Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Nature ⋅
- Environment

Why Is Photosynthesis Important for All Organisms?

How Does a Plant Convert Light Energy to Chemical Energy?
Photosynthesis is important to living organisms because it is the number one source of oxygen in the atmosphere. Without photosynthesis, the carbon cycle could not occur, oxygen-requiring life would not survive and plants would die. Green plants and trees use photosynthesis to make food from sunlight, carbon dioxide and water in the atmosphere: It is their primary source of energy. The importance of photosynthesis in our life is the oxygen it produces. Without photosynthesis there would be little to no oxygen on the planet.
TL;DR (Too Long; Didn't Read)
Photosynthesis is important for all living organisms because it provides the oxygen needed by most living creatures for survival on the planet.
Reasons Why Photosynthesis Is Important
- It is the number one source of oxygen in the atmosphere.
- It contributes to the carbon cycle between the earth, the oceans, plants and animals.
- It contributes to the symbiotic relationship between plants, humans and animals.
- It directly or indirectly affects most life on Earth.
- It serves as the primary energy process for most trees and plants.
How Photosynthesis Works
Photosynthesis uses light energy from the sun and carbon dioxide and water in the atmosphere to make food for plants, trees, algae and even some bacteria. It releases oxygen as a byproduct. The chlorophyll in these living organisms, which also contributes to their green hues, absorbs the sunlight and combines it with carbon dioxide to convert these compounds into an organic chemical called adenosine triphosphate (ATP). ATP is crucial in the relationship between energy and living things, and is known as the "energy currency for all life."
Importance of Cellular Respiration to Photosynthesis
Cellular respiration allows all living cells to extract energy in the form of ATP from food and offer that energy for the vital processes of life. All living cells in plants, animals and humans take part in cellular respiration in one form or another. Cellular respiration is a three-step process. In step one, the cytoplasm of the cell breaks down glucose in a process called glycolysis, producing two pyruvate molecules from one glucose molecule and releasing a bit of ATP. In the second step, the cell transports the pyruvate molecules into the mitochondria, the energy center of the cells, without using oxygen, This is known as anaerobic respiration. The third step of cellular respiration involves oxygen and is called aerobic respiration, in which the food energy enters an electron transport chain where it produces ATP.
Cellular respiration in plants is essentially the opposite of photosynthesis. Living creatures breathe in oxygen and release carbon dioxide as a byproduct. A plant uses the carbon dioxide exhaled by animals and humans in combination with the sun's energy during cellular respiration to produce the food that it requires. Plants eventually release oxygen back into the atmosphere, resulting in a symbiotic relationship between plants, animals and humans.
Non-Photosynthetic Plants
While most plants use photosynthesis to produce energy, there are some that are non-photosynthetic. Plants that do not use photosynthesis to produce food are usually parasitic, which means they rely on a host for nutrient generation. Examples include Indian pipe ( Monotropa uniflora ) – also known as the ghost or corpse plant – and beechdrops ( Epifagus americana ), which steals nutrients found in beech tree roots. The Indian pipe plant is a ghostly white color because it contains no chlorophyll. Plants in the fungi kingdom – mushrooms, molds and yeasts – rely on their environment for food instead of photosynthesis.
Related Articles
What is the sun's role in photosynthesis, what provides electrons for the light reactions, how do plants store energy during photosynthesis, organelles involved in photosynthesis, is the krebs cycle aerobic or anaerobic, structural characteristics of blue-green algae, what are the functions of photosynthesis, key differences between c3, c4 and cam photosynthesis, how do plants make their own food, what is produced as a result of photosynthesis, what is the photosynthesis equation, the structure of a eukaryotic cell, what is the role of pigments in photosynthesis, how are photosynthesis & cellular respiration related, difference between heterotrophs & autotrophs, what are the reactants of photosynthesis, why are cells important for living organisms, what are the five subdivisions of kingdoms.
- University of California Santa Barbara: How Does Photosynthesis Affect Other Organisms?
- Columbia University: The Carbon Cycle and Earth's Climate
- State University of New York Cortland: Non-Photosynthetic Plants
- California State University, Sacramento: Kingdom Fungi
About the Author
As a journalist and editor for several years, Laurie Brenner has covered many topics in her writings, but science is one of her first loves. Her stint as Manager of the California State Mining and Mineral Museum in California's gold country served to deepen her interest in science which she now fulfills by writing for online science websites. Brenner is also a published sci-fi author. She graduated from San Diego's Coleman College in 1972.
Find Your Next Great Science Fair Project! GO
We Have More Great Sciencing Articles!
ENCYCLOPEDIC ENTRY
Photosynthesis.
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar.
Loading ...
Learning materials, instructional links.
- Photosynthesis (Google doc)
Most life on Earth depends on photosynthesis .The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2 ) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.
The process
During photosynthesis, plants take in carbon dioxide (CO 2 ) and water (H 2 O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose. The plant then releases the oxygen back into the air, and stores energy within the glucose molecules.
Chlorophyll
Inside the plant cell are small organelles called chloroplasts , which store the energy of sunlight. Within the thylakoid membranes of the chloroplast is a light-absorbing pigment called chlorophyll , which is responsible for giving the plant its green color. During photosynthesis , chlorophyll absorbs energy from blue- and red-light waves, and reflects green-light waves, making the plant appear green.
Light-dependent Reactions vs. Light-independent Reactions
While there are many steps behind the process of photosynthesis, it can be broken down into two major stages: light-dependent reactions and light-independent reactions. The light-dependent reaction takes place within the thylakoid membrane and requires a steady stream of sunlight, hence the name light- dependent reaction. The chlorophyll absorbs energy from the light waves, which is converted into chemical energy in the form of the molecules ATP and NADPH . The light-independent stage, also known as the Calvin cycle , takes place in the stroma , the space between the thylakoid membranes and the chloroplast membranes, and does not require light, hence the name light- independent reaction. During this stage, energy from the ATP and NADPH molecules is used to assemble carbohydrate molecules, like glucose, from carbon dioxide.
C3 and C4 Photosynthesis
Not all forms of photosynthesis are created equal, however. There are different types of photosynthesis, including C3 photosynthesis and C4 photosynthesis. C3 photosynthesis is used by the majority of plants. It involves producing a three-carbon compound called 3-phosphoglyceric acid during the Calvin Cycle, which goes on to become glucose. C4 photosynthesis, on the other hand, produces a four-carbon intermediate compound, which splits into carbon dioxide and a three-carbon compound during the Calvin Cycle. A benefit of C4 photosynthesis is that by producing higher levels of carbon, it allows plants to thrive in environments without much light or water. The National Geographic Society is making this content available under a Creative Commons CC-BY-NC-SA license . The License excludes the National Geographic Logo (meaning the words National Geographic + the Yellow Border Logo) and any images that are included as part of each content piece. For clarity the Logo and images may not be removed, altered, or changed in any way.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
March 20, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Biology
Course: ap®︎/college biology > unit 3.
- Photosynthesis
Intro to photosynthesis
- Breaking down photosynthesis stages
- Conceptual overview of light dependent reactions
- The light-dependent reactions
- The Calvin cycle
- Photosynthesis evolution
- Photosynthesis review
Introduction
What is photosynthesis.
- Energy. The glucose molecules serve as fuel for cells: their chemical energy can be harvested through processes like cellular respiration and fermentation , which generate adenosine triphosphate— ATP , a small, energy-carrying molecule—for the cell’s immediate energy needs.
- Fixed carbon. Carbon from carbon dioxide—inorganic carbon—can be incorporated into organic molecules; this process is called carbon fixation , and the carbon in organic molecules is also known as fixed carbon . The carbon that's fixed and incorporated into sugars during photosynthesis can be used to build other types of organic molecules needed by cells.
The ecological importance of photosynthesis
- Photoautotrophs use light energy to convert carbon dioxide into organic compounds. This process is called photosynthesis.
- Chemoautotrophs extract energy from inorganic compounds by oxidizing them and use this chemical energy, rather than light energy, to convert carbon dioxide into organic compounds. This process is called chemosynthesis.
- Photoheterotrophs obtain energy from sunlight but must get fixed carbon in the form of organic compounds made by other organisms. Some types of prokaryotes are photoheterotrophs.
- Chemoheterotrophs obtain energy by oxidizing organic or inorganic compounds and, like all heterotrophs, get their fixed carbon from organic compounds made by other organisms. Animals, fungi, and many prokaryotes and protists are chemoheterotrophs.
Leaves are sites of photosynthesis
The light-dependent reactions and the calvin cycle.
- The light-dependent reactions take place in the thylakoid membrane and require a continuous supply of light energy. Chlorophylls absorb this light energy, which is converted into chemical energy through the formation of two compounds, ATP —an energy storage molecule—and NADPH —a reduced (electron-bearing) electron carrier. In this process, water molecules are also converted to oxygen gas—the oxygen we breathe!
- The Calvin cycle , also called the light-independent reactions , takes place in the stroma and does not directly require light. Instead, the Calvin cycle uses ATP and NADPH from the light-dependent reactions to fix carbon dioxide and produce three-carbon sugars—glyceraldehyde-3-phosphate, or G3P, molecules—which join up to form glucose.
Photosynthesis vs. cellular respiration
Attribution.
- “ Overview of Photosynthesis ” by OpenStax College, Biology, CC BY 3.0 . Download the original article for free at http://cnx.org/contents/5bb72d25-e488-4760-8da8-51bc5b86c29d@8 .
- “ Overview of Photosynthesis ” by OpenStax College, Concepts of Biology, CC BY 3.0 . Download the original article for free at http://cnx.org/contents/[email protected] .
Works cited:
- "Great Oxygenation Event." Wikipedia. Last modified July 17, 2016. https://en.wikipedia.org/wiki/Great_Oxygenation_Event .
Additional references
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page


- Next Article
Cover Image

- PDF Icon PDF Link Table of Contents
- PDF Icon PDF Link Editorial Board
An overview of photosynthesis
How the photosystems work, other electron transfer chain components, abbreviations, competing interests, recommended reading and key publications, photosynthesis.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Matthew P. Johnson; Photosynthesis. Essays Biochem 31 October 2016; 60 (3): 255–273. doi: https://doi.org/10.1042/EBC20160016
Download citation file:
- Ris (Zotero)
- Reference Manager
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide–adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin–Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
Introduction
Photosynthesis is the ultimate source of all of humankind's food and oxygen, whereas fossilized photosynthetic fuels provide ∼87% of the world's energy. It is the biochemical process that sustains the biosphere as the basis for the food chain. The oxygen produced as a by-product of photosynthesis allowed the formation of the ozone layer, the evolution of aerobic respiration and thus complex multicellular life.
Oxygenic photosynthesis involves the conversion of water and CO 2 into complex organic molecules such as carbohydrates and oxygen. Photosynthesis may be split into the ‘light’ and ‘dark’ reactions. In the light reactions, water is split using light into oxygen, protons and electrons, and in the dark reactions, the protons and electrons are used to reduce CO 2 to carbohydrate (given here by the general formula CH 2 O). The two processes can be summarized thus:
Light reactions:

Dark reactions:

The positive sign of the standard free energy change of the reaction (Δ G °) given above means that the reaction requires energy ( an endergonic reaction ). The energy required is provided by absorbed solar energy, which is converted into the chemical bond energy of the products ( Box 1 ).

Photosynthesis converts ∼200 billion tonnes of CO 2 into complex organic compounds annually and produces ∼140 billion tonnes of oxygen into the atmosphere. By facilitating conversion of solar energy into chemical energy, photosynthesis acts as the primary energy input into the global food chain. Nearly all living organisms use the complex organic compounds derived from photosynthesis as a source of energy. The breakdown of these organic compounds occurs via the process of aerobic respiration, which of course also requires the oxygen produced by photosynthesis.

Unlike photosynthesis, aerobic respiration is an exergonic process (negative Δ G °) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste). The use of exergonic reactions to power endergonic ones associated with biosynthesis and housekeeping in biological organisms such that the overall free energy change is negative is known as ‘ coupling’.
Photosynthesis and respiration are thus seemingly the reverse of one another, with the important caveat that both oxygen formation during photosynthesis and its utilization during respiration result in its liberation or incorporation respectively into water rather than CO 2 . In addition, glucose is one of several possible products of photosynthesis with amino acids and lipids also being synthesized rapidly from the primary photosynthetic products.
The consideration of photosynthesis and respiration as opposing processes helps us to appreciate their role in shaping our environment. The fixation of CO 2 by photosynthesis and its release during breakdown of organic molecules during respiration, decay and combustion of organic matter and fossil fuels can be visualized as the global carbon cycle ( Figure 1 ).
The global carbon cycle

The relationship between respiration, photosynthesis and global CO 2 and O 2 levels.
At present, this cycle may be considered to be in a state of imbalance due to the burning of fossil fuels (fossilized photosynthesis), which is increasing the proportion of CO 2 entering the Earth's atmosphere, leading to the so-called ‘greenhouse effect’ and human-made climate change.
Oxygenic photosynthesis is thought to have evolved only once during Earth's history in the cyanobacteria. All other organisms, such as plants, algae and diatoms, which perform oxygenic photosynthesis actually do so via cyanobacterial endosymbionts or ‘chloroplasts’. An endosymbiotoic event between an ancestral eukaryotic cell and a cyanobacterium that gave rise to plants is estimated to have occurred ∼1.5 billion years ago. Free-living cyanobacteria still exist today and are responsible for ∼50% of the world's photosynthesis. Cyanobacteria themselves are thought to have evolved from simpler photosynthetic bacteria that use either organic or inorganic compounds such a hydrogen sulfide as a source of electrons rather than water and thus do not produce oxygen.
The site of photosynthesis in plants
In land plants, the principal organs of photosynthesis are the leaves ( Figure 2 A). Leaves have evolved to expose the largest possible area of green tissue to light and entry of CO 2 to the leaf is controlled by small holes in the lower epidermis called stomata ( Figure 2 B). The size of the stomatal openings is variable and regulated by a pair of guard cells, which respond to the turgor pressure (water content) of the leaf, thus when the leaf is hydrated, the stomata can open to allow CO 2 in. In contrast, when water is scarce, the guard cells lose turgor pressure and close, preventing the escape of water from the leaf via transpiration.
Location of the photosynthetic machinery

( A ) The model plant Arabidopsis thaliana . ( B ) Basic structure of a leaf shown in cross-section. Chloroplasts are shown as green dots within the cells. ( C ) An electron micrograph of an Arabidopsis chloroplast within the leaf. ( D ) Close-up region of the chloroplast showing the stacked structure of the thylakoid membrane.
Within the green tissue of the leaf (mainly the mesophyll) each cell (∼100 μm in length) contains ∼100 chloroplasts (2–3 μm in length), the tiny organelles where photosynthesis takes place. The chloroplast has a complex structure ( Figure 2 C, D) with two outer membranes (the envelope), which are colourless and do not participate in photosynthesis, enclosing an aqueous space (the stroma) wherein sits a third membrane known as the thylakoid, which in turn encloses a single continuous aqueous space called the lumen.
The light reactions of photosynthesis involve light-driven electron and proton transfers, which occur in the thylakoid membrane, whereas the dark reactions involve the fixation of CO 2 into carbohydrate, via the Calvin–Benson cycle, which occurs in the stroma ( Figure 3 ). The light reactions involve electron transfer from water to NADP + to form NADPH and these reactions are coupled to proton transfers that lead to the phosphorylation of adenosine diphosphate (ADP) into ATP. The Calvin–Benson cycle uses ATP and NADPH to convert CO 2 into carbohydrates ( Figure 3 ), regenerating ADP and NADP + . The light and dark reactions are therefore mutually dependent on one another.
Division of labour within the chloroplast

The light reactions of photosynthesis take place in the thylakoid membrane, whereas the dark reactions are located in the chloroplast stroma.
Photosynthetic electron and proton transfer chain
The light-driven electron transfer reactions of photosynthesis begin with the splitting of water by Photosystem II (PSII). PSII is a chlorophyll–protein complex embedded in the thylakoid membrane that uses light to oxidize water to oxygen and reduce the electron acceptor plastoquinone to plastoquinol. Plastoquinol in turn carries the electrons derived from water to another thylakoid-embedded protein complex called cytochrome b 6 f (cyt b 6 f ). cyt b 6 f oxidizes plastoquinol to plastoquinone and reduces a small water-soluble electron carrier protein plastocyanin, which resides in the lumen. A second light-driven reaction is then carried out by another chlorophyll protein complex called Photosystem I (PSI). PSI oxidizes plastocyanin and reduces another soluble electron carrier protein ferredoxin that resides in the stroma. Ferredoxin can then be used by the ferredoxin–NADP + reductase (FNR) enzyme to reduce NADP + to NADPH. This scheme is known as the linear electron transfer pathway or Z-scheme ( Figure 4 ).
The photosynthetic electron and proton transfer chain

The linear electron transfer pathway from water to NADP + to form NADPH results in the formation of a proton gradient across the thylakoid membrane that is used by the ATP synthase enzyme to make ATP.
The Z-scheme, so-called since it resembles the letter ‘Z’ when turned on its side ( Figure 5 ), thus shows how the electrons move from the water–oxygen couple (+820 mV) via a chain of redox carriers to NADP + /NADPH (−320 mV) during photosynthetic electron transfer. Generally, electrons are transferred from redox couples with low potentials (good reductants) to those with higher potentials (good oxidants) (e.g. during respiratory electron transfer in mitochondria) since this process is exergonic (see Box 2 ). However, photosynthetic electron transfer also involves two endergonic steps, which occur at PSII and at PSI and require an energy input in the form of light. The light energy is used to excite an electron within a chlorophyll molecule residing in PSII or PSI to a higher energy level; this excited chlorophyll is then able to reduce the subsequent acceptors in the chain. The oxidized chlorophyll is then reduced by water in the case of PSII and plastocyanin in the case of PSI.
Z-scheme of photosynthetic electron transfer

The main components of the linear electron transfer pathway are shown on a scale of redox potential to illustrate how two separate inputs of light energy at PSI and PSII result in the endergonic transfer of electrons from water to NADP + .
The water-splitting reaction at PSII and plastoquinol oxidation at cyt b 6 f result in the release of protons into the lumen, resulting in a build-up of protons in this compartment relative to the stroma. The difference in the proton concentration between the two sides of the membrane is called a proton gradient. The proton gradient is a store of free energy (similar to a gradient of ions in a battery) that is utilized by a molecular mechanical motor ATP synthase, which resides in the thylakoid membrane ( Figure 4 ). The ATP synthase allows the protons to move down their concentration gradient from the lumen (high H + concentration) to the stroma (low H + concentration). This exergonic reaction is used to power the endergonic synthesis of ATP from ADP and inorganic phosphate (P i ). This process of photophosphorylation is thus essentially similar to oxidative phosphorylation, which occurs in the inner mitochondrial membrane during respiration.
An alternative electron transfer pathway exists in plants and algae, known as cyclic electron flow. Cyclic electron flow involves the recycling of electrons from ferredoxin to plastoquinone, with the result that there is no net production of NADPH; however, since protons are still transferred into the lumen by oxidation of plastoquinol by cyt b 6 f , ATP can still be formed. Thus photosynthetic organisms can control the ratio of NADPH/ATP to meet metabolic need by controlling the relative amounts of cyclic and linear electron transfer.

