Kiel I, Hopkins WG, Harrison CL, et al. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front Physiol. 2020;11:606.
dos Santos IK, Ashe MC, Cobucci RN, Soares GM, de Oliveira Maranhão TM, Dantas PMS. The effect of exercise as an intervention for women with polycystic ovary syndrome: A systematic review and meta-analysis. Medicine. 2020;99(16):e19644.
dos Santos IK, Nunes F, Queiros VS, Cobucci RN, Dantas PB, Soares GM, et al. Effect of high-intensity interval training on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2021;16(1):e0245023.
Richards CT, Meah VL, James PE, Rees DA, Lord RN. HIIT'ing or MISS'ing the Optimal Management of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of High-Versus Moderate-Intensity Exercise Prescription. Front Physiol. 2021;12:715881.
Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23(3):642–50.
Spahn JM, Reeves RS, Keim KS, Laquatra I, Kellogg M, Jortberg B, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010;110(6):879–91.
Saelens BE, Gehrman CA, Sallis JF, Calfas KJ, Sarkin JA, Caparosa S. Use of self-management strategies in a 2-year cognitive-behavioral intervention to promote physical activity. Behav Ther. 2000;31(2):365–79.
Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82(2):421–9.
Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–306.
Geier L, Bekx M, Connor E. Factors contributing to initial weight loss among adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2012;25(6):367–70.
Pirotta S, Joham AJ, Moran LJ, Skouteris H, Lim SS. Implementation of evidence-based PCOS lifestyle management guidelines: Perceived barriers and facilitators by consumers using the Theoretical Domains Framework and COM-B Model. Patient Educ Couns. 2021;104(8):2080–8.
Oberg E, Lundell C, Blomberg L, Gidlöf SB, Egnell PT, Hirschberg AL. Psychological well-being and personality in relation to weight loss following behavioral modification intervention in obese women with polycystic ovary syndrome: a randomized controlled trial. Eur J Endocrinol. 2020;183(1):1–11.
Larsson I, Hulthén L, Landén M, Pålsson E, Janson P, Stener-Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35(1):213–8.
Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod. 2013;28(8):2276–83.
Alvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol Endocrinol. 2011;27(12):978–81.
de Angelis C, Nardone A, Garifalos F, Pivonello C, Sansone A, Conforti A, et al. Smoke, alcohol and drug addiction and female fertility. Reprod Biol Endocrinol. 2020;18(1):1–26.
Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Cueto HT, et al. Alcohol consumption and fecundability: prospective Danish cohort study. BMJ. 2016;354:i4262.
Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology (Cambridge, Mass). 2009;20(3):374.
Grodstein F, Goldman MB, Cramer DW. Infertility in women and moderate alcohol use. Am J Public Health. 1994;84(9):1429–32.
Mo L, Mansfield DR, Joham A, Cain SW, Bennett C, Blumfield M, et al. Sleep disturbances in women with and without polycystic ovary syndrome in an Australian National cohort. Clin Endocrinol. 2019;90(4):570–8.
Legro RS, Chen G, Kunselman AR, Schlaff WD, Diamond MP, Coutifaris C, et al. Smoking in infertile women with polycystic ovary syndrome: baseline validation of self-report and effects on phenotype. Hum Reprod. 2014;29(12):2680–6.
Zhang B, Zhou W, Shi Y, Zhang J, Cui L, Chen Z-J. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr Disord. 2020;20(1):1–7.
Tao Y, Liu B, Chen Y, Hu Y, Zhu R, Ye D, et al. Genetically Predicted Cigarette Smoking in Relation to Risk of Polycystic Ovary Syndrome. Clin Epidemiol. 2021;13:527.
Cupisti S, Häberle L, Dittrich R, Oppelt PG, Reissmann C, Kronawitter D, et al. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94(2):673–7.
Xirofotos D, Trakakis E, Peppa M, Chrelias C, Panagopoulos P, Christodoulaki C, et al. The amount and duration of smoking is associated with aggravation of hormone and biochemical profile in women with PCOS. Gynecol Endocrinol. 2016;32(2):143–6.
