March 7, 2020

Do Essential Oils Work? Here’s What Science Says
Every time you turn around someone is suggesting aromatherapy. Essential oils are a $1 billion industry, but are they effective?
By Everyday Einstein Sabrina Stierwalt

Madeleine Steinbach Getty Images

Your friend suggests that you use a lotion infused with peppermint essential oil to help combat your nausea. Your coworker insists that he has never slept so well since starting to sprinkle a little lavender oil on his pillow at night. Last year alone consumers in the United States spent $1 billion on essential oil products and is expected to exceed $11 billion by the year 2022. But what does the research say? Do essential oils really work?
What is aromatherapy?
On supporting science journalism.
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Essential oils are oils, typically fragrant ones, that have been extracted from the roots, flowers, leaves, or seeds of plants using steam or applied pressure. The qualifier “essential” refers to the fact that the oil contains the “essence” of the plant (i.e. the natural chemicals that provide a distinct odor or flavor). In the practice of aromatherapy, these oils—once diluted—are applied to the skin, smelled, dabbed on a pillow or in a bath, or heated so that their aroma is dispersed into the air. Some soaps and lotions can also be made with essential oils and used as aromatherapy products.
The use of essential oils is cross-cultural and dates back thousands of years. Many know the story of frankincense being offered as one of the gifts of the Magi. Even if you haven’t purchased an essential oil roller or diffuser, chances are you may have used them anyway. Vick’s Vaporub , typically rubbed on the chest as a cough suppressant, contains the essential eucalyptus, cedarleaf, and nutmeg oils (among others) suspended in petroleum jelly.
Do essential oils and aromatherapy work?
The National Institute of Health provides a thorough summary via the US National Library of Medicine of research conducted into the efficacy of essential oils . Currently, there is no evidence-backed research showing any illnesses that can be cured through the use of essential oils or the practice of aromatherapy. The results on the other possible benefits of essential oils as, for example, mood elevators or stress relievers, are more mixed. But most are still inconclusive.
One of the scientific studies that have revealed positive results from essential oils involves patients with dementia. Although, contrary to common lore, drinking a tablespoon of fish oil every day won’t likely stave off dementia , there is evidence that balm from lemon oil reduces agitation in patients with dementia according to a study in the Journal of Clinical Psychiatry.
There are other proven success stories for essential oils, such as the treatment of acne with tea tree oil and the treatment of alopecia areata or hair loss with oils like thyme, rosemary, lavender and cedarwood.
Research into the use of essential oils found in citrus fruits is particularly intriguing due to their natural antibacterial qualities. For example, citrus oil, particularly when combined with Dead Sea salts, was shown to inhibit bacterial growth in mice and act as an anti-inflammatory agent. The citrus essential oil bergamot could help fight the growth of common causes of food poisoning like listeria, e coli, and staphylococcus.
However, most of these studies have not yet extended to clinical trials, meaning there is still much more work to do before essential oils would be potentially prescribed by physicians. Given the strong public interest in essential oils, whether it be to target things other medicines have so far failed to fix (like migraines, anxiety, stress, insomnia, and memory) or to control what goes into their medicine cabinet without a prescription, more research into the possible benefits of essential oils is clearly worthwhile.
There are very few noted side effects associated with the use of essential oils, although in the US they do not require approval from the FDA . One exception is the estrogen-like effects noted for lavender and tea tree oils which have been linked to breast enlargement in pre-pubescent boys when applied over long periods of time.
So if you’re looking to relieve stress, adding a few drops of diluted essential oils to a warm bath probably doesn’t hurt. But before you spend $40 on a 15-mL bottle, you might want to try a scented candle first.
»Continue reading “Do Essential Oils Work? Here’s What Science Says” on QuickAndDirtyTips.com
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Efficacy of Essential Oils in Pain: A Systematic Review and Meta-Analysis of Preclinical Evidence
Damiana scuteri, kengo hamamura, tsukasa sakurada, chizuko watanabe, shinobu sakurada, luigi antonio morrone, laura rombolà, paolo tonin, giacinto bagetta, maria tiziana corasaniti.
- Author information
- Article notes
- Copyright and License information
Edited by: Francesca Guida , University of Campania Luigi Vanvitelli, Italy
Reviewed by: Franca Marino , University of Insubria, Italy
Agnieszka Barbara Najda , University of Life Sciences of Lublin, Poland
*Correspondence: Giacinto Bagetta, [email protected] ; Laura Rombolà, [email protected]
This article was submitted to Inflammation Pharmacology, a section of the journal Frontiers in Pharmacology
Received 2020 Dec 10; Accepted 2021 Jan 18; Collection date 2021.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Background: The demand for essential oils (EOs) has been steadily growing over the years. This is mirrored by a substantial increase in research concerned with EOs also in the field of inflammatory and neuropathic pain. The purpose of this present systematic review and meta-analysis is to investigate the preclinical evidence in favor of the working hypothesis of the analgesic properties of EOs, elucidating whether there is a consistent rational basis for translation into clinical settings.
Methods: A literature search has been conducted on databases relevant for medical scientific literature, i.e., PubMed/MEDLINE, Scopus, and Web of Science from database inception until November 2, 2020, following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) criteria for systematic reviews and meta-analyses.
Results: The search was conducted in order to answer the following PICOS (participants/population, interventions, comparisons, outcomes, and study design) question: are EOs efficacious in reducing acute nociceptive pain and/or neuropathic pain in mice experimental models? The search retrieved 2,491 records, leaving 954 studies to screen after the removal of duplicates. The title and abstract of all 954 studies were screened, which left 127 records to evaluate in full text. Of these, 30 articles were eligible for inclusion.
Conclusion: Most studies (27) assessed the analgesic properties of EOs on acute nociceptive pain models, e.g. the acetic acid writhings test, the formalin test, and the hot plate test. Unfortunately, efficacy in neuropathic pain models, which are a more suitable model for human conditions of chronic pain, had fewer results (only three studies). Moreover, some methodologies raised concerns in terms of the risk of bias. Therefore, EOs with proven efficacy in both types of pain were corroborated by methodologically consistent studies, like the EO of bergamot, which should be studied in clinical trials to enhance the translational impact of preclinical modeling on clinical pain research.
Keywords: essential oils, pain models, inflammatory pain, neuropathic pain, chronic pain, systematic review, meta-analysis
1 Introduction
1.1 rationale.
Essential oils (EOs) containing components in exact proportion contributing synergically to the whole plant effect, have been used in traditional medicine for centuries since The Divine Farmer’s Materia Medica , the first text of Chinese Traditional Medicine, representing a form of combinatorial medicine ( Li and Weng, 2017 ). The search for natural and green products is constantly increasing the use of essential oils and the demand for these products from developing countries. There has been a remarkable increase in the import of EOs by the European market from 2011–2018 (Eurostat) and it is estimated that the demand for essential oils in the global market will grow by 7.5% from 2020 to 2027 ( GVR, 2020 ). These data are mirrored by the steady increase of research on EOs that pave the way for the development of these products.
Identifying the year 1880 as this field emerged ( Wood and Reichut, 1880 ), we found a remarkable increase in publications concerned with EOs up to 2020 ( Figures 1A,B ) (see also ( Scuteri et al., 2017a )). EOs have shown several beneficial properties, many of which concern the treatment of neurologic diseases, mood disturbances, and pain. Modulation of the γ-aminobutyric acid (GABA) neurotransmission and blockade of neuronal voltage-gated sodium channels (Na + channels) as well as activity on serotonergic neurotransmission are proposed as mechanisms involved in the action of EOs endowed with anxiolytic and anti-nociceptive properties like bergamot essential oil (BEO) ( Rombolà et al., 2017 ; Scuteri et al., 2018a ; Scuteri et al., 2019a ; Scuteri et al., 2019b ; Rombolà et al., 2019 ; Rombola et al., 2020 ), lavender essential oil (LEO) ( Lopez et al., 2017 ), and melissa (lemon balm) ( Abuhamdah et al., 2008 ; Awad et al., 2009 ). The cholinergic system is targeted by extracts of plants as sage ( Perry et al., 2000 ; Savelev et al., 2003 ; Savelev et al., 2004 ), ginkgo ( Stein et al., 2015 ; Zhang et al., 2018 ), and lemon balm ( Dastmalchi et al., 2009 ; Guginski et al., 2009 ), showing therapeutic potential for diseases like dementia. The gathered evidence shows the potential benefits of EOs in the treatment of pain in fragile patients for whom several drugs can be more harmful, e.g. in aging or chronic neurologic diseases such as dementia ( Achterberg et al., 2020 ). Pain is associated with mood disturbances ( Evans, 1987 ; Husebo et al., 2011 ) influenced by aging ( Hamm and Knisely, 1985 ; Scuteri et al., 2020a ) and neuropathology ( Scherder et al., 2003 ) and its treatment represents a field of strong inappropriateness in patients suffering from Alzheimer’s disease. ( Scuteri et al., 2017b ; Scuteri et al., 2018b ; Achterberg et al., 2020 ; Scuteri et al., 2020f ). Therefore, aromatherapy represents a fundamental tool for the safer handling of pain.
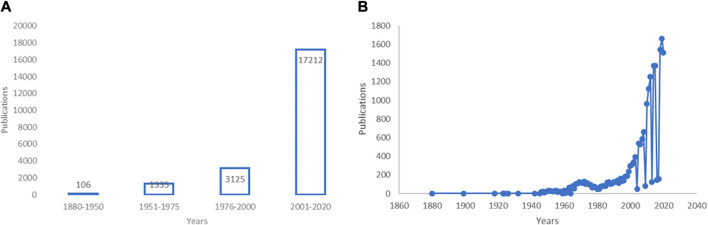
Research in the field of essential oils (EOs) over the years. (A,B) Increase of research in the field of essential oils (EOs). (A) A PubMed advanced search using the key word “essential oils” combined with the dates of publication from 1880 to present through the Boolean operator AND has retrieved an increase from 106 to 17,212 (date of last search November 19, 2020) of results. The first interval “1880–1950” is wider because no great amount of research in this field has been detected up until the 1950s. (B) Data are presented per year of publication based on search query “essential oils” (date of last search November 25, 2020). Modified from ( Scuteri et al., 2017a ).
Despite a large amount of continuously growing research on EOs, a real translation of aromatherapy into clinical settings and the treatment of pain has not occurred. Research efforts have aimed to discover the mechanisms at the root of the analgesic activity of EOs, often focusing on the single components commonly present in different plant oils e.g., linalool, limonene, pinene, eugenol, and cinnamal. For instance, linalool, limonene, and pinene contribute to the anxiolytic and antidepressant properties of some EOs (see ( Lizarraga-Valderrama, 2020 )). In particular, some natural components of plants have been suggested as possible candidates for an analgesic action in neuropathic pain ( Quintans et al., 2014 ). However, the strongest effect of EOs is due to the whole phytocomplex made up of various plant components that need to be present in a precise ratio to exert the so called entourage effect ( Ribeiro, 2018 ). Definite combination of the constituents of EOs is necessary, but further studies are needed to highlight the exact active composition for each EO. The EOs of the species Citrus contain volatile components (85–99%), most abundantly terpenoids, and a non-volatile fraction including coumarins i.e. bergapten inducing phototoxicity ( Zaynoun et al., 1977 ). Thus, the EO of bergamot has been deprived of bergapten ( Bagetta et al., 2010 ), but is still endowed with its characteristics. The EO of bergamot can modulate the synaptic level of glutamate and this occurs when it is used as a bergapten-free fraction ( Morrone et al., 2007 ). Hence, a mixture of monoterpene hydrocarbons within the volatile fraction may be responsible for bergamot analgesic activity since glutamate is significantly involved in the pain descending pathway due to metabotropic glutamate receptors mGluR7 and mGluR8 ( Boccella et al., 2020 ). The novel phytocannabinoid cannabidihexol, with the terpenophenolic core of cannabidiol and Δ9-tetrahydrocannabinol, has proven to significantly reduce the late phase of the formalin test at low doses in C57BL/6J mice ( Linciano et al., 2020 ). Cannabidiol oil has been demonstrated to reduce traumatic brain injury-induced allodynia ( Belardo et al., 2019 ). Certain EOs have been proven to have enhanced efficacy if combined: e.g., peppermint and caraway oil are significantly effective on post-inflammatory visceral hyperalgesia only when used in combination ( Adam et al., 2006 ). Likewise, the route of administration and the time of exposure can influence the effects of EOs ( Scuteri et al., 2018a ; Koyama and Heinbockel, 2020 ). Moreover, some EOs are efficacious in a preclinical setting ( Sarmento-Neto et al., 2015 ), but often only in a definite model of pain, usually acute e.g. the acetic acid-induced writhings, that does not find a significant counterpart in clinic. Furthermore, EOs are often administered as gavage or for inhalation not always allowing an exact determination of the dose.
Clinical trials in aromatherapy are few, small and methodologically limited, hence it is not always possible to draw rigorous conclusions, particularly in dementia. As recently demonstrated in a Cochrane systematic review by Ball et al. (2020) , the design, reporting and consistency of outcome measurement have been identified as the weakest points and need to be improved in the future. Thus, despite accumulating preclinical and clinical evidence for EOs ( Scuteri et al., 2020d ) and nutraceuticals ( Scuteri et al., 2020e ) in lots of forms and supplements, which have been studied in several neurodegenerative conditions, a sound rationale for their clinical use, especially in treating chronic pain ( Lakhan et al., 2016 ), has not yet emerged.
2.1 Objectives
The present systematic review and meta-analysis aimed to verify the working hypothesis that EOs have analgesic properties by investigating preclinical evidence in favor of the latter, to understand whether there is a consistent rational basis for clinical translation. For this purpose, the objective was to assess the efficacy of EOs in preclinical models of both nociceptive and neuropathic pain through the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) ( Liberati et al., 2009 ; Moher et al., 2009 ) criteria for systematic reviews and meta-analyses. The systematic review and meta-analysis focuses on the following PICOS (participants/population, interventions, comparisons, outcomes, and study design) question: are EOs efficacious in reducing acute nociceptive pain and/or neuropathic pain in mice experimental models? In particular, this work aimed at evaluating:
analgesic effectiveness (outcome);
of EOs with a known composition (interventions), and not single components or extracts, administered intraperitoneally (i.p.) or subcutaneously (s.c.) to allow determination of the exact dose and reproducibility;
in male mice subjected to nociceptive or neuropathic pain models (participants/population);
with respect to providing a vehicle or other treatments (comparators);
in studies designed according to legislation to minimize the suffering of animals (study design).
To the best of our knowledge, this is the first meta-analysis of preclinical studies on the analgesic effects of EOs interventions in models of both nociceptive and neuropathic pain.
2.2 Protocol
The search strategy and extraction of data to answer to PICOS question followed the PRISMA ( Liberati et al., 2009 ; Moher et al., 2009 ) criteria. Due to the nature of preclinical animal intervention systematic review and meta-analysis, the latter aims at investigating the consistency of the body of evidence for clinical translation without an outcome of clear human relevance. For this reason, it has not been registered in the International prospective register of systematic reviews PROSPERO. However, statistically analyzing basic research independent studies testing the same hypothesis with comparable parameters can: determine its consistency allowing to study that phenomenon in a larger sample surmounting the issues concerned with small sample sizes; correct confounders; improve reproducibility ( Editorial, 2016 ). Thus, a systematic review and meta-analysis is fundamental to establish a real possible clinical translation of a preclinically studied effect since it can highlight whether it has been consistently proven with the most reliable human disease modelled approach. Two independent researchers ran the search in agreement with the previously established protocol and inclusion and exclusion criteria, including double-checking the retrieved results, and any conflicts found by them were resolved by a third author.
2.3 Eligibility Criteria
2.3.1 inclusion criteria.
The analysis included studies assessing the antinociceptive effect of EOs, administered i. p. or s. c. to allow determination of the exact dose and reproducibility, with a known percentage of components on male mice subjected to nociceptive or neuropathic pain models. Compliance with animal welfare regulations was an inclusion criterion of the utmost importance. The studies included needed to be designed according to legislation to minimize animal suffering. Either acute nociceptive or neuropathic pain models are included. Independently of the model used, the outcome of the study had to be antinociception for eligibility.
2.3.2 Exclusion Criteria
Studies on species different from mice or any strains and female gender were not eligible. The use of different species and genders would not allow comparison and the number of papers examining pain in non-rodent species is very small. Papers in which extracts or single plant components are used were excluded. Studies that did not consider ethics were excluded. Narrative or systematic reviews and meta-analysis, in vitro studies, abstracts and congress communications, proceedings, editorials, book chapters, and studies not published in English and not available in full text were not eligible.
2.4 Information Sources
A literature search was performed on PubMed/MEDLINE, Scopus, and Web of Science. Embase could not be searched as it was not freely/institutionally available. No restriction of publication date was applied and databases were searched for records matching the search strings used from their inception. The date of the last search was November 2, 2020. After the elimination of duplicate records, the first screening evaluated the title and abstract, and then the full text was assessed to define inclusion in qualitative and/or in quantitative synthesis.
2.5 Search Strategy
The following search terms and modifications were used as key words in combination: essential oils, pain, animal pain models, antinociceptive activity, allodynia, Von Frey (‘s test), hyperalgesia, Hargreaves (‘test), hot plate, capsaicin test, formalin test, tail flick test, acetone test, complete Freund's adjuvant, streptozocin, chemotherapy(-induced), oxaliplatin, cisplatin, paclitaxel, docetaxel, vincristine, vinblastine, eribulin, bortezomib, thalidomide, neuropathy, mice.
2.6 Data Collection Process
The eligibility of the studies was assessed independently by two authors to minimize the risk of excluding relevant records. The references list of the articles was examined to extend and refine the search. A complete consensus was reached and no relevant conflicts were raised. The selection process is illustrated in the PRISMA flow diagram ( Figure 2 ).