Light absorption by pigments
Photosynthesis begins with the absorption of light by pigments molecules located in the thylakoid membrane. The most well-known of these is chlorophyll, but there are also carotenoids and, in cyanobacteria and some algae, bilins. These pigments all have in common within their chemical structures an alternating series of carbon single and double bonds, which form a conjugated system π–electron system ( Figure 6 ).
Major photosynthetic pigments in plants

The chemical structures of the chlorophyll and carotenoid pigments present in the thylakoid membrane. Note the presence in each of a conjugated system of carbon–carbon double bonds that is responsible for light absorption.
The variety of pigments present within each type of photosynthetic organism reflects the light environment in which it lives; plants on land contain chlorophylls a and b and carotenoids such as β-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin and neoxanthin ( Figure 6 ). The chlorophylls absorb blue and red light and so appear green in colour, whereas carotenoids absorb light only in the blue and so appear yellow/red ( Figure 7 ), colours more obvious in the autumn as chlorophyll is the first pigment to be broken down in decaying leaves.
Basic absorption spectra of the major chlorophyll and carotenoid pigments found in plants

Chlorophylls absorb light energy in the red and blue part of the visible spectrum, whereas carotenoids only absorb light in the blue/green.
Light, or electromagnetic radiation, has the properties of both a wave and a stream of particles (light quanta). Each quantum of light contains a discrete amount of energy that can be calculated by multiplying Planck's constant, h (6.626×10 −34 J·s) by ν, the frequency of the radiation in cycles per second (s −1 ):

The frequency (ν) of the light and so its energy varies with its colour, thus blue photons (∼450 nm) are more energetic than red photons (∼650 nm). The frequency (ν) and wavelength (λ) of light are related by:

where c is the velocity of light (3.0×10 8 m·s −1 ), and the energy of a particular wavelength (λ) of light is given by:

Thus 1 mol of 680 nm photons of red light has an energy of 176 kJ·mol −1 .
The electrons within the delocalized π system of the pigment have the ability to jump up from the lowest occupied molecular orbital (ground state) to higher unoccupied molecular electron orbitals (excited states) via the absorption of specific wavelengths of light in the visible range (400–725 nm). Chlorophyll has two excited states known as S 1 and S 2 and, upon interaction of the molecule with a photon of light, one of its π electrons is promoted from the ground state (S 0 ) to an excited state, a process taking just 10 −15 s ( Figure 8 ). The energy gap between the S 0 and S 1 states is spanned by the energy provided by a red photon (∼600–700 nm), whereas the energy gap between the S 0 and S 2 states is larger and therefore requires a more energetic (shorter wavelength, higher frequency) blue photon (∼400–500 nm) to span the energy gap.
Jablonski diagram of chlorophyll showing the possible fates of the S 1 and S 2 excited states and timescales of the transitions involved

Photons with slightly different energies (colours) excite each of the vibrational substates of each excited state (as shown by variation in the size and colour of the arrows).
Upon excitation, the electron in the S 2 state quickly undergoes losses of energy as heat through molecular vibration and undergoes conversion into the energy of the S 1 state by a process called internal conversion. Internal conversion occurs on a timescale of 10 −12 s. The energy of a blue photon is thus rapidly degraded to that of a red photon. Excitation of the molecule with a red photon would lead to promotion of an electron to the S 1 state directly. Once the electron resides in the S 1 state, it is lower in energy and thus stable on a somewhat longer timescale (10 −9 s). The energy of the excited electron in the S 1 state can have one of several fates: it could return to the ground state (S 0 ) by emission of the energy as a photon of light (fluorescence), or it could be lost as heat due to internal conversion between S 1 and S 0 . Alternatively, if another chlorophyll is nearby, a process known as excitation energy transfer (EET) can result in the non-radiative exchange of energy between the two molecules ( Figure 9 ). For this to occur, the two chlorophylls must be close by (<7 nm), have a specific orientation with respect to one another, and excited state energies that overlap (are resonant) with one another. If these conditions are met, the energy is exchanged, resulting in a mirror S 0 →S 1 transition in the acceptor molecule and a S 1 →S 0 transition in the other.
Basic mechanism of excitation energy transfer between chlorophyll molecules

Two chlorophyll molecules with resonant S 1 states undergo a mirror transition resulting in the non-radiative transfer of excitation energy between them.
Light-harvesting complexes
In photosynthetic systems, chlorophylls and carotenoids are found attached to membrane-embedded proteins known as light-harvesting complexes (LHCs). Through careful binding and orientation of the pigment molecules, absorbed energy can be transferred among them by EET. Each pigment is bound to the protein by a series of non-covalent bonding interactions (such as, hydrogen bonds, van der Waals interactions, hydrophobic interaction and co-ordination bonds between lone pair electrons of residues such as histidine in the protein and the Mg 2+ ion in chlorophyll); the protein structure is such that each bound pigment experiences a slightly different environment in terms of the surrounding amino acid side chains, lipids, etc., meaning that the S 1 and S 2 energy levels are shifted in energy with respect to that of other neighbouring pigment molecules. The effect is to create a range of pigment energies that act to ‘funnel’ the energy on to the lowest-energy pigments in the LHC by EET.
Reaction centres
A photosystem consists of numerous LHCs that form an antenna of hundreds of pigment molecules. The antenna pigments act to collect and concentrate excitation energy and transfer it towards a ‘special pair’ of chlorophyll molecules that reside in the reaction centre (RC) ( Figure 10 ). Unlike the antenna pigments, the special pair of chlorophylls are ‘redox-active’ in the sense that they can return to the ground state (S 0 ) by the transfer of the electron residing in the S 1 excited state (Chl*) to another species. This process is known as charge separation and result in formation of an oxidized special pair (Chl + ) and a reduced acceptor (A − ). The acceptor in PSII is plastoquinone and in PSI it is ferredoxin. If the RC is to go on functioning, the electron deficiency on the special pair must be made good, in PSII the electron donor is water and in PSI it is plastocyanin.
Basic structure of a photosystem

Light energy is captured by the antenna pigments and transferred to the special pair of RC chlorophylls which undergo a redox reaction leading to reduction of an acceptor molecule. The oxidized special pair is regenerated by an electron donor.
It is worth asking why photosynthetic organisms bother to have a large antenna of pigments serving an RC rather than more numerous RCs. The answer lies in the fact that the special pair of chlorophylls alone have a rather small spatial and spectral cross-section, meaning that there is a limit to the amount of light they can efficiently absorb. The amount of light they can practically absorb is around two orders of magnitude smaller than their maximum possible turnover rate, Thus LHCs act to increase the spatial (hundreds of pigments) and spectral (several types of pigments with different light absorption characteristics) cross-section of the RC special pair ensuring that its turnover rate runs much closer to capacity.
Photosystem II
PSII is a light-driven water–plastoquinone oxidoreductase and is the only enzyme in Nature that is capable of performing the difficult chemistry of splitting water into protons, electrons and oxygen ( Figure 11 ). In principle, water is an extremely poor electron donor since the redox potential of the water–oxygen couple is +820 mV. PSII uses light energy to excite a special pair of chlorophylls, known as P680 due to their 680 nm absorption peak in the red part of the spectrum. P680* undergoes charge separation that results in the formation of an extremely oxidizing species P680 + which has a redox potential of +1200 mV, sufficient to oxidize water. Nonetheless, since water splitting involves four electron chemistry and charge separation only involves transfer of one electron, four separate charge separations (turnovers of PSII) are required to drive formation of one molecule of O 2 from two molecules of water. The initial electron donation to generate the P680 from P680 + is therefore provided by a cluster of manganese ions within the oxygen-evolving complex (OEC), which is attached to the lumen side of PSII ( Figure 12 ). Manganese is a transition metal that can exist in a range of oxidation states from +1 to +5 and thus accumulates the positive charges derived from each light-driven turnover of P680. Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light and is known as the S-state cycle ( Figure 12 ). After the fourth turnover of P680, sufficient positive charge is built up in the manganese cluster to permit the splitting of water into electrons, which regenerate the original state of the manganese cluster, protons, which are released into the lumen and contribute to the proton gradient used for ATP synthesis, and the by-product O 2 . Thus charge separation at P680 provides the thermodynamic driving force, whereas the manganese cluster acts as a catalyst for the water-splitting reaction.
Basic structure of the PSII–LHCII supercomplex from spinach

The organization of PSII and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 3JCU
S-state cycle of water oxidation by the manganese cluster (shown as circles with roman numerals representing the manganese ion oxidation states) within the PSII oxygen-evolving complex

Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light. Each of the electrons given up by the cluster is eventually repaid at the S 4 to S 0 transition when molecular oxygen (O 2 ) is formed. The protons extracted from water during the process are deposited into the lumen and contribute to the protonmotive force.
The electrons yielded by P680* following charge separation are not passed directly to plastoquinone, but rather via another acceptor called pheophytin, a porphyrin molecule lacking the central magnesium ion as in chlorophyll. Plastoquinone reduction to plastoquinol requires two electrons and thus two molecules of plastoquinol are formed per O 2 molecule evolved by PSII. Two protons are also taken up upon formation of plastoquinol and these are derived from the stroma. PSII is found within the thylakoid membrane of plants as a dimeric RC complex surrounded by a peripheral antenna of six minor monomeric antenna LHC complexes and two to eight trimeric LHC complexes, which together form a PSII–LHCII supercomplex ( Figure 11 ).
Photosystem I
PSI is a light-driven plastocyanin–ferredoxin oxidoreductase ( Figure 13 ). In PSI, the special pair of chlorophylls are known as P700 due to their 700 nm absorption peak in the red part of the spectrum. P700* is an extremely strong reductant that is able to reduce ferredoxin which has a redox potential of −450 mV (and is thus is, in principle, a poor electron acceptor). Reduced ferredoxin is then used to generate NADPH for the Calvin–Benson cycle at a separate complex known as FNR. The electron from P700* is donated via another chlorophyll molecule and a bound quinone to a series of iron–sulfur clusters at the stromal side of the complex, whereupon the electron is donated to ferredoxin. The P700 species is regenerated form P700 + via donation of an electron from the soluble electron carrier protein plastocyanin.
Basic structure of the PSI–LHCI supercomplex from pea

The organization of PSI and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 4XK8.
PSI is found within the thylakoid membrane as a monomeric RC surrounded on one side by four LHC complexes known as LHCI. The PSI–LHCI supercomplex is found mainly in the unstacked regions of the thylakoid membrane ( Figure 13 ).
Plastoquinone/plastoquinol
Plastoquinone is a small lipophilic electron carrier molecule that resides within the thylakoid membrane and carries two electrons and two protons from PSII to the cyt b 6 f complex. It has a very similar structure to that of the molecule ubiquinone (coenzyme Q 10 ) in the mitochondrial inner membrane.
Cytochrome b 6 f complex
The cyt b 6 f complex is a plastoquinol–plastocyanin oxidoreductase and possess a similar structure to that of the cytochrome bc 1 complex (complex III) in mitochondria ( Figure 14 A). As with Complex III, cyt b 6 f exists as a dimer in the membrane and carries out both the oxidation and reduction of quinones via the so-called Q-cycle. The Q-cycle ( Figure 14 B) involves oxidation of one plastoquinol molecule at the Qp site of the complex, both protons from this molecule are deposited in the lumen and contribute to the proton gradient for ATP synthesis. The two electrons, however, have different fates. The first is transferred via an iron–sulfur cluster and a haem cofactor to the soluble electron carrier plastocyanin (see below). The second electron derived from plastoquinol is passed via two separate haem cofactors to another molecule of plastoquinone bound to a separate site (Qn) on the complex, thus reducing it to a semiquinone. When a second plastoquinol molecule is oxidized at Qp, a second molecule of plastocyanin is reduced and two further protons are deposited in the lumen. The second electron reduces the semiquinone at the Qn site which, concomitant with uptake of two protons from the stroma, causes its reduction to plastoquinol. Thus for each pair of plastoquinol molecules oxidized by the complex, one is regenerated, yet all four protons are deposited into the lumen. The Q-cycle thus doubles the number of protons transferred from the stroma to the lumen per plastoquinol molecule oxidized.

( A ) Structure drawn from PDB code 1Q90. ( B ) The protonmotive Q-cycle showing how electrons from plastoquinol are passed to both plastocyanin and plastoquinone, doubling the protons deposited in the lumen for every plastoquinol molecule oxidized by the complex.
Plastocyanin
Plastocyanin is a small soluble electron carrier protein that resides in the thylakoid lumen. The active site of the plastocyanin protein binds a copper ion, which cycles between the Cu 2+ and Cu + oxidation states following its oxidation by PSI and reduction by cyt b 6 f respectively.
Ferredoxin is a small soluble electron carrier protein that resides in the chloroplast stroma. The active site of the ferredoxin protein binds an iron–sulfur cluster, which cycles between the Fe 2+ and Fe 3+ oxidation states following its reduction by PSI and oxidation by the FNR complex respectively.
Ferredoxin–NADP + reductase
The FNR complex is found in both soluble and thylakoid membrane-bound forms. The complex binds a flavin–adenine dinucleotide (FAD) cofactor at its active site, which accepts two electrons from two molecules of ferredoxin before using them reduce NADP + to NADPH.
ATP synthase
The ATP synthase enzyme is responsible for making ATP from ADP and P i ; this endergonic reaction is powered by the energy contained within the protonmotive force. According to the structure, 4.67 H + are required for every ATP molecule synthesized by the chloroplast ATP synthase. The enzyme is a rotary motor which contains two domains: the membrane-spanning F O portion which conducts protons from the lumen to the stroma, and the F 1 catalytic domain that couples this exergonic proton movement to ATP synthesis.
Membrane stacking and the regulation of photosynthesis
Within the thylakoid membrane, PSII–LHCII supercomplexes are packed together into domains known as the grana, which associate with one another to form grana stacks. PSI and ATP synthase are excluded from these stacked PSII–LHCII regions by steric constraints and thus PSII and PSI are segregated in the thylakoid membrane between the stacked and unstacked regions ( Figure 15 ). The cyt b 6 f complex, in contrast, is evenly distributed throughout the grana and stromal lamellae. The evolutionary advantage of membrane stacking is believed to be a higher efficiency of electron transport by preventing the fast energy trap PSI from ‘stealing’ excitation energy from the slower trap PSII, a phenomenon known as spillover. Another possible advantage of membrane stacking in thylakoids may be the segregation of the linear and cyclic electron transfer pathways, which might otherwise compete to reduce plastoquinone. In this view, PSII, cyt b 6 f and a sub-fraction of PSI closest to the grana is involved in linear flow, whereas PSI and cyt b 6 f in the stromal lamellae participates in cyclic flow. The cyclic electron transfer pathway recycles electrons from ferredoxin back to plastoquinone and thus allows protonmotive force generation (and ATP synthesis) without net NADPH production. Cyclic electron transfer thereby provides the additional ATP required for the Calvin–Benson cycle (see below).
Lateral heterogeneity in thylakoid membrane organization

( A ) Electron micrograph of the thylakoid membrane showing stacked grana and unstacked stromal lamellae regions. ( B ) Model showing the distribution of the major complexes of photosynthetic electron and proton transfer between the stacked grana and unstacked stromal lamellae regions.
‘Dark’ reactions: the Calvin–Benson cycle
CO 2 is fixed into carbohydrate via the Calvin–Benson cycle in plants, which consumes the ATP and NADPH produced during the light reactions and thus in turn regenerates ADP, P i and NADP + . In the first step of the Calvin–Benson cycle ( Figure 16 ), CO 2 is combined with a 5-carbon (5C) sugar, ribulose 1,5-bisphosphate in a reaction catalysed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The reaction forms an unstable 6C intermediate that immediately splits into two molecules of 3-phosphoglycerate. 3-Phosphoglycerate is first phosphorylated by 3-phosphoglycerate kinase using ATP to form 1,3-bisphosphoglycerate. 1,3-Bisphosphoglycerate is then reduced by glyceraldehyde 3-phosphate dehydrogenase using NADPH to form glyceraldehyde 3-phosphate (GAP, a triose or 3C sugar) in reactions, which are the reverse of glycolysis. For every three CO 2 molecules initially combined with ribulose 1,5-bisphopshate, six molecules of GAP are produced by the subsequent steps. However only one of these six molecules can be considered as a product of the Calvin–Benson cycle since the remaining five are required to regenerate ribulose 1,5-bisphosphate in a complex series of reactions that also require ATP. The one molecule of GAP that is produced for each turn of the cycle can be quickly converted by a range of metabolic pathways into amino acids, lipids or sugars such as glucose. Glucose in turn may be stored as the polymer starch as large granules within chloroplasts.
The Calvin–Benson cycle

Overview of the biochemical pathway for the fixation of CO 2 into carbohydrate in plants.
A complex biochemical ‘dance’ ( Figure 16 ) is then involved in the regeneration of three ribulose 1,5-bisphosphate (5C) from the remaining five GAP (3C) molecules. The regeneration begins with the conversion of two molecules of GAP into dihydroxyacetone phosphate (DHAP) by triose phosphate isomerase; one of the DHAP molecules is the combined with another GAP molecule to make fructose 1,6-bisphosphate (6C) by aldolase. The fructose 1,6-bisphosphate is then dephosphorylated by fructose-1,6-bisphosphatase to yield fructose 6-phosphate (6C) and releasing P i . Two carbons are then removed from fructose 6-phosphate by transketolase, generating erythrose 4-phosphate (4C); the two carbons are transferred to another molecule of GAP generating xylulose 5-phosphate (5C). Another DHAP molecule, formed from GAP by triose phosphate isomerase is then combined with the erythrose 4-phosphate by aldolase to form sedoheptulose 1,7-bisphosphate (7C). Sedoheptulose 1,7-bisphosphate is then dephosphorylated to sedoheptulose 7-phosphate (7C) by sedoheptulose-1,7-bisphosphatase releasing P i . Sedoheptulose 7-phosphate has two carbons removed by transketolase to produce ribose 5-phosphate (5C) and the two carbons are transferred to another GAP molecule producing another xylulose 5-phosphate (5C). Ribose 5-phosphate and the two molecules of xylulose 5-phosphate (5C) are then converted by phosphopentose isomerase to three molecules of ribulose 5-phosphate (5C). The three ribulose 5-phosphate molecules are then phosphorylated using three ATP by phosphoribulokinase to regenerate three ribulose 1,5-bisphosphate (5C).
Overall the synthesis of 1 mol of GAP requires 9 mol of ATP and 6 mol of NADPH, a required ratio of 1.5 ATP/NADPH. Linear electron transfer is generally thought to supply ATP/NADPH in a ratio of 1.28 (assuming an H + /ATP ratio of 4.67) with the shortfall of ATP believed to be provided by cyclic electron transfer reactions. Since the product of the Calvin cycle is GAP (a 3C sugar) the pathway is often referred to as C 3 photosynthesis and plants that utilize it are called C 3 plants and include many of the world's major crops such as rice, wheat and potato.
Many of the enzymes involved in the Calvin–Benson cycle (e.g. transketolase, glyceraldehyde-3-phosphate dehydrogenase and aldolase) are also involved in the glycolysis pathway of carbohydrate degradation and their activity must therefore be carefully regulated to avoid futile cycling when light is present, i.e. the unwanted degradation of carbohydrate. The regulation of the Calvin–Benson cycle enzymes is achieved by the activity of the light reactions, which modify the environment of the dark reactions (i.e. the stroma). Proton gradient formation across the thylakoid membrane during the light reactions increases the pH and also increases the Mg 2+ concentration in the stroma (as Mg 2+ flows out of the lumen as H + flows in to compensate for the influx of positive charges). In addition, by reducing ferredoxin and NADP + , PSI changes the redox state of the stroma, which is sensed by the regulatory protein thioredoxin. Thioredoxin, pH and Mg 2+ concentration play a key role in regulating the activity of the Calvin–Benson cycle enzymes, ensuring the activity of the light and dark reactions is closely co-ordinated.
It is noteworthy that, despite the complexity of the dark reactions outlined above, the carbon fixation step itself (i.e. the incorporation of CO 2 into carbohydrate) is carried out by a single enzyme, Rubisco. Rubisco is a large multisubunit soluble protein complex found in the chloroplast stroma. The complex consists of eight large (56 kDa) subunits, which contain both catalytic and regulatory domains, and eight small subunits (14 kDa), which enhance the catalytic function of the L subunits ( Figure 17 A). The carboxylation reaction carried out by Rubisco is highly exergonic (Δ G °=−51.9 kJ·mol- 1 ), yet kinetically very slow (just 3 s −1 ) and begins with the protonation of ribulose 1,5-bisphosphate to form an enediolate intermediate which can be combined with CO 2 to form an unstable 6C intermediate that is quickly hydrolysed to yield two 3C 3-phosphoglycerate molecules. The active site in the Rubisco enzyme contains a key lysine residue, which reacts with another (non-substrate) molecule of CO 2 to form a carbamate anion that is then able to bind Mg 2+ . The Mg 2+ in the active site is essential for the catalytic function of Rubisco, playing a key role in binding ribulose 1,5-bisphosphate and activating it such that it readily reacts with CO 2.. Rubisco activity is co-ordinated with that of the light reactions since carbamate formation requires both high Mg 2+ concentration and alkaline conditions, which are provided by the light-driven changes in the stromal environment discussed above ( Figure 17 B).

