Research Projects

The Division of Diabetes Translation (DDT) conducts and supports studies, often in collaboration with partners, to develop and apply sound science to reduce the burden of diabetes and to address the research needs of DDT programs and the diabetes community.
Analyses from various studies, such as LEAD and SEARCH, to help improve diabetes surveillance and guide decision-making.
Research, including the Diabetes Prevention Program Outcome Study (DPPOS), that evaluates the success of diabetes interventions and strategies.
Reviews of the effect of type 2 diabetes-related health policies on various populations, such as the NEXT-D2 study.
Collection of the most recent publications and access to archived ones.
- US Diabetes Surveillance System
- Chronic Kidney Disease Surveillance System
- Vision and Eye Health Surveillance System
- Diabetes State Burden Toolkit
- Diabetes Prevention Impact Toolkit
To receive updates about diabetes topics, enter your email address:
- Diabetes Home
- State, Local, and National Partner Diabetes Programs
- National Diabetes Prevention Program
- Native Diabetes Wellness Program
- Chronic Kidney Disease
- Vision Health Initiative
- Heart Disease and Stroke
- Overweight & Obesity

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How old is too old to run?

America’s graying. We need to change the way we think about age.

Can we talk?
“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
No such thing, specialist says — but when your body is trying to tell you something, listen

Experts say instead of disability, focus needs to shift to ability, health, with greater participation, economically and socially

Study finds that conversation – even online – could be an effective strategy to help prevent cognitive decline and dementia
When math is the dream
Dora Woodruff was drawn to beauty of numbers as child. Next up: Ph.D. at MIT.
Three will receive 2024 Harvard Medal
In recognition of their extraordinary service
So what exactly makes Taylor Swift so great?
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records.
Sharing links
Milestones | 17 June 2021
Milestones in diabetes
Milestone 1 1922
A history of insulin: initial discovery to first use in the treatment of T1D
Frederick Banting declared that “insulin is not a cure for diabetes; it is a treatment” in his 1923 Nobel lecture. The year 2021 marks 100 years since the discovery of insulin, which revolutionized the management of patients with type 1 diabetes. The past 100 years have seen seismic shifts in our understanding of the pathogenesis of the different types of diabetes, leading to advances in patient care. In this Nature Milestones in Diabetes , we highlight some of these key discoveries, which lay a path to the elusive goal of finding a cure for diabetes. Read more.

Insulin and islets: understanding diabetes
Type 1 and type 2 diabetes are characterized by increased blood glucose levels. They affect almost half a billion people around the globe, and this number is projected to rise as we reach the middle of the century. In most individuals, blood glucose levels are kept within a healthy range by a hormone called insulin, which is secreted by the pancreas, but this fine-tuned regulation can go wrong in type 1 and type 2 diabetes. In this animation, we lay out our current understanding of these diseases and explore active areas of research that aim to restore the body's blood glucose control.
Please visit YouTube to view this video.
In the early 1920s, Banting, Best, Macleod and Collip identified and purified a pancreatic extract — insulin. Subsequently, insulin was successfully used to regulate glucose levels in patients with type 1 diabetes, revolutionizing the treatment of these patients. Read more.
By Claire Greenhill

The discovery of insulin
Nearly 100 years since insulin was first used in the treatment of diabetes, Professor Chantal Mathieu, Professor of Medicine at the Katholieke Universiteit Leuven, Belgium, takes us through the history, development and future of this life saving drug.
Nobel Prize in Physiology or Medicine

Frederick Banting and John Macleod were awarded the Nobel Prize in Physiology or Medicine for their role in the discovery of insulin. Banting and Macleod went on to share the prize money with Charles Best and James Bertram Collip.
Related articles:
- The Nobel Prize: Frederick G. Banting facts
- The Nobel Prize: John Macleod facts
Nobel Prize in Chemistry
Frederick Sanger was awarded a share of the Nobel Prize in Chemistry for his work determining the structure of proteins, particularly insulin. He had determined the amino acid sequence of insulin.
Related article: The Nobel Prize: Frederick Sanger facts

Chemical synthesis of insulin

Several laboratories independently developed methods to chemically synthesize insulin.
- Insulin Peptides. IX. The Synthesis of the A-Chain of Insulin and its Combination with Natural B-Chain to Generate Insulin Activity
- Insulin Peptides. X. The Synthesis of the B-Chain of Insulin and Its Combination with Natural or Synthetis A-Chin to Generate Insulin Activity
- Synthese der Insulinketten und ihre Kombination zu insulin aktiven Präparaten (pdf)
- Total synthesis of crystalline bovine insulin
Milestone 2 1965
Islet pathology in diabetes
Early autopsy studies of individuals who died at onset of type 1 diabetes have helped inform us of the pathological changes occurring in the pancreas. In type 1 diabetes, a progressive and immune-mediated deterioration of β-cells occurs within pancreatic islets, eventually leading to an almost complete absence of insulin secretion. In type 2 diabetes, β-cell number is reduced owing to increased β-cell apoptosis. Read more.
By Shimona Starling

Proinsulin identified

Proinsulin, the precursor of insulin, was identified by Donald F. Steiner and colleagues at the University of Chicago. This, together with subsequent works, showed that insulin is derived from a larger prohormone, through processing inside insulin-secreting cells.
- The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma (pdf)
- Insulin Biosynthesis: Evidence for a Precursor
X-ray crystallography of insulin
Nobel Prize-winning chemist Dorothy Hodgkin and colleagues used X-ray crystallography to create a 3D electron density map of insulin.
Related article: Structure of Rhombohedral 2 Zinc Insulin Crystals

Glycated haemoglobin

Glycated haemoglobin (HbA 1c ), a form of haemoglobin that is chemically linked to glucose, was identified as a stable indicator of diabetes status.
Related article: Hemoglobin Components in Patients with Diabetes Mellitus
Milestone 3 1974
The genetic underpinnings of T1D
Nerup et al. demonstrated that the types of HLA protein present on white blood cells associate with type 1 diabetes as well as with the occurrence of anti-pancreatic autoantibodies in patients, thereby pinpointing that the HLA system genes contribute to the heritability of type 1 diabetes. Read more.
By Anna Kriebs

Rosalyn Yalow was awarded a share in the Nobel Prize in Physiology or Medicine for her work on developing radioimmunoassays of peptide hormones. The radioimmunoassay enabled Yalow and her colleagues to track insulin in a patient, leading to the demonstration that type 2 diabetes was caused by the body being unable to use insulin effectively, rather than a lack of insulin.
Related article: The Nobel Prize: Rosalyn Yalow facts
Milestone 4 1978
Animal models of T1D
The BB rat and the non-obese diabetic (NOD) mouse, two animal models of type 1 diabetes, spontaneously develop the disease. These models have enabled the study of mechanisms underlying type 1 diabetes onset and progression and the development of therapeutic interventions. Read more.
By Aline Lueckgen

Milestone 5 1978
A pioneering study of diabetes complications

A ground-breaking longitudinal study, conducted by the Belgian physician Jean Pirart over a period of more than 30 years, established a clear link between poor glycaemic control and degenerative complications such as neuropathy, retinopathy and nephropathy in patients with diabetes. Read more.
By Heather Wood
Milestone 6 1979
Insulin gets an upgrade
Concerns about the immunogenicity of animal-derived insulin led to a search for alternative methods of insulin production. The first successful generation of fully synthetic human insulin was reported in 1979 and the product was approved by the FDA just 3 years later, providing a less immunogenic form of insulin for the millions of people around the world who need it. Read more.
By Sarah Lemprière

Milestone 7 1982
Autoantibodies emerge on the scene

The 1970s and 1980s saw the discovery of islet cell antibodies and their various targets, providing evidence of type 1 diabetes as an autoimmune disease, as well as spurring on research into disease prevention and the development of antibody assays that remain in use today. Read more.
By Jessica McHugh
Bruce Merrifield was awarded the Nobel Prize in Chemistry for his development of a methodology for chemical synthesis on a solid matrix, which included work on insulin.
Related article: The Nobel Prize: Bruce Merrifield facts

Milestone 8 1986
Cytokines directly implicated in T1D

A study published in Science showed a direct role for the cytokine IL-1 in mediating β-cell death, heralding a new understanding of the mechanisms involved in insulitis and providing a new approach for targeted therapies. Read more.
By Joanna Clarke
Milestone 9 1987
Illuminating the incretin effect
In 1987, the laboratories of Habener and Holst established intestinal GLP1(7–37) as a key regulator of pancreatic insulin secretion. These studies laid the foundations for further exploration of the role of GLP1 in glucose homeostasis, which ultimately led to the development of incretin-based therapies for the treatment of type 2 diabetes. Read more.
By Sarah Crunkhorn

Milestone 10 1988
GLUT4 traffic control

Insulin is essential for glucose homeostasis, and one of its key functions is to drive glucose uptake into adipocytes and myocytes. Owing to work in the late 1980s, we now know that the insulin-stimulated glucose uptake in these tissues relies on the expression of a unique glucose transporter, GLUT4, whose intracellular trafficking is regulated by insulin signalling. Read more.
By Paulina Strzyz
Insulin analogues
The first insulin analogues were developed in 1988. These analogues are absorbed much faster than previous forms of insulin, which enables patients to achieve an insulin plasma profile that more closely reflects the profile in people without diabetes.
Related article: Monomeric insulins obtained by protein engineering and their medical implications

Milestone 11 1992
The discovery of monogenic diabetes

In the early 1990s, genetic linkage analysis studies identified a new form of diabetes, known as monogenic diabetes, in which single gene mutations that interfere with β-cell function lead to disease. This discovery is considered an important landmark in the field owing to its profound implications for the clinical care and prognosis of patients. Read more.
By Deepitha Maennich
Milestone 12 1993
TNF short-circuits the insulin receptor
Key studies in the 1990s from Gökhan Hotamisligil, Bruce Spiegelman and colleagues showed how the pro-inflammatory cytokine TNF drives insulin resistance and provided key insight into the mechanisms of obesity-associated diabetes. Read more.
By Yvonne Bordon

Milestone 13 1993
Findings from DCCT — glycaemic control prevents diabetes complications

The publication of the results of the Diabetes Control and Complications Trial marked a change in the treatment of diabetes. This trial demonstrated that intensive therapy — aimed at achieving glycaemic control as close to the non-diabetic range as safely possible — substantially reduced the complications of diabetes. From this point on, intensive therapy became the new standard therapy for patients with insulin-dependent diabetes. Read more.
By Megan Cully
Milestone 14 1995
Role of bariatric surgery in T2D
In 1995, Pories et al. reported the remarkable remission of type 2 diabetes in a cohort of individuals with obesity undergoing bariatric surgery. Bariatric surgery remains one of the most effective treatment options for this disease. Read more.
By Isobel Leake

Milestone 15 1997
Better living (not) through chemistry

In 1997, the Da Qing IGT and Diabetes Study reported a statistically significant reduction in incident type 2 diabetes following a 6-year behavioural intervention of diet and/or exercise compared with placebo in individuals with impaired glucose tolerance. Read more.
By Jennifer Sargent
Milestone 16 2000
Genetics of T2D
In 2000, a ground-breaking genetic association study was published that confirmed PPARG as a type 2 diabetes susceptibility gene. The field has now advanced so far that >550 type 2 diabetes risk signals have been identified. The valuable knowledge gained from these genetic factors has been used to inform disease mechanisms and research into therapeutics and might be used to form the basis of future precision medicine approaches. Read more.

