Research Journal of Medicinal Plants
Research Journal of Medicinal Plants is an international, double-blind, peer-reviewed, open-access journal published by the Science International with one issue(s) per year.

Aims & Scope
Research Journal of Medicinal Plants is an international peer-reviewed scientific journal dedicated to publishing significant research work in the entire field of medicinal plants. Scope of the journal includes but is not limited to traditional medicine, ethnobotany, herbal therapeutics, phytomedicine, herbal preparations, medicinal plants in healthcare, clinical trials and pilot studies, biological and pharmacological effects of plant extracts, medicinal plants, and their anti-inflammatory, anti-cholesterol, hypotensive, antispasmodic, anti-diabetic, anticancer, antiviral, antibacterial and antifungal activity.
Why Publish in this journal?
- Fast decisions and rapid online publication
- Discoverable in the leading indexing databases
- Global reach with impact
- Expert Editorial Board to manage submitted paper
- Personalised service from in-house staff
- Discoverable research read by millions of people
Abstracting and Indexing
Research Journal of Medicinal Plants has gained significant recognition within the academic community and has been indexed in several prestigious platforms:
- Asian Digital Library
- ASCI-Database
- Google Scholar
- JournalsPedia
- Open Access Asia
These indexing services ensure that the content of our journal is widely accessible and discoverable by researchers and scholars worldwide. Our published articles, reviews, and perspectives receive increased visibility and reach through these reputable databases, fostering collaboration and knowledge dissemination within the global scientific community.
Acceptance Criteria
To be published in Research Journal of Medicinal Plants, a paper must demonstrate scientific validity and technical soundness in its methodology and analysis. As a peer-reviewed scientific journal, Research Journal of Medicinal Plants evaluates submitted manuscripts based on research methods, relevance to readers, writing style, presentation clarity, and adherence to high ethical standards, irrespective of perceived novelty.
Editorial Board
The Editorial Board serves as the backbone of a successful journal. The Editorial Board of Research Journal of Medicinal Plants comprises the Editor-in-Chief and Editorial Board members. All Editorial Board Members are selected based on strict criteria, ensuring their active and productive contribution to the journal. The Editorial Team of Research Journal of Medicinal Plants is responsible for maintaining the quality of published research, enhancing the journal's impact, and contributing to the advancement of the broader scientific community.
Reviewer's Database
Research Journal of Medicinal Plants strives to make decisions on all submitted manuscripts, including commissioned content, based on the advice provided by at least two independent reviewers. To facilitate this process, Research Journal of Medicinal Plants maintains a reviewer database. This database assists us in identifying the most suitable subject experts who can thoroughly review the manuscript.
Ethics in Publication
Research Journal of Medicinal Plants fully adheres to and complies with the policies and principles established by the Committee on Publication Ethics and the Asian Council of Science Editors . The Research Journal of Medicinal Plants editors enforce a rigorous peer-review process, along with strict ethical policies and standards, to ensure the publication of high-quality scientific works in the field.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 May 2021
Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources
- Manyou Yu 1 ,
- Irene Gouvinhas 1 ,
- João Rocha 2 &
- Ana I. R. N. A. Barros 1 , 3
Scientific Reports volume 11 , Article number: 10041 ( 2021 ) Cite this article
25k Accesses
107 Citations
2 Altmetric
Metrics details
- Biochemistry
Plants with medicinal properties play an increasingly important role in food and pharmaceutical industries for their functions on disease prevention and treatment. This study characterizes the phenolic composition and antioxidant activity of seven medicinal and food plants, including the leaves of Salvia officinalis L., Rosmarinus officinalis L., Olea europaea L., and Punica granatum L., as well as the leaves and young stems of Ruta graveolens L., Mentha piperita L., and Petroselinum crispum , Mill., by using colorimetric, chromatographic, and spectrophotometric assays. Results revealed that the hydro-methanolic leaf extracts of P. granatum (pomegranate) displayed the highest content of total phenols (199.26 mg gallic acid per gram of plant dry weight), ortho -diphenols (391.76 mg gallic acid per gram of plant dry weight), and tannins (99.20 mg epicatechin per gram of plant dry weight), besides a higher content of flavonoids (24 mg catechin per gram of plant dry weight). The highest antioxidant capacity measured by ABTS, DPPH, and FRAP (2.14, 2.27, and 2.33 mM Trolox per gram of plant dry weight, respectively) methods was also obtained in pomegranate leaf extracts, being 4–200 times higher than the other species. Such potent antioxidant activity of pomegranate leaves can be ascribed to the presence of different types of phenolic compounds and the high content in tannins, whilst phenolic acids and flavonoids were found to be the dominant phenolic classes of the other six plants. Consequently, despite the well-known antioxidant properties of these plant species, our study suggests pomegranate leaf can stand out as a relatively more valuable plant source of natural bioactive molecules for developing novel functional food-pharma ingredients, with potential for not only promoting human health but also improving bio-valorization and environment.
Similar content being viewed by others

Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial
Minju Sim, Chong-Su Kim, … Dong-Mi Shin
The role of fines in espresso extraction dynamics
Samo Smrke, André Eiermann & Chahan Yeretzian

Nanopore analysis of cis-diols in fruits
Pingping Fan, Zhenyuan Cao, … Shuo Huang
Introduction
The recent development of functional foods and pharmaceutical products based on medicinal and food (namely fruits and vegetables) plants has brought improvements to all aspects of life, including the alleviation of physical disorders, the reduction in the use of synthetic antibiotics, and the increase in life expectancy 1 , 2 . Indeed, these plants have long been used as safe, effective and sustainable sources of natural antioxidants or free radical scavengers, particularly phenolic compounds, such as phenolic acids, flavonoids, tannins, stilbenes, and anthocyanins 2 . Those phenolics are mostly regarded to confer upon the antioxidant activity of medicinal and food plants, making a marked contribution in the fight against many pathological conditions such as cancer, diabetes, aging, cardiovascular, and other degenerative diseases 2 , 3 , 4 , 5 .
Salvia officinalis L., Rosmarinus officinalis L., and Mentha piperita L. commonly named as sage, rosemary, and peppermint, respectively, belongs to the family of Lamiaceae. They are well-known herbs and spices used in foods for flavors and aromas. Infusions, leaves or essential oils of its each species are reported to possess therapeutics in anti-cancer, anti-microbial, anti-diabetes, and gastrointestinal diseases, etc. 3 , 6 , 7 , 8 . Several bioactivities of sage like antinociceptive, hypolipidemic, and memory-enhancing effects have been demonstrated with clinical trials 7 . Rosmarinic acid is abundant both in sage and rosemary, contributing to their anti-inflammatory properties 3 , 6 , 7 . Flavonoids, phenolic lignans and stilbenes, and essential oils are expected to be responsible for the aroma effects of peppermint 8 .
Rue ( Ruta graveolens L.) has been one of the key plants of the European pharmacopoeia since ancient times for the use in tremors, paralysis, nervine disorders, and joint pain 9 . And nowadays, it becomes medicine in Mediterranean region, due to its prominent biological activities, especially neuroprotection 9 , 10 . Rutin, psoralen, limonene, and pinene are reported as main constituents in this plant extracts or rue oils 9 , 10 .
Olive ( Olea europaea L.) oil is one of the major components of the Mediterranean diets. Recently, phenolics present in olive leaves, especially the oleuropein, are reviewed to be potential economic and renewable source of natural by-products, attributed to its antioxidant, antihypertensive, hypoglycemic, hypocholesterolemic and cardioprotective activity 11 , 12 .
Parsley ( Petroselinum crispum Mill.), used as culinary and medicinal herb, is originated from Mediterranean region. Phytochemicals particularly apigenin, coumarins, myristicin, and apiol are active compounds rich in parsley leaves, exhibiting diverse pharmacological properties, such as cyto-, gastro-, brain-, nephron-protective effects, and so on 13 , 14 , 15 .
Pomegranate ( Punica granutum L.) a deciduous shrub in the family of Lythraceae, is one of the oldest known plants. Both the edible (namely fruit juice) and non-edible parts (including seeds, peels, leaves, roots and bark) of this plant have been evidenced to have a wide range of health benefits, largely resulting from its abundant phenolic acid, flavonoids, tannins, amino acids, and alkaloids 16 , 17 . However, the importance of pomegranate leaves, as agricultural and industrial waste, is of great interest and value to be emphasized by means of describing its beneficial effects and studies performed on this field.
Within the frame, materials from the seven medicinal and food plants aforementioned, that is, leaves and young stems (easy for picking) of rue, peppermint, and parsley, as well as the leaves of sage, rosemary, olive, and pomegranate are outstanding for their higher levels of phenolic contents and antioxidant capacities, along with relatively lower (dose-dependent) or inexistent toxicity 6 , 7 , 8 , 9 , 11 , 13 , 15 , 17 . Therefore, in an attempt to explore plant-based alternative solutions in promoting health, as well as paving the way towards our future pre-clinical and clinical studies, we aimed to analyze the phenolic classes (total phenols, ortho -diphenols, flavonoids, and tannins) and antioxidant activities of different plant species under the same evaluation condition. Furthermore, the principal phenolic constituents were chromatographically characterized to investigate the relationship between the phenolic content and antioxidant activity.
Results and discussion
Phenolic content of tested medicinal and food plants.
Results of colorimetric and spectrophotometric analysis of seven medicinal and food plants were showed in Table 1 . In general, the total phenolic content of the selected plant species was found to be at the highest level in pomegranate leaf extracts at 199.26 mg of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW), followed by three Lamiaceae species, including peppermint (70.06 mg GAE g −1 DW), sage (50.89 mg GAE g −1 DW) and rosemary (48.48 mg GAE g −1 DW). On the contrary, parsley displayed the lowest value of total phenols (6.94 mg GAE g −1 DW). The same trend was observed concerning the content of ortho -diphenols and tannins of all investigated samples, reporting the following sequence: pomegranate > peppermint > sage > rosemary > rue > olive > parsley. The ortho -diphenol and tannin content of the methanolic extracts ranged from 26.40 to 391.76 mg GAE g −1 DW, and from 1.33 to 99.20 mg of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW), respectively. Moreover, results on total flavonoids content showed a different pattern compared to other phenolic classes, with peppermint showing maximum values at 70.14 mg of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW), following with rosemary (49.14 mg CATE g −1 ), sage (43.92 mg CATE g −1 ), and pomegranate (24.34 mg CATE g −1 ). Furthermore, the flavonoid content of olive leaf was higher than that of rue, in contrast to the trend of the other phenolic classes. Rosemary and sage had comparatively high levels of flavonoids, while the minimum values were reported for parsley.
Different phenolic contents of different plant samples have been reported in the literature 12 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 . For instance, the total phenol content of sage and peppermint was 27.94 and 45.25 mg GAE 100 g −1 DW, meanwhile the flavonoid content of them was 27.54 and 25.17 mg catechin per 100 g, which were much lower than that of our results 19 . Parsley extracts had 1.583 GAE mL −1 of total phenols, 0.091 mg catechin mL −1 of flavonoids, and 1.167 mg catechin mL −1 of condensed tannins 26 . Salama et al. 12 described significant differences in the amounts of total phenolics, flavonoids, and tannins of olive leaves, under different extraction solvents, ranging from 42.02 to 85.50 mg GAE g −1 , 31.22 to 105.19 mg quercetin g −1 , and 30.92 to 51.03 mg tannic acid g −1 , respectively. The contents of phenolic and flavonoid compounds in rue were 14.1 GAE g −1 and 15.8 mg rutin g −1 of dry extracts 20 . Some studies 27 , 28 , 29 have evidenced considerably high level of phenolics in pomegranate leaf extracts, up to 328 mg GAE g −1 DW. Interestingly, pomegranate leaves are characterized by carbohydrates, reducing sugars, sterols, saponins, flavonoids, ellagitannins, piperidine alkaloids, flavones, glycosidic compounds, which are the richest source of phytochemicals when considering the non-edible parts of this species, some food products (red wine, green tea, etc.), and another 109 medicinal plants 30 , 31 , 32 . Our results disclosed that tannins were the main phenolic compounds of pomegranate leaf extract, which has also been corroborated by other studies 33 .
As shown in data (Table 1 ), significant differences ( p < 0.001) around 29, 15, 92 and 75 times were observable respectively for total phenols, ortho -diphenols, flavonoids and tannins in the seven plant extracts, indicating that each phenolic classes exhibited considerably different content among the studied plants. This result was in agreement with other authors 34 , who found that depending on the plant species and botanical family, strong differences were found among 10 medicinal herbs and 11 spices. Meanwhile, the same authors 34 observed a wide variance of phenolics in different samples of the same species, such as the total phenolic content of nine independent samples of peppermint was from 18.3 to 284.3 mg GAE g −1 . Moreover, contents of total phenolics, flavonoids, and condensed tannins of 13 different provenances of rosemary, collected in different seasons ranged from 22.46 to 44.57 mg GAE g −1 DW, from 1.49 to 5.01 mg quercetin g −1 , and from 0.81 to 1.71 mg CATE g −1 DW, respectively 18 . Our results showed inconsistency with this observation, probably attributed to the varieties, or geographical differences, as well as to the collection time, agroclimatic conditions and other relevant factors 24 , 25 . However, to some extent, pomegranate leaf was supposed to have a relatively higher phenolic content than many other medicinal plants. Therefore, it can be inferred that pomegranate leaf could be an important valuable source of bioactive compounds for medicinal purposes and health care.
In addition, in the current study, the colorimetric analysis of flavonoids varied between pomegranate leaf (orange-yellowish) with other plants (pink) and the standard (catechin, pink) under the same conditions (as below described in the methods). This visual observation may be related to the fact that leaves from pomegranate have different predominant sub-classes of flavonoids, different from that existing in the other studied plants 32 . So, the methodology, especially to normalize the use of standards such as quercetin or rutin 35 should be modified to accurately quantify the amount of flavonoids.
In vitro antioxidant activity
The in vitro antioxidant activity assays were carried out to assess the capacity of plant extracts to scavenge free radicals including 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid radical cation (ABTS +· ) and 2,2‐di(4‐tert‐octylphenyl)‐1‐picrylhydrazyl radical (DPPH·), as well as the ability to reduce ferric (III) iron to ferrous (II) iron. Overall, Table 1 revealed that all the species displayed high antioxidant capacities, although significant differences were observed ( p < 0.001), ranging from 0.01 to 2.14 mM Trolox per gram of plant dry weight (mM Trolox g −1 ) for ABTS, from 0.01 to 2.27 mM Trolox g −1 for DPPH, and from 0.01 to 2.33 mM Trolox g −1 for FRAP (ferric reducing antioxidant power), with large variation over 210-fold. It was found that pomegranate always exhibited the highest antioxidant properties (2.14–2.33 mM Trolox g −1 ) throughout the three measurements, followed by peppermint (0.35–0.50 mM Trolox g −1 ), sage (0.27–0.40 mM Trolox g −1 ), rosemary (0.27–0.42 mM Trolox g −1 ), rue (0.10–0.16 mM Trolox g −1 ), and olive leaf (0.11–0.15 mM Trolox g −1 ). No significant difference was observed between sage and rosemary, and between rue and olive leaf. However, parsley extracts reported the lowest antioxidant potential (0.01 mM Trolox g −1 ).
