Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
The risk factors, presentation, management, and outcome of neonatal cerebellar hemorrhage will be discussed here. An overview of cerebellar development and cerebellar disorders is presented separately. (See "Overview of cerebellar injury and malformations in neonates" .)
PATHOLOGY AND PATHOGENESIS
Preterm infants — In very preterm infants (gestational age [GA] <32 weeks), there is a wide range of pathology due to CBH, ranging from small punctate lesions detected by magnetic resonance imaging (MRI), focal unilateral bleeds, and massive bleeds involving both hemispheres and including the vermis [ 1 ].
Traditionally, two pathologic patterns of preterm CBH were described. The first consists of extensive CBH occurring in the sickest and most immature infants (<28 weeks gestation and/or <750 g) ( image 1 ) [ 2,3 ]. These large lesions often lead to a destruction of cerebellar parenchyma and subsequent volume reduction and are associated with long-term neurodevelopmental disabilities in surviving infants [ 4 ]. The second pattern includes single or multiple small, punctate (<3 to 4 mm) hemorrhages detected by MRI ( image 2 ). These small lesions are usually not detected by cranial ultrasound and are frequently encountered as a chance finding on MRI. They usually do not lead to cerebellar volume reduction and are associated with a more favorable prognosis [ 5,6 ].
In more recent studies, a third category of limited/focal lesions (ie, larger than punctate hemorrhages but smaller than the extensive CBH) has been described [ 4 ]. Outcomes tend to be more favorable with these lesions than in cases with extensive CBH, but this also depends on size/location and additional complications. (See 'Outcome' below.)
- Introduction
- Conclusions
- Article Information
International Classification of Diseases, Ninth Revision ( ICD - 9 ) and International Classification of Diseases, Tenth Revision ( ICD - 10 ) code searching was performed by 2 independent biomedical informatics experts separately accessing the universal provincial hospital inpatient and outpatient systems respectively. Analysts were blinded to each other’s findings, which were then cross-referenced to exclude duplicates and create the final sample. APrON indicates Alberta Pregnancy Outcomes and Nutrition; APSP, Alberta Perinatal Stroke Program; CSVT+HT, hemorrhagic transformation of cerebral sinovenous thrombosis; HIE+HT, hemorrhagic transformation of hypoxic ischemic encephalopathy; HT, hemorrhagic transformation; NAIS+HT, hemorrhagic transformation of neonatal arterial ischemic stroke; NHS, neonatal hemorrhagic stroke; PI, principal investigator; PPHS, presumed perinatal hemorrhagic stroke; and REDCap, Research Electronic Data Capture.
Arrowheads designate location of injury. A, Neonatal hemorrhagic stroke is a hematoma in the brain parenchyma (gradient echocardiogram shown). B, Neonatal arterial ischemic stroke with hemorrhagic transformation is blood within an area of arterial territory infarction (T1 shown). C, Cerebral sinovenous thrombosis with hemorrhagic transformation is blood within an area of venous infarction confirmed by parenchymal and venous imaging (diffusion image shown). D, Hypoxic-ischemic encephalopathy with hemorrhagic transformation is clinical and radiographic confirmation of blood within areas of global infarction (T1 shown). E, Presumed perinatal hemorrhagic stroke is a focal area of remote parenchymal damage with demonstration of hemorrhage (susceptibility shown).
Long-term neurological outcome categories by Pediatric Stroke Outcome Measure were available for 50 cases. Mean Pediatric Stroke Outcome Measure scores varied widely across subgroups (A). The error bars indicate the greatest and lowest value excluding outliers, the horizontal lines within the boxes are median lines, and the circles indicate outliers (> or < 1.5 times the quartile value). Classification of poor outcome by Pediatric Stroke Outcome Measure >1 or ≥1 was generally comparable across groups with hemorrhagic transformation of neonatal arterial ischemic stroke (HT+NAIS) and hemorrhagic transformation of cerebral sinovenous thrombosis (HT+CSVT) having the highest and lowest proportions, respectively (B). For the incidence rate of poor outcome, HT+NAIS, 80% had Pediatric Stroke Outcome Measure >1 and 100% had ≥1 and HT+CSVT, 20% had Pediatric Stroke Outcome Measure >1 and 60% ≥1. Examination of Pediatric Stroke Outcome Measure subcategories for the neonatal hemorrhagic stroke (NHS) group demonstrated wide-ranging morbidity with sensorimotor deficits being most common (C). PPHS indicates presumed perinatal hemorrhagic stroke; HIE+HT, hemorrhagic transformation of hypoxic ischemic encephalopathy.
- Neonatal Hemorrhagic Stroke JAMA Pediatrics Editorial March 1, 2017 Catherine Amlie-Lefond, MD; Jeffrey G. Ojemann, MD
- Errors in Figure and Table JAMA Pediatrics Correction June 1, 2017
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Cole L , Dewey D , Letourneau N, et al. Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke : A Population-Based Case-Control Study . JAMA Pediatr. 2017;171(3):230–238. doi:10.1001/jamapediatrics.2016.4151
Manage citations:
© 2024
- Permissions
Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke : A Population-Based Case-Control Study
- 1 Calgary Pediatric Stroke Program, University of Calgary, Calgary, Alberta, Canada
- 2 Department of Pediatrics, Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Alberta, Canada
- 3 Department of Community Health Services, Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Alberta, Canada
- 4 Department of Clinical Neuroscience, Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Alberta, Canada
- Editorial Neonatal Hemorrhagic Stroke Catherine Amlie-Lefond, MD; Jeffrey G. Ojemann, MD JAMA Pediatrics
- Correction Errors in Figure and Table JAMA Pediatrics
Question What are the incidence, types, presentations, associated factors, and long-term outcomes of neonatal hemorrhagic stroke?
Findings This population-based case-control study found a neonatal hemorrhagic stroke incidence of 1 in 6300 live births and independent associations between idiopathic neonatal hemorrhagic stroke and lower maternal age, primiparity, prior spontaneous abortion, difficult fetal transition, and small for gestational age. Outcomes were poor in approximately 50% of infants including sensorimotor delays and epilepsy.
Meaning Neonatal hemorrhagic stroke is more common than previously reported, and clinical risk factors associated with idiopathic neonatal hemorrhagic stroke do not support a primary acquired pathophysiology.
Importance Hemorrhage into the brain of term newborns often results in major injury and lifelong disability. The clinical epidemiology of neonatal hemorrhagic stroke (NHS) remains undefined, hindering the development of strategies to improve outcomes.
Objective To characterize the incidence, types, presentations, associated factors, and outcomes of neonatal hemorrhagic stroke.
Design, Setting, and Participants Population-based, nested case-control study. The Alberta Perinatal Stroke Project, a provincial registry, ascertained NHS cases using exhaustive diagnostic code searching (1992-2010, >2500 medical record reviews). Prospective cases were captured through the Calgary Pediatric Stroke Program (2007-2014). Participants included term neonates with magnetic resonance imaging–confirmed NHS including primary and secondary intracerebral hemorrhage, hemorrhagic transformation of ischemic injury, and presumed perinatal hemorrhagic stroke. Control infants with common data were recruited from a population-based study (4 to 1 ratio).
Main Outcomes and Measures Infants with NHS underwent structured medical record review using data-capture forms and blinded scoring of neuroimaging. Clinical risk factor common data elements were explored using logistic regression. Provincial live births were obtained from Statistics Canada. Outcomes were extrapolated to the Pediatric Stroke Outcome Measure.
Results We identified 86 cases: 51 infants (59%) with NHS, of which 32 (67%) were idiopathic, 30 (35%) were hemorrhagic transformation of primary ischemic injuries (14 with neonatal cerebral sinovenous thrombosis, 11 with hypoxic ischemic encephalopathy, and 5 with neonatal arterial ischemic stroke), and 5 were presumed perinatal hemorrhagic stroke. Sixty-two percent were male. Incidence of pure NHS was 1 in 9500 live births and 1 in 6300 for all forms. Most presented in the first week of life with seizures and encephalopathy. Acute neurosurgical intervention was rare (3 of 86 total cases; 3.5%). Temporal lobe was the most common NHS location (16 of 51 pure NHS cases; 31%). A primary cause was evident in 19 of the 51 cases of non–hemorrhagic transformation NHS (37%). Idiopathic NHS was independently associated with lower maternal age (odds ratio [OR], 0.87; 95% CI, 0.78-0.94), primiparity (OR, 2.98; 95% CI, 1.18-7.50), prior spontaneous abortion (OR, 0.11; 95% CI, 0.02-0.53), difficult fetal transition (bradycardia [OR, 15.0; 95% CI, 2.19-101.9] and low Apgar [OR, 14.3; 95% CI, 2.77-73.5]), and small for gestational age (OR, 14.3; 95% CI, 1.62-126.1). Follow-up of 50 cases at a median of 37 months demonstrated poor neurological outcomes in 21 patients (44%).
Conclusions and Relevance Neonatal hemorrhagic stroke is more common than previously reported, occurring in at least 1 in 6300 live births. Etiologies are approximately equally distributed between idiopathic, secondary, and hemorrhagic transformation. Clinical associations do not suggest a common mechanism or predictability of NHS. Recurrence is rare. Outcomes are often poor, mandating attention to prevention and rehabilitation.
Perinatal stroke is a leading cause of cerebral palsy and lifelong neurological morbidity. Compared with perinatal ischemic stroke diseases, which are now well defined, term neonatal hemorrhagic stroke (NHS) research has been limited. Neonatal hemorrhagic stroke is frequently encountered clinically, but only 1 retrospective study of 20 patients could suggest an incidence of 6.2 per 100 000 live births. 1 The lack of well-powered, population-based studies has limited understanding of presentations, possible risk factors, treatment strategies, and long-term outcomes.
Classification of perinatal intracerebral hemorrhage is challenging, and variable terminology represents another barrier to progress. Perinatal intracerebral hemorrhage is defined within the National Institutes of Health Common Data Elements as a term neonate with encephalopathy, seizures, altered mental status, and/or neurological deficit within the first 28 days of life with a focal collection of blood within the brain parenchyma confirmed by neuroimaging or autopsy. 2 This is distinct from intracranial hemorrhage in preterm infants, where germinal matrix hemorrhages are common. 3 The definition does not address overlapping hemorrhages (intraventricular and subarachnoid), does not capture nongerminal matrix hemorrhages in the fetus, and excludes presentation beyond the neonatal period.
The epidemiology of NHS is poorly described. Modestly powered, single-center, uncontrolled, retrospective, non–population-based studies constitute the bulk of current knowledge. 1 , 4 - 6 Studies report variable proportions of different syndromes. 1 , 6 - 8 Minimal case-control data limit the exploration of risk factors, a successful approach in perinatal ischemic stroke. 9 , 10 Suggested risk factors include vascular malformations, bleeding diatheses, and possibly trauma, although these account for few cases. “Excessive” trauma is notoriously difficult to define, and studies suggesting associations with NHS are uncontrolled. 11 , 12 Well-powered, population-based, case-control data are required to explore potential risk factors.
Modern neuroimaging has facilitated the classification and study of specific perinatal stroke disease states. Hemorrhagic transformation (HT) of ischemic injuries, such as neonatal arterial ischemic stroke (NAIS), neonatal cerebral sinovenous thrombosis (CSVT), and hypoxic ischemic encephalopathy (HIE), also creates intraparenchymal hemorrhage. Magnetic resonance imaging sequences are exquisitely sensitive to hemorrhage, including detection of hemosiderin deposition years later, creating the ability to retrospectively diagnose NHS that was asymptomatic at birth, analogous to presumed perinatal ischemic stroke. 9 , 13
With no controlled population-based studies to our knowledge, most cases unexplained, poor outcomes lasting decades, and no treatment or prevention strategies, there is a clear need for improved understanding of NHS epidemiology. We used a large, population-based perinatal stroke cohort to perform a nested case-control study to define NHS incidence, classification, presentations, possible risk factors, and outcomes.
This was a population-based, nested case-control study. Neonatal hemorrhagic stroke cases were identified via retrospective and prospective methods within the Alberta Perinatal Stroke Project. This research cohort was designed to capture all perinatal stroke cases in Southern Alberta, Canada (population of approximately 2.1 million), leveraging universal health care at a single tertiary care pediatric center. Established in 2008, the Alberta Perinatal Stroke Project includes more than 900 magnetic resonance imaging–confirmed perinatal stroke cases. Methods were approved by the institutional research ethics board at University of Calgary and written informed consent was obtained.
Case ascertainment is outlined in Figure 1 . Exhaustive retrospective analysis of at least 140 International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes was performed across inpatient and outpatient populations for 1992-2010. A standardized data collection form extracted medical records and imaging report data. Imaging was then reviewed in person with an expert investigator (A.K.) to confirm perinatal stroke diagnosis and subtype. Inclusion criteria were (1) radiographic NHS, (2) term birth (≥35 weeks to exclude germinal matrix bleeding), and (3) resident of Southern Alberta. Prospectively, all cases were identified through active Alberta Perinatal Stroke Project case surveillance from 2007 through June 2015.