Milestone 17 2002
Anti-CD3: the agonist and the ecstasy

Immunomodulating CD3-specific antibodies were shown for the first time to slow the loss of β-cell function in patients with type 1 diabetes. Read more.
By Zoltan Fehervari
Milestone 18 2006
Towards a stem cell therapy for diabetes
Transplantation of insulin-producing pancreatic β-cells to replenish diminishing populations in patients with type 1 diabetes might provide the ultimate therapy or even cure for the disease. This milestone study reported, for the first time, the generation of hormone-expressing endocrine pancreatic cells from differentiating human embryonic stem cells in vitro. Some of these cells expressed β-cell markers and synthesized and secreted insulin, although they showed a minimum response to glucose. Read more.
By Anna Melidoni

Milestone 19 2007
Islet inflammation in T2D

A 2007 study by Marc Donath and colleagues showed that insulitis is a pathological feature of type 2 diabetes, as well as type 1 diabetes, paving the way for further exploration of inflammasome activation and anti-inflammatory therapies in type 2 diabetes. Read more.
By Kirsty Minton
Milestone 20 2012
T reg cells to the rescue: the first clinical studies
The first clinical trials using regulatory T cells in children and adults with diabetes were reported in 2012 and 2014, respectively. These trials showed that the approach is safe and tolerable, with promising first indications of efficacy. Read more.
By Alexandra Flemming

Milestone 21 2014
Technology will set you free

A paper published in 2014 provided the first demonstration of the use of a bihormonal closed-loop system under free-living conditions in adults and adolescents with type 1 diabetes. Read more.
The artificial pancreas: a bridge to a cure
Tight control of blood glucose levels is vital for people with diabetes to lead healthy lives. But this challenge is no small undertaking, requiring careful monitoring of the diet and blood glucose levels, and regular insulin injections. One solution might be the development of the artificial pancreas; a device that monitors blood glucose levels and administers insulin automatically. Dr Helen Murphy, Clinical Professor in Medicine at Norwich Medical School, University of East Anglia, UK, takes us through her work with such devices — could they represent a bridge to a cure for people with diabetes?
Islet transplantation
A phase III trial reported improved glucose control, improved hypoglycaemia awareness and fewer severe hypoglycaemic events in patients with type 1 diabetes following a transplant of purified human pancreatic islets.
Related article: Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia

T2D in sub-Saharan Africa

The protocol for one of the first large studies to assess the prevalence of type 2 diabetes in sub-Saharan Africa, as well as the environmental and genetic determinants of the disease, was published in 2016. The study is part of the Human Heredity and Health in Africa (H3Africa) initiative.
Related article: H3Africa multi-centre study of the prevalence and environmental and genetic determinants of type 2 diabetes in sub-Saharan Africa: study protocol
Diabetes in sub-Saharan Africa
It is estimated that over 19 million adults in Africa have diabetes, an enormous problem that Professor Jean Claude Mbanya, Professor of Medicine and Endocrinology at the University of Yaoundé I, Cameroon, understands too well. Here he takes us through the unique challenges and pitfalls of treating patients with diabetes in sub-Saharan Africa.
Milestone 22 2017
Incretin drugs for glycaemic control
Incretin drugs, which include glucagon-like peptide 1 receptor agonists (GLP1RAs) and dipeptidyl peptidase 4 inhibitors, exploit the effects of GLP1 on insulin production to improve glycaemic control. In addition to their proven benefits in the treatment of hyperglycaemia, clinical studies have shown that GLP1RAs promote weight loss, and can improve cardiovascular and kidney outcomes in patients with type 2 diabetes, expanding the therapeutic potential of these agents. Read more.
By Monica Wang

Milestone 23 2019
An infectious cause for T1D?

In 2002, clinical centres across the USA and Europe began recruiting young children to study the environmental causes of type 1 diabetes (T1D) — the TEDDY study. From this cohort, a paper by Vehik et al. in 2019 pinpointed a role for prolonged enteroviral B infection in T1D development in young children, representing a major advance in our understanding of the links between the virome and T1D. Read more.
By Lucy Bird
Milestone 24 2019
Getting to the heart of the matter
2019 saw the publication of meta-analyses demonstrating beneficial effects for cardiovascular and renal outcomes in patients with type 2 diabetes given glucose-lowering therapies — sodium–glucose co-transporter 2 inhibitors and glucagon-like peptide 1 receptor agonists. Read more.

Patients with diabetes were found to have an increased risk of severe COVID-19.
- Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study
- Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy
- Clinical Characteristics of Covid-19 in New York City

New Report Highlights Diabetes Research Advances and Achievements

Today, the American Diabetes Association® (ADA) released its 2023 Research Report , highlighting investments in advancing diabetes research and clinical practice. ADA research grants focused on innovative projects with high impact and helped researchers establish collaborative networks to move their innovations into the hands of people living with diabetes.
“Research at the ADA is the engine that drives clinical advances by catapulting them into practice. 2023 has brought many prominent achievements. We are incredibly proud of our legacy of highlighting science and eager to build on this research to move even closer to a world free of diabetes and all its burdens,” said Charles “Chuck” Henderson, the ADA’s chief executive officer.
The report highlights include:
- Support behavioral and mental health of people with diabetes
- Tackle the epidemic of youth-onset type 2 diabetes
- Improve the lives of women living with diabetes
- Increased investment in early career researchers by expanding funding opportunities for postdoctoral fellowship awards to ensure these researchers can stay within the field of diabetes.
- Takeaways from the 2023 Scientific Sessions, where researchers from all over the world shared the latest progress and study results with the global diabetes community.
- Identify and address disparities in access and outcomes for Hispanic/Latino communities
- Implement virtual interventions for those living with type 1 diabetes
- Improve outcomes for the deaf community through specially designed diabetes self-management education and support (DSMES)
In addition, the report provides an update on the Pathway to Stop Diabetes® (Pathway) program, which pairs talented early-career scientists with mentorship from world-renowned diabetes scientists to drive research innovation free from traditional project constraints. This year, through the Pathway program, ADA dedicated over $4.8 million dollars in new grant funding to support breakthroughs in translation and clinical science, technology, care, and potential cures in the field of diabetes.
To learn more about the ADA’s research findings and ongoing areas of study, visit professional.diabetes.org .
About the American Diabetes Association The American Diabetes Association (ADA) is the nation’s leading voluntary health organization fighting to bend the curve on the diabetes epidemic and help people living with diabetes thrive. For 83 years, the ADA has driven discovery and research to treat, manage, and prevent diabetes while working relentlessly for a cure. Through advocacy, program development, and education we aim to improve the quality of life for the over 136 million Americans living with diabetes or prediabetes. Diabetes has brought us together. What we do next will make us Connected for Life ® . To learn more or to get involved, visit us at diabetes.org or call 1-800-DIABETES (1-800-342-2383). Join the fight with us on Facebook ( American Diabetes Association ), Spanish Facebook ( Asociación Americana de la Diabetes ), LinkedIn ( American Diabetes Association ), Twitter ( @AmDiabetesAssn ), and Instagram ( @AmDiabetesAssn ).
Contact Virginia Cramer for press-related questions.
Donate Today and Change Lives!
- Clinical and Research Advances
- Current Issues
- Forecast of Major Research Advances

