An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data
Julie-anne quinn, flor m munoz, bernard gonik, lourdes frau, clare cutland, tamala mallett-moore, aimee kissou, frederick wittke, wendy watson, ana-maria alguacil ramos, jose f cordero, wan-ting huang, sonali kochhar, jim buttery.
- Author information
- Copyright and License information
Corresponding author at: Monash Children's Hospital, Victoria, Australia. Tel.: +61 3 95944828.Monash Children's HospitalVictoriaAustralia [email protected]
Brighton Collaboration homepage: http://www.brightoncollaboration.org .
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Preterm birth is commonly defined as any birth before 37 weeks completed weeks of gestation. An estimated 15 million infants are born preterm globally, disproportionately affecting low and middle income countries (LMIC). It contributes directly to estimated one million neonatal deaths annually and is a significant contributor to childhood morbidity. However, in many clinical settings, the information available to calculate completed weeks of gestation varies widely. Accurate dating of the last menstrual period (LMP), as well as access to clinical and ultrasonographic evaluation are important components of gestational age assessment antenatally. This case definition assign levels of confidence to categorisation of births as preterm, utilising assessment modalities which may be available across different settings. These are designed to enable systematic safety evaluation of vaccine clinical trials and post-implementation programmes of immunisations in pregnancy.
Keywords: Preterm birth, Adverse event, Antenatal, Immunisation, Guidelines, Case definition
1. Preamble
1.1. need for developing case definitions and guidelines for data collection, analysis, and presentation for preterm birth as an adverse event following immunisation.
Preterm birth has been defined as any birth before 37 weeks completed weeks of gestation. An estimated 15 million infants are born preterm, with resulting complications. It is the principal cause of an estimated one million neonatal deaths annually and a significant contributor to childhood morbidities. Low and middle income countries (LMIC) carry a higher burden of disease attributed to preterm birth.
The World Health Organisation (WHO) defines preterm birth as any birth before 37 completed weeks of gestation, or fewer than 259 days since the first day of the woman's last menstrual period (LMP). This is further subdivided on the basis of gestational age (GA):
extremely preterm (<28 weeks);
very preterm (28–<32 weeks);
moderate or late preterm (32–<37 completed weeks of gestation).
This is the most extensively used and accepted definition of preterm birth [1] .
The ability to accurately determine the completed weeks of gestation varies widely between pregnancies, with the most precise assessment methods not uniformly available across different settings. Vaccination in pregnancy has been widely implemented to protect women and their babies from tetanus and pertussis in recent years, with an increasing number of vaccines being developed and trialled for use in pregnancy against a variety of bacterial and viral infections. As preterm birth is such an important pregnancy outcome that may represent an adverse event, it is important to establish a case definition for use across vaccine studies and post-licensure surveillance that is able to make use of all methodologies used to calculate gestational age, and that incorporates a hierarchy based upon the precision of the various methods used.
The nomenclature of GA is typically discussed in terms of the number of completed weeks (e.g., 33 weeks and 2 days, or 33 2/7 weeks). Defining GA has been considered useful in terms of neonatal outcome. In the past, three groups have been classified and utilised according to delivery following the onset of the last menstrual period. Pre-term : less than 259 days (37 weeks), term : 259–293 days (37–41 weeks). Post-term : 294 days (42 weeks) or more.
A term birth has been defined as between 37 and 42 weeks and used to describe the optimal timing for a good outcome for the mother and baby. The International Classification of Diseases defines term pregnancy as a delivery from 37 completed weeks to less than 42 completed weeks (259–293 days) of gestation. However, neonatal outcomes vary within this wide gestational age range, with a 2012 international stakeholder working group recommending sub-categorisation of term birth to more accurately describe deliveries and their outcomes. These sub-categories are: early term (37 0/7 weeks of gestation through 38 6/7 weeks gestation); full term (39 0/7 weeks of gestation through 40 6/7 weeks of gestation); late term (41 0/7 weeks of gestation through 41 6/7 weeks of gestation); and, post term (42 0/7 weeks of gestation and beyond). The American College of Obstetricians and Gynaecologists (ACOG) and the Society for Maternal–Foetal Medicine (SMFM) has endorsed this recommendation and encourages its use for categorising GA [2] , [3] , [4] , [5] .
1.1.1. Pathophysiology of preterm birth
Causes of preterm birth are complex and the pathophysiology that triggers preterm birth is largely unknown, however, contributing maternal, foetal and placental predisposing factors have been identified. The most common of these include: antepartum haemorrhage or abruption; mechanical factors such as uterine over-distention and cervical incompetence; hormonal changes; and, bacterial infection and inflammation [6] , [7] .
Over the past 20 years the access to assisted reproduction technology (ART) in many high income countries has contributed to the rise in the number of multiple births and an overall increase in the rates of preterm delivery. Infants born from multiple pregnancies are more likely to be born preterm due to spontaneous labour or premature rupture of membranes (PROM), or as a result of maternal conditions such as pre-eclampsia or foetal disorders [8] , [9] . Changes to policies which limit the number of embryos implanted as part of ART have led to a decline in the number of preterm births due to assisted fertility [10] , [11] .
Epidemiologic studies have identified preterm birth risk factors as maternal age of less than 17 years or more than 35 years, being underweight, having an overweight pre-pregnancy body mass index, and short stature. Preterm birth rates vary geographically and within ethnic origins, with LMIC consistently having higher rates [7] , [12] . Physical and psychosocial stress and smoking have also been associated with higher preterm risk as does a previous preterm birth.
The assessment and diagnosis of preterm birth has remained problematic since it is not a defined disease and the WHO definition does not contain universally recognised reference standards. Different methodologies are used for assessing GA and because reporting rates vary widely between and within countries, accurate comparison of reporting rates of preterm birth and trending data is difficult to analyse [13] , [14] , [15] , [16] , [17] .
1.1.2. Preterm birth categorisation
Preterm birth defined as less than 37 completed weeks encompasses a wide gestational age range with rates varying across countries. The WHO subcategories of ‘extremely preterm’, ‘very preterm’ and ‘moderate or late preterm’ are recommended to improve comparability of preterm birth data in relation to immunisation.
A limitation of the WHO definition is that there is no boundary between spontaneous abortion and a viable birth, complicating the assessment of preterm birth in the extremely preterm group of babies. A comparison between and within countries becomes complex with varying gestational lower limits of viability over time and across different settings. Determining a lower limit is complex as it is variably defined and arbitrary. It is often described in terms of risk factors and its causes, and is predominately developed according to postnatal viability and data quality in different settings [17] , [18] , [19] , [20] .
Preterm births are reported only for live born infants. The pregnancy outcomes differ across countries where the upper limit for national or regional criteria for registration of a foetal death range from 16 weeks to 28 weeks, this impacting on the proportion of preterm births [21] .
The registrations of births in LMIC often do not routinely record GA and the data on birthweight (BW) is often not recorded or compiled. It has been reported that 58% of babies in these countries are not weighed at birth and home based births are not represented [20] , [22] , [23] .
1.1.3. Preterm birth following immunisation: what is known in literature?
Pregnant women are at increased risk of morbidity and mortality and adverse pregnancy outcomes, including preterm birth, due to vaccine preventable diseases. Vaccination in pregnancy is a recognised preventive measure for protecting the mother, foetus and infant [24] , [25] , [26] , [27] .
Until the 1960s vaccines, including polio, influenza, diphtheria and tetanus toxoid vaccines, were routinely administered to pregnant women in maternal immunisation programmes. Studies in a variety of developed settings detected no increase in adverse consequences for the mother or foetus in vaccinated women [28] , [29] . However, the thalidomide teratogenicity disaster in pregnant women resulted in widespread concerns about the safety of all medicine use in pregnancy, including vaccines. Vaccines were then recommended to be only administered in the third trimester of pregnancy to prevent any attribution of teratogenicity risk, as well as to minimise the potential risk to the course of normal gestation such as induction of premature labour [30] , [31] .
Over recent decades, with further development of safe and immunogenic vaccines, as well as improved ability to explore pregnancy outcome datasets, ongoing studies have provided important information on vaccine safety. Immunisation with inactivated vaccines and toxoids during pregnancy has not been associated with any increased risk to the mother or baby. The extensive use of Tetanus Toxoid (TT) and Tetanus diphtheria (Td) in pregnant women, to prevent neonatal tetanus, has shown no clinically significant adverse events and no adverse pregnancy outcomes for women who have received the Tetanus, diphtheria, acellular pertussis (Tdap) vaccine during pregnancy [32] , [33] , [34] .
The US Advisory Committee on Immunisation Practices (ACIP) in 2012 updated their recommendations to providers of prenatal care to implement a Tdap immunisation programme for all pregnant women to reduce the burden of pertussis in infants. Its recommendation is for its use with every pregnancy. Similarly, in October 2012, the United Kingdom Department of Health recommended a temporary Tdap programme in pregnancy in response to an outbreak [35] , [36] , [37] , [38] . An observational cohort study linking more than 20,000 vaccinated women with pregnancy outcomes showed no increase in stillbirth or other major complications, including preterm birth [39] . Immunisation of pregnant women with inactivated trivalent influenza vaccine has also been recommended and endorsed for more than a decade showing no increase in adverse events. Pregnant women who received H1N1 influenza vaccine during the 2009 H1N1 influenza pandemic were in fact less likely to give birth preterm [40] , [41] , [42] , [43] , [44] , [45] , [46] , [47] .
Live viral vaccines, such as measles, mumps, rubella (MMR); varicella; intranasal live-attenuated influenza; Yellow Fever, and BCG however are contraindicated and not recommended during pregnancies, with a theoretical risk that the vaccine virus could be transmitted to the foetus [48] . Follow up of inadvertent vaccinations of pregnant women with live vaccines have not demonstrated significant adverse effects but these limited data have not been sufficient to change recommendations. The risk benefit to the mother and neonate needs to be taken into account.
1.1.4. Existing case definitions for preterm birth
Historically, preterm birth was determined using neonatal physical examination, reviewing clinical history and socio-demographics [49] , [50] . Early definitions of prematurity relied on BW, using a birth weight category of less than 2300 or 2500 g. One of the earliest working definitions was introduced by the World Health Assembly (WHA) in 1948 using a birth weight of 2500 g (5 pounds, 8 ounces) or less as a determinant [49] .
Early epidemiological studies of prematurity tended to include all low birth weight babies irrespective of gestation. BW was used as a criterion alone as it was objective, easily measured, and the survival of the very low birth weight (VLBW) neonates was described in birth weight specific categories [51] , [52] , [53] , [54] . BW however, can only be used as a surrogate in the lower gestational age babies where it has been identified to be a specific and sensitive method in assessing the early preterm. Babies weighing less than 1500 g are predominately assessed as being preterm [55] , [56] . The lack of standardised birthweight categories makes it difficult to analyse and compare data from different regions [22] , [57] , [58] , [59] .
However, BW based standards for preterms are complicated by physiological variables that occur more commonly pregnancies complicated by preterm birth [60] , [61] . Preterm infants are often growth restricted and conventional BW charts are limited as they do not reflect the degree of growth restriction. The Foetal Growth Longitudinal Study (FGLS), part of the INTERGROWTH-21 Project developed international growth and size standards for foetuses. The growth standards are recommended for the clinical interpretation of ultrasound measurements and for comparisons across populations [62] . Foetal growth standards for preterm infants will determine the precise incidence of foetal growth restriction when gestation is known [63] , [64] , [65] .
The World Health Organisation (WHO) definition of preterm birth remains the most widely utilised and accepted definition. The International Classification of Diseases (ICD-9, ICD-10) defines preterm birth as less than 37 completed weeks (less than 259 days) of gestation. The duration of gestation is measured from the first day of the LMP. GA is expressed in completed days or completed weeks (e.g., events occurring 280–286 completed days after the onset of the LMP are considered to have occurred at 40 weeks of gestation). Where the date of the LMP is not available, GA is based on the best clinical estimate [2] .
A search of terminology databases, including the Global Alliance on Prevention of Prematurity (GAPPS), the National Institutes of Health, and the Common Terminology Criteria for Adverse Events (CTCAE) show a consistency with the WHO definition as the onset of labour before 37 completed weeks of pregnancy (full term is 40 completed weeks) [7] , [66] .
Essential in any definition or the sub classification of preterm birth is the need for accurate dating. GA has evolved as the stand alone parameter for determining preterm birth. In 1949 the U.S. National Centre for Health Statistics of the Centres for Disease Control and Prevention revised the World Health Assembly definition and deleted the reference to BW, to include only the reporting of the length of pregnancy in weeks. In 1956 this was further revised to specify the reporting of completed weeks of gestation [67] .
A 1961 report by the Expert Committee on Maternal and Child Health of the World Health Organisation highlighted the difference between premature and those infants of low birth weight (LBW) and in 1970 a working party of obstetricians and paediatricians at the Second European Congress of Perinatal Medicine set the boundary between preterm and term birth at 37 weeks of gestation [5] .
This is the basis of the most recent widely utilised and accepted definition of prematurity [68] .
Before the development of more accurate methods of estimating gestation, the LMP remains as the most widely available measure. This method is used where ultrasound (US) is not available or accessible and is recommended by the WHO for determining preterm birth [12] , [69] , [70] .
When available, in the clinical context, it is a valid and applicable measure, especially when estimating gestation of less than or equal to 33 weeks. Its limitations are discussed as being a determinant based on self-reporting and therefore felt to be imprecise. Studies have shown, however, that women who were certain of their LMP were accurate in their assessment of pregnancy duration compared with their ultrasound dating [71] .
When information about the LMP is absent or uncertain, estimates of gestational age can be determined from a clinical assessment including the description of pregnancy symptoms such as nausea, fatigue, tender swollen breasts, frequent urination, a pelvic examination when performed in the first trimester and fundal height (FH) ascertainment. One study demonstrated a high correlation between early pregnancy dating up to 9 completed weeks by a clinician based on an examination and history and that determined by ultrasound but this is influenced by the skill and experience of the clinician [72] .
Fundal height (FH) is often used in conjunction with LMP and/or the BW of the neonate, especially in low resource settings. Women from many traditional societies however often do not record their LMP date and can present late in the first trimester. Variability in FH measurements relate to previous caesarean section (C/S), multiple pregnancy, race, maternal height, intrauterine growth retardation, maternal obesity, polyhydramnios, and a difference in examination techniques. FH measurement is not standardised and use beyond 16 weeks reduces in accuracy, affecting its reliability and the precision of dating [58] , [73] , [74] . Its use in combination with more sensitive assessment methods is recommended.
Through the advent of US, the use of early home pregnancy tests, ART such as intrauterine insemination (IUI), and home ovulation test kits, the actual timing of conception can be determined and therefore accurate dating of gestational age performed. Pregnancies achieved through ART represent the most accurate method.
Outside the use of ART, an ultrasound performed in the first trimester (≤13 6/7 weeks), is viewed as the most accurate and reliable measure. There has been a shift from using LMP to using US for predicting an actual date of delivery. Estimation of the foetal crown rump length ± biparietal diameter/femur length between the gestational age of 6–18 weeks shows an accuracy within 5–7 days. In women with uncertain dates an early US is recommended for optimal dating [3] .
The methodology for US gestational age assessment, however, is not standardised and tends to give a transitory increase in preterm births when compared to the use of LMP alone. In addition, US accessibility in LMIC is limited for the majority of women and therefore cannot be considered a universal measure for determining preterm birth [20] , [57] , [75] , [76] , [77] , [78] . Defining preterm delivery for LMIC would therefore be strengthened by the use of one or both measures when available [4] , [79] , [80] , [81] , [82] , [83] .
Where measurements such as LMP, US or antenatal clinical assessment are absent or likely to be inaccurate, a recommended criterion for determining preterm birth is a clinical estimation of gestational age based on the physical and neurological examination of the neonate [84] , [85] . A review of methods used identified tools based on neurological and physical criteria, or physical criteria alone. Methods that use neurologic criteria are proven, reliable measures with expert operators but the feasibility for use is compromised especially in LMIC, being limited by complexity, and requiring skill and experience to perform [70] , [86] , [87] , [88] , [89] . Ascertainment of neurologic signs and external characteristics used in the assessment need to be precise and accurate to ensure correct correlation with actual GA [51] , [90] , [91] .
The physical examination based systems have been refined and modified to improve their applicability and accuracy. Some methods now use external characteristics alone, enabling gestational age to be determined and estimated in all settings. Using the available methods ranging in the current criteria, there is a correlation with LMP based estimation of over 90%, but an acknowledged range of error for predicting clinical maturity of ±2.4 weeks. Physical examination tools alone, when used for neonates under 28–33 weeks are recognised to be inaccurate and are therefore are not recommended to be used as a measure to accurately estimate the gestation of neonates within the lower limit of viability [92] , [93] , [94] , [95] , [96] , [97] .
The Ballard Maturational Score, known as the New Ballard Score, uses both physical and neurological assessment and has been refined and expanded to include extremely premature neonates and is described as a valid and accurate gestational assessment tool [98] .
1.2. Methods for the development of the case definition and guidelines for data collection, analysis, and presentation for preterm birth as an adverse events following immunisation
Following the process described in the overview paper as well as on the Brighton Collaboration Website http://www.brightoncollaboration.org/internet/en/index/process.html , the Brighton Collaboration Preterm Birth Working Group was formed in 2015 and included members of clinical, academic, public health, and industry background. The composition of the working and reference group as well as results of the web-based survey completed by the reference group with subsequent discussions in the working group can be viewed at: http://www.brightoncollaboration.org/internet/en/index/workinggroups.html .