Neonatal hemorrhagic stroke was defined as imaging evidence of blood within the brain parenchyma with or without intraventricular or subarachnoid blood ( Figure 2 ). Cases where blood was exclusively extra-axial were not included. Imaging confirmed concurrent neonatal arterial ischemic stroke (NAIS), neonatal cerebral sinovenous thrombosis (CSVT), and global hypoxic-ischemic encephalopathy (HIE). Neonatal hemorrhagic stroke categories were hemorrhagic transformation (HT) of a primary ischemic injury (NAIS+HT, CSVT+HT, or HIE+HT), 14 secondary NHS (sNHS) explained by a highly probably cause (vascular malformation and bleeding diathesis), or idiopathic NHS (iNHS). Major trauma was defined by concurrent skull fracture or severe bruising/soft tissue injury. Presumed perinatal hemorrhagic stroke was defined as a term child with no perinatal neurological history demonstrating remote NHS on imaging performed in childhood.
Using a standardized data capture form, medical and birth record review collected the following: (1) demographics and clinical presentation (age, sex, presenting signs, and acute neurosurgical intervention); (2) potential risk factors classified using common data elements including maternal health, pregnancy, labor and delivery, and neonatal factors; (3) neuroimaging analysis using a standardized scoring tool (location, mass effect, restricted diffusion, hydrocephalus, or concurrent NAIS/CSVT/HIE) 15 , 16 ; and (4) neurological outcomes measured by the Pediatric Stroke Outcome Measure 13 , 17 and epilepsy outcomes according to the Modified Engel Classification. 18 The Pediatric Stroke Outcome Measure, based on the most recent clinical assessment at older than 12 months of age by the same pediatric neurologist (A.K.), generated summative scores (normal/mild/moderate/severe) for sensorimotor, language production, language comprehension, and cognitive deficits. Total scores were dichotomized into good vs poor based on cutoffs of more than 1 and at least 1. The Modified Engel Classification classified epilepsy outcomes as follows: 0 = seizure free and no antiepileptic drugs for 6 months, 1 = seizure free for 6 months with medication or seizure free without medication less than 6 months, 2 = less than 1 seizure/mo with medication, 3 = 1 to 4 seizures/mo with medication, 4 = 5 to 30 seizures/mo with medication, and 5 = 30 or more seizures/mo.
Control data were obtained from the Alberta Pregnancy Outcomes and Nutrition study, a prospective cohort study of more than 2000 mother-infant dyads followed up from early pregnancy beyond the perinatal period ( Figure 1 ). 19 This sample was optimal because it was population-based from the same geographical region with precise matching of common data elements. A blinded coordinator randomly selected 4 control dyads per NHS case (n = 204).
Data were entered and managed through Research Electronic Data Capture hosted at the University of Calgary. Incidence rates were calculated annually by dividing the number of cases into the number of live births in Southern Alberta (Statistics Canada). Categorical and continuous variables were compared using χ 2 , Fisher exact, and t tests. Logistic regression modeling used a reverse elimination method where the maximum number of variables was entered into the model, and variables not associated with iNHS were removed one by one until only significant variables (or confounders) remained. 20 Variables were grouped into blocks according to clinical significance. Significant associations were expressed as odds ratios (ORs) with 95% CIs. Analysis used Stata, version 13 (StataCorp).
The final sample consisted of 86 cases. Fifty-one (59%) were pure NHS (iNHS/sNHS), with 32 (67%) being idiopathic. Thirty (35%) were HT including CSVT (14), HIE (11), and NAIS (5). Five cases were presumed perinatal hemorrhagic stroke. A significant male predominance was observed (62%). Additional population characteristics are available in Table 1 .
Mean live births in Southern Alberta were 24 650 per year, increasing steadily during the study period. This yielded an incidence for all forms of NHS of 15.9 in 100 000 per year or approximately 1 in 6300 live births. For pure NHS and presumed perinatal hemorrhagic stroke, the incidence was 10.5 in 100 000 per year or approximately 1 in 9500 live births. Incidence rates did not differ between the retrospective and prospective ascertainment periods. Prevalence estimates suggest there are currently at least 1000 Canadian children living with NHS.
Most presented within the first 28 days of life (n = 81; 94%) and were imaged in the first week (n = 59; 69%). Median age at diagnosis or first imaging did not differ between subgroups. Common clinical presentations consisted of seizures (n = 54; 67%) and/or encephalopathy (n = 67; 83%) and/or hypotonia (n = 34; 42%). Apgar scores were less than 5 at 1 minute in 36 cases (47%) and at 5 minutes in 12 cases (16%). Neonatal intensive care unit admission was common (88%). There were no differences in proportions of clinical presentations between subgroups. No cases of neonatal vitamin K refusal were documented. Given the decades of study, neonatal electroencephalogram and cerebral monitoring were variable and data were not consistently available. Neonatal neurosurgery was rare, with 1 infant having a hematoma evacuation and aneurysm clipping and another requiring external ventricular drain placement.
Imaging results are summarized in Table 2 . Initial modalities included computed tomography in 31 patients (36%) and magnetic resonance imaging in 55 patients (64%). No difference in median age at imaging was seen between subgroups aside from presumed perinatal hemorrhagic stroke. The temporal lobe was the most common location (31% compared with <20% for other locations). Infratentorial hemorrhage was uncommon (cerebellar in 11 patients [13%]). Multifocal hemorrhage was described in 22% and comparable across subgroups. Mass effect was documented in a minority (24%). Restricted diffusion within and surrounding hemorrhage was observed in 30% (n = 6 iNHS, n = 4 sNHS, n = 3 nAIS+HT, n = 5 CSVT+HT, and n = 8 HIE+HT). Intraventricular hemorrhage was described in 49% but acute hydrocephalus was uncommon (11%). Cerebral sinovenous thrombosis + HT and HIE+HT demonstrated higher rates of thalamic involvement, intraventricular hemorrhage, and acute hydrocephalus.
In the 51 patients with NHS, 19 (37%) had a highly probable primary etiology. Blood disorders were most common including 7 cases of severe thrombocytopenia, 1 of unspecified coagulopathy, and 1 of hemophilia A. Vascular abnormalities in 6 cases included arteriovenous malformation (n = 3), cavernoma (n = 2), and aneurysm (n = 1). Major trauma was identified by skull fracture, soft tissue injury, and extra-axial hemorrhage in 4 cases. When these 19 sNHS cases were compared with the remaining 32 idiopathic cases, the only differences observed involved only 1 to 2 patients and were not considered clinically significant ( Table 2 ).
The maternal and infant variable exploratory logistic regression model demonstrated independent associations between maternal age, spontaneous abortion, and primiparity with iNHS. The odds of iNHS were reduced by a factor of 0.87 (95% CI, 0.78-0.94) for every year of increase in maternal age. When controlling for age and spontaneous abortion, the odds of iNHS were increased with primiparity by 2.98 times (95% CI, 1.18-7.50). Controlling for age and parity, the odds of iNHS among mothers with prior spontaneous abortion were 0.11 times the odds of those without (95% CI, 0.02-0.53). The labor and delivery model found that bradycardia or variable decelerations were both independently associated with iNHS, controlling for maternal age (OR, 15.0; 95% CI, 2.19-101.9 and OR, 14.3; 95% CI, 2.77-73.5, respectively). Among neonatal factors, the odds of being small for gestational age were 14.3 times higher (95% CI, 1.62-126.1) in iNHS compared with control infants when controlling for maternal age and 5-minute Apgar score. Apgar scores were independently associated with iNHS, controlling for maternal age and small for gestational age. For every unit increase in 5-minute Apgars, the odds of iNHS were 86% lower (OR, 0.24; 95% CI, 0.12-0.48).
There were 50 cases of infants with Pediatric Stroke Outcome Measure at older than 12 months of age. Median (SD) age at last Pediatric Stroke Outcome Measure was 37 (51) months with follow-up ranging from 1 to 15 years. There were no recurrences of hemorrhagic stroke. Neurosurgical interventions outside the neonatal period included ventriculoperitoneal shunt insertion (n = 4), epilepsy surgery (n = 3), and arteriovenous malformation embolization (n = 1). There were 3 deaths (4%). All occurred beyond the neonatal period including 5 weeks (sNHS with malignant arteriovenous malformation), 5 years (sNHS with shunt failure), and 7 years (HIE with severe disability and intercurrent illness).
Outcomes are summarized in Figure 3 . Mean total Pediatric Stroke Outcome Measure scores varied across subgroups ( Figure 3 A). Mean Pediatric Stroke Outcome Measure scores were significantly higher in NAIS+HT compared with CSVT+HT. Proportions of poor outcome (Pediatric Stroke Outcome Measure ≥1 or >1) are compared in Figure 3 B. Outcomes were categorized as poor (Pediatric Stroke Outcome Measure >1) in 21 cases (42%) compared with 28 cases (56%) for Pediatric Stroke Outcome Measure of at least 1. Both poor outcome categories were significantly different, with NAIS+HT having higher proportions compared with CSVT+HT. In the iNHS and sNHS groups, approximately 40% had poor outcome regardless of the cutoff value. Breakdown of Pediatric Stroke Outcome Measure subcategories in the iNHS and sNHS groups demonstrated a wide range of morbidities with sensorimotor deficits being most common ( Figure 2 C). The iNHS group had 16 cases with follow-up at greater than 12 months of age, and there were 6 cases (38%) with poor outcome. Thirty-nine case patients (78%) required physical, occupational, and/or speech therapy at last follow-up.
Epilepsy occurred in 11 cases (13%). Rates of epilepsy by subgroup were 3 with iNHS or sNHS (6%), 1 with NAIS+HT (20%), 3 with CSVT+HT (21%), 2 with HIE+HT (18%), and 2 with presumed perinatal hemorrhagic stroke (40%). Mean (SD) age at seizure onset was 2.5 (3.2) years (range, 0.1-13 years). The mean (SD) number of anticonvulsants was 1.3 (1.4) (range, 0-8). Outcomes according to modified Engel Classification were 0 (n = 3; 27%), 1 (n = 3; 27%), 2 (n = 1; 9%), 3 (n = 2; 18%), 4 (n = 1; 9%), and 5 (n = 1; 9%). Three (23%) had epilepsy surgery with postoperative Engel classifications of 0, 1, and 5.
Five cases presented outside the first 28 days of life with imaging confirming remote focal hemorrhage consistent with presumed perinatal hemorrhagic stroke. Clinical presentations included seizures and developmental delay in 2 cases and early hand preference or motor asymmetry with developmental delay in 2 others. Positive family history led to imaging and diagnosis without symptoms in the fifth case. Etiologies included hereditary hemorrhagic telangiectasia in 2 and hemophilia B in 1, while the other 2 were idiopathic.
We provide population-based, controlled data, including original imaging classification, common data elements, and standardized outcomes, for children with NHS. The incidence of all forms of NHS approximates 1 in 6000 live births. Cases are often explained by primary conditions or hemorrhagic transformation of ischemic injury, but many remain idiopathic. Neonatal hemorrhagic stroke is associated with difficult neonatal transition, but no primary causative associations are suggested for idiopathic NHS. Outcomes are poor, with long-term morbidity in most.
Our methods advance the levels of evidence for NHS. Most previous studies have been uncontrolled case series with inconsistent classification, variable inclusion criteria, and other limitations. 1 , 6 - 8 A notable exception is a case-control study using administrative data from Northern California. 1 We have tried to overcome some of the limitations of that study with truly population-based data, broader International Classification of Diseases code searching, and original neuroimaging review. Many findings appear comparable, including clinical presentations and associated factors, but notable discrepancies are discussed here.
Inconsistent terminology and classification has complicated NHS studies to date. 1 , 6 - 8 We focused on NHS with direct parenchymal brain injury 6 and maximal risk of long-term morbidity. Isolated hemorrhage in the subdural, subarachnoid, and epidural spaces does not usually cause brain injury and is common in normal newborns. 21 Classification must also consider hemorrhagic transformation of ischemic injuries with our findings supporting previous evidence 7 that this is a common cause of intraparenchymal blood. A final classification advance is our description of presumed perinatal hemorrhagic stroke, analogous to its ischemic counterpart where such terminology has advanced studies. 9 , 13
Precise incidence and prevalence estimates for term intracerebral hemorrhage are not established. In the retrospective, 20-case, administrative study, the estimated incidence was 6.2 per 100 000 (approximately 1 in 16 000) live births for combined intraparenchymal and subarachnoid hemorrhage. 1 Our population-based calculations, including prospective ascertainment and original imaging, may be more accurate. Our rate of 1 in 9000 live births for pure NHS (iNHS and sNHS) and 1 in 6000 for all NHS is at least 3 times higher. While we are confident our methods captured a very high proportion of cases without false positives, this still reflects a minimum incidence.
Acute clinical presentations of seizures and encephalopathy are consistent with previous studies, 7 , 8 , 22 and proportions were similar across groups compared with ischemic perinatal stroke. 23 This suggests NHS should be considered in any neonate with encephalopathy, but that specific clinical predictors are unlikely. Although we could not investigate seizure features in detail, previous studies suggest lesion-specific (eg, temporal) and subclinical seizures often occur, suggesting a role for electroencephalogram monitoring. 24 Precise diagnosis and assessment of cause requires magnetic resonance imaging.
