
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


Experiment_602_Empirical Formula of MgO 1_4_2
- Last updated
- Save as PDF
- Page ID 302985
Experiment 60 2 : Empirical Formula
Section 1: Purpose and Summary
Determine the empirical formula of magnesium oxide.
Calculate the mass of oxygen using weighing-by-difference.
Calculate the mole of a sample from its mass.
In this experiment, students will conduct the reaction between magnesium and oxygen gas. Students will determine the mass of magnesium sample before and after the reaction, and the mass of magnesium and oxygen in the product. Students will learn how to convert mass to mole of a given sample and determine empirical formula of a substance from mass and mole data.
Section 2: Safety Precautions and Waste Disposal
Safety Precautions:
Do not look directly at the burning magnesium ribbon. The flame is bright enough to damage your eye. Use of eye protection is required for all experimental procedures.
A hot crucible will break if placed directly on a cold surface. Set hot crucibles on to wire screens to cool.
A hot crucible will break if splashed with water directly. Let crucibles cool prior to adding water.
Waste Disposal:
The solid product from the reaction can be disposed into the regular garbage can in the lab.
Section 3: Procedure
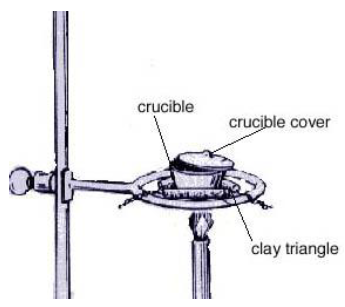
Part 1: Preparation of the c rucible
Part 2 : Preparation of m agnesium s ample
Part 3: Heating the m agnesium s ample
Section 4: Calculations
Post Lab Questions:
1. There are some experimental errors that could lead to high or low mole ratio between Mg and O. In each case below, decide whether the situation described would lead to a calculated ratio of too much oxygen, or too little oxygen, and explain your answer.
(a) You forgot to do the initial drying step and proceeded right away to weighing the crucible and lid you obtained from the stockroom.
(b) Your magnesium ribbon is not shiny. But you did not polish it with steel wool prior to use as indicated in the experiment.
(c) You added more laboratory water than is needed in Part 3 Step 7, and you did not dry it out completely.
(d) After strong heating of the crucible you removed the lid but dropped it and broke. You then obtained a new lid for the final weighing.
2. A similar experiment is performed to determine the empirical formula of an oxide of copper, and the following data were collected. Predict the empirical formula of the copper oxide from these data.
Mass of crucible, cover, and copper sample 21.53 g
Mass of empty crucible with cover 19.66 g
Mass of crucible and cover and sample (after heating) 21.76 g
How can the experiment for the determination of the empirical formula of an oxide of copper be improved?
Usually requires both the moles of magnesium and the moles of oxygen to be divided by whichever is the lowest number.
⚛ with the symbol for magnesium (Mg) written before the symbol for oxygen (O) ⚛ using the lowest whole number ratio of moles of magnesium (x) to moles of oxygen (y), the subscripts for Mg and O are added to give a formula of the type Mg x O y Note that a subscript number is NOT included in the formula if it would be a 1
1. Magnesium oxide is best thought of as an ionic compound containing magnesium ions and oxide ions. So, strictly speaking we should not talk of magnesium atoms and oxygen atoms in this compound, nor should we talk of a molecule of magnesium oxide because a molecule refers to a compound in which atoms are covalently bonded to each other. However, in order to keep the explanation as clear as possible, we shall refer to "atoms" and "molecules". 2. In order to save time in class, it is quite likely that your crucible has already been prepared for you. You should still be aware of how this is done and why. 3. Alternatively you can place a pipe clay triangle over a tripod in a "star of David" formation. You may need to use a heat resistant mat or tiles under the Bunsen Burner to maintain the correct position. 4. It is better to cool the crucible in a dessicator to remove any moisture, however, in a school laboratory it is not advisable for students to be walking around with hot crucibles, so either keep the crucible in the pipe clay triangle, or, remove the hot crucible using tongs and place it on a nearby heat resistant mat to cool. 5. If time is limited, it is possible to light the magnesium strip inside the crucible and quickly position the lid using tongs before heating the crucible. However, it should be noted that some magnesium oxide will probably be lost as the fine powder quickly escapes from the crucible before the lid is positioned. It is possible to heat the crucible containing the magnesium without the lid in order to start the combustion of the magnesium, then replace the lid very quickly, repeating this several times until all the magnesium has been converted to magnesium oxide. This method works well to convert the magnesium to magnesium oxide, but once again, while the lid is off the crucible some of the fine-powdery magnesium oxide tends be lost. 6. The magnesium nitride can be converted to magnesium oxide to remove this source of error. When the combustion reaction appears to have been completed, and the crucible is cool, add a few drops of water to wet the entire sample to convert magnesium nitride to magnesium hydroxide and ammonia gas: Mg 3 N 2 + 6H 2 O → 3Mg(OH) 2 + 2NH 3 Heat the crucible very gently until the product appears to be dry, then heat it strongly to remove excess water: Mg(OH) 2 → MgO + H 2 O

- Michael Jansen's blog
The Empirical Formula of Magnesium Oxide Lab: A Successful Failure, Next Steps—and an Important Lesson

PART 1: Why this lab is no good
For many years I was troubled by this commonly used, straight-forward, interesting-to-carry-out, and engaging experiment 1 . This analytical, mole concept-based activity can be found in pretty much any chemistry lab manual, from Grade 11 to first-year university.
Procedure-wise, students set out to quantitatively combust a piece of magnesium ribbon in a covered crucible, over a rip-roaring Bunsen burner flame to produce magnesium oxide. A typical apparatus is illustrated in figure 1 2 . During the “cooking”, after the bottom of the crucible becomes red-hot, I visit each student-station, carefully admitting a small quantity of air to the crucible as required for combustion, while simultaneously allowing minimal loss of smoke, which is, after all, magnesium oxide.
burning_mg_chemix.jpg