Previous data regarding the antioxidant capacities of sage, rosemary, rue, olive leaf, peppermint, parsley, and pomegranate leaf have been reported by several authors 12 , 14 , 18 , 22 , 26 , 31 , 36 . The IC 50 values of ABTS and DPPH radical scavenging activity, as well as the EC 50 values of reducing powder regarding olive leaves ranged from 20.13 to 190.95 µg mL −1 , from 17.97 to 41.64 µg mL −1 , and from 90 to 216 µg mL −1 , arising from diverse extraction solvents 12 . Rosemary leaves displayed 75.04 and 9.08 µg mL −1 of IC 50 by ABTS and DPPH assay, along with 4.12 µM by FRAP method 18 . Farnad et al. 22 reported the methanol-ethanol (1:1) extract of peppermint had the best DPPH radical scavenging ability (10.05 mg mL −1 of IC 50 ) and ferric reducing power (184.22 µmol per 100 g powder). The ethanolic extract of parsley displayed 0.34 mg AAE mL −1 (milligrams of ascorbic acid equivalents per milliliter) of DPPH and 0.942 mg AAE mL −1 of FRAP, which was correlated with the anti-glycation activity of this extract 26 . The best antioxidant capacities conducted by DPPH (17.09% of IC 50 ) and FRAP (458.26 mmol Fe II L −1 ) were determined for sage leaves which were collected in May 36 . Cefali et al. 14 stated the rue extracts exhibited antioxidant potential against DPPH (281.02 µg mL −1 of IC 50 ) and ABTS (587.98 µg mL −1 of IC 50 ) radicals, indicating the premature aging protective effect.
Importantly, several studies in vitro and in vivo have recorded the superior antioxidant capacity of pomegranate leaves by contrast with its non-edible parts, of which leaves are as effective as peels in the anti-bacterial, analgesic, acute and chronic anti-inflammatory effects 37 , 38 , while more potent than flowers, stems, and seeds 31 , 39 , 40 , 41 , 42 . Authors proved the potency of pomegranate leaf was higher than that of flower in the prevention of ethylene glycol-induced nephrolithiasis, in the inhibition of DPPH and hydroxyl radicals, and in the reduction of ferric iron 39 , 40 . Data 41 highlighted leaves worked more effectively than stems and led to the most loss of MMP (mitochondrial membrane permeability) potential, consequently suggested as an anti-cancer and anti-proliferative agent. Elfalleh et al. 31 illustrated the highest reducing power (348.68 µg mL −1 of EC 50 ) occurring in the aqueous extract of pomegranate leaf. Furthermore, a higher antioxidant and enzyme inhibitory activity was exposed in two extracts (methanolic and water) of pomegranate leaves among different fruit tree leaves 28 . The ethanolic extracts of pomegranate leaf also exhibited remarkable antioxidant and anti-glycation ability of twenty edible and medicinal plants 29 . The level of anti-radical and ferric reducing properties of pomegranate leaves in our results was similar to some authors 42 . However, comprehensively comparative research involving in the phytochemical and antioxidant properties between pomegranate leaves and other numerous medicinal plants is still scarce; Widely practical application of pomegranate leaf hasn’t come into being, although different biological activities of this material extracts are studied increasingly. Many authors have deeply reviewed for sage, rosemary, peppermint, rue, parsley, and olive leaf. Thus it is worth stressing on the brilliant phenolics and antioxidant property of pomegranate leaves, and developing high added-value products from these materials in the food, pharmaceutical, or even nutraceutical and cosmeceutical industries.
Chromatographic analysis of phenolic compounds
With the development of chromatographic techniques, the phenolic chemistry of many plants has been explored and analyzed to a certain degree, providing us important reference data. To obtain a more complete picture of the quality and quantity of phenolic constituents in the selected plants, 64 phenolic compounds were identified (Table 2 ), of which 59 were quantified with authentic standards relying on RP-HPLC-DAD, as well as by comparison with the literature (retention time, UV/Visible λ max , and spectra). Concentrations of identified phenolics were expressed as milligram per gram dry weight of plant (mg g −1 ).
As shown in Table 2 and Fig. S1 , phenolic profile of diverse plants was significantly different. Leaf extracts of both sage and rosemary were characterized by a high proportion of rosmarinic acid (4.61 mg g −1 or 4.31 mg g −1 , respectively). Rue presented the highest content of rutin (26.10 mg g −1 ), followed by epicatechin gallate (7.82 mg g −1 ). The major phenolic components in olive leaves were oleuropein and its derivatives. Flavanones, especially eriodictyol glycosides, following rutin were found as predominant in the leaf and stem extracts of peppermint. Parsley was described in high amount of apigenin-7- O -apiosylglucoside also called apiin (4.04 mg g −1 ) and epicatechin (3.72 mg g −1 ) in its leaf and stem extracts. The principal phenolic constituents in pomegranate leaves were hydrolyzable tannins, particularly ellagitannin I (56.06 mg g −1 ) and ellagitannin II (45.16 mg g −1 ), ranking the highest concentrations among all identified compounds.
On the other hand, results from Table 2 and Fig. S1 also showed that the most abundant phenolic classes in the tested samples were phenolic acids, flavonoids, tannins, and phenylethanoids. A considerable variation of phenolics was found, ranging, for instance, from 0.03 mg g −1 of 2,3-hydrocybenzoic acid to 56.06 mg g −1 of ellagitannin I. For each identified compound, significant differences were observed ( p < 0.05), such as gallocatechin. The most widespread phenolic acids present in the studied samples included hydroxybenzoic acids (gallic acid and its derivative, vanillic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, and neochlorogenic acid), and their ester derivatives (e.g. rosmarinic acid). Significantly high contents of chlorogenic acid (1.54 mg g −1 ) and neochlorogenic acid (1.96 mg g −1 ), and the presence of coumaric acid were perceptible in rue extracts. Ellagic acid and its derivatives were abundant in pomegranate leaves. Except in sage and rosemary, rosmarinic acid was also found in peppermint (0.23 mg g −1 ), but its concentration was lower than that in literature 43 . The special existence of rosmarinic acid, rosmanol, epirosmanol, carnosol, and carnosic acid in sage and rosemary was consistent with other authors 23 , 25 , 44 , 45 .
Besides flavanols including gallocatechin, catechin and epicatechin gallate, then various flavones (luteolin and apigenin) and flavonols (quercetin and diosmetin), mainly in the forms of their derivatives were widely distributed in the most of the studied species. Among them, the highest content of gallocatechin (2.10 mg g −1 ), catechin (3.61 mg g −1 ) and epicatechin gallate (7.82 mg g −1 ) was detected in parsley, rosemary, and rue, respectively. Epicatechin was only found in parsley with a good quantity (3.72 mg g −1 ). Furthermore, the main flavonoids from our data present in sage, rosemary, rue, peppermint, and parsley were apigenin glycosides, luteolin glycosides, quercetin glycosides, flavanone glycosides, and apigenin glycosides, respectively. In addition, peppermint also had comparative amounts of luteolin and quercetin glycosides. Likewise, pomegranate leaves possessed several apigenin and luteolin glycosides. Particularly, rutin presented the highest proportion (26.10 mg g −1 ) in rue, followed by peppermint (9.90 mg g −1 ), while the lowest (0.86 mg g −1 ) in olive leaf.
An important observation is that pomegranate leaf extracts held the greatest number of hydrolyzable tannins, especially ellagitannins. Nevertheless, no ellagitannins were detected by the HPLC method in the other six plants, while condensed tannins were present by the spectrophotometric approach. This was possibly caused by the lack of authentic standards involving different tannins, which need to be performed in the chromatographic analysis. In practice, certain studies have reported the tannins present in sage, rosemary, peppermint, rue, parsley, and olive leaves 12 , 18 , 19 , 26 , 46 , 47 , 48 , mainly in the form of condensed tannins.
In some cases, phenylethanoids, which are phenethyl alcohol-structured phenolic antioxidants, were abundantly found in olive leaves, including oleuropein and its derivatives, followed by tyrosol and verbascoside. These molecules may conduct to its high antioxidant properties 11 , 49 . Tyrosol existed in highest concentration in rosemary (4.56 mg g −1 ), but in small quantity in olive leaf (0.75 mg g −1 ) and rue (0.19 mg g −1 ).
Many studies have described the domination of rosmarinic acid in sage and rosemary, detected in varied amounts depending on phenophase, genotypes, extraction methods, and geographical conditions 23 , 24 , 25 , 36 , 44 , 45 , 50 . A concentration ranging from 0.27 to 2.49% of rosmarinic acid was determined in rosemary leaf extract, according to regions 44 . Khaleel et al. 45 reported 4.5 µg mL −1 of rosmarinic acid in aqueous extract of rosemary, whereas 17.3 µg mL −1 was measured in our methanolic extract of this plant. Exceptionally high content of rosmarinic acid was found in May extract (19.375 mg L −1 ) of sage leaves described by Generalić et al. 36 , very close to our data (18.653 mg L −1 ). Roby et al. 23 declared that the predominant phenolic compounds in sage methanolic extract were ferulic acid (18%), rosmarinic acid (17%) and apigenin (14%) of the total extracted phenols, while in our results, rosmarinic acid and apigenin glycoside III were primary and accounted for 9% and 13% of the total phenolics of sage. Among more than one-hundred active ingredients of rue, rutin, as one of its major compounds, has been a topic of interest for researchers 9 , 20 . Asgharian et al. 20 detected a high level of rutin (40.15 mg g −1 ) by extraction with 70% ethanol, which was higher than that of our study. Melnyk et al. 46 identified rutin as the highest content of phenolics in the rue methanolic extract, consistent with the present work. Several studies 48 , 49 have reported oleuropein and its derivatives as the dominant phenolics in the olive leaf, according with our results. As shown in data (Table 2 and Fig. S1 ), up to 20 phenolic compounds were identified in methanolic extract of olive leaf, more than those identified in other six plants, evidencing it as a rich source of bioactive compounds. However, the composition of olive leaf shows a remarkable variability due to location, climatic-seasonal factors, and cultivation practices, suggesting a trend to understand the factors that control the composition of olive leaves. This can be worthy for the harvesting and production of suitable extracts to be applied in human health. Kapp et al. 43 demonstrated eriocitrin, as a powerful bioactive compound, was the most abundant phenolics in peppermint, in accord with our records, composed of 38% of its aqueous extract, or reaching from 19.9 to 68.1% in 26 peppermint tea samples, respectively. However, the same authors 43 reported that rosmarinic acid accounted for a highest proportion (54.2%) of phenolics in one peppermint tea sample which was originated from Estonia. Additionally, other authors 22 , 47 , 51 also pointed out different dominant phenolics in peppermint, such as epicatechin, naringenin, caffeic acid, chlorogenic acid, 4-hydroxybenzoic acid, which can be attributed to diverse varieties, growing environment, and extraction conditions. The main finding of the present work performed on parsley corresponded to several studies 15 , 52 that apiin extractability was maximum when the solvent was ethanol, methanol or acetone. Yet Hozayen et al. 53 and Aissani et al. 21 conducted rosmarinic acid and quinic acid as the most abounded constituent in aqueous and methanol extracts of parsley, respectively. Fourteen phenolic constituents (Figure S1 ) of pomegranate leaf extracts were preliminarily identified and quantified by reference to chromatographic parameters and the literature. These results are agreeable to other researchers 33 , 54 , 55 , 56 , highlighting that ellagic acid and its derivatives, ellagitannins (punicalin, granatin A and B, etc.), flavone (apigenin, luteolin) and its glycosides, and flavonol (kaempferol) and its glycosides, are the principal phenolics in pomegranate leaves. In addition, many ellagitannins (such as punicalagins, punicafolin, castalagin, corilagin, strictinin, tercatain, brevifolin), and their galloyl and/or hexahydroxydiphenoyl (HHDP) substitutions, have been isolated from the leaf 57 . Other flavonoid derivatives like kaempferol, gossypin, quercetin, and rutin were also detected as major constituents in hydro-methanolic or hydro-ethanolic leaf extracts of pomegranate leaves 33 , 57 . However, the detailed structures of tannins and flavonoids of pomegranate leaf will require further identification by mass spectrometry and nuclear magnetic resonance spectroscopy.
Correlation analysis
In order to better understand the relationship between the antioxidant activity (by ABTS, DPPH, FRAP assays) and the phenolic composition (total phenols, ortho -diphenols, flavonoids, tannins) of the studied plants, correlation coefficients ( r ) were determined (Fig. 1 ). Strong relationships were characterized between antioxidant capacities with total phenols and ortho -diphenols (Fig. 1 a,b), indicating that phenolic compounds contribute to the inhibition of oxidative processes. The content of tannins was well correlated with antioxidant potential (Fig. 1 d). No correlation of antioxidant activities was found with flavonoid content (Fig. 1 c). However, a better relationship of flavonoids (Fig. 1 e) or tannins (Fig. 1 f) can be obtained with the antioxidant activity if excluding pomegranate or peppermint from the data, respectively. The above analysis demonstrated that the antioxidant potential from different plants was dependent on both the concentrations and the structures of phenolic compounds, in line with Cai et al. 30 . Compared to radical scavenging assays (ABTS and DPPH), the stronger correlation between reducing power and phenolic contents confirmed that FRAP was more closely related to total phenols, ortho -diphenols and tannins, which was also mentioned by Li et al. 1 .
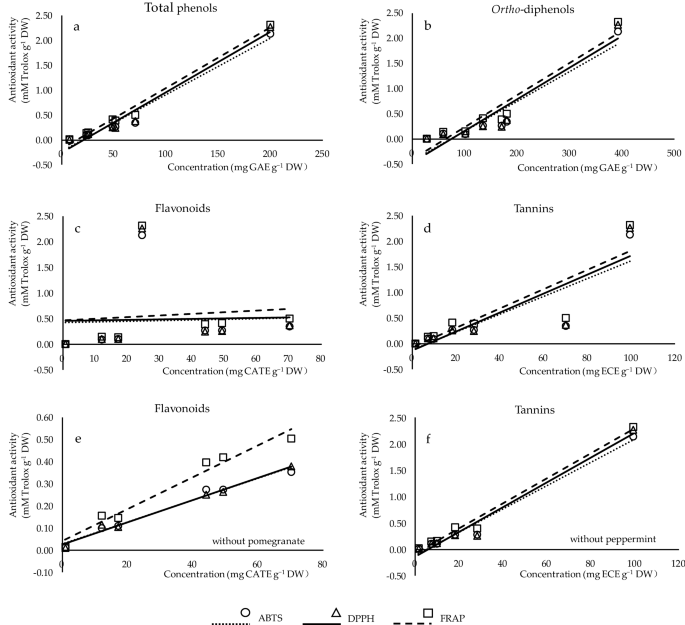
Correlation analysis between the contents of phenolic classes (x-axis) and antioxidant capacities (y-axis) measured by ABTS (circles), DPPH (triangles), and FRAP (squares). ( a – d ) The correlation of total phenols ( r ABTS , DPPH , FRAP = 0.985***, 0.984***, 0.993***), ortho -diphenols ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**), flavonoids ( r ABTS , DPPH , FRAP = 0.038, 0.031, 0.098), and tannins ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**) of the studied plants with their antioxidant activity, respectively. ( e ) The correlation of flavonoids ( r ABTS , DPPH , FRAP = 0.989***, 0.992***, 0.983***) of studied plants excluding pomegranate with their antioxidant activity. ( f ) The correlation of tannins of studied plants excluding peppermint ( r ABTS , DPPH , FRAP = 0.989***, 0.987***, 0.993***) with their antioxidant activity.