To guide the decision-making for the case definition and guidelines, a literature search was performed using Medline, Embase, PubMed, Cinahl, Clincal Key and the Cochrane Libraries for published work relevant to this review with the search terms including: ‘prematurity’; ‘preterm birth’; ‘neonatal outcomes’; ‘birth outcomes’; ‘gestational age’; ‘premature labour’; ‘preterm delivery’; ‘spontaneous labour’; ‘antenatal’; ‘low birth weight’; ‘neonatal mortality’; ‘stillbirth’; ‘extremely preterm’; ‘moderate preterm’; ‘late preterm’; ‘neurological assessment’; ‘vaccination’; ‘immunisation’; ‘viability’; ‘spontaneous labour’; ‘epidemiology’; ‘perinatal outcomes’; ‘last menstrual period’; ‘LMP’; ‘ultrasound’; ‘US’; ‘pregnancy duration’; ‘obstetrics’; ‘morbidity’; ‘small for gestational age’; ‘birthweight’; ‘foetal growth’; ‘multiple pregnancy’; ‘pregnancy’; ‘risk factors’; ‘fundal height’; and terms in combination. We focused on work published published in English language, but also included commonly referenced older publications. The search resulted in the identification of references, 262 articles with potentially relevant material were reviewed in more detail in order to identify studies using case definitions or, in their absence, providing clinical descriptions of the case material. This review resulted in a detailed summary of 99 articles, including information on the diagnostic criteria or case definition put forth.
1.3. Rationale for selected decisions about the case definition of preterm birth as an adverse event following immunisation
An ideal standardised case definition aims to improve reliability and comparability of data collected from immunised mothers that deliver full term or preterm infants across all health care settings. A functional case definition is important for the evaluation and assessment of data to help determine whether a vaccine administered during pregnancy may or may not be implicated in a subsequent preterm birth. The definition must be applicable in regions that are geographically and administratively diverse, regardless of available health care personnel, training and resources.
There has never been a single unanimously accepted definition of prematurity. The literature identified was notable for inconsistent definitions and numerous descriptions for preterm birth. Existing definitions categorise preterm birth by clinical presentation, BW and GA.
There is hence no uniformly accepted definition of preterm birth that is able to be employed to evaluate its occurrence following antenatal immunisations. This poses a missed opportunity and a potential risk to immunisation trials and programmes as well as pregnant women, as potential associations may be falsely raised or dismissed without an agreed case definition. Data comparability across a myriad of trials and varying surveillance systems would facilitate data interpretation and improve the ability of investigators and regulators to confidently detect or rule out any association between immunisations in pregnancy and preterm birth. The work group recommended the use of the current definition for Preterm Birth with the subcategories of extremely preterm, very preterm and moderate or late preterm. Accurate dating is essential for the definition, with GA assessment criteria implicit for categorising.
1.3.1. Formulating a case definition that reflects diagnostic certainty: weighing specificity versus sensitivity
It needs to be re-emphasised that the grading of definition levels is entirely about diagnostic certainty of the event, not its clinical severity. Thus, a very severe clinical event, like Preterm Birth, may appropriately be classified as Level Two or Three rather than Level One, based upon the level confidence the birth truly occurred preterm.
The amount of information, including number of symptoms and/or signs, that will be documented for each case may vary considerably. The case definition has been formulated such that the Level 1 definition is highly specific (or confident) for preterm birth. As maximum specificity normally implies a loss of sensitivity, two additional diagnostic levels have been included in the definition, offering a stepwise increase of sensitivity from Level One down to Level Three, while retaining an acceptable level of specificity at all levels. In this way it is hoped that all possible cases of Preterm Birth can be captured.
1.3.2. Precision of gestational age assessment
The most accurate methods for GA assessment are included in Level 1 of diagnostic certainty. The GAIA Preterm Birth working group considered that pregnant women with a certain menstrual date or those who have undergone IUI or Embryo Transfer (ET) with a confirmatory 1st trimester scan (≤13 6/7 weeks) or a 1st trimester ultrasound established date (≤13 6/7 weeks) alone, as representing the “gold standard” of diagnostic certainty of GA assessment, and therefore the likelihood of preterm birth. If the LMP date and U/S date do not correlate, defaulting to U/S for GA assessment is required.
Where there is no 1st trimester scan or ART performed, Level 2A of diagnostic certainty is used to describe gestational dating. With a certain menstrual date, either a confirmatory 2nd trimester ultrasound established date (14 0/7 weeks to 27 6/7 weeks) or a 1st trimester pelvic bimanual examination are considered the next most precise measurement methodologies. Where there is no menstrual date, it is recommended that the 2nd trimester ultrasound established date be used and categorised as Level 2B.
Level 3A of diagnostic certainty represents a certain menstrual date with a 3rd trimester scan of 28 0/7 weeks+, or a confirmatory 2nd trimester fundal height, or birth weight. Where there is no menstrual date, a 1st trimester pelvic bimanual examination would meet the requirement. A separate level 3B category was recommended where there is an uncertain or no menstrual date. In this case a fundal height, or newborn physical assessment or birth weight would meet the requirement for this level.
1.3.2.1. Timing post immunisation in pregnancy
In the absence of existing data supporting an association between any vaccine and preterm birth, the working group did not feel it was appropriate to define a ‘risk window’ following vaccination during pregnancy and subsequent possible Preterm Birth. The case definition of the outcome (Preterm Birth) is independent from the exposure (e.g., immunisations). Therefore, to avoid selection bias, a restrictive time interval from immunisation to onset of Preterm Birth has not been included. Instead, where feasible, details of this interval should be assessed and reported as described in the data collection guidelines.
Further, Preterm Birth often occurs outside the controlled settings of clinical trials or hospitals. In some settings it may be impossible to obtain a clear timeline of the event, particularly in less developed or rural settings. In order to avoid excluding such cases, the Brighton Collaboration case definition avoids setting arbitrary time frames.
1.4. Guidelines for data collection, analysis and presentation
As mentioned in the overview paper, the case definition is accompanied by guidelines which are structured according to the steps of conducting a clinical trial, i.e. data collection, analysis and presentation. Neither case definition nor guidelines are intended to guide or establish criteria for management of ill infants, children, or adults. Both were developed to improve data comparability.
1.5. Periodic review
Similar to all Brighton Collaboration case definitions and guidelines, review of the definition with its guidelines is planned on a regular basis (i.e. every three to five years) or more often if needed.
2. Case definition of preterm birth
2.1. prematurity and assessment of gestational age criteria.
Definitions of terms used:
Intrauterine insemination (IUI) – A procedure in which a fine catheter is inserted through the cervix into the uterus to deposit a sperm sample directly into the uterus, to achieve fertilisation and pregnancy.
Embryo transfer – The procedure in which one or more embryos are placed in the uterus or fallopian tube.
Ultrasound (U/S) [62] :
1st trimester (≤13 6/7 weeks).
2nd trimester scan (14 0/7–27 6/7 weeks).
3rd trimester (28 0/7 + weeks).
LMP (last menstrual period) – Gestational age is calculated from the first day of the mother's last menstrual period.
If LMP and U/S do not correlate, default to U/S GA assessment
*Certain LMP: (LMP date + 280 days): Use LMP if within 7 days at ≤14 weeks; within 14 days at ≤26 weeks; within 21 days beyond 26 weeks.
*Uncertain LMP – first trimester (≤13 6/7 weeks by LMP): Use the approximate date of the last menstrual period (LMP) if corroborated by physical exam, or a first trimester ultrasound. If there is a discrepancy of >7 days between the LMP and the first trimester ultrasound, the ultrasound-established dates will take preference over LMP for gestational age dating.
*Uncertain LMP – second trimester (14 0/7–27 6/7 weeks by LMP): Use the approximate date of the LMP if corroborated by physical exam including fundal height, or a second trimester ultrasound. If there is a discrepancy of >10 days between the LMP and the second trimester ultrasound, the ultrasound-established dates will take preference over LMP for gestational age dating.
*Uncertain LMP – third trimester >28 weeks – third trimester ultrasound.
*No LMP date: If menstrual dates are unknown, the ultrasound-established dates will be used for gestational age dating or 2nd trimester fundal height and/or newborn physical examination.
Pregnancy symptoms – nausea, fatigue, tender swollen breasts, frequent urination.
Antenatal Physical Examination – pelvic bimanual examination confirming enlarged uterus [63] .
Newborn Physical Examination – New Ballard Score – physical and neurological assessment – Appendix 1.
Fundal Height (FH) in cms – Appendix 2.
Birth Weight (BW) in grams – Appendix 2.
2.2. Prematurity and assessment of gestational age
Level 1: (highest level of certainty)
Certain LMP* or intrauterine insemination (IUI) date or embryo transfer (ET) date with confirmatory 1st trimester scan (≤13 6/7 weeks).
1st trimester scan (≤13 6/7 weeks).
Level 2A
Certain LMP* with 2nd trimester scan (14 0/7 weeks to 27 6/7 weeks). If LMP and U/S do not correlate, default to U/S GA assessment.
Certain LMP* with 1st trimester physical examination.
Level 2B
Uncertain LMP with 2nd trimester scan (14 0/7 weeks to 27 6/7 weeks).
Level 3A
Certain LMP with 3rd trimester scan – 28 0/7 weeks +.
Certain LMP with confirmatory 2nd trimester FH.
Certain LMP with birth weight.
Uncertain LMP with 1st trimester physical examination.
Level 3B
Uncertain LMP with FH.
Uncertain LMP with newborn physical assessment.
Uncertain LMP with Birth weight.
3. Guidelines for data collection, analysis and presentation of preterm birth
It was the consensus of the Brighton Collaboration Working Group for Preterm Birth to recommend the following guidelines to enable meaningful and standardised collection, analysis, and presentation of information about Preterm Birth. However, implementation of all guidelines might not be possible in all settings. The availability of information may vary depending upon resources, geographical region, and whether the source of information is a prospective clinical trial, a post-marketing surveillance or epidemiological study, or an individual report of Preterm Birth. Also, as explained in more detail in the overview paper in this volume, these guidelines have been developed by this working group for guidance only, and are not to be considered a mandatory requirement for data collection, analysis, or presentation.
3.1. Data collection
These guidelines represent a desirable standard for the collection of data on availability following immunisation to allow for comparability of data, and are recommended as an addition to data collected for the specific study question and setting. The guidelines are not intended to guide the primary reporting of Preterm Birth to a surveillance system or study monitor. Investigators developing a data collection tool based on these data collection guidelines also need to refer to the criteria in the case definition, which are not repeated in these guidelines.
Guidelines numbers below have been developed to address data elements for the collection of adverse event information as specified in general drug safety guidelines by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [99] , and the form for reporting of drug adverse events by the Council for International Organisations of Medical Sciences [100] . These data elements include an identifiable reporter and patient, one or more prior immunisations, and a detailed description of the adverse event, in this case, Preterm Birth following maternal immunisation. The additional guidelines have been developed as guidance for the collection of additional information to allow for a more comprehensive understanding of Preterm Birth following maternal immunisation.
3.1.1. Source of information/reporter
For all cases and/or all study participants, as appropriate, the following information should be recorded:
Date of report.
Name and contact information of person reporting 2 and/or diagnosing the Preterm Birth as specified by country-specific data protection law.
Name and contact information of the investigator or clinician responsible for the subject, as applicable.
Relation to the patient (e.g., immuniser [clinician, nurse], family member [indicate relationship], other).
3.1.2. Vaccinee/control
3.1.2.1. demographics.
Case/study participant identifiers (e.g., first name initial followed by last name initial) or code (or in accordance with country-specific data protection laws).
Date of birth, age, and sex.
For infants: gestational age and birth weight.
3.1.2.2. Clinical and immunisation history
For all cases and/or all study participants, as appropriate, the following information regarding the immunised woman should be recorded:
Past medical history, including hospitalisations, underlying diseases/disorders, pre-immunisation signs and symptoms including identification of indicators for, or the absence of, a history of allergy to vaccines, vaccine components or medications; food allergy; allergic rhinitis; eczema; asthma.
Any medication history (other than treatment for the event described) prior to, during, and after immunisation including prescription and non-prescription medication as well as medication or treatment with long half-life or long term effect (e.g., immunoglobulins, blood transfusion and immunosuppressants).
Immunisation history (i.e. previous immunisations and any adverse event following immunisation (AEFI)), in particular occurrence of Preterm Birth after a previous immunisation.
3.1.3. Details of the immunisation
Date and time of maternal immunisation(s).
Description of vaccine(s) (name of vaccine, manufacturer, lot number, dose (e.g., 0.25 mL, 0.5 mL, etc.) and number of dose if part of a series of immunisations against the same disease).
The anatomical sites (including left or right side) of all immunisations (e.g., vaccine A in proximal left lateral thigh, vaccine B in left deltoid).
Route and method of administration (e.g., intramuscular, intradermal, subcutaneous, and needle-free (including type and size), other injection devices).
Needle length and gauge.
3.1.4. The adverse event
For all cases at any level of diagnostic certainty and for reported events with insufficient evidence, the criteria fulfilled to meet the case definition should be recorded.
Specifically document
Clinical description of signs and sympt oms of Preterm Birth in immunised woman and newborn and if there was medical confirmation of the event (i.e. patient seen by physician).
Date/time of onset, 3 first observation 4 and diagnosis, 5 end of episode 6 and final outcome. 7
Concurrent signs, symptoms, and diseases in immunised woman and newborn.
Measurement/testing
Values and units of routinely measured parameters (e.g., temperature, blood pressure) – in particular those indicating the severity of the event.
Method of measurement (e.g., type of thermometer, oral or other route, duration of measurement, etc.).
Results of laboratory examinations, surgical and/or pathological findings and diagnoses if present.
Treatment given for Preterm Birth to mother and/or newborn, especially specify what medication and dosing, or specific interventions.
Outcome 8 at last observation.
Objective clinical evidence supporting classification of the event as “serious”. 9
Exposures other than the immunisation 24 h before and after immunisation (e.g., food, environmental) considered potentially relevant to the reported event.
3.1.5. Miscellaneous/general
The duration of surveillance for Preterm Birth should be until the pregnancy has been completed, but specific surveillance may be further predefined based on
Biologic characteristics of the vaccine e.g., live attenuated versus inactivated component vaccines.
Biologic characteristics of the vaccine-targeted disease.
Biologic characteristics of Preterm Birth including patterns identified in previous trials (e.g., early-phase trials).
Biologic characteristics of the vaccinee (e.g., nutrition, underlying disease like immunodepressing illness).
The duration of follow-up reported during the surveillance period should be predefined likewise. It should aim to continue to resolution of the event.
Methods of data collection should be consistent within and between study groups, if applicable.
Follow-up of cases should attempt to verify and complete the information collected as outlined in data collection guidelines 1–24.
Investigators of patients with Preterm Birth should provide guidance to reporters to optimise the quality and completeness of information provided.
Reports of Preterm Birth should be collected throughout the study period regardless of the time elapsed between immunisation and the adverse event. If this is not feasible due to the study design, the study periods during which safety data are being collected should be clearly defined.
3.2. Data analysis
The following guidelines represent a desirable standard for analysis of data on Preterm Birth to allow for comparability of data, and are recommended as an addition to data analysed for the specific study question and setting.
Reported events should be classified in one of the following five categories including the three levels of diagnostic certainty. Events that meet the case definition should be classified according to the levels of diagnostic certainty as specified in the case definition. Events that do not meet the case definition should be classified in the additional categories for analysis.
Event classification in 5 categories 10
Event meets case definition
Level 1: Criteria as specified in the Preterm Birth case definition.
Level 2: Criteria as specified in the Preterm Birth case definition.
Level 3: Criteria as specified in the Preterm Birth case definition.
Event does not meet case definition
Additional categories for analysis
Reported Preterm Birth with insufficient evidence to meet the case definition. 11
Not a case of Preterm Birth.
The interval between immunisation and reported Preterm Birth could be defined as the date/time of immunisation to the date/time of onset 12 of the newborn delivery. If few cases are reported, the concrete time course could be analysed for each; for a large number of cases, data can be analysed in the following increments:
Subjects with preterm birth by interval to presentation
The duration of a possible Preterm Birth could be analysed as the interval between the date/time of onset 1 of the first symptoms and/or signs consistent with the definition and the end of episode 5 and/or final outcome. 6 Whatever start and ending are used, they should be used consistently within and across study groups.
If more than one measurement of a particular criterion is taken and recorded, the value corresponding to the greatest magnitude of the adverse experience could be used as the basis for analysis. Analysis may also include other characteristics like qualitative patterns of criteria defining the event.
The distribution of data (as numerator and denominator data) could be analysed in predefined increments (e.g., measured values, times), where applicable. Increments specified above should be used. When only a small number of cases is presented, the respective values or time course can be presented individually.
Data on Preterm Birth obtained from subjects receiving a vaccine should be compared with those obtained from an appropriately selected and documented control group(s) to assess background rates of Preterm Birth in non-exposed populations, and should be analysed by study arm and dose where possible, e.g., in prospective clinical trials.
