How Do Clinical Trials Work?
Clinical trials are designed to work in phases that follow strict guidelines, including who can participate. Learning how clinical trials work can help you decide if you want to join.

Who Is Eligible for Cancer Clinical Trials?

"I want to participate in the clinical trial to advance our research and technology. I want to contribute in saving our women in the coming generations." —Madhu Sood, NCI clinical trial participant
Every clinical trial has requirements that must be met for you to join. These requirements are called eligibility criteria.
Common eligibility criteria address things such as your:
- medical history
- family medical history
- risk factors
- treatment history
- tumor’s genetic changes
These criteria help reduce the medical differences among people in the trial, reduce the risk that people will be harmed, and limit people in the trial to those most likely to benefit.
When people taking part in a trial are alike in specific ways, researchers can be more certain that the results are due to the intervention or drug being tested and not to other factors.
What Are the Phases of Clinical Trials?
Clinical trials to test new cancer treatments involve a series of steps, called phases. Depending on the results of each phase, a treatment may move to testing in the next phase.

What Are Clinical Trial Phases?
This video explains the main phases of clinical trials.
The Four Phases of Clinical Trials Early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. Later phases (phase 3 and 4) compare the treatment to current standard treatments.
In a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects are, whether people can tolerate it, and the highest dose that people can tolerate. These trials are done in a small group of people (around 15 to 30). They also make sure a treatment affects the cancer.
A phase 2 clinical trial includes more people (50 to 100) to see if the new treatment seems to work against the cancer, such as by shrinking tumors or slowing their growth. Researchers want to see how the new treatment affects the body and fights cancer. In this phase, teams continue to study safety, including short-term side effects.
In a phase 3 clinical trial, researchers compare the treatment to the current standard therapy to see which works better. They also compare the side effects of the treatments. Participants are randomly assigned to one of the treatments to ensure that any differences are real and not the result of differences in the people in each group. Phase 3 trials include large numbers of people (from 100 to several thousand) to make sure that the result is valid.
Results from phase 1–3 trials are used to make decisions about approving new treatments or existing treatments for new conditions by agencies like the US Food and Drug Administration (FDA).
A phase 4 clinical trial looks at long-term safety and effectiveness that take place after a new treatment has been approved by the FDA and is available to the public. Treatment effectiveness and safety are monitored in large, diverse populations. More information is gathered as more people use the drug or device over a longer period of time.
Randomization and Bias in Cancer Clinical Trials

Randomization in Clinical Trials
Learn how researchers randomly assign clinical trial participants to different treatment groups in order to prevent bias in the results.
Clinical trial randomization is the process of assigning people by chance to groups that receive different interventions or drugs in later phase trials. A computer is most often used to assign people to groups.
In the simplest trial design, the investigational group receives the study intervention or drug and the control group receives standard treatment.
At several points during and at the end of the clinical trial, researchers compare the groups to see which intervention or drug is more effective or has fewer side effects.
Randomization, in which people are assigned to groups by chance alone, helps prevent bias. Bias occurs when a trial's results are affected by human choices or other factors not related to the treatment being tested.
For example, if doctors could choose which patients to assign to which groups, some might assign healthier patients to the treatment group and sicker patients to the control group even without meaning to. This might make the treatment group appear better than the control group even if it isn’t. Randomization helps avoid biases of this type.
If you are thinking about joining a clinical trial that includes randomization, it is important to understand that neither you nor your doctor can choose which group you will be assigned to.
Use of Placebos in Clinical Trials
Placebos are another way to help prevent bias in research. The placebo is designed to look like the medicine being tested, but it is not active. Using a placebo in this way can help prevent you and your doctors from figuring out which group you are assigned to. If doctors know which group you are in, it may affect how they assess your response without meaning to.
Placebos are rarely used in cancer treatment clinical trials. If placebos are used it is likely because no standard treatment exists. Or they may be used in a trial that compares standard treatment plus a placebo, with standard treatment plus the study treatment. You always will be told ahead of time if a study uses a placebo.
Placebos may be used in other types of trials, such as prevention trials.
Research Team Members
Designing and running a clinical trial requires the skills of many experts. Different sites of the same trial may set up their teams differently. Typical team members and their duties include:
Where Do Cancer Clinical Trials Take Place?
Cancer clinical trials take place in cities and towns across the United States and throughout the world.
They take place in doctors’ offices, cancer centers, medical centers, community hospitals and clinics, and veterans’ and military hospitals. A single trial may take place in one or two places, or at hundreds of different sites.
Trials that are funded in full or in part by NCI, include trials that take place at NCI-Designated Cancer Centers and at the NIH Clinical Center in Bethesda, Maryland .

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Find NHLBI Clinical Trials
- How Clinical Trials Work
- Benefits, Risks, and Safety Measures
- Who Can Participate
MORE INFORMATION
Clinical Trials How Clinical Trials Work
Language switcher, what are clinical trials.
Clinical trials are medical studies that involve people like you. They help find new ways to prevent, detect, or treat diseases that are safe and effective. The National Heart, Lung, and Blood Institute (NHLBI) leads and supports many studies aimed at preventing, diagnosing, and treating heart, lung, blood, and sleep disorders.
Clinical trials are an important part of the research spectrum. The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As studies about new treatments move through a series of steps called phases, researchers learn more information about the treatment, its risks, and its effectiveness.
Each clinical trial has criteria describing who can join. Children as well as adults, patients and healthy volunteers, and people of a diverse range of ethnic and racial backgrounds can and are encouraged to participate in clinical trials.
Clinical trials follow a protocol, a carefully designed plan to safeguard your health and answer specific research questions. The protocol describes what you will be doing and what you can expect from the research team. It is important to understand the risks and benefits of participation before joining. You also have rights and protections as a participant in clinical trials.
National Institutes of Health (NIH) Institutes and Centers, including the NHLBI, support many types of clinical trials that contribute to medical knowledge and practice. Clinical trials can be described in a number of different ways, including by their purpose or by phase.
Purpose of clinical trials
Clinical trials have different purposes. What that purpose is helps define the type of trial it is.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Quality-of-life trials, or supportive care trials, explore and measure ways to improve the comfort and quality of life for people with conditions or illnesses.
- Screening trials test new ways for detecting diseases or health conditions.
- Treatment trials test new treatments, new combinations of medicines, or new approaches to surgery or radiation therapy.
Clinical trial phases
Researchers conduct clinical trials in a series of steps called phases. Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials: Researchers test a medicine or other treatment in a small group of people for the first time. The purpose is to learn about the best dosage for a medicine or other treatment and to learn about the safety and side effects.
- Phase II trials: Researchers study the new medicine or treatment in a larger group of people to determine its effectiveness and to further study its safety.
- Phase III trials: Researchers give the new medicine or treatment to an even larger group of participants to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments or a placebo , and collect information that will allow the new medicine or treatment to be used safely.
- Phase IV trials: After the U.S. Food and Drug Administration (FDA) approves a medicine or treatment and it is made available to the public, researchers track its safety in the general population, seeking more information about the medicine or treatment’s benefits and optimal use.
Clinical trial experience
As a participant in a clinical trial, you may work with a healthcare team, and you may need to go to a hospital or other location. Everything that happens throughout your experience follows a plan called a clinical trial protocol.
Governing bodies called Institutional Review Boards (IRBs) approve protocols and are responsible for ensuring your safety. The research team will also operate by other national and international standards that protect you and help produce reliable study results. The NHLBI is one of many types of organizations that support clinical trials.
Before you join a clinical trial, you will be told all about the study, what procedures you will be undergoing, how much time you will be spending on aspects of the study, and any other information you need to know. Once your questions have been answered and you are comfortable, you will be asked to give your consent to participate.
During a clinical trial, you may see doctors, nurses, social workers, and other healthcare providers who will monitor your health closely. You may have more tests and medical exams than you would if you were not taking part in a clinical trial. You may also be asked to do other tasks, such as keeping a log about your health or filling out forms about how you feel.
You may need to travel or stay in a hospital to take part in clinical trials. For example, the NIH Clinical Center in Bethesda, Maryland, runs clinical trials. It is the largest research hospital in the world. Many other clinical trials take place in medical centers and doctors’ offices around the country. If you decide that a trial is not for you, it is important to remember that you can withdraw at any time. Whether you participate will not affect your regular medical care.
Clinical trial protocols
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits of a trial with the risks to participants. It also answers specific research questions. A protocol describes the following:
- Details about tests, procedures, and treatments
- Eligibility requirements
- Expected duration, or how long the study will last
- Goals of the study
- Information to be gathered
- Protections against risks to participants
A clinical trial team is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
Clinical trial designs
There are different types of clinical trials and different trial designs. However, many clinical trials include standard design elements.
- In single-blind (single-masked) studies, you are not told what you are being given, but the research team knows.
- In double-blind studies, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
- Randomization is the process by which participants are randomly assigned a treatment instead of being selected for one or the other. This is done to avoid bias when making assignments. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the study is stopped so that all the volunteers receive the more beneficial treatment.
When the study is finished
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a Phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a Phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in scientific journals in articles that have gone through peer review . Results that are particularly important may be featured in the news and discussed at scientific meetings and by patient advocacy groups. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice. In many cases, if you participated in a blinded or masked study, you will get information about the treatment you received
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study’s official name or Protocol ID number in the National Library of Medicine’s PubMed® database .
Participate in an NHLBI clinical trial
Search our list of research studies by topic, location, and age to see whether you or someone you know is eligible to join.
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

