Clinical Trial Data Management and Professionals
By Andy Marker | January 16, 2020 (updated September 16, 2021)
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
Link copied
This guide provides professionals with everything they need to understand clinical data management, offering expert advice, templates, graphics, and a sample clinical data management plan.
Included on this page, you'll find information on how to become a clinical trial data manager , a clinical data management plan template , a clinical data validation plan template , and much more.

What Is Clinical Trial Management?
Clinical trial management refers to the structured, organized regulatory approach that managers take in clinical trial projects to produce timely and efficient project outcomes. It includes developing and maintaining specified or general software systems, processes, procedures, training, and protocols.
What Is a Clinical Trial Management System (CTMS)?
A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether across one or several institutions.
Companies use CTMS for their clinical data management to ensure they build trust with regulatory agencies. Trust is earned as the companies collect, integrate, and validate their clinical trial data with integrity over time. A comprehensive system helps them do so.
In a 2017 paper, “ Artificial intelligence based clinical data management systems: A review ,” Gazali discusses CTMS and what makes it worthwhile for investigators — namely, that it helps to authenticate data. Accurate study results and a trail of data collection, as collected through a quality CTMS, lend credence to research study data. Clinical trial data management systems enable researchers to adhere to quality standards and provide assurance that they are appropriately collecting, cleaning, and managing the data.
A clinical data management system also offers remote data monitoring. The sponsor, or principal investigator, may want to monitor the trial from a distance, especially if the organization has many sites. Since the FDA mandates monitoring in clinical trials, and many studies generally consider it a large cost, remote monitoring offers a lower-priced option in which sponsors can identify issues and outliers and mitigate them quickly.
Many data management systems are also incorporating artificial intelligence (AI). AI-based clinical data management systems support process automation, data insights analysis, and critical decision making. All of this can happen as your staff inputs the research data. According to a review of clinical data management systems , researchers note that automating all dimensions of clinical data management in trials can take them from mere electronic data capture to something that helps with findings in clinical trials.
The most helpful strategies for implementing clinical data management systems balance risk reduction and lead time. All trial managers want to have their software deployed rapidly. However, it is best to set up the databases thoroughly before the trial. When staff must make software changes during the trial, it can be costly and have implications on the trial data’s validity.
Other strategies that help organizations implement a new system include making sure that, prior to deployment, the intended users give input. These users include entities such as the contract research organization (CRO), the sponsor, staff at the investigator site, and any onsite technical support. Staff should respond well to the graphical user interface (GUI). Additionally, depending on software support, the staff can gradually expand the modules to include more functionality, perform module-based programming, and duplicate the hardware. These actions give the staff the most functionality and the software the best chance at success.
How to Compare Clinical Data Management Systems
When deciding which clinical data management system to use, compare the program’s available features and those that your clinical sites need. Additionally, you can compare clinical data management systems by reviewing the installation platforms, pricing, technical support, and number of allowed users.
For programs that collect data on paper and send it to data entry staff, the data entry portal should be simple and allow for double entry and regular oversight.
In general, here are the main features to compare in a clinical data management system:
- 21 CFR Part 11 Compliance: Electronic systems must provide assurance of authentic records.
- Document Management: All documents should be in a centralized location, linked to their respective records.
- Electronic Data Capture (EDC): Direct clinical trial data collection, as opposed to paper forms.
- Enrollment Management: Research studies can use this data (from interactive web or voice response systems) to enroll, randomize, and manage patients.
- HIPAA compliance: Ensure compliance with the Health Insurance Portability and Accountability Act to protect patients’ information.
- Installation: Identify whether you want a cloud-based or on-premises solution and if you need mobile deployment (iOS or Android).
- Investigator and Site Profiling: Use this function to rapidly identify the feasibility of possible investigators and sites.
- Monitoring: The system should offer a calendar, scheduling capabilities, adverse and severe adverse event tracking, trip reporting, site communication, and triggers.
- Number of Users: How many users can the software can handle? Is there a minimum number of required users? Does the software provide levels of accessibility and price based on the number of users?
- Patient Database: Separate from recruitment and enrollment, the patient database is a record of previous contacts that you can potentially draw from for future trials.
- Payment Portal: Pay out stipends, contracts, and other finances related to the research project.
- Pricing: Check whether the software company offers free trials, free or premium options, monthly subscriptions, annual subscriptions, or a one-time license.
- Recruiting Management: This function helps streamline recruitment by targeting potential trial patients with web recruitment and prescreening.
- Scheduling: Use this feature to keep track of visits and events.
- Study Planning and Workflows: This function enables you to track all required study pieces from the beginning and optimize each piece with workflows.
- Support: Check if the software company offers 24-hour issue support and training on the software.
What Is Clinical Data Management?
Clinical data management (CDM) is the part of clinical trial management that deals specifically with information that comes out of the trials. In order to yield ethical, repeatable results, researchers must document their patients’ medical status — including everything relative to that status — and the trial’s interventions.
Clinical data management evolved from drug companies’ need for an honest path from their research to their findings; in short, their data had to be reproducible. CDM helps evolve a standards-based approach, and many regulators are continually imposing their requirements on it. For instance, paper is no longer favored as a collection method; most clinical trials prefer software systems that improve the timeliness and quality of data.
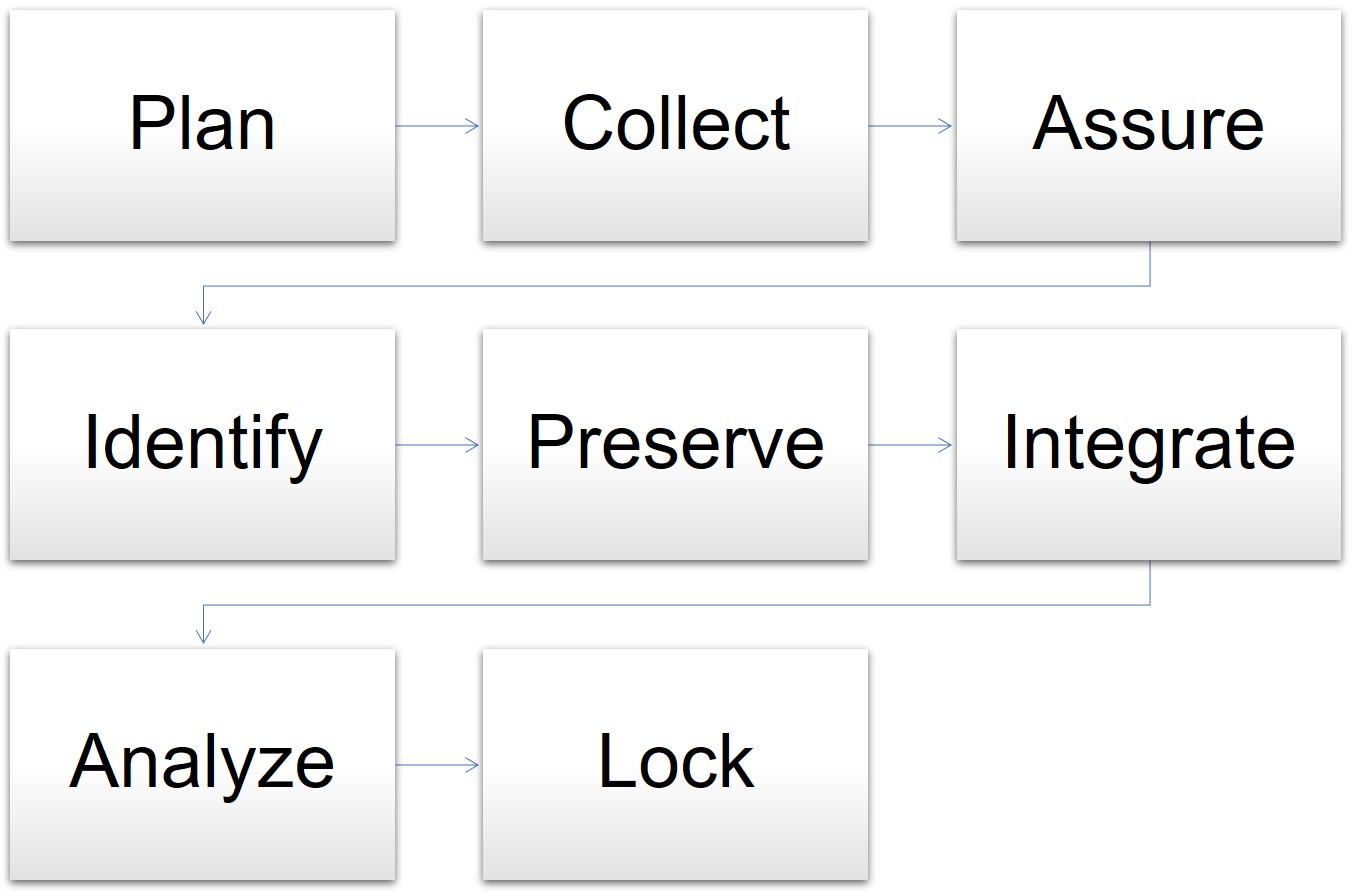
In one model for data management, the cycle begins when the clinical trial is in the planning stages and goes through the final analysis and lockdown of the data. The stages for data management are as follows:
- Plan: The data manager prepares the database, forms, and overall plan.
- Collect: Staff gathers data in the course of the trial.
- Assure: The data manager determines if the data plan and tools meet the requirements.
- Identify: Staff and the data manager identify any issues or risks.
- Preserve: The data manager preserves the data already collected and mitigates risks.
- Integrate: The data manager oversees different datasets and information mapped together for consistency.
- Analyze: The statisticians analyze the mapped data trends and outcomes.
- Lock: The data manager locks the database for integrity.
Model for Data Management in Clinical Trials
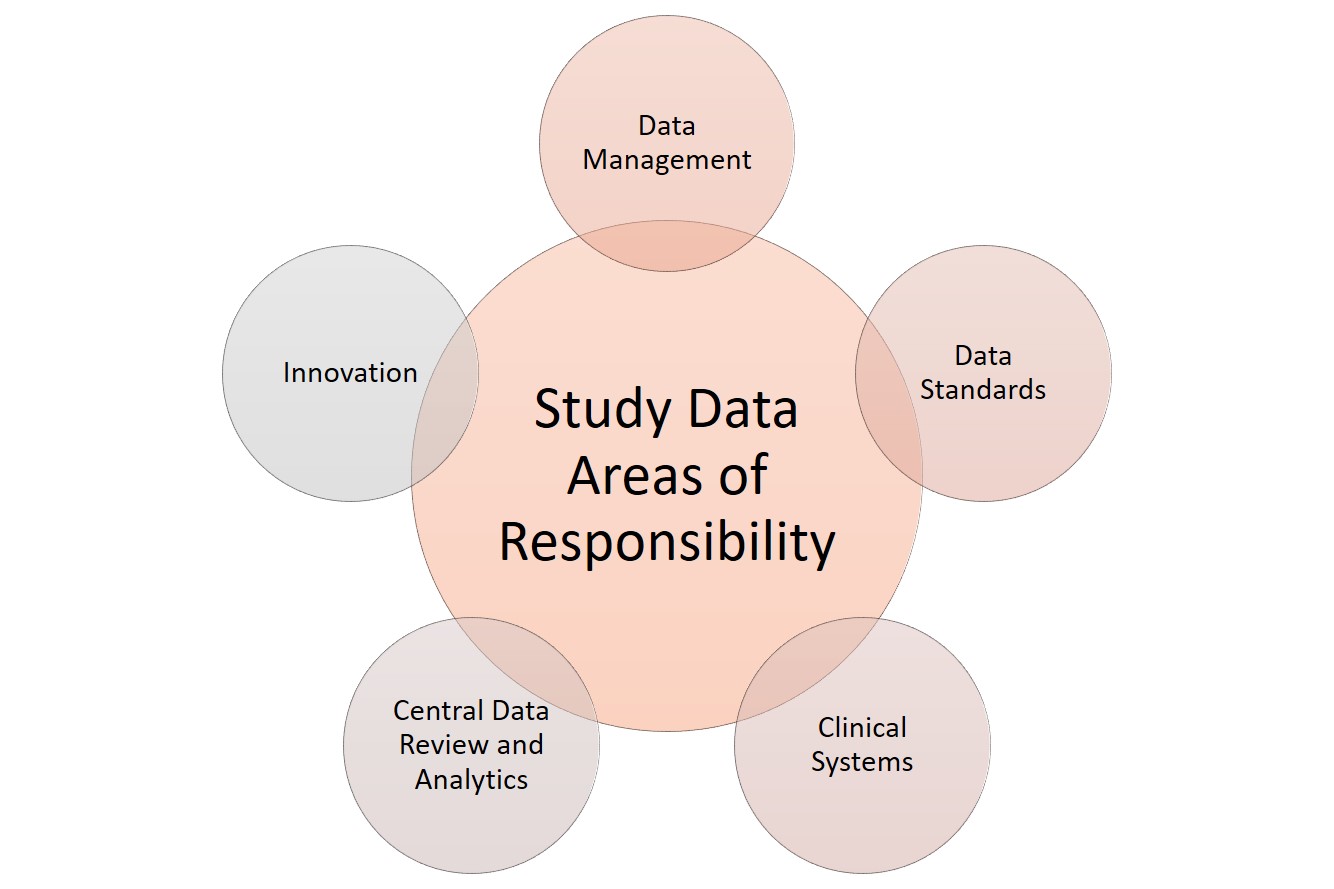
When it comes to data, clinical research has several areas of responsibility. Sponsors can split these functions among several staff or, in smaller studies, assign them to the main data manager. These functions include the following:
Clinical systems: Any software or technology used.
Data management: Data acquisition, coding, and standardization.
Data review and analytics: Quality management, auditing, and statistical analysis of the collected data.
Data standards: Checking against regulatory requirements.
Innovation: Using tools and theory that coordinate with the developing field. For more innovative templates to use in clinical trials, see “ Clinical Trial Templates to Start Your Clinical Research .”
Clinical Research Data Areas of Responsibility
Clinical data management is one of the most critical functions in overall clinical trial management. Staff collects data from many different sources in a clinical trial — some will necessarily be from paper forms filled out by the patient, their representative, or a staff member on their behalf. However, instead of paper, some clinics may use devices such as tablets or iPads to fill out this direct-entry data electronically.
Clinical data management also includes top-line data , such as the demographic data summary, the primary endpoint data, and the safety data. Together, this constitutes the executive summary for clinical trials. Companies often issue this data as a part of press releases. Additional clinical trial data management activities include the following:
- Case report form (CRF) design, annotation, and tracking
- Central lab data
- Data abstraction and extraction
- Data archiving
- Data collection
- Data entry and validation
- Data extraction
- Data queries and analysis
- Data storage and privacy
- Data transmission
- Database design, build, and testing
- Database locking
- Discrepancy management
- Medical data coding and processing
- Patient recorded data
- Severe adverse event (SAE) reconciliation
- Study metrics and tracking
- Quality control and assurance
- User acceptance testing
- Validation checklist
Since there are many different types of data coming from many different sources, some data managers have become experts in hybrid data management — the synchronization required to not only make disparate data relate to each other, but also to adequately manage each type of data. For example, one study could generate data on paper from both the trial site and from a contract research organization, electronic data from the site, and clinical data measurements from a laboratory.
The Roles and Responsibilities in Clinical Data Management
Clinical data management software assigns database access limitations based on the assigned roles and responsibilities of the users. This coding ensures there is an audit trail and the users can only access their respective required functionalities, without the ability to make other changes.
All staff members, whether a manager, programmer, administrator, medical coder, data coordinator, quality control staff, or data entry person, have differing levels of access to the software system, as delineated in the protocol. The principle investigator can use the CDMS to restrict these access levels.
What Is Clinical Trial Data Management (CDM)?
Clinical trial data management (CDM) is the process of a program or study collecting, cleaning, and managing subject and study data in a way that complies with internal protocols and regulatory requirements. It is simultaneously the initial phase in a clinical trial, a field of study, and an aspirational model.
With properly collected data in clinical trials, the study can progress and result in reliable, high-quality, statistically appropriate conclusions. Proper data collection also decreases the time from drug development to marketing. Further, proper data collection involves a multidisciplinary team, such as the research nurses, clinical data managers, investigators, support personnel, biostatisticians, and database programmers. Finally, CDM enables high-quality, understandable research, which can be capitalized on in its field and across many disciplines, according to the National Institutes of Health (NIH).
In clinical trials, data managers perform setup during the trial development phase. Data comes from the primary sources, such as site medical records, laboratory results, and patient diaries. If the project uses paper-based CRFs, staff members must transcribe them, then enter this source data into a clinical trial database. They enter paper-based forms twice, known as double data entry, and compare them, per best practice. This process significantly decreases the error rate from data entry mistakes. Electronic CRFs (eCRFs) enable staff to enter source data directly into the database.
As with any project, the financial and human resources in clinical trials are finite. Coming up with and sticking to a solid data management plan is crucial — it should include structure for the research personnel, resources, and storage. A clinical trial is a huge investment of time, people, and money. It warrants expert-level management from its inception.
Clinical Data Management Plans
Clinical data management plans (DMPs) outline all the data management work needed in a clinical research project. This includes the timeline, any milestones, and all deliverables, as well as strategies for how the data manager will deal with disparate data sets.
Regulators do not require a DMP, but they expect and audit them in clinical research. Thus, the DMPs should be comprehensive and all stakeholders should agree on them. They should also be living documents that staff regularly updates as the study evolves and the various study pieces develop.
For example, during one study, the study manager might change the company used for laboratory work. This affects the DMP in two ways: First, staff needs to develop the data sharing agreement with the new company, and second, they need to integrate the data from both laboratories into one dataset at the end of the trial. The DMP should describe both.
When creating DMPs, you should also bear in mind any industry data standards, so the research can also be valuable outside of the discrete study. The Clinical Data Acquisitions Standards Harmonization (CDASH) recommends 16 standards for data collection fields for consistency in data across different studies.
The final piece of standardization in DMPs is the use of a template, which provides staff with a solid place to start developing a DMP specific to their study. Sponsors may have a standard template they use across their projects to help reduce the complexity inherent in clinical trials.
Data Management Plan Template for Clinical Trials
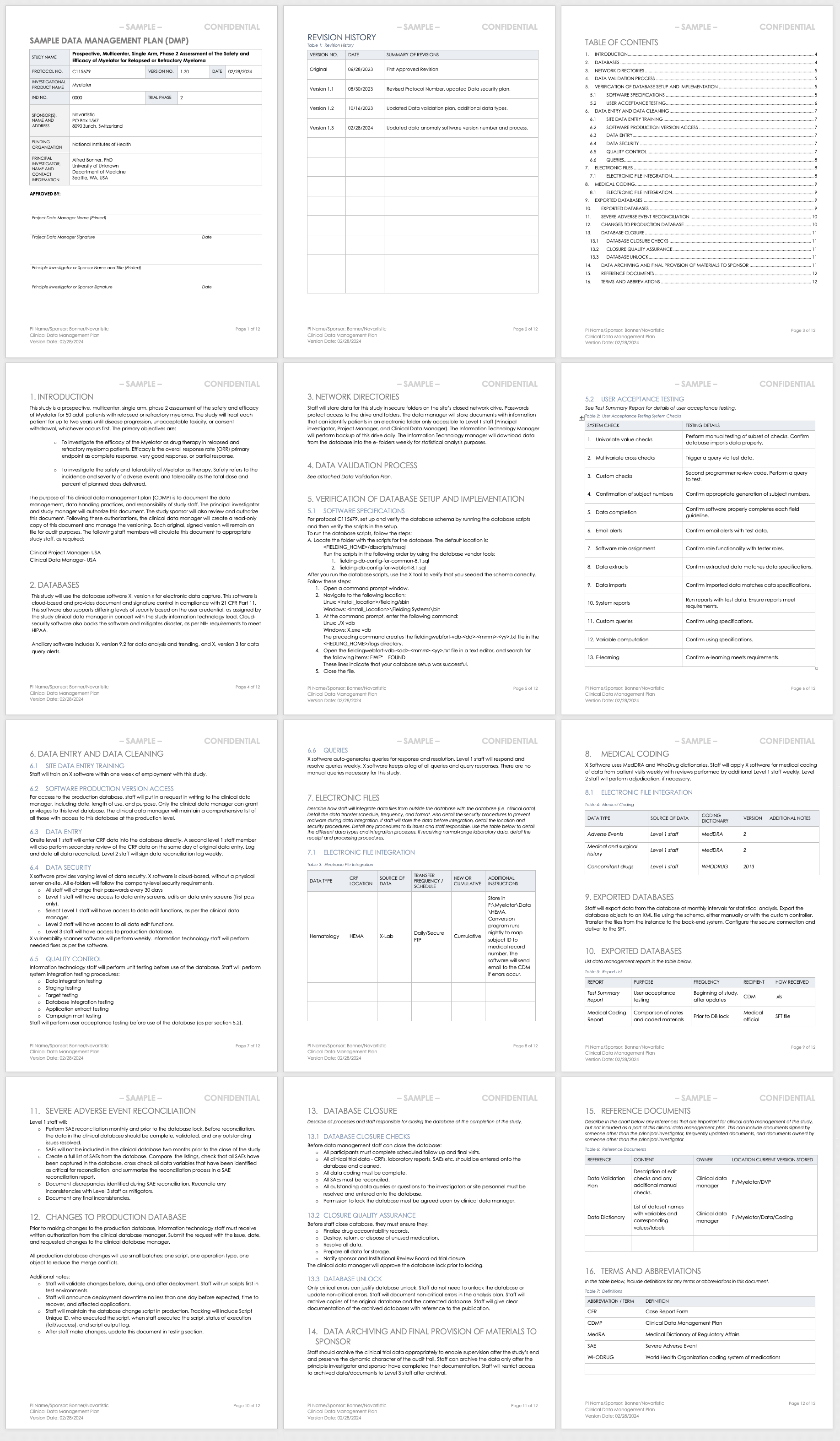
This data management plan template provides the required contents of a standard clinical trial data management plan, with space and instructions to input elements such as the data validation process, the verification of database setup and implementation processes, and the data archival process.
Download Data Management Plan Template - Word
Sample Data Management Plan for Clinical Trials
This sample data management plan shows a fictitious prospective, multicenter, single-arm study and its data management process needs. In two years of study, the data manager should regularly update this plan to demonstrate the study’s evolving needs, and document each change and update. Examples of sections include the databases used, how data will be entered and cleaned, and how staff will integrate different data sets collected in the study.
Download Sample Data Management Plan - Word
Clinical Trial Data Validation Plan
Data validation involves resolving database queries and inconsistencies by checking the data for accuracy, quality, and completeness. A data validation plan in clinical trials has all the variable calculations and checks that data managers use to identify any discrepancies in the dataset.
When the data is final, the database administrator locks it to ensure no further changes are made, as they could interrupt the integrity of the data. During reporting and analysis, experts may copy the data and reformat it into tables, lists, and graphs. Once the analysts complete their work, they report the results. When they have significant findings, they may create additional tables, lists, and graphs to present as part of the results. They then integrate these results into higher-level findings documentation. Examples of this type of documentation include investigator’s brochures or clinical case study reports (CSRs). Finally, the data manager archives the database.
The above steps are important because they preserve the integrity of the data in the database. However, managers do not need to perform them in a strict order. Some studies may need more frequent data validation, due to the high volume of data they produce, while other studies may produce intermediate analysis and reporting as part of their predetermined requirements. Finally, due to the complexity of some studies, the data manager or analyst may need to query , which means running a data request in a database and determining cursory results so that they may adjust the protocol.
Use this template to develop your own data validation plan. This Word template includes space and instructions for you to develop a data validation plan that you can include in your data management plan or use as a stand-alone document. Examples of sections include selecting and classifying the computer systems, validation protocol, and validation reporting.