Waylen A, Metwally M, Jones G, Wilkinson A, Ledger W. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15(1):31–44.
Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, et al. Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–99.
Alur-Gupta S, Lee I, Chemerinski A, Liu C, Lipson J, Allison K, et al. Racial differences in anxiety, depression, and quality of life in women with polycystic ovary syndrome. F S Rep. 2021;2(2):230–7.
Khan NN, Vincent A, Boyle JA, Burggraf M, Pillay M, Teede H, et al. Development of a question prompt list for women with polycystic ovary syndrome. Fertil Steril. 2018;110(3):514–22.
Rosen CB, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208.
Roessler KK, Glintborg D, Ravn P, Birkebaek C, Andersen M. Supportive relationships-psychological effects of group counselling in women with polycystic ovary syndrome (PCOS). Commun Med. 2012;9(2):125.
Abdollahi L, Mirghafourvand M, Babapour JK, Mohammadi M. Effectiveness of cognitive-behavioral therapy (CBT) in improving the quality of life and psychological fatigue in women with polycystic ovarian syndrome: a randomized controlled clinical trial. J Psychosom Obstet Gynecol. 2019;40(4):283–93.
Cooney LG, Milman LW, Hantsoo L, Kornfield S, Sammel MD, Allison KC, et al. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil Steril. 2018;110(1):161–71 e1.
Jiskoot G, Dietz de Loos A, Beerthuizen A, Timman R, Busschbach J, Laven J. Long-term effects of a three-component lifestyle intervention on emotional well-being in women with Polycystic Ovary Syndrome (PCOS): A secondary analysis of a randomized controlled trial. PLoS One. 2020;15(6):e0233876.
Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction for overweight/obese women with and without polycystic ovary syndrome: design and methods of a pilot randomized controlled trial. Contemp Clin Trial. 2015;41:287–97.
Stefanaki C, Bacopoulou F, Livadas S, Kandaraki A, Karachalios A, Chrousos GP, et al. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: a randomized controlled trial. Stress. 2015;18(1):57–66.
Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry. 2016;15(3):245–58.
Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive–behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: a pilot study. J Pediatr Psychol. 2009;34(2):156–63.
Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. Jama. 2008;300(11):1350–2.
Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction in women with overweight or obesity: a randomized clinical trial. Obesity. 2017;25(8):1349–59.
Young CC, Monge M, Minami H, Rew L, Conroy H, Peretz C, et al. Outcomes of a Mindfulness-Based Healthy Lifestyle Intervention for Adolescents and Young Adults with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol. 2021;35:305–13.
Jiskoot G, Timman R, Beerthuizen A, Dietz de Loos A, Busschbach J, Laven J. Weight reduction through a cognitive behavioral therapy lifestyle intervention in PCOS: the primary outcome of a randomized controlled trial. Obesity. 2020;28(11):2134–41.
Roessler KK, Birkebaek C, Ravn P, Andersen MS, Glintborg D. Effects of exercise and group counselling on body composition and VO 2max in overweight women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2013;92(3):272–7.
Sam S, Ehrmann DA. Pathogenesis and consequences of disordered sleep in PCOS. Clin Med Insights. 2019;13:1179558119871269.
Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86(2):517–20.
Ehrmann DA. Metabolic dysfunction in PCOS: relationship to obstructive sleep apnea. Steroids. 2012;77(4):290–4.
Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill C, et al. Poor sleep in PCOS; is melatonin the culprit. Hum Reprod. 2013;28(5):1348–53.
Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–43.
Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228(2-3):333–43.
Wei D, Zhang C, Xie J, Song X, Yin B, Liu Q, et al. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J Assist Reprod Genet. 2013;30(7):933–8.
Sirotkin AV, Schaeffer HJ. Direct regulation of mammalian reproductive organs by serotonin and melatonin. J Endocrinol. 1997;154(1):1–5.
Hu KL, Ye X, Wang S, Zhang D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Front Endocrinol (Lausanne). 2020;11:160.
Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(1):191–6.
Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3(1):37–46.
