
- Search by keyword
- Search by citation
Page 1 of 13

Physiochemical analyses and molecular characterization of heavy metal-resistant bacteria from Ilesha gold mining sites in Nigeria
The contribution of the processes involved and waste generated during gold mining to the increment of heavy metals concentration in the environment has been well established. While certain heavy metals are req...
- View Full Text
Acetaminophen-traces bioremediation with novel phenotypically and genotypically characterized 2 Streptomyces strains using chemo-informatics, in vivo, and in vitro experiments for cytotoxicity and biological activity
We isolated two novel bacterial strains, active against the environmental pollutant acetaminophen/Paracetamol®. Streptomyces chrestomyceticus (symbol RS2) and Flavofuscus (symbol M33) collected from El-Natrun Val...
Biosoftening of banana pseudostem fiber using cellulase and pectinase enzyme isolated from Aspergillus niger for textile industry
Nowadays, farmers are facing a lot of problems for the disposal of banana pseudostem waste after the harvesting of banana. Banana pseudostem is a rich source of fiber, which is an alternative source of other n...
FolE gene expression for folic acid productivity from optimized and characterized probiotic Lactobacillus delbrueckii
Lactobacillus delbrueckii was one of the most common milk lactic acid bacterial strains (LAB) which characterized as probiotic with many health influencing properties.

Reverse transcription loop-mediated isothermal amplification (RT-LAMP) primer design based on Indonesia SARS-CoV-2 RNA sequence
The COVID-19 pandemic has highlighted the importance of tracking cases by using various methods such as the Reverse transcription loop-mediated isothermal amplification (RT-LAMP) which is a fast, simple, inexp...
In silico analysis of HLA-1 and HLA-2 recognition of a designed recombinant human papillomavirus vaccine based on L1 protein HPV subtype 45
Human leukocyte antigen (HLA) can bind and present the processed antigenic peptide derived from the vaccine to the T cell receptor, and this capability is crucial in determining the effectivity of the vaccine ...
In silico design of an epitope-based vaccine against PspC in Streptococcus pneumoniae using reverse vaccinology
Streptococcus pneumoniae is a major pathogen that poses a significant hazard to global health, causing a variety of infections including pneumonia, meningitis, and sepsis. The emergence of antibiotic-resistant st...
A scalable overexpression of a thermostable recombinant poly-histidine tag carboxyl esterase under lambda promoter: purification, characterization, and protein modelling
As a white biotechnological trend, esterases are thought to be among the most active enzymes’ classes in biocatalysis and synthesis of industrially importance organic compounds. Esterases are used in many appl...
Correction: Mycosynthesis of silver nanoparticles using marine fungi and their antimicrobial activity against pathogenic microorganisms
The original article was published in Journal of Genetic Engineering and Biotechnology 2023 21 :127
Whole genome sequence and comparative genomics analysis of multidrug-resistant Staphylococcus xylosus NM36 isolated from a cow with mastitis in Basrah city
Staphylococcus xylosus is a coagulase-negative, gram-positive coccus that is found in the environment and as a commensal organism on the skin and mucosal surfaces of animals. Despite the fact that S. xylosus is c...
Immunoinformatics-aided rational design of multiepitope-based peptide vaccine (MEBV) targeting human parainfluenza virus 3 (HPIV-3) stable proteins
Human parainfluenza viruses (HPIVs) are common RNA viruses responsible for respiratory tract infections. Human parainfluenza virus 3 (HPIV-3) is particularly pathogenic, causing severe illnesses with no effect...
Isolation of plant growth-promoting rhizobacteria from the agricultural fields of Tattiannaram, Telangana
Plant probiotics bacteria are live microbes that promote soil health and plant growth and build the stress-tolerant capacity to the plants. They benefit the plants by increasing nutrient absorption and release...
Exploring structural antigens of yellow fever virus to design multi-epitope subunit vaccine candidate by utilizing an immuno-informatics approach
Yellow fever is a mosquito-borne viral hemorrhagic disease transmitted by several species of virus-infected mosquitoes endemic to tropical regions of Central and South America and Africa. Earlier in the twenti...
Short tandem repeat (STR) variation from 6 cities in Iraq based on 15 loci
One thousand sixty-one individuals were sampled from the cities of Anbar, Baghdad, Basra, Diyala, Najaf, and Wasit in Iraq and typed for 15 forensic STRs to explore the genetic structure of Iraq and develop a ...
The hepato- and neuroprotective effect of gold Casuarina equisetifolia bark nano-extract against Chlorpyrifos-induced toxicity in rats
The bark of Casuarina equisetifolia contains several active phytoconstituents that are suitable for the biosynthesis of gold nanoparticles (Au-NPs). These nanoparticles were subsequently evaluated for their effec...
Cloning and characterization of an acidic lipase from a lipolytic bacterium in tempeh
Lipases have emerged as essential biocatalysts, having the ability to contribute to a wide range of industrial applications. Microbial lipases have garnered significant industrial attention due to their stabil...
Recent advances in genome annotation and synthetic biology for the development of microbial chassis
This article provides an overview of microbial host selection, synthetic biology, genome annotation, metabolic modeling, and computational methods for predicting gene essentiality for developing a microbial ch...
In-silico analysis of potent Mosquirix vaccine adjuvant leads
World Health Organization recommend the use of malaria vaccine, Mosquirix, as a malaria prevention strategy. However, Mosquirix has failed to reduce the global burden of malaria because of its inefficacy. The ...
Influenza vaccine: a review on current scenario and future prospects
Vaccination is a crucial tool in preventing influenza, but it requires annual updates in vaccine composition due to the ever-changing nature of the flu virus. While healthcare and economic burdens have reduced...
Endophytic bacteria Klebsiella spp. and Bacillus spp . from Alternanthera philoxeroides in Madiwala Lake exhibit additive plant growth-promoting and biocontrol activities
The worldwide increase in human population and environmental damage has put immense pressure on the overall global crop production making it inadequate to feed the entire population. Therefore, the need for su...
Immunoinformatics analysis of Brucella melitensis to approach a suitable vaccine against brucellosis
Brucellosis caused by B. melitensis is one of the most important common diseases between humans and livestock. Currently, live attenuated vaccines are used for this disease, which causes many problems, and unfort...
Enhancement effect of AgO nanoparticles on fermentative cellulase activity from thermophilic Bacillus subtilis Ag-PQ
Cellulase is an important bioprocessing enzyme used in various industries. This study was conducted with the aim of improving the biodegradation activity of cellulase obtained from the Bacillus subtilis AG-PQ str...