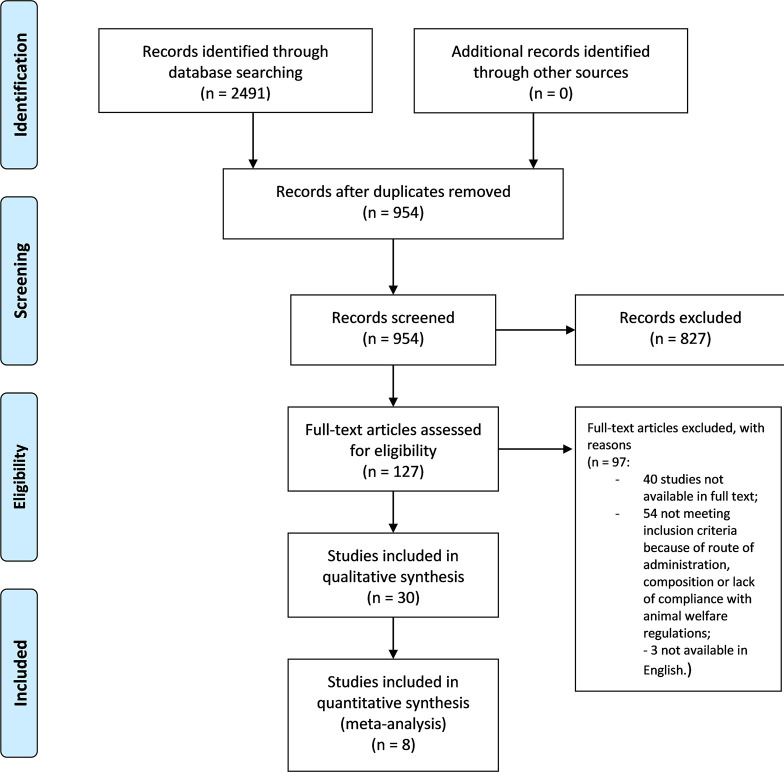
Literature search and screening of retrieved records. PRISMA flow diagram ( Moher et al., 2009 ) of the selection process of the studies eligible for qualitative and quantitative synthesis.
2.7 Synthesis, Risk of Bias, and Statistical Analysis
A systematic and narrative synthesis of the results, according to the Cochrane Consumers and Communication Review Group guidelines ( Ryan, 2019 http://cccrg.cochrane.org , March 13, 2019 (accessed DATE).) was carried out. The risk of bias (internal validity) and the quality of the studies was assessed by two independent researchers through tools specific to preclinical animal studies like the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE’s) risk of bias (RoB) tool ( Hooijmans et al., 2014 ) and the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist for study quality ( Macleod et al., 2004 ). Any discrepancies were resolved through consensus or with the help of a third author.
Meta-analyses were conducted using Cochrane Review Manager 5.3 (RevMan5.3; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration). A minimum of five articles per outcome measure was required according to the systematic review protocol for animal intervention studies by SYRCLE. When the tests included in articles were multiple and performed at different times and doses, only the most significant time point for pain development and progression in the specific model was considered for meta-analysis and only data related to the most efficacious dose were included. Studies expressing the analgesic outcome in a comparable way were included in the meta-analysis. Data available and comparable, but not expressed with the same measure of effect size as proportional reduction of outcome in treated animals relative to untreated controls were converted in mean and standard deviation to allow statistical comparison. Data not available and not extractable from graphs using digital ruler software, e.g., PlotDigitizer 2.6.9, were excluded from quantitative analysis. The Higgins I 2 value was calculated to assess the heterogeneity of studies ( Higgins and Thompson, 2002 ). Differences were presented as risk ratios (RR) including 95% confidence intervals (CI), using a random effect model ( DerSimonian and Kacker, 2007 ) to manage the eventual heterogeneity of the studies and to assess intra- and inter-study variation. Publication bias was assessed through Egger’s linear regression test to measure funnel plot asymmetry, adjusted through the “trim and fill” method ( Egger et al., 1997 ; Duval and Tweedie, 2000 ; Sterne and Egger, 2001 ).
3.1 Selection Process and Data Collection
The search retrieved 2,491 results from databases and there were no results from additional searches. The records were screened for duplicates, leaving 954 studies. Title and abstract screening led to an initial exclusion of narrative or systematic reviews and meta-analysis, in vitro studies, abstracts and congress communications, proceedings, editorials, book chapters. This left 127 records in full text. Among these, two had to be excluded because the text was in Chinese ( Li et al., 1991 ; Chen et al., 2011 ) and one was excluded because it was written in Spanish ( Do Nascimento Silva et al., 2018 ). After full text screening, 30 studies were included in qualitative analysis: 40 studies were not available in full text and 54 were excluded because they did not meet inclusion criteria because of species used, route of administration, composition, or lack of compliance with animal welfare regulations. For instance, the study by Ali et al. (2012) in which the EO of Nepeta pogonosperma Jamzad et Assadi was proven to have significant efficacy at different doses in the tail-flick and formalin test in Wistar rats was therefore not eligible. Among the records included in qualitative analysis, eight were included in quantitative synthesis, reporting comparable outcomes and the exact number of animals used. The process of literature search and screening was illustrated in the PRISMA flow diagram ( Moher et al., 2009 ) in Figure 2 .
3.2 Qualitative Synthesis
The data obtained from the 46 studies included in the qualitative analysis were grouped according to Cochrane Consumers and Communication Review Group guidelines ( Ryan, 2019 http://cccrg.cochrane.org , March 13, 2019 (accessed DATE).). These groups were based on the experimental pain model used in 1) EOs showing analgesia in nociceptive models, and 2) EOs with analgesic properties in neuropathic pain. The majority (27/30) of the studies used an acute nociceptive model. Studies providing a range and not an exact number of animals per group were not considered eligible for quantitative analysis. Studies expressing the analgesic outcome in a not comparable manner to the majority were excluded from the meta-analysis. The main characteristics of the studies with reference to the PICOS question are reported in Tables 1 , 2 .
Main characteristics of the studies included showing the efficacy of EOs in nociceptive models.
Studies characteristics in response to PICOS (participants/population, interventions, comparisons, outcomes, and study design) question for records including acute nociceptive pain models; n = number of animals. The order of references in the table follows that in the text.
Main characteristics of the studies included showing efficacy of EOs in neuropathic models.
Studies characteristics relative to PICOS (participants/population, interventions, comparisons, outcomes, and study design) question for retrieved records about neuropathic pain models; n = number of animals. The order of references in the table follows that in the text.
3.2.1 Essential Oils Endowed With Efficacy in Acute Nociceptive Models
Based on the obtained results, several EOs showed analgesic activity in acute nociceptive tests like the acetic acid writhings test, hot-plate test, and the formalin test, with the latter very useful since it includes features of both peripheral and central pain. In the study by Anaya-Eugenio et al. (2016) the EO of artemisia ludoviciana Nutt (Asteraceae) exerted dose-dependent antinociceptive activity in the hot-plate and the formalin test. It was less potent than the reference drug morphine and antagonism studies have revealed that it was inhibited by the non-selective opioid receptor antagonist naloxone. Inula britannica L (Asteraceae) has shown analgesia in the acetic acid writhings test, in the formalin test, in the tail-flick, and the glutamate test ( Zarei et al., 2018 ). This effect is reversed by naloxone and potentiated by l -arginine, therefore all the studies performed with negative and positive controls highlighted the involvement of the opioid system and NO pathway ( Zarei et al., 2018 ).
The EO of Myrcia pubiflora DC., Myrtaceae ( Andrade et al., 2012 ) has demonstrated analgesic efficacy in the acetic acid writhings test and the formalin test, but not in the hot plate test. From the same family, the EO of Eugenia candolleana DC (Myrtaceae) reduced the number of writhings and licking behavior in the second phase of the formalin test in a dose-dependent manner (only at the dose of 100 mg/kg in the first phase, but not the nociceptive reaction in the hot-plate test ( Guimaraes et al., 2009 )).
Clove bud oil (Eugenia caryophyllata, Myrtaceae) significantly reduced formalin-induced pain behavior but affected tail-flick response in a variable way ( Halder et al., 2012 ). The study by Bae et al. (2020) considered basil for its i. p. administration and demonstrated analgesic properties linked to action on δ- and µ-opioid pathways. Moreover, it provides orofacial antinociception at high doses ( Venâncio et al., 2011 ). Aristolochia trilobata L. demonstrated strong analgesia in the formalin test and was comparable to morphine in the acetic acid test ( Quintans et al., 2017 ).
The EO of Croton conduplicatus Kunth (Euphorbiaceae) has shown efficacy ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ): in the acetic acid test; on the formalin‐induced nociceptive behavior at all the doses and in both phases, with effect antagonized by naloxone; on nociception in term of latency time at the highest dose (50 ( deOliveira et al., 2018 ) and 100 ( de Oliveira Júnior et al., 2017 ) mg/kg) in the hot‐plate test. In particular, the mechanism of action of this EO has been proposed to be influenced by ATP-sensitive K+ channels, opioid and cholinergic systems ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ).
Croton cordiifolius Baill (Euphorbiaceae) also had effective results in acetic acid, formalin, and glutamate but not the capsaicin test. This antinociceptive effect was independent on naloxone ( Nogueira et al., 2015 ). Croton adamantinus Müll. Arg. showed a strongly effective comparison with morphine in reducing licking and was more efficacious than indomethacin in decreasing abdominal contortions ( Ximenes et al., 2013 ). Of the study by Hajhashemi et al. (2009) only the experiments using the EO i. p. and on mice could be included in the analysis, showing the effectiveness of Heracleum persicum to be almost comparable to indomethacin in the reduction of the number of writhings.
In the study by Jahandar et al. (2018) only the experiments performed on mice were considered. Pycnocycla bashagardiana (Apiaceae) has not proven analgesic but anti-inflammatory properties. In another study by Ulku Karabay–Yavasoglu et al. (2006) only experiments with the formalin test in mice were considered. The EO of Satureja thymbra L (Lamiaceae) was demonstrated to have antinociceptive efficacy in both the early and late (also at a lower dose) phases of the formalin test ( Ulku Karabay–Yavasoglu et al., 2006 ).
In the study by Katsuyama et al. (2015) the EO of bergamot (Citrus bergamia Risso) demonstrated significant dose-dependent analgesia in both phases of the formalin test, only when administered in the ipsilateral hindpaw and antagonized by naloxone hydrochloride and methiodide (not able to cross the blood brain barrier), suggesting the involvement of peripheral opioid mechanisms. This was earlier observed in the capsaicin test in which it also enhanced morphine analgesia ( Sakurada et al., 2011 ).
Neroli (Citrus aurantium L.) significantly increases reaction time (at 40 mg/kg) in the hot-plate test and significantly decreased the number of writhings in the study by Khodabakhsh et al. (2015) , with the latter effect potentiated by L-nitro arginine methyl ester ( l -NAME). In the study by Khalid et al. (2011) the EO of Zingiber zerumbet (L.) Smith, dose-dependent and comparable to acetylsalicylic acid, inhibited the nociceptive response to capsaicin, acetic acid, glutamate, and phorbol 12-myristate 13-acetate (PMA). Eucalyptus EO has significantly reduced licking time in the second phase of the formalin test in the study by Lee et al. (2019) , and this effect was mediated by the opioid system. It also reduced the number of writhings in a dose-dependent manner but did not display activity on thermal hyperalgesia ( Lee et al., 2019 ). In the study by Lima et al. (2012) the EO of Chrysopogon zizanioides L (Roberty, Poaceae) produced antinociception similar to morphine in the acetic acid test, and this effect was partially reversed by naloxone. Moreover, it reduced the licking time in the second phase of the formalin test, but it did not demonstrate any effects in the Hargreaves’ test.
A common trait is the presence of antiinflammatory analgesia devoid of thermal anti-hyperalgesic effect. The EO of Zhumeria majdae Rech. F. and Wendelbo (Lamiaceae) has displayed dose-related antinociceptive properties in the acetic acid and in the hot-plate test ( Miraghazadeh et al., 2015 ). Chamaecyparis obtuse has also shown analgesia in the writhings and in the formalin, but not in the hot-plate test ( Park et al., 2015 ). Furthermore, in the study by Mishra et al. (2010) Senecio rufinervis D.C. (Asteraceae) produced significant and dose-dependent inhibition of writhes and thermal hyperalgesia. In the study by Sharif et al. (2020) Tanacetum balsamita (Compositae) presented an anti-hyperalgesic effect. The antinociceptive properties exerted by Xylopia laevigata (Annonaceae) in the acetic acid and in the formalin test have not proven dependency on opioid pathways ( Queiroz et al., 2014 ). The antinociceptive effect of Bunium persicum (Boiss.) is reversed by naloxone and attenuated by chlorpheniramine and cimetidine ( Zendehdel et al., 2015 ), thus confirming the complex neuromodulation and the involvement of histamine in nociception ( Hayashi et al., 2020 ). The main features of the studies on EOs analgesia in nociceptive models are summarized in Table 1 .
3.2.2 Essential Oils Endowed With Efficacy in Neuropathic Models
Studies assessing the analgesic properties of EOs in neuropathic pain models are fundamental because these painful conditions are the most appropriate to model chronic neuropathic pain in humans. In the study by Komatsu et al. (2018) the EO of bergamot (Citrus bergamia Risso) was demonstrated to reduce partial sciatic nerve ligation (PSNL)-induced mechanical allodynia on the seventh post-operative day, in which it peaks ( Kusunose et al., 2010 ). In the study by Kuwahata et al. (2013) the EO of bergamot increased mechanical thresholds dose-dependently and significantly at a dose of 20 μg/paw ( Kuwahata et al., 2013 ). Moreover, this anti-allodynic effect is stronger than that of comparable doses of morphine, of which the EO of bergamot enhances the activity ( Kuwahata et al., 2013 ), and it was reversed by naloxone methiodide, peripherally μ-opioid receptor preferring antagonist, β-funaltrexamine hydrochloride, selective μ-opioid receptor antagonist, and β-endorphin antiserum, but not by the non-selective δ-opioid receptor antagonist naltrindole and by the selective κ-opioid receptor antagonist nor-binaltorphimine. Importantly, the study by Hamamura et al. (2020) in which the EO of bergamot was administered s. c. with an osmotic pump to allow a continuous delivery devoid of smell during PSNL, demonstrated that the anti-allodynic effect of this EO is systemic and does not depend on olfactory stimulation. In this study ( Hamamura et al., 2020 ) the increase of planar activity during the light period induced by PSNL, with the maximum effect at the seventh post-operative day and like allodynia, was shown to be abolished by continuously administered EO. This effect is antagonized by naloxone hydrochloride. Observation lasting 14 days with a theoretical duration of the osmotic pump of one week can mimic administration during chronic pain. The main features of the studies on EOs anti-allodyinic properties are summarized in Table 2 .
3.3 Risk of Bias Assessment
The studies included in the qualitative analysis were assessed for methodological quality according to the SYRCLE’s RoB tool ( Hooijmans et al., 2014 ) and the CAMARADES checklist ( Macleod et al., 2004 ; Hooijmans et al., 2014 ; Suokas et al., 2014 ), based on the Cochrane RoB ( Sterne et al., 2019 ). These items comprise all the possible forms of bias. 1) Selection bias–sequence generation (allocation sequence able to produce comparable groups). 2) Selection bias–baseline characteristics (comparable and not adjusted for confounders in the analysis). 3) Selection bias–allocation concealment (during the enrollment). 4) Performance bias–random housing and randomization during the study. 5) Performance bias–blinding of investigators during the study. 6) Detection bias–random outcome assessment. 7) Detection bias–blinding of outcome assessors. 8) Attrition bias (animals eventually excluded from outcome assessment). 9) Reporting bias–reports free of selective outcome reporting. Finally, 10) other sources of bias: lack of evidence of induced pain using the selected behavioral outcome measure before EO administration and examination (i.e., sham procedure), clear description of methods with number of animals used, attention to circadian regulation for behavioral studies, use of the same observer for behavioral tests, use of control and positive and negative control drugs, sample size calculation, statement of conflict of interest, statement of compliance with animal welfare regulations and attention to ethics.
In terms of the two items regarding selection bias, no study reported the method of allocation and, even though they conducted baseline measures, none of the studies describe how experimental groups were composed to ensure homogeneity and consistency. Only the study by Lima and collaborators ( Lima et al., 2012 ) in which mice with baseline latencies of more than 10 s, and studies by de Oliveira Júnior and colleagues ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ) of more than 20 s, at the hot-plate were excluded from the experiments.
As reported in Table 1 , five studies ( Guimaraes et al., 2009 ; Andrade et al., 2012 ; Lima et al., 2012 ; de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ) adopted random housing of mice. The paper by Khodabakhsh et al. reported no randomization of mice but only of rats, which are not included in this systematic review and meta-analysis ( Khodabakhsh et al., 2015 ). Mice were tested in a randomized order in studies by Sakurada and collaborators and Katsuyama et al., 2015 ( Sakurada et al., 2011 ; Katsuyama et al., 2015 ). In the study by Bae et al. (2020) mice were randomly assigned to groups. The study by Khalid et al. (2011) used a blind, randomized design. Mice were randomly assigned to groups and experiments were performed in a blind manner in the study by Quintans and coworkers ( Quintans et al., 2017 ). Moreover, in the study by Ximenes et al. (2013) , the observation was conducted by a blind observer, but the number of animals used for behavioral testing was not reported, only for histological assays. Otherwise, the number of animals per group was reported, but studies that provided a range and not an exact number were not considered eligible for quantitative analysis. Attrition and reporting biases cannot be assessed from the full text of the included studies. Importantly, sham procedure and the certainty of exact execution of the pain model is present only in studies on allodynia, i.e., the studies by Hamamura et al. (2020) , Komatsu et al. (2018a) , and Kuwahata et al. (2013) .
Attention to the circadian rhythm in behavioral testing was reported by the following studies: ( Andrade et al., 2012 ; Quintans et al., 2017 ; Guimaraes et al., 2009 ; Halder et al., 2012 ; Katsuyama et al., 2015 ; Khodabakhsh et al., 2015 ; Miraghazadeh et al., 2015 ; Sakurada et al., 2011 ; Sharif et al., 2020 ; Zarei et al., 2018 ; Zendehdel et al., 2015 ; Komatsu et al., 2018b ; Kuwahata et al., 2013 ). All the studies used control and positive and negative modulators. Importantly, multiple controls were used in the following studies ( Katsuyama et al., 2015 ; Komatsu et al., 2018b ; Kuwahata et al., 2013 ; Sakurada et al., 2011 ). Behavioral testing was conducted by the same observers in the following studies ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ; Venãncio et al., 2011 ). Sample size calculation was not reported and the conflict of interest statement is present only in eight studies ( Queiroz et al., 2014 ; Nogueira et al., 2015 ; de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ; Jahandar et al., 2018 ; Zarei et al., 2018 ; Lee et al., 2019 ; Hamamura et al., 2020 ). This could be due to the lack of requirement of these aspects in journals in the last few years. A statement of compliance with animal welfare regulations is reported in all the studies since it is an inclusion criterion. Moreover, six studies ( Ulku Karabay–Yavasoglu et al., 2006 ; Venâncio et al., 2011 ; Queiroz et al., 2014 ; Khodabakhsh et al., 2015 ; Miraghazadeh et al., 2015 ; Sharif et al., 2020 ) also stated that they used each mouse only once, thus proving particular attention to animal welfare. Importantly, only the study by Hamamura et al. (2020) reported acclimatization to lighting conditions for one week and that an observation period of 14 days can model examination during chronic pain.
