- FCUS Courses
- Register for FCUS Course

- What is the ICN?
- The ICN Story
- ICN Hot cases
- Semantic sMatter
- ECG Proving Ground
- ICU Radiology Cases
- Game Changing Evidence
- Pharmacology
- Past Papers
- Approaches to Questions
- Clinical Exam
- Clinical Governance
- Past Papers with Answers
- The ICN meta feed
- Conferences
- Websites & Blogs
- Books & Journals
- Clinical Calculators

Clinical Cases
This section is a collection of critical care clinical cases to test yourself and hopefully get some new ideas.
Please leave feedback and comments, and if you want to put your own hot cases up, please get in touch and we can make it happen.

Can you work out the cause of this DVT?

Pulmonary Artery Catheters: PAC traps for young snake charmers

End-o-bed-o-gram – Case 3

Spot Diagnosis #2
End-o-bed-o-gram – case 2.

The NEJM Critical Care Challenge: Case 11 (Answer)
Spot diagnosis #1, the nejm critical care challenge: case 11 (end of life care), nejm critical care challenge case 10 (sdh) answer, the nejm critical care challenge: case 10 (acute sdh), icu-acquired weakness: nejm critical care challenge case 9 (question and answer).

Nutrition: NEJM Critical Care Challenge Case 8 Answer
End-o-bed-o-gram 1, the nejm critical care challenge: case 8, coagulopathy: nejm critical care challenge case 7 answer, coagulopathy: nejm critical care challenge case 7, icu sedation: nejm critical care challenge case 6 answer, icu sedation: nejm critical care challenge case 6, acute liver failure: nejm critical care challenge case 5 answer, acute liver failure: nejm critical care challenge case 5, ventilation: nejm critical care challenge case 4 answer.

Haemodynamic Monitoring: NEJM Critical Care Challenge Case 3 ANSWER
Haemodynamic monitoring: the nejm critical care challenge case 3, resuscitation fluid: nejm critical care challenge case 2 answer.

The NEJM Critical Care Challenge: Case 2
Septic shock: nejm critical care challenge case 1 answer, septic shock: nejm critical care challenge case 1.

The NEJM Critical Care Challenge: Case 1
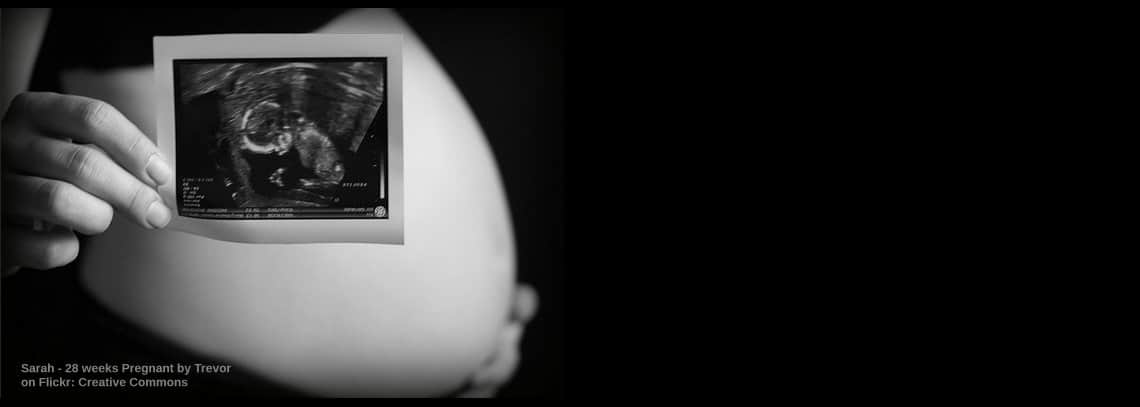
Hot Case #14 – I’m pregnant … HELLP!

Hot Case #13 – To bust or not to bust?

ICN Hot Case #12 – Pull my finger!

Hot Case #11 – Testing testing

ICN Hot Case #10

ICN Hot Case #9

ICN Hot Case #8

ICN Hot Case #7

ICN Hot Case # 6
The nejm critical care challenge: case 4.
ICN Hot Case #5

September Case of the month
ICN Hot Case #4
ICN Hot Case #3

- What is ICN
- The Team
- ICN NSW
- ICN QLD
- ICN VIC
- ICN WA
- ICN NZ
- ICN UK
- Lung US
- Exam Help
- Clinical Cases
- Echo Cases
- Echo Guide
- ICU Radiology
- Game Changing Evidence
- ICN Metafeed
- Simulation Resouces
- SMACC Posters
- Audio
- Video
- Pecha Kuchas
- End-o-Bed-o-Gram
- ICU Primary Exam
- CICM Fellowship
- ANZCA Fellowship
- Paeds Fellowship
- Emergency Primary
- Anaesthetics Primary
® 2024 The Intensive Care Network || All rights reserved || Disclaimer || Site Map || Contact ICN Support
Log in with your credentials
or Create an account
Forgot your details?
Create account.
LOGIN
Annual Report
- Board of Directors
- Nomination Process
- Organizational Structure
- ATS Policies
- ATS Website
- MyATS Tutorial
- ATS Experts
- Press Releases
Member Newsletters
- ATS in the News
- ATS Conference News
- Embargo Policy
ATS Social Media
Breathe easy podcasts, ethics & coi, health equity, industry resources.
- Value of Collaboration
- Corporate Members
- Advertising Opportunities
- Clinical Trials
- Financial Disclosure
In Memoriam
Global health.
- International Trainee Scholarships (ITS)
- MECOR Program
- Forum of International Respiratory Societies (FIRS)
- 2019 Latin American Critical Care Conference
Peer Organizations
Careers at ats, affordable care act, ats comments and testimony, forum of international respiratory societies, tobacco control, tuberculosis, washington letter.
- Clinical Resources
- ATS Quick Hits
- Asthma Center
Best of ATS Video Lecture Series
- Coronavirus
- Critical Care
- Disaster Related Resources
- Disease Related Resources
- Resources for Patients
- Resources for Practices
- Vaccine Resource Center
- Career Development
- Resident & Medical Students
- Junior Faculty
- Training Program Directors
- ATS Reading List
- ATS Scholarships
- ATS Virtual Network
ATS Podcasts
- ATS Webinars
- Professional Accreditation
Pulmonary Function Testing (PFT)
- Calendar of Events
Patient Resources
- Asthma Today
- Breathing in America
- Fact Sheets: A-Z
- Fact Sheets: Topic Specific
- Patient Videos
- Other Patient Resources
Lung Disease Week
Public advisory roundtable.
- PAR Publications
- PAR at the ATS Conference
Assemblies & Sections
- Abstract Scholarships
- ATS Mentoring Programs
- ATS Official Documents
- ATS Interest Groups
- Genetics and Genomics
- Medical Education
- Terrorism and Inhalation Disasters
- Allergy, Immunology & Inflammation
- Behavioral Science and Health Services Research
- Clinical Problems
- Environmental, Occupational & Population Health
- Pulmonary Circulation
- Pulmonary Infections and Tuberculosis
- Pulmonary Rehabilitation
- Respiratory Cell & Molecular Biology
- Respiratory Structure & Function
- Sleep & Respiratory Neurobiology
- Thoracic Oncology
- Joint ATS/CHEST Clinical Practice Committee
- Clinicians Advisory
- Council of Chapter Representatives
- Documents Development and Implementation
- Drug/Device Discovery and Development
- Environmental Health Policy
- Ethics and Conflict of Interest
- Health Equity and Diversity Committee
- Health Policy
- International Conference Committee
- International Health
- Members In Transition and Training
- View more...
- Membership Benefits
- Categories & Fees
- Special Membership Programs
- Renew Your Membership
- Update Your Profile
- ATS DocMatter Community
- Respiratory Medicine Book Series
- Elizabeth A. Rich, MD Award
- Member Directory
- ATS Career Center
- Welcome Trainees
- ATS Wellness
- Thoracic Society Chapters
- Chapter Publications
- CME Sponsorship
Corporate Membership
Clinical cases, professionals.
- Respiratory Health Awards
- Clinicians Chat
- Ethics and COI
- Pulmonary Function Testing
- ATS Resources
- Live from the CCD
- Pediatric Division Directors
Reviewed By Behavioral Science Assembly
Submitted by
Lokesh Venkateshaiah, MD
Division of Pulmonary, Critical Care and Sleep Medicine
The MetroHealth System, Case Western Reserve University
Cleveland, Ohio
Bruce Arthur, MD
J. Daryl Thornton, MD, MPH
Assistant Professor
Division of Pulmonary, Critical Care and Sleep Medicine, Center for Reducing Health Disparities
Submit your comments to the author(s).
A 60-year-old man presented to the emergency department complaining of persistent right-sided chest pain and cough. The chest pain was pleuritic in nature and had been present for the last month. The associated cough was productive of yellow sputum without hemoptysis. He had unintentionally lost approximately 30 pounds over the last 6 months and had nightly sweats. He had denied fevers, chills, myalgias or vomiting. He also denied sick contacts or a recent travel history. He recalled childhood exposures to persons afflicted with tuberculosis.
The patient smoked one pack of cigarettes daily for the past 50 years and denied recreational drug use. He reported ingesting twelve beers daily and had had delirium tremens, remote right-sided rib fractures and a wrist fracture as a result of alcohol consumption. He had worked in the steel mills but had discontinued a few years previously. He collected coins and cleaned them with mercury.
The patient’s past medical history was remarkable for chronic “shakes” of the upper extremities for which he had not sought medical attention. Other than daily multivitamin tablets, he took no regular medications.
Hospital course He was initially admitted to the general medical floor for treatment of community-acquired pneumonia (see Figure 1) and for the prevention of delirium tremens. He was initiated on ceftriaxone, azithromycin, thiamine and folic acid. Diazepam was initiated and titrated using the Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWAS-Ar), a measure of withdrawal severity (1). By hospital day 5, his respiratory status continued to worsen, requiring transfer to the intensive care unit (ICU) for hypoxemic respiratory failure. His neurologic status had also significantly deteriorated with worsening confusion, memory loss, drowsiness, visual hallucinations (patient started seeing worms) and worsening upper extremity tremors without generalized tremulousness despite receiving increased doses of benzodiazepines.
Physical Exam
White blood cell count was 11,000/mm 3 with 38% neutrophils, 8% lymphocytes, 18 % monocytes and 35% bands
Hematocrit 33%
Platelet count was 187,000/mm 3
Serum sodium was 125 mmol/L, potassium 3 mmol/L, chloride 91 mmol/L, bicarbonate 21 mmol/L, blood urea nitrogen 14 mg /dl, serum creatinine 0.6 mg/dl and anion gap of 14.
Urine sodium <10 mmol/L, urine osmolality 630 mosm/kg
Liver function tests revealed albumin 2.1 with total protein 4.6, normal total bilirubin, aspartate transaminase (AST) 49, Alanine transaminase (ALT) 19 and alkaline phosphatase 47.
Three sputum samples were negative for acid-fast bacilli (AFB).
Bronchoalveolar lavage (BAL) white blood cell count 28 cells/µl, red blood cell count 51 cells/µl, negative for AFB and negative Legionella culture. BAL gram stain was without organisms or polymorphonuclear leukocytes.
Blood cultures were negative for growth.
Sputum cultures showed moderate growth of Pasteurella multocida.
2D transthoracic ECHO of the heart showed normal valves and an ejection fraction of 65% with a normal left ventricular end-diastolic pressure and normal left atrial size. No vegetations were noted.
Purified protein derivative (PPD) administered via Mantoux testing was 8 mm in size at 72 hr after placement.
Human immunodeficiency virus (HIV) serology was negative.
Arterial blood gas (ABG) analysis performed on room air on presentation to the ICU: pH 7.49, PaCO 2 29 mm Hg, PaO 2 49 mm Hg.

After admission to the ICU, the patient was noted to be in acute lung injury (ALI), a subset of acute respiratory distress syndrome (ARDS). The diagnosis of ALI requires all three of the following: (a) bilateral pulmonary infiltrates, (b) a PaO 2 :FiO 2 ratio of ≤ 300 and (c) echocardiographic evidence of normal left atrial pressure or pulmonary-artery wedge pressure of ≤ 18 mm Hg (2).
While patients with ALI and ARDS can be maintained with pressure-limited or volume-limited modes of ventilation, only volume assist-control ventilation was utilized in the ARDS Network multicenter randomized controlled trial that demonstrated a mortality benefit.
Noninvasive ventilation has not been demonstrated to be superior to endotracheal intubation in the treatment of ARDS or ALI and is not currently recommended (4).
This is a case of heavy metal poisoning with mercury. The patient used mercury to clean coins. Family members who had visited his house while he was hospitalized found several jars of mercury throughout his home. The Environmental Protection Agency (EPA) was notified and visited the home. They found aerosolized mercury levels of > 50,000 PPM and had the home immediately demolished.
Alcoholic hallucinosis is a rare disorder occurring in 0.4 - 0.7% of alcohol-dependent inpatients (5). Affected persons experience predominantly auditory but occasionally visual hallucinations. Delusions of persecution may also occur. However, in contrast to alcohol delirium, other alcohol withdrawal symptoms are not present and the sensorium is generally unaffected.
Delerium tremens (DT) occurs in approximately 5% of patients who withdraw from alcohol and is associated with a 5% mortality rate. DT typically occurs between 48 and 96 hr following the last drink and lasts 1-5 days. DT is manifested by generalized alteration of the sensorium with vital sign abnormalities. Death often results from arrhythmias, pneumonia, pancreatitis or failure to identify another underlying problem (6). While DT certainly could have coexisted in this patient, an important initial step in the management of DT is to identify and treat alternative diagnoses.
Delirium is frequent among older patients in the ICU (7), and may be complicated by pneumonia and sepsis. However, pneumonia and sepsis as causes for delirium are diagnoses of exclusion and should only be attributed after other possibilities have been ruled out.
Frontal lobe stroke is unlikely, given the absence of other findings in the history or physical examination present to suggest an acute cerebrovascular event.
In 1818, Dr. John Pearson coined the term erethism for the characteristic personality changes attributed to mercury poisoning (8). Erethism is classically the first symptom in chronic mercury poisoning (9). It is a peculiar form of timidity most evident in the presence of strangers and closely resembles an induced paranoid state. In the past, when mercury was used in making top hats, the term “mad as a hatter” was used to describe the psychiatric manifestations of mercury intoxication. Other neurologic manifestations include tremors, especially in patients with a history of alcoholism, memory loss, drowsiness and lethargy. All of these were present in this patient.
Acute respiratory failure (ALI/ARDS) can occur following exposure to inhalation of mercury fumes (10). Mercury poisoning has also been associated with acute kidney injury (11).
Although all of the options mentioned above could possibly contribute to the development of delirium, only mercury poisoning would explain the constellation of findings of confusion, upper extremity tremors, visual hallucinations, somnolence and acute respiratory failure (ALI/ARDS).
Knowledge of the form of mercury absorbed is helpful in the management of such patients, as each has its own distinct characteristics and toxicity. There are three types of mercury: elemental, organic and inorganic. This patient had exposure to elemental mercury from broken thermometers.
Elemental mercury is one of only two known metals that are liquid at room temperature and has been referred to as quicksilver (12). It is commonly found in thermometers, sphygmomanometers, barometers, electronics, latex paint, light bulbs and batteries (13). Although exposure can occur transcutaneously or by ingestion, inhalation is the major route of toxicity. Ingested elemental mercury is poorly absorbed and typically leaves the body unchanged without consequence (bioavailability 0.01% [13]). However, inhaled fumes are rapidly absorbed through the pulmonary circulation allowing distribution throughout the major organ systems. Clinical manifestations vary based on the chronicity of the exposure (14). Mercury readily crosses the blood-brain barrier and concentrates in the neuronal lysosomal dense bodies. This interferes with major cell processes such as protein and nucleic acid synthesis, calcium homeostasis and protein phosphorylation. Acute exposure symptoms manifest within hours as gastrointestinal upset, chills, weakness, cough and dyspnea.
Inorganic mercury salts are earthly-appearing, red ore found historically in cosmetics and skin treatments. Currently, most exposures in the United States occur from exposure through germicides or pesticides (15). In contrast to elemental mercury, inorganic mercury is readily absorbed through multiple routes including the gastrointestinal tract. It is severely corrosive to gastrointestinal mucosa (16). Signs and symptoms include profuse vomiting and often-bloody diarrhea, followed by hypovolemic shock, oliguric renal failure and possibly death (12).
Organic mercury, of which methylmercury is an example, has garnered significant attention recently following several large outbreaks as a result of environmental contamination in Japan in 1956 (17) and grain contamination in Iraq in 1972 (18). Organic mercury is well absorbed in the GI tract and collects in the brain, reaching three to six times the blood concentration (19). Symptoms may manifest up to a month after exposure as bilateral visual field constriction, paresthesias of the extremities and mouth, ataxia, tremor and auditory impairments (12). Organic mercury is also present in a teratogenic agent leading to development of a syndrome similar to cerebral palsy termed "congenital Minamata disease" (20).
The appropriate test depends upon the type of mercury to which a patient has been exposed. After exposure to elemental or inorganic mercury, the gold standard test is a 24-hr urine specimen for mercury. Spot urine samples are unreliable. Urine concentrations of greater than 50 μg in a 24-hr period are abnormal (21). This patient’s 24-hr urine level was noted to be 90 μg. Elemental and inorganic mercury have a very short half-life in the blood.
Exposure to organic mercury requires testing hair or whole blood. In the blood, 90% of methyl mercury is bound to hemoglobin within the RBCs. Normal values of whole blood organic mercury are typically < 6 μg/L. This patient’s whole blood level was noted to be 26 μg/L. This likely reflects the large concentration of elemental mercury the patient inhaled and the substantial amount that subsequently entered the blood.
Mercury levels can be reduced with chelating agents such as succimer, dimercaprol (also known as British anti-Lewisite (BAL)) and D-penicillamine, but their effect on long-term outcomes is unclear (22-25).
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84:1353-1357.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-824.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-1308.
- Agarwal R, Reddy C, Aggarwal AN, et al. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006;100:2235-2238.
- Soyka M. Prevalence of alcohol-induced psychotic disorders. Eur Arch Psychiatry Clin Neurosci 2008;258:317-318.
- Tavel ME, Davidson W, Batterton TD. A critical analysis of mortality associated with delirium tremens. Review of 39 fatalities in a 9-year period. Am J Med Sci 1961;242:18-29.
- McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591-598.
- Bateman T. Notes of a case of mercurial erethism. Medico-Chirurgical Transactions 1818;9:220-233.
- Buckell M, Hunter D, Milton R, et al. Chronic mercury poisoning. 1946. Br J Ind Med 1993;50:97-106.
- Rowens B, Guerrero-Betancourt D, et al. Respiratory failure and death following acute inhalation of mercury vapor. A clinical and histologic perspective. Chest 1991;99:185-190.
- Aguado S, de Quiros IF, Marin R, et al. Acute mercury vapour intoxication: report of six cases. Nephrol Dial Transplant 1989;4:133-136.
- Ibrahim D, Froberg B, Wolf A, et al. Heavy metal poisoning: clinical presentations and pathophysiology. Clin Lab Med 2006;26:67-97, viii.
- A fact sheet for health professionals - elemental mercury. Available from: http://www.idph.state.il.us/envhealth/factsheets/mercuryhlthprof.htm
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury - current exposures and clinical manifestations. N Engl J Med 2003;349:1731-1737.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol 2000;43:81-90.
- Dargan PI, Giles LJ, Wallace CI, et al. Case report: severe mercuric sulphate poisoning treated with 2,3-dimercaptopropane-1-sulphonate and haemodiafiltration. Crit Care 2003;7:R1-6.
- Eto K. Minamata disease. Neuropathology 2000;20:S14-9.
- Bakir F, Damluji SF, Amin-Zaki L, et al. Methylmercury poisoning in Iraq. Science 1973;181:230-241.
- Berlin M, Carlson J, Norseth T. Dose-dependence of methylmercury metabolism. A study of distribution: biotransformation and excretion in the squirrel monkey. Arch Environ Health 1975;30:307-313.
- Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology 1978;18:285-288.
- Graeme KA, Pollack CVJ. Heavy metal toxicity Part I: Arsenic and mercury. J Emerg Med 1998;16:45-56.
- Aaseth J, Frieheim EA. Treatment of methylmercury poisoning in mice with 2,3-dimercaptosuccinic acid and other complexing thiols. Acta Pharmacol Toxicol (Copenh) 1978;42:248-252.
- Archbold GP, McGuckin RM, Campbell NA. Dimercaptosuccinic acid loading test for assessing mercury burden in healthy individuals. Ann Clin Biochem 2004;41:233-236.
- Kosnett MJ. Unanswered questions in metal chelation. J Toxicol Clin Toxicol 1992;30:529-547.
- Zimmer LJ, Carter DE. The efficacy of 2,3-dimercaptopropanol and D-penicillamine on methyl mercury induced neurological signs and weight loss. Life Sci 1978;23:1025-1034.

The American Thoracic Society improves global health by advancing research, patient care, and public health in pulmonary disease, critical illness, and sleep disorders. Founded in 1905 to combat TB, the ATS has grown to tackle asthma, COPD, lung cancer, sepsis, acute respiratory distress, and sleep apnea, among other diseases.
AMERICAN THORACIC SOCIETY 25 Broadway New York, NY 10004 United States of America Phone: +1 (212) 315-8600 Fax: +1 (212) 315-6498 Email: [email protected]
Privacy Statement | Term of Use | COI Conference Code of Conduct