Our high proportion of idiopathic cases is consistent with existing literature. 1 , 22 Our exploratory regression analysis for possible associations in this subset was an advantage over previous studies that often considered all types together. Consistent with previous studies 1 was an association between difficult transition including fetal bradycardia, decelerations, and low Apgar scores. As suggested by NHS 7 and perinatal ischemic stroke 25 - 27 studies, such difficulty may be secondary to brain injury that has already occurred. Associations between maternal factors including age, primiparity, and abortion have only been inconsistently described in perinatal ischemic stroke. Additional possible risk factors not supported by our data include gestational age and postmaturity. 1 Trauma, an often-suggested but usually unproven consideration, was rare. Uncontrolled studies have suggested increased rates of assisted deliveries in NHS, but we did not observe this. 4 , 6 Our results support long-standing evidence dissociating routine trauma, assisted delivery, and neonatal hemorrhage. 12
Long-term outcomes were often poor and comparable with previous reports. 6 , 28 , 29 Our length of follow-up was long, although young children may grow into their deficits over decades. One study found that 56% of NHS children required physical, occupational, and/or speech therapy services, a rate comparable with our population. 7 Careful clinical and imaging classification may assist in predicting some outcomes. For example, hemorrhage associated with deep CSVT was associated with hemorrhage pattern (thalamic and intraventricular extension) while thalamic hemorrhage has been associated with long-term epilepsy. 30 - 32 The recurrence risk of NHS appears to be very low.
Limitations include potential reporter bias during medical record review, although these were completed prior to final imaging confirmation by the lead investigator. Completeness and consistency of data collection across multiple Southern Alberta centers was challenging but facilitated by universal health care, unified medical records systems across sites, and tertiary level neonatal and pediatric care at only a single center. Neurological outcome data were only available from the most recent clinic visit and more complete data (eg, neuropsychological testing) at longer follow-up intervals may demonstrate additional morbidities that we could not measure. Our strict case criteria that excluded subdural and subarachnoid hemorrhages were considered more specific but limits comparability with some previous studies.
What causes idiopathic NHS? A small weakness in an artery that ruptures with the large surge in blood pressure that accompanies transition to extrauterine life is purely speculative. However, this would be consistent with existing evidence including the lack of associations mentioned in previous paragraphs, known risk of larger arteriovenous malformations, lack of lesions on follow-up imaging, and minimal recurrence. Why this would occur in 1 of every 6000 to 10 000 live births may relate to unique vascular development or anatomy. An increasing number of genetic disorders may lead to perinatal hemorrhage. 33 Quantified arterial tortuosity may be an imaging biomarker of vascular biology in children with stroke, 34 and we have recently demonstrated increased variability of tortuosity in NHS compared with controls (unpublished). Collectively, evidence suggests NHS pathophysiology involves rare events occurring in uniquely susceptible individuals rather than any controllable external factors, limiting opportunities for prevention.
Corresponding Author: Adam Kirton, MD, Alberta Children’s Hospital, University of Calgary, 2888 Shaganappi Trail NW, Calgary, AB T3B6A8, Canada ( [email protected] ).
Accepted for Publication: October 19, 2016.
Correction: This article was corrected on April 3, 2017, to correct errors in Figure 1 and Table 1.
Published Online: January 17, 2017. doi:10.1001/jamapediatrics.2016.4151
Author Contributions: Dr Kirton had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Cole, Letourneau, Kirton.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Cole, Letourneau, Kirton.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Cole, Letourneau, Chaput, Kirton.
Obtained funding: Cole, Dewey, Letourneau, Kaplan, Kirton.
Administrative, technical, or material support: Cole, Dewey, Letourneau, Kaplan, Hodge, Floer, Kirton.
Study supervision: Letourneau, Hodge, Kirton.
Conflict of Interest Disclosures: None reported.
Funding/Support: Funding was provided by Alberta Innovates Health Solutions.
Role of the Funder/Sponsor: The funding source did not influence the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 28 October 2022
Neonatal subgaleal hemorrhage: twenty years of trends in incidence, associations, and outcomes
- Thomas R. Christensen 1 ,
- Timothy M. Bahr ORCID: orcid.org/0000-0002-7946-0290 2 , 3 ,
- Erick Henry 2 ,
- Con Yee Ling 2 , 3 ,
- Taylor H. Hanton ORCID: orcid.org/0000-0002-0570-8767 3 ,
- Jessica M. Page 2 ,
- Sarah J. Ilstrup 4 ,
- Nicholas R. Carr 2 , 3 ,
- Robin K. Ohls ORCID: orcid.org/0000-0003-2865-8878 2 , 3 &
- Robert D. Christensen ORCID: orcid.org/0000-0001-5872-582X 2 , 3
Journal of Perinatology volume 43 , pages 573–577 ( 2023 ) Cite this article
724 Accesses
8 Altmetric
Metrics details
- Outcomes research
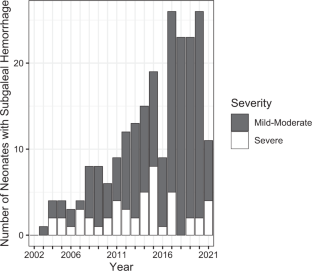
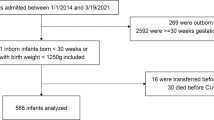
In 2011, we reported 38 neonates with subgaleal hemorrhage (SH), relating an increasing incidence. It is unclear whether the incidence in our hospitals continued to rise and which risk factors and outcomes are associated with this condition.
We retrospectively analyzed every recognized case of SH in our hospitals from the end of our previous report (2010) to the present (2022). We redescribed the incidence, scored severity, tabulated blood products transfused, and recorded outcomes.
Across 141 months, 191 neonates were diagnosed with SH; 30 after vacuum or forceps. The incidence (one/1815 births) was higher than in our 2011 report (one/7124 births). Also, severe SH (requiring transfusion) was more common (one/10,033 births vs. one/20,950 births previously). Four died (all with severe SH) and 12 had neurodevelopmental impairment.
Recognized cases of SH are increasing in our system without a clear explanation. Adverse outcomes are rare but continue to occur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Encephalopathy in neonates with subgaleal hemorrhage is a key predictor of outcome
Mohamed El-Dib, Melanie P Parziale, … Terrie Inder
Effectiveness of a care bundle for primary prevention of intraventricular hemorrhage in high-risk neonates: a Bayesian analysis
Benjamin J. S. al-Haddad, Brittany Bergam, … Kendell German

The prevalence of cardiac complications and their impact on outcomes in patients with non-traumatic subarachnoid hemorrhage
Maarit Lång, Stephan M. Jakob, … Stepani Bendel
Data availability
A deidentified dataset is available by written request to the corresponding author.
Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal haematoma: associated risk factors, complications and outcome. J Paediatr Child Health. 1996;32:228–32.
Article CAS PubMed Google Scholar
Levin G, Mankuta D, Eventov-Friedman S, Ezra Y, Koren A, Yagel S, et al. Factors associated with the severity of neonatal subgaleal haemorrhage following vacuum assisted delivery. Eur J Obstet Gynecol Reprod Biol. 2020;245:205–9.
Article PubMed Google Scholar
Levin G, Elchalal U, Yagel S, Eventov-Friedman S, Ezra Y, Sompolinsky Y, et al. Risk factors associated with subgaleal hemorrhage in neonates exposed to vacuum extraction. Acta Obstet Gynecol Scand. 2019;98:1464–72.
Boo NY, Foong KW, Mahdy ZA, Yong SC, Jaafar R. Risk factors associated with subaponeurotic haemorrhage in full-term infants exposed to vacuum extraction. BJOG 2005;112:1516–21.
Lee SJ, Kim JK, Kim SJ. The clinical characteristics and prognosis of subgaleal hemorrhage in newborn. Korean J Pediatr. 2018;61:387–91.
Article PubMed PubMed Central Google Scholar
Kilani RA, Wetmore J. Neonatal subgaleal hematoma: presentation and outcome—radiological findings and factors associated with mortality. Am J Perinatol. 2006;23:41–8.
Uchil D, Arulkumaran S. Neonatal subgaleal hemorrhage and its relationship to delivery by vacuum extraction. Obstet Gynecol Surv. 2003;58:687–93.
Swanson AE, Veldman A, Wallace EM, Malhotra A. Subgaleal hemorrhage: risk factors and outcomes. Acta Obstet Gynecol Scand 2012;91:260–3.
Liu LY, Antaya RJ. Neonatal subgaleal hematoma from trauma during vaginal delivery without instrument use. Pediatr Dermatol 2017;34:e40–e41.
El-Dib M, Parziale MP, Johnson L, Benson CB, Grant PE, Robinson J, et al. Encephalopathy in neonates with subgaleal hemorrhage is a key predictor of outcome. Pediatr Res. 2019;86:234–41.
Levin G, Mankuta D, Eventov-Friedman S, Ezra Y, Elchalal U, Yagel S, et al. Neonatal subgaleal hemorrhage unrelated to assisted vaginal delivery: clinical course and outcomes. Arch Gynecol Obstet. 2020;301:93–9.
Rohyans JA, Miser AW, Miser JS. Subgaleal hemorrhage in infants with hemophilia: report of two caes and review of the literature. Pediatrics 1982;70:306–7.
Wetzel EA, Kingma PS. Subgaleal hemorrhage in a neonate with factor X deficiency following a non-traumatic cesarean section. J Perinatol 2012;32:304–5.
Christensen RD, Baer VL, Henry E. Neonatal subgaleal hemorrhage in a multihospital healthcare system: prevalence, associations, and outcomes. E J Neo Res. 2011;1:1–8.
Google Scholar
Bahr TM, Kerry BB, Baserga MC, Christensen RD. Improving thermoregulation in transported preterm infants. J Perinatol. 2021;41:356–7.
Hulse W, Bahr TM, Fredrickson L, Canfield CM, Friddle K, Pysher TJ, et al. Warming blood products for transfusion to neonates: In vitro assessments. Transfusion 2020;60:1924–8.
PubMed Google Scholar
Plauche. Subgaleal hematoma. A complication of instrumental delivery. JAMA 1980;244:1597–8.
Hünseler C, Kribs A, Eifinger F, Roth B. Recombinant activated factor seven in acute life-threatening bleeding in neonates: report on three cases and review of literature. J Perinatol. 2006;26:706–13.
Strauss T, Kenet G, Schushan-Eisen I, Mazkereth R, Kuint J. Rescue recombinant activated factor VII for neonatal subgaleal hemorrhage. Isr Med Assoc J. 2009;11:639–40.
Legge N, Guaran R. Critical bleeding protocol for infants used for a catastrophic subgaleal hemorrhage. J Paed Child Health. 2022;58:542–5.
Article Google Scholar
Gertler R, Grubler M, Grassin-Delyle, Urien S, Martin K, Tassani-Prell P, et al. Pharmacokinetics of tranexamic acid in neonates and infants undergoing cardiac surgery. Br J Clin Pharm. 2017;83:1745–57.
Article CAS Google Scholar
Devereaux PJ, Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, et al. Tranexamic acid in patients undergoing noncardiac surgery. N. Engl J Med. 2022;26:386 1986–97.
Vanderspurt CK, Spinella PC, Cap AP, Hill R, Matthews SA, Corley JB, et al. The use of whole blood in US military operations in Iraq, Syria, and Afghanistan since the introduction of low-titer Type O whole blood: feasibility, acceptability, challenges. Transfusion 2019;59:965–70.
Bahr TM, DuPont TL, Morris DS, Pierson SE, Esplin MS, Brown SM, et al. First report of using low-titer cold-stored type O whole blood in massive postpartum hemorrhage. Transfusion 2019;59:3089–92.
Carr NR, Henry E, Bahr TM, Ohls RK, Page JM, Ilstrup SJ, et al. Fetomaternal hemorrhage: Evidence from a multihospital healthcare system that up to 40% of severe cases are missed. Transfusion 2022;62:60–70.
Carr NR, Hulse WL, Bahr TM, Davidson JM, Ilstrup SJ, Christensen RD First report of transfusing low-titer cold-stored type O whole blood to an extremely-low-birth-weight neonate after acute blood loss. Transfusion. 2022. https://doi.org/10.1111/trf.17034 .
Jacquot C, Mo YD, Luban NLC Transfusion Practices. In Neonatal Hematology, 3 rd Edition. Eds, de Alarcon PA, Werner EJ, Christensen RD, Sola-Visner MC Cambridge Medicine, Cambridge UK, 2021; pp 329–66.
Leeper CM, Yazer MH, Triulzi DJ, Neal MD, Gaines BA. Whole blood is superior to component transfusion for injured children: A propensity matched analysis. Ann Surg. 2020;272:590–4.
Leeper CM, Yazer MH, Cladis FP, Saladino R, Triulzi DJ, Gaines BA. Cold-stored whole blood platelet function is preserved in injured children with hemorrhagic shock. J Trauma Acute Care Surg. 2019;87:49–53.
Leeper CM, Yazer MH, Morgan KM, Triulzi DJ, Gaines BA. Adverse events after low titer group O whole blood versus component product transfusion in pediatric trauma patients: A propensity-matched cohort study. Transfusion. 2021;61:2621–8.
Morgan KM, Yazer MH, Triulzi DJ, Strotmeyer S, Gaines BA, Leeper CM. Safety profile of low-titer group O whole blood in pediatric patients with massive hemorrhage. Transfusion. 2021;61:S8–s14.
Salazar EG, Handley SC, Greenberg LT, Edwards EM, Lorch SA Measuring quality of care in moderate and late preterm infants. J Perinatol. 2022. https://doi.org/10.1038/s41372-022-01 .