Studying the pathogenicity of 26 variants characterized in the first molecular analyses of Egyptian aplastic anemia patients
Aplastic anemia (AA) is a bone marrow disorder characterized by peripheral pancytopenia and marrow hypoplasia which can lead to life-threatening complications. Our objective was to study the telomerase genes ( TER...
Optimizing the generation of mature bone marrow-derived dendritic cells in vitro: a factorial study design
Factorial design is a simple, yet elegant method to investigate the effect of multiple factors and their interaction on a specific response simultaneously. Hence, this type of study design reaches the best opt...
Biodiversity and biological applications of marine actinomycetes—Abu-Qir Bay, Mediterranean Sea, Egypt
The ability of actinomycetes to produce bioactive secondary metabolites makes them one of the most important prokaryotes. Marine actinomycetes are one of the most important secondary metabolites producers used...
A computational simulation appraisal of banana lectin as a potential anti-SARS-CoV-2 candidate by targeting the receptor-binding domain
The ongoing concern surrounding coronavirus disease 2019 (COVID-19) primarily stems from continuous mutations in the genome of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to the e...
Metagenomic analysis reveals diverse microbial community and potential functional roles in Baner rivulet, India
The health index of any population is directly correlated with the water quality, which in turn depends upon physicochemical characteristics and the microbiome of that aquatic source. For maintaining the water...
Mapping of conserved immunodominant epitope peptides in the outer membrane porin (Omp) L of prominent Enterobacteriaceae pathogens associated with gastrointestinal infections
Members of Enterobacteriaceae such as Escherichia coli O 157:H7, Salmonella sp., Shigella sp., Klebsiella sp., and Citrobacter freundii are responsible for the outbreak of serious foodborne illness and other muco...
Dual action of epigallocatechin-3-gallate in virus-induced cell Injury
Viral infections cause damage and long-term injury to infected human tissues, demanding therapy with antiviral and wound healing medications. Consequently, safe phytochemical molecules that may control viral i...
Designing a novel and combinatorial multi-antigenic epitope-based vaccine “MarVax” against Marburg virus—a reverse vaccinology and immunoinformatics approach
Marburg virus (MARV) is a member of the Filoviridae family and causes Marburg virus disease (MVD) among humans and primates. With fatality rates going up to 88%, there is currently no commercialized cure or va...
Bioinformatics study of phytase from Aspergillus niger for use as feed additive in livestock feed
Phytase supplementation in rations can reduce their phytic acid composition in order to enhance their nutritional value. Aspergillus niger is a fungus that can encode phytase. This study aims to determine the cha...
Improved production of Bacillus subtilis cholesterol oxidase by optimization of process parameters using response surface methodology
Cholesterol oxidase has numerous biomedical and industrial applications. In the current study, a new bacterial strain was isolated from sewage and was selected for its high potency for cholesterol degradation ...
Microsatellite diversity and complexity in the viral genomes of the family Caliciviridae
Microsatellites or simple sequence repeats (SSR) consist of 1–6 nucleotide motifs of DNA or RNA which are ubiquitously present in tandem repeated sequences across genome in viruses: prokaryotes and eukaryotes....
Prevalence of Extended Spectrum β-Lactamase Producers (ESBLs) with antibiotic resistance pattern of Gram negative pathogenic bacteria isolated from door handles in hospitals of Pokhara, Western Nepal
The presence of drug-resistant Gram-negative pathogenic bacteria and Extended Spectrum β-Lactamase Producers (ESBLs) in hospital associated fomites like door handles can serve as vehicles in transmission and m...
Application of statistical methodology for the optimization of l -glutaminase enzyme production from Streptomyces pseudogriseolus ZHG20 under solid-state fermentation
Actinomycetes are excellent microbial sources for various chemical structures like enzymes, most of which are used in pharmaceutical and industrial products. Actinomycetes are preferred sources of enzymes due ...
Investigating marine Bacillus as an effective growth promoter for chickpea
Microorganisms have characteristics that aid plant growth and raise the level of vital metabolites in plants for better growth including primary and secondary metabolites as well as several developmental enzym...
The pectinolytic activity of Burkholderia cepacia and its application in the bioscouring of cotton knit fabric
Enzymatic catalysis in different industrial applications is often preferred over chemical methods due to various advantages, such as higher specificity, greater efficiency, and less environmental footprint. Pe...
In silico analysis of a novel hypothetical protein (YP_498675.1) from Staphylococcus aureus unravels the protein of tryptophan synthase beta superfamily (Try-synth-beta_ II)
Staphylococcus aureus is a gram-positive spherical bacteria and the most common cause of nosocomial infections in the world. Given its clinical significance, the genome sequence of S. aureus has been elucidated t...
Nutrigenomics and microbiome shaping the future of personalized medicine: a review article
The relationship between nutrition and genes has long been hinted at and sometimes plainly associated with certain diseases. Now, after many years of research and coincidental findings, it is believed that thi...
Alpha-glucan: a novel bacterial polysaccharide and its application as a biosorbent for heavy metals
This study identified an extracellular bacterial polysaccharide produced by Bacillus velezensis strain 40B that contains more than 90% of the monosaccharide glucose as alpha-glucan. A prominent peak at 1074 cm −1 ,...
De novo assembly and comparative genome analysis for polyhydroxyalkanoates-producing Bacillus sp. BNPI-92 strain
Certain Bacillus species play a vital role in polyhydroxyalkanoate (PHA) production. However, most of these isolates did not properly identify to species level when scientifically had been reported.
Adverse effect of Tamarindus indica and tamoxifen combination on redox balance and genotoxicity of breast cancer cell
Breast cancer is the most significant threat to women worldwide. Most chemotherapeutic drugs cause cancer cell death and apoptosis by inducing oxidative stress and producing reactive oxygen species (ROS). Canc...
In silico molecular and functional characterization of a dual function antimicrobial peptide, hepcidin (GIFT-Hep), isolated from genetically improved farmed tilapia (GIFT, Oreochromis niloticus )
Antimicrobial peptides (AMPs), innate immune response molecules in organisms, are also known for their dual functionality, exemplified by hepcidin—an immunomodulator and iron regulator. Identifying and studyin...
Codon optimization of a gene encoding DNA polymerase from Pyrococcus furiosus and its expression in Escherichia coli
DNA polymerase is an essential component in PCR assay for DNA synthesis. Improving DNA polymerase with characteristics indispensable for a powerful assay is crucial because it can be used in wide-range applica...
Immunoinformatics study to explore dengue (DENV-1) proteome to design multi-epitope vaccine construct by using CD4+ epitopes
Immunoinformatics is an emerging interdisciplinary field which integrates immunology, bioinformatics, and computational biology to study the immune system. In this study, we apply immunoinformatics approaches ...
Mycosynthesis of silver nanoparticles using marine fungi and their antimicrobial activity against pathogenic microorganisms
At the present time, there is a persistent need to get rid of environmental contaminants by eco-friendly, sustainable, and economical technologies. Uncontrolled disposal practices of domestic and industrial so...
The Correction to this article has been published in Journal of Genetic Engineering and Biotechnology 2023 21 :164
Expression, purification, and characterization of self-assembly virus-like particles of capsid protein L1 HPV 52 in Pichia pastoris GS115
Cervical cancer caused by the human papillomavirus (HPV) is one of the most frequent malignances globally. HPV 52 is a high-risk cancer-causing genotype that has been identified as the most prevalent type in I...
Pangenome diversification and resistance gene characterization in Salmonella Typhi prioritized RfaJ as a significant therapeutic marker
Salmonella Typhi stands as the etiological agent responsible for the onset of human typhoid fever. The pressing demand for innovative therapeutic targets against S. Typhi is underscored by the escalating prevale...
Association between polymorphisms of immune response genes and early childhood caries — systematic review, gene-based, gene cluster, and meta-analysis
Early childhood caries is a significant public health concern affecting about 600 million children globally. The etiology of early childhood caries can be explained as an interplay between genetic and environm...
Experimental and hypothetical appraisal on inhibition of glucose-induced glycation of bovine serum albumin by quercetin
The specificity of protein functions depends on its folding ability into a functional structure. Protein folding is an essential systemic phenomenon that prevents incorrect folding which could result in harmfu...
- ISSN: 2090-5920 (electronic)
- Search Menu
- Volume 12, Issue 1, 2024 (In Progress)
- Volume 11, Issue 1, 2023
- Advance articles
- Editor's Choice
- Virtual Issues
- Clinical Briefs
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- Why submit?
- About Evolution, Medicine, and Public Health
- About the International Society for Evolution, Medicine and Public Health
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- For Reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, human enhancement, genetic engineering, conclusions.
- < Previous
Human enhancement: Genetic engineering and evolution
- Article contents
- Figures & tables
- Supplementary Data
Mara Almeida, Rui Diogo, Human enhancement: Genetic engineering and evolution, Evolution, Medicine, and Public Health , Volume 2019, Issue 1, 2019, Pages 183–189, https://doi.org/10.1093/emph/eoz026
- Permissions Icon Permissions
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context. In summarizing key open questions, we highlight the importance of acknowledging multiple effects (pleiotropy) and complex epigenetic interactions among genotype, phenotype and ecology, and the need to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). We also propose that a practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations. Overall, we suggest that it is essential for ethical, philosophical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
Lay Summary: This Commentary explores genetic enhancement in an evolutionary context. We highlight the multiple effects associated with germline heritable genetic intervention, the need to consider the unit of impact to human populations and their natural environment, and propose that a practicable distinction between ‘therapy’ and ‘enhancement’ is needed.
There are countless examples where technology has contributed to ameliorate the lives of people by improving their inherent or acquired capabilities. For example, over time, there have been biomedical interventions attempting to restore functions that are deficient, such as vision, hearing or mobility. If we consider human vision, substantial advances started from the time spectacles were developed (possibly in the 13th century), continuing in the last few years, with researchers implanting artificial retinas to give blind patients partial sight [ 1–3 ]. Recently, scientists have also successfully linked the brain of a paralysed man to a computer chip, which helped restore partial movement of limbs previously non-responsive [ 4 , 5 ]. In addition, synthetic blood substitutes have been created, which could be used in human patients in the future [ 6–8 ].
The progress being made by technology in a restorative and therapeutic context could in theory be applied in other contexts to treat non-pathological conditions. Many of the technologies and pharmaceutical products developed in a medical context to treat patients are already being used by humans to ‘enhance’ some aspect of their bodies, for example drugs to boost brain power, nutritional supplements, brain stimulating technologies to control mood or growth hormones for children of short stature. Assistive technology for disabled people, reproductive medicine and pharmacology, beside their therapeutic and restorative use, have a greater potential for human ‘enhancement’ than currently thought. There are also dual outcomes as some therapies can have effects that amount to an enhancement as for example, the artificial legs used by the South African sprinter Oscar Pistorius providing him with a competitive advantage.
This commentary will provide general ethical considerations on human enhancement, and within the several forms of so-called human biomedical enhancement, it will focus on genetic engineering, particularly on germline (heritable) genetic interventions and on the insights evolutionary biology can provide in rationalizing its likely impact. These insights are a subject often limited in discussions on genetic engineering and human enhancement in general, and its links to ethical, philosophical and policy discussions, in particular [ 9 ]. The rapid advances in genetic technology make this debate very topical. Moreover, genes are thought to play a very substantial role in biological evolution and development of the human species, thus making this a topic requiring due consideration. With this commentary, we explore how concepts based in evolutionary biology could contribute to better assess the implications of human germline modifications, assuming they were widely employed. We conclude our brief analysis by summarizing key issues requiring resolution and potential approaches to progress them. Overall, the aim is to contribute to the debate on human genetic enhancement by looking not only at the future, as it is so often done, but also at our evolutionary past.
The noun ‘enhancement’ comes from the verb ‘enhance’, meaning ‘to increase or improve’. The verb enhance can be traced back to the vulgar Latin inaltiare and late Latin inaltare (‘raise, exalt’), from ‘ altare ’ (‘make high’) and altus (‘high’), literally ‘grown tall’. For centuries human enhancement has populated our imagination outlined by stories ranging from the myths of supernormal strengths and eternal life to the superpowers illustrated by the 20th century comic books superheroes. The desire of overcoming normal human capacities and the transformation to an almost ‘perfect’ form has been part of the history of civilization, extending from arts and religion to philosophy. The goal of improving the human condition and health has always been a driver for innovation and biomedical developments.
In the broadest sense, the process of human enhancement can be considered as an improvement of the ‘limitations’ of a ‘natural version’ of the human species with respect to a specific reference in time, and to different environments, which can vary depending on factors such as, for example, climate change. The limitations of the human condition can be physical and/or mental/cognitive (e.g. vision, strength or memory). This poses relevant questions of what a real or perceived human limitation is in the environment and times in which we are living and how it can be shifted over time considering social norms and cultural values of modern societies. Besides, the impact that overcoming these limitations will have on us humans, and the environment, should also be considered. For example, if we boost the immune system of specific people, this may contribute to the development/evolution of more resistant viruses and bacteria or/and lead to new viruses and bacteria to emerge. In environmental terms, enhancing the longevity of humans could contribute to a massive increase in global population, creating additional pressures on ecosystems already under human pressure.
Two decades ago, the practices of human enhancement have been described as ‘biomedical interventions that are used to improve human form or functioning beyond what is necessary to restore or sustain health’ [ 10 ]. The range of these practices has now increased with technological development, and they are ‘any kind of genetic, biomedical, or pharmaceutical intervention aimed at improving human dispositions, capacities, or well-being, even if there is no pathology to be treated’ [ 11 ]. Practices of human enhancement could be visualized as upgrading a ‘system’, where interventions take place for a better performance of the original system. This is far from being a hypothetical situation. The rapid progress within the fields of nanotechnology, biotechnology, information technology and cognitive science has brought back discussions about the evolutionary trajectory of the human species by the promise of new applications which could provide abilities beyond current ones [ 12 , 13 ]. If such a possibility was consciously embraced and actively pursued, technology could be expected to have a revolutionary interference with human life, not just helping humans in achieving general health and capabilities commensurate with our current ones but helping to overcome human limitations far beyond of what is currently possible for human beings. The emergence of new technologies has provided a broader range of potential human interventions and the possibility of transitioning from external changes to our bodies (e.g. external prosthesis) to internal ones, especially when considering genetic manipulation, whose changes can be permanent and transmissible.
The advocates of a far-reaching human enhancement have been referred to as ‘transhumanists’. In their vision, so far, humans have largely worked to control and shape their exterior environments (niche construction) but with new technologies (e.g. biotechnology, information technology and nanotechnology) they will soon be able to control and fundamentally change their own bodies. Supporters of these technologies agree with the possibility of a more radical interference in human life by using technology to overcome human limitations [ 14–16 ], that could allow us to live longer, healthier and even happier lives [ 17 ]. On the other side, and against this position, are the so-called ‘bioconservatives’, arguing for the conservation and protection of some kind of ‘human essence’, with the argument that it exists something intrinsically valuable in human life that should be preserved [ 18 , 19 ].
There is an ongoing debate between transhumanists [ 20–22 ] and bioconservatives [ 18 , 19 , 23 ] on the ethical issues regarding the use of technologies in humans. The focus of this commentary is not centred on this debate, particularly because the discussion of these extreme, divergent positions is already very prominent in the public debate. In fact, it is interesting to notice that the ‘moderate’ discourses around this topic are much less known. In a more moderate view, perhaps one of the crucial questions to consider, independently of the moral views on human enhancement, is whether human enhancement (especially if considering germline heritable genetic interventions) is a necessary development, and represents an appropriate use of time, funding and resources compared to other pressing societal issues. It is crucial to build space for these more moderate, and perhaps less polarized voices, allowing the consideration of other positions and visions beyond those being more strongly projected so far.
Ethical and societal discussions on what constitutes human enhancement will be fundamental to support the development of policy frameworks and regulations on new technological developments. When considering the ethical implications of human enhancement that technology will be available to offer now and in the future, it could be useful to group the different kinds of human enhancements in the phenotypic and genetic categories: (i) strictly phenotypic intervention (e.g. ranging from infrared vision spectacles to exoskeletons and bionic limbs); (ii) somatic, non-heritable genetic intervention (e.g. editing of muscle cells for stronger muscles) and (iii) germline, heritable genetic intervention (e.g. editing of the C–C chemokine receptor type 5 (CCR5) gene in the Chinese baby twins, discussed later on). These categories of enhancement raise different considerations and concerns and currently present different levels of acceptance by our society. The degree of ethical, societal and environmental impacts is likely to be more limited for phenotypic interventions (i) but higher for genetic interventions (ii and iii), especially for the ones which are transmissible to future generations (iii).
The rapid advances in technology seen in the last decades, have raised the possibility of ‘radical enhancement’, defined by Nicholas Agar, ‘as the improvement of human attributes and abilities to levels that greatly exceed what is currently possible for human beings’ [ 24 ]. Genetic engineering offers the possibility of such an enhancement by providing humans a profound control over their own biology. Among other technologies, genetic engineering comprises genome editing (also called gene editing), a group of technologies with the ability to directly modify an organism’s DNA through a targeted intervention in the genome (e.g. insertion, deletion or replacement of specific genetic material) [ 25 ]. Genome editing is considered to achieve much greater precision than pre-existing forms of genetic engineering. It has been argued to be a revolutionary tool due to its efficiency, reducing cost and time. This technology is considered to have many applications for human health, in both preventing and tackling disease. Much of the ethical debate associated with this technology concerns the possible application of genome editing in the human germline, i.e. the genome that can be transmitted to following generations, be it from gametes, a fertilized egg or from first embryo divisions [ 26–28 ]. There has been concern as well as enthusiasm on the potential of the technology to modify human germline genome to provide us with traits considered positive or useful (e.g. muscle strength, memory and intelligence) in the current and future environments.
Genetic engineering: therapy or enhancement and predictability of outcomes
To explore some of the possible implications of heritable interventions we will take as an example the editing (more specifically ‘deletion’ using CRISPR genome editing technology) of several base pairs of the CCR5 gene. Such intervention was practised in 2018 in two non-identical twin girls born in China. Loss of function mutations of the CCR5 had been previously shown to provide resistance to HIV. Therefore, the gene deletion would be expected to protect the twin baby girls from risk of transmission of HIV which could have occurred from their father (HIV-positive). However, the father had the infection kept under control and the titre of HIV virus was undetectable, which means that risk of transmission of HIV infection to the babies was negligible [ 29 ].
From an ethical ground, based on current acceptable practices, this case has been widely criticized by the scientific community beside being considered by many a case of human enhancement intervention rather than therapy [ 29 , 30 ]. One of the questions this example helps illustrate is that the ethical boundary between a therapy that ‘corrects’ a disorder by restoring performance to a ‘normal’ scope, and an intervention that ‘enhances’ human ability outside the accepted ‘normal’ scope, is not always easy to draw. For the sake of argument, it could be assumed that therapy involves attempts to restore a certain condition of health, normality or sanity of the ‘natural’ condition of a specific individual. If we take this approach, the question is how health, normality and sanity, as well as natural per se, are defined, as the meaning of these concepts shift over time to accommodate social norms and cultural values of modern societies. It could be said that the difficulty of developing a conceptual distinction between therapy and enhancement has always been present. However, the potential significance of such distinction is only now, with the acceleration and impact of technological developments, becoming more evident.
Beyond ethical questions, a major problem of this intervention is that we do not (yet?) know exactly the totality of the effects that the artificial mutation of the CCR5 may have, at both the genetic and phenotypic levels. This is because we now know that, contrary to the idea of ‘one gene-one trait’ accepted some decades ago, a gene—or its absence—can affect numerous traits, many of them being apparently unrelated (a phenomenon also known as pleiotropy). That is, due to constrained developmental interactions, mechanisms and genetic networks, a change in a single gene can result in a cascade of multiple effects [ 31 ]. In the case of CCR5, we currently know that the mutation offers protection against HIV infection, and also seems to increase the risk of severe or fatal reactions to some infectious diseases, such as the influenza virus [ 32 ]. It has also been observed that among people with multiple sclerosis, the ones with CCR5 mutation are twice as likely to die early than are people without the mutation [ 33 ]. Some studies have also shown that defective CCR5 can have a positive effect in cognition to enhance learning and memory in mice [ 34 ]. However, it’s not clear if this effect would be translated into humans. The example serves to illustrate that, even if human enhancement with gene editing methods was considered ethically sound, assessing the totality of its implications on solid grounds may be difficult to achieve.
Genetic engineering and human evolution: large-scale impacts
Beyond providing the opportunity of enhancing human capabilities in specific individuals, intervening in the germline is likely to have an impact on the evolutionary processes of the human species raising questions on the scale and type of impacts. In fact, the use of large-scale genetic engineering might exponentially increase the force of ‘niche construction’ in human evolution, and therefore raise ethical and practical questions never faced by our species before. It has been argued that natural selection is a mechanism of lesser importance in the case of current human evolution, as compared to other organisms, because of advances in medicine and healthcare [ 35 ]. According to such a view, among many others advances, natural selection has been conditioned by our ‘niche-construction’ ability to improve healthcare and access to clean water and food, thus changing the landscape of pressures that humans have been facing for survival. An underlying assumption or position of the current debate is that, within our human species, the force of natural selection became minimized and that we are somehow at the ‘end-point’ of our evolution [ 36 ]. If this premise holds true, one could argue that evolution is no longer a force in human history and hence that any human enhancement would not be substituting itself to human evolution as a key driver for future changes.
However, it is useful to remember that, as defined by Darwin in his book ‘On the Origin of the Species’, natural selection is a process in which organisms that happen to be ‘better’ adapted to a certain environment tend to have higher survival and/or reproductive rates than other organisms [ 37 ]. When comparing human evolution to human genetic enhancement, an acceptable position could be to consider ethically sound those interventions that could be replicated naturally by evolution, as in the case of the CCR5 gene. Even if this approach was taken, however, it is important to bear in mind that human evolution acts on human traits sometimes increasing and sometimes decreasing our biological fitness, in a constant evolutionary trade-off and in a contingent and/or neutral—in the sense of not ‘progressive’—process. In other worlds, differently from genetic human enhancement, natural selection does not ‘ aim ’ at improving human traits [ 38 ]. Human evolution and the so-called genetic human enhancement would seem therefore to involve different underlying processes, raising several questions regarding the implications and risks of the latter.
But using genetic engineering to treat humans has been proposed far beyond the therapeutic case or to introduce genetic modifications known to already occur in nature. In particular, when looking into the views expressed on the balance between human evolution and genetic engineering, some argue that it may be appropriate to use genetic interventions to go beyond what natural selection has contributed to our species when it comes to eradicate vulnerabilities [ 17 ]. Furthermore, when considering the environmental, ecological and social issues of contemporary times, some suggest that genetic technologies could be crucial tools to contribute to human survival and well-being [ 20–22 ]. The possible need to ‘engineer’ human traits to ensure our survival could include the ability to allow our species to adapt rapidly to the rate of environmental change caused by human activity, for which Darwinian evolution may be too slow [ 39 ]. Or, for instance, to support long-distance space travel by engineering resistance to radiation and osteoporosis, along with other conditions which would be highly advantageous in space [ 40 ].
When considering the ethical and societal merits of these propositions, it is useful to consider how proto-forms of enhancement has been approached by past human societies. In particular, it can be argued that humans have already employed—as part of our domestication/‘selective breeding’ of other animals—techniques of indirect manipulation of genomes on a relatively large scale over many millennia, albeit not on humans. The large-scale selective breeding of plants and animals over prehistoric and historic periods could be claimed to have already shaped some of our natural environment. Selective breeding has been used to obtain specific characteristics considered useful at a given time in plants and animals. Therefore, their evolutionary processes have been altered with the aim to produce lineages with advantageous traits, which contributed to the evolution of different domesticated species. However, differently from genetic engineering, domestication possesses inherent limitations in its ability to produce major transformations in the created lineages, in contrast with the many open possibilities provided by genetic engineering.
When considering the impact of genetic engineering on human evolution, one of questions to be considered concerns the effects, if any, that genetic technology could have on the genetic pool of the human population and any implication on its resilience to unforeseen circumstances. This underlines a relevant question associated with the difference between ‘health’ and biological fitness. For example, a certain group of animals can be more ‘healthy’—as domesticated dogs—but be less biologically ‘fit’ according to Darwin’s definition. Specifically, if such group of animals are less genetically diverse than their ancestors, they could be less ‘adaptable’ to environmental changes. Assuming that, the human germline modification is undertaken at a global scale, this could be expected to have an effect, on the distribution of genetically heritable traits on the human population over time. Considering that gene and trait distributions have been changing under the processes of evolution for billions of years, the impact on evolution will need to be assessed by analysing which genetic alterations have been eventually associated with specific changes within the recent evolutionary history of humans. On this front, a key study has analysed the implications of genetic engineering on the evolutionary biology of human populations, including the possibility of reducing human genetic diversity, for instance creating a ‘biological monoculture’ [ 41 ]. The study argued that genetic engineering will have an insignificant impact on human diversity, while it would likely safeguard the capacity of human populations to deal with disease and new environmental challenges and therefore, ensure the health and longevity of our species [ 41 ]. If the findings of this study were considered consistent with other knowledge and encompassing, the impact of human genetic enhancements on the human genetic pool and associated impacts could be considered secondary aspects. However, data available from studies on domestication strongly suggests that domestication of both animals and plans might lead to not only decreased genetic diversity per se, but even affect patterns of variation in gene expression throughout the genome and generally decreased gene expression diversity across species [ 42–44 ]. Given that, according to recent studies within the field of biological anthropology recent human evolution has been in fact a process of ‘self-domestication’ [ 45 ], one could argue that studies on domestication could contribute to understanding the impacts of genetic engineering.
Beyond such considerations, it is useful to reflect on the fact that human genetic enhancement could occur on different geographical scales, regardless of the specific environment and geological periods in which humans are living and much more rapidly than in the case of evolution, in which changes are very slow. If this was to occur routinely and on a large scale, the implications of the resulting radical and abrupt changes may be difficult to predict and its impacts difficult to manage. This is currently highlighted by results of epigenetics studies, and also of the microbiome and of the effects of pollutants in the environment and their cumulative effect on the development of human and non-human organisms alike. Increasingly new evidence indicates a greater interdependence between humans and their environments (including other microorganisms), indicating that modifying the environment can have direct and unpredictable consequences on humans as well. This highlight the need of a ‘systems level’ approach. An approach in which the ‘bounded body’ of the individual human as a basic unit of biological or social action would need to be questioned in favour of a more encompassing and holistic unit. In fact, within biology, there is a new field, Systems Biology, which stresses the need to understand the role that pleiotropy, and thus networks at multiple levels—e.g. genetic, cellular, among individuals and among different taxa—play within biological systems and their evolution [ 46 ]. Currently, much still needs to be understood about gene function, its role in human biological systems and the interaction between genes and external factors such as environment, diet and so on. In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of human evolution enable us to better understand the implications of genetic interventions.