- Search Menu
- Volume 2024, Issue 3, March 2024 (In Progress)
- Volume 2024, Issue 2, February 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Answer to part 1, answer to part 2, answer to part 3, answer to part 4, answer to part 5.
- < Previous
Educational Case: A 57-year-old man with chest pain
Contributed equally.
- Article contents
- Figures & tables
- Supplementary Data
Nikhil Aggarwal, Subothini Selvendran, Vassilios Vassiliou, Educational Case: A 57-year-old man with chest pain, Oxford Medical Case Reports , Volume 2016, Issue 4, April 2016, Pages 62–65, https://doi.org/10.1093/omcr/omw008
- Permissions Icon Permissions
This is an educational case report including multiple choice questions and their answers. For the best educational experience we recommend the interactive web version of the exercise which is available via the following link: http://www.oxfordjournals.org/our_journals/omcr/ec01p1.html
A 57 year-old male lorry driver, presented to his local emergency department with a 20-minute episode of diaphoresis and chest pain. The chest pain was central, radiating to the left arm and crushing in nature. The pain settled promptly following 300 mg aspirin orally and 800 mcg glyceryl trinitrate (GTN) spray sublingually administered by paramedics in the community. He smoked 20 cigarettes daily (38 pack years) but was not aware of any other cardiovascular risk factors. On examination he appeared comfortable and was able to complete sentences fully. There were no heart murmurs present on cardiac auscultation. Blood pressure was 180/105 mmHg, heart rate was 83 bpm and regular, oxygen saturation was 97%.
What is the most likely diagnosis?
An ECG was requested and is shown in figure 1.
How would you manage the patient? (The patient has already received 300 mg aspirin).
30 minutes later the patient's chest pain returned with greater intensity whilst waiting in the emergency department. Now, he described the pain as though “an elephant is sitting on his chest”. The nurse has already done an ECG by the time you were called to see him. This is shown in figure 2.
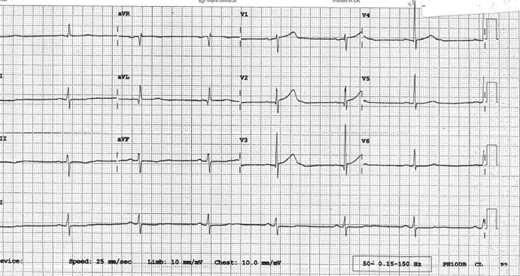
ECG on admission.
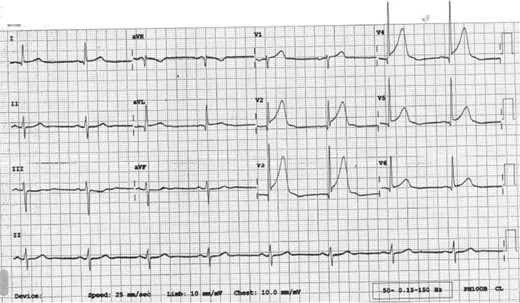
ECG 30 minutes after admission.
What would be the optimal management for this patient?
He was taken to the catheterization lab where the left anterior descending coronary artery (LAD) was shown to be completely occluded. Following successful percutaneous intervention and one drug eluding stent implantation in the LAD normal flow is restored (Thrombosis in myocardial infarction, TIMI = 3). 72 hours later, he is ready to be discharged home. The patient is keen to return to work and asks when he could do so.
When would you advise him that he could return to work?
One week later, he receives a letter informing him that he is required to attend cardiac rehabilitation. The patient is confused as to what cardiac rehabilitation entails, although he does remember a nurse discussing this with him briefly before he was discharged. He phones the hospital in order to get some more information.
Which of the following can be addressed during cardiac rehabilitation?
A - Acute coronary syndrome
Although the presentation could be attributable to any of the above differential diagnoses, the most likely etiology given the clinical picture and risk factors is one of cardiac ischemia. Risk factors include gender, smoking status and age making the diagnosis of acute coronary syndrome the most likely one. The broad differential diagnosis in patients presenting with chest pain has been discussed extensively in the medical literature. An old but relevant review can be found freely available 1 as well as more recent reviews. 2 , 3
C - Atorvastatin 80 mg, Clopidogrel 300 mcg, GTN 500 mcg, Ramipril 2.5 mg,
In patients with ACS, medications can be tailored to the individual patient. Some medications have symptomatic benefit but some also have prognostic benefit. Aspirin 4 , Clopidogrel 5 , Atenolol 6 and Atorvastatin 7 have been found to improve prognosis significantly. ACE inhibitors have also been found to improve left ventricular modeling and function after an MI. 8 , 9 Furthermore, GTN 10 and morphine 11 have been found to be of only significant symptomatic benefit.
Oxygen should only to be used when saturations <95% and at the lowest concentration required to keep saturations >95%. 12
There is no evidence that diltiazem, a calcium channel blocker, is of benefit. 13
His ECG in figure 1 does not fulfil ST elevation myocardial infarction (STEMI) criteria and he should therefore be managed as a Non-STEMI. He would benefit prognostically from beta-blockade however his heart rate is only 42 bpm and therefore this is contraindicated. He should receive a loading dose of clopidogrel (300 mg) followed by daily maintenance dose (75 mg). 14 , 15 He might not require GTN if he is pain-free but out of the available answers 3 is the most correct.
D - Proceed to coronary angiography
The ECG shows ST elevation in leads V2-V6 and confirms an anterolateral STEMI, which suggests a completely occluded LAD. This ECG fulfils the criteria to initiate reperfusion therapy which traditionally require one of the three to be present: According to guidance, if the patient can undergo coronary angiography within 120 minutes from the onset of chest pain, then this represents the optimal management. If it is not possible to undergo coronary angiography and potentially percutaneous intervention within 2 hours, then thrombolysis is considered an acceptable alternative. 12 , 16
≥ 1 mm of ST change in at least two contiguous limb leads (II, III, AVF, I, AVL).
≥ 2 mm of ST change in at least two contiguous chest leads (V1-V6).
New left bundle branch block.
GTN and morphine administration can be considered in parallel but they do not have a prognostic benefit.
E - Not before an exercise test
This patient is a lorry driver and therefore has a professional heavy vehicle driving license. The regulation for driving initiation in a lorry driver following a NSTEMI/ STEMI may be different in various countries and therefore the local regulations should be followed.
In the UK, a lorry driver holds a category 2 driving license. He should therefore refrain from driving a lorry for at least 6 weeks and can only return to driving if he completes successfully an exercise evaluation. An exercise evaluation is performed on a bicycle or treadmill. Drivers should be able to complete 3 stages of the standard Bruce protocol 17 or equivalent (e.g. Myocardial perfusion scan) safely, having refrained from taking anti-anginal medication for 48 hours and should remain free from signs of cardiovascular dysfunction during the test, notably: angina pectoris, syncope, hypotension, sustained ventricular tachycardia, and/or electrocardiographic ST segment shift which is considered as being indicative of myocardial ischemia (usually >2 mm horizontal or down-sloping) during exercise or the recovery period. 18
For a standard car driving license (category 1), driving can resume one week after successful intervention providing that no other revascularization is planned within 4 weeks; left ventricular ejection fraction (LVEF) is at least 40% prior to hospital discharge and there is no other disqualifying condition.
Therefore if this patent was in the UK, he could restart driving a normal car one week later assuming an echocardiogram confirmed an EF > 40%. However, he could only continue lorry driving once he has passed the required tests. 18
E - All of the above
Cardiac rehabilitation bridges the gap between hospitals and patients' homes. The cardiac rehabilitation team consists of various healthcare professions and the programme is started during hospital admission or after diagnosis. Its aim is to educate patients about their cardiac condition in order to help them adopt a healthier lifestyle. This includes educating patients' about their diet, exercise, risk factors associated with their condition such as smoking and alcohol intake and finally, about the medication recommended. There is good evidence that adherence to cardiac rehabilitation programmes improves survival and leads to a reduction in future cardiovascular events. 19 , 20
Oille JA . Differential diagnosis of pain in the chest . Can Med Assoc J . 1937 ; 37 (3) : 209 – 216 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC536075/ .
Google Scholar
Lee TH , Goldman L . Evaluation of the patient with acute chest pain . N Engl J Med . 2000 ; 342 (16) : 1187 – 1195 . http://www.nejm.org/doi/full/10.1056/NEJM200004203421607 .
Douglas PS , Ginsburg GS . The evaluation of chest pain in women . N Engl J Med . 1996 ; 334 (20) : 1311 – 1315 . http://www.nejm.org/doi/full/10.1056/NEJM199605163342007 .
Baigent C , Collins R , Appleby P , Parish S , Sleight P , Peto R . ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. the ISIS-2 (second international study of infarct survival) collaborative group . BMJ . 1998 ; 316 (7141) : 1337 – 1343 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC28530/ .
Yusuf S , Zhao F , Mehta S , Chrolavicius S , Tognoni G , Fox K . Clopidogrel in unstable angina to prevent recurrent events trail investigators . effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation . N Engl J Med . 2001 ; 345 (7) : 494 – 502 . http://www.nejm.org/doi/full/10.1056/NEJMoa010746#t=articleTop .
Yusuf S , Peto R , Lewis J , Collins R , Sleight P . Beta blockade during and after myocardial infarction: An overview of the randomized trials . Prog Cardiovasc Dis . 1985 ; 27 (5) : 335 – 371 . http://www.sciencedirect.com/science/article/pii/S0033062085800037 .
Schwartz GG , Olsson AG , Ezekowitz MD et al. . Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial . JAMA . 2001 ; 285 (13) : 1711 – 1718 . http://jama.jamanetwork.com/article.aspx?articleid=193709 .
Pfeffer MA , Lamas GA , Vaughan DE , Parisi AF , Braunwald E . Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction . N Engl J Med . 1988 ; 319 (2) : 80 – 86 . http://content.onlinejacc.org/article.aspx?articleid=1118054 .
Sharpe N , Smith H , Murphy J , Hannan S . Treatment of patients with symptomless left ventricular dysfunction after myocardial infarction . The Lancet . 1988 ; 331 (8580) : 255 – 259 . http://www.sciencedirect.com/science/article/pii/S0140673688903479 .
Ferreira JC , Mochly-Rosen D . Nitroglycerin use in myocardial infarction patients . Circ J . 2012 ; 76 (1) : 15 – 21 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3527093/ .
Herlitz J , Hjalmarson A , Waagstein F . Treatment of pain in acute myocardial infarction . Br Heart J . 1989 ; 61 (1) : 9 – 13 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1216614/ .
Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, et al . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation . Eur Heart J . 2012 ; 33 (20) : 2569 – 2619 . http://eurheartj.oxfordjournals.org/content/33/20/2569 .
The effect of diltiazem on mortality and reinfarction after myocardial infarction . the multicenter diltiazem postinfarction trial research group . N Engl J Med . 1988 ; 319 (7) : 385 – 392 . http://www.nejm.org/doi/full/10.1056/NEJM198808183190701 .
Jneid H , Anderson JL , Wright RS et al. . 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non–ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update) A report of the american college of cardiology foundation/american heart association task force on practice guidelines . J Am Coll Cardiol . 2012 ; 60 (7) : 645 – 681 . http://circ.ahajournals.org/content/123/18/2022.full .
Hamm CW , Bassand JP , Agewall S et al. . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC) . Eur Heart J . 2011 ; 32 (23) : 2999 – 3054 . http://eurheartj.oxfordjournals.org/content/32/23/2999.long .
O'Gara PT , Kushner FG , Ascheim DD et al. . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines . J Am Coll Cardiol . 2013 ; 61 (4) : 485 – 510 . http://content.onlinejacc.org/article.aspx?articleid=1486115 .
BRUCE RA , LOVEJOY FW Jr . Normal respiratory and circulatory pathways of adaptation in exercise . J Clin Invest . 1949 ; 28 (6 Pt 2) : 1423 – 1430 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC439698/ .
DVLA . Https://Www.gov.uk/current-medical-guidelines-dvla-guidance-for-professionals-cardiovascular-chapter-appendix .
British Heart Foundation . Http://Www.bhf.org.uk/heart-health/living-with-heart-disease/cardiac-rehabilitation.aspx .
Kwan G , Balady GJ . Cardiac rehabilitation 2012: Advancing the field through emerging science . Circulation . 2012 ; 125 (7) : e369–73. http://circ.ahajournals.org/content/125/7/e369.full .
Author notes
- knowledge acquisition
Email alerts
Citing articles via, affiliations.
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Case report
- Open access
- Published: 03 May 2021
Severe COVID-19 in the intensive care unit: a case series
- Hori Hariyanto ORCID: orcid.org/0000-0001-6746-4406 1 , 3 ,
- Corry Quando Yahya 2 &
- Ronald Christian Agustinus Aritonang 1 , 3
Journal of Medical Case Reports volume 15 , Article number: 259 ( 2021 ) Cite this article
3958 Accesses
1 Citations
7 Altmetric
Metrics details
Coronavirus disease 2019 (COVID-19) was first identified in Indonesia in March 2020, and the number of infections has grown exponentially. The situation is at its worst, overwhelming intensive care unit (ICU) resources and capacity.
Case presentation
This is a single-center observational case study of 21 confirmed COVID-19 patients admitted to the ICU from March 20, 2020, to April 31, 2020. Demographics, baseline comorbidities, clinical symptoms, laboratory tests, electrocardiogram (ECG) and chest imaging were obtained consecutively during patient care. We identified 21 patients with confirmed COVID-19 severe infection in our ICU. The mean (± standard deviation) age of the patients was 54 ± 10 years; 95% were men, with shortness of breath (90.6%) the most common symptom. Hypertension was identified as a comorbidity in 28.6% of patients. The most common reason for admission to the ICU was hypoxemic respiratory failure, with 80% (17 patients) requiring mechanical ventilation. Half of the patients (10) died between day 1 and day 18, with septic shock as the primary cause of death. Of the 11 surviving patients, five were discharged home, while six were discharged from the ICU but remained in the hospital ward. Even then, the median length of ICU stay amongst survivors was 18 days.
Conclusions
To date, there are no known effective antiviral agents or specific therapy to treat COVID-19. As severe systemic inflammatory response and multiple organ failure seems to be the primary cause of death, supportive care in maintaining oxygenation and hemodynamic stability remain the mainstay goals in treating critically ill COVID-19 patients.
Peer Review reports
Coronavirus disease 2019 (COVID-19) has spread from a single city to the entire globe with alarming speed. Arising from China, this virus has expanded rapidly to all parts of the world, knowing no geopolitical boundaries in infecting the human population. The first case of COVID-19 in Indonesia was identified in March 2020. Since then, the number of cases in Indonesia has grown exponentially; as of October 7, 2020, there had been 315,714 confirmed COVID-19 cases and 11,472 deaths [ 1 ]. While most patients with COVID-19 are asymptomatic or experience only mild symptoms, some individuals develop acute respiratory distress syndrome (ARDS) requiring mechanical ventilation, while some succumb to septic shock. Reports describing patients admitted to the intensive care unit (ICU) in Indonesia are sparse; therefore, it is our aim to share our early experience of COVID-19 pandemic care amongst ICU patients.
Study design and participants
This is a single-center observational case series study. All patients completed an informed consent form that was approved by the Ethical Committee at Siloam Hospital Kelapa Dua (Study protocol: 19-03-0317). Data were collected consecutively during admission. Enrollment included all patients admitted to the ICU starting with the first patient in March 20, 2020 up to April 31, 2020. All 21 cases enrolled in this study were confirmed COVID-19 from double-gene polymerase chain reaction (PCR) detection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using a nasopharyngeal swab in line with the diagnostic criteria guideline established by the Indonesian Ministry of Health.
Data collection
Demographics, baseline comorbidities, clinical symptoms, laboratory tests, chest imaging and electrocardiogram (ECG) changes were obtained consecutively during patient visits to the ICU. Diagnoses during the hospital course, inpatient medications, treatments including invasive mechanical ventilation and kidney replacement therapy, and outcomes including length of stay, discharge and mortality were also recorded. To quantify the extent of infection, a severity score was calculated using the CURB-65 [confusion, urea, respiratory rate, blood pressure, and 65 years of age or older] pneumonia risk score and Acute Physiology Assessment and Chronic Health Evaluation II (APACHE II) score.
Statistical analysis
Variables are reported as frequency, percentage (%), mean (SD) if they were normally distributed, and median with range (min–max) for non-normal distribution. Laboratory results are presented as actual data, and all data analysis was carried out using STATA version 12 software (StataCorp LLC, College Station, TX, USA).
Patient characteristics
During the period from March 20, 2020, through April 31, 2020, we identified 21 critically ill patients with confirmed COVID-19 infection admitted to the ICU. The demographic and clinical characteristics of the patients are shown in Table 1 . The mean (± SD) age of the patients was 54 ± 10 years (range 31–79); 20 (95%) were male and one (4.8%) was female. The mean duration of symptoms before hospital admission was 8 ± 3 days. All patients were Indonesian citizens of Malay ethnicity, and none had recently traveled to a country with known transmission such as China, South Korea, Iran or Italy. However, the majority of patients confirmed recent contact exposure from various cluster sites including family and religious gatherings. Comorbidities of patients in this critically ill population included diabetes 1 (4.8%), hypertension 6 (28.6%) and cerebrovascular disease 1 (4.8%). One (4.8%) patient was documented to be a former smoker, and another patient (4.8%) had chronic obstructive pulmonary disease.
Symptoms presented upon admission included fever [18 (85.7%) of 21 patients], cough [18 (85.7%)] and shortness of breath [19 (90.4%)]. Other symptoms reported were fatigue [3 (14.2%)], sore throat [2 (9.5%)] and myalgia [2 (9.4%)]. Upon admission, the mean APACHE score was 10–14 in seven patients (33.3%), 15–19 in 10 (47.6%), 20–24 in two (9.5%) and greater than 25 in two (9.5%). The mean CURB-65 score was 0 was nine patients (42.9%); 1 in nine patients (42.9%) and 2 in three patients (14.3%).
In this study, all patients received hydroxychloroquine, azithromycin, meropenem and antifungal prophylaxis; eight patients (38%) received compassionate-use tocilizumab, and no patients received systemic steroids. Thromboprophylaxis was given with heparin 250 U/hour, intravenously.
Laboratory findings
Table 2 shows the laboratory and radiologic findings of patients upon admission to the ICU. On admission, lymphocytopenia was common (in 86% of the patients), with a mean leukocyte count of 11.056 ± 6.604 × 10 3 /μL and low median lymphocyte count of 13.5% (interquartile range 1–19%). Inflammation markers including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and lactate dehydrogenase were also measured, and all values were dramatically elevated. Mean lactate dehydrogenase was uniformly elevated at 951 ± 140, along with mean CRP level of 217 ± 122. Hepatic alanine aspartate enzyme was 40 U/L or higher in all patients.
Chest radiographs were obtained in all 21 patients, all of which showed bilateral pulmonary opacities, while pleural effusion was seen in 12 (57.1%) of the patients (Fig. 1 ). A computed tomography (CT) scan of the chest was obtained in six patients (29%); five of which showed bilateral ground glass opacities and one consolidation. Overall, 17 patients progressed to respiratory distress and required mechanical ventilation, while the other four were discharged to the ward after a mean of 13 days in the ICU.
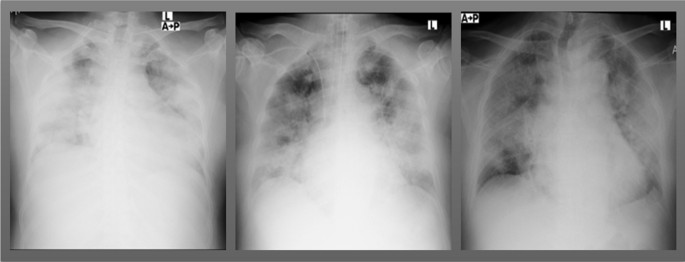
Chest films of severe COVID-19 patients upon admission to the intensive care unit
- Respiratory failure
Seventeen patients (80.9%) received invasive mechanical ventilation, as their ratios of arterial oxygen partial pressure to fraction of inspired oxygen [PaO 2 :FiO 2 (p/f ratio)] were consistent with severe acute respiratory distress syndrome (ARDS): mean p/f ratio 100 ± 36. The time to initiation of mechanical ventilation was 4 ± 3 days, and all patients were placed in the prone position starting day 2 of mechanical ventilation.
The median FiO 2 on day 1 of mechanical ventilation was 0.9 (interquartile range 0.7–1.0); on day 3, median FiO 2 was 0.6 (interquartile range 0.5–0.7), and on day 5 median FiO 2 was 0.4 (interquartile range 0.35–0.55). The median driving pressure [the difference between plateau pressure and positive end-expiratory pressure (PEEP)] on day 1 of mechanical ventilation was 23 ± 5 cmH 2 O, with median pulmonary compliance of 20 mL/cmH 2 O (interquartile range, 13–27). Initial PEEP was set at 11 ± 2 cmH 2 O. Throughout 5 days of mechanical ventilation, the median driving pressure was gradually lowered to 15 ± 3 cmH 2 O, pulmonary compliance improved to 42 mL/cmH 2 O (interquartile range, 28–52), and PEEP was maintained at 9 ± 1 cmH 2 O. The mean p/f ratio was 150 ± 62 on day 1, 193 ± 112 on day 3, and 235 ± 109 on day 5. Out of 17 patients, two (13%) developed progressive ARDS and died. Seven (41%) patients survived, with a mean duration of mechanical ventilation of 10 ± 4.8 days. Amongst these, one underwent bronchoscopy due to atelectasis; three encountered pneumothorax, and two underwent tracheostomy due to difficulty in weaning and prolonged mechanical ventilation support (greater than 20 days of mechanical ventilation).
Twelve patients (75%) presented with concurrent hypotension requiring vasopressors without clear evidence of secondary infection. Of these patients, three (18%) had transient hypotension after intubation; nine (56%) had hypotension that was unrelated to intubation or that persisted for more than 12 hours after intubation. Six patients (38%) developed septic shock and died; one (6%) experienced cardiac arrest upon prone positioning, and another patient (6%) experienced cardiac arrest due to intractable hyperkalemia and persistent acidosis, despite undergoing hemodialysis.
As of May 31, out of the 21 patients cared for in the ICU, 10 (47%) had died and 11 survived, with six (23%) patients who had been discharged from the ICU but remained in the hospital and five (23%) who had been discharged from the hospital (Fig. 2 ). The median length of ICU stay among survivors was 18 days (interquartile range, 7–36), while the median length of ward stay after ICU discharge was 11 days (interquartile range, 7–25). Fitness for discharge was based on the absence of fever for at least 7 days, improvement in chest radiograph and negative nasopharyngeal PCR test.
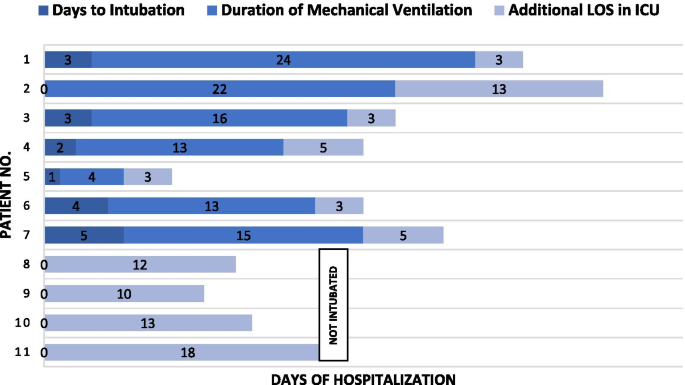
Duration of therapy amongst 11 intensive care unit survivors of severe COVID-19. LOS length of stay
Discussion and conclusion
The majority of patients admitted to our ICU were men, with a mean age of 54 ± 10 years, and had hypertension as a comorbidity. Clinical manifestations were fever, cough and shortness of breath. No gastrointestinal, renal or cerebrovascular manifestations were documented in our study. All 21 patients had abnormal blood test results with elevated CRP and liver enzymes, decreased lymphocytes, increased D-dimer and coagulation abnormalities, all of which were similar to reports from China [ 2 , 3 ]. Six chest CT scans were performed showing ground-glass opacities and/or consolidation similar to other reports [ 4 ].
Recent studies have highlighted two phenotypes in COVID-19 pneumonia. The L-type lung is characterized by normal compliance, low ventilation-to-perfusion ratio and low lung weight. Over time, the lungs may either improve or evolve into an H-type pneumonia characterized by low compliance, high right-to-left shunt and increasing pulmonary edema, which contribute to the deadly cycle of hypoxemia and strain on body organs [ 5 ]. In this report, the majority [18 (85.8%)] of the 21 patients had an admission CURB score of 0–1. Nevertheless, more than half progressed to severe ARDS and respiratory failure as evidenced by hypoxemia, progressive bilateral infiltrates and decreased respiratory system compliance (H-type COVID-19 pneumonia). Out of 17 patients receiving mechanical ventilation, two rapidly progressed to severe ARDS and died.
High-flow nasal cannula was initially used to improve oxygenation, but promptly escalated to mechanical ventilation once increased work of breathing was observed. Notably, high oxygen requirements and poor lung compliance were observed soon after initiation of mechanical ventilation. In severe ARDS, damage to type II alveolar cells not only renders surfactant inactive, but these edematous alveoli also compress alveoli in dependent regions, thereby contributing to alveolar collapse [ 6 ]. Prone positioning has the benefit of reopening collapsed alveoli, as the heart rests on the sternum and exerts less pressure on the pleura and lung [ 7 ]. This together with the lung recruitment maneuver opens the dorsal parts of the lung and allows more homogeneous ventilation and perfusion [ 8 ]. Therefore, a high initial PEEP (10-12 cmH 2 O) was given and patients were placed in the prone position for 6 hours per day. Prone positioning started on day 2 of mechanical ventilation, and an increased p/f ratio was observed from day 3 onwards.
Early in the clinical course, sputum production was minimal and sterile. As mechanical ventilation continued, coexisting lower respiratory bacterial infections were identified, further complicating the course of disease and resulting in longer ICU stays. Seven patients survived, with two encountering pneumothorax and placed on tracheostomy due to prolonged ventilator support, while the other five were successfully liberated from mechanical ventilation without any long-term sequelae. Even then, the median ICU stay among the survivors was a lengthy 18 days (interquartile range, 7–36 days).
In this study, all 21 patients received hydroxychloroquine, azithromycin, meropenem and antifungal prophylaxis, with eight patients (38%) receiving compassionate-use tocilizumab. Unfortunately, one of our patients experienced Torsades de pointes and died. Such fatal arrhythmia may have been caused by the direct effect of hydroxychloroquine and azithromycin on ventricular repolarization, thus prolonging the QT interval [ 9 ]. Hence, hydroxychloroquine and azithromycin use was terminated halfway through the course of ICU care. None of our patients received steroids, as studies during that time were inconclusive for the use of systemic glucocorticoids.
Upon admission, the majority of our patients had an APACHE score of 10–19 (mortality score of 12–22%); nevertheless, six (35%) of 17 patients who received mechanical ventilation died due to septic shock. Symptoms were similar to septic shock caused by bacterial infections, but one distinctive difference was the deterioration that occurred within a very short time (< 24 hours). This might be attributable to the massive explosive release of viral antigens, thus creating a violent inflammatory response and sudden hemodynamic collapse, as others have speculated [ 10 , 11 ]. Taken together, this suggests that no severity scores seem to aid in predicting the future course and prognosis of COVID-19 infection.
To date, there are still no solid markers for predicting disease progression, and various treatments with immunomodulators, antivirals and interleukin inhibitors are given with hopes of halting the progression of the disease, but no consensus guidelines have yet been developed. To make matters worse, this virus possesses remarkable mimicry capability, as it displays atypical presentation ranging from gastrointestinal symptoms, neurologic complications, antiphospholipid syndrome and acute myocardial injury to fatal ventricular arrhythmia [ 12 , 13 , 14 , 15 ], all of which may lead to a false diagnosis, delay treatment and postpone isolation measures within a community.
COVID-19 has emerged as a complex disease that appears to have many “faces.” Despite evidence of extensive damage both in radiologic and laboratory findings, the clinical presentation does not always seem to conform. In the midst of this pandemic, we would like to share our experience of caring for those with the greatest severity of illness: the ICU population. We understand the limitations of our study relating to its small sample size and limited laboratory investigations. However, our experience in caring for these patients has reminded us that supportive therapy remains the hallmark in fighting this self-limiting disease. Until new evidence becomes available, physicians can expect mechanical ventilation to be a lengthy journey, with bacterial co-infections, sepsis and pneumothorax encountered along the course of ICU stay.
Availability of data and materials
Please contact the author for data requests.
Abbreviations
Coronavirus disease 2019
Intensive care unit
Standard deviation
World Health Organization
Acute respiratory distress syndrome
Polymerase chain reaction
Confusion, urea, respiratory rate, blood pressure, and 65 years of age or older pneumonia risk score
Acute Physiology and Chronic Health Evaluation II
Erythrocyte sedimentation rate
C-Reactive protein
Computed tomography
PaO 2 :FiO 2
Fraction of inspired oxygen
Positive end-expiratory pressure
World Health Organization (WHO). Coronavirus Disease (COVID-19) Situation Reports. 2020.
Yang X, Yu Y, Xu J, Shu H, Ja X, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Article CAS Google Scholar
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China. JAMA. 2020;323(11):1061–9.
Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–34.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–102.
Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213(2):54-6.e1.
Article Google Scholar
Gattinoni L, Pesenti A, Carlesso E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med. 2013;39(11):1909–15.
Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–24.
Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020;17(9):1472–9.
Coz Yataco AO, Simpson SQ. Coronavirus Disease 2019 Sepsis: a nudge toward antibiotic stewardship. Chest.
Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–20.
The LR. High-stakes heterogeneity in COVID-19. Lancet Rheumatol. 2020;2(10):e577.
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382(17):e38.
Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020.
Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19(9):713–5.
Download references
Acknowledgements
Not applicable.
None received.
Author information
Authors and affiliations.
Faculty of Medicine, Department of Anesthesiology and Intensive Care, Universitas Pelita Harapan, Jl. M. H. Thamrin Boulevard 1100, Lippo Village Tangerang, Tangerang, Banten, 15811, Indonesia
Hori Hariyanto & Ronald Christian Agustinus Aritonang
Faculty of Medicine, Universitas Indonesia, Jalan Diponegoro No 77, Jakarta Pusat, 10430, Indonesia
Corry Quando Yahya
Siloam Hospitals Kelapa Dua, Jl. Kelapa Dua Raya No.1001, Kelapa Dua, Tangerang, Banten, 15810, Indonesia
You can also search for this author in PubMed Google Scholar
Contributions
HH and CY contributed to the conceptualization, data curation, formal analysis and investigation of the patients. RA contributed to data curation, formal analysis, investigation, project administration and resources. CY and HH contributed in writing this report and coordinated to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Hori Hariyanto .
Ethics declarations
Ethics approval.
All patients completed an informed consent form that was approved by the Ethical Committee at Siloam Hospitals, Kelapa Dua (Study protocol: 19-03-0317).
Consent for publication
Written informed consent was obtained from the patients’ or from the next of kin (in case the patient deceased) for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hariyanto, H., Yahya, C.Q. & Aritonang, R.C.A. Severe COVID-19 in the intensive care unit: a case series . J Med Case Reports 15 , 259 (2021). https://doi.org/10.1186/s13256-021-02799-1
Download citation
Received : 06 November 2020
Accepted : 18 March 2021
Published : 03 May 2021
DOI : https://doi.org/10.1186/s13256-021-02799-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Septic shock
- Mechanical ventilation
- Severe infection
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Care of the critically ill patient
The care of critically ill patient within the intensive care unit requires a multidisciplinary approach. An understanding of the main principles of intensive care medicine is essential for surgeons, both for participating in the management of their own critically ill patients and also because surgical complications of critical care are well recognized. This article describes the main principles of intensive care medicine within the context of the COVID-19 pandemic, giving an overview of a systematic approach to assessment and treatment of organ dysfunction, and highlights some of the complex ethical and organizational challenges.
Principles of critical care
Critical care is the process of looking after patients who either suffer from life-threatening conditions or are at risk of developing them. The intensive care unit (ICU) is a distinct geographical entity in which high staffing ratios, advanced monitoring and organ support can be offered to improve patient morbidity and mortality. However, effective intensive care demands an integrated approach that stretches beyond the boundaries of the ICU. It requires prevention, early warning and response systems, a multidisciplinary approach before and during an ICU stay, as well as comprehensive follow-up or good quality palliative care.
The cornerstones of intensive care management are the optimization of a patient's physiology, the provision of advanced organ support, and the identification and treatment of underlying pathological processes. This is best achieved through a multidisciplinary team approach, with shared responsibility between the admitting ‘parent’ team and a specialized critical care team coordinated by a critical care physician.
The surgeon on ICU
The role of the surgeon within the critical care team is crucial for advice on individual aspects of the patient care such as specific management of the surgical condition, wound care, nutrition and management of anticoagulation in the immediate postoperative period. Moreover, strategic decisions on the overall care of surgical patients, and a duty to communicate these to patients and relatives, rest jointly on both the surgeon and critical care physician. Difficult decisions regarding the need for treatment limitations and the recognition of failing treatments and burdensome treatments should be explored between both teams, the patient and family.
Organization of critical care services
Prevention and ‘critical care without walls’.
Early recognition of acutely ill patients in hospitals is a challenging task but can potentially improve outcomes. The use of early warning scores and ‘track and trigger' systems has now been widely implemented in many countries. Rapid optimization of care on the ward and early senior involvement are essential to minimize any deterioration and reduce the need for subsequent critical care admission. Medical emergency and critical care outreach teams may play an important role in facilitating early aggressive ward care as well as helping with education and development of skilled ward staff.
Referral and admission to the ICU
The decision to admit an acutely deteriorating patient to the ICU is complex and warrants senior involvement, both from the parent specialty and a critical care physician. The primary question is whether an ICU admission and escalation of care is in the patient's best interest. While considerable effort has been spent to predict outcomes with scoring systems – based on disease process, physiological parameters prior to admission, age and comorbidities – these do not necessarily apply to individual patients and may not be relevant in the acute setting. An increasingly referenced concept is that of patient frailty, as this may be an important determinant of outcome in ICU. The assessment of frailty may add important information to the decision making process in the perioperative period.
Frailty can be quantified quickly using tools such as the Clinical Frailty Scale (CFS), which gives a numerical score between 1 and 9 equating to the patient's pre-morbid activity and dependence levels. A higher score has been associated with increased mortality in surgical patients. 1 It should be noted that the CFS is not validated in patients under the age of 65 and so should be used with caution in this age group. The CFS is not designed for use in those with stable long term disability or those with a learning disability.
For each emergency referral the following issues need to be considered:
- • Is there a reversible pathological process?
- • Does the patient have the physiological reserve to withstand the insults of their illness and the necessary treatment?
- • Is there a reasonable chance of recovery with the prospect of return to an acceptable quality of life, as viewed by the patient?
- • Has the patient expressed any wishes regarding their care? Do they have an advanced directive?
For any admission, a balance must be reached between the available technical ICU interventions and the potential to cause considerable distress to the patient, with both physical and psychological impact during and beyond their ICU stay. The inherent ethical conflicts of beneficence (chance of good outcome), non-maleficence (ICU often involves distressing/painful interventions), autonomy (patients often do not have the capacity to express their wishes) and justice (responsibility with resource allocation) need to be carefully considered. These factors are complex and need individual, careful, and experienced consideration for each patient.
Broadly speaking, two types of critical care admissions are recognized:
- • Planned admissions: patients requiring optimization and monitoring of their physiological condition before or usually after an intervention, e.g. the postoperative care of the high-risk major general surgical patient to monitor for complications of the surgical procedure, anaesthetic or exacerbation of known comorbidities.
- • Emergency admissions: patients with potential or established organ failure needing monitoring and support of one or more vital organ functions, e.g. a patient with septic shock secondary to four quadrant peritonitis requiring invasive ventilation and haemodynamic support post operatively.
Overall, surgical patients requiring critical care appear to have lower acute hospital mortality than medical patients. Recent UK data estimated this at 2.4% for planned and 13.6% for emergency surgery, with 27% for non-surgical patients. 2
Levels of care
Modern critical care medicine offers a large variety of advanced monitoring and organ support capabilities ( Table 1 ). These depend on the design and scope of individual units. Below, two levels of critical care are described:
Overview of some critical care organ support and monitoring options
High-dependency unit (HDU) or ‘level 2’: Admission for single-organ support (not including invasive ventilation) and should not require a dedicated critical care nurse for each patient. Provides an environment for close monitoring of patients with or at risk of developing organ failure:
- • respiratory: non-invasive ventilation, arterial blood gases
- • cardiovascular: low dose vasopressors, invasive arterial pressure monitoring
- • renal: close fluid balance control, certain renal replacement therapies.
Intensive care unit (ICU) or ‘level 3’: Admission for multi-organ support or delivery of advanced monitoring techniques requiring at least one dedicated critical care nurse for each patient:
- • respiratory: invasive and non-invasive ventilation, extra-corporeal membrane oxygenation (ECMO) or carbon dioxide removal (ECCO 2 R) in selected centres
- • cardiovascular: vasopressor and inotropic support, advanced cardiac output monitoring, intra-aortic balloon pump, ventricular assist devices, ECMO
- • renal: renal replacement therapies
- • neurological: intracranial pressure monitoring, EEG, advanced neurological monitoring.
Post critical care
Discharge from ICU does not terminate the involvement of the critical care team and many units are developing processes to ensure high-quality in-patient follow-up with some hospitals having established RaCI (Recovery after critical illness) clinics. These may help to understand, alleviate and prevent the detrimental long-term effects of critical illness. With more patients surviving to hospital discharge it is only recently that the long-term burden and reduction in quality of life post-critical illness is being understood. 3
Sepsis is a major cause of morbidity and mortality around the world, and affects a large proportion of ICU patients either at the point of admission or as a complication during their ICU stay. Sepsis is defined as ‘life-threatening organ dysfunction caused by a dysregulated host response to infection ( Table 2 ). 3 Septic shock is sepsis complicated by hypotension despite volume resuscitation and raised serum lactate >2 mmol/L. It is worth noting that sepsis is no longer defined in terms of the systemic inflammatory response syndrome (SIRS) as this may in fact represent an appropriate response to inflammation, infection or a combination of the two. 4
Common definitions in relation to Sepsis-3 3
Over the past decade there has been a significant improvement in survival from sepsis in the developed world. This has been attributed to the fact that the basic principles of sepsis have become widely accepted, in part by global initiatives such as the Surviving Sepsis Campaign. 5
The main principles of progressive sepsis care are:
- • early recognition of sepsis
- • appropriate balanced resuscitation
- • rapid identification of the source of infection
- • timely source control
- • early and effective antimicrobial therapy
- • haemodynamic support, consideration of adjunctive therapies and high-quality supportive care.
Critical care organ support
Comprehensive care for critically ill patients usually requires a systems-based approach and integration of complex information. To provide a consistently high standard of care, some interventions have been grouped into ‘care bundles’, which have been shown to improve outcome when implemented together.
Airway and respiratory support
A significant proportion of critically ill patients will need some form of advanced respiratory support during their admission. The decision to commence mechanical ventilation must not be taken lightly as it may be associated with significant patient morbidity. On the other hand, it should not be delayed unnecessarily until the patient is in extremis. However, technical interventions do not replace good quality basic respiratory therapy, which often features input from a variety of specialties, most crucially the physiotherapist.
High flow oxygen therapy: is now widely used perioperatively, for single system ward-based support in medical and surgical patients, and in the ICU. An air–oxygen blender is used to deliver very high flows of warmed humidified oxygen at a set oxygen fraction to patients via a nasal or facial interface. The high flows of up to 60 litres/min are thought to reduce work of breathing and improve respiratory mechanics by providing a small amount of positive end expiratory pressure (PEEP) and washing out dead space gases. This combination with humidification acts to prevent drying of the mucous membranes, aids tolerability and promotes secretion clearance. High flow nasal cannula (HFNC) has been shown to be beneficial in the management of patients with severe acute hypoxic respiratory failure in comparison to non-invasive ventilation or face mask oxygen. 6
Non-invasive ventilation (NIV): is a form of respiratory support that obviates the need for endotracheal intubation. It is most commonly delivered by application of positive airway pressure via a facial interface utilizing either continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP).
CPAP refers to maintaining a constant positive pressure throughout the respiratory cycle. This is similar to PEEP in invasively ventilated patients. The benefits include a reduction in the work of breathing, reversal of hypoxia through alveolar recruitment and correction of pulmonary shunt as well as a reduction in cardiac afterload (via reduced left ventricular transmural pressure). CPAP is delivered either via a tight-fitting facemask or via a dedicated CPAP hood or helmet. Great care must be taken to avoid pressure damage, particularly to the nasal bridge.
Bi-level positive airway pressure (BiPAP) allows separate settings for positive airway pressure during the inspiratory (IPAP) and expiratory (EPAP) phase of the respiratory cycle. It maintains the benefits of CPAP but has the added benefits of augmenting the patient's tidal volume and overcoming respiratory muscle insufficiency. NIV BiPAP is most commonly provided through tight-fitting facemasks.
Successful delivery of NIV depends on many factors including patient co-operation and the absence of contraindications such as: an unprotected airway; the inability to clear secretions; marked haemodynamic instability; or the presence of an untreated pneumothorax.
NIV is well established in the treatment of respiratory failure secondary to cardiogenic pulmonary oedema and COPD. However, it is now also being used successfully in asthma, pneumonia (particularly in the immuno-compromised patient), other forms of acute lung injury, in postoperative respiratory failure and as a tool to assist weaning from mechanical ventilation.
Surgical opinion may be requested when commencing NIV in patients who have had recent upper GI or head and neck surgery, or those who have pathology in these areas due to the risk of surgical emphysema associated with delivery of positive pressure. In these cases balancing the risks of respiratory or surgical complications must be carefully considered.
Invasive ventilation: mandates tracheal intubation in one form or another. Securing the airway in critically ill patients poses significant additional challenges compared with the controlled environment of an elective theatre list. This may be due to profound physiological derangement (often paired with a rapid decline), the presence of anatomical difficulties (e.g. airway burns), external factors (e.g. cervical in-line stabilization in trauma), significant time pressures, suboptimal positioning, unfamiliar environments, and limited availability of equipment and help. Thus, thorough preparation and excellent communication of airway plans are paramount for patient safety. Some indications for tracheal intubation are outlined in Table 3 .
Indications for tracheal intubation
The mechanical ventilators used in most UK intensive care units are increasingly sophisticated and allow a wide variety of different modes that can be selected based on the patients underlying physiology and acute pathology. The more advanced machines can monitor the patient's respiratory mechanics and automatically adjust to optimize ventilation.
Broadly speaking, when intubated, a patient may be fully ventilated by the machine, may trigger breaths spontaneously, or a combination of the two. The process of reducing the support given by the ventilator to allow the patient to be safely extubated is known as weaning. When reviewing a patient who is intubated it is worth noting the amount of oxygen they require (the FiO 2 ), whether the patient is breathing spontaneously, and basic setting such as the level of PEEP they require.
Advanced techniques of respiratory support include prone ventilation (may confer mortality benefit in severe acute respiratory distress syndrome (ARDS)), extra-corporeal carbon dioxide removal (ECCO 2 R) and ECMO.
Mechanical ventilation can itself induce lung injury, presumably through barotrauma and volutrauma, but also through repeat inflation and deflation of collapsed lung areas (atelectotrauma) and through triggering the release of inflammatory mediators (biotrauma). A lung-protective ventilation strategy has been extrapolated from ventilatory management in ARDS 7 and has now been widely adopted in clinical practice. It includes the following ventilator goals:
- • Aim for tidal volumes of 6–8 ml/kg (of ideal body weight).
- • Limit plateau pressures to ≤30 cmH 2 O.
- • Apply PEEP ≥5 cmH 2 O to avoid alveolar collapse.
Complications of mechanical ventilation can be divided into those related to tracheal intubation such as damaged lips and teeth, and vocal cord injury; those resulting from equipment problems, for example ventilator malfunction or contamination; those from mechanical ventilation itself such as cardiovascular instability, ventilator-associated lung injury or pneumonia, and oxygen toxicity; and complications stemming from prolonged immobilization and sedative use in the critically ill, for example pressure sores, peripheral and respiratory muscle weakness, deep vein thrombosis, delirium, gastrointestinal tract erosions with bleeding, and so on. To reduce the likelihood and severity of these complications a ‘ventilator care bundle’ has been established featuring the following components:
- • Elevation of the head of the bed to between 30° and 45°.
- • Daily sedative interruption or reduction and assessment for readiness to extubate.
- • Peptic ulcer disease prophylaxis.
- • Thromboembolic disease prophylaxis.
Respiratory support is usually guided by clinical and laboratory findings, supplemented by chest radiographs and computer tomography. Lung ultrasound is now widely used as a non-invasive bedside diagnostic tool for the assessment of pleural effusions, pneumothoraces and lung pathology (consolidation, pulmonary oedema etc.), without using radiation or transferring the patient from the safety of the ICU.
Cardiovascular support
Haemodynamic management of critically ill patients aims to optimize tissue perfusion and oxygen delivery to the various organ systems. The cornerstones of this approach are appropriate fluid management and use of vasoactive drugs based on frequent assessment of cardiovascular changes.
Haemodynamic monitoring: Haemodynamic derangements in critical illness are complex and their assessment is notoriously difficult. Clinicians must consider pathophysiological insults to both macrocirculation and microcirculation, and to integrate complex information from various sources. These include history, physical examination, clinical observations and various monitoring modalities. The latter often assess either pressures (such as arterial or central venous pressure monitors) or blood flow (such as cardiac output monitors). Invasive cardiac output monitoring devices such as the pulmonary artery flotation catheters have drifted out of favour (outside specialized cardiac ICUs) due to their associated significant risks in the absence of improved clinical outcomes. However, several less invasive techniques, such as arterial pulse contour analysis (i.e. LiDCO™) or oesophageal Doppler devices have been developed. As most values are derived rather than measured, they are best used in a dynamic fashion, for example to assess response to a fluid challenge. Specialized investigations such as echocardiography are finding an increasing role in the haemodynamic assessment at the bedside and many intensive care physicians are now trained to perform focussed echocardiography exams.
Fluid management: the goal of fluid management is restoration of an adequate circulating volume to support tissue perfusion. However, endothelial damage and capillary leakage can lead to significant tissue oedema, which can in turn adversely affect diffusion of oxygen and nutrients. The presence of a cumulative positive fluid balance during a patients admission to ICU has been associated with worse clinical outcomes. 8 Fluid management is therefore a delicate balance of potentially conflicting requirements and the importance of monitoring fluid balance cannot be overstated.
There is ongoing controversy over the optimal type of intravenous fluid, crystalloid or colloid, used for resuscitation and maintenance during critical illness. Balanced crystalloid solutions are most commonly used in sepsis, blood products in severe trauma and, should a colloid be chosen, albumin is an accepted safe alternative in sepsis and liver disease. 9 Starch solutions are avoided due to potential nephrotoxic side effects and possible increased mortality, 10 and gelatins lack evidence of benefit or harm.
Blood transfusion in all patients, including the general critical care population, is not recommended until the haemoglobin is less than 70 g/L and then optimally should be administered in single unit aliquots. 11 A higher threshold may however be considered in the presence or anticipation of bleeding, or in patients with previous myocardial infarction or unstable angina.
Vasoactive drugs and principles of use: Vasopressor and inotropic agents are short-term to medium-acting drugs that are used to enhance vascular tone or cardiac output in a variety of critical illness conditions. They are used as a temporary measure until sufficient cardiovascular function returns on resolution of the pathological process.
Vasopressors trigger smooth muscle contraction in peripheral blood vessels, leading to increased systemic vascular resistance as well as vasoconstriction in venous capacitance vessels. The observed net effect is often an increase in blood pressure. Frequently used vasopressors include norepinephrine, epinephrine, metaraminol, phenylephrine, dopamine (via α-adrenoceptor effect) and vasopressin (via vasopressin V 1 receptors).
Inotropes increase the contractility of the myocardium, thereby leading to a rise in cardiac output. Examples of commonly used inotropes are epinephrine, dobutamine, dopamine (via β-adrenoceptor effects) and milrinone (a phosphodiasterase inhibitor). Levosimendan is a newer type of inotrope which works by increasing myocardial sensitivity to calcium.
Septic patients often require escalating haemodynamic support. Aggressive fluid resuscitation is followed by increasing doses of vasopressors (e.g. norepinephrine followed by the addition of vasopressin). Hydrocortisone may be added to reduce the dose and duration of vasopressor support. If sepsis-induced myocardial dysfunction is suspected, temporary inotropic support (e.g. with dobutamine or epinephrine) may be required.
Central nervous system
Admission to ICU may be triggered by an altered sensorium, most commonly a reduced level of consciousness and often reported using the Glasgow Coma Scale (GCS). ICU care is required as the patient may be at risk of airway compromise and need higher nursing input.
Neuroprotective management is essential for patients with intracranial or spinal pathology (e.g. traumatic brain injury, ischaemic strokes, intracranial bleeds, spinal cord ischaemia). It requires a multisystem approach with critical importance of meticulous ventilatory and haemodynamic support. Advanced imaging and monitoring techniques such as intracranial pressure or neurological function monitoring are now available to inform treatment decisions. In addition, extracranial disease processes pose a risk of causing secondary brain injury, which may be preventable. An example is targeted temperature management and prevention of hyperthermia, potentially leading to improved neurological outcome in patients surviving out of hospital cardiac arrest. 12
Renal support
Acute kidney injury (AKI) is a major complication of critical illness occurring in up to 67% in the general ICU population and represents a significant therapeutic challenge for the intensive care team as the mortality of critically ill patients with AKI remains high (40–50%). The KDIGO definition of AKI classifies severity based on serum creatinine and urine output to give a stage between 1 and 3.
Pre-renal causes (hypotension, sepsis, low cardiac output) are commonly the initial precipitant of AKI in critical illness. However, renal dysfunction often becomes multifactorial during the ICU stay, for example through parenchymal damage from nephrotoxic drugs. Post-renal causes are rare in the critically ill but need to be excluded.
Treatment of AKI relies on timely diagnosis, adequate fluid management, haemodynamic support and elimination of the underlying and contributing causes. When AKI is severe, renal replacement therapy (RRT) may be necessary to maintain homeostasis of fluid, electrolytes and metabolic waste products. Indications for RRT in critical illness include:
- • oliguria/anuria
- • urea >35 mmol/L or uraemic complications (pericarditis, encephalopathy)
- • creatinine >400 μmol/L
- • K + > 6.5 mmol/L or rapidly rising
- • pulmonary oedema
- • uncompensated metabolic acidosis (pH < 7.1)
- • severe hyperthermia (>40°C)
- • overdose with dialysable toxins
RRT relies on removal of unwanted solutes and water through a semi-permeable membrane. The techniques of intermittent haemodialysis (IHD; relying on diffusion) and continuous veno–veno haemofiltration (CVVH; based on convection; can be combined with dialysis) are widespread. CVVH modes are preferred in the UK for cardiovascular stability but there is lack of strong evidence comparing different modalities. The optimal dose of CVVH is controversial and currently 25–30 mL/kg/h are recommended unless dictated by specialized circumstances. Both IHD and CVVH require insertion of a large-bore double lumen venous catheter into a large central vein and some form of anticoagulation is required. The anticoagulant of choice is changing from a heparin-based to a citrate-based regime to further improve circuit lifespan and reduce bleeding risk.
Gastrointestinal support and nutrition
Many patients are malnourished on admission to the ICU. This has a profound impact on their ability to withstand the physiological stresses of critical illness. Moreover, critical illness can directly affect gut function and contribute to an impaired nutritional balance. Malnutrition may lead to an increased risk of infection, poor wound healing and loss of muscle bulk. A thorough nutritional assessment on admission to identify high-risk patients and timely institution of nutritional support are therefore crucial to improve patient outcome. The surgeon has a key role in decision making regarding the initiation of feeding in the post-surgical patient. The decision of whether to start early enteral feeding, to delay feeding or to start parenteral nutrition is not straightforward.
The preferred route of nutritional support has been an area of great controversy. Enteral feeding often involves nasogastric or nasojejunal feeding tubes, frequently facilitated using prokinetics (metoclopramide and erythromycin). Parenteral nutrition is usually administered through a central vein and provides an alternative route in persistent gut failure. Both modalities are associated with a significant number of complications. At present, early enteral nutrition is the favoured approach as early parenteral nutrition does not confer clear patient benefits and has additional risks. 13 The role of nutritional supplements and immunonutrients such as glutamine and arginine remains controversial. Glycaemic control is now a cornerstone of good critical care practice. However, blood sugar targets have been relaxed (aim ≤10 mmol/L) due to the significant risk of hypoglycaemia with the previously advocated intensive insulin therapy. 14
Neuromuscular considerations
A significant proportion of critically ill patients suffer from profound muscle weakness. In addition to distinct disease entities that may precipitate critical care admission (i.e. Guillain-Barré syndrome, myasthenia gravis), many patients acquire neuropathies, myopathies or a combination of both. These are often collectively described as intensive care unit-acquired weakness (ICUAW). Several factors play a contributing role, including muscle disuse atrophy, sepsis, multiple organ dysfunction syndrome, exposure to certain drugs (i.e. corticosteroids, neuromuscular blocking agents) and malnutrition. This is a common finding in patients who have survived a significant complication of surgery such as an anastomotic leak, these patient have often required multiple trips to theatre and received significant organ support. ICUAW may lead to significant delays in weaning from mechanical ventilation and discharge from ICU. Furthermore, patients are prone to developing muscle contractures. The role of early mobilization and regular and intensive physiotherapy is crucial in ameliorating the consequences of critical illness weakness.
Sleep and delirium
Critical illness is often associated with profound disturbance in the patient's natural sleep–wake cycle. The ideal situation of daytime wakefulness and night time rest is difficult to achieve on the ICU. Contributing factors are the underlying disease process, medication side effects, frequent interventions, pain, mechanical organ support, and high noise and lighting levels. Efforts to minimize the detrimental effects of sleep disturbance therefore focus on minimizing the above risk factors and promoting sleep hygiene by re-establishing a normal circadian rhythm. Furthermore, pharmacological efforts including sedation breaks, analgesia-based sedation regimes and melatonin are being used in some units. Awareness of psychological aspects of critical illness and recovery can improve the patient and relative experience, and the return to function after discharge.
Delirium is an acute and fluctuating confusional state, with features of inattention and disorganized thinking. It affects up to 69% of ventilated patients, is frequently under-recognized (especially hypoactive delirium), and is an independent predictor of mortality. Consequently, daily delirium-screening assessments such as the CAM-ICU are now advocated.
Risk factors for postoperative delirium include age, existing cognitive impairment, depression, sensory impairment, medical co-morbidity and psychotropic drug use. Precipitating factors for postoperative delirium include surgery, critical care admission, polypharmacy (including sedatives, benzodiazepines and opiates), infection, hypoxia, dehydration, poor nutritional status, metabolic derangement, pain, constipation and sleep deprivation. 15 The development of delirium in the post-surgical patient requires careful assessment as this may be the first sign of a complication of surgery such as an anastomotic leak or the development of a postoperative pneumonia. A high index of suspicion is required in patients with a hypoactive delirium as the presentation is more subtle than the hyperactive delirium patient with agitation. Management depends on the type (hyperactive, hypoactive or mixed) and comprises treatment of the underlying cause, avoidance of precipitants, reassurance for the patient as well as judicious and targeted pharmacological intervention to ensure the patient and staff remain safe.
Further critical care considerations
Critical care is a multidisciplinary endeavour. In addition to the resident critical care staff, there is invaluable input from many specialties including physiotherapy, pharmacy, nutrition, microbiology, radiology, psychology, and speech and language teams.
Infection control
Critically ill patients are at increased risk of acquiring infections with multi-resistant organisms. The prevalence of these ‘super bugs’ is often specific to the country, hospital or individual critical care unit. Antimicrobial stewardship is a major part of day-to-day management of the critically ill patient. In surgical patients it is important to ensure that appropriate prophylaxis is given intraoperatively but also that postoperative antibiotics are only prescribed in accordance with the principles of good antibiotic stewardship based on the best available evidence.
Effective infection control needs to focus on prevention, screening and avoidance of cross-contamination. As a visiting team to the intensive care unit, it is important to follow the local protocols such as appropriate personal protective equipment (PPE) when examining patients. Most measures are similar to other hospital wards but specific measures on critical care units include:
- • selective gut decontamination in ventilated patients
- • isolation of contagious patients
- • frequent microbiology input to rationalize antibiotic use
- • meticulous attention to asepsis on insertion and handling of invasive lines.
The Matching Michigan initiative aims to reduce central venous line infection rates and is founded on strong evidence that strict asepsis can lead to a significant reduction in mortality. 16
End-of-life care
Inevitably, a proportion of patients will die on the ICU from their underlying illness. Recent UK figures suggest a critical care unit mortality of around 13% in general ICUs but this will vary depending on case mix. 2 Prognostication is imperfect; therefore, the intended benefits of continued treatment need to be balanced against the potential burden for the patient. Once it becomes apparent that escalation or continuation of treatment is not in the patient's best interest, decisions on limitation or withholding of life-sustaining therapy are required. Often, patients are not able to directly express their wishes. Therefore, this decision rests on the critical care and parent specialty team. Respectful discussions with the patient, when possible, and family are essential in this process. If withdrawal of ongoing active treatment is deemed appropriate, the main focus becomes palliative, addressing symptom relief, comfort and dignity at the end of life.
Organ donation
An international shortage of organ donors necessitates consideration of organ donation in all dying critical care patients. Two different pathways are recognized in the UK: donation after circulatory death (DCD) and donation after brain death (DBD). The latter requires a process of formal brain-stem death testing for diagnosis. Some countries routinely supplement this with ancillary testing (imaging of cerebral blood flow or electro-encephalographic response to external stimuli). Early physiological stabilization and donor optimization improves transplant outcomes after DBD. Dedicated organ donation teams can facilitate this process, help to support the donor's family and coordinate retrieval of organs by the receiving teams.
Critical care in 2020: COVID-19
It is hard to understate the impact of the COVID-19 pandemic on intensive care medicine.
Ventilatory support is the most common indication for critical care admission in COVID-19 with approximately 75% of admitted patients requiring advanced respiratory support which may be prolonged. 17 Patients receiving critical care with a diagnosis of COVID-19 in England, Wales and Northern Ireland have an approximately 39% mortality. 17
Current practice is best supportive care with basic and advanced ventilatory support as required; treatment of any co-existing bacterial infection; strict fluid management to avoid lung injury; thromboprophylaxis; and adherence to infection control. To date, only dexamethasone has been shown to reduce mortality in patients with severe respiratory complications of COVID-19. 18
The associated pro-coagulant state found in COVID-19 makes complications such as venous thromboembolism and ischaemic stroke more common in this patient group and may be the reason for intensive care admission.
Elective surgical patients who develop symptomatic COVID-19 are at increased mortality risk and have higher rates of pulmonary complications. 19
Practice points
- • Intensive care medicine is the provision of advanced organ support and monitoring to patients who are critically ill or at high risk of deterioration
- • Surgeons should be aware of the principles of intensive care medicine to understand the progress of their own patients, but also as they may be asked to assist in the management of surgical complications of critical illness
- • A multidisciplinary approach is essential, from admission through to rehabilitation following discharge
- • Morbidity from critical illness is high, so when considering intensive care admission careful thought must be given to the best interests of the patient
Advertisement
Delirium in critical illness: clinical manifestations, outcomes, and management
- Narrative Review
- Published: 16 August 2021
- Volume 47 , pages 1089–1103, ( 2021 )
Cite this article
- Joanna L. Stollings ORCID: orcid.org/0000-0001-6792-1646 1 , 2 ,
- Katarzyna Kotfis 3 ,
- Gerald Chanques 4 ,
- Brenda T. Pun 1 , 5 ,
- Pratik P. Pandharipande 1 , 5 , 6 &
- E. Wesley Ely 1 , 5 , 7 , 8
57k Accesses
95 Citations
402 Altmetric
Explore all metrics
Delirium is the most common manifestation of brain dysfunction in critically ill patients. In the intensive care unit (ICU), duration of delirium is independently predictive of excess death, length of stay, cost of care, and acquired dementia. There are numerous neurotransmitter/functional and/or injury-causing hypotheses rather than a unifying mechanism for delirium. Without using a validated delirium instrument, delirium can be misdiagnosed (under, but also overdiagnosed and trivialized), supporting the recommendation to use a monitoring instrument routinely. The best-validated ICU bedside instruments are CAM-ICU and ICDSC, both of which also detect subsyndromal delirium. Both tools have some inherent limitations in the neurologically injured patients, yet still provide valuable information about delirium once the sequelae of the primary injury settle into a new post-injury baseline. Now it is known that antipsychotics and other psychoactive medications do not reliably improve brain function in critically ill delirious patients. ICU teams should systematically screen for predisposing and precipitating factors. These include exacerbations of cardiac/respiratory failure or sepsis, metabolic disturbances (hypoglycemia, dysnatremia, uremia and ammonemia) receipt of psychoactive medications, and sensory deprivation through prolonged immobilization, uncorrected vision and hearing deficits, poor sleep hygiene, and isolation from loved ones so common during COVID-19 pandemic. The ABCDEF (A2F) bundle is a means to facilitate implementation of the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) Guidelines. In over 25,000 patients across nearly 100 institutions, the A2F bundle has been shown in a dose–response fashion (i.e., greater bundle compliance) to yield improved survival, length of stay, coma and delirium duration, cost, and less ICU bounce-backs and discharge to nursing homes.
Similar content being viewed by others