Bi SY, Yu YH, Li C, Xu P, Xu HY, Li JH, et al. A standardized implementation of multicenter quality improvement program of very low birth weight newborns could significantly reduce admission hypothermia and improve outcomes. BMC Pediatr. 2022;14:281. 22.
Hausfeld K, Baker RB, Boettcher-Prior P, Hancock D, Helms C, Jablonski T, et al. Randomized prospective clinical trial comparing room temperature and warmed intravenous fluid boluses on pediatric patients’ comfort. J Pediatr Nurs. 2015;30:e3–9.
Blumenberg A. Dosing heat: expected core temperature change with warmed or cooled intravenous fluids. Ther Hypothermia Temp Manag 2021;11:223–9.
Nair SS, Sreedevi V, Nagesh DS. Warming of blood and intravenous fluids using low-power infra-red light-emitting diodes. J Med Eng Technol. 2021;45:614–26.
Mattson MK, Groves C, Smith MM, Christensen JM, Chen D, Stubbs JR, et al. Platelet transfusion: The effects of a fluid warmer on platelet function. Transfusion 2021;61:52–6.
Patel S, Ohls RK. Darbepoetin administration in term and preterm neonates. Clin Perinatol. 2015;42:557–66.
Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller JA, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin or placebo. Pediatrics 2014;133:1023–30.
Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, et al. Preschool assessment of preterm infants treated with darbepoetin and erythropoietin. Pediatrics 2016;137:1–9.
Burd J, Gomez J, Berghella V, Bellussi F, de Vries B, Phipps H, et al. Prophylactic rotation for malposition in the second stage of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol MFM 2022;4:100554 https://doi.org/10.1016/j.ajogmf.2021.100554 . Epub 2022 Feb 6.
Download references
Acknowledgements
The authors thank Molly Adams, Intermountain Healthcare Women and Newborn’s Research Department, for assistance with Institutional Review Board communications.
Author information
Authors and affiliations.
University of Utah, Student, Salt Lake City, UT, USA
Thomas R. Christensen
Obstetric and Neonatal Operations, Intermountain Healthcare, Salt Lake City, UT, USA
Timothy M. Bahr, Erick Henry, Con Yee Ling, Jessica M. Page, Nicholas R. Carr, Robin K. Ohls & Robert D. Christensen
Division of Neonatology, Department of Pediatrics, University of Utah, Salt Lake City, UT, USA
Timothy M. Bahr, Con Yee Ling, Taylor H. Hanton, Nicholas R. Carr, Robin K. Ohls & Robert D. Christensen
Transfusion Medicine, Intermountain Medical Center, Murray, UT, USA
Sarah J. Ilstrup
You can also search for this author in PubMed Google Scholar
Contributions
TRC: conceptualization, investigation, data curation, and review of final manuscript. TMB: conceptualization, investigation, data curation, data analysis, and review of final manuscript. EH: investigation, data curation, and review of final manuscript. CL: conceptualization and review of final manuscript. THH: conceptualization, investigation, data curation, and review of final manuscript. JMP: conceptualization and review of final manuscript. SJL: investigation, data curation, and review of final manuscript. NRC: conceptualization and review of final manuscript. RKO: conceptualization and review of final manuscript. RDC: conceptualization, investigation, data curation, initial draft of manuscript, and review of final manuscript.
Corresponding author
Correspondence to Timothy M. Bahr .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Treatments administered to 47 neonates with a severe subgaleal hemorrhage, emergency recognition and treatment of severe subgaleal hemorrhage in a newborn infant, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Christensen, T.R., Bahr, T.M., Henry, E. et al. Neonatal subgaleal hemorrhage: twenty years of trends in incidence, associations, and outcomes. J Perinatol 43 , 573–577 (2023). https://doi.org/10.1038/s41372-022-01541-z
Download citation
Received : 02 August 2022
Revised : 05 October 2022
Accepted : 12 October 2022
Published : 28 October 2022
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41372-022-01541-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Birth trauma.
Vikramaditya Dumpa ; Ranjith Kamity .
Affiliations
Last Update: August 28, 2023 .
- Continuing Education Activity
This activity reviews common birth-related injuries. The mechanism of injury, clinical features, and management of various birth-related traumatic events are described. An interprofessional team's role in the assessment, management, and prevention of birth-injuries is also highlighted.
- Describe the pathophysiology and clinical course of the common extracranial and intracranial hemorrhages.
- Review the mechanism of injury, clinical features, and prognosis of brachial plexus injuries.
- Outline the most common soft tissue injuries related to birth with a special emphasis on subcutaneous fat necrosis and its complications.
- Summarize the importance of an interprofessional team in the management and long term follow up of infants who sustain neurodevelopmental impairment from birth trauma.
- Introduction
The National Vital Statistics Report defines birth injury as "an impairment of the neonate's body function or structure due to an adverse event that occurred at birth." These injuries include a wide range of minor to major injuries due to various mechanical forces during labor and delivery. Birth injuries are different from birth defects or malformations and are often easily distinguishable from congenital defects by a focused clinical assessment. Birth trauma rates have steadily declined over the last few decades due to refinements in obstetrical techniques and the increased use of cesarean delivery in cases of dystocia or difficult vaginal deliveries. The birth trauma rate fell from 2.6 per 1000 live births in 2004 to 1.9 per 1000 live births in 2012. The rates of instrumental deliveries have also gradually declined over the past three decades, reducing the number of both forceps and vacuum-assisted deliveries. [1]
- Issues of Concern
The risk factors associated with birth trauma can group into those related to the fetus, pregnancy, mother, or iatrogenic factors (use of instrumentation during delivery). [2] [3]
- Macrosomia (estimated fetal weight greater than 4000g)
- Macrocephaly
- Very low birth weight and extreme prematurity
- Fetal congenital anomalies
- Oligohydramnios and malpresentation, including breech presentation as well as other abnormal presentations (such as the face, brow, or transverse)
- Maternal obesity,
- Maternal diabetes,
- Dephalopelvic disproportion
- Small maternal stature
- Primiparity
- Dystocia and difficult extraction
- Use of vacuum or forceps
- Prolonged or rapid labor
- Clinical Significance
The clinical management and prognosis of infants with birth injuries vary widely depending on the injury's type and severity. The common sites for birth trauma can include the head, neck, and shoulders. Other less common locations include the face, abdomen, and lower limbs. A summary of the common traumatic clinical conditions occurring related to birth is listed below.
Head Trauma
Head trauma can include superficial lesions, extracranial and intracranial hemorrhages, and fractures of the skull bones.
Caput Succedaneum: Caput succedaneum is a common scalp swelling in newborns. It is a subcutaneous swelling and edema of the scalp between the skin and the periosteum due to local venous congestion from the birth canal's pressure on the presenting part. The edema is above the plane of the periosteum, and hence the scalp swelling crosses suture lines. No intervention is required, and it typically resolves over the first few postnatal days. Rare complications include bruising of the skin over the swelling with necrosis resulting in scarring and alopecia, and rarely systemic infection.
Skull Fractures: Skull fractures from birth trauma are most often a result of instrumented vaginal delivery. These fractures could be linear or depressed and are usually asymptomatic unless associated with an intracranial injury. Plain film radiographs of the skull usually clarify the diagnosis. Still, computed tomography (CT) or magnetic resonance imaging (MRI) of the brain is recommended if there is suspicion of intracranial injury or neurologic symptoms.
Extracranial Hemorrhages:
Cephalohematoma: Cephalohematoma is a localized sub-periosteal collection of blood resulting from the rupture of blood vessels traversing from the skull to the periosteum. Limited by the periosteal attachment to the underlying skull bones, the swelling does not cross suture lines and is often unilateral. It is more common in deliveries involving vacuum or forceps and occurs in up to 2.5% of all deliveries. [5] The usual course is a spontaneous resolution in 2 weeks to 3 months without intervention. However, complications such as calcification, deformities of the skull, infection, and osteomyelitis can occur.
Subgaleal hemorrhage: subgaleal hemorrhage is a collection of blood in the loose areolar tissue space between the galea aponeurotica and the skull's periosteum. The injury occurs when traction pulls the scalp away from the stationary bony calvarium, resulting in the shearing or severing of the bridging vessels. A difficult vaginal delivery resulting in the use of forceps or vacuum is the most common predisposing event in the formation of subgaleal hemorrhage. It has been estimated to occur in 4 of 10000 spontaneous vaginal deliveries and 59 of 10000 vacuum-assisted deliveries. [6] Since the subgaleal space is a significant potential space extending over the entire area of the scalp from the anterior attachment of the galea aponeurosis near the frontal bones to the posterior attachment at the nape of the neck, there is a potential for massive bleeding into this space that could result in acute hypovolemic shock, multi-organ failure, and death. Treatment includes supportive care with early recognition and restoration of blood volume using blood or fresh frozen plasma to correct the acute onset hypovolemia. The hemorrhage itself is not drained and allowed to resorb over time. A workup for bleeding disorders may be considered in selected cases if the degree of bleeding is out of proportion to the trauma at birth.
Intracranial Hemorrhages:
Traumatic intracranial hemorrhages include epidural, subdural, subarachnoid, intraventricular, and less frequently intracerebral and intracerebellar hemorrhages.
Epidural hemorrhage is rare in neonates and usually accompanies linear skull fractures in the parietal-temporal region following an operative delivery. Signs include bulging fontanelle, bradycardia, hypertension, irritability, altered consciousness, hypotonia, seizures. Diagnosis is via CT or MRI of the head, which shows a convex appearance of blood collection in the epidural space. Prompt neurosurgical intervention is necessary due to the potential to deteriorate rapidly.
Subdural Hemorrhage is the most common type of intracranial hemorrhage in neonates. Operative vaginal delivery is a major risk factor, and hemorrhage over the cerebral convexities is the most common site. Presenting signs/symptoms include bulging fontanelle, altered consciousness, irritability, respiratory depression, apnea, bradycardia, altered tone, and seizures. Subdural hemorrhages can occasionally be found incidentally in asymptomatic neonates. Management depends on the location and extent of the bleeding. Surgical evacuation is reserved for extensive hemorrhages causing raised intracranial pressure and associated clinical signs.
Subarachnoid Hemorrhage is the second most common type of neonatal intracranial hemorrhage and is usually the result of the rupture of bridging veins in the subarachnoid space. Operative vaginal delivery is a risk factor, and the infants are typically asymptomatic unless the hemorrhage is extensive. Ruptured vascular malformations are a rare cause of subarachnoid hemorrhages, even in the neonatal population. Treatment is usually conservative.
Intraventricular hemorrhage even though most commonly seen in premature infants, can also occur in term infants depending on the nature and extent of the birth injury. [7]
Intracerebral and intracerebellar hemorrhages are less common and occur as a result of occipital diastasis.
Cranial Nerve injuries:
Facial nerve: Facial nerve is the most common cranial nerve-injured with a traumatic birth. It occurs in up to 10 per 1000 live births and is usually a result of pressure on the facial nerve by forceps or from a prominent maternal sacral promontory during descent. Clinical manifestations include diminished movement or loss of motion on the affected side of the face. Facial nerve palsy requires differentiation from asymmetric crying facies, which results from congenital hypoplasia of the depressor anguli oris muscle and causes a localized movement abnormality of the corner of the mouth. Although forceps delivery has a strong association, facial palsy can occur in the newborn without apparent trauma. [8] The prognosis in traumatic facial nerve injury is good, with spontaneous resolution usually noted within the first few weeks of life.
Peripheral Nerve and Spinal Cord Injuries
Brachial plexus injuries: These occur in up to 2.5 per 1000 live births and result from stretching of the cervical nerve roots during the process of delivery. These injuries are usually unilateral, and risk factors include macrosomia, shoulder dystocia, difficult delivery, breech position, multiparity, and assisted deliveries. [9]
- Injury involving the fifth and sixth cervical nerve roots results in Erbs-Duchenne palsy manifested by the upper arm's weakness. Adduction and internal rotation of the arm with flexion of the fingers are presenting symptoms; this is by far the most common form of brachial plexus injury.
- Injury to the eighth cervical and first thoracic nerves results in Klumpke's palsy manifested by paralysis of the hand's muscles, absent grasp reflex, and sensory impairment along the ulnar side of the forearm and arm.
- Injury to all the nerve roots can result in total arm paralysis.
- Injury to the phrenic nerve can be an associated feature of brachial palsy. Clinical manifestations include tachypnea with asymmetric chest motion and diminished breath sounds on the affected side.
The majority of brachial plexus injuries are stretch injuries, and treatment is conservative, with physical therapy playing a major role in the return of gradual function. [10] Rare, severe cases of brachial plexus injuries result in lasting weakness on the affected side.
Spinal cord: Spinal cord injuries are infrequent in the neonatal period and are usually a result of excessive traction or rotation of the spinal cord during extraction. [11] The clinical manifestations depend on the type and location of the lesion. Higher lesions (cervical/upper thoracic) are associated with a high mortality rate, and lower lesions (lower thoracic, lumbosacral) may result in significant morbidity with bladder and bowel dysfunction. Diagnosis is via ultrasonography or MRI of the spinal cord. The management aims towards presenting clinical symptomatology with cardiorespiratory stabilization as needed.