Figure 1: The apparatus for the determination of the empirical (simplest) formula of magnesium oxide (image created using Chemix ).
Some lab manuals talk about only the reaction of magnesium with oxygen 3 :
Mg(s) + O 2 (g) à Mg x O y (s) (1)
More advanced instructions discuss the reaction of magnesium with N 2 (g), which comprises 78% (v/v) of the earth’s atmosphere 4 , to produce magnesium nitride, and the subsequent decomposition of Mg 3 N 2 to give magnesium oxide 5 :
Mg(s) + N 2 (g) + O 2 (g) à MgO(s) + Mg 3 N 2 (s) (2)
MgO(s) + Mg 3 N 2 (g) + H 2 O(l) à MgO(s) + Mg(OH) 2 (s) + NH 3 (g) (3)
MgO(s) + Mg(OH) 2 (s) à Mg x O y (s) + H 2 O(g) (4)
No matter how I’ve had students analyze their experimental data, results were invariably lack-lustre, with just enough decent values for the simplest formula of magnesium oxide to keep this activity on the roster.
Mg burning in air
Video 1: Reaction of Magnesium with Oxygen (derived from Jerrold J Jacobsen and John W. Moore. Chemistry Comes Alive! Vol. 3: Abstract of Special Issue 23 on CD ROM. Journal of Chemical Education 1997 74 (5), p 607-608. DOI: 10.1021/ed074p607), ChemEd Xchange on Vimeo. (accessed 8/12/21)
Several years ago, I was hit by multiple (figurative) lightning bolts:
As part of a pre-lab discussion/demonstration, I burn a piece of magnesium. Students conclude that both the pure white smoke and the pure white ash are magnesium oxide 6 . This shows the importance of not allowing smoke to escape during the combustion. We’re collecting magnesium oxide quantitatively, after all.
a) The “magnesium oxide” produced in the crucible is not pure white—it is grey 7 . The typical procedure generally notes that the formation of the grey powdery substance signals that the reaction is complete and directs students to cool and weigh the crucible.
b) Magnesium reacts with carbon dioxide to produce carbon and magnesium oxide 8 :
2 Mg(s) + CO 2 (g) à 2 MgO(s) + C(s) (5)
The Mg in the crucible is bathed in CO 2 (g) from the combustion products of methane, used to fuel the Bunsen burner.
CH 4 (g) + 2 O 2 (g) à CO 2 (g) + 2 HOH(g) 9 (6)
This explains the grey product—it’s a mixture of MgO and C.
Reaction of Magnesium with Carbon Dioxide
Video 2: Reaction of Magnesium with Carbon Dioxide (derived from Jerrold J Jacobsen and John W. Moore. Chemistry Comes Alive! Vol. 3: Abstract of Special Issue 23 on CD ROM. Journal of Chemical Education 1997 74 (5), p 607-608. DOI: 10.1021/ed074p607), ChemEd Xchange on Vimeo. (accessed 8/12/21)
See reaction 5 in the Chemistry Comes Alive #3 video from our ChemEd X video collection above 10 . Reaction (5) is also beautifully demonstrated by Sir Professor Doctor Martyn Poliakoff’s people in the Periodic Table of Videos clip on Carbon Dioxide (Part II) 11 . The simultaneous production of magnesium oxide and carbon is apparent when magnesium is burned in a dry ice “sandwich” 12 . In a less spectacular, but equally effective demonstration, I simply lower a piece of burning magnesium into a 2-L beaker full of CO 2 (g) 13 . After the reaction, white MgO and black C are apparent.
As for Mg 3 N 2 , it is a greenish-yellow powder 14 , which I have not, in over 30 years, observed with the naked eye in the reaction products. To be fair, it’s not a vibrant color; perhaps there isn’t enough of it to see.
To sum-up, this activity does not produce what it claims to produce. The empirically observed result—MgO and C—does not line-up with the expected product—pure MgO.
PART 2: Use this experiment as a successful failure
The fact that the “MgO” produced (grey) does not resemble pure MgO (white) is an eye-opener. I’m not proud to say that it took me over 25 years to interpret the plain-as-day empirical evidence. It was, after all, in the lab manual—it had to be true.
In the “Make Lemonade from Lemons” department, I continue to use this experiment—as a successful failure. It is an important teachable moment; I milk it for everything it’s worth.
This is Chemistry, for Pete’s sake—AN EMPIRICAL SCIENCE!!!!!
A favourite post-lab question, which I use as a “soft introduction” 15 to stoichiometry, asks students to calculate the total mass of MgO and C produced when 1.00 g of Mg reacts 50/50 by mass in each of reactions (1) and (5).
PART 3: And then . . .
a) I don’t stop there. I ask students how we could modify this experiment. After some back-and-forth, I reveal a combustion chamber (figure 2) that I built by taping two 2-L beakers together, with the “lips” of the beaker co-incident, to create a small opening.
magnesium_combusion_chamber.png

Figure 2: The magnesium combustion chamber
I don’t discard the MgO and C from the failed experiment. In a future experiment, I have students gravimetrically analyze the MgO/C mixture. Students can determine the % C in the mixture of MgO and C.
I ignite a weighed piece of Magnesium, which I hold with tongs, and insert it, without delay, into the weighed, empty combustion chamber. If all goes well—inserting the burning magnesium into the opening is tricky—we wait for the magnesium oxide “smoke” to settle before recording the final mass of the combustion chamber.
If I’m not able to accomplish this task after a few attempts, I simple refer my students to the data in Table 1.
Table 1. Reaction of Mg(s) + O 2 (g) à Mg x O y (s)
reaction_of_mg_with_oxygen.png