There is a highly correlation between the phenolic composition and antioxidant properties of plants. High anti-radical activity of rosemary leaf in summer was strongly related to high amounts of total phenols, total flavonoids, condensed tannins, and carnosic acid 18 . It is suggested that intraperitoneal of hydroalcoholic extract of rue increased serum and brain antioxidant capacity, due to their potent antioxidant activities of total phenolic and flavonoids content, especially rutin, caffeic acid, and apigenin 20 . Parsley methanolic extract inhibited human glioblastima cancer and oxidative stress owing to its antioxidant properties primarily related to phenolic content 21 . Peppermint extracted by various alcoholic solvents are found to have different levels of antioxidant potential, attributed to the presence of vast flavonoids, anthocyanins, and total phenols 22 . The strong reducing power, free radical scavenging capacity, and the inhibition of hydro-peroxide radicals activity of sage leaves can be linked to the high quantity of phenolic acids, especially rosmarinic acid, and certain flavonoids like catechins and flavanols 36 . Makowska-Wąs et al. 49 revealed considerable antioxidant and cytotoxic properties of olive leaf against several human cancers, largely concerned in the existence of phenolic acids, flavonoids, oleuropein, fatty acids, and volatile oils. The high concentration of phenolic components in pomegranate leaf extracts such as tannins, flavonoids, phyto-steroids, terpenoids, and saponins can be responsible for its high antioxidant activity in vitro and in vivo 27 , 28 , 29 , 32 , 58 .
To date, amount of studies have reported the close relationship not only between the phenolic contents but also between the phenolic structures and the antioxidant capacities 28 , 30 , 59 . The level of antioxidant potential of plants mainly depends on the presence and hydroxyl groups of (poly)phenolic compounds. Specifically, the antioxidant ability of phenolic acids is firstly related to the number and position of phenolic hydroxyls, and secondly to the methoxy and carboxylic acid groups 59 . Rosmarinic acid which was mainly detected in sage, rosemary, and peppermint in our work, is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid, comprising two catechol moieties, thus having two pairs of ortho hydroxyl groups grafted on two phenolic rings 18 . Gallic and chlorogenic acid are well-known antioxidant agents, due to three and two active hydroxyl groups on the aromatic ring, respectively 59 . Moreover, the catechol structure in the B-ring, the 2,3-double bond conjugated to a 4-oxo functionality, and the available of both 3- and 5-hydroxyl groups of flavonoids are essential for assessing their antioxidant properties 28 . Rutin is a rutinoside of quercetin with one of the four hydroxyl groups at position C-3 substituted with glucose and rhamnose sugar groups 20 . Apiin or eriocitrin is a apigenin or eriodictyol glycoside, on which the different glycoside moiety is located at position C-7 via a glycosidic linkage along with two or three residual hydroxyl groups on the phenolic rings 15 , 43 . Furthermore, phenylethanoids are characterized by a phenethyl alcohol (C6–C2) moiety attached to a β-glucopyranose/β-allopyranose via a glycosidic bond. Studies indicated the ortho -dihydroxyphenyl groups were the most significant, and the steric hindrance, the number and the position of phenolic hydroxyls were also thought to play an important role 60 . Oleuropein with two hydroxyl groups is an ester of elenolic acid and hydroxytyrosol, and has a oleosidic skeleton that is common to the secoiridoid glucosides of Oleaceae 49 . The strong correlation of antioxidant property with well-identified phenolic acids, flavonoids, and oleuropein present in sage, rosemary, peppermint, rue, parsley, and olive leaves has been individually demonstrated to explain their diverse biological functions 6 , 7 , 8 , 9 , 11 , 13 . In addition, ellagic acid and tannins, defined as polyphenols, are complex chemical substances, possessing plentiful hydroxyl groups, especially ortho -dihydroxyl or galloyl groups 61 . Bigger tannin molecules appear more galloyl and ortho -dihydroxyl groups, consequently, their activities are stronger 61 . Ellagitannins, ellagic acid, and their metabolites have been reported to exhibit numerous beneficial effects on human health including antioxidant, anti-inflammatory, anti-cancer, prebiotic, and cardio-protective properties 61 . Thus they deserve to be part of a healthy diet as functional foods.
The researches on the structure–activity relationship between phenolics and their antioxidant activities have focused on phenolic acids and flavonoids, as well as oleuropein and its derivatives owing to their partially acknowledged health-promoting effects 2 , 30 . However, the benefits of medicinal and food plants may arise from the action of some less well-studied antioxidant molecules or from a synergy of certain antioxidants 30 . Cai et al. 30 found some anticancer-related medicinal plants contained higher quantities and more sorts of tannins, quinones, phenolic terpenoids and special phenolic glycosides than that of phenolic acids and flavonoids. Regarding pomegranate leaves, some authors detected kaempferol 54 or kaempferol 3- O -glycoside 33 as the main compound in ethanolic extracts, while others found as ellagic acid 55 . The principal ellagitannins of pomegranate leaves also differed from one another, considered as granatin B 56 , or castalagin derivative 33 , or undefined galloy-HHDP derivatives 55 . This difference may be induced by varieties, phenology, and growing conditions. In our study, the potent antioxidant capacity of pomegranate leaves was highly correlated with the content of tannins, which can be considered as the key antioxidant contributors of this plant material. However, the chemical structures of the tentatively identified ellagitannins were not determined, and studies on these constituents are also incomplete. Therefore, it is important to note although this is a preliminary study to provide a baseline of data for future investigations, a major limitation is that identified phyto-constituents were neither isolated, nor separately analyzed for their bioactivities. Moreover, the association between these compounds and antioxidant effect of pomegranate leaf is yet to be well understood. In this regard, it is necessary to further characterize the structure of these less-exploited phenolics (tannins) and their associated biological properties within pomegranate leaf. Hence, the results presented in our study confirm pomegranate leaf as a promising natural alternative in the development of antioxidant products, thereby assisting in the prevention and treatment of some diseases.
Conclusions
The level of different phenolic classes, antioxidant capacities and the phenolic profiles of seven medicinal and food plants were evaluated and correlated, including the leaves of sage, rosemary, olive, and pomegranate, as well as the leaves and young stems of rue, peppermint, and parsley. This study compared and demonstrated these plant extracts as valuable sources of bioactive compounds, likely for preparing novel functional products in various industries. High correlations of phenolic composition with antioxidant potential were investigated in our analysis. Different kinds of phenolic acids and flavonoids along with their derivatives were found widespread in the studied plant materials. Phenylethanoids especially oleuropein and its derivatives were characterized as the most abundant constituents of olive leaf extracts, probably contributing to its beneficial biological properties. While tannins particularly ellagitannins were supposed to be the main contributor to the features of pomegranate leaf. Interestingly, our results highlighted that the hydro-methanolic extracts of Punica granatum L. (pomegranate) leaves displayed the greatest levels of free radical scavenging capacity and ferric reducing antioxidant power, as well as the highest contents of total phenols, ortho -diphenols and tannins; a relatively high content of flavonoids was also found. Studies have increasingly evidenced the close association of tannins and less-studied compounds with antioxidant activity in medicinal and food plants 12 , 18 , 19 , 26 , 48 . Thus it is expected that richer phenolic types, namely tannins and phenolic glycosides, and their higher concentrations, are maintained in pomegranate leaves, making it possible to explore active ingredients and bioavailable products in the food-pharm, nutraceutical or cosmeceutical industries.
Moreover, only a limited number of researches have pointed out the comparison of biological activities and phenolic components of the tested plant organs, which belong to tree plants or shrub plants with large or small leaves. Many authors have stated the importance of vegetables, fruits, medicinal and aromatic plants in the current dietary patterns 2 , 3 , 4 , 5 , 29 , 30 , 50 . However, it doesn’t mean the agricultural and industrial waste like the tree leaves are useless for application. Extracts of olive leaves have attracted more attention recently, being reviewed as promising cheap, renewable and plenty source of bio-phenols for by-products. Some articles proved pomegranate leaf as a safe substrate due to its lower or inexistent toxicity 17 , 35 . In addition, ellagitannins as effective ingredients in teas are considered to be more abundant in the large-leaf tree than those from the small-leaf tree 61 , 62 . Therefore, as per olive leaf, research into finding new uses for by-products of pomegranate leaf may be proved as a strong argument for not only promoting human health but also improving bio-valorization and environment. However, samples of pomegranate leaves were not collected from different varieties or different seasons. Hence, studies on these issues would be of much interest in the future, in order to select the most promising matrix of the wasted bio-phenol materials.
Materials and methods
Chemicals and standards.
Compounds: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS ·+ ), (±)-6-hydroxy-2,5,7,8-tetramethylchromone-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhidrazyl radical (DPPH · ), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), sodium carbonate, sodium molybdate, potassium persulfate, and hydrochloric acid, all extra pure (> 99%) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Reagents: ferric chloride, methanol, aluminum chloride, sodium nitrite, all extra pure (> 99%), and methyl cellulose (1500 centipoises viscosity at 2%) were acquired from Merck (Merck, Darmstadt, Germany). Sodium hydroxide, ammonium sulfate, Folin-Ciocalteu’s reagent and acetic acid, all extra pure (> 99%) were purchased from Panreac (Panreac Química S.L.U., Barcelona, Spain). Authentic standards of phenolic compounds used in the chromatographic analysis, including that protocatechuic acid (> 97%), p -hydroxybenzoic acid (> 99%), benzoic acid (> 99.5%) were obtained from Fluka (Fluka Chemika, Neu-Ulm, Switzerland), and caffeic acid (> 98%) was from Panreac (Panreac Química S.L.U., Barcelona, Spain). Standards: neochlorogenic acid (> 95%), chlorogenic acid (> 99%), vanillic acid (> 97%), syringic acid (≥ 99%), myricitin-3- O -glucoside (≥ 99%), p -coumaric acid (> 99%), rutin (quercetin-3-rutinoside) (≥ 94%), ellagic acid (≥ 95%), ferulic acid (> 99%), apigenin-7- O -glucoside (≥ 95%), rosmarinic acid (≥ 98%), luteolin (≥ 98%), quercetin (> 95%), trans -cinnamic acid (> 95%), and kaempferol (> 90%) were purchased from Chem-Lab (Chem-Lab N.V., Zedelgem, Belgium). Gallic acid (> 97.5%), tyrosol (> 98%), caftaric acid (≥ 97%), catechin (≥ 98%), gentisic acid (≥ 98%), epicatechin (≥ 98%), 4-hydrocinnamic acid (> 95%), luteolin-7- O -glucoside (≥ 98%), isorhamnetina-3- O -glucoside (> 95%), oleuropein (> 98%), resveratrol (≥ 99%), and trans -stilben (> 96%) were acquired from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Chromatography solvents were of RP-HPLC-DAD grade according to the analysis performed. Ultrapure water was obtained using a Water Purification System (Arioso Power, Human Corporation, Seoul, Korea).
Plant materials
From about one-hundred common medicinal and food plants reported in literature references, we have selected seven medicinal and food plants (Table S1 ) in this study according to following criteria: (1) higher phenolic content and antioxidant capacity, (2) lower or inexistent toxicity. Plant species were botanically authenticated by Prof. António Crespí (Department of Biology and Environment, University of Trás-os-Montes e Alto Douro, UTAD, Portugal) and Dr. João Rocha (Chemistry Centre-Vila Real, UTAD, Portugal). Samples of each species were hand-picked randomly from a pool of individual specimens (n > 10) that are naturally growing in the Botanical Garden of UTAD (Vila Real, Portugal), which belongs to the international network of botanical gardens. Sage, rosemary, rue, peppermint, and parsley are present in the Aromatic and Medicinal Plants collection; olive is present in the Mediterranean Calcareous collection; pomegranate is present in the Garden Fruits collection (more detailed information of each plant species can be checked at http://jb.utad.pt/ ). Thus, a mixture sample for each species was obtained and used for the subsequent analysis. The collected samples were immediately dried at 40 ℃ (Drying Cabinet, LEEC, Nottingham, UK) for 72 h, before being ground into a fine powder with a blender (MB 800, KINEMATICA AG, Malters, Switzerland), and hermetically stored in the dark, at room temperature (RT) until analysis. Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material have complied with relevant institutional, national, and international guidelines and legislation.
Preparation of plant phenolic extracts
The sample powder of each species was weighed and extracted in triplicate with 40 mg of dry weight (DW). The extraction was performed by agitating (30 min, 200 rpm, RT) the mixture of the powder and 1.5 mL of a hydro-methanolic solution (methanol:H 2 O, 70:30, v/v) in an orbital shaker (GFL 3005, GEMINI, Apeldoorn, Netherlands). Afterwards, the suspensions were centrifuged (10,000 rpm, 4 ℃) for 15 min (Sigma 2-16KL Refrigerated Centrifuges, Sigma Laborzentrifugen, Berlin, Germany). The supernatants were collected in a 5 mL volumetric flask, and the solid residues were then extracted twice via the same procedure. All the three supernatants from successive extractions were kept together and the final volume came to 5 mL with the above-mentioned extraction solvent.
Content of different phenolic classes
The content of total phenols, ortho -diphenols, and flavonoids was determined by colorimetric and spectrophotometric approaches according to the literature 63 . The content of tannins was evaluated by the methyl cellulose (MC) methodology previously reported by Dambergs et al. 64 .
For the determination of total phenol content, 20 μL of diluted sample, 100 μL of diluted Folin-Ciocalteu reagent (90%, v/v), and 80 μL aqueous sodium carbonate (7.5%, w/v) were mixed in sequence. The mixture was incubated for 30 min at 42 ℃ in the dark and measured at 750 nm, using gallic acid as standard. Results were expressed in milligrams of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW).
For the assessment of ortho -diphenols content, 40 μL of sodium molybdate solution (5%, w/v) prepared with hydro-methanol (50%, v/v) was added to 160 μL of diluted extract. The mixture was stood for 15 min at RT, protected from light, before the absorbance at 375 nm was read. The content was quantified using gallic acid as standard. Results were defined in mg GAE g −1 DW.
For the quantification of total flavonoids content, 24 μL of diluted extract and 28 μL of sodium nitrite (5%, w/v) were mixed. After 5 min at RT, 28 μL of a 10% (w/v) aluminum chloride solution was added in the mixture and reacted for 6 min. Afterwards, 120 μL of sodium hydroxide (1 M) was added and the final mixture was read at 520 nm after agitation for 30 s in a microplate reader. The results were expressed in milligrams of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW).
The above-mentioned assays were undertaken with a microplate reader (Multiskan FC Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland) in 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) with a final volume of 200 µL.