A randomized clinical trial to evaluate the effect of post-intensive care multidisciplinary consultations on mortality and the quality of life at 1 year
Tarek Sharshar, Lamiae Grimaldi-Bensouda, … on behalf of the Suivi-Rea Investigators

Guidelines for Neuroprognostication in Comatose Adult Survivors of Cardiac Arrest
Venkatakrishna Rajajee, Susanne Muehlschlegel, … Panayiotis N. Varelas

Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients
Aaron M. Cook, G. Morgan Jones, … Lori Shutter
Avoid common mistakes on your manuscript.
Introduction and rationale
Delirium is a commonly neglected manifestation of organ dysfunction in the ICU. It is commonly unmonitored and not discussed on rounds [ 1 ]. This is often because the ICU team feels there is nothing that can be done about delirium since we are already treating the patient’s main diseases, or because it might seem logical that a sedated patient would have a cognitive dysfunction [ 2 ]. Beyond that, there seems to be a perception that the patient “needs” the sedatives and is too sick to get out of the bed anyway. On one hand the sedatives enable mechanical ventilation, but on the other they contribute to delirium development. Also, temporal, and spatial disorientation is often considered as the norm in patients sedated for several days and nights, who may be in ICU rooms without windows or with a direct view of the outside [ 3 ]. These factors lead to an indifference about this form of brain dysfunction that results in the patient’s suffering well beyond ICU discharge. It is linked to a greater risk of demise and imposes additional burden on the family and caregivers.
In this narrative review, we aim to describe the current state of evidence for diagnostic, preventive and therapeutic measures that can mitigate the course of delirium. It is essential that as an ICU community, we consider the importance of delirium prevention and treatment in our daily management of critically ill patients. With higher acuity of patients and increased complexity of care, we must find ways to avoid over-sedation and prolonged immobilization to help patients have more complete and intact survival.
Definition and prevalence
According to the Diagnostic and statistical manual of mental disorders: DSM-5 delirium is defined as disturbance in attention (top mandatory feature) that develops over a short period of time, is associated with additional disturbances in cognition that are not better explained by another preexisting, established or evolving neurocognitive disorder, and do not occur in the context of a severely reduced level of arousal, and evidence from the history, physical examination or laboratory findings that indicate that the disturbance is a direct physiological consequence of another medical condition, substance intoxication, or withdrawal [ 4 , 5 ]. If one or more of the delirium criteria are lacking, a diagnosis of subsyndromal delirium [ 6 , 7 ] can be made for which the management is quite similar to that for delirium, but it is also important to consider several differential diagnoses, e.g. alcohol withdrawal syndrome [ 8 ] that usually begins with hallucinations and delusion before the well-known “delirium” tremens, interruption of antipsychotics in patients suffering from mental illness [ 9 ], isolated hallucinations associated with the use of opioids [ 10 ], as well as the sleep deprivation that is frequent in ICU patients and associated with isolated hallucinations without cognitive dysfunction experimentally [ 11 ].
Historically, delirium was reported in 60–80% of mechanically ventilated patients [ 12 , 13 , 14 ] and 20–50% of lower severity of illness ICU patients [ 15 , 16 ]. With increased utilization of validated diagnostic tools globally, using translations of these tools into over 30 languages (see translations at https://cibs.webflow.io/medical-professionals/downloads/resource-language-translations ), and modifications of routine management in ICUs to reduce the culture of over-sedation and immobility, delirium rates in many ICUs are now down by about 25% [ 12 , 17 , 18 ]. In fact, delirium prevalence was reported to be 48% in a large, 21 center, prospective study including only mechanically ventilated and shock patients, a population that for > 15 years had consistently shown delirium rates ~ 75% using the same methodology [ 17 ]. In the ICU, delirium may present as hyperactive (agitated and restless), hypoactive (flat affect, apathy, lethargy, decreased responsiveness), or mixed hyper/hypoactive states, where patients fluctuate among these states. Hypoactive delirium is the most difficult to detect. Unless a validated screening tool is used, detection can be missed due to the clinical presentation being misinterpreted as fatigue or depression. Hypoactive delirium portends more dangerous outcomes [ 19 , 20 , 21 ].
Additionally, delirium has been classified as rapidly reversible sedation-related delirium. Rapidly reversible sedation-associated delirium is defined as delirium while receiving sedation that resolved within 2 h after stopping sedation during a spontaneous awakening trial (SAT). Rapidly reversible delirium was found in 12% of the 102 patients while 75% of these patients had persistent delirium (their delirium persisted for more than 2 h after sedative interruption). Thus, assessing patient’s mental status through serial assessments of delirium throughout the day both before and after SAT will give the best picture of the patient’s mental status [ 22 ]. In the case of persistent delirium after SAT, or if SAT cannot be performed for some reason, all predisposal factors of delirium (other than analgesia sedation) should be screened and managed.
A multicenter, prospective cohort study of adult medical and surgical ICU patients with respiratory failure and/or shock within two parallel studies (BRAIN-ICU) and Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU) was conducted to determine the association between the duration of clinical phenotypes of delirium and Repeatable Battery for Assessment of Neuropsychological Status (RBANS) score, an instrument to assess global cognitive function in adults, at 3 and 12 months following critical illness [ 2 , 23 , 24 ]. The clinical phenotypes of delirium were defined as hypoxic, septic, sedative-associated, or metabolic (renal of liver dysfunction) delirium [ 13 ]. Sedative-associated delirium was the most common phenotype of delirium, which was present during 2634 (63%) of delirium days (Figs. 1 ) [ 2 ]. A worse RBANS global cognition score 12 months later was predicted by a longer duration of sedative-associated delirium after adjusting for covariates (difference in score comparing 3 days vs 0 days: − 4.03, 95% CI − 7.80 to − 0.26). Worse cognitive function at 12 months was predicted by longer durations of hypoxic delirium (− 3.76, 95% CI − 7.16 to − 0.37), septic delirium (− 3.67 − 7·13 to − 0.22), and unclassified delirium (− 4.70, − 7.16 to − 2.25). However, the duration of metabolic delirium did not predict worse cognitive function at 12 months (1.14, − 0.12–3.01) [ 2 ].
“CONFIRM or EXCLUDE DELIRIUM: To diagnose any organ dysfunction it is necessary to identify the fact, the degree and the causes of this dysfunction. With brain dysfunction, active screening for delirium, using the CAM-ICU or ICDSC is critical. In this case, it is necessary to identify if the patient can pay attention and organize thoughts. Assessments of inattention, such as falling asleep in the middle of a conversation or missing details of the conversation can be used. Then ask the patient to hold up two fingers of one hand and repeat this action with the other hand. Failing to perform these easy tasks is a highly specific screen for delirium. The next step is to identify the cause of brain dysfunction.” [ 25 ]
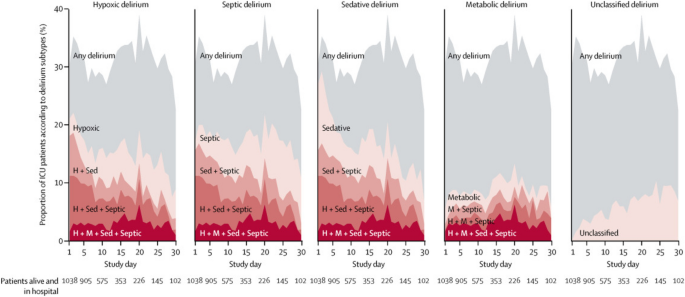
Prevalence of delirium phenotypes. Each plot area (on the y-axis) shows the percentage of the study participatnets in the hospital who had any delirium, a single delirium phenotype, or a combination of multiple delirum phenotypes according to the study day (shown on the x-axis). The grey shading indicates the overall percentage of participants with delirium on each study day. The red lines and shaded area represent the numbe of phenotypes of delirum present, with darker reds respresenting the presence of more phenotypes of delirum. The lighetest red regions show the percentage of participants with a given single phenotype ( H hypoxic, M metabolic, Sed sedative, Sep septic)
Delirium detection
Using rigorous psychometric evaluation, the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) Guidelines recommend routine monitoring of delirium in adult ICU patients and using with either the Confusion Assessment Method for the ICU (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC) [ 25 , 26 ]. The CAM-ICU was originally validated in 96 adult patients at Vanderbilt University Medical Center (USA) in a medical or coronary ICU. Critical care study nurses performed 471 paired evaluations and compared with assessments by delirium experts using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. Compared with the reference standard used for diagnosing delirium, the CAM-ICU had a sensitivity of 100% and 93%, specificity of 98% and 100%, and high interrater reliability ( κ = 0.96; 95% CI, 0.92–0.99) [ 12 ]. The CAM-ICU can be conducted in under 1 min, including in non-verbal patients, has been modified and validated in pediatric, emergency department, and neurocritical care patients, and has been translated into over 30 languages [ 27 , 28 , 29 ]. The CAM-ICU provides a result for delirium at the time of the test performance. It should be conducted at least once per shift, and if at all possible, each time changes in consciousness occur (e.g., before and after sedation cessation) [ 30 ]. The updated version of the CAM-ICU that was published in 2014 was also validated against the fifth version of DSM-5 using strict and standardized neuropsychological evaluation [ 3 ]. The ICDSC was originally validated in 93 patients at Hôpital Maisonneuve-Rosemont in Montreal, Quebec, Canada. A psychiatry evaluation was compared to an ICU physician evaluation. The sensitivity and specificity of the ICDSC was evaluated using a receiver operating characteristic (ROC) analysis. The area under the ROC curve was 0.9017; the ICDSC’s predicted sensitivity was 99% and specificity was 64% [ 15 ]. The ICDSC is well suited to patients that are non-communicative and includes data obtained during routine bedside care over the whole nursing shift. Both the CAM-ICU and the ICDSC can recognize patients that have subsyndromal delirium (i.e., have some abnormal features in their delirium assessment, but not meeting all criteria for delirium diagnosis). A recent systematic review of studies in ICU patients using the CAM-ICU demonstrated pooled sensitivity of 80% and specificity of 96%, while the ICDSC demonstrated a pooled sensitivity of 74% and specificity of 82% [ 31 ]. However, the sensitivity of both tools many decrease when performed by bedside personnel as compared to researchers [ 32 ]. This highlights the importance of education [ 30 ]. These two tools, the CAM-ICU and the ICDSC, are widely used in all types of critical care settings all over the world (i.e., medical, surgical, neurological, and cardiac).
Lastly, there are a small number of assessment tools designed to quantify the severity of delirium, which has been linked to increased mortality and the possibility of a nurse home placement [ 33 , 34 , 35 ]. While mainly used for research in the ICU population, delirium severity scores have been used in both in clinical settings and in research outside of the ICU. The Delirium Rating Scale (DRS) [ 33 ], CAM-S [ 34 ] and the CAM-ICU-7 [ 35 ] are the three tools used to rate the severity of delirium. The DRS [ 33 ] was designed for research, while the CAM-S [ 34 ] has been validated in general medicine and in elective, major, noncardiac surgery patients. The CAM-ICU-7, a severity scoring adapted from the CAM-ICU assessment, is an ICU delirium severity score that was validated in 518 adult medical, surgical, and progressive ICUs at three academic medical centers. Patients received the CAM-ICU and Richmond Agitation-Sedation Scale (RASS) assessments twice daily. Patients were assessed with both the CAM-ICU and the Delirium Rating Scale-Revised (DRS-R)-98. A 7-point scale rated 0–7 was derived from responses to the CAM-ICU and RASS assessments. High internal consistency (Cronbach’s alpha = 0.85) and good correlation with DRS-R-98 scores (correlation coefficient = 0.64) was found with the CAM-ICU-7. Good predictive validity was found with the CAM-ICU-7 showing higher odds (OR = 1.47; 95% CI = 1.30–1.66) of in-hospital mortality, and lower odds (OR = 0.8; 95% CI = 0.72–0.9) of being discharged home after adjusting for co-factors. Increased length of ICU stay was also associated with higher CAM-ICU-7 scores ( p = 0.001). Further studies need to be conducted with this tool to determine if it could be utilized to correlate delirium severity with long-term complications of delirium. Additionally, studies should compare this tool to other delirium severity measures and provide validation in a various populations of critically ill patients prior to utilization in clinical practice [ 35 ].
The Prediction Model for Delirium (PRE-DELIRIC), The Early Prediction Model for Delirium (E-PRE-DELIRIC), and the Lanzhou model are 3 prediction models that could aid clinicians in preventing or treating delirium. The PRE-DELIRIC includes 10 predictors [age, APACHE II score, admission group (medical, surgical, trauma, neurologic), emergency admission, infection, coma, sedation, morphine use, urea level, and metabolic acidosis], the E-PRE-DELIRIC includes 9 predictors [age, history of cognitive impairment, history of alcohol abuse, blood urea nitrogen, admission group (medical, surgical, trauma, neurologic), emergency admission, mean arterial blood pressure, use of corticosteroids, and respiratory failure], and the Lanzhou Model includes 11 predictors (age, APACHE II score, mechanical ventilation, emergency surgery, coma, multiple trauma, metabolic acidosis, history of hypertension, history of delirium, history of dementia, and use of dexmedetomidine). A prospective observational study of 455 ICU patients validated these predictive models in routine clinical practice. The PRE-DELIRIC showed an area under the receiver operating characteristic (AUROC) curve of 0.79 (95% CI, 0.75–0.83), the E-PRE-DELIRIC showed an AUROC curve of 0.72 (95% CI, 0.67–0.77), and the Lanzhou Model showed an AUROC curve of 0.77 (95% CI, 0.72–0.81). However, the outputs from these models are often not pragmatic and make real time action by clinicians limited, especially if calculated in patients that have been in the ICU more than 24 h [ 36 ]. They can be used for screening for high-risk delirium patients before enrollment in clinical trials on delirium management, and/or for comparing baseline characteristics of these patients.
The role of magnetic resonance imagining (MRI) in the evaluation and management of delirium is unclear [ 37 ]. MRIs may provide diagnostic information for structural problems such as strokes or abscesses that guides therapy. MRI may also provide information on long-term cognitive prognosis [ 38 ]. Pre-operative deep and white matter and thalamic abnormalities on diffusion tension imaging have been shown in elderly patients with postoperative delirium [ 39 ]. A case series of 8 patients that underwent MRI showed white mater hyperintensities (WMH) and atrophy in 6 patients. Smaller WMH were found in younger patients. Six patients had a 3-month neuropsychological follow up which showed memory impairment, executive dysfunction, and attention impairment [ 40 ]. There is no obvious role of MRI in standard delirium care, as scanning adds burden for patients, uses a lot of resources and is likely to yield motion artifacts. However, in case of persistent delirium after having managed all potential causes, MRI can be indicated to look for any brain injury that cannot be seen with brain CT (small ischemic stroke, bleeding, encephalitis, etc.) By contrast, MRI is an excellent tool for research purposes in delirium.
EEG is a potentially useful tool to assess for delirium. Inflammatory mediators cross the blood–brain barrier and increase vascular permeability and result in EEG changes [ 41 , 42 ]. A prospective cohort of non-intubated patients underwent delirium assessment with the 3D-CAM within 1 h of an EEG. Generalized theta or delta slowing was the EEG finding most strongly associated with delirium (odds ratio 10.3, 95% CI 5.3–20.1). Prevalence of delirium severity correlated with overall delirium severity ( R 2 = 0.907) and each of the individual features of the CAM. After adjustment for delirium presence or severity, EEG slowing was associated with longer hospitalizations, worse functional outcomes, and increased mortality. However, larger studies need to be conducted to confirm these findings [ 43 ]. EEG is also indicated to eliminate non-convulsive seizures that can be associated with delirium, especially in ICU septic patients [ 44 ].