New forms of human enhancement are increasingly coming to play due to technological development. If phenotypic and somatic interventions for human enhancement pose already significant ethical and societal challenges, germline heritable genetic intervention, require much broader and complex considerations at the level of the individual, society and human species as a whole. Germline interventions associated with modern technologies are capable of much more rapid, large-scale impacts and seem capable of radically altering the balance of humans with the environment. We know now that beside the role genes play on biological evolution and development, genetic interventions can induce multiple effects (pleiotropy) and complex epigenetics interactions among genotype, phenotype and ecology of a certain environment. As a result of the rapidity and scale with which such impact could be realized, it is essential for ethical and societal debates, as well as underlying scientific studies, to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). An important practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations, although a distinct line between the two may be difficult to draw.
In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of humans and other organisms, including domesticated ones, enable us to better understand the implications of genetic interventions. In particular, effective regulation of genetic engineering may need to be based on a deep knowledge of the exact links between phenotype and genotype, as well the interaction of the human species with the environment and vice versa .
For a broader and consistent debate, it will be essential for technological, philosophical, ethical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) of Portugal [CFCUL/FIL/00678/2019 to M.A.].
Conflict of interest : None declared.
Pham P , Roux S , Matonti F et al. Post-implantation impedance spectroscopy of subretinal micro-electrode arrays, OCT imaging and numerical simulation: towards a more precise neuroprosthesis monitoring tool . J Neural Eng 2013 ; 10 : 046002 .
Google Scholar
Maghami MH , Sodagar AM , Lashay A et al. Visual prostheses: the enabling technology to give sight to the blind . J Ophthal Vis Res 2014 ; 9 : 494 – 505 .
Weitz AC , Nanduri D , Behrend MR et al. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration . Sci Transl Med 2015 ; 7 : 318ra203.
Bouton CE , Shaikhouni A , Annetta NV et al. Restoring cortical control of functional movement in a human with quadriplegia . Nature 2016 ; 533 : 247 – 50 .
Geddes L. First paralysed person to be ‘reanimated’ offers neuroscience insights. Technique moves man’s arm by decoding his thoughts and electrically stimulating his own muscles . Nat News 2016 ; 533 .
Squires JE. Artificial blood . Science 2002 ; 295 : 1002 – 5 .
Lowe KC. Blood substitutes: from chemistry to clinic . J Mater Chem 2006 ; 16 : 4189 – 96 .
Moradi S , Jahanian-Najafabadi A , Roudkenar MH. Artificial blood substitutes: first steps on the long route to clinical utility . Clin Med Insights Blood Disord 2016 ; 9 : 33 – 41 .
Powell R , Kahane G , Savulescu J. Evolution, genetic engineering, and human enhancement . Philos Technol 2012 ; 25 : 439 – 58 .
Parens E (ed.). Enhancing Human Traits: Ethical and Social Implications . Washington, DC : Georgetown University Press , 1998 .
Google Preview
Giubilini A , Sanyal S. Challenging human enhancement. In: Clarke S , Savulescu J , Coady T et al. (eds). The Ethics of Human Enhancement: Understanding the Debate . Oxford : Oxford University Press , 2016 .
Elliott C. Better Than Well: American Medicine Meets the American Dream . New York, NY : WWW Norton & Company, Inc ., 2003 .
Kramer P. Listening to Prozac . London : Fourth Estate , 1994 .
Moravec H. Mind Children: The Future of Robot and Human Intelligence . Cambridge : Harvard University Press , 1990 .
Bostrom N. Human genetic enhancements: a transhumanist perspective . J Value Inq 2003 ; 37 : 493 – 506 .
Kurzweil R. The Singularity is Near: When Humans Transcend Biology . New York, NY : Viking , 2005 .
Harris J. Enhancing Evolution: The Ethical Case for Making Better People . Princeton, NJ : Princeton University Press , 2010 .
Fukuyama F. Our Posthuman Future: Consequences of the Biotechnology Revolution . New York, NY : Picador , 2002 .
Sandel M. The Case Against Perfection: Ethics in the Age of Genetic Engineering . Cambridge : The Belknap Press of Harvard University Press , 2007 .
Savulescu J , Persson I. The perils of cognitive enhancement and the urgent imperative to enhance the moral character of humanity . J Appl Philos 2008 ; 25 : 162 – 77 .
Buchanan A. Beyond Humanity . Oxford : Oxford University Press , 2011 .
Persson I , Savulescu J. Moral enhancement, freedom, and the god machine . Monist 2012 ; 95 : 399 – 421 .
Leon K. Ageless bodies, happy souls: biotechnology and the pursuit of perfection . New Atlantis 2003 ; 1 : 9 – 28 .
Agar N. Humanity’s End: Why We Should Reject Radical Enhancement . Cambridge : MIT Press , 2010 .
Gaj T , Gersbach CA , Barbas CF III ,. ZFN, TALEN, and CRISPR/Cas based methods for genome engineering . Trends Biotechnol 2013 ; 3 : 397 – 405 .
Baltimore D , Berg P , Botchan M et al. Biotechnology. A prudent path forward for genomic engineering and germline gene modification . Science 2015 ; 348 : 36 – 8 .
Otieno MO. CRISPR/Cas9 human genome editing: challenges, ethical concerns and implications . J Clin Res Bioeth 2015 ; 6 : 253 .
Ishii T. Germline genome-editing research and its socio-ethical implications . Trends Mol Med 2015 ; 21 : 473 – 81 .
Bionews.org.uk. First Genome-edited Babies: A Very Different Perception of Ethics , 2018 . https://www.bionews.org.uk/page_140060 (27 August 2019, date last accessed).
Cyranoski D. CRISPR-baby scientist fails to satisfy his critics . Nat News 2018 ; 564 : 13 – 4 .
Galis F , Metz JA. Evolutionary novelties: the making and breaking of pleiotropic constraints . Integr Comp Biol 2007 ; 47 : 409 – 19 .
Falcon A , Cuevas MT , Rodriguez-Frandsen A et al. CCR5 deficiency predisposes to fatal outcome in influenza virus infection . J Gen Virol 2015 ; 96 : 2074 – 8 .
Gade-Andavolu R , Comings DE , MacMurray J et al. Association of CCR5 Δ32 deletion with early death in multiple sclerosis . Genet Med 2004 ; 6 : 126 – 31 .
Zhou M , Greenhill S , Huang S et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory . eLife 2016 ; 5 : e20985 .
Tibayrenc M , Ayala FJ (eds). On Human Nature: Biology, Psychology, Ethics, Politics, and Religion . London : Academic Press , 2017 .
Baldi P. The Shattered Self: The End of Natural Evolution . Cambridge : MIT Press , 2001 .
Darwin C. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life . London : J. Murray , 1859 .
Gould SJ. The Structure of Evolutionary Theory . Belknap, NY : Harvard University Press , 2002 .
Rees M. Our Final Century: Will the Humans Race Survive the Twenty-first Century? Eastbourne : Gardners Books , 2003 .
Nuffield Council on Bioethics. Genome Editing: An Ethical Review . London : Nuffield Council on Bioethics , 2016 .
Powell R. The evolutionary biological implications of human genetic engineering . J Med Philos 2012 ; 37 : 204 – 26 .
Liu W , Chen L , Zhang S et al. Decrease of gene expression diversity during domestication of animals and plants . BMC Evol Biol 2019 ; 19 : 1 – 11 .
Fages A , Hanghøj K , Khan N et al. Tracking five millennia of horse management with extensive ancient genome time series . Cell 2019 ; 177 : 1419 – 35 .
Zhang J , Wang X , Yao J et al. Effect of domestication on the genetic diversity and structure of Saccharina japonica populations in China . Sci Rep 2017 ; 7 : 42158 .
Theofanopoulou C , Gastaldon S , O’Rourke T et al. Self-domestication in Homo sapiens : insights from comparative genomics . PLoS One 2018 ; 13 : e0196700 .
Capra F , Luisi PL. The Systems View of Life . Cambridge : Cambridge University Press , 2014
- genetic engineering
Email alerts
Citing articles via, affiliations.
- Online ISSN 2050-6201
- Copyright © 2024 International Society for Evolution, Medicine, and Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Precautionary Reasoning in Environmental and Public Health Policy pp 165–240 Cite as
Genetic Engineering
- David B. Resnik 13
- First Online: 28 March 2021
214 Accesses
Part of the book series: The International Library of Bioethics ((ILB,volume 86))
In this chapter I will apply the PP to ethical and policy issues related to genetic engineering of microbes, plants, animals, and human beings. I will argue that the PP can provide some useful insights into these issues, due to the scientific and morally uncertainty surrounding the consequences of genetic engineering for public health, the environment, society, and patients.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
By “genetic engineering” I mean technologies that involve direct modification or alteration of the genomes of cells or organisms. Changes brought about by genetic engineering might or might not be inheritable, depending on the type of change and the organism. Modification of the genomes of somatic cells in humans (discussed below) does not normally result in inheritable genetic changes, but modification of human germ cells, sperm, eggs, or embryos does (Resnik et al. 1999 ). Modification of bacterial genomes always results in inheritable genetic changes because bacteria are unicellular organisms. Ooplasm transfer, nuclear transfer, and reproductive cloning in human beings raise important ethical and social issues, but these procedures are not genetic engineering, according to my definition, because their purposes is not modify genomes, even though they involve the manipulation of genetic material. Synthetic biology uses genetic engineering methods to design cells, organisms, and biological system that do not already exist in the natural world (Biotechnology Innovation Organization 2020b ).
Some viruses encode their genetic information in RNA (ribonucleic acid).
A polymer is a large molecule.
James Watson (1928–) and Francis Crick (1916–2004) won the Nobel Prize in Physiology of Medicine in 1962 for discovering the structure of DNA. Their model was confirmed by Rosalind Franklin’s x-ray crystallography data, Watson and Crick did not name Franklin as an author on the paper that described their model of the structure of DNA. Franklin (1920–1958) was also not awarded the Nobel Prize for her contribution, because she died of ovarian cancer in 1958, and the Nobel Prize is not awarded posthumously (Maddox 2003 ).
Because mitochondria have their own DNA, scientists have speculated that mitochondria were at one time independent organisms that became incorporated into primordial, unicellular organisms (Alberts et al. 2015 ).
Prokaryotes are single-celled organisms with no distinct cell nucleus or organelles.
Mitochondria replicate independently of the cell.
Most higher life forms, including most plants, mammals, and human beings, are diploid (Alberts et al. 2015 ).
Many species of plants and animals that reproduce sexually can also propagate asexually. Growing a new plant from a cutting is a form of asexual propagation.
Plant stem cells can also generate different tissue types.
Berg, Gilbert, and Sanger won the Nobel Prize in chemistry in 1980 for their development of recombinant DNA techniques (Nobel Prize.org 2021 ).
Doudna and Charpentier won the Nobel Prize in Chemistry in 2000 for the discovery of CRISPR (Ledford and Callaway 2020 ).
Laboratory animals are used to produce monoclonal antibodies. An antigen is introduced into the animal, which produces antibodies in its lymphocyte cells. These cells are cultured and then antibodies are isolated. Since these antibodies would be rejected by the human immune system, the cells are genetically modified so that they produce antibodies with a human protein component, or humanized antibodies. The genetically modified cells are then cultured and humanized antibodies are isolated for production (GenScript 2020 ).
Somatic cells are cells other than the reproductive or germ cells, such as skin, nerve, muscle, liver or bone marrow cells.
Monsanto has developed GM crops (known as Bt crops) that produce Bacillus thuringiensis toxins, which are deadly to insects. Farmers were already using these toxins as pesticides were Bt crops were developed (Resnik 2012 ).
Monsanto has developed GM crops (known as “Roundup Ready” crops) that are immune to the effects of glyphosate, the active ingredient in the widely-used herbicide Roundup ™. Farmers can control weeds with damaging their crops by spraying their crops with Roundup (Resnik 2012 ).
Golden rice, for example, contains more beta carotene than normal rice (McDivitt 2019 ).
In 2018, 228 million people worldwide contracted malaria and 405,000 people died from the disease (World Health Organization 2020a ). About 390 million people contract the dengue virus each year and about 4000 die from the disease (World Health Organization 2020b ).
Oxitec has also genetically engineered diamondback moths (Plutella xylostella) to control these populations. Diamondback moths are a destructive pests that feed on cauliflower, cabbage, broccoli and canola (Campbell 2020a ).
E.g. Bt crops. See Footnote 12.
These are the sorts of problems encountered by the natural law approaches to morality, discussed in Chapter 3 .
Most defenders of the slippery slope argument in genetic only apply it to using genome editing in humans, but it could be applied to other applications of genetic engineering.
I am assuming that GM microbes will not be intentionally released into the environment, which would create risks not discussed here. Scientists have developed GM microbes to clean up oil spills but have not deployed them yet, mostly due to regulatory issues. In nature, microbes already play an important role in cleaning up oil spills (Ezezika and Singer 2010 ).
The reproduction rate is how many people infected persons infect. R 0 = 1 means that an infected person infects one more person on average; R 0 = 2 means an infected person infects two people on average.
It is worth noting, however, that a voluntary moratorium was a reasonable option when this technology was emerging in the 1970s.
As noted in Chapter 6 , a black market for alcohol emerged during Prohibition era in the US (1919–1933). The desire to avoid creating a black market for any product is an relevant to regulatory actions that involve prohibitions.
As a side note, members of Greenpeace broke into a research farm in Australia in 2011 and destroyed an entire crop of GM wheat. Members of another environmental damaged a crop of golden rice in the Philippines (Zhang et al. 2016 ).
To date, 156 Nobelists have signed the petition (Nobel Prize Winners 2016 ).
For a review of the GM food safety literature, also see Domingo ( 2016 ).
It is worth noting the long-term animal studies pose some scientific and technical challenges because most of the rodent species used in these types of experiments have a lifespan of about three years and normally develop tumors and other health problems as they age. So, it can be difficult to determine whether an adverse effect in a laboratory animal is due to an exposure to a GM food or the natural aging process. A two-year study published by Séralini et al. ( 2012 ) claiming that mice fed a diet of Roundup Ready GM corn had more tumors than mice fed the normal diet (the control group) was later retracted by the journal due to serious methodological flaws that undermined the validity of the data (Resnik 2015a ).
See Footnote 12.
Davidson ( 2001 ) defends a principle of charity for interpreting language. The basic idea here is that one should interpret a speaker’s statements as being rational, other things being equal. Interpreting disagreements about GM foods/crops as based on differing value priorities portrays these disagreements as rational, rather than based on irrational fear or ignorance.
It is also worth noting that bans on GM plants can create black markets because of the high demand for these products.
As of the writing of this book, Kenya is currently rethinking its ban on GM crops (Meeme 2019 ).
Most of the debate about chimeras so far has focused on inserting human cells into early animal embryos (or blastocysts), not on inserting human genes into animals.
It is also worth noting that a ban would probably create a black market because demand for GM animals and animal products it high.
There is a potential regulatory gap in the genetic engineering of animals for meat or animal products. Although regulations and ethical guidelines require IACUCs to review and oversee genetic engineering of animals for research conducted at academic institutions, there are no such requirements for genetic engineering of animals for non-research purposes, such as meat production. One could argue that companies that genetically engineer animals for non-research purposes should form ethics committees similar to IACUCs to oversee these activities.
Anderson led the research team that conducted the world’s first human gene therapy clinical trial. The experiment used an adenovirus vector to insert the adenosine deaminase gene into the T-cells of two young children with combined immunodeficiency. The trial showed that the procedure was safe and effective even if did not cure the patients (Blaese et al. 1995 ). In 2006, Anderson was convicted of molesting and sexually abusing a girl over a four-year period, beginning when she was 10 years old, and he served 12 years in prison. Anderson maintains that he is innocent and that his conviction was based on falsified evidence (Begley 2018 ).
See Footnote 29.
An example of somatic genetic enhancement would be a transferring a gene to an adult male to stimulate production of testosterone to enhance athletic and sexual performance.
It is worth noting that not everyone regards genetic enhancement immoral or morally questionable. The transhumanist movement embraces various forms of enhancement to benefit mankind and allow people to express creative freedom (Harris 2007 ; Bostrom 2008 , 2010 ; More and Vita-More 2013 ; Porter 2017 ; Rana and Samples 2019 ).
Some have attempted to define health in terms of a normal range of variation for an organism. In medicine, a normal physiological trait is a trait that falls within a range of variation for healthy functioning of the organism (Boorse 1977 ; Schaffner 1993 ). For example, normal fasting blood sugar levels range from 60 mg/dL to 100 mg/dL (WebMD 2020 ). Fasting blood sugar levels that are too high cause diabetes and levels that are too low cause hypoglycemia, both of which are unhealthy conditions. However, normality cannot be equated with the statistical norm for a population, since the statistical norm might be unhealthy. If most people in a population have a fasting blood sugar greater than 100 mg/dL, we would not say that a fasting blood sugar greater than 100 mg/dL is normal, even though it would be the statistical norm for that population. Thus, the concept of a normal range of variation cannot be defined statistically and depends on a broader concept of health, which may be influenced by moral, social, and cultural factors.
Some argue that “gene therapy” is a misleading term because it implies that the genetic interventions are likely to benefit the patient or human subject, when often they do not (Henderson et al. 2006 ).
See Resnik ( 2018a ) for discussion of additional safety protections for subjects enrolled in clinical research.
In 1996, the US Congress passed a ban, known as the Dickey-Wicker amendment, on the use of federal funds to create human embryos for research (Green 2001 ). Though the ban has been interpreted differently by different administrations, it is still in effect.
For further discussion of creating embryos for research, see Green ( 2001 ).
I will assume that parents who are willing to use medical technology to prevent the birth of children with genetic diseases view abortion as morally acceptable, at least for this purpose.
Prenatal genetic testing can also be used to avoid giving birth to children with chromosomal abnormalities, such as Trisomy 21 (Down Syndrome).
Embryos that are not implanted would be destroyed. I am assuming that parents would view this as morally acceptable.
See Resnik et al. ( 1999 ) and National Academies of Sciences, Engineering, and Medicine ( 2017 ) for additional examples of monogenic disorders that GGE might be used to prevent.
The concept of a parent can be confusing here, because people who related to the child genetically might not be related socially. The concept of a parent can be even more confusing when surrogate pregnancy is used to produce children, since woman who gestates and gives birth to the child might not be genetically related to the child, if she is carrying a fetus created by another couple in vitro.
This is one of the themes of the science fiction movie GATTACA.
This cost estimate is based on dividing the total cost of the Human Genome Project--$3 billion—by three. The Human Genome Project was a US-funded research project that took place from 1990 to 2003. Although sequencing the human genome was the primary goal of the project, it also included other activities, such as studies of human diseases, model organisms, genetic technologies, computational methods, and ethical issues (Human Genome Project 2020 ).
Interestingly, two of the scientists who called for the moratorium, David Baltimore and Paul Berg, participated in the Asilomar conference on recombinant DNA (discussed earlier).
These studies could include the creation of human embryos to study the safety and efficacy of GGE methods and techniques (Liang et al. 2015 ).
This is an example of the problem of incoherence discussed in Chapter 4 .
Alopecia areata is a condition that leads to hair loss. It is thought to have a genetic basis (McIntosh 2017 ).
The moratorium would not apply to GGE for research purposes.
The moratorium would not apply to research on embryos created by GGE, which would be necessary to obtain the knowledge needed to better understand the safety and efficacy of using GGE to produce children (Liang et al. 2015 ; Baltimore et al. 2015 ).
Agar, N. 2014. Truly Human Enhancement: A Philosophical Defense of Limits . Cambridge, MA: MIT Press.
Book Google Scholar
Alberts, B., A.D. Johnson, J. Lewis, D. Morgan, M. Raff, K. Roberts, and P. Walter. 2015. Molecular Biology of the Cell , 6th ed. New York, NY: W. W. Norton.
Google Scholar
American Association for the Advancement of Science. 2000. Human Inheritable Genetic Modifications: Assessing Scientific, Ethical, Religious, and Policy Issues . Washington, DC: American Association for the Advancement of Science.
American Association for the Advancement of Science. 2012. Statement by the AAAS Board of Directors on labeling of genetically modified foods, October 2012. Available at: http://www.aaas.org/sites/default/files/AAAS_GM_statement.pdf . Accessed 18 Jan 2021.
American College of Obstetricians and Gynecologists. 2019. Prenatal genetic screening tests. Available at: https://www.acog.org/Patients/FAQs/Prenatal-Genetic-Screening-Tests?IsMobileSet=false . Accessed 18 Jan 2021.
Anderson, W.F. 1985. Human Gene Therapy: Scientific and Ethical Considerations. Journal of Medicine and Philosophy 10 (3): 275–291.
Article Google Scholar
Anderson, W.F. 1989. Human Gene Therapy: Why Draw a Line? Journal of Medicine and Philosophy 14 (6): 81–93.
Annas, G.J., L.B. Andrews, and R.M. Isasi. 2002. Protecting the Endangered Human: Toward an International Treaty Prohibiting Cloning and Inheritable Alterations. American Journal of Law and Medicine 28: 151–178.
Araki, A., and T. Ishii. 2016. Providing Appropriate Risk Information on Genome Editing for Patients. Trends in Biotechnology 34 (2): 86–90.
Arms Control Association. 2018. The Biological Weapons Convention (BWS) at a Glance. Available at: https://www.armscontrol.org/factsheets/bwc . Accessed 18 Jan 2021.
Baeshen, N.A., M.N. Baeshen, A. Sheikh, R.S. Bora, M.M. Ahmed, H.A. Ramadan, K.S. Saini, and E.M. Redwan. 2014. Cell Factories for Insulin Production. Microbial Cell Factories 13: 141.
Baltimore, D., P. Berg, M. Botchan, D. Carroll, R.A. Charo, G. Church, J.E. Corn, G.Q. Daley, J.A. Doudna, M. Fenner, H.T. Greely, M. Jinek, G.S. Martin, E. Penhoet, J. Puck, S.H. Sternberg, J.S. Weissman, and K.R. Yamamoto. 2015. A Prudent Path Forward for Genomic Engineering and Germline Gene Modification. Science 348 (6230): 36–38.
Bates, K.G. 2014. A Chosen Exile: Black People Passing in White America. NRP, October 7. Available at: https://www.npr.org/sections/codeswitch/2014/10/07/354310370/a-chosen-exile-black-people-passing-in-white-america . Accessed 18 Jan 2021.
Baylis, F. 2019. Altered Inheritance: CRISPR and the Ethics of Human Genome Editing . Cambridge, MA: Harvard University Press.
BBC News. 2015. Is Opposition to Genetically Modified Food Irrational? BBC News , June 3. Available at: https://www.bbc.com/news/science-environment-32901834 . Accessed 18 Jan 2021.
Beauchamp, T.L., and D. DeGrazia. 2020. Principles of Animal Research Ethics . New York, NY: Oxford University Press.
Begley S. 2018. Out of Prison, the ‘Father of Gene Therapy’ Faces a Harsh Reality: A Tarnished Legacy and an Ankle Monitor. STAT , July 23. Available at: https://www.statnews.com/2018/07/23/w-french-anderson-father-of-gene-therapy/ . Accessed 18 Jan 2021.
Berger, E., and B. Gert. 1991. Genetic Disorders and the Ethical Status of Germ-Line Gene Therapy. Journal of Medicine and Philosophy 16 (6): 667–683.
Beriain, I. 2018. Human Dignity and Gene Editing: Using Human Dignity as an Argument Against Modifying the Human Genome and Germline Is a Logical Fallacy. EMBO Reports 19 (10): e46789.
Berry, R. 2013. The Ethics of Genetic Engineering . New York, NY: Routledge.
Biello, D. 2010. Genetically Modified Crops on the Loose and Evolving in the U.S. Midwest. Scientific American , August 6. Available at: https://www.scientificamerican.com/article/genetically-modified-crop/ . Accessed 18 Jan 2021.
Billings, L.K., and J.C. Florez. 2010. The Genetics of Type 2 Diabetes: What Have We Learned from GWAS? Annals of New York Academy of Science 1212: 59–77.
Biofuels International. 2018. GM Yeast Could Fix Food vs. Fuel Debate Around Bioethanol. Biofuels International , April 4. Available at: https://biofuels-news.com/news/gm-yeast-could-fix-food-vs-fuel-debate-around-bioethanol/ . Accessed 26 Feb 2020.
Biotechnology Innovation Organization. 2020b. Genetically Engineered Animals: Frequently Asked Questions. Available at: https://archive.bio.org/articles/genetically-engineered-animals-frequently-asked-questions . Accessed 18 Jan 2021.
Blackford, R. 2014. Humanity Enhanced: Genetic Choice and the Challenge for Liberal Democracies . Cambridge, MA: MIT Press.
Blaese, R.M., K.W. Culver, A.D. Miller, C.S. Carter, T. Fleisher, M. Clerici, G. Shearer, L. Chang, Y. Chiang, P. Tolstoshev, J.J. Greenblatt, S.A. Rosenberg, H. Klein, M. Berger, C.A. Mullen, W.J. Ramsey, L. Muul, R.A. Morgan, and W.F. Anderson. 1995. T Lymphocyte-Directed Gene Therapy for ADA-SCID: Initial Trial Results After 4 Years. Science 270 (5235): 475–480.
Blancke, S. 2015. Is Opposition to Genetically Modified Food Irrational? Scientific American , August 18. Available at: https://www.scientificamerican.com/article/why-people-oppose-gmos-even-though-science-says-they-are-safe/ . Accessed 18 Jan 2021.
Blendon, R.J., M.T. Gorski, and J.M. Benson. 2016. The Public and the Gene-Editing Revolution. New England Journal of Medicine 374 (15): 1406–1411.
Boone, C.K. 1988. Bad axioms in Genetic Engineering. Hastings Center Report 18 (4): 9–13.
Bodner, A. 2015. Preventing Escape of GMO Salmon. Biology Fortified , November 20. Available at: https://biofortified.org/2015/11/gmo-salmon/ . Accessed 18 Jan 2021.
Boorse, C. 1977. Health as a Theoretical Concept. Philosophy of Science 44: 542–573.
Borges, B.J., O.M. Arantes, A.A. Fernandes, J.R. Broach, and P.M. Fernandes. 2018. Genetically Modified Labeling Policies: Moving Forward or Backward? Frontiers in Bioengineering and Biotechnology 6: 181.
Bostrom, N. 2010. Letter from Utopia (Version 1.9). Studies in Ethics, Law, and Technology 2: 1–7.
Bostrom, N. 2008. Why I Want to Be a Posthuman When I Grow Up. In Medical Enhancement and Posthumanity , ed. B. Gordijn and R. Chadwick, 107–137. Dordrecht, Netherlands: Springer.
Buchanan, A., D.W. Brock, N. Daniels, and D. Wikler. 2000. From Chance to Choice: Genetics and Justice . Cambridge, UK: Cambridge University Press.
Callahan, D. 1995. Setting Limits: Medical Goals in an Aging Society with “A Response to My Critics” . Washington, DC: Georgetown University Press.
Campbell, M. 2020a. World’s First Genetically Engineered Moth Is Released into an Open Field. Technology Networks , January 29. Available at: https://www.technologynetworks.com/genomics/news/world-first-genetically-engineered-moth-is-released-into-an-open-field-329960 . Accessed 18 Jan 2021.
Campbell, M. 2020b. Genetically Engineered Bacteria Protect Honey Bees Against Parasites. Technology Networks , February 24. Available at: https://www.technologynetworks.com/genomics/news/genetically-engineered-bacteria-protect-honey-bees-against-parasites-331209 . Accessed 18 Jan 2021.
Caplan, A. 1995. Moral Matters . New York, NY: Wiley.
Caplan, A. 1997. The Concepts of Health, Illness, and Disease. In Medical Ethics , 2nd ed, ed. R. Veatch, 57–74. Sudbury, MA: Jones and Bartlett.
Carlson, E.A. 2001. The Unfit: A History of a Bad Idea . Cold Spring Harbor, NY: Cold Spring Harbor Press.
Centers for Disease Control and Prevention. 2019. Heart Disease Facts. Available at: https://www.cdc.gov/heartdisease/facts.htm . Accessed 18 Jan 2021.
Centers for Disease Control and Prevention and National Institutes of Health. 2009. Biosafety in Microbiological and Biomedical Laboratories, 5th ed. Available at: https://www.cdc.gov/labs/pdf/CDC-BiosafetyMicrobiologicalBiomedicalLaboratories-2009-P.PDF . Accessed 18 Jan 2021.
Christensen J. 2018. The Five Most Expensive Drugs in the United States. CNN , May 11. Available at: https://www.cnn.com/2018/05/11/health/most-expensive-prescription-drugs/index.html . Accessed 18 Jan 2021.
Cilluffo, A., and N.G. Ruiz. 2019. World’s Population Is Projected to Nearly Stop Growing by the End of the Century. Pew Research Center , June 17. Available at: https://www.pewresearch.org/fact-tank/2019/06/17/worlds-population-is-projected-to-nearly-stop-growing-by-the-end-of-the-century/ . Accessed 18 Jan 2021.
Coelho, A.C., and J.D. García. 2015. Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot Be Ignored in Health Biotechnology. Frontiers in Bioengineering and Biotechnology 3: 56.
Cohen J. 2019a. China’s CRISPR Push in Animals Promises Better Meat, Novel Therapies, and Pig Organs for People. Science , July 31. Available at: https://www.sciencemag.org/news/2019/07/china-s-crispr-push-animals-promises-better-meat-novel-therapies-and-pig-organs-people . Accessed 18 Jan 2021.
Cohen, J. 2019b. Deaf Couple May Edit Embryo’s DNA to Correct Hearing Mutation. Science , October 21. Available at: https://www.sciencemag.org/news/2019/10/deaf-couple-may-edit-embryo-s-dna-correct-hearing-mutation . Accessed 18 Jan 2021.
Cole-Turner, R. 1997. Genes, Religion and Society: The Developing Views of the Churches. Science and Engineering Ethics 3: 273–288.
Collins, M., and A. Thrasher. 2015. Gene Therapy: Progress and Predictions. Proceedings of Biological Sciences 282: 1821.
Conrow, J. 2018. Developing Nations Lead the Growth of GMO Crops. Alliance for Science , June 29. Available at: https://allianceforscience.cornell.edu/blog/2018/06/developing-nations-lead-growth-gmo-crops/ . Accessed 18 Jan 2021.
Convention on Biological Diversity. 2020. Available at: https://www.cbd.int/ . Accessed 18 Jan 2021.
Cornish, L. 2018. Understanding the Continued Opposition to GMOs. Devex , January 22. Available at: https://www.devex.com/news/understanding-the-continued-opposition-to-gmos-91888 . Accessed 18 Jan 2021.
Cossins, D. 2015. Will We Ever See GM Meat? BBC Future . March 9. Available at: https://www.bbc.com/future/article/20150309-will-we-ever-eat-gm-meat . Accessed 18 Jan 2021.
Costa, J.R., B.E. Bejcek, J.E. McGee, A.I. Fogel, K.R. Brimacombe, and R. Ketteler. 2017. Genome Editing Using Engineered Nucleases and Their Use in Genomic Screening. In Assay Guidance Manual , ed. S. Sittampalam et al. Bethesda, MD: Eli Lilly and Company and the National Center for Advancing Translational Sciences. Available at: https://www.ncbi.nlm.nih.gov/books/NBK464635/ . Accessed 18 Jan 2021.
Cummings, J.P. 2018. The Lifetime Economic Burden of Monogenic Diseases and the Social Motivations for Their Treatment with Genetic Therapy. Thesis. Rochester Institute of Technology. Available at: https://scholarworks.rit.edu/cgi/viewcontent.cgi?article=10984&context=theses . Accessed 18 Jan 2021.
Cyranoski, D. 2020. What CRISPR-Baby Prison Sentences Mean for Research. Nature 577: 154–155.
Daniell, H. 2002. Molecular Strategies for Gene Containment in Transgenic Crops. Nature Biotechnology 20 (6): 581–586.
Darwin, C. 1859. The Origin of Species by Means of Natural Selection . London, UK: John Murray.
Davidson, D. 2001. Inquiries into Truth and Interpretation , 2nd ed. Oxford, UK: Clarendon Press.
Davis, D.S. 2001. Genetic Dilemmas: Reproductive Technology, Parental Choices, and Children’s Futures . New York, NY: Routledge.
De Wert, G., B. Heindryckx, G. Pennings, A. Clarke, U. Eichenlaub-Ritter, C.G. van El, F. Forzano, M. Goddijn, H.C. Howard, D. Radojkovic, E. Rial-Sebbag, W. Dondorp, B.C. Tarlatzis, M.C. Cornel, and European Society of Human Genetics and the European Society of Human Reproduction and Embryology. 2018. Responsible Innovation in Human Germline Gene Editing: Background Document to the Recommendations of ESHG and ESHRE. European Journal of Human Genetics 26 (4): 450–470.
Domingo, J.L. 2016. Safety Assessment of GM Plants: An Updated Review of the Scientific Literature. Food and Chemical Toxicology 95: 12–18.
Doyle, A., M.P. McGarry, N.A. Lee, and J.J. Lee. 2012. The Construction of Transgenic and Gene Knockout/Knockin Mouse Models of Human Disease. Transgenic Research 21 (2): 327–349.
Duan, J.J., M. Marvier, J. Huesing, G. Dively, and Z.Y. Huang. 2008. A Meta-Analysis of Effects of Bt Crops on Honey Bees (Hymenoptera: Apidae). PLoS One 3 (1): e1415.
Dubljević, V. 2019. Neuroethics, Justice and Autonomy: Public Reason in the Cognitive Enhancement Debate . Cham, Switzerland: Springer.
Dunn, S.E., J.L. Vicini, K.C. Glenn, D.M. Fleischer, and M.J. Greenhawt. 2017. The Allergenicity of Genetically Modified Foods from Genetically Engineered Crops: A Narrative and Systematic Review. Annals of Allergy, Asthma and Immunology 119 (3): 214–222.
Environmental Protection Agency. 2020b. EPA’s Regulation of Biotechnology for Use in Pest Management. Available at: https://www.epa.gov/regulation-biotechnology-under-tsca-and-fifra/epas-regulation-biotechnology-use-pest-management . Accessed 18 Jan 2021.
European Commission. 2020. GMO Legislation. Available at: https://ec.europa.eu/food/plant/gmo/legislation_en . Accessed 18 Jan 2021.