Helvaci N, Karabulut E, Demir AU, Yildiz BO. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. Endocrine Connect. 2017;6(7):437–45.
Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med. 2014;62(6):868–74.
Thannickal A, Brutocao C, Alsawas M, Morrow A, Zaiem F, Murad MH, et al. Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Clin Endocrinol. 2020;92(4):338–49.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91.
Kahal H, Kyrou I, Tahrani AA, Randeva HS. Obstructive sleep apnoea and polycystic ovary syndrome: a comprehensive review of clinical interactions and underlying pathophysiology. Clin Endocrinol. 2017;87(4):313–9.
Franik G, Krysta K, Madej P, Gimlewicz-Pięta B, Oślizło B, Trukawka J, et al. Sleep disturbances in women with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(12):1014–7.
Kutenaee MA, Amirjani S, Asemi Z, Taghavi S-A, Allan H, Kamalnadian S-N, et al. The impact of depression, self-esteem, and body image on sleep quality in patients with PCOS: a cross-sectional study. Sleep Breath. 2019;1:1–8.
Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod. 2015;30(2):466–72.
Bennett CJ, Mansfield DR, Mo L, Joham A, Cain SW, Blumfield ML, et al. Sleep disturbances in women with and without polycystic ovary syndrome and their association with lifestyle factors (diet, physical activity and sitting time). Br J Nutr. 2021; Accepted in press.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26.
Cappuccio FP, D'elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20.
Cappuccio FP, Cooper D, D'elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92.
Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructve sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(10):3878–84.
Nandalike K, Strauss T, Agarwal C, Coupey SM, Sin S, Rajpathak S, et al. Screening for sleep-disordered breathing and excessive daytime sleepiness in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2011;159(4):591–6.
Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153(7):435–41.
Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68.
Unfer V, Facchinetti F, Orru B, Giordani B, Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect. 2017;6(8):647–58.
Pundir J, Psaroudakis D, Savnur P, Bhide P, Sabatini L, Teede H, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG. 2018;125(3):299–308.
Montanino Oliva M, Buonomo G, Calcagno M, Unfer V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J Ovarian Res. 2018;11(1):38.
Asbaghi O, Ghanavati M, Ashtary-Larky D, Bagheri R, Rezaei Kelishadi M, Nazarian B, et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants (Basel). 2021;10:6.
Gunalan E, Yaba A, Yilmaz B. The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunctions: A critical review. J Turk Ger Gynecol Assoc. 2018;19(4):220–32.
Kazerooni T, Asadi N, Dehbashi S, Zolghadri J. Effect of folic acid in women with and without insulin resistance who have hyperhomocysteinemic polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;101(2):156–60.
Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23(9):505–10.
Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, et al. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod. 2005;20(6):1521–8.
Bahmani F, Karamali M, Shakeri H, Asemi Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin Endocrinol. 2014;81(4):582–7.
Asemi Z, Karamali M, Esmaillzadeh A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Mol Nutr Food Res. 2014;58(7):1465–73.
Guo S, Tal R, Jiang H, Yuan T, Liu Y. Vitamin D Supplementation Ameliorates Metabolic Dysfunction in Patients with PCOS: A SystematicReview of RCTs and Insight into the Underlying Mechanism. Int J Endocrinol. 2020;2020:7850816.
Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. 2020;19(4):2641–9.
Gao H, Li Y, Yan W, Gao F. The Effect of Vitamin D Supplementation on Blood Lipids in Patients with Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2021;2021:8849688.
Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J Assist Reprod Genet. 2012;29(4):325–8.
Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, et al. Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(2):319–27.
Tarkesh F, Namavar Jahromi B, Hejazi N, Tabatabaee H. Beneficial health effects of Menaquinone-7 on body composition, glycemic indices, lipid profile, and endocrine markers in polycystic ovary syndrome patients. Food Sci Nutr. 2020;8(10):5612–21.
Oh JS, Ahn MJ, Han CJ, Kim H, Kwon O, Chung HW, et al. Relationship between flavonoids intake and metabolic syndrome in Korean women with polycystic ovary syndrome. J Nutr Health. 2014;47(3):176–85.