See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Olefsky JM. Prospects for Research in Diabetes Mellitus. JAMA. 2001;285(5):628–632. doi:10.1001/jama.285.5.628
Manage citations:
© 2024
- Permissions
Prospects for Research in Diabetes Mellitus
Author Affiliation: Department of Medicine, Division of Endocrinology and Metabolism, University of California, San Diego, La Jolla, and Department of Veterans Affairs, San Diego.
Diabetes mellitus is the sixth leading cause of death in the United States, and morbidities resulting from diabetes-related complications such as retinopathy, kidney disease, and limb amputation cause a huge burden to the national health care system. Identification of the genetic components of type 1 and type 2 diabetes is the most important area of research because elucidation of the diabetes genes will influence all efforts toward a mechanistic understanding of the disease, its complications, and its treatment, cure, and prevention. Also, the link between obesity and type 2 diabetes mandates a redoubled effort to understand the genetic and behavioral contributions to obesity.
Diabetes mellitus affects between 6% and 7% of the US population equating to about 16 million people. It is projected that there will be 800 000 new cases per year and a total of 23 million affected people within 10 years. 1 Diabetes occurs in all populations and age groups but is increasing in prevalence in the elderly and in blacks, Hispanics, Native Americans, and Asians. 2 Although deaths due to cancer, stroke, and cardiovascular disease are declining, the death rates due to diabetes have increased by about 30% in the past 12 years ( Figure 1 ), and life expectancy for persons with diabetes is approximately 15 years less than in those who do not have diabetes. Diabetes is the sixth leading cause of death in the United States and accounted for more than 193 000 deaths in the US in 1997. However, this is an underestimate because diabetes contributes substantially to many deaths that are ultimately ascribed to other causes, such as cardiovascular disease. 3
Due to its complications, diabetes causes an enormous national burden of morbidity. For example, diabetic retinopathy is the leading cause of blindness in adults aged 20 through 74 years, 4 and diabetic kidney disease accounts for 40% of all new cases of end-stage renal disease. 5 Diabetes is the leading cause for amputation of limbs in the country. 6 Heart disease and strokes occur 2 to 4 times more frequently in adults with diabetes than in those who are healthy. Diabetes causes special problems during pregnancy, and the rate of congenital malformations can be 5 times higher in the offspring of women with diabetes. In aggregate diabetes mellitus costs $105 billion annually and involves 1 of every 10 US health care dollars and 1 of every 4 Medicare dollars. 7 (pp746-757)
Diabetes mellitus refers to a number of disorders that share the cardinal characteristic feature of elevated blood glucose levels. The 2 most common general categories of this disease are termed type 1 and type 2 diabetes. 8 Research has enormously increased our understanding of type 1 and type 2 diabetes, but much more remains to be done.
Documentation that elevated blood glucose levels are a direct cause of long-term complications of diabetes has been a major accomplishment. The Diabetes Control and Complications Trial (DCCT) 9 and the United Kingdom Prospective Diabetes Study (UKPDS) 10 both showed that control of blood glucose levels as close to normal as possible prevents and retards development of diabetic retinopathy, nephropathy, neuropathy, and macrovascular disease. The fact that each increment of improved control of blood glucose levels reduces complications has focused clinical and research efforts to elucidate disease mechanisms and to design new therapies. This insight coincided with the development of home glucose monitoring systems that make it possible to measure blood glucose levels throughout the day and coincided with the availability of new insulin preparations; insulin delivery devices, such as insulin pumps; and oral antidiabetic agents. 11
Likewise, fetal malformations and perinatal morbidity are now known to be due to elevated maternal glucose levels, and blood glucose control before and after conception can reduce these risks to normal. 7 (pp863-870) As a consequence, intensive efforts are now being made to diagnose and control glucose levels in pregnant women with diabetes. Although these advances have certainly helped improve the lives of patients, they do not provide an answer because most patients with diabetes do not obtain adequate blood glucose control.
Type 1 diabetes accounts for 5% to 10% of diabetes, usually occurs in children or young adults, and was formerly termed insulin-dependent diabetes mellitus (IDDM) or juvenile-onset diabetes . 12 This disease is caused by autoimmune destruction of the pancreatic β cells that secrete insulin. 12 The process involves a smoldering destructive process that can persist for several years and ultimately leads to failure of insulin secretion. This autoimmune process is due to genetic and environmental factors, and many genes contribute to the pathogenesis. During the preclinical phase, a variety of autoimmune antibodies directed against β-cell antigens serve as markers for the prediabetic state, allowing for early detection and possible prevention strategies. Patients with type 1diabetes require insulin therapy for survival, but blood glucose is still difficult to control, and most patients ultimately develop devastating complications of this disease. The present need is for improved means of treating type 1 diabetes until it is practical to prevent its development.
New methods to achieve tight glucose control are needed that are practical and can be administered to all persons with type 1 diabetes, including methods of insulin delivery, better forms of insulin, and practical, affordable methods of noninvasive self monitoring that can be coupled to patient-specific insulin treatment regimens. Cure of diabetes will require permanent replacement of lost β-cell function, which could involve islet cell transplantation, regeneration of β cells, or development of immortalized insulin secreting cell line. The ultimate aim in preventing disease onset will require a major multidisciplinary effort to identify the genes that predispose to type 1 diabetes and to identify the interacting environmental factors that trigger the disease. A thorough understanding of the cellular and molecular causes of the autoimmune destructive process will also be necessary.
Type 2 diabetes accounts for 90% to 95% of all patients with diabetes and is increasing in prevalence, especially in minority populations. 13 Type 2 diabetes is a heterogeneous, polygenic disorder, and the responsible genes have been identified in selected subtypes of this disease. 7 (pp691-705) Multiple diabetes genes exist, and more than 1 gene is likely to be involved in an individual patient. Some of the known environmental factors are obesity, a sedentary lifestyle, and aging. Obesity probably is the major environmental factor contributing to the increasing incidence of type 2 diabetes, and some of the hormonal, genetic, and environmental factors that predispose to obesity have been identified.
Insulin resistance is a characteristic metabolic defect in the great majority of patients with type 2 diabetes, and this defect can be demonstrated in the prediabetic state many years prior to the development of hyperglycemia. 14 As a consequence of insulin resistance, the β cell produces increased amounts of insulin, and, if sufficient, the compensatory hyperinsulinemia maintains glucose levels within the normal range ( Figure 2 ). In those individuals destined to develop diabetes, β-cell function eventually declines, and relative insulin insufficiency occurs. 15 Thus, insulin resistance combined with β-cell failure leads to the decompensated hyperglycemic diabetic state.
A number of the molecular steps in the insulin action cascade have been identified, and several components of the β-cell insulin secretion pathway have been elucidated. Researchers are beginning to understand the complex heterogeneous, genetic determinants of type 2 diabetes susceptibility. Efforts to understand genetic variation, gene expression profiling, and the interaction between genetic factors and environmental triggers must be intensified. This information will reveal new targets for pharmacologic intervention. Researchers also must continue work to understand the basic mechanisms that cause insulin resistance and limitation of compensatory insulin secretion. Truly effective treatments for type 2 diabetes will only come about when drugs are developed to target and correct the 2 underlying defects.
Obesity is the major environmental risk factor promoting the rise in type 2 diabetes incidence, and obesity is an increasing problem in the United States. The genetic and environmental factors that control food intake and energy expenditure must be identified so that we can improve the ability to effect beneficial lifestyle changes and eventually develop drugs to treat obese patients who are refractory to lifestyle modifications.
Much has been learned about the basic biology, epidemiology, and treatment of diabetes, and extraordinary opportunities exist to understand, treat, cure, and prevent diabetes. Coupled with these opportunities are substantial challenges and hurdles. The Diabetes Research Working Group 3 has identified several research areas that present unique opportunities for major advances and changes that will have to be made in the scientific infrastructure to implement this research endeavor.
Identification of the genetic components of types 1 and 2 diabetes is the single most important area of research because elucidation of the diabetes genes (alleles) will influence all efforts toward a mechanistic understanding of the disease, its complications, and its treatment, cure, and prevention. Completion of the Human Genome Project, the identification of a large number of single nucleotide polymorphisms—which will make genome-wide association studies for complex multigenic diseases feasible—the availability of new technologies such as DNA gene chips and genetic manipulation of animals have provided a solid foundation for rapid and tremendous advances in the study of diabetes genetics.
The new knowledge and technology are available for application to diabetes research, and a rigorous, multidisciplinary, well-funded effort is needed to achieve these goals. Increased funding for individual scientists should be a cornerstone of this approach, but new enhancements to the scientific infrastructure are equally important. A multidisciplinary approach will require coordination of many centers and different disciplines to identify the diabetes genes. This will necessitate the establishment and availability of repositories of DNA samples from phenotypically well-characterized diabetes patients spanning a number of ethnic groups. A coordinating and planning agency should be established to bring together and integrate the efforts of the National Institutes of Health and of nongovernment organizations such as the American Diabetes Association and Juvenile Diabetes Foundation International so that information is broadly disseminated as rapidly as possible. Once the diabetes genes are identified, it will be necessary to deal with the ethical, legal, and social issues involved in the availability of such information.