3.4 Meta-Analysis
This meta-analysis comprises eight studies for a total of 140 mice. The studies were considered comparable when the analgesic outcome was expressed as mean ± standard error of the mean (SEM) since these measures could be converted for meta-analysis in mean and standard deviation (SD). Moreover, only studies reporting the exact number of animals per group were included in quantitative analysis. Studies investigating the same pain model were considered. The formalin test pain model was chosen since it provides a biphasic nociceptive response. Due to the sensitization processes occurring during the second phase, the study on mechanical allodynia expressed has been included ( Komatsu et al., 2018b ). The results of the forest plot favor the analgesic efficacy of EO (Mean difference MD −59.77; 95% CI (−93.32) - (−26.22); I 2 = 94%; p < 0.00001; Figure 3 ), but need to be carefully examined because of the extremely high heterogeneity, which is also confirmed by the asymmetry of the funnel plot analysis standing for high risk of publication bias, small studies and high differences in study precision.
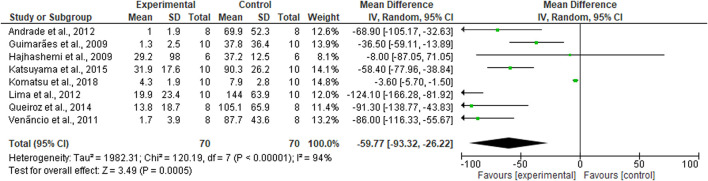
Forest plot for EOs-induced analgesia. The results of the meta-analysis favor the efficacy of the EOs, but they are affected by high heterogeneity (Mean difference MD −59.77; 95% CI (−93.32) - (−26.22); I 2 = 94%; p < 0.00001).
4 Discussion
Interest in the use of EOs and aromatherapy has been continuously growing during the last few decades in parallel with preclinical research. However, in spite of all this effort of preclinical research, it is necessary to establish whether there is a strong rationale for the clinical use of EOs. This issue is even more controversial in the field of pain relief since the use of aromatherapy could reduce the dose of painkillers endowed with serious side effects, particularly in under studied areas of neuropathic pain, like opioids ( Morrone et al., 2017 ; Scuteri et al., 2020b ). Alternative pain treatments could increase time in treatment before the loss of efficacy. This is relevant to fragile populations, e.g., patients suffering from dementia, who are often undertreated compared to cognitively intact counterparts, more so during the Sars-CoV2 pandemic ( Scuteri et al., 2020c ).
This systematic review and meta-analysis assesses the efficacy of EOs in preclinical models of acute inflammatory nociception and neuropathic pain to understand if there is a rational basis for clinical translation. Several EOs from multiple families were found to be efficacious, in particular, croton and bergamot EOs have been extensively studied. It is noteworthy that 27 out of the 30 studies included in the qualitative analysis were only performed on acute pain models like writhings and the hot plate test. These tests are very useful since they are easy to conduct and provide fast results, but they do not resemble clinical pain conditions. Taking this into account, the quantitative analysis only includes studies on formalin test, which is more similar to clinical conditions due to its biphasic nature, and the only study on mechanical allodynia that could compare to the other seven included.
All these studies included in this review have a different experimental design and most of them present serious concerns in terms of selection, performance, and detection biases. Most studies do not adhere to the guidelines for Animal Research: Reporting In Vivo Experiments (ARRIVE), which are fundamental for accurate in vivo preclinical research ( Rice et al., 2013 ). Another methodological aspect responsible for bias in the meta-analysis is that control groups were often used in more than one experiment, and studies including multiple comparisons can introduce errors. Thus, this systematic review and meta-analysis points to the importance of appropriate in vivo modeling to enhance the translational impact of pain research. Future research is necessary to improve the methodological quality and homogeneity of studies.
The results of the meta-analysis highlighted the efficacy of EOs in preclinical pain, but these data are downgraded due to the high heterogeneity of the studies. In particular, the analyzed EOs present the analgesic efficacy required by the recommendations of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) ( Turk et al., 2003 ), according to which a decrease in pain is defined as clinically meaningful if it accounts for a 30% to 36% reduction. However, this is referred to chronic pain and this systematic review and meta-analysis have found that only the EO of bergamot had proven efficacy both in nociceptive and in neuropathic pain models. Moreover, it was also studied for 14 days, an experimental setting suitable for modeling chronic pain ( Hamamura et al., 2020 ).
Another important issue is that the consolidated data come from hypothesis-generating completely original preclinical studies and that they are then confirmed by hypothesis-driven studies ( Mikolajewicz and Komarova, 2019 ). In this case, the EO of bergamot was confirmed to have strong analgesic properties in some of the most used and reliable models of inflammatory pain, i.e., formalin and capsaicin test in different experiments, sharing with most EO mechanisms involving opioid neurotransmission, and also in the PSNL. To the best of our knowledge, this is the first meta-analysis of preclinical studies on the analgesic effects of EOs and its working hypothesis was verified for bergamot EO, which could represent an important pharmacological tool for pain management in clinical settings. Along with clinical translations, more efforts are required to standardize in vivo preclinical studies in the field of pain research to allow for consistent research able to elucidate the mechanisms responsible for the analgesic properties of EOs.
Data Availability Statement
The original contributions presented in the study are included in the article.
Author Contributions
DS, GB, TS, SS, and MTC. conceived the study. All Authors participated in preparation and read and approved the final manuscript.
DS is a post-doc recipient of a research grant salary as part of the research project (Tutor: GB) “Pharmacoepidemiology of drugs used in the treatment of neuropsychiatric symptoms and pain in people aged (over 65) with dementia” funded by Calabria Region (POR Calabria FESR-FSE 2014/2020—Linea B) Azione 10.5.12.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Abuhamdah S., Huang L., Elliott M. S., Howes M. J., Ballard C., Holmes C., et al. (2008). Pharmacological profile of an essential oil derived from Melissa officinalis with anti-agitation properties: focus on ligand-gated channels. J. Pharm. Pharmacol. 60 (3), 377–384. 10.1211/jpp.60.3.0014 [ DOI ] [ PubMed ] [ Google Scholar ]
- Achterberg W., Lautenbacher S., Husebo B., Erdal A., Herr K. (2020). Pain in dementia. Pain Rep. 5, e803. 10.1097/PR9.0000000000000803 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adam B., Liebregts T., Best J., Bechmann L., Lackner C., Neumann J., et al. (2006). A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scand. J. Gastroenterol. 41 (2), 155–160. 10.1080/00365520500206442 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ali T., Javan M., Sonboli A., Semnanian S. (2012). Evaluation of the antinociceptive and anti-inflammatory effects of essential oil of Nepeta pogonosperma Jamzad et Assadi in rats. Daru 20, 48. 10.1186/2008-2231-20-48 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Anaya-Eugenio G. D., Rivero-Cruz I., Bye R., Linares E., Mata R. (2016). Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol 179, 403–411. 10.1016/j.jep.2016.01.008 [ DOI ] [ PubMed ] [ Google Scholar ]
- Andrade G. S., Guimaraes A. G., Santana M. T., Siqueira R. S., Passos L. O., Machado S. M. F., et al. (2012). Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Revista Brasileira De Farmacognosia 22 (1), 181–188. 10.1590/s0102-695x2011005000205 [ DOI ] [ Google Scholar ]
- Awad R., Muhammad A., Durst T., Trudeau V. L., Arnason J. T. (2009). Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother Res. 23 (8), 1075–1081. 10.1002/ptr.2712 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bae A. H., Kim G., Seol G. H., Lee S. B., Lee J. M., Chang W., et al. (2020). Delta- and mu-opioid pathways are involved in the analgesic effect of Ocimum basilicum L in mice. J. Ethnopharmacol 250, 112471. 10.1016/j.jep.2019.112471 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bagetta G., Morrone L. A., Rombolà L., Amantea D., Russo R., Berliocchi L., et al. (2010). Neuropharmacology of the essential oil of bergamot. Fitoterapia 81 (6), 453–461. 10.1016/j.fitote.2010.01.013 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ball E. L., Owen-Booth B., Gray A., Shenkin S. D., Hewitt J., McCleery J. (2020). Aromatherapy for dementia. Cochrane Database Syst. Rev. 8, CD003150. 10.1002/14651858.CD003150.pub3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Belardo C., Iannotta M., Boccella S., Rubino R. C., Ricciardi F., Infantino R., et al. (2019). Oral cannabidiol prevents allodynia and neurological dysfunctions in a mouse model of mild traumatic brain injury. Front. Pharmacol. 10, 352. 10.3389/fphar.2019.00352 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boccella S., Marabese I., Guida F., Luongo L., Maione S., Palazzo E. (2020). The modulation of pain by metabotropic glutamate receptors 7 and 8 in the dorsal striatum. Curr. Neuropharmacol. 18 (1), 34–50. 10.2174/1570159X17666190618121859 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen Y., Zhao Y. Y., Wang X. Y., Liu J. T., Huang L. Q., Peng C. S. (2011). [GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of Cyperus rotundus]. Zhong Yao Cai 34 (8), 1225–1229. [ PubMed ] [ Google Scholar ]
- Dastmalchi K., Ollilainen V., Lackman P., Boije af Gennäs G., Dorman H. J., Järvinen P. P., et al. (2009). Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem. 17 (2), 867–871. 10.1016/j.bmc.2008.11.034 [ DOI ] [ PubMed ] [ Google Scholar ]
- de Oliveira Júnior R. G., Ferraz C. A. A., Silva J. C., de Oliveira A. P., Diniz T. C., E Silva M. G., et al. (2017). Antinociceptive effect of the essential oil from Croton conduplicatus Kunth (euphorbiaceae). Molecules 22 (6), 900. 10.3390/molecules22060900 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- de Oliveira R. G., Ferraz C. A. A., Silva J. C., Teles R. B. D., Silva M. G., Diniz T. C., et al. (2018). Neuropharmacological effects of essential oil from the leaves of Croton conduplicatus Kunth and possible mechanisms of action involved. J. Ethnopharmacology 221, 65–76. 10.1016/j.jep.2018.04.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- DerSimonian R., Kacker R. (2007). Random-effects model for meta-analysis of clinical trials: an update. Contemp. Clin. Trials 28 (2), 105–114. 10.1016/j.cct.2006.04.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- Do Nascimento Silva A., Bomfim H. F., Magalhães A. O., Da Rocha M. L., Lucchese A. M. (2018). Chemical composition and antinociceptive activity of essential oil from myrcia rostrata dc. (myrtaceae) in animal models. Quimica Nova 41 (9), 982–988. 10.21577/0100-4042.20170274 [ DOI ] [ Google Scholar ]
- Duval S., Tweedie R. (2000). Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2), 455–463. 10.1111/j.0006-341x.2000.00455.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Editorial . (2016). Meta-analysis in basic biology. Nat. Methods 13 (12), 959. 10.1038/nmeth.4102 [ DOI ] [ Google Scholar ]
- Egger M., Davey Smith G., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. 10.1136/bmj.315.7109.629 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans L. K. (1987). Sundown syndrome in institutionalized elderly. J. Am. Geriatr. Soc. 35 (2), 101–108. 10.1111/j.1532-5415.1987.tb01337.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Guginski G., Luiz A. P., Silva M. D., Massaro M., Martins D. F., Chaves J., et al. (2009). Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol. Biochem. Behav. 93 (1), 10–16. 10.1016/j.pbb.2009.03.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- Guimaraes A. G., Melo M. S., Bonfim R. R., Passos L. O., Machado S. M. F., Ribeiro A. D., et al. (2009). Antinociceptive and anti-inflammatory effects of the essential oil of Eugenia candolleana DC., Myrtaceae, on mice. Revista Brasileira De Farmacognosia 19 (4), 883–887. 10.1590/s0102-695x2009000600016 [ DOI ] [ Google Scholar ]
- GVR (2020). Report No.: 978-1-68038-549-6. 1-153. Essential oils market size, share & trends analysis report by application (food & beverages, spa & relaxation), by product (orange, peppermint), by sales channel, and segment forecasts, 2020–2027. [ Google Scholar ]
- Hajhashemi V., Sajjadi S. E., Heshmati M. (2009). Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J. Ethnopharmacol 124 (3), 475–480. 10.1016/j.jep.2009.05.012 [ DOI ] [ PubMed ] [ Google Scholar ]
- Halder S., Mehta A. K., Mediratta P. K., Sharma K. K. (2012). Acute effect of essential oil of Eugenia caryophyllata on cognition and pain in mice. Naunyn Schmiedebergs Arch. Pharmacol. 385 (6), 587–593. 10.1007/s00210-012-0742-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hamamura K., Katsuyama S., Komatsu T., Scuteri D., Bagetta G., Aritake K., et al. (2020). Behavioral effects of continuously administered bergamot essential oil on mice with partial sciatic nerve ligation. Front. Pharmacol. 11, 1310. 10.3389/fphar.2020.01310 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hamm R. J., Knisely J. S. (1985). Environmentally induced analgesia: an age-related decline in an endogenous opioid system. J. Gerontol. 40 (3), 268–274. 10.1093/geronj/40.3.268 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hayashi T., Watanabe C., Katsuyama S., Agatsuma Y., Scuteri D., Bagetta G., et al. (2020). Contribution of histamine to nociceptive behaviors induced by intrathecally administered cholecystokinin-8. Front. Pharmacol. 11, 590918. 10.3389/fphar.2020.590918 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Higgins J. P., Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. 10.1002/sim.1186 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hooijmans C. R., Rovers M. M., de Vries R. B., Leenaars M., Ritskes-Hoitinga M., Langendam M. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 (1), 43. 10.1186/1471-2288-14-43 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Husebo B. S., Ballard C., Sandvik R., Nilsen O. B., Aarsland D. (2011). Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 343, d4065. 10.1136/bmj.d4065 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jahandar F., Asgarpanah J., Najafizadeh P., Mousavi Z. (2018). Anti-inflammatory activity and chemical composition of Pycnocycla bashagardiana fruit’s essential oil in animal models. Iran J. Basic Med. Sci. 21 (2), 188–193. 10.22038/ijbms.2017.20860.5426 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jun Y. S., Kang P., Min S. S., Lee J. M., Kim H. K., Seol G. H. (2013). Effect of eucalyptus oil inhalation on pain and inflammatory responses after total knee replacement: a randomized clinical trial. Evid. Based Complement. Alternat Med. 2013, 502727. 10.1155/2013/502727 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Katsuyama S., Otowa A., Kamio S., Sato K., Yagi T., Kishikawa Y., et al. (2015). Effect of plantar subcutaneous administration of bergamot essential oil and linalool on formalin-induced nociceptive behavior in mice. Biomed. Res. 36 (1), 47–54. 10.2220/biomedres.36.47 [ DOI ] [ PubMed ] [ Google Scholar ]
- Khalid M. H., Akhtar M. N., Mohamad A. S., Perimal E. K., Akira A., Israf D. A., et al. (2011). Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: possible mechanisms. J. Ethnopharmacol 137 (1), 345–351. 10.1016/j.jep.2011.05.043 [ DOI ] [ PubMed ] [ Google Scholar ]
- Khodabakhsh P., Shafaroodi H., Asgarpanah J. (2015). Analgesic and anti-inflammatory activities of Citrus aurantium L. blossoms essential oil (neroli): involvement of the nitric oxide/cyclic-guanosine monophosphate pathway. J. Nat. Med. 69 (3), 324–331. 10.1007/s11418-015-0896-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- Komatsu T., Katsuyama S., Uezono Y., Sakurada C., Tsuzuki M., Hamamura K., et al. (2018a). Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 686, 127–132. 10.1016/j.neulet.2018.08.053 [ DOI ] [ PubMed ] [ Google Scholar ]
- Komatsu T., Katsuyama S., Uezono Y., Sakurada C., Tsuzuki M., Hamamura K., et al. (2018b). Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 686, 127–132. 10.1016/j.neulet.2018.08.053 [ DOI ] [ PubMed ] [ Google Scholar ]
- Koyama S., Heinbockel T. (2020). The effects of essential oils and terpenes in relation to their routes of intake and application. Int. J. Mol. Sci. 21 (5), 1558. 10.3390/ijms21051558 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kusunose N., Koyanagi S., Hamamura K., Matsunaga N., Yoshida M., Uchida T., et al. (2010). Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Mol. Pain 6, 83. 10.1186/1744-8069-6-83 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kuwahata H., Komatsu T., Katsuyama S., Corasaniti M. T., Bagetta G., Sakurada S., et al. (2013). Peripherally injected linalool and bergamot essential oil attenuate mechanical allodynia via inhibiting spinal ERK phosphorylation. Pharmacol. Biochem. Behav. 103 (4), 735–741. 10.1016/j.pbb.2012.11.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lakhan S. E., Sheafer H., Tepper D. (2016). The effectiveness of aromatherapy in reducing pain: a systematic review and meta-analysis. Pain Res. Treat. 2016, 8158693. 10.1155/2016/8158693 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee G., Park J., Kim M. S., Seol G. H., Min S. S. (2019). Analgesic effects of eucalyptus essential oil in mice. Korean J. Pain 32 (2), 79–86. 10.3344/kjp.2019.32.2.79 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li F. S., Weng J. K. (2017). Demystifying traditional herbal medicine with modern approach. Nat. Plants 3 (8), 17109. 10.1038/nplants.2017.109 [ DOI ] [ PubMed ] [ Google Scholar ]
- Li W., Chen Y., Wang X., Qu S. (1991). [Pharmacological studies on the volatile oil isolated from the leaves of Pinus pumila (Pall.) Regel]. Zhongguo Zhong Yao Za Zhi 16 (3), 172–192. [ PubMed ] [ Google Scholar ]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLos Med. 6, e1000100. 10.1371/journal.pmed.1000100 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lima G. M., Quintans-Júnior L. J., Thomazzi S. M., Almeida E. M. S. A., Melo M. S., Serafini M. R., et al. (2012). Phytochemical screening, antinociceptive and anti-inflammatory activities of Chrysopogon zizanioides essential oil. Braz. J. Pharmacognosy 22 (2), 443–450. 10.1590/S0102-695X2012005000002 [ DOI ] [ Google Scholar ]