The content of tannins was evaluated both in treatment and control groups simultaneously, by adding 600 μL of methyl cellulose (MC) solution (treatment) or water (control) to 200 μL of sample in a 2 mL Eppendorf. The mixture was stirred manually for 2–3 min at RT. Four hundred μL of saturated ammonium sulfate and 800 μL of water were added successively both in the treatment and control groups until 2 mL of total volume was reached. The final mixture was vortexed and kept for 10 min. After centrifugation (10,000 rpm, 16 ℃, 5 min), the absorbance was read at 280 nm, by using a conventional spectrophotometer (Helios Gamma UV Spectrophotometer, Thermo Electron Corporation, Warwickshire, UK). The absorbance of tannins was obtained by subtracting the treatment absorbance from the value registered from the control, using epicatechin as standard. The results were described in milligrams of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW).
Evaluation of in vitro antioxidant activity
The antioxidant activity of sample extracts was determined by ABTS, DPPH and FRAP (ferric reducing antioxidant power) spectrophotometric methods, reported by Mena et al. 65 , with some modifications.
The ABTS + radicals were produced by mixing 5 mL of ABTS stock solution (7.0 mM) with 88 μL of potassium persulfate (148 mM), and diluted to a working solution with sodium acetate buffer (20 mM, pH 4.5), showing an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 188 μL of ABTS working solution and 12 μL of sample dilutions (water used as blank) were mixed and reacted for 30 min at RT, and then the absorbance was read at 734 nm.
The DPPH radicals (8.87 mM) were formed with methanol (99.9%) and diluted in a working solution with hydro-methanol (70%, v/v), achieving an absorbance of 1000 at 520 nm. A mixture of 190 μL of DPPH working solution and 10 μL of sample dilutions (70% hydro-methanol used as blank) was incubated for 15 min at RT, reading the absorbance at 520 nm.
The FRAP working solution was prepared by mixing 10-volume acetate buffer (300 mM, pH 3.6), 1-volume TPTZ (10 mM dissolved in hydrochloric acid), and 1-volume ferric chloride (20 mM in water). The mixture was maintained at 37 ℃ for 10 min before use. The reaction of FRAP working solution (180 μL) and sample dilutions (20 μL) was kept at 37 ℃ for 30 min and the absorbance read at 593 nm.
The three antioxidant assays were adapted to microscale using 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) and microplate readers (Multiskan GO Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland), using Trolox as standard. All the results were expressed in millimoles of Trolox per gram of plant dry weight (mM Trolox g −1 DW).
Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) system (Thermo Finnigan, San Diego, CA, USA) was carried out to determine the (poly)phenolic profile of each plant extract, as previously described 63 . The analysis equipment is composed of three parts, including LC pump (Surveyor), autosampler (Surveyor), and PDA detector (Surveyor). Sample extracts, in triplicate, and 31 pure standard compounds (all in HPLC grade), including 17 phenolic acids, 10 flavonoids, 2 phenylethanoids and 2 stilbenoids, were prepared and filtered through 0.45 μm PVDF filters (Millex-HV Syringe Filter Unit, Merck Millipore, Bedford, MA, USA) and injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE, Aberdeen, Scotland), using a mobile phase composed of water/formic acid (99.9:0.1, v/v) (solvent A) and acetonitrile/formic acid (99.9:0.1, v/v) (solvent B). The linear gradient program (t in min and %B) was: t = 0–0%; t = 5–0%; t = 20–20%; t = 35–50%; t = 40–100%; t = 45–0%; and t = 65–0%. The injection volume was 20 μL and the flow rate was kept at 1.0 mL min −1 . UV/Vis detection was recorded from 200 to 600 nm range. Peaks were monitored at 280 and 330 nm, and identified by congruent retention time compared with standards. Data acquisition, peak integration and analysis were performed using Chromeleon software (Version 7.1; Thermo Scientific, Dionex, USA). The three extracts of each medicinal plant were chromatographed and results were expressed in milligram per liter of sample extracts (mg L −1 ).
Data and statistical analysis
All the measurements of phenolic phytochemicals and antioxidant activity of the plant extracts were conducted in triplicate. The results of phenolic content and antioxidant activity are presented as mean ± standard deviation (SD). Concentrations of individual identified phenolic compounds are presented as mean (n = 3) with the determination of the Least Significant Difference (LSD) for a p value < 0.05. The obtained data were subjected to analysis of variance (ANOVA) and a multiple range test (Tukey’s test) with IBM SPSS statistics 21.0 software (SPSS Inc., Chicago, USA). Pearson ( r ) analysis was carried out to establish correlations between phenolic chemical classes and antioxidant activity.
Li, Q. et al. Cholinesterase, β -amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 108 , 512–519. https://doi.org/10.1016/j.indcrop.2017.07.001 (2017).
Article CAS Google Scholar
Nollet, L. M. & Gutierrez-Uribe, J. A. Phenolic Compounds in Food: Characterization and Analysis 1st edn, 167 (CRC Press, 2018).
Book Google Scholar
Uritu, C. M. et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018 , 7801543. https://doi.org/10.1155/2018/7801543 (2018).
Article PubMed PubMed Central Google Scholar
Etkin, N. L. Plants and Indigenous Medicine and Diet: Biobehavioral Approaches eBook, 336 (Taylor & Francis, 2019).
Watson, R. R. & Preedy, V. R. Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion 1st edn. (Elsevier Academic Press, 2016).
Google Scholar
Alavi, M. S., Fanoudi, S., Ghasemzadeh Rahbardar, M., Mehri, S. & Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. https://doi.org/10.1002/ptr.6894 (2020).
Article PubMed Google Scholar
Ghorbani, A. & Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7 , 433–440. https://doi.org/10.1016/j.jtcme.2016.12.014 (2017).
Mahendran, G. & Rahman, L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint ( Mentha × piperita L.)—a review. Phytother. Res. 34 , 2088–2139. https://doi.org/10.1002/ptr.6664 (2020).
Shamal Badhusha, P. A. et al. Traditional uses, phytochemistry and ethanopharmacology of Ruta graveolens Linn: A review. Int. J. Pharm. Drug Anal. 20 , 1–4 (2020).
Colucci-D’Amato, L. & Cimaglia, G. Ruta graveolens as a potential source of neuroactive compounds to promote and restore neural functions. J. Tradit. Complement. Med. 10 , 309–314. https://doi.org/10.1016/j.jtcme.2020.05.002 (2020).
Şahin, S. & Bilgin, M. Olive tree ( Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 98 , 1271–1279. https://doi.org/10.1002/jsfa.8619 (2018).
Article CAS PubMed Google Scholar
Salama, Z. A. et al. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves ( Musa acuminata ) and olive leaves ( Olea europaea L.) as by-products. Res. J. Pharm. Technol. 13 , 687–696. https://doi.org/10.5958/0974-360X.2020.00132.8 (2020).
Article Google Scholar
Farzaei, M. H., Abbasabadi, Z., Ardekani, M. R. S., Rahimi, R. & Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 33 , 815–826. https://doi.org/10.1016/S0254-6272(14)60018-2 (2013).
Cefali, L. C. et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L.. Plants 8 , 453. https://doi.org/10.3390/plants8110453 (2019).
Article CAS PubMed Central Google Scholar
Mara de Menezes Epifanio, N. et al. Chemical characterization and in vivo antioxidant activity of parsley ( Petroselinum crispum ) aqueous extract. Food Funct. 11 , 5346–5356. https://doi.org/10.1039/D0FO00484G (2020).
Vučić, V., Grabež, M., Trchounian, A. & Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 25 , 1817–1827. https://doi.org/10.2174/1381612825666190708183941 (2019).
Viswanatha, G. L., Venkataranganna, M. V., Prasad, N. B. L. & Ashok, G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J. Intercult. Ethnopharmacol. 5 , 415–421. https://doi.org/10.5455/jice.20160904102857 (2016).
Article CAS PubMed PubMed Central Google Scholar
Yeddes, W., Chalghoum, A., Aidi-Wannes, W., Ksouri, R. & Saidani Tounsi, M. Effect of bioclimatic area and season on phenolics and antioxidant activities of rosemary ( Rosmarinus officinalis L.) leaves. J. Essential Oil Res. 31 , 432–443. https://doi.org/10.1080/10412905.2019.1577305 (2019).
Christova-Bagdassarian, V. L., Bagdassarian, K. S., Atanassova, M. S. & Ahmad, M. A. Comparative analysis of total phenolic and total flavonoid contents, rutin, tannins and antioxidant capacity in Apiaceae and Lamiaceae families. Indian Hortic. J. 4 , 131–140 (2014). https://www.academia.edu/15503276/Comparative_Analysis_of_Total_Phenolic_and_Total_Flavonoid_Contents_Rutin_Tannins_and_Antioxidant_Capacity_in_Apiaceae_and_Lamiaceae_families?source=swp_share
Asgharian, S. et al. Ruta graveolens and rutin, as its major compound: Investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 58 , 447–453. https://doi.org/10.1080/13880209.2020.1762669 (2020).
Aissani, N., Albouchi, F. & Sebai, H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1842894 (2020).
Farnad, N., Heidari, R. & Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint ( Mentha piperita ). J. Food Meas. Charact. 8 , 113–121. https://doi.org/10.1007/s11694-014-9171-x (2014).
Roby, M. H. H., Sarhan, M. A., Selim, K.A.-H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme ( Thymus vulgaris L.), sage ( Salvia officinalis L.), and marjoram ( Origanum majorana L.) extracts. Ind. Crops Prod. 43 , 827–831. https://doi.org/10.1016/j.indcrop.2012.08.029 (2013).
Dent, M., Dragović-Uzelac, V., Penić, M., Bosiljkov, T. & Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage ( Salvia officinalis L.) extracts. Food Technol. Biotech. 51 , 84–91 (2013).
CAS Google Scholar
Mulinacci, N. et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 85 , 167–176. https://doi.org/10.1016/j.talanta.2011.03.050 (2011).
Ramkissoon, J. S., Mahomoodally, M. F., Ahmed, N. & Subratty, A. H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 6 , 561–569. https://doi.org/10.1016/S1995-7645(13)60097-8 (2013).
Mestry, S. N., Dhodi, J. B., Kumbhar, S. B. & Juvekar, A. R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 7 , 273–280. https://doi.org/10.1016/j.jtcme.2016.06.008 (2017).
Uysal, S., Zengin, G., Aktumsek, A. & Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 22 , 518–532. https://doi.org/10.1016/j.jff.2016.02.006 (2016).
Kaewnarin, K., Niamsup, H., Shank, L. & Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 41 , 105–116 (2014).
Cai, Y., Luo, Q., Sun, M. & Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74 , 2157–2184. https://doi.org/10.1016/j.lfs.2003.09.047 (2004).
Elfalleh, W. et al. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6 , 4724–4730 (2012).
Fellah, B. et al. Untargeted metabolomics reveals changes in phenolic profile following in vitro large intestine fermentation of non-edible parts of Punica granatum L.. Food Res. Int. 128 , 108807. https://doi.org/10.1016/j.foodres.2019.108807 (2020).
Pinheiro, A. J. M. C. R. et al. Punica granatum L. leaf extract attenuates lung inflammation in mice with acute lung injury. J. Immunol. Res. 2018 , 1–11. https://doi.org/10.1155/2018/6879183 (2018).
Ulewicz-Magulska, B. & Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Food Hum. Nutr. 74 , 61–67. https://doi.org/10.1007/s11130-018-0699-5 (2019).
Ankita, P., Deepti, B. & Nilam, M. Flavonoid rich fraction of Punica granatum improves early diabetic nephropathy by ameliorating proteinuria and disturbed glucose homeostasis in experimental animals. Pharm. Biol. 53 , 61–71. https://doi.org/10.3109/13880209.2014.910533 (2015).
Generalić, I. et al. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 127 , 427–433. https://doi.org/10.1016/j.foodchem.2011.01.013 (2011).
Salwe, K. J. & Sachdev, D. Evaluation of antinociceptive and anti-inflammatory effect of the hydroalcoholic extracts of leaves and fruit peel of P. granatum in experimental animals. Asian J. Pharm. Clin. Res. 7 , 137–141 (2014).
AlFadel, F., Al Laham, S. & Alkhatib, R. The anti-bacterial activity of various parts of Punica granatum on antibiotics resistance Escherichia coli . Seeds 21 , 76 (2014).
Gheith, I. & El-Mahmoudy, A. Potent anti-oxidant and anti-inflammatory potentials of Punica granatum leaf and flower hydromethanolic extracts in vitro. Biosci. J. https://doi.org/10.14393/BJ-v33n2-33736 (2017).
Pararin, S., Rouhi, L. & Ghasemi Pirbalouti, A. The beneficial effect of hydro-alcoholic extract of Punica granatum L. leaves and flower on ethylene glycol-induced kidney calculi in RATS. J. Herb. Drugs (Int. J. Med. Herbs) 7 , 59–64 (2016).
Kiraz, Y., Neergheen-Bhujun, V. S., Rummun, N. & Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37 , 1803–1815. https://doi.org/10.1007/s13277-015-3962-5 (2016).
Rummun, N., Somanah, J., Ramsaha, S., Bahorun, T. & Neergheen-Bhujun, V. S. Bioactivity of nonedible parts of Punica granatum L.: A potential source of functional ingredients. Int. J. Food Sci. 2013 , 602312. https://doi.org/10.1155/2013/602312 (2013).
Kapp, K. et al. Commercial peppermint ( Mentha × piperita L) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 53 , 758–766. https://doi.org/10.1016/j.foodres.2013.02.015 (2013).
Meziane-Assami, D., Tomao, V., Ruiz, K., Meklati, B. Y. & Chemat, F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Method 6 , 282–288. https://doi.org/10.1007/s12161-012-9430-6 (2013).
Khaleel, I. R., Kadhim, M. I. & Subhi, J. H. Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary ( Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). Al-Nahrain J. Sci. 14 , 20 (2018).
Melnyk, M., Vodoslavskyi, V. & Obodianskyi, M. Research of phenolic compounds of ruta graveolens l and stellaria media (l.) vill. Asian J. Pharm. Clin. Res. 11 , 152–156 (2018).
Figueroa-Pérez, M. G. et al. Diabetic nephropathy is ameliorated with peppermint ( Mentha piperita ) infusions prepared from salicylic acid-elicited plants. J. Funct. Foods 43 , 55–61. https://doi.org/10.1016/j.jff.2018.01.029 (2018).
Dekanski, D. et al. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 74 , 367–377. https://doi.org/10.2298/JSC0904367D (2009).
Makowska-Wąs, J. et al. Identification of predominant phytochemical compounds and cytotoxic activity of wild olive leaves ( Olea europaea L. ssp. sylvestris) harvested in South Portugal. Chem. Biodiv. 14 , e1600331. https://doi.org/10.1002/cbdv.201600331 (2017).
Skotti, E. et al. Biological activity of selected Greek medicinal and aromatic plants extracts on Alternaria alternata. Emirates J. Food Agric. 28 , 796–804. https://doi.org/10.9755/ejfa.2016-06-618 (2016).
Arruda, M. O. et al. The hydroalcoholic extract obtained from Mentha piperita L. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. J. Immunol. Res. 2017 , 2078794. https://doi.org/10.1155/2017/2078794 (2017).
Luthria, D. L., Mukhopadhyay, S. & Kwansa, A. L. A systematic approach for extraction of phenolic compounds using parsley ( Petroselinum crispum ) flakes as a model substrate. J. Sci. Food Agric. 86 , 1350–1358. https://doi.org/10.1002/jsfa.2521 (2006).