Skeletal injuries:
Most of the fractures resulting from birth trauma are associated with difficult extractions or abnormal presentations. Clavicular fractures are the most common bone fracture during delivery and can occur in up to 15 per 1000 live births. The clinical presentation is significant for crepitus at the site of fracture, tenderness, and decreased movement of the affected arm with an asymmetric Moro reflex. Clavicular fractures have a good prognosis with spontaneous healing occurring in the majority of infants. The humerus is the most common long bone to fracture during birth, which can be associated with a brachial plexus injury. The clinical presentation could be similar to a clavicular fracture with an asymmetric Moro reflex, inability to move the affected arm. Also, a significant deformity might be noted on the affected arm with swelling and tenderness at the fracture site. Rare conditions may involve a distal humeral epiphyseal separation due to birth trauma requiring expert orthopedic intervention. [12] In general, immobilization for 3 to 4 weeks is necessary and often heals well without deformities. Other fractures, such as femur fracture, rib fractures, can occur during birth but are rare. [13] On the other hand, femur fractures are extremely rare in newborns and may be seen in difficult vaginal breech extraction deliveries. Diagnosis is made by clinical exam with tenderness, swelling, and deformity of the thigh and confirmed further on plain radiographs. Orthopedic consultation is the recommendation for long bone fractures for appropriate immobilization.
Facial injuries:
Ocular injuries: Subconjunctival hemorrhages (SCH) are superficial hematomas seen under the bulbar conjunctiva, commonly seen in infants born after going through labor. It is suggested to be due to ruptured subconjunctival capillaries from venous congestion, occurring from increased back pressure in the head and neck veins. This injury can result from either a nuchal cord or increased abdominal or thoracic compression during uterine contractions. [14] SCH is a benign condition in the newborn and resolves without intervention. A more significant ocular injury may occur with the use of instrumentation during delivery (forceps), resulting in corneal abrasions, vitreous hemorrhages, etc. that require immediate attention and referral to an ophthalmologist to prevent long term visual defects. [15] [16]
Soft tissue injuries:
Soft tissue injuries resulting from birth trauma include petechiae, bruising, ecchymoses, lacerations, and subcutaneous fat necrosis. Subcutaneous fat necrosis is thought to result from ischemic injury to the adipose tissue and characterized by palpation of soft, indurated nodules in the subcutaneous plane. These lesions resolve gradually over a few weeks. Hypercalcemia is one of the complications; therefore, it is recommended to monitor serum calcium. [17] There are reports of accidental lacerations during cesarean section deliveries, with an Italian study showing a 3% incidence of accidental lacerations during cesarean sections, and a higher incidence in emergent deliveries compared to scheduled cesarean deliveries.[18]
Visceral injuries:
Birth trauma resulting in abdominal visceral injuries is uncommon and primarily consists of hemorrhage into the liver, spleen, or adrenal gland. The clinical presentation depends on the volume of blood loss and can include pallor, bluish discoloration of the abdomen, distension of the abdomen, and shock. Treatment is supportive with volume resuscitation and surgical intervention if needed.
- Enhancing Healthcare Team Outcomes
Birth trauma in a newborn has a varied presentation depending on the type of injury sustained. The affected newborns may present with mild benign signs and symptoms to severe life-threatening signs and symptoms. Injuries occur at birth for various reasons, secondary to maternal, fetal, or external risk factors. The prognosis of the birth injuries also depends on the type and severity of the initial injury. A detailed physical exam of the newborn is warranted at birth to identify birth injuries and differentiate them from congenital malformations or birth defects. Extracranial hemorrhages typically heal well with occasional complications of hyperbilirubinemia, infections. Subgaleal hemorrhage requires careful clinical observation and monitoring due to the potential for life-threatening acute severe hypovolemia. If large, intracranial hemorrhages can result in focal neurological damage and required prompt intervention, including a neurosurgical evaluation. The prognosis of spinal cord injuries depends on the level of injury. Lesions below T4 have a comparatively better prognosis. Superficial soft tissue injuries generally heal well without any residual sequelae. The majority of brachial plexus injuries resolve within a few weeks, but physical therapy and close follow-up are mandated. Occasional cases of brachial plexus injuries can have long-lasting weakness or dysfunction (level III evidence). [18] The prognosis is also good for facial nerve injuries, with a complete recovery expected within a few weeks in a majority of infants.
A vital element in this context is the prevention of birth trauma in the first place using an interprofessional team involving obstetricians, neonatologists, pediatricians, radiologists, and specialty trained nurses. Advances in antenatal care have resulted in increased awareness of fetal malformations and malpresentation. Potential problems can be anticipated before delivery, thereby leading to improved preparation for a high-risk delivery. Hence pregnant women need to adhere to prenatal care recommendations for optimal outcomes. A coordinated educational effort involving the clinician, nurse midwife, and specialty-trained nurse reinforcing good prenatal efforts will decrease the incidence of untoward events. It is also important to acknowledge that not all birth-related injuries are iatrogenic or preventable. Infants who sustain birth injuries that place them at risk for neurodevelopmental impairment should be monitored closely by an interprofessional team to appropriately attain developmental milestones. This team should include a pediatrician, labor and delivery nurse, physical and occupational therapist, and a developmental-behavioral pediatrician supported by the clinical and nursing staff's coordination of care. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Vikramaditya Dumpa declares no relevant financial relationships with ineligible companies.
Disclosure: Ranjith Kamity declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Dumpa V, Kamity R. Birth Trauma. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. [Semin Perinatol. 2006] Review Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Hankins GD, Clark SM, Munn MB. Semin Perinatol. 2006 Oct; 30(5):276-87.
- Temporal trends in severe maternal and neonatal trauma during childbirth: a population-based observational study. [BMJ Open. 2018] Temporal trends in severe maternal and neonatal trauma during childbirth: a population-based observational study. Wen Q, Muraca GM, Ting J, Coad S, Lim KI, Lisonkova S. BMJ Open. 2018 Mar 2; 8(3):e020578. Epub 2018 Mar 2.
- Severe maternal and neonatal morbidity after attempted operative vaginal delivery. [Am J Obstet Gynecol MFM. 2021] Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Panelli DM, Leonard SA, Joudi N, Girsen AI, Judy AE, El-Sayed YY, Gilbert WM, Lyell DJ. Am J Obstet Gynecol MFM. 2021 May; 3(3):100339. Epub 2021 Feb 23.
- Mode of delivery in nulliparous women and neonatal intracranial injury. [Obstet Gynecol. 2011] Mode of delivery in nulliparous women and neonatal intracranial injury. Werner EF, Janevic TM, Illuzzi J, Funai EF, Savitz DA, Lipkind HS. Obstet Gynecol. 2011 Dec; 118(6):1239-1246.
- Morbidity and Mortality Associated With Forceps and Vacuum Delivery at Outlet, Low, and Midpelvic Station. [J Obstet Gynaecol Can. 2019] Morbidity and Mortality Associated With Forceps and Vacuum Delivery at Outlet, Low, and Midpelvic Station. Muraca GM, Sabr Y, Lisonkova S, Skoll A, Brant R, Cundiff GW, Joseph KS. J Obstet Gynaecol Can. 2019 Mar; 41(3):327-337. Epub 2018 Oct 23.
Recent Activity
- Birth Trauma - StatPearls Birth Trauma - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Face presentation at term: incidence, risk factors and influence on maternal and neonatal outcomes
- Maternal-Fetal Medicine
- Published: 09 April 2024
Cite this article
- Yongqing Zhang 1 na1 ,
- Tiantian Fu 1 na1 ,
- Luping Chen 1 ,
- Yinluan Ouyang 1 ,
- Xiujun Han 1 &
- Danqing Chen ORCID: orcid.org/0000-0002-0201-7215 1
The incidence, diagnosis, management and outcome of face presentation at term were analysed.
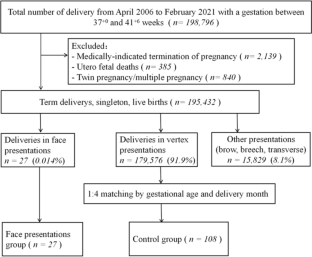
A retrospective, gestational age-matched case–control study including 27 singletons with face presentation at term was conducted between April 2006 and February 2021. For each case, four women who had the same gestational age and delivered in the same month with vertex position and singletons were selected as the controls (control group, n = 108). Conditional logistic regression was used to assess the risk factors of face presentation. The maternal and neonatal outcomes of the face presentation group were followed up.
The incidence of face presentation at term was 0.14‰. After conditional logistic regression, the two factors associated with face presentation were high parity (adjusted odds ratio [aOR] 2.76, 95% CI 1.19–6.39)] and amniotic fluid index > 18 cm (aOR 2.60, 95% CI 1.08–6.27). Among the 27 cases, the diagnosis was made before the onset of labor, during the latent phase of labor, during the active phase of labor, and during the cesarean section in 3.7% (1/27), 40.7% (11/27), 11.1% (3/27) and 44.4% (12/27) of cases, respectively. In one case of cervical dilation with a diameter of 5 cm, we innovatively used a vaginal speculum for rapid diagnosis of face presentation. The rate of cesarean section and postpartum haemorrhage ≥ 500 ml in the face presentation group was higher than that of the control group (88.9% vs. 13.9%, P < 0.001, and 14.8% vs. 2.8%, P = 0.024), but the Apgar scores were similar in both sets of newborns. Among the 27 cases of face presentation, there were three cases of adverse maternal and neonatal outcomes, including one case of neonatal right brachial plexus injury and two cases of severe laceration of the lower segment of the uterus with postpartum haemorrhage ≥ 1000 ml.
Conclusions
Face presentation was rare. Early diagnosis is difficult, and thus easily neglected. High parity and amniotic fluid index > 18 cm are risk factors for face presentation. An early diagnosis and proper management of face presentation could lead to good maternal and neonatal outcomes.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
The original data can be provided by E-mail if needed.
Code availability
Cunningham FG, Williams JW (2018) Williams obstetrics, 25th edn. McGraw-Hill Medical, New York, pp 450–452
Google Scholar
Arsène E, Langlois C, Clouqueur E, Deruelle P, Subtil D (2019) Prognosis for deliveries in face presentation: a case-control study. Arch Gynecol Obstet 300:869–874. https://doi.org/10.1007/s00404-019-05241-6
Article PubMed Google Scholar
Cruikshank DP, Cruikshank RN (1981) Face and brow presentation. A review. Clin Obstet Gynecol 24:333–351. https://doi.org/10.1097/00003081-198106000-00003
Article CAS PubMed Google Scholar
Schwartz Z, Dgani R, Lancet M, Kessler I (1986) Face presentation. Aust N Z J Obstet Gynaecol 26:172–176. https://doi.org/10.1111/j.1479-828x.1986.tb01560.x
Bashiri A, Burstein E, Bar-David J, Levy A, Mazor M (2008) Face and brow presentation: independent risk factors. J Matern Fetal Neonatal Med 21:357–360. https://doi.org/10.1080/14767050802037647
Arsene E, Langlois C, Garabedian C, Clouqueur E, Deruelle P, Subtil D (2016) Prenatal factors related to face presentation: a case–control study. Arch Gynecol Obstet 294:279–284. https://doi.org/10.1007/s00404-015-3992-7
Tapisiz OL, Aytan H, Altinbas SK, Arman F, Tuncay G, Besli M et al (2014) Face presentation at term: a forgotten issue. J Obstet Gynaecol Res 40:1573–1577. https://doi.org/10.1111/jog.12369
Lau WL, Cho LY, Leung WC (2011) Intrapartum translabial ultrasound demonstration of face presentation during first stage of labor. J Obstet Gynaecol Res 37:1868–1871. https://doi.org/10.1111/j.1447-0756.2011.01650.x
Shaffer BL, Cheng YW, Vargas JE, Laros RK Jr, Caughey AB (2006) Face presentation: predictors and delivery route. Am J Obstet Gynecol 194:e10-12
Benedetti TJ, Lowenshon RI, Truscott AM (1980) Face presentation at term. Obstet Gynecol 55:199–202
CAS PubMed Google Scholar
Pilliod RA, Caughey AB (2017) Fetal malpresentation and malposition: diagnosis and management. Obstet Gynecol Clin N Am 44:631–643. https://doi.org/10.1016/j.ogc.2017.08.003
Article Google Scholar
Bellussi F, Ghi T, Youssef A, Salsi G, Giorgetta F, Parma D et al (2017) The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol 217:633–641. https://doi.org/10.1016/j.ajog.2017.07.025
Ducarme G, Ceccaldi PF, Chesnoy V, Robinet G, Gabriel R (2006) Face presentation: retrospective study of 32 cases at term. Gynecol Obstet Fertil 34:393–396. https://doi.org/10.1016/j.gyobfe.2005.07.042
De Bernardo G, Svelto M, Giordano M, Sordino D (2017) Face presentation in delivery room: what is strategy? BMJ Case Rep. https://doi.org/10.1136/bcr-2016-219114
Article PubMed PubMed Central Google Scholar
Manning JB, Tolcher MC, Chandraharan E, Rose CH (2015) Delivery of an impacted fetal head during cesarean: a literature review and proposed management algorithm. Obstet Gynecol Surv 70:719–724. https://doi.org/10.1097/OGX.0000000000000248
Download references
Acknowledgements
The authors wish to acknowledge Menglin Zhou, Zhengyun Chen and Guohui Yan for their valuable assistance for the manuscript.