- Linciano P., Citti C., Russo F., Tolomeo F., Laganà A., Capriotti A. L., et al. (2020). Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: cannabidihexol. Sci. Rep. 10 (1), 22019. 10.1038/s41598-020-79042-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lizarraga-Valderrama L. R. (2020). Effects of essential oils on central nervous system: focus on mental health. Phytother Res. [Epub ahead of print]. 10.1002/ptr.6854 [ DOI ] [ PubMed ] [ Google Scholar ]
- López V., Nielsen B., Solas M., Ramírez M. J., Jäger A. K. (2017). Exploring pharmacological mechanisms of lavender (lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 8, 280. 10.3389/fphar.2017.00280 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Macleod M. R., O’Collins T., Howells D. W., Donnan G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35 (5), 1203–1208. 10.1161/01.STR.0000125719.25853.20 [ DOI ] [ PubMed ] [ Google Scholar ]
- Mikolajewicz N., Komarova S. V. (2019). Meta-analytic methodology for basic research: a practical guide. Front. Physiol. 10, 203. 10.3389/fphys.2019.00203 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miraghazadeh S. G., Shafaroodi H., Asgarpanah J. (2015). Analgesic and antiinflammatory activities of the essential oil of the unique plant Zhumeria majdae. Nat. Prod. Commun. 10 (4), 669–672. 10.1590/s2175-97902019000217011 [ DOI ] [ PubMed ] [ Google Scholar ]
- Mishra D., Bisht G., Mazumdar P. M., Sah S. P. (2010). Chemical composition and analgesic activity of Senecio rufinervis essential oil. Pharm. Biol. 48 (11), 1297–1301. 10.3109/13880209.2010.491083 [ DOI ] [ PubMed ] [ Google Scholar ]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 6, e1000097. 10.1371/journal.pmed.1000097 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morrone L. A., Rombolà L., Pelle C., Corasaniti M. T., Zappettini S., Paudice P., et al. (2007). The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: implication of monoterpene hydrocarbons. Pharmacol. Res. 55 (4), 255–262. 10.1016/j.phrs.2006.11.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- Morrone L. A., Scuteri D., Rombolà L., Mizoguchi H., Bagetta G. (2017). Opioids resistance in chronic pain management. Curr. Neuropharmacol 15 (3), 444–456. 10.2174/1570159X14666161101092822 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Neves I. A., Rezende S. R. F., Kirk J. M., Pontes E. G., de Carvalho M., Gamble A. (2017). Composition and larvicidal activity of essential oil of Eugenia candolleana DC. (MYRTACEAE) against Aedes aegypti . Rev. Virtual Quim. 9 (6), 2305–2315. 10.21577/1984-6835.20170138 [ DOI ] [ Google Scholar ]
- Nogueira Lde. M., Da Silva M. R., Dos Santos S. M., De Albuquerque J. F., Ferraz I. C., de Albuquerque T. T., et al. (2015). Antinociceptive effect of the essential oil obtained from the leaves of croton cordiifolius baill. (Euphorbiaceae) in mice. Evid. Based Complement. Alternat Med. 2015, 620865. 10.1155/2015/620865 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park Y., Jung S. M., Yoo S. A., Kim W. U., Cho C. S., Park B. J., et al. (2015). Antinociceptive and anti-inflammatory effects of essential oil extracted from Chamaecyparis obtusa in mice. Int. Immunopharmacol 29 (2), 320–325. 10.1016/j.intimp.2015.10.034 [ DOI ] [ PubMed ] [ Google Scholar ]
- Perry N. S., Houghton P. J., Theobald A., Jenner P., Perry E. K. (2000). In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 52 (7), 895–902. 10.1211/0022357001774598 [ DOI ] [ PubMed ] [ Google Scholar ]
- Queiroz J. C., Antoniolli A. R., Quintans-Júnior L. J., Brito R. G., Barreto R. S., Costa E. V., et al. (2014). Evaluation of the anti-inflammatory and antinociceptive effects of the essential oil from leaves of xylopia laevigata in experimental models. Sci. World J. 2014, 816450. 10.1155/2014/816450 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Quintans J. S., Alves R. D., Santos D. A., Serafini M. R., Alves P. B., Costa E. V., et al. (2017). Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents. Z. Naturforsch C J. Biosci. 72 (3-4), 93–97. 10.1515/znc-2016-0053 [ DOI ] [ PubMed ] [ Google Scholar ]
- Quintans J. S., Antoniolli A. R., Almeida J. R., Santana-Filho V. J., Quintans-Júnior L. J. (2014). Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin. Pharmacol. Toxicol. 114 (6), 442–450. 10.1111/bcpt.12178 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ribeiro S. (2018). “Whole organisms or pure compounds? Entourage effect versus drug specificity,” in Plant medicines, healing and psychedelic science. Editors Labate B., Cavnar C. (Cham: Springer; ). [ Google Scholar ]
- Rice A. S. C., Morland R., Huang W., Currie G. L., Sena E. S., Macleod M. R. (2013). Transparency in the reporting of in vivo pre-clinical pain research: the relevance and implications of the ARRIVE (Animal Research: reporting in Vivo Experiments) guidelines. Scand. J. Pain 4 (2), 58–62. 10.1016/j.sjpain.2013.02.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- Rombolà L., Tridico L., Scuteri D., Sakurada T., Sakurada S., Mizoguchi H., et al. (2017). Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules 22 (4), 614. 10.3390/molecules22040614 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rombolà L., Scuteri D., Adornetto A., Straface M., Sakurada T., Sakurada S., et al. (2019). Anxiolytic-like effects of bergamot essential oil are insensitive to flumazenil in rats. Evid. Based Complement. Alternat Med. 2019 , 2156873. 10.1155/2019/2156873 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rombolà L., Scuteri D., Watanabe C., Sakurada S., Hamamura K., Sakurada T., et al. (2020). Role of 5-HT1A receptor in the anxiolytic-relaxant effects of bergamot essential oil in rodent. Int. J. Mol. Sci. 21 (7), 2597. 10.3390/ijms21072597 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ryan R. (2019). Cochrane Consumers and Communication Review Group: data synthesis and analysis. Available at: http://cccrg.cochrane.org (Accessed March 13, 2019).
- Sakurada T., Mizoguchi H., Kuwahata H., Katsuyama S., Komatsu T., Morrone L. A., et al. (2011). Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 97 (3), 436–443. 10.1016/j.pbb.2010.09.020 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sarmento-Neto J. F., do Nascimento L. G., Felipe C. F., de Sousa D. P. (2015). Analgesic potential of essential oils. Molecules 21 (1), E20. 10.3390/molecules21010020 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Savelev S., Okello E., Perry N. S., Wilkins R. M., Perry E. K. (2003). Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 75 (3), 661–668. 10.1016/s0091-3057(03)00125-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- Savelev S. U., Okello E. J., Perry E. K. (2004). Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 18 (4), 315–324. 10.1002/ptr.1451 [ DOI ] [ PubMed ] [ Google Scholar ]
- Scherder E. J., Sergeant J. A., Swaab D. F. (2003). Pain processing in dementia and its relation to neuropathology. Lancet Neurol. 2 (11), 677–686. 10.1016/s1474-4422(03)00556-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Morrone L. A., Rombolà L., Avato P. R., Bilia A. R., Corasaniti M. T., et al. (2017a). Aromatherapy and aromatic plants for the treatment of behavioural and psychological symptoms of dementia in patients with alzheimer's disease: clinical evidence and possible mechanisms. Evid. Based Complement. Alternat Med. 2017, 9416305. 10.1155/2017/9416305 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Piro B., Morrone L. A., Corasaniti M. T., Vulnera M., Bagetta G. (2017b). The need for better access to pain treatment: learning from drug consumption trends in the USA. Funct. Neurol. 22 (4), 229–230. 10.11138/fneur/2017.32.4.229 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Crudo M., Rombolà L., Watanabe C., Mizoguchi H., Sakurada S., et al. (2018a). Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 129, 20–24. 10.1016/j.fitote.2018.06.007 [ DOI ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Garreffa M. R., Esposito S., Bagetta G., Naturale M. D., Corasaniti M. T. (2018b). Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regen. Res. 13 (9), 1619–1621. 10.4103/1673-5374.237125 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Rombolà L., Morrone L. A., Bagetta G., Sakurada S., Sakurada T., et al. (2019a). Neuropharmacology of the neuropsychiatric symptoms of dementia and role of pain: essential oil of bergamot as a novel therapeutic approach. Int. J. Mol. Sci. 20 (13), 3327. 10.3390/ijms20133327 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Rombolá L., Tridico L., Mizoguchi H., Watanabe C., Sakurada T., et al. (2019b). Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain-related BPSDs. Curr. Med. Chem. 26 (20), 3764–3774. 10.2174/0929867325666180307115546 [ DOI ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Berliocchi L., Rombolà L., Morrone L. A., Tonin P., Bagetta G., et al. (2020a). Effects of aging on formalin-induced pain behavior and analgesic activity of gabapentin in C57BL/6 mice. Front. Pharmacol. 11, 663. 10.3389/fphar.2020.00663 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Mantovani E., Tamburin S., Sandrini G., Corasaniti M. T., Bagetta G., et al. (2020b). Opioids in post-stroke pain: a systematic review and meta-analysis. Front. Pharmacol. [Epub ahead of print]. 10.3389/fphar.2020.587050 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Matamala-Gomez M., Bottiroli S., Corasaniti M. T., De Icco R., Bagetta G., et al. (2020c). Pain assessment and treatment in dementia at the time of coronavirus disease COVID-19. Front. Neurol. 11, 890. 10.3389/fneur.2020.00890 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Rombolà L., Morrone L. A., Monteleone D., Corasaniti M. T., Sakurada T., et al. (2020d). “Exploitation of aromatherapy in dementia-impact on pain and neuropsychiatric symptoms,” in The neuroscience of dementia: diagnosis and management in dementia. Editors Preedy V. R., Martin C. R. (San Diego: Academic Press; ), 713–726. [ Google Scholar ]
- Scuteri D., Rombolà L., Watanabe C., Sakurada S., Corasaniti M. T., Bagetta G., et al. (2020e). Impact of nutraceuticals on glaucoma: a systematic review. Prog. Brain Res. 257, 141–154. 10.1016/bs.pbr.2020.07.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- Scuteri D., Vulnera M., Piro B., Bossio R. B., Morrone L. A., Sandrini G., et al. (2020f). Pattern of treatment of behavioural and psychological symptoms of dementia and pain: evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. Eur. J. Clin. Pharmacol. [Epub ahead of print]. 10.1007/s00228-020-02995-w [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sharif M., Najafizadeh P., Asgarpanah J., Mousavi Z. (2020). In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 56, e18357. 10.1590/s2175-97902019000418357 [ DOI ] [ Google Scholar ]
- Sofi P. A., Zeerak N. A., Singh P. (2009). Kala zeera (Bunium persicum Bioss.): a Kashmirian high value crop. Turkish J. Biol. 33, 249–258. 10.3906/biy-0803-18 [ DOI ] [ Google Scholar ]
- Stein C., Hopfeld J., Lau H., Klein J. (2015). Effects of ginkgo biloba extract EGb 761, donepezil and their combination on central cholinergic function in aged rats. J. Pharm. Pharm. Sci. 18 (4), 634–646. 10.18433/j3wc8v [ DOI ] [ PubMed ] [ Google Scholar ]
- Sterne J. A., Egger M. (2001). Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 54 (10), 1046–1055. 10.1016/s0895-4356(01)00377-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. 10.1136/bmj.l4898 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sulaiman M. R., Tengku Mohamad T. A., Shaik Mossadeq W. M., Moin S., Yusof M., Mokhtar A. F., et al. (2010). Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Med. 76 (2), 107–112. 10.1055/s-0029-1185950 [ DOI ] [ PubMed ] [ Google Scholar ]
- Suokas A. K., Sagar D. R., Mapp P. I., Chapman V., Walsh D. A. (2014). Design, study quality and evidence of analgesic efficacy in studies of drugs in models of OA pain: a systematic review and a meta-analysis. Osteoarthr Cartil 22 (9), 1207–1223. 10.1016/j.joca.2014.06.015 [ DOI ] [ PubMed ] [ Google Scholar ]
- Todorova M., Trendafilova A., Ivanova V., Danova K., Dimitrov D. (2017). Essential oil composition of Inula britannica L. from Bulgaria. Nat. Prod. Res. 31 (14), 1693–1696. 10.1080/14786419.2017.1285295 [ DOI ] [ PubMed ] [ Google Scholar ]
- Turk D. C., Dworkin R. H., Allen R. R., Bellamy N., Brandenburg N., Carr D. B., et al. (2003). Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106 (3), 337–345. 10.1016/j.pain.2003.08.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ulku Karabay-Yavasoglu N., Baykan S., Ozturk B., Apaydin S., Tuglular I. (2006). Evaluation of the antinociceptive and anti-inflammatory activities of Satureja thymbra. L. Essential oil. Pharm. Biol. 44 (8), 585–591. 10.1080/13880200600896827 [ DOI ] [ Google Scholar ]
- Venâncio A. M., Marchioro M., Estavam C. S., Melo M. S., Santana M. T., Onofre A. S. C., et al. (2011). Ocimum basilicum leaf essential oil and (-)-linalool reduce orofacial nociception in rodents: a behavioral and electrophysiological approach. Braz. J. Pharmacognosy 21 (6), 1043–1051. 10.1590/S0102-695X2011005000147 [ DOI ] [ Google Scholar ]
- Wood H. C., Reichut E. T. (1880). Note on the action upon the circulation of certain volatile oils. J. Physiol. 2, 446. 10.1113/jphysiol.1880.sp000073 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ximenes R. M., De Morais Nogueira L., Cassundé N. M., Jorge R. J., Dos Santos S. M., Magalhães L. P., et al. (2013). Antinociceptive and wound healing activities of Croton adamantinus Müll. Arg. essential oil. J. Nat. Med. 67 (4), 758–764. 10.1007/s11418-012-0740-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zarei M., Mohammadi S., Komaki A. (2018). Antinociceptive activity of Inula britannica L. and patuletin: in vivo and possible mechanisms studies. J. Ethnopharmacol 219, 351–358. 10.1016/j.jep.2018.03.021 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zaynoun S. T., Johnson B. E., Frain-Bell W. (1977). A study of oil of bergamot and its importance as a phototoxic agent. I. Characterization and quantification of the photoactive component. Br. J. Dermatol. 96 (5), 475–482. 10.1111/j.1365-2133.1977.tb07149.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Zendehdel M., Torabi Z., Hassanpour S. (2015). Antinociceptive mechanisms of Bunium persicum essential oil in the mouse writhing test: role of opioidergic and histaminergic systems. Veterinarni Medicina 60 (2), 63–70. 10.17221/7988-VETMED [ DOI ] [ Google Scholar ]
- Zhang L., Li D., Cao F., Xiao W., Zhao L., Ding G., et al. (2018). Identification of human acetylcholinesterase inhibitors from the constituents of EGb761 by modeling docking and molecular dynamics simulations. Comb. Chem. High Throughput Screen. 21 (1), 41–49. 10.2174/1386207320666171123201910 [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
REVIEW article
Essential oils: a systematic review on revolutionizing health, nutrition, and omics for optimal well-being.

- 1 NovaVita, Guayaquil, Ecuador
- 2 Laboratorio para Investigaciones Biomédicas, Facultad de Ciencias de la Vida, Escuela Superior Politécnica del Litoral (ESPOL), Guayaquil, Ecuador
Purpose: Essential oils from various plants have diverse therapeutic properties and are researched extensively. They have applications in medicine, aromatherapy, microbiology, agriculture, livestock, and the food industry, benefiting the population.
Methods: This systematic review followed the PRISMA verification protocol. The study focused on the anti-inflammatory effects, nutraceutical properties, antioxidant and antibacterial activity of essential oils in lemon, orange, cumin, cinnamon, coriander, rosemary, thyme, and parsley. We also looked at their presence in the diet, their effect, their mechanism of action on health, and the most important active compounds. The search was conducted in the PubMed database for the last 12 years of publications, including in vitro , in vivo , and online cell model tests.
Results: Essential oils have been shown to have multiple health benefits, primarily due to their antimicrobial and anti-inflammatory effects. The mechanism of action of cinnamon oil alters bacterial membranes, modifies lipid profiles, and inhibits cell division, giving a potential benefit in protection against colitis. On the other hand, a significant improvement was observed in the diastolic pressure of patients with metabolic syndrome when supplementing them with cumin essential oil. The antimicrobial properties of coriander essential oil, especially its application in seafood like tilapia, demonstrate efficacy in improving health and resistance to bacterial infections. Cumin essential oil treats inflammation. Parsley essential oil is an antioxidant. Orange peel oil is antibacterial, antifungal, antiparasitic, and pro-oxidative. Lemon essential oil affects mouse intestinal microbiota. Thyme essential oil protects the colon against damage and DNA methylation. Carnosic acid in rosemary oil can reduce prostate cancer cell viability by modifying the endoplasmic reticulum function.
Conclusion and discussion: Essential oils have many therapeutic and antiparasitic properties. They are beneficial to human health in many ways. However, to understand their potential benefits, more research is needed regarding essential oils such as coriander, parsley, rosemary, cumin, and thyme. These research gaps are relevant since they restrict understanding of the possible benefits of these crucial oils for health-related contexts.
1 Introduction
Essential oils (EOs) are fragrant extracts obtained from various plants. Their composition varies depending on the plant species from which they are extracted. It is estimated that more than 200 compounds may be present in these oils. In recent years, essential oils have gained significant popularity in various industries, such as aromatherapy, food flavoring, and natural pharmacological treatments, due to their numerous uses, primary components, and respective properties. Consequently, several applications have been studied, including their antimicrobial, anti-inflammatory, analgesic, and antioxidant properties ( 1 ).
The main bioactive compounds of EOs are terpenes and terpenoids, which are responsible for the biological activities mentioned above ( 2 ). So, the properties of EOs contribute to the prevention of diseases through different mechanisms of action. In vitro studies are generally carried out, and it has been shown that the anti-inflammatory components of EOs inhibit free radicals that can generate mutations alone. In the DNA. Likewise, when there is prolonged oxidative stress, an excessive accumulation of reactive oxygen species (ROS) can trigger chronic disorders such as metabolic syndrome, cardiovascular diseases, diabetes, and even cancer for the same reason that there are mutations in the DNA. EOs rich in polyphenols and their antioxidant properties act as therapeutic agents for these diseases ( 3 ).