Ezezika, O.C., and P.A. Singer. 2010. Genetically Engineered Oil-Eating Microbes for Bioremediation: Prospects and Regulatory Challenges. Technology in Society 32 (4): 331–335.
Fagan, J., M. Antoniou, and C. Robinson. 2014. GMO Myths and Truths , 2nd ed. London, UK: Earth Open Source.
Fernandez-Cornejo, J., S. Wechsler, M. Livingston, and L. Mitchell. 2014. Genetically Engineered Crops in the United States. U.S. Department of Agriculture, Economic Research Report 162, February. Available at: https://www.ers.usda.gov/webdocs/publications/45179/43668_err162.pdf . Accessed 18 Jan 2021.
Food and Drug Administration. 2020a. Animals with Intentional Genomic Alterations: Consumer Q & A. Available at: https://www.fda.gov/animal-veterinary/animals-intentional-genomic-alterations/consumer-qa . Accessed 19 Jan 2021.
Food and Drug Administration. 2020b. Oxitec Mosquito. Available at: https://www.fda.gov/animal-veterinary/animals-intentional-genomic-alterations/oxitec-mosquito . Accessed 19 Jan 2021.
Food and Drug Administration. 2020c. Therapeutic Cloning and Genome Modification. Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/therapeutic-cloning-and-genome-modification . Accessed 19 Jan 2021.
Food and Drug Administration. 2020d. What Is the Approval Process for Generic Drugs? Available at: https://www.fda.gov/drugs/generic-drugs/what-approval-process-generic-drugs . Accessed 19 Jan 2021.
Forabosco, F., M. Löhmus, L. Rydhmer, and L.F. Sundström. 2013. Genetically Modified Farm Animals and Fish in Agriculture: A Review. Livestock Science 153 (1–3): 1–9.
Frank, S.A. 2014. Somatic Mosaicism and Disease. Current Biology 24 (2): R577–R581.
Fukuyama, F. 2002. Our Posthuman Future: Consequences of the Biotechnology Revolution . New York: Picador.
Funk, C., and M. Hefferon. 2018. Public Views of Gene Editing for Babies Depend on How It Would Be Used. Pew Research Center , July 26. Available at: https://www.pewresearch.org/science/2018/07/26/public-views-of-gene-editing-for-babies-depend-on-how-it-would-be-used/ . Accessed 19 Jan 2021.
Gallo, A.M., D. Wilkie, M. Suarez, R. Labotka, R. Molokie, A. Thompson, P. Hershberger, and B. Johnson. 2010. Reproductive Decisions in People with Sickle Cell Disease or Sickle Cell Trait. Western Journal of Nursing Research 32 (8): 1073–1090.
Geib, C. 2018. Changing Regulations Mean Genetically Modified Meat Could Soon Be on Your Plate. Futurism , March 14. Available at: https://futurism.com/genetically-modified-meat-fda-usda . Accessed 19 Jan 2021.
Genetic Literacy Project. 2018. New Generation of GMO Crops Could Dramatically Boost Biofuel Production. Available at: https://geneticliteracyproject.org/2018/01/15/new-generation-gmo-crops-dramatically-boost-biofuel-production/ . Accessed 19 Jan 2021.
Genetic Literacy Project. 2020. GMO FAQs. Available at: https://gmo.geneticliteracyproject.org/FAQ/where-are-gmos-grown-and-banned/ . Accessed 19 Jan 2021.
GenScript. 2020. What Are Monoclonal Antibodies? Available at: https://www.genscript.com/how-to-make-monoclonal-antibodies.html . Accessed 19 Jan 2021.
GM Watch. 2019. International Scientists Urge Precaution with Gene Drives: New Study. GM Watch , May 21. https://www.gmwatch.org/en/news/latest-news/18951-international-scientists-urge-precaution-with-gene-drives-new-study . Accessed 19 Jan 2021.
GMO Answers. 2020a. What GMO Crops Are Currently Available on the Market? Available at: https://gmoanswers.com/current-gmo-crops?gclid=CjwKCAiAhc7yBRAdEiwAplGxX265z5GBxlV4Y4pqKVOfiooF2qfFs91eOW8InUo3yuJGH_B39BkoDxoCY2gQAvD_BwE . Accessed 19 Jan 2021.
GMO Answers. 2020b. Nine Things You Need to Know About GMO Salmon. Available at: https://gmoanswers.com/nine-9-things-you-need-know-about-gmo-salmon . Accessed January.
Gonzaludo, N., J.W. Belmont, V.G. Gainullin, and R.J. Taft. 2019. Estimating the Burden and Economic Impact of Pediatric Genetic Disease. Genetics in Medicine 21: 1781–1789.
Green, R.M. 2001. The Human Embryo Research Debates: Bioethics in the Vortex of Controversy . New York, NY: Oxford University Press.
Guillemaud, T., E. Lombaert, and D. Bourguet. 2016. Conflicts of Interest in GM Bt Crop Efficacy and Durability Studies. PLoS One 11 (12): e0167777.
Gurevich, R. 2020. How Much Does IVF Really Cost? Very Well Family, March 5. Available at: https://www.verywellfamily.com/how-much-does-ivf-cost-1960212 . Accessed 19 Jan 2021.
Harmon, A. 2016. Fighting Lyme Disease in the Genes of Nantucket’s Mice. New York Times , June 7, A15.
Harris, J. 1992. Wonderwoman and Superman: The Ethics of Human Biotechnology . Oxford, UK: Oxford University Press.
Harris, J. 2007. Enhancing Evolution: The Ethical Case for Making Better People . Princeton, NJ: Princeton University Press.
He, K., L.R. Wilkens, D.O. Stram, L.N. Kolonel, B.E. Henderson, A.H. Wu, L. Le Marchand, and C.A. Haiman. 2011. Generalizability and Epidemiologic Characterization of Eleven Colorectal Cancer GWAS Hits in Multiple Populations. Cancer Epidemiology and Biomarkers and Prevention 20 (1): 70–81.
Henderson, G.E., M.M. Easter, C. Zimmer, N.M. King, A.M. Davis, B.B. Rothschild, L.R. Churchill, B. Wilfond, and D.K. Nelson. 2006. Therapeutic Misconception in Early Phase Gene Transfer Trials. Social Science and Medicine 62 (1): 239–253.
Henkel, R.D., T. Miller, and R.S. Weyant. 2012. Monitoring Select Agent Theft, Loss and Release Reports in the United States—2004–2010. Applied Biosafety 18: 171–180.
Hjältén, J., and E.P. Axelsson. 2015. GM Trees with Increased Resistance to Herbivores: Trait Efficiency and Their Potential to Promote Tree Growth. Frontiers in Plant Science , May 1. Available at: https://doi.org/10.3389/fpls.2015.00279 . Accessed 19 Jan 2021.
Holdrege, C. 2008. Understanding the Unintended Effects of Genetic Manipulation. The Nature Institute . Available at: https://natureinstitute.org/txt/ch/nontarget.php . Accessed 19 Jan 2021.
Horgan, J. 2017. Has the Era of Gene Therapy Finally Arrived? Scientific American , September 1. Available at: https://blogs.scientificamerican.com/cross-check/has-the-era-of-gene-therapy-finally-arrived/ . Accessed 19 Jan 2021.
Hou, Z., and Z. Zhang. 2019. Inserting DNA with CRISPR. Science 365 (6448): 25–26.
House, K. 2019. China Quietly Confirms Birth of Third Gene-Edited Baby. Futurism , December 30. Available at: https://futurism.com/neoscope/china-confirms-birth-third-gene-edited-baby . Accessed 19 Jan 2021.
Hryhorowicz, M., J. Zeyland, R. Słomski, and D. Lipiński. 2017. Genetically Modified Pigs as Organ Donors for Xenotransplantation. Molecular Biotechnology 59 (9–10): 435–444.
Hübner, D. 2018. Human-animal Chimeras and Hybrids: An Ethical Paradox Behind Moral Confusion? The Journal of Medicine and Philosophy 43 (2): 187–210.
Human Fertilisation and Embryology Authority. 2020. About Us. Available at: https://www.hfea.gov.uk/about-us/ . Accessed 19 Jan 2021.
Human Genome Project. 2020. Human Genome Project Budget. Available at: https://web.ornl.gov/sci/techresources/Human_Genome/project/budget.shtml . Accessed 19 Jan 2021.
International Service for the Acquisition of Agri-biotech Applications. 2018. Gm Crops and the Environment. Available at: https://www.isaaa.org/resources/publications/pocketk/4/default.asp . Accessed: 19 Jan 2021.
Johnston, T. 2005. In One’s Own Image: Ethics and the Reproduction of Deafness. Journal of Deaf Studies and Deaf Education 10 (4): 426–441.
Juengst, E. 1997. Can Enhancement Be Distinguished from Prevention in Genetic Medicine? Journal of Medicine and Philosophy 22 (2): 125–142.
Justlabelit.org. 2020. Labelling Around the World. Available at: http://www.justlabelit.org/right-to-know-center/labeling-around-the-world/ . Accessed 19 Jan 2021.
Kaebnick, G.E., E. Heitman, J.P. Collins, J.A. Delborne, W.G. Landis, K. Sawyer, L.A. Taneyhill, and D.E. Winickoff. 2016. Precaution and Governance of Emerging Technologies. Science 354 (6313): 710–711.
Kaemmerer, W.F. 2018. How Will the Field of Gene Therapy Survive Its Success? Bioengineering and Translational Medicine 3 (2): 166–177.
Kaiser Family Foundation. 2016. Medicaid Coverage of Family Planning Benefits: Results from a State Survey. Available at: https://www.kff.org/report-section/medicaid-coverage-of-family-planning-benefits-results-from-a-state-survey-fertility-services/ . Accessed 19 Jan 2021.
Kelle, A. 2013. Beyond Patchwork Precaution in the Dual-Use Governance of Synthetic Biology. Science and Engineering Ethics 19 (3): 1121–1139.
Kevles, D.J. 1985. In the Name of Eugenics: Genetics and the Uses of Human Heredity . Cambridge, MA: Harvard University Press.
Kids Health. 2018. Osteogenesis Imperfecta (Brittle Bone Disease). Available at: https://kidshealth.org/en/parents/osteogenesis-imperfecta.html . Accessed 19 Jan 2021.
Kimman, T.G., E. Smit, and M.R. Klein. 2008. Evidence-Based Biosafety: A Review of the Principles and Effectiveness of Microbiological Containment Measures. Clinical Microbiology Reviews 21 (3): 403–425.
Kimmelman, J. 2010. Gene Transfer and the Ethics of First-in-Human Research: Lost in Translation . Cambridge, UK: Cambridge University Press.
Kitcher, P. 1996. The Lives to Come: the Genetic Revolution and Human Possibilities . New York, NY: Simon and Schuster.
Koch, T. 2020. Transhumanism, Moral Perfection, and Those 76 Trombones. Journal of Medicine and Philosophy 45 (2): 179–192.
Koplin, J.J., C. Gyngell, and J. Savulescu. 2020. Germline Gene Editing and the Precautionary Principle. Bioethics 34 (1): 49–59.
Koplin, J.J., and D. Wilkinson. 2019. Moral Uncertainty and the Farming of Human-Pig Chimeras. Journal of Medical Ethics 45 (7): 440–446.
Kriebel, D., J. Tickner, P. Epstein, J. Lemons, R. Levins, E.L. Loechler, M. Quinn, R. Rudel, T. Schettler, and M. Stoto. 2001. The Precautionary Principle in Environmental Science. Environmental Health Perspectives 109 (9): 871–876.
Kumar, P., J. Radhakrishnan, M.A. Chowdhary, and P.F. Giampietro. 2001. Prevalence and Patterns of Presentation of Genetic Disorders in a Pediatric Emergency Department. Mayo Clinic Proceedings 76 (8): 777–783.
Kumar, S.R.P., D.M. Markusic, M. Biswas, K.A. High, and R.W. Herzog. 2016. Clinical Development of Gene Therapy: Results and Lessons from Recent Successes. Molecular Therapy—Methods and Clinical Development 3: 16034.
Kuzma, J. 2016. A Missed Opportunity for U.S. Biotechnology Regulation. Science 353 (6305): 1211–1213.
Lander, E.S., F. Baylis, F. Zhang, E. Charpentier, P. Berg, C. Bourgain, B. Friedrich, J.K. Joung, J. Li, D. Liu, L. Naldini, J.B. Nie, R. Qiu, B. Schoene-Seifert, F. Shao, S. Terry, W. Wei, and E.L. Winnacker. 2019. Adopt a Moratorium on Heritable Genome Editing. Nature 567 (7747): 165–168.
Lanphier, E., F. Urnov, S.E. Haecker, M. Werner, and J. Smolenski. 2015. Don’t Edit the Human Germ Line. Nature 519: 410–411.
Ledford, H., and E. Callaway. 2020. Pioneers of CRISPR Gene Editing Win Nobel in Chemistry. Nature 586: 346–347.
Lee, B. 2018. What Are Biologics? 5 Examples of Biological Drugs You May Already Be Taking. Good RX , June 13. Available at: https://www.goodrx.com/blog/biologics-biological-drugs-examples/ . Accessed 19 Jan 2021.
Le Page, M. 2020. Human Genes Have Been Added to Pigs to Create Skin for Transplants. New Scientist , January 29. Available at: https://www.newscientist.com/article/2231579-human-genes-have-been-added-to-pigs-to-create-skin-for-transplants/#ixzz6GPggXYEP . Accessed 19 Jan 2021.
Liang, P., Y. Xu, X. Zhang, C. Ding, R. Huang, Z. Zhang, J. Lv, X. Xie, Y. Chen, Y. Li, Y. Sun, Y. Bai, Z. Songyang, W. Ma, C. Zhou, and J. Huang. 2015. CRISPR/Cas9-Mediated Gene Editing in Human Tripronuclear Zygotes. Protein and Cell 6 (5): 363–372.
Losey, J.E., L.S. Rayor, and M.E. Carter. 1999. Transgenic Pollen Harms Monarch Larvae. Nature 399: 214.
Lucht, J.M. 2015. Public Acceptance of Plant Biotechnology and GM Crops. Viruses 7 (8): 4254–4281.
Maddox, B. 2003. Rosalind Franklin: The Dark Lady of DNA . New York, NY: HarperCollins.
Main, D. 2017. USDA Agrees to Not Regulate Genetically Modified GRASS on the Loose in Oregon. Newsweek , January 31. Available at: https://www.newsweek.com/usda-agrees-not-regulate-gmo-grass-loose-oregon-550942 . Accessed 19 Jan 2021.
Mamcarz, E., S. Zhou, T. Lockey, H. Abdelsamed, S.J. Cross, G. Kang, Z. Ma, J. Condori, J. Dowdy, B. Triplett, C. Li, G. Maron, J.C. Aldave Becerra, J.A. Church, E. Dokmeci, J.T. Love, A.C. da Matta Ain, H. van der Watt, X. Tang, W. Janssen, B.Y. Ryu, S.S. De Ravin, M.J. Weiss, B. Youngblood, J.R. Long-Boyle, S. Gottschalk, M.M. Meagher, H.L. Malech, J.M. Puck, M.J. Cowan, and B.P. Sorrentino. 2019. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. New England Journal of Medicine 380 (16): 1525–1534.
Marshall, D.A., E.I. Benchimol, A. MacKenzie, D.D. Duque, K.V. MacDonald, T. Hartley, H. Howley, A. Hamilton, M. Gillespie, F. Malam, and K. Boycott. 2019. Direct Health-Care Costs for Children Diagnosed with Genetic Diseases Are Significantly Higher Than for Children with Other Chronic Diseases. Genetics in Medicine 21: 1049–1057.
Maslen, H., N. Faulmüller, and J. Savulescu. 2014. Pharmacological Cognitive Enhancement-How Neuroscientific Research Could Advance Ethical Debate. Frontiers in Systems Neuroscience 8: 107.
Maziarz, R.T. 2019. CAR T-Cell Therapy Total Cost Can Exceed $1.5 Million Per Treatment. Healio , May 29. Available at: https://www.healio.com/hematology-oncology/cell-therapy/news/online/%7B124396e7-1b60-4cff-a404-0a2baeaf1413%7D/car-t-cell-therapy-total-cost-can-exceed-15-million-per-treatment . Accessed 19 Jan 2021.
McDivitt, P. 2019. Golden Rice: The GMO Crop Loved by Humanitarians, Opposed by Greenpeace. Genetic Literacy Project , November 8. Available at: https://geneticliteracyproject.org/2019/11/08/golden-rice-the-gmo-crop-loved-by-humanitarians-opposed-by-greenpeace/ . Accessed 19 Jan 2021.
McDonald, J. 2007. Could Genetically Modified Crops Be Killing Honeybees? SF Gate , March 10. Available at: https://www.sfgate.com/homeandgarden/article/Could-genetically-modified-crops-be-killing-bees-2611496.php . Accessed 19 Jan 2021.
McGee, G. 2000. The Perfect Baby: Parenthood in the New World of Cloning and Genetics , 2nd ed. Lanham, MD: Rowman and Littlefield.
McIntosh, J. 2017. What’s to Know About Alopecia Areata? Medical News Today , December 22. Available at: https://www.medicalnewstoday.com/articles/70956#home-remedies . Accessed 19 Jan 2021.
Meeme, V. 2019. Kenya Reconsidering GMO Crop Ban for Food Security. Alliance for Science , April 30. Available at: https://allianceforscience.cornell.edu/blog/2019/04/kenya-reconsidering-gmo-crop-ban-support-food-security/ . Accessed 19 Jan 2021.
Mehlman, M.J. 2009. The Price of Perfection: Individualism and Society in the Era of Biomedical Enhancement . Baltimore, MD: Johns Hopkins University Press.
Merler, S., M. Ajelli, L. Fumanelli, and A. Vespignani. 2013. Containing the Accidental Laboratory Escape of Potential Pandemic Influenza Viruses. BMC Medicine 11: 252.
Messer, K.D., S. Bligh, M. Costanigro, and H.M. Kaiser. 2015. Process Labeling of Food: Consumer Behavior, the Agricultural Sector, and Policy Recommendations. Council for Agricultural Science and Technology 10: 1–16.
Miller, F.G., and S. Joffe. 2009. Benefit in Phase 1 Oncology Trials: Therapeutic Misconception or Reasonable Treatment Option? Clinical Trials 5 (6): 617–623.
Miliotou, A.N., and L.C. Papadopoulou. 2018. CAR T-Cell Therapy: A New Era in Cancer Immunotherapy. Current Pharmaceutical Biotechnology 19 (1): 5–18.
Mitchell, C.B., E.D. Pellegrino, J.B. Elshtain, J.F. Kilner, and S.B. Rae. 2007. Biotechnology and the Human Good . Washington, DC: Georgetown University Press.
Molteni, M. 2018. Now You Can Sequence Your Whole Genome for Just $200. Wired , November 11. Available at: https://www.wired.com/story/whole-genome-sequencing-cost-200-dollars/ . Accessed 19 Jan 2021.
More, M., and N. Vita-More (eds.). 2013. The Transhumanist Reader: Classical and Contemporary Essays on the Science, Technology, and Philosophy of the Human Future . New York, NY: Wiley-Blackwell.
Moritz, R. 2020. Community Engagement on Pathogen Research. Presentation to the National Science Advisory Board for Biosecurity, January 24. Bethesda, MD.
Murphy, D. 2020. Concepts of Health and Disease. Stanford Encyclopedia of Philosophy . Available at: https://plato.stanford.edu/entries/health-disease/ . Accessed 19 Jan 2021.
National Academies of Sciences, Engineering, and Medicine. 2016a. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values . Washington, DC: National Academies Press.
National Academies of Sciences, Engineering, and Medicine. 2016b. Genetically Engineered Crops: Experiences and Prospects . Washington, DC: National Academies Press.
National Academies of Sciences, Engineering, and Medicine. 2017. Human Genome Editing: Science, Ethics, and Governance . Washington, DC: National Academies Press.
National Conference of State Legislatures. 2019. State Laws Related to Insurance Coverage for Infertility Treatment . Available at: https://www.ncsl.org/research/health/insurance-coverage-for-infertility-laws.aspx . Accessed 19 Jan 2021.
National Heart, Lung, and Blood Institute. 2020. Cell Sickle Disease. Available at: https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease . Accessed 19 Jan 2021.
National Human Genome Research Institute. 2017. How Does Genome Editing Work? Available at: https://www.genome.gov/about-genomics/policy-issues/Genome-Editing/How-genome-editing-works . Accessed 19 Jan 2021.
National Institutes of Health. 2020a. Stem Cell Information. Available at: https://stemcells.nih.gov/ . Accessed 19 Jan 2021.
National Research Council. 2004. Biotechnology in the Age of Terrorism . Washington, DC: National Academies Press.
National Research Council. 2011. Guide for the Care and Use of Laboratory Animals , 8th ed. Washington, DC: National Academies Press.
Neuhaus, C.P. 2018. Community Engagement and Field Trials of Genetically Modified Insects and Animals. Hastings Center Report 48 (1): 25–36.
Nobel Prize.org. 2021. The Nobel Prize in Chemistry 1980. Available at: https://www.nobelprize.org/prizes/chemistry/1980/berg/lecture/ . Accessed 10 Jan 2021.
Nobel Prize Winners. 2016. Letter to Greenpeace, June 26. Available at: https://www.supportprecisionagriculture.org/nobel-laureate-gmo-letter_rjr.html . Accessed 19 Jan 2021.
Nogrady, B. 2020. What the Data Say About Asymptomatic COVID Infections. Nature 587: 534–535.
Norero, D. 2016. Genetically Modified Crops and the Exaggeration of “Interest Conflict.” Cornell Alliance for Science , November 3. Available at: https://allianceforscience.cornell.edu/blog/2016/11/genetically-modified-crops-and-the-exaggeration-of-interest-conflict/ . Accessed 19 Jan 2021.
Normile, D. 2004. Infectious Diseases: Mounting Lab Accidents Raise SARS Fears. Science 304: 659–661.
Normile, D. 2018. Shock Greets Claim of CRISPR-Edited Babies. Science 362 (6418): 978–979.
Normile, D. 2019. China Tightens Rules on Gene Editing. Science 363 (6431): 1023.
Nozick, R. 1974. Anarchy, State, Utopia . New York, NY: Basic Books.
Nuffield Council on Bioethics. 2016. Genome Editing: An Ethical Review. Available at: https://www.nuffieldbioethics.org/publications/genome-editing-an-ethical-review . Accessed 13 Mar 2020.
Organizing Committee of the Second International Summit on Human Genome Editing. 2018. Concluding Statement. Available at: http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11282018b . Accessed 19 Jan 2021.
Ormandy, E.H., J. Dale, and G. Griffin. 2011. Genetic Engineering of Animals: Ethical Issues, Including Welfare Concerns. The Canadian Veterinary Journal 52 (5): 544–550.
Parens, E. (ed.). 1998. Enhancing Human Traits: Ethical and Social Implications . Washington, DC: Georgetown University Press.
Parens, E., and A. Asch. 1999. The Disability Rights Critique of Prenatal Genetic Testing: Reflections and Recommendations. Hastings Center Report 29 (5): S1–22.
Park, A. 2019. Experts Are Calling for a Ban on Gene Editing of Human Embryos. Time Magazine , March 13. Available at: https://time.com/5550654/crispr-gene-editing-human-embryos-ban/ . Accessed 19 Jan 2021.
Pew Research Center. 2016. Public Opinion About Genetically Modified Foods and Trust in Scientists Connected with These Foods. Pew Research Center , December 1. Available at: https://www.pewresearch.org/science/2016/12/01/public-opinion-about-genetically-modified-foods-and-trust-in-scientists-connected-with-these-foods/ . Accessed 19 Jan 2021.
Poppy, G. 2000. GM Crops: Environmental Risks and Non-target Effects. Trends in Plant Science 5 (1): 4–6.
Porter, A. 2017. Bioethics and Transhumanism. Journal of Medicine and Philosophy 42 (3): 237–260.
Porterfield, A., and J. Entine. 2018. ‘Substantial Equivalence’: Are GMOs as Safe as Other Conventional and Organic Foods? Genetic Literacy Project , May 11. Available at: https://geneticliteracyproject.org/2018/05/11/substantial-equivalence-are-gmos-as-safe-as-other-conventional-organic-foods/ . Accessed 19 Jan 2021.
President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. 1982. Washington, DC: President’s Commission.
President’s Council on Bioethics. 2002. Human Cloning and Human Dignity: An Ethical Inquiry . Washington, DC: President’s Council on Bioethics.
President’s Council on Bioethics. 2003. Beyond Therapy: Biotechnology and the Pursuit of Happiness . New York, NY: Harper Perennial.
Proctor, R. 1988. Racial Hygiene: Medicine Under the Nazis . Cambridge, MA: Harvard University Press.
Public Health Emergency. 2015. Biosafety Levels. Available at: https://www.phe.gov/s3/BioriskManagement/biosafety/Pages/Biosafety-Levels.aspx . Accessed 19 Jan 2021.
Ragnedda, M., and G.W. Muschert (eds.). 2015. The Digital Divide . New York, NY: Routledge.
Rana, F.R., and K.R.Samples. 2019. Humans 2.0: Scientific, Philosophical, and Theological Perspectives on Transhumanism . Covina, CA: Reasons to Believe.
Rasko, J.E., G.M. O’Sullivan, and R.A. Ankeny (eds.). 2006. The Ethics of Inheritable Genetic Modification: a Dividing Line? Cambridge, UK: Cambridge University Press.
Rawls, J. 2005. Political Liberalism , 2nd ed. New York: Columbia University Press.
Regan, T. 1983. The Case for Animal Rights . Berkeley, CA: University of California Press.
Reiss, M.J., and R. Straughan. 1996. Improving Nature? The Science and Ethics of Genetic Engineering . Cambridge, UK: Cambridge University Press.
Resnik, D.B. 1993. Debunking the Slippery Slope Argument Against Human Germ-Line Gene Therapy. Journal of Medicine and Philosophy 19 (1): 23–40.
Resnik, D.B. 2000a. The Moral Significance of the Therapy/Enhancement Distinction in Human Genetics. Cambridge Quarterly of Healthcare Ethics 9 (3): 365–377.
Resnik, D.B. 2000b. Of Maize and Men: Reproductive Control and the Threat to Genetic Diversity. Journal of Medicine and Philosophy 25 (4): 451–467.
Resnik, D.B. 2001. DNA Patents and Human Dignity. Journal of Law, Medicine, and Ethics 29 (2): 153–165.
Resnik, D.B. 2007. Embryonic Stem Cell Patents and Human Dignity. Health Care Analysis 15 (3): 211–222.
Resnik, D.B. 2011. Ethical Issues Concerning Transgenic Animals in Biomedical Research. In The Ethics of Animal Research: Exploring the Controversy , ed. J. Garrett, 169–179. Cambridge, MA: MIT Press.
Resnik, D.B. 2012. Environmental Health Ethics . Cambridge, UK: Cambridge University Press.
Resnik, D.B. 2015a. Retracting Inconclusive Research: Lessons from the Séralini GM Maize Feeding Study. Journal of Agricultural and Environmental Ethics 28 (4): 621–633.
Resnik, D.B. 2015b. Food and Beverage Policies and Public Health Ethics. Health Care Analysis 23 (2): 122–133.
Resnik, D.B. 2018a. The Ethics of Research with Human Subjects: Protecting People, Advancing Science, Promoting Trust . Cham, Switzerland: Springer.
Resnik, D.B. 2018b. Ethics of Community Engagement in Field Trials of Genetically Modified Mosquitoes. Developing World Bioethics 18 (2): 135–143.
Resnik, D.B. 2019a. Two Unresolved Issues in Community Engagement for Field Trials of Genetically Modified Mosquitoes. Pathogens and Global Health 113 (5): 238–245.
Resnik, D.B. 2019b. How Should Engineered Nanomaterials Be Regulated for Public and Environmental Health? AMA Journal of Ethics 21 (4): E363–369.
Resnik, D.B., and D. Vorhaus. 2006. Genetic Modification and Genetic Determinism. Philosophy, Ethics, and Humanities in Medicine 1: 9.
Resnik, D.B., H. Steinkraus, and P. Langer. 1999. Human Germ-Line Gene Therapy: Scientific, Moral and Political Issues . Georgetown, TX: RG Landes.
Resnik, D.B., and P. Langer. 2001. Human Germline Gene Therapy Reconsidered. Human Gene Therapy 12 (11): 1449–1458.
Ridley, M. 2000. Genome: The Autobiography of a Species in 23 Chapters . New York, NY: Harper Collins.
Rifkin, J. 1983. Algeny . New York, NY: Viking Press.
Rigby, B. 2017. Growth Hormones in Meat: Myths and Reality. Climbing Nutrition , February 24. Available at: https://www.climbingnutrition.com/diet/growth-hormones-meat-myths-reality/ . Accessed 19 Jan 2021.
Robert, J.S., and F. Baylis. 2003. Crossing Species Boundaries. American Journal of Bioethics 3 (3): 1–13.
Robertson, J.A. 1994. Children of Choice: Freedom and the New Reproductive Technologies . Princeton, NJ: Princeton University Press.
Rollin, B. 1995. The Frankenstein Syndrome: Ethical and Social Issues in the Genetic Engineering of Animals . Cambridge, UK: Cambridge University Press.
Russell, W., and R. Birch. 1959. Principles of Humane Animal Experimentation . Springfield, IL: Charles C. Thomas.
Sandel, M.J. 2009. The Case Against Perfection: Ethics in the Age of Genetic Engineering . Cambridge, MA: Harvard University Press.
Savulescu, J. 2002. Education and Debate: Deaf Lesbians, “Designer Disability,” and the Future of Medicine. British Medical Journal 325 (7367): 771–773.
Schaffner, K.F. 1993. Discovery and Explanation in Biology and Medicine . Chicago, IL: University of Chicago Press.
Schuppli, C., D. Fraser, and M. McDonald. 2004. Expanding the Three Rs to Meet New Challenges in Humane Animal Experimentation. Alternative to Laboratory Animals 32: 515–532.
Science and Environmental Health Network. 1998. Wingspread Statement on the Precautionary Principle. Available at: http://www.who.int/ifcs/documents/forums/forum5/wingspread.doc . Accessed: 19 Jan 2021.
Sears, M.K., R.L. Hellmich, D.E. Stanley-Horn, K.S. Oberhauser, J.M. Pleasants, H.R. Mattila, B.D. Siegfried, and G.P. Dively. 2001. Impact of Bt Corn Pollen on Monarch Butterfly Populations: A Risk Assessment. Proceedings of the National Academy of Sciences of the United States of America 98 (21): 11937–11942.
Séralini, G.E., E. Clair, R. Mesnage, S. Gress, N. Defarge, M. Malatesta, D. Hennequin, and J.S. de Vendômois. 2012. Long Term Toxicity of a Roundup Herbicide and a Roundup-Tolerant Genetically Modified Maize. Food and Chemical Toxicology 50 (11): 4221–4231. Retraction in: Food and Chemical Toxicology 63: 244.
Shamoo, A.E., and D.B. Resnik. 2015. Responsible Conduct of Research , 3rd ed. New York, NY: Oxford University Press.
Shendure, J., G.M. Findlay, and M.W. Snyder. 2019. Genomic Medicine–Progress, Pitfalls, and Promise. Cell 177 (1): 45–57.
Simmons, D. 2008. The Use of Animal Models in Studying Genetic Disease: Transgenesis and Induced Mutation. Nature Education 1 (1): 70.
Singer, P. 2009. Animal Liberation , reissue ed. New York, NY: Harper Perennial.
Spinello, R.A. 2016. Cyberethics: Morality and Law in Cyberspace , 6th ed. Boston: MA: Jones and Bartlett.
Stöppler, M.C. 2019. Genetic Diseases. Medicine.net . Available at: https://www.medicinenet.com/genetic_disease/article.htm . Accessed 19 Jan 2021.
Streiffer, R. 2005. At the Edge of Humanity: Human Stem Cells, Chimeras, and Moral Status. Kennedy Institute of Ethics Journal 15 (4): 347–370.
Szasz, T. 1961. The Myth of Mental Illness . New York, NY: Harper.
Tait, J. 2001. More Faust Than Frankenstein: The European Debate About the Precautionary Principle and Risk Regulation for Genetically Modified Crops. Journal of Risk Research 4 (2): 175–189.
The Business Research Company. 2019. Global Biologic Market Size and Segments, March 20. Available at: https://www.globenewswire.com/news-release/2019/03/27/1774114/0/en/Global-Biologics-Market-Size-and-Segments.html . Accessed 20 Jan 2021.
Thompson, P.B. 1993. Genetically Modified Animals: Ethical Issues. Journal of Animal Science 71 (Suppl. 3): 51–56.
Tratar, U.L., S. Horvat, and M. Cemazar. 2018. Transgenic Mouse Models in Cancer Research. Frontiers in Oncology 8 (July 20): 268.
Treatment Solutions. 2017. Are GMO Bacteria Safe for Wastewater Treatment? Available at: https://aosts.com/gmo-bacteria-safe-wastewater-treatment/ . Accessed 26 Feb 2020.
United Nations Educational, Scientific, and Cultural Organization. 2020. UNESCO Panel of Experts Calls for Ban on “Editing” of Human DNA to Avoid Unethical Tampering with Hereditary Traits. Available at: https://en.unesco.org/news/unesco-panel-experts-calls-ban-editing-human-dna-avoid-unethical-tampering-hereditary-traits . Accessed 20 Jan 2021.
United States Department of Agriculture. 2018. Establishing the National Bioengineered Food Disclosure Standard. Available at: https://www.usda.gov/media/press-releases/2018/12/20/establishing-national-bioengineered-food-disclosure-standard . Accessed 20 Jan 2021.
United States Department of Agriculture. 2020. Biotechnology Frequently Asked Questions. Available at: https://www.usda.gov/topics/biotechnology/biotechnology-frequently-asked-questions-faqs . Accessed 20 Jan 2021.
United States Department of Homeland Security. 2008. National Bio and Agro-Defense Facility Final Environmental Impact Statement, Appendix B . Washington, DC: US Department of Homeland Security.
Urry, L.A., M.L. Cain, S.A. Wasserman, P.V. Minorsky, and J.B. Reece. 2016. Campbell Biology , 11th ed. New York, NY: Pearson.
Walters, L., and J.G. Palmer. 1997. The Ethics of Human Gene Therapy . New York, NY: Oxford University Press.
Walton, D. 2017. The Slippery Slope Argument in the Ethical Debate on Genetic Engineering of Humans. Science and Engineering Ethics 23 (6): 1507–1528.
Wang, H., and H. Yang. 2019. Gene-Edited Babies: What Went Wrong and What Could Go Wrong. PLoS Biology 17 (4): e3000224.
Wareham, C., and C. Nardini. 2015. Policy on Synthetic Biology: Deliberation, Probability, and the Precautionary Paradox. Bioethics 29 (2): 118–125.
Warwick, S.I., H.J. Beckie, and L.M. Hall. 2009. Gene Flow, Invasiveness, and Ecological Impact of Genetically Modified Crops. Annals of the New York Academy of Sciences 1168 (1): 72–99.
WebMD. 2020. What Are Normal Blood Sugar Levels? Available at: https://www.webmd.com/diabetes/qa/what-are-normal-blood-sugar-levels . Accessed 20 Jan 2021.
Werth, J., L. Boucher, D. Thornby, S. Walker, and G. Charles. 2013. Changes in Weed Species Since the Introduction of Glyphosate-Resistant Cotton. Crop and Pasture Science 64 (8): 791–798.
Whiteside, K. 2006. Precautionary Politics: Principle and Practice in Confronting Environmental Risk . Cambridge, MA: MIT Press.
Whitlock J. 2019. Gender Reassignment Surgery. Very Well Health, November 8. Available at: https://www.verywellhealth.com/sex-reassignment-surgery-srs-3157235 . Accessed 20 Jan 2021.
Wolinetz, C.D., and F.S. Collins. 2019. NIH Pro Germline Editing Moratorium. Nature 567: 175.
World Health Organization. 2020a. Malaria. Available at: https://www.who.int/malaria/en/ . Accessed 20 Jan 2021.
World Health Organization. 2020b. Dengue and Severe Dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue . Accessed 20 Jan 2021.
World Health Organization. 2020c. Determinants of Health. Available at: https://www.who.int/hia/evidence/doh/en/ . Accessed 20 Jan 2021.
Yabroff, K.R., J. Lund, D. Kepka, and A. Mariotto. 2011. Economic Burden of Cancer in the United States: Estimates, Projections, and Future Research. Cancer Epidemiology, Biomarkers and Prevention 20 (10): 2006–2014.
Yourgenome.org. 2020. What Are Single Gene Disorders? Available at: https://www.yourgenome.org/facts/what-are-single-gene-disorders . Accessed 20 Jan 2021.
Zhang, C., R. Wohlhueter, and H. Zhang. 2016. Genetically Modified Foods: A Critical Review of Their Promise and Problems. Food Science and Human Wellness 5 (3): 116–123.
Zhang, X.H., L.Y. Tee, X.G. Wang, Q.S. Huang, and S.H. Yang. 2015. Off-Target Effects in CRISPR/Cas9-Mediated Genome Engineering. Molecular Therapy—Nucleic Acids 4: e264.
Download references
Author information
Authors and affiliations.
National Institutes of Health, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
David B. Resnik
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to David B. Resnik .
Rights and permissions
Reprints and permissions
Copyright information
© 2021 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply
About this chapter
Cite this chapter.
Resnik, D.B. (2021). Genetic Engineering. In: Precautionary Reasoning in Environmental and Public Health Policy. The International Library of Bioethics, vol 86. Springer, Cham. https://doi.org/10.1007/978-3-030-70791-0_7
Download citation
DOI : https://doi.org/10.1007/978-3-030-70791-0_7
Published : 28 March 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-70790-3
Online ISBN : 978-3-030-70791-0
eBook Packages : Religion and Philosophy Philosophy and Religion (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Libraries | Research Guides
Genetic engineering, journals and databases.
- ACS Synthetic Biology ACS Synthetic Biology is a monthly peer-reviewed journal dedicated to research in synthetic biology and biological systems. The journal is particularly interested in studies on the design and synthesis of new genetic circuits and gene products; computational methods in the design of systems; and integrative applied approaches to understanding disease and metabolism.
- Gene Reports Gene Reports publishes papers that focus on the regulation, expression, function and evolution of genes in all biological contexts, including all prokaryotic and eukaryotic organisms, as well as viruses. Some general topics include: DNA organization, replication & evolution; expression and function; and regulation.
- Journal of Genetic Engineering and Biotechnology Journal of Genetic Engineering and Biotechnology is devoted to rapid publication of full-length research papers that leads to significant contribution in advancing knowledge in genetic engineering and biotechnology and provide novel perspectives in this research area. JGEB includes all major themes related to genetic engineering and recombinant DNA.
- PLoS Genetics This link opens in a new window PLoS Genetics reflects the full breadth and interdisciplinary nature of genetics and genomics research by publishing outstanding original contributions in all areas of biology - human studies as well as research on model organisms - from mice and flies, to plants and bacteria.
- GenETHX: Genetics and Ethics Database This link opens in a new window A database of citations to literature in books, journal articles, book chapters, bills, laws, court decisions, reports, news articles and audiovisuals, relating to ethics and public policy issues in genetics. Links to full-text and abstracts are provided when available.
- PubMed This link opens in a new window PubMed was developed by the National Center for Biotechnology Information (NCBI). It was developed in conjunction with publishers of biomedical literature as a search tool for accessing literature citations and linking to full-text journals at web sites of participating publishers.
Your Librarian