( A ) Structure of the Rubisco enzyme (the large subunits are shown in blue and the small subunits in green); four of each type of subunit are visible in the image. Drawn from PDB code 1RXO. ( B ) Activation of the lysine residue within the active site of Rubisco occurs via elevated stromal pH and Mg 2+ concentration as a result of the activity of the light reactions.
In addition to carboxylation, Rubisco also catalyses a competitive oxygenation reaction, known as photorespiration, that results in the combination of ribulose 1,5-bisphosphate with O 2 rather than CO 2 . In the oxygenation reaction, one rather than two molecules of 3-phosphoglycerate and one molecule of a 2C sugar known as phosphoglycolate are produced by Rubisco. The phosphoglycolate must be converted in a series of reactions that regenerate one molecule of 3-phosphoglycerate and one molecule of CO 2 . These reactions consume additional ATP and thus result in an energy loss to the plant. Although the oxygenation reaction of Rubisco is much less favourable than the carboxylation reaction, the relatively high concentration of O 2 in the leaf (250 μM) compared with CO 2 (10 μM) means that a significant amount of photorespiration is always occurring. Under normal conditions, the ratio of carboxylation to oxygenation is between 3:1 and 4:1. However, this ratio can be decreased with increasing temperature due to decreased CO 2 concentration in the leaf, a decrease in the affinity of Rubisco for CO 2 compared with O 2 and an increase in the maximum rate of the oxygenation reaction compared with the carboxylation reaction. The inefficiencies of the Rubisco enzyme mean that plants must produce it in very large amounts (∼30–50% of total soluble protein in a spinach leaf) to achieve the maximal photosynthetic rate.
CO 2 -concentrating mechanisms
To counter photorespiration, plants, algae and cyanobacteria have evolved different CO 2 -concentrating mechanisms CCMs that aim to increase the concentration of CO 2 relative to O 2 in the vicinity of Rubisco. One such CCM is C 4 photosynthesis that is found in plants such as maize, sugar cane and savanna grasses. C 4 plants show a specialized leaf anatomy: Kranz anatomy ( Figure 18 ). Kranz, German for wreath, refers to a bundle sheath of cells that surrounds the central vein within the leaf, which in turn are surrounded by the mesophyll cells. The mesophyll cells in such leaves are rich in the enzyme phosphoenolpyruvate (PEP) carboxylase, which fixes CO 2 into a 4C carboxylic acid: oxaloaceatate. The oxaloacetate formed by the mesophyll cells is reduced using NADPH to malate, another 4C acid: malate. The malate is then exported from the mesophyll cells to the bundle sheath cells, where it is decarboxylated to pyruvate thus regenerating NADPH and CO 2 . The CO 2 is then utilized by Rubisco in the Calvin cycle. The pyruvate is in turn returned to the mesophyll cells where it is phosphorylated using ATP to reform PEP ( Figure 19 ). The advantage of C 4 photosynthesis is that CO 2 accumulates at a very high concentration in the bundle sheath cells that is then sufficient to allow Rubisco to operate efficiently.
Diagram of a C 4 plant leaf showing Kranz anatomy

The C 4 pathway (NADP + –malic enzyme type) for fixation of CO 2

Plants growing in hot, bright and dry conditions inevitably have to have their stomata closed for large parts of the day to avoid excessive water loss and wilting. The net result is that the internal CO 2 concentration in the leaf is very low, meaning that C 3 photosynthesis is not possible. To counter this limitation, another CCM is found in succulent plants such as cacti. The Crassulaceae fix CO 2 into malate during the day via PEP carboxylase, store it within the vacuole of the plant cell at night and then release it within their tissues by day to be fixed via normal C 3 photosynthesis. This is termed crassulacean acid metabolism (CAM).
This article is a reviewed, revised and updated version of the following ‘Biochemistry Across the School Curriculum’ (BASC) booklet: Weaire, P.J. (1994) Photosynthesis . For further information and to provide feedback on this or any other Biochemical Society education resource, please contact [email protected]. For further information on other Biochemical Society publications, please visit www.biochemistry.org/publications .
adenosine diphosphate
adenosine triphosphate
carbohydrate
cytochrome b 6 f
dihydroxyacetone phosphate
excitation energy transfer
ferredoxin–NADP + reductase
glyceraldehyde 3-phosphate
light-harvesting complex
nicotinomide–adenine dinucleotide phosphate
phosphoenolpyruvate
inorganic phosphate
reaction centre
ribulose-1,5-bisphosphate carboxylase/oxygenase
I thank Professor Colin Osborne (University of Sheffield, Sheffield, U.K.) for useful discussions on the article, Dr Dan Canniffe (Penn State University, Pennsylvania, PA, U.S.A.) for providing pure pigment spectra and Dr P.J. Weaire (Kingston University, Kingston-upon-Thames, U.K.) for his original Photosynthesis BASC article (1994) on which this essay is partly based.
The Author declares that there are no competing interests associated with this article.
Get Email Alerts
- Online ISSN 1744-1358
- Print ISSN 0071-1365
- Submit Your Work
- Language-editing services
- Recommend to Your Librarian
- Request a free trial
- Accessibility
- Sign up for alerts
- Sign up to our mailing list
- The Biochemist Blog
- Biochemical Society Membership
- Publishing Life Cycle
- Biochemical Society Events
- About Portland Press
- Portland Press Tel
- +44 (0)20 3880 2795
- Portland Press Company no. 02453983
- Biochemical Society Tel
- +44 (0)20 3880 2793
- Email: [email protected]
- Biochemical Society Company no. 00892796
- Registered Charity no. 253894
- VAT no. GB 523 2392 69
- Privacy and cookies
- © Copyright 2024 Portland Press
This Feature Is Available To Subscribers Only
Sign In or Create an Account
8.1 Overview of Photosynthesis
Learning objectives.
In this section, you will explore the following questions:
- What is the relevance of photosynthesis to living organisms?
- What are the main cellular structures involved in photosynthesis?
- What are the substrates and products of photosynthesis?
Connection for AP ® Courses
As we learned in Chapter 7, all living organisms, from simple bacteria to complex plants and animals, require free energy to carry out cellular processes, such as growth and reproduction. Organisms use various strategies to capture, store, transform, and transfer free energy, including photosynthesis. Photosynthesis allows organisms to access enormous amounts of free energy from the sun and transform it to the chemical energy of sugars. Although all organisms carry out some form of cellular respiration, only certain organisms, called photoautotrophs, can perform photosynthesis. Examples of photoautotrophs include plants, algae, some unicellular eukaryotes, and cyanobacteria. They require the presence of chlorophyll, a specialized pigment that absorbs certain wavelengths of the visible light spectrum to harness free energy from the sun. Photosynthesis is a process where components of water and carbon dioxide are used to assemble carbohydrate molecules and where oxygen waste products are released into the atmosphere. In eukaryotes, the reactions of photosynthesis occur in chloroplasts; in prokaryotes, such as cyanobacteria, the reactions are less localized and occur within membranes and in the cytoplasm. (The structural features of the chloroplast that participate in photosynthesis will be explored in more detail later in The Light-Dependent Reactions of Photosynthesis and Using Light Energy to Make Organic Molecules.) Although photosynthesis and cellular respiration evolved as independent processes—with photosynthesis creating an oxidizing atmosphere early in Earth’s history—today they are interdependent. As we studied in Cellular Respiration, aerobic cellular respiration taps into the oxidizing ability of oxygen to synthesize the organic compounds that are used to power cellular processes.
Information presented and the examples highlighted in the section support concepts and learning objectives outlined in Big Idea 1 and Big Idea 2 of the AP ® Biology Curriculum Framework, as shown in the table. The learning objectives listed in the Curriculum Framework provide a transparent foundation for the AP ® Biology course, an inquiry-based laboratory experience, instructional activities, and AP ® exam questions. A learning objective merges required content with one or more of the seven science practices.
Teacher Support
Use this first part of the chapter to present an overview that will be filled out and completed in the later two portions. This will introduce the students to the biochemistry that they need to know and give them a chance to build up their understanding of the material.
Importance of Photosynthesis
Use this section to stress the importance of the interdependence between different species and the role played by photosynthesis in bringing energy to the living organisms. A number of terms, such as photoautotroph, heterotrophy, and chemoautotroph will be introduced here.
Photosynthesis is essential to all life on earth; both plants and animals depend on it. It is the only biological process that can capture energy that originates in outer space (sunlight) and convert it into chemical compounds (carbohydrates) that every organism uses to power its metabolism. In brief, the energy of sunlight is captured and used to energize electrons, whose energy is then stored in the covalent bonds of sugar molecules. How long lasting and stable are those covalent bonds? The energy extracted today by the burning of coal and petroleum products represents sunlight energy captured and stored by photosynthesis almost 200 million years ago.
Plants, algae, and a group of bacteria called cyanobacteria are the only organisms capable of performing photosynthesis ( Figure 8.2 ). Because they use light to manufacture their own food, they are called photoautotrophs (literally, “self-feeders using light”). Other organisms, such as animals, fungi, and most other bacteria, are termed heterotrophs (“other feeders”), because they must rely on the sugars produced by photosynthetic organisms for their energy needs. A third very interesting group of bacteria synthesize sugars, not by using sunlight’s energy, but by extracting energy from inorganic chemical compounds; hence, they are referred to as chemoautotrophs .
The importance of photosynthesis is not just that it can capture sunlight’s energy. A lizard sunning itself on a cold day can use the sun’s energy to warm up. Photosynthesis is vital because it evolved as a way to store the energy in solar radiation (the “photo-” part) as energy in the carbon-carbon bonds of carbohydrate molecules (the “-synthesis” part). Those carbohydrates are the energy source that heterotrophs use to power the synthesis of ATP via respiration. Therefore, photosynthesis powers 99 percent of Earth’s ecosystems. When a top predator, such as a wolf, preys on a deer ( Figure 8.3 ), the wolf is at the end of an energy path that went from nuclear reactions on the surface of the sun, to light, to photosynthesis, to vegetation, to deer, and finally to wolf.
Science Practice Connection for AP® Courses
Think about it.
- Why do scientists think that photosynthesis evolved before aerobic cellular respiration?
- Why do carnivores, such as lions, depend on photosynthesis to survive? What evidence supports the claim that photosynthesis and cellular respiration are interdependent processes?
- The first Think About It question is an application of Learning Objective 1.15 and Science Practice 7.2 because students are describing the evolution of two energy-procuring processes that today are present in different organisms.
- The second Think About It question is an application of Learning Objective 2.5 and Science Practice 6.2 because you are explaining how the interdependent processes of photosynthesis and cellular respiration allow organisms to capture, store, and use free energy.
Possible answers:
- Aerobic cellular respiration requires free oxygen, which was not available in the Earth’s atmosphere until photosynthetic organisms produced enough oxygen as waste to support developing aerobic respiration.
- Carnivores at the top of the food chain eat herbivores that eat photoautotrophs. So no matter where you are in the food chain, every species depends on photosynthesis to convert light energy to chemical energy. In ecosystems that lack photosynthetic organisms (such as by forests burned by forest fire), organisms on all levels of the food chain die off.
The structures, substrates and products of photosynthesis are introduced in this section. Remind them that Figure 8.5 can also be read from right to left, if cellular respiration is the subject. This should help the students to connect the two pathways of photosynthesis and cellular respiration.
Obtain diagrams of leaf structures to illustrate the content of this section. Try to bring in some leaves for students to look at. They have all seen lots of leaves, but probably never examined them for structural detail. A simple magnifying glass should allow them to see the inner structures discussed in this section.
Main Structures and Summary of Photosynthesis
Photosynthesis is a multi-step process that requires sunlight, carbon dioxide (which is low in energy), and water as substrates ( Figure 8.4 ). After the process is complete, it releases oxygen and produces glyceraldehyde-3-phosphate (G3P), simple carbohydrate molecules (which are high in energy) that can subsequently be converted into glucose, sucrose, or any of dozens of other sugar molecules. These sugar molecules contain energy and the energized carbon that all living things need to survive.
The following is the chemical equation for photosynthesis ( Figure 8.5 ):
Although the equation looks simple, the many steps that take place during photosynthesis are actually quite complex. Before learning the details of how photoautotrophs turn sunlight into food, it is important to become familiar with the structures involved.
In plants, photosynthesis generally takes place in leaves, which consist of several layers of cells. The process of photosynthesis occurs in a middle layer called the mesophyll . The gas exchange of carbon dioxide and oxygen occurs through small, regulated openings called stomata (singular: stoma), which also play roles in the regulation of gas exchange and water balance. The stomata are typically located on the underside of the leaf, which helps to minimize water loss. Each stoma is flanked by guard cells that regulate the opening and closing of the stomata by swelling or shrinking in response to osmotic changes.
In all autotrophic eukaryotes, photosynthesis takes place inside an organelle called a chloroplast . For plants, chloroplast-containing cells exist in the mesophyll. Chloroplasts have a double membrane envelope (composed of an outer membrane and an inner membrane). Within the chloroplast are stacked, disc-shaped structures called thylakoids . Embedded in the thylakoid membrane is chlorophyll, a pigment (molecule that absorbs light) responsible for the initial interaction between light and plant material, and numerous proteins that make up the electron transport chain. The thylakoid membrane encloses an internal space called the thylakoid lumen . As shown in Figure 8.6 , a stack of thylakoids is called a granum , and the liquid-filled space surrounding the granum is called stroma or “bed” (not to be confused with stoma or “mouth,” an opening on the leaf epidermis).
Visual Connection
- Rate of photosynthesis will be inhibited as the level of carbon dioxide decreases.
- Rate of photosynthesis will be inhibited as the level of oxygen decreases.
- The rate of photosynthesis will increase as the level of carbon dioxide increases.
- Rate of photosynthesis will increase as the level of oxygen increases.
The Two Parts of Photosynthesis
There are different terms that have been used for these reactions. Go over each pair of terms and discuss how they apply to the pathways.
Photosynthesis takes place in two sequential stages: the light-dependent reactions and the light independent-reactions. In the light-dependent reactions , energy from sunlight is absorbed by chlorophyll and that energy is converted into stored chemical energy. In the light-independent reactions , the chemical energy harvested during the light-dependent reactions drives the assembly of sugar molecules from carbon dioxide. Therefore, although the light-independent reactions do not use light as a reactant, they require the products of the light-dependent reactions to function. In addition, several enzymes of the light-independent reactions are activated by light. The light-dependent reactions utilize certain molecules to temporarily store the energy: These are referred to as energy carriers. The energy carriers that move energy from light-dependent reactions to light-independent reactions can be thought of as “full” because they are rich in energy. After the energy is released, the “empty” energy carriers return to the light-dependent reaction to obtain more energy. Figure 8.7 illustrates the components inside the chloroplast where the light-dependent and light-independent reactions take place.
Link to Learning
Click the link to learn more about photosynthesis.
- The light reactions produces ATP and NADPH, which are then used in the Calvin cycle.
- The light reactions produces NADP + and ADP, which are then used in the Calvin cycle.
- The light reactions uses NADPH and ATP, which are produced by the Calvin cycle.
- The light reactions produce only NADPH, which is produced by the Calvin cycle.
Everyday Connection for AP® Courses
Photosynthesis at the grocery store.
Major grocery stores in the United States are organized into departments, such as dairy, meats, produce, bread, cereals, and so forth. Each aisle ( Figure 8.8 ) contains hundreds, if not thousands, of different products for customers to buy and consume.
Although there is a large variety, each item links back to photosynthesis. Meats and dairy link, because the animals were fed plant-based foods. The breads, cereals, and pastas come largely from starchy grains, which are the seeds of photosynthesis-dependent plants. What about desserts and drinks? All of these products contain sugar—sucrose is a plant product, a disaccharide, a carbohydrate molecule, which is built directly from photosynthesis. Moreover, many items are less obviously derived from plants: For instance, paper goods are generally plant products, and many plastics (abundant as products and packaging) are derived from algae. Virtually every spice and flavoring in the spice aisle was produced by a plant as a leaf, root, bark, flower, fruit, or stem. Ultimately, photosynthesis connects to every meal and every food a person consumes.
- at the base
- near the top
- in the middle, but generally closer to the top
- in the middle, but generally closer to the base
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Authors: Julianne Zedalis, John Eggebrecht
- Publisher/website: OpenStax
- Book title: Biology for AP® Courses
- Publication date: Mar 8, 2018
- Location: Houston, Texas
- Book URL: https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Section URL: https://openstax.org/books/biology-ap-courses/pages/8-1-overview-of-photosynthesis
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.1: Introduction to Photosynthesis
- Last updated
- Save as PDF
- Page ID 75475

The processes in all organisms—from bacteria to humans—require energy. To get this energy, many organisms access stored energy by eating, that is, by ingesting other organisms. But where does the stored energy in food originate? All of this energy can be traced back to photosynthesis.
Contributors and Attributions
Connie Rye (East Mississippi Community College), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Community College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Island College) among other contributing authors. Original content by OpenStax (CC BY 4.0; Download for free at http://cnx.org/contents/[email protected] ).

MSU Extension
The important role of photosynthesis.
Bill Cook, Michigan State University Extension - April 09, 2013
Photosynthesis is not just about oxygen production it is also about energy production.
Most people would agree that photosynthesis is a great thing. I’ve never heard anyone argue against it. However, some folks have missed the purpose of photosynthesis. It’s not oxygen production.
The primary function of photosynthesis is to convert solar energy into chemical energy and then store that chemical energy for future use. For the most part, the planet’s living systems are powered by this process. It’s not particularly efficient by human engineering standards, but it does the job. Photosynthesis happens in regions of a cell called chloroplasts. The chemistry and physics are complex.
It’s a bit humbling to consider that the energy in our bodies travels 93 million miles in a little more than eight minutes, and that life has tapped into that energy stream. For a short time that energy is tied up in biological systems before it continues on its merry way into the dark of space.
In essence, green plants take carbon, hydrogen and oxygen from the molecules of carbon dioxide and water, and then recombine them into a new molecule called glucose. This happens in the presence of sunlight, of course. Energy is stored in the bonds of the glucose molecule. Glucose is a fairly simple sugar, easy to break down. Ever wonder why kids bounce off the walls and ceilings soon after a good dose of sugar?
Chemically speaking, the inputs to photosynthesis are six carbon atoms, 12 hydrogen atoms and 18 oxygen atoms. Glucose uses six carbon, 12 hydrogen, and six oxygen molecules. Simple math shows 12 leftover oxygen atoms, or six oxygen molecules. Oxygen atoms prefer mates.
Interestingly, and not coincidentally, the process of respiration breaks apart the glucose molecule. Respiration occurs in the cells of nearly all living things. The released energy is then used for all sorts of metabolic activity, including the energy that you are using to read this article. Respiration happens in regions of a cell called mitochondria. The chemical reactions are the reverse of photosynthesis, using a glucose molecule and six oxygen molecules (12 atoms) as inputs. Energy is released along with some carbon dioxide and water.
But this is enough chemistry.
Trees and other green plants practice respiration, too, just like animals, but they also practice photosynthesis. This is why ecologists categorize green plants as “producers” and most every other life form as a “consumer.” It’s about the energy. OK, there are decomposers, too, but that’s another story and they’re still dependent upon the energy captured by the producers.
Oxygen is a byproduct of photosynthesis and, correspondingly, carbon dioxide the byproduct of respiration. Trees are often credited as the major oxygen generator for the planet, but that would be false. Most of the planet is covered with water and the collective photosynthesis of lowly algae is the true oxygen machine.
Nevertheless, trees and forests are, indeed, significant oxygen producers. However, if oxygen was the only benefit of trees and forests, we could easily live without them. And some forests actually produce more carbon dioxide than oxygen. Fortunately, the benefits of both trees and forests extend far beyond something as narrow as oxygen production.
Much of the basic structural material of plants and wood is cellulose, which is an especially complex sugar. The constituent molecules of carbon, hydrogen and oxygen can be recombined to form lots of useful chemicals such as ethanol, perfumes, bioplastics, clothing fabrics and a range of industrial ingredients. It’s generally agreed that sources from within renewable living ecosystems have distinct advantages over using the ancient materials that make up fossil fuels.
Plants and photosynthesis are the basis of fossil fuels, too, but from millions and millions of years ago. Bringing huge volumes of those molecules back into living ecosystems has a few drawbacks that science has gotten pretty good at measuring and describing.
Trees, forests, forest soils and forest products are mighty important in the cycling of carbon and the relative size of various carbon pools. There are other elements that also cycle through forests. Science has a pretty good handle on these relationships, too. Michigan residents might do well to place a bit more weight on these service benefits of trees, forests, and forest management.
As for photosynthesis itself, maybe it’s better if we think more about the energy capture and less about the oxygen production.
This article was published by Michigan State University Extension . For more information, visit https://extension.msu.edu . To have a digest of information delivered straight to your email inbox, visit https://extension.msu.edu/newsletters . To contact an expert in your area, visit https://extension.msu.edu/experts , or call 888-MSUE4MI (888-678-3464).
Did you find this article useful?
Check out the Environmental Studies & Sustainability B.S. program!
Check out the Sustainable Parks, Recreation & Tourism B.S. program!
new - method size: 3 - Random key: 0, method: tagSpecific - key: 0
You Might Also Be Interested In

AC3 Podcast episode 3
Published on June 30, 2021

MIFruitcast: Meet the Educators
Published on January 18, 2024
MIFruitcast: Biological Controls with Jackie Perkins
Published on December 1, 2023

Meet Up and Eat Up with Ken Kujawski
Published on September 16, 2020

MSU Dairy Virtual Coffee Break: What are lenders looking for?
Published on April 7, 2021


Ep. 38: Land Acknowledgement with Emily Proctor
Published on April 21, 2021
- msu extension
- natural resources
- msu extension,