In this experiment, it is obvious that the pure white product is indeed magnesium oxide; data support the formula of MgO.
b) I don’t discard the MgO and C from the failed experiment. In a future experiment, I have students gravimetrically analyze the MgO/C mixture. When excess 2.0 mol/L HCl(aq) is added, the MgO reacts as follows:
MgO(s) + HCl(aq) à MgCl 2 (aq) + HOH(l) (7)
Mercifully, C does not react, and so after filtering off the aqueous magnesium chloride, and drying and washing, the C collected in the filter paper can be weighed. This can be used to determine the % C in the mixture of MgO and C.
PART 4: Next Steps
After this experiment and the post-lab work, I have students carry out a separate determination of the empirical formula of zinc chloride, according to the reaction:
Zn(s) + 2 HCl(aq) à ZnCl 2 (aq) + H 2 (g) (8)
In the fumehood, students react a weighed piece of clean zinc with a slight excess of concentrated (12 mol/L) HCl(aq) in a weighed beaker atop a hot plate. The H 2 (g) and unreacted HCl(aq) exit via the chimney. Students simply weigh the dried product, which is ZnCl 2 . Student results support the accepted formula.
This experiment may not be appropriate for your students: At Crescent School we have plenty of fumehood space; students wear lab aprons and nitrile gloves. I’m okay with letting them use a wee bit of 12 mol/L HCl. If that’s not an option for you, a teacher demonstration might be a good idea.
PART 5: The most important part
It is the zenith of irony when an empirical formula experiment is empirically bogus.
This may sound like it belongs in the “Department of Redundancy Department”, but a post-lab discussion MUST include the essential ingredient of science: EMPIRICAL EVIDENCE.
Now, more than ever, students—everyone—must be made aware:
Just because an experiment “looks good” or is widely accepted doesn’t make it good.
Just because an experiment “looks good” doesn’t mean it’s carried out properly.
And just because an experiment “looks good” doesn’t mean that it is interpreted properly.
We must teach the importance of asking questions. Questions are more important than answers. I want my students to question everything I do or say. Sometimes—okay, many times—I don’t know the answer; sometimes the explanation will take too long; and sometimes the answer is “because I said so”. The point is—they need to ASK QUESTIONS 16 .
May peace be with you.
- https://uwaterloo.ca/chem13-news-magazine/april-2015/feature/vive-science
- https://edu.rsc.org/experiments/the-change-in-mass-when-magnesium-burns/...
- McGraw-Hill Ryerson chemistry text, 2011
- https://en.wikipedia.org/wiki/Nitrogen
- https://www.webassign.net/question_assets/ucscgencheml1/lab_2/manual.html
- https://en.wikipedia.org/wiki/Magnesium_oxide
- http://www.dynamicscience.com.au/tester/solutions1/chemistry/moleandempi...
- Yehoshua Sivan, “Burning magnesium in a Bunsen blame and other flame experiments”, Chem 13 News, February 2015, pages 12 – 13
- One of my many quirks as a Chemistry teacher is to write the formula for water as HOH, rather than H 2 O, to emphasize the bonding order of the atoms. I do not compel my students to adopt this irregular approach. (Be thankful you don’t live with me . . . )
- https://vimeo.com/419694043
- https://www.youtube.com/watch?v=0dSMzg0UPPo&t=332s
- https://pubs.acs.org/doi/pdf/10.1021/ed055p450.2
- I prepare the CO 2 by reacting HCl(aq) with NaHCO 3 (s)
- https://en.wikipedia.org/wiki/Magnesium_nitride
- I’m a big fan of “foreshadowing” or “soft launching” concepts. See https://uwaterloo.ca/chem13-news-magazine/february-2017/pedagogy-opinion...
- An upcoming test question will present some information: a table or a graph or something. Students will be required to ask an intelligent question.
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016) .
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations .
Other Safety resources
RAMP : Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
Science Practice: Analyzing and Interpreting Data
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Science Practice: Asking Questions and Defining Problems
Asking questions and defining problems in grades 9–12 builds from grades K–8 experiences and progresses to formulating, refining, and evaluating empirically testable questions and design problems using models and simulations.
questions that challenge the premise(s) of an argument, the interpretation of a data set, or the suitability of a design.
Scientific questions arise in a variety of ways. They can be driven by curiosity about the world (e.g., Why is the sky blue?). They can be inspired by a model’s or theory’s predictions or by attempts to extend or refine a model or theory (e.g., How does the particle model of matter explain the incompressibility of liquids?). Or they can result from the need to provide better solutions to a problem. For example, the question of why it is impossible to siphon water above a height of 32 feet led Evangelista Torricelli (17th-century inventor of the barometer) to his discoveries about the atmosphere and the identification of a vacuum.
Questions are also important in engineering. Engineers must be able to ask probing questions in order to define an engineering problem. For example, they may ask: What is the need or desire that underlies the problem? What are the criteria (specifications) for a successful solution? What are the constraints? Other questions arise when generating possible solutions: Will this solution meet the design criteria? Can two or more ideas be combined to produce a better solution?
Science Practice: Constructing Explanations and Designing Solutions
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Science Practice: Engaging in Argument from Evidence
Science practice: obtaining, evaluating, and communicating information.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science. Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments.
Science Practice: Planning and Carrying out Investigations
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
Science Practice: Using Mathematics and Computational Thinking
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
HS-PS1-2 Chemical Reactions
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org .
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.
HS-PS1-7 Mathematical Representations
Students who demonstrate understanding can use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Assessment does not include complex chemical reactions.
Emphasis is on using mathematical ideas to communicate the proportional relationships between masses of atoms in the reactants and the products, and the translation of these relationships to the macroscopic scale using the mole as the conversion from the atomic to the macroscopic scale. Emphasis is on assessing students’ use of mathematical thinking and not on memorization and rote application of problem - solving techniques.
All comments must abide by the ChemEd X Comment Policy , are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Use crown bottle tops and make the experiment work.

Go to https://microchemuk.weebly.com/3-blog-is-this-supposed-to-happen/it-is-n... and see how you can get this experiment to work every time(as reactions should). It is so easy and enjoy collecting the bottle tops. There is an old CLEAPS video here Finding the formula of magnesium oxide . If you have not got the small pipe clay triangles, then place the bottle tops on the gauze section of the gauze, not the ceramic centre. As well as MgO there is Mg₃N₂. To note its presence, place the product, in a vial, add hot water, and put moist red litmus over the top and it goes blue with the ammonia Note I only use 0.12 to 0.2g of magnesium ribbon, coiled around a pencil so it fits in the bottle top sandwich. Cheers Bob Worley in the UK.

Analía Bellizzi – Chemistry Classes
Ronald Reagan Senior High School
Empirical Formula of Magnesium Oxide
Empirical Formula of Magnesium Oxide – LAB
Word Document
Purpose: to determine the experimental empirical formula of a compound, magnesium oxide, and compare it to its theoretical empirical formula, MgO.
Materials: (list the materials you have on your table)
Procedures:
- Clean a crucible and lid, rinsing thoroughly with deionized water as a last step. (It will not be possible to get a used crucible completely clean.) Dry the crucible and lid with a paper towel. Check the crucible for cracks. Your instructor will tell you how.
- Place the clean, dry crucible and lid on a clay triangle on a ring stand and heat strongly with a Bunsen burner (blue cone) for 5 minutes to remove any volatile material. Use crucible tongs to carefully move the hot crucible and lid to a tile on the counter to cool completely. USE CAUTION TO AVOID BURNS! Hot ceramic and hot metal look just like cold ceramic and cold metal. Once the crucible and lid are completely cool (room temperature), weigh them on the milligram balance and record the exact mass on the data table.
- Obtain a piece of Magnesium ribbon that weighs about 0.3 g. (Do not take time to measure a piece of exactly 0.300 g, just get close and record the exact mass used in the data table.) Crumple the ribbon and pack it into the bottom of the preheated and pre-weighed crucible. Weigh the crucible, crucible lid, and magnesium on the digital balance and record the exact mass on the data table.
- Place the crucible in the triangle as illustrated, with the crucible lid tilted slightly to allow air into the crucible. Heat strongly with a Bunsen burner (blue cone) . NEVER LOOK DIRECTLY AT BURNING MAGNESIUM . If the magnesium starts to burn (very bright light emitted) and “smoke” is given off, use crucible tongs to cover the crucible completely with the crucible lid for a short period of time. After several minutes, tilt the crucible lid open again. Repeat the same procedure until no more “smoke” is observed and the contents of the crucible no longer glow brightly as the crucible is heated. Heat the crucible for several more minutes with the lid off completely so that the bottom of the crucible glows a dull red. Remove heat and allow the crucible to cool completely on the clay triangle.
- When the crucible has cooled, add 6 to 8 drops of distilled water to the contents of the crucible, using a medicine dropper. Water is added to react with and then remove any nitrogen that may have been added to your sample from the air during heating. Heat the crucible gently with the cover off for 5 more minutes. Remove from heat and allow the crucible to cool. Use crucible tongs to move the crucible and lid to a tile on the counter to cool completely. Weigh the crucible, crucible lid, and product on the milligram balance, and record the exact mass on the data table.