Clinical Trials Guide

Published: 24 June 2019
Introduction
Clinical trials, compared to observational studies, are considered by many to be the gold standard method for evaluation of healthcare interventions. They contribute significantly to relevant research evidence developed by the NIHR to support the NHS in England and other care providers. However, clinical trials are complex and many researchers, particularly those in the early stages of their career, find it challenging to know where to start, either to contribute to or lead a trial.
After conducting an internal report on trainee engagement in clinical trials,the NIHR Trainees Coordinating Centre began a project to develop a source of information to support individuals interested in pursuing a research career that involves the delivery of clinical trials.
Version 1 of this booklet, released in 2015, was the outcome of the first stage of the project which was to determine which questions aspiring trialists need answering ahead of starting their journeys into clinical trials.
This latest version (version 2) contains additional guidance for people looking to develop their career in clinical trials, developed by an NIHR task and finish group set up to consider how to increase capacity and capability in clinical trials. This latest version also contains case studies both of trainees who have started to develop their career in clinical trials and also case studies from CTUs showing how they can provide support to trainees.
If you have any suggestions or feedback then please email [email protected] or tweet us @NIHRcommunity
General Information
What exactly is meant by the term ‘clinical trial’.
A clinical trial is a research project that compares two or more treatments in patients with a particular condition or at risk of a condition to help generate high quality evidence about which is the more effective treatment or preventative strategy. The treatment being investigated in a clinical trial can be a medicinal product, a procedure, a device or another type of therapeutic intervention. Clinical trials are an essential part of the process of evidenced based practice and can help guide treatment decisions for both health care professionals and patients. Clinical trials are an important part of the pathway by which new medicinal products can obtain a licence from MHRA and become available for use as a new treatment in patients.
I like the idea of becoming involved in clinical trials, but don’t know how to go about this. Where do I start?
Clinical trials are performed widely across the NHS and the Research and Development Department in your local NHS Trust will have a record of all the clinical trials active in your hospital. Many trials are also registered on national and international databases that are searchable and can identify trials in specific diseases or using specific treatments. These include the NIHR research network databases, Clinical Trials.gov, UK Clinical Trials Gateway and EudraCT.
Many clinical trials, especially those involving a new medicinal product or involving multiple sites, are supervised by a Clinical Trials Unit (CTU) or a Contract Research Organisation. Your local registered CTU will also be a good point of contact about clinical trials being performed in your area.
What is generally involved in conducting a clinical trial?
A clinical trial should be considered when there is uncertainty as to which of a range of treatment options or preventative strategies is more effective.A team of investigators are responsible for conducting a clinical trial and this requires meticulous planning. Once the case for a new clinical trial has been made on medical, ethical and financial grounds then the trial needs to be designed so that it will provide the highest possible quality of evidence to guide future decision making. Trial design is a multi-disciplinary activity involving input from clinicians, trial methodologists, pharmacists, statisticians and health economists among others. After the clinical trial is designed, the funding to pay for the trial to be conducted must be identified either from industry, who may fund a clinical trial as part of the development pathway for a new medicinal product, device or technology or from a research funding body such as NIHR or Medical Research Council or from a charity such as Cancer Research UK or the British Heart Foundation. After funding is secured, then all the necessary permissions such as research ethics approval and NHS research governance approval must be sought. Training on the legal responsibilities when conducting a trial can be provided locally as a Good Clinical Practice (GCP) course which is offered by your local NIHR Clinical Research Network.
How will I know whether or not a clinical trial is appropriate for my research?
Before embarking on a clinical trial it is important to establish whether a new trial is indeed needed. You should check what research has already been done. Are there existing trials that may provide enough evidence to answer the question that you wish to address? The NIHR is committed to avoiding waste in research and unjustified duplication of a trial is unlikely to be funded. The NIHR and other research funders recommend that all clinical trials should start with a systematic review of the existing research evidence. This may reveal that there is already sufficient high-quality research evidence to answer your research question (in which case you will need to think of a new trial or project) or provide sound information to justify your research,and potentially help with your trial design.
If a clinical trial isn’t appropriate, what are my other options?
Clinical trials are not always the most appropriate option to further your research and to support the development and evaluation of new treatments. A pilot study to assess the feasibility of conducting a clinical trial is often needed. The pilot study will allow the team of investigators to determine the likely difficulties in performing a full clinical trial and also inform the calculations on sample sizes in a full clinical trial to be done. An observational study may be a more appropriate option if there is uncertainty about the most robust endpoints to use in a clinical trial, or if the mechanism of a potential new treatment has not been established.
What would be a realistic timescale for a clinical trial and does this differ at all?
The time required to design a clinical trial, produce the detailed trial protocol and secure all permissions is substantial and can take 6-12 months to complete. The time required to perform the clinical trial will vary widely and will depend on the sample sizes needed, the frequency by which participants are recruited and the follow up period for each participant in the study.
How many projects should I become involved in?
If new to clinical trials, it is best to get involved in one clinical trial initially and fully understand the processes involved in more detail.
What pitfalls should I be aware of in general?
By working with an experienced CTU and experienced trial methodologists then the risk of pitfalls can be reduced. However, common pitfalls include underestimating the time it takes to develop the trial protocol and secure all the permissions before starting.
Failing to recruit participants in an appropriate time-frame is also a significant pitfall. There is a risk in over predicting the ease by which specific groups of patients will be willing to participate and this can lead to unrealistic milestones being set.
Careful monitoring for serious adverse events is essential when conducting a clinical trial. If these events occur they will be reviewed by the research ethics committee and by an independent data monitoring committee who have the power to terminate a study early if there is potential that the intervention being assessed is causing harm.
The following simple checklist may help you decide whether a clinical trial is appropriate for your research:
Step 1 – establish whether a relevant systematic review already exists
- If yes, and this resolves the clinical uncertainty – stop;
- If yes, and it demonstrates continued uncertainty – continue to design and justify your trial, using information from the systematic review as part of your justification;
- If yes, but the review is out of date, or of poor quality – consider updating the review;
- If no – consider doing a systematic review.
Step 2 – establish whether relevant clinical trials exist Are there already clinical trials that address your research question;
- If no, continue to design and justify your trial
- If yes, but there is clearly insufficient evidence to answer the clinical question robustly e.g. a single trial with uncertain results - use this information to justify the need for and inform the design of your trial
- If yes, consider carrying out the systematic review as a first step.
Even if similar reviews or trials exist, if these are in a different context or setting, or address a slightly different question, your trial may still be relevant – but it will be important to be clear why it is different and still needed.
When searching for systematic reviews or clinical trials it is helpful to enlist the help or advice of a trained information specialist or medical librarian.
Useful places to search:
You can find examples of completed systematic reviews at the Cochrane Library and University of York Centre for Reviews and Dissemination . Examples of ongoing systematic reviews can be found at University of York PROSPERO and ongoing clinical trials can be accessed at Be Part of Research , the ISRCTN Registry , the U.S National Library of Medicine Clinical Trials , and WHO International Clinical Trials Registry Platform .
Fellowships and Clinical Trials
Do clinical trials fit within the remit of nihr research training awards.
NIHR spends a large proportion of its research programme budget on clinical trials. The importance of clinical trials to the NIHR means that it is very keen to attract and develop future clinical trial leaders. NIHR research training awards support outstanding individuals to become health research leaders of the future and supporting people who will lead NIHR funded trials is very much within this remit. The schematic below highlights how you may utilise NIHR’s suite of research training awards to start or further your career in clinical trials.
What aspects of a clinical trial can realistically be included within a Fellowship application to NIHR? Does this change depending on the level of award I apply for?
Applicants do need to consider:
- the type (e.g. clinical trial of investigational medicinal product (CTIMP),trial of surgical intervention or trial of complex intervention)
- the scope (single or multi-centre)
- feasibility / pilot trial (the NIHR Glossary can provide you with additional resources related to the feasibility/pilot trial)
- phase of trial (I to IV), and
- risk level of the trial (see GOV.UK Clinical trials for medicines: manage your authorisation, report safety issues in respect of CTIMPs )
Applicants should also ensure it is commensurate with the level of award and experience of the applicant. For example, we would not normally expect a doctoral level applicant to propose leading a multi-centre randomised controlled trial of an investigational medicinal product. Fellowship applications, especially at doctoral and early post-doctoral level, will tend to focus on feasibility and pilot trials or may form a distinct add-on to an existing trial (in this case it must be clear the trial is a distinct, standalone piece of work and the role of the applicant must be clear).
Applicants are strongly encouraged to read the additional guidance from page 46, which outlines the expectations NIHR has for what a research training award based around clinical trials should deliver, and should be read in conjunction with guidance specific to the scheme you are applying to.
What is the difference between running a clinical trial in an NIHR Fellowship and an NIHR project grant?
It is very important that applicants keep in mind that the proposed research project in a Fellowship application is a vehicle for training and this needs to be clearly demonstrated as part of the application.
Applications for a fellowship can’t just look like a project grant application.
Applicants should also consider the feasibility of the trial within the scope of a fellowship award. NIHR research training awards are personal fellowships and not project or programme grants; therefore awards will not be extended to allow completion of a trial. Please bear in mind the lead in time for clinical trial set-up vis-à-vis the time available within the course of a fellowship.
Run-in time for drug and placebo procurement, manufacture and packaging for CTIMPs and the fact these activities must be completed, before regulatory approval can be sought, must be taken into account when planning the fellowship schedule and completing the application form. Regulatory, ethical and R&D approval can take several months and appropriate advice on the processes and timelines should be sought from the outset.
How do I move from hypothesis-generating to clinical trials hypothesis-testing under an NIHR funded career pathway?
There are a number of pathways, depending on a person’s experience and the sort of trial needed. This might be a doctoral fellowship, for a hypothesis answered within a small single centre trial, or the doctoral/postdoctoral fellowship might be a pilot/feasibility study, then a later fellowship might support a full trial. If in doubt, please contact your local Clinical Trials Unit, Research Support Service or the NIHR Academy.
What parts do I need to seek approvals for in my research? Where do I obtain approval from?
On 31 March 2016, Health Research Authority (HRA) Approval became the route for applying for approval to conduct research in the NHS in England. HRA Approval brings together the assessment of governance and legal compliance, undertaken by dedicated HRA staff, with the independent REC opinion provided through the UK research ethics service.
HRA Approval removes the need for NHS permission to be issued by each participating organisation and replaces the local R&D approval process previously delivered through NIHR CSP. As a result the NIHR CSP system was withdrawn from service on 5 August 2016, following its closure to new applications in March.
HRA Approval will provide a single approval for project based research in the NHS in England.
Local organisations will now concentrate on assessing, arranging and confirming that they have the capacity and capability to participate in the study.
If your study is eligible to access our Clinical Research Network (CRN) support, we will support you with setting up and delivering your study within the NHS through the Study Support Service .
Please ensure that you apply to the CRN as early as possible, ideally before submitting any other regulatory approvals, including your application for HRA Approval, using the Portfolio Application Form Integrated Research Application System.
Is there a pathway I can follow in order to obtain regulatory approval? Where can I find out information on trial governance?
Our workforce development team has developed Good Clinical Practice training which is free to researchers and may be useful for trainees.
How much monitoring should I be doing?
Monitoring and governance information can be found within the HRA research community website , the monitoring of a clinical trial is usually done by the Clinical Trials Unit and/or the Sponsor institution.
How do I, and can I, publish the protocol for a clinical trial?
It is a condition of Research Ethic Committee (REC) favourable opinion that trials are registered, you can find guidance at the Health Research Authority (HRA) In addition, many trial protocols are published in journals, such as the online BioMed Central (BMC) series.
Please note that trial registration is not quite the same as publication of the protocol. Open access journals will often publish trial protocols and they can also be made available on a study or unit website. Further information on approvals and when they will be required can be found by using the Clinical Trials Toolkit
Designs, Types and Planning
What are the different types of trials.
There are many clinical trial designs and the exact type depends on your research question. The optimum design is the one that is least likely to incur bias and will have the best chance of answering your research question. For example, if the intervention under investigation is delivered in groups, it might be most appropriate to choose a cluster randomised trial. Alternatively, if you are testing a drug to treat a chronic disease, you are more likely to consider an individually randomised design. When considering trial ‘phase’, the most common phases in clinical trials are phase II trials, feasibility trials, pilot trials and phase III trials of effectiveness/efficacy.
The term ‘phase’ usually refers to I-V:
- I = first in man
- II = proof of concept/efficacy
- III-V = effectiveness
- Pilot or feasibility studies could be done for any phase of trial.
For further information, please see the following:
What is a pilot or feasibility study? A review of current practice and editorial policy. Arain M, Campbell MJ, Cooper CL, Lancaster GA. BMC Medical Research Methodology 2010, 10:67. doi:10.1186/1471-2288-10-67
Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials : Development of a Conceptual Framework. Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, Bond CM. PLoS ONE 2016, 11(3): e0150205.
I’ve heard other trainees talk about trial designs, what does this mean?
Trial designs incorporate many aspects to optimise the ability for teams to answer their research questions. The term can refer to all aspects of the study design and how it is implemented, including all methodological aspects and the patient pathway.
Commonly used designs in trials include:
Parallel group trials – groups or individuals randomised to one of two interventions (A or B) with outcomes compared at the final endpoint (either by comparing differences in a pre-specified primary outcome at a pre-specified time point, or by comparing the disease severity between baseline and follow-up).
Factorial trials – groups or individuals randomised to single treatments (A or B), or a combination of treatments (A and B). This design allows you to answer two or three questions at once (e.g. is treatment A more effective that treatment B / Is the combination of treatments better than a single treatment A etc.) and enables you to consider potential interactions.
Cross over trials – groups or individuals randomised to one of two treatments (A or B), followed by a wash-out period (not always needed) then switching of treatments (B or A). These are only possible in trials of chronic conditions; they are also carried out for other conditions; however whether or not this is useful is a different question.
Whilst the above gives a brief overview, it should not be taken that this is all that needs to be considered in study design. For more information refer to the NIHR Clinical trials toolkit
How would I know which design to choose? What do I need to consider when choosing my design?
It is important to talk to people with expertise and experience in trial design and methods to help you design your study.
If you are involving a CTU, there will be experts here to support you. Otherwise, your supervisory or mentoring team should include someone with experience and/or expertise in trial design, and the your local Research Support Service will be able to provide additional advice. Trial statisticians are very important collaborators that can help with planning and designing your study.
Once you think you have chosen the best design, consider all of the ways that bias might be introduced in that design. For more information refer to the NIHR clinical trials toolkit .
How can I minimise the potential for bias in my clinical trial design?
Each type of possible bias should be considered and appropriate approaches/designs implemented, such as participation bias reduced by the recruitment methods(e.g. recruited by independent researchers/clinicians).The Cochrane handbook is a resource commonly used for systematic reviewers.
Are there any study designs that can be used in situations where it wouldn’t be appropriate/feasible to conduct a clinical trial?
Trials are no longer restrictive and many innovative approaches and study designs are possible, even with the most complex of interventions.
What are the advantages and disadvantages of a multi-centred trial?
Multi-site trials make results more generalizable to the population. They also increase the ability to recruit. Multi-centred trials require a different set of skills and expertise to single site studies and are often more challenging to conduct, e.g. more work getting sites to agree, set-up, approvals and monitoring.
Can NIHR trainees or fellows lead a multi-centred trial?
From the point of view of the NIHR a trainee can lead a multi-centre study and it may be very beneficial for their training and development to do so, provided they have appropriate experience of clinical trials and the right support around them. Please be aware that there are both large multi-centre and small multi-centre studies; this is not a single entity.
Any decision about the role a trainee will have on a clinical trial and the size and scope of that trial should be taken in discussion with supervisors and/or mentors bearing in mind the scope of the research training award in which the trial will be included and the experience and expertise of the individual. For instance, a senior fellowship holder with significant clinical trials experience may well be very suitable to lead a multi-centred trial, whereas a doctoral level fellow with limited trial experienced would be more likely to focus on smaller scale/feasibility studies.
Methodologies
Where would i go to find help with analysing data produced by the trial.
Analysis plans are usually written by trial statisticians with input from the study team, and the data analysis is likewise conducted by the trial statistician. This is in part to ensure robust blinding to the study intervention throughout the trial. However, where a clinical trial forms part of a higher degree, especially where a pilot or feasibility study is conducted, it would be very suitable for the PhD student to be involved in the data analysis.
The level of input will depend on the level of the fellowship that you’re applying for and what can be requested in terms of the scheme. It is not recommended that fellows seek to do this on their own unless they have expertise in this area. Given that most applications will be for pilot or feasibility studies, most trainees will only be looking at descriptive analysis; however most will also involve sample size calculations, which will require input from an expert.
Where can I get statistical assistance, such as how to do a power calculation?
At the pre-funding application stage, this support can be sought either from the trials unit that you are collaborating with or from the Research Support Service.
What should I be looking out for when interpreting data from clinical trials?
The analysis plan (which should be written during the set-up period) should clearly indicate how data will be analysed and will state what the measure of efficacy/effectiveness will be. Collaboration with a statistician is essential for writing the analysis plan. Once the analysis is complete, interpretation of the data should involve the full trial team including all stakeholders. Involving patient and public involvement groups will help ensure a patient perspective in interpretation of the data.
Teams and management
Are there any managerial and/or structural frameworks available for managing a clinical trials team.
Things to bear in mind when managing a team are:
- make sure you choose the right people (provision of expertise and those conducting the research)
- ensure accountability (e.g. contracts)
- schedule meetings in advance
- standing agenda items related to co-applicant involvement
- publication strategy and plan in advance (linked to protocol)
Visit the managing clinical trials page of the NCBI website for further information on clinical trials management
Patient and public involvement
What is public involvement in research.
INVOLVE defines public involvement in research as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. The NIHR expects patients and the public to be actively involved in all stages of the research process from project design to disseminating the findings in any research it funds. When using the term ‘public’ we include patients, potential patients, carers and people who use health and social care services as well as people from organisations that represent people who use services. Whilst all of us are actual, former or indeed potential users of health and social care services, there is an important distinction to be made between the perspectives of the public and the perspectives of people who have a professional role in health and social care services.
Where do I start with patient and public involvement?
To help you plan and undertake public involvement in your research we suggest you consider the following points:
- involve people as early as possible
- be clear with those you involve about what their role will be
- be accessible
- resource public involvement in research
- offer training and support
- clarify organisational responsibilities
- document and record public involvement in your research
Guidance for patient and public involvement can be found at the NIHR public involvement briefing notes for researchers
What are the practical issues regarding selection? Who should I involve and how do I find them?
In deciding who best to involve it is important to think about the knowledge and perspective that you are looking for from members of the public, and what support you are able to give to people who you plan to involve.
Even if your research is about informing practitioners about approaches to practice, the end user of the research will be the person receiving the practice. In some research projects you will want to consider involving both practitioners and members of the public.
Once you have considered who you would like to involve, you then need to think about how to make contact with them. Speak with colleagues and members of the public and ask for their views on how to find the people you want to involve. Allow time to make contact with organisations and individuals as finding people will nearly always take longer than you think.
Clinical trials unit
What does a clinical trials unit (ctu) do.
CTUs are specialist units which have been set up with a specific remit to design, conduct, analyse and publish clinical trials and other well-designed studies. They have the capability to provide specialist expert statistical, epidemiological and other methodological advice and coordination to undertake successful clinical trials. In addition, most CTUs will have expertise in the coordination of trials involving investigational medicinal products which must be conducted in compliance with the UK Regulations governing the conduct of clinical trials resulting from the EU Directive for Clinical Trials.
Units awarded UKCRC Registration are required to provide evidence to an international panel of experts of their capability to centrally coordinate multi-centre clinical trials (i.e. having overall responsibility for the design, development, recruitment, data management, publicity and analysis of a portfolio of trials), and that they have established robust systems to ensure conduct and delivery of clinical trials to the highest quality standards. More information on the UKCRC CTU Network and unit registration can be found at UKRC Registered Clinical Trials Units Network
In what ways will a CTU help me with my clinical trial?
CTUs collaborate with you to play a key role in providing the dedicated expertise and support necessary for the design, development, management, analysis and publication of high quality clinical trials. Registered CTUs will usually work with the Chief Investigator on the following:
Coordination and preparation of the grant application including
- Trial development (including the question identification and appropriate design)
- Systematic reviews (when appropriate)
- Trial costing and staff planning
- Discussion with disciplines required for different trial components e.g. quality of life, health economics, associated translational research
- Sub-study development. Communication with research networks regarding feasibility and levels of interest
- Conduct of the trial including
- Regulatory and governance issues
- Negotiation with international collaborators and/or industry (if applicable)
- Management of funded trials
- Protocol development and Case Report Forms (CRFs) design
- Liaising with potential centres and sites, identifying and initiating participating centres, and maintaining good communications throughout to deliver required patient identification and recruitment
- Trial set-up and permissions (e.g. ethics, MHRA etc.)
- Central coordination and management of essential trial documents and patient data
- Data monitoring
- Analysis and publication including
- Interim and final analyses
- Report preparation (e.g. for funding bodies, MHRA, Data Monitoring Committee, Trial Steering Committee)
How do I gain access to a Clinical Trials Unit?
The UKCRC CTU Network contains a variety of information on registered units, including a resource finder. You are able to search for CTUs that are interested in supporting fellowships and other research training award applications and also search based on the disease area, study type and methodological expertise of the CTU.
The NIHR recognises the important and crucial role played by CTUs in helping the design, development and delivery of quality research projects.
If you are interested in working with a CTU, you should contact them as early as possible in the process. Ideally, this should be at least three months before a research grant application deadline (although many units prefer longer than this for open calls) in order to provide adequate time to schedule the work required and ensure the CTU is able to offer the full benefit of its experience and knowledge from the initial stages of study development. You will need to provide the CTU with information about your study and your requirements. Some CTUs will have their own collaboration request form.
What are NHS Service Support Costs?
The Department of Health guidance ‘ Attributing the costs of health and social care Research and Development (AcoRD) ’ defines NHS support costs as ‘the additional patient care costs associated with the research, which would end once the R&D study in question had stopped, even if the patient care involved continued to be provided’. For the purpose of attributing costs during a research study, an assumption is made that the care/treatment under review will become standard. For example, if during a clinical trial patients require additional tests to pick up any adverse effects to the new treatment which wouldn’t need to be continued if the treatment later became standard care in the NHS, the costs of these additional tests would be classed as service support costs.
What is classified as an Excess Treatment Cost?
AcoRD guidelines classify NHS treatment costs as ‘the patient care costs, which would continue to be incurred if the patient care service in question continued to be provided after the R&D study had stopped.’ So continuing the example from the above the NHS treatment costs would be the costs of treating the patients in the clinical trial that would continue if the new treatment later became standard care in the NHS. Excess treatment costs arise when the new treatment being trialled is more expensive than standard care.
The difference between the treatment costs of the new intervention and standard care is classed as the excess treatment costs. Of course the new intervention may be cheaper than standard care in which case a saving in treatment costs to the NHS will be observed.
How do I cost a clinical trial for an NIHR Fellowship application?
All NIHR application forms are accompanied by extensive guidance notes which detail what costs can and cannot be included in a particular application. Again this is another area where expert advice must be sought.
Additional support
What support can the research support service offer.
The Research Support Service (RSS) supports research teams to develop and submit high quality applied health and social care grant applications to NIHR and other national peer-reviewed funding programmes.
The RSS offers specialist advice on all aspects of an application including:
- designing a research study
- research methods (qualitative and quantitative)
- identifying suitable sources of funding
- involving patients and public in research design
- identifying potential academic, clinical and lay collaborators
Their advice is confidential and free of charge.
Who can I contact for specific information about how to fit a clinical trial into an award?
Please direct all queries [email protected] and a relevant member of the team will contact you.
Summary of useful links
- Completed systematic reviews can be found at the Cochrane Library and the Centre for Reviews and Dissemination
- Ongoing systematic reviews can be found at PROSPERO
- Ongoing clinical trials information can be found on the following websites, Be part of research , the ISRCTN Registry , the U.S National Library of Medicine – Clinical Trials and WHO – International Clinical Trials Registry Platform
- Information and guidance for feasibility and pilot studies can be found on the NIHR Guidance on applying for feasibility studies webpage
- More information about trial risk levels can be found by visiting the clinical trials for medicines page on the government website
- Regulatory body approval information can be found at HRA approval and amendments (link) and MHRA – how we regulate
- Information about trial governance can be found at the NIHR Good Clinical Practice (GCP) and HRA Managing your approval websites
- Guidance for the publishing protocol of a clinical trial can be found on the Health Research Authority (HRA) Research planning webpage and the NIHR clinical trials toolkit routemap
- Resources for the planning and design of your trial can be found in the NIHR clinical trials toolkit - Trial Planning & Design
- Information about minimising bias can be found in the Cochrane handbook for systematic reviews of interventions
- Support for managing a team in a clinical trial can be found in the managing clinical trials article
- Guidance for patient and public involvement can be found at the NIHR public involvement briefing notes for researchers
- Information about Clinical Trials Units (CTUs) can be accessed at UKCRC Registered Clinical Trials Units Network
- Information relating to NHS support costs can be found at the Attributing the costs for health and social care research and development (AcoRD) guidance notes
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
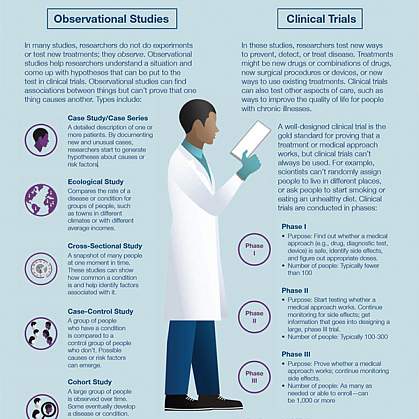
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you.
The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial.
What are clinical trials and why do people participate?
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:

- Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups.
- Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
- Health services, which looks at how people access health care providers and health care services, how much care costs, and what happens to patients as a result of this care.
- Clinical trials, which evaluate the effects of an intervention on health outcomes.
What are clinical trials and why would I want to take part?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future
Why is diversity and inclusion important in clinical trials?
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
See Diversity & Inclusion in Clinical Trials for more information.
How does the research process work?
The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness.
What are clinical trial protocols?
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
- The goal of the study
- Who is eligible to take part in the trial
- Protections against risks to participants
- Details about tests, procedures, and treatments
- How long the trial is expected to last
- What information will be gathered
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
What is an Institutional Review Board?
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB.
What is a clinical trial sponsor?
Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research.
What is informed consent?
Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What are the types of clinical trials?
There are different types of clinical trials.
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
What are the phases of clinical trials?
Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials : Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials : The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials : The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials : After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
What do the terms placebo, randomization, and blinded mean in clinical trials?
In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value).
Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial.
Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials .
" Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Who takes part in clinical trials?
Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research.
Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers.
Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks.
A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants.
Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition.
Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need.
What do I need to know if I am thinking about taking part in a clinical trial?