Essay on Photosynthesis
Students are often asked to write an essay on Photosynthesis in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Photosynthesis
What is photosynthesis.
Photosynthesis is how plants make their own food using sunlight. It happens in the leaves of plants. Tiny parts inside the leaves, called chloroplasts, use sunlight to turn water and carbon dioxide from the air into sugar and oxygen. The sugar is food for the plant.
The Ingredients
The main things needed for photosynthesis are sunlight, water, and carbon dioxide. Roots soak up water from the soil. Leaves take in carbon dioxide from the air. Then, using sunlight, plants create food and release oxygen.
The Process
In the chloroplasts, sunlight energy is changed into chemical energy. This energy turns water and carbon dioxide into glucose, a type of sugar. Oxygen is made too, which goes into the air for us to breathe.
Why It’s Important
Photosynthesis is vital for life on Earth. It gives us food and oxygen. Without it, there would be no plants, and without plants, animals and people would not survive. It also helps take in carbon dioxide, which is good for the Earth.
250 Words Essay on Photosynthesis
Photosynthesis is a process used by plants, algae, and some bacteria to turn sunlight, water, and carbon dioxide into food and oxygen. Think of it like a recipe that plants use to make their own food. This happens in the leaves of plants, which have a green substance called chlorophyll.
Why is Photosynthesis Important?
This process is very important because it is the main way plants make food for themselves and for us, too. Without photosynthesis, plants could not grow, and without plants, animals and humans would not have oxygen to breathe or food to eat.
How Photosynthesis Works
Photosynthesis happens in two main stages. In the first stage, the plant captures sunlight with its leaves. The sunlight gives the plant energy to split water inside its leaves into hydrogen and oxygen. The oxygen is released into the air, and the hydrogen is used in the next stage.
In the second stage, the plant mixes the hydrogen with carbon dioxide from the air to make glucose, which is a type of sugar that plants use for energy. This energy helps the plant to grow, make flowers, and produce seeds.
The Cycle of Life
Photosynthesis is a key part of the cycle of life on Earth. By making food and oxygen, plants support life for all creatures. When animals eat plants, they get the energy from the plants, and when animals breathe, they use the oxygen that plants release. It’s a beautiful cycle that keeps the planet alive.
500 Words Essay on Photosynthesis
Photosynthesis is a process used by plants, algae, and some bacteria to turn sunlight, water, and carbon dioxide into food and oxygen. This happens in the green parts of plants, mainly the leaves. The green color comes from chlorophyll, a special substance in the leaves that captures sunlight.
The Ingredients of Photosynthesis
To make their food, plants need three main things: sunlight, water, and carbon dioxide. Sunlight is the energy plants use to create their food. They get water from the ground through their roots. Carbon dioxide, a gas found in the air, is taken in through tiny holes in the leaves called stomata.
The Photosynthesis Recipe
When sunlight hits the leaves, the chlorophyll captures it and starts the food-making process. The energy from the sunlight turns water and carbon dioxide into glucose, a type of sugar that plants use for energy, and oxygen, which is released into the air. This process is like a recipe that plants follow to make their own food.
The Importance of Photosynthesis
Photosynthesis is very important for life on Earth. It gives us oxygen, which we need to breathe. Plants use the glucose they make for growth and to build other important substances like cellulose, which they use to make their cell walls. Without photosynthesis, there would be no food for animals or people, and no oxygen to breathe.
The Benefits to the Environment
Photosynthesis also helps the environment. Plants take in carbon dioxide, which is a gas that can make the Earth warmer when there is too much of it in the air. By using carbon dioxide to make food, plants help keep the air clean and the Earth’s temperature just right.
Photosynthesis and the Food Chain
All living things need energy to survive, and this energy usually comes from food. Plants are at the bottom of the food chain because they can make their own food using photosynthesis. Animals that eat plants get energy from the glucose in the plants. Then, animals that eat other animals get this energy too. So, photosynthesis is the start of the food chain that feeds almost every living thing on Earth.
Photosynthesis in Our Lives
Photosynthesis affects our lives in many ways. It gives us fruits, vegetables, and grains to eat. Trees and plants also give us wood, paper, and other materials. Plus, they provide shade and help make the air fresh and clean.
In conclusion, photosynthesis is a vital process that allows plants to make food and oxygen using sunlight, water, and carbon dioxide. It is the foundation of the food chain and has a big impact on the environment and our lives. Understanding photosynthesis helps us appreciate how important plants are and why we need to take care of them and the environment they live in.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Gender Equality And Women’s Empowerment
- Essay on Gender Equality And Sustainable Development
- Essay on Exciting Cricket Match
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Photosynthesis
Affiliation.
- 1 Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield S10 2TN, U.K. [email protected].
- PMID: 27784776
- PMCID: PMC5264509
- DOI: 10.1042/EBC20160016
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide-adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin-Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
Keywords: membrane; photosynthesis; thylakoid.
© 2016 The Author(s).
Publication types
- Electron Transport*
- Photosynthesis*
- Photosynthetic Reaction Center Complex Proteins / chemistry
- Photosynthetic Reaction Center Complex Proteins / genetics
- Photosynthetic Reaction Center Complex Proteins / metabolism*
- Plants / metabolism*
- Photosynthetic Reaction Center Complex Proteins
Home — Essay Samples — Science — Light — Photosynthesis Process
Photosynthesis Process
- Categories: Light Photosynthesis
About this sample

Words: 423 |
Published: Feb 12, 2019
Words: 423 | Page: 1 | 3 min read
Works Cited
- Campbell, N. A., & Reece, J. B. (2008). Photosynthesis and cellular respiration. In Biology (8th ed., pp. 190-220). Benjamin-Cummings Publishing Company.
- Taiz, L., & Zeiger, E. (2010). Photosynthesis: Carbon reactions. In Plant physiology (5th ed., pp. 174-207). Sinauer Associates.
- Raven, P. H., Evert, R. F., & Eichhorn, S. E. (2016). Photosynthesis and respiration. In Biology of Plants (8th ed., pp. 186-229). W. H. Freeman and Company.
- Niyogi, K. K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 333-359. doi:10.1146/annurev.arplant.50.1.333
- Siedow, J. N., & Day, D. A. (2000). Respiration and photorespiration. In Plant physiology (3rd ed., pp. 500-548). Academic Press.
- Allen, J. F. (2002). Photosynthesis and cellular respiration considered as coupled redox cycles: A chemiosmotic bridge linking two epochs. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 357(1426), 707-717. doi:10.1098/rstb.2001.0993
- Geigenberger, P. (2003). Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology, 6(3), 247-256. doi:10.1016/S1369-5266(03)00038-8
- Foyer, C. H., & Noctor, G. (2005). Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. The Plant Cell, 17(7), 1866-1875. doi:10.1105/tpc.105.033589
- Sharkey, T. D. (2005). Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment, 28(3), 269-277. doi:10.1111/j.1365-3040.2005.01324.x
- Sweetlove, L. J., & Fernie, A. R. (2018). The impact of oxidative stress on metabolism: A compartmental analysis. Frontiers in Plant Science, 9, 1647. doi:10.3389/fpls.2018.01647

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
6 pages / 2684 words
5 pages / 2673 words
1 pages / 596 words
1 pages / 639 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online

Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Light
The light bulb greatly changed the world in many ways that continue to affect how individuals experience their lives today. Long ago using natural sources, for example, candles, lamps, and firewood were common ways of [...]
Light arrives on our planet after a speedy trip from the Sun, 149 million km (93 million miles away). Light travels at 186,000 miles (300,000 km) per second, so the light you’re seeing now was still tucked away in the Sun about [...]
Lightning or illumination: Lighting or illumination is the purposeful use of light to achieve a practical or visual effect. Lighting includes the use of both artificial light sources like lamps and light fixtures, as well as [...]
Throughout centuries many authors have written great novels. However, as time moves on certain pieces of literature may be forgotten or become irrelevant as it no longer appears to current generations. At times certain topics [...]
The function of the diode is regulating the voltage at a particular current. 1. Small Signal Diode It is a small device with disproportional characteristics and whose applications are mainly involved at high frequency and [...]
Have you ever thought that the color of the light can affect the plant growth? I believe that color will affect the plant growth. Light is essential during plant growth and photosynthesis. Light powers and gives energy to the [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Portland Press Opt2Pay

Photosynthesis
Matthew p. johnson.
Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield S10 2TN, U.K.
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide–adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin–Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
An overview of photosynthesis
Introduction.
Photosynthesis is the ultimate source of all of humankind's food and oxygen, whereas fossilized photosynthetic fuels provide ∼87% of the world's energy. It is the biochemical process that sustains the biosphere as the basis for the food chain. The oxygen produced as a by-product of photosynthesis allowed the formation of the ozone layer, the evolution of aerobic respiration and thus complex multicellular life.
Oxygenic photosynthesis involves the conversion of water and CO 2 into complex organic molecules such as carbohydrates and oxygen. Photosynthesis may be split into the ‘light’ and ‘dark’ reactions. In the light reactions, water is split using light into oxygen, protons and electrons, and in the dark reactions, the protons and electrons are used to reduce CO 2 to carbohydrate (given here by the general formula CH 2 O). The two processes can be summarized thus:
Light reactions:
Dark reactions:
The positive sign of the standard free energy change of the reaction (Δ G °) given above means that the reaction requires energy ( an endergonic reaction ). The energy required is provided by absorbed solar energy, which is converted into the chemical bond energy of the products ( Box 1 ).
Standard free energy change

Photosynthesis converts ∼200 billion tonnes of CO 2 into complex organic compounds annually and produces ∼140 billion tonnes of oxygen into the atmosphere. By facilitating conversion of solar energy into chemical energy, photosynthesis acts as the primary energy input into the global food chain. Nearly all living organisms use the complex organic compounds derived from photosynthesis as a source of energy. The breakdown of these organic compounds occurs via the process of aerobic respiration, which of course also requires the oxygen produced by photosynthesis.
Unlike photosynthesis, aerobic respiration is an exergonic process (negative Δ G °) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste). The use of exergonic reactions to power endergonic ones associated with biosynthesis and housekeeping in biological organisms such that the overall free energy change is negative is known as ‘ coupling’.
Photosynthesis and respiration are thus seemingly the reverse of one another, with the important caveat that both oxygen formation during photosynthesis and its utilization during respiration result in its liberation or incorporation respectively into water rather than CO 2 . In addition, glucose is one of several possible products of photosynthesis with amino acids and lipids also being synthesized rapidly from the primary photosynthetic products.
The consideration of photosynthesis and respiration as opposing processes helps us to appreciate their role in shaping our environment. The fixation of CO 2 by photosynthesis and its release during breakdown of organic molecules during respiration, decay and combustion of organic matter and fossil fuels can be visualized as the global carbon cycle ( Figure 1 ).

The relationship between respiration, photosynthesis and global CO 2 and O 2 levels.
At present, this cycle may be considered to be in a state of imbalance due to the burning of fossil fuels (fossilized photosynthesis), which is increasing the proportion of CO 2 entering the Earth's atmosphere, leading to the so-called ‘greenhouse effect’ and human-made climate change.
Oxygenic photosynthesis is thought to have evolved only once during Earth's history in the cyanobacteria. All other organisms, such as plants, algae and diatoms, which perform oxygenic photosynthesis actually do so via cyanobacterial endosymbionts or ‘chloroplasts’. An endosymbiotoic event between an ancestral eukaryotic cell and a cyanobacterium that gave rise to plants is estimated to have occurred ∼1.5 billion years ago. Free-living cyanobacteria still exist today and are responsible for ∼50% of the world's photosynthesis. Cyanobacteria themselves are thought to have evolved from simpler photosynthetic bacteria that use either organic or inorganic compounds such a hydrogen sulfide as a source of electrons rather than water and thus do not produce oxygen.
The site of photosynthesis in plants
In land plants, the principal organs of photosynthesis are the leaves ( Figure 2 A). Leaves have evolved to expose the largest possible area of green tissue to light and entry of CO 2 to the leaf is controlled by small holes in the lower epidermis called stomata ( Figure 2 B). The size of the stomatal openings is variable and regulated by a pair of guard cells, which respond to the turgor pressure (water content) of the leaf, thus when the leaf is hydrated, the stomata can open to allow CO 2 in. In contrast, when water is scarce, the guard cells lose turgor pressure and close, preventing the escape of water from the leaf via transpiration.

( A ) The model plant Arabidopsis thaliana . ( B ) Basic structure of a leaf shown in cross-section. Chloroplasts are shown as green dots within the cells. ( C ) An electron micrograph of an Arabidopsis chloroplast within the leaf. ( D ) Close-up region of the chloroplast showing the stacked structure of the thylakoid membrane.
Within the green tissue of the leaf (mainly the mesophyll) each cell (∼100 μm in length) contains ∼100 chloroplasts (2–3 μm in length), the tiny organelles where photosynthesis takes place. The chloroplast has a complex structure ( Figure 2 C, D) with two outer membranes (the envelope), which are colourless and do not participate in photosynthesis, enclosing an aqueous space (the stroma) wherein sits a third membrane known as the thylakoid, which in turn encloses a single continuous aqueous space called the lumen.
The light reactions of photosynthesis involve light-driven electron and proton transfers, which occur in the thylakoid membrane, whereas the dark reactions involve the fixation of CO 2 into carbohydrate, via the Calvin–Benson cycle, which occurs in the stroma ( Figure 3 ). The light reactions involve electron transfer from water to NADP + to form NADPH and these reactions are coupled to proton transfers that lead to the phosphorylation of adenosine diphosphate (ADP) into ATP. The Calvin–Benson cycle uses ATP and NADPH to convert CO 2 into carbohydrates ( Figure 3 ), regenerating ADP and NADP + . The light and dark reactions are therefore mutually dependent on one another.

The light reactions of photosynthesis take place in the thylakoid membrane, whereas the dark reactions are located in the chloroplast stroma.
Photosynthetic electron and proton transfer chain
The light-driven electron transfer reactions of photosynthesis begin with the splitting of water by Photosystem II (PSII). PSII is a chlorophyll–protein complex embedded in the thylakoid membrane that uses light to oxidize water to oxygen and reduce the electron acceptor plastoquinone to plastoquinol. Plastoquinol in turn carries the electrons derived from water to another thylakoid-embedded protein complex called cytochrome b 6 f (cyt b 6 f ). cyt b 6 f oxidizes plastoquinol to plastoquinone and reduces a small water-soluble electron carrier protein plastocyanin, which resides in the lumen. A second light-driven reaction is then carried out by another chlorophyll protein complex called Photosystem I (PSI). PSI oxidizes plastocyanin and reduces another soluble electron carrier protein ferredoxin that resides in the stroma. Ferredoxin can then be used by the ferredoxin–NADP + reductase (FNR) enzyme to reduce NADP + to NADPH. This scheme is known as the linear electron transfer pathway or Z-scheme ( Figure 4 ).

The linear electron transfer pathway from water to NADP + to form NADPH results in the formation of a proton gradient across the thylakoid membrane that is used by the ATP synthase enzyme to make ATP.
The Z-scheme, so-called since it resembles the letter ‘Z’ when turned on its side ( Figure 5 ), thus shows how the electrons move from the water–oxygen couple (+820 mV) via a chain of redox carriers to NADP + /NADPH (−320 mV) during photosynthetic electron transfer. Generally, electrons are transferred from redox couples with low potentials (good reductants) to those with higher potentials (good oxidants) (e.g. during respiratory electron transfer in mitochondria) since this process is exergonic (see Box 2 ). However, photosynthetic electron transfer also involves two endergonic steps, which occur at PSII and at PSI and require an energy input in the form of light. The light energy is used to excite an electron within a chlorophyll molecule residing in PSII or PSI to a higher energy level; this excited chlorophyll is then able to reduce the subsequent acceptors in the chain. The oxidized chlorophyll is then reduced by water in the case of PSII and plastocyanin in the case of PSI.

The main components of the linear electron transfer pathway are shown on a scale of redox potential to illustrate how two separate inputs of light energy at PSI and PSII result in the endergonic transfer of electrons from water to NADP + .
Relationship between redox potentials and standard free energy changes

The water-splitting reaction at PSII and plastoquinol oxidation at cyt b 6 f result in the release of protons into the lumen, resulting in a build-up of protons in this compartment relative to the stroma. The difference in the proton concentration between the two sides of the membrane is called a proton gradient. The proton gradient is a store of free energy (similar to a gradient of ions in a battery) that is utilized by a molecular mechanical motor ATP synthase, which resides in the thylakoid membrane ( Figure 4 ). The ATP synthase allows the protons to move down their concentration gradient from the lumen (high H + concentration) to the stroma (low H + concentration). This exergonic reaction is used to power the endergonic synthesis of ATP from ADP and inorganic phosphate (P i ). This process of photophosphorylation is thus essentially similar to oxidative phosphorylation, which occurs in the inner mitochondrial membrane during respiration.
An alternative electron transfer pathway exists in plants and algae, known as cyclic electron flow. Cyclic electron flow involves the recycling of electrons from ferredoxin to plastoquinone, with the result that there is no net production of NADPH; however, since protons are still transferred into the lumen by oxidation of plastoquinol by cyt b 6 f , ATP can still be formed. Thus photosynthetic organisms can control the ratio of NADPH/ATP to meet metabolic need by controlling the relative amounts of cyclic and linear electron transfer.
How the photosystems work
Light absorption by pigments.
Photosynthesis begins with the absorption of light by pigments molecules located in the thylakoid membrane. The most well-known of these is chlorophyll, but there are also carotenoids and, in cyanobacteria and some algae, bilins. These pigments all have in common within their chemical structures an alternating series of carbon single and double bonds, which form a conjugated system π–electron system ( Figure 6 ).

The chemical structures of the chlorophyll and carotenoid pigments present in the thylakoid membrane. Note the presence in each of a conjugated system of carbon–carbon double bonds that is responsible for light absorption.
The variety of pigments present within each type of photosynthetic organism reflects the light environment in which it lives; plants on land contain chlorophylls a and b and carotenoids such as β-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin and neoxanthin ( Figure 6 ). The chlorophylls absorb blue and red light and so appear green in colour, whereas carotenoids absorb light only in the blue and so appear yellow/red ( Figure 7 ), colours more obvious in the autumn as chlorophyll is the first pigment to be broken down in decaying leaves.

Chlorophylls absorb light energy in the red and blue part of the visible spectrum, whereas carotenoids only absorb light in the blue/green.
Light, or electromagnetic radiation, has the properties of both a wave and a stream of particles (light quanta). Each quantum of light contains a discrete amount of energy that can be calculated by multiplying Planck's constant, h (6.626×10 −34 J·s) by ν, the frequency of the radiation in cycles per second (s −1 ):
The frequency (ν) of the light and so its energy varies with its colour, thus blue photons (∼450 nm) are more energetic than red photons (∼650 nm). The frequency (ν) and wavelength (λ) of light are related by:
where c is the velocity of light (3.0×10 8 m·s −1 ), and the energy of a particular wavelength (λ) of light is given by:
Thus 1 mol of 680 nm photons of red light has an energy of 176 kJ·mol −1 .
The electrons within the delocalized π system of the pigment have the ability to jump up from the lowest occupied molecular orbital (ground state) to higher unoccupied molecular electron orbitals (excited states) via the absorption of specific wavelengths of light in the visible range (400–725 nm). Chlorophyll has two excited states known as S 1 and S 2 and, upon interaction of the molecule with a photon of light, one of its π electrons is promoted from the ground state (S 0 ) to an excited state, a process taking just 10 −15 s ( Figure 8 ). The energy gap between the S 0 and S 1 states is spanned by the energy provided by a red photon (∼600–700 nm), whereas the energy gap between the S 0 and S 2 states is larger and therefore requires a more energetic (shorter wavelength, higher frequency) blue photon (∼400–500 nm) to span the energy gap.

Photons with slightly different energies (colours) excite each of the vibrational substates of each excited state (as shown by variation in the size and colour of the arrows).
Upon excitation, the electron in the S 2 state quickly undergoes losses of energy as heat through molecular vibration and undergoes conversion into the energy of the S 1 state by a process called internal conversion. Internal conversion occurs on a timescale of 10 −12 s. The energy of a blue photon is thus rapidly degraded to that of a red photon. Excitation of the molecule with a red photon would lead to promotion of an electron to the S 1 state directly. Once the electron resides in the S 1 state, it is lower in energy and thus stable on a somewhat longer timescale (10 −9 s). The energy of the excited electron in the S 1 state can have one of several fates: it could return to the ground state (S 0 ) by emission of the energy as a photon of light (fluorescence), or it could be lost as heat due to internal conversion between S 1 and S 0 . Alternatively, if another chlorophyll is nearby, a process known as excitation energy transfer (EET) can result in the non-radiative exchange of energy between the two molecules ( Figure 9 ). For this to occur, the two chlorophylls must be close by (<7 nm), have a specific orientation with respect to one another, and excited state energies that overlap (are resonant) with one another. If these conditions are met, the energy is exchanged, resulting in a mirror S 0 →S 1 transition in the acceptor molecule and a S 1 →S 0 transition in the other.

Two chlorophyll molecules with resonant S 1 states undergo a mirror transition resulting in the non-radiative transfer of excitation energy between them.
Light-harvesting complexes
In photosynthetic systems, chlorophylls and carotenoids are found attached to membrane-embedded proteins known as light-harvesting complexes (LHCs). Through careful binding and orientation of the pigment molecules, absorbed energy can be transferred among them by EET. Each pigment is bound to the protein by a series of non-covalent bonding interactions (such as, hydrogen bonds, van der Waals interactions, hydrophobic interaction and co-ordination bonds between lone pair electrons of residues such as histidine in the protein and the Mg 2+ ion in chlorophyll); the protein structure is such that each bound pigment experiences a slightly different environment in terms of the surrounding amino acid side chains, lipids, etc., meaning that the S 1 and S 2 energy levels are shifted in energy with respect to that of other neighbouring pigment molecules. The effect is to create a range of pigment energies that act to ‘funnel’ the energy on to the lowest-energy pigments in the LHC by EET.
Reaction centres
A photosystem consists of numerous LHCs that form an antenna of hundreds of pigment molecules. The antenna pigments act to collect and concentrate excitation energy and transfer it towards a ‘special pair’ of chlorophyll molecules that reside in the reaction centre (RC) ( Figure 10 ). Unlike the antenna pigments, the special pair of chlorophylls are ‘redox-active’ in the sense that they can return to the ground state (S 0 ) by the transfer of the electron residing in the S 1 excited state (Chl*) to another species. This process is known as charge separation and result in formation of an oxidized special pair (Chl + ) and a reduced acceptor (A − ). The acceptor in PSII is plastoquinone and in PSI it is ferredoxin. If the RC is to go on functioning, the electron deficiency on the special pair must be made good, in PSII the electron donor is water and in PSI it is plastocyanin.

Light energy is captured by the antenna pigments and transferred to the special pair of RC chlorophylls which undergo a redox reaction leading to reduction of an acceptor molecule. The oxidized special pair is regenerated by an electron donor.
It is worth asking why photosynthetic organisms bother to have a large antenna of pigments serving an RC rather than more numerous RCs. The answer lies in the fact that the special pair of chlorophylls alone have a rather small spatial and spectral cross-section, meaning that there is a limit to the amount of light they can efficiently absorb. The amount of light they can practically absorb is around two orders of magnitude smaller than their maximum possible turnover rate, Thus LHCs act to increase the spatial (hundreds of pigments) and spectral (several types of pigments with different light absorption characteristics) cross-section of the RC special pair ensuring that its turnover rate runs much closer to capacity.
Photosystem II
PSII is a light-driven water–plastoquinone oxidoreductase and is the only enzyme in Nature that is capable of performing the difficult chemistry of splitting water into protons, electrons and oxygen ( Figure 11 ). In principle, water is an extremely poor electron donor since the redox potential of the water–oxygen couple is +820 mV. PSII uses light energy to excite a special pair of chlorophylls, known as P680 due to their 680 nm absorption peak in the red part of the spectrum. P680* undergoes charge separation that results in the formation of an extremely oxidizing species P680 + which has a redox potential of +1200 mV, sufficient to oxidize water. Nonetheless, since water splitting involves four electron chemistry and charge separation only involves transfer of one electron, four separate charge separations (turnovers of PSII) are required to drive formation of one molecule of O 2 from two molecules of water. The initial electron donation to generate the P680 from P680 + is therefore provided by a cluster of manganese ions within the oxygen-evolving complex (OEC), which is attached to the lumen side of PSII ( Figure 12 ). Manganese is a transition metal that can exist in a range of oxidation states from +1 to +5 and thus accumulates the positive charges derived from each light-driven turnover of P680. Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light and is known as the S-state cycle ( Figure 12 ). After the fourth turnover of P680, sufficient positive charge is built up in the manganese cluster to permit the splitting of water into electrons, which regenerate the original state of the manganese cluster, protons, which are released into the lumen and contribute to the proton gradient used for ATP synthesis, and the by-product O 2 . Thus charge separation at P680 provides the thermodynamic driving force, whereas the manganese cluster acts as a catalyst for the water-splitting reaction.