- Last Updated: Jul 6, 2022 11:17 AM
- URL: https://libguides.northwestern.edu/geneng
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Scientists develop a rapid gene-editing screen to find effects of cancer mutations
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."

Previous image Next image
Tumors can carry mutations in hundreds of different genes, and each of those genes may be mutated in different ways — some mutations simply replace one DNA nucleotide with another, while others insert or delete larger sections of DNA.
Until now, there has been no way to quickly and easily screen each of those mutations in their natural setting to see what role they may play in the development, progression, and treatment response of a tumor. Using a variant of CRISPR genome-editing known as prime editing, MIT researchers have now come up with a way to screen those mutations much more easily.
The researchers demonstrated their technique by screening cells with more than 1,000 different mutations of the tumor suppressor gene p53, all of which have been seen in cancer patients. This method, which is easier and faster than any existing approach, and edits the genome rather than introducing an artificial version of the mutant gene, revealed that some p53 mutations are more harmful than previously thought.
This technique could also be applied to many other cancer genes, the researchers say, and could eventually be used for precision medicine, to determine how an individual patient’s tumor will respond to a particular treatment.
“In one experiment, you can generate thousands of genotypes that are seen in cancer patients, and immediately test whether one or more of those genotypes are sensitive or resistant to any type of therapy that you’re interested in using,” says Francisco Sanchez-Rivera, an MIT assistant professor of biology, a member of the Koch Institute for Integrative Cancer Research, and the senior author of the study.
MIT graduate student Samuel Gould is the lead author of the paper , which appears today in Nature Biotechnology .
Editing cells
The new technique builds on research that Sanchez-Rivera began 10 years ago as an MIT graduate student. At that time, working with Tyler Jacks, the David H. Koch Professor of Biology, and then-postdoc Thales Papagiannakopoulos, Sanchez-Rivera developed a way to use CRISPR genome-editing to introduce into mice genetic mutations linked to lung cancer.
In that study, the researchers showed that they could delete genes that are often lost in lung tumor cells, and the resulting tumors were similar to naturally arising tumors with those mutations. However, this technique did not allow for the creation of point mutations (substitutions of one nucleotide for another) or insertions.
“While some cancer patients have deletions in certain genes, the vast majority of mutations that cancer patients have in their tumors also include point mutations or small insertions,” Sanchez-Rivera says.
Since then, David Liu, a professor in the Harvard University Department of Chemistry and Chemical Biology and a core institute member of the Broad Institute, has developed new CRISPR-based genome editing technologies that can generate additional types of mutations more easily. With base editing, developed in 2016, researchers can engineer point mutations, but not all possible point mutations. In 2019, Liu, who is also an author of the Nature Biotechnology study, developed a technique called prime editing, which enables any kind of point mutation to be introduced, as well as insertions and deletions.
“Prime editing in theory solves one of the major challenges with earlier forms of CRISPR-based editing, which is that it allows you to engineer virtually any type of mutation,” Sanchez-Rivera says.
When they began working on this project, Sanchez-Rivera and Gould calculated that if performed successfully, prime editing could be used to generate more than 99 percent of all small mutations seen in cancer patients.
However, to achieve that, they needed to find a way to optimize the editing efficiency of the CRISPR-based system. The prime editing guide RNAs (pegRNAs) used to direct CRISPR enzymes to cut the genome in certain spots have varying levels of efficiency, which leads to “noise” in the data from pegRNAs that simply aren’t generating the correct target mutation. The MIT team devised a way to reduce that noise by using synthetic target sites to help them calculate how efficiently each guide RNA that they tested was working.
“We can design multiple prime-editing guide RNAs with different design properties, and then we get an empirical measurement of how efficient each of those pegRNAs is. It tells us what percentage of the time each pegRNA is actually introducing the correct edit,” Gould says.
Analyzing mutations
The researchers demonstrated their technique using p53, a gene that is mutated in more than half of all cancer patients. From a dataset that includes sequencing information from more than 40,000 patients, the researchers identified more than 1,000 different mutations that can occur in p53.
“We wanted to focus on p53 because it’s the most commonly mutated gene in human cancers, but only the most frequent variants in p53 have really been deeply studied. There are many variants in p53 that remain understudied,” Gould says.
Using their new method, the researchers introduced p53 mutations in human lung adenocarcinoma cells, then measured the survival rates of these cells, allowing them to determine each mutation’s effect on cell fitness.
Among their findings, they showed that some p53 mutations promoted cell growth more than had been previously thought. These mutations, which prevent the p53 protein from forming a tetramer — an assembly of four p53 proteins — had been studied before, using a technique that involves inserting artificial copies of a mutated p53 gene into a cell.
Those studies found that these mutations did not confer any survival advantage to cancer cells. However, when the MIT team introduced those same mutations using the new prime editing technique, they found that the mutation prevented the tetramer from forming, allowing the cells to survive. Based on the studies done using overexpression of artificial p53 DNA, those mutations would have been classified as benign, while the new work shows that under more natural circumstances, they are not.
“This is a case where you could only observe these variant-induced phenotypes if you're engineering the variants in their natural context and not with these more artificial systems,” Gould says. “This is just one example, but it speaks to a broader principle that we’re going to be able to access novel biology using these new genome-editing technologies.”
Because it is difficult to reactivate tumor suppressor genes, there are few drugs that target p53, but the researchers now plan to investigate mutations found in other cancer-linked genes, in hopes of discovering potential cancer therapies that could target those mutations. They also hope that the technique could one day enable personalized approaches to treating tumors.
“With the advent of sequencing technologies in the clinic, we'll be able to use this genetic information to tailor therapies for patients suffering from tumors that have a defined genetic makeup,” Sanchez-Rivera says. “This approach based on prime editing has the potential to change everything.”
The research was funded, in part, by the National Institute of General Medical Sciences, an MIT School of Science Fellowship in Cancer Research, a Howard Hughes Medical Institute Hanna Gray Fellowship, the V Foundation for Cancer Research, a National Cancer Institute Cancer Center Support Grant, the Ludwig Center at MIT, the Koch Institute Frontier Research Program via the Casey and Family Foundation Cancer Research Fund, Upstage Lung Cancer, and the Michael (1957) and Inara Erdei Cancer Research Fund, and the MIT Research Support Committee.
Share this news article on:
Related links.
- Francisco Sanchez-Rivera
- Koch Institute
- Department of Biology
Related Topics
- Broad Institute
Related Articles
Gene-editing technique could speed up study of cancer mutations