Risks and potential benefits
Clinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
- The possible harms that could result from taking part in the study
- The level of harm
- The chance of any harm occurring
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits.
Potential benefits
Well-designed and well-executed clinical trials provide the best approach for you to:
- Help others by contributing to knowledge about new treatments or procedures.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
Risks to taking part in clinical trials include the following:
- There may be unpleasant, serious, or even life-threatening effects of experimental treatment.
- The study may require more time and attention than standard treatment would, including visits to the study site, more blood tests, more procedures, hospital stays, or complex dosage schedules.
What questions should I ask if offered a clinical trial?
If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
- What is the purpose of the study?
- Why do researchers think the approach may be effective?
- Who will fund the study?
- Who has reviewed and approved the study?
- How are study results and safety of participants being monitored?
- How long will the study last?
- What will my responsibilities be if I take part?
- Who will tell me about the results of the study and how will I be informed?
Risks and possible benefits
- What are my possible short-term benefits?
- What are my possible long-term benefits?
- What are my short-term risks, and side effects?
- What are my long-term risks?
- What other options are available?
- How do the risks and possible benefits of this trial compare with those options?
Participation and care
- What kinds of therapies, procedures and/or tests will I have during the trial?
- Will they hurt, and if so, for how long?
- How do the tests in the study compare with those I would have outside of the trial?
- Will I be able to take my regular medications while taking part in the clinical trial?
- Where will I have my medical care?
- Who will be in charge of my care?
Personal issues
- How could being in this study affect my daily life?
- Can I talk to other people in the study?
Cost issues
- Will I have to pay for any part of the trial such as tests or the study drug?
- If so, what will the charges likely be?
- What is my health insurance likely to cover?
- Who can help answer any questions from my insurance company or health plan?
- Will there be any travel or child care costs that I need to consider while I am in the trial?
Tips for asking your doctor about trials
- Consider taking a family member or friend along for support and for help in asking questions or recording answers.
- Plan what to ask — but don't hesitate to ask any new questions.
- Write down questions in advance to remember them all.
- Write down the answers so that they’re available when needed.
- Ask about bringing a tape recorder to make a taped record of what's said (even if you write down answers).
This information courtesy of Cancer.gov.
How is my safety protected?

Ethical guidelines
The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science.
Informed consent
Informed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits.
If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time.
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB.
Further reading
For more information about research protections, see:
- Office of Human Research Protection
- Children's Assent to Clinical Trial Participation
For more information on participants’ privacy and confidentiality, see:
- HIPAA Privacy Rule
- The Food and Drug Administration, FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective
For more information about research protections, see: About Research Participation
What happens after a clinical trial is completed?
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice.
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database .
How does clinical research make a difference to me and my family?

Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers.
This page last reviewed on October 3, 2022
Connect with Us
- More Social Media from NIH
UW Health recently identified and investigated a security incident regarding select patients’ information. Learn more
- Find a Doctor
- Conditions & Services
- Locations & Clinics
- Patients & Families
- Refer a Patient
- Refill a prescription
- Price transparency
- Obtain medical records
- Clinical Trials
- Order flowers and gifts
- Volunteering
- Send a greeting card
- Make a donation
- Find a class or support group
- Priority OrthoCare
- Emergency & Urgent care
April 16, 2024
Your guide to understanding phases of cancer clinical trials

Clinical trials can be an important resource for patients at every stage of their cancer diagnosis, but understanding the scientific terms, study protocols and process can be intimidating to many patients.
That’s why UW Health | Carbone Cancer Center prioritizes educational resources, including a team of Clinical Trial Nurse Navigators and the new uwhealth.org/cancertrials webpage, to ensure patients have accurate information about how clinical trials work and how their care and safety will be prioritized at every step.
“Having a good, educational source of information about clinical trials is so important because there are a lot of myths and misconceptions out there,” said Sarah Kotila, Clinical Trials Navigation Team Manager at Carbone Cancer Center.
One of the most common questions patients have is what the different phases of a clinical trial mean. Read more about each step in the important process of approving new cancer treatments.
In this initial step of a clinical trial, the research staff is looking at the safety and appropriate dosage of giving a new treatment. They also watch for side effects. The number of patients enrolled on phase I trials are small — there are typically fewer than 50 patients involved.
Kotila often hears patients ask if phase 1 trials are safe. She explains that any cancer treatment comes with potential risks and benefits, whether it’s established clinical care or treatments being tested in clinical trials. With clinical trials, a team of experts is closely monitoring the patient’s care and frequently checking in to see how the patient is doing and feeling.
“Patients should know there can be risks and benefits to any cancer treatment they receive, and this is the same with clinical trials,” she said.
Kotila adds that to get Food and Drug Administration approval to start a clinical trial there has been considerable lab and preclinical research already done to prepare their research for this important next step.
Once a study has cleared its phase I benchmarks, it can move into phase 2. Researchers at this stage continue to monitor safety and are focused on whether the new treatment method is effective for certain diagnoses.
“In phase II, they’re having more people enroll to see if the treatment is effective in specific types of cancer” she said. “It’s still a smaller number, usually less than 100 people.”
Because they are measuring whether the approach is effective, phase 2 typically lasts several months to two years to measure changes over time. Researchers also continue to monitor for side effects that were not seen in phase I with the smaller group.
If the treatment proves to be effective for certain types of cancer, it can advance to phase 3 status.
This is the final step of testing before a treatment can be approved by the FDA for standard clinical use. The new treatment is being compared directly to existing standard of care treatments to determine if the new treatment is as good or better than our current treatments. The patient pool is at least several hundred people to get a widespread view of patient effects and validate the findings.
Placebos, which are inactive substances designed to look like study medication, can be used to randomize patient effects in the study and help preserve integrity of results when evaluating a new treatment. Placebos are rarely used in cancer treatment clinical trials. Kotila reassures patients who are afraid of placebos that they will still get treatment when needed.
“It would be unethical to not treat a cancer patient that needs treatment. Patients in these phase III randomized drug trials may get standard of care plus a placebo or standard of care plus the new treatment being tested, but they will always be treated,” she said.
FDA approval
So what happens if you’re part of a clinical trial and the study medication receives FDA approval? Dr. Mark Burkard , a physician-scientist who leads several clinical trials at Carbone, said the study’s sponsor can choose to stop the trial or continue studying long-term effects.
“In most cases, the sponsor will continue the trial to collect additional information about how the drug works, allowing the patient to choose (if they continue),” Burkard said. “Most patients choose to continue the trial. Some choose to stop the trial, if for example, they live far away and can get the same medicine from an oncologist close to home.”

Learning more
Kotila said patients who are considering clinical trials and have more questions can contact the Clinical Trials Navigation Team at (608) 262-0439 or [email protected] . If a patient would like to schedule an appointment or be seen for a clinical trial at UW Carbone, please contact our intake team at (608) 262-5223 .
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
- Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Introduction to Cancer Research
- About Cancer Clinical Trials
- Patient Safety in Clinical Trials
- Phases of Clinical Trials
- Health Insurance Coverage of Clinical Trials
- Placebos in Cancer Clinical Trials
- Questions to Ask About Clinical Trials
- Finding a Clinical Trial
- What is the TAPUR Study?
- ASCO Annual Meetings
- Health Disparities and Cancer
- For Patient Advocates
- Public Policy Advocacy
- Cancer Awareness Dates
- Survivorship
Clinical trials are research studies that include human volunteers. Before they start a clinical trial , doctors must prove there is a chance that the new treatment will work better than the one available now. They do research until they can prove this. For example, they might test the treatment on laboratory animals. They do this to make sure it is safe to test in people.
What happens in different phases of clinical trials?
Once clinical trials are approved to start, each one must follow certain steps in order. The steps are called "phases." They are designed to keep volunteers safe. Making sure all the steps are done helps protect patients and give accurate results about what the clinical trial is testing.
You may join any phase of a clinical trial. The clinical trial just needs to be appropriate for you, your health, and your cancer. Here is a chart about the different phases of clinical trials .
Phase I clinical trials
Doctors do a phase I clinical trial to learn if a new drug, treatment, or treatment combination is safe for people. They may have already tested it in laboratory animals.
In a phase I clinical trial, doctors collect information on:
The dose or treatment
When you take it, and how often
Any side effects or problems
How the treatment affects you, such as how it affects the cancer or side effects
In a phase I clinical trial, you could be one of the first people to get the new drug or treatment.
Phase I clinical trials each last several months to a year. They usually have 10 to 30 volunteers. The treatment might help the cancer. Also, information from the clinical trial may help other people in the future.
Phase II clinical trials
A phase II clinical trial tells doctors more about how safe the treatment is and how well it works. Doctors also test whether a new treatment works for a specific cancer. They might measure the tumor, take blood samples, or check how well you can do certain activities. Or you might keep a log of your daily activities and symptoms. These are all ways to learn how well the treatment works.
A Phase II clinical trial lasts about 2 years. Volunteers sometimes receive different treatments. For example, a phase II trial could have 2 groups.
Group 1 – People who receive the usual treatment for the condition. This is also called the standard treatment. It is the best treatment known.
Group 2 – People who receive the usual treatment plus the new treatment doctors are studying.
Or a phase II clinical trial could have 3 groups. Volunteers in each group get a different dose of the treatment doctors are studying.
If the phase II clinical trial shows the treatment works and is as safe as the regular treatment, doctors can do a phase III trial.
How do doctors put volunteers into groups in a clinical trial?
Doctors use a computer program to put volunteers into different groups. The computer does this at random, which means by chance. Each volunteer has an equal chance of going in any of the groups. The name for this process is "randomization."
Using a computer to put volunteers in groups keeps the research staff from possibly changing the clinical trial results. They might do this if they chose who went in which group. For example, they might think a certain volunteer would benefit from the new treatment. So they might put that person in the new-treatment group. But this could change the clinical trial results. Randomization helps avoid this. It is very important to use randomization when a clinical trial compares 2 treatments or more.
Phase III clinical trials
A phase III clinical trial tests a treatment that worked well for volunteers in a phase II clinical trial. Doctors use phase III to compare the new treatment with the standard treatment. They want to know if the new treatment is better, has fewer side effects, or both. So they put volunteers in different groups. The volunteers in each group get a different treatment.
Phase III clinical trials can take many years. They may have several thousand volunteers. These must include men, women, and people of different ages and ethnic groups, if possible. This helps doctors learn how the treatment works in different people.
If a phase III clinical trial shows the treatment works well, doctors might begin using it with people outside the clinical trial. For example, if they learn that a certain amount of exercise lowers your cancer risk, they publish a report. This shares the information with other doctors. If the researchers or sponsor learn a new medicine is safe and effective, they can ask the government to approve it for people to use. In the United States, they ask the Food and Drug Administration (FDA). The FDA looks at the results of the clinical trial's phases. They approve the treatment if the results meet their standards.
Phase IV clinical trials
Doctors can prescribe a drug for their patients after the FDA approves it. But the FDA may require the sponsor to keep studying that approved treatment. In these clinical trials, doctors may check if the treatment benefits people as much as it did earlier. They also look for more possible side effects. These clinical trials are called phase IV clinical trials.
In a Phase IV clinical trial, doctors might study the drug or treatment in different doses, or with other drugs or treatments. Or they might study how it works if people take it at different times. They might study it in different people than earlier clinical trials did. For example, they might study how well it works for children or older adults. Doctors can also study how well a drug or treatment works over time.
Drug makers may do phase IV clinical trials even if the FDA does not ask them to. They might do this to get FDA approval to use the drug in a new way. For example, they might want to use it for another type of cancer.
Phase IV clinical trials can also check the safety of drugs or treatments being used now. They do this to make sure drug makers report any new or serious side effects. The FDA may take away a drug's approval if new research shows it is not as safe or effective as earlier testing showed. Doctors cannot prescribe it any longer if this happens.
How are clinical trial phases different from cancer stages?
It is easy to confuse cancer "stages" and clinical trial "phases." They use similar numbers. Clinical trial phases are numbered I, II, III, and IV (1, 2, 3, and 4). Cancer stages are 0, I, II, III, and IV (0, 1, 2, 3, and 4). But the numbers describe different things. A clinical trial's phase number tells you what doctors are testing in that phase. It also tells you how many volunteers are in the study. The stage of a person’s cancer tells you:
How much the cancer has grown and spread
What type of cancer cells are present. Some types mean the cancer is likely to get worse and some do not.
You can join any phase of a clinical trial with any stage of cancer, depending on the clinical trial's rules. The phase does not have to match your cancer stage. For example, you might join a phase II trial when you have stage IV cancer.
Do I need to be in all the phases of a clinical trial?
No. You can join any phase of a clinical trial if you qualify to join. For example, you may join a phase II clinical trial whether you were in phase I, or not.
Sometimes different phases are done at the same time. If so, the research staff will let you know. You always have a choice to be in the clinical trial, and you may leave at any time.
Learn more with free videos
You can watch a free series of educational videos on Cancer.Net. The series is called Preparatory Education About Clinical Trials, or PRE-ACT.
For a personalized video series, answer questions about your own situation and create an account. You may also watch the whole series. With your account, you can start and stop watching any time.creating an account.
Related Resources
Drug Discovery and Development
More Information
Clinicaltrials.gov
Research and Advocacy
More in this section.
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
- Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

- Phases of clinical trials
This page is about the different phases of clinical trials. It has information about
What are trial phases? Trial phases at a glance Phase 0 trials Phase 1 trials Phase 2 trials Phase 3 trials Phase 4 trials Trials covering more than one phase
What are trial phases?
Clinical trials testing new treatments are divided into different stages, called phases. The earliest phase trials may look at whether a drug is safe or the side effects it causes. Later phase trials aim to test whether a new treatment is better than existing treatments.
There are 3 main phases of clinical trials – phases 1 to 3. Phase 1 trials are the earliest phase trials and phase 3 are later phase trials.
Some trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been licensed.
Some trials are randomised . This means the people taking part are put into one of the treatment groups at random. Doing this means the results are more reliable.
Trial phases at a glance
Phase 0 trials.

- whether the drug reaches the cancer cells
- what happens to the drug in the body
- how cancer cells in the body respond to the drug
Phase 1 trial
- how much of the drug is safe to give
- what the side effects are
- what happens to the drug in the body
- if the treatment helps shrink the cancer
Phase 2 trials
- if the new treatment works well enough to be tested in a larger phase 3 trial
- which types of cancer the treatment works for
- more about side effects and how to manage them
- more about the best dose to give
Phase 3 trials
- which treatment works better for a particular type of cancer
- more about the side effects
- how the treatment affects people’s quality of life
- a completely new treatment
- different doses of the same treatment
- having the same treatment more, or less, often
- a new way of giving a standard treatment (radiotherapy for example)
Phase 4 trials
- more about the side effects including the rarer side effects and safety of the drug
- what the long term risks and benefits are
- how well the drug works when it’s used more widely for people not included in the phase 3 trial
Trials covering more than one phase
Oxford Handbook of Clinical and Healthcare Research (1st edition) R Sumantra, S Fitzpatrick, R Golubic and others Oxford University Press, 2016
Related information
Last reviewed, find a trial, about cancer.
- Spot cancer early
- Talking to your doctor
- What is cancer?
- Types of clinical trials
- Randomised trials
- How to find a clinical trial
- How to join a clinical trial
- What you should be told about a clinical trial
- How clinical trials are planned and organised
- Clinical trial results
- What to ask your doctor about clinical trials
- Clinical trial organisations
- Children's Cancers
- Sign up for relevant cancer information and support
- How do I check for cancer?
- Welcome to Cancer Chat

Rate this page:

- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.11(2); 2019 Feb

Planning and Conducting Clinical Research: The Whole Process
Boon-how chew.
1 Family Medicine, Universiti Putra Malaysia, Serdang, MYS
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question. Engagement with clinical experts, experienced researchers, relevant stakeholders of the research topic, and even patients can enhance the research question’s relevance, feasibility, and efficiency. Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental or non-experimental, sampling or census, and time features of the variables to be studied. The ultimate aims of research reporting are to present findings succinctly and timely. Concise, explicit, and complete reporting are the guiding principles in clinical studies reporting.
Introduction and background
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices. There are many ways a clinical research's findings can become invalid or less impactful including ignorance of previous similar studies, a paucity of similar studies, poor study design and implementation, low test agent efficacy, no predetermined statistical analysis, insufficient reporting, bias, and conflicts of interest [ 1 - 4 ]. Scientific, ethical, and moral decadence among researchers can be due to incognizant criteria in academic promotion and remuneration and too many forced studies by amateurs and students for the sake of research without adequate training or guidance [ 2 , 5 - 6 ]. This article will review the proper methods to conduct medical research from the planning stage to submission for publication (Table (Table1 1 ).
a Feasibility and efficiency are considered during the refinement of the research question and adhered to during data collection.
Epidemiologic studies in clinical and medical fields focus on the effect of a determinant on an outcome [ 7 ]. Measurement errors that happen systematically give rise to biases leading to invalid study results, whereas random measurement errors will cause imprecise reporting of effects. Precision can usually be increased with an increased sample size provided biases are avoided or trivialized. Otherwise, the increased precision will aggravate the biases. Because epidemiologic, clinical research focuses on measurement, measurement errors are addressed throughout the research process. Obtaining the most accurate estimate of a treatment effect constitutes the whole business of epidemiologic research in clinical practice. This is greatly facilitated by clinical expertise and current scientific knowledge of the research topic. Current scientific knowledge is acquired through literature reviews or in collaboration with an expert clinician. Collaboration and consultation with an expert clinician should also include input from the target population to confirm the relevance of the research question. The novelty of a research topic is less important than the clinical applicability of the topic. Researchers need to acquire appropriate writing and reporting skills from the beginning of their careers, and these skills should improve with persistent use and regular reviewing of published journal articles. A published clinical research study stands on solid scientific ground to inform clinical practice given the article has passed through proper peer-reviews, revision, and content improvement.
Systematic literature reviews
Systematic literature reviews of published papers will inform authors of the existing clinical evidence on a research topic. This is an important step to reduce wasted efforts and evaluate the planned study [ 8 ]. Conducting a systematic literature review is a well-known important step before embarking on a new study [ 9 ]. A rigorously performed and cautiously interpreted systematic review that includes in-process trials can inform researchers of several factors [ 10 ]. Reviewing the literature will inform the choice of recruitment methods, outcome measures, questionnaires, intervention details, and statistical strategies – useful information to increase the study’s relevance, value, and power. A good review of previous studies will also provide evidence of the effects of an intervention that may or may not be worthwhile; this would suggest either no further studies are warranted or that further study of the intervention is needed. A review can also inform whether a larger and better study is preferable to an additional small study. Reviews of previously published work may yield few studies or low-quality evidence from small or poorly designed studies on certain intervention or observation; this may encourage or discourage further research or prompt consideration of a first clinical trial.
Conceptual framework
The result of a literature review should include identifying a working conceptual framework to clarify the nature of the research problem, questions, and designs, and even guide the latter discussion of the findings and development of possible solutions. Conceptual frameworks represent ways of thinking about a problem or how complex things work the way they do [ 11 ]. Different frameworks will emphasize different variables and outcomes, and their inter-relatedness. Each framework highlights or emphasizes different aspects of a problem or research question. Often, any single conceptual framework presents only a partial view of reality [ 11 ]. Furthermore, each framework magnifies certain elements of the problem. Therefore, a thorough literature search is warranted for authors to avoid repeating the same research endeavors or mistakes. It may also help them find relevant conceptual frameworks including those that are outside one’s specialty or system.
Conceptual frameworks can come from theories with well-organized principles and propositions that have been confirmed by observations or experiments. Conceptual frameworks can also come from models derived from theories, observations or sets of concepts or even evidence-based best practices derived from past studies [ 11 ].
Researchers convey their assumptions of the associations of the variables explicitly in the conceptual framework to connect the research to the literature. After selecting a single conceptual framework or a combination of a few frameworks, a clinical study can be completed in two fundamental steps: study design and study report. Three study designs should be planned in sequence and iterated until satisfaction: the theoretical design, data collection design, and statistical analysis design [ 7 ].
Study designs
Theoretical Design
Theoretical design is the next important step in the research process after a literature review and conceptual framework identification. While the theoretical design is a crucial step in research planning, it is often dealt with lightly because of the more alluring second step (data collection design). In the theoretical design phase, a research question is designed to address a clinical problem, which involves an informed understanding based on the literature review and effective collaboration with the right experts and clinicians. A well-developed research question will have an initial hypothesis of the possible relationship between the explanatory variable/exposure and the outcome. This will inform the nature of the study design, be it qualitative or quantitative, primary or secondary, and non-causal or causal (Figure (Figure1 1 ).