Download Data Validation Plan - Word
Data Management Workflow
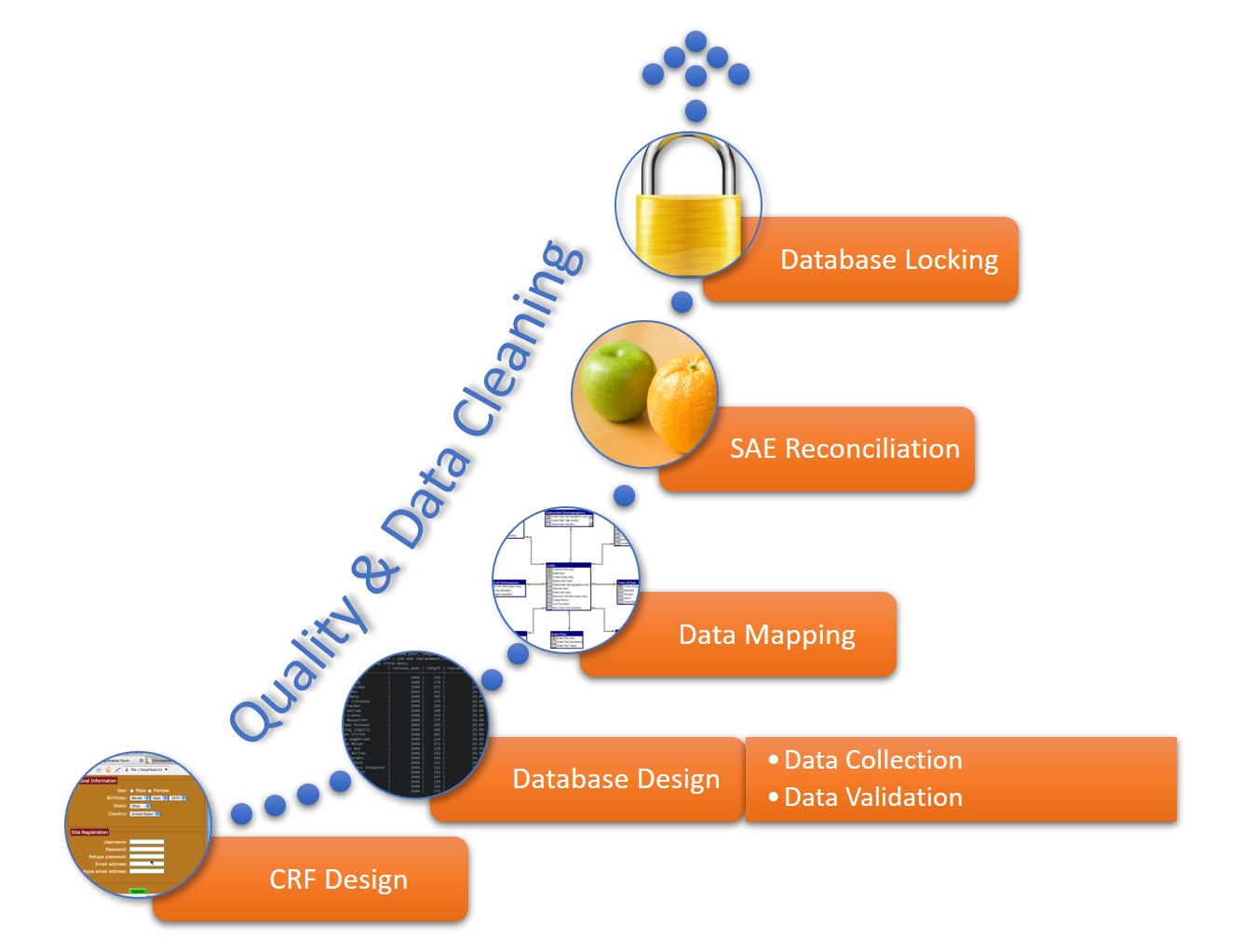
A data management workflow is the process clinical research uses to deal with their data, from the data collection design to the electronic archival and findings presentation. This includes getting through the entry process, any batch validation, discrepancy management, coding, reconciliations, and quality control plans.
This workflow starts when researchers generate a CRF, whether manually or electronically, and continues through the final lock on the database. The data manager should perform quality checks and data cleaning throughout the workflow. The workflow steps for a data manager are as follows:
- CRF Design: This initial design step forms the basis of initial data collection.
- Database Design: The database should include space for all data collected in the study.
- Data Mapping: This step integrates data from different forms or formats so researchers can consistently report it.
- SAE Reconciliation: Data managers should regularly review and correct severe adverse events and potential events.
- Database Locking: Once a study is complete, the database manager should lock the database so that no one can change the data.
Clinical Trial Data Audits
A clinical trial data audit is a review of the information collected in order to ensure the quality, accuracy, and appropriateness for the stated research requirements, per the study protocol. Regulatory authorities, sponsors, and internal study staff can conduct two varieties of audit: overall and database-specific.
Regulators use database audits to ensure that no one has tampered with the data. In general, there must be an audit trail to know which user made changes to what and when in the database. For example, the auditors will look at record creation, modification, and deletion, noting the usernames, dates, and times. FDA 21 CFR Part 11 includes this as a part of fraud detection, and requires that there is a complete history of the recordkeeping system and clinical trial data transparency.
The data manager develops templates for auditing the study during the study development phase and performs their own internal audits as a part of its quality management.
This free clinical trial data management audit checklist template will help you develop your own checklist. This Excel template lets you show the status of your audit in an easy color-coded display, the category and tasks to review, and what criteria you require. It brings all your audit requirements and results together.
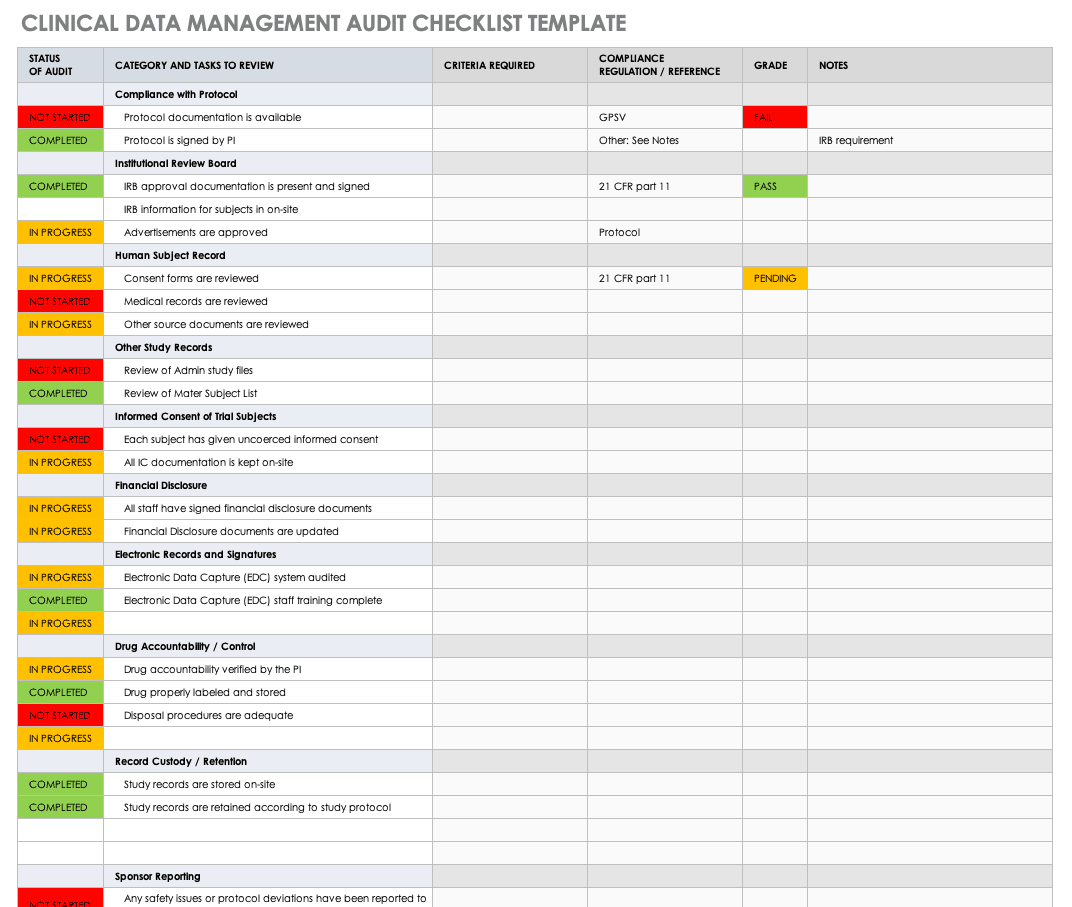
Download Clinical Data Management Audit Checklist - Excel
Quality Management in Clinical Trials
Data quality management (DQM) refers to the practices that ensure clinical information is of high value. In a clinical trial, DQM starts when staff first acquires the information and continues until the findings are distributed. DQM is critical in providing accurate outcomes.
The factors that influence the quality of clinical data include how well the study investigators develop and implement each of the following data pieces:
- Case Report Forms (CRF): Design the CRF in parallel with the protocol so that the data collected by staff is complete, accurate, and consistent.
- Data Conventions: Data conventions include dates, times, and acronyms. Data managers should set these conventions during study development, especially if there are multiple study locations and investigators.
- Guidelines for Monitoring: The overall data quality is contingent on the quality of the monitoring guidelines established.
- Missing Data: Missing data are those values not available that could change the analysis findings. During study development, investigators and analysts should determine how they will handle missing data.
- Verification of Source Data: Staff must verify that the source data is complete and accurate during data validation.
Regulations, Guidelines, and Standards in Clinical Data Management
Different regulations, guidelines, and standards govern clinical data management industry. The Clinical Data Interchange Standards Consortium (CDISC) is a global organization that holds clinical studies accountable to clinical trial data standards, international regulations, institutional and sponsor standard operating procedures (SOPs), and state laws.
There are standard operating procedures and best practices in clinical trial data management that are widespread. CDISC has two standards, the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG), mandated by the U.S. Food and Drug Administration (FDA), and the Clinical Data Acquisition Standards Harmonization (CDASH). Also, in the industry, the Society for Clinical Data Management (SCDM) releases the Good Clinical Data Management Practices (GCDMP) guidelines and administers the International Association for Continuing Education and Training (IACET) credential for certified clinical data managers. The National Accreditations Board of Hospitals Health (NABH) provides additional guidance, such as pharmaceutical study auditing checklists. Finally, Good Clinical Practices (GCP) guidelines discuss ethical and quality standards in clinical research.
A trial conducted under the appropriate standards ensures that staff has followed the protocol and treated the patients according to that protocol. Ultimately, this shows the integrity and reproducibility of the study and acceptance in the industry.
Case Report Forms in Data Management
In data management, CRFs are the main tool researchers use to collect information from their participants. Researchers design CRFs based on the study protocol; in them, they document all patient information per the protocol for the duration of the study’s requirements.
CRFs should comply with all regulatory requirements and enable efficient analysis to decrease the need for data mapping during any data exchange. When longer than one page, the CRF is known as a CRF book, and each visit adds to the book. The main parts of a CRF are the header, the efficacy-related modules, and the safety-related modules:
- CRF Header: This portion includes the patient identification information and study information, such as the study number, site number, and subject identification number.
- Efficacy-Related Modules: This portion includes the baseline measurements, any diagnostics, the main efficacy endpoints of the trial, and any additional efficacy testing.
- Safety-Related Modules: The portion contains the patient’s demographic information, any adverse events, medical history, physical history, medications, confirmation of eligibility, and any reasons for release from the study.
What Is the Role of a Clinical Data Manager?
In a clinical trial, the data manager is the person who ensures that the research staff collects, manages, and prepares the resulting information accurately, comprehensively, and securely. This is a key role in clinical research, as the person is involved in the study setup, conduct, closeout, and some analysis and reporting.