Fast modeling of cancer mutations
Previous item Next item
More MIT News

Making the clean energy transition work for everyone
Read full story →

3 Questions: What you need to know about audio deepfakes

Study finds lands used for grazing can worsen or help climate change

Envisioning a time when people age without fear of dementia

2024 MacVicar Faculty Fellows named

Study finds workers misjudge wage markets
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Genomic engineering articles from across Nature Portfolio
Genomic engineering is the synthetic assembly of complete chromosomal DNA that is more or less derived from natural genomic sequences. Genomic engineering is the top-down, global approach to synthetic biology; to be distinguished from bottom-up, local genetic circuit engineering.
Latest Research and Reviews
Synthetic reversed sequences reveal default genomic states
Introduction of a long synthetic DNA into yeast genomic loci results in high default transcriptional activity in yeast but low activity in mouse, suggesting distinct default levels of genomic activity in these organisms.
- Brendan R. Camellato
- Jef D. Boeke
Predicting transcriptional outcomes of novel multigene perturbations with GEARS
GEARS predicts transcriptional changes of multigene perturbations even for genes that were never experimentally perturbed.
- Yusuf Roohani
- Kexin Huang
- Jure Leskovec
CasTuner is a degron and CRISPR/Cas-based toolkit for analog tuning of endogenous gene expression
Understanding dosage-sensitive processes requires quantitative modulation of protein abundance. Here the authors report a CRISPR-based methodology for analog tuning of endogenous gene expression, CasTuner, and show homogeneous gene expression tuning across mouse and human cells.
- Gemma Noviello
- Rutger A. F. Gjaltema
- Edda G. Schulz
Heterologous production of the D-cycloserine intermediate O-acetyl-L-serine in a human type II pulmonary cell model
- Laurel Robbins
- Ariane Balaram
- William H. Conrad
Multimodal perturbation analyses of cyclin-dependent kinases reveal a network of synthetic lethalities associated with cell-cycle regulation and transcriptional regulation
- Brenton P. Munson
- Trey Ideker
A universal deep-learning model for zinc finger design enables transcription factor reprogramming
Zinc finger design is facilitated with a deep-learning model.
- David M. Ichikawa
- Osama Abdin
- Marcus B. Noyes
News and Comment
Machine learning predicts cellular response to genetic perturbation
GEARS, a machine learning model informed by biological knowledge of gene–gene relationships, effectively predicts transcriptional responses to multi-gene perturbations. GEARS can predict the effects of perturbing previously unperturbed genes and detects non-additive interactions, such as synergy, when predicting combinatorial perturbation outcomes. Thus, GEARS expands insights gained from perturbational screens.
Engineered yeast tune down gut inflammation
A yeast strain engineered to regulate levels of pathogenic metabolites in the gut provides protection against colitis in mouse models.
- Cathryn R. Nagler
The RASER’s edge
- Grant Miura

Genome editing for disease locus dissection
A new study uses haplotype editing to dissect the functions of a major genetic risk locus for cardiovascular disease.
- Darren J. Burgess
SCRaMbLEing to understand and exploit structural variation in genomes
- Jan Steensels
- Anton Gorkovskiy
- Kevin J. Verstrepen
MAGE in yeast is a go
- Susan Jones
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
DNA And Genetic Engineering Research Paper