Romualdi D, Constantini B, Campagna G, Lanzone A, Guido M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril. 2008;90(5):1826–33.
Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23(7):1602–6.
Dunning KR, Robker RL. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci. 2012;134(1-2):69–75.
Jamilian H, Jamilian M, Samimi M, Afshar Ebrahimi F, Rahimi M, Bahmani F, et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Gynecol Endocrinol. 2017;33(6):442–7.
Expression of concern: Comparison of myo-inositol and metformin on mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial and The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosom Obstet Gynaecol. 2020;41(4):I.
Masharani U, Gjerde C, Evans JL, Youngren JF, Goldfine ID. Effects of controlled-release alpha lipoic acid in lean, nondiabetic patients with polycystic ovary syndrome. J Diabetes Sci Technol. 2010;4(2):359–64.
Cianci A, Panella M, Fichera M, Falduzzi C, Bartolo M, Caruso S. d-chiro-Inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol Endocrinol. 2015;31(6):483–6.
Guler I, Himmetoglu O, Turp A, Erdem A, Erdem M, Onan MA, et al. Zinc and homocysteine levels in polycystic ovarian syndrome patients with insulin resistance. Biol Trace Elem Res. 2014;158(3):297–304.
Gedik O, Zileli MS. Effects of hypocalcemia and theophylline on glucose tolerance and insulin release in human beings. Diabetes. 1977;26(9):813–9.
Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res. 2016;170(2):271–8.
Szczuko M, Skowronek M, Zapalowska-Chwyc M, Starczewski A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Rocz Panstw Zakl Hig. 2016;67(4):419–26.
Shojaeian Z, Sadeghi R, Latifnejad RR. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ocvary syndrome: A systematic review and meta-analysis. Caspian J Intern Med. 2019;10(4):359–69.
Nasiadek M, Stragierowicz J, Klimczak M, Kilanowicz A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients. 2020;12:8.
Hajizadeh-Sharafabad F, Moludi J, Tutunchi H, Taheri E, Izadi A, Maleki V. Selenium and Polycystic Ovary Syndrome; Current Knowledge and Future Directions: A Systematic Review. Horm Metab Res. 2019;51(5):279–87.
Hamilton KP, Zelig R, Parker AR, Haggag A. Insulin Resistance and Serum Magnesium Concentrations among Women with Polycystic Ovary Syndrome. Curr Dev Nutr. 2019;3(11):nzz108.
Fazelian S, Rouhani MH, Bank SS, Amani R. Chromium supplementation and polycystic ovary syndrome: A systematic review and meta-analysis. J Trace Elem Med Biol. 2017;42:92–6.
Tang XL, Sun Z, Gong L. Chromium supplementation in women with polycystic ovary syndrome: Systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44(1):134–43.
Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16(1):27.
Thakker D, Raval A, Patel I, Walia R. N-acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Obstet Gynecol Int. 2015;2015:817849.
Samimi M, Zarezade Mehrizi M, Foroozanfard F, Akbari H, Jamilian M, Ahmadi S, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2017;86(4):560–6.
Liao D, Zhong C, Li C, Mo L, Liu Y. Meta-analysis of the effects of probiotic supplementation on glycemia, lipidic profiles, weight loss and C-reactive protein in women with polycystic ovarian syndrome. Minerva Med. 2018;109(6):479–87.
Shamasbi SG, Ghanbari-Homayi S, Mirghafourvand M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Nutr. 2020;59(2):433–50.
Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–11.
Lindheim L, Bashir M, Munzker J, Trummer C, Zachhuber V, Leber B, et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12(1):e0168390.
Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, Jafari-Vayghan H, Alizadeh M, Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res. 2020;13(1):11.
Shojaei-Zarghani S, Rafraf M. Resveratrol and markers of polycystic ovary syndrome: a systematic review of animal and clinical studies. Reprod Sci. 2021;26:1–1.
Jamilian M, Foroozanfard F, Mirhosseini N, Kavossian E, Aghadavod E, Bahmani F, et al. Effects of Melatonin Supplementation on Hormonal, Inflammatory, Genetic, and Oxidative Stress Parameters in Women With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2019;10:273.