A study is qualitative if the research question aims to explore, understand, describe, discover or generate reasons underlying certain phenomena. Qualitative studies usually focus on a process to determine how and why things happen [ 12 ]. Quantitative studies use deductive reasoning, and numerical statistical quantification of the association between groups on data often gathered during experiments [ 13 ]. A primary clinical study is an original study gathering a new set of patient-level data. Secondary research draws on the existing available data and pooling them into a larger database to generate a wider perspective or a more powerful conclusion. Non-causal or descriptive research aims to identify the determinants or associated factors for the outcome or health condition, without regard for causal relationships. Causal research is an exploration of the determinants of an outcome while mitigating confounding variables. Table Table2 2 shows examples of non-causal (e.g., diagnostic and prognostic) and causal (e.g., intervention and etiologic) clinical studies. Concordance between the research question, its aim, and the choice of theoretical design will provide a strong foundation and the right direction for the research process and path.
A problem in clinical epidemiology is phrased in a mathematical relationship below, where the outcome is a function of the determinant (D) conditional on the extraneous determinants (ED) or more commonly known as the confounding factors [ 7 ]:
For non-causal research, Outcome = f (D1, D2…Dn) For causal research, Outcome = f (D | ED)
A fine research question is composed of at least three components: 1) an outcome or a health condition, 2) determinant/s or associated factors to the outcome, and 3) the domain. The outcome and the determinants have to be clearly conceptualized and operationalized as measurable variables (Table (Table3; 3 ; PICOT [ 14 ] and FINER [ 15 ]). The study domain is the theoretical source population from which the study population will be sampled, similar to the wording on a drug package insert that reads, “use this medication (study results) in people with this disease” [ 7 ].
The interpretation of study results as they apply to wider populations is known as generalization, and generalization can either be statistical or made using scientific inferences [ 16 ]. Generalization supported by statistical inferences is seen in studies on disease prevalence where the sample population is representative of the source population. By contrast, generalizations made using scientific inferences are not bound by the representativeness of the sample in the study; rather, the generalization should be plausible from the underlying scientific mechanisms as long as the study design is valid and nonbiased. Scientific inferences and generalizations are usually the aims of causal studies.
Confounding: Confounding is a situation where true effects are obscured or confused [ 7 , 16 ]. Confounding variables or confounders affect the validity of a study’s outcomes and should be prevented or mitigated in the planning stages and further managed in the analytical stages. Confounders are also known as extraneous determinants in epidemiology due to their inherent and simultaneous relationships to both the determinant and outcome (Figure (Figure2), 2 ), which are usually one-determinant-to-one outcome in causal clinical studies. The known confounders are also called observed confounders. These can be minimized using randomization, restriction, or a matching strategy. Residual confounding has occurred in a causal relationship when identified confounders were not measured accurately. Unobserved confounding occurs when the confounding effect is present as a variable or factor not observed or yet defined and, thus, not measured in the study. Age and gender are almost universal confounders followed by ethnicity and socio-economic status.

Confounders have three main characteristics. They are a potential risk factor for the disease, associated with the determinant of interest, and should not be an intermediate variable between the determinant and the outcome or a precursor to the determinant. For example, a sedentary lifestyle is a cause for acute coronary syndrome (ACS), and smoking could be a confounder but not cardiorespiratory unfitness (which is an intermediate factor between a sedentary lifestyle and ACS). For patients with ACS, not having a pair of sports shoes is not a confounder – it is a correlate for the sedentary lifestyle. Similarly, depression would be a precursor, not a confounder.
Sample size consideration: Sample size calculation provides the required number of participants to be recruited in a new study to detect true differences in the target population if they exist. Sample size calculation is based on three facets: an estimated difference in group sizes, the probability of α (Type I) and β (Type II) errors chosen based on the nature of the treatment or intervention, and the estimated variability (interval data) or proportion of the outcome (nominal data) [ 17 - 18 ]. The clinically important effect sizes are determined based on expert consensus or patients’ perception of benefit. Value and economic consideration have increasingly been included in sample size estimations. Sample size and the degree to which the sample represents the target population affect the accuracy and generalization of a study’s reported effects.
Pilot study: Pilot studies assess the feasibility of the proposed research procedures on small sample size. Pilot studies test the efficiency of participant recruitment with minimal practice or service interruptions. Pilot studies should not be conducted to obtain a projected effect size for a larger study population because, in a typical pilot study, the sample size is small, leading to a large standard error of that effect size. This leads to bias when projected for a large population. In the case of underestimation, this could lead to inappropriately terminating the full-scale study. As the small pilot study is equally prone to bias of overestimation of the effect size, this would lead to an underpowered study and a failed full-scale study [ 19 ].
The Design of Data Collection
The “perfect” study design in the theoretical phase now faces the practical and realistic challenges of feasibility. This is the step where different methods for data collection are considered, with one selected as the most appropriate based on the theoretical design along with feasibility and efficiency. The goal of this stage is to achieve the highest possible validity with the lowest risk of biases given available resources and existing constraints.
In causal research, data on the outcome and determinants are collected with utmost accuracy via a strict protocol to maximize validity and precision. The validity of an instrument is defined as the degree of fidelity of the instrument, measuring what it is intended to measure, that is, the results of the measurement correlate with the true state of an occurrence. Another widely used word for validity is accuracy. Internal validity refers to the degree of accuracy of a study’s results to its own study sample. Internal validity is influenced by the study designs, whereas the external validity refers to the applicability of a study’s result in other populations. External validity is also known as generalizability and expresses the validity of assuming the similarity and comparability between the study population and the other populations. Reliability of an instrument denotes the extent of agreeableness of the results of repeated measurements of an occurrence by that instrument at a different time, by different investigators or in a different setting. Other terms that are used for reliability include reproducibility and precision. Preventing confounders by identifying and including them in data collection will allow statistical adjustment in the later analyses. In descriptive research, outcomes must be confirmed with a referent standard, and the determinants should be as valid as those found in real clinical practice.
Common designs for data collection include cross-sectional, case-control, cohort, and randomized controlled trials (RCTs). Many other modern epidemiology study designs are based on these classical study designs such as nested case-control, case-crossover, case-control without control, and stepwise wedge clustered RCTs. A cross-sectional study is typically a snapshot of the study population, and an RCT is almost always a prospective study. Case-control and cohort studies can be retrospective or prospective in data collection. The nested case-control design differs from the traditional case-control design in that it is “nested” in a well-defined cohort from which information on the cohorts can be obtained. This design also satisfies the assumption that cases and controls represent random samples of the same study base. Table Table4 4 provides examples of these data collection designs.
Additional aspects in data collection: No single design of data collection for any research question as stated in the theoretical design will be perfect in actual conduct. This is because of myriad issues facing the investigators such as the dynamic clinical practices, constraints of time and budget, the urgency for an answer to the research question, and the ethical integrity of the proposed experiment. Therefore, feasibility and efficiency without sacrificing validity and precision are important considerations in data collection design. Therefore, data collection design requires additional consideration in the following three aspects: experimental/non-experimental, sampling, and timing [ 7 ]:
Experimental or non-experimental: Non-experimental research (i.e., “observational”), in contrast to experimental, involves data collection of the study participants in their natural or real-world environments. Non-experimental researches are usually the diagnostic and prognostic studies with cross-sectional in data collection. The pinnacle of non-experimental research is the comparative effectiveness study, which is grouped with other non-experimental study designs such as cross-sectional, case-control, and cohort studies [ 20 ]. It is also known as the benchmarking-controlled trials because of the element of peer comparison (using comparable groups) in interpreting the outcome effects [ 20 ]. Experimental study designs are characterized by an intervention on a selected group of the study population in a controlled environment, and often in the presence of a similar group of the study population to act as a comparison group who receive no intervention (i.e., the control group). Thus, the widely known RCT is classified as an experimental design in data collection. An experimental study design without randomization is referred to as a quasi-experimental study. Experimental studies try to determine the efficacy of a new intervention on a specified population. Table Table5 5 presents the advantages and disadvantages of experimental and non-experimental studies [ 21 ].
a May be an issue in cross-sectional studies that require a long recall to the past such as dietary patterns, antenatal events, and life experiences during childhood.
Once an intervention yields a proven effect in an experimental study, non-experimental and quasi-experimental studies can be used to determine the intervention’s effect in a wider population and within real-world settings and clinical practices. Pragmatic or comparative effectiveness are the usual designs used for data collection in these situations [ 22 ].
Sampling/census: Census is a data collection on the whole source population (i.e., the study population is the source population). This is possible when the defined population is restricted to a given geographical area. A cohort study uses the census method in data collection. An ecologic study is a cohort study that collects summary measures of the study population instead of individual patient data. However, many studies sample from the source population and infer the results of the study to the source population for feasibility and efficiency because adequate sampling provides similar results to the census of the whole population. Important aspects of sampling in research planning are sample size and representation of the population. Sample size calculation accounts for the number of participants needed to be in the study to discover the actual association between the determinant and outcome. Sample size calculation relies on the primary objective or outcome of interest and is informed by the estimated possible differences or effect size from previous similar studies. Therefore, the sample size is a scientific estimation for the design of the planned study.
A sampling of participants or cases in a study can represent the study population and the larger population of patients in that disease space, but only in prevalence, diagnostic, and prognostic studies. Etiologic and interventional studies do not share this same level of representation. A cross-sectional study design is common for determining disease prevalence in the population. Cross-sectional studies can also determine the referent ranges of variables in the population and measure change over time (e.g., repeated cross-sectional studies). Besides being cost- and time-efficient, cross-sectional studies have no loss to follow-up; recall bias; learning effect on the participant; or variability over time in equipment, measurement, and technician. A cross-sectional design for an etiologic study is possible when the determinants do not change with time (e.g., gender, ethnicity, genetic traits, and blood groups).
In etiologic research, comparability between the exposed and the non-exposed groups is more important than sample representation. Comparability between these two groups will provide an accurate estimate of the effect of the exposure (risk factor) on the outcome (disease) and enable valid inference of the causal relation to the domain (the theoretical population). In a case-control study, a sampling of the control group should be taken from the same study population (study base), have similar profiles to the cases (matching) but do not have the outcome seen in the cases. Matching important factors minimizes the confounding of the factors and increases statistical efficiency by ensuring similar numbers of cases and controls in confounders’ strata [ 23 - 24 ]. Nonetheless, perfect matching is neither necessary nor achievable in a case-control study because a partial match could achieve most of the benefits of the perfect match regarding a more precise estimate of odds ratio than statistical control of confounding in unmatched designs [ 25 - 26 ]. Moreover, perfect or full matching can lead to an underestimation of the point estimates [ 27 - 28 ].
Time feature: The timing of data collection for the determinant and outcome characterizes the types of studies. A cross-sectional study has the axis of time zero (T = 0) for both the determinant and the outcome, which separates it from all other types of research that have time for the outcome T > 0. Retrospective or prospective studies refer to the direction of data collection. In retrospective studies, information on the determinant and outcome have been collected or recorded before. In prospective studies, this information will be collected in the future. These terms should not be used to describe the relationship between the determinant and the outcome in etiologic studies. Time of exposure to the determinant, the time of induction, and the time at risk for the outcome are important aspects to understand. Time at risk is the period of time exposed to the determinant risk factors. Time of induction is the time from the sufficient exposure to the risk or causal factors to the occurrence of a disease. The latent period is when the occurrence of a disease without manifestation of the disease such as in “silence” diseases for example cancers, hypertension and type 2 diabetes mellitus which is detected from screening practices. Figure Figure3 3 illustrates the time features of a variable. Variable timing is important for accurate data capture.

The Design of Statistical Analysis
Statistical analysis of epidemiologic data provides the estimate of effects after correcting for biases (e.g., confounding factors) measures the variability in the data from random errors or chance [ 7 , 16 , 29 ]. An effect estimate gives the size of an association between the studied variables or the level of effectiveness of an intervention. This quantitative result allows for comparison and assessment of the usefulness and significance of the association or the intervention between studies. This significance must be interpreted with a statistical model and an appropriate study design. Random errors could arise in the study resulting from unexplained personal choices by the participants. Random error is, therefore, when values or units of measurement between variables change in non-concerted or non-directional manner. Conversely, when these values or units of measurement between variables change in a concerted or directional manner, we note a significant relationship as shown by statistical significance.
Variability: Researchers almost always collect the needed data through a sampling of subjects/participants from a population instead of a census. The process of sampling or multiple sampling in different geographical regions or over different periods contributes to varied information due to the random inclusion of different participants and chance occurrence. This sampling variation becomes the focus of statistics when communicating the degree and intensity of variation in the sampled data and the level of inference in the population. Sampling variation can be influenced profoundly by the total number of participants and the width of differences of the measured variable (standard deviation). Hence, the characteristics of the participants, measurements and sample size are all important factors in planning a study.
Statistical strategy: Statistical strategy is usually determined based on the theoretical and data collection designs. Use of a prespecified statistical strategy (including the decision to dichotomize any continuous data at certain cut-points, sub-group analysis or sensitive analyses) is recommended in the study proposal (i.e., protocol) to prevent data dredging and data-driven reports that predispose to bias. The nature of the study hypothesis also dictates whether directional (one-tailed) or non-directional (two-tailed) significance tests are conducted. In most studies, two-sided tests are used except in specific instances when unidirectional hypotheses may be appropriate (e.g., in superiority or non-inferiority trials). While data exploration is discouraged, epidemiological research is, by nature of its objectives, statistical research. Hence, it is acceptable to report the presence of persistent associations between any variables with plausible underlying mechanisms during the exploration of the data. The statistical methods used to produce the results should be explicitly explained. Many different statistical tests are used to handle various kinds of data appropriately (e.g., interval vs discrete), and/or the various distribution of the data (e.g., normally distributed or skewed). For additional details on statistical explanations and underlying concepts of statistical tests, readers are recommended the references as cited in this sentence [ 30 - 31 ].
Steps in statistical analyses: Statistical analysis begins with checking for data entry errors. Duplicates are eliminated, and proper units should be confirmed. Extremely low, high or suspicious values are confirmed from the source data again. If this is not possible, this is better classified as a missing value. However, if the unverified suspicious data are not obviously wrong, they should be further examined as an outlier in the analysis. The data checking and cleaning enables the analyst to establish a connection with the raw data and to anticipate possible results from further analyses. This initial step involves descriptive statistics that analyze central tendency (i.e., mode, median, and mean) and dispersion (i.e., (minimum, maximum, range, quartiles, absolute deviation, variance, and standard deviation) of the data. Certain graphical plotting such as scatter plot, a box-whiskers plot, histogram or normal Q-Q plot are helpful at this stage to verify data normality in distribution. See Figure Figure4 4 for the statistical tests available for analyses of different types of data.