Melissa Peda , Clinical Data Manager at Fred Hutch Cancer Research Center , says, “Being a clinical data manager, you have to be very detail-oriented. We write up very specific instructions for staff. For example, the specifications to a program’s database include one document that could easily have 1,000 rows in Excel, and it needs to be perfect for queries to fire in real time. Code mistakes can put your project behind, so they must do their review with a close eye. You must also be logical and think through the project setup. A good clinical data manager must be detailed, so the programmers and other staff can do their thing.”
Krishnankutty, et al. , developed an overview of best practices for data management in clinical research. In their article, published in the Indian Journal of Pharmacology, they say that the need for strong clinical data management has sprung up from the pharmaceuticals industry wanting to fast-track drug development by having high-quality data, regardless of the type of data. Clinical data managers can expect to work with many different types of clinical data; the most common types include the following:
- Administrative data
- Case report forms (CRFs)
- Electronic health records
- Laboratory data
- Patient diaries
- Patient or disease registries
- Safety data
The clinical data managers often must oversee the analysis of the data as well. Data analysis conducted in clinical trial data management is very delicate: It requires a solid dataset and an analyst who can explain the findings. Regulatory agencies, along with other companies and professionals, check the findings and analysis, so they need to be accurate and understandable.
Education and Credentials of a Clinical Data Manager
Professionals in clinical data management receive data management in clinical trials training, and often have the Certified Clinical Data Manager (CCDM) credential. Their studies can have optimized outcomes since they are executed by a competent CDM team with validated skill sets and continued professional development.
To become certified, the applicant must have the appropriate education and experience, including the following:
- A bachelor’s degree and two or more years of full-time data management experience.
- An associate’s degree and three or more years of full-time data management experience.
- Four years of full-time data management experience.
- Part-time data management experience that adds up to the requirements above.

Raleigh Edelstein , a clinical data manager and EDC programmer, discusses the credentialing in this field. “Anyone can excel in this profession,” she says. “A CRA — a clinical research associate, also called a clinical monitor or a trial monitor — may need this credential more, as their profession is more competitive, and their experience is more necessary in trials. But if the credential makes you more confident, then I say go for it. Your experience and confidence matter.”
There are several degrees with an emphasis on clinical research that can also teach the necessary technical skills. In addition to many online options, these include the following, or a combination of the following:
- Associate of Science in biology, mathematics, or pharmacy.
- Bachelor of Science in one of the sciences.
- Post-Master's certificate in clinical data management, or a certificate related to medical device and drug development.
- Master of Science in clinical research, biotechnology, bioinformatics.
- Doctor of Nursing Practice.
- Doctor of Philosophy in any clinical research area.
These degree programs include concepts that help data managers understand what clinical studies need. They especially focus on survey design and data collection, but also include the following:
- Biostatistics
- Clinical research management and safety surveillance
- Compliance, ethics, and law
- New product business and strategic planning
- New product research and development
These degree programs offer coursework that improves the relevant clinical research skills. Many of the courses are introductory to clinical research, trials, and pharmacology, and others include the following:
- Business processes
- Clinical outsourcing
- Clinical research biostatistics
- Clinical trial design
- Compliance and monitoring
- Data collection strategies
- Data management
- Electronic data capture
- Epidemiology
- Ethics in research
- Federal regulatory issues
- Health policy and economics
- Human research protection
- Medical devices and product regulation
- Patient recruitment and informed consent
- Pharmaceutical law
- Review boards
- Worldwide regulations for submission
Clinical data managers can get involved with several professional organizations worldwide, including the following:
- The Association for Clinical Data Management (ACDM): This global professional organization supports the industry by providing additional resources and promoting best practices.
- The Association Française de Data Management Biomédicale (DMB): This French data management organization shares information and practices among professionals.
- International Network of Clinical Data Management Associations (INCDMA): Based in the United Kingdom, this professional network exchanges information and discusses relevant professional issues.
- The Society for Clinical Data Management (SCDM): This global organization awards CCDM credential to professionals, provides additional education, and facilitates conferences in clinical data management.
FAQs about Clinical Trial Managers
The field of clinical management is quickly expanding in many forms to support the need for new research. Below are some frequently asked questions.
How do I become a clinical trial manager?
To become a clinical trial manager, you must obtain the appropriate education, experience, and credentialing, as detailed above.
What is better: a Master’s in Health Administration or a Master’s in Health Sciences?
To work as a clinical data manager, either degree program is appropriate. Your choice depends on your interest.
What can you do with a degree in biotechnology or bioenterprise?
Biotechnology is involved in the technology that aids in biological research, and bioenterprise takes the products of biotechnology and markets and sells them.
What is a clinical application analyst?
A clinical application analyst is a professional who helps clinics evaluate software systems and vendors.
What is a clinical data analyst?
A clinical data analyst is a professional who analyzes data from clinical trials, and develops and maintains databases.
Contract Research Organizations for Data Management Services
Contract research organizations (CROs) are companies that provide outsourced research services to industries such as pharmaceutical, biotechnology, and research development. Designed to keep costs low, studies can hire them to perform everything from overall project management and data management to technical jobs.
Studies can hire CROs that specialize as clinical trial data management companies so they don’t have to worry about having all the necessary skills in-house. According to Melissa Peda, “A consultant may have the expertise that someone already working in the organization may not have, so they make sense to bring in.” Further, a contractor outside of the business can bring a lack of bias to the project.
According to Raleigh Edelstein, “A third-party person in charge of data management may be necessary because you don’t have to worry about the lack of company loyalty that the data may need.”
CROs can offer skills such as the following:
- Annotation and review
- Coding and validation
- Database export, transfer, and locking
- Database integration
- Database setup and validation
- Double data entry and third-party review of discrepancies
- Form design
- Planning, such as project management and data management plans
- Quality assessments and auditing
- Software implementation and training
- SAE reconciliation
Related Topics in Clinical Data Management
The following are related topics to clinical data management:
- Application Analyst: This position deals with the software side of clinical trials. Examples of their work include choosing software, designing databases, and writing queries.
- Clinical Data Analyst: A professional who examines and verifies that clinical study data is appropriate and means what it is supposed to mean.
- Clinical Research Academic Programs: Entry-level professional positions in clinical trials often require a minimum of a bachelor’s degree.
- Clinical Research Associate: This clinical trial staff member designs and performs clinical studies.
- Laboratory Informatics: The field of data and computational systems specialized for laboratory work.
- Laboratory Information Management System (LIMS): LIMS enables collection and analysis of data from laboratory work. LIMS is specialized to work in different environments, such as manufacturing and pharmaceuticals.
- Scientific Management: This management theory studies workflows, applying science to process engineering and management.
Improve Clinical Trial Data Management with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Any articles, templates, or information provided by Smartsheet on the website are for reference only. While we strive to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability, or availability with respect to the website or the information, articles, templates, or related graphics contained on the website. Any reliance you place on such information is therefore strictly at your own risk.
These templates are provided as samples only. These templates are in no way meant as legal or compliance advice. Users of these templates must determine what information is necessary and needed to accomplish their objectives.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.
Data management in clinical research: An overview
Affiliation.
- 1 Global Medical Affairs, Dr. Reddy's Laboratories Ltd., Ameerpet, Hyderabad, India.
- PMID: 22529469
- PMCID: PMC3326906
- DOI: 10.4103/0253-7613.93842
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right from inception to completion. They should have adequate process knowledge that helps maintain the quality standards of CDM processes. Various procedures in CDM including Case Report Form (CRF) designing, CRF annotation, database designing, data-entry, data validation, discrepancy management, medical coding, data extraction, and database locking are assessed for quality at regular intervals during a trial. In the present scenario, there is an increased demand to improve the CDM standards to meet the regulatory requirements and stay ahead of the competition by means of faster commercialization of product. With the implementation of regulatory compliant data management tools, CDM team can meet these demands. Additionally, it is becoming mandatory for companies to submit the data electronically. CDM professionals should meet appropriate expectations and set standards for data quality and also have a drive to adapt to the rapidly changing technology. This article highlights the processes involved and provides the reader an overview of the tools and standards adopted as well as the roles and responsibilities in CDM.
Keywords: Clinical data interchange standards consortium; clinical data management systems; data management; e-CRF; good clinical data management practices; validation.
Redirect Notice

Writing a Data Management & Sharing Plan
Learn what NIH expects Data Management & Sharing Plans to address, as well as how to submit your Plan.
- Applications for Receipt Dates BEFORE Jan 25 2023
- Applications for Receipt Dates ON/AFTER Jan 25 2023
Writing a Data Sharing Plan
Under its 2003 data sharing policy , NIH expects investigators to submit a data sharing plan with requests for funding or grants, cooperative agreements, intramural research, contracts, or other funding agreements of $500,000 or more per year.
Data sharing plans should describe how an applicant will share their final research data. The specifics of the plan will vary on a case-by-case basis, depending on the type of data to be shared and how the investigator plans to share the data.
Examples of information to cover in a data sharing plan include:
- The expected schedule for data sharing
- The format of the dataset
- The documentation to be provided with the dataset
- Whether any analytic tools also will be provided
- A brief description of such an agreement
- Criteria for deciding who can receive the data
- Whether or not any conditions will be placed on their use
- Investigators choosing to handle their own data sharing may wish to enter into a data-sharing agreement.
Generating large-scale genomic data? NIH’s Genomic Data Sharing (GDS) policy may also apply to your research. See our GDS Policy Overview to learn more.
Examples of Data Sharing Plans
The exact content and level of detail to be included in a data sharing plan depends on the specifics of the project, such as how the investigator is planning to share data, or the size and complexity of the dataset. The examples below give a sense of what a data sharing plan can look like.
Example 1 This application requests support to collect public-use data from a survey of more than 22,000 Americans over the age of 50 every 2 years. Data products from this study will be made available without cost to researchers and analysts. User registration is required in order to access or download files. As part of the registration process, users must agree to the conditions of use governing access to the public release data, including restrictions against attempting to identify study participants, destruction of the data after analyses are completed, reporting responsibilities, restrictions on redistribution of the data to third parties, and proper acknowledgment of the data resource. Registered users will receive user support, as well as information related to errors in the data, future releases, workshops, and publication lists. The information provided to users will not be used for commercial purposes, and will not be redistributed to third parties.
Example 2 The proposed research will include data from approximately 500 subjects being screened for three bacterial sexually transmitted diseases (STDs) at an inner city STD clinic. The final dataset will include self-reported demographic and behavioral data from interviews with the subjects and laboratory data from urine specimens provided. Because the STDs being studied are reportable diseases, we will be collecting identifying information. Even though the final dataset will be stripped of identifiers prior to release for sharing, we believe that there remains the possibility of deductive disclosure of subjects with unusual characteristics. Thus, we will make the data and associated documentation available to users only under a data-sharing agreement that provides for: (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; and (3) a commitment to destroying or returning the data after analyses are completed.
Example 3 The proposed research will involve a small sample (less than 20 participants) recruited from clinical facilities in the New York City area with Williams syndrome. This rare craniofacial disorder is associated with distinguishing facial features. Even with the removal of all identifiers, we believe that it would be difficult if not impossible to protect the identities of subjects given the physical characteristics of subjects, the type of clinical data (including imaging) that we will be collecting, and the relatively restricted area from which we are recruiting subjects. Therefore, we are not planning to share the data.
What data that will be shared:
I will share phenotypic data associated with the collected samples by depositing these data at ________________ which is an NIH-funded repository. Genotype data will be shared by depositing these data at ________________. Additional data documentation and de-identified data will be deposited for sharing along with phenotypic data, which includes demographics, family history of XXXXXX disease, and diagnosis, consistent with applicable laws and regulations. I will comply with the NIH GWAS Policy and the funding IC’s existing policies on sharing data on XXXXXX disease genetics to include secondary analysis of data resulting from a genome wide association study through the repository. Meta-analysis data and associated phenotypic data, along with data content, format, and organization, will be available at ____________. Submitted data will confirm with relevant data and terminology standards.
Who will have access to the data:
I agree that data will be deposited and made available through ________________ which is an NIH-funded repository, and that these data will be shared with investigators working under an institution with a Federal Wide Assurance (FWA) and could be used for secondary study purposes such as finding genes that contribute to process of XXXXXX. I agree that the names and Institutions of persons either given or denied access to the data, and the bases for such decisions, will be summarized in the annual progress report. Meta-analysis data and associated phenotypic data, along with data content, format, and organization, will be made available to investigators through ____________.
Where will the data be available:
I agree to deposit and maintain the phenotypic data, and secondary analysis of data (if any) at ________________, which is an NIH-funded repository and that the repository has data access policies and procedures consistent with NIH data sharing policies.
When will the data be shared:
I agree to deposit genetic outcome data into ________________ repository as soon as possible but no later than within one year of the completion of the funded project period for the parent award or upon acceptance of the data for publication, or public disclosure of a submitted patent application, whichever is earlier.
How will researchers locate and access the data:
I agree that I will identify where the data will be available and how to access the data in any publications and presentations that I author or co-author about these data, as well as acknowledge the repository and funding source in any publications and presentations. As I will be using ________________, which is an NIH-funded repository, this repository has policies and procedures in place that will provide data access to qualified researchers, fully consistent with NIH data sharing policies and applicable laws and regulations.
How to Submit Data Sharing Plans
The plan should be included in the Resource Sharing section of the application. See the How to Apply – Application Guide for form instructions.
Writing a Data Management and Sharing Plan
Under the 2023 Data Management and Sharing (DMS) Policy , NIH expects researchers to maximize the appropriate sharing of scientific data, taking into account factors such as legal, ethical, or technical issues that may limit the extent of data sharing and preservation.
NIH requires all applicants planning to generate scientific data to prepare a DMS Plan that describes how the scientific data will be managed and shared. For more on what constitutes scientific data, see Research Covered Under the Data Management & Sharing Policy .
Applications subject to NIH’s Genomic Data Sharing (GDS) Policy should also address GDS-specific considerations within the elements of a DMS Plan (see NOT-OD-22-189 and details below).
Submitting Data Management and Sharing Plans
The DMS Plan should be submitted as follows:
- DMS Plans should be included within the “Other Plan(s)” field on the PHS 398 Research Plan or PHS 398 Career Development Award Supplemental Form as indicated in the Application Instructions . See below for details on developing and formatting Plans.
- A brief summary and associated costs should be submitted as part of the budget and budget justification (see Budgeting for Data Management and Sharing and the Application Instructions for details).
- Extramural (contracts) : as part of the technical evaluation
- Intramural : determined by the Intramural Research Program
- Other funding agreements : prior to the release of funds
Data Management and Sharing Plan Format
DMS Plans are recommended to be two pages or less in length.
NIH has developed an optional DMS Plan format page that aligns with the recommended elements of a DMS Plan.
Important: Do not include hypertext (e.g., hyperlinks and URLs) in the DMS Plan attachment.