ICU delirium and patient outcomes
“DELIRIUM IS A MANIFESTATION OF BRAIN DYSFUNCTION: The longer a patient suffers from organ dysfunction, the greater is the chance for prolonged and irreversible impairment. This holds true for delirium as a marker of acute brain dysfunction.” [ 23 ]
Delirium in hospitalized patients is a strong independent predictor of mortality, increased hospital length of stay, subsequent hospitalizations, long-term cognitive impairment, and cost of care. A prospective cohort study of 275 adult medical and coronary ICUs sought to determine the effect of delirium on mortality and length of stay. During the ICU stay, 183 (81.7%) patients developed delirium. Following adjustment for age, severity of illness, comorbid conditions, coma, and use of sedation or analgesia, delirium was independently associated with higher 6-month mortality (adjusted hazard ratio [HR], 3.2; 95% confidence interval [CI], 1.4–7.7; p = 0.008), longer hospital stay (adjusted HR, 2.0; 95%CI, 1.4–3.0; p < 0.001), and a longer post-ICU stay (adjusted HR, 1.6; 95% CI, 1.2–2.3; p = 0.009) [ 14 ]. The true attributable risk of mortality to delirium has been evaluated in other studies [ 45 ] specifically evaluating the differential severity of illness prior to delirium onset demonstrating that delirium is not casually related to mortality. This study specifically found that in patients with > 2 days of delirium in the ICU, there was a true risk of mortality attributable to delirium. This study brings to light the differences between associations and causality. Causality requires the following criteria: strength of association, consistency, temporality, biological gradient, plausibility, and lastly an experiment demonstrating that treatment of delirium decreases mortality.
Further the incident risk of delirium mortality was recently evaluated in 1495 critically ill adults. Incident delirium and days spent with delirium were not significantly associated with mortality. Both, days spent with coma and days spent with delirium or coma were significantly associated with mortality [ 46 ]. A retrospective cohort study of 6323 ICU patients evaluated the association between delirium subtypes and 90-day mortality following adjustment for covariates. Only mixed delirium, not hyperactive, hypoactive, or rapidly reversible delirium was associated with 90-day mortality [1.57 (95%CI: 1.51–2.14)] [ 47 ].
The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study, a large, multicenter, prospective observational cohort study of 821 adult medical ICU and surgical ICU patients with respiratory failure, cardiogenic or septic shock was conducted to determine the prevalence of long-term cognitive impairment following critical illness. At 3 months following discharge, RBANS score similar to Alzheimer’s disease was found in 26% of patients, and a score similar to moderate traumatic brain injury was found in 40% of patients (Fig. 2 ). Both young and older adults, with and without comorbidities, experienced these impairments, which were still present at 12 months in 24% and 34% of these individuals [ 23 ]. A subgroup analysis of 402 patients that received surgery with anesthesia exposure had similar global cognition scores to those who did not, at 3 and 12 months even after in-hospital or baseline covariates [ 48 ]. Delirium was the strongest independent predictor of cognitive impairment in this cohort. Delirium does not always precede cognitive impairment and there are no randomized, clinical trials to date demonstrating that long-term cognitive impairment is improved through treatment of delirium [ 49 ].
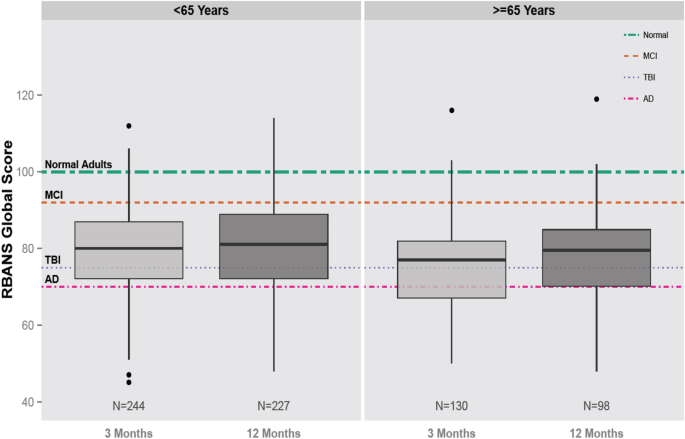
Global cognition scores in survivors of critical illness
Delirium also has a high cost. In a subgroup analysis within the BRAIN-ICU study, the patient-level 30-day cumulative cost attributable to higher resource utilization of ICU delirium was $17,838 (95% confidence interval, $11,132–$23,497). This cost could have been even higher if not for the early mortality associated with delirium in some patients that resulted in a reduction in cost of care of $4654 (95% confidence interval, $2056–7869) [ 50 ]. Direct 1-year health care costs associated with delirium are predicted to range between $143 to $152 billion, assuming delirium occurs in 20% of elderly patients hospitalized annually [ 51 ].
Prevention and management of ICU delirium
“NO SINGLE PHARMACOLOGICAL AGENT CAN PREVENT DELIRIUM: No single pharmacological agent can prevent brain dysfunction in the form of delirium. It is necessary to actively monitor for delirium and pay attention to the details that may put patients at risk for delirium.” [ 53 ]
Pharmacologic prevention of ICU delirium
The neurotransmitter hypothesis has led to studies that have evaluated the benefit of antipsychotic medications in delirium. Haloperidol primarily acts by blocking dopamine and atypical antipsychotics block serotonin, dopamine, alpha-1 adrenergic receptors, and histamine. Multiple studies have been conducted targeting this hypothesis, as well as other central nervous receptors, yet none have consistently demonstrated a significant reduction in delirium. The PADIS guidelines therefore suggest not using haloperidol, atypical antipsychotics, dexmedetomidine, statins, or ketamine to prevent delirium in all critically ill adults [ 17 ]. The main studies supporting this recommendation are described below.
A double-blind placebo-controlled randomized trial (HOPE ICU) was conducted to determine the effect of haloperidol on the prevention of delirium. Patients were randomized to receive haloperidol 2.5 mg or 0.9% saline placebo intravenously every 8 h if receiving mechanical ventilation within 7 h of admission. The number of days alive and without delirium and coma were similar between the haloperidol group and the placebo group (median 5 days [IQR 0–10] vs. 6 days [0–11] days; p = 0.53) [ 52 ].
The Prophylactic Haloperidol Use for Delirium in Patients at High Risk for Delirium (REDUCE) trial was a randomized, double-blind, placebo-controlled study of 1789 critically ill patients who received prophylactic haloperidol 1 mg, haloperidol 2 mg, or placebo. The 1-mg haloperidol group was prematurely stopped because of futility. No difference occurred in the median survival during 28 days in the 2-mg haloperidol group compared with 28 days in the placebo group (95% CI, 0–0; p = 0.93) with a hazard ratio of 1.003 (95% CI, 0.78–1.30; p = 0.82). None of the 15 secondary outcomes were statistically different between the three groups. These outcomes included delirium incidence (mean difference 1.5%; 95% CI, − 3.6% to 6.7%), delirium- and coma-free days (mean difference 0 days; 95% CI, 0–0 days), and duration of mechanical ventilation, ICU, and hospital length of stay (mean difference 0 days; 95% CI, 0–0 days for all three measures). Adverse events did not differ between the groups [ 53 ].
A randomized, double-blind, placebo-controlled study was conducted to determine the effect of risperidone on postoperative delirium following cardiac surgery in 126 patients. Patients were randomized to receive 1 mg of risperidone or placebo. The incidence of postoperative delirium was lower in the risperidone group than the placebo group (11.1% vs. 31.7%, p = 0.009, relative risk = 0.35, 95% confidence interval = 0.16–0.77) [ 54 ]. A randomized, double-blind, placebo-controlled trial was conducted in 700 elderly patients admitted to the ICU after non-cardiac surgery at two tertiary-care hospitals in China. Patients were randomized to receive either dexmedetomidine from ICU admission on the day of surgery until 0800 h on postoperative day 1 or placebo. During the first seven days postoperatively, the incidence of delirium was significantly lower in the dexmedetomidine group (32 [9%] of 350 patients) as compared to the placebo group (79 [23%] of 350 patients; odds ratio [OR] 0.35, 95% CI 0.22–0.54; p < 0.0001) [ 55 ]. A multicenter, double-blind, placebo-controlled trial randomized 100 critically ill patients without delirium to nocturnal dexmedetomidine or placebo. A greater proportion of patients in the dexmedetomidine group remained delirium-free during the ICU stay (dexmedetomidine [40 (80%) of 50 patients] compared to placebo [27 (54%) of 50 patients]; relative risk, 0.44; 95% confidence interval, 0.23–0.82; p = 0.006) [ 56 ]. The authors of the PADIS guidelines considered the delirium incidence and duration, duration of mechanical ventilation, ICU length of stay, and mortality the most critical outcomes. Although there was a consistent decrease in delirium incidence, the PADIS guideline committee deemed that none of the studies reported a meaningful difference for any of the other important clinical outcomes. Additionally, many of these studies contained primarily surgical patients that have a lower severity of illness than medical patients. Given the strong association of delirium and severity of illness, and that many critically ill patients may have delirium when admitted to the ICU, further research needs to be conducted to assess pharmacologic prevention of delirium [ 21 , 26 ].
The association between statin cessation during critical illness and an increased occurrence of delirium has been shown in three cohort studies [ 56 , 57 , 58 ]. Conversely, a randomized study in cardiac surgery patients found that pre-operative atorvastatin did not decrease delirium [ 59 ]. Similarly, delirium was not decreased in older adults following major surgery in a large, randomized study following a single dose of ketamine [ 60 ]. However, a double-blinded RCT in 162 patients (26% surgical) compared ketamine to placebo in order to reduce the dose of remifentanil used for analgosedation (primary outcome) and reported unexpectedly significant lower incidence and duration of delirium with ketamine but no other differences in patients’ outcomes, which deserves further investigation [ 61 ].
Pharmacologic treatment of ICU delirium
“NO SINGLE PHARMACOLOGICAL AGENT CAN TREAT DELIRIUM: No single pharmacological agents can treat delirium. However, currently, clinicians need to focus on predisposal factors of delirium. Non-pharmacologic strategies should be used. There may be situations where the use of drugs may be necessary to manage hyperactive behavior of delirious patients, but it is essential to realize that it is not treating the delirium.” [ 17 ]
Similar to the data on ICU delirium prevention, no large trials have shown that the use of any pharmacologic agents can treat delirium in the ICU stetting thus leading the PADIS guidelines to suggest against routine using haloperidol, atypical antipsychotics, or statins to treat delirium. The guidelines do underscore that there may be situations where the use of these drugs may be warranted to manage the hyperactive behavior of delirious patients, or stress related symptoms (anxiety, hallucinations, delusion, fear, etc.), but it is essential to realize that it is not treating the delirium. If antipsychotics are chosen for these situations, it should be in the smallest doses and shortest duration that is necessary [ 24 , 26 ]. It is also important to note the conceptual limitation that disambiguating delirium prevention from delirium treatment is exceptionally challenging in the real-world setting.
The MIND study, a randomized, double-blind placebo-controlled trial compared the use of haloperidol, ziprasidone, or placebo every 6 h for up to 14 days in 101 mechanically ventilated ICU patients. Patients in the haloperidol group had a similar number of days alive without delirium or coma compared to the ziprasidone and placebo groups (14 [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ] days, 15 [9.1–18] days, 12.5 [1.2–17.2]) [ 62 ].
The Modifying the Impact of the ICU-Associated Neurological Dysfunction-USA (MIND USA) Study, a multicenter, randomized, placebo-controlled study of 566 patients with acute respiratory failure or shock compared haloperidol, ziprasidone, and placebo for the treatment of delirium. The adjusted median number of days alive without delirium or coma was 8.5 (95% CI, 5.6–9.9) in the placebo group, as compared with 7.9 (95% CI, 4.4–9.6) in the haloperidol group and 8.7 (95% CI, 5.9–10.0) in the ziprasidone group, p = 0.26. Within the study, 60% of patients had hypoactive delirium and 40% of patients had hyperactive delirium at some point in the study. There was no difference between the groups in mechanical ventilation duration, ICU or hospital length of stay, days to ICU readmission, death at 30 days, or death at 90 days compared with placebo. Arrhythmias, Parkinsonism (extrapyramidal symptoms), neuroleptic malignant syndrome, study drug discontinuation, and other safety concerns were extremely low across all three groups [ 17 ].
However, the results of a multinational European cohort study by Collet et al. [ 63 ], the AID-ICU study, including 1260 patients from 99 ICUs in 13 countries have shown that haloperidol was the most common pharmacological intervention for delirium regarding delirium subtype. In this study the use of haloperidol within 24 h of ICU admission [aOR 1.2 (0.5–2.5); p = 0.66] and within 72 h of ICU admission [aOR 1.9 (1.0–3.9); p = 0.07], was not associated with increased 90-day mortality, yet at 72 h after admission to the ICU the use of haloperidol was associated with the need for circulatory support [aOR 2.6 (1.1–6.9)].
Antipsychotics remain viable for the short-term control of severe agitation to prevent the risk of patient’s self-removing of ICU devices, fall, or aggressive behavior against the ICU team, severe anxiety with the need to avoid respiratory suppression (e.g., heart failure, COPD, or asthma), or symptomatic delirium features such as hallucinations or delusions [ 26 ]. If an antipsychotic is initiated, low starting doses should be considered, and daily review of drug interactions, adverse effects, dosing titration, and need for the antipsychotic should be completed.
In addition to antipsychotics, other drugs have been investigated to treat delirium such as statins and dexmedetomidine. A randomized, double-blind placebo-controlled trial of 142 patients found that high dose simvastatin (80 mg daily) does not increase days alive without delirium and without coma at day 14 (5.7 days (SD 5.1) with simvastatin and 6.1 days (5.2) with placebo (mean difference 0.4 days, 95% CI-1.3–2.1; p = 0.66) [ 64 ]. While there is no recommendation for statin use, the PADIS guidelines recommend using dexmedetomidine for patients with delirium in which agitation is preventing extubation or weaning off the ventilator [ 26 ]. The Dexmedetomidine to Lessen ICU Agitation (DAHLIA) study was a double-blind placebo-controlled trial in 15 ICUs in Australia and New Zealand in which 39 patients were randomized to dexmedetomidine and 32 to receive placebo. In the first 7 days after study randomization, dexmedetomidine was associated with a small increase in ventilator-free hours compared to placebo (median, 144.8 h vs. 127.5 h, 95% CI 4–33.2 h, p = 0.01). Dexmedetomidine use did not affect ICU or hospital length of stay or the patient’s discharge disposition. Patients did not receive opioids commonly and the prevalence of alcohol withdrawal was not reported [ 65 ]. Future studies need to evaluate the role of dexmedetomidine in patients with hypoactive delirium or those in which agitation is not preventing extubation, as well as in non-intubated patients [ 66 ].
Future studies evaluating pharmacologic therapy of delirium need to concentrate on long-term cognitive and functional outcomes. Additionally, agents such a valproic acid, which have only been included in small studies, need to be thoroughly evaluated in prospective randomized trials [ 22 ].
Nonpharmacologic prevention and management
While no pharmacologic agents have been shown to significantly impact delirium, bundling of non-pharmacologic strategies have and thus this bundle concept has become a mainstay of ICU care. One mnemonic to consider when thinking about the differential causes of delirium is DR.DRE, composed of Disease Remediation, Drug Removal (screening for both drug related delirium and withdrawal syndromes), and Environment. The use of this mnemonic helps consider the most common risk factors and may be particularly useful for communication within the whole therapeutic team (medical, nursing, physiotherapy, pharmacology personnel). However, the use of mnemonics depends on the ICU culture and clinicians’ preference and should not replace an exhaustive screening for all frequent causes of delirium (e.g., bladder retention, hypoglycemia, lack of bowel movement). Bright light therapy, family participation in care, and psychoeducational programs are the three single-component interventions that have been evaluated in the ICU. Three studies evaluated the effects of bright light therapy and did not find a reduction in delirium or ICU length of stay [ 67 , 68 , 69 ], so the PADIS guidelines made a conditional recommendation against its use. The PADIS guidelines recommend using multicomponent interventions such reorientation, cognitive stimulation, use of clocks, sleep enhancement, increased wakefulness, early mobility, and use of hearing aids and eyeglasses when indicated. Many multicomponent bundles have shown improved outcomes in critically ill adults including reduction in delirium, ICU length of stay and hospital mortality [ 67 , 70 , 71 , 72 , 73 , 74 ].
One example of a multi-component strategy is the A2F bundle (A, assess, prevent, and manage pain; B, both spontaneous awakening and spontaneous breathing trials; C, choice of analgesic and sedation; D, delirium: assess, prevent, and manage; E, early mobility, and exercise; and F, family engagement). This easy to memorize bundle is a 6-step approach, created to facilitate implementation of the recommendations of multiple guidelines [ 24 , 25 , 26 , 75 ]. This bundle has been shown to improve a spectrum of patient outcomes in a single center study, a multiple hospital/single regional system study, and a large nationwide collaborative. However, notably all the below discussed trials are non-randomized and did not have concurrent controls. While it is widely believed to be effective, there is currently not a single RCT demonstrating the benefit of the A2F bundle which is the gold standard in terms of demonstration of therapeutic efficacy.
A prospective, cohort quality improvement study in ventilated and non-ventilated patients was conducted in 6,064 patients at seven community hospitals. Patients had a 7% higher odds of hospital survival for every 10% increase in total bundle compliance (odds ratio, 1.07; 95% CI, 1.04–1.11; p < 0.001). Patients had a 15% higher hospital survival for every 10% increase in partial bundle compliance (odds ratio, 1.15; 95% CI, 1.09–1.22; p < 0.001). With total bundle compliance (incident rate ratio, 1.02; 95% CI, 1.01–1.04; p = 0.004) and partial bundle compliance (incident rate ratio, 1.15; 95% CI, 1.09–1.22; p < 0.001), patients had more days alive and free of delirium and coma [ 76 ].
In a prospective, multicenter, quality improvement collaborative from 68 academic, community, and federal ICUs during a 20-month collection period, performance of the complete A2F bundle was associated with a lower likelihood of death within 7 days (HR 0.32; CI, 0.17–0.62), next-day mechanical ventilation (OR 0.28; CI, 0.22–0.36), coma (OR 0.35; CI, 0.22–0.56), delirium (OR 0.60; CI, 0.49–0.72), physical restraint use (OR 0.37; CI,0.30–0.46), ICU readmission (OR 0.54; CI, 0.37–0.79), and discharge to a facility other than home (OR 0.64; CI. 0.510.80). There was a dose response between a higher proportional bundle performance and improvement in each clinical outcome ( p < 0.002). Pain was more commonly reported as bundle performance increased ( p = 0.0001), probably because more patients were awake [ 77 ]. Members of the collaborative faculty published two subsequent papers to aid in implementation of the A2F bundle [ 78 , 79 ].
Lastly, a prospective cohort study of 1855 mechanically ventilated patients was conducted to evaluate staged implementation of the A2F bundle. Implementation of the full versus partial bundle resulted in reduced mechanical ventilation duration (− 22.3%; 95% CI, − 22.5% to − 22.0%; p < 0.001), ICU length of stay (− 10.3%; 95% CI, − 15.6% to − 4.7%; p = 0.028), and hospital length of stay (− 7.8%; 95% CI, − 8.7% to − 6.9%; p = 0.006) after adjustment for patient-level covariates. ICU and hospital costs were also decreased by 24.2% (95% CI, − 41.4% to − 2.0%; p = 0.03) and 30.2% (95% CI, − 46.1% to − 9.5%; p = 0.007), respectively [ 80 ].
Quite contrary in a recent meta-analysis of randomized controlled trials by Bannon et al. [ 81 ], concentrating on the effectiveness of non-pharmacological interventions versus standard care in reducing the incidence and duration of delirium in the ICU the authors identified 15 trials (2812 participants) with results indicating that current evidence is too weak to support the use of non-pharmacological interventions (principally single interventions) in reducing incidence and duration of delirium in critically ill patients. However, to support the importance of the F (Family) element of the A2F bundle, a trial of reorientation using a family voice showed a beneficial effect [ n = 30, MD (days) − 1.30, 99% CI − 2.41 to − 0.19, p = 0.003 [ 81 ]. The future goals to be achieved in ICU Delirium research and care have been identified recently and should include all the above mentioned non-pharmacological interventions and practices, including the A2F bundle [ 49 ].
Important to note, the “A” element of the A2F bundle stands for assess, prevent, and treat pain. Untreated pain can predispose patients to delirium. However, utilization of opioids can also result in delirium [ 82 ]. This highlights the importance of using validated tool such as the Numeric Rating Scale, Critical Care Pain Observational Tool, or the Behavioral Pain Scale to diagnose pain in critically ill patients [ 26 ].
Also, important to note, the “C’ element of the A2F bundle stands for choice of sedation and focuses on constant vigilance to ensure that patients receive the best sedative agent at the least amount. The PADIS guidelines suggest using either propofol or dexmedetomidine over benzodiazepines for sedation in critically ill mechanically ventilated adults [ 83 ]. These recommendations were based on observational studies demonstrating increased risk of delirium when receiving benzodiazepines [ 84 , 85 ], and comparator studies of either propofol or dexmedetomidine versus benzodiazepines, where each of the studies showed worse outcomes in the benzodiazepine group [ 86 , 87 , 88 , 89 ]. No significant differences have been found in time to extubation or other important secondary outcomes in three randomized trials containing a total of 850 patients comparing dexmedetomidine and propofol [ 86 , 87 , 88 , 89 ].
SPICE III is an open label, randomized controlled trial comparing dexmedetomidine as primary sedation to usual care (propofol, midazolam or other sedation) in patients receiving less than 12 h of mechanical ventilation who are expected to be mechanically ventilated for at least one additional day that was conducted following publication of the PADIS guidelines. The target RASS goal was − 2 to + 1. The target RASS goal was − 2 to + 1. Death at 90 days occurred in 569 of 1956 (29.1%) of the usual care group and 566 of 1956 (29.1%) in the dexmedetomidine group (adjusted risk difference, 0.0 percentage points; 95% confidence interval, − 2.9 to 2.8). In the dexmedetomidine group, 64% of the patients received propofol, 3% received midazolam, and 7% received both during the first 2 days following randomization. Noteworthy, 60% of patients received propofol, 12% received midazolam, and 20% received both in the dexmedetomidine group. Given the multiple sedatives administered in both groups, the application of the results of this study is difficult. In the dexmedetomidine group, bradycardia and hypotension occurred in 5.1% and 2.7% patients respectively. The median number of days free from coma or delirium was 1 day longer in the dexmedetomidine as compared to the usual care group 24 (11–26) vs. 23 (10–26), adjusted risk difference, 1 (95% confidence interval, 0.5–1.5) [ 90 ].
The Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS 2), is a multicenter, double-blind, randomized controlled trial of 432 patients randomized to dexmedetomidine or propofol for up to 14 days or extubation. No difference was found between dexmedetomidine and propofol in the number of days alive without delirium or coma (odds ratio [OR], 0.96; 95% CI, 0.74–1.26), ventilator-free days (OR, 0.98; 95% CI, 0.63–1.51), or death at 90 days (HR, 1.06; 95% CI, 0.74–1.52) [ 91 ].
Finally, the whole A2F bundle is driven toward a reduction of sedatives use. In this way, the best non-pharmacological prevention of delirium could be to completely avoid sedation when unnecessary. A RCT in 137 postoperative ICU patients mostly admitted for peritonitis and septic shock reported as secondary outcomes a significant reduction in delirium incidence (72% vs. 43%; − 29 (− 50 to − 14)%, p < 0.001) and delirium duration [2 (0–4) days vs. 0 (0–2); − 0.5 (− 1.0–0.0) days, p = 0.003] in the group where the sedatives were immediately stopped compared to the group where a moderate sedation (RASS -3) was sustained for one day and half [ 92 ].
The optimal sedation strategies for mechanically ventilated patients with severe respiratory failure and adult respiratory distress syndrome (ARDS) and prevention of ICU delirium have become especially important in the light of COVID-19 pandemic [ 93 ]. The recently published COVID-D study, an observational cohort of 2,088 COVID-19 positive ICU patients from 69 sites and 14 countries, reported 81% of patients had coma for a median of 10 [IQR 6–15] days, and 55% were delirious for a median of 3 [IQR 2–6] days [ 94 ]. Deep sedative levels and prolonged sedatives infusions while on mechanical ventilation was common with 64% receiving benzodiazepines for 7 [ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ] days and 71% receiving propofol for 7 [ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ] days and the median RASS score was − 4 [− 5 to − 3]. Benzodiazepines were associated with increased risk of delirium development and fewer days alive without either delirium or coma. Additionally, visits (in-person or virtual) with family or friends were associated with decreased risk of delirium. These data support the management goals behind the A2F Bundle in every clinical and epidemiological situation in the ICU: use of lighter sedation, avoidance of benzodiazepines, involvement of family, friends, and caregivers to provide targeted humane care, even in patients with ARDS, including patients suffering from COVID-19.
Recently, an expert panel recommended the A2F bundle for these patients and proposed to update the bundle adding a R for respiratory drive control (A2F-R bundle) [ 95 ]. In the A2F perspective to prefer non pharmacological intervention in order to reduce sedatives and the risk of cognitive dysfunction, it would be especially meaningful in mechanically ventilated patients to prioritize the optimization of the ventilator setting, preferring a more comfortable ventilation mode, allowing for reducing opioids and sedatives, along with the screening and management of patient’s associated factors of high respiratory demand (metabolic acidosis, fever, stressful symptoms, e.g., pain, anxiety, dyspnea). This strategy would benefit from patient’s outcomes as well as the sparing of analgesics, sedatives and neuromuscular blocking agents which is crucial in times of pandemics and high requirement of ICU resources. Figure 3 is an algorithm to guide clinicians in evaluating patients exhibiting an acute change in mental status.
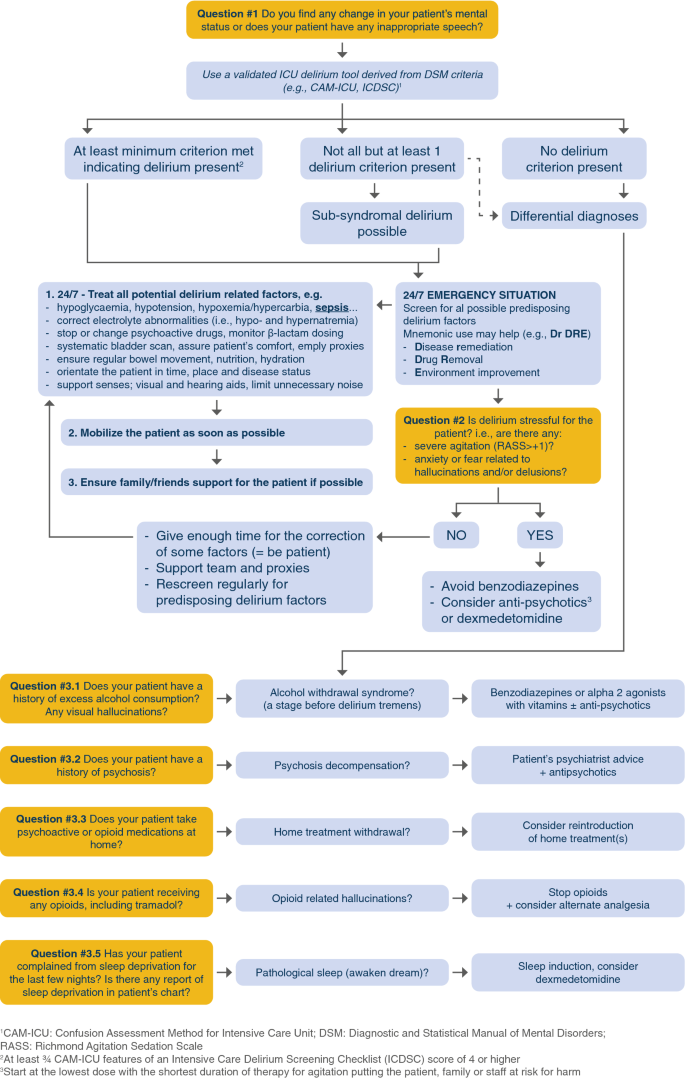
Diagnosis and treatment algorithm for critically ill patients. Exhibiting a sudden change in their mental status including inappropriate speech
Future directions
Knowledge gaps and research agenda for the next 10 years.
Validation and development of objective tools for delirium diagnosis such as EEG or computer-based apps.
Mastering delirium pathophysiology and its association with long-term cognitive impairment.
Development of new delirium phenotyping models.
Beyond casual inference, understanding the association of delirium with outcomes.
Further understanding delirium biomarkers and their practical use in predictive models.
Conduction of large, randomized clinical trials in critically ill patients evaluating the effects of sleep optimization, cognitive/physical training, alternate safety practices, and family engagement/non-pharmacological interventions on delirium and long-term outcomes [ 49 ].
Future directions include assessing diagnostic tools including EEG, CSF studies, and imaging studies (MRI) and utilizing prediction models in diverse patient populations. Additionally, studies need to assess the effects of antipsychotics on the symptoms of hallucinations and delusions in ICU patients with delirium. Lastly, larger, randomized studies need to be conducted to assess the non-pharmacologic prevention and treatment of delirium and its burden including clinically meaningful and long-term outcomes.
Delirium is an acute organ dysfunction independently predictive of mortality and multiple morbidities including increased ICU and hospital length of stay, cognitive dysfunction, and cost. Multiple tools have been validated for diagnosis of delirium in the ICU. The CAM-ICU and the ICDSC are the two tools that are recommended for diagnosing delirium in the ICU by PADIS guidelines. Antipsychotics, dexmedetomidine, statins, and ketamine are not recommended to prevent delirium. Antipsychotics are also not recommended for use to treat delirium. However, antipsychotics can be considered for short-term control of severe agitation or stressful symptoms (anxiety, hallucinations, delusion, fear). Non-pharmacologic therapy including the A2F Bundle is the main means of delirium prevention or treatment, including the suggestion of adding a “R” to underline the role of better management of Respiratory drive control and ventilator setting in patients with ARDS and more generally in all mechanically ventilated patients. Diagnostic tools, effects of antipsychotics on hallucinations and delusions, and non-pharmacological prevention and treatment of delirium and association with long-term outcomes are some of the top study areas to be conquered next in the field of ICU delirium.
Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, Azoulay E, Bellelli G, Csomos A, Fan E et al (2017) Worldwide survey of the “assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) bundle. Crit Care Med 45(11):e1111–e1122
Article PubMed PubMed Central Google Scholar
Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM et al (2018) Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 6(3):213–222
Chanques G, Ely EW, Garnier O, Perrigault F, Eloi A, Carr J, Rowan CM, Prades A, de Jong A, Moritz-Gasser S et al (2018) The 2014 updated version of the confusion assessment method for the intensive care unit compared to the 5th version of the diagnostic and statistical manual of mental disorders and other current methods used by intensivists. Ann Intensive Care 8(1):33
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association
Book Google Scholar
Slooter AJC, Otte WM, Devlin JW, Arora RC, Bleck TP, Claassen J, Duprey MS, Ely EW, Kaplan PW, Latronico N et al (2020) Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med 46(5):1020–1022
Brummel NE, Boehm LM, Girard TD, Pandharipande PP, Jackson JC, Hughes CG, Patel MB, Han JH, Vasilevskis EE, Thompson JL et al (2017) Subsyndromal delirium and institutionalization among patients with critical illness. Am J Crit Care 26(6):447–455
Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y (2007) Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med 33(6):1007–1013
Article PubMed Google Scholar
Wood E, Albarqouni L, Tkachuk S, Green CJ, Ahamad K, Nolan S, McLean M, Klimas J (2018) Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The rational clinical examination systematic review. JAMA 320(8):825–833
Salomon C, Hamilton B, Elsom S (2014) Experiencing antipsychotic discontinuation: results from a survey of Australian consumers. J Psychiatr Ment Health Nurs 21(10):917–923
Article CAS PubMed Google Scholar
Sivanesan E, Gitlin MC, Candiotti KA (2016) Opioid-induced hallucinations: a review of the literature, pathophysiology, diagnosis, and treatment. Anesth Analg 123(4):836–843
Article CAS PubMed PubMed Central Google Scholar
Babkoff H, Sing HC, Thorne DR, Genser SG, Hegge FW (1989) Perceptual distortions and hallucinations reported during the course of sleep deprivation. Percept Mot Skills 68(3 Pt 1):787–798
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R et al (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286(21):2703–2710
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA et al (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 371(9607):126–134
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291(14):1753–1762
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y (2001) Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 27(5):859–864
Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, Putensen C (2010) Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care 25(1):144–151
Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ et al (2018) Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 379(26):2506–2516
Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB, Stevens RD (2015) Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 350:2538
Article Google Scholar
Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW (2006) Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 54(3):479–484
Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, Dittus R, Ely EW (2007) Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med 33(10):1726–1731
Hayhurst CJ, Marra A, Han JH, Patel MB, Brummel NE, Thompson JL, Jackson JC, Chandrasekhar R, Ely EW, Pandharipande PP et al (2020) Association of hypoactive and hyperactive delirium with cognitive function after critical illness. Crit Care Med 48(6):e480–e488
Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP (2014) Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 189(6):658–665
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14):1306–1316
Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE (2020) Perioperative quality initiative w: american society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg 130(6):1572
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM et al (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41(1):263–306
Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B et al (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 46(9):e825–e873
Han JH, Wilson A, Shintani AK, Graves AJ, Schnelle J, Vernon J, Dittus RS, Storrow AB, Ely EW (2011) The validation of the brief confusion assessment method in older emergency department patients. Ann Emerg Med 60(Suppl):S28
Google Scholar
Smith HA, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, Rutherford MT, Denton D, Thompson JL, Chandrasekhar R et al (2016) The preschool confusion assessment method for the ICU: valid and reliable delirium monitoring for critically Ill infants and children. Crit Care Med 44(3):592–600
Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, Eden SK, Terrell MK, Boswell T, Wolfram K et al (2011) Diagnosing delirium in critically ill children: validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit Care Med 39(1):150–157
Pun B, Devlin J (2013) Delirium monitoring in the ICU: strategies for initiating and sustaining screening efforts. Semin Respir Crit Care Med 34(02):179–188
Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC (2012) The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 16(4):R115
van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A et al (2011) Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med 184(3):340–344
Trzepacz PT (1999) The delirium rating scale - its use in consultation-liaison research. Psychosomatics 40(3):193–204
Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN (2014) The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 160(8):526–533
Khan BA, Perkins AJ, Gao S, Hui SL, Campbell NL, Farber MO, Chlan LL, Boustani MA (2017) The confusion assessment method for the ICU-7 delirium severity scale: a novel delirium severity instrument for use in the ICU. Crit Care Med 45(5):851–857
Green C, Bonavia W, Toh C, Tiruvoipati R (2018) Prediction of ICU delirium: validation of current delirium predictive models in routine clinical practice. Crit Care Med 47(3):428–435
Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM (2008) Neuroimaging studies of delirium: a systematic review. J Psychosom Res 65(3):239–248
Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR 3rd et al (2012) The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med 40(7):2022–2032
Shioiri A, Kurumaji A, Takeuchi T, Matsuda H, Arai H, Nishikawa T (2010) White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriat Psyc 18(8):743–753
Morandi A, Gunther ML, Vasilevskis EE, Girard TD, Hopkins RO, Jackson JC, Pandharipande P, Ely EW (2010) Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont) 7(9):28–33
Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L (1984) Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol 246(6 Pt 2):R994-999
CAS PubMed Google Scholar
van der Kooi AW, Zaal IJ, Klijn FA, Koek HL, Meijer RC, Leijten FS, Slooter AJ (2015) Delirium detection using EEG: what and how to measure. Chest 147(1):94–101
Kimchi EY, Neelagiri A, Whitt W, Sagi AR, Ryan SL, Gadbois G, Groothuysen D, Westover MB (2019) Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology 93(13):e1260–e1271
Gilmore EJ, Gaspard N, Choi HA, Cohen E, Burkart KM, Chong DH, Claassen J, Hirsch LJ (2015) Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 41(4):686–694
Klein Klouwenberg PM, Zaal IJ, Spitoni C, Ong DS, van der Kooi AW, Bonten MJ, Slooter AJ, Cremer OL (2014) The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ 349:g6652
Duprey MS, van den Boogaard M, van der Hoeven JG, Pickkers P, Briesacher BA, Saczynski JS, Griffith JL, Devlin JW (2020) Association between incident delirium and 28- and 90-day mortality in critically ill adults: a secondary analysis. Crit Care 24(1):161
Rood PJT, van de Schoor F, van Tertholen K, Pickkers P, van den Boogaard M (2019) Differences in 90-day mortality of delirium subtypes in the intensive care unit: a retrospective cohort study. J Crit Care 53:120–124
Hughes CG, Patel MB, Jackson JC, Girard TD, Geevarghese SK, Norman BC, Thompson JL, Chandrasekhar R, Brummel NE, May AK et al (2017) Surgery and anesthesia exposure is not a risk factor for cognitive impairment after major noncardiac surgery and critical illness. Ann Surg 265(6):1126–1133
Pandharipande PP, Ely EW, Arora RC, Balas MC, Boustani MA, La Calle GH, Cunningham C, Devlin JW, Elefante J, Han JH et al (2017) The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med 43(9):1329–1339
Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, Patel MB, Hughes CG, Cao A, Pandharipande PP et al (2018) The cost of ICU delirium and coma in the intensive care unit patient. Med Care 56(10):890–897
Leslie DL, Inouye SK (2011) The importance of delirium: economic and societal costs. J Am Geriatr Soc 59(Suppl 2):S241-243
Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF (2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 1(7):515–523
van den Boogaard M, Slooter AJC, Bruggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW et al (2018) Effect of haloperidol on survival among critically Ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 319(7):680–690
Article PubMed PubMed Central CAS Google Scholar
Prakanrattana U, Prapaitrakool S (2007) Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care 35(5):714–719
Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D (2016) Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 388(10054):1893–1902
Skrobik Y, Duprey MS, Hill NS, Devlin JW (2018) Low-dose nocturnal dexmedetomidine prevents ICU delirium a randomized, placebo-controlled trial. Am J Respir Crit Care Med 197(9):1147–1156
Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP (2011) Statins and brain dysfunction a hypothesis to reduce the burden of cognitive impairment in patients who are critically III. Chest 140(3):580–585
Mather JF, Corradi JP, Waszynski C, Noyes A, Duan Y, Grady J, Dicks R (2017) Statin and its association with delirium in the medical ICU. Crit Care Med 45(9):1515–1522
Billings FTT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ (2016) High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA 315(9):877–888
Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM et al (2017) Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 390(10091):267–275
Perbet S, Verdonk F, Godet T, Jabaudon M, Chartier C, Cayot S, Guerin R, Morand D, Bazin JE, Futier E et al (2018) Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med 37(6):589–595
Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY et al (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med 38(2):428–437
Collet MO, Caballero J, Sonneville R, Bozza FA, Nydahl P, Schandl A, Woien H, Citerio G, van den Boogaard M, Hastbacka J et al (2018) Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med 44(7):1081–1089
Page VJ, Casarin A, Ely EW, Zhao XB, McDowell C, Murphy L, McAuley DF (2017) Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 5(9):727–737
Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, Davies A, Delaney A, Ghosh A, van Haren F et al (2016) Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA 315(14):1460–1468
Louis C, Godet T, Chanques G, Bourguignon N, Morand D, Pereira B, Constantin JM (2018) network A: effects of dexmedetomidine on delirium duration of non-intubated ICU patients (4D trial): study protocol for a randomized trial. Trials 19(1):307
Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y (2011) The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs 27(3):158–166
Taguchi T, Yano M, Kido Y (2007) Influence of bright light therapy on postoperative patients: a pilot study. Intensive Crit Care Nurs 23(5):289–297
Simons KS, Laheij RJ, van den Boogaard M, Moviat MA, Paling AJ, Polderman FN, Rozendaal FW, Salet GA, van der Hoeven JG, Pickkers P et al (2016) Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med 4(3):194–202
Foster J, Kelly M (2013) A pilot study to test the feasibility of a nonpharmacologic intervention for the prevention of delirium in the medical intensive care unit. Clin Nurse Spec 27(5):231–238
Moon KJ, Lee SM (2015) The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud 52(9):1423–1432
Colombo R, Corona A, Praga F, Minari C, Giannotti C, Castelli A, Raimondi F (2012) A reorientation strategy for reducing delirium in the critically ill. Results of an interventional study. Minerva Anestesiol 78(9):1026–1033
Hanison J, Conway D (2015) A multifaceted approach to prevention of delirium on intensive care. BMJ Qual Improv Rep 4(1).
Rivosecchi RM, Kane-Gill SL, Svec S, Campbell S, Smithburger PL (2016) The implementation of a nonpharmacologic protocol to prevent intensive care delirium. J Crit Care 31(1):206–211
Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, Cox CE, Wunsch H, Wickline MA, Nunnally ME et al (2017) Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med 45(1):103–128
Barnes-Daly MA, Phillips G, Ely EW (2017) Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6064 patients. Crit Care Med 45(2):171–178
Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, Byrum D, Carson SS, Devlin JW, Engel HJ et al (2019) Caring for critically Ill patients with the ABCDEF BUNDLE: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 47(1):3–14
Stollings JL, Devlin JW, Pun BT, Puntillo KA, Kelly T, Hargett KD, Morse A, Esbrook CL, Engel HJ, Perme C et al (2019) Implementing the ABCDEF bundle: top 8 questions asked during the ICU liberation ABCDEF bundle improvement collaborative. Crit Care Nurse 39(1):36–45
Balas MC, Pun BT, Pasero C, Engel HJ, Perme C, Esbrook CL, Kelly T, Hargett KD, Posa PJ, Barr J et al (2019) Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse 39(1):46–60
Hsieh SJ, Otusanya O, Gershengorn HB, Hope AA, Dayton C, Levi D, Garcia M, Prince D, Mills M, Fein D et al (2019) Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med 47(7):885–893
Bannon L, McGaughey J, Verghis R, Clarke M, McAuley DF, Blackwood B (2019) The effectiveness of non-pharmacological interventions in reducing the incidence and duration of delirium in critically ill patients: a systematic review and meta-analysis. Intensive Care Med 45(1):1–12
Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, Briesacher BA, Saczynski JS, Griffith JL, Devlin JW, Slooter AJC (2021) Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM et al (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30(1):119–141
Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA Jr, Dittus R, Ely EW (2008) Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 65(1):34–41
PubMed PubMed Central Google Scholar
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW (2006) Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 104(1):21–26
Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA et al (2007) Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 298(22):2644–2653
Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW et al (2009) Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301(5):489–499
Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J (2012) Dexmedetomidine for long-term sedation I: dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 307(11):1151–1160
Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, Bourdet S, Ivanova A, Henderson AG, Pohlman A et al (2006) A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 34(5):1326–1332
Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC et al (2019) Early sedation with dexmedetomidine in critically Ill patients. N Engl J Med 380(26):2506–2517
Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, Jackson JC, Hoskins AS, Pun BT, Orun OM et al (2021) Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med 384(15):1424–1436
Chanques G, Conseil M, Roger C, Constantin JM, Prades A, Carr J, Muller L, Jung B, Belafia F, Cisse M et al (2017) Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-Ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med 5(10):795–805
Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW (2020) COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care 24(1):176
Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, Simpson BK, Wilson-Linville S, Hinojal OB, Vallejo de la Cueva A et al (2021) Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D) a multicentre cohort study. Lancet Respir Med 9(3):239–250
Chanques G, Constantin JM, Devlin JW, Ely EW, Fraser GL, Gelinas C, Girard TD, Guerin C, Jabaudon M, Jaber S et al (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med 46(12):2342–2356
Download references
Acknowledgements
Dr Ely has received honoraria for CME activities sponsored by Pfizer, Orion, and Abbott. Dr Pandharipande has received a research grant from Pfizer (previously Hospira Inc.). Dr Chanques received fees for speaker (Orion Pharma, Aspen Medical) and for expert board (Orion Pharma). None of the other authors have any conflicts of interest to disclose.
BTP is supported in part by National Heart Lung and Blood Institute (R01HL14678-01). EWE is currently receiving grant funding from National Institute on Aging (1R01AG058639-02A1 and 3R01AG058639-02S1) and the Veteran's Administration. PPP is supported by the National Institute of Health AG061161, AG058639, AG054259 and GM120484.
Author information
Authors and affiliations.
Critical Illness Brain Dysfunction Survivorship Center, Nashville, Vanderbilt University Medical Center, 1211 Medical Center Drive, B-131 VUH, Nashville, TN, 37232-7610, USA
Joanna L. Stollings, Brenda T. Pun, Pratik P. Pandharipande & E. Wesley Ely
Department of Pharmaceutical Services, Vanderbilt University Medical Center, Nashville, TN, USA
Joanna L. Stollings
Department Anesthesiology, Intensive Therapy and Acute Intoxications, Pomeranian Medical University, Szczecin, Poland
Katarzyna Kotfis
Department of Anaesthesia and Critical Care Medicine, Saint Eloi Hospital, Montpellier University Hospital Center, and PhyMedExp, University of Montpellier, INSERM, CNRS, Montpellier, France
Gerald Chanques
Division of Pulmonary and Critical Care Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA
Brenda T. Pun, Pratik P. Pandharipande & E. Wesley Ely
Division of Anesthesiology Critical Care Medicine, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA
Pratik P. Pandharipande
Center for Health Services Research, Vanderbilt University Medical Center, Nashville, TN, USA
E. Wesley Ely
Geriatric Research, Education and Clinical Center Service, Department of Veterans Affairs Medical Center, Tennessee Valley Health Care System, Nashville, TN, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Joanna L. Stollings .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Stollings, J.L., Kotfis, K., Chanques, G. et al. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med 47 , 1089–1103 (2021). https://doi.org/10.1007/s00134-021-06503-1
Download citation
Received : 03 January 2021
Accepted : 29 July 2021
Published : 16 August 2021
Issue Date : October 2021
DOI : https://doi.org/10.1007/s00134-021-06503-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antipsychotics
- ICU Liberation
- Cognitive impairment
- Critical care
- Find a journal
- Publish with us
- Track your research
- Radiomics Advances Prognostication in Advance
- Cryocure-VT Trial - Clinical Ventricular...
Artificial Intelligence in Critical Care:...
- UK to Ban Tobacco Use For Young Generation
- Navigating Connectivity Challenges in Hospita

SCCM Guidelines on Glycaemic Control for...