3.3. Data presentation
These guidelines represent a desirable standard for the presentation and publication of data on Preterm Birth following maternal immunisation to allow for comparability of data, and are recommended as an addition to data presented for the specific study question and setting. Additionally, it is recommended to refer to existing general guidelines for the presentation and publication of randomised controlled trials, systematic reviews, and meta-analyses of observational studies in epidemiology (e.g., statements of Consolidated Standards of Reporting Trials (CONSORT), of Improving the quality of reports of meta-analyses of randomised controlled trials (QUORUM), and of Meta-analysis Of Observational Studies in Epidemiology (MOOSE), respectively [101] , [102] , [103] ).
All reported events of Preterm Birth should be presented according to the categories listed in guideline 31.
Data on possible Preterm Birth events should be presented in accordance with data collection guidelines 1–24 and data analysis guidelines 31–36.
Terms to describe Preterm Birth such as “low-grade”, “mild”, “moderate”, “high”, “severe” or “significant” are highly subjective, prone to wide interpretation, and should be avoided, unless clearly defined.
Data should be presented with numerator and denominator ( n / N ) (and not only in percentages), if available.
Although immunisation safety surveillance system denominator data are usually not readily available, attempts should be made to identify approximate denominators. The source of the denominator data should be reported and calculations of estimates be described (e.g., manufacturer data like total doses distributed, reporting through Ministry of Health, coverage/population based data, etc.).
The incidence of cases in the study population should be presented and clearly identified as such in the text.
If the distribution of data is skewed, median and range are usually the more appropriate statistical descriptors than a mean. However, the mean and standard deviation should also be provided.
Any publication of data on Preterm Birth should include a detailed description of the methods used for data collection and analysis as possible. It is essential to specify:
The study design.
The method, frequency and duration of monitoring for Preterm Birth.
The trial profile, indicating participant flow during a study including drop-outs and withdrawals to indicate the size and nature of the respective groups under investigation.
The type of surveillance (e.g., passive or active surveillance).
The characteristics of the surveillance system (e.g., population served, mode of report solicitation).
The search strategy in surveillance databases.
Comparison group(s), if used for analysis.
The instrument of data collection (e.g., standardised questionnaire, diary card, report form).
Whether the day of immunisation was considered “day one” or “day zero” in the analysis.
Whether the date of onset 2 and/or the date of first observation 3 and/or the date of diagnosis 4 was used for analysis.
Use of this case definition for Preterm Birth, in the abstract or methods section of a publication. 11
Acknowledgements
The authors are grateful for the support and helpful comments provided by the Brighton Collaboration (Jan Bonhoeffer, Jorgen Bauwens) and the reference group (see https://brightoncollaboration.org/public/what-we-do/setting-standards/case-definitions/groups.html for reviewers), as well as other experts consulted as part of the process, including professor Euan Wallace. The authors are also grateful to the Brighton Collaboration Secretariat and to the members of the ISPE Special Interest Group in Vaccines (VAX SIG) for their review and constructive comments on this document. Finally, we would like to acknowledge the Global Alignment of Immunisation Safety Assessment in Pregnancy (GAIA) project, funded by the Bill and Melinda Gates Foundation.
Disclaimer: The findings, opinions and assertions contained in this consensus document are those of the individual scientific professional members of the working group. They do not necessarily represent the official positions of each participant's organisation (e.g., government, university, or corporation). Specifically, the findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their respective institutions.
If the reporting centre is different from the vaccinating centre, appropriate and timely communication of the adverse event should occur.
The date and/or time of onset is defined as the time post immunisation, when the first sign or symptom indicative for Preterm Birth occurred. This may only be possible to determine in retrospect.
The date and/or time of first observation of the first sign or symptom indicative for Preterm Birth can be used if date/time of onset is not known.
The date of diagnosis of an episode is the day post immunisation when the event met the case definition at any level.
The end of an episode is defined as the time the event no longer meets the case definition at the lowest level of the definition.
E.g., recovery to pre-immunisation health status, spontaneous resolution, therapeutic intervention, persistence of the event, sequelae, death.
An AEFI is defined as serious by international standards if it meets one or more of the following criteria: (1) it results in death, (2) is life-threatening, (3) it requires inpatient hospitalisation or results in prolongation of existing hospitalisation, (4) results in persistent or significant disability/incapacity, (5) is a congenital anomaly/birth defect, (6) is a medically important event or reaction.
To determine the appropriate category, the user should first establish, whether a reported event meets the criteria for the lowest applicable level of diagnostic certainty, e.g., Level three. If the lowest applicable level of diagnostic certainty of the definition is met, and there is evidence that the criteria of the next higher level of diagnostic certainty are met, the event should be classified in the next category. This approach should be continued until the highest level of diagnostic certainty for a given event could be determined. Major criteria can be used to satisfy the requirement of minor criteria. If the lowest level of the case definition is not met, it should be ruled out that any of the higher levels of diagnostic certainty are met and the event should be classified in additional categories four or five.
If the evidence available for an event is insufficient because information is missing, such an event should be categorised as “Reported Preterm Birth with insufficient evidence to meet the case definition”.
An event does not meet the case definition if investigation reveals a negative finding of a necessary criterion (necessary condition) for diagnosis. Such an event should be rejected and classified as “Not a case of Preterm Birth”.
Use of this document should preferably be referenced by referring to the respective link on the Brighton Collaboration website ( http://www.brightoncollaboration.org ).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.03.045 .

Appendices 1 and 2. Supplementary data
The following are the supplementary data to this article:
- 1. Howson C.P., Kinney M.V., Lawn J. March of Dimes, PMNCH, Save the Children, WHO; 2012. Born Too Soon: the global action report on preterm birth. [ Google Scholar ]
- 2. World Health Organization . 2010. ICD-10: international statistical classification of diseases and related health problems, tenth revision. [ Google Scholar ]
- 3. Spong C.Y. Defining term pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309:2445–2446. doi: 10.1001/jama.2013.6235. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. ACOG Committee Opinion No 579 Definition of term pregnancy. Obstet Gynecol. 2013;122:1139–1140. doi: 10.1097/01.AOG.0000437385.88715.4a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Working party to discuss nomenclature based on gestational age and birthweight. Arch Dis Child. 1970;45:730. doi: 10.1136/adc.45.243.730. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Villar J., Papageorghiou A.T., Knight H.E., Gravett M.G., Iams J., Waller S.A. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206:119–123. doi: 10.1016/j.ajog.2011.10.866. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Wijnans L., de Bie S., Dieleman J., Bonhoeffer J., Sturkenboom M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine. 2011;29:7559–7571. doi: 10.1016/j.vaccine.2011.08.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Crider K.S., Whitehead N., Buus R.M. Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005;7:593–604. doi: 10.1097/01.gim.0000187223.69947.db. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Blondel B., Kogan M.D., Alexander G.R., Dattani N., Kramer M.S., Macfarlane A. The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: an international study. Am J Public Health. 2002;92:1323–1330. doi: 10.2105/ajph.92.8.1323. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Felberbaum R.E. Multiple pregnancies after assisted reproduction – international comparison. Reprod Biomed Online. 2007;15(Suppl. 3):53–60. doi: 10.1016/s1472-6483(10)62252-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Blondel B., Macfarlane A., Gissler M., Breart G., Zeitlin J., Group P.S. Preterm birth and multiple pregnancy in European countries participating in the PERISTAT project. BJOG. 2006;113:528–535. doi: 10.1111/j.1471-0528.2006.00923.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Simmons L.E., Rubens C.E., Darmstadt G.L., Gravett M.G. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34:408–415. doi: 10.1053/j.semperi.2010.09.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Calderon-Margalit R., Sherman D., Manor O., Kurzweil Y. Adverse perinatal outcomes among immigrant women from Ethiopia in Israel. Birth. 2015;42:125–131. doi: 10.1111/birt.12163. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Segal S., Gemer O., Yaniv M. The outcome of pregnancy in an immigrant Ethiopian population in Israel. Arch Gynecol Obstet. 1996;258:43–46. doi: 10.1007/BF01370931. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Alexander G.R., Tompkins M.E., Altekruse J.M., Hornung C.A. Racial differences in the relation of birth weight and gestational age to neonatal mortality. Public Health Rep. 1985;100:539–547. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Lawn J.E., McClure E.M., Blencowe H. Elsevier; 2014. Birth outcomes: a global perspective. Jekel's epidemiology, biostatistics, preventive medicine, and public health; pp. 272–287. [ Google Scholar ]
- 17. Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.B., Narwal R. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Froen J.F., Cacciatore J., McClure E.M., Kuti O., Jokhio A.H., Islam M. Stillbirths: why they matter. Lancet. 2011;377:1353–1366. doi: 10.1016/S0140-6736(10)62232-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Kramer M.S., Papageorghiou A., Culhane J., Bhutta Z., Goldenberg R.L., Gravett M. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206:108–112. doi: 10.1016/j.ajog.2011.10.864. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Goldenberg R.L., Gravett M.G., Iams J., Papageorghiou A.T., Waller S.A., Kramer M. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol. 2012;206:113–118. doi: 10.1016/j.ajog.2011.10.865. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Lawn J.E., Gravett M.G., Nunes T.M., Rubens C.E., Stanton C., Group G.R. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(1 Suppl.):S1. doi: 10.1186/1471-2393-10-S1-S1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Fedrick J., Anderson A.B. Factors associated with spontaneous pre-term birth. Br J Obstet Gynaecol. 1976;83:342–350. doi: 10.1111/j.1471-0528.1976.tb00840.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Swamy G.K., Heine R.P. Vaccinations for pregnant women. Obstet Gynecol. 2015;125:212–226. doi: 10.1097/AOG.0000000000000581. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Glezen W.P., Alpers M. Maternal immunization. Clin Infect Dis. 1999;28:219–224. doi: 10.1086/515122. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Munoz F.M. Maternal immunization: an update for pediatricians. Pediatr Ann. 2013;42:153–158. doi: 10.3928/00904481-20130723-09. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Fischer G.W., Ottolini M.G., Mond J.J. Prospects for vaccines during pregnancy and in the newborn period. Clin Perinatol. 1997;24:231–249. [ PubMed ] [ Google Scholar ]
- 28. Heinonen O.P., Shapiro S., Monson R.R., Hartz S.C., Rosenberg L., Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol. 1973;2:229–235. doi: 10.1093/ije/2.3.229. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Freda V.J. A preliminary report on typhoid, typhus, tetanus, and cholera immunizations during pregnancy. Am J Obstet Gynecol. 1956;71:1134–1136. doi: 10.1016/0002-9378(56)90743-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Brent R.L. Risks and benefits of immunizing pregnant women: the risk of doing nothing. Reprod Toxicol. 2006;21:383–389. doi: 10.1016/j.reprotox.2005.09.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Munoz F.M., Englund J.A. A step ahead. Infant protection through maternal immunization. Pediatr Clin N Am. 2000;47:449–463. doi: 10.1016/s0031-3955(05)70217-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Bricks L.F. Vaccines in pregnancy: a review of their importance in Brazil. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:263–274. doi: 10.1590/s0041-87812003000500006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Kharbanda E.O., Vazquez-Benitez G., Lipkind H.S., Klein N.P., Cheetham T.C., Naleway A. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312:1897–1904. doi: 10.1001/jama.2014.14825. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Sukumaran L., McCarthy N.L., Kharbanda E.O., McNeil M.M., Naleway A.L., Klein N.P. Association of Tdap vaccination with acute events and adverse birth outcomes among pregnant women with prior tetanus-containing immunizations. JAMA. 2015;314:1581–1587. doi: 10.1001/jama.2015.12790. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Lindsey B., Kampmann B., Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis. 2013;26:248–253. doi: 10.1097/QCO.0b013e3283607a58. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Zheteyeva Y.A., Moro P.L., Tepper N.K., Rasmussen S.A., Barash F.E., Revzina N.V. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207:591e–597e. doi: 10.1016/j.ajog.2012.05.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Healy C.M. Vaccines in pregnant women and research initiatives. Clin Obstet Gynecol. 2012;55:474–486. doi: 10.1097/GRF.0b013e31824f3acb. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Castillo-Solorzano C., Reef S.E., Morice A., Vascones N., Chevez A.E., Castalia-Soares R. Rubella vaccination of unknowingly pregnant women during mass campaigns for rubella and congenital rubella syndrome elimination, the Americas 2001–2008. J Infect Dis. 2011;204(Suppl. 2):S713–S717. doi: 10.1093/infdis/jir489. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., Donegan K. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384:1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Donegan K., King B., Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349:g4219. doi: 10.1136/bmj.g4219. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Fell D.B., Platt R.W., Lanes A., Wilson K., Kaufman J.S., Basso O. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG. 2015;122:17–26. doi: 10.1111/1471-0528.12977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Richards J.L., Hansen C., Bredfeldt C., Bednarczyk R.A., Steinhoff M.C., Adjaye-Gbewonyo D. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56:1216–1222. doi: 10.1093/cid/cit045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Moro P.L., Broder K., Zheteyeva Y., Walton K., Rohan P., Sutherland A. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol. 2011;204:146 e1–e1467. doi: 10.1016/j.ajog.2010.08.050. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Brydak L.B., Nitsch-Osuch A. Vaccination against influenza in pregnant women. Acta Biochim Pol. 2014;61:589–591. [ PubMed ] [ Google Scholar ]
- 46. Steinhoff M.C., MacDonald N., Pfeifer D., Muglia L.J. Influenza vaccine in pregnancy: policy and research strategies. Lancet. 2014;383:1611–1613. doi: 10.1016/S0140-6736(14)60583-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Munoz F.M., Greisinger A.J., Wehmanen O.A., Mouzoon M.E., Hoyle J.C., Smith F.A. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192:1098–1106. doi: 10.1016/j.ajog.2004.12.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Guidelines for Vaccinating Pregnant Women . 2014. Centers for Disease Control and Prevention (CDC): Guidelines for vaccinating pregnant women from the Advisory Committee for Immunization Practices (ACIP) [ Google Scholar ]
- 49. Ballantyne J.W. Antenatal clinics and prematernity practice at the Edinburgh Royal Maternity Hospital in the years 1909–1915. Br Med J. 1916;1:189–192. doi: 10.1136/bmj.1.2875.189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Ballantyne J.W. The problem of the premature infant. Br Med J. 1902;1:1196–1200. doi: 10.1136/bmj.1.2159.1196. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Phelps D.L., Brown D.R., Tung B., Cassady G., McClead R.E., Purohit D.M. 28-day survival rates of 6676 neonates with birth weights of 1250 grams or less. Pediatrics. 1991;87:7–17. [ PubMed ] [ Google Scholar ]
- 52. Berkowitz G.S. An epidemiologic study of preterm delivery. Am J Epidemiol. 1981;113:81–92. doi: 10.1093/oxfordjournals.aje.a113068. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Battaglia F.C., Lubchenco L.O. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–163. doi: 10.1016/s0022-3476(67)80066-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Tucker J., McGuire W. Epidemiology of preterm birth. BMJ. 2004;329:675–678. doi: 10.1136/bmj.329.7467.675. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Salomon L.J., Bernard J.P., Ville Y. Estimation of fetal weight: reference range at 20–36 weeks’ gestation and comparison with actual birth-weight reference range. Ultrasound Obstet Gynecol. 2007;29:550–555. doi: 10.1002/uog.4019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Geerts L.T., Brand E.J., Theron G.B. Routine obstetric ultrasound examinations in South Africa: cost and effect on perinatal outcome – a prospective randomised controlled trial. Br J Obstet Gynaecol. 1996;103:501–507. doi: 10.1111/j.1471-0528.1996.tb09796.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.B. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(1 Suppl.):S2. doi: 10.1186/1742-4755-10-S1-S2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Wilcox A.J. On the importance – and the unimportance – of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Gardosi J., Chang A., Kalyan B., Sahota D., Symonds E.M. Customised antenatal growth charts. Lancet. 1992;339:283–287. doi: 10.1016/0140-6736(92)91342-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Gardosi J., Mongelli M., Wilcox M., Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–174. doi: 10.1046/j.1469-0705.1995.06030168.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Papageorghiou A.T., Ohuma E.O., Altman D.G., Todros T., Cheikh Ismail L., Lambert A. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384:869–879. doi: 10.1016/S0140-6736(14)61490-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Cooke R.W. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F189–F192. doi: 10.1136/adc.2005.089698. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Hutcheon J.A., Zhang X., Cnattingius S., Kramer M.S., Platt R.W. Customised birthweight percentiles: does adjusting for maternal characteristics matter. BJOG. 2008;115:1397–1404. doi: 10.1111/j.1471-0528.2008.01870.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Hutcheon J.A., Platt R.W. The missing data problem in birth weight percentiles and thresholds for small-for-gestational-age. Am J Epidemiol. 2008;167:786–792. doi: 10.1093/aje/kwm327. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Eunice Kennedy Shriver National Institute of Child Health and Human Development. Bethesda MEKSNIoCHaHD. Preterm Labor and Birth: Overview.