Zhou K, Zhang J, Xu L, Wu T, Lim CE. Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst Rev. 2016;10(10):Cd007535.
Arentz S, Smith CA, Abbott J, Bensoussan A. Nutritional supplements and herbal medicines for women with polycystic ovary syndrome; a systematic review and meta-analysis. BMC Complement Altern Med. 2017;17(1):500.
Zhang D-W, Fu M, Gao S-H, Liu J-L. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053.
Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. 2021;80:153395.
Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201.
Ghowsi M, Yousofvand N, Moradi S. Effects of Salvia officinalis L. (common sage) leaves tea on insulin resistance, lipid profile, and oxidative stress in rats with polycystic ovary: An experimental study. Avicenna J Phytomed. 2020;10(3):263–72.
Amini L, Mojab F, Jahanfar S, Sepidarkish M, Raoofi Z, Maleki-Hajiagha A. Efficacy of Salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: A double-blinded placebo-controlled clinical trial. Complement Ther Med. 2020;48:102245.
Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674.
Mokaberinejad R, Rampisheh Z, Aliasl J, Akhtari E. The comparison of fennel infusion plus dry cupping versus metformin in management of oligomenorrhoea in patients with polycystic ovary syndrome: a randomised clinical trial. J Obstet Gynaecol. 2019;39(5):652–8.
Minnetti M, De Alcubierre D, Bonaventura I, Pofi R, Hasenmajer V, Tarsitano MG, et al. Effects of licorice on sex hormones and the reproductive system. Nutrition. 2022;103-104:111727.
Armanini D, Mattarello MJ, Fiore C, Bonanni G, Scaroni C, Sartorato P, et al. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69(11-12):763–6.
Armanini D, Castello R, Scaroni C, Bonanni G, Faccini G, Pellati D, et al. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007;131(1):61–7.
Sadeghi Ataabadi M, Alaee S, Bagheri MJ, Bahmanpoor S. Role of Essential Oil of Mentha Spicata (Spearmint) in Addressing Reverse Hormonal and Folliculogenesis Disturbances in a Polycystic Ovarian Syndrome in a Rat Model. Adv Pharm Bull. 2017;7(4):651–4.
Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108.
Chen Z-T, Chu H-L, Chyau C-C, Chu C-C, Duh P-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012;135(4):2119–27.
Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–20.
Ainehchi N, Khaki A, Farshbaf-Khalili A, Hammadeh M, Ouladsahebmadarek E. The Effectiveness of Herbal Mixture Supplements with and without Clomiphene Citrate in Comparison to Clomiphene Citrate on Serum Antioxidants and Glycemic Biomarkers in Women with Polycystic Ovary Syndrome Willing to be Pregnant: A Randomized Clinical Trial. Biomolecules. 2019;9(6):215.
Stener-Victorin E, Jedel E, Manneras L. Acupuncture in polycystic ovary syndrome: current experimental and clinical evidence. J Neuroendocrinol. 2008;20(3):290–8.
Stener-Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol (1985). 2006;101(1):84–91.
Wu J, Chen D, Liu N. Effectiveness of acupuncture in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(22):e20441.
Qu F, Wu Y, Hu X-Y, Barry JA, Zhou J, Wang F-F, et al. The effects of acupuncture on polycystic ovary syndrome: A systematic review and meta-analysis. Eur J Integr Med. 2016;8(1):12–8.
Monro R. Yoga therapy. J Bodyw Mov Ther. 1997;1(4):215–8.
Thakur D, Saurabh Singh DS, Tripathi DM, Lufang D. Effect of yoga on polycystic ovarian syndrome: A systematic review. J Bodyw Mov Ther. 2021;27:281–6.
Verma A, Upadhyay V, Saxena V. Effect of Yoga Therapy on Health Outcomes in Women With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Am J Lifestyle Med. 2021;1:15598276211029221.
Mohseni M, Eghbali M, Bahrami H, Dastaran F, Amini L. Yoga Effects on Anthropometric Indices and Polycystic Ovary Syndrome Symptoms in Women Undergoing Infertility Treatment: A Randomized Controlled Clinical Trial. Evid Based Complement Alternat Med. 2021;2021:5564824.