Hozayen, W. G., El-Desouky, M. A., Soliman, H. A., Ahmed, R. R. & Khaliefa, A. K. Antiosteoporotic effect of Petroselinum crispum , Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. Bmc. Complement. Altern. Med. 16 , 165. https://doi.org/10.1186/s12906-016-1140-y (2016).
Marques, L. C. et al. Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med. 82 , 1463–1467. https://doi.org/10.1055/s-0042-108856 (2016).
Swilam, N. & Nematallah, K. A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 10 , 14851. https://doi.org/10.1038/s41598-020-71847-5 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Akkawi, M., Abu-Lafi, S. & Abu-Remeleh, Q. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 7 , 193–200 (2019).
Pinheiro, A. C. et al. Galloyl-hexahydroxydiphenoyl (HHDP)-glucose isolated from Punica granatum L. leaves protects against lipopolysaccharide (LPS)-induced acute lung injury in BALB/c mice. Front. Immunol. 10 , 1978. https://doi.org/10.3389/fimmu.2019.01978 (2019).
Lakshminarayanashastry Viswanatha, G., Venkatanarasappa Venkataranganna, M. & Lingeswara Prasad, N. B. Methanolic leaf extract of Punica granatum attenuates ischemia-reperfusion brain injury in Wistar rats: Potential antioxidant and anti-inflammatory mechanisms. Iran. J. Basic Med. Sci. 22 , 187–196. https://doi.org/10.22038/ijbms.2018.30660.7389 (2019).
Chen, J. et al. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 10 , 2611. https://doi.org/10.1038/s41598-020-59451-z (2020).
Xue, Z. & Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules (Basel, Switzerland) 21 , 991. https://doi.org/10.3390/molecules21080991 (2016).
Fraga-Corral, M. et al. Technological application of tannin-based extracts. Molecules 25 , 614. https://doi.org/10.3390/molecules25030614 (2020).
Yang, X. & Tomás-Barberán, F. A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 67 , 5394–5404. https://doi.org/10.1021/acs.jafc.8b05010 (2019).
Gouvinhas, I. et al. Monitoring the antioxidant and antimicrobial power of grape ( Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 126 , 83–91. https://doi.org/10.1016/j.indcrop.2018.10.006 (2018).
Dambergs, R. G., Mercurio, M. D., Kassara, S., Cozzolino, D. & Smith, P. A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 66 , 656–664. https://doi.org/10.1366/11-06516 (2012).
Article ADS CAS PubMed Google Scholar
Mena, P. et al. Phytochemical characterisation for industrial use of pomegranate ( Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 91 , 1893–1906. https://doi.org/10.1002/jsfa.4411 (2011).
Download references
Acknowledgements
The experiments were approved by the FCT-Portuguese Foundation for Science and Technology (PD/BD/135333/2017), under the Doctoral Programme “Agricultural Production Chains-from fork to farm” (PD/00122/2012).
This research was funded by the FCT (Portuguese Foundation for Science and Technology) Grant number UIDB/04033/2020.
Author information
Authors and affiliations.
Centre for the Research and Technology of Agro-Environmental and Biological Sciences, CITAB, University de Trás-os-Montes e Alto Douro, UTAD, 5000-801, Vila Real, Portugal
Manyou Yu, Irene Gouvinhas & Ana I. R. N. A. Barros
Chemistry Centre-Vila Real, CQ-VR, UTAD, 5000-801, Vila Real, Portugal
Department of Chemistry, School of Life Sciences and Environment, UTAD, Quinta de Prados, 5001-801, Vila Real, Portugal
Ana I. R. N. A. Barros
You can also search for this author in PubMed Google Scholar
Contributions
M.Y. carried out data analysis, wrote the manuscript, and participated in all experimental measurements. I.G. developed and performed the chromatographic analysis. J.R. supervised botanical identification and sample collection. A.I.R.N.A.B. conceived all experiments, performed theoretical calculations, and supervised data analysis and interpretation. All authors reviewed the manuscript and participated in editing the manuscript.
Corresponding authors
Correspondence to Manyou Yu or Ana I. R. N. A. Barros .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, M., Gouvinhas, I., Rocha, J. et al. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11 , 10041 (2021). https://doi.org/10.1038/s41598-021-89437-4
Download citation
Received : 04 January 2021
Accepted : 27 April 2021
Published : 11 May 2021
DOI : https://doi.org/10.1038/s41598-021-89437-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated metabolomic and transcriptomic dynamic profiles of endopleura coloration during fruit maturation in three walnut cultivars.
- Hengzhao Liu
- Huijuan Zhou
BMC Plant Biology (2024)
Rheological characteristics of wheat flour fortified with Musaceae flours and physicochemical properties of layered flat-bread (Parotta)
- K. Ashwath Kumar
- Crassina Kasar
- P. Prabhasankar
Journal of Food Measurement and Characterization (2024)
Microwave Assisted Extraction of Polyphenols from Pithecellobium dulce Benth Fruit Peels and Evaluation of its Anticancer and Antioxidant Activity
- Selvakumar Murugesan
- Prakash Maran
- Ronaldo Anuf Alexander
Waste and Biomass Valorization (2024)
Natural flavonols: actions, mechanisms, and potential therapeutic utility for various diseases
- Aar Rafi Mahmud
- Tanzila Ismail Ema
- Md. Furkanur Rahaman Mizan
Beni-Suef University Journal of Basic and Applied Sciences (2023)
Boesenbergia rotunda displayed anti-inflammatory, antioxidant and anti-apoptotic efficacy in doxorubicin‐induced cardiotoxicity in rats
- Linye Zhang
- Qihong Jiang
- Opeyemi Joshua Olatunji
Scientific Reports (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Brief Bioinform
- v.24(1); 2023 Jan

From single- to multi-omics: future research trends in medicinal plants
Lifang yang.
Kunming University of Science and Technology, China
the academician of the Chinese Academy of Engineering, studies the development of traditional Chinese medicine, Chinese Academy of Chinese Medical Sciences, China
Xiuming Cui
Associated data.
Medicinal plants are the main source of natural metabolites with specialised pharmacological activities and have been widely examined by plant researchers. Numerous omics studies of medicinal plants have been performed to identify molecular markers of species and functional genes controlling key biological traits, as well as to understand biosynthetic pathways of bioactive metabolites and the regulatory mechanisms of environmental responses. Omics technologies have been widely applied to medicinal plants, including as taxonomics, transcriptomics, metabolomics, proteomics, genomics, pangenomics, epigenomics and mutagenomics. However, because of the complex biological regulation network, single omics usually fail to explain the specific biological phenomena. In recent years, reports of integrated multi-omics studies of medicinal plants have increased. Until now, there have few assessments of recent developments and upcoming trends in omics studies of medicinal plants. We highlight recent developments in omics research of medicinal plants, summarise the typical bioinformatics resources available for analysing omics datasets, and discuss related future directions and challenges. This information facilitates further studies of medicinal plants, refinement of current approaches and leads to new ideas.
Introduction
Medicinal plants (MPs) are the main source of natural metabolites such as pigments, condiments, insecticides and medicines. MPs have been used to treat diverse diseases in China, India and Egypt for 5000 years and are still used today, despite the availability of pharmaceuticals [ 1 ]. Plant-derived monomers (morphine, artemisinin, taxol, digitali, vinblastine, etc.) are essential for chemical drug development, and mixed secondary metabolites such as total saponins and tanshinones exert strong therapeutic effects [ 2 ]. In addition, various well-known MPs, such as Panax ginseng and Panax quiquefolium , which enhance physical function and improve memory, have been widely used as supplements and in healthcare products [ 3 ].
Discovering novel and pharmacologically relevant compounds and determining their biosynthetic pathways in MPs are challenging. The continuous introduction of novel omics concepts and rapid development of sequencing technologies has greatly facilitated the comprehensive dissection of biological processes occurring in plants at the genetic, transcriptional and metabolic levels, leading to the rapid development of omics-based plant studies over the last two decades ( Figure 1 ). Meanwhile, omics studies of MPs are gradually transitioning from single- to multi-omics, the integrated multi-omics studies are becoming abundant and the number of omics-based MPs studies is increasing rapidly ( Figure 2 ). Most omics studies of MPs have focused on (i) identifying DNA and chemical markers for classifying MPs [ 4 , 5 ], (ii) locating functional genes controlling specific agronomic traits [ 6–8 ], (iii) identifying key metabolic pathways involved in the biosynthesis of active compounds [ 9–11 ] and (iv) determining the molecular mechanisms of stress responses [ 12–14 ]. These studies provide a theoretical basis for obtaining large quantities of specific compounds through synthetic biology and can enhance the molecular breeding of MPs.

Timeline for omics technology development and typical omics-based plant studies over the past two decades. The proposal of omics concepts is shown in yellow, key events related to the development of omics technologies are indicated in green boxes and typical omics-based plant studies were illustrated by blue. Abbreviations: NGS, next-generation sequencing; SMRT, single molecule real-time; MSI, mass spectrometry imaging; Smart-seq, switching mechanism at 5′ end of the RNA transcript sequencing; ATAC-seq, assay for transposase-accessible chromatin with high-throughput sequencing; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/CRISPR-associated9; ONT, Oxford Nanopore Technology.

Summary of research pattern and bibliometrics of omics studies on medicinal plants. (A) The pattern of omics studies on medicinal plants: (I) Taxonomics mainly involves identification and classification of medicinal plants based on phenotyping, DNA markers and chemical markers. (II) Transcriptomics studies contain bulk RNA-seq, single-cell RNA-seq (scRNA-seq), spatial RNA-seq (spRAN-seq), as well as degradome and ncRNAs. (III) Metabolomics mainly involves targeted, widely targeted, untargeted metabolome and spatial metabolome studies on secondary metabolites. (IV) Proteomics focuses on structures, functions and protein–protein interaction of protein molecules. (V) Genomics can be divided into structural and functional genomics studies. (VI) Pangenomics lays particular emphasis on the effects of SNPs, indels and SVs. (VII) Epigenomics mainly involves three aspects: DNA methylation, histone modification and chromatin remodeling. (VII) Mutagenomics aims at gaining desired species by random mutagenesis, targeted genome modifications and reverse genetics strategy. (B) The number of articles of omics studies on medicinal plants published from 2000 to 2022 from PubMed database. Keywords of medicinal plant taxonomic, transcriptome, metabolomic, proteomic, genomic, pangenome, DNA methylation and mutagenesis are searched, under the Title/Abstract term in the query box.
Here, we comprehensively review recent advances and future trends in omics studies of MPs to promote the development of novel ideas and methods related to integrated multi-omics research.
Phenotypes and DNA markers are used in taxonomy
Phenotyping is the most intuitive approach for identifying and classifying plants but is time-consuming, laborious and often destructive to plants. High-throughput phenotyping platforms with high-resolution, advanced sensors and fully automatic data collection systems are promising advancements in plant phenotyping [ 15 ]. Bioinformatics tools and image databases have also been developed for handling the massive amounts of phenotypic data and plant images collected using high-throughput phenotyping platforms ( Table 1 ; [ 16 , 17 ]).
The list of typical bioinformatics resources available for omics studies on medicinal plants
URL, uniform resource locator;Null represents no URL or Reference.
However, delimitation of certain taxa derived from congeneric species is difficult because of the existence of morphological intermediates. Therefore, many DNA barcodes such as 5S ribosomal RNA, 18S ribosomal RNA, internal transcribed spacer, matK , rbcL , trnH-psbA and trnL-F have been widely applied since 2008 for analysing the taxonomy of MPs [ 4 ]. In addition, specific types of DNA markers, such as single-nucleotide polymorphisms (SNPs) and simple sequence repeats, can be used to identify MPs. Currently, an interactive database of DNA barcodes from medicinal materials is regularly updated to support medicinal material identification and MP taxonomy studies [ 18 ]. Combining DNA barcodes with metabolomics data has been recommended for more accurately taxonomizing MPs and identifying their subspecies or varieties [ 12 , 59 ].
The availability of bioinformatics resources for taxonomic studies of MPs remains limited; thus, it is necessary to construct a standardised taxonomic system that combines phenotypic images with DNA markers and specific metabolites. Accurate taxonomic classification of MP species can not only confirm the authenticity of medicinal raw materials but also ensure the high quality of medicinal products produced from these materials.
Transcriptomics is the most widely used approach for studying gene expression
Transcriptomics can be divided into microarrays based on hybridization and RNA sequencing (RNA-seq) based on sequencing methods. The major difference between these approaches is that microarray can only detect the expression levels of known genes in samples, whereas RNA-seq can detect the expression information of all genes. In microarray analysis, the roles of specific mRNAs and microRNAs (miRNAs) can be determined under given stress conditions and identify molecular markers of specific compositions in plants [ 60 , 61 ]. RNA-seq can provide a dynamic genetic map of the spatiotemporal expression patterns of genes in different parts and developmental stages of plants. The transcriptomes of MPs with multiple medicinal parts have been sequenced using next-generation sequencing (NGS) platforms to investigate the organ- and tissue-specific expression patterns of genes [ 62–64 ]. Dynamic transcriptional changes in MPs under different stress conditions [ 65 ] and at different developmental stages [ 66 ] have been extensively studied. Compared with NGS, long-read sequencing technologies, such as PacBio and Oxford Nanopore Technologies, can reveal the complexity of transcriptomes, including post-transcriptional modifications, alternative splicing and fusion transcripts; thus, combining NGS and PacBio platforms can provide a finer transcriptome landscape of complex gene expression [ 67 ]. Two mainstream methods for transcriptome assembly, the combination of HISAT and StringTie [ 19 ] and Trinity [ 20 ], are applicable to the availability and non-availability of reference genomes, respectively. Currently, two databases of plant transcriptome data, PPRD and ARS, have important reference value for studying MP transcriptomes ( Table 1 ; [ 21 , 22 ]).
Novel advancements in technology have improved the resolution of transcriptomic research from bulk RNA-seq to single-cell RNA-seq (scRNA-seq). Although limited by reference genomes and current technologies, scRNA-seq has been applied in plants such as Zea mays , Oryza sativa , Solanum lycopersicum and Arabidopsis thaliana , and a single-nucleus transcriptome atlas of S. lycopersicum and A. thaliana was reported [ 68 ]. Application of scRNA-seq and target genome-editing techniques has been proposed for supporting precise crop breeding, as clustered regularly interspaced short palindromic repeat droplet sequencing (CRISPR-seq) depends on a guide RNA vector with a unique barcode that can be detected using scRNA-seq [ 69 ]. Moreover, scRNA-seq can be combined with transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) for multi-omics co-labelling, which can simultaneously capture information on transcripts and open chromatin from each cell. A pre-trained deep learning-based method, scDeepSort, can be used to annotate cell types in single-cell transcriptomic datasets [ 23 ]. The online tool Plant Single Cell Transcriptome Hub and continuously updated PlantscRNAdb were developed for plant scRNA-seq research [ 24 , 25 ]. These resources promoted scRNA-seq studies on MPs and pave the way for the combined application of scRNA-seq with other omics or techniques.