No specific funding was obtained for this study.
Author information
Yongqing Zhang and Tiantian Fu have contributed equally to this work.

Authors and Affiliations
Department of Obstetrics, School of Medicine, Women’s Hospital, Zhejiang University, 1st Xueshi Road, Hangzhou, 310006, Zhejiang, People’s Republic of China
Yongqing Zhang, Tiantian Fu, Luping Chen, Yinluan Ouyang, Xiujun Han & Danqing Chen
You can also search for this author in PubMed Google Scholar
Contributions
YZ: conceptualization, methodology, writing—original draft. TF: conceptualization, formal analysis, writing—original draft. LC: data collection, follow-up. YO: investigation, resources. XH: investigation, formal analysis, supervision. DC: conceptualization, writing—review and editing, supervision. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Xiujun Han or Danqing Chen .
Ethics declarations
Conflict of interest.
All authors have no conflicts of interest to disclose.
Ethics approval
Our study was planned in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Local Ethics Committee of the Women’s hospital, school of medicine, Zhejiang university ( Ethical No. IRB-20210211-R ).
Consent for publication
Consent to participate.
As this was a retrospective study, written informed consents were not obtained, but all patients’ records/information were anonymized before analysis. The images used in this paper (Figs. 1 , 2 , 3 ) were given informed consent by the pregnant woman.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Zhang, Y., Fu, T., Chen, L. et al. Face presentation at term: incidence, risk factors and influence on maternal and neonatal outcomes. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07406-4
Download citation
Received : 09 July 2023
Accepted : 28 January 2024
Published : 09 April 2024
DOI : https://doi.org/10.1007/s00404-024-07406-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Face presentation
- Risk factors
- Brachial plexus injury
- Postpartum haemorrhage
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 08 April 2024
Clinical analysis of complete uterine rupture during pregnancy
- Jing Xie 1 , 2 ,
- Xuefang Lu 3 &
- Miao Liu 1 , 2
BMC Pregnancy and Childbirth volume 24 , Article number: 255 ( 2024 ) Cite this article
76 Accesses
Metrics details
Uterine rupture in pregnant women can lead to serious adverse outcomes. This study aimed to explore the clinical characteristics, treatment, and prognosis of patients with complete uterine rupture.
Data from 33 cases of surgically confirmed complete uterine rupture at Chenzhou No.1 People’s Hospital between January 2015 and December 2022 were analyzed retrospectively.
In total, 31,555 pregnant women delivered in our hospital during the study period. Of these, approximately 1‰ ( n = 33) had complete uterine rupture. The average gestational age at complete uterine rupture was 31 +4 weeks (13 +1 –40 +3 weeks), and the average bleeding volume was 1896.97 ml (200–6000 ml). Twenty-six patients (78.79%) had undergone more than two deliveries. Twenty-five women (75.76%) experienced uterine rupture after a cesarean section, two (6.06%) after fallopian tube surgery, one (3.03%) after laparoscopic cervical cerclage, and one (3.03%) after wedge resection of the uterine horn, and Fifteen women (45.45%) presented with uterine rupture at the original cesarean section incision scar. Thirteen patients (39.39%) were transferred to our hospital after their initial diagnosis. Seven patients (21.21%) had no obvious symptoms, and only four patients (12.12%) had typical persistent lower abdominal pain. There were 13 cases (39.39%, including eight cases ≥ 28 weeks old) of fetal death in utero and two cases (6.06%, both full term) of severe neonatal asphyxia. The rates of postpartum hemorrhage, blood transfusion, hysterectomy were 66.67%, 63.64%, and 21.21%. Maternal death occurred in one case (3.03%).
Conclusions
The site of the uterine rupture was random, and was often located at the weakest point of the uterus. There is no effective means for detecting or predicting the weakest point of the uterus. Rapid recognition is key to the treatment of uterine rupture.
Peer Review reports
Uterine rupture(UR) is a serious complication that directly jeopardizes the life of the mother and the fetus [ 1 ]. It refers to the rupture of the uterine body or the lower uterine segment in late pregnancy or during labor [ 2 ], requiring a cesarean section to terminate the pregnancy as soon as the diagnosis is confirmed. The most common risk factors of UR are a history of previous cesarean section (CS), myomectomy, multiparity, malpresentation, breech extraction, and instrumental deliveries [ 3 ].
The incidence of uterine rupture in China has recently been reported to range from 0.1% to 0.55% [ 4 ]; although this incidence rate is low, UR is highly likely to lead to serious adverse outcomes.
Currently, there are no effective means for detecting or predicting the weakest points of the uterus. Therefore, in this study, we aimed to provide reference information and practical experience for the early recognition, management and emergency treatment of uterine rupture.
The Department of Obstetrics and Gynaecology of the First people’s Hospital of Chenzhou is a critical care center wherein treatment, consultation, referral, and technical guidance are proviede to pregnant women in Southern Hunan and the city with acute and critical illnesses. This was a retrospective study aimed at exploring the clinical characteristics, treatment, and prognosis of patients with complete uterine rupture between January 2015 and December 2022. The data (complete clinical data, medical history, and surgical records) of all patients with complete uterine rupture admitted to our hospital were retrospectively analyzed.
Diagnostic criteria
Complete uterine rupture was defined as rupture of the entire wall of the uterine myometrium, with the uterine cavity communicating with the abdominal cavity during late pregnancy or labor [ 1 ].
Postpartum hemorrhage (PPH) was defined as bleeding of ≥ 500 ml for vaginal delivery and ≥ 1000 ml for cesarean delivery within 24 h after delivery of the fetus [ 1 ].
Research methods
Basic maternal information (age, pregnancies, number of deliveries), previous pregnancy and surgery-related indicators (risk factor, causes and clinical manifestations, comorbidities, distance between periconceptional caesarean section scar and vesicovaginal fold), situation at the time of uterine rupture (gestational age, interval between the current pregnancy and previous cesarean section delivery, rupture site and length, bleeding volume and number of required blood transfusions, minimum hemoglobin level), mode of the current delivery (induced delivery, transvaginal delivery, or cesarean section), and outcomes of the mother and child (postpartum hemorrhage, hysterectomy, maternal death, perinatal deaths, severe neonatal asphyxia (Apgar scores are recorded at 1, 5, and 10 min after birth, with a score below or equal to 3 indicating severe asphxia) were cllected from the patients’ medical records. This study meticulously adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines.
General information
This was a retrospective study aimed at exploring the clinical characteristics, treatment, and prognosis of all patients with complete uterine rupture between January 2015 and December 2022. The data (complete clinical data, medical history, and surgical records) of all patients with uterine rupture admitted to our hospital were retrospectively analyzed. Thirty-three patients with surgically confirmed complete uterine rupture were included into the study.
Incidence of uterine rupture in our hospital
The total number of pregnant women who delivered in our hospital during the study was 31,555, with 33 cases of complete uterine rupture, accounting for approximately 0.1%(Table 1 ). The average gestational age at complete uterine rupture was 31 +4 weeks (13 +1 –40 +3 weeks), and the average bleeding volume was 1896.97 ml (200–6000 ml) (Table 2 ).
Causes of uterine rupture
The causes of uterine rupture are shown in Table 3 . Ten patients (30.3%) had a history of cesarean section and rupture at an incision site other than the original cesarean section. Patient 2 was involved in a car accident. Patient 11 underwent an elective cesarean section, and uterine rupture was found intraoperatively: the blood flow around the rupture was not rich, the bleeding was not much, and there were no obvious symptoms. Patient 14 had a history of laparoscopic right tubal surgery with poor symptomatology due to adhesion coverage. Patient 16 had a history of cesarean section and wedge resection of the right uterine horn (> 5 years prior), two artificial abortions (AA2), and one induction of labor in middle pregnancy (20 + weeks gestation, fetal anomaly, postpartum evacuation), with poor symptomatology due to adhesion coverage. Patient 27 has a cesarean section after transabdominal cerclage (intraoperative discovery of placental implantation), with uterine rupture in the cerclage line. Patient 33 had a history of two cesarean sections; this time, she was treated with ritodrine for fetal preservation and low molecular heparin in an outside hospital due to the presence of contractions, small vaginal bleeding, fast heart rhythm, and incomplete suppression of contractions, which were not taken seriously. She was transferred to our hospital for shock and stillbirth where she underwent an emergency cesarean section.
Six patients (18.18%) had a history of uterine operation. Patient 17 had a history of one AA and two vaginal births (VB). She was involved in coitus multiple times in the week prior to the delivery, and the night before delivery, resulting in premature rupture of the membranes; she did not notify the medical staff, and the labor did not come to term. The cervical canal did not open, HS-1 (the lowest point of the fetal skull is 1 cm below the sciatic ischiadica), and 10 min after using oxytocin, cervical dilatation was at 3 cm. Oxytocin was discontinued once abnormal fetal presentation was observed. A cesarean section was performed immediately fetal heart monitoring revealed a deceleration. Patient 20 underwent a breech vaginal trial of labor, with difficulty delivering the fetal head, vaginal rupture, and uterine rupture.
Clinical signs and symptoms of uterine rupture
The clinical signs and symptoms associated with complete uterine rupture are shown in Table 4 .
There were a few special cases. Patient 1 had an intellectual disability and was unable to express her discomfort accurately. Patient 25 had a metal ring at the breach site. Patient 10 had a cesarean scar pregnancy (CSP) with abdominal blood accumulation (mass) of approximately 3200 ml. Four (12.21%) placental implantation at the incision sites. Three patients presented with severe postpartum hemorrhage, and two of them underwent hysterectomies. The third patient had a repeat vaginal delivery after three VB, two cesarean sections (CS), and one vaginal birth after cesarean (VBAC), and was transferred to our hospital with hemorrhage after delivery; intraoperative rupture of the original cesarean section incision and placenta implantation at the rupture site were observed. Patient 21 had a post-VBAC.
Treatment of uterine rupture and maternal and fetal outcomes
The fetal outcomes and treatment of uterine rupture are shown in Table 5 . Postpartum hemorrhage did not occur in 11 patients (33.33%); six (18.18%) were found to have uterine rupture during full-term, elective surgery, with little blood flow around the rupture, and little bleeding with no obvious symptoms. Two patients (6.06%) had severe adhesions. One (Patient 21) was promptly delivered by cesarean section due to abnormal fetal heart rate monitoring; and two (Patients 28 and 29) had uterine tenderness after ethacridine administration and promptly underwent cesarean section.
Incidence of complete uterine rupture
Since the opening of the separate two-child policy in 2013, full liberalization of the two-child policy in 2016, and opening of the three-child policy in 2021, the cesarean section rate in China has increased from 34.9% (2014) to 41.1% (2016) [ 15 ]. Following this, the rate of scarred uterus has increased from 9.8% (2012) to 17.7% (2016) [ 16 ], which is far beyond the World Heallth Organizations ideal range.
The incidence of uterine rupture has been reported in several countries and regions; it is not consistent across countries and regions. This rate is related not only to the high-risk factors of the pregnant women themselves, but also to the economic level of each country, number of years of occurrence (which is related to the country's policy at that time), level of medical care, and transportation status (referral time). The reported incidence of uterine rupture: is 0.06% in Northern Europe [ 17 ], 0.67% in the University of Pakistan Teaching Hospital [ 18 ], 0.01% in the First Maternity and Infant Hospital of Shanghai [ 19 ], 0.2% in the Jiangxi Maternity and Child Healthcare Hospital [ 20 ], and 0.05% in the Women's Hospital of the Medical College of Zhejiang University [ 21 ]. Following the latest data [ 22 ], the total incidence of uterine rupture in China is 0.13%, consistent with 0.1% found in this study.
Analysis of the etiology and risk factors for complete uterine rupture
Due to the low cesarean section rate and the large number of patients with two or multiple deliveries between 1960 and 1990, uterine rupture was the predominantly primary. After 1990, with the implementation of family planning policies, the cesarean section rate increased, and subsequently, cesarean section scar rupture became the primary cause of uterine rupture [ 23 ]. Therefore, with the improvement in medical standards, doctors' awareness of uterine rupture, and repeated emergency drills, the main etiology has changed.
The known etiologies and risk factors for uterine rupture are as follows [ 1 , 23 ].
Previous uterine injury or history of abnormalities.