Since type 1 diabetes is an autoimmune disease, the mechanisms underlying this process must be thoroughly understood. Expanded efforts are needed to identify the environmental triggers and how they interact with the genetic predispositions. The basic cell biology of the immune destructive process must be solved, and the specific β-cell autoantigens must be identified. Hopefully this will lead to development of highly specific immunosuppressive agents that will produce relatively few adverse effects.
Insulin resistance and impaired insulin secretion are the key metabolic defects in type 2 diabetes. Increased efforts are necessary to dissect the molecular components involved in insulin signaling, insulin secretion, and β-cell growth and development. This research coupled with the efforts to identify the diabetes genes, will provide a mechanistic understanding of the specific defects in these pathways in type 2 diabetes, which should lead to the development of more specific, and more effective, pharmaceutical agents directed against defined molecular targets.
It is also essential to redouble efforts to understand the genetic and behavioral contributions to obesity. Excess body weight is a widespread and increasing problem in the United States and contributes to the high and increasing incidence of type 2 diabetes. A thorough understanding of basic mechanisms will enhance development of new methods of prevention and treatment. To facilitate the country's ability to make rapid progress in these areas of scientific opportunity, the Diabetes Research Working Group has recommended changes in the infrastructure. These include the following:
Create new mechanisms and modify existing programs to maximize recruitment, training, and career development of diabetes investigators.
Substantially strengthen and enhance National Institutes of Health–sponsored diabetes centers by increasing the funding levels and expanding their mission.
Create new regional centers for advanced technologies required for metabolic and functional imaging studies, such as nuclear magnetic resonance and positron emission tomography.
Enhance efforts to develop and characterize small- and large-animal models of type 1 and type 2 diabetes and establish regional centers for these animal models.
Expand procurement of human tissues, DNA samples, and organs for diabetes research.
If aggressive efforts across the broad front of diabetes research are accompanied by increased research funding in the areas of exceptional opportunity, the future does indeed look promising and it is likely that major accomplishments over the next 25 years will change the picture of diabetes prevention, treatment, and cure. ( Figure 3 )
For patients with type 1 diabetes, the procedures of cadaveric islet cell transplants will be largely perfected so that this can be performed either without the need for immunosuppression or with the use of specific highly focused immunosuppressive agents that will produce minimal adverse effects. However, that supply of freshly isolated human islets will be insufficient to provide transplants for all patients with type 1 diabetes. Replenishable sources of β cells for replacement could be derived from xenografts, possibly from genetically modified animals, or by creating a relatively inexhaustible, functional insulin secreting β–cell line. Such cell lines will be developed by learning to expand and grow large amounts of β cells from progenitor cells or by genetically engineering immortalized β cells.
Identification of the genes that predispose to type 1 diabetes will make it possible to identify individuals destined to develop the disease. Coupled with the elucidation of the basic immunologic mechanisms that cause autoimmune β-cell destruction and the development of specific targeted treatments to interrupt this process, the prevention of type 1 diabetes will become a reality. On the way to reaching these goals, substantial advances in glucose monitoring and insulin delivery mechanisms, which will lead to patient-specific treatment algorithms, will improve the outlook for patients with type 1 diabetes.
The genes responsible for the predisposition to type 2 diabetes and the mechanisms by which environmental factors bring out this predisposition will be identified. In parallel with this genetic information, identification of the cellular defects responsible for insulin resistance and impaired insulin secretion in type 2 diabetes will lead to development of new drugs that will be specific for defined molecular targets and that will be relatively free of unwanted adverse effects. This should include new ways to prevent or treat obesity. Once the predisposing diabetes genes are identified, it will be a straightforward matter to genotype individuals for diabetes susceptibility. The availability of new pharmaceutical treatments, together with the ability to predict diabetes susceptibility will provide a sound basis for early intervention and will lead to the prevention of type 2 diabetes in susceptible individuals. If an appropriate health care delivery system can disseminate these new therapeutic modalities to all diabetic patients, then control or prevention of diabetes will be a reality. In this event, the burden of diabetes complications will gradually diminish and ultimately disappear. Advances in methods of gene therapy may make genetic interventions a reality for this disorder.
The surest way to treat diabetic complications is to prevent them by glycemic control in patients with established diabetes or preferably by prevention of diabetes. While moving toward these goals over the next 25 years, it is critical to improve treatment and prevention of the microvascular and macrovascular complications of diabetes because these complications account for the excessive morbidity and mortality associated with this disease.
All of these predictions are fully achievable if adequate resources (financial and human) are applied to the field of diabetes. With appropriate effort, future generations could be freed from the scourge of diabetes.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 24 April 2024
Effects of sodium-glucose cotransporter 2 inhibitors on bone metabolism in patients with type 2 diabetes mellitus: a systematic review and meta-analysis
- Jing Wang 1 ,
- Yang Li 3 &
- Chen Lei 4
BMC Endocrine Disorders volume 24 , Article number: 52 ( 2024 ) Cite this article
76 Accesses
Metrics details
Sodium glucose cotransporter 2 (SGLT2) inhibitors are widely used in type 2 diabetes mellitus (T2DM) therapy. The impact of SGLT2 inhibitors on bone metabolism has been widely taken into consideration. But there are controversial results in the study on the effect of SGLT2 inhibitors on bone metabolism in patients with T2DM. Therefore, we aimed to examine whether and to what extent SGLT2 inhibitors affect bone metabolism in patients with T2DM.
A literature search of randomized controlled trials (RCTs) was conducted through PubMed, Web of Science, Embase, Cochrane databases, and Scopus from inception until 15 April 2023. Eligible RCTs compared the effects of SGLT2 inhibitors versus placebo on bone mineral density and bone metabolism in patients with T2DM. To evaluate the differences between groups, a meta-analysis was conducted using the random effects inverse-variance model by utilizing standardized mean differences (SMD).
Through screening, 25 articles were finally included, covering 22,828 patients. The results showed that, compared with placebo, SGLT2 inhibitors significantly increased parathyroid hormone (PTH, SMD = 0.13; 95%CI: 0.06, 0.20), and cross-linked C-terminal telopeptides of type I collagen (CTX, SMD = 0.11; 95%CI: 0.01, 0.21) in patients with T2DM, decreased serum alkaline phosphatase levels (ALP, SMD = -0.06; 95%CI: -0.10, -0.03), and had no significant effect on bone mineral density (BMD), procollagen type 1 N-terminal propeptide (P1NP), 25-hydroxy vitamin D, tartrate resistant acid phosphatase-5b (TRACP-5b) and osteocalcin.
Conclusions
SGLT2 inhibitors may negatively affect bone metabolism by increasing serum PTH, CTX, and decreasing serum ALP. This conclusion needs to be verified by more studies due to the limited number and quality of included studies.
Systematic review registration
PROSPERO, identifier CRD42023410701
Peer Review reports
Research in context
SGLT2 inhibitors have been widely used in clinical practice for their good cardiorenal protective and hypoglycemic effects. However, their effects on bones are still controversial. The drug has been shown to have a potential adverse effect on bone in multiple animal experiments. However, in the latest meta-analysis, it was not found that the risk of fracture increased in patients with type 2 diabetes mellitus (T2DM) treated with SGLT2 inhibitors.
Can SGLT2 inhibitors affect bone mineral density and bone metabolism in patients with T2DM?
We found that SGLT2 inhibitors may have a negative effect on bone in patients with T2DM.
When T2DM is treated in clinical work, doctors will pay more attention to the monitoring of bone safety. And we provided a reference for the use of SGLT2 inhibitors.
Introduction
It is well known that type 2 diabetes mellitus (T2DM) is characterized by persistently elevated blood glucose or elevated postprandial blood glucose containing carbohydrates [ 1 ]. As a chronic non-communicable disease, its prevalence is increasing worldwide, especially related to the gradual entry of people into an aging society, high calorie intake, and a sedentary lifestyle [ 2 ]. Recent studies have shown that in addition to the cardiovascular, ocular, renal and neurological complications of the disease in patients, bone strength is also impaired and leads to an increased risk of fractures [ 3 ]. The presence of T2DM is associated with a prevalent metabolic disorder that has detrimental effects on bone metabolism, leading to an increased susceptibility to fractures [ 4 , 5 ]. Among the various types of osteoporotic fractures, individuals with T2DM face a heightened risk for hip fractures, which are considered the most severe, as well as limb fractures such as those occurring in the leg or ankle [ 6 ].
The anti-diabetic drugs currently applied clinically have certain effects on the bone metabolism of patients [ 7 ]. Sodium–glucose cotransporter 2 (SGLT2) inhibitor is one of the new hypoglycemic drugs. It can reduce glucose re-absorption by inhibiting SGLT2 in proximal tubules of the kidney, thus promoting urine glucose excretion and reducing blood glucose [ 8 ]. In recent years, studies on the effects of SGLT2 inhibitors on bone metabolism have been continuously released, and the existing relationship between the two is still controversial. Theoretically, SGLT2 inhibitors increase renal tubular reabsorption of phosphate and serum parathyroid hormone concentration [ 9 ].
Considering the significant economic and social burden caused by bone health issues and associated fracture risks, it is imperative to conduct a comprehensive evaluation of the impact of SGLT2 inhibitors on fractures and bone metabolism. In view of the fact that there are still controversial results in the study on the effect of SGLT2 inhibitors on bone metabolism in patients with T2DM, we conducted a systematic and comprehensive analysis of the existing research results in order to provide reference for the selection of SGLT2 inhibitors in the treatment of T2DM in clinical work.
Protocol and registration
The protocol of this systematic review and meta-analysis has been registered in PROSPERO (registration no. CRD42023410701).
Eligibility criteria