Molecular Phenotypes to Advance Precision...

Early Systemic Insults Following Traumatic...
The intensive care unit of tomorrow: a case study of patient-centred care.
- Fri, 4 May 2012
ICU Management & Practice, ICU Volume 12 - Issue 1 - Spring 2012
The University Medical Center Utrecht (UMC Utrecht) is a 1,042 bed hospital, which admits approximately 30,000 inpatients per year. All academic specialties are present and the hospital provides a core service in heart and lung transplantations, ventricular assist devices, trauma, neurosurgery, oncology, haematology and AIDS patients. In 2004, an independent survey measuring the quality of care and effectiveness of intensive care units in the Netherlands concluded that UMC Utrecht performed adequately in this field, a stature which the hospital board subsequently felt could be improved. The first change to be made was in the overall structure of the ICU. Originally, the intensive care department was divided into four separate sectors: internal, surgical, cardio surgical and neurological/neurosurgical units, all of which were located in different parts of the hospital. In the new arrangement, these divisions were re-organised as a multidisciplinary medical department, marking the birth of a revolutionary project headed by the authors of this article.
Introduction
The vision generated by this team was to create an intensive care department that produces the best possible environment for critically ill patients and their relatives, allowing doctors and nursing staff to focus on the patient. Patient-centred care, safety, functionality, innovation and future-proof concepts evolved as the main aspects of the new ICU design. Former patients and their family members were interviewed to assess opinions on the present condition of the department, including its shortcomings, and significant issues to be considered when building the new ICU were identified. Daylight, tranquil surroundings, patient privacy, adequate space, family comfort, ergonomics, logistics and safety were communicated as important, and concepts for improvements in these areas were developed. Two courses were implemented, with the first centred on architecture and interior design dictated by patient-centred care, and the second focused on functionality, safety and innovation.
Patient-Centred Care
In designing patient rooms, the well-being and orientation of the patient were considered of main concern; therefore, a key focus was the formation of a day and night rhythm. Each of the 36 single-patient rooms measure 25m 2 and have a view either to either one of the four specially designed 60m 2 Dutch gardens or to other well lit spaces, providing sufficient daylight to the department.
The rooms are also designed to make patients feel at home, featuring comfortable armchairs and fashionable lamps among other attributes, all coordinated with soft warm colours. A whiteboard with the names of attending personnel was placed inside each room to forge a more personable atmosphere as well as to provide another method for sharing information, while a clear glass wall and door separates the room from the nursing staff, replacing what were old-fashioned curtains. The glass doors close automatically, unless kept deliberately open, providing a quiet environment for the patient. Also, since the glass is electrostatic, meaning it instantly becomes opaque at the touch of a button, privacy is guaranteed whenever required.
The ceiling of each room is painted in a soft blue with as few irregularities as possible, increasing the relaxing tone of patients’ surroundings in the hope of lessening feelings of disorientation by patients suffering from delirium, a frequent occurrence in ICUs. Such patients commonly imagine strange phenomena emerging from the ceiling whereas in fact it may simply be an air conditioning duct. Simple aspects such as this are often overseen but can make a significant difference in patient comfort levels.
The Needs of the Patient’s Family
The benefits that could be generated for visiting relatives were not overlooked when forming the new ICU scheme, including from the perspective of the positive effects they have on the patient. It was recognised that comfort and peace of mind have a significant effect on the overall atmosphere. A large area with catering and Internet facilities is reserved for family use, while a 24-hour visiting policy is applied, with no restrictions on visiting time.
With family values in mind, six double bedrooms, each with a bathroom and shower, were built in the unit for those relatives who live far away, or for specific cases where the patient is particularly ill. From the provision of PCs with Internet connection, cable TV and telephones, to outside meeting space and their own cafeteria, relatives are made to feel as welcome and as comfortable as possible. The family area is situated in a quiet corner of the floor so that members are not exposed to the daily activities of the intensive care department, giving them an atmosphere of privacy, security and trust.
Functionality, Safety and Innovation
Before buying any required medical equipment, concepts were developed concerning functionality, safety and innovation, with these set to shape subsequent choices on medical apparatus. The main themes considered when making decisions were the availability of a physician and nurse at the patient’s bedside or nearby throughout their stay, ergonomics, safety and silence.
Physician and Nurse at the Bedside or Nearby
Simplicity, ease of use and minimal alarms were all features high on the list with regard to selection of medical equipment. The chosen system with remote control use of monitors from outside patients’ rooms resulted in fewer interactions; however, an innovative solution was found. As the patient rooms were divided in pairs (36 beds in all) with a small nurse station outside each pair, the monitor at each desk allowed the staff to do exactly what was required: view, control, review and record, all from outside the patient’s room. With these measures, the patients are not exposed to unnecessary noise and presence of personnel in the room.

Patient rooms have ceiling service units for the medical apparatus and PDMS, allowing for convenience and comfort. The routinely used medical equipment is off the floor, and flexible positioning of a patient’s bed is possible. The bed can be positioned easily towards the gardens or the outside view without extensive re positioning of the other equipment.
Each nurse carries a bleeper which receives specified patient alarms; the device can also be used to call for help in emergency situations. Medications and supplies are prepared and delivered to patients by the pharmacy personnel and logistics department, with most medicines prepared and delivered ready to use in syringes. The pharmacy located within the ICU prepares more than 80% of the medicines used by its patients, reducing errors significantly.
In another section of the department, infection control rooms were fitted with a double door positive pressure system, with a sophisticated delivery system for supplies, to reduce the risk of cross infection.
The Sound of Silence
The new intensive care department, especially the patient rooms, has carefully been provided with a quiet and peaceful atmosphere. This can be summarised with new unit and concept design, spreading the medical and nursing personnel evenly across the floor instead of gathering them at the central post, and keeping the doors of the rooms mostly closed. An ongoing project aims to improve the alarm systems, filtering the alarms to be sent selectively to the cell phones of the nurses, and avoiding alarms within patients’ rooms.
An organisation structured around the needs of the patient is mandatory in designing an effective intensive care department. Materials, apparatus and buildings will eventually get old, but the concepts such as daylight, privacy and safety will always be the future. Concepts should, however, be developed and used in choosing medical equipment so that the entire scheme developed will survive the test of time.
As a result of these innovative developments, UMC Utrecht was selected as the recipient of the 2011 ICU Design Citation Award, which is co-sponsored by the Society of Critical Care Medicine, the American Association of Critical Care Nurses and the American Institute of Architects Academy on Architecture for Health.
«« HAMILTON MEDICAL – Your Ventilation Solution Provider from Mobile to Clinical ICU
Multi-Resistant Skin Bacteria Spreading in Hospitals in Sweden, Researcher Finds »»

Margriet Schneider, MD, PhD

Prof. Jozef Kesecioglu, MD, PhD
Latest articles, pharmacist in the icu.
- ICU Journal Article
Pharmacists play a crucial role in the ICU, where patients often require complex medication regimens, including multiple medications and intravenous therapies. The role of a critical care pharmacist is multifaceted and vital to ensuring optimal patient care in the critical care setting. Cr
Defining Medicines Optimisation in the Intensive Care Unit
Complex polypharmacy and pathophysiology are common in the intensive care unit (ICU). Medicines optimisation is essential to deliver safe, effective, and individualised pharmacotherapy. This is ideally performed by a specialised ICU pharmacist. Introduction Medicines ar
Critical Care Pharmacists Save Lives
The purpose of this review is to discuss the role of critical care pharmacists on the interprofessional healthcare team in the care of critically ill patients and explore current gaps in the provision of comprehensive medication management. Introduction “The patient’s
Latest News

- Movers & Shakers

Former KPMG Chairman and CEO Lynne Doughtie...

Fresenius Medical Care Appoints Jörg Häring...

Abionic Appoints Patrick Pestalozzi as New...

Intensive Care Consultant to Lead NIHR's...

12th EuroELSO Congress 2024

20th Annual Critical Care Symposium

13th Ultrasound In Acute Care 2024

Urgent Care Updates 2024

34th ECCMID 2024
- Advertising
- Submit Article
- Author Guide
- Privacy Policy
- Cookie Policy
- Terms and conditions
- Copyright and permissions
- Editorial Board
- White Papers & Case Studies
- IMAGING Highlights
- ICU Highlights
- EXEC Highlights
- IT Highlights
- CARDIO Highlights
- HealthManagement
- ICU Management
- (E)Hospital
- Imaging Management
- Imaging Management French
- Healthcare IT
- Cardiology Management
- IQ - Interventional Quarter
- List your event
- Past Events
- International Association
- National Association
- Universities - Institutions
- Paid Guest Posts
- I-I-I DigiFlash
Communities
- Decision Support
- Women's Health
- Enterprise Imaging
- Artificial Intelligence
- Finance Management
- Cybersecurity
- Sustainability
- Digital Transformation