Once data characteristics are ascertained, further statistical tests are selected. The analytical strategy sometimes involves the transformation of the data distribution for the selected tests (e.g., log, natural log, exponential, quadratic) or for checking the robustness of the association between the determinants and their outcomes. This step is also referred to as inferential statistics whereby the results are about hypothesis testing and generalization to the wider population that the study’s sampled participants represent. The last statistical step is checking whether the statistical analyses fulfill the assumptions of that particular statistical test and model to avoid violation and misleading results. These assumptions include evaluating normality, variance homogeneity, and residuals included in the final statistical model. Other statistical values such as Akaike information criterion, variance inflation factor/tolerance, and R2 are also considered when choosing the best-fitted models. Transforming raw data could be done, or a higher level of statistical analyses can be used (e.g., generalized linear models and mixed-effect modeling). Successful statistical analysis allows conclusions of the study to fit the data.
Bayesian and Frequentist statistical frameworks: Most of the current clinical research reporting is based on the frequentist approach and hypotheses testing p values and confidence intervals. The frequentist approach assumes the acquired data are random, attained by random sampling, through randomized experiments or influences, and with random errors. The distribution of the data (its point estimate and confident interval) infers a true parameter in the real population. The major conceptual difference between Bayesian statistics and frequentist statistics is that in Bayesian statistics, the parameter (i.e., the studied variable in the population) is random and the data acquired is real (true or fix). Therefore, the Bayesian approach provides a probability interval for the parameter. The studied parameter is random because it could vary and be affected by prior beliefs, experience or evidence of plausibility. In the Bayesian statistical approach, this prior belief or available knowledge is quantified into a probability distribution and incorporated into the acquired data to get the results (i.e., the posterior distribution). This uses mathematical theory of Bayes’ Theorem to “turn around” conditional probabilities.
The goal of research reporting is to present findings succinctly and timely via conference proceedings or journal publication. Concise and explicit language use, with all the necessary details to enable replication and judgment of the study applicability, are the guiding principles in clinical studies reporting.
Writing for Reporting
Medical writing is very much a technical chore that accommodates little artistic expression. Research reporting in medicine and health sciences emphasize clear and standardized reporting, eschewing adjectives and adverbs extensively used in popular literature. Regularly reviewing published journal articles can familiarize authors with proper reporting styles and help enhance writing skills. Authors should familiarize themselves with standard, concise, and appropriate rhetoric for the intended audience, which includes consideration for journal reviewers, editors, and referees. However, proper language can be somewhat subjective. While each publication may have varying requirements for submission, the technical requirements for formatting an article are usually available via author or submission guidelines provided by the target journal.
Research reports for publication often contain a title, abstract, introduction, methods, results, discussion, and conclusions section, and authors may want to write each section in sequence. However, best practices indicate the abstract and title should be written last. Authors may find that when writing one section of the report, ideas come to mind that pertains to other sections, so careful note taking is encouraged. One effective approach is to organize and write the result section first, followed by the discussion and conclusions sections. Once these are drafted, write the introduction, abstract, and the title of the report. Regardless of the sequence of writing, the author should begin with a clear and relevant research question to guide the statistical analyses, result interpretation, and discussion. The study findings can be a motivator to propel the author through the writing process, and the conclusions can help the author draft a focused introduction.
Writing for Publication
Specific recommendations on effective medical writing and table generation are available [ 32 ]. One such resource is Effective Medical Writing: The Write Way to Get Published, which is an updated collection of medical writing articles previously published in the Singapore Medical Journal [ 33 ]. The British Medical Journal’s Statistics Notes series also elucidates common and important statistical concepts and usages in clinical studies. Writing guides are also available from individual professional societies, journals, or publishers such as Chest (American College of Physicians) medical writing tips, PLoS Reporting guidelines collection, Springer’s Journal Author Academy, and SAGE’s Research methods [ 34 - 37 ]. Standardized research reporting guidelines often come in the form of checklists and flow diagrams. Table Table6 6 presents a list of reporting guidelines. A full compilation of these guidelines is available at the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network website [ 38 ] which aims to improve the reliability and value of medical literature by promoting transparent and accurate reporting of research studies. Publication of the trial protocol in a publicly available database is almost compulsory for publication of the full report in many potential journals.
Graphics and Tables
Graphics and tables should emphasize salient features of the underlying data and should coherently summarize large quantities of information. Although graphics provide a break from dense prose, authors must not forget that these illustrations should be scientifically informative, not decorative. The titles for graphics and tables should be clear, informative, provide the sample size, and use minimal font weight and formatting only to distinguish headings, data entry or to highlight certain results. Provide a consistent number of decimal points for the numerical results, and with no more than four for the P value. Most journals prefer cell-delineated tables created using the table function in word processing or spreadsheet programs. Some journals require specific table formatting such as the absence or presence of intermediate horizontal lines between cells.
Decisions of authorship are both sensitive and important and should be made at an early stage by the study’s stakeholders. Guidelines and journals’ instructions to authors abound with authorship qualifications. The guideline on authorship by the International Committee of Medical Journal Editors is widely known and provides a standard used by many medical and clinical journals [ 39 ]. Generally, authors are those who have made major contributions to the design, conduct, and analysis of the study, and who provided critical readings of the manuscript (if not involved directly in manuscript writing).
Picking a target journal for submission
Once a report has been written and revised, the authors should select a relevant target journal for submission. Authors should avoid predatory journals—publications that do not aim to advance science and disseminate quality research. These journals focus on commercial gain in medical and clinical publishing. Two good resources for authors during journal selection are Think-Check-Submit and the defunct Beall's List of Predatory Publishers and Journals (now archived and maintained by an anonymous third-party) [ 40 , 41 ]. Alternatively, reputable journal indexes such as Thomson Reuters Journal Citation Reports, SCOPUS, MedLine, PubMed, EMBASE, EBSCO Publishing's Electronic Databases are available areas to start the search for an appropriate target journal. Authors should review the journals’ names, aims/scope, and recently published articles to determine the kind of research each journal accepts for publication. Open-access journals almost always charge article publication fees, while subscription-based journals tend to publish without author fees and instead rely on subscription or access fees for the full text of published articles.
Conclusions
Conducting a valid clinical research requires consideration of theoretical study design, data collection design, and statistical analysis design. Proper study design implementation and quality control during data collection ensures high-quality data analysis and can mitigate bias and confounders during statistical analysis and data interpretation. Clear, effective study reporting facilitates dissemination, appreciation, and adoption, and allows the researchers to affect real-world change in clinical practices and care models. Neutral or absence of findings in a clinical study are as important as positive or negative findings. Valid studies, even when they report an absence of expected results, still inform scientific communities of the nature of a certain treatment or intervention, and this contributes to future research, systematic reviews, and meta-analyses. Reporting a study adequately and comprehensively is important for accuracy, transparency, and reproducibility of the scientific work as well as informing readers.
Acknowledgments
The author would like to thank Universiti Putra Malaysia and the Ministry of Higher Education, Malaysia for their support in sponsoring the Ph.D. study and living allowances for Boon-How Chew.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The materials presented in this paper is being organized by the author into a book.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- What are the four...
What are the four phases of clinical research trials?
- Related content
- Peer review
- Philip Sedgwick , reader in medical statistics and medical education
- 1 Centre for Medical and Healthcare Education, St George’s, University of London, London, UK
- p.sedgwick{at}sgul.ac.uk
Children with neurological and developmental disorders often experience chronic sleep disturbances. Melatonin has been commonly prescribed because of its hypnotic properties. However, trials have had conflicting results. Therefore, researchers assessed the effectiveness of melatonin in treating severe sleep problems in children with neurodevelopmental disorders. A randomised double blind placebo controlled multicentre trial study design was used. The intervention was immediate release melatonin capsules given 45 minutes before the child’s bedtime for a period of 12 weeks. Treatment started with a 0.5 mg capsule, and escalated through 2 mg, 6 mg, and 12 mg, depending on the child’s response to treatment. 1
Participants were 146 children who had a severe sleep problem and had not responded to standardised sleep behaviour advice provided to parents four to six weeks before randomisation. Children were recruited from 19 hospitals across England and Wales. The children were randomised to melatonin (n=70) or placebo (n=76).
The outcome measures included subjective (as assessed from sleep diaries completed by the parents) and objective (as recorded by actigraphy) measures of sleep. The researchers reported that children gained little additional sleep on melatonin compared with placebo. However, the children receiving melatonin fell asleep significantly more quickly and they awoke earlier.
Which one of the following best describes the phase of the above clinical trial?
b) Phase II
c) Phase III
d) Phase IV
The above trial is best described as a phase III trial (answer c ).
The development of a drug follows a well established and lengthy process. It may take between 10 and 15 years to develop a new drug from discovery to when it is available for treating patients. Drugs are tested in humans only after they have undergone laboratory testing. Testing in humans is divided into a series of successive clinical trials known as phase I, phase II, phase III, and phase IV trials. These phases are separate clinical studies, and each has a unique objective. Generally, phase I trials establish safety and tolerability in healthy volunteers; phase II trials determine the drugs’ efficacy and adverse effects at different dosages in patients; phase III trials establish the effectiveness and safety of the drug compared with placebo or current standard treatment; and phase IV trials determine general risks and benefits after the drug has been licensed. The phases are described in further detail below. As drug development progresses from one phase to the next, the number of participants will typically increase. Drugs that are found to be unsafe or ineffective during development will not progress through all four phases.
Phase I trials are the first stage of drug development in humans. They are conducted on a small number, possibly 20, of healthy volunteers on an inpatient basis. The main aim of a phase I trial is to obtain early indications of the pharmacological actions, safety, and adverse effects of a drug. The drug is typically tested in a single dose to start with. Through the exposure of participants to increasing doses of the drug, a range of safe doses is determined. This allows testing of the pharmacological actions of the drug, and enables researchers to determine how it is processed and tolerated at multiple doses.
Once the initial safety of the drug has been established, the drug will undergo a phase II trial. The purpose of a phase II trial is to investigate the short term safety and therapeutic efficacy of the drug in patients with the disease or condition that the drug is intended to treat. Patients are given the different drug dosages found to be safe in the phase I trial, allowing the drug’s efficacy and adverse effects to be compared between different dosages. When the efficacy and safety of the drug have been demonstrated in patients with the disease or condition that the drug is intended to treat, the drug will proceed to a phase III trial.
Phase III trials are the final stage before a new drug is licensed for treating patients. Phase I and II trials tend to be exploratory. By contrast, phase III trials provide confirmation of the properties of the drug discovered in the earlier phases of the drug’s development. A randomised placebo controlled trial study design will be used. However, if it is unethical to treat patients with a placebo, the current gold standard treatment may be used instead. Phase III trials involve many more participants than previous stages and are often multicentre. The aim is to generate statistically significant data about the safety, efficacy, and dosage of the drug in a large group of patients for whom the drug is intended. Dosages will be modified to determine the one that provides the most beneficial effects with the fewest adverse effects. As in earlier phases, patients are closely monitored for adverse effects, and if serious ones are reported then the trial will be stopped. The phase III trial provides the necessary information for evaluating whether the drug can be approved and licensed. A drug will tend to be licensed only if it shows a worthwhile contribution to medical treatment.
Phase IV trials take place once the drug has been licensed. The drug is evaluated for long periods of time in larger numbers of patients, and possibly subgroups of patients within the population. The aim is to establish the long term efficacy and safety of the drug. The drug may be compared or combined with other available standard treatments. In many respects phase IV trials are similar to phase III trials, although they tend not to be placebo controlled.
The definitions of the four phases of clinical trials are not distinct and there is some overlap between them. Often clinical trials do not fit neatly into one of the four phases described above, and it may not be that simple to discern which phase a trial comes under. The aim of the above trial was to establish the effectiveness of melatonin in treating severe sleep problems in children with neurodevelopmental disorders who had not responded to standardised sleep behaviour advice. Previous trials had had conflicting results. The trial focused on confirming the properties of melatonin that were discovered in earlier trials and therefore it is probably best described as a phase III trial. The trial would not be a phase IV trial because melatonin had not been shown to be effective in treating severe sleep problems in children with neurodevelopmental disorders, and therefore would not be licensed for use as described.
Cite this as: BMJ 2014;348:g3727
Competing interests: None declared.
- ↵ Gringras P, Gamble C, Jones AP, Wiggs L, Williamson PR, Sutcliffe A, et al; on behalf of the MENDS Study Group. Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. BMJ 2012 ; 345 : e6664 . OpenUrl Abstract / FREE Full Text
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Market Research Research Tools and Apps
Research Process Steps: What they are + How To Follow

There are various approaches to conducting basic and applied research. This article explains the research process steps you should know. Whether you are doing basic research or applied research, there are many ways of doing it. In some ways, each research study is unique since it is conducted at a different time and place.
Conducting research might be difficult, but there are clear processes to follow. The research process starts with a broad idea for a topic. This article will assist you through the research process steps, helping you focus and develop your topic.
Research Process Steps
The research process consists of a series of systematic procedures that a researcher must go through in order to generate knowledge that will be considered valuable by the project and focus on the relevant topic.
To conduct effective research, you must understand the research process steps and follow them. Here are a few steps in the research process to make it easier for you:
Step 1: Identify the Problem
Finding an issue or formulating a research question is the first step. A well-defined research problem will guide the researcher through all stages of the research process, from setting objectives to choosing a technique. There are a number of approaches to get insight into a topic and gain a better understanding of it. Such as:
- A preliminary survey
- Case studies
- Interviews with a small group of people
- Observational survey
Step 2: Evaluate the Literature
A thorough examination of the relevant studies is essential to the research process . It enables the researcher to identify the precise aspects of the problem. Once a problem has been found, the investigator or researcher needs to find out more about it.
This stage gives problem-zone background. It teaches the investigator about previous research, how they were conducted, and its conclusions. The researcher can build consistency between his work and others through a literature review. Such a review exposes the researcher to a more significant body of knowledge and helps him follow the research process efficiently.
Step 3: Create Hypotheses
Formulating an original hypothesis is the next logical step after narrowing down the research topic and defining it. A belief solves logical relationships between variables. In order to establish a hypothesis, a researcher must have a certain amount of expertise in the field.
It is important for researchers to keep in mind while formulating a hypothesis that it must be based on the research topic. Researchers are able to concentrate their efforts and stay committed to their objectives when they develop theories to guide their work.
Step 4: The Research Design
Research design is the plan for achieving objectives and answering research questions. It outlines how to get the relevant information. Its goal is to design research to test hypotheses, address the research questions, and provide decision-making insights.
The research design aims to minimize the time, money, and effort required to acquire meaningful evidence. This plan fits into four categories:
- Exploration and Surveys
- Data Analysis
- Observation
Step 5: Describe Population
Research projects usually look at a specific group of people, facilities, or how technology is used in the business. In research, the term population refers to this study group. The research topic and purpose help determine the study group.
Suppose a researcher wishes to investigate a certain group of people in the community. In that case, the research could target a specific age group, males or females, a geographic location, or an ethnic group. A final step in a study’s design is to specify its sample or population so that the results may be generalized.
Step 6: Data Collection
Data collection is important in obtaining the knowledge or information required to answer the research issue. Every research collected data, either from the literature or the people being studied. Data must be collected from the two categories of researchers. These sources may provide primary data.
- Questionnaire
Secondary data categories are:
- Literature survey
- Official, unofficial reports
- An approach based on library resources
Step 7: Data Analysis
During research design, the researcher plans data analysis. After collecting data, the researcher analyzes it. The data is examined based on the approach in this step. The research findings are reviewed and reported.
Data analysis involves a number of closely related stages, such as setting up categories, applying these categories to raw data through coding and tabulation, and then drawing statistical conclusions. The researcher can examine the acquired data using a variety of statistical methods.
Step 8: The Report-writing
After completing these steps, the researcher must prepare a report detailing his findings. The report must be carefully composed with the following in mind:
- The Layout: On the first page, the title, date, acknowledgments, and preface should be on the report. A table of contents should be followed by a list of tables, graphs, and charts if any.
- Introduction: It should state the research’s purpose and methods. This section should include the study’s scope and limits.
- Summary of Findings: A non-technical summary of findings and recommendations will follow the introduction. The findings should be summarized if they’re lengthy.
- Principal Report: The main body of the report should make sense and be broken up into sections that are easy to understand.
- Conclusion: The researcher should restate his findings at the end of the main text. It’s the final result.
LEARN ABOUT: 12 Best Tools for Researchers
The research process involves several steps that make it easy to complete the research successfully. The steps in the research process described above depend on each other, and the order must be kept. So, if we want to do a research project, we should follow the research process steps.
QuestionPro’s enterprise-grade research platform can collect survey and qualitative observation data. The tool’s nature allows for data processing and essential decisions. The platform lets you store and process data. Start immediately!
LEARN MORE FREE TRIAL
MORE LIKE THIS

21 Best Customer Advocacy Software for Customers in 2024
Apr 19, 2024

10 Quantitative Data Analysis Software for Every Data Scientist
Apr 18, 2024
11 Best Enterprise Feedback Management Software in 2024

17 Best Online Reputation Management Software in 2024
Apr 17, 2024
Other categories
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
The Four Phases of Clinical Research
Clinical trials are comprised of a series of phases, focused on comprehensively investigating an experimental treatment or medication’s side effects, effectiveness and safety. All medications or treatments must sequentially complete the clinical research phases before moving on to FDA consideration for release to the general public.
When an experimental medication comes into a clinic, researchers assess its benefits and shortcomings. Some of the questions researchers are looking to answer may include:
- How does the medication affect the participant’s body?
- Do the benefits of the medication outweigh the side effects?
- How does this medication react when combined with other prescription medications or over-the-counter treatments?
- How does this medication affect a certain segment of the population?
- What is the recommended dosage recommendation breakdown?
The breakdown of the four clinical trial phases is as follows:
Phase I: Initial small group testing to determine safety and dosing, and identify side effects
During this phase, researchers pay particularly close attention to the way study volunteers metabolize the administered medication. Phase I trials are designed for a relative small number of healthy participants and typically last a few weeks to several months. Certain clinical trials may call for volunteers who are impacted by a specific cancers or conditions.
Phase II: Further safety and efficacy evaluation on a larger group
Phase II of a clinical trial may span several months to approximately two years. During this period, researchers evaluate the wellbeing of a group of volunteers who receive the experimental medication against a “control group” who received a placebo. This is done to ensure statistically valid data and to help rule out alternative explanations for study results.
Phase III: Large group testing to determine safety, effectiveness, efficacy and monitor side effects
According to the U.S. National Library of Medicine, the primary goal of phase III is “to confirm the experimental medication’s effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.” Phase III studies may last anywhere from 1-4 years and offer the FDA and manufacturers a deeper understanding of the benefits and caveats of a treatment.
Phase IV: Post-market studies focused on long-term usage side effects
Phase IV trials, otherwise known as post-marketing surveillance , occur after the experimental drug has been approved marketed. This stage offers manufacturers further insight into the medication’s comparison against other treatments currently on the market. Phase IV trials also serve to highlight the long-term benefits of the treatment in a much larger patient population size.
Help shape the future of modern medical treatment
At OCRC, we offer a variety of clinical trials and research studies in which you can participate in our state-of-the-art clinical research facility. The Phase I-IV clinical trials that we specialize in range from 6-7 months, and test investigational medications being developed by pharmaceutical and biotech companies. All participants receive all treatments and medical care related to the trial. Additionally, participants may be compensated for time and travel. Health insurance is never a requirement.
If you are interested in participating, please tell us a little more about yourself in the Current studies section of the home page and we will respond to you to determine your eligibility for current and future studies.