The organization of PSII and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 3JCU

Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light. Each of the electrons given up by the cluster is eventually repaid at the S 4 to S 0 transition when molecular oxygen (O 2 ) is formed. The protons extracted from water during the process are deposited into the lumen and contribute to the protonmotive force.
The electrons yielded by P680* following charge separation are not passed directly to plastoquinone, but rather via another acceptor called pheophytin, a porphyrin molecule lacking the central magnesium ion as in chlorophyll. Plastoquinone reduction to plastoquinol requires two electrons and thus two molecules of plastoquinol are formed per O 2 molecule evolved by PSII. Two protons are also taken up upon formation of plastoquinol and these are derived from the stroma. PSII is found within the thylakoid membrane of plants as a dimeric RC complex surrounded by a peripheral antenna of six minor monomeric antenna LHC complexes and two to eight trimeric LHC complexes, which together form a PSII–LHCII supercomplex ( Figure 11 ).
Photosystem I
PSI is a light-driven plastocyanin–ferredoxin oxidoreductase ( Figure 13 ). In PSI, the special pair of chlorophylls are known as P700 due to their 700 nm absorption peak in the red part of the spectrum. P700* is an extremely strong reductant that is able to reduce ferredoxin which has a redox potential of −450 mV (and is thus is, in principle, a poor electron acceptor). Reduced ferredoxin is then used to generate NADPH for the Calvin–Benson cycle at a separate complex known as FNR. The electron from P700* is donated via another chlorophyll molecule and a bound quinone to a series of iron–sulfur clusters at the stromal side of the complex, whereupon the electron is donated to ferredoxin. The P700 species is regenerated form P700 + via donation of an electron from the soluble electron carrier protein plastocyanin.

The organization of PSI and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 4XK8.
PSI is found within the thylakoid membrane as a monomeric RC surrounded on one side by four LHC complexes known as LHCI. The PSI–LHCI supercomplex is found mainly in the unstacked regions of the thylakoid membrane ( Figure 13 ).
Other electron transfer chain components
Plastoquinone/plastoquinol.
Plastoquinone is a small lipophilic electron carrier molecule that resides within the thylakoid membrane and carries two electrons and two protons from PSII to the cyt b 6 f complex. It has a very similar structure to that of the molecule ubiquinone (coenzyme Q 10 ) in the mitochondrial inner membrane.
Cytochrome b 6 f complex
The cyt b 6 f complex is a plastoquinol–plastocyanin oxidoreductase and possess a similar structure to that of the cytochrome bc 1 complex (complex III) in mitochondria ( Figure 14 A). As with Complex III, cyt b 6 f exists as a dimer in the membrane and carries out both the oxidation and reduction of quinones via the so-called Q-cycle. The Q-cycle ( Figure 14 B) involves oxidation of one plastoquinol molecule at the Qp site of the complex, both protons from this molecule are deposited in the lumen and contribute to the proton gradient for ATP synthesis. The two electrons, however, have different fates. The first is transferred via an iron–sulfur cluster and a haem cofactor to the soluble electron carrier plastocyanin (see below). The second electron derived from plastoquinol is passed via two separate haem cofactors to another molecule of plastoquinone bound to a separate site (Qn) on the complex, thus reducing it to a semiquinone. When a second plastoquinol molecule is oxidized at Qp, a second molecule of plastocyanin is reduced and two further protons are deposited in the lumen. The second electron reduces the semiquinone at the Qn site which, concomitant with uptake of two protons from the stroma, causes its reduction to plastoquinol. Thus for each pair of plastoquinol molecules oxidized by the complex, one is regenerated, yet all four protons are deposited into the lumen. The Q-cycle thus doubles the number of protons transferred from the stroma to the lumen per plastoquinol molecule oxidized.

( A ) Structure drawn from PDB code 1Q90. ( B ) The protonmotive Q-cycle showing how electrons from plastoquinol are passed to both plastocyanin and plastoquinone, doubling the protons deposited in the lumen for every plastoquinol molecule oxidized by the complex.
Plastocyanin
Plastocyanin is a small soluble electron carrier protein that resides in the thylakoid lumen. The active site of the plastocyanin protein binds a copper ion, which cycles between the Cu 2+ and Cu + oxidation states following its oxidation by PSI and reduction by cyt b 6 f respectively.
Ferredoxin is a small soluble electron carrier protein that resides in the chloroplast stroma. The active site of the ferredoxin protein binds an iron–sulfur cluster, which cycles between the Fe 2+ and Fe 3+ oxidation states following its reduction by PSI and oxidation by the FNR complex respectively.
Ferredoxin–NADP + reductase
The FNR complex is found in both soluble and thylakoid membrane-bound forms. The complex binds a flavin–adenine dinucleotide (FAD) cofactor at its active site, which accepts two electrons from two molecules of ferredoxin before using them reduce NADP + to NADPH.
ATP synthase
The ATP synthase enzyme is responsible for making ATP from ADP and P i ; this endergonic reaction is powered by the energy contained within the protonmotive force. According to the structure, 4.67 H + are required for every ATP molecule synthesized by the chloroplast ATP synthase. The enzyme is a rotary motor which contains two domains: the membrane-spanning F O portion which conducts protons from the lumen to the stroma, and the F 1 catalytic domain that couples this exergonic proton movement to ATP synthesis.
Membrane stacking and the regulation of photosynthesis
Within the thylakoid membrane, PSII–LHCII supercomplexes are packed together into domains known as the grana, which associate with one another to form grana stacks. PSI and ATP synthase are excluded from these stacked PSII–LHCII regions by steric constraints and thus PSII and PSI are segregated in the thylakoid membrane between the stacked and unstacked regions ( Figure 15 ). The cyt b 6 f complex, in contrast, is evenly distributed throughout the grana and stromal lamellae. The evolutionary advantage of membrane stacking is believed to be a higher efficiency of electron transport by preventing the fast energy trap PSI from ‘stealing’ excitation energy from the slower trap PSII, a phenomenon known as spillover. Another possible advantage of membrane stacking in thylakoids may be the segregation of the linear and cyclic electron transfer pathways, which might otherwise compete to reduce plastoquinone. In this view, PSII, cyt b 6 f and a sub-fraction of PSI closest to the grana is involved in linear flow, whereas PSI and cyt b 6 f in the stromal lamellae participates in cyclic flow. The cyclic electron transfer pathway recycles electrons from ferredoxin back to plastoquinone and thus allows protonmotive force generation (and ATP synthesis) without net NADPH production. Cyclic electron transfer thereby provides the additional ATP required for the Calvin–Benson cycle (see below).

( A ) Electron micrograph of the thylakoid membrane showing stacked grana and unstacked stromal lamellae regions. ( B ) Model showing the distribution of the major complexes of photosynthetic electron and proton transfer between the stacked grana and unstacked stromal lamellae regions.
‘Dark’ reactions: the Calvin–Benson cycle
CO 2 is fixed into carbohydrate via the Calvin–Benson cycle in plants, which consumes the ATP and NADPH produced during the light reactions and thus in turn regenerates ADP, P i and NADP + . In the first step of the Calvin–Benson cycle ( Figure 16 ), CO 2 is combined with a 5-carbon (5C) sugar, ribulose 1,5-bisphosphate in a reaction catalysed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The reaction forms an unstable 6C intermediate that immediately splits into two molecules of 3-phosphoglycerate. 3-Phosphoglycerate is first phosphorylated by 3-phosphoglycerate kinase using ATP to form 1,3-bisphosphoglycerate. 1,3-Bisphosphoglycerate is then reduced by glyceraldehyde 3-phosphate dehydrogenase using NADPH to form glyceraldehyde 3-phosphate (GAP, a triose or 3C sugar) in reactions, which are the reverse of glycolysis. For every three CO 2 molecules initially combined with ribulose 1,5-bisphopshate, six molecules of GAP are produced by the subsequent steps. However only one of these six molecules can be considered as a product of the Calvin–Benson cycle since the remaining five are required to regenerate ribulose 1,5-bisphosphate in a complex series of reactions that also require ATP. The one molecule of GAP that is produced for each turn of the cycle can be quickly converted by a range of metabolic pathways into amino acids, lipids or sugars such as glucose. Glucose in turn may be stored as the polymer starch as large granules within chloroplasts.

Overview of the biochemical pathway for the fixation of CO 2 into carbohydrate in plants.
A complex biochemical ‘dance’ ( Figure 16 ) is then involved in the regeneration of three ribulose 1,5-bisphosphate (5C) from the remaining five GAP (3C) molecules. The regeneration begins with the conversion of two molecules of GAP into dihydroxyacetone phosphate (DHAP) by triose phosphate isomerase; one of the DHAP molecules is the combined with another GAP molecule to make fructose 1,6-bisphosphate (6C) by aldolase. The fructose 1,6-bisphosphate is then dephosphorylated by fructose-1,6-bisphosphatase to yield fructose 6-phosphate (6C) and releasing P i . Two carbons are then removed from fructose 6-phosphate by transketolase, generating erythrose 4-phosphate (4C); the two carbons are transferred to another molecule of GAP generating xylulose 5-phosphate (5C). Another DHAP molecule, formed from GAP by triose phosphate isomerase is then combined with the erythrose 4-phosphate by aldolase to form sedoheptulose 1,7-bisphosphate (7C). Sedoheptulose 1,7-bisphosphate is then dephosphorylated to sedoheptulose 7-phosphate (7C) by sedoheptulose-1,7-bisphosphatase releasing P i . Sedoheptulose 7-phosphate has two carbons removed by transketolase to produce ribose 5-phosphate (5C) and the two carbons are transferred to another GAP molecule producing another xylulose 5-phosphate (5C). Ribose 5-phosphate and the two molecules of xylulose 5-phosphate (5C) are then converted by phosphopentose isomerase to three molecules of ribulose 5-phosphate (5C). The three ribulose 5-phosphate molecules are then phosphorylated using three ATP by phosphoribulokinase to regenerate three ribulose 1,5-bisphosphate (5C).
Overall the synthesis of 1 mol of GAP requires 9 mol of ATP and 6 mol of NADPH, a required ratio of 1.5 ATP/NADPH. Linear electron transfer is generally thought to supply ATP/NADPH in a ratio of 1.28 (assuming an H + /ATP ratio of 4.67) with the shortfall of ATP believed to be provided by cyclic electron transfer reactions. Since the product of the Calvin cycle is GAP (a 3C sugar) the pathway is often referred to as C 3 photosynthesis and plants that utilize it are called C 3 plants and include many of the world's major crops such as rice, wheat and potato.
Many of the enzymes involved in the Calvin–Benson cycle (e.g. transketolase, glyceraldehyde-3-phosphate dehydrogenase and aldolase) are also involved in the glycolysis pathway of carbohydrate degradation and their activity must therefore be carefully regulated to avoid futile cycling when light is present, i.e. the unwanted degradation of carbohydrate. The regulation of the Calvin–Benson cycle enzymes is achieved by the activity of the light reactions, which modify the environment of the dark reactions (i.e. the stroma). Proton gradient formation across the thylakoid membrane during the light reactions increases the pH and also increases the Mg 2+ concentration in the stroma (as Mg 2+ flows out of the lumen as H + flows in to compensate for the influx of positive charges). In addition, by reducing ferredoxin and NADP + , PSI changes the redox state of the stroma, which is sensed by the regulatory protein thioredoxin. Thioredoxin, pH and Mg 2+ concentration play a key role in regulating the activity of the Calvin–Benson cycle enzymes, ensuring the activity of the light and dark reactions is closely co-ordinated.
It is noteworthy that, despite the complexity of the dark reactions outlined above, the carbon fixation step itself (i.e. the incorporation of CO 2 into carbohydrate) is carried out by a single enzyme, Rubisco. Rubisco is a large multisubunit soluble protein complex found in the chloroplast stroma. The complex consists of eight large (56 kDa) subunits, which contain both catalytic and regulatory domains, and eight small subunits (14 kDa), which enhance the catalytic function of the L subunits ( Figure 17 A). The carboxylation reaction carried out by Rubisco is highly exergonic (Δ G °=−51.9 kJ·mol- 1 ), yet kinetically very slow (just 3 s −1 ) and begins with the protonation of ribulose 1,5-bisphosphate to form an enediolate intermediate which can be combined with CO 2 to form an unstable 6C intermediate that is quickly hydrolysed to yield two 3C 3-phosphoglycerate molecules. The active site in the Rubisco enzyme contains a key lysine residue, which reacts with another (non-substrate) molecule of CO 2 to form a carbamate anion that is then able to bind Mg 2+ . The Mg 2+ in the active site is essential for the catalytic function of Rubisco, playing a key role in binding ribulose 1,5-bisphosphate and activating it such that it readily reacts with CO 2.. Rubisco activity is co-ordinated with that of the light reactions since carbamate formation requires both high Mg 2+ concentration and alkaline conditions, which are provided by the light-driven changes in the stromal environment discussed above ( Figure 17 B).

( A ) Structure of the Rubisco enzyme (the large subunits are shown in blue and the small subunits in green); four of each type of subunit are visible in the image. Drawn from PDB code 1RXO. ( B ) Activation of the lysine residue within the active site of Rubisco occurs via elevated stromal pH and Mg 2+ concentration as a result of the activity of the light reactions.
In addition to carboxylation, Rubisco also catalyses a competitive oxygenation reaction, known as photorespiration, that results in the combination of ribulose 1,5-bisphosphate with O 2 rather than CO 2 . In the oxygenation reaction, one rather than two molecules of 3-phosphoglycerate and one molecule of a 2C sugar known as phosphoglycolate are produced by Rubisco. The phosphoglycolate must be converted in a series of reactions that regenerate one molecule of 3-phosphoglycerate and one molecule of CO 2 . These reactions consume additional ATP and thus result in an energy loss to the plant. Although the oxygenation reaction of Rubisco is much less favourable than the carboxylation reaction, the relatively high concentration of O 2 in the leaf (250 μM) compared with CO 2 (10 μM) means that a significant amount of photorespiration is always occurring. Under normal conditions, the ratio of carboxylation to oxygenation is between 3:1 and 4:1. However, this ratio can be decreased with increasing temperature due to decreased CO 2 concentration in the leaf, a decrease in the affinity of Rubisco for CO 2 compared with O 2 and an increase in the maximum rate of the oxygenation reaction compared with the carboxylation reaction. The inefficiencies of the Rubisco enzyme mean that plants must produce it in very large amounts (∼30–50% of total soluble protein in a spinach leaf) to achieve the maximal photosynthetic rate.
CO 2 -concentrating mechanisms
To counter photorespiration, plants, algae and cyanobacteria have evolved different CO 2 -concentrating mechanisms CCMs that aim to increase the concentration of CO 2 relative to O 2 in the vicinity of Rubisco. One such CCM is C 4 photosynthesis that is found in plants such as maize, sugar cane and savanna grasses. C 4 plants show a specialized leaf anatomy: Kranz anatomy ( Figure 18 ). Kranz, German for wreath, refers to a bundle sheath of cells that surrounds the central vein within the leaf, which in turn are surrounded by the mesophyll cells. The mesophyll cells in such leaves are rich in the enzyme phosphoenolpyruvate (PEP) carboxylase, which fixes CO 2 into a 4C carboxylic acid: oxaloaceatate. The oxaloacetate formed by the mesophyll cells is reduced using NADPH to malate, another 4C acid: malate. The malate is then exported from the mesophyll cells to the bundle sheath cells, where it is decarboxylated to pyruvate thus regenerating NADPH and CO 2 . The CO 2 is then utilized by Rubisco in the Calvin cycle. The pyruvate is in turn returned to the mesophyll cells where it is phosphorylated using ATP to reform PEP ( Figure 19 ). The advantage of C 4 photosynthesis is that CO 2 accumulates at a very high concentration in the bundle sheath cells that is then sufficient to allow Rubisco to operate efficiently.

Plants growing in hot, bright and dry conditions inevitably have to have their stomata closed for large parts of the day to avoid excessive water loss and wilting. The net result is that the internal CO 2 concentration in the leaf is very low, meaning that C 3 photosynthesis is not possible. To counter this limitation, another CCM is found in succulent plants such as cacti. The Crassulaceae fix CO 2 into malate during the day via PEP carboxylase, store it within the vacuole of the plant cell at night and then release it within their tissues by day to be fixed via normal C 3 photosynthesis. This is termed crassulacean acid metabolism (CAM).
Acknowledgments
I thank Professor Colin Osborne (University of Sheffield, Sheffield, U.K.) for useful discussions on the article, Dr Dan Canniffe (Penn State University, Pennsylvania, PA, U.S.A.) for providing pure pigment spectra and Dr P.J. Weaire (Kingston University, Kingston-upon-Thames, U.K.) for his original Photosynthesis BASC article (1994) on which this essay is partly based.
Abbreviations
This article is a reviewed, revised and updated version of the following ‘Biochemistry Across the School Curriculum’ (BASC) booklet: Weaire, P.J. (1994) Photosynthesis . For further information and to provide feedback on this or any other Biochemical Society education resource, please contact [email protected]. For further information on other Biochemical Society publications, please visit www.biochemistry.org/publications .
Competing Interests
The Author declares that there are no competing interests associated with this article.
Recommended reading and key publications
- Blankenship R.E. Early evolution of photosynthesis. Plant Physiol. 2010; 154 :434–438. doi: 10.1104/pp.110.161687. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blankenship R.E. Molecular Mechanisms of Photosynthesis. Chichester: Wiley–Blackwell Publishing; 2014. [ Google Scholar ]
- Nelson N., Ben Shem A. The complex architecture of oxygenic photosynthesis. Nat. Rev. 2004; 5 :1–12. doi: 10.1038/nrm1525. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raines C. The Calvin cycle revisited. Photosynth. Res. 2003; 75 :1–10. doi: 10.1023/A:1022421515027. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ruban A.V. Evolution under the sun: optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015; 66 :7–23. doi: 10.1093/jxb/eru400. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sage R.F. The evolution of C 4 photosynthesis. New Phytol. 2004; 161 :341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
10. Explain the significance of photosynthesis?
Significance of photosynthesis are as follows:- 1. provide food:- photosynthesis helps to prepare food in plants and they prepare their food not only for themselves but all living beings which are lived on this earth because each and every organism is dependent on plants, either directly or indirectly. photosynthesis play vital role for the growth and sustenance of the biosphere. 2. provide oxygen:- photosynthesis is the only process by which oxygen is evolved which is used by living organisms photosynthesis in plants is necessary to maintain the oxygen levels in the atmosphere..