Elements to Include in a Data Management and Sharing Plan
As outlined in NIH Guide Notice Supplemental Policy Information: Elements of an NIH Data Management and Sharing Plan , DMS Plans should address the following recommended elements and are recommended to be two pages or less in length. As described in the Application Guide, the DMS Plan should be attached to the application as a PDF file. See NIH’s Format Attachments page.
1. Data Type
Briefly describe the scientific data to be managed and shared:
- Summarize the types (for example, 256-channel EEG data and fMRI images) and amount (for example, from 50 research participants) of scientific data to be generated and/or used in the research. Descriptions may include the data modality (e.g., imaging, genomic, mobile, survey), level of aggregation (e.g., individual, aggregated, summarized), and/or the degree of data processing.
- Describe which scientific data from the project will be preserved and shared. NIH does not anticipate that researchers will preserve and share all scientific data generated in a study. Researchers should decide which scientific data to preserve and share based on ethical, legal, and technical factors. The plan should provide the reasoning for these decisions.
A brief listing of the metadata, other relevant data, and any associated documentation (e.g., study protocols and data collection instruments) that will be made accessible to facilitate interpretation of the scientific data
For data subject to the GDS Policy: Data types expected to be shared under the GDS Policy should be described in this element. Note that the GDS Policy expects certain types of data to be shared that may not be covered by the DMS Policy’s definition of “scientific data”. For more information on the data types to be shared under the GDS Policy, consult Data Submission and Release Expectations .
2. Related Tools, Software and/or Code
Indicate whether specialized tools are needed to access or manipulate shared scientific data to support replication or reuse, and name(s) of the needed tool(s) and software. If applicable, specify how needed tools can be accessed.
3. Standards
Describe what standards, if any, will be applied to the scientific data and associated metadata (i.e., data formats, data dictionaries, data identifiers, definitions, unique identifiers, and other data documentation).
4. Data Preservation, Access, and Associated Timelines
Give plans and timelines for data preservation and access, including:
- The name of the repository(ies) where scientific data and metadata arising from the project will be archived. See Selecting a Data Repository for information on selecting an appropriate repository.
- How the scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other standard indexing tools.
When the scientific data will be made available to other users and for how long. Identify any differences in timelines for different subsets of scientific data to be shared.
- Note that NIH encourages scientific data to be shared as soon as possible, and no later than the time of an associated publication or end of the performance period, whichever comes first. NIH also encourages researchers to make scientific data available for as long as they anticipate it being useful for the larger research community, institutions, and/or the broader public.
For data subject to the GDS Policy: For human genomic data: Investigators are expected to submit data to a repository acceptable under the Genomic Data Sharing Policy. See Where to Submit Genomic Data . Human genomic data is expected to be shared according to NIH’s Data Submission and Release Expectations , but no later than the end of the performance period, whichever comes first. For Non-human genomic data: Investigators may submit data to any widely used repository. Non-human genomic data is expected to be shared as soon as possible, but no later than the time of an associated publication, or end of the performance period, whichever is first.
5. Access, Distribution, or Reuse Considerations
Describe any applicable factors affecting subsequent access, distribution, or reuse of scientific data related to:
- Informed consent
- Privacy and confidentiality protections consistent with applicable federal, Tribal, state, and local laws, regulations, and policies
- Whether access to scientific data derived from humans will be controlled
- Any restrictions imposed by federal, Tribal, or state laws, regulations, or policies, or existing or anticipated agreements
Any other considerations that may limit the extent of data sharing. Any potential limitations on subsequent data use should be communicated to the individuals or entities (for example, data repository managers) that will preserve and share the scientific data. The NIH ICO will assess whether an applicant’s DMS plan appropriately considers and describes these factors. For more examples, see Frequently Asked Questions for examples of justifiable reasons for limiting sharing of data.
Expectations for human genomic data subject to the GDS Policy: Informed Consent Expectations: For research involving the generation of large-scale human genomic data from cell lines or clinical specimens that were created or collected AFTER the effective date of the GDS Policy (January 25, 2015): NIH expects that informed consent for future research use and broad data sharing will have been obtained. This expectation applies to de-identified cell lines or clinical specimens regardless of whether the data meet technical and/or legal definitions of de-identified (i.e. the research does not meet the definition of “human subjects research” under the Common Rule). For research involving the generation of large-scale human genomic data from cell lines or clinical specimens that were created or collected BEFORE the effective date of the GDS Policy: There may or may not have been consent for research use and broad data sharing. NIH will accept data derived from de-identified cell lines or clinical specimens lacking consent for research use that were created or collected before the effective date of this Policy. Institutional Certifications and Data Sharing Limitation Expectations: DMS Plans should address limitations on sharing by anticipating sharing according to the criteria of the Institutional Certification . In cases where it is anticipated that Institutional Certification criteria cannot be met (i.e., data cannot be shared as expected by the GDS Policy), investigators should state the institutional Certification criteria in their DMS Plan, explaining why the element cannot be met, and indicating what data, if any, can be shared and how to enable sharing to the maximal extent possible (for example, sharing data in a summary format). In some instances, the funding NIH ICO may need to determine whether to grant an exception to the data submission expectation under the GDS Policy. Genomic Summary Results: Investigators conducting research subject to the GDS Policy should indicate in their DMS Plan if a study should be designated as “sensitive” for the purposes of access to Genomic Summary Results (GSR), as described in NOT-OD-19-023 .
6. Oversight of Data Management and Sharing
Indicate how compliance with the DMS Plan will be monitored and managed, the frequency of oversight, and by whom (e.g., title, roles). This element refers to oversight by the funded institution, rather than by NIH. The DMS Policy does not create any expectations about who will be responsible for Plan oversight at the institution.
Sample Plans
NIH has provided sample DMS Plans as examples of how a DMS Plan could be completed in different contexts, conforming to the elements described above. These sample DMS Plans are provided for educational purposes to assist applicants with developing Plans but are not intended to be used as templates and their use does not guarantee approval by NIH.
Note that the sample DMS Plans provided below may reflect additional expectations established by NIH or specific NIH Institutes, Centers, or Offices that go beyond the DMS Policy. Applicants will need to ensure that their Plan reflects any additional, applicable expectations (including from NIH policies and any ICO- or program-specific expectations as stated in the FOA).
Assessment of Data Management and Sharing Plans
Program staff at the proposed NIH Institute or Center (IC) will assess DMS Plans to ensure the elements of a DMS Plan have been adequately addressed and to assess the reasonableness of those responses. Applications selected for funding will only be funded if the DMS Plan is complete and acceptable.
During peer review, reviewers will not be asked to comment on the DMS Plan nor will they factor the DMS Plan into the Overall Impact score, unless sharing data is integral to the project design and specified in the funding opportunity (see NOT-OD-22-189 ).
If data sharing is integral to the project and tied to a scored review criterion in the funding opportunity, program staff will assess the adequacy of the DMS Plan per standard procedure, but peer reviewers will also be able to view the DMS Plan attachment and may factor that information into scores as outlined in the evaluation criteria.
For information about budget assessment by peer reviewers, see Budgeting for Data Management and Sharing .
Revising Data Management and Sharing Plans
Pre-Award Plan Revisions: If the DMS Plan provided in the application cannot be approved based on the information provided, applicants will be notified that additional information is needed. This will occur through the Just-in-Time (JIT) process. Applicants will be expected to communicate with their Program Officer and/or Grants Management Specialist to resolve any issues that prevent the funding IC from approving the DMS Plan. If needed, applicants should submit a revised DMS Plan. Refer to NIH Grants Policy Statement Section 2.5.1 Just-in-Time Procedures for additional guidance.
Post-Award Plan Revisions: Although investigators submit plans before research begins, plans may need to be updated or revised over the course of a project for a variety of reasons for example, if the type(s) of data generated change(s), a more appropriate data repository becomes available, or if the sharing timeline shifts. If any changes occur during the award or support period that affects how data is managed or shared, investigators should update the Plan to reflect the changes. It may be helpful to discuss potential changes with the Program Officer. In addition, the funding NIH ICO will need to approve the updated Plan. NIH staff will monitor compliance with approved DMS Plans during the annual RPPR process as well. For more details, please refer to NOT-OD-23-185: Prior Approval Requests for Revisions to an Approved Data Management and Sharing (DMS) Plan Must be Submitted Using the Prior Approval Module .
Additional Considerations
Note that funding opportunities or ICs may have specific expectations (for example: scientific data to share, relevant standards, repository selection). View a list of NIH Institute or Center data sharing policies . Investigators are encouraged to reach out to program officers with questions about specific ICO requirements.
Please note that a Plan is part of an application, and, as such, an institution takes responsibility for the Plan and the rest of the application's contents when submitting an application. Although part of the official submission, when not considered during peer review the attachment is maintained as a separate “Data Management and Sharing (DMS) Plan” document in the grant folder viewable via the Status Information screen in eRA Commons. This document is viewable by authorized users and is not part of the assembled e-Application.
New Data Management & Sharing Policy Effective January 25, 2023!
Related resources.
Selecting a Data Repository
Budgeting for Data Management & Sharing
Data Management
NIH Institute or Center Data Sharing Policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Appl Clin Inform
- v.9(1); 2018 Jan

Exploring Data Quality Management within Clinical Trials
Lauren houston.
1 School of Medicine, Faculty of Science, Medicine and Health, University of Wollongong, Wollongong, Australia
2 Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, Australia
Yasmine Probst
3 School of Computing and Information Technology, Faculty of Engineering and Information Sciences, University of Wollongong, Wollongong, Australia
Allison Martin
Background Clinical trials are an important research method for improving medical knowledge and patient care. Multiple international and national guidelines stipulate the need for data quality and assurance. Many strategies and interventions are developed to reduce error in trials, including standard operating procedures, personnel training, data monitoring, and design of case report forms. However, guidelines are nonspecific in the nature and extent of necessary methods.
Objective This article gathers information about current data quality tools and procedures used within Australian clinical trial sites, with the aim to develop standard data quality monitoring procedures to ensure data integrity.
Methods Relevant information about data quality management methods and procedures, error levels, data monitoring, staff training, and development were collected. Staff members from 142 clinical trials listed on the National Health and Medical Research Council (NHMRC) clinical trials Web site were invited to complete a short self-reported semiquantitative anonymous online survey.
Results Twenty (14%) clinical trials completed the survey. Results from the survey indicate that procedures to ensure data quality varies among clinical trial sites. Centralized monitoring (65%) was the most common procedure to ensure high-quality data. Ten (50%) trials reported having a data management plan in place and two sites utilized an error acceptance level to minimize discrepancy, set at <5% and 5 to 10%, respectively. The quantity of data variables checked (10–100%), the frequency of visits (once-a-month to annually), and types of variables (100%, critical data or critical and noncritical data audits) for data monitoring varied among respondents. The average time spent on staff training per person was 11.58 hours over a 12-month period and the type of training was diverse.
Conclusion Clinical trial sites are implementing ad hoc methods pragmatically to ensure data quality. Findings highlight the necessity for further research into “standard practice” focusing on developing and implementing publicly available data quality monitoring procedures.
Background and Significance
Clinical trials are an important research method for improving medical knowledge and patient care. Evidence has linked poor data quality to incorrect conclusions and recommendations; 1 2 3 4 therefore, data quality is of paramount importance for acquiring reliable research findings from clinical trials. 5 6 As poor data quality may stem from error; consequently, preventing data error is just as important as the development, design, and collection of clinical trial data. 7 Assessment of all possible sources of error, including data recording, abstraction, transcription, entry, coding, and/or cleaning processes, contributes to improving data quality for clinical trials. 8
Many strategies and interventions have been developed aiming at reducing error in clinical trials, including standard operating procedures (SOPs), personnel training, data monitoring, and design of case report forms (CRFs). Additionally, multiple international and national guidelines stipulate the need for data quality and assurance; however, they are nonspecific in the nature and extent of the necessary methods. These guidelines include the International Conference on Harmonisation Good Clinical Practice (ICHGCP) guideline E6 (1996) 9 updated in 2015, guideline E6(R2); 10 the International Standards Organization (ISO) Clinical Investigation of Medical Devices for Human Subjects—GCP (2011) (ISO 14155:2011); 11 the Food and Drug Administration (FDA) Guidelines for Monitoring of Clinical Investigations (1998) updated in 2013; 12 the European Union's Clinical Trial Directive 2001/20/EC (2001), 13 updated in 2009; and the Australian Government's National Statement on Ethical Conduct in Human Research (1999), updated, in 2007. 14
According to ICHGCP, clinical trials must protect the rights and safety of all patients and ensure trial results are legible and valid. Onsite monitoring is important to achieve high data quality and to ensure the method of source data verification a (SDV) fulfills the original objectives. 15 To optimize the efficacy of monitoring, there has been an emphasis on data audits and reducing onsite monitoring. 10 11 However, the ICHGCP guidelines are flexible in interpretation and do not provide specific details on how and when to conduct audits, or how much or how little monitoring is required to maintain data integrity. 16 17 There is a lack of evidence to support intensive monitoring for data quality; in fact, updated guidelines promote alternative monitoring methods, such as risk-based approaches b , centralized monitoring c and remote monitoring d , which complement trial procedures by improving the use of resources available. 12 18 19 20 Although a reduction in onsite monitoring is suggested (updated ICHGCP guidelines re 21 22 ), the majority of clinical trials continue to conduct traditional 100% SDV. 23 Lack of clear guidance on which monitoring method is valid and cost-effective to ensure data integrity creates confusion within the clinical research community. A reduction in onsite SDV and the risk of missing critical issues are tradeoffs that more efficient, modern monitoring approaches need to consider.
Due to a growing concern about the effectiveness and efficiency of monitoring procedures, Brosteanu et al conducted a clustered randomized study comparing intensive onsite monitoring and risk-adapted monitoring. Results found the benefit of intensive onsite SDV to be small (8.2%) when compared with risk-based monitoring, which utilized less than 50% of resources while ensuring the same level of GCP compliance. 18 It is evident that a standardized approach needs to be adopted for monitoring data quality in clinical research. In support of this notion, a survey conducted by the Clinical Trials Transformation Initiative (CTTI) investigated the intensity, focus, and methodology of monitoring practices by clinical research sponsors over a range of trial settings. 24 Survey results found that there is heterogeneity within and between organizational types including academic/government, clinical research organizations, and industry.
Evidence of the effect of nonstandardized data quality checks within clinical trials is the online blog of publication retractions due to fraudulent data. 25 This Web site lists 562 publications in 2017 alone that had to be withdrawn due to incorrect data/analysis. There appears to be a lack of knowledge about systematic methods and procedures for data quality assessment in clinical trials. 1 To ensure data integrity in clinical research, it is imperative to introduce a “gold standard methodology” so that manuscripts can be published referencing their methods employed and the broader research community can be reassured the data was valid.
The objective of this feasibility study was to gather information about current data quality tools and procedures used within Australian clinical trial sites, with the aim to develop a standard data quality monitoring procedures to ensure data integrity.
Clinical trial sites listed on the Australian Government National Health and Medical Research Council (NHMRC) Australian Clinical Trial site list were invited to participate in this survey ( n = 148). 5 26 Staff members targeted to complete the survey included the manager/employee in charge of trial-related data quality assurance processes. The NHMRC clinical trial list was determined as a representative sample of Australian clinical trials including all phases (I–IV) and types (treatment, diagnostic/screening, and prevention) of clinical trials.
Clinical trial sites that were identified to have an affiliation with the University of Wollongong (UoW), the organization that the researchers were employed, were excluded from the study to avoid the potential risk of bias. Any overlapped sites, which may cause duplication, were also excluded. Several clinical trial networks on the list responded that they did not run clinical trials independently. In this case, permission was given for these networks to forward the survey to their collaborating organizations that run clinical trials. Informed tacit consent was obtained by completion and return of the online questionnaire survey form.
Development of the Online Questionnaire Survey Form
Eleven survey questions were adapted in short form from the published and validated survey questions. 24 27 Information gathered from the survey included data quality management methods and procedures, error levels, data monitoring, staff training, and development (see Appendix ).
Construct validation was completed by a convenience sample of 10 UoW researchers who reviewed survey questions to ensure that the intended concept was assessed. Participants were also asked to comment on any procedural, usability, and transparency issues faced in completing the survey. Expert advice from a data management manager was sought to ensure content validity, question clarity, and answers fully address the research questions. The online questionnaire survey was designed using the Research Electronic Data Capture (REDCap) tool hosted at the University of Wollongong. 28
Survey Administration
A cross-sectional study design was applied to get an overview of the current quality tools and practices implemented in Australian clinical trials. Invitations for participation were sent to the identified contact person for each clinical trial site via email. The email contained a brief introduction and a direct link to the survey. Each clinical trial site was provided with an individual identification code and three email reminders were sent over a 4-month period to nonrespondents. Clinical trial sites were asked to forward the survey to their collaborating sites using the same individual identification code if they were identified to be clinical institute network.
Data Analysis
Questionnaire responses were standardized into categorical options and numerically coded for analysis. Statistical analyses were conducted using the IMB SPSS software (Version 22, IMB Australia, Lane Cove, NSW, Australia). Data were explored via descriptive statistical analyses. Free text responses were analyzed using the six phases of thematic analysis 29 and conducted using the NVivo qualitative data analysis software (QRS International Pty Ltd., Version 10, 2012). Ethics approval was obtained from the University of Wollongong Human Research Ethics Committee (HE16/131).
Of the 148 clinical trial sites identified, 142 initial invitations were sent out, see Fig. 1 . A total of 34 clinical trial sites consented to participate in the online survey, yielding a response rate of 24%. Of the 34 responses, 14 were excluded from the analyses due to missing data for > 25% of survey questions. Three clinical networks asked to forward the survey invitation email to respondents that were more appropriate as well as to their collaborating sites. Finally, 20 clinical trial site employees completed the survey in full and were included in data analysis. Survey respondents were all female, majority had completed a university degree, and the mean duration of current employment was 5.74 ± 5.15 years (range, 0.5–22) (see Table 1 ). At each clinical trial site, more than one type of clinical trial was conducted at any point in time. Table 2 lists the number and types of clinical trial sites.