A history of myometrial surgery and short or long intervals between surgeries, which include cesarean section (incidence of uterine rupture was 0.071% [ 21 ], 0.095% for a history of one cesarean Sect [ 24 ]., and 1.92% for a history of two or more cesarean Sects [ 24 ].), history of repair of uterine rupture (33% [ 25 ]), myomectomy, wedge resection of the uterine horn (incidence of uterine rupture is up to 30% [ 26 ]), tubal surgery, hysteroscopic septum resection (incidence of uterine rupture is 1.0%–2.7% [ 27 , 28 ]), and separation of adhesions in the uterine cavity. Human Immunodeficiency Virus (HIV) infection may increase the incidence of cesarean delivery complications [ 29 ]. Current research suggests that the uterine wound healing takes 12 weeks [ 30 ], while myometrial incision healing and scar formation takes 6–12 months [ 31 ]; however, wound healing does not mean that it is able to withstand the pressure of pregnancy immediately. It also undergoes a process of tissue reconstruction, which further strengthens the elasticity of the uterine myometrial wall in the area of the scar. Therefore, less than 12–18 months is a high-risk factor for uterine rupture [ 32 ], and 2–3 years after cesarean section is the optimal period for uterine incision healing [ 33 ]. After > 5 years [ 6 ], the uterine scar’s degree of muscularization will gradually deteriorate and it will lose elasticity, making uterine rupture more likely during another pregnancy. The risk of uterine rupture for second pregnancies has been reported in the literature, even when tubal surgery does not involve the mesosalpinx or uterine horn, with a higher risk in the presence of electrocoagulation injuries, injury to or absence of part of the myometrial layer of the uterine horn, localized unsutures, and short intervals between pregnancies. The incidence of uterine rupture in our study was 0.79 per 1000 in those with a history of cesarean section, 0.057% in those with a history of one cesarean section, and 0.022% in those with a history of two or more cesarean sections. In our study, 11 patients (33.33%) had an interval of pregnancy out of 1.5 and 5 years, two (6.06%) had a history of tubal surgery, one (3.03%) had a history of wedge resection of the uterine horn, and one (3.03%) was a person living with HIV. It is important to note that in patients with a history of uterine rupture, the rupture was not at the same location. Uterine injuries include abortion, curettage, as well as sharp or blunt contusions such as car accidents, knives, and hidden uterine ruptures. In the present study, six (18.18%) patients had a history of intrauterine manipulation as a risk factor. Congenital abnormalities include uterine dysplasia, and connective tissue defects. In the present study, two patients (6.06%) had a double uterus. Furthermore, one patient (case 7) had a history of transabdominal cervical cerclage. During pregnancy, the cerclage line increases with the uterus, creating a chronic transverse cutting effect on the cervix, Uterine rupture occurs at the site of the cervical cut once there is significant uterine contraction.
Combined uterine injuries or abnormalities in this pregnancy
Postnatal etiologies include advanced age, muliple pregnancies and deliveries, spontaneous tonic uterine contractions, excessive contractions due to the use of oxytocin or prostaglandins and maternal sensitivity to drugs, prostaglandin or saline intra-amniotic infusion, sharp forceps contusion, external inversion, amniotic fluid overload, or multiple pregnancies. In this study, one patient (3.03%) was treated with uterotonics and three (9.09%) underwent induction of labor.
Intrapartum etiologies include any mechanism leading to obstruction of fetal descent, such as pelvic stenosis, cephalopelvic disproportion, obstruction of the soft birth canal, abnormal fetal position, and macrosomia; internal inversion; forceps delivery; emergency labor; breech traction; fetus destruction; excessive dilatation of the uterus in the lower part of the uterus caused by fetal anomalies; excessive pressure in the uterine cavity during delivery; implantation of the placenta or severe adhesion; and difficulty in manually stripping the placenta. One patient (case 20) in this study had uterine rupture due to breech traction during vaginal delivery and eight (24.24%) had placenta implantation.
Acquired etiologies include gestational trophoblastic disease, adenomyosis, posterior flexion uterine implantation, and uterine artery embolization surgery. One patient in this study had adenomyosis.
CSP involves a poorly healed uterine incision, wide scarring, and inflammation, leading to the development of microscopic fissures through which the fertilized ovum is deposited into the myometrium. Most cases have a poor prognosis [1]. Only one case of CSP was reported in this study.
Placental implantation: when it occurs at the site of the original cesarean section scar is caused by a structural defect in the endometrium [ 23 ] that allows the placenta to attach abnormally to the uterine myometrium. In our study, four patients (12.12%) had placental implantation, with three who had severe postpartum hemorrhage and two who underwent hysterectomies.
Trial of labor after cesarean (TOLAC), during a second pregnancy or request for a vaginal trial of labor; A meta-analysis showed that TOLAC results in a 0.27% higher risk of uterine rupture [ 9 ]. The management of TOLAC is a multifactorial. Factors [ 34 , 35 ] such as a previous vaginal delivery, use of epidural anesthesia, indication for previous cesarean section, pregnancy complications (such as preeclampsia, and placental anomalies), fetal weight above 4000 g, dose of oxytocin used, induction of labor with prostaglandins, women who delivered at > 41 +0 weeks of gestation, ethnicity, cervical length, head-perineum distance, maternal age (maybe), inter-delivery interval, body mass index (maybe), and prolonged second stage of labor (maybe) contribute to uterine rupture during TOLAC. However, there are no data or literature supporting whether to perform a TOLAC and assess the risk of uterine rupture in a second pregnancy after a history of two cesarean deliveries and after one VBAC.
Endometriosis causes tissue adhesions. Surgical separation of these adhesions results in localized myometrial destruction and thinning, affecting the healing, brittleness, and elasticity of the scar. Patient 6 had this clinical presentation. It has also been shown that the distance from the cesarean scar to the vesicovaginal fold (suggestive of the horizontal position of the uterine incision from the previous cesarean section) is significantly increased in patients with a gestational age > 22 weeks and antepartum uterine rupture, and may be predictive of uterine rupture [ 9 ].
Regardless of how the uterus is damaged, scarring occurs during the repair process, which constitutes non-normal muscle tissue (connective and scar tissue) [ 1 ]. This forms a weak site of the uterus during pregnancy. We obseerved that uterine rupture occurred at a random site, mainly at the weakest point of the uterus. There is no effective means for detecting or predicting the weakest point of the uterus. In addition, the uterus may have ruptured in more than one location (Patient 2 had three ruptures).
Clinical manifestations and early diagnosis of uterine rupture
In general, most uterine ruptures progress from uterine rupture precursors, with the main clinical manifestations being abdominal pain (especially during the intervals between contractions), uterine tenderness (reported in the literature to be approximately 36.0% [ 21 ]), fetal abnormalities, abnormal vaginal bleeding, pathological contractions, hematuria, disappearance of contractions, hemodynamic instability (tachycardia, hypotension, or shock), change of fetal position, signs of uterine rupture detected by ultrasound, and changes in abdominal contour [ 1 ]. Some symptoms are asymptomatic; however, typical symptoms are rare (less than 10% [ 36 ]). Currently, pregnancy relies on the co-monitoring of history, clinical presentation, signs, and ultrasonography or magnetic resonance imaging (MRI). It is very difficult to rely on pregnancy management to prevent uterine rupture, which may be due to the following: the timing of uterine rupture is random, the rupture may be unrelated to the original surgical site, most patients have multiple risk factors, and the weakest part of the uterus may change with gestational age and cannot be predicted in advance. The time window for uterine rupture is difficult to control. The time may be longer in patients with thick abdominal fat and varying pain tolerance. If referral is required (long travel time), the optimal time for resuscitation is easily delayed. Abdominal pain, fetal distress, and vaginal bleeding do not allow us to initially consider uncommon uterine ruptures. Clinicians have limitations in considering abdominal pain, which is prone to misdiagnosis as other acute abdominal conditions or labor precursors. Furthermore, multiple pregnancies, literacy levels, and family economic status may lead to irregularities during obstetrical tests. Uterine rupture most often occurs suddenly most ofter, with immediate surgery performed upon diagnosis, failure to achieve continuous fetal heart monitoring, or fetal death at the time of presentation. Other conditions that do not directly lead to uterine rupture but can interfere with early recognition, such as mental retardation, history of frequent coitus, history of abdominal trauma, unawareness of the condition by family members (inability to provide an accurate history when the patient is in shock), use of ritodrine (rapid heart rate) and vomiting during pregnancy, can mask the early signs of shock. Color ultrasonography and MRI can be affected by the level of the examiner, thickness of abdominal fat in the pregnant woman, measurement site, number of measurements, bladder filling, rupture site (posterior wall rupture is difficult to diagnose), fetal movement, dynamic monitoring or not, and clarity of the ultrasound machine. In addition, the presence of unknown previous surgery (inverted T-shaped incision, uterine monolayer suture, weak local myometrium, infection, poor healing of incision, cause of postpartum hemorrhage, and method used to stop bleeding), diverticulum of the uterine incision (occurs in approximately 60% of patients after a primary cesarean section and 100% after three cesarean Sects [ 37 ].), any perforation during uterine manipulation, artificial placental removal during previous delivery, and subsequent follow-ups can affect the diagnosis of uterine rupture. In particular, the healing of the original cesarean section scar is unknown; a study showed that the use of synthetic absorbable monofilament sutures for uterine closure was associated with increased residual myometrial thickness, with respect to synthetic absorbable multifilament sutures. A uterine segment thickness after cesarean section below 2.0 mm between 35 and 38 gestational weeks has been repetitively associated with a greater risk of uterine rupture or scar dehiscence [ 37 ]. Furthermore, when the breach is small and there are no blood vessels at the breach, there may be no obvious symptoms or imaging changes. Atypical symptoms, difficult diagnosis of intra-abdominal hemorrhage, and unsupportive ancillary tests plague surgical decision-making. There is limited data on some factors that may affect the healing of the uterine incision [ 23 ] (previous history of postpartum hemorrhage, gestational diabetes mellitus or diabeties as a comorbidity, embryo transplantation, hypertensive disorders of pregnancy, and hypoproteinemia), and factors that may provide local protection, such as the severity of the pelvic-abdominal adhesions (three patients in this study had little hematochezia or peritoneal hemorrhage). Therefore, the education of pregnant women and their families, as well as the rapid recognition of uterine rupture after it occurs, are key to early diagnosis.
Complications of complete uterine rupture
Uterine rupture can cause severe postpartum hemorrhage, shock, disseminated intravascular coagulation, impaired organ function (ischemia–reperfusion), bladder injury, massive blood transfusion, hysterectomy, maternal death, neonatal asphyxia, ischemic-hypoxic encephalopathy, perinatal death (fetal or neonatal death), and other serious adverse outcomes.
Good prenatal and pregnancy care (contraceptive promotion, previous surgical records, control of diet weight gain, etc.), graded management (all women need to be risk-graded, strict control of high-risk factors, and timely referral), and early hospitalization of patients with high-risk factors for uterine rupture are key to the early diagnosis and treatment of uterine rupture. For patients with reproductive requirements, strict control of surgical indications, strengthening of suturing skills, guidance on postoperative precautions, strict control of indications for uterotonics and close monitoring are important. Correct management of the labor process, mastery of the indications for obstetric surgically assisted delivery and operation norms, and strict inspection during surgery (e.g., abdominal cervical cerclage patients to check the integrity of the lower segment of the uterus in the posterior wall) are also required. Regardless of high-risk factors, vigilance for uterine rupture, early recognition, proactive management, and training of rapid response teams should be strengthened to achieve favorable maternal and fetal outcomes.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Artificial Abortion
Caesarean Section
Caesarean Scar Pregnancy
Complete Placenta Previa
Caesarean Scar to Vesicovaginal Fold Distance
Chronic Hypertension
Chronic Hypertension with Superimposed Preeclampsia
Ectopic Pregnancy
Endometriosis
Gestational Diabetes Mellitus
Pregestational Diabetes Mellitus
Hypertensive Disorders of Pregnancy
Induce Childbirth
In Vitro Fertilization and Embryo Transfer
Low Molecular Weight Heparin
Marginal Placenta Previa
Placenta Accreta
Placenta Increta
Pernicious Placenta Previa
Postpartum Hemorrhage
Trial Of Labor After Cesarean
Uterine Myomectomy
Vaginal Birth
Vaginal Birth after Cesarean
Xie Xing, Kong Beihua, Duan Tao. Obstetrics and gynecology. 9th ed. Beijing: people’s health press, 2018:212–213
Zhe L, Huixia Y, Hong X, et al. National multicenter survey on the status of uterine rupture and analysis of outcomes. Chinese Journal of Obstetrics and Gynecology. 2019;54(6):363–8.
Google Scholar
Andrea Tinelli, Loannis P. Kosmas, Jose Tony Carugno, et at. Uterine rupture during pregnancy: The URIDA (uterine rupture international data acquisition) study[J]. Int J Gynecol Obstet 2021,1(157):76–84, DOI: https://doi.org/10.1002/IJGO.13810
Jian-Ping Z, Juhua W. Diagnosis and treatment of uterine rupture. Chinese Journal of Practical Gynecology and Obstetrics. 2011;27(2):118–20.
Tiantian C, Chunrong L, Yijun L, Meng C, Xinghui L. Analysis of clinical characteristics of 86 cases of complete uterine rupture[J]. Journal of Practical Obstetrics and Gynecology. 2020;36(12):926–30.
Perdue M, Felder L, Berghella V. First-trimester uterine rupture: a case report and systematic review of the literature [J]. Am J Obstet Gynecol. 2022;227(2):209–17.
Article PubMed Google Scholar
Savukyne E, Bykovaite·stankeviciene R, Machtejeviene E, et al. Symptomatic uterine rupture: a fifteen year review [J]. Medicina (Kaunas). 2020;56(11):574.
Sharon N, Maymon R, Pekar-Zlotin M, et al. Midgestational prelabor spontaneous uterine rupture: a systematic review [J]. J Matern Fetal Neonatal Med. 2022;35(25):5155–60.
Vimercati A, Dellino M, Crupano FM, Gargano G, Cicinelli E. Ultrasonic Assessment Of Cesarean Section Scar To Vesicovaginal Fold Distance: An Instrument To Estimate Pre-Labor Uterine Rupture Risk. J Matern Fetal Neonatal Medicine. 2021;4:1–5. https://doi.org/10.1080/14767058.2020.1849121 .