We included randomized controlled trials (RCTs) comparing the efficacy of SGLT2 inhibitors versus placebo, in English only. Eligible participants were adults with T2DM, regardless of background hypoglycemic therapy. Interventions should last for at least 12 weeks and the outcomes should include at least one of bone mineral density or bone metabolism.
Search strategy
We searched PubMed, Web of Science, Embase, Cochrane databases, and Scopus on 15 April 2023 for English-language studies. Detailed information about our search strategy was presented in the electronic supplementary material (Table S1 ). To avoid omitting any eligible studies, any terms related to “SGLT2 inhibitor” were searched.
Selection process
All search results were downloaded into EndNote (version X9, Thomson Reuters, Philadelphia, PA, USA) to eliminate duplication. Two reviewers independently performed a preliminary screening of the title and abstract. Remaining articles were read through the full text to determine inclusion, and the reasons for excluded articles were recorded. Any disagreements were resolved by a third reviewer. Articles that could not get the required data were also excluded. Articles for which the required data were not available after contacting the corresponding author were also excluded.
Data collection and risk of bias assessment
Data extraction was done by two independent reviewers and arbitrated by a third reviewer. The relevant information extracted from the included articles mainly included: (1) Basic information: first author, publication year, sample size, and the number of experimental and control groups. (2) Characteristics of research subjects: gender, age, glycated hemoglobin, BMI, SGLT2 inhibitor type and dose, and duration of treatment; (3) Outcomes: Mean ± standard deviation (SD) of post-treatment relative baseline changes in bone mineral density (BMD) and bone metabolism-related indicators including parathyroid hormone (PTH), cross-linked C-terminal telopeptides of type I collagen (CTX), alkaline phosphatase (ALP), 25-hydroxy vitamin D, procollagen type 1 N-terminal propeptide (P1NP), osteocalcin, and Tartrate resistant acid phosphatase-5b (TRACP-5b); (4) Relevant information described in the literature that can be used to assess the risk of bias.
The risk of bias will be assessed by two authors independently using the RoB2 tool for the included RCTs [ 10 ]. Using the RoB2 tool, we will assess domains such as randomization process, assignment and adhering to intervention, missing data and measurement of outcome, and finally categorize the studies as having a low, some concern, or high risk of bias.
Statistical analysis
We will pool the results using a random-effects meta-analysis, using standard mean difference (SMD) for continuous outcomes, and calculate 95% confidence interval (CI). A p -value < 0.05 was considered statistically significant. The Chi-square test combined with I-value analysis was used to judge the heterogeneity among the articles. When the heterogeneity of the studies in each group was relatively large ( P < 0.05, I 2 ≥ 50%), the source of heterogeneity needed to be clarified. Subsequent subgroup analysis or sensitivity analysis was conducted to explain the reasons for heterogeneity. Egger’s tests were performed to assess publication bias. R (version 4.2.3) and the statistical package ‘meta’ were used for analysis.
Search results
According to the established retrieval strategy, we screened a total of 8554 studies from 5 databases. After a series of screenings, 25 studies ultimately met the eligibility criteria, totaling 22,828 unique participants. Twenty-three studies included in the analysis were RCTs [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ], and two studies were for RCTs Pooled analysis [ 34 , 35 ] (Fig. 1 ).
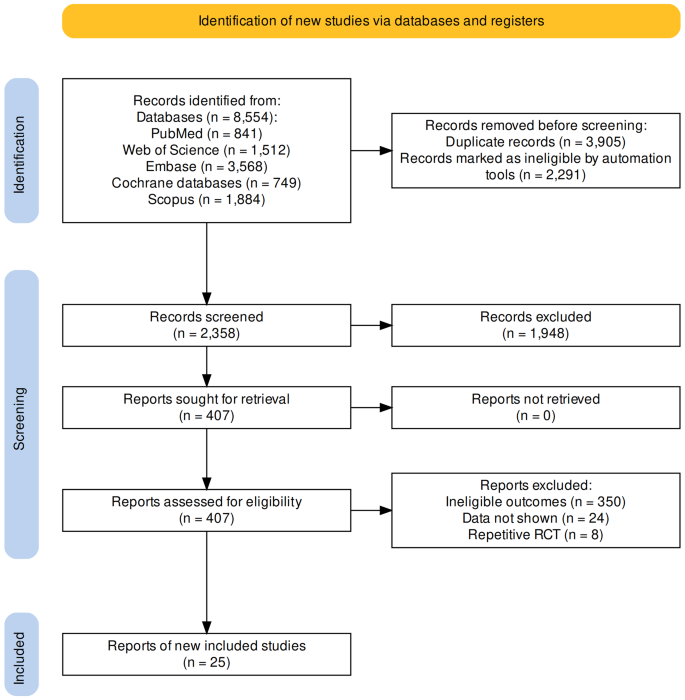
Flow diagram of the identification of eligible trials
Study characteristics
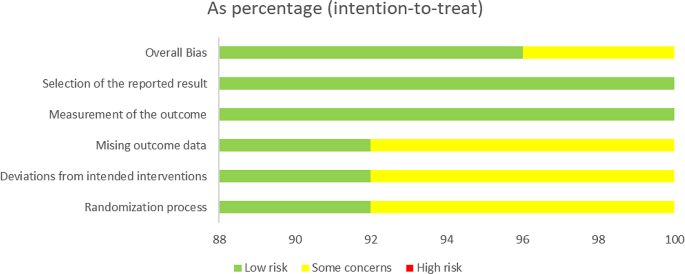
The study characteristics were summarized in Table 1 . A total of 22,828 participants from 25 RCTs were randomly assigned to one of five SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and ertugliflozin) or placebo. Sample sizes for individual trials ranged from 40 to 12,620 participants, and the average trial duration was 55 weeks (range 12–104 weeks).
The risk of bias in the 25 RCTs is summarized in Fig. 2 . Most of the trials included in the meta-analysis were judged to have a low risk of bias.
Risk of bias assessments of included studies
Meta-analysis results
- Bone mineral density
A total of 3 studies [ 11 , 24 , 26 ] reported the effects of SGLT2 inhibitors on BMD in patients with T2DM. The results of the overall and subgroup meta-analysis are presented in Fig. 3 . There was no significant difference in BMD after treatment between the SGLT2 inhibitor group and the placebo group (SMD = -0.02; 95%CI: -0.09, 0.05). In subgroup analyses of bone sites, there was also no significant change in BMD in the two groups (lumbar spine, SMD = − 0.02, 95%CI: −0.13, 0.10; femoral neck, SMD = 0.05, 95%CI: −0.11, 0.22; total hip, SMD = -0.08, 95%CI: −0.27, 0.12; and distal forearm, SMD = − 0.06, 95%CI: −0.18, 0.06). No evidence of publication bias was observed (Table S2 ).
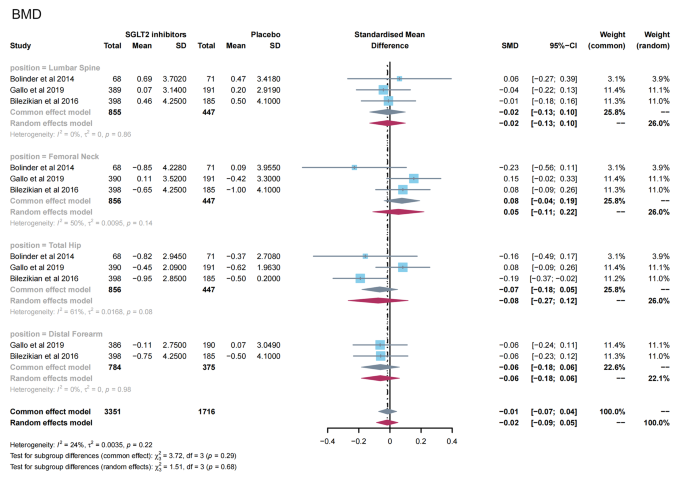
Meta-analysis of the effect of Sodium–glucose cotransporter 2 (SGLT2) inhibitors on BMD compared with placebo. BMD, bone mineral density
- Bone metabolism
13 studies [ 11 , 12 , 13 , 14 , 15 , 16 , 19 , 23 , 24 , 28 , 31 , 32 , 35 ] reported PTH levels after SGLT2 inhibitor treatment (Fig. 4 ). 7 papers compared CTX [ 11 , 19 , 23 , 24 , 26 , 28 , 32 ] and 25-hydroxy vitamin D [ 11 , 14 , 15 , 23 , 31 , 32 , 35 ] levels after treatment (Fig. 5 A-B). 15 papers [ 11 , 15 , 16 , 18 , 20 , 21 , 22 , 25 , 27 , 29 , 30 , 34 , 35 ] reported ALP levels after treatment (Fig. 6 ). 3 papers compared P1NP [ 11 , 14 , 24 ] and osteocalcin [ 14 , 26 , 32 ] levels after treatment (Fig. 7 A-B). 2 papers [ 23 , 28 ] reported TRACP-5b levels after treatment (Fig. 7 C). Except for osteocalcin ( P = 0.02, I 2 = 75%), no significant heterogeneity was observed. Meta results showed that, compared with placebo, SGLT2 inhibitors significantly increased PTH levels (SMD = 0.13; 95%CI: 0.06, 0.20) and CTX levels (SMD = 0.11; 95%CI: 0.01, 0.21), while significantly decreased ALP levels (SMD = -0.06; 95%CI: -0.10, -0.03). However, there was no significant difference in 25-hydroxy vitamin D (SMD = 0.09; 95%CI: 0.00, 0.18), P1NP (SMD = 0.13; 95%CI: -0.02, 0.28), osteocalcin (SMD = 0.19; 95%CI: -0.16, 0.54), and TRACP-5b (SMD = 0.05; 95%CI: -0.17, 0.28) after treatment between the SGLT2 inhibitor group and the placebo group.
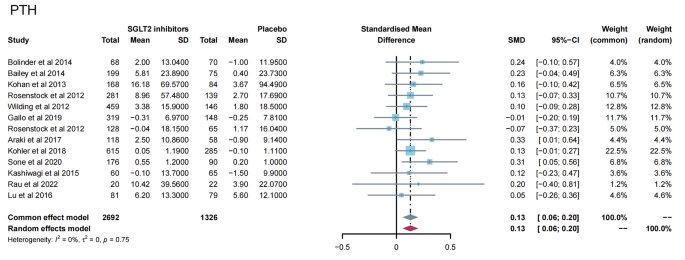
Meta-analysis of the effect of Sodium–glucose cotransporter 2 (SGLT2) inhibitors on PTH compared with placebo. PTH, parathyroid hormone
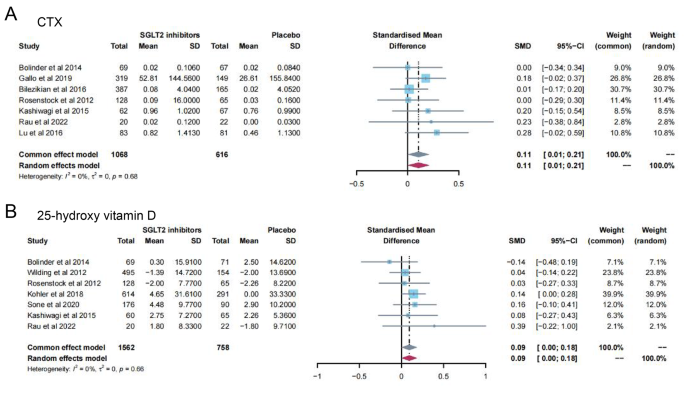
Meta-analysis of the effect of Sodium–glucose cotransporter 2 (SGLT2) inhibitors on CTX ( A ) and 25-hydroxy vitamin D ( B ) compared with placebo. CTX, Cross-linked C-terminal telopeptides of type I collagen
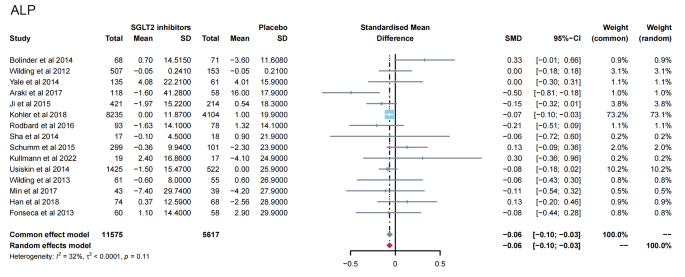
Meta-analysis of the effect of Sodium–glucose cotransporter 2 (SGLT2) inhibitors on ALP compared with placebo. ALP, Alkaline phosphatase
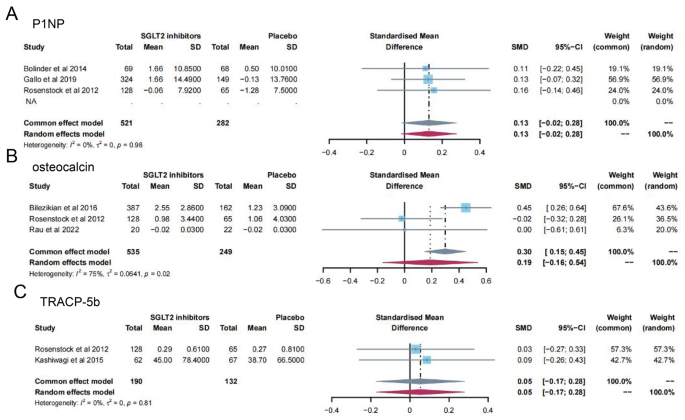
Meta-analysis of the effect of Sodium–glucose cotransporter 2 (SGLT2) inhibitors on P1NP ( A ), osteocalcin ( B ) and TRACP-5b ( C ) compared with placebo. P1NP, Procollagen type 1 N-terminal propeptide; TRACP-5b, Tartrate resistant acid phosphatase-5b
In addition, no evidence of publication bias was observed for any of the above outcomes (Table S2 ).
The combined detection of BMD and bone turnover markers can be used to evaluate bone metabolism in patients. However, the changes of bone turnover markers are more sensitive [ 36 ]. In this study, after a comprehensive literature search and analysis, 25 studies were finally included for meta-analysis. Our results suggested that SGLT2 inhibitors had no significant effect on BMD in patients with T2DM compared to placebo. However, due to the short follow-up period and limited number of the RCTs included in the studies, more long-term studies are needed to accurately determine the impact of SGLT2 inhibitors on BMD.
In terms of bone metabolism, we observed that SGLT2 inhibitors significantly increased serum PTH and CTX levels and decreased serum ALP levels in patients with T2DM. This presents a seemingly paradoxical situation, as it is traditionally understood that elevated levels of PTH normally stimulate bone formation, which in turn increases levels of ALP, the active marker of bone formation [ 37 ]. This reflects the discrepancy between increased PTH levels and decreased ALP levels in patients using SGLT2 inhibitors underscores the complexity of the drugs’ impact on bone metabolism. It suggests a multifactorial influence involving immediate metabolic changes, differential effects on bone remodeling phases, the intricate role of RAAS activation, and the body’s broader compensatory responses [ 38 ]. In addition, no statistically significant effect of SGLT2 inhibitors on P1NP, TRACP-5b, 25-hydroxy vitamin D, and osteocalcin was observed in this study. However, although CTX and ALP levels change significantly in the meta-analysis, no single report shows a significant increase in CTX and only one study found a significant reduction of ALP. The reason for these phenomena can be attributed to the short duration of the study. The studies included this time are up to just over 3 months (104 days). Current research suggests that short-term studies (3 months) may not sufficiently capture significant changes in bone metabolism markers due to the physiological lag between alterations in glucose metabolism and their impact on bone remodeling processes []. In contrast, studies extending beyond 6 to 12 months are considered more likely to demonstrate meaningful changes in these markers [ 37 , 39 ]. Further research, particularly studies with longer follow-up periods and detailed analyses of bone quality and turnover markers, is needed to fully elucidate these relationships.
The exact mechanism of the negative effects of SGLT2 inhibitors on bone health remains unknown. A study has shown that SGLT2 is not expressed in either the osteoblast lineage or the osteoclast lineage [ 40 ]. SGLT1 was detected in MC3T3-E1 differentiated osteoblasts, but its expression level was low. Therefore, the effects of these drugs on bone may be indirect [ 41 ]. SGLT2 inhibitors destroy serum calcium, phosphate, and vitamin D homeostasis [ 42 ]. As reabsorption of sodium in the proximal renal tubules decreases, the activity of sodium-phosphate co-transporters at the apical membrane increases. Serum phosphate levels further increase, inducing parathyroid cells and osteoblasts to secrete PTH and fibroblast growth factor 23 (FGF23). PTH causes bone resorption. While FGF23 promotes urinary phosphate excretion, inhibition of 1-αhydroxylase causes a decrease in 1,25-dihydroxvitamin D levels [ 43 ]. The decrease in blood sodium concentration can also directly affect osteoclasts, leading to an increase in bone fragility [ 44 ]. In the opposite way, calcium is reabsorbed by sodium-calcium cotransporters. The inhibition of SGLT2 leads to increased excretion of urine glucose and urine calcium, and the decrease of serum calcium causes secondary hyperparathyroidism [ 9 ]. It has been verified that the main results in our study suggested SGLT2 inhibitors could significantly increase serum PTH. Unfortunately, there are no more clinical studies reporting the effects of SGLT2 inhibitors on FGF23 in patients with T2DM.