- 67. Blackmore C.A., Rowley D.L., Kiely J.L. From data to action: CDC's public health surveillance for women, infants and children. US Department of Health and Human Services Public Health Service Centers for Disease Control and Prevention; 1994. Preterm birth. [ Google Scholar ]
- 68. Fleischman A.R., Oinuma M., Clark S.L. Rethinking the definition of term pregnancy. Obstet Gynecol. 2010;116:136–139. doi: 10.1097/AOG.0b013e3181e24f28. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Pereira A.P., Dias M.A., Bastos M.H., da Gama S.G., Leal Mdo C. Determining gestational age for public health care users in Brazil: comparison of methods and algorithm creation. BMC Res Notes. 2013;6:60. doi: 10.1186/1756-0500-6-60. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Rosenberg R.E., Ahmed A.S., Ahmed S., Saha S.K., Chowdhury M.A., Black R.E. Determining gestational age in a low-resource setting: validity of last menstrual period. J Health Popul Nutr. 2009;27:332–338. doi: 10.3329/jhpn.v27i3.3375. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Ellertson C., Elul B., Ambardekar S., Wood L., Carroll J., Coyaji K. Accuracy of assessment of pregnancy duration by women seeking early abortions. Lancet. 2000;355:877–881. doi: 10.1016/S0140-6736(99)10170-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Bracken H., Clark W., Lichtenberg E.S., Schweikert S.M., Tanenhaus J., Barajas A. Alternatives to routine ultrasound for eligibility assessment prior to early termination of pregnancy with mifepristone-misoprostol. BJOG. 2011;118:17–23. doi: 10.1111/j.1471-0528.2010.02753.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. DiPietro J.A., Allen M.C. Estimation of gestational age: implications for developmental research. Child Dev. 1991;62:1184–1199. [ PubMed ] [ Google Scholar ]
- 74. Chauhan K.P. Assessment of symphysio-fundal height (SFH) and its implication during antenatal period. Int J Reprod Contracept Obstet Gynecol. 2013;2:363–366. [ Google Scholar ]
- 75. Yang H., Kramer M.S., Platt R.W., Blondel B., Breart G., Morin I. How does early ultrasound scan estimation of gestational age lead to higher rates of preterm birth. Am J Obstet Gynecol. 2002;186:433–437. doi: 10.1067/mob.2002.120487. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Goldenberg R.L., Davis R.O., Cutter G.R., Hoffman H.J., Brumfield C.G., Foster J.M. Prematurity, postdates, and growth retardation: the influence of use of ultrasonography on reported gestational age. Am J Obstet Gynecol. 1989;160:462–470. doi: 10.1016/0002-9378(89)90473-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Blondel B., Morin I., Platt R.W., Kramer M.S., Usher R., Breart G. Algorithms for combining menstrual and ultrasound estimates of gestational age: consequences for rates of preterm and postterm birth. BJOG. 2002;109:718–720. doi: 10.1111/j.1471-0528.2002.01068.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Breart G., Blondel B., Tuppin P., Grandjean H., Kaminski M. Did preterm deliveries continue to decrease in France in the 1980. Paediatr Perinat Epidemiol. 1995;9:296–306. doi: 10.1111/j.1365-3016.1995.tb00146.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Henriksen T.B., Wilcox A.J., Hedegaard M., Secher N.J. Bias in studies of preterm and postterm delivery due to ultrasound assessment of gestational age. Epidemiology. 1995;6:533–537. doi: 10.1097/00001648-199509000-00012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Kramer M.S., McLean F.H., Boyd M.E., Usher R.H. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations. JAMA. 1988;260:3306–3308. [ PubMed ] [ Google Scholar ]
- 81. Rowlands S., Royston P. Estimated date of delivery from last menstrual period and ultrasound scan: which is more accurate. Br J Gen Pract. 1993;43:322–325. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Buekens P., Delvoye P., Wollast E., Robyn C. Epidemiology of pregnancies with unknown last menstrual period. J Epidemiol Community Health. 1984;38:79–80. doi: 10.1136/jech.38.1.79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Gardosi J., Geirsson R.T. Routine ultrasound is the method of choice for dating pregnancy. Br J Obstet Gynaecol. 1998;105:933–936. doi: 10.1111/j.1471-0528.1998.tb10253.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Farr V., Kerridge D.F., Mitchell R.G. The value of some external characteristics in the assessment of gestational age at birth. Dev Med Child Neurol. 1966;8:657–660. doi: 10.1111/j.1469-8749.1966.tb01823.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Dubowitz L.M., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77:1–10. doi: 10.1016/s0022-3476(70)80038-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Mitchell D. Accuracy of pre- and postnatal assessment of gestational age. Arch Dis Child. 1979;54:896–897. doi: 10.1136/adc.54.11.896. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Parkin J.M., Hey E.N., Clowes J.S. Rapid assessment of gestational age at birth. Arch Dis Child. 1976;51:259–263. doi: 10.1136/adc.51.4.259. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Ounsted M.K., Chalmers C.A., Yudkin P.L. Clinical assessment of gestational age at birth: the effects of sex, birthweight, and weight for length of gestation. Early Hum Dev. 1978;2:73–80. doi: 10.1016/0378-3782(78)90053-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Capurro H., Konichezky S., Fonseca D., Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Dubowitz L.M., Dubowitz V., Palmer P., Verghote M. A new approach to the neurological assessment of the preterm and full-term newborn infant. Brain Dev. 1980;2:3–14. doi: 10.1016/s0387-7604(80)80003-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Farr V., Mitchell R.G., Neligan G.A., Parkin J.M. The definition of some external characteristics used in the assessment of gestational age in the newborn infant. Dev Med Child Neurol. 1966;8:507–511. doi: 10.1111/j.1469-8749.1966.tb01796.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Spinnato J.A., Sibai B.M., Shaver D.C., Anderson G.D. Inaccuracy of Dubowitz gestational age in low birth weight infants. Obstet Gynecol. 1984;63:491–495. [ PubMed ] [ Google Scholar ]
- 93. von Voss H., Trampisch H.J., Grittern M. Simple and practical method for the determination of gestational age of newborns. J Perinat Med. 1985;13:207–217. doi: 10.1515/jpme.1985.13.5.207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Nicolopoulos D., Perakis A., Papadakis M., Alexiou D., Aravantinos D. Estimation of gestational age in the neonate: a comparison of clinical methods. Am J Dis Child. 1976;130:477–480. doi: 10.1001/archpedi.1976.02120060023005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Eregie C.O. A new method for maturity determination in newborn infants. J Trop Pediatr. 2000;46:140–144. doi: 10.1093/tropej/46.3.140. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Ballard J.L., Novak K.K., Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95:769–774. doi: 10.1016/s0022-3476(79)80734-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Verhoeff F.H., Milligan P., Brabin B.J., Mlanga S., Nakoma V. Gestational age assessment by nurses in a developing country using the Ballard method, external criteria only. Ann Trop Paediatr. 1997;17:333–342. doi: 10.1080/02724936.1997.11747907. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Ballard J.L., Khoury J.C., Wedig K., Wang L., Eilers-Walsman B.L., Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Guidelines for Clinical Safety assessment (E2a–e). [ DOI ] [ PMC free article ] [ PubMed ]
- 100. Council for International Organizations of Medical Sciences (CIOMS). Reporting form for International Reporting of Adverse Drug Reactions.
- 101. Schulz K.F., Altman D.G., Moher D.S., Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Moher D., Cook D.J., Eastwood S., Olkin I., Rennie D., Stroup D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (417.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
6 Preterm Labor Nursing Care Plans

Preterm labor is regular uterine contractions after 20 weeks and before 37 weeks of pregnancy that cause cervical change or regular contractions with an initial presentation with cervical dilation of 2 cm or more. Preterm birth is birth after 20 weeks gestation and before 37 completed weeks gestation. Preterm birth affects over 15 million babies and their mothers and families worldwide. In 2019, in the United States, the preterm birth rate rose for the fifth year in a row to 10.23% from 10.02% in 2018, and the highest level was reported in more than a decade. Preterm babies are at risk for a multitude of complications that account for 36.3% of reported infant deaths (Griggs et al., 2020).
Table of Contents
Nursing problem priorities, nursing assessment, nursing diagnosis, nursing goals, 1. reducing anxiety, 2. managing activity restriction and bed rest, preventing maternal injury, preventing fetal injury, 4. managing pain, 5. initiating patient education and health teachings, 6. administer medications and provide pharmacologic support, recommended resources, nursing care plans and management.
One goal of Healthy People 2030 is that 90% of all women will receive prenatal care starting in the first trimester. Early prenatal care allows clients to be educated concerning signs of preterm labor so that interventions can occur early. Management involves suppression of preterm labor when tests show immature fetal pulmonary development, cervical dilation is less than 2 cm, and the absence of factors that contraindicate the continuation of pregnancy. The nurse should monitor closely for signs of fetal or maternal distress, and provide comprehensive supportive care for clients in preterm labor.
The following are the nursing priorities for patients in preterm labor:
- Assess fetal well-being
- Monitor maternal vital signs
- Evaluate cervical dilation and effacement
- Provide emotional support to the mother
- Prepare for potential emergency delivery
- Monitor and manage uterine contractions
- Initiate or continue IV fluids as needed
Assess for the following subjective and objective data :
- Vaginal bleeding or spotting
- Regular or frequent contractions (more than four contractions in one hour)
- Menstrual-like cramps or lower abdominal pain
- Pressure or a feeling of heaviness in the pelvis
- Backache, especially in the lower back
- Change in vaginal discharge (increase in amount or change in consistency)
- Pelvic pressure or the sensation that the baby is pushing down
- Abdominal tightening or a feeling of the uterus hardening and then relaxing
- Intermittent or continuous dull lower back pain
- Increase in pelvic pressure or a feeling of the baby “dropping”
- Fluid leakage or gush of fluid from the vagina (rupture of membranes)
- Increase in pelvic pressure or a feeling that the baby is pushing down
- Flu -like symptoms, such as nausea , vomiting , diarrhea , or fever
- Unusual or persistent abdominal pain or cramping
- Change in fetal movement patterns (increase or decrease in fetal activity
Following a thorough assessment , a nursing diagnosis is formulated to specifically address the challenges associated with preterm labor based on the nurse’s clinical judgment and understanding of the patient’s unique health condition. While nursing diagnoses serve as a framework for organizing care, their usefulness may vary in different clinical situations. In real-life clinical settings, it is important to note that the use of specific nursing diagnostic labels may not be as prominent or commonly utilized as other components of the care plan. It is ultimately the nurse’s clinical expertise and judgment that shape the care plan to meet the unique needs of each patient, prioritizing their health concerns and priorities.
Goals and expected outcomes may include:
- The client will verbalize understanding of the individual situation and possible outcomes.
- The client will report anxiety is reduced and/or manageable.
- The client will appear relaxed; with maternal vital signs within normal limits.
- The client will identify and/or engage in activities appropriate to the situation.
- The client will demonstrate reduction and/or cessation of uterine contractions.
- The client will prevent complications that arise from complete bed rest .
- The client will display no evidence of untoward effects of tocolytic therapy.
- The client will prevent or minimize maternal injury .
- The client will demonstrate cessation of uterine contractions, dependent on fetal well-being.
- The client will maintain pregnancy at least to the point of fetal maturity.
- The client will deliver a preterm but complication-free neonate.
- The client will report discomfort is minimized or controlled.
- The client will use relaxation techniques, effectively.
- The client will appear relaxed and will rest appropriately.
- The client will express feelings and worries to healthcare personnel.
- The client will state the absence of responsibility for preterm labor.
- The client will verbalize awareness of the implications and possible outcomes of preterm labor.
- The client will identify signs and symptoms requiring evaluation and intervention.
- The client will demonstrate an understanding of home therapy and/or self-care needs.
Nursing Interventions and Actions
Therapeutic interventions and nursing actions for patients in preterm labor may include:
Anxiety has both short-term and long-term effects on maternal, pregnancy and fetal outcomes. With increased anxiety , the cortisol hormone appears to cross the placenta and affect the fetus, disrupting ongoing processes, affecting the limbic and prefrontal cortex, and releasing chemicals such as acetylcholine and adrenaline in the mother’s body. These chemicals pass through the placenta into the fetus and have detrimental effects on proper fetal growth . Anxiety can also lead to inappropriate maternal responses to the fetus during pregnancy and decrease the ability to play a motherly role. Accordingly, it is essential to find some efficient therapeutic plans to reduce maternal anxiety during pregnancy (Bazrafshan et al., 2020).
Assess support systems available to the client or couple, whether the client remains hospitalized or is to return home to await delivery. The assistance and caring of significant others, including caregivers , are extremely important during this time of uncertainty and stress. If the client is to return home, additional support will be required to meet self-care needs and homemaker activities as well as child care, as appropriate. Fostering an environment of intentional support and empathetic dialogue can build mutual trust, and assist the nurse in understanding the client’s perceptions of their experiences of preterm labor (Griggs et al., 2020).
Monitor maternal signs of preterm labor. The nurse should be aware of the symptoms of preterm labor because they may occur in any pregnant woman, with or without risk factors. Common symptoms of early preterm labor are persistent, dull, and low backache; vaginal spotting; a feeling of pelvic pressure or abdominal tightening; menstrual-like cramping; increased vaginal discharge; uterine contractions; and intestinal cramping.
Continuously monitor maternal and fetal vitals. Monitor the client’s vital signs and neurologic status closely. Respirations should be at least 12 breaths/min. FHR should be at 120-160 beats/min. Decreasing FHR can indicate fetal distress.
Explain the procedures, nursing interventions , and treatment regimen. Information and knowledge of the reasons for these activities can decrease fear of the unknown. Clients desire honest and complete information, an opportunity to answer questions, and an explanation of medical terms, procedures, and expectations of care conferred in the language they understand and at the appropriate literacy level (Griggs et al., 2020).
Answer questions honestly, especially information regarding contraction patterns and fetal status. The provision of clear information can help the client or couple understand what is happening and may reduce anxiety. The healthcare team should avoid using terms with negative connotations such as fetus, nonviable, incompatible with life, spontaneous abortion , and miscarriage as these terms might trivialize or dehumanize the client’s experiences (Griggs et al., 2020).
Encourage the use of relaxation techniques. Application of relaxation exercises among 60 hospitalized primiparous pregnant clients at risk of preterm labor could significantly lessen the pregnancy anxiety level. The relaxation exercises were trained through the educational booklet and CD, and face-to-face and question-and-answer communications (Bazrafshan et al., 2020).
Encourage verbalization of fears or concerns. The nurse should assess the client’s views of their pregnancy at the time of preterm labor. Incorporating this type of assessment will help provide individualized empathetic care (Griggs et al., 2020). Saisto et al., (2006) found that support interventions for pregnant women, which included discussions of concerns and feelings about birth, taught positive birth imagery (Kao et al., 2017).
Encourage the client to engage in complementary and alternative medicine (CAM). Mind-body interventions, which constitute a major portion of the overall use of CAM, can provide support to alleviate or reduce feelings of stress. These include autogenic training, hypnotherapy, imagery, prayer, auto-suggestion, tai-chi, and yoga. These complementary therapies can have physiological as well as psychological benefits, which may consequently reduce the physio-pathological impact of stress (Kao et al., 2017).
Provide relaxation-focused nursing care to the client. Relaxation-focused nursing care (RFNC) was created using Hypnobirthing and the Transactional Model. Hypnobirthing philosophy aims to reduce stress, fear , and tension in the pregnant woman by creating positive thoughts and emotions using the laws of mind. The Transactional Model defines stress as a special relationship between a person and the environment. It identifies the stressors of the person and indicates how they should be handled. RFNC is formed by using these two models and comprises positive language, a positive environment, and reducing stressors. RFNC may reduce the client’s state of anxiety, lower cortisol levels, and thus prevent preterm labor by lowering the severity of uterine contractions (Özberk et al., 2020).
Arrange psychotherapy sessions for the client as indicated. The results of a study indicated that adding six sessions of individual face-to-face psychotherapy, one hour per day for six consecutive days, to inpatient medical care of clients successfully treated for preterm labor, reduced anxiety, and pregnancy stress, while enhancing their perceived control. Psychotherapy involves supportive elements focusing on responsiveness to needs for helping others through the insight obtained via empathic immersion in the therapy. This approach focuses on the client’s emotions (Mirtabar et al., 2020).
Assess individual strengths and identify previous positive coping behaviors. Ascertaining the client’s coping behaviors and strengths helps to build on the qualities already available for the client to use in coping with the current situation.
Assess for aspects of behavioral distress in the client.
Maternal mental health problems can be measured using the standardized psychometric methods Impact of Event Scale (IES), General Health Questionnaire (GHQ), and State/Trait Anxiety Inventory. The predictors for maternal mental health gleaned through these assessment tools are related to pregnancy, preterm delivery, the infant’s gestational age, maternal trait anxiety, and parity (Misund et al., 2014).
Assess for the presence of a support person/s. The client needs a strong support person with her during labor because if she has not yet taken preparation for labor class, she may be more concerned than the average woman.
Encourage the client to verbalize feelings and concerns. The client in preterm labor is undergoing an extreme crisis situation. She cannot help asking herself, “What did I do to cause this?” Time spent taking the initial history or timing contractions presents an opportunity to not only bring that concern out in the open but any others the client may have as well.
Provide assurance to the client and partner during labor until the postpartum period. Offer the client and her partner frequent reassurance during labor that she is breathing well with contractions or just is “doing well”. During the postpartum period, she may need continued reassurance as she is being asked to care for a very small and vulnerable appearing infant. Helping rebuild self- esteem this way not only prepares her better for the stress of labor but also prepares her to be a parent to her preterm infant.
Provide accurate information, reinforcing information previously given. Receiving accurate information regarding her situation enhances the client’s trust in the healthcare personnel and also provides an opportunity to question and assimilate information.
Arrange for an appointment with a psychiatric clinical nurse specialist or other preofessionals for counseling, as appropriate. The client may need additional help to resolve her feelings about the loss of control of her pregnancy. Referring her to professionals may help her gradually come to terms with the situation and assist her in creating future plans for herself and her infant.