Survey invitation flow diagram.
Data Management and Monitoring
At the time of the survey, 10 sites (50%) reported having a clinical data management plan in place and the majority ( n = 19) had implemented at least one or more procedures to ensure data quality (see Table 3 ). Only two sites set an error acceptance level, <5% and 5 to 10%, respectively, both responding that no follow-up or further monitoring was conducted if the error rate was found to be higher than the error acceptance level.
Centralized monitoring: Data collected through an electronic data capture and queries identified by monitor that may need further attention to alleviate problems.
Remote monitoring: Data monitored off-site, includes delivering documents via email, fax, or snail mail to monitors to conduct source data verification.
Logic, range, and consistency check: Logic check, flag indicator results that fail a common-sense comparison to other indicator or other disaggregation; Range check, check the value of data to see if it is within a certain range; Consistency check, performed to determine if the data has an internal conflict and data field correspond.
Onsite SDV: At the site comparing source data (original or certified copy) document to data recorded or entered to a case report form or electronic record or database.
Statistic technique: For example, cluster and outlier analysis.
Risk-based targeted monitoring: Focus on a certain data point that has been identified to have the most risk.
Risk-based triggered monitoring: After a certain event like a large number of adverse events or deviations further detailed monitoring occurs.
The structure of data monitoring was reported in terms of variables to be selected, its coverage and amount, and time of execution. Monitoring 100% of the data points was the most common ( n = 7) response, although the procedures implemented varied greatly, and the amount of data included in monitoring ranging from 10 to 100%. The timing of data monitoring varied and was specific to the clinical trial and study design. The frequency of data monitoring varied among the six sites (30%) from monthly to annually. The variables included in data monitoring were completed on all (100%) data points ( n = 5), only critical data points ( n = 1), critical and noncritical data points defined by each study ( n = 3), or were dependent on the clinical trial ( n = 3). When asked about how their data were monitored, seven sites (35%) reported that they implemented at least one or more sampling techniques to extract data points, seven sites (35%) did not know, and one site (5%) did not implement sampling techniques at all, see Table 3 for further details.
Training and Development
A certain type of staff training and development devoted to data quality were conducted at all clinical trial sites (100%, see Table 4 ). The average amount of time spent on staff training and development per person, per clinical trial was 11.58 ± 9.01 hours, (range, 2–30) over a 12-month period.
Abbreviations: ICH-GCP, International Conference on Harmonisation and Good Clinical Practice; SOP, Standard operating procedure.
The personnel responsible for reviewing the reports of data quality and consistency varied from chief investigators (65%), auditor/monitor (60%), the data manager (55%), and sponsor (50%). In total, 75% of respondents answered that more than one person reviewed the reports.
This feasibility study highlights the heterogeneity of data quality management practices within Australian clinical trials. Only 50% of the respondent clinical trial sites currently had a clinical data management plan in place, confirming our proposition that developing and maintaining a data management system is a challenge for clinical trials. 30 This is also in accordance with a recently published survey. 27 This survey reported considerable variation in data management, with over 50% of clinical research centers having a data management system but many did not comply with guidelines and legal requirements (GCP and FDA). 27 There are many reasons for this, such as individual clinical trials implementing different procedures dictated by the sponsor, or monetary constraints in academic versus pharmaceutical clinical trials. 31
Centralized and remote monitoring were found to be the most common data monitoring methods utilized, although there appeared to be a lack of credible literature to suggest the advantage of these “newer” methods over the more traditional approaches. 20 This study identified that 50% of sites still use traditional data monitoring methods such as 100% onsite SDV, which is an expensive, labor-intensive activity 32 and does not guarantee error-free results. 33 Andersen et al 34 compared the effect of partial SDV and traditional 100% SDV using post hoc analyses of three-phase III randomized control trials. Because completing traditional 100% SDV monitoring only reduced error marginally (0.26%) compared with partial SDV, the authors challenged the belief that a 0% error rate is not an achievable goal. Only 2 out of 20 trial sites in our survey reported having a set error acceptance level, being ≤10%, which is in line with published literature. 8 35 36 One of the two clinical trial sites stated that they implemented a 5 to 10% threshold range; however, no further comment on why or when a different threshold for data validation was adhered to. As the survey was administered to clinical trial sites, the researchers have assumed that it might be possible that different clinical trial types have a tighter threshold than others; for example, a 5% threshold for a phase IV trials compared with 10% for epidemiological trials. Future research is required to explore the rationale for different levels of error acceptance within clinical trials.
The major quality assurance activity reported to “prevent” data errors was regular education and training of data collectors throughout the clinical trial. Although the majority of survey respondents reported that staff training and development was undertaken, the amount of training time varied greatly. Many researchers receive little to no training in regards to best practice for attaining, evaluating, and controlling the quality of data collected. This is in line with the literature that reported due to the limitation of time and resources, not all research trials implement all the necessary data quality management tools and procedures. 37
Within the pharmaceutical/private industries 38 and information sciences literature, data quality tools and procedures are well developed in which many frameworks acknowledge the multiple dimensions of data quality. 39 40 41 42 43 44 However, only a small body of clinical and health researchers have described the use of data quality frameworks, 38 45 46 47 48 and fewer have identified appropriate methods to quantify the quality of data. 8 Although many data quality dimensions and attributes have been determined within the clinical and health literature, the majority provides no usable definitions. Public sharing of this knowledge is crucial in developing a standardized approach that can be implemented across the clinical and broader research community to improve the rigor of clinical trials.
Study Limitations
The results of this feasibility study are limited to clinical trials listed on the Australian NHMRC clinical trial site list. The survey results are subject to potential bias in a positive direction as the staff member who completed the survey may be more knowledgeable about their organization's data quality management procedures than those who chose not to participate. As clinical trial sites were recruited as an organization, it is impossible for us to track if the staff member who completed the survey was best positioned to do so in the organization. In addition, the tools and procedures differed among clinical trials, which were influenced by overarching site policies. All these cause difficulty in interpreting results. The reason for low response rate might be clinical data audits and data management procedures are usually considered highly confidential by many research organizations and kept in-house. 49 As it is impossible to collect data about how many forwarding emails were sent by the clinical trial sites listed on the NHMRC Web site, this data was not accounted for in calculating a response rate. The low response rate means that the results of this survey should be used with caution. They may not be generalizable as a representative sample of clinical trial sites. Future research should include qualitative analysis through key-informant interviews with the provision of SOPs. This feasibility study was not designed to assess which data management tool is more effective, but rather to gather information about current data quality tools and procedures used in Australian clinical trials. Further research is required to fully examine the best method of monitoring data quality to assure and control data integrity in clinical research.
At the conclusion of three studies, OPTI-misation of MONitoring (OPTIMON), 23 Strategic Timing of AntiRetroviral Treatment (START) trial Monitoring Substudy, 50 and TargetEd Monitoring: Prospective Evaluation and refinement (TEMPER) study, 51 the scientific community will have a better understanding of effective monitoring strategies. At the conclusion of all three studies, empirical evidence will be provided and aid in improving the currently limited published procedures, as all the three studies have different aims and designs in developing audit methodology.
This research is part of a collaborative project and an important opportunity for clinical trial sites to bring together their existing experience to improve data quality management systems. The authors' highlight that this was not a validated survey and formal validation of the instrument is required in future trials. It is recommended to further compare what is described in the literature and what is currently happening at a site level to identify the gaps, the facilitators, and the barriers to implementing data quality management systems. To achieve the objective of informing the data quality improvement initiative over a broad spectrum of clinical trials, this critical information needs to be made freely available in the published literature.
This is the first survey gathering information about current data quality tools and procedures within Australian clinical trial sites. This survey found that clinical trial sites were implementing newer approaches such as centralized and remote monitoring despite the majority were still completing 100% SDV, a labor-intensive and cost-inefficient method. It is clear that data quality management procedures vary greatly between clinical trials sites, with only 50% of the trial sites with a data management plan in place. Further research is required to assess differences between data management tools and procedures between clinical trials within a clinical trial site. This will allow researchers to investigate what is “standard practice” and focus on developing and implementing publicly available data quality monitoring procedures to ensure data integrity. Data quality is essential for the reliability of scientific findings generated from the investment in clinical trials, adequate infrastructure, staff skills, management support, and resources need to be in place to ensure data are effectively managed. It is time that quality assurance and quality control tools and procedures implemented in clinical trials are cited in all publications.
Clinical Relevance Statement
It is vital to ensure the scientific rigor of clinical trials to evaluate data quality management procedures, assure the accuracy of findings, and reduce error. This survey highlights the heterogeneity of clinical trial's data quality management practices in Australia. It thus suggests that “best practices” need to be made freely available in the published literature. Adequate infrastructure, staff skills, management support, and resources need to be considered and implemented to ensure high quality data and research are produced and managed effectively.
Multiple Choice Question
Which of the following data monitoring methods is the traditional approach that fulfills the International Conference on Harmonisation Good Clinical Practice (ICH-GCP) E6 guideline (1996)?
- Remote monitoring
- Source data verification
- Central monitoring
- Risk-based approach
Correct Answer : The correct answer is b, source data verification. In 1996, the International Conference on Harmonisation (ICH) guideline E6 on Good Clinical Practice (GCP) reported there is a need for onsite monitoring before, during, and after a clinical trial. The practice of source data verification (SDV) fulfills the ICHGCP requirements and is a process of comparing data collected on original source documents to data recorded on a CRF or electronic record. Source documents are considered the “gold standard” from which data are obtained in clinical trials. Therefore, SDV is considered the traditional approach to monitoring data utilized by clinical trial auditors.
Acknowledgment
This research has been conducted with the support of the Australian Government Research Training Program Scholarship.
Funding Statement
Funding None.
Conflict of Interest None.
Authors' Contributions
L.H. conceptualized and formulated the research question, designed the study, performed the study, evaluated the data, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. Y.P., P.Y., and A.M. made substantial contributions to the study design, analysis, and interpretation of the data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Protection of Human and Animal Subjects
This study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and was approved by the University of Wollongong Human Research Ethics Committee (HE16/131).
a Source data verification (SDV) defines the process of comparing data collected on original source documents to data recorded on a case report form (CRF) either on paper or electronic records.
b Risk-based approach includes a mixed method approach focused on the critical data points and processes that are identified to have the most risk via a targeted or triggered assessment.
c Centralized monitoring is a remote evaluation carried out by the sponsor personnel or representatives at a location other than the sites at which the clinical investigation is being carried out on a real time basis.
d Remote monitoring is off-site monitoring of activities previously conducted on-site. Documents are delivered via email, fax or snail mail to a clinical research associate (CRA) to conduct SDV and satisfy queries.
Clinical Data Management: Process, Benefits, and Software Solutions
03 Jan 2024