Data Table:

Calculations: SHOW YOUR WORK
a) Based on the data in your Table, what mass of magnesium is contained in your compound? Show your calculation.
b) Based on the data in your Table, what mass of oxygen is contained in your compound? Show your calculation.
c) Compare the mass of the Mg ribbon with the mass of the magnesium oxide (rows 2 and 3 in your Data Table). Notice that the mass of the magnesium oxide is greater than the mass of the Mg. How do you account for this apparent increase in mass?
d) Now that you have the mass of magnesium and oxygen in your compound, you can find moles of each element in the compound and you can determine your experimental empirical formula. Show your calculations and your empirical formula below.
Analysis Questions: Show your work!
- The percent by mass composition of a salt was found to be 56.58% potassium, 8.68% carbon, and 34.73% oxygen. What is the empirical formula of this salt?
- A compound containing iron and sulfur was formed by combining 2.233 g of iron with 1.926 g of sulfur. What is the empirical formula of the compound?
- Propylene has a molar mass of 42.00 g/mole and is composed of 14.3 % hydrogen and 85.7 % carbon. What is the molecular formula of propylene?
- A sample of an 10 g of oxide of nitrogen is found to contain 3.04g nitrogen. What is its empirical formula?
- A sample of an oxide of arsenic is found to contain 75.74% arsenic. What is its empirical formula?
- What is the empirical formula for a compound containing 26.57g potassium, 35.36g chromium, and 38.07g oxygen?
- What is the empirical formula of a compound comprised of 1.8% hydrogen, 56.1% sulfur and 42.1% oxygen?
- A borane is a compound containing only boron and hydrogen. If a borane is found to contain 88.45% boron, what is its empirical formula?
- Find the empirical formula for a compound containing 40.6% carbon, 5.1% hydrogen, and 54.2% oxygen.
- What is the empirical formula of a compound containing 47.37% carbon, 10.59% hydrogen and 42.04% oxygen?
Lab 2 – Determination of the Empirical Formula of Magnesium Oxide
Goal and overview.
The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. The resulting masses are used to calculate the experimental empirical formula of magnesium oxide, which is then compared to the theoretical empirical formula. A crucible and Bunsen burner will be used to heat magnesium metal to burning.
Objectives and Science Skills
- • Determine the empirical formula and percent yield of the ionic oxide produced by the reaction of Mg with O 2 based on experimental data.
- • Quantitatively and qualitatively evaluate experimental results relative to those theoretically predicted based on known chemical principles and stoichiometric calculations.
- • Identify and discuss factors or effects that may contribute to deviations between theoretical and experimental results and formulate optimization strategies.
SUGGESTED REVIEW AND EXTERNAL READING
- • Data analysis introduction (online), reference materials
- • Textbook information on ionic compounds and empirical formulas
A great deal of chemical knowledge has been amassed by using simple combustion experiments conducted with crucibles, burners, and balances. In this experiment, you are using this technique to experimentally determine the empirical formula of magnesium oxide. This lab illustrates (1) the law of conservation of mass and (2) the law of constant composition.
- 1 The total mass of the products of a reaction must equal the total mass of the reactants.
- 2 Any portion of a compound will have the same ratio of masses as the elements in the compound.
Molecular composition can be expressed three ways:
- 1 In terms of the number of each type of atom per molecule or per formula unit (the formula).
- 2 In terms of the mass of each element per mole of compound.
- 3 In terms of the mass of each element present to the total mass of the compound (mass percent).
The empirical formula of a compound gives the lowest whole-number ratio of the constituent atoms that is consistent with the mass ratios measured by experiment. In this lab, magnesium metal (an element) is oxidized by oxygen gas to magnesium oxide (a compound). Magnesium reacts vigorously when heated in the presence of air. The Mg-O 2 reaction is energetic enough to allow some Mg to react with gaseous N 2 . Although there is a higher percentage of N 2 gas in the atmosphere than O 2 , O 2 is more reactive and the magnesium oxide forms in a greater amount than the nitride. The small amount of nitride that forms can be removed with the addition of water, which converts the nitride to magnesium hydroxide and ammonia gas. Heating the product again causes the loss of water and conversion of the hydroxide to the oxide. The unbalanced equations are:
Balancing the reactions is not necessary because the theoretical reaction product and yield is based on the amount of Mg available to react. The expected product is MgO, so the 1-to-1 mole ratio Mg to O in the product is all that is required. Based on the masses of the solid reactant (Mg) and product (Mg x O y ), the mass in grams and the amount in moles of Mg and O in the product can be determined. Recall that the conversion factor relating grams to moles is molar mass.
where w is the grams of Mg used and z is the grams of O incorporated. The empirical formula of magnesium oxide, Mg x O y , is written as the lowest whole-number ratio between the moles of Mg used and moles of O consumed. This is found by determining the moles of Mg and O in the product; divide each value by the smaller number; and, multiply the resulting values by small whole numbers (up to five) until you get whole number values (with 0.1 of a whole number). For example, if 0.0109 moles of Mg are combined with 0.0103 moles of O:
For example, if 0.0129 moles of Mg are combined with 0.0103 moles of O:
In the first case, the ratio of Mg-to-O is close enough to 1-to-1. In the second example, the product is slightly magnesium-rich; the ratio of Mg-to-O is greater than the 1-to-1 expected. A magnesium-poor product would have a ratio of Mg-to-O that is less than the 1-to-1 expected.
EXPERIMENTAL NOTES
Equipment list.
- Safety goggles
- Magnesium ribbon, Mg
- Balance (to 0.0001g)
- Ring stand
- Bunsen burner
- Ring support/ clay triangle
- Crucible/ lid
- Clay tile
Crucible Use
- • Crucibles are used to heat substances to high temperatures (like those encountered with burning metals) without risk of breakage. However, they are ceramic and can break. Please be careful — if your crucible breaks, please inform your TA and get help with the clean-up and disposal. Please know that it is now chemical waste and must be placed in the solid waste container.
- • Do not touch the crucible with your hands (oils contaminate it and/or you could be severely burned).
- • Do not place a hot crucible on a lab bench (the temperature difference may cause it to break). Use the clay triangle.