Bed rest was often prescribed for women at risk for preterm birth. However, the benefits of bed rest are not clear, and many adverse maternal effects can occur. Therefore total bed rest is prescribed less frequently than it was in the past. Activity restrictions are often more moderate, such as resting in a semi-Fowler’s position or partial bed rest.
Assess uterine contractions and fetal response per protocol. Assessment of uterine activity occurs with fetal heart monitoring to evaluate the presence or absence of contractions, frequency, duration, and palpated strength to inform the plan of care for preterm labor. The nurse may also assess uterine activity by observing the client’s demeanor, listening to the client on frequency and discomfort experienced with contractions, and uterine palpation to evaluate contractions (Griggs et al., 2020).
Assess the client’s vital signs and history of events prior to preterm labor. Assess the vital signs of the client and obtain the history of events leading to the beginning of labor. The assessment provides a baseline for future comparisons.
Assess for the availability of psychosocial support. High-risk pregnancy and the subsequent need for complete bed rest are stressful conditions in the normal course of family life. Therefore, the pregnant client and her partner need psychosocial support from their friends and relatives. Women in the study stated that psychosocial support from their families and families-in-law made it easier for them to endure bed rest. In addition to the pregnant woman, the spouse experiences tension due to the conditions associated with bed rest. The family’s problems during a high-risk pregnancy and maternal bed rest put the partner in need of getting support from relatives and friends (Janighorban et al., 2018).
Provide comforts measures (e.g., back rubs, changes of position, decreased stimuli in the room) Comfort measures and relaxation techniques decrease muscle tension and fatigue and help promote a sense of well-being. Instruction in applying relaxation techniques can improve the state of a person’s psycho-physiological well-being (Griggs et al., 2020).
Explain the reasons for requiring bed rest and activity restrictions. The prescription of bed rest is based on the assumption that it is effective in preventing preterm birth and safe for both the mother and fetus. However, Hatch et al (1998) report that evidence indicates that vigorous maternal exercise does not increase the risk of preterm birth. Leisure-time exercise may, in fact, improve high-risk pregnancy outcomes and reduce the risk of preterm birth (Maloni, 2010).
Place the patient in a lateral recumbent position (i.e., side-lying position ) and decrease activity. These measures are intended to keep the fetus off the cervix and may enhance uterine perfusion, bedrest may decrease uterine irritability. Nursing care should include positioning the client on her side for better placental blood flow, assessing vital signs frequently, and notifying the healthcare provider if tachycardia occurs.
Cluster nursing care: group activities together as much as possible, such as medication administration , vital signs, and assessment. Clustering nursing care promotes longer opportunities for clients to rest between interruptions. Holistic care provided by the nurse focuses on facilitating the client’s psychological well-being and helping them cope with the impact of physiological stress brought by complete bed rest (Kao et al., 2017).
Provide uninterrupted periods for rest and/or sleep . This helps promote rest, prevent fatigue , and may enhance relaxation. Additional efforts to minimize the adverse effects of long-term bed rest and prolonged hospitalization include the provision of privacy in a family unit and more organized activities to relieve boredom by recreation activities such as scrapbooking, blanket making, and knitting supplies during hospitalization (Maloni, 2010).
Offer diversional activities (e.g., reading, watching TV) The distractions incorporated into the support intervention allowed participants in a study to engage in activities involving the care of their unborn infant and focused attention on the positive aspects of their pregnancy, rather than the restrictions in activities resulting from antepartum bed rest. Preparing clothing for a newborn , recording fetal growth, and maintaining a diary about their feelings were distractions that assisted the participants in recognizing they had a responsibility to their unborn child and were capable of performing well in the role of motherhood (Maloni, 2010).
3. Preventing Injuries
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide, with an estimated incidence of 11.54% in North America. Tocolytics have been used to delay preterm labor, allowing sufficient time to transfer clients to tertiary care facilities and to administer antenatal corticosteroids. Rare and severe side effects such as pulmonary edema , associated with the use of magnesium sulfate and calcium channel blockers, have emerged as the number of clients taking these medications increased (Xiao et al., 2014). In 2011, Cantwell reported that pulmonary edema is the fourth most common form of maternal morbidity, therefore it is important to pay attention to this condition due to tocolytic administration (Zeraati & Naghibi, 2019).
Monitor vital signs and investigate cardiac irregularities. Beta-adrenergic drugs such as terbutaline have cardiac side effects such as increased pulse rate and blood pressure . If calcium channel blockers were given as tocolytics, the nurse should closely monitor the pulse rate and blood pressure because the drug causes maternal flushing and hypotension .
Auscultate lung sounds and investigates reports of dyspnea and/or chest tightness. When magnesium sulfate (MgSO₄) is used, the nurse should monitor for respiratory rate and lung sounds, and signs of fluid overload . One study found that the combination of nifedipine and MgSO₄ increases the risk of pulmonary edema . Nifedipine enhances the neuromuscular-blocking effect of MgSO₄ which in turn can alter cardiac and pulmonary functions (Xiao et al., 2014).
Measure intake and output . Magnesium levels must be monitored frequently by checking serum levels every 6 to 8 hours or clinically by checking the urine output (Hicks & Tyagi, 2022). This prevents fluid excess, especially when MgSO₄ is administered which is excreted through the kidneys, therefore urine output must be maintained.
Weight client daily. Weighing the client upon admission and monitoring regularly detects potential alteration in urinary functioning and/or retention of fluid. Fluid redistribution may be caused by sympathetic activation in the stress status which could lead to weight changes in pulmonary edema (Zeraati & Naghibi, 2019).
Monitor for drowsiness, hot flashes, visual disturbances, respiratory depression , and depressed tendon reflexes. This may indicate neuromuscular depression, indicating increasing serum levels of magnesium sulfate. Clients most commonly complain of minor facial flushing and warmth with the administration; however, symptoms typically resolve spontaneously. Patellar reflexes should be checked frequently to monitor magnesium levels (Hicks & Tyagi, 2022).
Monitor serum magnesium levels per protocol during the administration of magnesium sulfate. The toxic effects of magnesium are inherently linked to the levels found in the serum. As magnesium levels rise, different symptoms start to manifest, and the fatality of those symptoms is proportional to the levels of magnesium found. Starting at 5 to 10 mEq/L, clients will begin to develop ECG changes. At 10 mEq/L, there will be a loss of deep tendon reflexes and muscle weakness . At 15 mEq/L, signs of abnormal conductivity surface as SA/AV node block (Ajib & Childress, 2021).
Monitor nifedipine levels. Note the development of tachycardia, hypotension , peripheral edema, or proteinuria . The therapeutic dosage of nifedipine for preterm labor has not been established. Periodic monitoring may avert or prevent the development of adverse effects (e.g., heart failure ). Nifedipine triggers hypotension and reflex sinus tachycardia . Depending on age, health, co-ingestion of other cardiovascular medications, and the magnitude of toxic ingestion, the presentation may vary from asymptomatic to sudden cardiovascular collapse and death (Reddy, 2022).
Assess uterine contractions and FHR electronically while IV tocolytics are administered, or at least twice a day when the oral route is used. Tactile electronic monitoring of uterine contractions and FHS provides a continuous fetal/uterine assessment and basis for altering or maintaining the rate of drug administration . The client may feel reassured by having an external fetal monitor during labor because the monitor screen shows evidence that, although her infant is going to be small, heart tones seem to be of good quality and the infant is reacting well to labor.
Encourage fluid intake between 2,000 and 3,000 ml/day, unless contraindicated (e.g., during the administration of magnesium sulfate). Following initial therapy and if contractions have ceased and there is evidence of fetal well-being, the client with arrested preterm labor can be safely cared for at home as long as they can dependably drink enough fluid to remain well hydrated. However, if the client is taking magnesium sulfate, increased fluid intake may not be appropriate as this may contribute to pulmonary edema (Hicks & Tyagi, 2022).
Place the client in a lateral recumbent position. Elevate head during infusion of IV drug. The lateral recumbent position decreases uterine irritability, increases placental perfusion, and reduces supine hypotension. Lying down on the left side encourages the blood to return to the uterus.
Have antidotes (calcium gluconate for magnesium sulfate; propranolol for terbutaline sulfate) readily available: See Pharmacological Management
Administer IV solution or fluid bolus as indicated. Hydration may decrease uterine activity. Before beginning drug therapy, hydration promotes renal clearance and minimizes hypotension. Intravenous therapy to keep the client well hydrated should be started because although not well documented, hydration may help stop contractions. This is thought to be effective because if the client is dehydrated, the pituitary gland will be activated to secrete the antidiuretic hormone , which might cause the pituitary gland to release oxytocin as well, strengthening uterine contractions.
Administer IV solutions containing tocolytic agents (e.g., magnesium sulfate, terbutaline sulfate) by infusion pumps or micro drip equipment, or by subcutaneous route. See Pharmacological Management
Obtain serum potassium level prior to initiation of IV terbutaline and periodically per protocol. Monitor serum glucose and potassium levels. Terbutaline sulfate causes the movement of potassium ions into cells, decreasing plasma levels; elevated blood glucose and plasma insulin levels, and release of glycogen from muscle and liver may result in hyperglycemia . The drug should be discontinued two hours before delivery to avoid side effects in the newborn.
Administer nifedipine to be chewed and swallowed with food or drink. Nifedipine may occasionally be alternated with terbutaline sulfate. Nifedipine, a calcium channel blocker, has been used experimentally when other drugs fail to suppress uterine activity. It is commonly used to stop labor contractions. Magnesium sulfate should not be used when nifedipine is used or when an intrauterine infection is suspected.
Apply anti embolic hose as indicated, and provide passive range of motion exercises to legs every 1-2 hours. Deep vein thrombosis and other emboli are 5 times more likely to occur during pregnancy, especially if the client is prescribed complete bed rest (Maloni, 2010). This prevents the pooling of blood in the lower extremities, which can occur because of smooth muscle relaxation.
Insert indwelling catheter, as indicated. Careful monitoring of urine output is warranted as magnesium sulfate is excreted through the kidneys (Griggs et al., 2020).
Assess for maternal conditions contraindicated to steroid therapy. Severe, fulminant chorioamnionitis is a contraindication to steroid therapy; immediate delivery is indicated in these cases. In the case of preeclampsia between 24 and 34 weeks of gestation, antenatal steroids can be administered as long as delivery can be safely delayed for at least 12 to 24 hours (Surbek et al., 2012).
Assess FHR; note the presence of uterine activity or cervical changes. Tocolytics can increase FHR. Overdose can affect the cardiorespiratory system. If the fetus is born during magnesium therapy, drowsiness may be present, and resuscitation may be required. Delivery may be extremely rapid with a small infant if persistent uterine contractions are unresponsive to tocolytics, or if cervical changes continue.
Assess the preterm infant immediately after birth. The nursery staff should be notified if magnesium sulfate therapy was used within two hours before delivery. The FDA recommends limiting the use of magnesium sulfate to fewer than 5 to 7 days because the fetus can develop low blood calcium, bone problems, and respiratory depression with prolonged use. Administration of prostaglandin synthesis inhibitors can stimulate the ductus arteriosus to close prematurely, causing fetal death. Close fetal monitoring is essential.
Provide information about the actions and side effects of the drug therapy . Information about the drugs’ side effects is important for the client or couple to know the purpose of the drugs being administered.
- Beta-agonist therapy: These drugs are administered subcutaneously to stop uterine contractions within minutes. Nasal stuffiness and hyperglycemia can occur, and the drug should be discontinued 2 hours before delivery to avoid side effects in the newborn.
- Steroid therapy: Steroids have significantly reduced the incidence of respiratory distress syndrome , intraventricular hemorrhage , necrotizing enterocolitis, and neonatal death by enhancing the formation of surfactant in fetal lungs (Griggs et al., 2020).
- Magnesium sulfate: Magnesium sulfate reduces cerebral palsy risk in premature infants and protects the fetal brain from injury through stabilization of neuronal axons, along with preventing intracranial bleeding .
Stress necessity of follow-up care for antenatal steroid therapy. If the fetus is not delivered within seven days of administration of steroids, the dose should be repeated weekly. It was assumed in certain clinical situations, namely, in acute deterioration with renewed threatened preterm delivery, that repeating antenatal steroids once (“rescue course”) could be advantageous. Although there has been limited evidence to date to support this, a recently published, randomized study with nearly 500 clients showed that the second course of antenatal steroids administered up to 32 weeks of gestation with a minimal interval of two weeks from the first course- can improve neonatal outcomes without increasing short-term risks (Surbek et al., 2012).
Educate the client thoroughly upon discharge. On discharge, the nurse should focus education on individualized client needs, home care, and provider follow-up instructions, and signs and symptoms that require seeking immediate medical attention such as ruptured membranes, bleeding. Increasing frequency and intensity of contractions as well as decreased fetal movement (Griggs et al., 2020).
Assist as needed with analysis of amniotic fluid from amniocentesis or vaginal pool specimen; test for ferning. L/S ratio, presence of PG, and shake test results indicate fetal lung status. Ferning indicates rupture of membranes with an increased risk of infection. The presence of amniotic fluid pooling, ferning pattern, and positive Nitrazine reading are all highly indicative of rupture of membranes (Griggs et al., 2020).
Administer betamethasone ( Celestone ) deep IM . See Pharmacological Management
Administer antibiotics , as indicated. See Pharmacological Management
Initiate tocolytic therapy, as ordered. The American College of Obstetricians and Gynecologists advocates for the use of a short course of tocolytic medications in cases of preterm labor less than 32 weeks of gestation. The goals are to inhibit contractions to have sufficient time for antenatal corticosteroid administration or for in utero transport to a hospital that can support a high-risk client (Griggs et al., 2020).
Assist in performing fetal fibronectin. Fetal fibronectin is an extracellular matrix glycoprotein that is produced by fetal cells and can be detected in maternal vaginal secretions from early gestation to the early second trimester, gradually decreasing to undetectable in pregnancy at low risk for preterm birth. When a sample of fluid is collected between 22- and 34 weeks gestation, and it is found to be negative for fetal fibronectin, this is a high-reliability indicator that preterm birth will not occur within 14 days (Griggs et al., 2020).
Preterm labor and birth can be either spontaneous or iatrogenic. Spontaneous preterm labor and preterm birth include preterm premature rupture of membranes, a natural onset of uterine contractions, and cervical dilation. The client is asked about pain , vaginal bleeding, amniotic fluid leakage, abdominal cramping or tightening, lower back pain or pelvic pressure, and specific activities that may have preceded symptoms (Griggs et al., 2020).
Assess the client’s level of pain. Pain perception differs from one person to the next and is influenced by the individual’s physical, psychological, and cultural conditions. Chemical and hormonal reactions taking place before, during, and after labor might influence pain thresholds. Anxiety and fear increase catecholamine concentration in the plasma, which is associated with enervated uterine contractions (Junge et al., 2018).
Monitor maternal and fetal vital signs. Assess the vital signs of the client and the fetus. An assessment provides a baseline for future comparisons. Attach a contraction and FHR monitors for continuous evaluation of contractions and fetal response. Uterine and fetal monitoring provides evidence of fetal well-being.
Assess and record the characteristics of uterine activities. Assessment of uterine activity occurs with FHR monitoring to evaluate the presence or absence of contractions, frequency, duration, and palpated strength to inform the plan of care for preterm labor. The nurse may assess uterine activity by observing the client’s demeanor; listening to the client on the frequency and discomfort experienced with contractions, and uterine palpation to evaluate contractions (Griggs et al., 2020).
Position the client in a lateral recumbent position. Position changes may promote comfort and increase venous return. A lateral recumbent position enhances placental circulation and helps the fetus adapt to the size and shape of the client’s pelvis.
Teach relaxation techniques (e.g., deep breathing exercises, visualization, guided imagery, soft music). Nursing care is believed to have a positive effect on anxiety by addressing stressors, informing about the preterm labor process, and providing relief through relaxation exercises. Nurses are recommended to apply relaxation exercises after evaluating their stress and anxiety levels. Uterine contraction severity may decrease if the stress and anxiety level of the client can be decreased by using relaxation-focused nursing care, causing an increase in gestational weeks. Thus, both maternal and fetal health can benefit from this (Özberk et al., 2020).
Use nursing comfort measures such as changes of linen and position, back rubs, and therapeutic touch . One of the most effective non-pharmacological methods to reduce pain is massage . The basis of this theory is the gate control theory proposed by Melzak and Wall. The gate control theory states that during labor, the impulse of pain labor travels from the uterus along the large nerve fibers toward the uterus to the gelatinous substance within the spinal column. The transmission cells project pain messages to the brain. The presence of stimulation (such as vibration, rubbing, or massage ) results in stronger and faster opposite messages along small nerve fibers. This opposite message closes the gate on the substance of gelatinosa and blocks pain messages, so the brain does not record the pain message (Oktriani et al., 2018).
Encourage routine inspection of mucous membranes for ulceration or reaction to chewing of nifedipine, if used. Nifedipine may be irritating to the oral cavity in which case it should be swallowed whole. Gingival overgrowth associated with nifedipine was first reported in 1984, with prevalence rates of 14.7-83%. Various possible associated pathogeneses include alteration in calcium metabolism, stimulation of interleukin -2 by activated T cells , and also the role of TGF-beta and heparan sulfate glycosaminoglycan (Sivaramakrishnan & Sridharan, 2016).