Rue Villain XIV 53-55
B-1050 brussels, belgium, tel: +357 86 870 007, e-mail: [email protected], emea & row office, 166, agias filaxeos, cy-3083, limassol, cyprus, headquarters, kosta ourani, 5, petoussis court, 5th floor, cy-3085 limassol, cyprus.
- Open access
- Published: 21 June 2022
Fluid challenge in critically ill patients receiving haemodynamic monitoring: a systematic review and comparison of two decades
- Antonio Messina 1 , 2 ,
- Lorenzo Calabrò 1 ,
- Luca Pugliese 1 ,
- Aulona Lulja 1 ,
- Alexandra Sopuch 1 ,
- Daniela Rosalba 3 ,
- Emanuela Morenghi 1 , 2 ,
- Glenn Hernandez 4 ,
- Xavier Monnet 5 , 6 , 7 &
- Maurizio Cecconi 1 , 2
Critical Care volume 26 , Article number: 186 ( 2022 ) Cite this article
11k Accesses
30 Citations
36 Altmetric
Metrics details
Introduction
Fluid challenges are widely adopted in critically ill patients to reverse haemodynamic instability. We reviewed the literature to appraise fluid challenge characteristics in intensive care unit (ICU) patients receiving haemodynamic monitoring and considered two decades: 2000–2010 and 2011–2021.
We assessed research studies and collected data regarding study setting, patient population, fluid challenge characteristics, and monitoring. MEDLINE, Embase, and Cochrane search engines were used. A fluid challenge was defined as an infusion of a definite quantity of fluid (expressed as a volume in mL or ml/kg) in a fixed time (expressed in minutes), whose outcome was defined as a change in predefined haemodynamic variables above a predetermined threshold.
We included 124 studies, 32 (25.8%) published in 2000–2010 and 92 (74.2%) in 2011–2021, overall enrolling 6,086 patients, who presented sepsis/septic shock in 50.6% of cases. The fluid challenge usually consisted of 500 mL (76.6%) of crystalloids (56.6%) infused with a rate of 25 mL/min. Fluid responsiveness was usually defined by a cardiac output/index (CO/CI) increase ≥ 15% (70.9%). The infusion time was quicker (15 min vs 30 min), and crystalloids were more frequent in the 2011–2021 compared to the 2000–2010 period.
Conclusions
In the literature, fluid challenges are usually performed by infusing 500 mL of crystalloids bolus in less than 20 min. A positive fluid challenge response, reported in 52% of ICU patients, is generally defined by a CO/CI increase ≥ 15%. Compared to the 2000–2010 decade, in 2011–2021 the infusion time of the fluid challenge was shorter, and crystalloids were more frequently used.
Fluid administration in the intensive care unit (ICU) is one of the most common and disputed interventions triggered at the bedside by several clinical variables [ 1 , 2 ].
Fluid therapy aims to increase stroke volume (SV) and cardiac output (CO) to optimise systemic blood flow and tissue perfusion. As with any therapeutic intervention, the final clinical effect elicited may vary because of a complex interplay between the patient's intrinsic conditions and the therapy itself.
Fluid responsiveness can occur only if both ventricles work on the ascending, steep part of the Frank–Starling curve, i.e. in cases where CO is preload dependent [ 3 , 4 ]. Preload dependency is assessed using a diagnostic test performed by infusing a fixed aliquot of fluid, the fluid challenge [ 5 , 6 , 7 ]. From a clinical perspective, this approach also allows titration of fluid administration (when the patient becomes no longer responsive to the fluid challenge) and reduces the risk of fluid overload, which worsens the outcome of ICU patients [ 8 , 9 ].
Several variables defining the characteristics of the fluid challenge have been further investigated in studies adopting continuous haemodynamic monitoring, showing that the amount of fluids given, the rate of administration, and the threshold adopted to define fluid responsiveness impact the outcome of a fluid challenge [ 10 , 11 , 12 ]. Moreover, despite conflicting results on shock reversal efficacy between crystalloids and colloids, crystalloids are now recommended as the first-line fluid type in patients with septic shock, being inexpensive and widely available. Also, the administration of colloids compared to crystalloids has not demonstrated any clear benefit in the literature [ 13 , 14 ].
However, neither the nature, mode of administration, and method to assess the effectiveness of the fluid challenge are standardised in current clinical practice, and the definition of fluid challenge responsiveness is also variable among studies [ 15 , 16 , 17 , 18 ].
Whether or not these findings have modified the modalities of fluid challenge and the definition of fluid responsiveness in published studies is uncertain. To address this issue, we systematically reviewed existing literature from the year 2000. We appraised the characteristics of fluid challenges in critically ill patients (i.e., amount and kind of fluid administration, time of infusion, hemodynamic variables, and thresholds for fluid responsiveness) enrolled in research studies receiving continuous haemodynamic monitoring and assessed the relationship between the reported fluid responsiveness and predefined independent variables. Secondarily, we compared data from studies published in 2011–2021 versus those published in 2000–2010.
Material and methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis-Protocols (PRISMA-P) guidelines (Additional file 1 : Table S1). The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) in November 2021 (CRD42021284761).
Search strategy
A systematic literature search was performed, including the following databases: PUBMED®, EMBASE®, and the Cochrane Controlled Clinical trials register. The following keywords and their related MeSh terms were used: “fluid challenge”, “fluid responsiveness”, “stroke volume variation”, “pulse pressure variation”, “dynamic indices OR indexes”, “passive leg raising”, OR “passive leg raising test”, “functional haemodynamic test OR tests”. Included papers were also examined to identify other studies of interest missed during the primary search.
Study selection and inclusion criteria
Articles enrolling at least 20 adult critically ill patients, written in English and published from 1st January 2000 to 31st December 2021 in indexed scientific journals, were considered. Editorials, commentaries, letters to the editor, opinion articles, reviews, and meeting abstracts were excluded. Studies enrolling paediatric or obstetric populations were excluded. References of selected papers, review articles, commentaries, and editorials on this topic were also reviewed to identify other studies of interest missed during the primary search. When multiple publications of the same research group/centre described potentially overlapping cohorts, the most recent publications were selected.
A fluid challenge was defined as an infusion of a definite quantity of fluid (expressed as a volume in mL or ml/kg) in a fixed time (expressed in minutes), whose outcome was defined as a change in one of the following haemodynamic variables above a predetermined threshold: CO, cardiac index (CI), SV, SV index (SVI), or surrogate of SV, i.e., velocity–time integral (VTI) in the left ventricular outflow tract and aortic blood flow (ABF), as assessed by transthoracic, transoesophageal echocardiography or oesophageal Doppler. We included studies adopting both a specific (i.e., Ringer lactate, saline, etc.) and a broad definition (i.e., crystalloids, colloids, etc.) of the fluid used for the fluid challenge. Studies adopting changes in systemic arterial pressure to define fluid responsiveness were excluded. Finally, we considered the predefined clinical reasons and triggers to start fluid challenge infusion.
Data extraction
Three couples of examiners independently evaluated titles and abstracts. The articles were then subdivided into three subgroups: “included” and “excluded” (if the two examiners agreed with the selection) or “uncertain” (in case of disagreement). In the case of “uncertain” classification, discrepancies were resolved by further examination performed by one of the three expert authors (A.M., X.M., or M.C.). We used a standardised electronic spreadsheet (Microsoft Excel, V 14.4.1; Microsoft, Redmond, WA) to extract data from all included studies, recording: the study setting (type of study, geographical area and time, where and when the study was carried out, and sample size), patient characteristics (gender, age, reason for admission, underlying diseases, ICU scores of severity, mode of ventilation, and inotropic/vasopressor support), criteria for haemodynamic instability, fluid challenge characteristics, pre- and post-fluid challenge haemodynamic variables. When necessary, the corresponding authors of the included studies were contacted to obtain missing data related to trial demographics, methods, and outcomes (Additional file 1 : Table S2).
Statistical analysis
Statistical analysis was conducted on the summary statistics described in the selected articles (e.g., means, medians, proportions) and, therefore, the statistical unit of observation for all the selected variables was the single study and not the patient. Due to the discrepancy between the overall patients enrolled in the trials over the two considered decaders, the comparisons were not weighted for study size.
Fluid challenge was the exposure variable, and clinical and haemodynamic characteristics were considered outcome variables. Descriptive statistics of individual studies used different statistical indicators for central tendency and variability, such as means and standard deviations (i.e., age, tidal volume, fluid responders, severity scores), whereas absolute and relative frequencies were adopted for qualitative variables. To show one indicator for the quantitative variables, we collected means with standard deviations (SD) or medians and inter-quartile ranges (IQR).
Student's t test or Mann–Whitney test in case of parametric or nonparametric distributions, respectively, were used to assess the difference in mean values between responders and non-responders.
Statistical analyses were conducted using GraphPad PRISM® 8 (GraphPad Software Inc., San Diego, CA, USA) and STATA®15 (StataCorp, College Station, TX, USA). For all comparisons, we considered p values < 0.05 significant.
The electronic search identified 3,963 potentially relevant studies. Figure 1 and Additional file 1 : Table S3 provide a detailed description of the selection process flow. After evaluating 160 full-text manuscripts, the inclusion criteria were met by 124 studies, 32 (25.8%) published in the period 2000–2010 and 92 (74.2%) in the period 2011–2021. Ten studies (8.1%) required revision by senior examiners because of disagreement regarding inclusion criteria between the initial examiners. We did not find any further relevant publications by reviewing the bibliography of the selected studies.
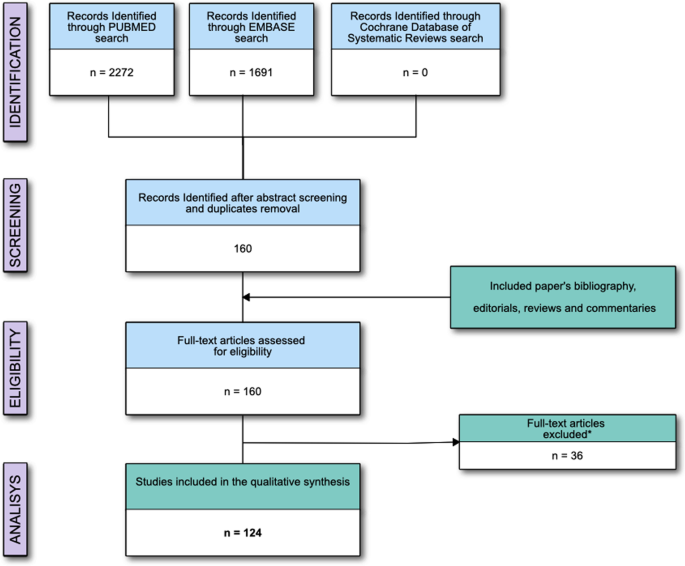
Flow of the studies; *Reasons for studies' exclusion are reported in the Supplementary materials
The general characteristics of the patients are reported in Table 1 . We included 6,086 patients, with a median (IQR) of 38 (30–59) patients enrolled in each study. Six studies (4.8%) [ 20 , 21 , 22 , 23 , 24 , 25 ] were retrospective, while the others were prospective. The median (IQR) period of enrolment [reported in 66 (52.8%) studies] was 12 (6–18) months. At baseline, 2,985 (49.0%) patients received norepinephrine, 179 (2.9%) dopamine, 416 (6.8%) dobutamine, and 177 (2.8%) epinephrine.
The reliability of a functional haemodynamic test in predicting fluid responsiveness was assessed in 46 (37.1%) studies. Comparing the two considered decades, no difference was found in the rate of FC administration [17 min (17–33) vs. 33 min (17–50); p = 0.39), in the percentage of fluid responders [52% (43–67) vs. 53% (45–60); p = 0.91], in the percentage of studies adopting crystalloids over colloids [63.6% vs. 67.9%; p = 1.00), or in the threshold of increase in CO or surrogates adopted to define fluid responsiveness (10% over 15%) [18.2% vs. 24.1%; p = 1.00).
Forty-four studies (35.4%) investigated the reliability of a dynamic index in predicting fluid responsiveness. Comparing the two considered decades, no differences were found in the rate of FC administration [17 min (17–25) vs. 29 min (13–33) p = 0.42), or in the rate of fluid responders [53% (41–62) vs. 50% (44–56) p = 0.81), or in the threshold of increase in CO or surrogates adopted to define fluid responsiveness (10% over 15%) (78.5% vs. 66.67 p = 0.42), as compared to studies in the decade 2000–2010. On the contrary, in the decade 2010-2021 we adopted more frequently crystalloids (21.4% vs. 60.0% p = 0.024).
Fluid challenge characteristics and haemodynamic monitoring
Overall, the included studies infused 6,333 fluid challenges. The median (IQR) proportion of fluid responders was 52 (44–62)% (Table 2 ).
In 19 studies (15.3%), the volume of the fluid challenge was reported in mL/kg, with a median (IQR) of 7 (6–8) mL/kg (Table 2 ). A fixed volume of 500 mL was administered in 95 (76.6)% of the included studies. The median (IQR) of the dispensed volume of fluid was 500 (500–500) mL, infused in a median (IQR) of 18 (11–30) min. Then, the median (IQR) infusion rate was 25 (17–33) mL/min.
CO/CI was used as target variables in 78 (62.9%) studies, while SV/SVI was used in 40 (32.2%) studies. The other six studies (4.8%) adopted SV surrogates (ABF in 4 studies and VTI in two studies). In 88 (70.9%) studies, the threshold adopted to define the fluid responsiveness was an increase of the considered variable ≥ 15% from baseline (Table 2 ).
Three studies (2.4%) [ 25 , 26 , 27 ] did not report the type of fluid used for the fluid challenge. Among the others, crystalloids were used in 68 (56.6)% studies, colloids in 52 (43.3) %, and blood in one (0.8)% (Table 2 ).
The majority of the studies [49 (39.5%)] used transpulmonary thermodilution/dye dilution calibrated haemodynamic monitoring; 22 (17.7%) studies adopted the pulmonary artery catheter monitoring. Echocardiography (either transthoracic or transoesophageal) was used in 31 (25.0)% of studies, and 5 (4.0%) used oesophageal doppler monitoring. Uncalibrated pulse wave analysis monitoring was used in the other 14 (11.2)% studies (Table 2 ). Finally, bioreactance was adopted in three studies (2.4%). Haemodynamic pre–post-fluid challenge variables in responders and non-responders populations are reported in Table 3 .
Trigger of fluid challenge administration.
Hypotension (i.e., systolic or mean arterial pressure below a fixed value or reduced by a fixed percentage from baseline) was used in 68 (62.4)% of studies. Oliguria (i.e. a drop in urine output below 0.5 mL/h for 2 or 3 consecutive hours) was used in 54 (49.5)% studies, skin mottling or peripheral hypoperfusion in 47 (43.1)% studies, tachycardia (i.e. an increase in heart rate above 100–110 beats/min) in 43 (39.4)%, the need for initiating the infusion or reducing the dose of vasoactive drugs in 41 (37.6)% studies, an increase in blood lactate in 34 (31.2)% studies, a diagnosis of sepsis/septic shock in 12 (11.0)% studies, and renal or hepatic dysfunction in seven (6.4)% studies. Fifteen studies (12.1%) did not report any trigger to start fluid challenge administration.
Comparison of publication periods 2011–2021 versus 2000–2010
The comparison between the 2000–2010 and 2011–2021 decades is reported in Table 4 . The percentage of fluid responders (52% for both the decades) and the volume infused (500 mL) were comparable. On the contrary, the infusion time was lower in the last decade (a median of 15 (10–30) min vs 30 (15–30) min, p = 0.03). Crystalloids were used in 61.9% of studies published between 2011–2021 and 34.3% in the 2000–2010 decade ( p = 0.007) (Figs. 2 and S1 in the Additional file 1 ).
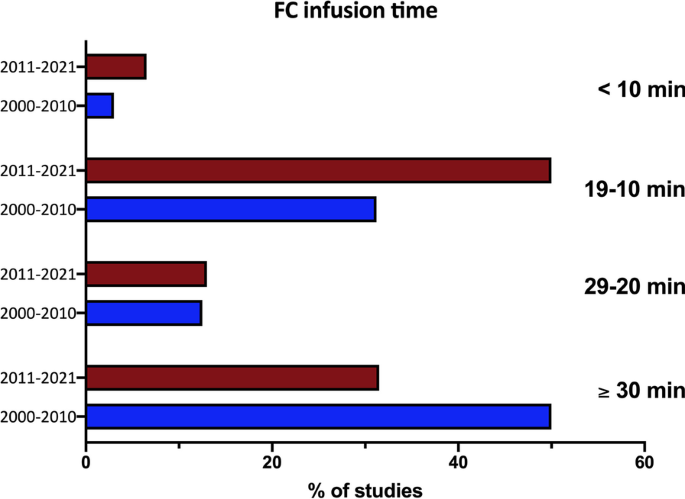
Percentage of studies in the two decades adopting different infusion timings. Fluid challenge, fluid challenge
CO/CI was used in 67% of the studies published in 2011–2021 and in 60% of those published in 2000–2010 ( p = 0.51). The threshold adopted was an increase in CO or surrogates ≥ 15% in 67.4% of the studies of the 2011–2021 decade and in 81.2% of the studies published in 2000–2010 ( p = 0.17) (Additional file 1 : Figure S1).
The results of this review, including research studies investigating the fluid challenge effect in critically ill adult patients receiving haemodynamic monitoring, may be summarised as follows: 1) fluid challenge is usually performed infusing a bolus of 500 mL of fluid, most often a crystalloid, in less than 20 min; 2) the response to fluid challenge is usually defined as a CI or CO increase ≥ 15% as compared to baseline; 3) positive response to fluid challenge is reported in about 50% of ICU patients; 4) the most common trigger for fluid challenge administration is usually the occurrence of hypotension, followed by oliguria and clinical signs of hypoperfusion; 5) the comparison between the 2000–2010 and 2011–2021 decades of publication showed no difference in the percentage of fluid responders (52% on average for both the decades), the volume infused (500 ml), and the criteria defining fluid responsiveness. On the contrary, compared to the 2000–2010 decade, in the period 2011–2021, the fluid challenge infusion time was lower, and crystalloids were more frequently used.
Fluid challenge characteristics
Among the included studies, the fluid challenge usually consisted of a median volume of 500 mL administered over a 20-min period and defined as a positive response by an increase ≥ 15% of CO or surrogate. These characteristics and responsiveness definition are to be considered good practice, for the response of CO to a fluid bolus is poorly followed by the simultaneous changes in arterial pressure [ 28 , 29 ] or heart rate [ 30 ]. However, this is not the case in clinical practice, where the fluid challenge effect is often assessed by a rise in arterial blood pressure [ 16 ].
Interestingly, 500 mL was also the median volume fluid challenge used in the FENICE study (an observational study including 311 centres across 46 countries) [ 16 ], whereas a fluid challenge of 250 mL is usually adopted in high-risk surgical patients undergoing goal-directed therapy optimisation [ 31 ]. The use of large volumes for fluid challenge optimisation should be balanced to the detrimental risk of fluid overload [ 9 ], primarily if safety limits (i.e. , increase in CVP) dynamically indicate fluid non-responsiveness are rarely used [ 19 ]. Since fluid challenge volume should be at least 4 mL/kg [ 32 ], smaller fluid challenge volumes may be considered for repetitive tests.
Moreover, the FENICE study reported a median of 24 min of infusion time and a rate of 17 mL/min [ 16 ]. Hence, the volume and rate of administration seem comparable between clinical and research settings. On the contrary, the infusion time was lower in the last decade (a median of 15 min vs 30 min, p = 0.03), indicating a trend towards the increase in the infusion rate in more recent studies. This global inception cohort study evaluated the clinical use of the fluid challenge in daily practice, whereas our review considered only research papers adopting the fluid challenge as a part of a protocol, limiting the comparison with the results of the FENICE. Moreover, in contrast with a previous metanalysis, including ICU studies up to 2014 [ 19 ], crystalloids are used in most studies. Crystalloids have been used in two-thirds of the studies from 2011 to 2021, compared to one-third from 2000 to 2010. These data indicate an alignment between research studies, recent guidelines, and metanalyses [ 13 , 14 ].
Limitations
Limitations of our review have to be considered when extrapolating the results to clinical practice. First, the present study does not report any outcome endpoints. A recent large randomised-controlled trial showed no difference in mortality rate among ICU patients receiving different fluid bolus infusion rates [ 33 ]. However, the faster rate adopted in this study (5.5 mL/min) is below the median rate found in the studies included in the present review (25 mL/min) [ 33 ]. The administration of aliquots of fluids at a slow rate should not probably be indicated as a fluid challenge. Moreover, all the included studies are research papers whose aim was to evaluate the haemodynamic changes after the fluid challenge infusion or assess the reliability of indexes or functional haemodynamic tests in predicting the response to a fluid challenge. We did not include studies on the fluid challenge clinical use in ICU patients.
Another potential source of bias is related to the different haemodynamic monitorings used to assess fluid challenge responsiveness. When considering the median cut-off value identifying responders from non-responders, the accuracy of measurement of the changes in CO, or its surrogates, is undoubtedly relevant. Additionally, the reliability of different monitorings in tracking the dynamic trends of CO may not be consistent and may be below the boundaries of accuracy and precision of the Critchley–Critchley criteria [ 34 ]. Hence, the reproducibility of CO measurements obtained by the different monitoring systems may be limited. Moreover, cut-off values and measurement techniques potentially induce heterogeneity in response to the fluid challenge administration. As confirmed, responders ranged from 23 to 100% across the included studies (Table 2 ). The use of echocardiography is associated with high proportions of fluid responders compared to other haemodynamic monitoring devices. The operator-dependent bias may affect the evaluation of SV changes after fluid challenge.
We excluded studies in which the fluid challenge response has been assessed without haemodynamic monitoring and, hence assessing changes in systemic arterial pressures, potentially limiting the whole comparability of the technique in the two considered decades. Finally, the overall number of patients enrolled in the trials of the two considered decades was considerably different. This could bias the comparisons between the two groups if weighted for study size.
This systematic review, including research studies on fluid challenge use in critically ill adult patients receiving haemodynamic monitoring, showed a positive response in 52% of the patients. This test was usually performed infusing a bolus of 500 mL fluid, more often a crystalloid, in less than 20 min, and fluid responsiveness was generally indicated as a CI or CO increase ≥ 15% compared to baseline. Fluid challenge administration is usually triggered by hypotension. In the 2011–2021, the infusion time was shorter, and crystalloids were more frequently used than in the 2000–2010 decade.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Cardiac index
Stroke volume index
Cardiac output
Stroke volume
Intensive care unit
Central venous pressure
Velocity–time integral in the left ventricular outflow tract
Aortic blood flow
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51.
Article CAS PubMed Google Scholar
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the european society of intensive care medicine. Intensive Care Med. 2014;40:1795–815.
Article PubMed PubMed Central Google Scholar
Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–34.
Magder S. Bench-to-bedside review: an approach to hemodynamic monitoring–guyton at the bedside. Crit Care. 2012;16:236.
Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17:290–5.
Article PubMed Google Scholar
Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1.
Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6:111.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettila V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the classic randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–705.
Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: An analysis of a large national database. Intensive Care Med. 2017;43:625–32.
Messina A, Palandri C, De Rosa S, Danzi V, Bonaldi E, Montagnini C, et al.: Pharmacodynamic analysis of a fluid challenge with 4 mL kg(-1) over 10 or 20 min: A multicenter cross-over randomized clinical trial. J Clin Monit Comput 2021
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45:e161–8.
Messina A, Sotgiu G, Saderi L, Cammarota G, Capuano L, Colombo D, et al.: Does the definition of fluid responsiveness affect passive leg raising reliability? A methodological ancillary analysis from a multicentric study. Minerva Anestesiol 2021
Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567.
PubMed Google Scholar
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185.
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: The fenice study: a global inception cohort study. Intensive Care Med. 2015;41:1529–37.
Messina A, Longhini F, Coppo C, Pagni A, Lungu R, Ronco C, et al.: Use of the fluid challenge in critically ill adult patients: a systematic review. Anesth Analg 2017
Messina A, Dell’Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: A systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019;23:264.
Messina A, Longhini F, Coppo C, Pagni A, Lungu R, Ronco C, et al. Use of the fluid challenge in critically ill adult patients: a systematic review. Anesth Analg. 2017;125:1532–43.
Vaquer S, Chemla D, Teboul JL, Ahmad U, Cipriani F, Oliva JC, et al. Volume infusion markedly increases femoral dp/dtmax in fluid-responsive patients only. Crit Care Med. 2020;48:1487–93.
Monge Garcia MI, Guijo Gonzalez P, Gracia Romero M, Gil Cano A, Oscier C, Rhodes A, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015;41:1247–55.
Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–8.
Velissaris D, Pierrakos C, Scolletta S, De Backer D, Vincent JL. High mixed venous oxygen saturation levels do not exclude fluid responsiveness in critically ill septic patients. Crit Care. 2011;15:R177.
Hu B, Xiang H, Liang H, Yu L, Xu T, Yang JH, et al. Assessment effect of central venous pressure in fluid resuscitation in the patients with shock: a multi-center retrospective research. Chin Med J (Engl). 2013;126:1844–9.
Google Scholar
De Santis P, De Fazio C, Franchi F, Bond O, Vincent JL, Creteur J, et al.: Incoherence between systemic hemodynamic and microcirculatory response to fluid challenge in critically ill patients. J Clin Med 2021;10
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–305.
Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a monitoring to select icu patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–9.
Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, et al. Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med. 2011;39:1394–9.
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–8.
Ait-Hamou Z, Teboul JL, Anguel N, Monnet X. How to detect a positive response to a fluid bolus when cardiac output is not measured? Ann Intensive Care. 2019;9:138.
Messina A, Pelaia C, Bruni A, Garofalo E, Bonicolini E, Longhini F, et al.: Fluid challenge during anesthesia: A systematic review and meta-analysis. Anesth Analg 2018
Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–91.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the basics randomized clinical trial. JAMA. 2021;326:830–8.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91.
Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–9.
Preau S, Dewavrin F, Soland V, Bortolotti P, Colling D, Chagnon JL, et al. Hemodynamic changes during a deep inspiration maneuver predict fluid responsiveness in spontaneously breathing patients. Cardiol Res Pract. 2012;2012: 191807.
Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–7.
Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A. Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med. 2008;34:1239–45.
Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143:364–70.
Mahjoub Y, Benoit-Fallet H, Airapetian N, Lorne E, Levrard M, Seydi AA, et al. Improvement of left ventricular relaxation as assessed by tissue doppler imaging in fluid-responsive critically ill septic patients. Intensive Care Med. 2012;38:1461–70.
Wyffels PA, Durnez PJ, Helderweirt J, Stockman WM, De Kegel D. Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg. 2007;105:448–52.
Wu Y, Zhou S, Zhou Z, Liu B. A 10-second fluid challenge guided by transthoracic echocardiography can predict fluid responsiveness. Crit Care. 2014;18:R108.
Jozwiak M, Depret F, Teboul JL, Alphonsine JE, Lai C, Richard C, et al. Predicting fluid responsiveness in critically ill patients by using combined end-expiratory and end-inspiratory occlusions with echocardiography. Crit Care Med. 2017;45:e1131–8.
Fellahi JL, Fischer MO, Rebet O, Massetti M, Gerard JL, Hanouz JL. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2012;26:217–22.
Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009;37:951–6.
Smorenberg A, Cherpanath TGV, Geerts BF, de Wilde RBP, Jansen JRC, Maas JJ, et al. A mini-fluid challenge of 150ml predicts fluid responsiveness using modelflow(r) pulse contour cardiac output directly after cardiac surgery. J Clin Anesth. 2018;46:17–22.
Monnet X, Bleibtreu A, Ferre A, Dres M, Gharbi R, Richard C, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–7.
Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: The mini-fluid challenge study. Anesthesiology. 2011;115:541–7.
Chen YH, Lai YJ, Huang CY, Lin HL, Huang CC. Effects of positive end-expiratory pressure on the predictability of fluid responsiveness in acute respiratory distress syndrome patients. Sci Rep. 2021;11:10186.
Article CAS PubMed PubMed Central Google Scholar
Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Arterial pressure changes during the valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009;35:77–84.
Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006;103:1478–84.
Messina A, Romano SM, Ozdemirkan A, Persona P, Tarquini R, Cammarota G, et al.: Multivariable haemodynamic approach to predict the fluid challenge response: a multicentre cohort study. Eur J Anaesthesiol 2020
Mahjoub Y, Pila C, Friggeri A, Zogheib E, Lobjoie E, Tinturier F, et al. Assessing fluid responsiveness in critically ill patients: False-positive pulse pressure variation is detected by doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37:2570–5.
Taccheri T, Gavelli F, Teboul JL, Shi R, Monnet X. Do changes in pulse pressure variation and inferior vena cava distensibility during passive leg raising and tidal volume challenge detect preload responsiveness in case of low tidal volume ventilation? Crit Care. 2021;25:110.
Fischer MO, Coucoravas J, Truong J, Zhu L, Gerard JL, Hanouz JL, et al. Assessment of changes in cardiac index and fluid responsiveness: a comparison of nexfin and transpulmonary thermodilution. Acta Anaesthesiol Scand. 2013;57:704–12.
Kaur KB, Nakra M, Mangal V, Singh S, Taank P, Marwah V. Comparative evaluation of stroke volume variation and inferior vena cava distensibility index for prediction of fluid responsiveness in mechanically ventilated patients. Ann Card Anaesth. 2021;24:327–32.
PubMed PubMed Central Google Scholar
Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53:534–42.
Biasucci DG, Cina A, Calabrese M, Antoniucci ME, Cavaliere C, Bevilacqua F, et al. Size and shape of the inferior vena cava before and after a fluid challenge: a pilot study. Minerva Anestesiol. 2019;85:514–21.
Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111:961–6.
Gavaud A, Nguyen LS, Caubel A, Grillet G, Donal E, Belliard G. Respiratory variability of pulmonary velocity-time integral as a new gauge of fluid responsiveness for mechanically ventilated patients in the icu. Crit Care Med. 2019;47:e310–6.
Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Brachial artery peak velocity variation to predict fluid responsiveness in mechanically ventilated patients. Crit Care. 2009;13:R142.
Depret F, Jozwiak M, Teboul JL, Alphonsine JE, Richard C, Monnet X. Esophageal doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit Care Med. 2019;47:e96–102.
Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, et al. Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim. 2012;31:e67-72.
Messina A, Colombo D, Barra FL, Cammarota G, De Mattei G, Longhini F, et al. Sigh maneuver to enhance assessment of fluid responsiveness during pressure support ventilation. Crit Care. 2019;23:31.
Soubrier S, Saulnier F, Hubert H, Delour P, Lenci H, Onimus T, et al. Can dynamic indicators help the prediction of fluid responsiveness in spontaneously breathing critically ill patients? Intensive Care Med. 2007;33:1117–24.
Vistisen ST, Krog MB, Elkmann T, Vallentin MF, Scheeren TWL, Solling C. Extrasystoles for fluid responsiveness prediction in critically ill patients. J Intensive Care. 2018;6:52.
Fellahi JL, Fischer MO, Dalbera A, Massetti M, Gerard JL, Hanouz JL. Can endotracheal bioimpedance cardiography assess hemodynamic response to passive leg raising following cardiac surgery? Ann Intensive Care. 2012;2:26.
Xu B, Yang X, Wang C, Jiang W, Weng L, Hu X, et al. Changes of central venous oxygen saturation define fluid responsiveness in patients with septic shock: a prospective observational study. J Crit Care. 2017;38:13–9.
Preau S, Bortolotti P, Colling D, Dewavrin F, Colas V, Voisin B, et al. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit Care Med. 2017;45:e290–7.
Lakhal K, Ehrmann S, Runge I, Benzekri-Lefevre D, Legras A, Dequin PF, et al. Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med. 2010;36:940–8.
Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med. 2011;26:116–24.
Oliveira-Costa CD, Friedman G, Vieira SR, Fialkow L. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics (Sao Paulo). 2012;67:773–8.
Article Google Scholar
Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–20.
Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, et al. Measuring aortic diameter improves accuracy of esophageal doppler in assessing fluid responsiveness. Crit Care Med. 2007;35:477–82.
Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39:689–94.
Ishihara H, Hashiba E, Okawa H, Saito J, Kasai T, Tsubo T. Neither dynamic, static, nor volumetric variables can accurately predict fluid responsiveness early after abdominothoracic esophagectomy. Perioper Med (Lond). 2013;2:3.
Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36:496–503.
Monge Garcia MI, Gil Cano A, Gracia Romero M, Monterroso Pintado R, Perez Madueno V, Diaz Monrove JC. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal co2 pressure during a passive leg-raising maneuver. Ann Intensive Care. 2012;2:9.
Muller L, Louart G, Bengler C, Fabbro-Peray P, Carr J, Ripart J, et al. The intrathoracic blood volume index as an indicator of fluid responsiveness in critically ill patients with acute circulatory failure: a comparison with central venous pressure. Anesth Analg. 2008;107:607–13.
Luzi A, Marty P, Mari A, Conil JM, Geeraerts T, Lepage B, et al. Noninvasive assessment of hemodynamic response to a fluid challenge using femoral doppler in critically ill ventilated patients. J Crit Care. 2013;28:902–7.
Heenen S, De Backer D, Vincent JL. How can the response to volume expansion in patients with spontaneous respiratory movements be predicted? Crit Care. 2006;10:R102.
Dong ZZ, Fang Q, Zheng X, Shi H. Passive leg raising as an indicator of fluid responsiveness in patients with severe sepsis. World J Emerg Med. 2012;3:191–6.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–23.
Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009;35:85–90.
Le Dorze M, Huche F, Coelembier C, Rabuel C, Payen D. Impact of fluid challenge increase in cardiac output on the relationship between systemic and cerebral hemodynamics in severe sepsis compared to brain injury and controls. Ann Intensive Care. 2018;8:74.
Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–25.
Wu J, Wang Z, Wang T, Yu T, Yuan J, Zhang Q, et al. Evaluation of the fluid responsiveness in patients with septic shock by ultrasound plus the passive leg raising test. J Surg Res. 2018;224:207–14.
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–7.
Si X, Cao DY, Chen J, Wu JF, Liu ZM, Xu HL, et al. Effect of systolic cardiac function on passive leg raising for predicting fluid responsiveness: a prospective observational study. Chin Med J (Engl). 2018;131:253–61.
Pouska J, Tegl V, Astapenko D, Cerny V, Lehmann C, Benes J. Impact of intravenous fluid challenge infusion time on macrocirculation and endothelial glycocalyx in surgical and critically ill patients. Biomed Res Int. 2018;2018:8925345.
Article PubMed PubMed Central CAS Google Scholar
Monnet X, Guerin L, Jozwiak M, Bataille A, Julien F, Richard C, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110:207–13.
Xu J, Peng X, Pan C, Cai S, Zhang X, Xue M, et al. Fluid responsiveness predicted by transcutaneous partial pressure of oxygen in patients with circulatory failure: a prospective study. Ann Intensive Care. 2017;7:56.
Loupec T, Nanadoumgar H, Frasca D, Petitpas F, Laksiri L, Baudouin D, et al. Pleth variability index predicts fluid responsiveness in critically ill patients. Crit Care Med. 2011;39:294–9.
Soussi S, Vallee F, Roquet F, Bevilacqua V, Benyamina M, Ferry A, et al. Measurement of oxygen consumption variations in critically ill burns patients: are the fick method and indirect calorimetry interchangeable? Shock. 2017;48:532–8.
Monnet X, Dres M, Ferre A, Le Teuff G, Jozwiak M, Bleibtreu A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109:330–8.
Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6:10.
Huang CC, Fu JY, Hu HC, Kao KC, Chen NH, Hsieh MJ, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36:2810–6.
Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by flotrac/vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–9.
Hamimy W, Mukhtar A, Zaghloul A, Salem M. Comparing transesophageal doppler corrected systolic flow time versus central venous pressure as a guide for fluid resuscitation in septic shock. Egypt J Anaesthesia. 2019;32:181–7.
Fischer MO, Pelissier A, Bohadana D, Gerard JL, Hanouz JL, Fellahi JL. Prediction of responsiveness to an intravenous fluid challenge in patients after cardiac surgery with cardiopulmonary bypass: a comparison between arterial pulse pressure variation and digital plethysmographic variability index. J Cardiothorac Vasc Anesth. 2013;27:1087–93.
Liu Y, Wei LQ, Li GQ, Yu X, Li GF, Li YM. Pulse pressure variation adjusted by respiratory changes in pleural pressure, rather than by tidal volume, reliably predicts fluid responsiveness in patients with acute respiratory distress syndrome. Crit Care Med. 2016;44:342–51.
Kramer A, Zygun D, Hawes H, Easton P, Ferland A. Pulse pressure variation predicts fluid responsiveness following coronary artery bypass surgery. Chest. 2004;126:1563–8.
Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monnet X. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care. 2015;19:411.
Yazigi A, Khoury E, Hlais S, Madi-Jebara S, Haddad F, Hayek G, et al. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26:387–90.
Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.
Wyler von Ballmoos M, Takala J, Roeck M, Porta F, Tueller D, Ganter CC, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14:R111.
Messina A, Colombo D, Cammarota G, De Lucia M, Cecconi M, Antonelli M, et al. Patient-ventilator asynchrony affects pulse pressure variation prediction of fluid responsiveness. J Crit Care. 2015;30:1067–71.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8.
Cecconi M, Monge Garcia MI, Gracia Romero M, Mellinghoff J, Caliandro F, Grounds RM, et al. The use of pulse pressure variation and stroke volume variation in spontaneously breathing patients to assess dynamic arterial elastance and to predict arterial pressure response to fluid administration. Anesth Analg. 2015;120:76–84.
Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15:R85.
Soliman RA, Samir S, el Naggar A, El Dehely K. Stroke volume variation compared with pulse pressure variation and cardiac index changes for prediction of fluid responsiveness in mechanically ventilated patients. The Egypt J Crit Care Med. 2015;3:9–16.
Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care. 2012;16:R188.
Nunes TS, Ladeira RT, Bafi AT, de Azevedo LC, Machado FR, Freitas FG. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care. 2014;4:25.
Giraud R, Siegenthaler N, Gayet-Ageron A, Combescure C, Romand JA, Bendjelid K. Scvo(2) as a marker to define fluid responsiveness. J Trauma. 2011;70:802–7.
CAS PubMed Google Scholar
Lakhal K, Ehrmann S, Perrotin D, Wolff M, Boulain T. Fluid challenge: Tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive). Intensive Care Med. 2013;39:1953–62.
Suehiro K, Rinka H, Ishikawa J, Fuke A, Arimoto H, Miyaichi T. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing airway pressure release ventilation. Anaesth Intensive Care. 2012;40:767–72.
Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50:1068–73.
Smorenberg A, Lust EJ, Beishuizen A, Meijer JH, Verdaasdonk RM, Groeneveld AB. Systolic time intervals vs invasive predictors of fluid responsiveness after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2013;44:891–7.
Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, et al. The estimation of cardiac output by the nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care. 2012;16:R212.
Schnell D, Camous L, Guyomarc’h S, Duranteau J, Canet E, Gery P, et al. Renal perfusion assessment by renal doppler during fluid challenge in sepsis. Crit Care Med. 2013;41:1214–20.
Yonis H, Bitker L, Aublanc M, Perinel Ragey S, Riad Z, Lissonde F, et al. Change in cardiac output during trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. 2017;21:295.
Xiao-ting W, Hua Z, Da-wei L, Hong-min Z, Huai-wu H, Yun L, et al. Changes in end-tidal co2 could predict fluid responsiveness in the passive leg raising test but not in the mini-fluid challenge test: a prospective and observational study. J Crit Care. 2015;30:1061–6.
Elsayed AI, Selim KA, Zaghla HE, Mowafy HE, Fakher MA. Comparison of changes in ppv using a tidal volume challenge with a passive leg raising test to predict fluid responsiveness in patients ventilated using low tidal volume. Indian J Crit Care Med. 2021;25:685–90.
Biais M, Vidil L, Sarrabay P, Cottenceau V, Revel P, Sztark F. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: Comparison between echocardiography and vigileo/flotrac device. Crit Care. 2009;13:R195.
Bataille B, de Selle J, Moussot PE, Marty P, Silva S, Cocquet P. Machine learning methods to improve bedside fluid responsiveness prediction in severe sepsis or septic shock: an observational study. Br J Anaesth. 2021;126:826–34.
Mallat J, Meddour M, Durville E, Lemyze M, Pepy F, Temime J, et al. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsiveness. Br J Anaesth. 2015;115:449–56.
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–8.
Kumar N, Malviya D, Nath SS, Rastogi S, Upadhyay V. Comparison of the efficacy of different arterial waveform-derived variables (pulse pressure variation, stroke volume variation, systolic pressure variation) for fluid responsiveness in hemodynamically unstable mechanically ventilated critically ill patients. Indian J Crit Care Med. 2021;25:48–53.
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–32.
Braun F, Proenca M, Wendler A, Sola J, Lemay M, Thiran JP, et al. Noninvasive measurement of stroke volume changes in critically ill patients by means of electrical impedance tomography. J Clin Monit Comput. 2020;34:903–11.
Silva E, De Backer D, Creteur J, Vincent JL. Effects of fluid challenge on gastric mucosal pco2 in septic patients. Intensive Care Med. 2004;30:423–9.
Huette P, Abou-Arab O, Longrois D, Guinot PG. Fluid expansion improve ventriculo-arterial coupling in preload-dependent patients: a prospective observational study. BMC Anesthesiol. 2020;20:171.
Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, et al. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78:527–33.
Mohammad Abdelfattah WoM, Saad-eldeen Elgammal S, Mohammad Elsayed K, Said Mowafy SM, Mohammad Abdalla R. Distensibility index of inferior vena cava and pulse pressure variation as predictors of fluid responsiveness in mechanically ventilated shocked patients. J Emerg Med Trauma Acute Care 2020;2020
Georges D, de Courson H, Lanchon R, Sesay M, Nouette-Gaulain K, Biais M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: an echocardiographic study. Crit Care. 2018;22:32.
Jacquet-Lagreze M, Bouhamri N, Portran P, Schweizer R, Baudin F, Lilot M, et al. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit Care. 2019;23:281.
Monnet X, Bataille A, Magalhaes E, Barrois J, Le Corre M, Gosset C, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med. 2013;39:93–100.
Beurton A, Teboul JL, Girotto V, Galarza L, Anguel N, Richard C, et al. Intra-abdominal hypertension is responsible for false negatives to the passive leg raising test. Crit Care Med. 2019;47:e639–47.
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Esophageal doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31:1195–201.
Roger C, Zieleskiewicz L, Demattei C, Lakhal K, Piton G, Louart B, et al. Time course of fluid responsiveness in sepsis: the fluid challenge revisiting (fcrev) study. Crit Care. 2019;23:179.
Biais M, Cottenceau V, Stecken L, Jean M, Ottolenghi L, Roullet S, et al. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med. 2012;40:1186–91.
Mukhtar A, Awad M, Elayashy M, Hussein A, Obayah G, El Adawy A, et al. Validity of mini-fluid challenge for predicting fluid responsiveness following liver transplantation. BMC Anesthesiol. 2019;19:56.
Trifi A, Abdellatif S, Daly F, Nasri R, Touil Y, Ben Lakhal S. Ultrasound stroke volume variation induced by passive leg raising and fluid responsiveness: An observational cohort study. Med Intensiva (Engl Ed). 2019;43:10–7.
Article CAS Google Scholar
Michard F, Alaya S, Zarka V, Bahloul M, Richard C, Teboul JL. Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest. 2003;124:1900–8.
Giraud R, Abraham PS, Brindel P, Siegenthaler N, Bendjelid K. Respiratory changes in subclavian vein diameters predicts fluid responsiveness in intensive care patients: a pilot study. J Clin Monit Comput. 2018;32:1049–55.
Download references
Acknowledgements
We are thankful to Dr. Katerina Negri for the linguistic revision of this manuscript.
This work has not been funded by an external source.
Author information
Authors and affiliations.
Department of Anesthesia and Intensive Care Medicine, Humanitas Clinical and Research Center—IRCCS, Via Alessandro Manzoni, 56, 20089, Rozzano, Milano, Italy
Antonio Messina, Lorenzo Calabrò, Luca Pugliese, Aulona Lulja, Alexandra Sopuch, Emanuela Morenghi & Maurizio Cecconi
Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milano, Italy
Antonio Messina, Emanuela Morenghi & Maurizio Cecconi
Università del Piemonte Orientale, Vercelli, Italy
Daniela Rosalba
Departamento de Medicina Intensiva, Facultad de Medicina, Pontificia Universidad Católica de Chile, Diagonal Paraguay 362, Santiago, Chile
Glenn Hernandez
Hôpitaux Universitaires Paris-Sud, Hôpital de Bicêtre, Medical Intensive Care Unit, Le Kremlin-Bicêtre, F-94270, Paris, France
Xavier Monnet
Université Paris-Saclay, AP-HP, Service de médecine intensive-réanimation, Hôpital de Bicêtre, Paris, France
DMU CORREVE, Inserm UMR S_999, FHU SEPSIS, Groupe de Recherche Clinique CARMAS, Le Kremlin-Bicêtre, Paris, France
You can also search for this author in PubMed Google Scholar
Contributions
AM designed the study, performed data analysis, and drafted the manuscript; EM: helped in data analysis and manuscript preparation; LC, LP, AL, AS, and DR substantially contributed to data collection and interpretation; MC, XM, and GE substantially contributed to data interpretation and manuscript draft. All the authors approved the final version of the paper and agreed to be accountable for all aspects of the work, thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Correspondence to Antonio Messina .
Ethics declarations
Humans ethics statement, adult consent to participate written and human accordance statement.
Not applicable.
Consent for publication
Competing interests.
Dr. Messina received travel expenses and registration for meetings, congresses, and courses and lecture fees from Vygon, Edwards, Philips, and Getinge. Xavier Monnet is a member of the medical advisory board of Pulsion Medical Systems (Getinge), and has given lectures for Baxter. Prof. Cecconi is a consultant of Edwards Lifesciences (Directed Systems Consultancy).
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Table S1. PRISMA-DTA checklist. Table S2. Extracted data in each study assessed for eligibility. Table S3. Full-text articles excluded, not fitting eligibility criteria. Table S4. Studies on functional haemodynamic tests or dynamic indexes of fluid responsiveness. Figure S1. Characteristics of fluid challenge administration and monitoring along the considered years.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Messina, A., Calabrò, L., Pugliese, L. et al. Fluid challenge in critically ill patients receiving haemodynamic monitoring: a systematic review and comparison of two decades. Crit Care 26 , 186 (2022). https://doi.org/10.1186/s13054-022-04056-3
Download citation
Received : 01 April 2022
Accepted : 07 June 2022
Published : 21 June 2022
DOI : https://doi.org/10.1186/s13054-022-04056-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fluid challenge
- Fluid bolus
- Fluid responsiveness
- Critically ill patients
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
April 15, 2024
An ICU Nurse Explains the Vital Role of Family Caregivers in Loved Ones' Health
Family caregivers provide critical support and familiarity to patients, but can also experience burnout
By Courtney Graetzer & The Conversation US