Heydarpour F, Hemati N, Hadi A, Moradi S, Mohammadi E, Farzaei MH. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: A systematic review and meta-analysis. J Ethnopharmacol. 2020;254:112741.
Kataoka J, Tassone EC, Misso M, Joham AE, Stener-Victorin E, Teede H, et al. Weight management interventions in women with and without PCOS: a systematic review. Nutrients. 2017;9(9):996.
Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2016;19(1):3–13.
Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Aalcohol Rev. 2006;25(6):515–36.
Mendelsohn C. Smoking and depression: a review. Aust Fam Physician. 2012;41(5):304–7.
Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118(4):330–41.
Al Wattar BH, Teede H, Garad R, Franks S, Balen A, Bhide P, et al. Harmonising research outcomes for polycystic ovary syndrome: an international multi-stakeholder core outcome set. Hum Reprod. 2020;35(2):404–12.
Theorell-Haglöw J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33(5):593.
Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14(4):402.
Dong Z, Xu X, Wang C, Cartledge S, Maddison R, Islam SMS. Association of overweight and obesity with obstructive sleep apnoea: a systematic review and meta-analysis. Obes Med. 2020;17:100185.
Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–61.
Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med. 2014;15(12):1456–62.
Chaput J-P, St-Onge M-P. Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation? Front Endocrinol. 2014;5:116.
Chaput J-P, Tremblay A. Insufficient sleep as a contributor to weight gain: an update. Curr Obes Rep. 2012;1(4):245–56.
Weaver MD, Vetter C, Rajaratnam SM, O’Brien CS, Qadri S, Benca RM, et al. Sleep disorders, depression and anxiety are associated with adverse safety outcomes in healthcare workers: A prospective cohort study. J Sleep Res. 2018;27(6):e12722.
Ham OK, Lee BG, Choi E, Choi SJ. Efficacy of cognitive behavioral treatment for insomnia: a randomized controlled trial. West J Nurs Res. 2020;42(12):1104–12.
Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–9.
Sharma MP, Andrade C. Behavioral interventions for insomnia: Theory and practice. Indian J Psychiatry. 2012;54(4):359.
Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Advanc Nutr. 2021;13:1243–66.
Raja-Khan N, Stener-Victorin E, Wu X, Legro RS. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2011;301(1):E1–E10.
Jazani AM, Azgomi HND, Azgomi AND, Azgomi RND. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). DARU J Pharm Sci. 2019;27(2):863–77.
Bargiota A, Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther Advanc Endocrinol Metab. 2012;3(1):27–47.
Arentz S, Smith CA, Abbott JA, Bensoussan A. A survey of the use of complementary medicine by a self-selected community group of Australian women with polycystic ovary syndrome. BMC Complement Altern Med. 2014;14(1):1–6.
Thomson P, Jones J, Evans JM, Leslie SL. Factors influencing the use of complementary and alternative medicine and whether patients inform their primary care physician. Complement Ther Med. 2012;20(1-2):45–53.
Milden SP, Stokols D. Physicians' attitudes and practices regarding complementary and alternative medicine. Behav Med. 2004;30(2):73–84.
Ge J, Fishman J, Vapiwala N, Li SQ, Desai K, Xie SX, et al. Patient-physician communication about complementary and alternative medicine in a radiation oncology setting. Int J Radiat Oncol Biol Phys. 2013;85(1):e1–6.
Berretta M, Rinaldi L, Taibi R, Tralongo P, Fulvi A, Montesarchio V, et al. Physician attitudes and perceptions of complementary and alternative medicine (CAM): a Multicentre Italian study. Front Oncol. 2020;10:594.
Barraco D, Valencia G, Riba AL, Nareddy S, Draus CB, Schwartz SM. Complementary and alternative medicine (CAM) use patterns and disclosure to physicians in acute coronary syndromes patients. Complement Ther Med. 2005;13(1):34–40.