Essential oils are used in healthcare to treat specific diseases or health conditions. They can be used to alleviate symptoms associated with conditions such as Alzheimer’s, cardiovascular diseases, sleep/stress disorders, and pain during childbirth ( 4 ). Research has shown that rosemary essential oil has potential anticholinesterase inhibitory and antioxidant effects that may help protect the brain from chronic anticholinesterase diseases such as Alzheimer’s. However, more studies are needed to determine the adverse effects or benefits. Additionally, aromatherapy has been found to improve cognitive function in patients with such conditions ( 5 ).
Antimicrobials are used for various purposes, including medical use as antiviral agents, immunomodulators, and antibiotics. They are also used as food preservatives due to their antimicrobial and antioxidant properties, which help counteract skin infections and, in addition to preventing food spoilage, are used to combat microorganisms that could be transmitted through food ( 4 ). According to Swamy et al. ( 6 ), lemon has antiviral properties against the influenza virus, while cinnamon is effective against enterobacteria.
Essential oils can affect bacteria differently, depending on their chemical components. Some oils can kill the bacteria (bactericidal action), while others can only slow their growth (bacteriostatic action). Essential oils can also affect cellular processes, such as nutrient processing, molecule synthesis, and regulation of biological processes between cells. Many plants with these characteristics are still being studied, and more findings are expected as they are widely used. These plants include cinnamon, thyme, rosemary, lemon, orange, cumin, and parsley ( 4 ). This systematic review identifies literature from the past 10 years to highlight their uses and effects on health, diet, microbiota, and mechanism of action.
This systematic review follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) verification protocol ( 7 ) with keywords in the search strategy as Essential oils, Nutrition, Omics, Bioactive Compounds, Well-being, properties, Application in Foods previously published experimental and/or clinical case trials in mouse models and/or cell lines and studies in human participants were used along with the use of search operators AND, OR. The study focuses on the anti-inflammatory effects, nutraceutical properties, antioxidant, and antibacterial activity, presence in food, effect and mechanism of action on health, and the most important active compounds of essential oils such as lemon, orange, cumin, cinnamon, coriander, rosemary, thyme, and parsley by searching for articles conducted in a cell line ( in vitro ), mouse model, in vivo specifically in PubMed database.
The study selection process was conducted in two stages. In the first stage, three authors independently reviewed the titles and abstracts of the studies based on the inclusion and exclusion criteria. They identified the studies that met the inclusion criteria and excluded the ones that did not. In the second stage, two authors thoroughly screened all the full articles and excluded the studies that did not meet the inclusion criteria. We developed custom search strategies for the PubMed bibliographic database. The search was conducted directly and included all articles published within the last 12 years without language restrictions. Additionally, we thoroughly examined the reference lists of the selected articles to identify any relevant research that may have been missed during the electronic database search. Three authors gathered the required data from the reports that had been selected. For all incorporated studies, the following details were recorded: author(s), year of publication, type of essential oil, its impact on health, its effects on health, and its presence in the diet. The authors independently reviewed all full articles.
3 Eligibility criteria
3.1 inclusion and exclusion criteria.
Studies were considered eligible if they met the following criteria: (1) retrospective experimental and clinical studies in human subjects and mouse models and/or cell lines, (2) studies investigating the health benefits of the essential oils described above, (3) studies with quantitative and qualitative data, (4) studies without any language restrictions, (5) systematic reviews, and (6) manuscripts in journals with an impact index.
The following studies were excluded: (1) not meeting the objectives of the article, (2) university theses, (3) not meeting the search criteria, (4) book chapters or books, (5) review articles published more than 12 years ago, and (6) studies not found in the database described above.
3.2 Search strategies
To refine the search, specific keywords such as “Essential oils” “Nutrition” “Omics” “Bioactive compounds” “Food application “Wellness” “properties” “mouse models ““experimental” “clinical” “cell lines” were used together with the use of search operators (Nutrition* OR “dietary” OR “nutritional* content” OR “macronutrients” OR “macronutrients” OR “diet”) AND (“Essential oils*” OR “volatile oils* “OR “Extracted oils* OR “Aromatherapy oils*” OR “Plant oils* “) AND (“Essential oils*” OR “Plant oils*” OR “macronutrients” OR “diet”) AND (“Essential oils*” OR “volatile oils* “OR “Extracted oils* Aromatherapy oils*” OR “Plant oils*) AND (Omics* OR “Microbiota*” OR “Genomics*” OR” Metabolomics*”) AND (Bioactive compounds* OR “Functional ingredient” OR “Major active compound *” OR “Active substance” OR “Health-promoting compound*”) AND (Food application * OR “Application in Food Safety “OR “Application in Food Industry”) AND (Wellness* OR “health “) AND (properties* OR “Aspects “OR “Traits”) performed in electronic databases such as Pubmed, ScienceDirect and Scopus.
4.1 Study selection
In the first phase of study selection, 107 citations were found in the PubMed electronic database. After thoroughly reviewing the abstracts, we excluded 27 articles with 12 duplicates. We then identified 85 additional articles through PubMed and reviewed their full text. Thus, this study included 80 references ( Figure 1 ).
Figure 1 . Flow diagram of literature search and selection criteria for essentials oils from PRISMA.
4.2 Study characteristics
The table below summarizes the key features of the studies analyzed in this research. These studies were conducted in different countries and published between 2011 and 2023 in both English and Spanish. It was observed that the number of publications increased significantly from 2019. All the selected studies were focused on essential oils, with most of them investigating the properties of these oils, such as their antimicrobial and anti-inflammatory effects. The studies have produced significant findings on the benefits of using essential oils, including inhibiting pathogenic microorganisms, preventing radical formation, acting as natural antioxidants and antimicrobial agents, exhibiting anticancer effects in the intestinal microbiota, and preserving food naturally.
Table 1 shows the type of food used, the analysis of the major active constituent identified, and relevant results in the essential oils performed in vivo or in vitro . The main groups of constituents were in cinnamon essential oil cinnamaldehyde, coriander essential oil β-linalool, camphor, geranyl acetate, and cymene; in cumin essential oil cumin aldehyde, and parsley essential oil myristicin, apiole, α-pinene, and β-pinene. Table 2 shows the most predominant bioactive compounds, their health effects, and tentative mechanisms. The main compounds in lemon and orange essential oils are limonene. In cinnamon essential oil, cinnamaldehyde; in Cilantro essential oil, linalool; in Parsley essential oil, myristicin; in Rosemary essential oil monoterpenes; in Thyme essential oil, thymol with antioxidant activity, antimicrobial, inflammatory activity, and innate immune responses.
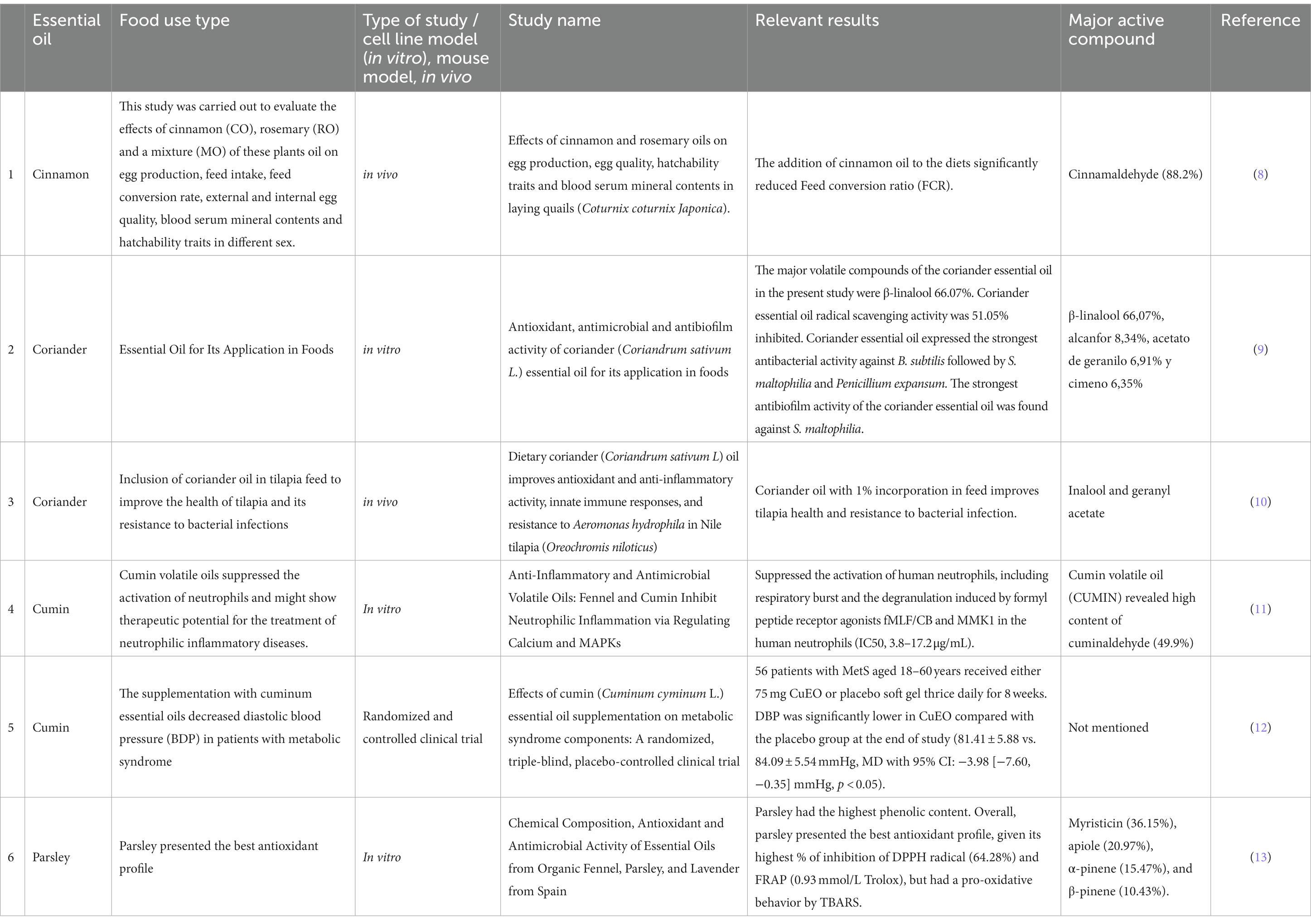
Table 1 . Essential oils and their used as food.
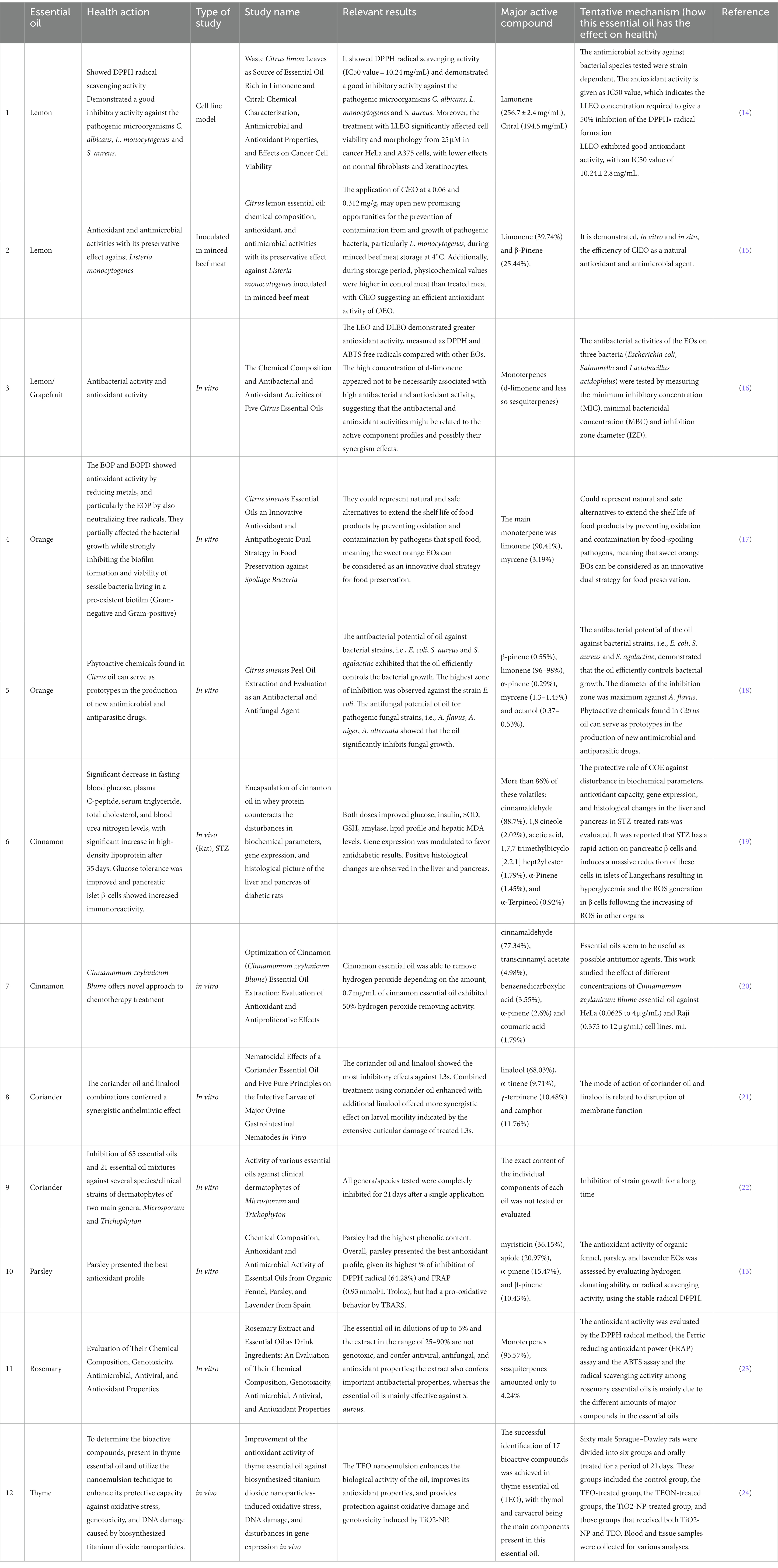
Table 2 . Actions in the health of the essential oils.
Table 2 shows how this essential oil affects health, the analysis of the major active constituent identified, and relevant results in the essential oils performed. The lemon essential oil has been found to contain a significant amount of limonene, which has strong antioxidant properties and can help eliminate DPPH radicals ( 16 ). Research has shown that it can also be effective as a food preservative, with concentrations of 0.06 and 0.312 mg/g potentially preventing the growth of pathogenic bacteria like L. monocytogenes ( 15 ). Limonene may also positively affect the immune system and intestinal microbiota ( 25 ). On the other hand, orange essential oil is also a natural citrus preservative in food due to its antimicrobial properties. Its main compounds, citral, and linalool, have been found to have superior effects on intestinal microbiota in mice when compared to lemon essential oil ( 17 ). It is important to note that further research is needed to fully understand the potential benefits of these essential oils in the areas mentioned. And findings regarding essential oils like parsley, cumin, coriander, thyme, and rosemary were relatively scarce, mostly showing effects on health. Mainly, parsley exhibits both antioxidant and prooxidative behavior, making it a promising subject for further investigation. Cumin, with volatile compounds inhibiting neutrophil activation, is strongly associated with treating inflammatory pathologies. Supplementation with cumin in patients with metabolic syndrome is estimated to decrease diastolic pressure ( 12 ). Coriander is known for its resistance to bacterial infections and tends to improve health in tilapia ( 10 ). Thyme demonstrated inhibition of cell proliferation, varying with the dose in all evaluated tumor cell lines ( 26 ).
Table 3 highlights the various omics mechanisms of the essential oils already mentioned above. The essential oils of lemon, orange, and cinnamon influence the intestinal microbiota in the same way the essential compounds of coriander inhibit the activity of gastrointestinal pathogenic bacteria. The essential compound of rosemary oil has an anti-Warburg effect on gastric carcinoma, dose, and time. And a thymol compound-dependent cytotoxic effect on cancer cell lines PC-3, DU145, MDA-MB-231, and KLN205.
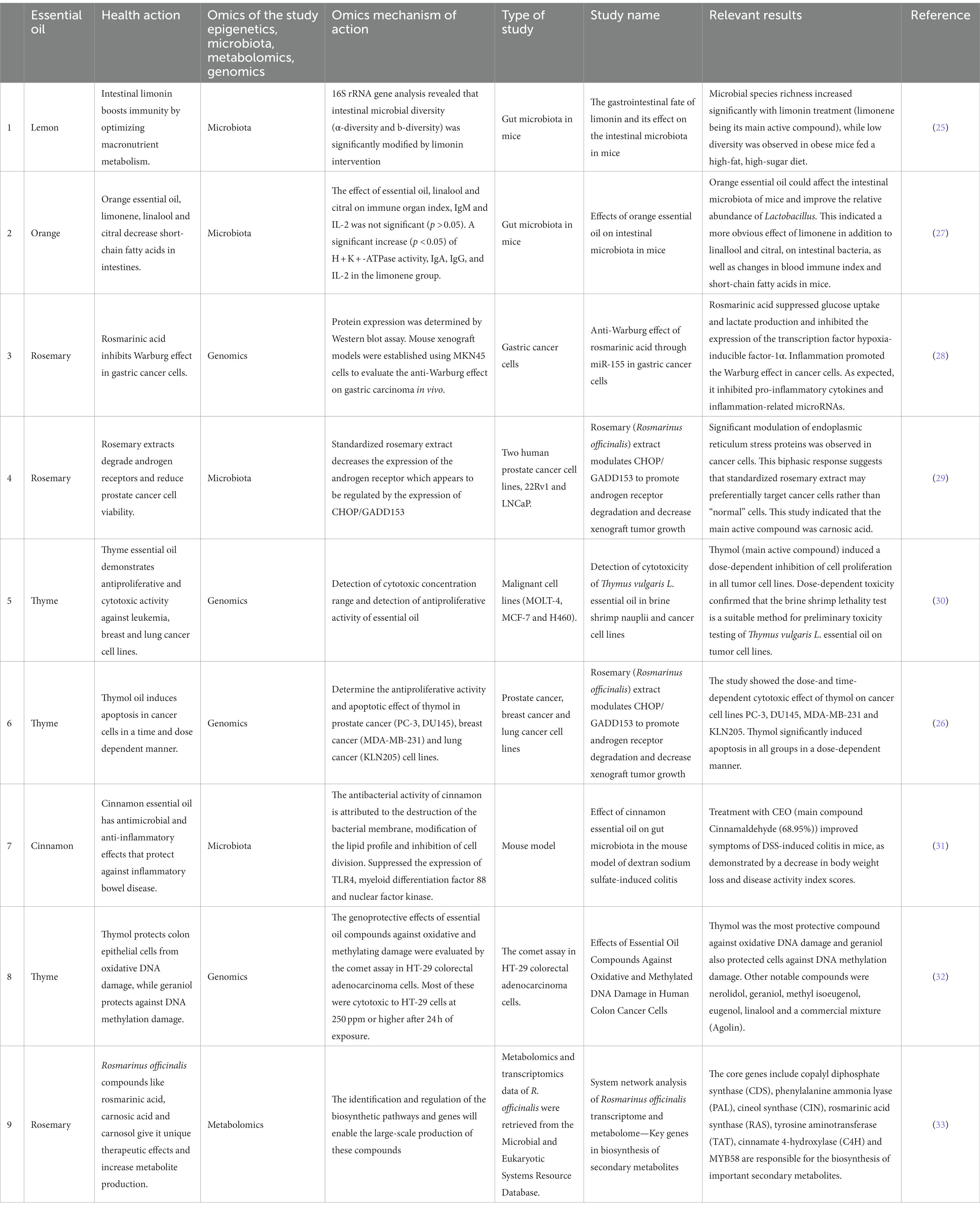
Table 3 . Essential oils and omics mechanism.