Want to get involved?
Tell us a little bit about yourself. We will contact you for further information to determine eligibility for current and future studies.
—Please choose an option— Diabetes Studies Kidney Studies Liver Studies Healthy Studies Other/General
Please add me to the mailing list
I am over 18 years of age


Office of Science Policy
Clinical Research
Of NIH’s more than $17B investment, clinical trials (~$7B ) reflect the point at which the public is most directly engaged in NIH’s clinical research activities, either as dedicated volunteer research participants or users of the resulting data and health interventions. Successful management and oversight of the clinical trial enterprise remains essential to NIH’s mission to translate basic biomedical discoveries into improved health outcomes. OSP works to ensure that NIH’s clinical trial policies enhance the design, conduct and oversight of clinical trials.
Clinical Trials
- Clinical Trials E-Protocol Tool
Informed Consent
Nih single irb (sirb) policy, data and safety monitoring, protections for participants in research (human subjects research protections), privacy and confidentiality in research, return of research results.
- sIRB Policy Implementation
NIH Definition of Clinical Trial
A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
- Notice of Revised NIH Definition of “Clinical Trial” Revised NIH Clinical Trial Definition
- NIH’s Clinical Trial Definition
- Does Your Human Subjects Research Study Meet the NIH Definition of a Clinical Trial?
Sharing Clinical Trial Information
HHS Final Rule for Clinical Trials Registration and Results Information Submission
- Federal Register Notice of Final Rule for Clinical Trials Registration and Results Submission
- NPRM Clinical Trials Registration and Results Submission (November 2014)
- Summary of the Final Rule and NIH Policy
- Key Elements of Final Rule and NIH Policy
- Summary Table of Changes from Current Practice Described in the Final Rule
- Federal Register Notice for NIH Policy on the Dissemination of NIH-Funded Clinical Trials
- NIH Guide Notice
- Federal Register Notice
- Compendium of Comments
GCP Training for NIH Awardees Involved in NIH-funded Clinical Trials
- Policy on Good Clinical Practice Training for NIH Awardees Involved in NIH-funded Clinical Trials
- FAQs_on_NIH_GCP_Policy
Clinical Trial Enrollment Workshop (July 2015)
NIH Workshop on the Enrollment and Retention of Participants in NIH-funded Clinical Trials July 25, 2014
- Poster Abstracts
- Poster Abstract for Poster Presentation
- Proceedings
Presentations:
- Moon Chen Ph.D.,M.P.H, University of California, Davis
- Jonathan Ellen M.D., John Hopkins University
- Maria Freire Ph.D., FNIH
- Joseph Unger Ph.D., Fred Hucthinson Cancer Research Center
- Jeffrey Vigne, Friends of NIH
- Mary Woolley, Research! America
- ClinicalTrials.gov
- Clinical Trial Requirements for Grants and Contracts
- ICH E6 (R2) Good Clinical Practice (GCP) Integrated Addendum to ICH E6 (R1)
- New Review Criteria for Research Project Applications Involving Clinical Trials
- NIH ClinRegs
- NIH Clinical Trials and You
- NIH Stem Cell Clinical Trials & Other Information
- Regulations: Good Clinical Practice and Clinical Trials
- Research Methods Resources
- OSP-OER Blog “Improving Visibility of NIH-Supported Clinical Trial Activities and Results Information”
- OSP-OER Blog “Building Better Clinical Trials through Enhanced Stewardship and Transparency”
- JAMA Article “Toward a New ERA of Trust and Transparency in Clinical Trials”
- NEJM Article “The Final Rule of U.S Clinical Trials Registration and Results Information Submission”
Clinical Trial E-Protocol Tool
Clinical trial e-protocol tool and template documents.
The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. The first type of trials are Phase 2 and 3 clinical trial protocols that require a Food and Drug Administration (FDA) Investigational New Drug (IND) or Investigational Device Exemption (IDE) application.
NIH developed a second protocol template to help behavioral and social science researchers prepare research protocols for human studies measuring a social or behavioral outcome or testing a behavioral or social science-based intervention.
Both templates found in the electronic protocol tool meet the standards outlined in the International Council on Harmonisation (ICH) Guidance for Industry, E6 Good Clinical Practice: Consolidated Guidance (ICH-E6) . These are international standards of good clinical practice that apply to all clinical trials, and their goals are to ensure research integrity and protect human subjects. In addition, use of the electronic protocol tool allows researchers to interface directly with clincialtrials.gov.
Take me to the Web-based e-Protocol Writing Tool
- Word Version of Final Template
- NIH Director’s Statement
- Under the Poliscope blog
- Under the Poliscope Blog
As a steward of the nation’s biomedical research enterprise, NIH is dedicated to ensuring that data and biospecimens are shared for research ethically, securely, and with respect for the privacy, autonomy, and well-being of research participants and the communities to which they belong. Responsible sharing of data and biospecimens derived from human participants relies on robust informed consent practices that uphold the principles of autonomy and trust in biomedical research. Fundamental to these practices are clear, efficient, and transparent communication strategies for conveying potential risks and benefits of sharing, enabling individuals to make informed decisions to participate in research, retain autonomy in decision making, and understand potential uses and contributions of their data and specimens.
OSP works to ensure robust informed consent practices are understood and in place across NIH, with the goal of protecting research participants altruistically donating data and specimens to advance the scientific enterprise.
Relevant Documents and Resources
- Informed Consent for Secondary Research with Data and Biospecimens: Points to Consider and Sample Language for Future Use and/or Sharing
- Request for Information: Developing Consent Language for Future Use of Data and Biospecimens
- Public Comments
The NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research establishes the expectation that all sites participating in multi-site studies involving non-exempt human subjects research funded by the National Institutes of Health (NIH) will use a single Institutional Review Board (sIRB) for all U.S. sites to conduct the ethical review required by the Department of Health and Human Services regulations for the Protection of Human Subjects. If you have any questions about this policy, please contact us here .
- NIH Single IRB Policy
- Final NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research
- Federal Register Notice on the Final NIH sIRB policy
- Federal Register Notice on sIRB Effective Date Extension
- NIH Guide Notice on the Final sIRB policy
- NIH Guide Notice on sIRB Effective Date Extension
- NIH Director’s Statement on the NIH sIRB policy
- OSP-OER Blog on the sIRB policy
- NCATS SMART IRB Reliance Platform
- Frequently Asked Questions about the Implementation of the sIRB policy
- NIH Guide Notice on Scenarios Illustrating the Use of Direct and Indirect Costs for Single IRB Review Under the sIRB Policy
- NIH Policy on the Use of a Single IRB for Multi-Site Research FAQs on Costs
- Request for Comments on the Draft NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research
- Public Comments on the Draft Policy
- New Federal Register Notice regarding the extension of the effective date for the Single IRB policy
Single IRB Review for Multi-Site Research Resource and Infrastructure Development Workshop (September 2018)
On September 12, 2018, NIH held a Workshop on Single IRB Resource and Infrastructure Development. The workshop focused on successful strategies and lessons learned for modifying and enhancing institutional IRB infrastructure for single IRB review of multi-site studies.
Agenda Biographies Relevant Information and Resources
Presentations: SESSION I – Customizing eIRB Systems to Review Multisite Studies Using a Single IRB Model, Medical University of South Carolina SESSION I – Models for Institutions Serving as sIRB of Record, Washington University SESSION I – Single IRB Standard Best Practices, New York University SESSION II – Models for Assisting Institution Relying on sIRB – Partnership and Innovation Exploring Single IRB Models to Support Clinical and Translational Research, Yale University SESSION II – Models for Assisting Institutions Relying on sIRB – Incorporating sIRB Procedures into an Existing Research Network, University of Rochester SESSION III – Facilitating Single IRB Review for Multi-site Research:The OneIRB IT Platform, University of Penslyvania SESSION III – Administrative Supplements for CTSA Awardees: Development of Resources to Facilitate Single IRB Review for Multi-Site Research, University of Cincinnati Webcast
Clinical trials must be conducted with a high standard of quality that assures the research question is answered in a reliable, valid, and unbiased manner, and that the rights and welfare of human subjects are protected.
In 1998, NIH issued a policy and further guidance was published in 2000. The NIH Data and Safety Monitoring policy states that each NIH Institute and Center (IC) should have a system for appropriate oversight and monitoring of clinical trials in order to ensure the safety of participants and the validity and integrity of the data. The 1998 and 2000 policies provide guidance on when monitoring should be conducted by a Data and Safety Monitoring Board (DSMB). The NIH requirement for data and safety monitoring –at a level commensurate with the risks, size and complexity of the trial–is separate and distinct from the requirement for protocol review and approval by an Institutional Review Board (IRB).
- Healthcare Research and Quality (AHRQ) Data and Safety Monitoring Policy
- Office for Human Research Protections (OHRP): Guidance on IRB Continuing Review of Research
- FDA Guidance for Clinical Trial Sponsors: Establishment and Operation of Clinical Trial Data Monitoring Committees
- NIH Policy for Data and Safety Monitoring, June 10, 1998
- Further Guidance on a Data and Safety Monitoring for Phase I and Phase II Trials, June 5, 2000
- Guideline on Data Monitoring Committees (European Medicines Agency, July 27, 2005)
- Operational Guidelines for the Establishment and Functioning of Data and Safety Monitoring Boards (World Health Organization)
Participants in NIH-funded clinical research studies receive a robust set of protections in accordance with standards established by the U.S. Department of Health and Human Services (HHS) in 45 Code of Federal Regulations (CFR) part 46 (“the Common Rule”), and, when applicable, Food and Drug Administration (FDA) Regulations for 21 CFR Part 50 and 56. Additional protections for research participants are also established under Section 2012 and 2013 of the 21st Century Cures Act, the NIH Certificates of Confidentiality policy, the Privacy Act, and the HIPAA Privacy Rule. Protections provided by the Common Rule ensure the safety, rights, and welfare of participants in research (what the Common Rule calls “human subjects”) under a framework that prioritizes the ethical principles enshrined in the Belmont Report (i.e., respect for persons, beneficence, and justice). These protections include oversight of research by Institutional Review Boards (IRBs), as well as requirements for informed consent, confidentiality, and privacy.
OSP serves as a resource for and advisor to the NIH research community with respect to the implementation of regulations, laws, and policies impacting the protection of participants in NIH research. OSP advises on harmonization and optimization, works with NIH Institute, Center, and Office (ICO) partners to identify and resolve policy issues, and recommends and develops new policies as needed. OSP works closely with colleagues across NIH and HHS, including NIH ICOs as well as FDA, the Department of Defense (DOD), the Department of Veteran’s Affairs (VA), the HHS Office for Human Research Protections (OHRP), and the HHS Secretary’s Advisory Committee on Human Research Protections (SACHRP).
- Common Rule: 45 CFR Part 46
- FDA Regulations for 21 CFR Parts 50 and 56
- NIH Human Subjects Research Policies and Policy Notices
- Office for Human Research Protections
- Secretary’s Advisory Committee on Human Research Protections (SACHRP)
Privacy and confidentiality are essential components of a robust research enterprise. NIH takes seriously the obligation to ensure privacy and confidentiality for individuals who have altruistically provided data and samples for research. Some of the obligations for these protections are outlined in the Common Rule, which describes a framework for protecting the privacy and confidentiality of sensitive, private information. Additional protections are provided by Section 2012 and 2013 of the 21st Century Cures Act and the NIH Certificates of Confidentiality Policy, which ensure appropriate protections for research when identifiable, sensitive information is collected or used. The Privacy Act and HIPAA Privacy Rule outline further protections for when individual data subject to these laws can be released.
OSP serves as a resource and advisor to the NIH research community on privacy and confidentiality regulations, laws, and policies. OSP advises NIH ICOs on these protections and works with ICOs to identify and resolve any policy issues.
- Common Rule: 45 CFR Part 46
- 21 CFR part 50 and 56 FDA Regulations for the Protection of Human Subjects
- Section 2012 and 2013 of the 21st Century Cures Act Provision on Certificates of Confidentiality
- NIH Certificates of Confidentiality policy
- NIH Certificates of Confidentiality website
- Privacy Act
- HIPAA Privacy Rule
- 2013 Privacy Rule Amendments
- HIPAA Privacy Rule and Its Impacts on Research (nih.gov)
NIH has long supported a movement in the research enterprise to engage participants as partners in research by informing research priorities and advising on clinical study designs to ensure that outcomes are meaningful to them. Research participants, for their part, have indicated the desire to have information returned to them about the studies in which they participate, including individual-level research results. In 2018, NIH co-sponsored a study by the National Academies of Sciences, Engineering, and Medicine (NASEM) to examine Returning Individual Research Results to Participants , specifically reviewing the current evidence on the benefits, harms, and costs of returning individual research results while considering the ethical, societal, regulatory, and operational issues related to the return of individual-specific research results generated from research on human biospecimens. In recognition of the evolving nature of research and the relationship between research participants and investigators, as well as the responsibility to uphold participant preferences, various programs and initiatives across NIH are moving towards creating a more participatory model of enabling greater return of individual-level research results.
OSP serves as a resource for and advisor to the NIH research community with regard to identifying and resolving policy issues and promoting best practices for the appropriate return of individual research results to participants.
Funding Opportunities Across NIH (updated 4/3/23)
- Strategies for Responsibly Reporting Back Environmental Health and Non-Genomic Research Results (R01 Clinical Trial Optional)
Select Return of Research Results Efforts at NIH
The below list of programs and initiatives is non-exhaustive and highlights select examples that include either a focus on returning research results from particular studies or promoting the development of tools and best practices to enable return of results.
- All of Us Research Program
- Return of Research Results webpage
- CLINSEQ®: A Large-Scale Medical Sequencing Clinical Research Pilot Study
- Clinical Sequencing Evidence-Generating Research (CSER) Consortium
- Electronic Medical Records and Genomics (eMERGE) Network
Select Resources
- Clinical Laboratory Improvement Amendments of 1988 (CLIA)
- The Secretary’s Advisory Committee on Human Research Protections (SACHRP) Recommendation for Return of Individual Research Results—Sharing Study Data and Results: Return of Individual Results
- OSP Blog: Recommendations for Moving to a Process for Returning Research Results
- OSP Blog: A Fresh Look at the Rules for Returning Research Results
Research Process: 8 Steps in Research Process

The research process starts with identifying a research problem and conducting a literature review to understand the context. The researcher sets research questions, objectives, and hypotheses based on the research problem.
A research study design is formed to select a sample size and collect data after processing and analyzing the collected data and the research findings presented in a research report.
What is the Research Process?
There are a variety of approaches to research in any field of investigation, irrespective of whether it is applied research or basic research. Each research study will be unique in some ways because of the particular time, setting, environment, and place it is being undertaken.
Nevertheless, all research endeavors share a common goal of furthering our understanding of the problem, and thus, all traverse through certain primary stages, forming a process called the research process.
Understanding the research process is necessary to effectively carry out research and sequence the stages inherent in the process.
How Research Process Work?

Eight steps research process is, in essence, part and parcel of a research proposal. It is an outline of the commitment that you intend to follow in executing a research study.
A close examination of the above stages reveals that each of these stages, by and large, is dependent upon the others.
One cannot analyze data (step 7) unless he has collected data (step 6). One cannot write a report (step 8) unless he has collected and analyzed data (step 7).
Research then is a system of interdependent related stages. Violation of this sequence can cause irreparable harm to the study.
It is also true that several alternatives are available to the researcher during each stage stated above. A research process can be compared with a route map.
The map analogy is useful for the researcher because several alternatives exist at each stage of the research process.
Choosing the best alternative in terms of time constraints, money, and human resources in our research decision is our primary goal.
Before explaining the stages of the research process, we explain the term ‘iterative’ appearing within the oval-shaped diagram at the center of the schematic diagram.
The key to a successful research project ultimately lies in iteration: the process of returning again and again to the identification of the research problems, methodology, data collection, etc., which leads to new ideas, revisions, and improvements.
By discussing the research project with advisers and peers, one will often find that new research questions need to be added, variables to be omitted, added or redefined, and other changes to be made. As a proposed study is examined and reexamined from different perspectives, it may begin to transform and take a different shape.
This is expected and is an essential component of a good research study.
Besides, examining study methods and data collected from different viewpoints is important to ensure a comprehensive approach to the research question.
In conclusion, there is seldom any single strategy or formula for developing a successful research study, but it is essential to realize that the research process is cyclical and iterative.
What is the primary purpose of the research process?
The research process aims to identify a research problem, understand its context through a literature review, set research questions and objectives, design a research study, select a sample, collect data, analyze the data, and present the findings in a research report.
Why is the research design important in the research process?
The research design is the blueprint for fulfilling objectives and answering research questions. It specifies the methods and procedures for collecting, processing, and analyzing data, ensuring the study is structured and systematic.
8 Steps of Research Process
Identifying the research problem.