Assist and educate the client with breathing techniques. Using consciously controlled breathing, or setting breathing patterns at specific rates, provides distraction as well as prevents the diaphragm from descending fully and putting pressure on the expanding uterus. To practice, after a cleansing breath, the client inhales comfortably but fully and then exhales, with her exhalation a little stronger than her inhalation to prevent hypoventilation .
Administer analgesics, as indicated. See Pharmacological Management
Assist with the administration of an epidural block. An epidural block is given by injecting anesthetic drugs so that they bathe the nerves as they emerge from the spinal cord . The spinal cord and nerves are not directly injected. An epidural block for labor is more accurately termed analgesia (reducing pain) than anesthesia (obliterating all sensation). An epidural block also decreases postpartum depression and reduces the partner’s feeling of helplessness, often increasing the partner’s participation.
Women desire honest and complete information, an opportunity to answer questions, explanation of medical terms, procedures, and expectations of care including resuscitative initiatives conferred in a language they understand and at the appropriate literacy level.
Ascertain the client’s knowledge about preterm labor and possible outcomes. Nurses, midwives , healthcare providers, and other members of the clinical team should avoid using terms with negative connotations such as fetus, nonviable, incompatible with life, spontaneous abortion , and miscarriage as these terms might trivialize or dehumanize the client’s experiences. More favorable language such as life-limiting condition, maternal and wellbeing, and referring to the baby’s name or what the parents prefer should be used (Griggs et al., 2020).
Assess client readiness to learn . Factors such as anxiety or lack of awareness of the need for information can interfere with readiness to learn . Retention of information is enhanced when the client is motivated and ready to learn. The nurse should assess the client’s views of their pregnancy at the time of the preterm labor. For example, do they perceive their unborn child as a son or daughter with a name or are they viewing this as early pregnancy. Involving this type of assessment will help provide individualized empathetic care (Griggs et al., 2020).
Include significant others in the teaching- learning process. Literature shows that partners can receive limited attention from midwives and that they are not always involved during labor and birth. Partners who are less involved can have a more negative birth experience. When the partner feels left out, this can be associated with panic and helplessness. Involving partners may result in a range of positive effects, such as being able to better manage their overwhelming feelings of helplessness or experiencing the first contact with their child more positively. Supportive partners during pregnancy can decrease the risk of preterm delivery by moderating the effects of maternal chronic stress (Eggermont et al., 2016).
Provide information about follow-up care when the client is discharged. The client may need to return on a regular basis for monitoring and/or treatments. On discharge, the nurse should focus education on individualized client needs, home care, provider follow-up instructions, and signs and symptoms that require seeking immediate medical attention such as ruptured membranes, bleeding, increasing frequency and intensity of contractions as well as decreased fetal movements (Griggs et al., 2020).
Identify signs and/or symptoms that should be reported immediately to the healthcare provider (e.g, sustained uterine contractions, clear drainage from the vagina, bleeding). Prompt evaluation and interventions may improve the outcome of the pregnancy and avert complications. Signs and symptoms of acute preterm labor include cramps that feel like menstrual cramps, feeling the baby balling up, regular and more frequent contractions (sometimes painless), increase in the amount of discharge, ruptured membranes or leaking fluid, constant low, dull backache, and pelvic or lower abdominal pressure (Griggs et al., 2020).
Review the signs and symptoms of early labor with the client. Because preterm labor signs and symptoms can be subtle, it is essential all pregnant women are offered counseling, education, and information early in pregnancy about signs and symptoms of preterm labor and what to do if these occur. Pregnant women need to know about the importance of immediately seeking medical care at the hospital for evaluation of preterm labor signs and symptoms (Griggs et al., 2020).
Demonstrate how the client is to evaluate contraction activity after discharge (e.g., lying down, tilted to the side with a pillow to the back, placing fingertips on the fundus for approximately 1 hour to note hardening or tightening of the uterus). Although uterine contractions commonly occur periodically, contractions occurring 10 mins or less apart for an hour can result in cervical dilation and labor without prompt intervention. Self-monitoring is usually adequate and has no cost; however, some healthcare providers may require electronic monitoring, which necessitates data being transmitted via telephone lines and interpreted by a nurse upon receipt. Self-monitored data on maternal and fetal well-being can be transferred electronically from the client to the hospital with a mobile device including a telemedicine platform as the primary source for communication and data transfer. A qualitative study showed that the pregnant women experienced less anxiety and more freedom and flexibility in the home monitoring setting than they thought they would have experienced under a conventional regime (Zizzo et al., 2021).
Arrange for the client to visit the neonatal intensive care unit (NICU). Investigators from Canada, Europe, Australia, and New Zealand recently published results from their multicenter cluster-randomized controlled trial that assessed the effect of family integrated care in the NICU on infant and parent outcomes. The intervention included a parental presence at the infant’s bedside for at least 6 hours per day, attendance at educational sessions, and active participation in infant care. In this study of 26 sites, compared to infants who received standard of care, infants in the family integrated care (FICare) group had better weight gain during birth hospitalization and exclusive breastmilk feeding at discharge. Parents in the FICare group had lower mean stress scores and lower mean anxiety scores (Klawetter et al., 2019).
Discuss the need to change lifestyle habits by smoking cessation and by restricting sexual activity and nipple stimulation. Smoking cessation- especially early in pregnancy- is associated with a reduced risk of preterm birth. Expectant mothers who smoke may face greater challenges in quitting compared with their counterparts who are not pregnant (Soneji & Beltran-Sanchez, 2019). Nicotine has an adverse effect on fetoplacental growth and on uterine circulation. Orgasm or release of oxytocin (from nipple stimulation) may stimulate uterine activity.
Encourage regular rest periods 2-3 times a day in a side-lying position. If the client is up and about, resting in the bedroom may maximize rest. If bed rest is to be continued after discharge, suggest the client spend part of the day on the couch or recliner. It is possible that antepartum bed rest might decrease preterm birth but evidence has not yet been found to support that conclusion. Experts agree that bed rest should not be a standard component of treatment for the prevention of preterm birth and, furthermore, that the practice should be eliminated (Maloni, 2010).
Review daily routine, employment, and activity schedule to identify alternatives and ways to compensate for limitations. Pacing activities, avoidance of heavy chores, lifting, and modification in work duties or cessation of employment may help prevent the recurrence of preterm labor. Of 300 women with a short cervix, women who exercised > 2 days per week (at least 20 min. duration), had lower rates of preterm birth compared to women who exercised less than this. The rate of preterm birth before 34 weeks was 9% in the ‘exercise’ group and 32% in the ‘no exercise’ group (Walsh, 2020).
Determine availability and level of commitment to supportive resources. Division of home care responsibilities helps reduce the risk of caregiver burnout when one individual attempts to take on responsibilities of the client in addition to their own role. Getting support from husbands, midwives and physicians, employers, and colleagues is an effective factor in building the capacity to cope with the problems during pregnancy and to achieve physical and mental balance. Moreover, the results of a study performed by Bawadi et al. demonstrated that husbands, by partaking in responsibilities, sympathy, attention, and protecting their wives play a supportive role during pregnancy. Weak support from a husband was associated with the pregnant woman’s anxiety and concern (Janighorban et al., 2018).
Advise client to empty bladder every two hours while awake. This prevents the pressure of a full bladder on the irritable uterus. Epidural or pudendal analgesia may interfere with sensations of fullness. The nurse should palpate the suprapubic area for a full bladder every 2 hours or more if a large IV solution was given.
Review daily fluid needs; avoid coffee. Dehydration and caffeine both lead to increased uterine muscle irritability. The client may drink two or three glasses of fluid to increase hydration. Hydration may also help stop contractions. This is thought to be effective because if a woman is dehydrated, the pituitary gland will be activated to secrete the antidiuretic hormone, which might cause the pituitary gland to release oxytocin as well, strengthening uterine contractions.
Stress avoidance of OTC drugs while tocolytic agents are being administered unless approved by the physician. Concurrent use of OTC drugs may cause deleterious effects, especially if OTC drug has similar side effects to tocolytic agents. Magnesium sulfate should not be used when nifedipine is used or when an intrauterine infection is suspected.
Provide information about taking oral tocolytics with food. Food improves tolerance to drugs and reduces side effects. Interactions between food and drugs may inadvertently reduce or increase the drug effect. The majority of clinically relevant food-drug interactions are caused by food-induced changes in the bioavailability of the drug (Bushra et al., 2011).
Identify drug side effects requiring medical evaluation. Magnesium sulfate causes a warm flush during the initiation of therapy. If the fetus is born during magnesium therapy, drowsiness may be present, and resuscitation may be required. Beta-adrenergic drugs cause cardiac side effects such as tachycardia, hypertension , nasal stuffiness, and hyperglycemia . Calcium channel blockers cause maternal flushing and hypotension due to their vasodilatory effects.
Establish a routine schedule for homecare nurse visitation. Provide regular telephone contact. Weekly or biweekly visits provide an opportunity for regular physical assessment , review of uterine activity records, and additional information for education. Most previous studies of home management report that high-risk pregnancies are monitored by daily or weekly visits either by healthcare professionals visiting the pregnant women in their homes or by pregnant women attending check-ups at the outpatient clinic (Zizzo et al., 2021).
Medications commonly used in preterm labor aim to delay or stop contractions and promote fetal lung maturation. Tocolytic drugs, such as beta- adrenergic agonists (e.g., terbutaline), calcium channel blockers (e.g., nifedipine), or prostaglandin inhibitors (e.g., indomethacin ), may be prescribed to inhibit uterine contractions. Other medications such as corticosteroids, (betamethasone or dexamethasone ), are administered to enhance fetal lung development and reduce the risk of respiratory distress syndrome in preterm infants.
IV solution or fluid bolus as indicated. Hydration may decrease uterine activity. Before beginning drug therapy, hydration promotes renal clearance and minimizes hypotension. Intravenous therapy to keep the client well hydrated should be started because although not well documented, hydration may help stop contractions. This is thought to be effective because if the client is dehydrated, the pituitary gland will be activated to secrete the antidiuretic hormone, which might cause the pituitary gland to release oxytocin as well, strengthening uterine contractions.
IV solutions containing tocolytic agents (e.g., magnesium sulfate, terbutaline sulfate) by infusion pumps or micro drip equipment, or by subcutaneous route.
- Magnesium sulfate Magnesium sulfate is the drug of choice. It acts directly on myometrial tissue to promote relaxation; therefore, there are fewer side effects than other drug choices. It is not a very effective tocolytic, but it is used to protect the fetus from developing cerebral palsy .
- Terbutaline sulfate This medication relaxes uterine muscles as well as bronchioles and blood vessel walls. These drugs are administered subcutaneously to stop uterine contractions within minutes.
Nifedipine Nifedipine, a calcium channel blocker, has been used experimentally when other drugs fail to suppress uterine activity. It is commonly used to stop labor contractions. Magnesium sulfate should not be used when nifedipine is used or when an intrauterine infection is suspected.
Betamethasone (Celestone) Betamethasone is synthetic cortisol that can accelerate fetal lung maturity by stimulating surfactant production and thereby preventing or decreasing the severity of respiratory distress syndrome. The standard regimen of antenatal corticosteroids involves 12 mg of betamethasone administered intramuscularly, repeated once after 24 hours (Surbek et al., 2012).
Antibiotics In the event of PROM and fetal lung immaturity, antibiotics may be used to prevent or reduce the risk of infection, while allowing an additional 24 hours to elapse after administration of Celestone. Antimicrobial therapy is often initiated in clients with preterm labor because studies have shown that subclinical chorioamnionitis is often present and for the prevention of group B streptococcus infection.
Analgesics Mild analgesics decrease muscle tension and discomfort. Analgesics agents are administered with caution because an immature infant will have enough difficulty breathing at birth without the additional burden of being sedated from a drug such as meperidine . If the client wants pharmaceutical pain management for labor, an epidural is preferable.
Antidotes (calcium gluconate for magnesium sulfate; propranolol for terbutaline sulfate) readily available: Administration of an antidote may be necessary to reverse or counteract the effects of tocolytic agents. In severe cases of magnesium toxicity , intravenous calcium gluconate can be used to displace and neutralize the effects of magnesium (Ajib & Childress, 2020)
Recommended books and resources for your NCLEX success:
Disclosure: Included below are affiliate links from Amazon at no additional cost from you. We may earn a small commission from your purchase. For more information, check out our privacy policy .
Saunders Comprehensive Review for the NCLEX-RN Saunders Comprehensive Review for the NCLEX-RN Examination is often referred to as the best nursing exam review book ever. More than 5,700 practice questions are available in the text. Detailed test-taking strategies are provided for each question, with hints for analyzing and uncovering the correct answer option.

Strategies for Student Success on the Next Generation NCLEX® (NGN) Test Items Next Generation NCLEX®-style practice questions of all types are illustrated through stand-alone case studies and unfolding case studies. NCSBN Clinical Judgment Measurement Model (NCJMM) is included throughout with case scenarios that integrate the six clinical judgment cognitive skills.

Saunders Q & A Review for the NCLEX-RN® Examination This edition contains over 6,000 practice questions with each question containing a test-taking strategy and justifications for correct and incorrect answers to enhance review. Questions are organized according to the most recent NCLEX-RN test blueprint Client Needs and Integrated Processes. Questions are written at higher cognitive levels (applying, analyzing, synthesizing, evaluating, and creating) than those on the test itself.

NCLEX-RN Prep Plus by Kaplan The NCLEX-RN Prep Plus from Kaplan employs expert critical thinking techniques and targeted sample questions. This edition identifies seven types of NGN questions and explains in detail how to approach and answer each type. In addition, it provides 10 critical thinking pathways for analyzing exam questions.

Illustrated Study Guide for the NCLEX-RN® Exam The 10th edition of the Illustrated Study Guide for the NCLEX-RN Exam, 10th Edition. This study guide gives you a robust, visual, less-intimidating way to remember key facts. 2,500 review questions are now included on the Evolve companion website. 25 additional illustrations and mnemonics make the book more appealing than ever.

NCLEX RN Examination Prep Flashcards (2023 Edition) NCLEX RN Exam Review FlashCards Study Guide with Practice Test Questions [Full-Color Cards] from Test Prep Books. These flashcards are ready for use, allowing you to begin studying immediately. Each flash card is color-coded for easy subject identification.

Other care plans related to the care of the pregnant mother and her baby:
- Abortion (Termination of Pregnancy) | 8 Care Plans
- Cervical Insufficiency (Premature Dilation of the Cervix) | 4 Care Plans
- Cesarean Birth | 11 Care Plans
- Cleft Palate and Cleft Lip | 7 Care Plans
- Gestational Diabetes Mellitus | 8 Care Plans
- Hyperbilirubinemia (Jaundice) | 4 Care Plans
- Labor Stages, Induced, Augmented, Dysfunctional, Precipitous Labor | 45 Care Plans
- Neonatal Sepsis | 8 Care Plans
- Perinatal Loss (Miscarriage, Stillbirth) | 6 Care Plans
- Placental Abruption | 4 Care Plans
- Placenta Previa | 4 Care Plans
- Postpartum Hemorrhage | 8 Care Plans
- Postpartum Thrombophlebitis | 5 Care Plans
- Prenatal Hemorrhage (Bleeding in Pregnancy) | 9 Care Plans
- Preeclampsia and Gestational Hypertension | 6 Care Plans
- Prenatal Infection | 5 Care Plans
- Preterm Labor | 7 Care Plans
- Puerperal & Postpartum Infections | 5 Care Plans
- Substance Abuse in Pregnancy | 9 Care Plans
Recommended journals, books, and other interesting materials to help you learn more about preterm labor nursing care plans and nursing diagnosis :
- Ajib, F. A., & Childress, J. M. (2021, November 14). Magnesium Toxicity – StatPearls . NCBI. Retrieved April 9, 2022.
- Bazrafshan, S., Kheirkhah, M., Inanlou, M., & Rasouli, M. (2020, August). Controlling the anxiety in Iranian pregnant women at risk of preterm labor by undergoing the counseling group intervention. Journal of Family Medicine and Primary Care , 9 (8), 4016-4025.
- Bushra, R., Aslam, N., & Yar Khan, A. (2011, March). Food-Drug Interactions. Oman Medical Journal, 26(2), 77-83.
- Eggermont, K., Beeckman, D., Van Hecke, A., Delbaere, I., & Verhaeghe, S. (2016). Needs of fathers during labor and childbirth: A cross-sectional study. Women and Birth .
- Griggs, K. M., Hrelic, D. A., Williams, N., McEwen-Campbell, M., & Cypher, R. (2020, November/December). Preterm Labor and Birth A Clinical Review. The American Journal of Maternal/Child Nursing , 45 (6), 328-337.
- Hicks, M. A., & Tyagi, A. (2022, January 31). Magnesium Sulfate – StatPearls . NCBI. Retrieved April 9, 2022.
- Janighorban, M., Heidari, Z., Dadkhah, A., & Mohammadi, F. (2018, January). Women’s Needs on Bed Rest during High-risk pregnancy and Postpartum Period: A Qualitative Study. Journal of Midwifery and Reproductive Health , 6 (3), 1327-1335. https://doi.org/10.22038/jmrh.2018.28162.1304
- Junge, C., von Soest, T., Weidner, K., Seidler, A., Eberhard-Gran, M., & Garthus-Niegel, S. (2018, February 19). Labor pain in women with and without severe fear of childbirth: A population-based, longitudinal stud. Birth , 45 , 469-477.