Adler SR, Wrubel J, Hughes E, Beinfield H. Patients' interactions with physicians and complementary and alternative medicine practitioners: older women with breast cancer and self-managed health care. Integr Cancer Ther. 2009;8(1):63–70.
Schiff E, Frenkel M, Shilo M, Levy M, Schachter L, Freifeld Y, et al. Bridging the physician and CAM practitioner communication gap: suggested framework for communication between physicians and CAM practitioners based on a cross professional survey from Israel. Patient Educ Couns. 2011;85(2):188–93.
Venkat Narayan K, Gregg E, Engelgau M, Moore B, Thompson T, Williamson D, et al. Translation research for chronic disease: the case of diabetes. Diabetes Care. 2000;23(12):1794–8.
Hiss RG. The concept of diabetes translation: addressing barriers to widespread adoption of new science into clinical care. Diabetes Care. 2001;24(7):1293–6.
Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–87.
Neven AC, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36(1):5–12.
Tay CT, Joham AE, Hiam DS, Gadalla MA, Pundir J, Thangaratinam S, et al. Pharmacological and surgical treatment of nonreproductive outcomes in polycystic ovary syndrome: An overview of systematic reviews. Clin Endocrinol. 2018;89(5):535–53.
Cinar N, Harmanci A, Demir B, Yildiz BO. Effect of an oral contraceptive on emotional distress, anxiety and depression of women with polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(6):1840–5.
Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–74.
Download references
Acknowledgements
CE is supported by an endowment from the Jacka Foundation of Natural Therapies. SL and AM are supported by National Health and Medical Research Council of Australia fellowships. LM is supported by a National Heart Foundation Future Leader Fellowship.
Author information
Authors and affiliations.
Monash Centre for Health Research and Implementation, Monash University, Clayton, Victoria, Australia
Stephanie Cowan, Chelsea Alycia, Melanie Gibson-Helm, Negar Naderpoor, Aya Mousa, Simon Alesi & Lisa Moran
Eastern Health Clinical School, Monash University, Box Hill, Victoria, Australia
Health and Social Care Unit, Monash University, Clayton, Victoria, Australia
Stephanie Pirotta
Robinson Research Institute, The University of Adelaide, North Adelaide, South Australia, Australia
Rebecca Thomson
Te Tātai Hauora o Hine – National Centre for Women’s Health Research Aotearoa, Te Herenga Waka - Victoria University of Wellington, Wellington, New Zealand
Melanie Gibson-Helm
Centre for Mental Health, Swinburne University of Technology, Hawthorn, Victoria, Australia
Rebecca Blackmore
Department of Nutrition, Dietetics and Food, Monash University, Notting Hill, Victoria, Australia
Christie Bennett
NICM Health Research Institute, Western Sydney University, Westmead, New South Wales, Australia
Carolyn Ee & Vibhuti Rao
You can also search for this author in PubMed Google Scholar
Contributions
SC, SL, CA, SP, RT, MG, RB, NN, CB, CE, VR, AM, SA and LM reviewed the literature and wrote the first draft of the manuscript. SC, SL, CA, SP, RT, MG, RB, NN, CB, CE, VR, AM, SA and LM revised and edited the manuscript. SC and LM conceptualised and determined the scope of the manuscript and had primary responsibility for the final content. LM supervised the review process. All authors meet ICMJE criteria for authorship and approved the final version for publication.
Corresponding author
Correspondence to Stephanie Cowan .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cowan, S., Lim, S., Alycia, C. et al. Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity. BMC Endocr Disord 23 , 14 (2023). https://doi.org/10.1186/s12902-022-01208-y
Download citation
Received : 20 April 2022
Accepted : 09 November 2022
Published : 16 January 2023
DOI : https://doi.org/10.1186/s12902-022-01208-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- physical activity
- cognitive behavioural therapy
- quality of life
- complementary medicine
BMC Endocrine Disorders
ISSN: 1472-6823
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
So, follow the steps below to write a research summary that sticks. 1. Read the parent paper thoroughly. You should go through the research paper thoroughly multiple times to ensure that you have a complete understanding of its contents. A 3-stage reading process helps.