Article CAS Google Scholar
Singh A, Shrivastava C. Uterine rupture: still a harsh reality![J]. J Obstet Gynaecol India. 2015;65(3):158–61.
Al-Zirqi I, Daltveit AK, Vangen S. Maternal outcome after complete uterine rupture[J]. Acta Obstet Gynecol Scand. 2019;98(8):1024–31.
Al-Zirqi I, Daltveit AK, Vangen S. Infant outcome after complete uterine rupture[J]. Am J Obstet Gynecol. 2018;219(1):109-e1-e8.
Article Google Scholar
Xinghui L, Jing He, Hongbo Qi. Midwifery. Beijing: People’s Health Publishing Co; 2018. p. 333–7.
Li HT, Luo S, Trasande L, et al. Geographic Variations and Temporal Trends in Cesarean Delivery Rates in China, 2008–2014. JAMA. 2017;317(1):69–76.
Liang J, Mu Y, Li X, et al. Relaxation of the one child policy and trends in caesarean section rates and birth outcomes in China between 2012 and 2016: Observational study of nearly seven million health facility births.BMJ,2018,360(5):k817
Colmorn LB, Petersen KB, Jakobsson M, et al. The Nordic Obstetric Surveillance Study: a study of complete uterine rupture, abnormally invasive placenta, peripartum hysterectomy, and severe blood loss at delivery. Acta Obstet Gynecol Scand. 2015;94(7):734–44.
Aziz N, Yousfani S. Analysis of uterine rupture at university teaching hospital Pakistan. Pak J Med Sci. 2015;31(4):920–4.
PubMed PubMed Central Google Scholar
Qingling K, Yang Z, Lei F, et al. Analysis of complete uterine rupture in middle and late pregnancy. Progress of modern obstetrics and gynecology. 2019;28(6):412–5.
Meilong Xu. Clinical analysis of 82 cases of uterine rupture in pregnancy. China Maternal and Child Health. 2018;33(20):4591–4.
Xiaoxia B, Zhengping W, Xiaofu Y. Clinical analysis of 67 cases of uterine rupture. Chinese Journal of Obstetrics and Gynecology. 2014;49(5):331–5.
Zhou Y, Mu Y, Chen P, et al. The incidence, risk factors and maternal and foetal outcomes of uterine rupture during different birth policy periods: an observational study in China. BMC Pregnancy Childbirth. 2021;21(1):360.
Article PubMed PubMed Central Google Scholar
Huixia Y, Hongbo C, Qintian Z, et al. Williams Obstetrics [M]. 25th ed. Beijing: People’s Health Publishing House; 2020. p. 648–9.
Suqin Wu, Ying W, Zhiming S, et al. Risk factor analysis of mode of delivery for second pregnancy in scarred uterus. Chinese Journal of Clinical Pharmacology. 2017;33(7):662–4.
Usta IM, Hamdi MA, Musa AA, et al. Pregnancy outcome in patients with previous uterine rupture[J]. Acta Obstet Gynecol Scand. 2007;86(2):172–6.
Liao CY, Tse J, Sung SY, et al. Cornual wedge resection for interstitial pregnancy and postoperative outcome[J]. Aust N Z JObstet Gynaecol. 2017;57(3):342–5.
Propst AM, Liberman RF, Harlow BL, et al. Complications of hysteroscopic surgery: predicting patients at risk[J]. Obstet Gynecol. 2000;96(4):517–20.
CAS PubMed Google Scholar
Jansen FW, Vredevoogd CB, van Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study[J]. Obstet Gynecol. 2000;96(2):266–70.
A Vimercati, P Greco, G Loverro, Pl Lopalco, V Pansini, L Selvaggi. Maternal Complications After Caesarean Section In Hiv-Infected Women. Europ J Obstet Gynecol Repr Biol (90) 2000:73–76
Tsuji S, Takahashi K, Imaoka I, et al. MRI evaluation of the uterine structure after myomectomy[J]. Gynecol Obstet Invest. 2006;61(2):106–10.
Chaoxia L, Danqing C. Timing and mode of delivery after uterine trauma. Journal of Practical Obstetrics and Gynecology. 2018;34(1):13–5.
Cunningham S, Algeo CE, Defranco EA. Influence of inter pregnancy interval on uterine rupture[J]. J Matern Fetal Neonatal Med. 2021;34(17):2848–53.
Hasbargen U, Margarita SM, Peter H, et al. Uterine dehiscence in a nullipara, diagnosed by MRI, following use of unipolar electrocautery during laparoscopic myomectomy[J]. Hum Reprod. 2002;17(8):2180–2.
Kui-lin F, WeiShe Z. Repregnancy in elderly women with scarred uterus. Chinese Journal of Practical Gynecology and Obstetrics. 2017;33(1):93–6.
Gitas G, Alkatout I, Ertan KA, et al. Risk factor analysis in women who underwent trial of labor after cesarean section: a multicenter study in Germany[J]. J Turk Ger Gynecol Assoc. 2022;3(23):137–44. https://doi.org/10.4274/JTGGA.GALENOS.2022.2022-1-2 .
Gitas G, Proppe L, Ertan AK, et al. Influence of the second stage of labor on maternal and neonatal outcomes in vaginal births after caesarean section: a multicenter study in Germany[J]. BMC Pregnancy Childbirth. 2021;1(21):356. https://doi.org/10.1186/S12884-021-03817-2 .
American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery[J]. Obstet Gynecol,2010,116(2Ptl):450–463
Antonio Simone Laganà, Antonella Cromi, Roberto Tozzi, et al. Uterine scar healing after cesarean section: managing an old surgery in an evidence-based Environment[J]. Journal of Investigative Surgery,2019,8(32):770–772, https://doi.org/10.1080/08941939.2018.1465145
Download references
Acknowledgements
We would like to express my gratitude to all those who helped me during the writing of this thesis.
Not applicable.
Author information
Authors and affiliations.
The Chenzhou No.1 People’s Hospital, Chenzhou, 423000, China
Jing Xie & Miao Liu
The First Affiliated Hospital of Xiangnan University, Chenzhou, 423000, China
Department of Radiology, Renmin Hospital of Wuhan University, No. 238 Jiefang Road, Wuchang District, Wuhan, 430060, China
You can also search for this author in PubMed Google Scholar
Contributions
JX performed the data analysis; ML performed the formal analysis; XFL performed the validation; JX wrote the manuscript. All authors read and approved the final manuscript.
Author’s information (Optional)
JX, female, 1989.06, resident physician, Master's Degree, Department of Obstetrics, the First People’s Hospital of Chenzhou/ the First Affiliated Hospital of Xiangnan University, Chenzhou 423000, China. Main research interest: Obstetric hemorrhage related diseases. XFL, female, 1988.10, attending physician, Master's Degree, Department of Radiology, Renmin Hospital of Wuhan University, Wuhan 430060, China. Main research interest: Radiodiagnosis. ML, female, 1975.11, head physician, Master's Degree, Department of Obstetrics, the First People’s Hospital of Chenzhou/ the First Affiliated Hospital of Xiangnan University, Chenzhou 423000, China. Main research interest: Obstetric hemorrhage related diseases.
Corresponding author
Correspondence to Xuefang Lu .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First People’s Hospital of Chenzhou City (Approval No.论2024006). The informed consent requirement was waived by the Ethics Committee of the First People’s Hospital of Chenzhou City because of the retrospective study design.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xie, J., Lu, X. & Liu, M. Clinical analysis of complete uterine rupture during pregnancy. BMC Pregnancy Childbirth 24 , 255 (2024). https://doi.org/10.1186/s12884-024-06394-2
Download citation
Received : 16 September 2023
Accepted : 05 March 2024
Published : 08 April 2024
DOI : https://doi.org/10.1186/s12884-024-06394-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Complete uterine rupture
- hysterectomy
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Hemorrhagic disorder of the newborn is a bleeding disorder that manifests in the first few weeks of life after delivery. The term hemorrhagic disorder of the newborn encompasses all hemorrhagic diseases, i.e., due to vitamin K deficiency, trauma, clotting factor deficiency, etc. When the cause is vitamin K deficiency, it is referred to as vitamin K deficiency bleeding or VKDB. Vitamin K is a ...
The risk factors, presentation, management, and outcome of neonatal cerebellar hemorrhage will be discussed here. An overview of cerebellar development and cerebellar disorders is presented separately. (See "Overview of cerebellar injury and malformations in neonates".)
subdural hemorrhage. in addition, neonates with subarach-noid hemorrhage may also present with seizures, as the blood from the S ah may irritate the meninges and adjacent cortex. 9 as with all neonatal seizures, these seizures may manifest as posturing, subtle movements, or apnea and require eeG for accurate diagnosis.
The online presentation reviews normal imaging findings at cranial US with an emphasis on a systematic approach and anatomic landmarks. Using a case-based approach, the online presentation also illustrates the gamut of imaging findings seen in routine pediatric radiology practice. Premature (<30-32 weeks gestation) and extremely low birth ...
Intracranial hemorrhage (ICH) is an important cause of neonatal morbidity and mortality. Many inherited and acquired disorders may cause neonatal ICH, although in a large proportion of cases no etiology can be identified. 1 An insult to the brain during the neonatal period often results in significant adverse neurodevelopmental outcomes as it is a critical window for brain development. 2 The ...
Neonatal Intracranial Hemorrhage. Infrequently, neonatal intracranial hemorrhage is the result of a vascular malformation—namely, aneurysms and arteriovenous malformations including vein of Galen malformations. ... Incidence, location, risk factors, presentation, and outcomes are presented side by side. Note that subdural hemorrhage is more ...
Key Points. Question What are the incidence, types, presentations, associated factors, and long-term outcomes of neonatal hemorrhagic stroke?. Findings This population-based case-control study found a neonatal hemorrhagic stroke incidence of 1 in 6300 live births and independent associations between idiopathic neonatal hemorrhagic stroke and lower maternal age, primiparity, prior spontaneous ...
Approximately 90% of GMH-IVH has occurred by day 4, and 95% of GMH-IVH will have occurred by 7 days. Up to 40% of infants show a progression of the hemorrhage over 3-5 days (strong evidence). Small cerebellar hemorrhages (CBH) (≤3 mm) are missed by HUS but detected with MRI (moderate evidence).
Subpial hemorrhagic stroke is a form of intracranial hemorrhage primarily in neonates that has been largely ignored until recently 1,2,3.Subpial hemorrhage was first described in infant autopsies ...
Neonatal Intracranial Hemorrhage - by dr. Sonali Mhatre. This presentation aims at discussion of the pathophysiology , clinical presentation and management of the different types of intracranial bleeds in a neonate. Special emphasis has been laid on intraventricular hemorrhage. The germinal matrix bleed in a preterm is discussed in depth along ...
In 2011, we reported 38 neonates with subgaleal hemorrhage (SH), relating an increasing incidence. ... Wetmore J. Neonatal subgaleal hematoma: presentation and outcome—radiological findings and ...
Noncontrast computerized tomography of the head for case 2 on initial presentation to the emergency department and before the operative evacuation of the hematoma. Images reveal right-sided intraparenchymal hemorrhage with midline shift and compression of the ventricular system. Download : Download high-res image (365KB)
Severe neonatal SH is an uncommon but high-acuity condition. Because severe SH can rapidly lead to hemorrhagic shock and death, or neurologic injury leading to disability, all professionals
Abstract. Stroke comprises acute focal brain injury due to arterial or venous occlusion, or due to primary hemorrhage. In the perinatal context its onset can be in utero, during delivery or at any time in the neonatal period. Although seizures and apnea are characteristic presentations, other signs and chance imaging finding are common.
The objective of the present retrospective study was to clarify the clinical presentation, management, and factors associated with morbidity and mortality in neonatal subgaleal hemorrhage. Background: Neonatal subgaleal hemorrhage (SGH) is a rare but potentially lethal medical emergency. The objective of the present retrospective study was to clarify the clinical presentation, management, and ...
Case report A 3-day-old male born at 33 weeks and four days of gestation who was admitted to the neonatal critical care unit due to prematurity and respiratory distress was noted to be febrile ...
Subdural Hemorrhage is the most common type of intracranial hemorrhage in neonates. Operative vaginal delivery is a major risk factor, and hemorrhage over the cerebral convexities is the most common site. ... Birth trauma in a newborn has a varied presentation depending on the type of injury sustained. The affected newborns may present with ...
The maternal and neonatal outcomes of the face presentation group were followed up. The incidence of face presentation at term was 0.14‰. After conditional logistic regression, the two factors associated with face presentation were high parity (adjusted odds ratio [aOR] 2.76, 95% CI 1.19-6.39)] and amniotic fluid index > 18 cm (aOR 2.60, 95 ...
Uterine rupture can cause severe postpartum hemorrhage, shock, disseminated intravascular coagulation, impaired organ function (ischemia-reperfusion), bladder injury, massive blood transfusion, hysterectomy, maternal death, neonatal asphyxia, ischemic-hypoxic encephalopathy, perinatal death (fetal or neonatal death), and other serious adverse ...
Part of the Bedside to Bench Lecture Series. Dial-In Information. Contact Sophy Aguilar: [email protected] for Zoom details. Friday, April 12 at 12:00pm to 1:00pm. Feil Family Research Building, Room RR-125B. 407 East 61st St., New York NY. Event Type. Lecture/Seminar.