This sample DNA and genetic engineering research paper features: 10100 words (approx. 33 pages), an outline, and a bibliography with 23 sources. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Introduction
An introduction to biotechnology and genetic engineering, early concepts of inheritance, gregor mendel: the father of genetics, hugo devries: the mutation theory of evolution, morgan and muller: the first genetic experiments, the discovery of the dna molecule, chromosomes: compact dna, dna structure: the double helix, dna replication, the rna molecule, transcription: dna to rna, translation: protein synthesis, dna sequencing, the human genome project: living in the postgenomic era, the origin of genetic engineering, modern genetic engineering, individualized medicine, dna consciousness, future directions.
- Bibliography
More Genetics Research Papers:
- Genetic Aspects Of Temperament Research Paper
- Genetic Aspects of Sexual Selection Research Paper
- Genetic Factors In Schizophrenia And Bipolar Disorder Research Paper
- Genetic Theories of Senescence Research Paper
- Risk Screening, Testing, And Diagnosis Research Paper
Anthropology has studied humankind in numerous capacities: morphologically, culturally, archaeologically, and philosophically. However, the knowledge gained by understanding the DNA molecule has increased our knowledge of humankind on a genetic and molecular level. In addition, with the completion of the Human Genome Project in 2003, the entire human genome has been sequenced and is now available for analysis. This is important to anthropologists because it allows the field to go beyond the bones and into the DNA.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code.
Genetic engineering may provide scientific ways to explore the chemical record provided by DNA. Anthropologists will be able to view and explore the past, the present, and conceivably the future of any species, including our own, by the scientific examination of DNA. In addition, understanding DNA and genetic engineering will potentially provide anthropologists with analytical data to explain our genetic relationship to other primates. This type of data will serve to strengthen and further clarify earlier DNA homology studies that have already provided empirical evidence of our close genetic relationships to chimpanzees and gorillas. In the future, this technology can be used to determine our genetic relationship to Neanderthals and Cro-Magnons.
The genetic study of Homo sapiens sapiens is possible because the DNA molecule provides a chemical record of humankind’s genetic makeup and evolutionary history as a process of time. This chemical record will allow examination because DNA is present in every cell in the body and is universal to all life-forms on this planet. All current conglomerations of DNA in all living species are a result of genetic variation and natural selection within populations throughout ongoing organic evolution.
The two terms biotechnology and genetic engineering are used somewhat synonymously. However, the two have different origins and initially they had slightly different applications. Biotechnology, by conventional definitions, is the intentional alteration of other living things (i.e., plants and animals) for the purpose of benefiting humankind. This has been done throughout the history of our species. In fact, the word clone is Greek for “twig,” because small sprouting twigs were removed from mature trees and planted in order to grow new trees.
Examples of early biotechnology include breeding animals that have desirable characteristics in order to increase the chances of producing offspring with those characteristics. It was noted even as far back as ancient times that if a fast male horse was bred with a fast female horse, most of the offspring would be fast.
Another example of early biotechnology would be the intentional pollination of specific crops that are more disease resistant and yield better fruition, while purposely not pollinating other crops lacking those desired characteristics.
Historically this method of biotechnology was limited to controlling what type of particular male specimen bred with a particular female specimen in an attempt to procure favorable genetic characteristics in the resulting offspring. In many ways, these practices were an early form of eugenics.
Genetic engineering is similar to biotechnology in that there is an alteration of an organism’s characteristics. In contrast to biotechnology, the process of genetic engineering denotes the intentional alteration of the actual DNA by using applications of new scientific technology that make changes at a molecular level. This means that a change is made in the actual genetic constitution of a cell by introducing, modifying, or eliminating specific genes by applying modern molecular-biologic techniques.
Another distinction is that biotechnology has traditionally been applied to agriculture for improving food products and livestock, whereas genetic engineering has more applications in medicine and anthropology. However, modern biotechnology has integrated genetic-engineering techniques as opposed to just utilizing breeding strategies to achieve those improvements. Due to the fact that biotechnology currently applies genetic-engineering techniques, the two terms are now frequently used interchangeably.
An alternative way to view the effects that biotechnology and genetic engineering could have on a modern population requires the natural manipulation of individuals through human intervention (using eugenics and euthenics or proliferagenics). The desired or beneficial genetic results can now be accelerated with genetic engineering.
From a historical perspective, humankind long ago began to alter the process of natural selection of animals and plants to yield beneficial results. Now, with the advent of genetic engineering, humankind has the ability to accelerate that process even more. In fact, one can speculate that humankind may eventually possess control over its own evolution.
The possibility that humankind may have direct control over its own evolution, by using genetic engineering and DNA nanotechnology, is known as emerging teleology. Emerging teleology is the theory that scientists can direct evolution by using genetic engineering and DNA nanotechnology—a technique that uses molecular recognition to create self-assembling branched DNA complexes, which in turn yields the engineering of functional systems at a molecular level. This concept of emerging teleology was first proposed by philosopher and anthropologist H. James Birx in 1991.
In conclusion, we have to ask ourselves, what is genetic engineering expected to accomplish for humankind? Or what has genetic engineering accomplished for humankind already? As mentioned earlier, understanding DNA can potentially help anthropologists to better understand the genetic relationships among species. Currently, several genetic-engineering techniques are already in use. Modern genetic-engineering applications include the use of genetically modified cells or microorganisms that can accomplish three major benefits:
- Cells or organisms can be engineered to producemedically beneficial substances. The most common example of this is the production of insulin. In 1987, the FDA approved the use of the first genetically engineered vaccine, which was used for Hepatitis B.
- Genetically modified organisms can be engineered thatwill help in the study of human diseases. An example of this is the use of “knockout mice,” which has helped scientists to understand diseases like cancer.
- Gene therapy allows the possibility of curing geneticallyinherited diseases by making corrections to the genetic defect at the level of the gene responsible. This can be achieved by inserting the correct gene(s) or by deleting the defective gene(s).
Although these three major benefits offer the potential to help millions of people—and already have—controversy will ultimately arise over the direct, nonmedical application of genetic engineering to enhance normal physiological functions in humans.
Genomics is the study of the genetic makeup of a species. A genome project of a species is a comprehensive identification and classification of a species’s genetic makeup. Genome projects of several microorganisms have been completed including many viral and bacterial genomes (e.g., Haemophilus influenza and Mycoplasma genitalium genomes were sequenced and completed in 1995). In addition, the Mouse Genome Project was completed in 1996 and the Human Genome Project was completed in 2003. Currently, other primate genome projects are underway, including the Chimpanzee Genome Project and the Neanderthal Genome Project.
In order to understand and conceptualize how understanding the DNA molecule and genetic engineering will impact many areas, including medicine and anthropology, one needs to first appreciate the history leading up to this marvelous technology. In addition, we need to stop and think about how the DNA molecule was discovered and what new technology enabled humankind to accomplish that important discovery. Finally, we need to be aware of the ideas that were proposed to be responsible for the phenomenon of inheritance before the discovery of the DNA molecule.
Before the DNA molecule was discovered, there were only ideas and theories about heredity and inheritance. The most enduring dogma was the idea of “pangenesis,” which held that all of the cells throughout the human body shed gemmules. These gemmules were believed to be able to collect in the reproductive organs periodically before fertilization and reproduction.
The term pangenesis came from the Greek word pan, meaning whole or encompassing, and genesis/genos, meaning birth/origin. Pangenesis was found in Greek writings in the 5th century BCE and was advocated (and in some ways espoused) by Hippocrates (460–370 BCE). This idea was accepted by fellow Greek thinkers Plato (428–347 BCE) and Aristotle (384–322 BCE). However, Aristotle later attempted to refute pangenesis with his idea of entelechy.
Aristotle proposed the concept of entelechy to explain the manner in which an organism inherits and expresses its traits, which according to this idea are determined by a “vital inner force.” He also noted the idea of “having one’s end within,” meaning that an organism’s essential potential can be actualized by its own vital inner force, or entelechy. Aristotle also believed that this vital force was possessed by males in their semen and that females merely possessed the raw material to be formed.
Pangenesis and entelechy were both prevalent and accepted as facts throughout the Middle Ages by great thinkers such as Albertus Magnus (1193–1280), his student Thomas Aquinas (1225–1274), and Roger Bacon (1220–1294). In the later part of the Middle Ages, a physician named Paracelsus (1493–1541), also known by the name Philippus Theophrastus Aureolus Bombastus von Hohenheim, proposed that semen was actually an extract of the human body, which contained all of the organs in what he called an “ideal form.” He believed that this was the biological link between parent and offspring. He was close.
Jean-Baptiste de Lamarck (1744–1829) proposed a theory that he called inheritance of acquired characters through use and disuse. In his theory, he proposed that changes in an organism’s physiology (over the course of its life span) were acquired and modified through the use of a particular function and then became a permanent and adaptable modification in what he called the germ-line. According to Lamarck, this modification was impressed on the parent form and then transmitted to the offspring, who would, as a result of this process, express this modification as a permanent characteristic that could be altered subsequently through use or disuse.
The acceptance of pangenesis and gemmules appeared as a provisional hypothesis by Charles Darwin (1809–1882) in his publication On the Origin of Species (1859), and later again in The Variation of Animals and Plants Under Domestication (1868). However, Darwin was unaware of the DNA molecule (which was not yet discovered) or of the works of Gregor Mendel (which were published during Darwin’s lifetime, and received but never read by him); therefore Darwin continued to comprehend his theory of evolution according to those concepts of his time— pangenesis and gemmules.
Thus, before any scientific explanation could account for the phenomenon of inheritance (or evolution), there were several unfounded ideas that were accepted. These ideas were mainly pangenesis, gemmules, and entelechy. Later theories such as the use and disuse of acquired characteristics were proposed and gained some popularity, but no theoretical model existed that could scientifically or mathematically account for how characteristics were inherited.
Gregor Johann Mendel (1822–1884) was a monk and a mathematician, and known as “the father of genetics” because his seminal works inspired others to study the phenomenon of inheritance. In 1857, he began conducting experiments using pea plants, Pisum sativum. He bred particular plants together and then he meticulously recorded the characteristics of the resulting offspring.
Mendel’s term character was a description of what we now call a phenotype. Typical characters that Mendel studied and measured were the height of the plants and the color of the pea plant’s flowers. Each character possessed different traits; for example, height was measured as tall, normal, or short; and color was measured as white, pink, or red. Therefore, traits were different varieties of phenotype (i.e., the measurable or observable characteristics of the plants).
Mendel’s experiments demonstrated that the traits of a character were distributed in a mathematically predictable pattern. He used these mathematically predictable patterns to devise two important laws known as Mendel’s laws, which he called the law of segregation and the law of independent assortment.
In general, the first law, which was the Mendelian law of segregation (of genetic factors), was hypothesized by Mendel to mean that each trait (e.g., height or color) must have two “factors.” Mendel would later call these factors “alleles.” One factor, or allele, was inherited from each parent, one from the mother and one from the father. Although two alleles are inherited, only one of the alleles was expressed, and therefore, according to Mendel, they were segregated. Today it is known that gametes are sperm and egg cells, which combine their genetic material during fertilization.
Mendel did not know the underlying biological process in cell replication and division at that time. However, it is now known that during the cell cycle, the DNA replicates itself and divides, yielding two identical cells, each with two sets of chromosomes, known as diploids. This process is known as mitosis. In addition, a specialized version of mitosis takes place with the production of the gametes, in which the gametes, known as haploids, have one set of chromosomes each. This process is known as meiosis. When the two separate gametes (or haploids) are joined during fertilization—one from the mother and one from the father—to form a zygote, the alleles (i.e., the DNA) then recombine.
During the course of his experiments, Mendel found that each allele was either dominant or recessive for a specific trait. He elaborated that there were three possibilities. First, if the two alleles were both dominant, then the trait inherited was considered to be homozygous dominant (AA). Second, if the two alleles were both recessive, then the inherited trait was considered to be homozygous recessive (aa). Third, if the two alleles were different, one dominant and one recessive, then the inherited trait was considered to be heterozygous (Aa), or a hybrid. The homozygous dominant, homozygous recessive, and heterozygous combinations could be crossbred and those results could be used to mathematically predict the probability of what type of offspring would result.
The Punnett square was devised by British geneticist Reginald Punnett (1875–1967), who published the first textbook on genetics, Mendelism (1905). He used these Punnett squares to predict the mathematical probability of the outcome of a particular breeding experiment. The results of the Punnett square could be used to predict the probability of possible genotypes of the offspring in a particular cohort given the genotype of the maternal allele and the makeup of the paternal allele.
Mendel’s second law, the Mendelian law of independent assortment (of genetic factors), is where he hypothesized that the inheritance pattern of one trait does not affect the inheritance pattern of another trait (i.e., they assort independently). He justified this with his concept that alleles segregate during gamete formation and then recombine independently of one another. He was incorrect in this assumption. It is now known that there is a multigene interaction and what is known as the blending of inherited traits. This was proven in the early 1900s by Thomas Hunt Morgan (1915/1978) and his colleagues in experiments involving fruit flies.
In essence, Mendel’s second law worked with pea plants because they are much simpler organisms, genetically, than mammals. In addition, the characteristics that he was measuring were not complicated. However, Mendel himself speculated that these laws may only apply to certain species, but he didn’t know why, because the DNA molecule had not been discovered yet. This is the reason why Mendel and others at his time could only study what was being expressed genetically. They did not understand or appreciate the genetic material itself.
In Example 1, a trait that is homozygous dominant (YY) is crossed with a trait that is homozygous recessive (yy). This example yields 100% heterozygous/ hybrid offspring (Yy). In Example 2, two hybrid traits are crossed. This example yields 50% heterozygous offspring, 25% homozygous dominant offspring, and 25% homozygous recessive offspring. This is a classical and simplified example of Punnett squares.
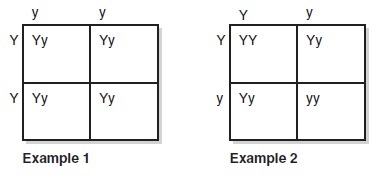
In addition to the two laws that Mendel devised, there are three other elements that made his work significant. First, he demonstrated the value of conducting controlled experiments. Second, he was a mathematician and applied mathematics to analyze and interpret his data. Third, he published his results, which is probably the most significant of all because his findings were not widely acknowledged during his time. However, his works were rediscovered after his death and had a profound effect on the study of inheritance and genetics. His work was of particular significance because this was the first successful mathematical model that had been proposed to explain inheritance.
Hugo Marie DeVries (1848–1935) was a Dutch botanist and is considered to be one of the first geneticists. He is known for his mutation theory of evolution, which was chiefly influenced by Gregor Mendel’s laws of heredity, which he rediscovered in the 1890s, and Charles Darwin’s theory of evolution.
DeVries’s (1905/2007) mutation theory of evolution speculated that new varieties of a species could appear in sudden, single jumps as opposed to slowly changing over time. His theory proposed that differences in an organism’s phenotype could change rapidly from one generation to the next; this also became known as saltationism. He based this theory on his experiments, which involved hybridizing plants. One particular observation was made by DeVries during these experiments that influenced and compelled his mutation theory of evolution. Occasionally an offspring appeared that had different characteristics than both the parents and was also different from the other offspring. Based on this finding, he postulated that new varieties of species could appear in nature spontaneously. By this, he in essence proposed that a mutated gene could equal a new species (i.e., mutation equals speciation). This was opposed to Darwin’s theory of gradualism.
DeVries’s mutation theory of evolution was supplanted in the late 1930s by modern evolutionary synthesis, initiated by Julian Huxley (1887–1975). Huxley first introduced this theory in his book Evolution: The Modern Synthesis (1942). At this time, he attempted to rationalize a unification of several biological specialties (e.g., genetics, systematics, morphology, cytology, botany, paleontology, and ecology) in order to postulate a more rational account of evolution. Julian Huxley’s work was stimulated by population genetics and served to clear up confusion and miscommunication between specialties existing at that time. In addition, modern evolutionary synthesis defended the notion that Mendelian genetics was more consistent with Darwin’s gradualism (and natural selection), as opposed to DeVries’s hypothesis of the mutation theory.
The mutation theory of evolution proposed by DeVries had nothing to do with what we currently acknowledge as genetic mutations. The current definition of a mutation is the process by which a gene undergoes a structural change to create a different form of the original allele, which results in a completely new allele. Therefore, spontaneous changes can occur in the DNA that can (but sometimes do not) cause changes in an organism’s physiology. This change does not give rise to the sudden appearance of a new species; rather it can produce a modification of the erstwhile species. This was later supported by genetic research done on white-eyed and red-eyed fruit flies by Thomas Morgan and colleagues (1915/1978).
DeVries was known for another accomplishment that arose from his experiments when he speculated that the inheritance of specific traits of an organism occured through a transfer of particles, which he termed pangenes (derived from the word pangenesis). The term pangenes was shortened 20 years later by Wilhelm Johannsen (1857–1927) to genes. The term gene is currently defined as a basic unit of inheritance.
There was some debate that surrounded the “rediscovery” of Mendel’s work. In DeVries’s publication on the topic of inheritance, he mentioned Mendel in a footnote but took credit for the concept of particles of inheritance with his idea of pangenes. It was Carl Erich Correns (1864–1933), a German botanist and geneticist, who pointed out Mendel’s priority, which DeVries eventually publicly acknowledged.
As it turned out, Carl Corren was a student of Karl Wilhelm von Nageli (1817–1891), a famous Swiss botanist, who had corresponded with Mendel regarding his findings years earlier. Corren was familiar with Mendel’s work as a result of this association. An even stranger twist to this was that when Nageli and Mendel were collaborating, Nageli had actually discouraged Mendel from doing any future work studying genetics, for what he considered religious and ethical reasons.
Thomas Morgan (1866–1945) was a geneticist who performed experiments on fruit flies ( Drosophila melanogaster ). He chose to conduct experiments on fruit flies because they required few resources, reproduced quickly, had observable characteristics that could be measured, and had only four chromosomes, which made them ideal for genetic research.
As a result of his experiments in the “fruit-fly lab,” Morgan established that genes were arranged in a line on what is known as a chromosome, which is present in every living cell. Since genes were believed to be responsible for inheritance and were now shown to exist on chromosomes, this became known as the chromosomal theory of inheritance, which had been alluded to prior to Morgan but had not been supported scientifically. He also noted that there was recombination of inherited characteristics resulting from the exchange of genes between two chromosomes of a pair, which he called “crossing over.” This of course disproved Mendel’s second law of independent assortment.
Morgan collaborated with three of his very important students: Hermann Muller, Alfred Sturtevant, and Calvin Bridges, all of whom continued performing genetic research on fruit flies. Collectively, from around 1908 to 1914, they were able to establish that chromosomes carry genes, those genes are distinct physical objects that are arranged on the chromosomes, the genes also could change place on the chromosomes, the genes could be mutated, and those mutated genes could be reliably inherited in future generations.
Morgan’s experimental proof that genes were discrete physical objects carried on chromosomes and they govern the patterns of inheritance was of major significance. Prior to this, the gene was a speculation with no scientific evidence to support it. Morgan’s research also illustrated that the sex of a species was inherited just as all other characteristics are inherited. He became aware that it was the chromosomal differences between the sperm and egg cells that correlated with the determination of gender. This was proven by his famous experiments with white-eyed male fruit flies and red-eyed female fruit flies.
A significant discovery, made by Hermann Muller (1890– 1967), was that mutations could be caused by exposure to high-energy radiation. This technique enabled them to perform those significant genetic experiments, and to give validity to the chromosomal theory of heredity. Hermann Muller received a Noble Prize for physiology and medicine for his discovery that X-rays induced mutations. He was also the first to visualize genes as the origin of life. The reason he believed this was because genes (or chromosomes) can replicate themselves. He further speculated that all of natural selection and evolution acted at the level of the gene.
Prior to Morgan and Muller, the first proof that chromosomes carried hereditary material came from American physician and geneticist Walter Sutton (1877–1916), based on his research on grasshopper cells. Sutton was the first to speculate that the Mendelian laws could be applied to the chromosomes at a cellular level, which is now known as the Boveri-Sutton chromosome theory. However, it was Morgan’s genetic research that provided enough reproducible scientific data to support the chromosomal theory of heredity, which became generally accepted by around 1915 (even though some geneticists, such as William Bateson, continued questioning it until about 1921).
In the early 1920s, it was generally accepted that genes were arranged on chromosomes and that this is how the inheritance of characteristics arose. However, no one was sure what chromosomes were chemically made of or how they worked.
In 1928, a British scientist named Frederick Griffith (1871–1941), who was influenced by Mendel’s hypothesis of units of inheritance, theorized that a molecule of inheritance must exist. He began conducting experiments on Streptococcus pneumonia and proposed that an inheritance molecule existed and could be passed on from one bacterium to another by a process called transformation. Griffith’s research on transformation proved how an inheritance molecule could be transferred from one bacterium to another; however, Griffith never discovered what the inheritance molecule was. Nevertheless, his work in turn inspired others to continue looking.
During this time, it was known that genes were arranged on chromosomes responsible for the phenomena of inheritance, but no one was able to prove their makeup. This dispute narrowed down to proteins, lipids, carbohydrates, and nucleic acids. The popular belief was that the inheritance molecule was protein because there were more proteins in existence, whereas only four nucleic acids were known (later a fifth nucleotide would be discovered in RNA). Some postulated that it was proteins and nucleic acids that made up the inheritance molecule, but there was no scientific proof to support any of these arguments.
Friedrich Miescher (1844–1895) discovered nucleic acids in 1868, while studying white blood cells. He called them nuclein because they were located in the nucleus, but no proof existed to support the fact that nucleic acids were responsible for the inheritance of characteristics.
In the early 1940s, a scientist named Oswald Avery (1877–1955) rediscovered Griffith’s work on transformation. Avery had the advantage of newer technology and advances in cellular biology. Avery had begun to conduct experiments that selectively destroyed different components (carbohydrates, proteins, lipids, and deoxyribonucleic acids) of a virulent bacterium, which he injected into a mouse. If the mouse died, he concluded that the bacterium had maintained its virulence (i.e., it was able to replicate its virulence). During his experiments, he found the bacteria were able to maintain their virulence when the carbohydrates, proteins, or lipids were destroyed. However, the bacteria were unable to be virulent when their deoxyribonucleic acids were destroyed. Therefore, Avery was the first scientist to prove that the genetic material responsible for inheritance was composed of nucleic acids.
Avery’s findings were very significant because they proved that genes, which are made out of nucleic acids (i.e., deoxyribonucleic acids or DNA), are responsible for the genetic inheritance of all organisms’ characteristics. However, at this time, no one knew what DNA’s structure was or how it functioned.
In 1952, Erwin Chargaff (1905–2002) published results based on his experiments involving the isolation of nucleic acids from three microorganisms: Serratia marcescens, Bacillus schatz, and Hemophilus influenza type C. He was able to separate the nucleic acids using a technique called adsorption chromatography. He discovered that DNA was composed of two purines, adenine (A) and guanine (G), and two pyrimidines, thymine (T) and cytosine (C).
In addition, Chargaff also pointed out that in any section of DNA, the number of A residues was always equal the number of T residues and that the number of C residues were always equal to the number of G residues. This became know as Chargaff’s rule. Later, Watson and Crick (1953) would correctly propose that A and T actually pair together and that G and C pair together (due to hydrogen bonding), which is known as Watson-Crick base pairing. By analyzing the chemical structures of these molecules, Watson and Crick pointed out that A and T both have two hydrogen bonds available, which is why they pair together. C and G have three hydrogen bonds available, which is the reason they pair together. Therefore, Watson and Crick were able to find the molecular explanation of Chargaff’s rule (A=T and C=G), but they went a step further with Watson-Crick base pairing to explain why this is true.
Shortly after Chargaff was making his discovery, another significant discovery was being made by scientists Maurice Wilkins (1916–2004) and Rosalind Franklin (1920–1958). Their research illustrated that the DNA molecule had a helical shape and was made up of two strands that were connected by ladderlike rungs. They were able to prove this by studying crystallized X-ray patterns of DNA.
On April 25, 1953, Watson and Crick published an article proposing a molecular structure of DNA. They had incorporated the findings of Chargaff, Wilkins, and Franklin, as well as their own, to propose that the helical strands were the sugar-phosphate backbone and that the ladderlike rungs were pairs of nucleotides (A=T and G=C). Therefore, Watson and Crick established that the molecular structure of DNA was in fact a double helix. This was significant because they were then able to explore and propose a model to explain how DNA worked.
It should be pointed out that the structure of DNA was discovered based on the research and results of many scientists. Watson and Crick definitely made this significant discovery, but they gained much insight from the works of Chargaff, Wilkins, and Franklin.
A chromosome is a long, single piece of DNA that contains several genes; in some species 10 to 40 genes and in other species thousands or more genes can be present in just one chromosome. In eukaryotes, the chromosomes are organized structures that consist of DNA and special structural proteins called histones that wind, coil, and compact large DNA sequences so that they fit efficiently in the nucleus. The chromosome does not always stay condensed, but periodically relaxes and uncoils for replication and for the transcription of proteins. Topoisomerase II is a DNA-nicking-closing enzyme that allows the uncoiling of the DNA supercoils during DNA replication and translation.
In prokaryotic cells, the DNA is either organized in clusters with no nucleus or into small circular DNA molecules called plasmids, which do not contain histones. In viral genomes, very simple chromosomes are found and can be made out of DNA or RNA, which are short, linear or circular chromosomes that usually lack structural proteins.
In all animals, DNA in the chromosomes is packed by histones into globular aggregates known as a nucleosomes. The amino-acid sequence of histones shows almost 100% homology across all species, which illustrates their importance in maintaining chromosomal integrity, structure, and function. In addition, it is now known that the genes that code for histones have no introns.
The DNA strand is wound up and packed with eight histones to form a nucleosome, or what is sometimes called “beaded strings.” These units are further coiled into what is called a solenoid, which contains five to six nucleosomes. The solenoids are then condensed into a chromatin fiber, which has histone H1 holding the core together. The chromatin fiber then folds into a series of loops that are held together by a central scaffold (made of nonhistone chromosomal protein), and this configuration is called a looped fiber. The looped fiber is then coiled to form the fully condensed heterochromatin of the chromosomes.
The coiled and condensed heterochromatin pairs up with an identical copy of itself, and each of the two are referred to as a chromatid. Two identical chromatids are attached to each other by a centromere. The centromere divides both chromatids into a long arm and a short arm. During cell division, microtubules attach to the centromere and align the chromosomes in the center of the dividing cell. The chromosomes are then split, yielding two identical cells—each with its own set of chromosomes. This process is known as mitosis.
All four arms of the chromosome (two long arms and two short arms) have a specialized cap known as a telomere, which has several functions (e.g., preventing the ends from sticking together). The chromosomes also show a distribution of two types of bands. G-bands are A-T rich regions and R-bands are G-C rich regions.
As mentioned earlier, the DNA molecule is composed of two purines (A and G) and two pyrimidines (T and C), and all four are nitrogenous bases. These nitrogenous bases are attached to a deoxyribose sugar, which is attached to a phosphate group to form a nucleotide.
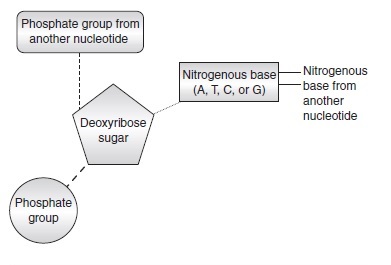
In DNA, a nucleotide is any of the four nitrogenous bases attached to a deoxyribose sugar, which, as explained, is attached to a phosphate group. Because there are four different nitrogenous bases (A, T, G, and C) in DNA, there are four different nucleotides. The deoxyribose sugar can bond with a phosphate group from another nucleotide to form a chain. The nitrogenous base portion of the nucleotide can bond with the nitrogenous base from another nucleotide.
Nucleotides attach side by side to make long strands of DNA. They are able to attach in this fashion by the phosphate group of one nucleotide to the deoxyribose sugar of another nucleotide. This strand is formed in what is known as the 5 prime to 3 prime direction and opposite of this strand is a complementary chain which goes in the 3 prime to 5 prime direction. The original strand is attached to the complementary strand by the hydrogen bond discussed earlier: A=T and C=G. Therefore if the original strand is
5 prime-ATGCTC-3 prime,
the complementary stand is
3 prime-TACGAG-5 prime.
When Watson and Crick proposed the structure of the DNA molecule, they stated that the molecule was a double helix held together by ladderlike projections. The backbone of the helix is the deoxyribose sugar and phosphate group. The ladderlike projections are the base pairs A=T, G=C, T=A, and C=G.
One of the phenomenal characteristics of the DNA molecule is that it not only stores genetic information but replicates itself. This process is simply known as replication. Replication starts when the double strand is opened up by a helicase enzyme, which exposes the base sequences. While the base pairs are exposed along the template strand, a new strand of DNA (a complementary strand) is synthesized by DNA polymerase.
Replication occurs continuously from the origin of one strand, called the leading strand, which follows the 3 prime to 5 prime direction. The other strand on the DNA molecule replicated is called the lagging strand and does not replicate continuously, but rather in small sections (~100– 200 bases), which are called Okazaki fragments. These fragments are linked together by DNA ligase. The leading strand replicates a complementary strand with DNA polymerase delta, while the lagging strand makes a complementary strand using DNA polymerase alpha.
The process of replication results in two identical copies (called daughter duplexes) of the original strand of DNA. Each daughter duplex contains one parental strand from the original DNA molecule and one newly synthesized strand. This is known as a semiconservative model. In 1958, Matthew Meselson and Franklin Stahl used a scientific technique with radio-labeled nitrogen bases to prove that the DNA molecule replicates using a semiconservative model.
In healthy cells, there is a set of postreplication-repair enzymes and base-mismatch proofreading systems. These systems remove and replace mistakes made during replication (e.g., a wrong base being inserted into a growing strand). Occasionally, a change in the nucleotide sequence takes place; this is known as a mutation.
In 1977, two scientists, Richard Roberts and Phil Sharp, discovered that there were many regions in the DNA that did not code for anything. Roberts and Sharp called these noncoding interruptions introns (short for intervening sequences ), and the sections that are coding regions are referred to as exons. They also found that mRNA, which was thought to be an exact copy of a transcribed section of DNA during protein synthesis, was actually missing these intron regions.
Introns are believed by some to be “junk DNA” or filler sequences. However, others believe that the extra sequences may stabilize the DNA molecule, or that the introns may be genetic remnants of evolution (vestigial DNA) and may have been expressed in the past but now lies dormant. In addition, it is conceivable that introns may have a function that presently eludes us.
RNA (ribonucleic acid) is a small, single-stranded nucleic acid that is involved in protein synthesis. Single-stranded RNA (and in rare cases double-stranded RNA) are also found in viruses. Besides being single stranded, RNA differs from DNA in two other important ways. First, DNA contains deoxyribose sugar in its backbone, whereas RNA contains ribose sugar. Secondly, in RNA there is no thymine; rather it is replaced with a different nitrogenous base called uracil, which pairs with A on the DNA molecule during protein synthesis (U=A), just as thymine does.
In the early 1980s, Thomas Cech did a significant amount of research on RNA. At that time it was believed that only proteins could act as biological catalysts. Cech was able to prove that RNA could function as a biological catalyst as well. He even discovered what he called ribozymes (later classified as species of RNA), which take part in the synthesis of mRNA, tRNA, and rRNA. Currently nine types of RNA have been identified:
- Heterogeneous nuclear RNA (hnRNA) is transcribed directly from DNA by an enzyme called RNA polymerase. This form of RNA contains all the coding regions (exons) and noncoding regions (introns). Then, hnRNA is processed to yield mRNA for protein synthesis.
- Messenger RNA (mRNA) is the modified version of hnRNA, which has had all of the introns removed, and possesses only the coding regions, which contain a code (the triplet code or codons) that is used for transcribing proteins.
- Transfer RNA (tRNA) is transcribed from coding sequences on the DNA molecule by RNA polymerase III. This type of RNA possesses an anticodon on one of its ends, which matches a particular section of mRNA. On the other end, tRNA has an amino acid attached. In a basic sense, tRNA serves as an adaptor between mRNA and amino acids during protein synthesis.
- Ribosomal RNA (rRNA) exists as several species of rRNA, which are categorized by their sedimentation coefficients that are recorded in Svedberg units (S). A ribosome is composed of two subunits: a large subunit (5S, 5.8S, and 28S) and a small subunit (18S). The ribosome’s function involves holding mRNA in place while the corresponding tRNA attaches amino acids together during protein synthesis.
- Small nuclear RNA (snRNA) is found in RNA-protein complexes called spliceosomes. Their function is to remove introns from hnRNA to produce mRNA. In the disease systemic lupus erythematus, the body produces antibodies to snRNA molecules.
- Small nucleolar RNA (snoRNA) functions in site-specific base modifications in rRNA and snRNA. These modifications include methylation and pseudouridylation.
- Signal recognition particle RNA functions by recognizing particular signal sequences on some proteins and assists in transporting them outside the cell, a process known as exocytosis.
- Micro-RNA (miRNA) is believed to control the
- translation of structural genes. They are proposed to do this by binding to the complementary sequences in the 3 prime untranslated regions of the mRNA.
- Mitochondrial RNA replicates and transcribes independently of the other nuclear DNA and RNA. However, mitochondrial DNA exists as a double-stranded loop (or circle) with an outer, heavy strand and an inner, light strand. Both strands are transcribed by mitochondrial-specific RNA polymerase to produce 37 separate mitochondrial RNA species (mitochondrial rRNA, mitochondrial tRNA, and mitochondrial mRNA).
Many scientists believe that RNA existed before DNA. This is mostly because small forms of RNA can support life (e.g., a virus). However, a virus needs to infect other cells to replicate itself and this is why viruses are known as “obligate intracellular parasites.” Small species of RNA (explained above) are also able to perform biologic activities independently (e.g., they are responsible for protein synthesis from strands of DNA). However, the DNA molecule is a far more stable repository for genetic information and it produces RNA. Therefore, the question of how RNA can exist without DNA to produce it arises. Finally, there is the possibility that both molecules arose at the same time, forming a symbiotic relationship.
The question now arises, how does DNA make RNA? Transcription is the process by which DNA makes a copy of a section of itself; that copy is RNA and is used for protein synthesis. In the DNA molecule, there are genes known as structural genes that code for proteins. Transcription begins when protein transcription factors attach to a promoter site on the DNA molecule. Next, RNA polymerase Pol II attaches to the transcription factors and then “unzips” the double helix. The complex of transcription factors and RNA polymerase Pol II move downstream (3 prime to 5 prime) along the template strand of the DNA, unzipping it as it moves forward and reconnecting the back portion of the double helix, and forming what is called a transcription bubble. During this process, nucleotides are linked together to form a complementary RNA copy of the coding strand of DNA. This is an exact copy of the DNA called hnRNA.
The hnRNA molecule is modified into mRNA, through ribonucleoprotein complexes called spliceosomes, which are several snRNA molecules that remove introns from hnRNA. The new mRNA molecule is then transported from the nucleus to the cytoplasm, where it will be used to make a peptide or peptides, which are used to make proteins and enzymes.
Translation is the process in which a strand of mRNA in conjunction with tRNA and rRNA produces peptides and polypeptides. The old central dogma of molecular biology was that DNA makes mRNA, and mRNA makes proteins.
The current theme is that DNA makes hnRNA, and that becomes mRNA (with the help of snRNA), which works with tRNA and rRNA to make polypeptides that are used to make proteins.
On a strand of mRNA, nucleotides pair up in sets of three (e.g., AUG and AAA, which are called triplet codons or codons). Each codon corresponds to an amino acid (e.g., GCA = alanine). There are 64 possible combinations of codons, but several codons represent the same amino acid (e.g., GCA, GCC, GCU, and GCG all represent alanine). Three of the codons—UAA, UAG, and UGA— represent a “stop” signal on the mRNA. AUG represents methionine, which is a “start” signal. The codons make up what is known as the genetic code. It works on the basis of tRNA, which contains and anticodon on one end and an amino acid on the other end.
A strand of mRNA is composed of a start signal (AUG), a coding region of codons, and a stop signal. Translation takes place in the cytoplasm within the endoplasmic reticulum. This process takes place in three main steps:
- Initiation: During this phase, the small rRNA subunit (which contains initiation factors) and tRNA, with the methionine (MET) amino acid, both attach to the start signal (AUG) on the mRNA. Then the large rRNA subunit attaches to the mRNA. When the small and large subunits are attached together they are referred to as a ribosome.
- Elongation: After initiation is complete and the first tRNA is attached to the strand of mRNA, a second tRNA attaches to the mRNA on the next codon. The second tRNA will correspond to that codon (e.g., the mRNA codon ACG would have tRNA with the anticodon [UGC] and the amino acid threonine [THR] attached). The existing MET amino acid on the mRNA would then form a bond to THR. This bond is a peptidyl transferase reaction, which creates a peptide bond between MET and THR. After that, a third, fourth (and possibly more) amino acids will be connected in this fashion, yielding an elongated chain of amino acids. This process continues throughout the entire coding message of the mRNA molecule.
- Termination: In this final phase, elongation continues until a stop codon is reached and enters into the ribosome (rRNA large and small subunits). When this takes place, a release factor disconnects the amino acid chain (called a peptide or polypeptide), and the ribosome splits into a small and large subunit, both of which separate from the mRNA molecule.
After translation is completed, posttranslational modification occurs, which involves the removal of methionine and peptide cleavage.
DNA sequencing is a scientific method for determining the order of the nucleotide bases in an unknown strand of DNA. The original method was devised in the early 1970s by Walter Gilbert and Allan Maxam, and called MaxamGilbert sequencing. Their method was very labor-intensive and involved the use of hazardous chemicals. In 1975, Frederick Sanger developed a quicker, more reliable, and less hazardous method of DNA sequencing called the Sanger method or chain-termination method. This method involves the use of dideoxynucleotides (ddATP, ddTTP, ddCTP, and ddGTP), which are different from normal nucleotides in that they lack a hydroxyl group. Because they lack a hydroxyl group, they interrupt and stop the normal sequence being produced in the complementary strand from the template DNA, which causes a termination in the chain. This termination takes place at that dideoxynucleotide’s spot (A, T, C, or G). This method is sometimes called the dideoxynucleotide DNA sequencing method, or chaintermination sequencing.
Utilizing this technology, a strand of unknown DNA can be taken, amplified using PCR (PCR is a process that rapidly replicates a piece of DNA), and sequenced. The single-stranded DNA of unknown sequence is used as the template and a complementary strand is made using radioactively labeled nucleotides. Next, the radioactively labeled complementary strand is placed in four separated mixes, each containing DNA polymerase and one of each of the four dideoxynucleotides. The four separate mixes are then run through a polyacrylamide of gel electrophoresis in four separate rows, which separates the small fragments of DNA. These four rows of fragments correspond to the particular dideoxynucleotide used. From this, a deduced sequence of the original template strand can be made. Currently a method using automated sequencing, which uses fluorescent markers instead of radioactively labeled markers, is used.
The groundbreaking technology of PCR and DNA sequencing made sequencing a genome a reality. Without this technology, the Human Genome Project would have taken several decades to complete or may have even been unattainable. DNA sequencing also has applications in diagnostic testing and forensics. It can also be used to identify a specific pathogenic mutation that causes a particular genetic disease.
The Human Genome Project (HGP) was completed in 2003. It had originated as an international project initiated in 1990 by the U.S. Department of Energy and the National Institute of Health. This project had six major goals:
- To identify all 20,000 to 25,000 genes in the human genome.
- To determine all of the sequences of the chemical basepairs that constitute the entire human genome. The approximate number of chemical pairs is estimated at around 3 billion. It is known that there is a large amount of repetition of these base pairs, and therefore an exact number of chemical base pairs at this time can only be estimated.
- To store all of this information and make it available in databases.
- To make improvements on computer-based tools for data analysis of biological problems. The field that currently deals with these issues is called bioinformatics.
- To transfer these related technologies to private biotechnology and genetic engineering sectors to stimulate further research and product development.
- To address the legal, social, and ethical issues that will appear as a result of the completion of the HGP and also the application of genetic engineering.
The completion of the HGP is significant for the field of anthropology because it will improve the study of topics such as germ-line mutations and assist in determining our genetic relationship with Cro-Magnons and Neanderthals, as well as establish the relationship between those two species. The Neanderthal Genome Project was launched in 2006 and upon its completion the Cro-Magnon Genome Project is likely to ensue.
With the completion of the HGP, there are many anticipated improvements in anthropology, medicine, energy, and the environment. However, many ethical and legal concerns will arise as well.
The first experiments involving genetic-engineering techniques were made possible by seminal works of three individuals: Paul Berg, Stanely Cohen, and Herbert Boyer. All three scientists were separately working on research and experiments involving DNA. Eventually, they collaborated, using all of their techniques to coordinate the very first experiments involving removal of a gene from one species and inserting it into the genome of another species.
During the years 1972 to 1973, Paul Berg at Stanford University was the first scientist to complete a successful gene splicing experiment. This research involved the removal of a gene from a viral genome called Simian Virus 40 (SV40), which was a monkey virus. He was initially interested in studying a particular gene because he found that SV40 could cause cancer in mice. The advantage of studying a viral genome was that it was very small— approximately a few hundred genes. This allowed him to easily identify and isolate this gene. He then attached this gene to the DNA of a lambda virus (known as a biological vector), which would then insert this gene into another cell. This was the very first time that a gene or genetic material from one organism, in this case a virus, was removed and spliced into the DNA of another organism, in this case a second virus. The new DNA, which had its original DNA along with the spliced DNA, would continue to function as normal and is known as recombinant DNA.
Recombinant DNA is the artificial or synthetic production of DNA, engineered by combining one or more strands of DNA from one source and attaching it to the strand of DNA of another source. This process yields a novel strand of DNA, known as recombinant DNA, that would normally not have existed. The recombinant DNA can then function normally, replicating itself and producing its sequenced products as all other DNA normally does.
Stanely Cohen was another scientist at Stanford University. His initial research was investigating how the genes in plasmids could make bacteria develop resistance to antibiotics. He implemented techniques allowing him to remove a plasmid, which was a small ring of DNA, from one bacterium and insert it into another bacterium. Once the plasmid was inside the other bacterium it could then produce the products that it normally made in the original bacterium. This process happens naturally between bacteria and was originally observed by Fredrick Griffith, who called it called transformation. However, Cohen was able to intentionally and selectively make this process take place.
Herbert Boyer, a scientist at the University of California, was doing research on a bacterium called Escherichia coli (or E. coli ), which is normally found in the human intestine. His research involved the use of restriction endonucleases (RE), which were originally discovered by Werner Arber, Daniel Nathans, and Hamilton Smith (they received a Nobel Prize in 1978 for isolating RE). It was discovered that bacteria produce RE to defend themselves against viruses, which work by snipping viral DNA into smaller pieces rendering the virus ineffective. Today there are over 800 RE that are used in laboratories for gene splicing and the production of recombinant DNA. The RE attach to very specific sites on the DNA and can be used to isolate and remove specific sections of DNA. After this technique was refined, Boyer later went on to genetically engineer human insulin, which was the first genetically engineered product approved by the FDA in 1978.
In 1973, the first animal gene was cloned, using the techniques refined by Berg, Cohen, and Boyer. Using Boyer’s RE, Cohen’s technique for removing plasmids, and Berg’s splicing techniques, they were then able to successfully remove and fuse a segment of DNA, which contained a gene from a frog ( Xenopus ) with the DNA of the bacterium E. coli.
In a basic sense, the frog gene was removed using RE, then spliced into a plasmid, and then inserted into E. coli. After the resulting DNA was inserted into E. coli, the frog gene was transcribed, producing a specific frog protein that was not previously produced by E. coli. This was the very first time that an animal’s gene was removed, inserted into a bacterial genome, and the product of that animal gene successfully produced.
The transfer of DNA from one organism into another organism is possible because DNA is universal among all organisms and cells on this planet. This means that the DNA in a bacterial cell is made up of the same components as the DNA in a human cell. The organism ( E. coli ) that successfully receives DNA from another organism (the frog gene) is known as a transgenic organism.
The fundamental steps in genetic engineering include the isolation of the DNA, the amplification of the DNA, and the transfer of that DNA into another cell. It is a very complicated process, but a simplification has been made here in order to establish an understanding of the process.
The DNA section of interest is called donor DNA and needs to be isolated from the rest of the DNA. This is done using restriction enzymes, which cut up the DNA into fragments. The restriction enzymes are very specific and cut the DNA at very specific points. Therefore, the DNA of interest can be located and removed.
After the desired section of DNA is isolated, it then needs to be amplified because the amount originally acquired is usually not enough to be effectively transferred. The donor DNA is amplified by a process called the polymerase chain reaction (PCR), invented by Kary Mullis. PCR is a process that rapidly replicates a piece of DNA by using Taq DNA polymerase.
Finally, the isolated and amplified DNA needs to be introduced into the host cell. This is accomplished with biological vectors and nonbiological vectors. Biological vectors are either plasmids or viruses, which were used in the original genetic engineering experiments by Berg and colleagues. Nonbiological vectors include electrochemical poration, biolistics, microinjections, and recombinasemediated cassette exchange (RMCE).
As mentioned, the DNA in all organisms and cells is made out of the same material (nucleotides and sugar phosphates). This is why it is possible to transfer DNA from one organism’s cells into another organism’s cells and this is also why DNA is able to produce its products normally within the new cell after this process is complete.
There are two types of genetic modifications; one involves the addition of genetic material and the other involves the deletion of genetic material or the products it expresses. Deletion is done in one of two ways: knockout and antisense genes.
Gene therapy is classified in one of two ways:
- Germ-line gene therapy: This is a genetic modification done on the sperm or the egg (germ cells), which are known as a haploids because they only contain one set of chromosomes, whereas all other cells in the body (somatic cells) contain two identical sets of chromosomes (two chromatids connected by a centromere). When this type of gene therapy occurs, the defective genes are no longer inherited (i.e., the genetic change is passed on from one generation to the next).
- Non-germ-line gene therapy: This is a genetic modification that is performed on the somatic cells as opposed to the germ cells. This is also called somatic gene therapy. When this type of gene therapy occurs the genetic disease can still be inherited by future generations. This is because the DNA from somatic cells is altered and these cells are not used for reproduction; therefore the defective genetic material in the haploid germ cells will continue to be passed on to future generations.
In addition to modifying germ cells and somatic cells, the techniques of genetic engineering can also be used to genetically clone species. Cloning is when two (and sometimes more) individuals or cells are produced from one genome.
On July 5, 1996, two scientists, Ian Wilmut and Keith Campbell, cloned the first animal from an adult somatic cell by using a technique called nuclear transfer. The animal was a ewe they named “Dolly.” This showed that one cell could be removed from the body of an animal and be used to re-create a second, identical individual animal.
Other projected use of genetic engineering is the possibility of individualized or genetic medicine. Individualized medicine is a futuristic style of medicine in which treatment will be tailored to the unique genetic needs of the patient. This is also known as personalized medicine. There are two major fields involved with the development of individualized medicine—pharmacogenetics and pharmacogenomics. Pharmacogenetics is an aspect of genetic medicine that studies the genetic sensitivity and differential response of a medication for a patient population. Pharmacogenomics is another aspect that is geared toward the manufacturing of pharmaceuticals with methods of genetic engineering.
In the near future, these two fields will change the way medicine is practiced. It is conceivable that during a typical office visit less time could be spent on deciphering somatic complaints and performing a physical exam, and more time on examining the genetics of the patient.
There is the possibility that the DNA molecule may in fact have a form of consciousness of its own, known as DNA consciousness or molecular/chemical consciousness (Grandy, 2006b, 2009a). This form of consciousness would of course be very different from neurological consciousness or human consciousness. In fact, DNA consciousness may underlie our very own conscious process.
This is a realistic possibility considering certain families of gene clusters; Hox and Pax genes are responsible for and oversee the development of our neurological consciousness. If those genes are altered or deleted, neurological consciousness does not develop.
Other ideas that support DNA consciousness are that the DNA molecule replicates itself, produces proteins freely, communicates chemically with other parts of the cell, and interacts with the external environment of the cell. It performs all of these functions independently. In addition, it is the first known molecule to discover itself (i.e., through Homo sapiens sapiens ) .
Genetic-engineering techniques may help us to explore this area by enabling scientists to explore how DNA interacts with itself, other molecules, and the environment; how it is able to freely self-replicate, and how it knows when and when not to produce certain products.
The future applications of genetic engineering are numerous indeed. Most of the immediate impact will be seen in the fields of anthropology and medicine. In medicine, there will be improvements in clinical therapeutics and individualized medicine. This will improve life spans and harness the potential to halt or reverse the aging process.
In anthropology, the completion of genome projects will assist in establishing genetic relationships between humankind and other species. In addition to studying our evolution, we could potentially control our evolution. Therefore, emerging teleology could become a reality.
The potential to alter human genomes could create the first transgenic Homo sapiens and provide the appearance of new species on this planet and elsewhere, a concept known as transhumanism. This could also give rise to new species such as Homo sapiens futurensis (the human of the future as proposed by Birx) or Homo sapiens genomicus (the transgenic human as proposed by Grandy).
It is also conceivable that genetic engineering could potentially equip our species with genes that could improve our ability to survive in outer space. This could give rise to Homo sapiens extraterrestrialis. During space travel, there is also the likelihood of encountering alien microorganisms as well as new types of diseases and aliments secondary to space exposure (Grandy, 2009d). This would open a new area of space medicine.
Ethical questions and fears will arise as well. We should be very cautious while considering the modification of our genome because we only understand a small fraction of the interworkings of the DNA molecule. Currently, scientists do not know how altering or modifying a gene in a genome as complicated as the human genome could affect us or future generations. Other ethical concerns will be raised; for example, what are good genes and what are bad genes? Who will make that determination? Originally, nature was in charge of deciding these issues. However, humankind began circumventing natural selection long ago without being prepared to address these questions.
In addition, our environment is not as dangerous (in a predatory sense) as it once was, and advances in medicine have increased human life spans while also allowing individuals—who would normally have died and not passed on their DNA to future generations—to survive and pass on inferior genes. This has given rise to a weaker gene pool or a failure to improve the species (Grandy, 2009c). Genetic engineering could potentially be a remedy to this situation. However, questions will arise over the nonmedical use of genome improvement.
With all of these possibilities on the horizon, we should always stop to remember the many great scientists and their pioneering research that made this a possibility. We should also keep our own humanity in mind as we attempt to tamper with something that we are only beginning to understand.
Bibliography:
- Birx, H. J. (1991). Interpreting evolution: Darwin & Teilhard de Chardin. Amherst, NY: Prometheus Books.
- Chargaff, E. (1952). On the deoxypentose nucleic acids from several microorganisms. Biochim et Biophys Aeta, 9,
- Chow, L. T., Roberts, J. M., Lewis, J. B., & Broker, T. R. (1977). A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA: DNA hybrids. Cell, 11 (4), 819–836.
- Darwin, C. (2000). Amherst, NY: Prometheus Books. (Original work published 1887)
- DeVries, H. (2007). Species and varieties: Their origin by mutation (Kindle ed.). New York: Evergreen Review. (Original work published 1905)
- Galton, F. (1990). Hereditary genius: An inquiry into its laws and consequences. New York: Peter Smith. (Original work published 1869)
- Grandy, J. (2006a). Bioinformatics. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 1, pp. 362–363). Thousand Oaks, CA: Sage.
- Grandy, J. (2006b). DNA molecule. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 2, pp. 753–756). Thousand Oaks, CA: Sage.
- Grandy, J. (2006c). Euthenics. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 2, pp. 873–875). Thousand Oaks, CA: Sage.
- Grandy, J. (2006d). Human Genome Project. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 3, pp. 1223–1226). Thousand Oaks, CA: Sage.
- Grandy, J. (2006e). RNA molecule. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 5, pp. 2026–2027). Thousand Oaks, CA: Sage.
- Grandy, J. (2009a). Consciousness. In H. J. Birx (Ed.), Encyclopedia of time (Vol. 1, pp. 212–216). Thousand Oaks, CA: Sage.
- Grandy, J. (2009b). DNA molecule. In H. J. Birx (Ed.), Encyclopedia of time (Vol. 1, pp. 333–335). Thousand Oaks, CA: Sage.
- Grandy, J. (2009c). Dying and death. In H. J. Birx (Ed.), Encyclopedia of time (Vol. 1, pp. 352–355). Thousand Oaks, CA: Sage.
- Grandy, J. (2009d). History of medicine. In H. J. Birx (Ed.), Encyclopedia of time (Vol. 2, pp. 842–845). Thousand Oaks, CA: Sage.
- Henig, R. M. (2001). The monk in the garden: The lost and found genius of Gregor Mendel, the father of genetics. New York: Mariner Books.
- Morgan, T. (1978). The mechanisms of Mendelian heredity. New York: Holt, Rinehart & Winston. (Original work published 1915)
- Pritchard, D., & Korf, B. (2008). Medical genetics at a glance (2nd ed.). Malden, MA: Blackwell.
- Siegetsleitner,A. (2006). Genetic engineering. In H. J. Birx (Ed.), Encyclopedia of anthropology (Vol. 3, pp.1036–1041). Thousand Oaks, CA: Sage.
- Watson, J. (1977). The double helix: A personal account of the discovery of the structure of DNA. NewYork: Mentor Books.
- Watson, J., & Crick, F. (1953). Molecular structure of nucleic acids. Nature, 171 (4356), 737–738.
- Wilkins, M. (1953). Crystallinity in sperm heads: Molecular structure of nucleoprotein in Vivo. Biochim et Biophys Aeta, 10,
- Yount, L. (2000). Biotechnology and genetic engineering. NewYork: Facts on File.
ORDER HIGH QUALITY CUSTOM PAPER