- Kao, M.-H., Hsu, P.-F., Tien, S.-F., & Chen, C.-P. (2017, November 27). Effects of Support Interventions in Women Hospitalized With Preterm Labor. Clinical Nursing Research , 1-18.
- Klawetter, S., Greenfield, J. C., Speer, S. R., Brown, K., & Hwang, S. S. (2019, May 5). An integrative review: maternal engagement in the neonatal intensive care unit and health outcomes for U.S.-born preterm infants and their parents. AIMS Public Health , 6 (2), 160-183.
- Leifer, G. (2018). Introduction to Maternity and Pediatric Nursing . Elsevier.
- Maloni, J. A. (2010, August 26). Antepartum Bed Rest for Pregnancy Complications: Efficacy and Safety for Preventing Preterm Birth. Biological Research for Nursing , 12 (2), 106-124.
- Mirtabar, S. M., Faramarzi, M., Khazaei, R., & Dini, M. (2020, August 10). Efficacy of psychotherapy for anxiety reduction in hospital management of women successfully treated for preterm labor: a randomized controlled trial. Women & Health , 60 (10), 1151-1163.
- Oktriani, T., Ermawati, & Bahctiar, H. (2018). The Difference Of Pain Labour Level With Counter Pressure And Abdominal Lifting On Primigravida In Active Phase Of First Stage Labor. Journal of Midwifery , 3 (2).
- Özberk, H., Mete, S., & Bektas, M. (2020, July 17). Effects of Relaxation-Focused Nursing Care in Women in Preterm Labor. Biological Research for Nursing .
- Reddy, R. (2022, February 2). Calcium Channel Blocker Toxicity – StatPearls . NCBI. Retrieved April 9, 2022.
- Silbert-Flagg, J., & Pillitteri, A. (2018). Maternal & Child Health Nursing: Care of the Childbearing & Childrearing Family . Wolters Kluwer.
- Sivaramakrishnan, G., & Sridharan, K. (2016, May 13). Adverse drug reactions in the oral cavity. Drug and Therapy Perspectives , 32 , 297-303.
- Soneji, S., & Beltran-Sanchez, H. (2019, April 19). Association of Maternal Cigarette Smoking and Smoking Cessation With Preterm Birth. JAMA Network Open , 2 (4).
- Surbek, D., Drack, G., Irion, O., Nelle, M., Huang, D., & Hoesli, I. (2012, April 29). Antenatal corticosteroids for fetal lung maturation in threatened preterm delivery: indications and administration. Archives of Gynecology and Obstetrics , 286 , 277-281.
- Walsh, C. A. (2020). Maternal activity restriction to reduce preterm birth: Time to put this fallacy to bed. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists , 1-3.
- Xiao, C., Gangal, M., & Abenhaim, H. A. (2014, February 25). Effect of magnesium sulfate and nifedipine on the risk of developing pulmonary edema in preterm births. Journal of Perinatal Medicine , 42 (5), 585-589.
- Zeraati, M. R., & Naghibi, T. (2019, July 10). Acute Pulmonary Edema Following Administration of Magnesium Sulfate in a Pregnant Patient. Journal of Advances in Medical and Biomedical Research , 27 (124), 43-46.
- Zizzo, A. R., Hvidman, L., Salvig, J. D., Holst, L., Kyng, M., & Petersen, O. B. (2021, December 7). Home management by remote self-monitoring in intermediate- and high-risk pregnancies: A retrospective study of 400 consecutive women. Acta Obstetricia et Gynecologica Scandinavica , 101 (1), 135-144.
Reviewed and updated by M. Belleza, R.N.
Leave a Comment Cancel reply
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
Pre-Term Labor Case Study Student Version
Maternal newborn nursing (nur446), university of sioux falls.
Students also viewed
- Chapter 1 Homework Solutions
- Liberal Arts Essay - Understanding and articulating a vision and purpose for your education can be
- World War 1 - Paper #2
- Col War Policies - Paper #3
- Biology Wrap-Up
- The Origin of Finch Species
Related documents
- Section 3 Questions for U.S History since 1877
- Section 2 Questions for U.S History since 1877
- Section 1 U.S History Notes
- Lab Practical Bio
- Bio Chem Test 3 - Test notes
- Bones NEED to know
Preview text
INSTRUCTIONS All questions apply to this case study. Your responses should be brief and to the point. When asked to provide several answers, list them in order of priority or significance. Do not assume information that is not provided.
P. is a married 30-year-old G4T0P2A1L2 at 28 weeks' gestation. She arrives in the labor and delivery unit at a level 2 hospital complaining of low back pain and frequency of urination. She states that she feels occasional uterine cramping and believes that her membranes have not ruptured.
1 are the charge nurse and admit P. Based on the information you have been given,
identify the two most likely diagnoses for P.
Uti or kidney infection or preterm labor
2 need additional information from P. to determine what you will do next. What
important questions do you need to ask to differentiate what is going on with P.?
Pain with urination, quality of cramping, timing and regularity of cramping, duration of
cramping, point to where it is, any bleeding or discharge, any pressure, diarrhea
- What actions would you take to help identify her underlying problem before calling the health care provider?
Vaginal exam, cbc (once provider sees them but you can prepare for this), fetal heart rate, UA,
Leopold’s maneuvers, vs,
- Early recognition of preterm labor is essential to successfully implement interventions. The diagnosis of preterm labor is based on what three major diagnostic criteria?
Before 37 weeks, consistent contraction pattern, beginning to dilate (progressive cervical change) PROGRESS P.'s history reveals that she had one preterm delivery 4 years ago at 31 weeks' gestation. The infant girl was in the neonatal intensive care unit (NICU) for 3 weeks and discharged without sequelae. The second pre- term infant, a boy, was delivered 2 years ago at 35 weeks' gestation and spent 4 days in the hospital before discharge. She has no other risk factors for preterm labor. Vital signs are normal. Her vaginal examination was essentially within normal limits: cervix long, closed, and thick; membranes intact. Abdominal examination revealed that the abdomen
was nontender, with fundal height at 29 cm, fetus in a vertex presentation.
- While you are waiting for laboratory results, what therapeutic measures do you consider?
Bbreathing, hydration, relaxation, watch her strip and monitor contractions STUDY PROGRESS While waiting for laboratory results, you consider that if P. is experiencing preterm labor, she would receive antenatal glucocorticoids.
- What is the rationale for the administration of antenatal glucocorticoids for preterm labor?
a. To accelerate fetal lung maturity
b. To stop uterine contractions
c. To soften the cervix
d. To prevent maternal infection
- How long do these drugs take to become effective?
They take 24-48 hrs, but after 1st dose 24 hrs
- If P. is in preterm labor, the nurse can expect that her provider will initiate medication therapy to stop the uterine contractions. Which class of medication is useful in stopping uterine contractions?
a. Tocolytics b. Antianxiety medications c. Cervical ripening agents d. Antibiotic therapy
- The provider orders indomethacin (Indocin) 100mg now, followed by 50mg every 8 hours PO for 3 days. Explain the purpose of this drug in this situation. Slow down uterine activity
Two hours later, the laboratory results indicate a urinary tract infection. The contraction monitor indicates infrequent, mild contractions. Her physician discharges her to home on an antibiotic for the UTI.
- What follow-up measures should be considered in providing P. discharge instructions?
- Multiple Choice
Course : Maternal Newborn Nursing (NUR446)
University : university of sioux falls.

- More from: Maternal Newborn Nursing NUR446 University of Sioux Falls 4 Documents Go to course
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
RISK FACTORS FOR PRETERM LABOUR; AN UNMATCHED CASE CONTROL STUDY

Background: Preterm labour continues to be the leading cause of perinatal and neonatal morbidity; representing one of the principal targets for obstetric health care and challenging the obstetricians and health policy makers to tackle this problem. Objectives: This work was designed to find out effects of selected sociodemographic, maternal, service-related and psychological risk factors forpreterm labour. Methods: This was a hospital based unmatched case control study Findings: Hypertensive disorders of pregnancy(OR 3.92:, 95% CI:2.72-5.65), maternal height < 153 cm(OR:2.30, 95% CI:1.61-3.29), polyhydroamnios(OR:2.45, 95% CI:1.69-3.54),twin gestation(OR:2.29, 95% CI:1.58-3.34),threatened abortions(OR:3.14, 95% CI:1.62-6.06) , maternal age < 38 years (OR:2.13, 95% CI:1.27-3.58),family history of prematurity(OR:1.5, 95% CI:1.34-4.73) Conclusion: Hypertensive disorders of pregnancy, maternal height < 153 cm, polyhydroamnios, twin gestation, threatened abortions, PROM,maternal age >38 years and family history of prematurity wererisk factors forpreterm labour.
Related papers
Port Said Scientific Journal of Nursing, 2015
A case-control design was selected in carrying out this study and a sample of 208 parturient women (104 with preterm labor being hospitalized or none hospitalized before delivery and 104 with normally vaginal delivery) were recruited for this study. The tools used for data collection were; an interview questionnaire and assessment sheet. Results: The results of this study revealed that (37.5%) were nulligravida, (39.4%) nulliparas, (29.8%) have history of previous preterm labor , previous CS (41.5%) and exposure to stress (71.2%), violence (29.8) were all risk factors for PTL. Recommendations: the study recommended that early diagnosis of preterm labor, identification of risk factors, adopting recent modalities of diagnoses and management in appropriate setting and with adequate resources may help in better outcome by reducing the fetal-maternal complications. Conclusion: It was concluded that more than one third of PTL women were nulligravida and nullipara. Higher percentage of them was exposed to violence and stress, passive smoking, medical and gynecological problems. PTL had adverse effects on fetal birth outcome lower Apgar score, low birth weight.
SBMU publishing, 2017
Introduction: Preterm birth is still a major health problem throughout the world, which results in 75% of neona-tal mortality. Preterm labor not only inflicts financial and emotional distress, it may also lead to permanent disability. The present study was conducted to determine therelated risk factors andpreventive measuresof preterm labor. Methods: This retrospective cross-sectional study assessed all preterm labors, as well as an equal number of term labors, during seven years, at an educational hospital. Probable risk factors of preterm labor were collected using medical profiles of participants by the aid of a pre-designed checklist. Significant related factors of preterm laborwere used for multivariate logistic regression analysis with SPSS 21.0. Results: 810 cases with the mean age of 28.33 ± 6.1 years were evaluated (48.7% preterm). Multipartite; fetal anomaly; prenatal care; smoking ; not consuming folic acid and iron supplements; in vitro fertilization; history of infertility, caesarian section, trauma, systemic disease, and hypertension; amniotic fluid leak; rupture of membranes; cephalic presentation; vaginal bleeding; placenta decolman; oligohydramnios; pre-eclampsia; chorioamnionitis; uterine abnormalities ; cervical insufficiency; intercourse during the previous week; short time since last delivery; and mother's weight significantly correlated with preterm labor. Conclusion: Based on the results of the present study, intercourse during the previous week, multipartite, short time from last delivery, preeclampsia, fetal anomaly, rupture of membranes, hypertension, and amniotic fluid leak, respectively, were risk factors for preterm labor. On the other hand, iron consumption, cephalic presentation, systematic disease, history of caesarian section, prenatal care, and mother's weight could be considered as protective factors.
BJOG: An International Journal of Obstetrics and Gynaecology, 2003
Socioeconomic factors associated with preterm labour include social class, (usually assessed by earnings and education), working conditions (professional status, ergonomic environment, working hours), physical and travelling activities, daily life activities, lifestyle, family status and psychosocial state as related to past and current pregnancy history together with current stress factors. A review of the association of these factors with preterm birth will be reported with an emphasis of the biological plausibility linking mostly emotional, and at a lesser degree, physical and psychological stress to the occurrence of preterm labour. A case control study, carried out in Quebec City among 101 women in preterm labour and 202 matched pregnancies for parity and gestational age, identified 7 risk factors in an explanatory multivariate model among 117 variables: Body mass index (BMI)
National Journal of Community Medicine, 2015
Objective: The objectives of this study were to study the various etiological factors responsible for onset of preterm labor and to evaluate the predictive value of these risk factors for spontaneous preterm labor. Methods: This prospective comparative study was conducted over a period of 1 year and included 240 women; of which 120 in preterm labor were enrolled in the study group and 120 with term pregnancy in labor were enrolled in the control group. History, clinical examination and investigations were carried out and risk factors for preterm labor were noted and analyzed to find out the predictive value of the risk factors for preterm labor. Results: Maximum numbers of patients (31.6%) were in the 35-36 weeks gestational age group. When both the groups were compared as regards risk factors, the cases showed a high incidence of infections; 21.7% incidence of chorioamnionitis, 32.5% incidence of bacterial vaginosis and a 12.5% incidence of urinary tract infections. Prior second trimester abortion was found in 13.3% cases and a history of prior preterm birth was found in 15% cases. Uterine over distension was observed in 20.83% cases. Maternal medical disorders were present in 60% cases. Conclusions: Multiple pregnancy, Prior preterm birth, Infections, Prior second trimester abortions and medical disorders in mother are strongly associated with an increased risk of preterm labor.
International Journal of General Medicine, 2012
Introduction: Premature labor is a serious worldwide problem that can cause neonatal death and other serious disorders. This study aimed to determine the most important factors related to preterm labor in Yasuj, Iran. Method: This case-control study was conducted in the maternity ward of Imam Sajjad Hospital, the obstetrics and gynecology center of Yasuj, in 2010. Among eligible samples, mothers with preterm labor were selected as the case group, and for each sample in the case group, one mother with full-term labor was selected by using clipper-matched sampling to make up the control group. Data were collected by a researcher-made questionnaire and the 28-item General Health Questionnaire. Finally, after deleting imperfect questionnaires, collected data of 52 subjects of case group and the same amount in control group were analyzed. Results: Among the 5400 live birth infants in Yasuj in 2010, 130 infants were premature (2.4%). The preterm labor risk in women with two or more pregnancies was 5.5 times more than women with less than two pregnancies, its risk in women with low general health status was 2.9 times more than in women with normal general health status, and the preterm labor risk in women with a history of diabetes mellitus/thyroid dysfunction/cardiac disease was 2.3 times more than healthy mothers (P 0.01). Conclusion: With respect to the above and due to the role and importance of mother-infant health in community health, it is necessary that the health-care system improve health education with regard to the appropriate number of pregnancies, diagnose and cure disease during pregnancy, especially diabetes and cardiovascular disease (hypertension and/or eclampsia), and recognize pregnant mothers with mental pressure or lack of sufficient support and help them.
Journal of Babol University of Medical Sciences, 2015
BACKGROUND AND OBJECTIVE: Premature delivery is the presence of progressive uterine contractions before reaching 37 weeks of pregnancy. Since it is associated with perinatal complications and high costs, this study aimed to study the risk factors for premature delivery. METHODS: This cross-sectional study was conducted on 377 pregnant women with preterm labor (23-37 weeks) and 423 pregnant women of term delivery referring to Ayatollah Rohani Hospital of Babol city, Iran. We extracted and investigated the subjects’ demographic data, history of infertility, smoking habits, use of drugs, fast food consumption, history or presence of maternal illnesses and surgery, Urinary Tract Infections (UTI), Oligohydramnios, intrauterine growth restriction (IUGR), embryonic anomalies, premature rupture of membranes, vaginal bleeding as well as the neonatal data. FINDINGS: As observed in the two groups of preterm and term delivery respectively, there was employment during pregnancy in 83 (22%) and 5...
International Journal of Biomedical Research, 2012
Background-Preterm labour is a common complication that contributes significantly to high perinatal morbidity and mortality. In India, the reported incidence of preterm labour is 10-15 percent. Premature babies are at risk of many immediate and long term complications. Material and Methods-Prospective observational study of various risk factors responsible for preterm labour and the perinatal outcome was done in 125 women, over one year period at tertiary care centre in rural area. Diagnosis of preterm labour was done by ACOG 197 criteria. Results-The incidence of preterm labour was 13.2%. It was observed that 95% women were unbooked cases from poor socio-economic class, staying in a rural area. Fifty percent cases had pregnancy duration of 32 to 34 weeks. Sixty percent cases had some associated risk factor responsible for preterm labour. Ante partum haemorrhage (22.53%) over distension of the uterus (16.90%), hypertension and maternal anaemia were common risk factors. Perinatal mortality in the study group was 42.4%. Early neonatal deaths accounted for 50% of the perinatal mortality. Antepartum haemorrhage, maternal anaemia, hepatitis, obstructed labour, and fetal congenital anomalies were common causes of stillbirths, where as respiratory distress, birth asphyxia and septicaemia were common causes of early neonatal deaths. Neonatal mortality was 100% in the babies with birth weight less than 1000 grams .Neonatal mortality was 63.33% in babies born before 31 weeks of gestation. Conclusion-Early detection of high risk factors, appropriate intervention, institutional delivery and good neonatal care back up facilities can improve the outcome of preterm labour.
An Act to consolidate and amend the laws relating to reorganisation and insolvency resolution of corporate persons, partnership firms and individuals in a time bound manner for maximisation of value of assets of such persons, to promote entrepreneurship, availability of credit and balance the interests of all the stakeholders including alteration in the order of priority of payment of Government dues and to establish an Insolvency and Bankruptcy Board of India, and for matters connected therewith or incidental thereto.