Write two significances of photosynthesis.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 March 2024
Keto-anthraquinone covalent organic framework for H 2 O 2 photosynthesis with oxygen and alkaline water
- Xiangcheng Zhang 1 ,
- Silian Cheng 1 ,
- Chao Chen ORCID: orcid.org/0000-0002-7083-2801 2 ,
- Xue Wen 1 ,
- Jie Miao 1 ,
- Baoxue Zhou ORCID: orcid.org/0000-0001-9691-3119 1 ,
- Mingce Long ORCID: orcid.org/0000-0002-5168-8330 1 &
- Lizhi Zhang ORCID: orcid.org/0000-0002-6842-9167 1
Nature Communications volume 15 , Article number: 2649 ( 2024 ) Cite this article
861 Accesses
Metrics details
- Catalyst synthesis
- Photocatalysis
Hydrogen peroxide photosynthesis suffers from insufficient catalytic activity due to the high energy barrier of hydrogen extraction from H 2 O. Herein, we report that mechanochemically synthesized keto-form anthraquinone covalent organic framework which is able to directly synthesize H 2 O 2 (4784 μmol h −1 g −1 at λ > 400 nm) from oxygen and alkaline water (pH = 13) in the absence of any sacrificial reagents. The strong alkalinity resulted in the formation of OH - (H 2 O) n clusters in water, which were adsorbed on keto moieties within the framework and then dissociated into O 2 and active hydrogen, because the energy barrier of hydrogen extraction was largely lowered. The produced hydrogen reacted with anthraquinone to generate anthrahydroquinone, which was subsequently oxidized by O 2 to produce H 2 O 2 . This study ultimately sheds light on the importance of hydrogen extraction from H 2 O for H 2 O 2 photosynthesis and demonstrates that H 2 O 2 synthesis is achievable under alkaline conditions.
Similar content being viewed by others