5.1 Health benefits
In a study by Mohammed et al. ( 19 ), six groups of male rats were treated orally for 4 weeks. The control and STZ-treated groups were compared with groups that received low or high doses of cinnamon oil emulsion (COE; 200 or 400 mg/kg Bw) and groups of STZ-treated rats that received COE in low or high doses. The results showed a significant decrease in fasting blood glucose levels, plasma C-peptide, serum triglycerides, total cholesterol, and blood urea nitrogen, with a substantial increase in high-density lipoproteins after 35 days. Glucose tolerance improved, and an increase in pancreatic islet β cells was observed. Both doses improved glucose, insulin, SOD, GSH, amylase, lipid profile, and hepatic MDA levels. Positive histological changes were also observed in the liver and pancreas. The current results revealed that cinnamon oil emulsion (COE) produced more than 1% of total volatile compounds, and GC/MS identified 16 compounds. More than 86% of these volatiles originated from 5 volatiles and included cinnamaldehyde (88.7%), 1,8 cineole (2.02%), acetic acid, 1,7,7trimethylbicyclo [2.2.1] heptyl ester (1.79%), α-Pinene (1.45%), and α-Terpineol (0.92%). This research aimed to examine the potential of essential oils as antitumor agents. In vitro models, including phosphomolybdenum, DPPH, and H2O2 methods, were used to achieve this. This study used BHT (butylhydroxytoluene) and ascorbic acid (vitamin C) as positive controls for comparison ( 20 ).
5.2 Food benefits
This review focuses on analyzing the effects of cinnamon oil when used as an additive in poultry feed, addressing its influence on various aspects such as bird performance, carcass characteristics, meat quality, its impact on cholesterol reduction, its antioxidant activity, its effects on immunity and considerations related to microbiology. The key results of this research indicate that including cinnamon essential oil extracts as additives in poultry feed carries notable benefits in terms of improved performance, reduced blood cholesterol levels, and increased activity: antioxidant, immunity booster, and favorable microbiological considerations. Cinnamon could represent a viable alternative to antibiotics, providing excellent safety in aspects related to animal health, the environment, and the economy in the poultry industry. Furthermore, it was observed that the main component of cinnamon oil is Cinnamaldehyde, which approximately represents Cinnamaldehyde (88.2%), eugenol (1.0%), and benzyl alcohol (8.0%) in its composition ( 34 ).

5.3 Actions in health
One of the potential health benefits is protection against colitis, which is an anti-inflammatory bowel disease. This is related to the mechanism of action, antimicrobial activity, and anti-inflammatory effect of cinnamon due to the alteration in the bacterial membrane, modifications in the lipid profile, and the inhibition of cell division, as shown in a mouse model study. Cinnamomum osmophloem reduced the expression of Toll-like receptor 4, myeloid adapter protein 88, and nuclear kinase in mice with colitis that had received endotoxin, suggesting an anti-inflammatory effect. In addition, it was identified that the predominant active component in cinnamon essential oil is cinnamaldehyde, which constitutes approximately 68.95% of its composition ( 31 ).
6.1 Health benefits
In a study conducted by Helal et al. ( 21 ) on the motility of third-instar larvae (L3) belonging to the Trichostrongylidaeindicate that these essential oils and their components, such as linalool (68.03%), α-tinene (9.71%), γ-terpinene (10.48%) and camphor (11.76%), could effectively combat infections caused by helminths.
Moreover, studies have shown that essential oils, including cilantro oil, can effectively hinder the growth of clinical strains of dermatophytes belonging to two primary genera, Microsporum and Trichophyton , for up to 21 days. It is worth noting that accurately identifying the specific individual is crucial ( 22 ).
6.2 Food benefits
The antioxidant, antimicrobial, and anti-biofilm properties of the essential oil obtained from cilantro ( Coriandrum sativum L. ) are the most studied concerning its possible application in food. The main volatile components identified in coriander essential oil in an in vitro study were mainly β-linalool, which accounted for 66.07% of the total content, showing a high antioxidant activity with an inhibition percentage of 51.05% in eliminating radicals. Its antibacterial activity presented the most effective against B. subtilis, followed by S. maltophilia and Penicillium expansum ( 9 ). An in vivo study assessed the effectiveness of adding 1% coriander oil to tilapia feed to enhance health and immunity against bacterial infections. The key compounds in this oil were linalool and geranyl acetate ( 10 ).
7.1 Health benefits
Recent studies have shown that cumin oil can potentially suppress neutrophil activation, which could be beneficial in treating inflammatory diseases that involve high levels of neutrophil activity. This includes respiratory burst and degranulation induced by formylpeptide receptor agonists fMLF/CB and MMK1 in human neutrophils. These effects’ mean inhibitory concentration (IC50) ranges from 3.8 to 17.2 μg/mL. Additionally, it has been noted that cumin oil contains a high percentage of cumin aldehyde, which makes up 49.9% of its composition ( 11 ). Morovati et al. ( 12 ) found that cumin essential oil significantly reduced diastolic blood pressure in patients with metabolic syndrome after 8 weeks of treatment.
8.1 Health benefits
Parsley essential oil exhibits an antioxidant profile due to its higher percentage of DPPH radical inhibition and FRAP value; however, it also showed pro-oxidative behavior according to the TBARS test. On the other hand, parsley essential oil demonstrates more significant bacterial activity after lavender. The main components were myristicin (36.15%), apiole (20.97%), α-pinene (15.47%), and β-pinene (10.43%). The presence of alkyltetramethoxybenzene, limonene and elemicin (6.45, 4.74 and 2.74%, respectively) was also relevant ( 13 ).
9.1 Health benefits
It has been shown that the essential oil obtained by hydrodistillation of discarded lemon leaves contains enough chemicals can inhibit the growth of harmful microorganisms such as C. albicans, L. monocytogenes , and S. aureus . The oil’s most abundant components are limonene (with a concentration of 260.7 mg/mL), followed by geranial (102.6 mg/mL) and neral (88.3 mg/mL). The study also found that a concentration of 25 μM of the oil led to a significant reduction in cell viability, with a 33% decrease in HeLa cells and a 27% decrease in A375 cells. This was also accompanied by notable changes in cellular morphology ( 14 ).
The main components of lemon essential oil responsible for its antibacterial and antioxidant properties are terpenoids, with d-limonene being the most abundant. D-limonene exhibits the highest antioxidant capacity and effectively removes DPPH radicals ( 16 ).
9.2 Food benefits
In the food industry, lemon essential oil’s antioxidant and antimicrobial properties can be used as a preservative. According to Ben Hsouna et al. ( 15 ), the application of this oil at concentrations of 0.06 and 0.312 mg/g presents promising potential for the prevention of contamination and the development of pathogenic bacteria, especially L. monocytogenes , which opens new perspectives in this field.
9.3 Action in health
The effect of limonin on the intestinal microbiota has been investigated in mice, and a significant increase in the diversity of the microbiota present in the colon of mice fed limonin has been observed. Thus, it was highlighted that the composition of the intestinal microbiota community was different compared to the control group. A prediction was made that limonin would positively affect the regulation of amino acid metabolism, lipids, and immune system function. It is highlighted that it can significantly suppress diseases related to the immune system and markers of infectious diseases based on its influence on the intestine ( 25 ).
10.1 Health benefits
The Citrus sinensis peel oil has potential antibacterial, antifungal, and antiparasitic effects, according to a study conducted by Anwar et al. ( 18 ). The analysis showed that the maximum inhibition zone diameter was 14 mm against E. coli , and the minimum was 10 mm against S. agalactiae . Moreover, the oil was 60% effective in inhibiting leishmaniasis at a 50 μg/mL concentration after 48 h of incubation. The oil’s antimicrobial properties were also demonstrated, suggesting its possible use as a natural food preservative or an effective treatment against various pathogenic organisms. The oil contains β-pinene (0.55%), limonene (96–98%), α-pinene (0.29%), myrcene (1.3–1.45%), and octanol (0.37–0.53%).
10.2 Food benefits
A study assessed commercial orange essential oils’ chemical compositions, antioxidant, and anti-pathogenic properties [ Citrus sinensis (L.) Osbeck ]. The study used cold pressing (EOP) and cold pressing followed by steam distillation (EOPD). The analysis revealed that both essential oils contained a high percentage of monoterpene hydrocarbons, mainly limonene (89.8 to 90.4%) and myrcene (3.1 to 3.2%). Although both essential oils had similar reducing capacities, EOP showed a more remarkable ability to eliminate free radicals. Regarding anti-pathogenic properties, both essential oils inhibited the biomass and cellular viability of Staphylococcus aureus and Pseudomonas aeruginosa in their biofilms. Additionally, both methods effectively reduced the production of elastase, pyocyanin, and quorum-sensing autoinducers, particularly in Gram-negative bacteria. These findings indicate that EOP and EOPD demonstrate significant antioxidant and anti-pathogenic properties ( 17 ).
10.3 Action in health
A recent study examined the effects of administering orange essential oil, as well as limonene, linalool, and citral, directly into the stomachs of mice. The researchers were interested in understanding how these substances might affect the mice’s intestinal microbiota and biochemical parameters. The study found that all four substances could influence the mice’s intestinal microbiota composition, with the relative proportion of Lactobacillus increasing in response to treatment. However, the mice that received limonene had a notably different bacterial composition in their cecum and colon than the other groups. These findings suggest that limonene may have a more pronounced effect on intestinal bacteria, which can lead to significant changes in blood immunological markers and short-chain fatty acid levels in mice ( 27 ).
11.1 Health benefits
Thyme essential oil (TEO) has health benefits due to its bioactive compounds. TEO nanoemulsion improves its biological activity and antioxidant properties and protects against oxidative damage and genotoxicity caused by TiO2-NP. TEO contains 17 bioactive compounds, with thymol and carvacrol being the main components ( 24 ).
11.2 Action in health
Thymol has been found to impact prostate cancer (PC-3), breast cancer (MDA-MB-231), and lung cancer cells. The antiproliferative activity of cancer cells varies depending on the dose and time of exposure. Thymol also significantly induces apoptosis in all groups, with the intensity of the response varying depending on the dose administered ( 26 ).
Likewise, the antiproliferative activity has been evaluated ( in vitro ) using three human tumor cell lines: MCF-7 (breast adenocarcinoma), H460 (lung carcinoma), and MOLT-4 (acute lymphoblastic leukemia) using the MTT assay, demonstrating a dose-dependent inhibition of cell proliferation in all tumor cell lines evaluated, and differential sensitivity between them. The main components of the essential oil included thymol (36.7%), p-cymene (30.0%), γ-terpinene (9.0%), and carvacrol (3.6%) ( 30 ).
Understanding oils’ effects on our health is crucial, particularly in preventing cellular toxicity and genotoxicity. A recent study found that essential oil compounds can offer protective benefits against oxidative and methylating damage, as seen through comet assays on colorectal adenocarcinoma HT-29 cells. While most of these compounds were found to be cytotoxic to HT-29 cells, they only reached cytotoxic levels at doses equal to or greater than 250 ppm after exposure for 24 h. The study identified thymol as the most effective component in protecting DNA against oxidative damage, while geraniol also showed promise in protecting against DNA methylation damage. This research highlights the potential of essential oil compounds, especially thymol, in protecting the colonic epithelium against oxidative DNA damage and geraniol against DNA methylation damage ( 32 ).
12 Rosemary
12.1 health benefits.
A study by Christopoulou et al. ( 23 ) assessed the chemical composition, genotoxicity, and antimicrobial, antiviral, and antioxidant properties of certain substances. The results showed that the essential oil, at concentrations of up to 5%, and the extract, ranging from 25 to 90%, did not exhibit any genotoxic effects. The essential oil and the extract also demonstrated antiviral, antifungal, and antioxidant properties. Specifically, the extract exhibited notable antibacterial properties, while the essential oil was primarily effective against S. aureus . The essential oil mainly comprised monoterpenes, constituting 95.57%, whereas sesquiterpenes only represented 4.24%.
12.2 Action in health
A study on rosmarinic acid (RA) and the anti-Warburg effect in gastric carcinoma suggested that rosemary oil may reduce glucose uptake and lactate production in cancer cells. It was found that RA inhibits the expression of hypoxia-inducible factor 1α, which is involved in the glycolytic pathway. In cancer cells, inflammation promotes the Warburg effect. However, RA reduces the production of pro-inflammatory cytokines and inflammation-related microRNAs, which suggests that RA could suppress the Warburg effect through an inflammatory pathway related to interleukin (IL)-6 and the transcription factor STAT3 ( 28 ).
Using essential oil to improve the functioning of the endoplasmic reticulum can be a helpful health action in reducing the viability of prostate cancer cells and promoting the degradation of androgen receptors. A study evaluated the effects of rosemary extract, standardized in carnosic acid, on two types of human prostate cancer cells, 22Rv1 and LNCaP, and prostate epithelial cells collected from two patients undergoing radical prostatectomy. The study found that cancer cells significantly altered endoplasmic reticulum stress proteins, while normal prostate epithelial cells did not suffer endoplasmic reticulum stress. This two-stage response suggests that standardized rosemary extract might be a preferable treatment option for cancer cells rather than normal cells.
On the other hand, some results suggest that the identified core genes, such as copalyl diphosphate synthase, phenylalanine ammonia-lyase, cineole synthase, rosmarinic acid synthase, tyrosine aminotransferase, cinnamate 4-hydroxylase, and MYB58, could play a crucial role in the metabolism of Rosmarinus officinalis . This genetic analysis provides valuable information for genetic and metabolic engineering research to improve the biosynthesis of secondary metabolites in Rosmarinus officinalis ( 33 ).
13 Discussion
This review emphasizes the significant advancements of essential oils in various fields, such as medicine and the food industry, achieved through in vitro , in vivo , and cell line studies. These breakthroughs provide valuable insights that support the continuation of research in these areas. For instance, the study evaluates the impact of rosemary essential oil extract on reducing prostate cancer cells ( 28 ). Moreover, the effects of cinnamon oil as a food additive for poultry are examined. The study explores its influences on avian performance, meat quality, cholesterol reduction, antioxidant activity, and immunity ( 35 ).
Insufficient literature exists on essential oils concerning innovative omics action mechanisms, more relevant applications in food to ensure quality, and their benefits and impact on health. Therefore, it is recommended that future research should conduct studies using cell lines ( in vivo ). This approach promotes more natural additives, thereby reducing the intake of processed additives that may adversely affect human health in the long run. These limitations are significant as they restrict understanding of essential oils’ potential benefits to improve the quality of life and address specific health-related needs in the food industry.
The potential health benefits of rosemary oil have been extensively studied and documented. Compounds included in rosemary oil, notably oleic and linoleic acid, are known to benefit cardiovascular health ( 36 ). Evidence is limited for the effects of rosemary oil on glucose. Further research, with more rigorous experimental designs and in larger populations, is required to reach more definitive conclusions about these properties ( 35 , 37 – 39 ). One of the main factors contributing to rosemary oil’s potential usage in pharmacological and biotechnological applications is its biological qualities ( 8 , 40 ). For example, natural antioxidants such as rosemary extracts have shown high thermal resistance and superior antioxidant activity compared to synthetic antioxidants ( 41 ). This makes them beneficial for preserving the quality of oils, particularly in high-heat cooking processes like frying ( 42 , 43 ). Beyond acting as food preservatives, rosemary oil components may have direct health advantages. The oil’s anti-inflammatory effects may also promote brain health by preventing neuronal cell damage ( 44 – 46 ). Ongoing research reveals rosemary oil’s multipronged therapeutic potential—from enhancing heart health to shielding brain function to inhibiting tumor growth ( 47 – 49 ).
Several studies have been conducted that provide convincing evidence of the usefulness of cinnamon essential oil, but most are in vitro or animal research. More large-scale, high-quality human clinical trials are still needed to confirm the effects, establish the optimal dose, assess long-term safety, etc. Mechanistic studies have elucidated certain pathways, such as modulation of glucose transporters, antioxidant enzymes and inflammatory markers that underlie the observed effects. However, further work is needed to elucidate the full pharmacokinetic and pharmacodynamic profile, especially of key actives such as cinnamaldehyde ( 50 – 53 ).
Most research has focused specifically on cinnamon essential oil or cinnamaldehyde-standardized bark extracts. Comparisons of potency, bioavailability, and synergies between components require further research. Despite promising gastrointestinal effects, more extensive studies through rigorous randomized controlled trials on outcomes such as ulcerative colitis, irritable bowel syndrome, and dysbiosis are needed before clinical use can be recommended ( 54 , 55 ). Safety, drug interactions, and contraindications have yet to be fully established.
Some studies demonstrate the efficacy of coriander oil against organisms such as fungi and helminths and agree that linalool e is the chemically essential compound ( 56 ). However, other chemotypes and oil compositions should also be examined, e.g., those richer in decanal, borneol, or geranyl acetate for which these comparative studies would demonstrate bioactivity that may reveal differential effects ( 57 , 58 ). In addition, more detailed analyses, especially in food matrices exploring lipid peroxidation, protein oxidation, effects on shelf life, etc., would provide additional information along with limited safety studies ( 59 – 61 ). Toxicity, pharmacokinetic, and residue level studies in food-producing animals could allow the administration of higher standardized doses to enhance health effects ( 62 – 65 ). Finally, few in vivo studies explore the bioavailability, metabolism, and excretion of key actives such as linalool and the impact of long-term repeated dosing. Such pharmacokinetic data can help correlate in vitro and clinical results ( 64 , 66 ).