The most logical way to structure quantitative results is to frame them around your research questions or hypotheses. For each question or hypothesis, share: A reminder of the type of analysis you used (e.g., a two-sample t test or simple linear regression). A more detailed description of your analysis should go in your methodology section.
Reporting Research Results in APA Style | Tips & Examples. Published on December 21, 2020 by Pritha Bhandari.Revised on January 17, 2024. The results section of a quantitative research paper is where you summarize your data and report the findings of any relevant statistical analyses.. The APA manual provides rigorous guidelines for what to report in quantitative research papers in the fields ...
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
The results chapter (also referred to as the findings or analysis chapter) is one of the most important chapters of your dissertation or thesis because it shows the reader what you've found in terms of the quantitative data you've collected. It presents the data using a clear text narrative, supported by tables, graphs and charts.
A summary must be coherent and cogent and should make sense as a stand-alone piece of writing. It is typically 5% to 10% of the length of the original paper; however, the length depends on the length and complexity of the article and the purpose of the summary. Accordingly, a summary can be several paragraphs or pages, a single paragraph, or ...
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and ...
Figure 14.1.a provides an example of a 'Summary of findings' table. Figure 15.1.b provides an alternative format that may further facilitate users' understanding and interpretation of the review's findings. Evidence evaluating different formats suggests that the 'Summary of findings' table should include a risk difference as a ...
Table of contents. Step 1: Restate the problem. Step 2: Sum up the paper. Step 3: Discuss the implications. Research paper conclusion examples. Frequently asked questions about research paper conclusions.
A typical research summary includes the following key sections: introduction (including the research question or objective), methodology (briefly describing the research design and methods), results (summarizing the key findings), discussion (highlighting the implications and significance of the findings), and conclusion (providing a summary of ...
Step 1: Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study. The guidelines will generally outline specific requirements for the results or findings section, and the published articles will ...
A Summary of Findings (SoF) table provides a summary of the main results of a review together with an assessment of the quality or certainty1 of the evidence (assessed using the GRADE tool) upon which these results are based. Assessing the certainty of the evidence for each outcome using GRADE is now compulsory in all new and updated reviews.
E. Research Methodology (Summary) In paragraph form, describe the basic elements of your research design. Use words that lay practitioners will understand. Word Limit: About 200 words for each separate experiment or study that is described (up to 500 words if three or more experiments or separate studies were conducted).
Draft Summary of Findings: Draft a paragraph or two of discussion for each finding in your study. Assert the finding. Tell the reader how the finding is important or relevant to your studies aim and focus. Compare your finding to the literature. Be specific in the use of the literature. The link or connection should be clear to the reader.
Research Summary Example 2. Below is another sample sketch, also from an imaginary article. Title - "The frequency of extreme weather events in US in 2000-2008 as compared to the '50s". Introduction - Weather events bring immense material damage and cause human victims.
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
5.3 Summary of Findings . ... The findings for Research Question 4 revealed ... A short presentation based on the first 2 Research Questions from my PhD thesis and examples of some of my findings.
Examples of Summaries. Here are a few examples that will help you get a clearer view of how to write a summary. Example 1: Summary of a News Article. Original Article: The article reports on the recent discovery of a rare species of frog in the Amazon rainforest. The frog, named the "Emerald Whisperer" due to its unique green hue and the ...
Here's a few steps on how to make a first draft: First, state the research question in the introduction of your summary. This holds the ground as to the summary's direction. Provide an explanation why your research is interesting and how it can help your target recipients. Second, state the hypothesis you wish to prove.
A more complete description of the design process, the parameters used in the design process, and typical values for the parameters is presented In the "Proposed Design Guidelines for Improving Pavement Surface Drainage" (2) alla in Appendix A. fIN1)INGS The following findings are based on the research accomplished during the project, a survey ...
Summary of findings and research gaps. The 2018 International Evidence-Based Guideline for the Assessment and Management of PCOS highlights lifestyle (diet, ... For example, to sustain patient engagement in women who express the desire to experiment with supplementation, healthcare providers could consider inositol supplementation, using a ...