Prior to Starting
- • Practice using the tongs to pick up the lid from the crucible and the crucible from the clay triangle.
- • Practice placing the lid partially over the crucible so that there is a gap of about 0.5 cm (the lid should rest on the crucible edge and two legs of the triangle).
- • Practice placing the crucible with lid on the clay tile (when carrying the crucible, always hold it with tongs and support it with the tile).
Your TA will demonstrate. Ask questions as needed.
REPORTING RESULTS
Complete your lab summary or write a report (as instructed). Report the following information. Show sample calculations in a separate section.
- 1 mass of Mg metal used to ±0.0001 g
- 2 theoretical yield of MgO from reaction: Mg( s ) + 1/2 O 2 ( g ) → MgO( s )to ±0.0001 g
- 3 mass of oxide product formed to ±0.0001 g
- 4 mass of O incorporated (by difference; see eq. 4a and 4b ) ±0.0001 g
- 5 mole ratio of Mg-to-O (four significant figures each)
- 6 empirical formula of the oxide (lowest whole-number subscripts)
- 6 percent by mass of Mg and O in the oxide (four significant figures)
- 7 percent yield of Mg + 1/2 O 2 → MgO (actual yield/theoretical yield) × 100% (four significant figures)
Discussion/Conclusions
- How does your experimental empirical formula compare to the theoretical empirical formula — do they match?
- What are primary sources of experimental error?
- 1 incomplete conversion of Mg 3 N 2 to MgO or
- 2 residual Mg(OH) 2 in the product affect your results?
- Does this method appear to be a valid way to determine the formula of metal oxides?
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

How can I calculate the empirical formula of magnesium oxide?

Explanation:
For example, you might heat a known mass of magnesium in a crucible and determine the mass of oxide formed.
Assume that you heated 0.297 g of magnesium and obtained 0.493 g of the oxide. What is the empirical formula of magnesium oxide?
The empirical formula is the simplest whole-number ratio of atoms in a compound.
The ratio of atoms is the same as the ratio of moles. So our job is to calculate the molar ratio of #"Mg"# to #"O"# .
#"Mass of Mg = 0.297 g"#
#"Mass of magnesium oxide = mass of Mg + mass of O"#
#"0.493 g = 0.297 g + mass of O"#
#"Mass of O = (0.493 – 0.297) g = 0.196 g"#
#"Moles of Mg" = 0.297 color(red)(cancel(color(black)("g Mg"))) × "1 mol Mg"/(24.3color(red)(cancel(color(black)( "g Mg")))) = "0.012 22 mol Mg"#
#"Moles of O "= 0.196 color(red)(cancel(color(black)("g O"))) × "1 mol O"/(16.00 color(red)(cancel(color(black)("g O")))) = "0.012 25 mol O"#
To get this into an integer ratio, we divide both numerator and denominator by the smaller value.
From this point on, I like to summarize the calculations in a table.
#"Element"color(white)(Mg) "Mass/g"color(white)(X) "Moles"color(white)(Xll) "Ratio"color(white)(mll)"Integers"# #stackrel(—————————————————-——)(color(white)(m)"Mg" color(white)(XXXm)0.297 color(white)(X)"0.012 22" color(white)(X)1color(white)(Xmmmm)1# #color(white)(m)"O" color(white)(XXXXll)0.196 color(white)(m)"0.012 25" color(white)(X)1.002 color(white)(XXX)1#
There is 1 mol of #"Mg"# for 1 mol of #"O"# .
The empirical formula of magnesium oxide is #"MgO"# .
Here is a video that illustrates how to determine an empirical formula.
Related questions
- How do empirical formulas and molecular formulas differ?
- How do you find molecular formula of a compound?
- What is the chemical formula of a diamond?
- What is the chemical formula of a carbohydrate?
- What is the empirical formula for valproic acid?
- What is the empirical formula of magnesium oxide?
- Why is the empirical formula not double that of the monosaccharides?
- Question #5c3b5
- What molecular formula represents a carbohydrate?
- What is the molecular formula of vinegar?
Impact of this question

Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Determining the Empirical Formula of Magnesium Oxide