Finally, lemon essential oil, the main component, D-limonene, boosts antimicrobial and antioxidant effects ( 15 , 67 , 68 ). However, further studies are needed on possible synergies with other components such as geranial ( 69 , 70 ). In addition, rigorous human clinical trials are required to validate efficacy as a natural food preservative and to determine optimal dosage ( 71 – 73 ). The effect on the gut microbiome is promising, but it is not yet clear what specific changes in bacterial composition drive the observed health outcomes ( 68 , 74 ).
14 Conclusion
Essential oils extracted from plants have been valued for their medicinal properties for centuries. Modern scientific research is now unraveling the composition and bioactivity of these complex natural extracts, providing insights into how essential oils might be used to help promote health and well-being. Based on the evidence summarized in the provided documents, several key conclusions regarding cinnamon, cilantro, cumin, parsley, lemon, orange, thyme, and rosemary essential oils can be drawn.
Firstly, these essential oils demonstrate varying degrees of antioxidant, antimicrobial, anti-inflammatory, or other beneficial biological effects. These effects are mediated by bioactive phytochemicals in the oils, such as cinnamaldehyde, linalool, limonene, thymol, and carnosic acid. The impacts of oils depend not just on their chemical composition but also on factors like genetics and growth conditions of the source plants. Standardization and quality control are thus important when studying and applying essential oil preparations. Secondly, many oils show promise as natural food preservatives—for example, inhibiting foodborne pathogens like Escherichia coli and Staphylococcus aureus . If efficacious and safe, plant-derived antimicrobials could replace synthetic additives. Areas needing more research include determining effects on food quality and nutritional content during storage.
Some of the main health benefits supported by current evidence are cinnamon oil improving glucose control and blood lipid levels; cilantro oil having parasite-killing properties; cumin oil reducing blood pressure; thyme oil protecting against oxidative cell damage and DNA mutations; and rosemary and thyme oils inhibiting cancer cell proliferation and viability. Further mechanisms of action are being elucidated—for instance, rosemary compounds may suppress altered metabolic pathways in tumor cells. More clinical trials are warranted to verify therapeutic efficacy and safety. An emerging area of research is how essential oil components like limonene and thymol influence intestinal microbiota populations.
So, essential oils are promising candidates for health promotion and disease treatment. However, converting traditional claims into evidence-based applications requires meticulous methodology. Key priorities in the future are clinical evaluations demonstrating efficacy, standardization, and quality control of oil preparations, untangling oils’ mechanisms of action, and further analyzing effects on human microbiota. With rigorous science illuminating their real therapeutic potential, essential oils could reveal themselves to be far more than just pleasant natural scents.
14.1 Limitations
This review is limited by the variability in the chemical composition of essential oils since these can vary according to the species/variety of the plant, growing conditions, time of harvest, extraction method, etc., making standardization and generalization of results difficult. Likewise, there is little information on the absorption, distribution, metabolism, and excretion (pharmacokinetics) of the components of essential oils in humans, and finally, there is not enough scientific evidence on interactions of essential oils with medications, nutrients, and other compounds - nutrient and drug interactions. For data analysis, the use of Cochrane tools to assess the risk of bias in randomized controlled trials, while for observational studies, the quality assessment tool of the US National Institutes of Health was not applied to the results since the included studies are in vitro .
Author contributions
CP-O: Conceptualization, Validation, Data curation, Investigation, Writing – original draft. FG: Conceptualization, Data curation, Investigation, Writing – original draft, Formal analysis, Methodology, Software. CM: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JM: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. AO-M: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing, Conceptualization, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by NovaVita S.A. grants for research. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors would like to express their gratitude toward Mary Young and Tamara Packer for their interest in the research and support.
Conflict of interest
CP-O and JLM were employed by company NovaVita.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Aziz, ZA, Abdul, AA, Setapar, SHM, Karakucuk, A, Azim, MM, et al. Essential oils: extraction techniques, pharmaceutical and therapeutic potential - a review. Curr Drug Metab . (2018) 19:1100–10. doi: 10.2174/1389200219666180723144850
Crossref Full Text | Google Scholar
2. Masyita, A, Sari, RM, Astuti, AD, Yasir, B, Rumata, NR, Emran, TB, et al. Terpenes and Terpenoids as Main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chemistry: X . (2022) 13:100217. doi: 10.1016/J.FOCHX.2022.100217
PubMed Abstract | Crossref Full Text | Google Scholar
3. Bunse, M, Daniels, R, Gründemann, C, Heilmann, J, Kammerer, DR, Keusgen, M, et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front Pharmacol . (2022) 13:956541. doi: 10.3389/fphar.2022.956541
4. Ramsey, JT, Shropshire, BC, Nagy, TR, Chambers, KD, Li, Y, and Korach, KS. Essential oils and health. Yale J. Biol. Med . 93, 291–305.
PubMed Abstract | Google Scholar
5. Benny, A, and Thomas, J. Essential oils as treatment strategy for Alzheimer’s disease: current and future perspectives. Planta Med . (2019) 85:239–48. doi: 10.1055/A-0758-0188
6. Swamy, MK, Akhtar, MS, and Sinniah, UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med . (2016) 2016:1–21. doi: 10.1155/2016/3012462
7. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev . (2021) 10:1–11. doi: 10.1186/S13643-021-01626-4/FIGURES/1
8. Aludatt, MH, Rababah, T, Alhamad, MN, Gammoh, S, Al-Mahasneh, MA, Tranchant, CC, et al. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: thyme, spearmint, and rosemary. Therapeutic, Probiotic, and Unconventional Foods . (2018):275–90. doi: 10.1016/B978-0-12-814625-5.00014-5
9. Kačániová, M, Galovičová, L, Ivanišová, E, Vukovic, NL, Štefániková, J, Valková, V, et al. Antioxidant, antimicrobial and Antibiofilm activity of coriander ( Coriandrum Sativum L.) essential oil for its application in foods. Food Secur . (2020, 2020) 9:282. doi: 10.3390/FOODS9030282
10. Das, S, Pradhan, C, and Pillai, D. Dietary coriander ( Coriandrum Sativum L) oil improves antioxidant and anti-inflammatory activity, innate immune responses and resistance to Aeromonas Hydrophila in Nile Tilapia ( Oreochromis Niloticus ). Fish Shellfish Immunol . (2023) 132:108486. doi: 10.1016/J.FSI.2022.108486
11. Korinek, M, Handoussa, H, Tsai, YH, Chen, YY, Chen, MH, Chiou, ZW, et al. Anti-inflammatory and antimicrobial volatile oils: fennel and cumin inhibit neutrophilic inflammation via regulating calcium and MAPKs. Front Pharmacol . (2021) 12:1–20. doi: 10.3389/FPHAR.2021.674095/FULL
12. Morovati, A, Gargari, BP, and Sarbakhsh, P. Effects of cumin ( Cuminum Cyminum L.) essential oil supplementation on metabolic syndrome components: a randomized, triple-blind, placebo-controlled clinical trial. Phytotherapy Res: PTR . (2019) 33:3261–9. doi: 10.1002/PTR.6500
13. Marín, I, Sayas-Barberá, E, Viuda-Martos, M, Navarro, C, and Sendra, E. Chemical composition, antioxidant and antimicrobial activity of essential oils from organic fennel, parsley, and lavender from Spain. Food Secur . (2016) 5:1–18. doi: 10.3390/FOODS5010018
14. Petretto, GL, Vacca, G, Addis, R, Pintore, G, Nieddu, M, Piras, F, et al. Waste Citrus Limon leaves as source of essential oil rich in limonene and Citral: chemical characterization, antimicrobial and antioxidant properties, and effects on Cancer cell viability. Antioxidants (Basel, Switzerland) . (2023) 12:1238. doi: 10.3390/ANTIOX12061238
15. Hsouna, AB, Halima, NB, Smaoui, S, and Hamdi, N. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria Monocytogenes inoculated in minced beef meat. Lipids Health Dis . (2017) 16:1–11. doi: 10.1186/S12944-017-0487-5
16. Li, Y, Liu, S, Zhao, C, Zhang, Z, Nie, D, Tang, W, et al. The chemical composition and antibacterial and antioxidant activities of five Citrus essential oils. Molecules (Basel, Switzerland) . (2022) 27:1–14. doi: 10.3390/MOLECULES27207044
17. Manzur, M, Luciardi, MC, Amparo Blázquez, M, Alberto, MR, Cartagena, E, and Arena, ME. Citrus Sinensis essential oils an innovative antioxidant and Antipathogenic dual strategy in food preservation against Spoliage Bacteria. Antioxidants (Basel, Switzerland) . (2023) 12:246. doi: 10.3390/ANTIOX12020246
18. Anwar, T, Qureshi, H, Fatima, A, Sattar, K, Albasher, G, Kamal, A, et al. Citrus Sinensis Peel oil extraction and evaluation as an antibacterial and antifungal agent. Microorganisms . (2023) 11:1662. doi: 10.3390/MICROORGANISMS11071662
19. Mohammed, KAA, Ahmed, HMS, Sharaf, HA, El-Nekeety, AA, Abdel-Aziem, SH, Mehaya, FM, et al. Encapsulation of cinnamon oil in whey protein counteracts the disturbances in biochemical parameters, gene expression, and histological picture of the liver and pancreas of diabetic rats. Environ Sci Pollut Res . (2020) 27:2829–43. doi: 10.1007/S11356-019-07164-W/FIGURES/3
20. Kallel, I, Hadrich, B, Gargouri, B, Chaabane, A, Lassoued, S, Gdoura, R, et al. Optimization of cinnamon ( Cinnamomum Zeylanicum Blume) essential oil extraction: evaluation of antioxidant and Antiproliferative effects. Evid Based Complement Alternat Med . (2019) 2019:1–11. doi: 10.1155/2019/6498347
21. Helal, MA, Abdel-Gawad, AM, Kandil, OM, Khalifa, MME, Cave, GWV, Morrison, AA, et al. Nematocidal effects of a coriander essential oil and five pure principles on the infective larvae of major ovine gastrointestinal nematodes in vitro. Pathogens . (2020) 9:740. doi: 10.3390/PATHOGENS9090740
22. Parrish, N, Fisher, SL, Gartling, A, Craig, D, Boire, N, Khuvis, J, et al. Activity of various essential oils against clinical dermatophytes of Microsporum and Trichophyton. Front Cell Infect Microbiol . (2020) 10:1–11. doi: 10.3389/FCIMB.2020.545913
23. Christopoulou, SD, Androutsopoulou, C, Hahalis, P, Kotsalou, C, Vantarakis, A, and Lamari, FN. Rosemary extract and essential oil as drink ingredients: an evaluation of their chemical composition, genotoxicity, antimicrobial, antiviral, and antioxidant properties. Foods (Basel, Switzerland) . (2021) 10:143. doi: 10.3390/FOODS10123143
24. Sallam, MF, Ahmed, HMS, Diab, KA, El-Nekeety, AA, Abdel-Aziem, SH, Sharaf, HA, et al. Improvement of the antioxidant activity of thyme essential oil against biosynthesized titanium dioxide nanoparticles-induced oxidative stress, DNA damage, and disturbances in gene expression in vivo. J Trace Elements in Med Biol: Organ of the Society for Minerals and Trace Elements (GMS) . (2022) 73:127024. doi: 10.1016/J.JTEMB.2022.127024
25. Gu, M, Sun, J, Qi, C, Cai, X, Goulette, T, Song, M, et al. The gastrointestinal fate of Limonin and its effect on gut microbiota in mice. Food Funct . (2019) 10:5521–30. doi: 10.1039/C9FO01274E
26. Elbe, E, Yigitturk, G, Cavusoglu, T, and Uyanikgil, Y. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of Cancer. Bratisl Lek Listy . (2020) 121:122–8. doi: 10.4149/BLL_2020_016
27. Wang, L, Zhang, Y, Fan, G, Ren, JN, Zhang, LL, and Pan, SY. Effects of Orange essential oil on intestinal microflora in mice. J Sci Food Agric . (2019) 99:4019–28. doi: 10.1002/JSFA.9629
28. Han, S, Yang, S, Cai, Z, Pan, D, Li, Z, Huang, Z, et al. Anti-Warburg effect of Rosmarinic acid via MiR-155 in gastric Cancer cells. Drug Des Devel Ther . (2015) 9:2695–703. doi: 10.2147/DDDT.S82342
29. Petiwala, SM, Berhe, S, Li, G, Puthenveetil, AG, Rahman, O, Nonn, L, et al. Rosemary ( Rosmarinus Officinalis ) extract modulates CHOP/GADD153 to promote androgen receptor degradation and decreases xenograft tumor growth. PloS One . (2014) 9:e89772. doi: 10.1371/JOURNAL.PONE.0089772
30. Niksic, H, Becic, F, Koric, E, Gusic, I, Omeragic, E, Muratovic, S, et al. Cytotoxicity screening of Thymus Vulgaris L. essential oil in brine shrimp Nauplii and Cancer cell lines. Sci Rep . (2021) 11:1–9. doi: 10.1038/S41598-021-92679-X
31. Li, A, Ni, WW, Zhang, QM, Li, Y, Zhang, X, Wu, HY, et al. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol . (2020) 64:23–32. doi: 10.1111/1348-0421.12749
32. Thapa, D, Richardson, AJ, Béatrice Zweifel, R, Wallace, J, and Gratz, SW. Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human Colon Cancer cells. J Food Sci . (2019) 84:1979–85. doi: 10.1111/1750-3841.14665
33. Moghadam, A, Foroozan, E, Tahmasebi, A, Taghizadeh, MS, Bolhassani, M, and Jafari, M. System network analysis of Rosmarinus Officinalis transcriptome and metabolome—key genes in biosynthesis of secondary metabolites. PloS One . (2023) 18:3. doi: 10.1371/JOURNAL.PONE.0282316
34. El-Hack, A, Mohamed, E, Alagawany, M, Abdel-Moneim, AME, Mohammed, NG, Khafaga, AF, et al. Cinnamon ( Cinnamomum Zeylanicum ) oil as a potential alternative to antibiotics in poultry. Antibiotics . (2020, 2020) 9:210. doi: 10.3390/ANTIBIOTICS9050210
35. Abdellatief, SA, Beheiry, RR, and El-Mandrawy, SAM. Peppermint essential oil alleviates hyperglycemia caused by Streptozotocin-nicotinamide-induced type 2 diabetes in rats. Biomed Pharmacother . (2017) 95:990–9. doi: 10.1016/J.BIOPHA.2017.09.020
36. Lahlou, S, Figueiredo, AF, Magalhães, PJC, and Leal-Cardoso, JH. Cardiovascular effects of 1,8-cineole, a Terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol . (2002) 80:1125–31. doi: 10.1139/Y02-142
37. Bungau, SG, Vesa, CM, Bustea, C, Purza, AL, Tit, DM, Brisc, MC, et al. Antioxidant and hypoglycemic potential of essential oils in diabetes mellitus and its complications. Int J Mol Sci . (2023) 24:1–22. doi: 10.3390/IJMS242216501
38. Naimi, M, Vlavcheski, F, Shamshoum, H, and Tsiani, E. Rosemary extract as a potential anti-hyperglycemic agent: current evidence and future perspectives. Nutrients . (2017) 9:1–19. doi: 10.3390/NU9090968
39. Selmi, S, Rtibi, K, Grami, D, Sebai, H, and Marzouki, L. Rosemary ( Rosmarinus Officinalis ) essential oil components exhibit anti-hyperglycemic, anti-Hyperlipidemic and antioxidant effects in experimental diabetes. Pathophysiology . (2017) 24:297–303. doi: 10.1016/J.PATHOPHYS.2017.08.002
40. Micić, D, Ðurović, S, Riabov, P, Tomić, A, Šovljanski, O, Filip, S, et al. Rosemary essential oils as a promising source of bioactive compounds: chemical composition, thermal properties, biological activity, and gastronomical perspectives. Food Secur . (2021) 10:1–16. doi: 10.3390/FOODS10112734/S1
41. Mira-Sánchez, MD, Castillo-Sánchez, J, and Morillas-Ruiz, JM. Comparative study of rosemary extracts and several synthetic and natural food antioxidants. Relevance of Carnosic acid/Carnosol ratio. Food Chem . (2020) 309:1–39. doi: 10.1016/J.FOODCHEM.2019.125688
42. Korkmaz, K, Tokur, B, and Ucar, Y. Does adding thyme and rosemary essential oils to sunflower oil during shallow-frying increase the lipid quality of Atlantic Bonito? Int J Gastronomy Food Sci . (2022) 28:100500. doi: 10.1016/J.IJGFS.2022.100500
43. Moufakkir, C, Kharbach, Y, Tanghort, M, Dassouli, A, and Remmal, A. Antioxidant effect of natural rosemary on the oxidation of mid-oleic sunflower frying oil on chicken wings. Food Sci Technol . (2022) 42:e70122. doi: 10.1590/FST.70122
44. MacHado, DG, Neis, VB, Balen, GO, Colla, A, Cunha, MP, Dalmarco, JB, et al. Antidepressant-like effect of Ursolic acid isolated from Rosmarinus Officinalis L. in mice: evidence for the involvement of the dopaminergic system. Pharmacol Biochem Behav . (2012) 103:204–11. doi: 10.1016/J.PBB.2012.08.016
45. Peng, G-j, Tian, J-s, Gao, X-x, Zhou, Y-z, and Qin, X-m. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol . (2015) 13:514–23. doi: 10.2174/1570159X1304150831120428
46. Rahbardar, MG, and Hosseinzadeh, H. Therapeutic effects of rosemary ( Rosmarinus Officinalis L.) and its active constituents on nervous system disorders. Iran J Basic Med Sci . (2020) 23:1100–12. doi: 10.22038/IJBMS.2020.45269.10541
47. Habtemariam, S . The therapeutic potential of rosemary ( Rosmarinus Officinalis ) Diterpenes for Alzheimer’s disease. Evid Based Complement Alternat Med . (2016) 2016:1–14. doi: 10.1155/2016/2680409
48. MacHado, DG, Cunha, MP, Neis, VB, Balen, GO, Colla, AR, Grando, J, et al. Rosmarinus Officinalis L. Hydroalcoholic extract, similar to fluoxetine, reverses depressive-like behavior without altering learning deficit in olfactory Bulbectomized mice. J Ethnopharmacol . (2012) 143:158–69. doi: 10.1016/J.JEP.2012.06.017
49. Moore, J, Yousef, M, and Tsiani, E. Anticancer effects of rosemary ( Rosmarinus Officinalis L.) extract and rosemary extract polyphenols. Nutrients . (2016) 8:1–32. doi: 10.3390/NU8110731
50. Berraaouan, A, Abid, S, and Bnouham, M. Antidiabetic oils. Curr Diabetes Rev . (2013) 9:499–505. doi: 10.2174/15733998113096660081
51. Mishra, A, Bhatti, R, Singh, A, and Ishar, MPS. Ameliorative effect of the cinnamon oil from Cinnamomum Zeylanicum upon early stage diabetic nephropathy. Planta Med . (2010) 76:412–7. doi: 10.1055/S-0029-1186237
52. Ping, H, Zhang, G, and Ren, G. Antidiabetic effects of cinnamon oil in diabetic KK-ay mice. Food Chem Toxicol . (2010) 48:2344–9. doi: 10.1016/J.FCT.2010.05.069
53. Stevens, N, and Allred, K. Antidiabetic potential of volatile cinnamon oil: a review and exploration of mechanisms using in silico molecular docking simulations. Molecules . (2022) 27:1–19. doi: 10.3390/MOLECULES27030853
54. Qi, L, Mao, H, Xiaohui, L, Shi, T, and Wang, J. Cinnamaldehyde promotes the intestinal barrier functions and reshapes gut microbiome in early weaned rats. Front Nutr . (2021) 8:748503. doi: 10.3389/FNUT.2021.748503/BIBTEX