Self-care, although often neglected by caregivers, is critical when looking after a loved one.
manassanant pamai/Getty Images
The floor nurse had just told me that my new patient – let’s call her Marie – would not stop screaming.
Marie landed in the intensive care unit where I am a bedside nurse because she was too agitated and needed more oxygen. We immediately tried to fit her with a more advanced oxygen mask, but the screaming continued and her oxygen level worsened. No matter how much I comforted her, it was not my hand she wanted to hold. She was screaming for her daughter, April, who was on her way.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
April had been Marie’s caregiver at home for the past few years after Marie was diagnosed with end-stage Alzheimer’s. April is Marie’s familiar face, her source of comfort when she gets disoriented. Now Marie had been admitted to the hospital for pneumonia, and April had not left her side.
As a seasoned bedside critical care nurse, I see firsthand the benefits that family caregivers bring to patient care in the hospital. I also witness the emotional stress that caregivers experience when their loved one comes to the ICU.
After years of helping families and physicians navigate the complicated course of an ICU hospital stay, I have some advice for caregivers to take with them.
Caregivers often battle anxiety and depression
From making medical decisions to advocating for their loved one, family caregivers have many important roles when their loved one is in the hospital. Their presence not only provides a sense of security, but also strongly influences a patient’s response to treatment .
For example, Marie refused to take walks during physical therapy until we found out from April that she felt safest in her pink New Balance shoes, which April brought to the hospital. April’s unique knowledge of Marie’s specific needs proved to be invaluable to guiding Marie’s treatment plan at the hospital.
Including the family in the patient’s treatment plan, also known as family-centered care, can help shorten a patient’s hospital stay and can even reduce hospital costs. However, caregivers carry heavy emotional burdens while supporting loved ones at the hospital .
In fact, family caregivers are at high risk of developing long-term psychological health problems . Up to 70% of first-degree relatives of ICU survivors suffer from anxiety symptoms, more than a third suffer from depression , and many can experience symptoms of post-traumatic stress disorder , or PTSD.
There are ways to help ease this emotional burden, and most of them come down to consistent and open communication between the patient, their caregivers and the medical team.
But how should you, as the caregiver without much medical knowledge, communicate with hospital staff when your loved one can’t speak for themself?
Communication is critical
First, exchange contact information with the primary medical team, which may include a passcode for patient privacy. This will ensure that you receive the most updated information about the patient and will give you the peace of mind knowing that you can call at any hour of the day or night to receive updates on your loved one.
Second, let the medical team know what the patient is normally like at home, which can include the patient’s medications, their baseline functional capabilities, any cultural or religious preferences, and their end-of-life wishes, just in case. With this information, the medical team can develop a reasonable treatment plan specific to your loved one, avoid unnecessary and uncomfortable tests, and provide a better insight into their prognosis and recovery.
As you provide information about the patient at home, the medical team should be giving you updates about the patient’s condition in the hospital. This is a good time for you to keep a diary to write down essential information and questions to ask them.
Knowing what to ask is essential to effective communication at the hospital. First, get yourself oriented to the hospital unit you are on: Ask about the visitation policy, unit phone number and even where the cafeteria and the bathrooms are.
Once familiar with your new environment, you may feel more at ease to truly be present for your loved one. Other important questions you can ask each day include:
What is happening to my loved one?
What is the plan for the next day?
What will the treatment be like for my loved one?
These are good first questions for setting daily expectations for the patient’s hospital stay. You can also find answers by participating in the patient’s clinical rounds. Every day, the interdisciplinary medical team sees each patient to discuss updates and treatment plans, and answers questions for the patient and their family. Research has also shown that rounds relieve anxiety and stress among family caregivers due to the consistent daily communication and emotional support that they provide.
Nurses can be helpful
After clinical rounds, the interdisciplinary team of doctors and nurses establishes a daily plan of care for your loved one, which will be carried out by your bedside nurse. The nurse will give the ordered medications, perform necessary clinical tasks and assess the patient for their response to the treatment. If you normally take care of the patient’s basic needs at home, offering to help your nurse with feeding or bathing may provide emotional reassurance to you and your loved one.
Nurses are the most accessible resource you have when your loved one is in the hospital. They can provide emotional support and coping strategies during this stressful time and can act as a translator between you and the physicians.
Once you establish a good relationship with your nurse and the medical team, spend quality time with your loved one. Even when the patient isn’t responsive, talk to them about familiar people in their life, FaceTime other family members, play their favorite music or TV show, and always remind them of the date and that they are in the hospital.
The importance of routines and familiarity
Since it’s easy for patients to lose track of the normal day-night cycle, they can be at high risk of ICU delirium , which is an acute and severe state of confusion. Preventing ICU delirium through reorientation and familiar faces can help prevent this serious complication and can even reduce their hospital stay.
Finally, one of the most important but often neglected task for you to do is self-care .
Research advises caregivers to tend to their own health and emotional needs by eating regularly, getting adequate sleep and taking breaks from the hospital. You have been strong for others and can continue to do so, but only if you take care of yourself as well.
Most families that come to the hospital describe the support they received from the medical team in a positive way . Your nurses and the rest of the medical team are all on your team, and we want the best possible outcome for your loved one.
This article was originally published on The Conversation . Read the original article .
Former smoking, but not active smoking, is associated with delirium in postoperative ICU patients: a matched case-control study
Affiliations.
- 1 Institute of Intensive Care Medicine, University Hospital Zurich & University of Zurich, Zurich, Switzerland.
- 2 Center of Clinical Nursing Sciences, University Hospital Zurich, Zurich, Switzerland.
- 3 Department for Quantitative Biomedicine, University of Zurich, Zurich, Switzerland.
- 4 Department of Anesthesia and Intensive Care Medicine, Tiefenau Hospital, Insel Group. University of Bern, Bern, Switzerland.
- PMID: 38559401
- PMCID: PMC10979642
- DOI: 10.3389/fpsyt.2024.1347071
Objective: To examine the relationship between current and former smoking and the occurrence of delirium in surgical Intensive Care Unit (ICU) patients.
Methods: We conducted a single center, case-control study involving 244 delirious and 251 non-delirious patients that were admitted to our ICU between 2018 and 2022. Using propensity score analysis, we obtained 115 pairs of delirious and non-delirious patients matched for age and Simplified Acute Physiology Score II (SAPS II). Both groups of patients were further stratified into non-smokers, active smokers and former smokers, and logistic regression was performed to further investigate potential confounders.
Results: Our study revealed a significant association between former smoking and the incidence of delirium in ICU patients, both in unmatched (adjusted odds ratio (OR): 1.82, 95% confidence interval (CI): 1.17-2.83) and matched cohorts (OR: 3.0, CI: 1.53-5.89). Active smoking did not demonstrate a significant difference in delirium incidence compared to non-smokers (unmatched OR = 0.98, CI: 0.62-1.53, matched OR = 1.05, CI: 0.55-2.0). Logistic regression analysis of the matched group confirmed former smoking as an independent risk factor for delirium, irrespective of other variables like surgical history (p = 0.010). Notably, also respiratory and vascular surgeries were associated with increased odds of delirium (respiratory: OR: 4.13, CI: 1.73-9.83; vascular: OR: 2.18, CI: 1.03-4.59). Medication analysis showed that while Ketamine and Midazolam usage did not significantly correlate with delirium, Morphine use was linked to a decreased likelihood (OR: 0.27, 95% CI: 0.13-0.55).
Discussion: Nicotine's complex neuropharmacological impact on the brain is still not fully understood, especially its short-term and long-term implications for critically ill patients. Although our retrospective study cannot establish causality, our findings suggest that smoking may induce structural changes in the brain, potentially heightening the risk of postoperative delirium. Intriguingly, this effect seems to be obscured in active smokers, potentially due to the recognized neuroprotective properties of nicotine. Our results motivate future prospective studies, the results of which hold the potential to substantially impact risk assessment procedures for surgeries.
Keywords: ICU delirium; case-control studies; critical care medicine; nicotine; postoperative delirium (POD); risk factors; smoking; withdrawal.
Copyright © 2024 Komninou, Egli, Rossi, Ernst, Krauthammer, Schuepbach, Delgado and Bartussek.
Grants and funding
- Open access
- Published: 17 April 2024
A data-driven combined prediction method for the demand for intensive care unit healthcare resources in public health emergencies
- Weiwei Zhang 1 &
- Xinchun Li 1
BMC Health Services Research volume 24 , Article number: 477 ( 2024 ) Cite this article
1 Altmetric
Metrics details
Public health emergencies are characterized by uncertainty, rapid transmission, a large number of cases, a high rate of critical illness, and a high case fatality rate. The intensive care unit (ICU) is the “last line of defense” for saving lives. And ICU resources play a critical role in the treatment of critical illness and combating public health emergencies.
This study estimates the demand for ICU healthcare resources based on an accurate prediction of the surge in the number of critically ill patients in the short term. The aim is to provide hospitals with a basis for scientific decision-making, to improve rescue efficiency, and to avoid excessive costs due to overly large resource reserves.
A demand forecasting method for ICU healthcare resources is proposed based on the number of current confirmed cases. The number of current confirmed cases is estimated using a bilateral long-short-term memory and genetic algorithm support vector regression (BILSTM-GASVR) combined prediction model. Based on this, this paper constructs demand forecasting models for ICU healthcare workers and healthcare material resources to more accurately understand the patterns of changes in the demand for ICU healthcare resources and more precisely meet the treatment needs of critically ill patients.
Data on the number of COVID-19-infected cases in Shanghai between January 20, 2020, and September 24, 2022, is used to perform a numerical example analysis. Compared to individual prediction models (GASVR, LSTM, BILSTM and Informer), the combined prediction model BILSTM-GASVR produced results that are closer to the real values. The demand forecasting results for ICU healthcare resources showed that the first (ICU human resources) and third (medical equipment resources) categories did not require replenishment during the early stages but experienced a lag in replenishment when shortages occurred during the peak period. The second category (drug resources) is consumed rapidly in the early stages and required earlier replenishment, but replenishment is timelier compared to the first and third categories. However, replenishment is needed throughout the course of the epidemic.
The first category of resources (human resources) requires long-term planning and the deployment of emergency expansion measures. The second category of resources (drugs) is suitable for the combination of dynamic physical reserves in healthcare institutions with the production capacity reserves of corporations. The third category of resources (medical equipment) is more dependent on the physical reserves in healthcare institutions, but care must be taken to strike a balance between normalcy and emergencies.
Peer Review reports
Introduction
The outbreak of severe acute respiratory syndrome (SARS) in 2003 was the first global public health emergency of the 21st century. From SARS to the coronavirus disease (COVID-19) pandemic at the end of 2019, followed shortly by the monkeypox epidemic of 2022, the global community has witnessed eight major public health events within the span of only 20 years [ 1 ]. These events are all characterized by high infection and fatality rates. For example, the number of confirmed COVID-19 cases worldwide is over 700 million, and the number of deaths has exceeded 7 million [ 2 ]. Every major public health emergency typically consists of four stages: incubation, outbreak, peak, and decline. During the outbreak and transmission, surges in the number of infected individuals and the number of critically ill patients led to a corresponding increase in the urgent demand for intensive care unit (ICU) medical resources. ICU healthcare resources provide material security for rescue work during major public health events as they allow critically ill patients to be treated, which decreases the case fatality rate and facilitates the prevention and control of epidemics. Nevertheless, in actual cases of prevention and control, the surge in patients has often led to shortages of ICU healthcare resources and a short-term mismatch of supply and demand, which are problems that have occurred several times in different regions. These issues can drastically impact anti-epidemic frontline healthcare workers and the treatment outcomes of infected patients. According to COVID-19 data from recent years, many infected individuals take about two weeks to progress from mild to severe disease. As the peak of severe cases tends to lag behind that of infected cases, predicting the changes in the number of new infections can serve as a valuable reference for healthcare institutions in forecasting the demand for ICU healthcare resources. The accurate forecasting of the demand for ICU healthcare resources can facilitate the rational resource allocation of hospitals under changes in demand patterns, which is crucial for improving the provision of critical care and rescue efficiency. Therefore, in this study, we combined a support vector regression (SVR) prediction model optimized by a genetic algorithm (GA) with bidirectional long-short-term memory (BILSTM), with the aim of enhancing the dynamic and accurate prediction of the number of current confirmed cases. Based on this, we forecasted the demand for ICU healthcare resources, which in turn may enable more efficient resource deployment during severe epidemic outbreaks and improve the precise supply of ICU healthcare resources.
Research on the demand forecasting of emergency materials generally employs quantitative methods, and traditional approaches mainly include linear regression and GM (1,1). Linear regression involves the use of regression equations to make predictions based on data. Sui et al. proposed a method based on multiple regression that aimed to predict the demand for emergency supplies in the power grid system following natural disasters [ 3 ]. Historical data was used to obtain the impact coefficient of each factor on emergency resource forecasting, enabling the quick calculation of the demand for each emergency resource during a given type of disaster. However, to ensure prediction accuracy, regression analysis needs to be supported by data from a large sample size. Other researchers have carried out demand forecasting for emergency supplies from the perspective of grey prediction models. Li et al. calculated the development coefficient and grey action of the grey GM (1,1) model using the particle swarm optimization algorithm to minimize the relative errors between the real and predicted values [ 4 ]. Although these studies have improved the prediction accuracy of grey models, they mainly involve pre-processing the initial data series without considering the issue of the excessively fast increase in predicted values by traditional grey GM (1,1) models. In emergency situations, the excessively fast increase in predicted values compared to real values will result in the consumption of a large number of unnecessary resources, thereby decreasing efficiency and increasing costs. As traditional demand forecasting models for emergency supplies have relatively poor perfect order rates in demand analysis, which result in low prediction accuracy, they are not mainstream.
At present, dynamic models of infectious diseases and demand forecasting models based on machine learning are at the cutting edge of research. With regard to the dynamic models of infectious diseases, susceptible infected recovered model (SIR) is a classic mathematical model employed by researchers [ 5 , 6 , 7 ]. After many years of development, the SIR model has been expanded into various forms within the field of disease transmission, including susceptible exposed infected recovered model (SEIR) and susceptible exposed infected recovered dead model (SEIRD) [ 8 , 9 ]. Nevertheless, with the outbreak of COVID-19, dynamic models of infectious diseases have once again come under the spotlight, with researchers combining individual and group variables and accounting for different factors to improve the initial models and reflect the state of COVID-19 [ 10 , 11 , 12 , 13 ]. Based on the first round of epidemic data from Wuhan, Li et al. predicted the time-delay distributions, epidemic doubling time, and basic reproductive number [ 14 ]. Upon discovering the presence of asymptomatic COVID-19 infections, researchers began constructing different SEIR models that considered the infectivity of various viral incubation periods, yielding their respective predictions of the inflection point. Based on this, Anggriani et al. further considered the impact of the status of infected individuals and established a transmission model with seven compartments [ 15 ]. Efimov et al. set the model parameters for separating the recovered and the dead as uncertain and applied the improved SEIR model to analyze the transmission trend of the pandemic [ 16 ]. In addition to analyzing the transmission characteristics of normal COVID-19 infection to predict the status of the epidemic, many researchers have also used infectious disease models to evaluate the effects of various epidemic preventive measures. Lin et al. applied an SEIR model that considered individual behavioral responses, government restrictions on public gatherings, pet-related transmission, and short-term population movements [ 17 ]. Cao et al. considered the containment effect of isolation measures on the pandemic and solved the model using Euler’s numerical method [ 18 ]. Reiner et al. employed an improved SEIR model to study the impact of non-pharmaceutical interventions implemented by the government (e.g., restricting population movement, enhancing disease testing, and increasing mask use) on disease transmission and evaluated the effectiveness of social distancing and the closure of public spaces [ 19 ]. These studies have mainly focused on modeling the COVID-19 pandemic to perform dynamic forecasting and analyze the effectiveness of control measures during the epidemic. Infectious disease dynamics offer good predictions for the early transmission trends of epidemics. However, this approach is unable to accurately estimate the spread of the virus in open-flow environments. Furthermore, it is also impossible to set hypothetical parameters, such as disease transmissibility and the recovery probability constant, that are consistent with the conditions in reality. Hence, with the increase in COVID-19 data, this approach has become inadequate for the accurate long-term analysis of epidemic trends.
Machine learning has shown significant advantages in this regard [ 20 , 21 ]. Some researchers have adopted the classic case-based reasoning approach in machine learning to make predictions. However, it is not feasible to find historical cases that fully match the current emergency event, so this approach has limited operability. Other researchers have also employed neural network training in machine learning to make predictions. For example, Hamou et al. predicted the number of injuries and deaths, which in turn were used to forecast the demand for emergency supplies [ 22 ]. However, this approach requires a large initial dataset and a high number of training epochs, while uncertainty due to large changes in intelligence information can lead to significant errors in data prediction [ 23 , 24 , 25 ]. To address these problems, researchers have conducted investigations that account (to varying degrees) for data characterized by time-series and non-linearity and have employed time-series models with good non-linear fitting [ 26 , 27 , 28 ]. The use of LSTM to explore relationships within the data can improve the accuracy of predicting COVID-19 to some extent. However, there are two problems with this approach. First, LSTM neural networks require extremely large datasets, and each wave of the epidemic development cycle would be insufficient to support a dataset suitable for LSTM. Second, neural networks involve a large number of parameters and highly complex models and, hence, are susceptible to overfitting, which can prevent them from achieving their true and expected advantages in prediction.
Overall, Our study differs from other papers in the following three ways. First, the research object of this paper focuses on the specific point of ICU healthcare resource demand prediction, aiming to improve the rate of critical care patient treatment. However, past research on public health emergencies has focused more on resource prediction , such as N95 masks, vaccines, and generalized medical supplies during the epidemic , to mitigate the impact of rapid transmission and high morbidity rates. This has led to less attention being paid to the reality of the surge in critically ill patients due to their high rates of severe illness and mortality.
Second, the idea of this paper is to further forecast resource needs based on the projected number of people with confirmed diagnoses, which is more applicable to healthcare organizations than most other papers that only predict the number of people involved. However, in terms of the methodology for projecting the number of people, this paper adopts a combined prediction method that combines regression algorithms and recurrent neural networks to propose a BILSTM-GASVR prediction model for the number of confirmed diagnoses. It capitalizes on both the suitability of SVR for small samples and non-linear prediction as well as the learning and memory abilities of BILSTM in processing time-series data. On the basis of the prediction model for the number of infected cases, by considering the characteristics of ICU healthcare resources, we constructed a demand forecasting model of emergency healthcare supplies. Past public health emergencies are more likely to use infectious disease models or a single prediction model in deep learning. some of the articles, although using a combination of prediction, but also more for the same method domain combination, such as CNN-LSTM, GRU-LSTM, etc., which are all recurrent neural networks.
Third, in terms of specific categorization of resources to be forecasted, considering the specificity of ICU medical resources, we introduce human resource prediction on the basis of previous studies focusing on material security, and classified ICU medical resources into three categories: ICU human resources, drugs and medical equipment. The purpose of this classification is to match the real-life prediction scenarios of public health emergencies and improve the demand forecasting performance for local ICU healthcare resources. Thus, it is easy for healthcare institutions to grasp the overall development of events, optimizing decision-making, and reducing the risk of healthcare systems collapsing during the outbreak stage.
In this section, we accomplish the following two tasks. Firstly, we introduce the idea of predicting the number of infected cases and show the principle of the relevant models. Secondly, based on the number of infected cases, ICU healthcare resources are divided into two categories (healthcare workers and healthcare supplies), and their respective demand forecasting models are constructed.
Prediction model for the number of infected cases
Gasvr model.
Support vector machine (SVM) is a machine-learning language for classification developed by Vapnik [ 29 ]. Suppose there are two categories of samples: H1 and H2. If hyperplane H is able to correctly classify the samples into these two categories and maximize the margin between the two categories, it is known as the optimal separating hyperplane (OSH). The sample vectors closest to the OSH in H1 and H2 are known as the support vectors. To apply SVM to prediction, it is essential to perform regression fitting. By introducing the \(\varepsilon\) -insensitive loss function, SVM can be converted to a support vector regression machine, where the role of the OSH is to minimize the error of all samples from this plane. SVR has a theoretical basis in statistical learning and relatively high learning performance, making it suitable for performing predictions in small-sample, non-linear, and multi-dimensional fields [ 30 , 31 ].
Assume the training sample set containing \(l\) training samples is given by \(\{({x}_{i},{y}_{i}),i=\mathrm{1,2},...,l\}\) , where \({x}_{i}=[{x}_{i}^{1},{x}_{i}^{2},...,{x}_{i}^{d}{]}^{\rm T}\) and \({y}_{i}\in R\) are the corresponding output values.
Let the regression function be \(f(x)=w\Phi (x)+b\) , where \(\phi (x)\) is the non-linear mapping function. The linear \(\varepsilon\) -insensitive loss function is defined as shown in formula ( 1 ).
Among the rest, \(f(x)\) is the predicted value returned by the regression function, and \(y\) is the corresponding real value. If the error between \(f(x)\) and \(y\) is ≤ \(\varepsilon\) , the loss is 0; otherwise, the loss is \(\left|y-f(x)\right|-\varepsilon\) .
The slack variables \({\xi }_{i}\) and \({\xi }_{i}^{*}\) are introduced, and \(w\) , \(b\) are solved using the following equation as shown in formula ( 2 ).
Among the rest, \(C\) is the penalty factor, with larger values indicating a greater penalty for errors > \(\varepsilon\) ; \(\varepsilon\) is defined as the error requirement, with smaller values indicating a smaller error of the regression function.
The Lagrange function is introduced to solve the above function and transformed into the dual form to give the formula ( 3 ).
Among the rest, \(K({x}_{i},{x}_{j})=\Phi ({x}_{i})\Phi ({x}_{j})\) is the kernel function. The kernel function determines the structure of high-dimensional feature space and the complexity of the final solution. The Gaussian kernel is selected for this study with the function \(K({x}_{i},{x}_{j})=\mathit{exp}(-\frac{\Vert {x}_{i}-{x}_{j}\Vert }{2{\sigma }^{2}})\) .
Let the optimal solution be \(a=[{a}_{1},{a}_{2},...,{a}_{l}]\) and \({a}^{*}=[{a}_{1}^{*},{a}_{2}^{*},...,{a}_{l}]\) to give the formula ( 4 ) and formula ( 5 ).
Among the rest, \({N}_{nsv}\) is the number of support vectors.
In sum, the regression function is as shown in formula ( 6 ).
when some of the parameters are not 0, the corresponding samples are the support vectors in the problem. This is the principle of SVR. The values of the three unknown parameters (penalty factor C, ε -insensitive loss function, and kernel function coefficient \(\sigma )\) , can directly impact the model effect. The penalty factor C affects the degree of function fitting through the selection of outliers in the sample by the function. Thus, excessively large values lead to better fit but poorer generalization, and vice versa. The ε value in the ε-insensitive loss function determines the accuracy of the model by affecting the width of support vector selection. Thus, excessively large values lead to lower accuracy that does not meet the requirements and excessively small values are overly complex and increase the difficulty. The kernel function coefficient \(\sigma\) determines the distribution and range of the training sample by controlling the size of inner product scaling in high-dimensional space, which can affect overfitting.
Therefore, we introduce other algorithms for optimization of the three parameters in SVR. Currently the commonly used algorithms are 32and some heuristic algorithms. Although the grid search method is able to find the highest classification accuracy, which is the global optimal solution. However, sometimes it can be time-consuming to find the optimal parameters for larger scales. If a heuristic algorithm is used, we could find the global optimal solution without having to trace over all the parameter points in the grid. And GA is one of the most commonly used heuristic algorithms, compared to other heuristic algorithms, it has the advantages of strong global search, generalizability, and broader blending with other algorithms.
Given these factors, we employ a GA to encode and optimize the relevant parameters of the model. The inputs are the experimental training dataset, the Gaussian kernel function expression, the maximum number of generations taken by the GA, the accuracy range of the optimized parameters, the GA population size, the fitness function, the probability of crossover, and the probability of mutation. The outputs are the optimal penalty factor C, ε-insensitive loss function parameter \(\varepsilon ,\) and optimal Gaussian kernel parameter \(\sigma\) of SVR, thus achieving the optimization of SVR. The basic steps involved in GA optimization are described in detail below, and the model prediction process is shown in Fig. 1 .
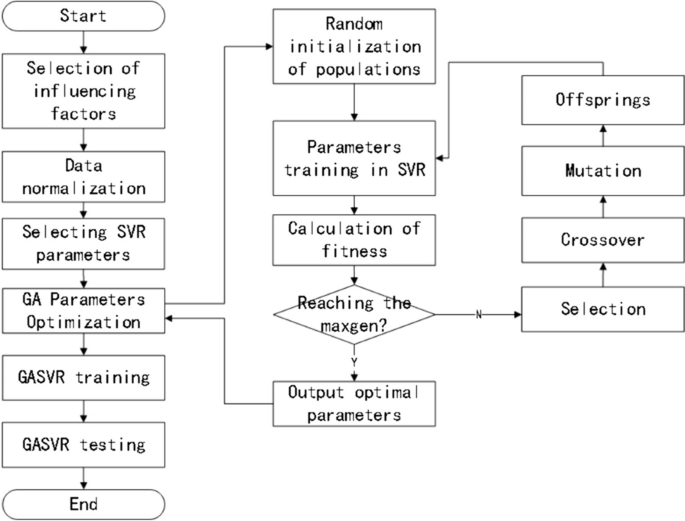
Prediction process of the GASVR model
Population initialization
The three parameters are encoded using binary arrays composed of 0–1 bit-strings. Each parameter consisted of six bits, and the initial population is randomly generated. The population size is set at 60, and the number of iterations is 200.
Fitness calculation
In the same dataset, the K-fold cross-validation technique is used to test each individual in the population, with K = 5. K-fold cross validation effectively avoids the occurrence of model over-learning and under-learning. For the judgment of the individual, this paper evaluates it in terms of fitness calculations. Therefore, combining the two enables the effective optimization of the model’s selected parameters and improves the accuracy of regression prediction.
Fitness is calculated using the mean error method, with smaller mean errors indicating better fitness. The fitness function is shown in formula ( 7 ) [ 32 ].
The individual’s genotype is decoded and mapped to the corresponding parameter value, which is substituted into the SVR model for training. The parameter optimization range is 0.01 ≤ C ≤ 100, 0.1 ≤ \(\sigma\) ≤ 20, and 0.001 ≤ ε ≤ 1.
Selection: The selection operator is performed using the roulette wheel method.
Crossover: The multi-point crossover operator, in which two chromosomes are selected and multiple crossover points are randomly chosen for swapping, is employed. The crossover probability is set at 0.9.
Mutation: The inversion mutation operator, in which two points are randomly selected and the gene values between them are reinserted to the original position in reverse order, is employed. The mutation probability is set at 0.09.
Decoding: The bit strings are converted to parameter sets.
The parameter settings of the GASVR model built in this paper are shown in Table 1 .
BILSTM model
The LSTM model is a special recurrent neural network algorithm that can remember the long-term dependencies of data series and has an excellent capacity for self-learning and non-linear fitting. LSTM automatically connects hidden layers across time points, such that the output of one time point can arbitrarily enter the output terminal or the hidden layer of the next time point. Therefore, it is suitable for the sample prediction of time-series data and can predict future data based on stored data. Details of the model are shown in Fig. 2 .
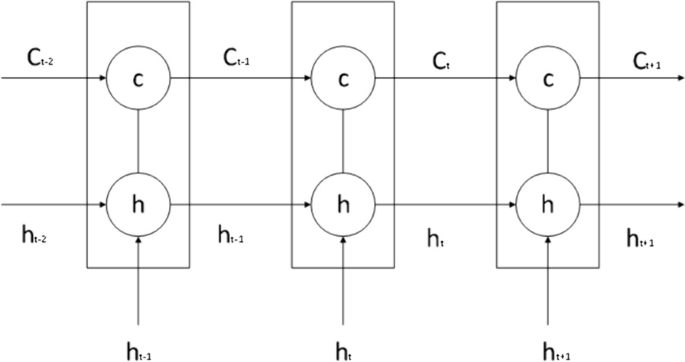
Schematic diagram of the LSTM model
LSTM consists of a forget gate, an input gate, and an output gate.
The forget gate combines the previous and current time steps to give the output of the sigmoid activation function. Its role is to screen the information from the previous state and identify useful information that truly impacts the subsequent time step. The equation for the forget gate is shown in formula ( 8 ).
Among the number, \(W_{f}\) is the weight of the forget gate, \({b}_{f}\) is the bias, \(\sigma\) is the sigmoid activation function, \({f}_{t}\) is the output of the sigmoid activation function, \(t-1\) is the previous time step, \(t\) is the current time step, and \({x}_{t}\) is the input time-series data at time step \(t\) .
The input gate is composed of the output of the sigmoid and tanh activation functions, and its role is to control the ratio of input information entering the information of a given time step. The equation for the input gate is shown in formula ( 9 ).
Among the number, \({W}_{i}\) is the output weight of the input gate, \({i}_{t}\) is the output of the sigmoid activation function, \({b}_{i}\) and \({b}_{C}\) are the biases of the input gate, and \({W}_{C}\) is the output of the tanh activation function.
The role of the output gate is to control the amount of information output at the current state, and its equation is shown in formula ( 10 ).
Among the number, \({W}_{o}\) is the weight of \({o}_{t}\) , and \({b}_{o}\) is the bias of the output gate.
The values of the above activation functions \(\sigma\) and tanh are generally shown in formulas ( 11 ) and ( 12 ).
\({C}_{t}\) is the data state of the current time step, and its value is determined by the input information of the current state and the information of the previous state. It is shown in formula ( 13 ).
Among the number, \(\widetilde{{C}_{t}}=\mathit{tan}h({W}_{c}[{h}_{t-1},{x}_{t}]+{b}_{c})\) .
\({h}_{t}\) is the state information of the hidden layer at the current time step, \({h}_{t}={o}_{t}\times \mathit{tan}h({c}_{t})\) .Each time step \({T}_{n}\) has a corresponding state \({C}_{t}\) . By undergoing the training process, the model can learn how to modify state \({C}_{t}\) through the forget, output, and input gates. Therefore, this state is consistently passed on, implying that important distant information will neither be forgotten nor significantly affected by unimportant information.
The above describes the principle of LSTM, which involves forward processing when applied. BILSTM consists of two LSTM networks, one of which processes the input sequence in the forward direction (i.e., the original order), while the other inputs the time series in the backward direction into the LSTM model. After processing both LSTM networks, the outputs are combined, which eventually gives the output results of the BILSTM model. Details of the model are presented in Fig. 3 .
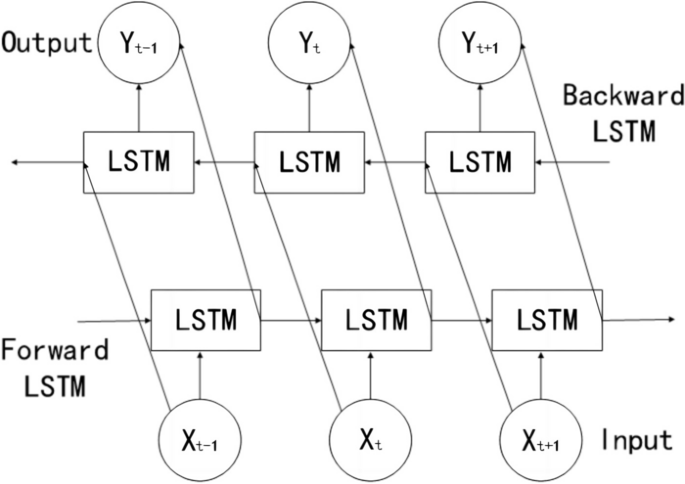
Schematic diagram of the BILSTM model
Compared to LSTM, BILSTM can achieve bidirectional information extraction of the time-series and connect the two LSTM layers onto the same output layer. Therefore, in theory, its predictive performance should be superior to that of LSTM. In BILSTM, the equations of the forward hidden layer( \(\overrightarrow{{h}_{t}}\) ) , backward hidden layer( \(\overleftarrow{{h}_{t}}\) ) , and output layer( \({o}_{t}\) ) are shown in formulas ( 14 ) , ( 15 ) and ( 16 ).
The parameter settings of the BILSTM model built in this paper are shown in Table 2 .
Informer model
The Informer model follows the compiler-interpreter architecture in the Transformer model, and based on this, structural optimizations have been made to reduce the computational time complexity of the algorithm and to optimize the output form of the interpreter. The two optimization methods are described in detail next.
With large amounts of input data, neural network models can have difficulty capturing long-term interdependencies in sequences, which can produce gradient explosions or gradient vanishing and affect the model's prediction accuracy. Informer model solves the existential gradient problem by using a ProbSparse Self-attention mechanism to make more efficient than conventional self-attention.
The value of Transformer self-attention is shown in formula ( 17 ).
Among them, \(Q\in {R}^{{L}_{Q}\times d}\) is the query matrix, \(K\in {R}^{{L}_{K}\times d}\) is the key matrix, and \(V\in {R}^{{L}_{V}\times d}\) is the value matrix, which are obtained by multiplying the input matrix X with the corresponding weight matrices \({W}^{Q}\) , \({W}^{K}\) , \({W}^{V}\) respectively, and d is the dimensionality of Q, K, and V. Let \({q}_{i}\) , \({k}_{i}\) , \(v_{i}\) represent the ith row in the Q, K, V matrices respectively, then the ith attention coefficient is shown in formula ( 18 ) as follows.
Therein, \(p({k}_{j}|{q}_{i})\) denotes the traditional Transformer's probability distribution formula, and \(k({q}_{i},{K}_{l})\) denotes the asymmetric exponential sum function. Firstly, q=1 is assumed, which implies that the value of each moment is equally important; secondly, the difference between the observed distribution and the assumed one is evaluated by the KL scatter, if the value of KL is bigger, the bigger the difference with the assumed distribution, which represents the more important this moment is. Then through inequality \(ln{L}_{k}\le M({q}_{i},K)\le {\mathit{max}}_{j}\left\{\frac{{q}_{i}{k}_{j}^{\rm T}}{\sqrt{d}}\right\}-\frac{1}{{L}_{k}}{\sum }_{j=1}^{{L}_{k}}\left\{\frac{{q}_{i}{k}_{j}^{\rm T}}{\sqrt{d}}\right\}+ln{L}_{k}\) , \(M({q}_{i},K)\) is transformed into \(\overline{M}({q}_{i},K)\) . According to the above steps, the ith sparsity evaluation formula is obtained as shown in formula ( 19 ) [ 33 ].
One of them, \(M({q}_{i},K)\) denotes the ith sparsity measure; \(\overline{M}({q}_{i},K)\) denotes the ith approximate sparsity measure; \({L}_{k}\) is the length of query vector. \(TOP-u\) quantities of \(\overline{M}\) are selected to form \(\overline{Q}\) , \(\overline{Q}\) is the first u sparse matrices, and the final sparse self-attention is shown in Formula ( 20 ). At this point, the time complexity is still \(O({n}^{2})\) , and to solve this problem, only l moments of M2 are computed to reduce the time complexity to \(O(L\cdot \mathit{ln}(L))\) .
Informer uses a generative decoder to obtain long sequence outputs.Informer uses the standard decoder architecture shown in Fig. 4 , in long time prediction, the input given to the decoder is shown in formula ( 21 ).
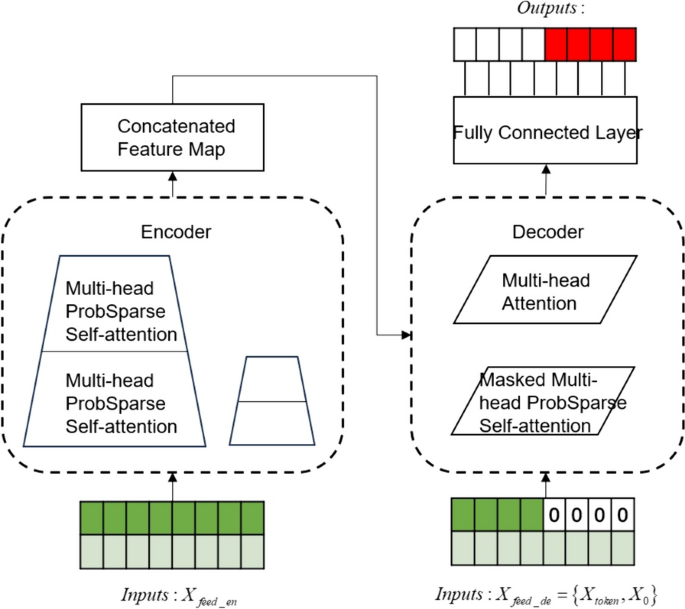
Informer uses a generative decoder to obtain long sequence outputs
Therein, \({X}_{de}^{t}\) denotes the input to the decoder; \({X}_{token}^{t}\in {R}^{({L}_{token}+{L}_{y})\times {d}_{\mathit{mod}el}}\) is the dimension of the encoder output, which is the starting token without using all the output dimensions; \({X}_{0}^{t}\in {R}^{({L}_{token}+{L}_{y})\times {d}_{\mathit{mod}el}}\) is the dimension of the target sequence, which is uniformly set to 0; and finally the splicing input is performed to the encoder for prediction.
The parameter settings of Informer model created in this paper are shown in Table 3 .
BILSTM-GASVR combined prediction model
SVR has demonstrated good performance in solving problems like finite samples and non-linearity. Compared to deep learning methods, it offers faster predictions and smaller empirical risks. BILSTM has the capacity for long-term memory, can effectively identify data periodicity and trends, and is suitable for the processing of time-series data. Hence, it can be used to identify the effect of time-series on the number of confirmed cases. Given the advantages of these two methods in different scenarios, we combined them to perform predictions using GASVR, followed by error repair using BILSTM. The basic steps for prediction based on the BILSTM-GASVR model are as follows:
Normalization is performed on the initial data.
The GASVR model is applied to perform training and parameter optimization of the data to obtain the predicted value \(\widehat{{y}_{i}}\) .
After outputting the predicted value of GASVR, the residual sequence between the predicted value and real data is extracted to obtain the error \({\gamma }_{i}\) (i.e., \({\gamma }_{i}={y}_{i}-\widehat{{y}_{i}}\) ).
The BILSTM model is applied to perform training of the error to improve prediction accuracy. The BILSTM model in this paper is a multiple input single output model. Its inputs are the true and predicted error values \({\gamma }_{i}\) and its output is the new error value \(\widehat{{\gamma }_{i}}\) predicted by BILSTM.
The final predicted value is the sum of the GASVR predicted value and the BILSTM residual predicted value (i.e., \({Y}_{i}=\widehat{{y}_{i}}+\widehat{{\gamma }_{i}}\) ).
The parameter settings of the BILSTM-GASVR model built in this paper are shown in Table 4 .
Model testing criteria