The first and foremost task in the entire process of scientific research is to identify a research problem .
A well-identified problem will lead the researcher to accomplish all-important phases of the research process, from setting objectives to selecting the research methodology .
But the core question is: whether all problems require research.
We have countless problems around us, but all we encounter do not qualify as research problems; thus, these do not need to be researched.
Keeping this point in mind, we must draw a line between research and non-research problems.
Intuitively, researchable problems are those that have a possibility of thorough verification investigation, which can be effected through the analysis and collection of data. In contrast, the non-research problems do not need to go through these processes.
Researchers need to identify both;
Non-Research Problems
Statement of the problem, justifying the problem, analyzing the problem.
A non-research problem does not require any research to arrive at a solution. Intuitively, a non-researchable problem consists of vague details and cannot be resolved through research.
It is a managerial or built-in problem that may be solved at the administrative or management level. The answer to any question raised in a non-research setting is almost always obvious.
The cholera outbreak, for example, following a severe flood, is a common phenomenon in many communities. The reason for this is known. It is thus not a research problem.
Similarly, the reasons for the sudden rise in prices of many essential commodities following the announcement of the budget by the Finance Minister need no investigation. Hence it is not a problem that needs research.
How is a research problem different from a non-research problem?
A research problem is a perceived difficulty that requires thorough verification and investigation through data analysis and collection. In contrast, a non-research problem does not require research for a solution, as the answer is often obvious or already known.
Non-Research Problems Examples
A recent survey in town- A found that 1000 women were continuous users of contraceptive pills.
But last month’s service statistics indicate that none of these women were using contraceptive pills (Fisher et al. 1991:4).
The discrepancy is that ‘all 1000 women should have been using a pill, but none is doing so. The question is: why the discrepancy exists?
Well, the fact is, a monsoon flood has prevented all new supplies of pills from reaching town- A, and all old supplies have been exhausted. Thus, although the problem situation exists, the reason for the problem is already known.
Therefore, assuming all the facts are correct, there is no reason to research the factors associated with pill discontinuation among women. This is, thus, a non-research problem.
A pilot survey by University students revealed that in Rural Town-A, the goiter prevalence among school children is as high as 80%, while in the neighboring Rural Town-A, it is only 30%. Why is a discrepancy?
Upon inquiry, it was seen that some three years back, UNICEF launched a lipiodol injection program in the neighboring Rural Town-A.
This attempt acted as a preventive measure against the goiter. The reason for the discrepancy is known; hence, we do not consider the problem a research problem.
A hospital treated a large number of cholera cases with penicillin, but the treatment with penicillin was not found to be effective. Do we need research to know the reason?
Here again, there is one single reason that Vibrio cholera is not sensitive to penicillin; therefore, this is not the drug of choice for this disease.
In this case, too, as the reasons are known, it is unwise to undertake any study to find out why penicillin does not improve the condition of cholera patients. This is also a non-research problem.
In the tea marketing system, buying and selling tea starts with bidders. Blenders purchase open tea from the bidders. Over the years, marketing cost has been the highest for bidders and the lowest for blenders. What makes this difference?
The bidders pay exorbitantly higher transport costs, which constitute about 30% of their total cost.
Blenders have significantly fewer marketing functions involving transportation, so their marketing cost remains minimal.
Hence no research is needed to identify the factors that make this difference.
Here are some of the problems we frequently encounter, which may well be considered non-research problems:
- Rises in the price of warm clothes during winter;
- Preferring admission to public universities over private universities;
- Crisis of accommodations in sea resorts during summer
- Traffic jams in the city street after office hours;
- High sales in department stores after an offer of a discount.
Research Problem
In contrast to a non-research problem, a research problem is of primary concern to a researcher.
A research problem is a perceived difficulty, a feeling of discomfort, or a discrepancy between a common belief and reality.
As noted by Fisher et al. (1993), a problem will qualify as a potential research problem when the following three conditions exist:
- There should be a perceived discrepancy between “what it is” and “what it should have been.” This implies that there should be a difference between “what exists” and the “ideal or planned situation”;
- A question about “why” the discrepancy exists. This implies that the reason(s) for this discrepancy is unclear to the researcher (so that it makes sense to develop a research question); and
- There should be at least two possible answers or solutions to the questions or problems.
The third point is important. If there is only one possible and plausible answer to the question about the discrepancy, then a research situation does not exist.
It is a non-research problem that can be tackled at the managerial or administrative level.
Research Problem Examples
Research problem – example #1.
While visiting a rural area, the UNICEF team observed that some villages have female school attendance rates as high as 75%, while some have as low as 10%, although all villages should have a nearly equal attendance rate. What factors are associated with this discrepancy?
We may enumerate several reasons for this:
- Villages differ in their socio-economic background.
- In some villages, the Muslim population constitutes a large proportion of the total population. Religion might play a vital role.
- Schools are far away from some villages. The distance thus may make this difference.
Because there is more than one answer to the problem, it is considered a research problem, and a study can be undertaken to find a solution.
Research Problem – Example #2
The Government has been making all-out efforts to ensure a regular flow of credit in rural areas at a concession rate through liberal lending policy and establishing many bank branches in rural areas.
Knowledgeable sources indicate that expected development in rural areas has not yet been achieved, mainly because of improper credit utilization.
More than one reason is suspected for such misuse or misdirection.
These include, among others:
- Diversion of credit money to some unproductive sectors
- Transfer of credit money to other people like money lenders, who exploit the rural people with this money
- Lack of knowledge of proper utilization of the credit.
Here too, reasons for misuse of loans are more than one. We thus consider this problem as a researchable problem.
Research Problem – Example #3
Let’s look at a new headline: Stock Exchange observes the steepest ever fall in stock prices: several injured as retail investors clash with police, vehicles ransacked .
Investors’ demonstration, protest and clash with police pause a problem. Still, it is certainly not a research problem since there is only one known reason for the problem: Stock Exchange experiences the steepest fall in stock prices. But what causes this unprecedented fall in the share market?
Experts felt that no single reason could be attributed to the problem. It is a mix of several factors and is a research problem. The following were assumed to be some of the possible reasons:
- The merchant banking system;
- Liquidity shortage because of the hike in the rate of cash reserve requirement (CRR);
- IMF’s warnings and prescriptions on the commercial banks’ exposure to the stock market;
- Increase in supply of new shares;
- Manipulation of share prices;
- Lack of knowledge of the investors on the company’s fundamentals.
The choice of a research problem is not as easy as it appears. The researchers generally guide it;
- own intellectual orientation,
- level of training,
- experience,
- knowledge on the subject matter, and
- intellectual curiosity.
Theoretical and practical considerations also play a vital role in choosing a research problem. Societal needs also guide in choosing a research problem.
Once we have chosen a research problem, a few more related steps must be followed before a decision is taken to undertake a research study.
These include, among others, the following:
- Statement of the problem.
- Justifying the problem.
- Analyzing the problem.
A detailed exposition of these issues is undertaken in chapter ten while discussing the proposal development.
A clear and well-defined problem statement is considered the foundation for developing the research proposal.
It enables the researcher to systematically point out why the proposed research on the problem should be undertaken and what he hopes to achieve with the study’s findings.
A well-defined statement of the problem will lead the researcher to formulate the research objectives, understand the background of the study, and choose a proper research methodology.
Once the problem situation has been identified and clearly stated, it is important to justify the importance of the problem.
In justifying the problems, we ask such questions as why the problem of the study is important, how large and widespread the problem is, and whether others can be convinced about the importance of the problem and the like.
Answers to the above questions should be reviewed and presented in one or two paragraphs that justify the importance of the problem.
As a first step in analyzing the problem, critical attention should be given to accommodate the viewpoints of the managers, users, and researchers to the problem through threadbare discussions.
The next step is identifying the factors that may have contributed to the perceived problems.
Issues of Research Problem Identification
There are several ways to identify, define, and analyze a problem, obtain insights, and get a clearer idea about these issues. Exploratory research is one of the ways of accomplishing this.
The purpose of the exploratory research process is to progressively narrow the scope of the topic and transform the undefined problems into defined ones, incorporating specific research objectives.
The exploratory study entails a few basic strategies for gaining insights into the problem. It is accomplished through such efforts as:
Pilot Survey
A pilot survey collects proxy data from the ultimate subjects of the study to serve as a guide for the large study. A pilot study generates primary data, usually for qualitative analysis.
This characteristic distinguishes a pilot survey from secondary data analysis, which gathers background information.
Case Studies
Case studies are quite helpful in diagnosing a problem and paving the way to defining the problem. It investigates one or a few situations identical to the researcher’s problem.
Focus Group Interviews
Focus group interviews, an unstructured free-flowing interview with a small group of people, may also be conducted to understand and define a research problem .
Experience Survey
Experience survey is another strategy to deal with the problem of identifying and defining the research problem.
It is an exploratory research endeavor in which individuals knowledgeable and experienced in a particular research problem are intimately consulted to understand the problem.
These persons are sometimes known as key informants, and an interview with them is popularly known as the Key Informant Interview (KII).
Reviewing of Literature

A review of relevant literature is an integral part of the research process. It enables the researcher to formulate his problem in terms of the specific aspects of the general area of his interest that has not been researched so far.
Such a review provides exposure to a larger body of knowledge and equips him with enhanced knowledge to efficiently follow the research process.
Through a proper review of the literature, the researcher may develop the coherence between the results of his study and those of the others.
A review of previous documents on similar or related phenomena is essential even for beginning researchers.
Ignoring the existing literature may lead to wasted effort on the part of the researchers.
Why spend time merely repeating what other investigators have already done?
Suppose the researcher is aware of earlier studies of his topic or related topics . In that case, he will be in a much better position to assess his work’s significance and convince others that it is important.
A confident and expert researcher is more crucial in questioning the others’ methodology, the choice of the data, and the quality of the inferences drawn from the study results.
In sum, we enumerate the following arguments in favor of reviewing the literature:
- It avoids duplication of the work that has been done in the recent past.
- It helps the researcher discover what others have learned and reported on the problem.
- It enables the researcher to become familiar with the methodology followed by others.
- It allows the researcher to understand what concepts and theories are relevant to his area of investigation.
- It helps the researcher to understand if there are any significant controversies, contradictions, and inconsistencies in the findings.
- It allows the researcher to understand if there are any unanswered research questions.
- It might help the researcher to develop an analytical framework.
- It will help the researcher consider including variables in his research that he might not have thought about.
Why is reviewing literature crucial in the research process?
Reviewing literature helps avoid duplicating previous work, discovers what others have learned about the problem, familiarizes the researcher with relevant concepts and theories, and ensures a comprehensive approach to the research question.
What is the significance of reviewing literature in the research process?
Reviewing relevant literature helps formulate the problem, understand the background of the study, choose a proper research methodology, and develop coherence between the study’s results and previous findings.
Setting Research Questions, Objectives, and Hypotheses

After discovering and defining the research problem, researchers should make a formal statement of the problem leading to research objectives .
An objective will precisely say what should be researched, delineate the type of information that should be collected, and provide a framework for the scope of the study. A well-formulated, testable research hypothesis is the best expression of a research objective.
A hypothesis is an unproven statement or proposition that can be refuted or supported by empirical data. Hypothetical statements assert a possible answer to a research question.
Step #4: Choosing the Study Design

The research design is the blueprint or framework for fulfilling objectives and answering research questions .
It is a master plan specifying the methods and procedures for collecting, processing, and analyzing the collected data. There are four basic research designs that a researcher can use to conduct their study;
- experiment,
- secondary data study, and
- observational study.
The type of research design to be chosen from among the above four methods depends primarily on four factors:
- The type of problem
- The objectives of the study,
- The existing state of knowledge about the problem that is being studied, and
- The resources are available for the study.
Deciding on the Sample Design

Sampling is an important and separate step in the research process. The basic idea of sampling is that it involves any procedure that uses a relatively small number of items or portions (called a sample) of a universe (called population) to conclude the whole population.
It contrasts with the process of complete enumeration, in which every member of the population is included.
Such a complete enumeration is referred to as a census.
A population is the total collection of elements we wish to make some inference or generalization.
A sample is a part of the population, carefully selected to represent that population. If certain statistical procedures are followed in selecting the sample, it should have the same characteristics as the population. These procedures are embedded in the sample design.
Sample design refers to the methods followed in selecting a sample from the population and the estimating technique vis-a-vis the formula for computing the sample statistics.
The fundamental question is, then, how to select a sample.
To answer this question, we must have acquaintance with the sampling methods.
These methods are basically of two types;
- probability sampling , and
- non-probability sampling .
Probability sampling ensures every unit has a known nonzero probability of selection within the target population.
If there is no feasible alternative, a non-probability sampling method may be employed.
The basis of such selection is entirely dependent on the researcher’s discretion. This approach is called judgment sampling, convenience sampling, accidental sampling, and purposive sampling.
The most widely used probability sampling methods are simple random sampling , stratified random sampling , cluster sampling , and systematic sampling . They have been classified by their representation basis and unit selection techniques.
Two other variations of the sampling methods that are in great use are multistage sampling and probability proportional to size (PPS) sampling .
Multistage sampling is most commonly used in drawing samples from very large and diverse populations.
The PPS sampling is a variation of multistage sampling in which the probability of selecting a cluster is proportional to its size, and an equal number of elements are sampled within each cluster.
Collecting Data From The Research Sample

Data gathering may range from simple observation to a large-scale survey in any defined population. There are many ways to collect data. The approach selected depends on the objectives of the study, the research design, and the availability of time, money, and personnel.
With the variation in the type of data (qualitative or quantitative) to be collected, the method of data collection also varies .
The most common means for collecting quantitative data is the structured interview .
Studies that obtain data by interviewing respondents are called surveys. Data can also be collected by using self-administered questionnaires . Telephone interviewing is another way in which data may be collected .
Other means of data collection include secondary sources, such as the census, vital registration records, official documents, previous surveys, etc.
Qualitative data are collected mainly through in-depth interviews, focus group discussions , Key Informant Interview ( KII), and observational studies.
Process and Analyze the Collected Research Data

Data processing generally begins with the editing and coding of data . Data are edited to ensure consistency across respondents and to locate omissions if any.
In survey data, editing reduces errors in the recording, improves legibility, and clarifies unclear and inappropriate responses. In addition to editing, the data also need coding.
Because it is impractical to place raw data into a report, alphanumeric codes are used to reduce the responses to a more manageable form for storage and future processing.
This coding process facilitates the processing of the data. The personal computer offers an excellent opportunity for data editing and coding processes.
Data analysis usually involves reducing accumulated data to a manageable size, developing summaries, searching for patterns, and applying statistical techniques for understanding and interpreting the findings in light of the research questions.
Further, based on his analysis, the researcher determines if his findings are consistent with the formulated hypotheses and theories.
The techniques used in analyzing data may range from simple graphical techniques to very complex multivariate analyses depending on the study’s objectives, the research design employed, and the nature of the data collected.
As in the case of data collection methods, an analytical technique appropriate in one situation may not be suitable for another.
Writing Research Report – Developing Research Proposal, Writing Report, Disseminating and Utilizing Results

The entire task of a research study is accumulated in a document called a proposal or research proposal.
A research proposal is a work plan, prospectus, outline, offer, and a statement of intent or commitment from an individual researcher or an organization to produce a product or render a service to a potential client or sponsor .
The proposal will be prepared to keep the sequence presented in the research process. The proposal tells us what, how, where, and to whom it will be done.
It must also show the benefit of doing it. It always includes an explanation of the purpose of the study (the research objectives) or a definition of the problem.
It systematically outlines the particular research methodology and details the procedures utilized at each stage of the research process.
The end goal of a scientific study is to interpret the results and draw conclusions.
To this end, it is necessary to prepare a report and transmit the findings and recommendations to administrators, policymakers, and program managers to make a decision.
There are various research reports: term papers, dissertations, journal articles , papers for presentation at professional conferences and seminars, books, thesis, and so on. The results of a research investigation prepared in any form are of little utility if they are not communicated to others.
The primary purpose of a dissemination strategy is to identify the most effective media channels to reach different audience groups with study findings most relevant to their needs.
The dissemination may be made through a conference, a seminar, a report, or an oral or poster presentation.
The style and organization of the report will differ according to the target audience, the occasion, and the purpose of the research. Reports should be developed from the client’s perspective.
A report is an excellent means that helps to establish the researcher’s credibility. At a bare minimum, a research report should contain sections on:
- An executive summary;
- Background of the problem;
- Literature review;
- Methodology;
- Discussion;
- Conclusions and
- Recommendations.
The study results can also be disseminated through peer-reviewed journals published by academic institutions and reputed publishers both at home and abroad. The report should be properly evaluated .
These journals have their format and editorial policies. The contributors can submit their manuscripts adhering to the policies and format for possible publication of their papers.
There are now ample opportunities for researchers to publish their work online.
The researchers have conducted many interesting studies without affecting actual settings. Ideally, the concluding step of a scientific study is to plan for its utilization in the real world.
Although researchers are often not in a position to implement a plan for utilizing research findings, they can contribute by including in their research reports a few recommendations regarding how the study results could be utilized for policy formulation and program intervention.
Why is the dissemination of research findings important?
Dissemination of research findings is crucial because the results of a research investigation have little utility if not communicated to others. Dissemination ensures that the findings reach relevant stakeholders, policymakers, and program managers to inform decisions.
How should a research report be structured?
A research report should contain sections on an executive summary, background of the problem, literature review, methodology, findings, discussion, conclusions, and recommendations.
Why is it essential to consider the target audience when preparing a research report?
The style and organization of a research report should differ based on the target audience, occasion, and research purpose. Tailoring the report to the audience ensures that the findings are communicated effectively and are relevant to their needs.