Intro The empirical formula of a substance is the simplest whole number ratio of the number of atoms of each element in the compound. This can be calculated knowing the mass of each element and using this to calculate the number of moles of each element. In this experiment, you will carry out the following reaction: Magnesium + Oxygen Magnesium Oxide Aim: To determine the empirical formula of Magnesium Oxide by burning magnesium. Hypothesis: The empirical formula will be MgO. Method: 1. Heat crucible + lid for five minutes and allow it to cool. (to remove water moisture for accurate mass) 2. Weigh crucible + lid. 3. Clean surface of a 5cm piece of Magnesium using steel wool. 4. Coil the Magnesium and place it in the crucible and record mass of Magnesium with crucible + lid. 5. Place crucible + contents onto a pipe clay triangle on top of a tripod. 6. Heat using blue flame until Magnesium burns. 7. Replace lid and heat for ten minutes. 8. Allow to cool then record mass. Results Object Mass (g) Crucible + lid 31.45g Crucible + lid + magnesium 31.62g Magnesium 0.17g Crucible + lid + magnesium oxide 31.70g Magnesium oxide 0.25g Oxygen 0.08g Steps to determine empirical formula of magnesium oxide: 1. Calculate moles of magnesium and oxygen. 2. Determine moles of magnesium to moles of oxygen. 3. Determine empirical formula of magnesium oxide. 4. Compare class results. 5. Compare actual ratio of practical. 6. Identify likely sources of error.
Related Papers
Folk Narongrit
The empirical formula for Magnesium Oxide is experimentally determined by the combustion of 17 samples of magnesium ribbons. The empirical formula is determined to be Mg5O3, in which the ratio is close to the accepted value of Mg1:O1
Wooyoung Jeong
Sintia Lestari
Klaus Mikaelson
Yuxuan Lang
Relative atomic, isotopic, molecular and formula masses: Relative atomic mass: the weighted average mass of the atoms of an element, taking into account the proportions of naturally occurring isotopes, measured on a scale on which an atom of the carbon-12 isotope has a mass of exactly 12 units. Relative formula mass: the mass of one formula unit of a compound measured on a scale on which an atom of the carbon-12 isotope has a mass of exactly 12 units. Relative isotopic mass: the mass of a particular isotope of an element on a scale in which an atom of the carbon-12 isotope has a mass of exactly 12 units. Relative molecular mass: the mass of a molecule measured on a scale in which an atom of the carbon-12 isotope has a mass of exactly 12 units. Mole and the Avogadro constant: One mole of a substance is the amount of that substance that has the same number of specific particles (atoms, molecules or ions) as there are atoms in exactly 12g of the carbon-12 isotope. The number of atoms in a mole of atom is 6.02×10 23 atoms. This number is called the Avogadro constant. The symbol for the Avogadro constant is L (or NA). Analyse mass spectra in terms of isotopic abundances: A mass spectrometer compares how much of each isotope is present-the relative abundance (isotopic abundance). The mass spectrum produced shows the relative abundance (isotopic abundance) on the vertical axis and the mass to ion charge ratio (m/e) on the horizontal axis. For single positively charged ions the m/e values give the nucleon number of the isotopes detected. Calculate the relative atomic mass of an element: To calculate the relative atomic mass: 1. Multiply each isotopic mass by its percentage abundance. 2. Add the figures together. 3. Divide by 100. ✽ Note: the number of significant figures should be consistent with the data given. Empirical and molecular formula: The empirical formula of a compound is the simplest whole number ratio of the elements present in one molecule or formula unit of the compound. The molecular formula of a compound shows the total number of atoms of each element present in a molecule. Perform calculations, including use of the mole concept, involving: One mole of a gas occupies 24 dm 3 at room temperature (25 ° C) and a pressure of 101 kPa (1 atm). Deduce stoichiometric relationships from calculations:
Lalit Kumar
J. Anal. At. Spectrom.
Jochen Vogl
Isotope mixtures for the ab initio-calibration of Mg isotope ratio measurements were prepared with unrivalled relative expanded uncertainties as low as 0.005%.
Savas Sahingoz
Journal of Analytical Atomic Spectrometry
RELATED PAPERS
British Journal of Haematology
SHELLEY CHAMBERS
Journal of molecular …
Ardeschir Vahedi-Faridi
Gholam-abbas Shirali
per hedfors
Einstein (São Paulo)
Maurício Campanelli Costas
Journal of Structural and Construction Engineering (Transactions of AIJ)
Shoji Nakazawa
Bulletin of Engineering Geology and the Environment
nouhad El Amrani
Cancer immunology, immunotherapy : CII
erni sukmawati
Revista de Chimie
Ibrahim Isildak
Ilham Maulana
European Neuropsychopharmacology
Giovanni Muscettola
Arturo San Feliciano
Revista Ibero-Americana de Estudos em Educação
Vitor Bremgartner
European Journal of Human Genetics
Angel Campos
Gideon Kruseman
2012 12th IEEE/ACM International Symposium on Cluster, Cloud and Grid Computing (ccgrid 2012)
Allergy & Rhinology
Marija Rowane
Rafael omar Fabrica mamani
IEEE Intelligent Systems
Edoardo Serra
La Ode Husen
Indian Journal of Animal Research
Omar AbdElkader
Journal of Informetrics
Giovanni Abramo
Nursing Management
Patricia Tuite
Moises Martín Betancor
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Teacher Resource Center
Pasco partnerships.

2024 Catalogs & Brochures
Empirical formula of magnesium oxide.
Students add enough heat to a sample of magnesium to produce magnesium oxide, then analyze the product to determine its empirical formula.
Supports NGSS Performance Expectation HS-PS1-7: Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Grade Level: High School
Subject: Chemistry
Student Files
Teacher Files
Sign In to your PASCO account to access teacher files and sample data.
Featured Equipment

OHAUS Scout SKX Balance 220g
Combines range, resolution and low cost, making it ideal for the student science lab. 220 g version.
Many lab activities can be conducted with our Wireless , PASPORT , or even ScienceWorkshop sensors and equipment. For assistance with substituting compatible instruments, contact PASCO Technical Support . We're here to help. Copyright © 2018 PASCO
Source Collection: Lab #06D
Essential Chemistry Teacher Lab Manual
More experiments.
Advanced Placement
- Shape Of Titration Curves
- What Does Acid Rain Do To Coral Reefs?
High School
- Conservation of Mass
- Boyle's Law
- Diprotic Titration: Multi-Step Chemical Reactions

- TOP CATEGORIES
- AS and A Level
- University Degree
- International Baccalaureate
- Uncategorised
- 5 Star Essays
- Study Tools
- Study Guides
- Meet the Team
Experiment - The Empirical Formula of Magnesium Oxide
The Empirical Formula of Magnesium Oxide
Experimental Design
Focus Question
What is the empirical formula for magnesium oxide?
The combustion of magnesium will generate data which can be used to calculate the empirical formula of magnesium oxide.
The following combination reaction was used in this experiment:
Magnesium + Oxygen → Magnesium Oxide
The Law of Conservation of Mass can be used to determine the amount of oxygen which has reacted with a given amount of magnesium in order to produce a measured amount of magnesium oxide. These masses can then be converted into moles in order to determine the simplest molar ratio and thus the empirical formula for magnesium oxide.
Apparatus and Materials
- Crucible and lid (1)
- Mg ribbon (1)
- Tripod stand (1)
- Bunsen Burner (1)
- Crucible tongs (1)
- Clay triangle (1)
- ±0.001g Electronic scale (1)
Safety Aspects
Protocol Diagram
Diagram 1 – apparatus as set up for Empirical Formula Experiment
Zero the scales, weigh the crucible and lid and record the mass.
Loosely coil the magnesium ribbon and place into the crucible while on the scales. replace the lid and record the mass., set up the bunsen burner (as shown in the diagram above) by placing the clay triangle on the tripod., place the magnesium into the crucible and position it on the tripod stand using the clay triangle., close the hole on the bunsen burner and light the burner., open the hole so that the bunsen burner is on the blue flame and place the tripod (with the crucible in it) over the bunsen burner., periodically lift the lid (using the tongs) to check if the reaction is complete., when the reaction is finished turn off the bunsen burner and allows the crucible to cool., once cooled, zero the scales and use the tongs to carefully place the crucible and lid on the scales. record the mass., repeat steps 1 – 9 (or collect results from other groups)..