55. Zobeiri, M, Parvizi, F, Shahpiri, Z, Heydarpour, F, Pourfarzam, M, Memarzadeh, MR, et al. Evaluation of the effectiveness of cinnamon oil soft capsule in patients with functional dyspepsia: a randomized double-blind placebo-controlled clinical trial. Evid Based Complement Alternat Med . (2021) 2021:1–7. doi: 10.1155/2021/6634115
56. Cantore, PL, Iacobellis, NS, De Marco, A, Capasso, F, and Senatore, F. Antibacterial activity of Coriandrum Sativum L. and Foeniculum Vulgare miller Var. Vulgare (miller) essential oils. J Agric Food Chem . (2004) 52:7862–6. doi: 10.1021/jf0493122
57. Ebrahimi, N, Samad, JH, and Ranjbar, H. Essential oil compositions of different accessions of Coriandrum Sativum L. from Iran. Nat Prod Res . (2010) 24:1287–94. doi: 10.1080/14786410903132316
58. Satyal, P, and Setzer, WN. Chemical compositions of commercial essential oils from Coriandrum Sativum fruits and aerial parts. Nat Prod Commun . (2020) 15:1934578X2093306. doi: 10.1177/1934578X20933067/SUPPL_FILE/10.1177_1934578X20933067-SUPPL2.XLSX
59. Duarte, A, Luís, Â, Oleastro, M, and Domingues, FC. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter Spp. Food Control . (2016) 61:115–22. doi: 10.1016/J.FOODCONT.2015.09.033
60. Rahim, MA, Imran, M, Khan, FA, Al-Asmari, F, Regenstein, JM, Alomar, SY, et al. Characterization and effect of optimized spray-drying conditions on spray-dried coriander essential oil. Ind Crop Prod . (2024) 209:117976. doi: 10.1016/J.INDCROP.2023.117976
61. Samojlik, I, Lakić, N, Mimica-Dukić, N, Daković-Švajcer, K, and Božin, B. Antioxidant and Hepatoprotective potential of essential oils of coriander ( Coriandrum Sativum L.) and caraway ( Carum Carvi L.) (Apiaceae). J Agric Food Chem . (2010) 58:8848–53. doi: 10.1021/JF101645N
62. Hajlaoui, H, Arraouadi, S, Noumi, E, Aouadi, K, Adnan, M, Khan, MA, et al. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum Carvi L. and Coriandrum Sativum L. essential oils alone and in combination. Molecules . (2021) 26:3625. doi: 10.3390/MOLECULES26123625
63. Horky, P, Skalickova, S, Smerkova, K, and Skladanka, J. Essential oils as a feed additives: pharmacokinetics and potential toxicity in Monogastric animals. Animals: Open Access J from MDPI . (2019) 9:352. doi: 10.3390/ANI9060352
64. Burdock, GA, and Carabin, IG. Safety assessment of coriander ( Coriandrum Sativum L.) essential oil as a food ingredient. Food Chem Toxicol . (2009) 47:22–34. doi: 10.1016/J.FCT.2008.11.006
65. López, MD, Jordán, MJ, and Pascual-Villalobos, MJ. Toxic compounds in essential oils of coriander, caraway and basil active against stored Rice pests. J Stored Prod Res . (2008) 44:273–8. doi: 10.1016/J.JSPR.2008.02.005
66. Önder, A, and Önder, A. Coriander and its Phytoconstituents for the beneficial effects. Potential of Essential Oils . (2018) 9:166–189. doi: 10.5772/INTECHOPEN.78656
67. Hung, YH, Ronny, HJ, Lin, EC, Lee, WJ, Ting, LY, Lin, BB, et al. Effect of lemon essential oil on the microbial control, physicochemical properties, and aroma profiles of peeled shrimp. LWT . (2023) 173:114340. doi: 10.1016/J.LWT.2022.114340
68. Zhao, C, Zhang, Z, Nie, D, and Li, Y. Protective effect of lemon essential oil and its major active component, D-limonene, on intestinal injury and inflammation of E. coli -challenged mice. Front Nutr . (2022) 9:843096. doi: 10.3389/FNUT.2022.843096
69. Pucci, M, Raimondo, S, Zichittella, C, Tinnirello, V, Corleone, V, Aiello, G, et al. Biological properties of a citral-enriched fraction of citrus limon essential oil. Foods (Basel, Switzerland) . (2020) 9:1290. doi: 10.3390/foods9091290
70. Rossi, P, Willnecker, A, Berti, J, Borgarello, AV, Mezza, GN, and Pramparo, M. D-limonene and geranial fractionation from lemon essential oil by molecular distillation. Lat Am Appl Res . (2011) 41:81–85. doi: 10.5772/intechopen.78656
71. Freche, E, Gieng, J, Pignotti, G, Ibrahim, SA, and Feng, X. Applications of lemon or cinnamon essential oils in strawberry fruit preservation: a review. J Food Process Preserv . (2022) 46:e16526. doi: 10.1111/JFPP.16526
72. Magalhães, D, Vilas-Boas, AA, Teixeira, P, and Pintado, M. Functional ingredients and additives from lemon by-products and their applications in food preservation: a review. Food Secur . (2023) 12:1095. doi: 10.3390/FOODS12051095
73. Meng, F-B, Gou, ZZ, Li, YC, Zou, LH, Chen, WJ, Liu, DY, et al. The efficiency of lemon essential oil-based Nanoemulsions on the inhibition of Phomopsis Sp. and reduction of postharvest decay of kiwifruit. Food Secur . (2022) 11:1510. doi: 10.3390/FOODS11101510
74. Lazar, V, Holban, AM, Curutiu, C, and Ditu, LM. Modulation of gut microbiota by essential oils and inorganic nanoparticles: impact in nutrition and health. Front Nutr . (2022) 9:920413. doi: 10.3389/FNUT.2022.920413
Keywords: essential oils, nutrition, omics, bioactive compounds, well-being
Citation: Pezantes-Orellana C, German Bermúdez F, Matías De la Cruz C, Montalvo JL and Orellana-Manzano A (2024) Essential oils: a systematic review on revolutionizing health, nutrition, and omics for optimal well-being. Front. Med . 11:1337785. doi: 10.3389/fmed.2024.1337785
Received: 13 November 2023; Accepted: 25 January 2024; Published: 16 February 2024.
Reviewed by:
Copyright © 2024 Pezantes-Orellana, German Bermúdez, Matías De la Cruz, Montalvo and Orellana-Manzano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Orellana-Manzano, YWtvcmVsbGFAZXNwb2wuZWR1LmVj
† These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Essential oils for clinical aromatherapy: A comprehensive review
Affiliations.
- 1 School of Pharmacy, Queen's University Belfast, 97 Lisburn Road, BT9 7BL, UK. Electronic address: [email protected].
- 2 Department of Pharmaceutics, St. John Institute of Pharmacy and Research, Palghar, 401404, Maharashtra, India.
- 3 Institute of Chemical Technology Mumbai, Marathwada Campus, Jalna, 431213, Maharashtra, India.
- 4 Molecular and Cellular Neuroscience Laboratory, Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research (NIPER)-Hyderabad, Telangana, 500037, India.
- 5 Department of Pharmaceutics and Pharmaceutical Technology, L. M. College of Pharmacy, Ahmedabad, Gujarat, India. Electronic address: [email protected].
- 6 Pharmacy Section, L. M. College of Pharmacy, Ahmedabad, Gujarat, India.
- 7 Molecular and Cellular Neuroscience Laboratory, Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research (NIPER)-Hyderabad, Telangana, 500037, India. Electronic address: [email protected].
- PMID: 38614262
- DOI: 10.1016/j.jep.2024.118180
Ethnopharmacological relevance: Aromatherapy, a holistic healing practice utilizing the aromatic essences of plant-derived essential oils, has gained significant attention for its therapeutic potential in promoting overall well-being. Use of phytoconstituent based essential oil has played a significant role in the evolving therapeutic avenue of aromatherapy as a complementary system of medicine.
Aim of the study: This comprehensive review article aims to explore the usage of essential oils for aromatherapy, shedding light on their diverse applications, scientific evidence, and safety considerations. Furthermore, the growing interest in using essential oils as complementary therapies in conjunction with conventional medicine is explored, underscoring the significance of collaborative healthcare approaches.
Materials and methods: Literature search was performed from databases like PubMed, ScienceDirect, Scopus, and Bentham using keywords like Aromatherapy, Aromatic Plants, Essential oils, Phytotherapy, and complementary medicine. The keywords were used to identify literature with therapeutic and mechanistic details of herbal agents with desired action.
Results: The integration of traditional knowledge with modern scientific research has led to a renewed interest in essential oils as valuable tools in contemporary healthcare. Various extraction methods used to obtain essential oils are presented, emphasizing their impact on the oil's chemical composition and therapeutic properties. Additionally, the article scrutinizes the factors influencing the quality and purity of essential oils, elucidating the significance of standardization and certification for safe usage. A comprehensive assessment of the therapeutic effects of essential oils is provided, encompassing their potential as antimicrobial, analgesic, anxiolytic, and anti-inflammatory agents, among others. Clinical trials and preclinical studies are discussed to consolidate the existing evidence on their efficacy in treating diverse health conditions, both physical and psychological. Safety considerations are of paramount importance when employing essential oils, and this review addresses potential adverse effects, contraindications, and best practices to ensure responsible usage.
Conclusions: This comprehensive review provides valuable insights into the exploration of essential oils for aromatherapy, emphasizing their potential as natural and potent remedies for a wide range of ailments. By amalgamating traditional wisdom and modern research, this article aims to encourage further investigation into the therapeutic benefits of essential oils while advocating for their responsible and evidence-based incorporation into healthcare practices.
Keywords: Aromatherapy; Aromatherapy mechanism; Essential oils; Mood disorders; Phytotherapy; Sleep disorders.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Publication types
- Aromatherapy* / methods
- Oils, Volatile* / therapeutic use
- Oils, Volatile
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

Aromatherapy and Essential Oils: A Map of the Evidence
Investigators: Michele Freeman , MPH, Chelsea Ayers , MPH, Carolyn Peterson , PhD, and Devan Kansagara , MD, MCR.
- Copyright and Permissions
Background:
The purpose of this review is to provide the Veterans Health Administration (VHA) with a broad overview of the effectiveness of aromatherapy and essential oils (EOs), and the health conditions for which these interventions have been examined.
Data Sources and Study Selection:
We searched multiple databases through February 2019 for systematic reviews (SRs) of aromatherapy and EOs for health conditions. Using pre-specified inclusion criteria, all abstracts and full-text articles were dual-screened for inclusion. When there were several qualified reviews for the same health condition, we selected a single review based on its recency, methods, scope, and applicability.
Data Abstraction:
From each review, we abstracted the focus of the SR, the number of controlled trials included, combined number of participants, duration of trials, condition treated, and relevant findings from controlled trials. We abstracted separate data for each of 5 outcome categories: psychological outcomes, nausea/vomiting, pain and other physical outcomes, sleep outcomes, and global health outcomes.
Data Synthesis:
For each review and outcome category we assigned values representing the effectiveness level of the intervention and confidence in the evidence and used these values to generate evidence maps. Additionally, we provide a narrative synthesis of the findings.
We included 26 SRs representing the most recent and comprehensive evidence available. There is moderate-confidence evidence that aromatherapy is beneficial for pain in dysmenorrhea. Aromatherapy is potentially effective for pain in labor/childbirth; blood pressure reduction in hypertension; stress, depression, and sleep in hemodialysis patients; stress in healthy adults; anxiety in perioperative patients; and sleep quality in various populations, with low to moderate confidence in the evidence. For EOs applied topically, there is moderate confidence in the potentially positive effect of tea tree oil for tinea pedis. There is insufficient evidence of efficacy for all other conditions examined.
- Collapse All
- Acknowledgments
- Introduction
- Conclusions
- Abbreviations Table
- Topic Development
- Search Strategy
- Study Selection
- Data Abstraction
- Quality Assessment
- Data Synthesis
- Rating the Body of Evidence
- Literature Flow
- KEY QUESTION: What evidence is available that examines the effectiveness of aromatherapy or essential oils for health-related indications?
- Limitations
- Research Gaps/Future Research
- APPENDIX A. Search Strategies
- APPENDIX B. Study Selection
- APPENDIX C. Assessment of Confidence in the Evidence from Systematic Reviews of Aromatherapy and Essential Oils
- APPENDIX D. Peer Review Comments/Author Responses
Suggested citation:
Freeman M, Ayers CK, Peterson C, and Kansagara D. Aromatherapy and Essential Oils: A Map of the Evidence. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #05-225; 2019. Available at: https://www.hsrd.research.va.gov/publications/esp/reports.cfm .
This report is based on research conducted by the Evidence Synthesis Program (ESP) Center located at the VA Portland Healthcare System, Portland, OR, funded by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development. The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the United States government. Therefore, no statement in this article should be construed as an official position of the Department of Veterans Affairs. No investigators have any affiliations or financial involvement ( eg , employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties) that conflict with material presented in the report.
This publication is in the public domain and is therefore without copyright. All text from this work may be reprinted freely. Use of these materials should be acknowledged.
- Cite this Page Freeman M, Ayers C, Peterson C, et al. Aromatherapy and Essential Oils: A Map of the Evidence. Washington (DC): Department of Veterans Affairs (US); 2019 Sep.
- PDF version of this title (1.4M)
Other titles in this collection
- VA Evidence-based Synthesis Program Reports
Related information
- NLM Catalog Related NLM Catalog Entries
Similar articles in PubMed
- Review Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [ 2017] Review Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. 2017 Jul
- Review Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the U.S. Preventive Services Task Force [ 2014] Review Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the U.S. Preventive Services Task Force Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Smith N, Webber E, Perdue LA, Bigler KD, Whitlock EP. 2014 Dec
- Review Telehealth Services Designed for Women: An Evidence Map [ 2017] Review Telehealth Services Designed for Women: An Evidence Map Goldstein KM, Gierisch JM, Zullig LL, Alishahi A, Brearly T, Dedert EA, Raitz G, Sata SS, Whited JD, Bosworth HB, et al. 2017 Nov
- Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [ 2013] Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force Lin JS, O'Connor E, Rossom RC, Perdue LA, Burda BU, Thompson M, Eckstrom E. 2013 Nov
- Effectiveness of Silexan oral lavender essential oil compared to inhaled lavender essential oil aromatherapy for sleep in adults: a systematic review. [JBI Database System Rev Implem...] Effectiveness of Silexan oral lavender essential oil compared to inhaled lavender essential oil aromatherapy for sleep in adults: a systematic review. Greenberg MJ, Slyer JT. JBI Database System Rev Implement Rep. 2018 Nov; 16(11):2109-2117.
Recent Activity
- Aromatherapy and Essential Oils: A Map of the Evidence Aromatherapy and Essential Oils: A Map of the Evidence
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Detailed insight on the antiviral action of essential oils still requires more research. Essential oils might interfere with virion envelopment, which is designed for entry into human host cells, synthesis of viral proteins, inhibition of the early gene expression process, glycosylation process of viral proteins, and inhibition of virus ...
The National Institute of Health provides a thorough summary via the US National Library of Medicine of research conducted into the efficacy of essential oils. Currently, there is no evidence ...
Essential oils (EOs) are highly concentrated, aromatic volatile oils of plant origin with numerous chemical constituents that are extracted by steam distillation, hydrodiffusion, or pressure . Aromatherapy is a field of complementary medicine that uses EOs to treat and prevent diseases via several routes of administration: Usually topical ...
Essential oils (EOs) have risen in popularity over the past decade. These oils function in society as holistic integrative modalities to traditional medicinal treatments, where many Americans substitute EOs in place of other prescribed medications. EOs are found in a multitude of products including …
Essential oils have many therapeutic and antiparasitic properties. They are beneficial to human health in many ways. However, to understand their potential benefits, more research is needed regarding essential oils such as coriander, parsley, rosemary, cumin, and thyme. These research gaps are relev …
This chapter explores the latest advancements and applications of essential oils, focusing on evidence-based research and practical insights. Beginning with an introduction to essential oils ...
Research in the field of essential oils (EOs) over the years.(A,B) Increase of research in the field of essential oils (EOs).(A) A PubMed advanced search using the key word "essential oils" combined with the dates of publication from 1880 to present through the Boolean operator AND has retrieved an increase from 106 to 17,212 (date of last search November 19, 2020) of results.
Essential oils extracted from plants have been valued for their medicinal properties for centuries. Modern scientific research is now unraveling the composition and bioactivity of these complex natural extracts, providing insights into how essential oils might be used to help promote health and well-being.
Results: The integration of traditional knowledge with modern scientific research has led to a renewed interest in essential oils as valuable tools in contemporary healthcare. Various extraction methods used to obtain essential oils are presented, emphasizing their impact on the oil's chemical composition and therapeutic properties.
Freeman M, Ayers CK, Peterson C, and Kansagara D. Aromatherapy and Essential Oils: A Map of the Evidence. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #05-225; 2019.