To test the effect of the model, the prediction results of the BILSTM-GASVR model are compared to those of GASVR, LSTM, BILSTM and Informer. The prediction error is mainly quantified using three indicators: mean squared error (MSE), root mean squared error (RMSE), and correlation coefficient ( \(R^{2}\) ). Their respective equations are shown in formulas ( 22 ), ( 23 ) and ( 24 ).
Demand forecasting model of ICU healthcare resources
ICU healthcare resources can be divided into human and material resources. Human resources refer specifically to the professional healthcare workers in the ICU. Material resources, which are combined with the actual consumption of medical supplies, can be divided into consumables and non-consumables. Consumables refer to the commonly used drugs in the ICU, which include drugs for treating cardiac insufficiency, vasodilators, anti-shock vasoactive drugs, analgesics, sedatives, muscle relaxants, anti-asthmatic drugs, and anticholinergics. Given that public health emergencies have a relatively high probability of affecting the respiratory system, we compiled a list of commonly used drugs for respiratory diseases in the ICU (Table 5 ).
Non-consumables refer to therapeutic medical equipment, including electrocardiogram machines, blood gas analyzers, electrolyte analyzers, bedside diagnostic ultrasound machines, central infusion workstations, non-invasive ventilators, invasive ventilators, airway clearance devices, defibrillators, monitoring devices, cardiopulmonary resuscitation devices, and bedside hemofiltration devices.
The demand forecasting model of ICU healthcare resources constructed in this study, as well as its relevant parameters and definitions, are described below. \({R}_{ij}^{n}\) is the forecasted demand for the \(i\) th category of resources on the \(n\) th day in region \(j\) . \({Y}_{j}^{n}\) is the predicted number of current confirmed cases on the \(n\) th day in region \(j\) . \({M}_{j}^{n}\) is the number of ICU healthcare workers on the \(n\) th day in region \(j\) , which is given by the following formula: number of healthcare workers the previous day + number of new recruits − reduction in number the previous day, where the reduction in number refers to the number of healthcare workers who are unable to work due to infection or overwork. In general, the number of ICU healthcare workers should not exceed 5% of the number of current confirmed cases (i.e., it takes the value range [0, \(Y_{j}^{n}\) ×5%]). \(U_{i}\) is the maximum working hours or duration of action of the \(i\) th resource category within one day. \({A}_{j}\) is the number of resources in the \(i\) th category allocated to patients (i.e., how many units of resources in the \(i\) th category is needed for a patient who need the \(i\) th unit of the given resource). \({\varphi }_{i}\) is the demand conversion coefficient (i.e., the proportion of the current number of confirmed cases who need to use the \(i\) th resource category). \({C}_{ij}^{n}\) is the available quantity of material resources of the \(i\) th category on the \(n\) th day in region \(j\) . At the start, this quantity is the initial reserve, and once the initial reserve is exhausted, it is the surplus from the previous day. The formula for this parameter is given as follows: available quantity from the previous day + replenishment on the previous day − quantity consumed on the previous day, where if \({C}_{ij}^{n}\) is a negative number, it indicates the amount of shortage for the given category of resources on the previous day.
In summary, the demand forecast for emergency medical supplies constructed in this study is shown in formula ( 25 ).
The number of confirmed cases based on data-driven prediction is introduced into the demand forecasting model for ICU resources to forecast the demand for the various categories of resources. In addition to the number of current confirmed cases, the main variables of the first demand forecasting model for human resources are the available quantity and maximum working hours. The main variable of the second demand forecasting model for consumable resources is the number of units consumed by the available quantity. The main variable of the third model for non-consumable resources is the allocated quantity. These three resource types can be predicted using the demand forecasting model constructed in this study.
Prediction of the number of current infected cases
The COVID-19 situation in Shanghai is selected for our experiment. A total of 978 entries of epidemic-related data in Shanghai between January 20, 2020, and September 24, 2022, are collected from the epidemic reporting platform. This dataset is distributed over a large range and belongs to a right-skewed leptokurtic distribution. The specific statistical description of data is shown in Table 6 . Part of the data is shown in Table 7 .
And we divided the data training set and test set in an approximate 8:2 ratio, namely, 798 days for training (January 20, 2020 to March 27, 2022) and 180 days for prediction (March 28, 2022 to September 24, 2022).
Due to the large difference in order of magnitude between the various input features, directly implementing training and model construction would lead to suboptimal model performance. Such effects are usually eliminated through normalization. In terms of interval selection, [0, 1] reflects the probability distribution of the sample, whereas [-1, 1] mostly reflects the state distribution or coordinate distribution of the sample. Therefore, [-1, 1] is selected for the normalization interval in this study, and the processing method is shown in formula ( 26 ).
Among the rest, \(X\) is the input sample, \({X}_{min}\) and \({X}_{max}\) are the minimum and maximum values of the input sample, and \({X}_{new}\) is the input feature after normalization.
In addition, we divide the data normalization into two parts, considering that the amount of data in the training set is much more than the test set in the real operating environment. In the first step, we normalize the training set data directly according to the above formula; in the second step, we normalize the test data set using the maximum and minimum values of the training data set.
The values of the preprocessed data are inserted into the GASVR, LSTM, Informer, BILSTM models and the BILSTM-GASVR model is constructed. Figures 5 , 6 , 7 , 8 and 9 show the prediction results. From Figs. 5 , 6 , and 7 , it can be seen that in terms of data accuracy, GASVR more closely matches the real number of infected people relative to BILSTM and LSTM. Especially in the most serious period of the epidemic in Shanghai (April 17, 2022 to April 30, 2022), the advantage of the accuracy of the predicted data of GASVR is even more obvious, which is due to the characteristics of GASVR for small samples and nonlinear prediction. However, in the overall trend of the epidemic, BILSTM and LSTM, which have the ability to learn and memorize to process time series data, are superior. It is clearly seen that in April 1, 2022-April 7, 2022 and May 10, 2022-May 15, 2022, there is a sudden and substantial increase in GASVR in these two time phases, and a sudden and substantial decrease in April 10, 2022-April 14, 2022. These errors also emphasize the stability of BILSTM and LSTM, which are more closely matched to the real epidemic development situation in the whole process of prediction, and the difference between BILSTM and LSTM prediction is that the former predicts data more accurately than the latter, which is focused on the early stage of prediction as well as the peak period of the epidemic. Informer is currently an advanced time series forecasting method. From Fig. 8 , it can be seen that the prediction data accuracy and the overall trend of the epidemic are better than the single prediction models of GASVR, LSTM and BILSTM. However, Informer is more suitable for long time series and more complex and large prediction problems, so the total sample size of less than one thousand cases is not in the comfort zone of Informer model. Figure 9 shows that the BILSTM-GASVR model constructed in this paper is more suitable for this smaller scale prediction problem, with the best prediction results, closest to the actual parameter (number of current confirmed cases), demonstrating small sample and time series advantages. In Short, the prediction effect of models is ranked as follows: BILSTM-GASVR> Informer> GASVR> BILSTM> LSTM.
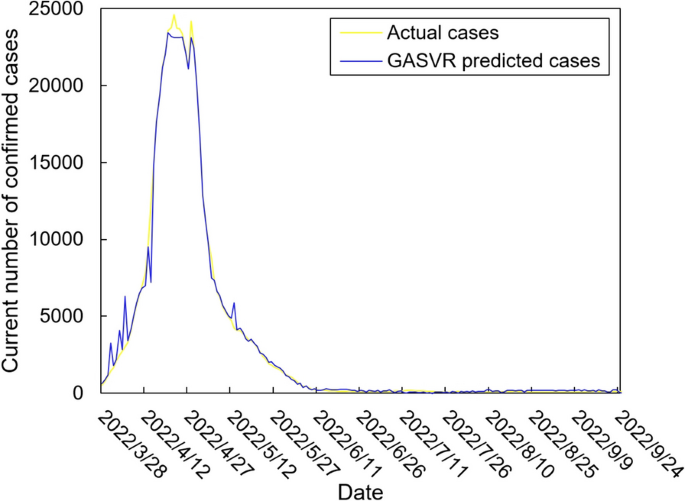
The prediction result of the GASVR model
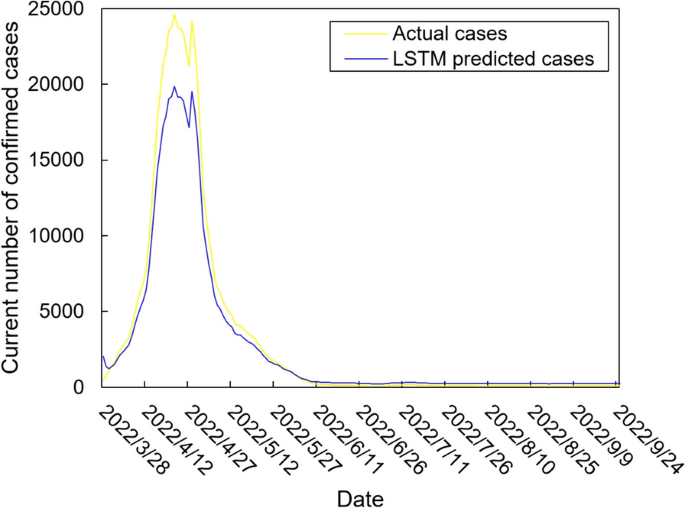
The prediction result of the LSTM model
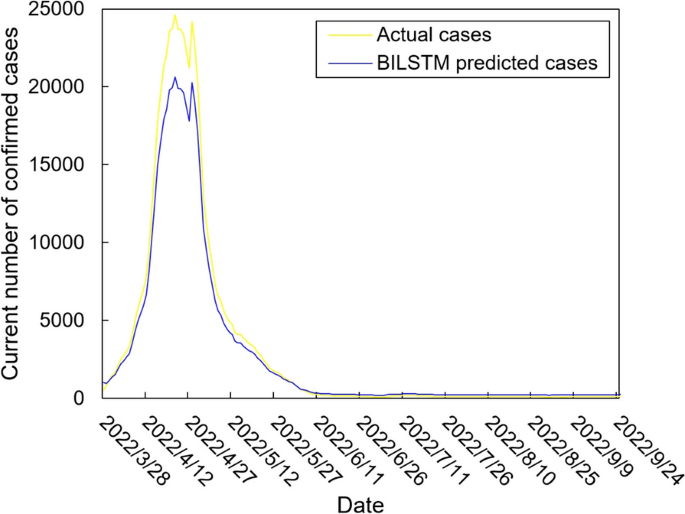
The prediction result of the BILSTM model
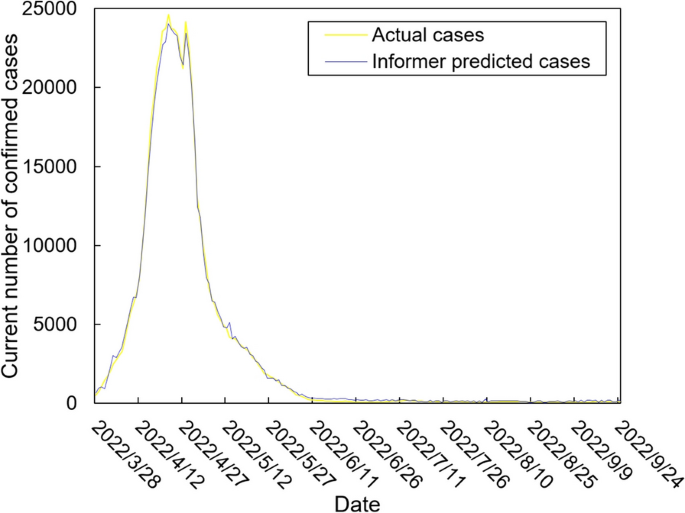
The prediction result of the Informer model
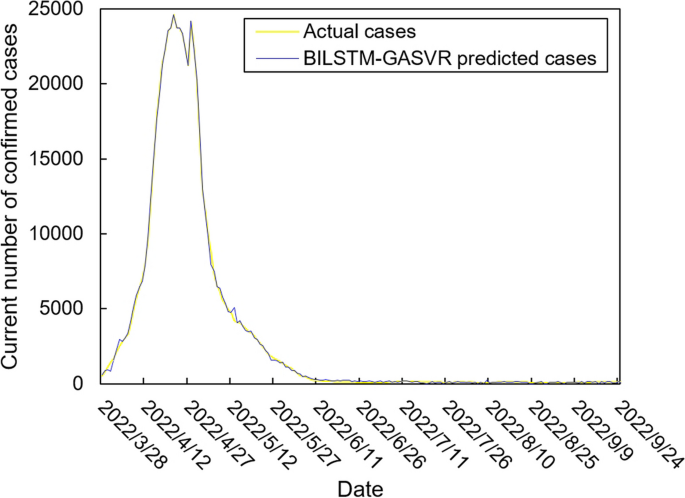
The prediction result of the BILSTM-GASVR model
The values of the three indicators (MSE, RMSE, and correlation coefficient \({R}^{2}\) ) for the five models are shown in Table 8 . MSE squares the error so that the larger the model error, the larger the value, which help capture the model's prediction error more sensitively. RMSE is MSE with a root sign added to it, which allows for a more intuitive representation of the order of magnitude difference from the true value. \({R}^{2}\) is a statistical indicator used to assess the overall goodness of fit of the model, which reflects the overall consistency of the predicted trend and does not specifically reflect the degree of data. The results in the Table 8 are consistent with the prediction results in the figure above, while the ranking of MSE, RMSE, and \({R}^{2}\) are also the same (i.e., BILSTM-GASVR> Informer> GASVR> BILSTM> LSTM).
In addition, we analyze the five model prediction data using significance tests as a way of demonstrating whether the model used is truly superior to the other baseline models. The test dataset with kurtosis higher than 4 does not belong to the approximate normal distribution, so parametric tests are not used in this paper. Given that the datasets predicted by each of the five models are continuous and independent datasets, this paper uses the Kruskal-Wallis test, which is a nonparametric test. The test steps are as follows.
Determine hypotheses (H0, H1) and significance level ( \(\alpha\) ).
For each data set, all its sample data are combined and ranked from smallest to largest. Then find the number of data items ( \({n}_{i}\) ), rank sum ( \({R}_{i}\) ) and mean rank of each group of data respectively.
Based on the rank sum, the test statistic (H) is calculated for each data set in the Kruskal-Wallis test. The specific calculation is shown in formula ( 27 ).
According to the test statistic and degrees of freedom, find the corresponding p-value in the Kruskal-Wallis distribution table. Based on the P-value, determine whether the original hypothesis is valid.
In the significance test, we set the significance setting original hypothesis (H0) as there is no significant difference between the five data sets obtained from the five predictive models. We set the alternative hypothesis (H1) as there is a significant difference between the five data sets obtained from the five predictive models. At the same time, we choose the most commonly used significance level taken in the significance test, namely 0.05. In this paper, multiple comparisons and two-by-two comparisons of the five data sets obtained from the five predictive models are performed through the SPSS software. The results of the test show that in the multiple comparison session, P=0.001<0.05, so H0 is rejected, which means that the difference between the five groups of data is significant. In the two-by-two comparison session, BILSTM-GASVR is less than 0.05 from the other four prediction models. The specific order of differences is Informer < GASVR < BILSTM < LSTM, which means that the BILSTM-GASVR prediction model does get a statistically significant difference between the dataset and the other models.
In summary, combined prediction using the BILSTM-GASVR model is superior to the other four single models in various aspects in the case study analysis of Shanghai epidemic with a sample size of 978.
Demand forecasting of ICU healthcare resources
Combined with the predicted number of current infected cases, representatives are selected from the three categories of resources for forecasting. The demand for nurses is selected as the representative for the first category of resources.
In view of the fact that there are currently no specific medications that are especially effective for this public health emergency, many ICU treatment measures involved helping patients survive as their own immune systems eliminated the virus. This involved, for example, administering antibiotics when patients developed a secondary bacterial infection. glucocorticoids are used to temporarily suppress the immune system when their immune system attacked and damaged lung tissues causing patients to have difficulty breathing. extracorporeal membrane oxygenation (ECMO) is used for performing cardiopulmonary resuscitation when patients are suffering from cardiac arrest. In this study, we take dexamethasone injection (5 mg), a typical glucocorticoid drug, as the second category of ICU resources (i.e., drugs); and invasive ventilators as the third category of ICU resources (i.e., medical equipment).
During the actual epidemic in Shanghai, the municipal government organized nine critical care teams, which are stationed in eight municipally designated hospitals and are dedicated to the treatment of critically ill patients. In this study, the ICU nurses, dexamethasone injections, and invasive ventilators in Shanghai are selected as the prediction targets and introduced into their respective demand forecasting models. Forecasting of ICU healthcare resources is then performed for the period from March 28, 2022, to April 28, 2022, as an example. Part of the parameter settings for the three types of resources are shown in Tables 9 , 10 , and 11 , respectively.
Table 12 shows the forecasting results of the demand for ICU nurses, dexamethasone injections, and invasive ventilators during the epidemic wave in Shanghai between March 28, 2022, and April 28, 2022.
For the first category (i.e., ICU nurses), human resource support is only needed near the peak period, but the supply could not be replenished immediately. In the early stages, Shanghai could only rely on the nurses’ perseverance, alleviating the shortage of human resources by reducing the number of shifts and increasing working hours. This situation persisted until about April 10 and is only resolved when nurses from other provinces and regions successively arrived in Shanghai.
The second category of ICU resources is drugs, which are rapidly consumed. The pre-event reserve of 30,000 dexamethasone injections could only be maintained for a short period and is fully consumed during the outbreak. Furthermore, daily replenishment is still needed, even when the epidemic has passed its peak and begun its decline.
The third category is invasive ventilators, which are non-consumables. Thus, the reserve lasted for a relatively long period of time in the early stages and did not require replenishment after its maximum usage during the peak period.
Demand forecasting models are constructed based on the classification of healthcare resources according to their respective features. We choose ICU nurses, dexamethasone injections, and invasive ventilators as examples, and then forecast demand for the epidemic wave in Shanghai between March 28, 2022, and April 28, 2022. The main conclusions are as follows:
A long period of time is needed to train ICU healthcare workers who can independently be on duty, taking at least one year from graduation to entering the hospital, in addition to their requiring continuous learning, regular theoretical training, and the accumulation of clinical experience during this process. Therefore, for the first category of ICU healthcare resources, in the long term, healthcare institutions should place a greater emphasis on their talent reserves. Using China as an example, according to the third ICU census, the ratio of the number of ICU physicians to the number of beds is 0.62:1 and the ratio of the number of nurses to the number of beds is 1.96:1, which are far lower than those stipulated by China itself and those of developed countries. Therefore, a fundamental solution is to undertake proactive and systematic planning and construction to ensure the more effective deployment of human resources in the event of a severe outbreak. In the short term, healthcare institutions should focus on the emergency expansion capacity of their human resources. In case there are healthcare worker shortages during emergencies, the situation can be alleviated by summoning retired workers back to work and asking senior medical students from various universities to help in the hospitals to prevent the passive scenario of severely compressing the rest time of existing staff or waiting for external aid. However, it is worth noting that to ensure the effectiveness of such a strategy of using retired healthcare workers or senior students of university medical faculties, it is necessary for healthcare organizations to provide them with regular training in the norm, such as organizing 2-3 drills a year, to ensure the professionalism and proficiency of healthcare workers who are temporarily and suddenly put on the job. At the same time, it is also necessary to fully mobilize the will of individuals. Medical institutions can provide certain subsidies to retired health-care workers and award them with honorable titles. For senior university medical students, volunteer certificates are issued and priority is given to their internships, so that health-care workers can be motivated to self-realization through spiritual and material rewards.
Regarding the second category of ICU resources (i.e., drugs), healthcare institutions perform the subdivision of drug types and carry out dynamic physical preparations based on 15–20% of the service recipient population for clinically essential drugs. This will enable a combination of good preparedness during normal times and emergency situations. In addition, in-depth collaboration with corporations is needed to fully capitalize on their production capacity reserves. This helps medical institutions to be able to scientifically and rationally optimize the structure and quantity of their drug stockpiles to prevent themselves from being over-stressed. Yet the lower demand for medicines at the end of the epidemic led to the problem of excess inventory of enterprises at a certain point in time must be taken into account. So, the medical institutions should sign a strategic agreement on stockpiling with enterprises, take the initiative to bear the guaranteed acquisition measures, and consider the production costs of the cooperative enterprises. These measures are used to truly safeguard the enthusiasm of the cooperative enterprises to invest in the production capacity.
Regarding the third category of ICU resources (i.e., medical equipment), large-scale medical equipment cannot be rapidly mass-produced due to limitations in the capacity for emergency production and conversion of materials. In addition, the bulk procurement of high-end medical equipment is also relatively difficult in the short term. Therefore, it is more feasible for healthcare institutions to have physical reserves of medical equipment, such as invasive ventilators. However, the investment costs of medical equipment are relatively high. Ventilators, for example, cost up to USD $50,000, and subsequent maintenance costs are also relatively high. After all, according to the depreciable life of specialized hospital equipment, the ventilator, as a surgical emergency equipment, is depreciated over five years. And its depreciation rate is calculated at 20% annually for the first five years, which means a monthly depreciation of $835. Thus, the excessively low utilization rate of such equipment will also impact the hospital. Healthcare institutions should, therefore, conduct further investigations on the number of beds and the reserves of ancillary large-scale medical equipment to find a balance between capital investment and patient needs.
The limitations of this paper are reflected in the following three points. Firstly, in the prediction of the number of infections, the specific research object in this paper is COVID-19, and other public health events such as SARS, H1N1, and Ebola are not comparatively analyzed. The main reason for this is the issue of data accessibility, and it is easier for us to analyze events that have occurred in recent years. In addition, using the Shanghai epidemic as a specific case may be more representative of the epidemic situation in an international metropolis with high population density and mobility. Hence, it has certain regional limitations, and subsequent studies should expand the scope of the case study to reflect the characteristics of epidemic transmission in different types of urban areas and enhance the generalizability.
Secondly, the main emphasis of this study is on forecasting the demand for ICU healthcare resources across the entire region of the epidemic, with a greater focus on patient demand during public emergencies. Our aims are to help all local healthcare institutions more accurately identify changes in ICU healthcare resource demand during this local epidemic wave, gain a more accurate understanding of the treatment demands of critically ill patients, and carry out comprehensive, scientifically based decision-making. Therefore, future studies can examine individual healthcare institutions instead and incorporate the actual conditions of individual units to construct multi-objective models. In this way, medical institutions can further grasp the relationship between different resource inputs and the recovery rate of critically ill patients, and achieve the balance between economic and social benefits.
Finally, for the BILSTM-GASVR prediction method, in addition to the number of confirmed diagnoses predicted for an outbreak in a given region, other potential applications beyond this type of medium-sized dataset still require further experimentation. For example, whether the method is suitable for procurement planning of a certain supply in production management, forecasting of goods sales volume in marketing management, and other long-period, large-scale and other situations.
Within the context of major public health events, the fluctuations and uncertainties in the demand for ICU resources can lead to large errors between the healthcare supply and actual demand. Therefore, this study focuses on the question of forecasting the demand for ICU healthcare resources. Based on the number of current confirmed cases, we construct the BILSTM-GASVR model for predicting the number of patients. By comparing the three indicators (MSE, MAPE, and correlation coefficient \(R^{2}\) ) and the results of the BILSTM, LSTM, and GASVR models, we demonstrate that our model have a higher accuracy. Our findings can improve the timeliness and accuracy of predicting ICU healthcare resources and enhance the dynamics of demand forecasting. Hence, this study may serve as a reference for the scientific deployment of ICU resources in healthcare institutions during major public events.
Given the difficulty in data acquisition, only the Shanghai epidemic dataset is selected in this paper, which is one of the limitations mentioned in Part 4. Although the current experimental cases of papers in the same field do not fully conform to this paper, the results of the study cannot be directly compared. However, after studying the relevant reviews and the results of the latest papers, we realize that there is consistency in the prediction ideas and prediction methods [ 34 , 35 ]. Therefore, we summarize the similarities and differences between the results of the study and other research papers in epidemic forecasting as shown below.
Similarities: on the one hand, we all characterize trends in the spread of the epidemic and predict the number of infections over 14 days. On the other hand, we all select the current mainstream predictive models as the basis and combine or improve them. Moreover, we all use the same evaluation method (comparison of metrics such as MSE and realistic values) to evaluate the improvements against other popular predictive models.
Differences: on the one hand, other papers focus more on predictions at the point of the number of patients, such as hospitalization rate, number of infections, etc. This paper extends the prediction from the number of patients to the specific healthcare resources. This paper extends the prediction from the number of patients to specific healthcare resources. We have divided the medical resources and summarized the demand regularities of the three types of information in the epidemic, which provides the basis for decision-making on epidemic prevention to the government or medical institutions. On the other hand, in addition to the two assessment methods mentioned in the same point, this paper assesses the performance of the prediction methods with the help of significance tests, which is a statistical approach to data. This can make the practicality of the forecasting methodology more convincing.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Yuan, R., Yang, Y., Wang, X., Duo, J. & Li, J. Study on the forecasting and allocation of emergency medical material needs in the event of a major infectious disease outbreak. J Saf Environ. https://doi.org/10.13637/j.issn.1009-6094.2023.2448 .
Total epidemic data (global). 2024. Retrieved March 30, 2024, from: https://www.sy72.com/world/ .
Sui, K., Wang, Y., Wang, S., Chen, C., & Sun, X. A multiple regression analysis-based method for forecasting the demand of emergency materials for power grids. Electron Technol Softw Eng. 2016; (23), 195-197.
Li K, Liu L, Zhai J, Khoshgoftaar TM, Li T. The improved grey model based on particle swarm optimization algorithm for time series prediction. Eng Appl Artif Intell. 2016;55:285–91. https://doi.org/10.1016/j.engappai.2016.07.005 .
Article Google Scholar
Fan R, Wang Y, Luo M. SEIR-based COVID-19 transmission model and inflection point prediction analysis. J Univ Electron Sci Technol China. 2020;49(3):369–74.
Google Scholar
Neves AGM, Guerrero G. Predicting the evolution of the COVID-19 epidemic with the A-SIR model: Lombardy, Italy and São Paulo state Brazil. Physica D. 2020;413:132693. https://doi.org/10.1016/j.physd.2020.132693 .
Article PubMed PubMed Central Google Scholar
Li R, Pei S, Chen B, Song Y, Zhang T, Wan Y, Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368(6490):489–93. https://doi.org/10.1126/science.abb3221 .
Article CAS PubMed PubMed Central Google Scholar
Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc R Soc Lond. 1927;115(772):700–21. https://doi.org/10.1098/rspa.1927.0118 .
Hethcote HW. The mathematics of infectious diseases. Siam Rev. 2000;42(4):599–653. https://doi.org/10.1137/SIREAD000042000004000655000001 .
Estrada E. COVID-19 and SARS-CoV-2. Modeling the present, looking at the future. Phys Rep. 2020;869:1–51. https://doi.org/10.1016/j.physrep.2020.07.005 .
Mizumoto K, Chowell G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect Dis Model. 2020;5:264–70. https://doi.org/10.1016/j.idm.2020.02.003 .
Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the Basic Reproduction Number. Emerg Infect Dis. 2019;25(1):1–4. https://doi.org/10.3201/eid2501.171901 .
Annas S, Isbar Pratama M, Rifandi M, Sanusi W, Side S. Stability analysis and numerical simulation of SEIR model for pandemic COVID-19 spread in Indonesia. Chaos Solit Fract. 2020;139:110072. https://doi.org/10.1016/j.chaos.2020.110072 .
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–209. https://doi.org/10.1056/NEJMoa2001316 .
Anggriani N, Ndii MZ, Amelia R, Suryaningrat W, Pratama MAA. A mathematical COVID-19 model considering asymptomatic and symptomatic classes with waning immunity. Alexandria Eng J. 2022;61(1):113–24. https://doi.org/10.1016/j.aej.2021.04.104 .
Efimov D, Ushirobira R. On an interval prediction of COVID-19 development based on a SEIR epidemic model. Ann Rev Control. 2021;51:477–87. https://doi.org/10.1016/j.arcontrol.2021.01.006 .
Lin Q, Zhao S, Gao D, Lou Y, Yang S, Musa SS, Wang MH. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int J Infect Dis. 2020;93:211–16.
Chao L, Feng P, Shi P. Study on the epidemic development of COVID-19 in Hubei. J Zhejiang Univ (Med Sci). 2020;49(2):178–84.
Reiner RC, Barber RM, Collins JK, et al. Modeling COVID-19 scenarios for the United States. Nat Med. 2021;27(1):94–105. https://doi.org/10.1038/s41591-020-1132-9 .
Article CAS Google Scholar
Shahid F, Zameer A, Muneeb M. Predictions for COVID-19 with deep learning models of LSTM GRU and Bi-LSTM. Chaos Solit Fract. 2020;140:110212. https://doi.org/10.1016/j.chaos.2020.110212 .
Chimmula VKR, Zhang L. Time series forecasting of COVID-19 transmission in Canada using LSTM networks. Chaos Solit Fract. 2020;135:109864. https://doi.org/10.1016/j.chaos.2020.109864 .
Hamou AA, Azroul E, Hammouch Z, Alaoui AAL. A fractional multi-order model to predict the COVID-19 outbreak in Morocco. Appl Comput Math. 2021;20(1):177–203.
Zhou, L., Zhao, C., Liu, N., Yao, X., & Cheng, Z. Improved LSTM-based deep learning model for COVID-19 prediction using optimized approach. Eng Appl Artif Intell. 2023; 122. https://doi.org/10.1016/j.engappai.2023.106157 .
Huang C, Chen Y, Ma Y, Kuo P. Multiple-Input Deep Convolutional Neural Network Model for COVID-19 Forecasting in China. medRxiv. 2020;74822–34.
Gautam Y. Transfer Learning for COVID-19 cases and deaths forecast using LSTM network. Isa Trans. 2022;124:41–56. https://doi.org/10.1016/j.isatra.2020.12.057 .
Article PubMed Google Scholar
Ghany KKA, Zawbaa HM, Sabri HM. COVID-19 prediction using LSTM algorithm: GCC case study. Inform Med Unlock. 2021;23:100566. https://doi.org/10.1016/j.imu.2021.100566 .
Devaraj J, Madurai Elavarasan R, Pugazhendhi R, Shafiullah GM, Ganesan S, Jeysree AK, Khan IA, Hossain E. Forecasting of COVID-19 cases using deep learning models: Is it reliable and practically significant? Results Phys. 2021;21:103817. https://doi.org/10.1016/j.rinp.2021.103817 .
Arora P, Kumar H, Panigrahi BK. Prediction and analysis of COVID-19 positive cases using deep learning models: a descriptive case study of India. Chaos Solit Fract. 2020;139:110017. https://doi.org/10.1016/j.chaos.2020.110017 .
Liu Q, Fung DLX, Lac L, Hu P. A Novel Matrix Profile-Guided Attention LSTM Model for Forecasting COVID-19 Cases in USA. Front Public Health. 2021;9:741030. https://doi.org/10.3389/fpubh.2021.741030 .
Ribeiro MHDM, Da Silva RG, Mariani VC, Coelho LDS. Short-term forecasting COVID-19 cumulative confirmed cases: perspectives for Brazil. Chaos Solit Fract. 2020;135:109853. https://doi.org/10.1016/j.chaos.2020.109853 .
Shoko C, Sigauke C. Short-term forecasting of COVID-19 using support vector regression: an application using Zimbabwean data. Am J Infect Control. 2023. https://doi.org/10.1016/j.ajic.2023.03.010 .
Feng T, Peng Y, Wang J. ISGS: A Combinatorial Model for Hysteresis Effects. Acta Electronica Sinica. 2023;09:2504–9.
Zhou H, Zhang S, Peng J, Zhang S, Li J, Xiong H, Zhang W. Informer: Beyond Efficient Transformer for Long Sequence Time-Series Forecasting. AAAI Conference Artif Intell. 2020;35(12):11106–15.
Rahimi I, Chen F, Gandomi AH. A review on COVID-19 forecasting models. Neural Comput Applic. 2023;35:23671–81. https://doi.org/10.1007/s00521-020-05626-8 .
Chen J, Creamer GG, Ning Y, Ben-Zvi T. Healthcare Sustainability: Hospitalization Rate Forecasting with Transfer Learning and Location-Aware News Analysis. Sustainability. 2023;15(22):15840. https://doi.org/10.3390/su152215840 .
Download references
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
No external funding received to conduct this study.
Author information
Authors and affiliations.
School of Logistics, Beijing Wuzi University, No.321, Fuhe Street, Tongzhou District, Beijing, 101149, China
Weiwei Zhang & Xinchun Li
You can also search for this author in PubMed Google Scholar
Contributions
WWZ and XCL conceived the idea and conceptualised the study. XCL collected the data. WWZ analysed the data. WWZ and XCL drafted the manuscript, then WWZ and XCLreviewed the manuscript. WWZ and XCL read and approved the final draft.
Corresponding author
Correspondence to Xinchun Li .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, W., Li, X. A data-driven combined prediction method for the demand for intensive care unit healthcare resources in public health emergencies. BMC Health Serv Res 24 , 477 (2024). https://doi.org/10.1186/s12913-024-10955-8
Download citation
Received : 21 September 2023
Accepted : 05 April 2024
Published : 17 April 2024
DOI : https://doi.org/10.1186/s12913-024-10955-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Public health emergency
- ICU healthcare resource demand
- Machine learning
- Combined prediction
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Case Presentation. The patient is a 60-year-old white female presenting to the emergency department with acute onset shortness of breath. Symptoms began approximately 2 days before and had progressively worsened with no associated, aggravating, or relieving factors noted. She had similar symptoms approximately 1 year ago with an acute, chronic ...
ECMO denotes extracorporeal membrane oxygenation, and ICU intensive care unit. Reference values are affected by many variables, including the patient population and the laboratory methods used.
In patients with Covid-19, an elevated d-dimer level has been associated with numerous poor outcomes, including ICU admission, the need for mechanical ventilation, thrombosis, bleeding, and death ...
On examination, the patient appeared to be in respiratory distress. The temperature was 35.9°C, the pulse 98 beats per minute, the blood pressure 129/58 mm Hg, the respiratory rate 24 breaths per ...
Case Study #1. Pt is a 36 y/o male who presented on 11/16 as a Code Trauma activation after sustaining GSW's to posterior head and R shoulder. Per EMS report patient was seated in the car when multiple gunshots were fired. Initially patient was responsive to painful stimuli with L-sided seizure activity, R side flaccid.
This section is a collection of critical care clinical cases to test yourself and hopefully get some new ideas. Please leave feedback and comments, and if you want to put your own hot cases up, please get in touch and we can make it happen. ... ICU-Acquired weakness: NEJM Critical Care Challenge Case 9 (Question and Answer) Lachlan Donaldson ...
Anticoagulation was initiated and the patient was transferred to the intensive care unit (ICU) for further management. ... which act as both inotropic agent and pulmonary vasodilator have shown promise in animal studies and case reports [12,13]. ... caution must be exercised in extrapolating the results of these small studies to patients with ...
Since this case was transferred to a regional hospital, the subsequent symptoms are unknown. In the future, it is necessary to investigate whether early rehabilitation for ICU patients affects the sequelae of COVID-19. Furthermore, since this is a case report of a single patient, the generalizability of the results is not known.
On arrival at the medical ICU, the patient appeared cachectic and dyspneic. He was unable to complete sentences. His blood pressure was 125/71 mm Hg, heart rate of 122/min, temperature 100 °F, respiratory rate 33/min, and oxygen saturation 77% on room air and 92% on 40% venti-mask.
Figure. Part 1 of this two-part series (Burn injuries in the ICU: A case scenario approach, March 2017) reviewed the various types of burn injuries and what critical care nurses need to know to provide initial resuscitative care for patients with severe burn injuries using the case study of a young Amish boy, Abe.This article is based on Abe's unfolding case scenario and conclusion and ...
A 57 year-old male lorry driver, presented to his local emergency department with a 20-minute episode of diaphoresis and chest pain. The chest pain was central, radiating to the left arm and crushing in nature. The pain settled promptly following 300 mg aspirin orally and 800 mcg glyceryl trinitrate (GTN) spray sublingually administered by ...
This article uses a case scenario to review various types of burn injuries, burn pathophysiology, and what nurses need to know to provide comprehensive assessment and resuscitative care to patients with this type of injury. Figure. Caring for a patient with severe burn injuries offers many unique challenges for critical care nurses.
Coronavirus disease 2019 (COVID-19) was first identified in Indonesia in March 2020, and the number of infections has grown exponentially. The situation is at its worst, overwhelming intensive care unit (ICU) resources and capacity. This is a single-center observational case study of 21 confirmed COVID-19 patients admitted to the ICU from March 20, 2020, to April 31, 2020.
Scenario 1. You are caring for a 56 year old man in the ICU who was admitted for chest pain to rule out myocardial infarction (heart attack). He has a history of high cholesterol, hypertension ...
Guiding FFICM and EDIC exam candidates through the intensive care medicine curriculum, this book provides 48 case studies mapped to eight key areas of study in the UK and European syllabuses. Cases include clinical vignettes, explanations and a list of key learning points, while also being formatted along the structure of FICM case reports.
Recent UK figures suggest a critical care unit mortality of around 13% in general ICUs but this will vary depending on case mix. 2 Prognostication is imperfect; therefore, the intended benefits of continued treatment need to be balanced against the potential burden for the patient. Once it becomes apparent that escalation or continuation of ...
CASE STUDY #3. THE PATIENT IS A 60-YEAR-OLD WHITE FEMALE PRESENTING TO THE EMERGENCY DEPARTMENT WITH ACUTE ONSET SHORTNESS OF BREATH. SYMPTOMS BEGAN APPROXIMATELY 2 DAYS BEFORE AND HAD PROGRESSIVELY WORSENED WITH NO ASSOCIATED, AGGRAVATING, OR RELIEVING FACTORS NOTED. SHE HAD SIMILAR SYMPTOMS APPROXIMATELY 1 YEAR AGO WITH AN ACUTE,CHRONIC ...
Dr. Lila M. Martin: A 73-year-old man was transferred to the intensive care unit (ICU) of an academic health center in Boston for acute hypoxemic respiratory failure in March 2020, during the ...
Delirium is a commonly neglected manifestation of organ dysfunction in the ICU. It is commonly unmonitored and not discussed on rounds [].This is often because the ICU team feels there is nothing that can be done about delirium since we are already treating the patient's main diseases, or because it might seem logical that a sedated patient would have a cognitive dysfunction [].
Owing to worsening respiratory status and SpO 2 <70%, the patient was intubated on the sixth day in the ICU, and every day, she was nursed in a prone position for >16 hours. We believe that the treatment and care activities under qualified and effective nursing care, such as providing appropriate respiratory support at the right time, early ...
More studies are needed in older ICU patients with morbidity outcomes that affect quality of life. Finally, there is a well-described need for geriatric specific education and further implementation of geriatric knowledge via multidisciplinary ICU teams as the population ages and ICU admissions increase. ... et al. The Cost of ICU Delirium and ...
Patient-Centred Care. In designing patient rooms, the well-being and orientation of the patient were considered of main concern; therefore, a key focus was the formation of a day and night rhythm. Each of the 36 single-patient rooms measure 25m2 and have a view either to either one of the four specially designed 60m2 Dutch gardens or to other ...
Fluid challenges are widely adopted in critically ill patients to reverse haemodynamic instability. We reviewed the literature to appraise fluid challenge characteristics in intensive care unit (ICU) patients receiving haemodynamic monitoring and considered two decades: 2000-2010 and 2011-2021. We assessed research studies and collected data regarding study setting, patient population ...
The impact of frailty and rapid response team activation on patients admitted to the intensive care unit: A case-control matched, observational, single-centre cohort study. ... Single-centre, retrospective observational case-control study of adult patients (>18 years) admitted to a medical-surgical ICU with (cases) or without (controls) a ...
Since it's easy for patients to lose track of the normal day-night cycle, they can be at high risk of ICU delirium, which is an acute and severe state of confusion. Preventing ICU delirium ...
Objective: To examine the relationship between current and former smoking and the occurrence of delirium in surgical Intensive Care Unit (ICU) patients. Methods: We conducted a single center, case-control study involving 244 delirious and 251 non-delirious patients that were admitted to our ICU between 2018 and 2022. Using propensity score analysis, we obtained 115 pairs of delirious and non ...
Presentation of Case. Dr. Jacqueline B. Henson (Medicine): A 54-year-old man was evaluated at this hospital after cardiac arrest associated with ventricular fibrillation. The patient had been in ...
A recent study developed a screening tool to systematically assess palliative care needs for ICU patients at the point of admission and initiate automatic referrals for palliative care ...
The intensive care unit (ICU) is the "last line of defense" for saving lives. And ICU resources play a critical role in the treatment of critical illness and combating public health emergencies. This study estimates the demand for ICU healthcare resources based on an accurate prediction of the surge in the number of critically ill patients ...
Several risk factors for increased hospital-acquired infections (HAIs) in patients with Covid-19 on invasive mechanical ventilation (IMV) have been proposed, such as prolonged IMV, the prone position, steroid treatment, severe mucus production, Covid-19's effect on the immune system, ICU understaffing and inexperienced staff. 6-11 Several studies have reported an increased risk of ...