Experimental Report
Data Collection and Processing
Photograph of Lab Setup
Photo 1: Laboratory Setup
- Crucible was used because magnesium could melt plastics when its temperature is high.
- In order to prevent the crucible from falling through the tripod, a clay triangle was employed.
- Bunsen burner was placed underneath the crucible so that the magnesium could be heated efficiently.
Qualitative Observation
Photo 2: Reaction taking place
This is a preview of the whole essay
At the beginning of the reaction, there were not any changes. Meanwhile, the lid was lifted twice. A few minutes later, the magnesium started glowing brilliantly (see the photo above). It glowed for a while until it stopped naturally. White powder was seen in the crucible.
Raw Data Table
*Ms. Crook’s class’ data was used because the data obtained in Table 1 did not have enough dots to draw a graph.
Data Processing
Overview
In order to calculate the empirical formula of magnesium oxide, the net masses of magnesium, magnesium oxide and oxygen were first to be determined. To reach molar ratios, the number of moles of magnesium and oxygen were reached and simplified into the lowest whole number ratios. The empirical formula of magnesium oxide was then decided. During data processing, the percentage uncertainties associated with masses were found so that the percentage uncertainties of the number of moles were concluded. They were later converted into absolute uncertainties. A scatter graph was drawn to show the compositions of magnesium and oxygen within magnesium oxide. The percentage yield of magnesium oxide was also found.
Sample Calculation
Presentation
* The percentage uncertainties of the number of moles were the percentage uncertainties of the mass of each element (explained in Sample Calculation Section). The final value was the greatest percentage errors in Table 2.
*The values of some of the absolute uncertainties are 0.000. This does not mean that there were no uncertainties; it was only because the uncertainties were so small that when they were rounded up to 3 decimal places, they became 0.000.
* Trial 4 seemed to be anomalous. Thus, when calculating the average, the results from Trial 4 were excluded. It is noticed that Ave. of n (Mg) is 0.006 and Ave. of n (O) is 0.004; their ratio seems to be 3:2. However, all the calculations were done by Excel and rounded up to 3 decimals. As a result, the Ave. ratio calculated is 1:1.
*Extrapolation was involved drawing the graph (magnesium: 0.000 mol, oxygen: 0.000 mol) since it is believed when there is no magnesium present, no oxygen will be reacting to form magnesium oxide.
This graph shows the relationship of the composition of magnesium and oxygen within the compound, magnesium oxide. It can be seen that the amount of magnesium is proportional to the amount of oxygen. The gradient of the line of best fit is 0.6005. The positive gradient indicates that an increase in the amount of magnesium will result in a proportional increase in the amount of oxygen. There are outlier points in the graph. The most obvious one could have affected the value of the gradient. In addition, the correlation coefficient (R2) is 0.5751, which is away from 1, indicating not all the points lie on the linear line.
This graph presents the same information as the graph shown above except that it excludes the most obvious outlier point (0.006, 0.001). As a result, the gradient increased (became 0.694) and the R 2 value got closer to 1.
Conclusion and Evaluation
Conclusion and Justification
According to the processed data, the hypothesis that the combustion of magnesium will generate data which can be used to calculate the empirical formula of magnesium oxide is supported because the molar ratios of magnesium and oxygen were managed. As it is shown in Table 3.1, the average ratio of magnesium and oxygen is 1:1, suggesting the empirical formula of magnesium oxide is MgO. However, it has to be noted that some of the results gained were controversial to each other, i.e., in one particular case, the calculated empirical formula of magnesium oxide is Mg 4 O. From Table 3 and Table 3.1, the possible empirical formulas for magnesium oxygen were: MgO, Mg 2 O or Mg 4 O.
Theoretically, the empirical formula for magnesium oxide should be MgO because MgO is an ionic compound. When magnesium atoms and oxygen atoms react with each other, magnesium atoms lose 2 electrons to become Mg 2+ (magnesium ions have a valence of 2+ charge) and oxygen ions gain 2 electrons to become O 2- (oxygen ions have a valence of 2- charge). The outer shells of both ions will then be full so that they become stable like the noble gases. That is to say, the molar ratio of magnesium and oxygen should be 1:1. Thus, the gradient of the scatter graph ought to be 1; though in the first graph the gradient is 0.6. After the elimination of the anomalous, the gradient became 0.7 which is closer to 1.0. It can be deduced that the off-true-value gradient was caused by some outlier points. From both Table 3.1 and Graph 1, it can be seen that the absolute uncertainties of the mass and the number of moles of oxygen were extremely large, indicating the unreliability of the experiment results. Comparing to Graph 1, the correlation coefficient in Graph 2 improved after the outlier point was excluded, i.e., it became 0.9, demonstrating that most of the dots lie on the linear line whose gradient is 0.7. In addition, the percentage yield in Table 4.1 shows the completion of the reaction. None of the results were close to 100%, suggesting magnesium did not react completely. Thus, the empirical formulas obtained were not accurate enough to determine the true formula.
In conclusion, the empirical formula of magnesium oxide can be calculated by the known; yet the accuracy may be affected by circumstances, such as the completion of the reaction. Nevertheless, the theoretical empirical formula of magnesium oxide is MgO.
Limitations of Experimental Design
Generally, the experiment went well since both qualitative and quantitative data had been produced. The empirical formula of magnesium oxide was deduced. Nonetheless, improvement can be made to achieve better results.
There were anomalies present (see Table 3 and Table 3.1): one of the molar ratios calculated was 4:1; some others were 2:1. They were considered outlier points because they are off the true value which is 1:1. The possible reasons could be: 1) the reactions were not fully completed; 2) the readings of the masses were incorrect since it took a while for the electronic balance to reach the real mass. Thus, if the experimenter did not wait till the readings stopped changing; the recorded data would be smaller than they were supposed to be. These could cause random errors. Another random error could be that when the crucible lid was lifted, the produced powdered magnesium oxide might have escaped the crucible. The mass would then be heavier than it was weighed. As shown on the graph, R 2 (the correlation coefficient) equals to 0.5751, indicating that the data was not very reliable since the value was away from 1. In addition, the data points did not spread out, i.e., they were gathering at 0.004 to 0.006 moles. Consequently, the gradient was influenced. The error bars were obviously significant, suggesting the recorded results were not precise.
Systematic errors could be caused by the precision of the equipment, e.g. the electronic balance. However, they can be avoided (see the next section).
Suggestions for Improvement

Document Details
- Word Count 2695
- Page Count 12
- Level International Baccalaureate
- Subject Chemistry
Related Essays