Your email address will not be published. Required fields are marked *
- National Headquarters
- 1-800-223-2732
- Select Location

American Parkinson Disease Association
- Mission & Leadership
- Strategic Plan
- Financial Reports
- APDA in the News
- Career Opportunities
- Virtual Events
2023 Update: New Parkinson’s Disease Treatments in the Clinical Trial Pipeline
New Parkinson’s Medication on the Horizon
The development of potential new medications for Parkinson’s disease (PD) medications remains very active, with multiple new medications in various stages of research development that are aiming to treat and slow down PD.
In past blogs, we have reviewed the various mechanisms of action that are being studied to see if they result in successful slowing of disease progression.
These treatment mechanisms include:
Targeting abnormal alpha-synuclein aggregation.
- Increasing activity of GLP-1, a strategy which may block activation of immune cells in the brain
- Other strategies of decreasing inflammation in the brain
- Increasing the activity of the enzyme glucocerebrosidase to enhance the cell’s lysosomal or garbage disposal system
- Decreasing activity of the proteins LRRK2 or c-Abl to decrease neurodegeneration
- Improving function of the mitochondria – the energy-producing element of the nerve cell – to support the health of the neurons
- Increasing neurotrophic factors to enhance nerve survival
- Using cell based therapies to restore healthy nerves in the brain
Decreasing oxidative stress in the brain
Most of the compounds presented in prior blogs are continuing to be studied in various stages of clinical trials.
You can view these past blogs below:
- Neuroprotective strategies in clinical trials – 2020
- Neuroprotective strategies in clinical trials – update 2021
- Medications in clinical trials – 2022
- Therapies for non-motor symptoms in clinical trials
- Repurposed medications being studied for PD
Here are additional medications that we are keeping our eye on in 2023 and into 2024

You can read more about each of the clinical trials mentioned by following the links provided. Each is associated with an NCT number on clinicaltrials.gov, a database of all the clinical trials for all diseases worldwide. Each link also provides the contact information for each trial if you would like to find out more about the possibility of participating in the trial.)
Decreasing activity of LRRK2
BIIB122: One compound that is successfully moving through the research pipeline is BIIB122. We previously reported on a Phase 1 study of a small molecule LRRK2 inhibitor known at the time as DNL151. The results of that study were published , and this molecule now called BIIB122, is being tested to see its efficacy in a much larger group of people.
Mutations (a change in the DNA sequence) in the LRRK2 (Leucine-rich repeat kinase 2) gene represent a common genetic cause of PD. LRRK2 plays several roles in the cell and mutations that increase its enzymatic activity are thought to cause neurodegeneration. BIIB122 is a small molecule that decreases the activity of LRRK2. The current study NCT05418673 is evaluating whether taking BIIB122 slows the progression of PD more than placebo in the early stages of PD. The study will focus on participants with specific genetic variants in their LRRK2 gene.
Butanetap : Buntanetap is a small molecule that suppresses the translation of DNA into messenger RNA of several neurotoxic proteins. This group of neurotoxic proteins produces insoluble clumps that accumulate in nerve cells, disrupting the cell’s normal function. One of these proteins is alpha-synuclein, which abnormally accumulates in PD. In early studies, Buntanetap showed reduction of inflammation and preservation of axonal integrity and synaptic function. The current study NCT05357989 is designed tomeasure safety and efficacy of Buntanetap compared with placebo in participants with early PD.
Sulfuraphane : Sulfuraphane is an antioxidant, found in dark green vegetables such as broccoli and brussel sprouts. It is currently being studied NCT05084365 to see if it improves motor and cognitive function in PD.
Decreasing activity of the c-Abl kinase
IKT-148009 : IKT-148009 is a small molecule that decreases the activity of c-Abl, an enzyme that acts on a wide range of targets within the cell, supporting many different cellular functions. Research suggests that overactivation of c-Abl is a downstream effect of oxidative stress and may play a role in neurodegeneration in PD. There is also research to suggest that increased c-Abl activation correlates with alpha-synuclein aggregation. These findings and others led to the possibility that inhibiting c-Abl may be a helpful strategy in PD therapy. The current study NCT05424276 is investigating whether decreasing the activity of c-Abl in early, untreated people with PD is safe and tolerable, and whether it improves motor and non-motor features of the disease.
Cell-based therapy
Bemdaneprocel (BRT-DA01, previously known as MSK-DA01): A recently-completed Phase 1 study investigated the surgical transplantation of dopaminergic neuron precursor cells into the brains of people with PD. In an open label study (one without a control group) of 12 people, the treatment was found to be safe and well-tolerated. Transplantation of the cells was feasible and resulted in successful cell survival and engraftment. A phase 2 study is currently being planned for early 2024.
Decreasing inflammation
RO-7486967/selnoflast: – RO-7486967 is a small molecule that inhibits the NLRP3 inflammasome, a complex of proteins involved in inflammation that is thought to be overactive in PD. The current study NCT05924243 will investigate whether this molecule is safe and tolerable in early stages of PD.
New mechanism of action: Targeting cell death
KM819: Apoptosis, a series of organized molecular steps that leads to programmed cell death, is a normal part of cell function. When this system goes awry however, cells may die when they are not supposed to. KM819 is a small molecule inhibitor of Fas-associated factor1 (FAF1), a key regulator of cell death. It is being investigated to see if decreasing the process of cell death will protect neurons in PD. The current study NCT05670782 is testing this compound in both healthy adults and people with PD.
The Parkinson’s Hope List
We continue to thank Dr. Kevin McFarthing, a biochemist and person with Parkinson’s for his efforts in creating and maintaining The Parkinson’s Hope List — a collation of all the compounds that are being explored as new therapies for PD at all stages of the research pipeline and is updated frequently. It is an excellent source of information for those interested in the current state of PD research focused on new potential treatments. APDA was privileged to host Dr. McFarthing as a special guest on our broadcast entitled Dr. Gilbert Hosts:Taking Research From the Lab to our Lives .
Dr. McFarthing and his colleagues put together a yearly review of the medications for Parkinson’s disease in clinical trials. The year 2023’s review can be accessed here . Dr. McFarthing and colleagues reported that as of January 2023, there were nearly 139 Parkinson’s therapies active in the clinical trial pipeline as registered on the www.clinicaltrials.gov website involving almost 17,000 participants. Of these drugs tested, 76 (55%) trials were focused on symptomatic treatment (STs), medications that attempt ameliorate the symptoms of PD; and 63 (45%) were disease-modifying therapies (DMTs), medications that attempt to slow the progression of the disease. The pipeline grew in the past year, with 35 newly registered trials (18 ST and 17 DMT trials). Most of these clinical trials (34%) are in Phase 1 (early-stage of clinical testing, primarily performed to assess for safety), while 52% have progressed to Phase 2 testing stage (mid-stage, performed in small numbers of people with PD to assess for efficacy), followed by 14% currently in Phase 3 (late-stage trials, performed in larger numbers of people with PD to assess for efficacy).
APDA proudly funds innovative work
APDA recently announced its newly-funded research grantees for the 2023-2024 academic year. Our new pool of grantees are working on many of the strategies discussed above and will continue to push the field of PD research forward, introducing new ideas to the field and new possibilities in PD therapy.
Here are some examples:
- Dr. Nikhil Panicker is investigating the NRLP3 inflammasome. He is exploring whether reducing the activation of the inflammasome within microglia can protect neurons from accumulating alpha-synuclein in a cell model of PD.
- Dr. William Zeiger is studying the mechanisms by which the abnormal accumulation of alpha-synuclein cause thinking and memory problems in PD.
- Dr. Naemeh Pourshafie is studying the relationship between tau and alpha-synuclein, two proteins that abnormally accumulate in neurodegenerative diseases.
We are so proud to help make this vital work possible!
Tips and takeaways
- There is hope in progress, with multiple treatment strategies in the PD research pipeline.
- Potential treatments are generally divided into two large categories: disease modifying therapies and symptomatic treatments.
- Mechanisms of action that are being studied to alter the progression of PD include: decreasing activity of LRRK2, decreasing aggregation of alpha-synuclein, decreasing oxidative stress in the brain, decreasing activity of c-Abl, introducing dopaminergic neurons into the brain, decreasing inflammation, and inhibiting programmed cell death.
- APDA supports essential research, bringing new ideas to fruition in the treatment of PD. Read more about past work we have funded, and the projects that we are funding this year.
- We need your support in order to continue this extremely valuable research. Click here to make a donation.
Support Our Mission
To support your local 2023 Update: New Parkinson’s Disease Treatments in the Clinical Trial Pipeline chapter please click the button below:
Insert/edit link
Enter the destination URL
Or link to existing content
chapter-content-page
training-courses
apda-in-the-news
css_apda_events
css_apda_event_recur
tutor_assignments
popupbuilder
Regions & Countries
Religious landscape study.

The RLS, conducted in 2007 and 2014, surveys more than 35,000 Americans from all 50 states about their religious affiliations, beliefs and practices, and social and political views. User guide | Report about demographics | Report about beliefs and attitudes
Explore religious groups in the U.S. by tradition, family and denomination
Explore religious affiliation data by state, region or select metro areas, northeastern states.
- Connecticut
- Massachusetts
- New Hampshire
- Pennsylvania
- Rhode Island
Southern States
- District of Columbia
- Mississippi
- North Carolina
- South Carolina
- West Virginia
Midwestern States
- North Dakota
- South Dakota
Western States
All metro areas.
- Atlanta Metro Area
- Baltimore Metro Area
- Boston Metro Area
- Chicago Metro Area
- Dallas/Fort Worth Metro Area
- Detroit Metro Area
- Houston Metro Area
- Los Angeles Metro Area
- Miami Metro Area
- Minneapolis/St. Paul Metro Area
- New York City Metro Area
- Philadelphia Metro Area
- Phoenix Metro Area
- Pittsburgh Metro Area
- Providence Metro Area
- Riverside, CA Metro Area
- San Diego Metro Area
- San Francisco Metro Area
- Seattle Metro Area
- St. Louis Metro Area
- Tampa Metro Area
- Washington, DC Metro Area
Topics & Questions
Demographic information.
- Race and Ethnicity
- Immigration Status
- Marital Status
- Parental Status
Beliefs and Practices
- Belief in God
- Importance of Religion
- Attendance at Religious Services
- Prayer Frequency
- Prayer Groups
- Feelings of Spiritual Wellbeing
- Feelings of Sense of Wonder
- Guidance on Right and Wrong
- Standards for Right and Wrong
- Reading Scripture
- Interpretation of Scripture
- Belief in Heaven
- Belief in Hell
Social and Political Views
- Political Party
- Political Ideology
- Size of Government
- Government Aid to the Poor
- Homosexuality
- Same-Sex Marriage
- Protecting the Environment
- Human Evolution
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
ORIGINAL RESEARCH article
[frontiers in microbiology] the high-throughput solid-phase extraction of cis-cyclo(l-leu-l-pro) and cis-cyclo(l-phe-l-pro) from lactobacillus plantarum demonstrates efficacy against multidrug-resistant bacteria and influenza a (h3n2) virus provisionally accepted.

- 1 Department of Food and Nutrition, Institute of Food and Nutrition Science, College of Bio-Convergence, Eulji University, Seongnam 13135, Republic of Korea, Republic of Korea
The final, formatted version of the article will be published soon.
2,5-diketopiperazines are the simplest forms of cyclic dipeptides (CDPs) and have diverse frameworks with chiral side chains that are useful for drug development. Previous research has investigated the antimicrobial properties of proline-linked CDPs and their combinations in the culture filtrate (CF) of Lactobacillus plantarum LBP-K10 using anion exchange chromatography (AEC). However, the quantity of CDPs showcasing notable anti-influenza virus activity derived from AECs was generally lower than those originating from Lactobacillus CF. To address this issue, the study aims to propose a more efficient method for isolating CDPs and to introduce the antiviral combinations of CDPs obtained using a new method. The study employed a novel technique entailing high-throughput C18-based solidphase extraction with a methanol gradient (MeSPE). The MeSPE method involved increasing the methanol concentration from 5% to 50% in 5% increments. The methanol SPE fractions (MeSPEfs) eluted with methanol concentrations between 35% and 45% evinced substantial efficacy in inhibiting the influenza A/H3N2 virus via plaque-forming assay. MeSPEf-45, the 45% MeSPEf, exhibited exceptional efficacy in preventing viral infections in Madin-Darby kidney cells, surpassing both individual CDPs and the entire set of MeSPEfs. To identify the specific antiviral components of MeSPEf-45, all MeSPEfs were further fractionated through preparative high-performance liquid chromatography (prep-HPLC). MeSPEf-45 fractions S8 and S11 presented the highest activity against multidrug-resistant bacteria and influenza A/H3N2 virus among all MeSPEfs, with 11 common fractions. Antiviral fractions S8 and S11 were identified as proline-based CDPs, specifically cis-cyclo(L-Leu-L-Pro) and cis-cyclo(L-Phe-L-Pro), using gas chromatography-mass spectrometry. The combination of MeSPEf-45 fractions S8 and S11 displayed superior antibacterial and anti-influenza virus effects compared to the individual fractions S8 and S11. High-throughput MeSPE-derived MeSPEfs and subsequent HPLC-fractionated fractions presents an innovative approach to selectively purify large amounts of potent antimicrobial CDPs from bacterial CF. The findings also show the effectiveness of physiologically bioactive combinations that utilize fractions not containing CDP. This study provides the initial evidence demonstrating the antimicrobial properties of CDPs acquired through high-throughput SPE techniques.
Keywords: Cyclic dipeptides, cis-cyclo(L-Leu-L-Pro), cis-cyclo(L-Phe-L-Pro), solid-phase extraction, Influenza A virus, Lactobacillus plantarum LBP-K10 AEC, anion exchange chromatography, cis-cyclo(L-Leu-L-Pro), cis-cyclo(Lleucine-L-proline), cis-cyclo(L-Phe-L-Pro), cis-cyclo(L-phenylalanine-L-proline)
Received: 02 Dec 2023; Accepted: 19 Apr 2024.
Copyright: © 2024 Son, Hong, Oh and Kwak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Prof. Min-Kyu Kwak, Department of Food and Nutrition, Institute of Food and Nutrition Science, College of Bio-Convergence, Eulji University, Seongnam 13135, Republic of Korea, Seongnam, Gyeonggi, Republic of Korea
People also looked at

IMAGES
VIDEO
COMMENTS
The purpose of this phase is to help speed up and streamline the drug approval process. Phase 0 studies may help researchers find out if the drugs do what they're expected to do. This may help save time and money that would have been spent on later phase trials. Phase 0 studies use only a few small doses of a new drug in a few people.
The Four Phases of Clinical Trials. Early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. Later phases (phase 3 and 4) compare the treatment to current standard treatments. In a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects ...
Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 . Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing ...
Researchers conduct clinical trials in a series of steps called phases. Each phase has a different purpose and helps researchers answer different questions. Phase I trials: Researchers test a medicine or other treatment in a small group of people for the first time. The purpose is to learn about the best dosage for a medicine or other treatment ...
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [1] For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study ...
When considering trial 'phase', the most common phases in clinical trials are phase II trials, feasibility trials, pilot trials and phase III trials of effectiveness/efficacy. The term 'phase' usually refers to I-V: ... For the purpose of attributing costs during a research study, an assumption is made that the care/treatment under ...
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II. ... When considering participation in a research study, carefully look at the benefits and risks. Benefits may include ...
Phase I trials: Researchers test a drug or treatment in a small group of people (20-80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects. ... Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy ...
Phase 1. In this initial step of a clinical trial, the research staff is looking at the safety and appropriate dosage of giving a new treatment. They also watch for side effects. The number of patients enrolled on phase I trials are small — there are typically fewer than 50 patients involved. Kotila often hears patients ask if phase 1 trials ...
Clinical trials are research studies that include human volunteers. Before they start a clinical trial, doctors must prove there is a chance that the new treatment will work better than the one available now. ... Phase II clinical trials. A phase II clinical trial tells doctors more about how safe the treatment is and how well it works. Doctors ...
describe 5 phases to the research process: the conceptual phase, the design and planning phase, the empirical phase, the analytic phase, and the dissemination phase (Table 1). This chapter will focus on the first two phases, conceptualizing and planning a study; however, all phases will be described.
There are 3 main phases of clinical trials - phases 1 to 3. Phase 1 trials are the earliest phase trials and phase 3 are later phase trials. Some trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been licensed. Some trials are randomised. This means the people taking part are put into one of ...
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
Clinical trials are carefully designed, reviewed and completed, and need to be approved before they can start. People of all ages can take part in clinical trials, including children. There are 4 phases of biomedical clinical trials: Phase I studies usually test new drugs for the first time in a small group of people to evaluate a safe dosage ...
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials. Read and share this infographic (PDF, 317K) to learn why researchers do different kinds of clinical studies. Observational studies monitor people in normal settings.
Watch this video to learn about the three phases of clinical trials. Clinical Research Phase Studies. Phase 1. Study Participants: 20 to 100 healthy volunteers or people with the disease/condition ...
A published clinical research study stands on solid scientific ground to inform clinical practice given the article has passed through proper peer-reviews, revision, and content improvement. ... In the theoretical design phase, a research question is designed to address a clinical problem, which involves an informed understanding based on the ...
Step 3: Formulate research questions. Next, based on the problem statement, you need to write one or more research questions. These target exactly what you want to find out. They might focus on describing, comparing, evaluating, or explaining the research problem.
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
What are the four phases of clinical research trials? Children with neurological and developmental disorders often experience chronic sleep disturbances. Melatonin has been commonly prescribed because of its hypnotic properties. However, trials have had conflicting results. Therefore, researchers assessed the effectiveness of melatonin in ...
Step 1: Identify the Problem. Finding an issue or formulating a research question is the first step. A well-defined research problem will guide the researcher through all stages of the research process, from setting objectives to choosing a technique. There are a number of approaches to get insight into a topic and gain a better understanding ...
Phase III studies may last anywhere from 1-4 years and offer the FDA and manufacturers a deeper understanding of the benefits and caveats of a treatment. Phase IV: Post-market studies focused on long-term usage side effects. Phase IV trials, otherwise known as post-marketing surveillance, occur after the experimental drug has been approved ...
Clinical Research. Of NIH's more than $17B investment, clinical trials (~$7B) reflect the point at which the public is most directly engaged in NIH's clinical research activities, either as dedicated volunteer research participants or users of the resulting data and health interventions.Successful management and oversight of the clinical trial enterprise remains essential to NIH's ...
These trials reflect diverse drug and mechanistic targets, as well as diversity in the stages of AD/ADRD they address. NIA's active trials include: Clinical Drug Development - Phase I and II (60), Clinical Drug Development - Phase II/III and Phase III (13), Nondrug (177), Dementia Care and Caregiving (228), Diagnostic Tools, Assessments ...
Setting Research Questions, Objectives, and Hypotheses. Step #4: Choosing the Study Design. Deciding on the Sample Design. Collecting Data From The Research Sample. Process and Analyze the Collected Research Data. Writing Research Report - Developing Research Proposal, Writing Report, Disseminating and Utilizing Results.
The pipeline grew in the past year, with 35 newly registered trials (18 ST and 17 DMT trials). Most of these clinical trials (34%) are in Phase 1 (early-stage of clinical testing, primarily performed to assess for safety), while 52% have progressed to Phase 2 testing stage (mid-stage, performed in small numbers of people with PD to assess for ...
KEEPsAKE 1 and KEEPsAKE 2 are ongoing phase 3 studies evaluating the efficacy and safety of risankizumab in patients with active PsA. ... AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. ...
Media Release. Centre for Human Drug Research (CHDR) will conduct Phase I trial to evaluate IMP761, a first-in-class LAG-3 agonist antibody designed to restore balance to the immune system and ...
Religious Landscape Study. The RLS, conducted in 2007 and 2014, surveys more than 35,000 Americans from all 50 states about their religious affiliations, beliefs and practices, and social and political views. ... About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and ...
2,5-diketopiperazines are the simplest forms of cyclic dipeptides (CDPs) and have diverse frameworks with chiral side chains that are useful for drug development. Previous research has investigated the antimicrobial properties of proline-linked CDPs and their combinations in the culture filtrate (CF) of Lactobacillus plantarum LBP-K10 using anion exchange chromatography (AEC).