Technical writer
Effective clinical data management is critical in modern healthcare to make better-informed decisions about diagnosis, treatment plans, and potential health risks. Healthcare organizations must meet the highest standards in data collection, processing, and storage, especially in clinical trials.
The importance of effective clinical data control manifests itself in improving the accuracy of diagnoses, ensuring patient safety, meeting regulatory compliance, and supporting research to enhance medical practice further.
In this article, we will explore the essence of the clinical data management process and its regulatory landscape, what software is used for it, and how to integrate the clinical data management system. You will also unveil why custom clinical data management software is better decision than ready-made one.
What Is Clinical Data Management and Why It’s Important
Clinical Data Management (CDM) is an integral component of clinical research operations allowing to manage all aspects of clinical trials including collecting, validating, storing, and managing data obtained during clinical trials. Clinical trials are scientific studies to assess the safety, efficacy, and quality of new medical treatments, drugs, or devices. Role of clinical data management is ensuring that the information generated from these trials is accurate, reliable, and adheres to regulatory standards.
Advantages of effective CDM
Clinical trial data management provides healthcare organizations with many advantages, including:
Data accuracy and integrity
Efficient document management in clinical trials minimizes errors in data entry, ensuring the precision and dependability of clinical trial information. Rigorous validation processes identify and rectify inconsistencies, upholding data integrity.
Compliance with regulatory standards
Adherence to health standards, including FDA (Food and Drug Administration) and ICH(International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) is paramount. Robust electronic clinical trial management solutions establish a compliant environment with audit trails that meticulously document data changes for regulatory scrutiny.
Timely and efficient data collection
Streamlined processes in clinical trials data management reduce the time and resources required for data entry and validation. Real-time monitoring enables swift issue identification and resolution, enhancing overall clinical trial study efficiency.
Patient safety
Effective clinical data management software facilitates early detection of adverse events, enabling prompt intervention to safeguard the well-being of study participants. Actionable insights support accurate assessments of treatment safety and efficacy.
Cost-efficiency
Data management in clinical research contributes to cost efficiency by automating and optimizing all aspects of clinical financial management processes. Fewer data queries and streamlined clinical operations minimize the need for additional resources, promoting resource optimization.
Data security and confidentiality
Integraton robust security measures in clinical data management solutions allows to protect sensitive patient information, ensuring compliance with privacy regulations. Access to study based on roles and permissions enhance data confidentiality in clinical applications.
Facilitated data analysis and reporting
Well-managed data sets form the basis for accurate statistical analysis, supporting meaningful interpretation of trial results. By merging and structuring data, clinical trial management system lead to faster generation of study reports and submission of regulatory documents.
Effective data review in clinical data management is pivotal for the success of clinical trials, providing a foundation of reliable, high-quality information essential for informed decision-making in healthcare and advancing medical knowledge.
Need advice on clinical data management system integration?
Book a consultation with our Healthcare matter experts to get advice on developing and integrating clinical data management solutions for better decision-making about care delivery
Clinical Data Management Process Step-By-Step
Understanding the clinical data management process flow chart is critical to successful clinical research. This familiarity with each step of CDM helps ensure data accuracy, reliability, and security, contributing to quality study outcomes.
Let's review each clinical research data management stage.
Data Management Plan (DMP)
The Data Management Plan (DMP) is a detailed document that outlines all procedures, tasks, milestones, and deliverables throughout the data management clinical trial lifecycle. This document provides a roadmap for handling information and managing potential risks. It also serves the vital function of clearly communicating what is happening in the study to everyone involved.
The DMP typically describes the following aspects:
- Data collection. Defining the information gathered from participants
- Data integration. Incorporating existing data into the process
- Data format. Establishing the format for collected data
- Metadata and standards. Determining metadata and its standards
- Storage and backup. Outlining methods for data storage and backup
- Security measures. Implementing measures to safeguard sensitive information
- Data quality procedures. Ensuring the quality of collected data
- Team responsibilities. Assigning roles and responsibilities within the team
- Access and sharing mechanisms. Defining mechanisms and restrictions for data sharing
- Long-term archiving. Detailing procedures for long-term data archiving
- Cost considerations. Evaluating the expenses related to data preparation and archiving
- Regulatory compliance. Ensuring adherence to healthcare industry regulations and standards (e.g., HIPAA, GDPR, FHIR, HL7 integration , etc.)
The DMP should be ready during the study management platform design phase before enrolling the first participant.
Design of the electronic case report form (eCRF)
The electronic case report form (eCRF) is a questionnaire for collecting data from study participants and submitting it to trial sponsors. This document is explicitly created for each research project by the study start-up protocol and Clinical Data Standardization Consortium (CDISC) guidelines to unify data sharing across the industry.
eCRF structure in clinical trial data management
Clinical trial database design
A clinical trial database is data gathered during a study and organized into rows and columns. It is designed with the structure of eCRFs kept in mind. Some questionnaire data may be coded into meaningful categories to save space in the database. In this case, database specialists create detailed decoding descriptions or techniques for mapping codes into CRF elements and standardize clinical data across trials. Before launching into the research environment, the database is tested with fictitious data in a secure setting.
Electronic data capture in clinical research
As mentioned earlier, CRFs (electronic or paper data collection forms) are the primary tool for obtaining information in clinical trials. Traditionally, clinicians or data entry specialists collected information for these forms from participants during their visits to healthcare facilities. However, current trends show that healthcare organizations are no longer the only research site. Details for content management are now also being extracted from various healthcare software and tools like Electronic Health Records (EHRs), medical devices, wearables, laboratory research management systems, etc.
Data validation
The validation phase of data management for clinical trials involves performing a series of tests to ensure the accuracy, consistency, readability, and integrity of data from different sources. This process includes such activities:
- Electronic checks. The database designer creates and embeds electronic checks into electronic CRFs to automatically compare input data against numeric and logical criteria, preventing unrealistic values from appearing in the document.
- Source Data Validation (SDV). This process involves verifying CRF data against original medical records and other sources to confirm that electronic CRFs contain all necessary information and faithfully represent the participant's profile.
- Data anonymization. Before submission, clinical data undergoes an anonymization process as required by law, removing all elements of protected health information (PHI) linking the document to a specific individual.
Database closure and data archiving
After the study, the database is locked to prevent the information from being altered. The clean data is then available to stakeholders for statistical analysis, reporting, and publication of results. However, all these steps are beyond the scope of clinical data management.
The necessary documents and study materials should be archived for at least three years, ensuring that the data can be monitored, evaluated, and recovered for future studies.
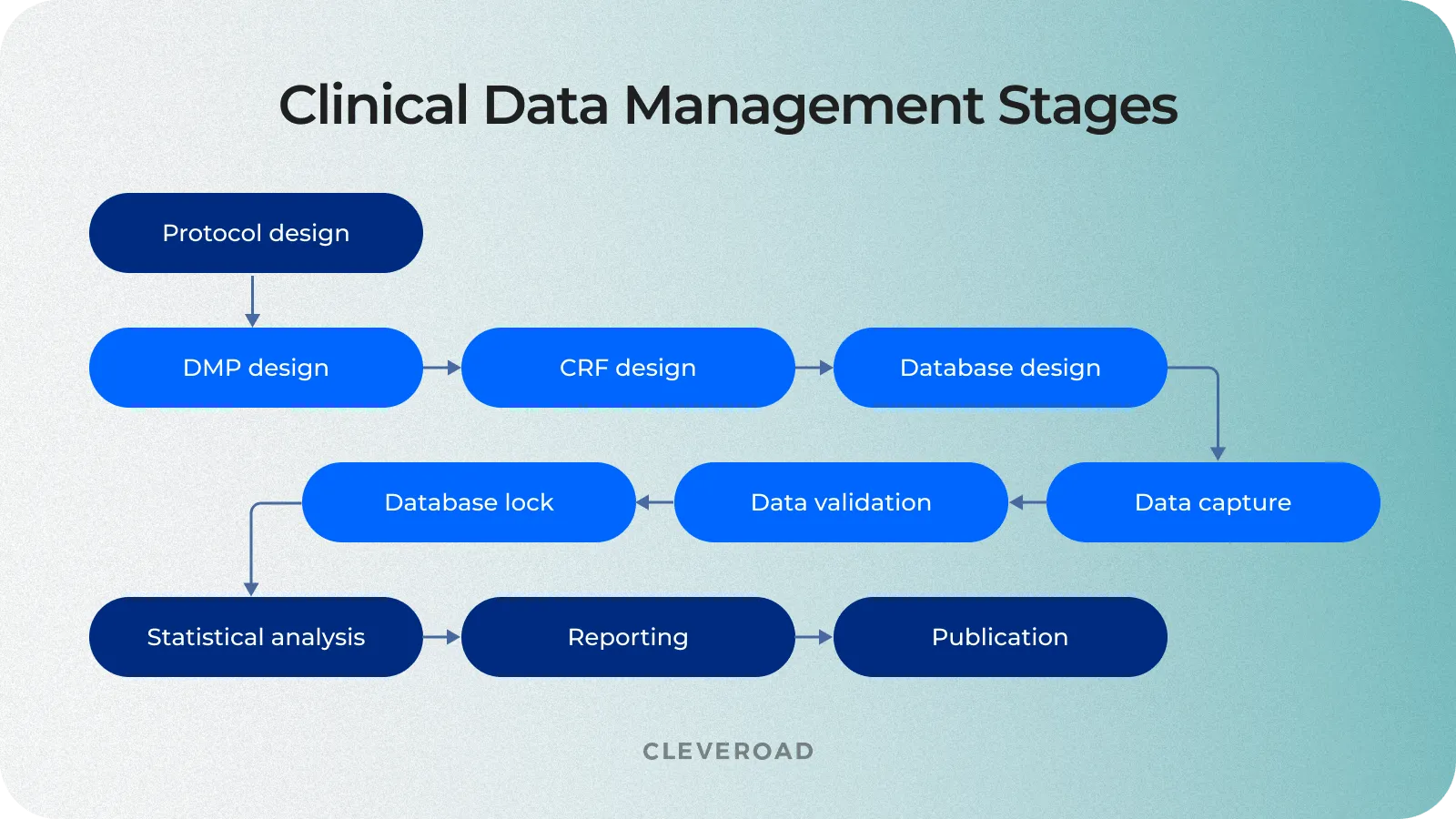
The process of clinical trial data management
Clinical Data Management Roles and Responsibilities
A well-coordinated team is crucial for smooth clinical trial operations. From project management to meticulous data entry and coding, each role ensures accurate and compliant data throughout the trial. This collaborative effort guarantees effective clinical suite data management, fostering transparency and precision in line with regulatory standards. The table below outlines the clinical data management roles and responsibilities .
The Most Successful Clinical Data Management Solutions
There is a large variety of software for clinical data management on the market, offering diverse tools. Let's take a look at some of the best clinical trial management software (CTMS) to explore their functionality and gain useful insights for creating your own solution:
IBM Clinical Development
IBM Clinical Development (ICD) is a leading clinical trial management cloud-based system designed for data capturing in large-scale, decentralized studies. This CTMS provides reliability, a rich library of pre-built forms, and a modular structure, allowing users to pay for specific features. The AI power of IBM Watson supports medical coding. However, users note drawbacks such as an archaic UI, slow customer support, and a higher cost of ownership, making it less accessible for startup projects.
Oracle Clinical
Oracle Clinical is a modern clinical trial solution distinguished by its extensive usage, providing reliability and adaptability to various clinical trials. With a customized pricing model, it offers rich functionality and seamless integration with the Oracle Health Sciences ecosystem. However, users highlight a steeper learning curve and potential costs associated with dedicated training.
Castor EDC is famous for its ease of use and adaptability, serving over 2000 clinical trials. Users are choosing a CTMS platform for its transparent subscription-based pricing ensures clarity and features a user-friendly interface with high customizability. Automated data validation enhances accuracy, but users note limitations in advanced features for complex trials.
Medidata Rave
Medidata Rave is a widely adopted cloud-based clinical trial platform for over 3500 clinical trials. With flexible pricing based on study characteristics, it offers cloud accessibility to manage your clinical data anywhere, risk-based monitoring, and eSource capabilities. However, users are cautious about higher initial costs and potential ongoing expenses, requiring careful budget management.
OpenClinica
OpenClinica, utilized in over 1500 clinical trials, stands out for its open-source nature, adhering to CDISC standards. Its open-source flexibility and role-based access enhance customization and manage clinical data securely. However, users mention limitations in advanced features and a reliance on community support, particularly for complex clinical studies.
Off-the-Shelf Vs. Custom CDMS
When deciding to build data management systems for clinical trials, you will be faced with a choice of two options :
- Use off-the-shelf CDM software
- Create custom clinical data management solution
Let's explore the features of each of these development approaches so that you can make an informed decision:
Off-the-shelf software
Off-the-shelf clinical trial software provides pre-packaged solutions for purchase and use. These solutions come with standard features and configurations for medical data administration, aiming to suit a broad range of users without extensive customization.
- Quick enrollment. Off-the-shelf solutions can be deployed quicker, saving time on development.
- Lower initial costs. These solutions come with lower upfront costs than custom alternatives.
- Limited customization. Lack of flexibility and customization options may lead to constraints in adapting to unique study requirements.
- Charges for unused functionality. You may have to pay for features that you don’t need, which, in the end, will be more expensive in the long run.
- Limited scalability. Even with top clinical trial management software, you can face challenges as the scale or complexity of the study increases.
- Generic user experience. Users may encounter a generic interface that does not cater specifically to their workflow.
Custom clinical data management software
Custom clinical data management software is tailored to the specific needs of your research organization or study. It involves developing a solution from scratch, ensuring that every aspect aligns with the study's unique protocols, workflows, and data formats.
- Tailored adaptability. Custom solutions can be adapted precisely to the unique protocols and workflows of the study, offering maximum flexibility.
- Scalability. Designed to scale seamlessly with evolving project needs, minimizing disruptions and accommodating growing data requirements.
- Enhanced security. Custom CTMS software provides robust safety measures to secure data from research systems.
- Optimized user experience. Interfaces can be designed to maximize user experience, improving overall usability.
- Longer development time. Building a custom solution may take longer due to the development process, testing, and fine-tuning. But such software will be tailored to your specific healthcare business needs, allowing you to boost clinical trial efficiency.
- Higher initial investment. The initial costs of developing a custom solution may be increased compared to off-the-shelf options. However, this can lead to potential long-term savings and efficiency gains.
In summary, while off-the-shelf software offers quick implementation and lower initial costs, the custom clinical data management solution provides tailored adaptability, scalability, security, and user experience, making it a preferred choice for organizations with specific and evolving needs in the clinical research domain.
Regulatory Landscape in Clinical Data Management
The regulatory framework for clinical data management is shaped by various industry standards, laws, and regulations designed to ensure ethical behavior, data integrity, and the rights of clinical trial participants. Compliance with these regulations is critical to confirm the validity of trial results and the well-being of participants.
Here is an example of healthcare laws and standards to comply with :
Good Clinical Practice (GCP). GCP is the global standard for the ethical and scientific conduct of clinical trials. With several standard requirements for GCP-compliant data management , it ensures participants' safety and the trial results' reliability through rigorous standards.
Health Insurance Portability and Accountability Act (HIPAA). HIPAA ensures the privacy and security of individually identifiable health information in the United States. The regulation prescribes measures to protect the confidentiality and integrity of participants' medical data.
Read our guide to HIPPAA-comliant software development with no mistakes
General Data Protection Regulation (GDPR). GDPR in the EU regulates the processing and protection of personal data. Particular attention is paid to consent, data anonymization, and the right to erasure, which affects data from clinical trials involving EU residents.
Clinical Data Interchange Standards Consortium (CDISC). CDISC sets global standards for the structure of clinical trial data. The main point of the regulation is to promote data standardization to facilitate exchange, reduce ambiguity, and simplify review by regulatory authorities.
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH promotes cooperation between regulatory authorities and the pharmaceutical industry. The primary purpose of harmonization is to interpret technical guidelines for drug registration.
21 CFR Part 11 - Electronic Records; Electronic Signatures. Part 11 of the US CFR establishes criteria for electronic records and signatures. It regulates electronic systems to ensure data integrity, security, and confidentiality in clinical trials.
Adhering to these healthcare laws and regulations is vital for clinical data managers, sponsors, and investigators. Compliance with these industry regulations validates trial results and advances medical knowledge and patient data safety.

Best Ways of Integrating a Robust Clinical Trial Data Management System
Now, let's consider the main points when designing and implementing clinical data management software.
Defining your healthcare business needs
Before creating a robust clinical trial data management system you must clearly define trial objectives, including identifying specific data, key performance indicators (KPIs), desired outcomes and other critical metrics. Understanding the trial's purpose is crucial for aligning data management efforts with overarching research goals. Also, early engagement with a study team and stakeholders, including researchers, clinicians, and data managers, ensures a comprehensive understanding of user needs.
Finding an experienced tech vendor
Selecting an experienced IT vendor providing quality healthcare software development services is a critical step. This includes researching the vendor's reputation, including checking reviews on platforms like Clutch and GoodFirms, reviewing previous projects, and evaluating their experience in creating solutions for healthcare and telemedicine. You should also ensure the vendor can build a custom clinical data management system that meets the unique needs of your healthcare organization.
You can review our Clutch profile to explore how we’ve helped our clients to solve their business challenges and their opinions about cooperation with Cleveroad.
Learn how to outsource healthcare software development from our detailed guide
Regulatory compliance and security
Compliance with industry and security standards is integral to implementing a clinical data management system. Data protection, privacy, and regulatory conformity are critical aspects of the process. Make sure your vendor is experienced in healthcare data security . For example, to ensure medical software safety, Cleveroad experts use security measures such as:
- Role-based access control and activity tracking
- Implementation of standards like HL7 and HTTPS/encrypted WebRTC
- Industry-standard data encryption at rest and on client devices
Ensuring adherence to healthcare laws and standards governing clinical trials is equally essential. This includes compliance with requirements for processing personal data (GDPR, HIPAA) and conformity with data storage and exchange standards, such as CDISC, HL7, and other relevant laws. Regulatory compliance ensures the legality and transparency of clinical trials, promotes trust among study participants, and supports ease of interaction with regulatory agencies.
With 11+ years of healthcare software development experience , our team will help you create clinical data management software in compliance with healthcare laws such as HIPAA, GDPR, CDISC, ICH, etc.
Technology stack selection
Identifying the right technologies is critical in implementing a clinical data management system. This includes deciding between cloud-based and on-premise options and assessing the selected technology's readiness for future innovations in clinical research. An experienced tech vendor specializing in creating medical software solutions will help you choose the best technologies to build reliable software for clinical data management for effective collection and recording of medical info.
Creation of a flexible system architecture
Developing a system architecture that is easy to change and scalable plays a crucial role in ensuring effective data management throughout the clinical trial lifecycle. This flexibility allows for rapid implementation of necessary changes, scaling to accommodate increasing workloads, and successfully adapting to the diverse requirements that may arise during the study process.
Development and implementation of a quality control system
Establishing a quality management system that includes data validation and auditing mechanisms is essential in ensuring the information's accuracy and validity. This step ensures that the data meet high industry standards, promoting confidence in the study results and ensuring their reliability.
The Cleveroad team has experience in developing Quality Management System - the solution for medical device manufacturers, aimed to automate document flow and processes needed for the FDA and ISO certification of medical devices’ production.
Staff training
For a Clinical Data Management System (CDMS), staff training is key. It's about giving clinical and data management teams the skills to use the system well. This includes hands-on sessions on data entry, validation, and compliance. Regular refreshers keep everyone up-to-date with the latest standards, fostering a culture of accurate and efficient clinical data handling.
Ongoing support
A solid support setup is vital for a Clinical Data Management System. This means having a dedicated team for CDMS-related issues. Using a ticketing system helps prioritize and solve problems quickly. Regular health checks and clear communication channels prevent and address technical hiccups. Continuous training ensures staff can handle new features or updates, keeping the CDMS running smoothly and in line with industry standards.
System monitoring and optimization
Systematic, real-time performance monitoring and optimization allows you to identify and fix problems, ensuring smooth operation. Analyzing utilization data will enable you to identify trends and make necessary changes to improve system efficiency, ensuring optimal system performance. You software vendor can provide you with professional support and maintenance services to ensure data management clinical trials software works properly.
Improve Your Clinical Data Management with Cleveroad
Cleveroad is a healthcare software development company from the CEE region. For more than 11 years now, we have been helping clinics and medical establishments improve patient care and increase efficiency by building custom and robust healthcare and telecare software solutions. Our healthcare software development services include creating various medical systems, such as telemedicine and remote patient monitoring software development, EHR/EMR and clinic management systems, medical billing software, health data visualization solutions, E-prescribing software, healthcare CRM’s, etc.
By cooperating with Cleveroad, you will get such benefits :
- Custom healthcare and telemedicine software development services according to your clinics particular needs
- Experience developing healthcare software compliant with industry laws and regulations, such as, HIPAA, GDPR, PIPEDA, ICH, HITECH, HL7, FDA, etc.
- Deep expertise in building robust healthcare solutions with industry-standard data security tools (RBAC, activity tracking, industry standard data encryption, etc.)
- Experience in healthcare legacy software modernization by implementing the latest technologies, advanced functionality, and enhanced security measures
- Cooperation with ISO 9001:2015 and ISO/IEC 27001:2013 certified company, ensuring quality and information security
- Flexible cooperation models that suit your needs: Staff Augmentation, Dedicated Team, Time & Materials, etc.
- Signing NDA per your request to ensure confidentiality of your business data from the first contact
To show you our experience, we would like to present one of our customers' successful case studies - Clinic Management System with EMR module .
Our client, a rehab clinic from the USA, approached us to help them build a custom clinic management solution for optimizing appointment management and overall workflow. Our team created a clinic management system and accompanying apps according to the client's requirements, user roles, and healthcare specifics. We also provided reliable data migration on AWS cloud hosting - the provider that meets all the requirements for HIPAA-compliant systems.
The developed solution included the following functionality :
- Patient profile and EMR with billing and user management
- Real-time booking for consultation appointments
- Admin panel for system management, including the ability to create users, manage user access, manage online consultations, appointments, access to them, and schedules
- A robust accounting system to structure payment data and information about all healthcare services provided and paid
- E-prescriptions solution helps select pharmacies, securely transmit recipes to them, and incorporate insurers' data
- Web and mobile applications for patients to access all clinic services online
As a result , our client received a bespoke medical solution covering appointment booking, real-time scheduling, e-prescriptions, and other functionality needed for effective management of administrative and operational activities in healthcare establishments. The clinic's data was transferred to new secure storage, meeting the requirements of HIPAA regulation. Integrated functionality allows the clinic to manage all the processes, optimize workflow, and exclude schedule conflicts' risk.
Clinic Management System developed by Cleveroad
Build clinical data management software with domain experts
Our team with 11+ years of experience in Healthcare software development is ready to create a robust healthcare system to simplify the storage, extraction, and management of clinical data
A Clinical Data Manager plays a pivotal role in clinical research, overseeing the end-to-end process of collecting, validating, and managing data derived from clinical trials. This involves designing and implementing data collection systems, ensuring data accuracy and integrity, and adhering to stringent regulatory standards. The Clinical Data Manager collaborates with cross-functional teams, including researchers, statisticians, and IT professionals, to guarantee the reliability of clinical trial data.
Clinical Data Management (CDM) is a comprehensive discipline within clinical research that focuses on the systematic organization, validation, and secure management of data collected during clinical trials. CDM encompasses several processes, including developing data collection instruments, data entry, verification, coding, and implementing measures to ensure data security and regulatory compliance. The ultimate goal is to generate high-quality, accurate data supporting evidence-based healthcare decision-making.
The Clinical Data Management process is a well-defined series of steps that begins with creating a detailed Data Management Plan (DMP). This plan outlines various stages' procedures, milestones, and deliverables, including data collection, integration, format determination, security measures, quality assurance, team responsibilities, and regulatory compliance. The process extends through data validation, analysis, and data preparation for reporting, contributing to clinical trials' overall success and validity.
Managing clinical data involves a systematic approach that encompasses several key components. It begins with the design of electronic case report forms (eCRFs) tailored to the unique requirements of each clinical trial. Data collection is then conducted, often electronically, with embedded electronic checks to ensure accuracy. Source Data Validation (SDV) verifies data against original records, and data anonymization is performed to remove personally identifiable information. A secure database stores the information, and stringent access controls are implemented to protect sensitive data.
The initial step in Clinical Data Management is the development of a comprehensive Data Management Plan (DMP). This document serves as a roadmap for the entire data management lifecycle, outlining critical aspects such as data collection methods, integration processes, data format, metadata standards, security measures, team responsibilities, access mechanisms, long-term archiving procedures, cost considerations, and regulatory compliance strategies. The DMP is a foundational document created during the study design phase.
Clinical Data Management is paramount in clinical trials due to its multifaceted contributions. It ensures the accuracy and integrity of trial data, which is critical for drawing valid conclusions and making informed decisions in healthcare. CDM plays a pivotal role in compliance with regulatory standards, including Good Clinical Practice (GCP) and the Health Insurance Portability and Accountability Act (HIPAA), safeguarding participant rights and data confidentiality. Moreover, efficient data management streamlines processes, enhances patient safety by enabling prompt detection of adverse events and contributes to the overall success and efficiency of clinical trials.