Ultra-fast green hydrogen production from municipal wastewater by an integrated forward osmosis-alkaline water electrolysis system
Gabriela Scheibel Cassol, Chii Shang, … Li Ling
Cage escape governs photoredox reaction rates and quantum yields
Cui Wang, Han Li, … Oliver S. Wenger
Phenol as proton shuttle and buffer for lithium-mediated ammonia electrosynthesis
Xianbiao Fu, Aoni Xu, … Ib Chorkendorff
Introduction
Hydrogen peroxide (H 2 O 2 ), a chemical with increasing market share, finds extensive applications in biomedicine, disinfection, bleaching, organic synthesis, and water treatment 1 , 2 , 3 , 4 . The well-known industrial production of H 2 O 2 is the anthraquinone (AQ) process, which suffers from intensive energy consumption and waste discharge 5 . As a green and carbon-neutral alternative, solar driven oxygen reduction strategy of H 2 O 2 synthesis from molecular oxygen and water attracts more and more attention 6 , 7 , 8 , 9 . Although many photocatalysts are effective for the H 2 O 2 synthesis, high dosages of organic sacrificial reagents such as isopropanol are always used to scavenge photogenerated holes and offer hydrogen for the H 2 O 2 formation, which obviously bring in undesired impurity and also increase the cost of H 2 O 2 synthesis 10 . In comparison with organic sacrificial reagents, water is more inexpensive and convenient hydrogen source, but an intrinsically poor hydrogen donor, because water molecules have a high O-H bond dissociation energy (BDE, 492 kJ mol −1 for homolytic cleavage) 11 , 12 . Thus, highly efficient H 2 O 2 photosynthesis only with molecular oxygen and water is of great significance, but remains a giant challenge.
It is well known that the hydrogen-bond (H-bond) in adsorbed water clusters plays critical role on water dissociation during photocatalysis 13 , 14 . At a “pseudodissociated” state 14 , the intermolecular H-bond facilitates the cleavage of water O-H bond at <1 monolayer coverage 15 , and interface H-bond also promotes photogenerated hole transfer and water oxidation by the strongly coupling of H-bond with holes 16 . Unfortunately, strong hydrogen bond network among water clusters inhibits the water dissociation 17 . Thus, an accurate control of H-bond network and adsorbed water monolayers over photocatalysts is vital for water dissociation 13 . Recently, scientists found that excess electrons of OH - anions in alkaline water could induce reorganization of hydrogen bond in water clusters, thus further diminishing the overall energy barrier of alkaline hydrogen evolution reaction (HER) 18 . However, it is still unknown whether this alkaline based H-bond network manipulation strategy is feasible for the H 2 O 2 photosynthesis.
Different from traditional metal oxide photocatalysts of poor interfacial H-bond modulation capacity, covalent organic frameworks (COFs), famous metal-free molecular photocatalysts possessing huge potential in H 2 O 2 photosynthesis, are very powerful to regulate H-bond at molecular levels because of their variable and designable organic units 19 , 20 . Among various organic units, AQ moieties is believed to be the optimal redox center for the H 2 O 2 synthesis, as the oxidation of the hydrogenated AQ (anthrahydroquinone, HAQ) by molecular oxygen can selectively produce H 2 O 2 , which is thermodynamically spontaneous and commercially used 5 . Recently, several AQ-containing COFs (such as TPE-AQ, TpAQ, AQTEE-COP, and AQTT-COP) were designed to promote photogenerated charge separation and facilitate WOR for efficient H 2 O 2 photosynthesis with pure water upon visible light irradiation (>400 nm), and their best activity reached 3221 μmol g −1 h −1 without manipulating H-bond network 21 , 22 , 23 , 24 .
As a typical AQ-containing COFs, TpAQ synthesized by β-ketoenamines links of 2,6-diaminoanthraquinone (AQ) and 2, 4, 6-triformylphloroglucinol (Tp), is often a mixture of keto- and enol- forms due to the formation of tautomerism during the polymerization (Fig. S 1 ). Different from the unstable enol-form that mainly form weak H-bond with oxygen in H 2 O 25 , keto-form AQ COFs (Kf-AQ) is a more favorable proton acceptor to combine with hydrogen in H 2 O via strong H-bond. Generally, traditional solvothermal method with acetic acid catalysis tends to produce enol-form dominant COFs. Although alkaline solution (such as OH − ) induces the transformation of enol-form into keto-form 26 , the NaOH addition disfavored the solvothermal synthesis of Kf-AQ, because the excessive solvents would consume NaOH to form carboxylates. Thus, the controlled synthesis of Kf-AQ is crucial for H 2 O 2 photosynthesis, but never reported previously.
Herein we demonstrate the mechanochemical synthesis of keto-form anthraquinone covalent organic framework (Kf-AQ) for direct H 2 O 2 photosynthesis with molecular oxygen and alkaline water (pH = 13), and this Kf-AQ could deliver a record H 2 O 2 production rate of 4784 μmol h −1 g −1 in the absence of any sacrificial reagents under visible light irradiation (λ > 400 nm). The critical roles of hydroxide anions and keto-form AQ moieties for efficient H 2 O 2 production are carefully clarified via in-situ characterization and theory calculations.
Results and discussion
Synthesis and structure characterization.
Kf-AQ was mechanochemically synthesized by a Schiff-base condensation reaction of Tp and AQ with CH 3 COONa (NaAc) as the catalysts (Fig. 1a ). Fourier-transformed infrared spectra (FT-IR) spectra clearly revealed a new C-N stretching band at 1260 cm −1 and a disappeared N-H stretching band at 3459~3151 cm −1 for NH 2 groups in AQ (Fig. S 2 ) 27 . The as-prepared Kf-AQ powder displays a red-black color, corresponding to its wide optical absorption with the edge extended to 900 nm (Fig. S 3 ), which is obviously red-shifted as compared to the absorption edge at 780 nm of TpAQ prepared by a traditional solvothermal method 28 . The simulated powder X-ray diffraction (PXRD) pattern of Kf-AQ with eclipsed AA stacking mode, whose fractional atomic coordinate data for the unit cell were presented in Table S1 , agreed with the experimental data in a large extent (Fig. 1b ), suggesting the validity of such structure in Kf-AQ. Particularly, the broad peak at 26.54 o was caused by the strong π-π stacking construction arisen from the existence of a multilayered COF structure with an interlayer distance of 3.48 Å. TEM and SEM images also displayed that Kf-AQ had a lamellar stacking structure and excellent crystallinity with an observable 0.33 nm lattice spacing (Fig. 1c, d and Fig. S 4 ) 28 ,very close to the simulated interlayer spacing (0.348 nm). Moreover, Kf-AQ had a specific surface area of 141.7 m 2 g −1 and a pore size of 2.2 nm (Fig. S 5 ), which was well matched with the simulated value (2.28 nm) in Fig. 1a .
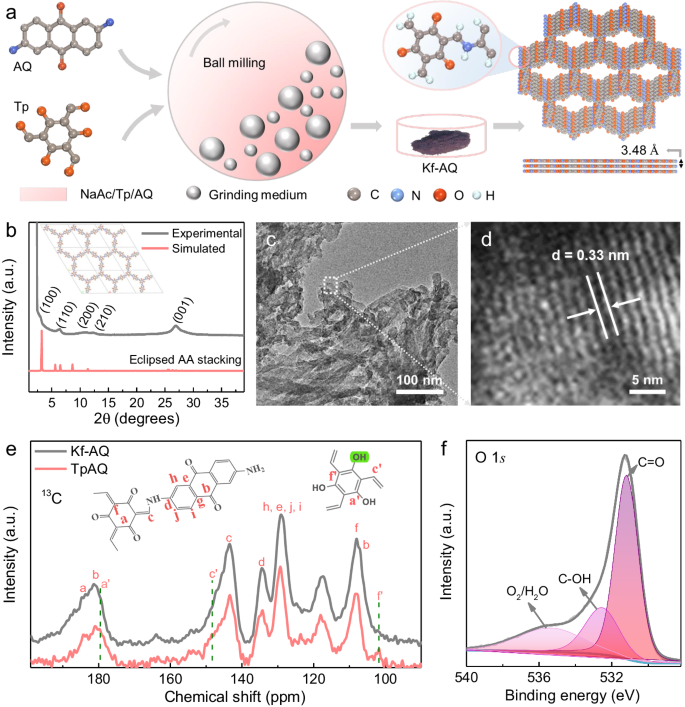
a Schematic of Kf-AQ condensation. b PXRD patterns of Kf-AQ, experimentally observed (dark) and simulated using eclipsed AA-stacking (red), the inset is the crystal structures of the eclipsed AA stacking model, the simulated cell parameters of ( a , b and c ) are 30.59, 30.59 and 3.51 Å, respectively. c , d TEM images of Kf-AQ. e 13 C CP-MAS solid state NMR spectra of Kf-AQ and TpAQ. f High-resolution O1 s XPS spectra for Kf-AQ.
Solid-state NMR spectra revealed that Kf-AQ had an almost exclusive keto-form structure (Fig. 1e ). The chemical shifts at 184 ppm, 144 ppm and 109 ppm in 13 C NMR spectra were all indexed to the keto-form structure of Kf-AQ 25 , 27 , 29 , while the chemical shift at 179 ppm (a’) and 101 ppm (f’) for the enol-form carbon and at 4.4 ppm for the enol-form hydrogen (c, C-OH) was absent in the 13 C and 1 H NMR spectra, respectively (Fig. 1e and Fig. S 6 ) 30 . Only C, O and N elements were present in the XPS survey spectrum of Kf-AQ, without any residual Na (Fig. S 7 ). More evidences of keto-form structure in Kf-AQ could be found in high resolution XPS spectra (Fig. S 8 ). The content of C=O in Kf-AQ was approximately 60%, about three times that of C-OH (22%) (Fig. 1f ). The content of C-N-H (56%), corresponding to the deconvolution peak at the binding energy of 400.4 eV in N 1 s spectra, was obviously higher than that of C = N (31%) (Fig. S 9a ) 31 , while C 1 s XPS spectra also illustrated more C-C (52%) and less C-O (38%) in Kf-AQ (Fig. S 9b ) 32 . All these above results supported the successful synthesis of an exclusive keto-form AQ COF.
The formation of Kf-AQ might be attributed to a NaAc-catalyzed Schiff-base condensation process as follows. Upon the heat generated from the collision of balls, the carbonyl oxygen on Tp monomer undergoes a nucleophilic addition with Na + to form aldehyde salts 33 , 34 , resulting in the neighbor carbon acquiring a positive charge to fulfill another nucleophilic addition with nitrogen atoms in AQ. The generated α-hydroxyl undergoes further dehydration with adjacent amino hydrogen to form an enol-form COF. Subsequently, Ac - anions as the Lewis base tend to bind with the hydrogen of hydroxyl group in enol moieties and then induce electron transfer from oxygen to alkene group, thus enabling the enol-form transformation into the thermodynamically more stable keto-form moiety (Fig. S 10 ). Such a transformation cannot be driven in the traditional solvothermal synthesis, but might partially occur in alkaline water to produce a keto-form dominated AQ COF 25 , 35 .
Efficient H 2 O 2 photosynthesis
The H 2 O 2 photosynthesis performance of Kf-AQ was evaluated by dispersing the powder in water at neutral and alkaline solutions (pH = 9, 11, 13, 14) with continuous O 2 purging. Upon visible light (λ > 400 nm) irradiation, the rate of H 2 O 2 production at pH 13 reached as high as 4784 μmol h −1 g −1 (Fig. 2a ), a record in H 2 O 2 photosynthesis of AQ containing COFs with water (Fig. 2b ) 21 , 22 , 23 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 . Upon a prolonged irradiation for 5 h, the H 2 O 2 production was steadily growing (Fig. S 11 ), and kept constant during five cycles of reaction (Fig. 2c ). The crystal structure and surface functional groups of the reacted Kf-AQ did not change (Fig. S 12 ), demonstrating its excellent stability for the H 2 O 2 photosynthesis. The contribution of Na + to the enhanced H 2 O 2 production was ruled out by the replacement of NaOH with NaCl and KOH (Fig. S 13 ), confirming the crucial promoting effect of hydroxide anions on the H 2 O 2 photosynthesis of Kf-AQ.
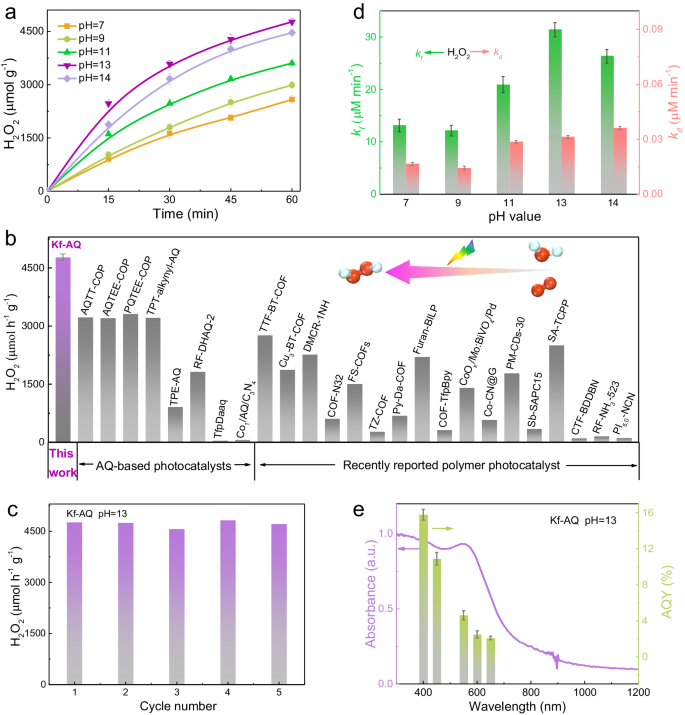
a Photocatalytic H 2 O 2 production at different pH conditions. Error bars are the standard deviations of three replicate measurements. b A comparison of photocatalytic H 2 O 2 production rates for photocatalysts in the absence of sacrificial regents. c The recycling tests of Kf-AQ at pH = 13. d The rate constants of H 2 O 2 formation ( k f ) and decomposition ( k d ). e Wavelength-dependent AQY of photocatalytic H 2 O 2 production on Kf-AQ at pH = 13.
The kinetic of H 2 O 2 production was analyzed by fitting the time-dependent H 2 O 2 production curves (Text S2). Kf-AQ exhibited the highest H 2 O 2 formation rate constant ( k f , 31.39 μM min −1 ), but a medium decomposition rate constant ( k d ) (0.031 μM min −1 ) at pH 13 (Fig. 2d and Table S2 ). Thus, the high H 2 O 2 photosynthesis performance of Kf-AQ with alkaline water was mainly attributed to its better H 2 O 2 formation ability. The apparent quantum efficiencies (AQY) of Kf-AQ at different wavelengths were well matched with its absorption spectrum, and the highest value appeared at 400 nm and reached 15.8% (Fig. 2e and Table S3 ). To the best of our knowledge, the AQY of Kf-AQ is higher than those of most reported H 2 O 2 synthesis photocatalysts 57 , 58 . The solar-to-chemical conversion (SCC) efficiency of Kf-AQ was estimated to be 0.70% at pH 13 (Fig. S 14 and Table S4 ), which was almost seven times of the average solar-to-biomass conversion (SBC) efficiency in nature 23 .
Mechanism investigation
We first checked the basic semiconductor properties of Kf-AQ to understand its high performance in H 2 O 2 photosynthesis. The Tauc plot showed that the band gap of Kf-AQ was 1.55 eV (Fig. S 15a ), and ultraviolet photoelectron spectroscopy (UPS) determined its valence band potential ( E VB ) as 1.90 V (Fig. S 15b ), suggesting that the conduction band potential ( E CB ) of Kf-AQ was accordingly calculated as 0.35 V. Therefore, both 2e − ORR (0.68 V vs. RHE) to produce H 2 O 2 and 4e − WOR (1.23 V vs. RHE) to evolute O 2 were thermodynamically feasible for Kf-AQ photocatalysis (Fig. 3a ) 59 . We further conducted density functional theory (DFT) calculations to elucidate the exciton dissociation in photocatalysis by using the dimer models of Kf-AQ and enol-form TpAQ. As depicted in Fig. 3b , the highest occupied molecular orbital (HOMO) of Kf-AQ dimer uniformly disperses in the whole structure, while the lowest unoccupied molecular orbital (LUMO) mainly localized at AQ units, without any residual LUMO on the benzene ring of the Tp moiety. Thus, the HOMO-LUMO transition under excitation can redistribute electron density from the Tp moieties to the adjacent AQ units, thus resulting in effective intramolecular charge transfer in Kf-AQ. In the contrast, the HOMO of enol-form TpAQ is uniformly distributed on the dimer, while the LUMO mainly localizes at the AQ moiety and overlaps with the HOMO, with a portion of LUMO remaining on the benzene ring of the Tp moiety (Fig. S 16 ). These results indicate that Kf-AQ is more favorable for the separation and transfer of photogenerated charges. The fluorescence spectra of TpAQ and Kf-AQ provided further evidence for their charge separation performance. Kf-AQ displayed much weaker fluorescence intensity than TpAQ in the steady-state fluorescence spectra (Fig. S 17a ), and the time-resolved fluorescence analysis showed that Kf-AQ had a longer relaxation time of electrons (6.82 ns) than TpAQ (6.07 ns) (Fig. S 17b ), thus proving the better performance of Kf-AQ in photogenerated-charge separation.
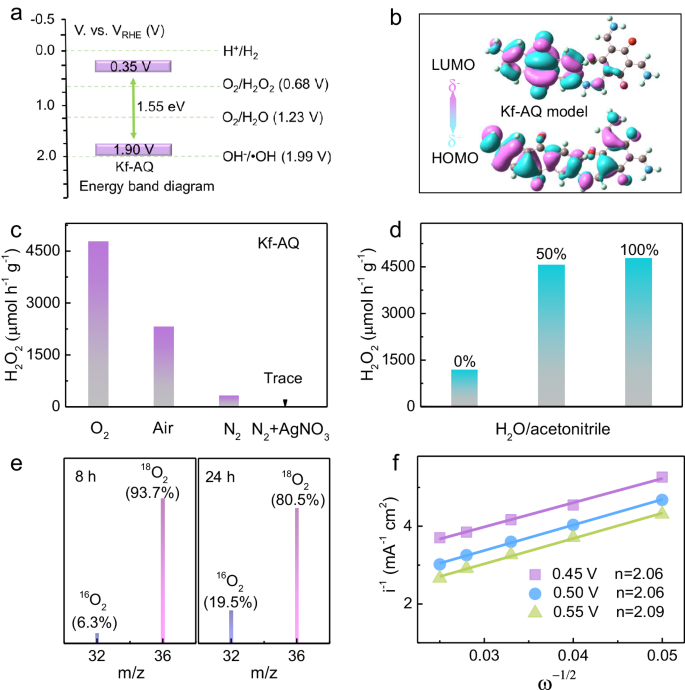
a Band edge potentials of Kf-AQ. b Calculated HOMO and LUMO for Kf-AQ dimer. c , d A comparison on H 2 O 2 production rates over Kf-AQ in different atmospheres and solutions. e Mass charts for O 2 evolved by decomposition of H 2 O 2 produced at pH 13 by isotopic experiments. f The Koutecky-Levich plots of Kf-AQ obtained by RDE measurements.
We then explored the sources of H and O for the H 2 O 2 production by various control experiments and isotopic labeling analysis. In comparison to oxygen atmosphere, either air or N 2 purging resulted in poor H 2 O 2 production (Fig. 3c ), and the O 2 concentration in an airtight oxygen saturated suspension decreased obviously during photocatalysis (Fig. S 18 ), suggesting the dominated contribution of ORR to the H 2 O 2 production. AgNO 3 was added as the electron scavenger in N 2 atmosphere to evaluate the contribution of water oxidation. The negligible amount of H 2 O 2 generated in the Kf-AQ suspension ruled out the direct contribution of WOR to the H 2 O 2 production. However, H 2 O 2 was obviously produced in case of N 2 purging and absence of AgNO 3 , suggesting that photocatalytically produced O 2 via 4e - WOR (Fig. 3c ) enabled the consequent ORR to produce H 2 O 2 (Fig. S 19 ). Significant H 2 O 2 was only detected in the mixed solution of H 2 O and acetonitrile ( v/v = 1:1) other than pure acetonitrile (Fig. 3d ), confirming that water was the exclusive hydrogen source for the H 2 O 2 photosynthesis.
We conducted the isotopic photoreaction experiments by purging H 2 16 O suspensions with 18 O 2 gas during the H 2 O 2 photosynthesis, and then used MnO 2 to catalytically decompose the as-synthesized H 2 O 2 into oxygen. After 8 h of photoreaction, strong 18 O 2 ( m/z = 36, 93.7%) and very weak 16 O 2 ( m/z = 32, 6.3%) signals appeared in the gas chromatography-mass spectra (GC-MS) of collected gas (Fig. 3e ), demonstrating that H 2 18 O 2 was the dominated product and mainly came from the reduction of 18 O 2 . Gradually, the signal of 18 O 2 peak decreased (80.5%), accompanying with an increased 16 O 2 signal (19.5%) at 24 h of reaction, because the photocatalytic oxidation of H 2 16 O produced 16 O 2 to increase the proportion of H 2 16 O 2 in the products. The electrons transfer number ( n ) of ORR was further measured to be about 2.06 ~ 2.09 by the RDE method (Figs. 3f and S 20 ) 24 . Thus, we suppose that both 2e - ORR and 4e - WOR take place during H 2 O 2 photosynthesis over Kf-AQ at pH 13.
To probe the active sites of Kf-AQ for the H 2 O 2 photosynthesis, we synthesized two control COFs by respectively replacing the monomers of Tp and AQ with 1,3,5-trimethylbenzaldehyde (LZU) and 2,6-diaminoanthracene (DA), namely LZUAQ and TpDA (Figs. S 21 , S 22 ). Their H 2 O 2 photosynthesis performance was much worse than that of Kf-AQ (Fig. S 23 ), suggesting that anthraquinone groups were the indispensable active sites for ORR, and the keto and AQ conjugated configuration accounted for the efficient WOR over Kf-AQ.
We then employed in-situ FTIR and Raman spectra to further understand the critical role of water adsorption and dehydrogenation in the H 2 O 2 photosynthesis of Kf-AQ. Upon irradiation, three obvious O-H stretching vibration bands appeared in the in-situ FTIR spectra of Kf-AQ (Fig. 4a ), corresponding to the water clusters including Na + (H 2 O) 3 or OH - (H 2 O) 3 (3540 cm −1 ), OH - (H 2 O) 4 (3410 cm −1 ), and OH - (H 2 O) 5 (3292 cm −1 ) 60 , 61 . These adsorbed water clusters were the proton precursors for H 2 O 2 photosynthesis, which can be further checked by in-situ Raman spectra. The notable O-H stretching bands in Raman spectra at around 3000–3700 cm −1 can be deconvoluted into three bands, corresponding to the four-coordinated hydrogen bonded water network (V 1 , ~3254 cm −1 ), the two-coordinated single donor hydrogen bonded water clusters (V 2 , ~3420 cm −1 ) and the Na + ion hydrated water (Na ⋅ H 2 O) clusters (V 3 , ~3553 cm −1 ), respectively (Fig. 4b ) 62 , 63 , 64 . The intensity of these bands for Kf-AQ was significantly higher than those for TpAQ, suggesting the formation of stronger hydrogen bond between keto moiety (-C=O) and OH - (H 2 O) n clusters 65 , possibly because the vibrational dipole moment (the direction of O-H bonds) in the clusters (such as Na ⋅ H 2 O) is parallel to the direction of the interfacial electric field, thus favoring the combination of hydrogen in the clusters with carbonyl groups of Kf-AQ. Simultaneously, V 2 and V 3 were the dominant forms in the Kf-AQ Raman spectrum, and generally had relatively weaker hydrogen bond network than V 1 , the dominant form in the TpAQ Raman spectrum. These differences can be attributed to the strong interaction between water clusters and carbonyl groups of Kf-AQ, resulting in the disorder and stretching of H-bonds in the arrangement of water molecules 66 , and the strong dipole-dipole force between Na + and H 2 O molecules in the Na + solvation structures further destroy the water-water interactions to form small water clusters of weak H-bonding environment 66 , thus favoring the photocatalytic dissociation of water and release of hydrogen. Therefore, the exclusive keto-form of Kf-AQ enhanced the adsorption and dissociation of water, thereby promoting hydrogen abstraction from water for the H 2 O 2 photosynthesis.
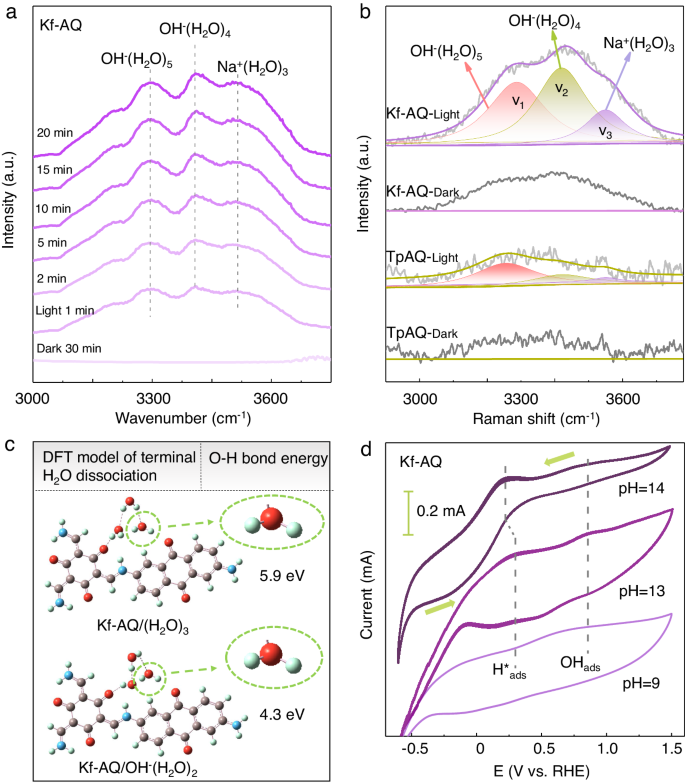
a In-situ FTIR spectra of Kf-AQ suspension for H 2 O 2 photosynthesis. b Raman spectra of Kf-AQ and TpAQ suspensions under visible light irradiation. c The H-O bond energy of the adsorbed terminal H 2 O over the Kf-AQ via DFT calculation. d Cyclic voltammogram (CV) curves of Kf-AQ in different pH electrolytes.
We compared water adsorption over Kf-AQ and enol-form TpAQ by DFT calculations. In case of one water molecule adsorption, the adsorption energy of Kf-AQ was −0.26 eV, much lower than that of enol-form TpAQ (−0.18 eV) (Fig. S 24 ). Increasing water cluster sizes to (H 2 O) 3 , the adsorption energy of Kf-AQ decreased to −0.35 eV, and further decreased to −0.44 eV for the OH - (H 2 O) 2 clusters, which was the dominant form of adsorbed water in alkaline water (Fig. S 25 ), suggesting the strong water adsorption capability of Kf-AQ. Moreover, the bond energy of terminal H-O in OH - (H 2 O) 2 form was 4.3 eV, much lower than that of (H 2 O) 3 (5.9 eV) (Fig. 4c ), suggesting the easier hydrogen dissociation from the terminal water, and thus favoring the subsequently combination with the neighboring H 2 O to form hydronium ion (H 3 O + ) 18 .
We detected the intermediates of H * ads and OH ads species by the cyclic voltammogram (CV) (Fig. 4d ). The H * ads species generated in the reduction stage by reducing hydronium ion (H 3 O + ) were oxidized, corresponding to an oxidative peak at about 0.25 V vs. RHE 67 , 68 . The oxidative peaks in the CV curves were more distinct with the increase of hydroxide anion concentrations, suggesting the increase of H * ads dosages at strong alkaline conditions 18 . Simultaneously, the reduction peak at 0.77 V was attributed to the reversible adsorbed OH ads species, which were produced via the loss of electrons in OH − (ref. 69 ). The OH ads species would be stabilized by forming hydroxyl-water-alkali metal cation cluster (OH ads -Na + -(H 2 O) n ), thus accordingly preventing its depletion by H 3 O + . Therefore, we propose that the dissociation of H 2 O into H * ads and OH ads species takes place in the 2e − ORR and 4e - WOR pathways.
These above results strongly suggest a synergism of keto and anthraquinone moieties in Kf-AQ for superior H 2 O 2 photosynthesis from water and oxygen, as depicted in Fig. 5 . Initially, OH − (H 2 O) n clusters preferentially adsorbs onto the keto-form moieties in Kf-AQ, thus weakening the H-O bond of the terminal H 2 O via forming the H-OH(H 2 O) n-1 OH − clusters and facilitating the dehydrogenation in water molecules. The detached protons then combine with the neighboring H 2 O to form H 3 O + , which was proved by the in-situ FTIR spectra of Kf-AQ under alkaline condition (Fig. S 26 ), as the absorption band at 3525 cm −1 for the stretching vibrations of the O-H group in H 3 O + was progressively intensified 69 , 70 . Upon visible light irradiation, surface H 3 O + on Kf-AQ can be reduced by interfacial electrons (e − ) to release H * ads species, which preferentially bind with the quinone groups (-C=O) in AQ and subsequently hydrogenate AQ to yield anthrahydroquinone (H 2 AQ). Afterwards, the parahydrogen atoms of H 2 AQ are abstracted to produce radicals, which react with O 2 to form 1,4-endoperoxide species, a well-known intermediate for the formation of H 2 O 2 , which was confirmed by the new Raman peak at 891 cm −1 (Fig. S 27 ). Then, 1,4-endoperoxide species couples the adjacent hydrogen in the hydroxyl group of H 2 AQ to release H 2 O 2 . Meanwhile, another dissociation product, OH ads intermediate, would not be dissociated as OH − within the interface layer, but form an adsorbed hydroxyl-water-alkali metal cation cluster (OH ads -Na + -(H 2 O) n ) 69 . Upon visible light irradiation, the photogenerated holes (h + ) oxidizes this OH ads to produce O 2 in a 4e − WOR pathway. Therefore, the formation of OH ads -Na + -(H 2 O) n and H 3 O + intermediates over Kf-AQ at high pH conditions facilitates water oxidation and hydrogen extraction from H 2 O molecules, resulting in its efficient photocatalytic H 2 O 2 production.
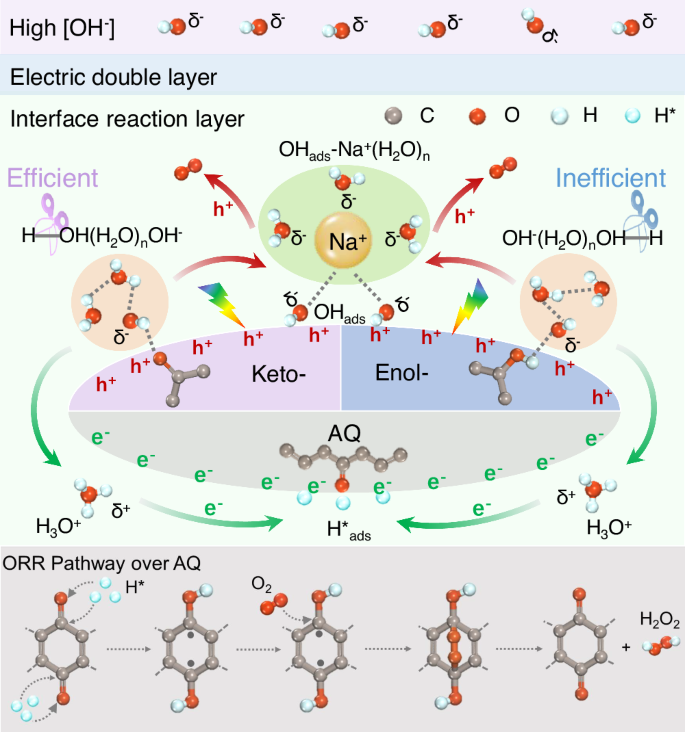
The photocatalytic pathway for H 2 O 2 production over Kf-AQ in alkaline conditions.
In summary, we have demonstrated the synthesis of a keto-form anthraquinone containing COF via a mechanochemical process and its efficient H 2 O 2 photosynthesis in alkaline water, with a record H 2 O 2 production rate of 4784 μmol h −1 g −1 under visible light irradiation in the absence of sacrificial reagents. The keto-form structure in Kf-AQ can promote the water adsorption through the formation of OH − (H 2 O) n clusters with weakened hydrogen bonds, which accordingly enhances the dehydrogenation of water and promotes efficient H 2 O 2 photosynthesis. The manipulating H-bond network of adsorbed water clusters represents a strategy to break the rate-limiting step of hydrogen extraction from water, and bring insights for the design of highly active photocatalysts to realize efficient H 2 O 2 photosynthesis from only water and oxygen.
Synthesis of Kf-AQ
Mechanochemical synthesis was conducted to produce Kf-AQ by use of a planetary ball mill (SFM-1, Hefei Kejing Material Technology Co., Ltd). Typically, 2,4,6-triformylphloroglucinol (Tp, 126 mg, 0.20 mmol), 2,6-diaminoanthraquinone (AQ, 213 mg, 0.30 mmol), and CH 3 COONa (5 mg) were placed in a 50 mL agate grinding jar, with fifteen 7 mm diameter and ten 5 mm diameter agate balls. Then, the mixture was ground at room temperature with a rotation speed of 400 rpm for 6 h. After that, the obtained precursors were washed with N, N-dimethylformamide and acetone, and then dried in a vacuum oven at 120 o C for 12 h. The obtained photocatalyst was denoted as Kf-AQ.
Synthesis of TpAQ
TpAQ was synthesized by Schiff-base condensation of Tp and AQ according to a modified previous method 28 . In a 10 mL Schlenk tube, 2,4,6-triformylphloroglucinol (71.4 mg, 0.20 mmol) and 2,6-diaminoanthraquinone (42.1 mg 0.30 mmol) were charged. Then, N, N-dimethylacetamide (2.0 mL) was added as the solvent, and the suspensions were sonicated for 10 min. Subsequently, 0.3 mL glacial acetic acid was added. After that, the ampoule was degassed by freeze-pump-thaw three times and then sealed on. The Schlenk tube was put into an oven and heated at 120 o C for 72 h. The obtained powder was washed with N, N-dimethylformamide and acetone, and then dried in a vacuum oven at 120 o C for 12 h.
H 2 O 2 photosynthesis
H 2 O 2 photosynthesis was conducted in a homemade quartz cuvette reactor. Generally, 5 mg of the photocatalysts were ultrasonically dispersed into 30 mL water whose initial pH was adjusted by 0.1 M NaOH solution. Then, the suspension was stirred for 15 min in the dark with continuously O 2 purging. After that, the reactor was illuminated by a 300 W Xe lamp (PLS-SXE300, Beijing Perfectlight) with a cut-off filter (λ > 400 nm). During the reaction, 1.5 mL of reaction mixture was withdrawn at every 15 min interval, and then filtrated through a 0.45 μm polyether sulfone (PES) filter for H 2 O 2 detection.
The H 2 O 2 concentration was measured by the N, N-diethylp-phenylenediamine (DPD)-horseradish peroxidase (POD) colorimetry method 71 . Typically, 3.0 mL of phosphate buffer (0.2 M, pH = 6) was added into a 15.0 mL colorimetric tube, and then 1.0 mL sample, 50 μL DPD, 50 μL POD were added into the mixture. Then, ultrapure water was added to set the volume to 10 mL. Finally, the absorbance was measured on a UV-vis spectrophotometer (TU-1810) at 551 nm to determine H 2 O 2 concentration according to the predetermined standard curve.
In-situ FTIR measurements
In-situ FTIR spectra were obtained by using a Thermo Scientific Nicolet Is50, equipped with a commercial chamber from Harrick Scientific. Typically, 5 mg of Kf-AQ was dispersed into 30 mL H 2 O at pH = 13. The formed uniform dispersion was bubbled with O 2 for 15 min in the dark, and then the background spectrum was collected. After that, the reaction chamber was irradiated by visible light (λ > 400 nm), and then the spectrum was collected at a one min interval.
Isotopic experiments
Specifically, 5 mg Kf-AQ was added into H 2 16 O (30 mL) within a glass tube (50 mL). The formed dispersion was sonicated for 10 min and bubbled with Ar for 30 min. Then, the reaction tube was sealed with rubber septum cap and vacuum. 18 O 2 gas was introduced to the tube by a syringe. The reaction tube was illuminated by a 300 W Xe lamp with a cut-off filter (λ > 400 nm). After photoreaction for 8 h and 24 h, the reaction solution was purged by Ar for 5 min to remove the residual 18 O 2 gas. The dispersion was filtered and injected into another clean tube, which was saturated with Ar and contained 200 mg MnO 2 powder. The generated gas was collected by an Aluminum foil air pocket (5 mL) and detected on a Shimadzu GC-MS system (Agilent 7890 A/ 5975 C).
DFT calculations
The DFT calculation used the method in previous ref. 72 . Briefly, geometry optimizations without symmetry restriction are performed by using the DFT/B3LYP/6-31 G(d, p) basis sets and scrf-smd solvent model. All calculations were performed on Gaussian 09. The enol-form TpAQ (Fig. S 28a ) and Kf-AQ (Fig. S 28b ) dimers were used as calculation models for H 2 O molecules and OH - (H 2 O)n clusters adsorption and dissociation.
The adsorption energy (Eads) of adsorbate (A, indicating H 2 O or OH − (H 2 O) n ) was defined as Eq. ( 1 ), wherein, E (A*), E (*) and E (A) are the energy of A adsorbed on the active site, the energy of active site, and the energy of isolated A, respectively. The O-H bond dissociation energy of H 2 O over Kf-AQ dimer was calculated by Eq. ( 2 ), wherein, E (Kf-AQ-H 2 O) is the energy of H 2 O adsorbed on the Kf-AQ, E (OH − ) is the energy of isolated OH - , and E (Kf-AQ-H + ) is the energy of H + adsorbed on the Kf-AQ.
Solvation has been a conscientious consideration in our study, and we employed an implicit solvation model for calculations.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Sun, X. et al. Molecular oxygen enhances H 2 O 2 utilization for the photocatalytic conversion of methane to liquid-phase oxygenates. Nat. Commun. 13 , 6677 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Shi, X. et al. Electrochemical synthesis of H 2 O 2 by two-electron water oxidation reaction. Chem 7 , 38–63 (2021).
Article CAS Google Scholar
Wang, X., Du, J. & Xu, C. Reactions in activated peroxide systems and their influences on bleaching performance. Mini-Rev. Org. Chem. 18 , 836–840 (2021).
Xu, J. et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H 2 O 2 . Nat. Sustain. 4 , 233–241 (2020).
Article PubMed PubMed Central Google Scholar
Campos-Martin, J. M., Blanco-Brieva, G. & Fierro, J. L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45 , 6962–6984 (2006).
Chen, Z., Yao, D., Chu, C. & Mao, S. Photocatalytic H 2 O 2 production systems: design strategies and environmental applications. Chem. Eng. J. 451 , 138489 (2023).
Kondo, Y., Kuwahara, Y., Mori, K. & Yamashita, H. Design of metal-organic framework catalysts for photocatalytic hydrogen peroxide production. Chem 8 , 2924–2938 (2022).
Wu, S. & Quan, X. Design principles and strategies of photocatalytic H 2 O 2 production from O 2 reduction. ACS ES&T Eng. 2 , 1068–1079 (2022).
Zeng, X., Liu, Y., Hu, X. & Zhang, X. Photoredox catalysis over semiconductors for light-driven hydrogen peroxide production. Green Chem 23 , 1466–1494 (2021).
Zhao, Y. et al. Efficient exciton dissociation in ionically interacted methyl viologen and polymeric carbon nitride for superior H 2 O 2 photoproduction. ACS Catal 5 , 2790–2801 (2023).
Article Google Scholar
Agarwal, R. G. et al. Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 122 , 1–49 (2022).
Article CAS PubMed Google Scholar
Zhang, J., Muck-Lichtenfeld, C. & Studer, A. Photocatalytic phosphine-mediated water activation for radical hydrogenation. Nature 619 , 506–513 (2023).
Ma, X. et al. Hydrogen-bond network promotes water splitting on the TiO 2 surface. J. Am. Chem. Soc. 144 , 13565–13573 (2022).
Du, Y. et al. Two pathways for water interaction with oxygen adatoms on TiO 2 (110). Phys. Rev. Lett. 102 , 096102 (2009).
Article ADS CAS PubMed Google Scholar
Tan, S. et al. Interfacial hydrogen-bonding dynamics in surface-facilitated dehydrogenation of water on TiO 2 (110). J. Am. Chem. Soc. 142 , 826–834 (2020).
Chu, W. et al. Ultrafast charge transfer coupled to quantum proton motion at molecule/metal oxide interface. Sci. Adv. 8 , eabo2675 (2022).
Wang, Y. et al. Enabling high-energy-density aqueous batteries with hydrogen bond-anchored electrolytes. Matter 5 , 162–179 (2022).
Wang, X., Xu, C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10 , 4876 (2019).
Wang, H., Yang, C., Chen, F., Zheng, G. & Han, Q. A Crystalline partially fluorinated triazine covalent organic framework for efficient photosynthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 61 , e202202328 (2022).
Article ADS CAS Google Scholar
Yang, C., Wan, S., Zhu, B., Yu, J. & Cao, S. Calcination-regulated microstructures of donor-acceptor polymers towards enhanced and stable photocatalytic H 2 O 2 production in pure water. Angew. Chem. Int. Ed. 61 , e202208438 (2022).
Xu, X. et al. Anthraquinone-based conjugated organic polymers containing dual oxidation centers for photocatalytic H 2 O 2 production from H 2 O and O 2 under visible-light irradiation. ACS Appl. Poly. Mater. 9 , 7571–7580 (2023).
Xu, X. et al. Conjugated organic polymers with anthraquinone redox centers for efficient photocatalytic hydrogen peroxide production from water and oxygen under visible light irradiation without any additives. ACS Catal 12 , 12954–12963 (2022).
Ye, Y. X. et al. A solar-to-chemical conversion efficiency up to 0.26% achieved in ambient conditions. Proc. Natl. Acad. Sci. USA 118 , e2115666118 (2021).
Article CAS PubMed PubMed Central Google Scholar
Zhang, X. et al. Keto-enamine-based covalent organic framework with controllable anthraquinone moieties for superior H 2 O 2 photosynthesis from O 2 and water. Chem. Eng. J. 466 , 143085 (2023).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134 , 19524–19527 (2012).
Wu, S. et al. The Keto‐switched photocatalysis of reconstructed covalent organic frameworks for efficient hydrogen evolution. Angew. Chem. Int. Ed. 135 , e202309026 (2023).
Li, Q. et al. Visible-light-responsive anthraquinone functionalized covalent organic frameworks for metal-free selective oxidation of sulfides: effects of morphology and structure. ACS Catal 10 , 6664–6675 (2020).
DeBlase, C. R., Silberstein, K. E., Truong, T. T., Abruna, H. D. & Dichtel, W. R. beta-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135 , 16821–16824 (2013).
Khayum, M. A. et al. Chemically delaminated free-standing ultrathin covalent organic nanosheets. Angew. Chem. Int. Ed. 55 , 15604–15608 (2016).
Dubois, M. et al. Solid state NMR study of nanodiamond surface chemistry. Solid State Nucl. Magn. Reson. 40 , 144–154 (2011).
Wu, Z. et al. Covalent-organic frameworks with keto-enol tautomerism for efficient photocatalytic oxidative coupling of amines to imines under visible light. Sci. Chi. Chem. 64 , 169–2179 (2021).
ADS Google Scholar
Chen, S. et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed. 133 , 16743–16750 (2021).
Article ADS Google Scholar
Zhang, L. et al. A facile solution-phase synthetic approach for constructing phenol-based porous organic cages and covalent organic frameworks. Green Chem 22 , 2498–2504 (2020).
Zhang, M. et al. Construction of flexible amine-linked covalent organic frameworks by catalysis and reduction of formic acid via the eschweiler-clarke reaction. Angew. Chem. Int. Ed. 60 , 12396–12405 (2021).
Chiang, Y., Kresge, A. J., Santaballa, J. A. & Wirz, J. Ketonization of acetophenone enol in aqueous buffer solutions. Rate-equilibrium relations and mechanism of the uncatalyzed reaction. J. Am. Chem. Soc. 110 , 5506–5510 (1988).
Wu, Q. et al. A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater. Nat. Commun. 12 , 483 (2021).
Shiraishi, Y. et al. Resorcinol-formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18 , 985–993 (2019).
Xu, X. et al. The construction of conjugated organic polymers containing phenanthrenequinone redox centers for visible-light-driven H 2 O 2 production from H 2 O and O 2 without any additives. Chem. Eng. J. 454 , 139929 (2023).
Yan, H. et al. Spontaneous exciton dissociation in organic photocatalyst under ambient conditions for highly efficient synthesis of hydrogen peroxide. Proc. Natl. Acad. Sci. USA 119 , e2202913119 (2022).
Zhao, C. et al. Molecular level modulation of anthraquinone-containing resorcinol-formaldehyde resin photocatalysts for H 2 O 2 production with exceeding 1.2% efficiency. Angew. Chem. Int. Ed. 62 , e202218318 (2023).
Kou, M. et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 61 , 202200413 (2022).
Chu, C. et al. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H 2 O 2 production. Proc. Natl. Acad. Sci. USA 117 , 6376–6382 (2020).
Chang, J. N. et al. Oxidation-reduction molecular junction covalent organic frameworks for full reaction photosynthesis of H 2 O 2 . Angew. Chem. Int. Ed. 62 , e202218868 (2023).
Chang, J. N. et al. Regulation of redox molecular junctions in covalent organic frameworks for H 2 O 2 photosynthesis coupled with biomass valorization. Angew. Chem. Int. Ed. 62 , e202303606 (2023).
Das, P. et al. Integrating bifunctionality and chemical stability in covalent organic frameworks via one-pot multicomponent reactions for solar-driven H 2 O 2 Production. J. Am. Chem. Soc. 145 , 2975–2984 (2023).
Liu, F. et al. Covalent organic frameworks for direct photosynthesis of hydrogen peroxide from water, air and sunlight. Nat. Commun. 14 , 4344 (2023).
Luo, Y. et al. Sulfone-modified covalent organic frameworks enabling efficient photocatalytic hydrogen peroxide generation via one-step two-electron O 2 reduction. Angew. Chem. Int. Ed. 62 , e202305355 (2023).
Mou, Y. et al. Linkage microenvironment of azoles-related covalent organic frameworks precisely regulates photocatalytic generation of hydrogen peroxide. Angew. Chem. Int. Ed. 62 , 202309480 (2023).
Sun, J. et al. Pyrene-based covalent organic frameworks for photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 19 , e202216719 (2023).
Google Scholar
Yang, T. et al. Covalent furan‐benzimidazole‐linked polymer hollow fiber membrane for clean and efficient photosynthesis of hydrogen peroxide. Adv. Func. Mater. 34 , 202300714 (2023).
Liu, T. et al. Overall photosynthesis of H 2 O 2 by an inorganic semiconductor. Nat. Commun. 13 , 1034 (2022).
Wang, W. et al. Photothermal-enabled single-atom catalysts for high-efficiency hydrogen peroxide photosynthesis from natural seawater. Nat. Commun. 14 , 2493 (2023).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4 , 374–384 (2021).
Zhang, Y. et al. H 2 O 2 generation from O 2 and H 2 O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 8 , 361–371 (2023).
Chen, L. et al. Acetylene and diacetylene functionalized covalent triazine frameworks as metal-free photocatalysts for hydrogen peroxide production: a new two-electron water oxidation pathway. Adv. Mater. 32 , e1904433 (2020).
Article PubMed Google Scholar
Yang, L. et al. Two-channel photocatalytic production of H 2 O 2 over g-C 3 N 4 nanosheets modified with perylene imides. J. Catal. 352 , 274–281 (2017).
Li, L., Xu, L., Hu, Z. & Yu, J. C. Enhanced mass transfer of oxygen through a gas-liquid-solid interface for photocatalytic hydrogen peroxide production. Adv. Funct. Mater. 22 , 2106120 (2021).
Chu, C. et al. Photocatalytic H 2 O 2 production driven by cyclodextrin-pyrimidine polymer in a wide pH range without electron donor or oxygen aeration. Appl. Catal. B: Environ. 314 , 121485 (2022).
Yu, F. Y., Zhou, Y. J., Tan, H. Q., Li, Y. G. & Kang, Z. H. Versatile photoelectrocatalysis strategy raising up the green production of hydrogen peroxide. Adv. Energy Mater. 13 , 202300119 (2023).
Mitsui, T., Rose, M. K., Fomin, E., Ogletree, D. F. & Salmeron, M. Water diffusion and clustering on Pd(111). Science 297 , 1850–1852 (2002).
Peng, J. et al. The effect of hydration number on the interfacial transport of sodium ions. Nature 557 , 701–705 (2018).
Nilsson, A. & Pettersson, L. G. The structural origin of anomalous properties of liquid water. Nat. Commun. 6 , 8998 (2015).
Shen, R. et al. Coupling oxygen vacancy and hetero-phase junction for boosting catalytic activity of Pd toward hydrogen generation. Appl. Catal. B: Environ. 328 , 122484 (2023).
Wang, Y. H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600 , 81–85 (2021).
Chen, S. et al. Aqueous rechargeable zinc air batteries operated at −110 °C. Chem 9 , 497–510 (2022).
Zeng, H. et al. pH-independent production of hydroxyl radical from atomic H*-Mediated electrocatalytic H 2 O 2 reduction: a green fenton process without byproducts. Environ. Sci. Technol. 54 , 14725–14731 (2020).
Liu, R. et al. Defect sites in ultrathin Pd nanowires facilitate the highly efficient electrochemical hydrodechlorination of pollutants by H* ads . Environ. Sci. Technol. 52 , 9992–10002 (2018).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1 , 466–472 (2009).
Tan, H. et al. Engineering a local acid-like environment in alkaline medium for efficient hydrogen evolution reaction. Nat. Commun. 13 , 2024 (2022).
Yu, W. et al. High-density frustrated lewis pair for high-performance hydrogen evolution. Adv. Energy Mater. 13 , 2203136 (2023).
Wei, Y. et al. Quantification of photocatalytically-generated hydrogen peroxide in the presence of organic electron donors: Interference and reliability considerations. Chemosphere 279 , 130556 (2021).
Gu, S. et al. Tunable redox chemistry and stability of radical intermediates in 2D covalent organic frameworks for high performance sodium ion batteries. J. Am. Chem. Soc. 141 , 9623–9628 (2019).
Download references
Acknowledgements
Financial supports from the National Natural Science Foundation of China (Nos. 52070128, 22206125, 22376138) are gratefully acknowledged.
Author information
Authors and affiliations.
School of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China
Xiangcheng Zhang, Silian Cheng, Xue Wen, Jie Miao, Baoxue Zhou, Mingce Long & Lizhi Zhang
School of Ecological and Environmental Science, Key Laboratory for Urban Ecological Processes and Eco-Restoration, East China Normal University, Shanghai, 200241, China
You can also search for this author in PubMed Google Scholar
Contributions
X.Z., M.L., B.Z., and L.Z. contributed to design of this study, X.Z., M.L., and L.Z. wrote the manuscript. X.Z., J.M., C.C., and S.C. conducted experiments and performed data analysis. X.W. provided DFT calculation and analysis.
Corresponding authors
Correspondence to Mingce Long or Lizhi Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Chuncheng Chen and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhang, X., Cheng, S., Chen, C. et al. Keto-anthraquinone covalent organic framework for H 2 O 2 photosynthesis with oxygen and alkaline water. Nat Commun 15 , 2649 (2024). https://doi.org/10.1038/s41467-024-47023-y
Download citation
Received : 28 November 2023
Accepted : 18 March 2024
Published : 26 March 2024
DOI : https://doi.org/10.1038/s41467-024-47023-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Share full article
Advertisement
Supported by
Guest Essay
‘If You See a Fox and I’ve Died, It Will Be Me’