Empirical Formula of Magnesium Oxide

Finding the empirical formula of magnesium oxide

Determining the empirical formula of magnesium oxide lab

IMAGES
VIDEO
COMMENTS
Experiment 602: Empirical Formula . Section 1: Purpose and Summary . Determine the empirical formula of magnesium oxide. Calculate the mass of oxygen using weighing-by-difference. Calculate the mole of a sample from its mass. In this experiment, students will conduct the reaction between magnesium and oxygen gas.
simplified giving an empirical formula of CH 2 O. (Example question - The molecular formula of hydrogen peroxide is H 2 O 2, what is the empirical formula?) To determine the empirical formula of magnesium oxide, you will react elemental magnesium (Mg (s)), with atmospheric oxygen (O 2 (g)), to form solid magnesium oxide (Mg x O y). 𝑴 (𝒔 ...
Empirical formula of magnesium oxide is written: ⚛ with the symbol for magnesium (Mg) written before the symbol for oxygen (O) ⚛ using the lowest whole number ratio of moles of magnesium (x) to moles of oxygen (y), the subscripts for Mg and O are added to give a formula of the type Mg x O y. Note that a subscript number is NOT included in ...
The goal of this experiment is to determine the empirical formula of magnesium oxide experimentally. Objectives. 1. To burn a sample of magnesium in air and measure the gain in mass. 2. To determine the empirical formula of magnesium oxide. 3. To find the theoretical and experimental yields of magnesium oxide, and report statistics on the ...
The object of this experiment is to determine the experimental empirical formula of a compound, magnesium oxide, and comparing it to its theoretical empirical formula, MgO. a table. Before coming to the lab, read the experiment and look at the measurements and calculations you will be making and list the data you will need to record on page 2. II.
Figure 1: The apparatus for the determination of the empirical (simplest) formula of magnesium oxide (image created using Chemix). Some lab manuals talk about only the reaction of magnesium with oxygen 3:. Mg(s) + O 2 (g) à Mg x O y (s) (1)
The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as H2O for water or HO for hydrogen peroxide). When magnesium and oxygen are heated together, they react: magnesium + oxygen magnesium oxide Rxn 1 From the masses of magnesium and oxygen that combine, we can calculate the ...
1 mol Mg. 24.31 g Mg. ( 5b ) mol O = z grams O ×. 1 mol O. 16.00 g O. , where w is the grams of Mg used and z is the grams of O incorporated.The empirical formula of magnesium oxide, MgxOy, is written as the lowest whole-number ratio between the moles of Mg used and moles of O consumed.
Chemistry 212 Lab 5 Empirical Formula of Magnesium Oxide (3.21 g S) 1 mole S 32.06 g = 0.100 mol S The empirical formula is the simplest whole number molar ratio of Al:S in the sample. The simplest ratio is easier to find if the smaller number of moles is placed in the denominator. mol S mol Al = 0.100 mol S 0.0667 mol Al = 1.5 mol S 1 mol Al
If it is already an empirical formula, put a check mark. C 4H 10O 6 Al(SO 3) 1.5 CH 3 Fe(NO 3) 3 Cr 2O 6 2) Based on the charges of magnesium and oxygen, what is the empirical formula for magnesium oxide? 3) Based on the predicted formula for magnesium oxide, state the mole Mg: mole O ratio (eg. 2:3, 1:2, etc.) 4) Based on the predicted formula ...
Explain. The correct formula for magnesium oxide is MgO, a 1.0 to 1.0 ratio. But sometimes in this experiment the ratio of Mg to O comes out too low. (Example: 0.9 to 1.0) In that case, it means that there was too much oxygen relative to the mass of magnesium. At other times it comes out that the ratio is too large.
A typical empirical formula problem is demonstrated. An empirical formula is a formula derived from lab data. Here, the empirical formula of magnesium oxide ...
The empirical formula is the simplest whole-number ratio of atoms in a compound. The ratio of atoms is the same as the ratio of moles. So our job is to calculate the molar ratio of Mg to O. Mass of Mg = 0.297 g. Mass of magnesium oxide = mass of Mg + mass of O. 0.493 g = 0.297 g + mass of O. Mass of O = (0.493 - 0.297) g = 0.196 g.
6. empirical formula of the oxide (individual; class) 7. percent by mass of Mg and O in Mg xOy 8. percent yield of the reaction: Mg + ½ O2 → MgO Discussion Compare experimental empirical formula to theoretical empirical formula Primary sources of error/deviation Effects of factors such as: incomplete conversion of Mg 3N2 to MgO, or
In this experiment, you will carry out the following reaction: Magnesium + Oxygen Magnesium Oxide Aim: To determine the empirical formula of Magnesium Oxide by burning magnesium. Hypothesis: The empirical formula will be MgO. Method: 1. Heat crucible + lid for five minutes and allow it to cool. (to remove water moisture for accurate mass) 2.
Experimental empirical formula of magnesium oxide. 7. Theoretical empirical formula of magnesium oxide. 9/29/15 7 IV. QUESTIONS: Give complete set-ups including all units and labels. Be sure your significant figures are correct. 1. The percent by mass composition of a salt was found to be 56.58% potassium, 8.68% carbon,
Experiment 7: Empirical Formulas 09/23/ Hypothesis: Using the combination and decomposition reactions on two separate samples would allow for an increase/decrease in the sample mass, which would allow to determine the mole ratio and empirical formula. ... Mole ratio of Mg to O 1 : 1 1 : 6. Consensus empirical formula of magnesium oxide. MgO Mg ...
Lab report experiment empirical formulas hypothesis when oxygen combines with magnesium during heating, the mole ratio will be creating the empirical formula to. Skip to document. ... (mol) 0 mol 0 mol Mole ratio of Mg to O 1: 1 mol 1 :1 mol Consensus empirical formula of magnesium oxide MgO Percent by mass (%) 60% Mg and 39% O Part C ...
Students add enough heat to a sample of magnesium to produce magnesium oxide, then analyze the product to determine its empirical formula. Supports NGSS Performance Expectation HS-PS1-7: Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction. Grade Level: High School.
Introduction Magnesium burns in the presence of oxygen to produce magnesium oxide. In this experiment you will determine the percentage composition of magnesium oxide and calculate its empirical. Percent Composition and Empirical Formula Lab Evaluation. Note - you will not be completing this lab - sample results will be provided for your use.
The combustion of magnesium will generate data which can be used to calculate the empirical formula of magnesium oxide. Theory. The following combination reaction was used in this experiment: Magnesium + Oxygen → Magnesium Oxide. The Law of Conservation of Mass can be used to determine the amount of oxygen which has reacted with a given ...
Chemistry Lab determination of the empirical formula of magnesium oxide : lazizjon negmatullaev ap chemistry periods purpose, hypothesis, and materials purpose
Hypothesis Predict the empirical formula for magnesium oxide. Material Solids Mg Apparatus crucible and cover, Bunsen burner, support stand, clay triangle, electronic balance, wire gauze, crucible tongs, sandpaper Safety • Safety goggles and lab aprons should always be worn when working with chemicals.