Spatial transcriptome sequencing (spRNA-seq) can compensate for the loss of spatial location information of cells evaluating using scRNA-seq. The first spatially resolved transcriptome profile of plant fields was obtained from A. thaliana in 2017 to determine the processes involved in plant development and evolution [ 70 ]. Subsequent spatial transcriptome studies of peanut tissue suggested that the spatial information of cells, independent of marker genes, is more useful for non-model species [ 71 ]. A spatiotemporal atlas of organogenesis of orchid flowers revealed that floral organ development is co-regulated by numerous specialised genes that function in different tissues and developmental stages [ 72 ]. Two spRNA-seq platforms (10X Visium, 10X Genomics, Pleasanton, CA, USA; and GeoMx DSP, NanoString Technologies, Seattle, WA, USA) have been commercially available since 2019; however, these platforms cannot achieve single-cell resolution. Subsequently, an excellent computational method, CellTrek, was developed that combines two datasets to perform single-cell spatial mapping [ 26 ]. Thus, a combination of spRNA-seq and scRNA-seq can accurately depict the spatiotemporal developmental trajectory and biological functions of certain cells of interest in MPs. In addition, a database for spatially resolved transcriptomes, SpatialDB, provides a repository for researchers studying the spatial cellular structure of tissues and the cellular microenvironment [ 27 ].
Degradome and non-coding RNAs (ncRNAs) sequencing, another direction for transcriptome data research, provides abundant information on RNA degradation, miRNAs and long ncRNAs and contributes to the identification of miRNA-mediated cleavage of target genes and functional studies of ncRNAs [ 73 ]. Combined analysis of degradome sequencing and miRNA profiles has been widely applied in MP research [ 14 , 74 ]. Corresponding bioinformatics tools and databases, such as psRNATarget, PLncPRO, PcircRNA_finder, PAREameters and MepmiRDB, have been developed to identify and determine the functions of novel ncRNAs in plants ( Table 1 ; [ 28–32 ]).
Metabolomics defines end-products of gene expression
The metabolome is a direct determinant of the authenticity and quality of MPs. Currently, studies based on metabolomics are focused in targeted, widely targeted and untargeted directions. The targeted metabolome is a suitable choice for distinguishing crude medicinal materials from congeneric species in compound preparations of traditional Chinese medicines [ 75 ]. A widely targeted metabolome study of Pueraria lobata and its varieties suggested that differences in the nutritional value among these species can be explained by changes in nutrient abundance, whereas medicinal quality can be assessed according to the contents of secondary metabolites [ 76 ]. Sixteen key metabolites useful for distinguishing different Ficus deltoidea varieties were identified in untargeted metabolome analysis and were stable regardless of the growth environment and geographical origin [ 77 ]. Therefore, chemical markers are important factors for MP authentication, whereas the contents of specific metabolites can be used to evaluate the quality of medicinal raw materials. Metabolite profiling of mutagenic lines with loss- or gain-of-function genes reveal specific metabolites that are synthesized under the control of target genes, thereby bridging the gap between genes and metabolites. In addition, a metabolomics-oriented reverse genetic approach can be used to further explore the genes responsible for the chemical structure diversity of secondary metabolites [ 78 ]. Therefore, analysis of biosynthetic regulation cascades involved in active metabolite production in MPs, as the first step toward molecular breeding and synthetic biology, has been largely driven by metabolomics-based analyses.
A deep learning framework, CRISP, was developed to identify, simulate and analyse contour regions of interest in metabolomic maps [ 33 ]. MAPPS is useful for metabolic network analysis and pathway prediction, whereas MetaboAnalyst 5.0 is a user-friendly platform for analysing raw metabolomics data and exploring metabolite functions [ 34 , 35 ]. The enormous structural diversity of plant-derived compounds suggests that medicinally relevant compounds can still be discovered in plants. METLIN, a highly annotated database containing over 850 000 molecular standards, is useful for screening plant-derived bioactive compounds [ 36 ].
Spatial metabolomics overcomes the limitations of bulk metabolomics and can accurately determine the types, contents and spatial distributions of metabolites, and then characterise the chemical makeup of a tissue or organ at spatial resolution [ 79 ]. Thus, spatial metabolomics can provide abundant spatial distribution albums of metabolites and achieve ‘real-time reporting’ of the metabolome in organisms. The in situ presentation and spatiotemporal transformation of metabolites can simplify various biological problems in MPs, such as the biosynthetic pathways of natural metabolites [ 80 ] and fruit development [ 81 ]. Combining spatial metabolomics with spRNA-seq is an exciting approach for investigating biological processes in specialised cell types of MPs.
Proteomics: a hub linking the transcriptome and metabolome
As proteins are directly involved in performing and controlling almost all biological processes, proteomics is essential for understanding the regulatory mechanisms responsible for the development and secondary metabolism of MPs [ 82 ]. iTRAQ quantitative proteomics of Rehmannia glutinosa roots revealed that many prenyltransferase present higher expression level at the expansion and maturation stage than the elongation stage [ 83 ]. Label-free quantitative proteomic study on P. ginseng leaves under heat revealed the molecular mechanism of stress and the influences of ginsenoside production at proteins level [ 84 ]. Proteins expressed in Chrysobalanus icaco , Bauhinia variegata and Bauhinia forficata have also been characterised and differentiated to determine the differences in their medicinal properties [ 85 ]. Notably, a recent study suggested that plant odorant-binding proteins bind specific metabolites, leading to changes in transcription activation, gene expression, protein function and metabolism, and play important roles in plant communication and defensive responses, which inspires researchers to further think about that whether the production and accumulation of desired metabolites can be induced by changing the expression and function of specific odourant-binding proteins [ 86 ]. The biological functions of a protein depend not only on the linear arrangement of the amino acid sequence but also on its spatial structure; post-translational modifications also have diverse effects on the activity and function of protein molecules [ 87 ].
Prosit is a proteome-wide prediction network based on deep learning that can enable larger numbers of identifications at >10x lower false discovery rates [ 37 ]. PiNET—a versatile web platform—is used for downstream analysis of proteomic data and visualisation of the results [ 38 ]. To date, there is no protein database specific for MPs; however, comprehensive protein databases, such as the continuously updated PRIDE and PPDB, are available for functional studies of proteins in MPs ( Table 1 ; [ 39 , 40 ]). A breakthrough in protein-structure prediction, the AlphaFold protein-structure database, an artificial intelligence (AI) system developed by DeepMind, enables state-of-the-art predictions of protein structures based on their amino acid sequences, allowing biomedical researchers to obtain 3D structural models for almost any protein sequence [ 41 ]. In addition, protein–protein interaction networks are useful for functional studies of proteins, in which protein functions can be inferred based on interactions between known and unknown proteins [ 88 ]. Information on protein–protein interactions in plants has been deposited in the STRING and BioGRID databases, which are open to the public for MP investigations ( Table 1 ; [ 42 , 43 ]).
Structural and functional genomics
Structural genomics relies on molecular markers that are available for tagging and mapping of candidate genes related to species traits. Currently, quantitative trait locus (QTLs) mapping and genome-wide association studies (GWAS) are the two most important approaches for studying traits in plants. QTLs has been widely applied in MPs to link complex phenotypes of interest to specific regions on chromosomes and then identifying the number, locations, interactions and functions of these regions [ 7 , 89 ]. GWAS focus on detecting genetic variations in multiple individuals from a population to determine genotypes, followed by statistical analyses between genotypes and phenotypes at the population level to screen genetic variations most likely to affect traits of interest. This method has been applied to evaluate the genes controlling the stem thickness and dry root weight of P. notogensing [ 8 ], amorpha-4,11-diene synthase gene expansion and ultimately results in higher artemisinin content [ 90 ] and high α-linolenic acid content in the seed oil of Perilla [ 91 ]. Studies of the relationship between the traits and genotypes of MPs based on GWAS and QTLs have contributed to subsequent utilisation of functional genomics in molecular breeding and genetic improvement.
After plant genome resources became available, a combination of genomics and breeding techniques resulted in development of the novel concept of ‘genomics-assisted breeding’ for crop improvement in 2005 [ 92 ]. The advent of NGS has greatly improved the throughput of genome sequencing, and the introduction of long-read sequencing and Hi-C has enabled chromosome-level genome assembly and research. The genome of Cannabis sativa was sequenced on Roche/454 (Basel, Switzerland) and Illumina (San Diego, CA, USA) platforms in 2011 [ 93 ], and that of Dendrobium officinale was sequenced on Illumina and PacBio (Menlo Park, CA, USA) platforms in 2015 [ 94 ]. Specifically, the number of chromosome-level genomes from various MPs, such as P. notoginseng [ 9 ], Artemisia annua [ 90 ], Opium poppy [ 95 ], Medicago sativa [ 96 ] and Bletilla striata [ 97 ], has sharply increased in the last few years. These studies suggest that chromosome-level genomes are important for delineating biological processes occurring in MPs, as they can be used to reduce the negative effects caused by false and incomplete genome assembly. Notably, gene duplication, rearrangement, introgression and fusion events may have directly relationship with the specialised secondary metabolites [ 95 ]. Thus, functional genomics is a prerequisite for the precise molecular breeding of MPs to improve their medicinal traits [ 97 ]. In addition, some pivotal transcription factors are indispensable for regulating the biosynthesis of active compounds in MPs [ 98 ].
SVision was developed to resolve complex structural variations (SVs) in the genome [ 44 ], and online bioinformatics tools and continually updated genome databases [ 45 , 46 ] have provided important support for genomic studies of MPs ( Table 1 ).
Pangenomics focuses on the dynamic genome
With the increasing of genomic studies, researchers realized that a single reference genome is insufficient to represent the genetic diversity of a species. Notably, a comparative genomic study of four Panax species illustrated how reshuffling of the ancestral core-eudicot genome results in a highly dynamic genome and causes metabolic diversification of extant eudicot plants [ 99 ]. Thus, a new era of pangenomic studies of MPs has emerged. The concept of the pangenome was initially proposed in 2005 and applied to bacteria to account for intraspecific variability. Pangenome refers to collection of all genes in a specific species, these genes can be divided into the core genes shared by all individuals and the dispensable genes present in a specific individual. Currently, pangenome studies of crops such as rice, maize, tomato, cucumber, wheat and soybean have demonstrated that dispensable genes are vital for maintaining the genetic diversity of species, because dispensable genes exhibit higher variability compared with core genes and contain higher-density SNPs and indels [ 100 , 101 ]. Large-scale structural variations (SVs), including copy number variants and presence/absence variants (PAVs) at the population level, are currently the most important focus of crop pangenome studies [ 102 ]. SVs directly affect dispensable genes in the pangenome of a species; these genes tend to be responsible for specific plant traits such as fruit traits, flowering time and seed size, environmental adaptation and disease resistance [ 103 ]. Moreover, SVs can be used to study gene expression divergence and quantitative trait variations, whereas PAVs can be used as markers in GWAS studies. Bioinformatics tools have also been developed for pangenome analysis ( Table 1 ; [ 47 , 48 ]). In addition, a comparative pangenomics database, GreenPhyIDB v5, was constructed for investigating gene families and homologous relationships among plant genomes [ 49 ].
Assembly of the plant genome and pangenome is challenging because of the occurrence of general polyploidization and presence of large number of repetitive sequences. However, long-read sequencing technologies are powerful for pangenome construction in plants with large genome sizes and can span complex repetitive regions in the genome to identify large-scale SVs. Notably, by combining differential gene identification and CRIPSR/Cas9, enables gene functions can be comprehensively dissected and validated. Pangenomic studies of crops have provided valuable references for constructing MP pangenomes. Pangenomes are expected to gradually replace single reference genomes and become a new standard for studying evolutionary clades and genetic variations in plants and MPs.
Epigenomics is an important supplement to genomics
Epigenetics involves changes in heritable traits caused by DNA methylation, histone modification and chromatin remodeling. Studies of epigenetic phenomena can be carried out on a genome-wide scale; thus, a new omics, epigenomics, combining epigenetics with genomics, has been developed [ 104 ]. Epigenomic studies have been performed to analyse epigenetic events occurring during the growth and development of plants, and to evaluate abnormalities caused by stress [ 105 ]. In addition, divergence in epigenetic regulation during polyploidization has led to high biochemical diversity among secondary metabolites in the Panax genus [ 99 ]. Since the DNA methylation pattern of the A. thaliana genome was reported in 2008 [ 106 ], DNA methylation studies have gradually become universally conducted to evaluate MPs. The pleiotropic roles of DNA methylation in MPs have been reviewed in detail [ 107 ]. Chromatin immunoprecipitation sequencing (ChIP-seq) can reveal information on histone modifications in studies of plant development and environmental memory [ 108 ], and ATAC-seq can be used to analyse genome-wide chromatin accessibility to explore the possible mechanisms of plant environmental adaptability [ 109 ]. Therefore, ChIP-seq and ATAC-seq are complementary methods that show highly consistent results [ 110 ]. Furthermore, ATAC-seq and RNA-seq can be combined to study differentially regulated transcription factors in key biological processes in plants [ 111 ]. The machine learning-based method chromatin interaction neural network (ChINN) is useful for predicting chromatin interactions based on DNA sequences, and PlantPan3.0 can be used to analyse the results of ChIP-seq experiments on MPs [ 50 , 51 ].
Currently, epigenomics is widely used to study epigenetic phenomena and the underlying epigenetic modification events in MPs. Several studies suggested that epigenetic modifications are closely related to the phenotypic traits of MPs and biosynthetic processes of secondary metabolites. These findings are expected to be applied in epigenetic engineering.
Mutagenomics for obtaining plant species with desired variations
Mutagenesis is one of the most effective approaches for obtaining species with desired variations and primarily involves random mutagenesis and targeted genome modifications. Random mutagenesis can produce many mutant individuals with diverse traits but requires large-scale screening, which is typically time-consuming and laborious because of the randomness of mutations. In the last two decades, several breakthroughs have been made in the genome-editing field, particularly in the CRISPR/Cas9 system, which is a site-directed mutagenesis technology for introducing targeted genome modifications. Using this system, targeted genome modifications were made in rice, tobacco and sorghum as early as 2013 [ 112 ]. Subsequently, this system was implemented in MPs ( S. miltiorrhiza , O. poppy , Camelina sativa and Dioscorea zingiberensis ) to produce pharmacologically bioactive metabolites through fine-scale targeted mutagenesis [ 113 ]. Transgenic herbal raw materials cannot be commercialised at present because of the specific nature of MPs (transgene introgression may lead to unpredictable changes in components and properties of herbal materials); thus, transgene-free genome editing may be important for avoiding transgene incorporation [ 114 ]. Transgene-free genome editing based on CRISPR/cas9 may be an optimal choice for improving the quality and yield of valuable MPs and achieving commercialisation. Notably, a machine learning-based approach, CRISPRidentify, can detect and differentiate true from false CRISPR arrays, greatly facilitating the application of CRISPR/Cas9 [ 52 ].
For genes with known functions, targeted genome modification is an excellent approach for rapidly and accurately obtaining a desired species. For genes with unknown or uncertain functions produced using genome sequencing and random mutagenesis, reverse genetics technologies can reveal associations between the differential genes and their functions and subsequently verify the functions of candidate genes. Integrated application of functional genomics and mutagenomics is currently the best approaches for improving species traits. Although mutagenomics has not been as widely used in MPs as in crops, its use in MP species is expected to increase with continuous improvements in MP genome resources and rapid development of suitable transformation and regeneration approaches.