By Sarah Wildman
Ms. Wildman is a staff writer and editor in Opinion.
A block from my house at the edge of Washington, there is a winding park with a road running through it. One Sunday recently, walking my regular loop along the trail, I heard leaves rustling on the wooded hill above me. I often see deer here; this time it was a bright young fox.
She paused. We stood there for a moment, she and I, aware. I wanted desperately for her to come closer, to stay in her orbit a moment longer. I lingered long after she left.
Sometime in my daughter Orli’s last months of life, she told me, lightly, “If you see a fox and I’ve died, it will be me.” I had never seen a fox in my neighborhood. Over the past several months, I have seen maybe a half dozen, here and elsewhere. Each time, I try to quell my desire to shout out, to ask the animal to stay, to call it by her name. It feels crazy; it feels sane.
I had never believed in signs. Now I notice when an interview runs exactly 1 hour and 13 minutes or when the hour is exactly 1:13. Orli was born on Jan. 13. It means nothing; it means something. A double rainbow stretched over a farm in Maine represents more than beauty.
March 17 will be one year since Orli died in our house, in her room, in my arms; March 20 a year since her burial. (In a quirk of this year’s Jewish calendar, the date of her yahrzeit, or memorial date, is some weeks farther on.)
A year is a strange and terrible marker of time, simultaneously endless and instant. A year of loss is a new form of permanence: This is the life we lead. It will not change. A year furthers us on the long march toward our altered future. In the life of a child, a year is transformative. Her peers have molted in the year from 14 to 15. They no longer attend the same school; they have begun new sports, met new friends, moved forward, moved on.
There is an immutability to a year of grief, a sense of solidity to the loss, a movement from the surreality of her absence into a hardened space. It’s not as though I believe she might return, but in the year between her death and now, I remain connected to her presence. My partner, Ian, has spent part of this year adding tattoos to his arms, each an ode to Orli, permanent signifiers of permanent loss. My younger daughter, Hana, has written through her grief; she notes, often, the lack of insight her peers have into the depth of losing a sister. Meanwhile, I wonder if I should keep every item of clothing I can picture Orli in, I wonder what she would say about each movie I see, each book I read. I yearn for her commentary.
On Orli’s birthday, one of her long-distance friends wrote to me, “Whether you consent or not, I bring Orli along in every escapade,” in good decisions, in hidden poor ones. She understood the essence of being human is to be mischievous, of both choosing well and of making bad decisions. I never craved a perfect child, just a living one.
The day before our first birthday without Orli, Hana, Ian and I — walking from separate directions — came upon a fox idling on a street corner, as though waiting for us.
Most of this year I have worked to center memories of Orli’s better moments, the joy she infused in each minute she got to live. One month after her first brain tumor surgery, when she’d rebounded better than any of us could have hoped, we met old friends from Spain for dinner. As we ate, a sudden, drenching storm came up. Orli got up and ran into the warm rain with our friends’ children, dancing, thrilled. It was, she told me, a “bucket list moment.”
She seemed to realize, far earlier than I, she had to lean into each experience, to expand it, to let it fuel her for whatever came next. In her journal she worried she might not see ninth grade. She did not share that with her friends.
Each of us in our rump family has felt an almost visceral physicality of these past few weeks — the slide from her birthday toward this anniversary, the terrible knowledge that we each hold of the last moments of her life, the good minutes we had, the harder hours, the terror of those final days.
In her last week, one doctor cornered me at the hospital to tell me Orli shouldn’t be here anymore. It was not clear if he meant “here, still receiving palliative treatment,” or “here, on earth.” She was fading, I knew. But it felt an awful thing to say — unforgivable, really. I thought of Abraham arguing with God to save the wicked towns . I wanted to ask: But what if I get 15 good minutes with her each hour? Or five? Orli was adamant she did not want to die.
In Judaism a child who is an avel, or mourner, is to stop saying Mourner’s Kaddish for her parent at 11 months as she re-emerges into the community. But because parents who have lost a child have no obligation beyond the first 30 days, this marker holds no meaning. And because those who have lost children are, in many ways, forever seen as mourners, forever noted for their loss, we remain on the margin — in the community but not entirely of it. Once, early in Orli’s illness, on the same path where I saw the fox, I overheard a woman, just slightly still within my earshot, who passed me. “That’s Sarah Wildman, the woman whose daughter …”
I tend to walk alone on this path. Grief of this kind is simultaneously universal and unshareable; loneliness is its inherent point of reference. I cannot conceive that March 18 will be drastically different from March 17.
When 2023 turned to 2024, I thought: It is a terrible thing to buy a calendar for a year Orli will not see. Still, I put up a calendar in her old room, the same feminist calendar she chose each year. As February turned to March, I found the page hard to flip over. Until this point, I have been able to look at the photos in my phone and say: This time last year, we were at this concert, we were at this movie, we had this meal. Now those memories slide farther back. These days Ian often sits in her room, working. He likes to be near her, and so, most nights, in homage to her, I straighten up after him — he is a mess, she craved order. I do it for her, I do it for me.
In early September, not quite six months after Orli died, I interviewed the actor Rob Delaney, who wrote a bracing, visceral book about his young son Henry’s life and death from brain cancer. “You probably at this point regularly — what, every day? — are shocked by the fact that she’s gone. Right?” Mr. Delaney asked me halfway through our call. “For better or for worse — I guess for the survival of the species, it’s for the better — but the acute physical pain will not go away. But it’ll weave itself into your life in a way where threads of Orli will be in the tapestry of your life forever,” he said.
“And in a few years, you’re going to wrap yourself in the tapestry of your life and marvel at the beauty of the threads of Hana and Orli and Ian, and it’ll all be — you will metabolize her life and her death, in a way where you feel a thousand things.” One of those things will always be “disbelief and pain,” he said. “That won’t go away.”
In the first days of March, Hana and I went to speak at Orli’s old school at a Women’s History Month assembly held in her honor. Orli had an “intuitive sense of justice, about doing what’s right in the world, about showing up for her friends and herself,” I told sixth, seventh and eighth graders, aware some of them would have known Orli only as that girl who died.
It was Hana who spoke best. “Orli was like an emotion,” she told the assembled children, all older than she. “I think I will never get over her. It might get less hard, but I will never not be sad.”
It wasn’t until that night, in bed, that I wept. The teachers still knew her as she was, I realized. I craved their memories.
“How are you?” each of them asked, as people often do. “Aquí estoy,” I said, as I have come to say. I’m here.
Sarah Wildman is a staff editor and writer in Opinion. She is the author of “Paper Love: Searching for the Girl My Grandfather Left Behind.”
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .

IMAGES
VIDEO
COMMENTS
Photosynthesis is critical for the existence of the vast majority of life on Earth. It is the way in which virtually all energy in the biosphere becomes available to living things. As primary producers, photosynthetic organisms form the base of Earth's food webs and are consumed directly or indirectly by all higher life-forms. Additionally ...
It is the main event in light reactions of photosynthesis. The function of light reactions is two fold —. (1) The photochemical splitting of water provides hydrogen atoms for the reduction of CO 2, and. (2) Producing of ATP which provides energy for the subsequent synthesis of carbohydrates.
Photosynthesis is important to living organisms because it is the number one source of oxygen in the atmosphere. Without photosynthesis, the carbon cycle could not occur, oxygen-requiring life would not survive and plants would die. Green plants and trees use photosynthesis to make food from sunlight, carbon dioxide and water in the atmosphere ...
The process. During photosynthesis, plants take in carbon dioxide (CO 2) and water (H 2 O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose.
The importance of photosynthesis is not just that it can capture sunlight's energy. A lizard sunning itself on a cold day can use the sun's energy to warm up. Photosynthesis is vital because it evolved as a way to store the energy in solar radiation (the "photo-" part) as high-energy electrons in the carbon-carbon bonds of carbohydrate ...
Photosynthesis ( / ˌfoʊtəˈsɪnθəsɪs / FOH-tə-SINTH-ə-sis) [1] is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities.
The ecological importance of photosynthesis. Photosynthetic organisms, including plants, algae, and some bacteria, play a key ecological role. They introduce chemical energy and fixed carbon into ecosystems by using light to synthesize sugars. Since these organisms produce their own food—that is, fix their own carbon—using light energy ...
Oxygenic photosynthesis involves the conversion of water and CO 2 into complex organic molecules such as carbohydrates and oxygen. Photosynthesis may be split into the 'light' and 'dark' reactions. In the light reactions, water is split using light into oxygen, protons and electrons, and in the dark reactions, the protons and electrons are used to reduce CO 2 to carbohydrate (given ...
The importance of photosynthesis is not just that it can capture sunlight's energy. A lizard sunning itself on a cold day can use the sun's energy to warm up. Photosynthesis is vital because it evolved as a way to store the energy in solar radiation (the "photo-" part) as energy in the carbon-carbon bonds of carbohydrate molecules (the ...
Figure 8.1.1 8.1. 1: This world map shows Earth's distribution of photosynthesis as seen via chlorophyll a concentrations. On land, this is evident via terrestrial plants, and in oceanic zones, via phytoplankton. (credit: modification of work by SeaWiFS Project, NASA/Goddard Space Flight Center and ORBIMAGE) The processes in all organisms ...
The chemical reactions are the reverse of photosynthesis, using a glucose molecule and six oxygen molecules (12 atoms) as inputs. Energy is released along with some carbon dioxide and water. But this is enough chemistry. Trees and other green plants practice respiration, too, just like animals, but they also practice photosynthesis.
Students are often asked to write an essay on Photosynthesis in their schools and colleges. And if you're also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic. ... The Importance of Photosynthesis. Photosynthesis is very important for life on Earth. It gives us oxygen, which we need to breathe.
Essay on Photosynthesis. Essay # 1. Meaning of Photosynthesis: Life on earth ultimately depends on energy derived from the Sun. Photosynthesis is the only process of biological importance that can harvest this energy. The term "Photosynthesis" literally means "synthesis using light". It is the ability of the green plants to utilise the ...
Photosynthesis Definition. Photosynthesis is the biochemical pathway which converts the energy of light into the bonds of glucose molecules. The process of photosynthesis occurs in two steps. In the first step, energy from light is stored in the bonds of adenosine triphosphate (ATP), and nicotinamide adenine dinucleotide phosphate (NADPH).
Essay # 1. Meaning of Photosynthesis: Although literary meaning of photosynthesis is 'synthesis with the help of light' but this term is usually applied to a very important vital process by which the green plants synthesize organic matter in presence of light. Photosynthesis is sometimes called as carbon assimilation and is represented by ...
Given its importance in making and keeping Earth lush, photosynthesis ranks high on the top-10 list of evolutionary milestones. In Science' s Origins essay this week, author Mitch Leslie describes how scientists are delving into ancient rocks and poring over DNA sequences to try to piece together how and when organisms first began to harness ...
Importance; Photosynthesis definition states that the process exclusively takes place in the chloroplasts through photosynthetic pigments such as chlorophyll a, chlorophyll b, carotene and xanthophyll. All green plants and a few other autotrophic organisms utilize photosynthesis to synthesize nutrients by using carbon dioxide, water and sunlight.
Abstract. Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried ...
Cellular respiration happens in the dark. In the process of photosynthesis, the plants need sunlight for energy. Photosynthesis is the process that plants use light energy from the sun to make their own food. Cellular respiration is a chemical process plants use to release the stored chemical energy from glucose as usable chemical energy so it ...
The chloroplast absorbs the light energy to convert to chemical energy such as ATP AND NADPH. Photosynthesis is the process of converting carbon dioxide to organic compounds, such as simple sugar, using the energy from sunlight (Smith, A.L.). The chemical reaction equation of photosynthesis is as followed: 6 C02 + 6 H20 + Light Energy → ...
Unlike photosynthesis, aerobic respiration is an exergonic process (negative ΔG°) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste).
Significance of Photosynthesis are as follows:-. 1. Provide food:-. Photosynthesis helps to prepare food in plants and they prepare their food not only for themselves but all living beings which are lived on this earth because each and every organism is dependent on plants, either directly or indirectly. Photosynthesis play vital role for the ...
Hydrogen peroxide photosynthesis is an important reaction that suffers from poor activity due to the high energy barrier of hydrogen extraction in water. Here, we report a keto-form anthraquinone ...
On April 8, North America will experience its second total solar eclipse in seven years. The moon will glide over the surface of our sun, casting a shadow over a swath of Earth below. Along this ...
We stood there for a moment, she and I, aware. I wanted desperately for her to come closer, to stay in her orbit a moment longer. I lingered long after she left. Sometime in my daughter Orli's ...