SGLT2 inhibitors provide modest weight loss. A reduction in mechanical pressure on the bone tissue may decrease bone density and enhance bone turnover [ 45 ]. This may partly explain the reduction in total hip bone density in T2DM patients with canagliflozin. Weight loss also decreases aromatase activity, resulting in decreased estradiol levels that severely affect bone density and bone turnover [ 46 , 47 ]. In addition to the indirect effects of SGLT2 inhibitors on bone metabolism, adverse events associated with these agents due to osmotic diuresis and volume consumption (orthostatic hypotension, postural dizziness, etc.) may increase the risk of falls and fractures [ 48 ].
There are some limitations to consider in this study. Most studies containing SGLT2 inhibitors focused on the cardiorenal effects. The main outcomes did not include bone health or relevant data were not shown. Therefore, some types of SGLT2 inhibitors received few articles and participants. Important confounding factors such as diet, exercise level, and solar radiation were not reported in some original studies and cannot be corrected. Since T2DM requires a combination of drugs in most cases, the background treatment for each patient cannot be unified, and there may be other drugs that also affect bones, leading to error in the results.
Although further studies are needed, the results of our study have demonstrated the possible negative effects of SGLT2 inhibitors on bone health in patients with T2DM. However, there is still a lack of human studies regarding the effects of SGLT2 inhibitors on bone microarchitectural changes in patients with T2DM. Further preclinical or clinical data are needed to elucidate the effects on bone matrix mineralization and collagen fiber distribution. SGLT2 inhibitors have a good hypoglycemic effect and cardiorenal protection, but they may have a secondary effect on bone turnover. The long-term safety of this effect on bones deserves continued monitoring as the use of this drug becomes more routine in patients with T2DM.
Data availability
Datasets used in this article are available from the corresponding author on reasonable request.
Abbreviations
Alkaline phosphatase
Cross-linked C-terminal telopeptides of type I collagen
Fibroblast growth factor 23
Procollagen type 1 N-terminal propeptide
Parathyroid hormone
Randomized controlled trials
Sodium–glucose cotransporter 2
Standard mean difference
Tartrate resistant acid phosphatase-5b
Vijan S. Type 2 diabetes. Ann Intern Med. 2019;171(9):ITC65–80.
Article PubMed Google Scholar
Picke AK, Campbell G, Napoli N, Hofbauer LC, Rauner M. Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr Connections. 2019;8(3):R55–70.
Article CAS Google Scholar
Koromani F, Ghatan S, van Hoek M, Zillikens MC, Oei EHG, Rivadeneira F, Oei L. Type 2 diabetes mellitus and vertebral fracture risk. Curr Osteoporos Rep. 2021;19(1):50–7.
Article PubMed PubMed Central Google Scholar
Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudang R, Zoulakis M, Wallander M, Darelid A, Lorentzon M. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Min Res. 2017;32(5):1062–71.
Article Google Scholar
Ahmad OS, Leong A, Miller JA, Morris JA, Forgetta V, Mujammami M, Richards JB. A Mendelian randomization study of the effect of type-2 diabetes and glycemic traits on bone mineral density. J Bone Min Res. 2017;32(5):1072–81.
Liu J, Cao L, Qian YW, Chen ZX, Guo SF, Sun WQ, He ZR. The association between risk of limb fracture and type 2 diabetes mellitus. Oncotarget. 2018;9(58):31302–10.
Mabilleau G, Bouvard B. Update on: effects of anti-diabetic drugs on bone metabolism. Expert Rev Endocrinol Metabolism. 2020;15(6):415–30.
Lupsa BC, Kibbey RG, Inzucchi SE. Ketones: the double-edged sword of SGLT2 inhibitors? Diabetologia. 2023;66(1):23–32.
Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol. 2018;14(8):473–4.
Article CAS PubMed PubMed Central Google Scholar
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metabolism. 2014;16(2):159–69.
Bailey CJ, Villegas ECM, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015;32(4):531–41.
Article CAS PubMed Google Scholar
Kohan DE. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–71.
Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473–8.
Wilding JPH, Woo V, Soler NG, Pahor AP, Sugg J, Rohwedder K, Parikh S. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin a randomized trial. Ann Intern Med. 2012;156(6):405–15.
Araki E, Onishi Y, Asano M, Kim H, Yajima T. Efficacy and safety of dapagliflozin over 1 year as add-on to insulin therapy in Japanese patients with type 2 diabetes: the DAISY (Dapagliflozin added to patients under InSulin therapY) trial. Diabetes Obes Metabolism. 2017;19(4):562–70.
Schumm-Draeger PM, Burgess L, Korányi L, Hruba V, Hamer-Maansson JE, de Bruin TWA. Twice-daily dapagliflozin co-administered with metformin in type 2 diabetes: a 16-week randomized, placebo-controlled clinical trial. Diabetes Obes Metabolism. 2015;17(1):42–51.
Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15(5):403–9.
Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J Diabetes Invest. 2016;7(3):366–73.
Han KA, Chon S, Chung CH, Lim S, Lee KW, Baik S, Jung CH, Kim DS, Park KS, Yoon KH, et al. Efficacy and safety of ipragliflozin as an add-on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: a randomized controlled trial. Diabetes Obes Metab. 2018;20(10):2408–15.
Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27(3):268–73.
Min KW, Ku BJ, Lee JH, Kim MS, Ahn KJ, Lee MK, Kokubo S, Yoshida S, Cho HJ, Cha BS. Addition of ipragliflozin to metformin treatment in Korean patients with type 2 diabetes mellitus: subgroup analysis of a phase 3 trial. Diabetes Metabolism J. 2017;41(2):135–45.
Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6(1):8–18.
Gallo S, Raji A, Calle RA, Pong A, Meyer C. The effects of ertugliflozin on β-cell function: pooled analysis from four phase 3 randomized controlled studies. Diabetes Obes Metabolism. 2020;22(12):2267–75.
Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, Qiu R, Meininger G. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17(1):23–31.
Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44–51.
Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–73.
Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35(6):1232–8.
Rodbard HW, Seufert J, Aggarwal N, Cao A, Fung A, Pfeifer M, Alba M. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18(8):812–9.
Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Mörschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16(11):1087–95.
Sone H, Kaneko T, Shiki K, Tachibana Y, Pfarr E, Lee J, Tajima N. Efficacy and safety of empagliflozin as add-on to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(3):417–26.
Rau M, Thiele K, Hartmann NUK, Möllmann J, Wied S, Hohl M, Marx N, Lehrke M. Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes– data from a randomized, placebo-controlled study. Bone Rep. 2022;16.
Kullmann S, Hummel J, Wagner R, Dannecker C, Vosseler A, Fritsche L, Veit R, Kantartzis K, Machann J, Birkenfeld AL, et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. 2022;45(2):398–406.
Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med. 2014;126(3):16–34.
Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I–III clinical trials. Adv Therapy. 2017;34(7):1707–26.
Brown JP, Don-Wauchope A, Douville P, Albert C, Vasikaran SD. Current use of bone turnover markers in the management of osteoporosis. Clin Biochem. 2022:109–110:1–10.
Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63(2):464–74.
Dong B, Lv R, Wang J, Che L, Wang Z, Huai Z, Wang Y, Xu L. The extraglycemic effect of SGLT-2is on mineral and bone metabolism and bone fracture. Front Endocrinol (Lausanne). 2022;13:918350.
Tu MY, Chen HL, Tung YT, Kao CC, Hu FC, Chen CM. Short-term effects of Kefir-fermented milk consumption on bone Mineral density and bone metabolism in a Randomized Clinical Trial of osteoporotic patients. PLoS ONE. 2015;10(12):e0144231.
Shaffner J, Chen B, Malhotra DK, Dworkin LD, Gong R. Therapeutic targeting of SGLT2: a new era in the treatment of diabetes and diabetic kidney disease. Front Endocrinol (Lausanne). 2021;12:749010.
Thrailkill KM, Nyman JS, Bunn RC, Uppuganti S, Thompson KL, Lumpkin CK, Kalaitzoglou E, Fowlkes JL. The impact of SGLT2 inhibitors, compared with insulin, on diabetic bone disease in a mouse model of type 1 diabetes. Bone. 2017;94:141–51.
Ye Y, Zhao C, Liang J, Yang Y, Yu M, Qu X. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2019;9.
Razzaque MS. Interactions between FGF23 and vitamin D. Endocr Connections. 2022;11(10):e220239.
Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss *. J Biol Chem. 2011;286(12):10864–75.
Watanabe-Takano H, Ochi H, Chiba A, Matsuo A, Kanai Y, Fukuhara S, Ito N, Sako K, Miyazaki T, Tainaka K, et al. Mechanical load regulates bone growth via periosteal osteocrin. Cell Rep. 2021;36(2):109380.
Schmitz D, Ek WE, Berggren E, Höglund J, Karlsson T, Johansson Å. Genome-wide association study of estradiol levels and the causal effect of estradiol on bone mineral density. J Clin Endocrinol Metab. 2021;106(11):e4471–86.
Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, Walkup MP, Hodges A, Williams CC, Bray GA. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the look AHEAD randomized trial. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2012;27(3):619–27.
Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metabolism. 2016;101(1):157–66.
Download references
Acknowledgements
Not applicable.
This study was supported by Key Science and technology project in Ningxia (2020BFG02011); Key Science and technology project in Ningxia (2023BEG02022); Ningxia natural science foundation (2023AAC03614) and Ningxia natural science foundation (2023AAC03597).
Author information
Authors and affiliations.
Office of Academic Research, General Hospital of Ningxia Medical University, 750004, Yinchuan, Ningxia, China
Department of Nutrition, General Hospital of Ningxia Medical University, 750004, Yinchuan, Ningxia, China
First Clinical Medical College, Ningxia Medical University, 750004, Yinchuan, Ningxia, China
Department of Geriatrics and Special Needs, General Hospital of Ningxia Medical University, No. 804 South Shengli Street, 750004, Yinchuan, Ningxia, China
You can also search for this author in PubMed Google Scholar
Contributions
XL, CL and JH designed the study. XL and YL identified and acquired reports of trials and extracted data. HT, QD, WS, SZ, YS and JH performed all data analyses, checked for statistical inconsistency, and interpreted data. HT, QD, WS, SZ, YS and JH contributed to data interpretation. HT drafted the report and all other authors critically reviewed the report. All authors approved the final version of manuscript. JH is the guarantor of this work.
Corresponding author
Correspondence to Chen Lei .
Ethics declarations
Ethics approval and consent to participate, patient consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, J., Li, X., Li, Y. et al. Effects of sodium-glucose cotransporter 2 inhibitors on bone metabolism in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. BMC Endocr Disord 24 , 52 (2024). https://doi.org/10.1186/s12902-024-01575-8
Download citation
Received : 14 December 2023
Accepted : 01 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s12902-024-01575-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes mellitus
- SGLT2 inhibitor
- Meta-analysis
BMC Endocrine Disorders
ISSN: 1472-6823
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci
- PMC10299511