Evgeniy Altynpara is a CTO and member of the Forbes Councils’ community of tech professionals. He is an expert in software development and technological entrepreneurship and has 10+years of experience in digital transformation consulting in Healthcare, FinTech, Supply Chain and Logistics
Give us your impressions about this article
Mar 14, 2024
Mar 11, 2024
Mar 07, 2024
Clinilaunch Research Institute

- Medical Transcription
- Career Fair
- Notification
- Pay Fees Online
- Certification
- Advance Diploma Course
- PG Diploma Course

Data Management in Clinical Trials: 5 Best Practices
- March 12, 2024
- No Comments

Effective data management helps you minimize potential errors while establishing processes and policies for usage, building trust and making decisions across your organization. When it comes to data management in Clinical trials, CTMS (Clinical Trial Management Systems), are designed to help clinical research studies meet their requirements. CDM plays a crucial role in every successful clinical trial as it directly affects treatment development decisions. This is the reason why regulatory bodies maintain strict guidelines and standards in clinical data. Now, data which is harnessed correctly facilitates the development of life-changing treatments. Therefore, effective data management is crucial for the success of clinical trials. Here are:
Top 5 Best Practices of Data Management in Clinical Trials

When it comes to best data management practices in Clinical Trials, effective management of data planning offers several advantages. A good plan helps safeguard the confidentiality and integrity of information during clinical research, such as patient records. Following best practices in clinical data management will also help publication managers ensure adherence to relevant regulatory guidelines around the use and collective distribution of clinical data. Clinical trials require intensive data collection, analysis, and generation efforts that collectively result in statistically significant and high-quality clinical findings.
These findings are specifically shared via established conferences and journals including distilled portions of collectively available raw data helping in arriving at findings. The data and findings are of high value to biomedical researchers, pharma companies, healthcare practitioners, and governmental, non-governmental, and private clinical trial sponsors. As distributors and facilitators of clinical findings through key channels, publication managers play a crucial role specifically in shaping how this data is stored, accessed and shared. This post will guide you through key data management practices that help publication managers handle data effectively.
Key Team Members for Data Management in Clinical Trials

Data Managers
A clinical data manager is specifically responsible for ensuring that the results and statistical information from clinical trials are recorded and reported accurately both after and during the completion of the research. Accurate and timely data management will be achieved through the careful design of clinical data management tools and methodology for interrogating data liaising it closely with other functions. Data managers approve CDM procedures and internal documents related to data management in clinical trials.
Clinical Study Administrator
Clinical study administrators assist clinical research associates, and regulatory and start-up teams in accurately updating and maintaining clinical documents and systems while tracking site compliance and performance with project timelines. CSA (Clinical Study Administrator) assists CRAs and RSU in preparing, handling, and distributing clinical trial supplies with tracking information maintenance while coordinating based on tracking and case report forms management, queries, and data flow.
The administrator acts as a central point of contact for designated project communication in the clinical team with corresponding and associated documentation. They may collaborate with the clinical team on the distribution, preparation, handling, filling and archiving of clinical documentation and reports based on the scope of work with standard operating procedures.
Database Developer/Programmer
Database developers or programmers are responsible for design, development, implementation, and database programming with information systems. Clinical database developers or programmers focus on organizing databases to encourage and enable efficiency in report generation and other uses. Once the databases are operational, it requires analysis to eliminate and modernize inefficient coding to maintain optimal performance. The database developer makes periodic alterations to accommodate the database software based on their changing needs.
Clinical Data Associate
Clinical data associates are responsible for documenting and recording data from clinical trial programs for validation, future studies and various purposes. While coordinating with different teams to gather accurate data, utilizing special tools and software, and preparing and processing data according to protocols and standards, the responsibilities of clinical data associates revolve around understanding every program’s requirements. Moreover, they typically work in a team setting that requires an active communication line to create a smooth and efficient workflow.
Data Management Standard Operating Procedures

SOPs (Standard Operating Procedures) are critical components of data management in a clinical setting. SOPs provide a clear set of instructions and guidelines for consistent and efficient execution of the processes in data management in clinical research practices. SOPs specifically ensure the quality and reliability of the clinical trial data. Here are some of the critical areas where SOPs are used in data management that include:
Data Collection
Standard Operating Procedures define data collection procedures from various sources, such as electronic data capture systems and case report forms.
Based on the data entry, SOPs guide the methods and processes, including manual data entry and electronic data capture.
Data Validation
SOPs can guide and define procedures for validating the completeness, accuracy and data consistency to resolve discrepancies and inconsistencies.
Data Cleaning
Standard operating procedures can guide cleaning and standardizing data to ensure consistency and accuracy.
Data Archiving
It can define the procedures for data preservation and long-term clinical research and trials.
Quality Control and Monitoring
In terms of quality control and monitoring, SOPs can guide the procedures for monitoring the trial data quality ensuring the data management process is being executed in compliance with the regulatory requirements.
Data Management Standard Operating Procedures is a tool that ensures consistency, efficiency, and quality in the data management processes of clinical trials. Effective implementation of SOPs helps clinical researchers minimize errors, ensure data quality, and increase confidence in the clinical trial results.
Clinical Data Management Process

Clinical data management is the collection, cleaning, and data management process based on the subject area in compliance with regulatory requirements. The primary objective of clinical data management is to provide high-quality data by reducing the number of errors and missing data and gathering maximum data for analysis. To specifically meet the minimum objective criteria of clinical data management, best practices are adopted to ensure that data are complete, reliable, and processed correctly.
Best practices have been facilitated by the software application that maintains clinical trial audits and provides easy identification and data discrepancies resolutions. Furthermore, start early to achieve efficient clinical trial outcomes before finalising the study protocols and consider aspects such as data collection methods, cleaning, validation and data setup.
Data Validation Plan (DVP) in Clinical Research and Data Management

According to the protocol specification, a clinical data validation plan is a document describing standard protocol to verify the validity of clinical trial data. The data validation plan includes the conditions the clinical data must meet to be considered valid specifically for analysis. Consistency in data is tested by using edit check programs depending on the dedication capability of discrepancies due to inconsistent information, out-of-range data, protocol deviations, and missing data. In the meantime, develop a comprehensive plan for data validation. It includes edit checks, query management, and ensuring data accuracy.
Discrepancy Management in Clinical Trials

Discrepancy management in clinical trials is defined as two or more statements that may signal problems with a trial report. The process of discrepancy management in trials entails all tasks related to working with discrepancies. It is critical to successful clinical data management that the collected patient data is free of errors and possible inaccuracies. Following the best practices of managing the discrepancies is systematically addressing these assigned discrepancies to a user role where they must identify the cause, and error access, and determine the appropriate action. The message is here to address discrepancies promptly with the implementation of resolving the data inconsistencies and query process.
In conclusion, effective data management is the backbone of successful clinical trials. By following best practices, while utilizing the right tools, and team members, we can ensure accuracy, completeness, and data reliability. Following the best practices, in turn, leads to trustworthy results that can shape the future of medicine. Remember, high-quality data is essential for developing life-changing treatments, and strong data management practices are the key to achieving that goal.
Remember that each of these aspects contributes to high-quality clinical trial data that ultimately impacts patient health and treatment decisions. If you have any further queries related to Clinical Data Management (CDM) or Biostatistics, feel free to register at www.clinilaunchresearch.in .
Unlock the Secrets of Cutting Edge Clinical Research
Download the brochure now, request for a free demo.


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Investigators should consider using this template when developing the Data and Safety Monitoring Plan (DSMP) for clinical studies funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
The goal of the DSMP is to provide a general description of a plan that you intend to implement for data and safety monitoring. The DSMP should specify the following:
- Primary and secondary outcome measures/endpoints
- Sample size and target population
- Inclusion and exclusion criteria
- A list of proposed participating sites and centers for multi-site trials
- Potential risks and benefits for participating in the study
- Procedures for data review and reportable events
- Project Director (PD)/Principal Investigator (PI) (required)
- Institutional Review Board (IRB) (required)
- Designated medical monitor
- Internal Committee or Board
- Independent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety Officer (SO) or Dual SOs
- Content and format of the safety report
- Data Management, Quality Control and Quality Assurance
Note that all sample text should be replaced with the study specific text. There is no need to include sections that are not relevant to the particular study. Please do not use the sample text verbatim .
TABLE OF CONTENTS
1.0 Study Overview
- 1.1 Study Description
- 1.2 Study Management
2.0 Participant Safety
- 2.1.1 Potential Risks
- 2.1.2 Potential Benefits
- 2.2.1 Informed Consent Process
3.0 Reportable Events
- 3.1.1 Adverse Events (AEs)
- 3.1.2 Serious Adverse Events (SAEs)
- 3.1.3 Unanticipated Problems (UPs)
- 3.1.4 Protocol Deviations
- 3.2 Collection and Assessment of AEs, SAEs, UPs, and Protocol Deviations
- 3.3.1 AE Reporting Procedures
- 3.3.2 SAE Reporting Procedures
- 3.3.3 UP Reporting Procedures
- 3.3.4 Protocol Deviation Reporting Procedures
- 3.3.5 Serious or Continuing Noncompliance
- 3.3.6 Suspension or Termination of IRB Approval
4.0 Interim Analysis & Stopping Rules
5.0 Data and Safety Monitoring
- 5.1 Frequency of Data and Safety Monitoring
- 5.2 Content of Data and Safety Monitoring Report
- 5.3 Monitoring Body Membership and Affiliation
- 5.4 Conflict of Interest for Monitoring Bodies
- 5.5 Protection of Confidentiality
- 5.6 Monitoring Body Responsibilities
6.0 Data Management, Quality Control, and Quality Assurance
1.0 Study Overview
1.1 study description.
This section outlines the overall goal of this project. It also describes the study design, primary and secondary outcome measures/endpoints, sample size/power calculation and target population, inclusion and exclusion criteria.
1.2 Study Management
This section includes the proposed participating sites and their responsibilities. In addition, this section should include the planned enrollment timetable (i.e. projected enrollment).
2.0 Participant Safety
2.1 potential risks and benefits for participants.
This section outlines the potential risks and benefits of the research for the study participants and for society. It should include a description of all expected adverse events (AEs), the known side effects of the intervention, and all known risks or complications of the outcomes being assessed.
2.1.1 Potential Risks
Outline potential risks for study participants including a breach of confidentiality.
{Begin sample text}
{End sample text}
2.1.2 Potential Benefits
Outline potential benefits for study participants or if there are no direct benefits to the participants.
2.2 Protection Against Study Risks
This section provides information on how risks to participants will be managed. It should specify any events that would preclude a participant from continuing in the study. In general, the format and content of this section are similar to the Human Participants section of the grant application.
In addition, this section describes measures to protect participants against study specific risks including the data security to protect the confidentiality of the data.
2.2.1 Informed Consent Process
This section explains the informed consent process. It should include, but not be limited to, who will be consenting the participant, how and under what conditions will a participant be consented, and that participation is voluntary. The informed consent process should meet the revised Common Rule requirements for consenting. For further details on this requirement, please visit: https://www.ecfr.gov/cgi-bin/text-idx?SID=921afb2e7909a2cf08c5f3ce160a0c96&mc=true&node=se45.1.46_1116 .
3.0 Data and Safety Monitoring
3.1 definitions.
This section should describe how to identify AEs, SAEs and UPs. In the case where the intervention is a Food and Drug Administration (FDA) regulated drug, device or biologic, it should include the FDA definition, grading scale and “study relatedness” criteria of AEs.
3.1.1 Adverse Events (AEs)
The definition of adverse event here is drawn from the OHRP guidance ( https://www.hhs.gov/ohrp/regulations-and-policy/guidance/reviewing-unanticipated-problems/index.html ); for some studies, the ICH E6 definition may be more appropriate. Expected and unexpected AEs should be listed in this section.
3.1.2 Serious Adverse Events (SAEs)
SAEs are a subset of all AEs.
3.1.3 Unanticipated Problems (UPs)
The OHRP definition of UPs can be accessed using the link provided in Section 3.1.1 above.
{End sample text}
3.1.4 Protocol Deviations
This section should include the study definition of protocol deviations and define the events placing the participant at increased risk of harm or compromising the integrity of the safety data.
3.2 Collection and Assessment of AEs, SAEs, UPs, and Protocol Deviations
The section should include who is responsible for collecting these events, how the information will be captured, where the information will be collected from (e.g., medical records, self-reported), and what study form(s) will be used to collect the information (e.g., case report forms, direct data entry). This section should also include what type of information will be collected (e.g., event description, time of onset, assessment of seriousness, relationship to the study intervention, severity, etc.). Note that it is the NIAMS requirement to collect all AEs regardless of the expectedness or relatedness.
This section should also describe who is responsible for assessing these events. The individual(s) responsible should have the relevant clinical expertise to make such an assessment (e.g., physician, nurse practitioner, physician assistant, nurse). When assessing AEs and SAEs, the following information should be included:
- Possibly/Probably (may be related to the intervention)
- Definitely (clearly related to the intervention)
- Not Related (clearly not related to the intervention)
3.3 Reporting of AEs, SAEs, UPs, Protocol Deviations, Serious or Continuing Noncompliance, and Suspension or Termination of IRB Approval
This section should describe who is responsible for reporting these events and the roles and responsibilities of each person on the clinical study team who is involved in the safety reporting to the IRB, FDA (if applicable), Monitoring Body, and NIAMS (through the NIAMS Executive Secretary). It should also include the Office for Human Research Protections (OHRP) and FDA reporting requirements. See NIAMS Reportable Events Requirements and Guidelines for more details.
3.3.1 AE Reporting Procedures
All non-serious AEs (regardless of expectedness or relatedness) are reported to the Monitoring Body and NIAMS (through the NIAMS Executive Secretary) semi-annually or as determined by the NIAMS.
3.3.2 SAE Reporting Procedures
All SAEs (regardless of expectedness or relatedness ) must be reported in an expedited manner to the NIAMS and the Monitoring Body. There may be different timeline for reporting SAE to the IRBs, FDA (if applicable), Monitoring Body and the NIAMS. The timeline for reporting SAEs to the Monitoring Body and NIAMS (through the NIAMS Executive Secretary) is within 48 hours of the investigator becoming aware of the event so that a real time assessment can be conducted, and the outcome shared in a timely manner.
3.3.3 UP Reporting Procedures
All events that meet the criteria of a UP must be reported in an expedited manner to the NIAMS and the Monitoring Body. There may be different timeline for reporting UPs to the IRBs, OHRP/FDA (if applicable), Monitoring Body, and the NIAMS. The timeline for reporting UPs to the Monitoring Body and NIAMS (through the NIAMS Executive Secretary) is within 48 hours of the investigator becoming aware of the event so that a real time assessment can be conducted, and the outcome shared in a timely manner.
3.3.4 Protocol Deviation Reporting Procedures
Protocol deviations impacting participant safety are subject to expedited reporting to the Monitoring Body and NIAMS (through the NIAMS Executive Secretary) within 48 hours of the investigator becoming aware of the even t so that a real time assessment can be conducted, and the outcome shared in a timely manner. All other events should be reported at the time of the routine DSMB meeting or submission of the safety report.
3.3.5 Serious or Continuing Noncompliance
This section should include the process in place at your institution to capture and report serious or continuing noncompliance. It should include who is responsible for reporting. Serious or continuing noncompliance must be reported to the NIAMS Program Officer and Grants Management Specialist within 3 business days of IRB determination. A copy of the IRB submission and determination must be submitted along with the report to the NIAMS. The guidance on reporting incidents to OHRP should also be followed to provide the timeline of reporting to this regulatory body.
3.3.6 Suspension or Termination of IRB Approval
This section should include the process for reporting study suspension or termination by the IRB. It should also include who is responsible for reporting to the NIAMS, OHRP, and the timeline for reporting of these events. Suspension or termination of IRB approval must include a statement of the reason(s) for the action and must be reported promptly to the NIAMS Program Officer and Grants Management Specialist within 3 business days of receipt by the PI.
4.0 Interim Analysis & Stopping Rules
This section provides information on planned interim analysis. Interim analysis may be conducted either due to pre-specified stopping rules as outlined in the protocol and at predetermined intervals, or as determined necessary by the Monitoring Body to assess safety concerns or study futility based upon accumulating data. An interim analysis may be performed for safety, efficacy and/or futility, and the reports are prepared by the unmasked study statistician or data coordinating center responsible for generating such reports. Rules for stopping the study, based on interim analysis, should be described.
If no interim analysis is planned, this should be noted within this section.
5.0 Data and Safety Monitoring
This section identifies the name of the individual or entity responsible for data and safety monitoring, what information will be reviewed, and frequency of such reviews. A brief general introduction regarding data and safety monitoring oversight should be provided in section 5.0, and further details should be provided in the subsequent sections.
5.1 Frequency of Data and Safety Monitoring
This section describes the frequency of data and safety monitoring reviews. As the reviews of reportable events (AEs, SAEs, UPs, and protocol deviations) are included in Section 3, this section should focus on the routine and ad hoc review of the full data and safety monitoring reports. {Begin sample text}
5.2 Content of Data and Safety Monitoring Report
This section describes the content of the data and safety monitoring reports. The specifics of the study and the requests of the Monitoring Body will guide requirements for additional tables and listings. Tables for multi-site studies will present aggregated data as well as data by site. For studies with more than one intervention group, this section should indicate the plans for providing data stratified by masked intervention group (i.e., Group A vs. Group B) as part of the closed report to the Monitoring Body, while the open report should have data presented in aggregate without stratification by groups. The complete data and safety monitoring report template should be included as an appendix. Refer to the NIAMS Data and Safety Monitoring Board Report Templates ( https://www.niams.nih.gov/grants-funding/conducting-clinical-research/trial-policies-guidelines-templates/data-safety-monitoring-guidelines-policies/clinical-study-templates-forms ) for guidance.
5.3 Monitoring Body Membership and Affiliation
This section includes a roster of the Monitoring Body’s name(s) and affiliation(s). For studies with a NIAMS-appointed Monitoring Body, the NIAMS Executive Secretary will provide the name(s) and affiliation(s) of the individual(s) serving once the Monitoring Body has been assembled. However, if this is an Internally-appointed Monitoring Body (i.e., PI-appointed), the study team should enter the information in this section once the NIAMS has confirmed that no conflicts of interest with the Monitoring Body member(s) are identified.
5.4 Conflict of Interest for Monitoring Bodies
This section describes the conflict of interest procedure for Monitoring Body members. For studies with a NIAMS-appointed Monitoring Body, the NIAMS Executive Secretary will conduct a conflict of interest check on each member prior to beginning their service and on an annual basis thereafter. For studies with an Internally-appointed Monitoring Body (i.e., PI-appointed), the study team should provide the name, affiliation, and curriculum vitae (if available) of the proposed Monitoring Body member(s) to the NIAMS Executive Secretary for a conflict of interest check to be conducted. Once the conflict of interest check is complete, this section should be updated to indicate that the NIAMS did not identify any conflicts of interest for the Monitoring Body member(s).
5.5 Protection of Confidentiality
This section describes how confidentiality of data presented to the Monitoring Body will be protected.
5.6 Monitoring Body Responsibilities
A charter provides a detailed list of the Monitoring Body’s responsibilities. Listed in the sample text below are the responsibilities for a NIAMS-appointed Monitoring Body. Please ensure that all of the items are applicable for this study. For studies with an Internally-appointed Monitoring Body, the study team should ensure that a detailed list of the Monitoring Body’s responsibilities are provided in this section.
6.0 Data Management, Quality Control, and Quality Assurance
This section describes how the site will collect, document, and review the data. Who will be responsible for data entry and ensure they are accurate and complete? Which database will be used? Does it have audit tracking capabilities? What is the data query process and frequencies? Are there any planned mitigation strategies in the event of non-compliance? What is the process for locking the final study datasets? Are there any procedures on data access and sharing as appropriate? Is there a description of security measures in place? (If you have a separate Clinical Monitoring and Data Management Plan, please reference it and utilize that information to help populate this section).
Each study should have standard operating procedures (SOPs) and/or a quality management plan that describe the following (if this is a multi-site study, each site should have SOPs and a plan):
- Staff training methods and how such training will be tracked
- How data will be evaluated for compliance with the protocol and for accuracy in relation to source documents
- The documents to be reviewed (e.g., case report forms, clinic notes, product accountability records, specimen tracking logs, questionnaires), who is responsible, and the frequency for reviews
- Who will be responsible for addressing quality assurance (QA) issues (correcting procedures that are not in compliance with protocol) and quality control issues (correcting data entry errors). It is anticipated that QA review and data verification will be performed by someone other than the individual originally collecting the data, or by double-data entry. The frequency of internal QA review and measures to be taken for corrective action (e.g., for trends in errors) should be included
- QA measures for participant recruitment, enrollment, enrollment targets, and for the validity and integrity of the data. E6 Good Clinical Practice (R1): 1.46 defines quality assurance as “All those planned and systematic actions that are established to ensure that the trial is performed and the data are generated, documented (recorded), and reported in compliance with Good Clinical Practice (GCP) and the applicable regulatory requirement(s)”
What is Clinical Data Management?
Updated: March 08, 2024
Published: October 03, 2022
How helpful is data that isn’t statistically sound, clean, and reliable? Not very. But, thanks to clinical data management , it’s possible to ensure that your data is up to par.