Multi-omics studies of medicinal plants are the future development trend
Rapid development of omics technologies is a prerequisite for successfully performing advanced omics studies. However, each omics technology, such as transcriptomics (including microarray technology, bulk RNA-seq, scRNA-seq and spRNA-seq), metabolomics (including bulk metabolomics and spatial metabolomics), proteomics (including iTRAQ quantitative and label-free quantitative technology) and genomics (including NGS and long-read sequencing technologies) has inherent advantages and disadvantages ( Table 2 ). Therefore, integrated analysis of multi-omics datasets, such as the integration of scRNA-seq and spRNA-seq, spRNA-seq and spatial metabolomics, bulk RNA-seq and metabolomics, and RNA-seq and proteomics, can compensate for the limitations of other methods when comprehensively studying biological processes. Currently, omics studies of MPs are gradually transitioning from single- to multi-omics, which has provided more comprehensive insights into biological processes of interest. Integrated multi-omics studies of MPs have mainly focused on four factors ( Figure 3 ). First, combined analysis of phenotypes, DNA markers and metabolomic data enables the accurate identification of MPs and processed medicinal materials [ 59 , 115 ]. Second, functional genes controlling the key agronomic traits of MPs can be located by linking extrinsic phenotypes to intrinsic genotype control [ 6 , 7 , 116 ]. Combining GWAS with other omics techniques may contribute to the identification of functional genes regulating complex traits [ 117 ]. Third, multi-omics integration can reveal the biosynthetic pathways of secondary metabolites in MPs [ 9–11 , 65 ]. Notably, integration of omics with gene editing tools is useful for the development of precision plant breeding [ 117 ]. Finally, multi-omics integration can explain the regulatory mechanisms involved in the responses of MPs to stress [ 12 , 13 , 118 ]. With the increasing diversity of omics technologies, researchers often obtain different types of omics datasets derived from the same or different samples, providing highly scientific and reliable access to specific biological processes in MPs. However, these findings also create challenges for the integrated and associated analysis of multiple omics data types.
Advantages and disadvantages of the leading technologies for omics

The application summary of integrated multi-omics approaches in medicinal plants. It is mainly involved in four aspects: (i) identifying medicinal plants species by integration of phenotype and DNA markers or chemical markers (purple box); (ii) locating function genes by combination of transcriptomics with degradome and ncRNAs, function genomics with mutagenomics, phenotype with structural genomics (green box); (iii) unearthing metabolic pathways by the integration of transcriptomics and genomics, proteomics, metabolomics, as well as the combination of genomics with transcriptomics and epigenomics (blue box) and (iv) unveiling regulation mechanisms response to stress by integration of transcriptomics and metabolomics, and physiological indices (red box).
Several bioinformatics tools for integrating and analysing multi-omics datasets were recently developed [ 53–56 ]. MPOD and 1 K-MPGD are specific for multi-omics studies of MPs, and will be continuously updated to provide long-term support for combined analysis of multi-omics datasets ( Table 1 ) [ 57 , 58 ]. Furthermore, data obtained using integrated multi-omics approaches can complement and validate each other when investigating changes in certain biological processes, making the analytical results more comprehensive and credible. Integrated multi-omics approaches will be widely applied in MP research to understand specific biological processes.
Recent developments in diverse omics technologies have provided an unprecedented opportunity for plant researchers to obtain considerable biological knowledge through integrated analysis of multiple omics datasets. Genomes, transcriptomes, proteomes, metabolomes and other omics datasets derived from various MPs have been reported, and corresponding bioinformatic tools and databases have been developed. Integrated analysis of multi-omics datasets is highly comprehensive for investigating MPs. Results based on multi-omics datasets not only provide a foundation for obtaining MP species with high yield, good quality and disease resistance through molecular breeding but also provide a theoretical basis for achieving steady biotransformation of desired secondary metabolites through synthetic biology. Notably, it is now feasible to identify functional genes controlling key biological traits and determine the catalytic mechanisms of key enzymes involved in biosynthetic pathways of active compounds by performing multi-omics and bioinformatic studies. However, there are many unsettled issues in genome editing and the knockout or overexpression of functional genes for MPs because of the lack of suitable transformation and regeneration approaches. Synthetic biology involves strain improvement, microbial system development and reconstruction and optimisation of metabolic models suitable for specific metabolite types, which are very challenging.
Although MPs have been widely examined in omics studies, further detailed examination is required. There have been few scRNA-seq and spRNA-seq studies of MPs because of the limitations of reference genomes and technologies. Furthermore, transgene-free genome modifications based on the CRISPR/Cas9 system have not been widely applied to MPs, as suitable transformation and regeneration approaches are lacking. Increasing evidence has shown that epigenetic modifications have non-negligible effects on gene expression; however, there are fewer epigenomic studies of MPs than of crops. In addition, ncRNAs play important roles in regulating gene expression; however, there is only one miRNA database specific for MPs, and no database exists for circRNAs and long ncRNAs in MPs. Finally, it remains challenging to integrate different results from multiple omics research, establish correlations between results and provide reasonable explanations for causalities because of differences in the representation of different omics datasets, particularly for more than three omics data types. The lack of bioinformatic tools and omics databases limits the interpretation of specific phenomena, inhibiting the understanding of certain biological processes. Therefore, more comprehensive bioinformatics sources for integrated analysis and visualisation of different omics datasets are urgently needed. Although a wide range of integrative bioinformatics tools have been proposed for analysing multi-omics datasets, biological interpretation is difficult because of the limitations of the tools themselves. Notably, machine learning and artificial intelligence are promising approaches for integrating and analysing multi-omics datasets based on their predictive performance, flexibility and capability to capture hierarchical and nonlinear features [ 119 ].
An increasing number of studies of MPs will lead to further omics databases and bioinformatics tools, enabling research to progress from single- to multi-omics. Integrated multi-omics studies on MPs are expected to expand and facilitate the development of molecular breeding of MPs as well as synthetic biology approaches.
Abbreviations
- We summarise research advances and future trends in current mainstream omics approaches in medicinal plants, including taxonomics, transcriptomics, metabolomics, proteomics, genomics, pangenomics, epigenomics and mutagenomics.
- We review typical bioinformatics tools and databases available for omics dataset analysis of medicinal plants.
- We highlight the integrated patterns of multi-omics studies of medicinal plants and discuss associated prospects and challenges.
- Omics studies of medicinal plants are gradually transitioning from single to multi-omics because of large advantages in integrated multi-omics.
Supplementary Material
Figure_information_bbac485.
Lifang Yang is a PhD student at Kunming University of Science and Technology, China. She is interested in the bulk RNA-seq, scRNA-seq and metabolomics studies on Panax genus .
Ye Yang is a professor at Kunming University of Science and Technology, China. His research interests mainly involve the molecular mechanisms of plants response to stresses.
Luqi Huang , the academician of the Chinese Academy of Engineering, studies the development of traditional Chinese medicine, Chinese Academy of Chinese Medical Sciences, China.
Xiuming Cui is a professor at Kunming University of Science and Technology, China. His research interests include sustainable development of germplasm resources of Panax genus.
Yuan Liu is an associate professor at Kunming University of Science and Technology, China, and is interested in developing bioinformatics analysis software and studying medicinal plant genomes.
Contributor Information
Lifang Yang, Kunming University of Science and Technology, China.
Ye Yang, Kunming University of Science and Technology, China.
Luqi Huang, the academician of the Chinese Academy of Engineering, studies the development of traditional Chinese medicine, Chinese Academy of Chinese Medical Sciences, China.
Xiuming Cui, Kunming University of Science and Technology, China.
Yuan Liu, Kunming University of Science and Technology, China.
Authors’ contributions
L.Y. wrote the manuscript; L.H., Y.Y. and X.C. provided valuable advice for the manuscript and Y.L. conceived the initial idea and reviewed the manuscript.
This work was supported by the Yunnan Major Scientific and Technological Projects [grant number KKAN20222025]; National Natural Science Foundation of China [grant number 31960134]; Establishment of Sustainable Use for Valuable Chinese Medicine Resources [grant number 2060302] and Major Science and Technology Special Project of Yunnan Province [grant number 202102AA310034].

- Proceedings
- Conferences
- Submit Manuscript
Journal of Medicinal Plants Research
- Abbreviation: J. Med. Plants Res.
- Language: English
- ISSN: 1996-0875
- DOI: 10.5897/JMPR
- Start Year: 2007
- Published Articles: 3829
- Instructions
- Articles In Press
Table of Content: May, 2023; 17(5)
- Sort by: Date
Sedative and hypnotic effects of the compatibility of Schisandra chinensis and Polygala tenuifolia on insomnia mice
The objective of this study is to study the sedative and hypnotic effects of the compatibility of Schisandra chinensis (SC) and Polygala tenuifolia (PT) on insomnia mice and its mechanisms. The 160 SPF KM mice were divided into 8 groups with 20 each group (10 female and 10 male mice), including control, model, estazolam, SC, PT, SC-PT 1:1, SC-PT 1:2, and SC-PT 2:1 group. Insomnia mice model was established with...
Author(s): Haifeng Sun, Han Wang, Na Li and Hongyu Yang
- https://doi.org/10.5897/JMPR2022.7260
- Article Number: 0B1D70B70679
Citrullus lanatus plant response to irrigation water magnetized in sandy soil
In this study, the productivity of the red watermelon (Citrullus lanatus) crop in arid regions with saline water is examined in relation to the effects of magnetic treatment of irrigation water. The greenhouse experiments compared two treatments (magnetic treatment of irrigation water) and (no magnetic treatment of irrigation water). Magnetic treatment device delta water with a power of 14500 gausses (1.45 T) that was...
Author(s): Djilani GHEMAM AMARA, Hacene LAOUEDJ, Ahmed Elkhalifa CHEMSA, Khaled KHERRAZ, Said TOUATI, Zeïd ALIA, TOUATIHAMAD larouaci, Ahmed IBEDand Hamza BOUGRINAT
- https://doi.org/10.5897/JMPR2022.7283
- Article Number: 49BF0C470683
Palyno-morphological assessment of Asteraceae taxa grown in Qarshi Botanical garden at Qarshi Industries (Pvt.) Ltd by using LM and SEM, Pakistan
The present study covers the micro-morphological characteristics of 32 taxa of Asteraceae family from Qarshi Botanical Garden at Qarshi Industries (Pvt.) Ltd., Hattar Haripur, Pakistan. The morpho-palynological characters studied were size, shape, polar and equatorial diameter and their exine ornamentation using light microscopy (LM) and scanning electron microscopy (SEM) for its taxonomic significance. In the present...
Author(s): Siraj Khan,, Sohail Anwer,, Muhammad Rashid,, Majid Iqbal, and Shabeer Ahmad,
- https://doi.org/10.5897/JMPR2022.7281
- Article Number: CEA6AE870699
HPLC determination of polyphenols of the flowers of Digitalis lamarckii, Xeranthemum annuum, Epilobium hirsutum and Silene compacta from Bolu (Turkey)
There have been a growing interest since decades in the medicinal plants in terms of preventive health care and treatment of various diseases. On the other hand, polyphenolic compounds present in medicinal plants are known to have antioxidant potency. In the current work, the quantitative HPLC analysis of the flowers of four plants grown in Bolu-Turkey, Silene compacta, Digitalis lamarckii, Xeranthemum annuum L., and...
Author(s): Nedime Dürüst, Yasar Dürüst, Nursel Ikinci, Seray Banko, Emir Zafer Hosgün and Berrin Bozan
- https://doi.org/10.5897/JMPR2022.7282
- Article Number: 15297AF70723
Evaluation of catechin, lupeol, and betulinic acid as markers for the chromatographic quality Control of Albizia coriaria raw materials; an experimental study
Albizia coriaria stembark is among the most common raw materials used to manufacture herbal products in Uganda. While the plant material is sourced from the wild, there are neither monographs nor chemical analytical methods for its standardisation. In addition, good cultivation, harvesting, and manufacturing practices are not mandatory. This leads to inconsistent quality of raw materials and products. This work...
Author(s): Bruhan Kaggwa, Edson Ireeta Munanura, Henry Kyeyune, Godwin Anywar, Hedmon Okella, Clement Olusoji Ajayi, Raphael Wangalwa, John Mulangwa, Crispin Duncan Sesaazi, Lynn K. Bagoloire, Casim Umba Tolo, Pakoyo Fadhiru Kamba and Patrick Engeu Ogwang
- https://doi.org/10.5897/JMPR2022.7285
- Article Number: 295FACA70745
- Open Access
- Creative Commons
- CrossMark policy
- Publication Ethics
- Peer Review
- Editorial Policies
- Reviewers Guidelines
- Publication Fees
- Waiver Policy
- Digital Archiving
- Self-Archiving
- Article Copyright
- Visit JMPR Facebook Page
Advertisement

Journal of Medicinal Plants

Subject Area and Category
- Complementary and Alternative Medicine
- Pharmacology
Publication type
Information.