Experimental Models to Study Diabetes Mellitus and Its Complications: Limitations and New Opportunities
Beatriz martín-carro.
1 Bone and Mineral Research Unit, Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Hospital Universitario Central de Asturias, 33011 Oviedo, Spain
2 Redes de Investigación Cooperativa Orientadas a Resultados en Salud (RICORS), RICORS2040 (Kidney Disease), Instituto de Salud Carlos III, 28029 Madrid, Spain
Javier Donate-Correa
3 Research Unit, Hospital Universitario Nuestra Señora de Candelaria, 38010 Santa Cruz de Tenerife, Spain
Sara Fernández-Villabrille
Julia martín-vírgala, sara panizo, natalia carrillo-lópez, laura martínez-arias, juan f. navarro-gonzález.
4 Nephrology Service, Hospital Universitario Nuestra Señora de Candelaria, 38010 Santa Cruz de Tenerife, Spain
Manuel Naves-Díaz
José l. fernández-martín, cristina alonso-montes, jorge b. cannata-andía.
5 Department of Medicine, Universidad de Oviedo, 33006 Oviedo, Spain
Associated Data
Not applicable.
Preclinical biomedical models are a fundamental tool to improve the knowledge and management of diseases, particularly in diabetes mellitus (DM) since, currently, the pathophysiological and molecular mechanisms involved in its development are not fully clarified, and there is no treatment to cure DM. This review will focus on the features, advantages and limitations of some of the most used DM models in rats, such as the spontaneous models: Bio-Breeding Diabetes-Prone (BB-DP) and LEW.1AR1- iddm , as representative models of type 1 DM (DM-1); the Zucker diabetic fatty (ZDF) and Goto-kakizaki (GK) rats, as representative models of type 2 DM (DM-2); and other models induced by surgical, dietary and pharmacological—alloxan and streptozotocin—procedures. Given the variety of DM models in rats, as well as the non-uniformity in the protocols and the absence of all the manifestation of the long-term multifactorial complications of DM in humans, the researchers must choose the one that best suits the final objectives of the study. These circumstances, added to the fact that most of the experimental research in the literature is focused on the study of the early phase of DM, makes it necessary to develop long-term studies closer to DM in humans. In this review, a recently published rat DM model induced by streptozotocin injection with chronic exogenous administration of insulin to reduce hyperglycaemia has also been included in an attempt to mimic the chronic phase of DM in humans.
1. Concept, Classification and the Role of Experimental Models in the Study of Diabetes Mellitus
Diabetes mellitus (DM) is a major global health problem not only because of its high and growing prevalence, which has tripled in the last 20 years, but also because of the number of premature deaths it causes. In the year 2021, worldwide records indicated that approximately 537 million adults were living with DM, and more than one in ten of global deaths from all causes (12%; 6.7 million adults) were related to complications derived from the disease [ 1 ]. The prevalence of DM in 2021 was approximately 10.5%, an increase of 3,9% compared to 2010. Moreover, DM generates a great economic impact on health systems, around 11.5% of the global health spending according to the World Health Organization [ 1 ].
The DM is a multifactorial disease triggered by a combination of genetic, epigenetic and environmental factors. The increase in life expectancy and unhealthy lifestyle habits, such as a sedentary lifestyle and the consumption of foods rich in saturated fats and added sugars, are risk factors for the development of obesity and associated comorbidities, such as metabolic syndrome, also called insulin resistance syndrome. This scenario is considered a predictor of DM [ 1 ]. In fact, the coexistence of diabetes and obesity, known as “diabesity”, shows an alarming rise [ 2 ].
Although the disorders associated with DM are diverse, the common feature to all of them is hyperglycaemia due to a relative or complete insulin resistance and/or deficiency. This hormone, which is produced in the pancreatic β-cell in the islets of Langerhans, is essential in the control of the glucose homeostasis by facilitating the glucose uptake and metabolism in peripheral tissues [ 3 ].
Chronic hyperglycaemia leads to a variety of complications, such as neuropathy, retinopathy and nephropathy [ 3 ]. The latter, known as diabetic kidney disease (DKD), is one of the most frequent long-term complications associated to DM, and its prevalence has increased in recent years in parallel to the substantial rise in the obese and diabetic population. The DKD, with different degrees of renal impairment, occurs in approximately 40% of diabetic patients, and today, it is the main cause of chronic kidney disease (CKD) that needs renal replacement therapy [ 4 , 5 ].
The main factors involved in the progression of DKD are uncontrolled hyperglycaemia, dyslipidaemia, hemodynamic (glomerular hypertension), inflammatory and profibrotic changes [ 6 ]. DM courses silently in early stages [ 7 , 8 ], but in the long-term, it damages several vital organs such as kidneys, blood vessels, heart and bones [ 1 , 3 , 9 , 10 ]. The high prevalence of the DKD, its lethal complications together with the still uncomplete knowledge of its pathogenesis [ 4 ], makes necessary the use of preclinical models to better understand the disease.
DM can be classified into four general categories: type 1 DM (DM-1), type 2 DM (DM-2), gestational DM and a group of specific types of DM due to other causes including monogenic DM syndromes, diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis) and drug- or chemical-induced DM (such as post-transplantation DM). DM-1 and DM-2 are the most common forms of the disease. DM-1, also called insulin-dependent DM, is present in 5–10% of patients and is due to autoimmune pancreatic β-cell destruction by T-cells and macrophages, usually leading to a widespread and irreversible insulin deficiency. Although DM-1 can appear at all ages, it is most often diagnosed in children and young people. DM-2, also called insulin-independent DM, is present in 90–95% of patients. DM-2 is due to a progressive loss of β-cell insulin secretion which leads to the inadequate response of the body to the action of insulin, known as insulin resistance [ 3 ].
Preclinical biomedical models of DM are fundamental to improving the knowledge and management of the disease. For research purposes, the spontaneous, induced and transgenic models of DM, currently using rodents, are the most used. However, unfortunately there is no animal model that presents all the phenotypic and/or genotypic alterations of DM in humans. Most of the studies of DM performed in rodent models are focused on the development of strategies for the prevention and early treatment of DM. However, in the experimental field, there is a gap in the knowledge of the long-term complications of DM. One of the main reasons is the difficulties of maintaining animal experiments long enough to be comparable with what happens in advanced stages of DM in humans. Therefore, there is an urgent need to carry out long-term studies to investigate the maintained effect of hyperglycaemia and its impact on the target organs of the disease [ 11 ].
The scientific and regulatory aspects of the use of experimental animals are subject to strict ethical guidelines around the world based on the principles of the 3Rs (replacement, refinement and reduction), which aim to improve both the quality of science and animal welfare when the use of animals is unavoidable [ 12 ]. In recent years, the trend has been towards a reduction in the use of experimental animals, improving alternative models such as 3D, computational and mathematical models of diseases, and every day important progress are made in this field. In relation to DM, there are some studies that have used this approach to study gestational DM [ 13 ] or to identify molecular markers to assess the glucose response [ 14 ]. However, research with animal models remains a fundamental tool to better understand biological processes and human diseases and to develop therapies, especially for systemic diseases such as DM.
2. Available Models for the Study of DM-1 and DM-2
2.1. the spontaneous dm rat models.
Spontaneous autoimmune DM has been observed in several rodent strains [ 15 ]. These rodent models have been widely used for the study of the pathogenesis of the insulitis of DM-1. The major rodent model of spontaneous DM-1 is the Bio-Breeding (BB) rat, which includes both the T-lymphopenic diabetes-prone (BB-DP) stock and the non-lymphopenic diabetes-resistant stock [ 16 ]. Other examples of strains of inbred rats include the LEW.1AR1- iddm rats, as representative models of DM-1, and Zucker diabetic fatty (ZDF) and Goto-kakizaki (GK) rats, as representative models of DM-2.
2.1.1. The Bio-Breeding Diabetic Rats
The Bio-Breeding diabetic-prone rats (BB-DP rats) were discovered in the 1970s at Bio-Breeding Laboratories in Canada. They originated from a spontaneous mutation in an outbred colony of Wistar rats affecting the major histocompatibility complex (MHC). The development frequency of DM-1 occurs in males and females in the same proportion, and between 50 and 90 days after birth, the rats show severe pancreatic insulitis, leading to a hypoinsulinemia state. The first manifestation of the disease is glycosuria at 8–16 weeks, and 90% of the rats develop overt DM-1 with hyperglycaemia, weight loss, polyuria, polydipsia and very severe ketoacidosis that requires exogenous insulin administration in order to survive [ 17 , 18 ]. Bio-Breeding diabetes-resistant (BB-DR) rats do not develop DM, and they are used as controls.
Even though the features of the BB-DP rats are similar to DM-1 in humans, an important limitation of this model is that DM is accompanied by a T-cell decrease, a disorder that does not occur in humans or in other animal models that makes it a questioned model. In addition, some promising antidiabetic drugs, such as anti-CD3 antidiabetic therapy, have shown the side effect of a decrease in the T-lymphocyte population, a finding that makes it unable to use this model for the study of this type of drugs [ 19 , 20 , 21 , 22 , 23 ]. Despite the mentioned limitations, the BB-DP rats have been widely used for to study the pathophysiology of DM and islet transplantation [ 24 ].
2.1.2. The LEW.1AR1- iddm Rats
The LEW.1 AR1- iddm rats were originated from the congenic strain LEW.1AR1 by a spontaneous mutation which also affects a gene associated with MHC. DM-1 occurs in males and females in the same proportion, showing intense pancreatic insulitis that causes subsequent hypoinsulinemia. The LEW.1AR1- iddm rats develop a prediabetic state for approximately one week [ 25 ], and by week 8 of life, they present many of the signs and symptoms of DM-1 such as hypoinsulinemia, weight loss, hyperglycaemia, polydipsia, polyuria, glycosuria and ketoacidosis. They have a long life expectancy, a fact that makes them an ideal model for long-term studies [ 17 , 24 , 26 ].
2.1.3. The Zucker Diabetic Fatty Rats
The Zucker fatty (ZF) rats are obese rats due to a mutation in the leptin receptor gene making them hyperphagic. They develop hyperlipidaemia and hyperinsulinemia; however, they maintain normal blood glucose levels and rarely progress to mild hyperglycaemia [ 27 ]. These alterations are also observed in the prediabetic state in humans, where obesity plays an important role as a risk factor for the development of DM-2. After selective crosses between ZF rats, Zucker diabetic fatty (ZDF) rats emerged which, unlike ZF, develop advanced insulin resistance and, progressively, hyperglycaemia that, at week 10, reaches values above 500 mg/dL.
The development of DM-2 occurs spontaneously, more frequently in male rats. Despite the genetic origin of the disease differing between these rats and humans, they develop similar complications as those observed in advanced stages of the human disease such as glomerular lesions, expansion of the mesangial matrix and tubulointerstitial fibrosis, among others. This model has been used to study the alterations associated to advanced stages of DM-2 [ 28 , 29 , 30 ].
2.1.4. The Goto-kakizaki Rats
The Goto-kakizaki (GK) rats constitute a very popular DM-2 model that, unlike the previous model, does not present obesity nor hyperlipidaemia. They result from selective inbreeding between Wistar rats with impaired glucose tolerance. They develop hyperglycaemia, hypoinsulinemia and peripheral insulin resistance at 12 weeks of age. The exposure of the foetus of the pregnant rat to a hyperglycaemic environment seems to affect the normal development of β-cells. Thus, at birth, the rats have a reduced number of pancreatic islets. Additionally, in these rats, exercise can reduce the increase of glycemia. Therefore, this model shares some environmental factors of the human DM, such as the hyperglycaemia “in utero” and the effect of the physical activity, making it an attractive model for studies related to the prevention and treatment of DM-2. This model develops retinal, kidney and peripheral nerves abnormalities, which is useful for studying the complications associated with the disease. A factor that limits the choice of this model for research purposes is the low rate of effective pregnancies and the decreased number of rats obtained per litter [ 30 , 31 , 32 ].
2.2. The Surgical Induced DM Rat Models
This model is obtained by ligation of the pancreatic ducts or by partial or total removal of the pancreas. They are not used frequently due to the traumatic nature of the technique, though it is used in research related to pancreas transplantation [ 33 , 34 , 35 ].
2.3. The Diet-Induced DM Rat Models
The dietary models are useful for studying the prodromal period of the diabetic syndrome, and they are considered more a model of obesity than of DM. As it is difficult to induce DM in rats just by feeding them only with hypercaloric diets, the use of this model often requires the combination with other techniques, such as pharmacological (streptozotocin, alloxan) or partial nephrectomy, to accelerate kidney damage an reduce the time of establishment and progression of the disease [ 11 ].
Different dietary interventions, such as the consumption of the Mediterranean diet [ 36 ], caloric restriction [ 37 , 38 ], intermittent fasting [ 39 ] and the therapeutic potential of various dietary supplements [ 40 ], have shown antioxidant, anti-inflammatory and metabolic profile improvement effects, constituting non-pharmacological complementary therapeutic strategies for the prevention and treatment of obesity and DM. Table 1 lists some therapeutic dietary strategies carried out in diet-induced DM-2 rat models.
Different dietary interventions strategies in diet-induced DM-2 rat models.
DM: Diabetes mellitus; DM-2; type 2 DM; SD: Sprague Dawley; STZ: Streptozotocin; HFD: High fat diet; Glc: glucose; NA: nicotinamide.
2.4. The Chemical-Induced DM Rat Models
Several chemical compounds have shown to be able to induce DM in animal models, and the two most widely used diabetogenic agents are alloxan [ 47 ] and streptozotocin (STZ) [ 48 , 49 ]. Both are cytotoxic glucose analogues that bind to pancreatic β-cell GLUT-2 transporters causing irreversible damage, leading to hyperglycaemia, β-cell necrosis and weight loss, without causing damage to other organs. These diabetogenic agents are very unstable, so the preparations must be prepared at the time they are injected (half-life: alloxan, 1–2 min; STZ, 1 h).
The main advantage of the chemically induced models is that they are simple and relatively cheap. In addition, following different protocols of the time of induction, route of administration and dose, it is possible to induce DM-1 or DM-2 [ 50 ]. The main disadvantages of these models are (a) that the human DM is rarely caused by a toxic substance; (b) the possibility that these compounds can cause toxicity in the liver and tubular cells where GLUT-2 is expressed; and (c) that a single dose can cause mortality due to ketosis associated with acute damage [ 51 , 52 ].
2.4.1. The Alloxan Model
Alloxan is a uric acid derivative that can selectively inhibit glucose-induced pancreatic insulin secretion by inhibiting glucokinase inducing insulin-dependent DM by promoting the formation of reactive oxygen species that cause selective β-cell necrosis. The diabetogenic dose range is very narrow, and even a mild overdose can cause systemic toxicity, especially to the kidney, although the damage is reversible in the surviving animals. It can be administered intraperitoneally (i.p.), intravenously (i.v.) and subcutaneously (s.c.), and the most frequent dose in rats is 45–65 mg/Kg i.v. [ 47 ].
2.4.2. The Streptozotocin Model
Streptozotocin (STZ) or [2-deoxy-2-(3-(methyl-3-nitrosoureido)-D-glucopyranose] is a nitrosourea analogue attached to a glucosamine moiety, isolated from Streptomyces achromogenes . The mechanism of action also involves the binding of the GLUT-2 transporter before entering in the β-cells and the nucleus where it causes alkylation and, consequently, fragmentation of the DNA promoting pancreatic β-cell necrosis, resulting in an insulin-dependent state due to insulin deprivation. Its sensitivity depends on the species, strain, sex (males are more susceptible), age and nutritional status of the animal.
The STZ can be administered i.p., i.v. and s.c., either as a single dose, between 35 and 65 mg/Kg (the most frequently used), or multiple doses, between 20 and 40 mg/Kg, during several days [ 49 , 53 ]. Adult rats are usually used to establish DM-1 by multiple doses (20–40 mg/Kg) during several days or a single dose of 40–200 mg/Kg. To establish DM-2, neonatal rats with a single dose (35–65 mg/Kg i.p.) or adult rats are used in which nicotinamide or fructose are added as antioxidants to protect the animals from the cytotoxic action of STZ obtaining a partial destruction of the pancreas. The use of STZ is preferred than alloxan in rats.
With the use of STZ or alloxan, the metabolic result is a DM-2 hyperglycaemia state which does not include other epigenetic/environmental factors, such as obesity, which play an important role in DM-2 in humans. To bring these models closer to the DM-2 in humans, the combination of these toxins with diets rich in fat/sugars can be used to better resemble the state of poor nutrition/overweight/obesity currently found in the DM-2 in humans [ 54 , 55 ].