Revista Labor
Ceskoslovenska psychologie, 2021
Open journal of business and management, 2024
E-flux, 2024
Democratization of Intelligence--Melding Strategic Intelligence and National Discourse, 2009
Transinformacao, 2012
Naukovì perspektivi, 2022
Jurnal Ilmiah Pendidikan Profesi Guru
Journal of the Mexican Chemical Society, 2019
Clinical Neurology and Neurosurgery, 1997
Building and Environment, 2015
Journal of Endocrinology, 2009
PLOS ONE, 2020
Anais do Congresso Nacional de Excelência em Gestão
The Developing Economies, 2014
Journal of Rehabilitation Medicine, 2019
Procedia Computer Science, 2015
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Miracle baby: managing extremely preterm birth in rural Uganda
Hannah katherine mitchell, rhianne thomas, michael hogan, carolin bresges.
- Author information
- Article notes
- Copyright and License information
Correspondence to Dr Hannah Katherine Mitchell, [email protected]
Series information
Case Report
Accepted 2014 May 9; Collection date 2014.
Preterm birth is an important cause of neonatal morbidity and mortality globally. Uganda has one of the highest rates of preterm birth in East Africa but few resources to care for these infants. This case highlights the clinical course of an extremely premature infant born at 26 weeks gestation to a nulliparous 24-year-old woman. Her mother was involved in her care and taught the principles of kangaroo mother care. After initial problems establishing feeds she progressed well and was discharged in the fifth week of life. The case describes some of the low technology conservative and medical measures which can be used to care for neonates, such as antenatal steroids and kangaroo care. The use of antibiotics and aminophylline are also discussed. The approach to the common challenges faced by premature infants such as respiratory disease, sepsis and necrotising enterocolitis in a resource-poor environment are discussed.
Case presentation
A 24-year-old primigravida with no known significant medical history and no specific risk factors for preterm birth was admitted to the maternity unit of a hospital in Uganda with a 2-day history of labour-like pain. Her membranes had ruptured 2 days previously. She was admitted to the ward for observation and given three doses of oral dexamethasone.
On examination her os was 7 cm dilated. One day later she gave birth to a live female infant by spontaneous vaginal delivery. The baby was born in a poor condition with an Apgar score of 2 at birth and 4 at 5 min. She was given inflation breaths, began to breathe spontaneously and her condition quickly improved.
According to the mother's last menstrual period, the pregnancy was dated at 26 weeks. The baby was scored according to the expanded New Ballard Score 1 for neuromuscular and physical parameters. A total score of 5 ( figure 1 ) correlated with the mother's menstruation dates and our estimated gestation date of 26 weeks, making the neonate extremely premature.
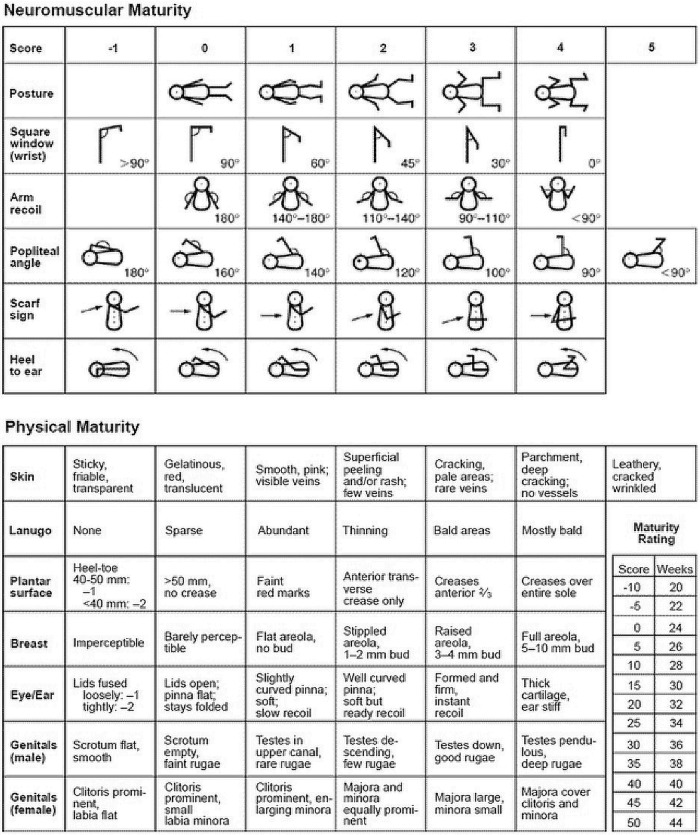
New Ballard Score. 1
Respiratory support facilities at the unit were limited with no capacity for ventilation and no surfactant available. The infant was given oxygen via nasal cannulas from an oxygen concentrator maintaining saturations between 88% and 92%. Prophylactic aminophylline was given to decrease the risk of apnoea. The baby fortunately remained stable from a respiratory point of view.
According to the hospital guidelines, premature babies are given 5 days of prophylactic ampicillin and gentamicin. The baby had an umbilical catheter inserted using aseptic technique which provided good intravenous access for the first 10 days of life.
As an extremely preterm and very low birth weight infant, the baby was at risk of developing necrotising enterocolitis. She was kept nil by mouth for the first 48 h and was maintained on intravenous fluids. On the third day of life the baby was started on 1 mL expressed breast milk every 2 h via nasogastric tube. The feeds were increased by 1 mL every other feed provided that aspirates were minimal. The neonate’s observations were monitored, with stool and urine output recorded on a chart. The milk volume was increased very slowly as there were a number of episodes of abdominal distention. Nevertheless the baby was on full oral feeds by day 14 of life.
Throughout the baby’s time in the unit her mother was kept up to date and involved in her care. Temperature maintenance was initially problematic. The baby's mother was taught about kangaroo care and encouraged to attend the unit as frequently as possible to provide care. Kangaroo care proved an effective method of stabilising the neonate’s temperature.
When the baby was on full oral feeds she was moved to the ‘kangaroo room’ ( figure 2 ) a warm room where the baby and mother were able to stay together. She was examined daily and her temperature monitored.

Kangaroo care room.
The baby was discharged from the unit in the fifth week of life, earlier than desired due to maternal financial constraints. The mother was well educated in the care of her preterm baby and was confident giving the oral aminophylline. She was advised of the symptoms which should prompt her to seek medical attention. The mother and infant returned for weekly weights and examinations until the child was 40 weeks corrected gestational age.
Global health problem list
Preterm birth rates are rising globally, no where more so than in the developing world. Uganda has one of the highest preterm birth rates in East Africa.
Preterm birth is one of the biggest risk factors for neonatal morbidity and mortality. Multiple complications are associated with preterm birth.
There is a lack of high-tech medical equipment and medication.
There is a good evidence base for low-tech and medical interventions but due to lack of knowledge these are often not implemented.
Global health problem analysis
Techniques in the management of preterm birth in the developed world have undergone significant advances, with outcomes for neonates born prematurely improving greatly over the past few decades. 2 However, these advances have not reached the developing world where access to high-tech equipment and drugs is extremely limited.
In 2005, the WHO estimated that globally 9.6% of births are preterm. 3 Preterm infants are disproportionately over-represented in neonatal mortality rates with estimates showing that a quarter of perinatal deaths are attributable to complications of prematurity. 4 5
Conservative and medical measures are often the only steps possible in a resource-poor environment. There are a number of pharmacological approaches which have shown demonstrable improvement in neonatal outcomes.
Administration of antenatal steroids to mothers going into preterm labour is routine practice in the developed world. Conversely, in the developing world they are often omitted. 6 Strong evidence exists for the role of antenatal steroids in reducing the incidence of respiratory distress syndrome, intraventricular haemorrhage, periventricular leukomalacia and necrotising enterocolitis in premature infants. 7 8 Even incomplete courses of antenatal steroids have been shown to give some benefits to extremely premature infants. 9 The importance of antenatal steroids cannot be overstated. They are generally widely available, easy to administer, even in the community, and have minimal risk of adverse effects to mother and baby. A recent study estimated that 500 000 neonatal lives could be saved annually if antenatal steroids were given appropriately to all mothers going into preterm labour. 6
Another pharmacological agent used to attempt to counteract some of the problems of prematurity is aminophylline. This drug has been shown to reduce the incidence of idiopathic apnoea in premature infants. 10 Owing to controversy regarding the use of aminophylline, caffeine is generally the preferred option in neonatal units in the UK. 11 In this hospital, however, caffeine was not available from the pharmacy. The long-term risk of neurodevelopmental disability associated with aminophylline administration needs to be taken into account. However in the context of providing medical care where respiratory support for these infants is not possible, aminophylline often represents the only available intervention.
Use of prophylactic antibiotics is controversial and guidelines for our institution recommend 5 days of prophylactic ampicillin and gentamicin for all new born infants. Evidence suggests that prophylactic antibiotics can reduce incidence of necrotising enterocolitis in low birthweight infants, 12 however the issue is fraught with difficulty with challenges of antibiotic resistance and antibiotic side effects.
In preterm prolonged rupture of membranes there is some evidence for giving antibiotics to the mother antenatally as this has been shown to prevent 4% of deaths due to complications of prematurity and 8% of deaths due to infection. 13
There are a number of conservative steps that can be taken in the management of preterm infants which can influence their outcomes. Poor weight gain, dehydration and hypothermia are problems particularly faced by preterm infants. There are challenges of attitudes and awareness both among parents and medical staff; all too often the assumption is made that the infant would not survive and few resources are dedicated to neonatal care. 14 Education of parents is paramount.
In low birthweight infants necrotising enterocolitis is a serious and often fatal problem. Feeding of mother’s milk has been shown to significantly reduce the risk of necrotising enterocolitis compared with formula feeding. 15 Monitoring for excessive or blood or bile-stained aspirates from the nasogastric tube can give indications that necrotising enterocolitis is starting to develop. 16 Feeding poses particular challenges in the community setting as nasogastric tubes may not be available and extremely premature infants are unable to breast feed effectively and risk dehydration. Other methods of feeding may need to be found.
‘Kangaroo mother care’ (KMC) has been estimated to reduce neonatal mortality and morbidity, particularly from infection. 17 In KMC the infant is tied to the front of the mother with a cloth. Skin to skin contact helps to maintain temperature and the mother may be able to recognise earlier when the infant is developing complications so a response can be started. KMC can be a useful tool for mother and baby in conventional neonatal care setting as well as being easily implemented in a community care setting. There is some evidence to support its use when more advanced care facilities are absent. 18 KMC is particularly relevant in the context of hospital care where infants remain in cots without heating facilities and become susceptible to hypothermia which is a significant problem potentially resulting in infant mortality. 19 20
The most basic practices such as good cord care, feeding and temperature control represent some of the most important elements and all too often these fundamentals are overlooked. 21 Parental involvement and maternal education regarding best practice in caring for their infants is of paramount importance.
There are particular challenges in caring for neonates in the developing world. Without the extensive array of equipment we have come to expect in the developed world, expectations can be low and there can be a reluctance to invest energy in caring for these infants. However as this report shows whether in hospital or the community there are still a number of steps that can be taken to help save the lives of these tiny infants.
Patient's perspective.
The baby was clinically stable on discharge from our unit. Initially, she returned for weekly weights and examinations and was growing well. When she was discharged from the follow-up of our unit her mother would continue to bring her back to talk to the nurses and midwifes and other mothers at the unit.
Learning points.
Antenatal steroids should always be given to mothers anticipating preterm delivery.
When gestational age is in doubt the New Ballard Score is a validated method of estimating maturity of the neonate.
Caffiene/aminophylline can help to reduce incidence of apnoea.
Kangaroo care can be done in all contexts and has demonstrable benefits.
Acknowledgments
The authors thank Wellbeing of Women for sponsoring Hannah Mitchell on her medical elective.
Contributors: HKM was involved in conception and design, drafting and final approval of submitted version. RT was involved in conception and design, drafting and revising the article, editing and final approval of submitted version. MH was involved in conception and design, drafting the article. CB was involved in conception and design, drafting and editing the article.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
- 1. Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr 1991;119:417–23 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004;329:675–8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Bulletin of the World Health Organization. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. 2010. http://www.who.int/bulletin/volumes/88/1/08–062554/en/ (accessed 21 Jul 2013). [ DOI ] [ PMC free article ] [ PubMed ]
- 4. Nankabirwa V, Tumwine JK, Tylleskär T, et al. Perinatal mortality in eastern Uganda: a community based prospective cohort study. PLoS ONE 2011;6:e19674. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Waiswa P, Kallander K, Peterson S, et al. Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health 2010;15:964–72 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Mwansa-Kambafwile J, Cousens S, Hansen T, et al. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol 2010;39 (Suppl 1):i122–33 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Elimian A, Verma U, Canterino J, et al. Effectiveness of antenatal steroids in obstetric subgroups. Obstetr Gynecol 1999;93:174–9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006;(3):CD004454. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Costa S, Zecca E, De Luca D, et al. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstetr Gynecol Reprod Biol 2007;131:154–7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev 2010;(12):CD000140. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Millar D, Schmidt B. Controversies surrounding xanthine therapy. Sem Neonatol 2004;9:239–44 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Bury RG, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev 2001;(1):CD000405. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Cousens S, Blencowe H, Gravett M, et al. Antibiotics for pre-term pre-labour rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. Int J Epidemiol 2010;39(Suppl 1):i134–43 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Waiswa P, Nyanzi S, Namusoko-Kalungi S, et al. ‘I never thought that this baby would survive; I thought that it would die any time’: perceptions and care for preterm babies in eastern Uganda. Trop Med Int Health 2010;15:1140–7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Tudehope DI. Human milk and the nutritional needs of preterm infants. J Pedriatr 2013;162(3 Suppl):S17–25 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271–83 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Lawn JE, Mwansa-Kambafwile J, Horta BL, et al. ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol 2010;39(Suppl 1):i144–54 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Conde-Agudelo A, Belizan JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011;(3):CD002771. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Manji KP, Kisenge R. Neonatal hypothermia on admission to a special care unit in Dar-es-Salaam, Tanzania: a cause for concern. Cent Afr J Med 2003;49:23–7 [ PubMed ] [ Google Scholar ]
- 20. Byaruhanga R, Bergstrom A, Okong P. Neonatal hypothermia in Uganda: prevalence and risk factors. J Trop Pediatr 2005;51:212–15 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Waiswa P, Peterson S, Tomson G, et al. Poor newborn care practices—a population based survey in eastern Uganda. BMC Pregnancy Childbirth 2010;10:9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.5 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

COMMENTS
Uma S, Nisha S, Shikha S. A prospective analysis of etiology and outcome of preterm labor. J Obstet Gynaecol India. 2007;57:48-52. [Google Scholar] 22. Singla S, Das B. Risk factor and perinatal outcome of preterm delivery in a tertiary care centre in rural Haryana. Int J Reprod Contracept Obstet Gynecol. 2020;9:4187-92. [Google Scholar] 23.
(33) The duration of a possible Preterm Birth could be analysed as the interval between the date/time of onset 1 of the first symptoms and/or signs consistent with the definition and the end of episode 5 and/or final outcome. 6 Whatever start and ending are used, they should be used consistently within and across study groups. (34) If more than one measurement of a particular criterion is ...
Preterm labor is regular uterine contractions after 20 weeks and before 37 weeks of pregnancy that cause cervical change or regular contractions with an initial presentation with cervical dilation of 2 cm or more. Preterm birth is birth after 20 weeks gestation and before 37 completed weeks gestation. Preterm birth affects over 15 million babies and their mothers and families worldwide.
Timing to obtain consent. Preterm labor is an unforeseen and acute complication that threatens neonatal survival. Pregnant women with premature uterine contractions are usually anxious and in pain, and may not be in the best condition to participate in a full consent procedure, particularly when there is limited time for decision-making [12, 13].The local IRB suggested that the maternity ward ...
PRETERM LABOR CASE STUDY. Christina Bloom. Mrs. Jones is a G4T1P1A1L2 at 32 weeks and 3 days. She came into labor triage for contractions that are happening every 2 to 4 minutes apart and lasting approximately 60 seconds long. Mrs. Jones delivered her last child at 34 weeks.
Pre term labor case study instructions all questions apply to this case study. your responses should be brief and to the point. when asked to provide several. Skip to document. University; High School. Books; Discovery. ... If P. is in preterm labor, the nurse can expect that her provider will initiate medication therapy to stop the uterine ...
Methods. Our case-control study included 467 term controls and 467 PTD cases. PTD was studied in aggregate and in subgroups (i.e., spontaneous preterm labor and delivery [SPTD], preterm premature rupture of membrane [PPROM], medically indicated preterm delivery [MIPTD], moderate preterm delivery [32-36 weeks], and very preterm delivery [<32 weeks]).
MATERIALS AND METHODS. This study used a case-control design because the incidence of preterm labor was relatively low. Apart from PROM, other variables studied included maternal characteristics (age, education, and work status), previous obstetric history (history of abortion and history of preterm labor), and variables related to the current pregnancy (parity, duration from previous delivery ...
Introduction: Preterm birth is still a major health problem throughout the world, which results in 75% of neona-tal mortality. Preterm labor not only inflicts financial and emotional distress, it may also lead to permanent disability. The present study was conducted to determine therelated risk factors andpreventive measuresof preterm labor.
Preterm birth is an important cause of neonatal morbidity and mortality globally. Uganda has one of the highest rates of preterm birth in East Africa but few resources to care for these infants. This case highlights the clinical course of an extremely premature infant born at 26 weeks gestation to a nulliparous 24-year-old woman.