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
- Remember me
- Create Account
- Reset Password
- About the Journal
- Editorial Board
- Aims& Scopes
- Journal News
- Current Issue
- Categorization of Subject Articles
- Submission Instruction & Guide to Submitting an Article
- Submission Form
- Publication Fee
- Guide to Send the Corrected Article (After Applying the Reviewers Comments)
- Key Points in Writing Articles
- Reviewers Section
- Decision Questions
- Registration Form
- Personal Page (User Panel)
- Contact Information
- Contact Form
- Ethical Consideration
- Advanced Search
- The Best of the Site
Journal of Medicinal Plants
Has a Scientific Research license from the Medical Sciences Publications Commission of the Country under Number 13375 dated 2002.11.09
2023، Volume 22، Number 87
Print ISSN: 2717-204X Electronic ISSN: 2717-2058
- Director-in-Charge: Dr. Reza Ghafarzadegan
- Editor-in-Chief: Dr Amirhossein Jamshidi
- Executive Manager: Dr. Mohammadreza Labbafi
View The Current Issue
- Herbal nano-formulations in lung cancer: Superiorities to original forms Javad Malakootikhah * , Mohammad Yahyaei, Reza Ghafarzadegan, Rezvan Ghafarzadegan
- Effectiveness of medicinal plant essential oils on drug-resistant bacteria in Iran: A systematic review Iman Khahan-Yazdi, Mohammadamin Shabani, Mohammadhassan Tajvidi-Monfared, Hassan Vahidi Emami, Faraz Mojab, Saeed Shams , Zahra Taheri Kharameh *
- Quantitative HPLC analysis of flavonoids and evaluation of antioxidant activity in two organs of Salvia tebesana Bunge Maryam Babaie, Monireh Cheniany * , Ali Ganjeali, Jamil Vaezi
- Bibliometric and subject analysis of articles in the ethnobotanical field of Iranian medicinal plants (1999 – 2022) Hossein Batooli, Zahra Batooli * , Somayyeh Nadi-Ravandi
- Variation of antioxidant activity and phenolic compositions of Marrubium vulgare L. as influenced by organic acids Maryam Zahedifar * , Sharareh Najafian
- Effects of topical Persian medicine Amygdalus communis L. var. Amara kernel oil on the symptoms of knee osteoarthritis: a randomized, triple-blind, active, and placebo-controlled clinical trial Saeed Kianbakht * , Farzaneh Nabati, Fataneh Hashem Dabaghian
- The optimization of Fenugreek seeds ( Trigonella foenum-graecum L.) extraction by response surface methodology based on β-Sitosterol Zahra Tofighi, Mohammad Reza Khoshayand, Masoud Besati, Mostafa Pirali Hamedani, Abbas Hadjiakhoondi, Ali Khorshidi, Arian Salimi, Saied Goodarzi *
- Quantitative HPLC analysis of flavonoids and evaluation of antioxidant activity in two organs of Salvia tebesana Bunge Maryam Babaie, Monireh Cheniany, Ali Ganjeali, Jamil Vaezi
- Herbal nano-formulations in lung cancer: Superiorities to original forms Javad Malakootikhah, Mohammad Yahyaei, Reza Ghafarzadegan, Rezvan Ghafarzadegan
- Effectiveness of medicinal plant essential oils on drug-resistant bacteria in Iran: A systematic review Iman Khahan-Yazdi, Mohammadamin Shabani, Mohammadhassan Tajvidi-Monfared, Hassan Vahidi Emami, Faraz Mojab, Saeed Shams , Zahra Taheri Kharameh
- Variation of antioxidant activity and phenolic compositions of Marrubium vulgare L. as influenced by organic acids Maryam Zahedifar, Sharareh Najafian
- Bibliometric and subject analysis of articles in the ethnobotanical field of Iranian medicinal plants (1999 – 2022) Hossein Batooli, Zahra Batooli, Somayyeh Nadi-Ravandi
- Effects of topical Persian medicine Amygdalus communis L. var. Amara kernel oil on the symptoms of knee osteoarthritis: a randomized, triple-blind, active, and placebo-controlled clinical trial Saeed Kianbakht, Farzaneh Nabati, Fataneh Hashem Dabaghian
- The optimization of Fenugreek seeds ( Trigonella foenum-graecum L.) extraction by response surface methodology based on β-Sitosterol Zahra Tofighi, Mohammad Reza Khoshayand, Masoud Besati, Mostafa Pirali Hamedani, Abbas Hadjiakhoondi, Ali Khorshidi, Arian Salimi, Saied Goodarzi
- Volume 22 - Issue 87
- Volume 22 - Issue 86
- Volume 22 - Issue 85
- Volume 21 - Issue 84
- Volume 21 - Issue 83
- Volume 21 - Issue 82
- Volume 21 - Issue 81
- Volume 20 - Issue 80
- Volume 20 - Issue 79
- Volume 20 - Issue 78
- Volume 20 - Issue 77
- Volume 19 - Issue 76
- Volume 19 - Issue 75
- Volume 19 - Issue 74
- Volume 19 - Issue 73
- Volume 18 - Issue 72 and S12 -Supplement 12
- Volume 18 - Issue 72
- Volume 18 - Issue 71
- Volume 18 - Issue 70
- Volume 18 - Issue 69
- 2024/02/17 - Appointment of the new editor of the journal of medicinal plants 2023/10/2 - New Issue of the Journal (Issue 86) 2023/03/1 - New Issue of the Journal (Issue 85) 2022/12/1 - New Issue of the Journal (Issue 84) 2022/09/1 - New Issue of the Journal (Issue 83) 2022/05/31 - New Issue of the Journal (Issue 82) 2022/03/1 - New Issue of the Journal (Issue 81) 2021/12/1 - New Issue of the Journal (Issue 80) 2021/09/19 - Indexing on the DOAJ site 2021/09/1 - New Issue of the Journal (Issue 79)
Accepted Articles
Journal information.
- Current Issue: 2023، Volume 22، Number 87
- Print ISSN: 2717-204X
- Electronic ISSN: 2717-2058
- Previous ISSN: 1684-0240
- Owner & Publisher: Institue of Medicinal Plants, ACECR
Best Quartile
Indexing Databases
- DOAJ (Open access)
Google Scholar
- Chemical Abstracts (CA)
- Index Copernicus
- Index Medicus for the Eastern Mediterranean Region (IMEMR)
- Scientific Information Database (SID)
- Islamic World Science Citation Center (ISC)
Site Statistics
- Registered users: 3703 users
- Online users: 0 users
- Guest users: 250 users
- All visits: 23307875 visits
- Visits in 24 Hours: 5885 visits
- Total articles: 3054 articles
- Published articles: 1258 Acceptance rate: 48.6 Rejection rate: 51.4 Average time to review: 235 days Average time to publish: 172 days
Newsletter subscription
Enter your email address to have site news and announcements delivered directly to your inbox.
Copyright Policy
Related Websites
- Scientific Publications Commission - Health Ministry
- Scientific Publications Commission - Science Ministry
- Institute of Medicinal Plants, ACECR
- Yektaweb Company
Site Keywords
© 2024 CC BY-NC 4.0 | Journal of Medicinal Plants
Designed & Developed by : Yektaweb
Journal of Medicinal Plants for Economic Development
- Editorial Team
- Submission Procedures
- Submission Guidelines
- Submit and Track Manuscript
- Publication fees
- Make a payment
- Journal Information
- Journal Policies
- Frequently Asked Questions
- Reviewer Guidelines
- Article RSS
- Support Enquiry (Login required)
- Aosis Newsletter
Quick links:
- Articles by Author
- Articles by Issue
- Articles by Section
- Find an Article
- Login / Log Out
Subscribe to our newsletter
Get specific, domain-collection newsletters detailing the latest CPD courses, scholarly research and call-for-papers in your field.
Journal of Medicinal Plants for Economic Development | ISSN: 2519-559X (PRINT) | ISSN: 2616-4809 (ONLINE)
Selection of Mexican Medicinal Plants by Identification of Potential Phytochemicals with Anti-Aging, Anti-Inflammatory, and Anti-Oxidant Properties through Network Analysis and Chemoinformatic Screening
Affiliations.
- 1 Department of Pharmacology, School of Medicine, Universidad Nacional Autónoma de México (UNAM), Mexico City 04510, Mexico.
- 2 Department of Basic Medical Sciences, Faculty of Health Sciences, Icesi University, Cali 760031, Colombia.
- PMID: 38002355
- PMCID: PMC10669844
- DOI: 10.3390/biom13111673
Many natural products have been acquired from plants for their helpful properties. Medicinal plants are used for treating a variety of pathologies or symptoms. The axes of many pathological processes are inflammation, oxidative stress, and senescence. This work is focused on identifying Mexican medicinal plants with potential anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence effects through network analysis and chemoinformatic screening of their phytochemicals. We used computational methods to analyze drug-like phytochemicals in Mexican medicinal plants, multi-target compounds, and signaling pathways related to anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence mechanisms. A total of 1373 phytochemicals are found in 1025 Mexican medicinal plants, and 148 compounds showed no harmful functionalities. These compounds displayed comparable structures with reference molecules. Based on their capacity to interact with pharmacological targets, three clusters of Mexican medicinal plants have been established. Curatella americana , Ximenia americana , Malvastrum coromandelianum , and Manilkara zapota all have anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence effects. Plumeria rubra , Lonchocarpus yucatanensis , and Salvia polystachya contained phytochemicals with anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence reported activity. Lonchocarpus guatemalensis , Vallesia glabra , Erythrina oaxacana , and Erythrina sousae have drug-like phytochemicals with potential anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence effects. Between the drug-like phytochemicals, lonchocarpin, vallesine, and erysotrine exhibit potential anti-oxidant, anti-inflammatory, anti-aging, and anti-senescence effects. For the first time, we conducted an initial virtual screening of selected Mexican medicinal plants, which was subsequently confirmed in vivo, evaluating the anti-inflammatory activity of Lonchocarpus guatemalensis Benth in mice.
Keywords: Mexican; anti-aging; anti-inflammatory; anti-oxidants; database; medicinal; native; network; phytochemicals; plants; senolytic.
Publication types
- Research Support, Non-U.S. Gov't
- Anti-Inflammatory Agents / pharmacology
- Antioxidants / pharmacology
- Cheminformatics
- Phytochemicals / chemistry
- Plant Extracts / chemistry
- Plants, Medicinal* / chemistry
- Antioxidants
- Anti-Inflammatory Agents
- Phytochemicals
- Plant Extracts
Grants and funding
- FM/DI/037/2022/División de Investigación, Facultad de Medicina, UNAM.

IMAGES
VIDEO
COMMENTS
Publisher: Science International. Research Journal of Medicinal Plant is now closed for new submissions effective by August 15, 2021. Science International has officially acquired this journal, and now it has been transferred to the new publisher. If you want to submit your research work to this journal, please visit the official website of ...
Aims & Scope. Research Journal of Medicinal Plants is an international peer-reviewed scientific journal dedicated to publishing significant research work in the entire field of medicinal plants. Scope of the journal includes but is not limited to traditional medicine, ethnobotany, herbal therapeutics, phytomedicine, herbal preparations ...
March, 2024 - Vol 18 Num. 2. Cultivation is cost effective for conservation and sustainable supply of rare and high value medicinal plants. Assessment of productivity and quality testing validates cultivation and improves trade prospective. Picrorhiza kurroa is a recently domesticated Himalayan medicinal herb.
The research of medicinal plants in Africa is greatly underdeveloped, in contrast with China and India. In fact, there is no African country among the countries that published the most in this field. ... The role of the South African Journal of Botany as a vehicle to promote medicinal plant research-A bibliometric appraisal. S. Afr. J. Bot ...
The following are examples of research that fits within the scope of the journal: All research along the primary production chain of medicinal and aromatic plants raw material and semi-finished products for herbal medicines, herbal teas, seasoning herbs, food and feed supplements and cosmetics
Different phenolic contents of different plant samples have been reported in the literature 12,18,19,20,21,22,23,24,25.For instance, the total phenol content of sage and peppermint was 27.94 and ...
Introduction. Medicinal plants (MPs) are the main source of natural metabolites such as pigments, condiments, insecticides and medicines. MPs have been used to treat diverse diseases in China, India and Egypt for 5000 years and are still used today, despite the availability of pharmaceuticals [].Plant-derived monomers (morphine, artemisinin, taxol, digitali, vinblastine, etc.) are essential ...
Research Journal of Medicinal Plant . Discontinued in Scopus as of 2016 ... SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a ...
The Rigveda says that man learnt to distinguish edible plants from the poisonous ones by observing animals. 7 Traditional medicinal plants are readily available and culturally acceptable. They offer an accessible and affordable health care regime and serve as an important source of livelihood for indigenous rural populations. 8 Research on plant and use of traditional medicinal information has ...
Journal of Medicinal Plants 2717-204X (Print) / 2717-2058 (Online) Website ISSN Portal About Articles About. Publishing with this journal. There are no publication fees (article processing ... Institue of Medicinal Plants, ACECR, Iran, Islamic Republic of
Journal of Medicinal Plants Research. Abbreviation: J. Med. Plants Res. Language: English; ISSN: 1996-0875; DOI: 10.5897/JMPR; Start Year: 2007; ... There have been a growing interest since decades in the medicinal plants in terms of preventive health care and treatment of various diseases. On the other hand, polyphenolic compounds present in ...
The Journal of Medicinal Plants Research (JMPR) provides researchers, students and academicians an avenue to present their findings on the value of medicinal plants, indigenous medications, ethnobotany and ethnomedicine, herbal medicines and the cultivation of aromatic and medicinal plants. The journal will consider for publication original ...
For centuries, plants have been part of human civilisation, serving as food, healing substances and treatments for various diseases. In recent years, advances in biological research have revealed the remarkable potential of plant compounds in the food and medicine sectors [].In the food industry, plant extracts are used to improve the safety, nutritional value and shelf life of food products.
The Journal of Medicinal Plants is published quarterly. This journal contains articles in the fields of basic and clinical sciences related to medicinal plants including pharmacognosy, basic and clinical pharmacology, basic and clinical toxicology, and pharmacology. Join the conversation about this journal. Quartiles.
March, 2024 - Vol 18 Num. 2. Cultivation is cost effective for conservation and sustainable supply of rare and high value medicinal plants. Assessment of productivity and quality testing validates cultivation and improves trade prospective. Picrorhiza kurroa is a recently domesticated Himalayan medicinal herb.
Has a Scientific Research license from the Medical Sciences Publications Commission of the Country under Number 13375 dated 2002.11.09. The Journal of Medicinal Plants is published quarterly (English language).This journal contains articles in the fields of basic and clinical sciences related to medicinal plants including pharmacognosy, basic and clinical pharmacology, basic and clinical ...
July 2021. Pachyrhizus spp. seed extracts control the Sclerotium rolfsii Sacc. mycelial growth. The present paper aimed to characterize the bioactivity being exerted on Sclerotium rolfsii Sacc. in vitro by seed aqueous extracts from 64 yam bean (Pachyrhizus spp.) genotypes as well as their maceration times (0, 48 and 72 h).
Journal of Medicinal Plants Research 5 (2), 171-175, 2011. 144: 2011: Blood Pressure Lowering Effect of Nigella sativa L. Seed Oil in Healthy Volunteers: A Randomized, Double‐Blind, Placebo‐controlled Clinical Trial.
A peer-reviewed, open access journal in pharmacognosy, pharmacology, toxicoloy, pharmaceutics, natural products & ethnobotany. ... Journal of Medicinal Plants 2717-204X (Print) / 2717-2058 (Online) Website ISSN Portal About ...
Journal of Medicinal Plants Research. Abbreviation: J. Med. Plants Res. Language: English; ISSN: 1996-0875; DOI: 10.5897/JMPR ... The use of plants and plant products in the treatment of various ailments has been with humanity since the dawn of time and the potentials of these plants are so enormous that the continuous search for their hidden ...
The Journal of Medicinal Plants for Economic Development is dedicated to the dissemination of research information on the use of plants and plant products for economic and social development especially in the developing world. It publishes original research papers, critical reviews and communications on the latest developments in phytomedicine with strong emphasis on originality and scientific ...
Phytotherapy Research is a medicinal chemistry journal for biochemists, pharmacologists, & toxicologists interested in medicinal plant research & clinical applications. ... Efficacy of plant-derived dietary supplements in improving overall menopausal symptoms in women: An updated systematic review and meta-analysis. Mi Ra Oh, ...
Albizia anthelmintica is a medicinal plant belonging to the Fabaceae family. It is widely used by smallholder farmers and pastoralists to treat internal parasites in their livestock. This study aimed to determine the antibacterial and antioxidant potential of A. anthelmintica on pathogenic veterinary isolates. 100% hexane (He100), 100% ...
A total of 1373 phytochemicals are found in 1025 Mexican medicinal plants, and 148 compounds showed no harmful functionalities. These compounds displayed comparable structures with reference molecules. Based on their capacity to interact with pharmacological targets, three clusters of Mexican medicinal plants have been established.
Academia Journal of Medicinal Plants (AJMP) ... Chair-Professor of Medical Research (2009 - Present) Chang Jung Christian University, Tainan City, Taiwan Professor of Pharmacology (1988 - 2009) Chairman of Research Center for Traditional Chinese Medicine (1996 -2009)