3. Are the Current Rat Models to Study the Human Diabetic Kidney Disease Enough?
The diabetic kidney disease (DKD) is a very important complication of the DM. In the human DKD, clinical and/or biochemical data, shown in Table 2 , are useful to determine the evolutionary course of the disease, avoiding invasive techniques [ 56 , 57 ].
Evolutionary course of human DKD in different stages.
DKD: Diabetic kidney disease; GBM: Glomerular basement membrane; UACR: Urine Albumin-Creatinine Ratio; BP: Blood pressure.
In animal models of DM, the beginning and progress of the signs and symptoms of the Table 2 are variable. Due to the multifactorial aetiology and complex pathogenesis of the human DM, there is no animal model that mimics all the structural and functional changes observed in humans [ 58 , 59 ]. These limitations led the Animal Models of Diabetic Complications Consortium (AMDCC) to propose three criteria to be met by a murine model to be considered as an acceptable DKD, listed in Table 3 [ 60 ].
Criteria to be met by a murine model of desirable DKD, according to the AMDCC.
DKD: Diabetic kidney disease; AMDCC: Animal Models of Diabetic Complications Consortium; GFR: glomerular filtration rate; GBM: Glomerular basement membrane.
The three criteria are not usually met in the same animal model; however, many of them are close to the changes observed in the human DKD. As it is unlikely that a single animal model will develop all the multifactorial complications of the DM in humans, it is advisable to use the different experimental models according to the main objectives of the planned study.
Thus, the first step is to choose the most suitable animal model to answer the research questions of the study [ 24 ].
4. Use of Rat Models for the Study of Antidiabetic Drugs
The very promising antidiabetic therapy with anti-CD3 antibodies induces a decrease in the lymphocyte population, a fact which makes it unable to use BB-DP rats due to their lymphopenia derived from the loss of GTPase function. This limitation is not present in the LEW.1AR1- iddm rat model which better resembles the pathophysiological characteristics of DM-1 in humans. In addition, LEW.1AR1- iddm rats develop DM rapidly, without a prolonged prodromal phase, a circumstance that, added to a longer life expectancy, makes it possible to use this strain to analyse the effect of therapies before developing age-related changes.
The new DM therapeutic approaches go far beyond only lowering blood glucose levels; they aim to act on key metabolic steps, such as sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) [ 61 ], which in turn shows clear benefits on the cardiorenal health of the DM [ 10 ]. Thus, to study the efficacy of SGLT2i and GLP-1RA, as well as their mechanisms of action, experimental models that develop cardiorenal complications because of the diabetic condition are necessary. Below is Table 4 , which compiles different models of DM in rats used in studies with SGLT2i and/or GLP-1RA.
The main characteristics of the rat DM models used in studies with SGLT2i and/or GLP-1RA.
DM: Diabetes mellitus; GK: Goto-kakizaki; SD: Sprague Dawley; STZ: Streptozotocin; HFD: High fat diet; Glc: glucose; SGLT2i: sodium-glucose cotransporter-2 inhibitors; GLP-1RA: Glucagon-like peptide-1 receptor agonist. Note: Only SGLT2i or GLP-1RA are detailed, although many of the referenced studies look at more drugs (monotherapy or multitherapy).
Most of the studies included in Table 4 concentrated on the general beneficial effects of the drugs used secondary to the damage associated with short-term hyperglycaemia. It is necessary to design long-term hyperglycaemia experimental models, closer to human DM, to further study their impact in the complications of DM.
5. Similarity of the Histological Finding in the Experimental and Human DKD
The histologic findings of the human DKD include glomerular hypertrophy, glomerular basement membrane thickening with absence of immune deposits, mesangial matrix expansion, loss of podocytes, glomerular capillary walls thickening, nodular sclerosis (±Kimmelstiel Wilson nodules), arteriolar hyalinosis and tubulointerstitial fibrosis [ 77 ].
The absence of a uniform classification has led the Renal Pathology Society to develop a consensus classification of the glomerular histological lesions present in the DKD, listed in Table 5 [ 58 ].
Consensus classification of glomerular histological lesions present in the DKD.
DKD: Diabetic kidney disease; OM: Optical microscopy; GBM: glomerular basement membrane.
Table 6 lists the kidney histological findings found in different DM rat models.
Histological findings at kidney level found in different models of DM in rats.
DM: Diabetes mellitus; STZ: streptozotocin; NA: nicotinamide; Glc: glucose; H&E: haematoxylin and eosin; PAS: Periodic acid–Schiff; TEM: transmission electron microscopy; ↑: Increased; GBM: glomerular basement membrane.
The non-uniformity in terms of the time of onset and severity of glomerular histological lesions found in the different studies in rats may be explained by the differences in the animal models/species of DM used and time of evolution of the DM, among others. Furthermore, compared to what happens in the DM in humans, the histological abnormalities are less than those observed in humans [ 59 , 82 ].
The DM model induced by STZ injection has been widely used to study the development and evolution of DKD. However, there is still no consensus regarding the age, dose of STZ used, the time to develop DKD, the parameters to consider a success the establishment of DKD and the end points of the experiments. Table 7 lists the details of the protocols used in studies with male Wistar rats and STZ.
Examples of protocols used in the induction of DKD by STZ.
DKD: Diabetic kidney disease; STZ: streptozotocin; DM: Diabetes mellitus; Glc: Glucose; UACR: Urine Albumin-Creatinine; BUN: Blood urea nitrogen; ↑: Increased.
The human DKD is a long-term complication of DM-1 and DM-2 that occurs in patients receiving insulin. However, none of the models previously described includes the use of insulin to correct or minimize the negative effects of hyperglycaemia. This circumstance has been acknowledged as a limitation to the study by several authors, in which potential biomarkers of platelet activation were analysed as instruments for the evaluation of thromboembolic risk in a model of long-term DM (28 weeks) induced by STZ (single dose of 60 mg/dL) [ 85 ].
To shed some light in this area, we recently developed a long-term rat model of DM in which we used long-term insulin administration in order to improve the control of the hyperglycaemia [ 86 ].
6. Experimental Model of DM and Partial Correction of the Hyperglycaemia Using Insulin
The model attempted to assess the effects of long-term hyperglycaemia in a chemically induced DM-1 model in which the blood glucose level was partially corrected during 24 weeks by the administration of exogenous insulin. Briefly, the experimental model of DM consisted in two groups of 4-month-old male Wistar rats (425 ± 43 g) and controls in which DM was obtained using STZ (55 mg/Kg). Rats were considered diabetics when weight loss, polyuria, hyperglycaemia, hyperglycosuria and elevated HbA1c levels were achieved. After the 24 weeks, the rats showed microalbuminuria (UACR > 30 mg/g) and hyperfiltration. Histologically, the kidneys showed structural changes, such as increased diameter of the proximal tubules, thickening of the glomerular basement membrane and denuded foot processes of the podocytes, with no changes in kidney fibrosis. The changes observed in this model allowed us to classify them as class I diabetic nephropathy, according to the classification of the Society of Renal Pathology [ 58 ]. In addition, the diabetic rats showed higher serum and urinary levels of advance glycation end-products (AGEs) and of their soluble receptors in urine (sRAGE) but lower soluble serum Klotho. A higher degree of fibrosis was observed in the heart. The control rats did not show any kind of changes in the kidney nor in the heart. In summary, despite a partial control of the hyperglycaemia using long-term administration of insulin, the diabetic rats showed kidney and heart important alterations.
7. Conclusions
Experimental models of DM are an essential biomedical research tool to better understand and improve the pathogenesis and management of the DM. However, unfortunately, so far there are no animal models that clearly resemble the disorders observed in the human DM. Furthermore, most investigations are centred in the early phase of DM. More studies are needed to mimic the long-term complications associated with the disease and the long-term effects of the treatment in the different organs damaged by DM. As a stimulus for hope, this review includes a summary of a recent long-term model of DM (24 weeks) induced by streptozotocin in which exogenous insulin was administered that can help to better understand some aspects of the pathogenesis and management of DM.
Funding Statement
This research was funded by Fondo Europeo de Desarrollo Regional (FEDER), Plan de Ciencia, Tecnología e Innovación 2013–2017 and 2018–2022 of the Principado de Asturias, grant numbers GRUPIN 14-028, IDI-2018-000-152 and IDI/2021/000080. Instituto de Salud Carlos III, Red Cooperativa en Salud REDinREN and RICORS2040, grant numbers RD12/0021/1023, RD16/0009/0017, RD21/0005/0013 and RD21/0005/0019. Instituto de Salud Carlos III and co-funded by the European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”, grant numbers: PI17/00384, PI19/00532, PI20/00753 and PI20/00633. B.M.-C. and S.F.-V. were supported by a graduate fellowship from the Gobierno del Principado de Asturias (“Severo Ochoa” program): BP19-057, BP20-081, J.M.-V. by a graduate fellowship from the Ministerio de Ciencia, Innovación y Universidades (FPU program): FPU2019-00483, S.P. was supported by Fundación para la Investigación Biosanitaria de Asturias (FINBA), C.A.-M. by RICORS2040 (Kidney Disease) and N.C.-L. by IDI-2018-000152 and IDI-2021-000080.
Author Contributions
Conceptualization, J.B.C.-A., J.L.F.-M., B.M.-C., C.A.-M., M.N.-D., S.P. and N.C.-L.; resources, J.D.-C., J.F.N.-G., M.N.-D., S.P., C.A.-M., N.C.-L., J.L.F.-M. and J.B.C.-A.; writing—original draft preparation, B.M.-C., J.D.-C., S.F.-V., J.M.-V., L.M.-A. and J.B.C.-A.; writing—review and editing, B.M.-C., J.D.-C., J.F.N.-G., S.F.-V., J.M.-V., L.M.-A., M.N.-D., S.P., C.A.-M., N.C.-L., J.L.F.-M. and J.B.C.-A.; supervision, J.B.C.-A., J.L.F.-M., C.A.-M., M.N.-D., N.C.-L. and S.P.; funding acquisition, M.N.-D., S.P., C.A.-M., N.C.-L., J.L.F.-M. and J.B.C.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Research Projects. Print. The Division of Diabetes Translation (DDT) conducts and supports studies, often in collaboration with partners, to develop and apply sound science to reduce the burden of diabetes and to address the research needs of DDT programs and the diabetes community.
Diabetes mellitus (DM) also known as simply diabetes, is a group of metabolic. diseases in which there are high blood sugar levels over a prolonged period This high. blood sugar produces the ...
Diabetes mellitus is a very common disorder in all over the world, often simply referred to as diabetes, is a group of metabolic diseases (Also known as metabolic syndrome and a slow poison) in ...
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the ...
Recent Advances. ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it.
Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-1585. Crossref. ... Chronicle of Diabetes Research and Practice, 3, 1, ...
The chronic complications of type 2 diabetes are a major cause of mortality and disability worldwide [ 1, 2 ]. Clinical research is the main way to gain knowledge about long-term diabetic complications and reduce the burden of diabetes. This allows for designing effective programs for screening and follow-up and fine-targeted therapeutic ...
In type 1 diabetes, a progressive and immune-mediated deterioration of β-cells occurs within pancreatic islets, eventually leading to an almost complete absence of insulin secretion. In type 2 ...
Diabetic keratopathy (DK) poses a significant challenge in diabetes mellitus, yet its molecular pathways and effective treatments remain elusive. The aim of our research was to explore the pyroptosis-related genes in the corneal epithelium of the streptozocin-induced diabetic rats. Methods. After sixteen weeks of streptozocin intraperitoneal ...
Different classes of diabetes mellitus, type 1, type 2, gestational diabetes and other types of diabetes mellitus are compared in terms of diagnostic criteria, etiology and genetics. The molecular genetics of diabetes received extensive attention in recent years by many prominent investigators and research groups in the biomedical field.
Changing our Future Through Research. The ADA is committed to innovation and breakthrough research that will improve the lives of all people living with diabetes. ADA Research: Science. Progress. Hope. ADA research provides critical funding for diabetes research. With 100% of donations directed to research, our goal is to ensure adequate ...
ADA research grants focused on innovative projects with high impact and helped researchers establish collaborative networks to move their innovations into the hands of people living with diabetes. "Research at the ADA is the engine that drives clinical advances by catapulting them into practice. 2023 has brought many prominent achievements.
Diabetes mellitus (DM), o r simply diabetes, is a group of metabolic diseases in which a person has. high blood sugar, either because the body does not produce enough insulin, or because cells do ...
Diabetes mellitus refers to a number of disorders that share the cardinal characteristic feature of elevated blood glucose levels. The 2 most common general categories of this disease are termed type 1 and type 2 diabetes. 8 Research has enormously increased our understanding of type 1 and type 2 diabetes, but much more remains to be done.
Type 1 diabetes cannot be prevented with current knowledge. Effective approaches are available to prevent type2 diabetes and to prevent the complications and premature death that can result from all types of diabetes. These include policies and practices across whole populations and within specific settings (school,
The health care system had participated in research projects employing CHWs to address maternal and infant mortality34 and was interested in expanding to chronic disease. ... diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2019; 42 (9):1661-1668. [PMC free article] [Google Scholar] 3.
Diabetes mellitus is characterised by chronic hyperglycemia. Over time, this can harm the cardiovascular, vasculature, ophthalmic, renal, and peripheral neurological systems (World Health Organization, Citation 2022). Type 2 diabetes has increased in all countries, regardless of income, over the past 30 years (Flood et al., Citation 2021). To ...
This DNP quality improvement project aimed to assess the feasibility and usability of educational videos to improve diabetic patients' knowledge of diabetes self-management. Background: Diabetes prevalence is growing and accounts for 20% of chronic diseases in the US, with 34.2 million people (10.5% of the population) diagnosed in 2018.
The present study utilized a correlational. design to examine the relationships among diabetes distress, social support, self-efficacy, and. performance of diabetes self-care activities. A total of 33 adults with T2DM participated in the. study by completing a battery of surveys regarding performance of diabetes self-care activities.
Trusted AI for Diabetes personalised self-management systems. Diabetes mellitus is a common cause of morbidity and mortality across the globe. Type 2 diabetes continues to rise in prevalence annually; from 1980 to 2004, Type 2 quadrupled in both prevalence and incidence [1]. This will be exacerbated further by the increase in obesity.
Sodium glucose cotransporter 2 (SGLT2) inhibitors are widely used in type 2 diabetes mellitus (T2DM) therapy. The impact of SGLT2 inhibitors on bone metabolism has been widely taken into consideration. But there are controversial results in the study on the effect of SGLT2 inhibitors on bone metabolism in patients with T2DM. Therefore, we aimed to examine whether and to what extent SGLT2 ...
Type 2 diabetes mellitus (T2D) is a common age-related disease, and is a major health concern, particularly in developed countries where the population is aging, including Europe. The multi-scale immune system simulator for the onset of type 2 diabetes (MISSION-T2D) is a European Union-funded project that aims to develop and validate an ...
We found direct associations between the risk of type 2 diabetes and exposures to various food additive emulsifiers widely used in industrial foods, in a large prospective cohort of French adults. Further research is needed to prompt re-evaluation of regulations governing the use of additive emulsifiers in the food industry for better consumer protection.
PROJECT OBJECTIVE: To review the literature on insulin-treated diabetes and driving for members of a Medical Advisory Board subcommittee formulating guidelines for DMV Driver Safety in cases of diabetes mellitus. SUMMARY: Research results are mixed. There is probably some increased risk of a crash caused by
INTRODUCTION. The incidence of diabetes mellitus (DM) is increasing substantially worldwide. Over the past three decades, the global burden of DM has swelled from 30 million in 1985 to 382 million in 2014, with current trends indicating that these rates will only continue to rise[].The latest estimates by the international diabetes federation project that 592 million (1 in 10 persons ...
Immune checkpoint inhibitor (CPI)-induced diabetes mellitus (CPI-DM) is a rare immune-related adverse event (irAE). Patients and providers fear that continuing CPIs puts patients at risk for additional irAEs and thus may discontinue therapy. Currently, there are little data to inform this decision. Therefore, this study aims to elucidate whether discontinuing CPIs after diagnosis of CPI-DM ...
Diabetes mellitus (DM) is a major global health problem not only because of its high and growing prevalence, which has tripled in the last 20 years, but also because of the number of premature deaths it causes. ... This research was funded by Fondo Europeo de Desarrollo Regional (FEDER), Plan de Ciencia, Tecnología e Innovación 2013-2017 ...