The medical research process includes the production of a lot of data, and in order to ensure that it’s used in the most effective manner possible, you’ll need a sound data management strategy.

So, what is clinical data management, and what does it look like in practice? In this post, we’ll answer these questions and share the best clinical data management tools, so you’re fully equipped with the skills and tools necessary to succeed.
What is clinical data management?
Why is clinical data management necessary, main objectives of clinical data management, what are clinical data management tools, what are the stages of a clinical data management cycle, clinical data management ensures data integrity.
Clinical data management (CDM) refers to the collection and management process of research data in accordance with regulatory standards. These standards ensure quality information that is error-free and complete. It must adhere to federal, state, and local guidelines.
Because clinical data management handles information from clinical trials, it’s essential that software systems, processes, training, procedures, databases, and protocols all support the proper collection, cleaning, and management of trial data.
CDM originated due to demands by regulatory authorities and the pharmaceutical industry. While the pharmaceutical industry wants to create and release products as rapidly as possible, regulatory bodies decided to enact rules of their own. They required that companies meet certain standards when collecting the data that’s used in the process of drug evaluation.
We can’t discuss CDM without mentioning two standards: the Clinical Data Acquisition Standards Harmonization (CDASH) and the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG). The Clinical Data Interchange Standards Consortium (CDISC) is to thank for these standards. (Can you tell they like their long titles, and acronyms?)
Clinical data management is essential as it ensures the data that is produced through a clinical trial is high-quality . When performed properly, clinical data management results in a dataset that is reliable, accurate, and analysis-ready when the study concludes.
Furthermore, CDM offers essential support for the evaluation process of regulated goods including cosmetics, food, medical devices, and pharmaceuticals. To guarantee that the products you’re getting are safe and will work as they should, adherence to regulatory standards is necessary.
Here are some of the benefits clinical data management offers:
- Data won’t get lost.
- Development is expedited.
- There is greater security.
- Data quality is guaranteed.
- Costs are reduced.
- Data collection is accurate and complete.
- The data is correctly formatted for optimal usability.
- The trial is accurately represented in the database.
- There’s a clean dataset available for reporting.
While there are various benefits associated with clinical data collection, its primary goals can be distilled into three main objectives. Let’s dive deeper into these.
1. Data Collection
Regardless of whether the data is in paper form or available electronically, clinical data management helps ensure that information is properly collected. Furthermore, correctly collecting and storing the data ensures that when it is needed later, it will be easily accessible.
2. Data Valuation (of data and system)
Valuation is the appraisal and estimate of an item’s worth. This includes manual review for additional oversight, plus user acceptance testing (UAT), programming completed with edit checks in place, and quality controls. The aim of this is to identify how useful the data is.
3. Data Integration
Lastly, data integration allows you to put all of the data into a singular database. Having all your information in one place supports correctness and consistency. Similar to data collection, this also simplifies the process of accessing the data at a later time.
CDM is made possible with specialized software applications. These applications help users conduct audits to detect discrepancies and minimize them — even in large, complex clinical trials. Some clinical data management tools you may be familiar with are Oracle Clinical, Clintrial, Rave, eClinical suite, and Macro.
With trials conducted in medical centers that produce massive amounts of data, it’s especially critical that effective clinical data management systems are used. These systems can be customized and often are by huge international pharmaceutical companies that want to address their specific requirements.
However, there are various clinical data management tools available. Some highly effective options are even open-source tools like OpenClinica, PhOSCo, open CDMS, and TrailDB.
Now that you know more about the tools needed for successful clinical data management, let’s dive into the cycle that is essential to keeping your data safe and secure.
1. Prepare.
First, collect all of your necessary forms — whether those be electronic or paper. Then, review your plan and ensure your database is ready to receive information. The more organized you are at this phase of the process, the more seamless the rest of it will be.
2. Collect data.
Now, you can begin the process of collecting your data. Continue to gather data throughout your study. Remember: accuracy is first and foremost. It’s not a bad idea to check in periodically to make sure that your data is accurate.
3. Ensure accuracy.
Make sure that the tools you’re using, your plan itself, and the data collected meet regulatory requirements . Data quality is a top priority, so if there are any discrepancies, the sooner you can figure it out the better.
4. Keep track and preserve your data.
It’s time to keep track of your data. Make sure that you’re monitoring it for potential issues or risks that may pop up. This is also the right time to think about what you can do to preserve your data’s quality.
5. Integrate your data.
You’ve monitored your data for issues, and preserved its integrity. Now, it’s time to map out datasets and information together. Consistency is critical — as it has been throughout the entirety of the process.
6. Analyze the outcomes.
Because your data is clean and consistent, you can now use it to analyze the outcome and feel secure about its accuracy. That brings us to our next point, that your clinical data management cycle isn’t done once you’ve analyzed the outcome. Now, it’s time to make sure your data is protected.
7. Protect your data.
After completing the clinical data management cycle, take time to secure your database so no information is misplaced or erroneously edited.
The main purpose of clinical data management is to ensure data integrity. If you follow the steps outlined and utilize the clinical data management tools to their fullest potential, you’ll obtain data that’s able to be analyzed and understood.
Don't forget to share this post!
Related articles.
Materialized View: What You Need to Know [+Best Practices]

What Is Data Hygiene?: Why You Need It & How to Do It Right
API Management: What Is It & Why Does It Matter?

5 Best Data Governance Tools

How to Create a Data Quality Management Plan

Single Source of Truth: Benefits, Challenges, & Examples

Data Governance (DG): A Straightforward Guide

What Is Event-Driven Architecture? Everything You Need to Know
Data Stream: Use Cases, Benefits, & Examples

ETL vs. ELT: What's the Difference & Which Is Better?
Get Started with HubSpot's Analytics Software for Free
Marketing software that helps you drive revenue, save time and resources, and measure and optimize your investments — all on one easy-to-use platform

VIDEO
COMMENTS
Data Management Plan (Model) Template - version 06.10.2021 Author: Khaled Mostaguir Page 1 / 10 Data Management Plan (DMP) for clinical research projects A model for submissions to the Swiss National Science Foundation (FNS) and/or to Ethics Committees Important Notes:
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right ...
7. Understand the reasons for performing research that is reproducible from data collection through publication of results. 9. Distinguish between variable types (e.g. continuous, binary, categorical) and understand the implications for selection of appropriate statistical methods. Extensively covered by required coursework.
A data management plan, or DMP, is a formal document that outlines how data will be handled during and after a research project. Many funding agencies, especially government sources, require a DMP as part of their application processes. Even if you are not seeking funding for your research, documenting a plan for your research data is a best ...
These data may and records including the medical record. Data quality management (DQM) is a formal process for managing the quality, validity and integrity of the research data captured throughout the study from the time it is collected, stored and transformed (processed) through analysis and publication.
A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether across one or several institutions.
Likewise, specific components of data management have been described in more narrowly scoped documents including research data security plans, data sharing plans, manuals of procedures, and manuals of operations, that cover some but not all aspects of the data lifecycle in a clinical study. The Society for Clinical Data Management (SCDM)
Data Management Guidance, Tools & Resources. As of Jan 2023 NIH now requires that all grants that generate research data include a Data Management and Sharing Plan. This page is a starting point to guide researchers through the data life cycle and highlight available tools for data organization and planning that can be included in this plan.
There are 6 modules in this course. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Understanding and implementing solid data management principles is critical for any scientific domain. Regardless of your current (or anticipated) role in the ...
Introduction to Clinical Data Clinical data is either collected during patient care or as part of a clinical trial program. Funding agencies, publishers, and research communities are encouraging researchers to share data, while respecting Institutional Review Board (IRB) and federal restrictions against disclosing identifiers of human subjects.
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right ...
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to ...
The clinical research community understands the importance of data standardization [26-29], data quality [30-33], and data collection [28, 34-36] and has established good clinical data management practices (GCDMP) to ensure that CRDM is conducted at the highest level of excellence.
The Plan is recommended not to exceed two pages. Text in italics should be deleted. There is no "form page" for the Data Management and Sharing Plan. The DMS Plan may be provided in the format shown below. Public reporting burden for this collection of information is estimated to average 2 hours per response, including the time for ...
Writing a Data Sharing Plan. Under its 2003 data sharing policy, NIH expects investigators to submit a data sharing plan with requests for funding or grants, cooperative agreements, intramural research, contracts, or other funding agreements of $500,000 or more per year.. Data sharing plans should describe how an applicant will share their final research data.
Clinical data management (CDM) is a critical process in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. Clinical data management ensures collection, integration and availability of data at appropriate quality and cost. It also supports the conduct, management and analysis of studies across the spectrum of clinical ...
Data management plan (DMP) is a crucial document that outlines how clinical data will be collected, managed, analyzed, and shared during a clinical trial. DMP serves as a roadmap for clinical data ...
Results Twenty (14%) clinical trials completed the survey. Results from the survey indicate that procedures to ensure data quality varies among clinical trial sites. Centralized monitoring (65%) was the most common procedure to ensure high-quality data. Ten (50%) trials reported having a data management plan in place and two sites utilized an ...
Data management is at the heart of the clinical research process. Although good data management practices cannot make up for poor study design, poor data management can render a perfectly executed trial useless. This book is about clinical research; the failure of a study to produce generalizable knowledge because of bad data management ...
The Data Management Plan (DMP) is a detailed document that outlines all procedures, tasks, milestones, and deliverables throughout the data management clinical trial lifecycle. ... The validation phase of data management for clinical trials involves performing a series of tests to ensure the accuracy, consistency, readability, and integrity of ...
The Data Management Plan (DMP) has multiple purposes and is used to comprehensively document the collection and handling of the data. This should represent the accountability ... in clinical research (nih.gov) System Integrations System integrations being used for this study are listed below: • [Safety database (PV)
5.5.4 Clinical Data Management Plan. The design of the research data lifecycle should be strategized in the clinical data management plan (CDMP). The exact content of the CDMP will vary on the type of trial, the number of sites involved, and the sponsor's specifications. Among the recommended items to include are: Clinical data management ...
To specifically meet the minimum objective criteria of clinical data management, best practices are adopted to ensure that data are complete, reliable, and processed correctly. Best practices have been facilitated by the software application that maintains clinical trial audits and provides easy identification and data discrepancies resolutions.
The goal of the DSMP is to provide a general description of a plan that you intend to implement for data and safety monitoring. The DSMP should specify the following: A brief description of the study design. Primary and secondary outcome measures/endpoints. Sample size and target population.
Clinical data management (CDM) refers to the collection and management process of research data in accordance with regulatory standards. These standards ensure quality information that is error-free and complete. It must adhere to federal, state, and local guidelines. Because clinical data management handles information from clinical trials, it ...