share this!
March 13, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Researchers use genetic engineering to create flood-tolerant plants
by Baylor College of Medicine
Adding too much water to your plants can damage them as much as not having enough water. In the environment, plants submerged under excessive rain have limited access to light and oxygen, which reduces or eliminates their ability to thrive. This poses an important problem for crops.
"Flooding is one of the biggest problems of present-day agriculture," said Dr. Kendal D. Hirschi, professor of pediatrics—nutrition at Baylor College of Medicine. "All crops have this problem, so if we could give plants the ability to be more flood-tolerant, we would provide an important solution to world agriculture."
Interestingly, it was a serendipitous finding that set Hirschi and his colleagues on a path to discover a potential way to make plants more flood tolerant.
They were running several experiments in the lab using plants with alterations in CAX1, a gene that transports calcium in plants, when they made an unexpected observation. When they removed CAX gene from plants using genetic engineering , the plants were more tolerant to low-oxygen stress (anoxia) and flooding than when the gene was active.
Next, the researchers further characterized plants lacking four functional calcium transporters. They reported in Plant, Cell & Environment that removing calcium transporters reduced calcium levels in leaves and increased the plant's resistance to low oxygen levels occurring under flooding conditions. The calcium transporter mutants sequentially altered plants' gene expression patterns and signaling pathways to promote anoxia tolerance.
Altered calcium transport in the mutants also was followed by changes in the abundance and distribution of other chemical elements in leaf tissue, such as zinc and potassium. The implications of these elemental changes are under investigation.
"These findings led to an original hypothesis that reduced endogenous calcium levels in plants confers anoxia tolerance," Hirschi said. "This hypothesis was supported by demonstrating that not-genetically modified plants grown in reduced calcium conditions had anoxia‐tolerant characteristics similar to those observed in calcium mutants."
The researchers conducted these studies in the lab with a simple weed called Arabidopsis. They are now exploring the possibility of giving crop plants , such as rice and tomatoes, an edge under flooding conditions by eliminating the gene.
This approach could be a part of solutions to limited food supplies affecting children and families worldwide living in areas prone to flooding.
Provided by Baylor College of Medicine
Explore further
Feedback to editors

Ultra-flat optics for broadband thermal imaging
11 hours ago

Shark-bitten orcas in the Northeastern Pacific could be a new population of killer whale

Einasto Supercluster: The new heavyweight contender in the universe
12 hours ago

Protein fragments ID two new 'extremophile' microbes—and may help find alien life
13 hours ago

Bridging the gap: Computer scientists develop model to enhance water data from satellites

Researchers develop a new strategy to enhance blue perovskite LED performance

Brighter, cheaper blue light could revolutionize screen technology

Gender and racial discrimination uncovered in leadership positions at Australia's leading universities

The 'baritone' of red giants refines cosmic distance measurements
14 hours ago

Even inactive deep-sea 'smokers' are densely colonized by microbial communities, study shows
Relevant physicsforums posts, potentially fatal dog parasite found in the colorado river.
16 hours ago
Biological culture and cultural biology
Mar 14, 2024
Electrical potential difference and charge separation
Are all biological catabolic reactions exergonic.
Mar 13, 2024
Nick Lanes on Sean Carroll's podcast
Mar 11, 2024
A First of Its Kind: A Calcium-based signal in the Human Brain
Mar 9, 2024
More from Biology and Medical
Related Stories

The gene helping submerged plants
Dec 17, 2018

Genome database for desiccation-tolerant plants released
Jan 5, 2024

Scientists identify gene that triggers dramatically increased root growth
Oct 26, 2023
Plants can measure the intensity of salt stress
Aug 25, 2022
Adding calcium to soils can help increase organic matter, trap more carbon
Nov 20, 2023

The signaling pathways that make plants more resistant to flooding
Jun 9, 2022
Recommended for you

Snakes: The new, high-protein superfood

Scientists generate new targeted protein degradation system that tunes a cell's own proteins

An invisible water surcharge: Climate warming increases crop water demand in the San Joaquin Valley
15 hours ago
New bioengineered protein design shows promise in fighting COVID-19

Shade-grown coffee demonstrates the benefits of combining agriculture and conservation

Alzheimer's drug fermented with help from AI and bacteria moves closer to reality
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- J Food Sci Technol
- v.50(6); 2013 Dec

Genetically modified foods: safety, risks and public concerns—a review
Defence Food Research Laboratory, Siddarthanagar, Mysore, 570011 India
K. R. Anilakumar
Genetic modification is a special set of gene technology that alters the genetic machinery of such living organisms as animals, plants or microorganisms. Combining genes from different organisms is known as recombinant DNA technology and the resulting organism is said to be ‘Genetically modified (GM)’, ‘Genetically engineered’ or ‘Transgenic’. The principal transgenic crops grown commercially in field are herbicide and insecticide resistant soybeans, corn, cotton and canola. Other crops grown commercially and/or field-tested are sweet potato resistant to a virus that could destroy most of the African harvest, rice with increased iron and vitamins that may alleviate chronic malnutrition in Asian countries and a variety of plants that are able to survive weather extremes. There are bananas that produce human vaccines against infectious diseases such as hepatitis B, fish that mature more quickly, fruit and nut trees that yield years earlier and plants that produce new plastics with unique properties. Technologies for genetically modifying foods offer dramatic promise for meeting some areas of greatest challenge for the 21st century. Like all new technologies, they also pose some risks, both known and unknown. Controversies and public concern surrounding GM foods and crops commonly focus on human and environmental safety, labelling and consumer choice, intellectual property rights, ethics, food security, poverty reduction and environmental conservation. With this new technology on gene manipulation what are the risks of “tampering with Mother Nature”?, what effects will this have on the environment?, what are the health concerns that consumers should be aware of? and is recombinant technology really beneficial? This review will also address some major concerns about the safety, environmental and ecological risks and health hazards involved with GM foods and recombinant technology.
Introduction
Scientists first discovered in 1946 that DNA can be transferred between organisms (Clive 2011 ). It is now known that there are several mechanisms for DNA transfer and that these occur in nature on a large scale, for example, it is a major mechanism for antibiotic resistance in pathogenic bacteria. The first genetically modified (GM) plant was produced in 1983, using an antibiotic-resistant tobacco plant. China was the first country to commercialize a transgenic crop in the early 1990s with the introduction of virus resistant tobacco. In 1994, the transgenic ‘Flavour Saver tomato’ was approved by the Food and Drug Administration (FDA) for marketing in the USA. The modification allowed the tomato to delay ripening after picking. In 1995, few transgenic crops received marketing approval. This include canola with modified oil composition (Calgene), Bacillus thuringiensis (Bt) corn/maize (Ciba-Geigy), cotton resistant to the herbicide bromoxynil (Calgene), Bt cotton (Monsanto), Bt potatoes (Monsanto), soybeans resistant to the herbicide glyphosate (Monsanto), virus-resistant squash (Asgrow) and additional delayed ripening tomatoes (DNAP, Zeneca/Peto, and Monsanto) (Clive 2011 ). A total of 35 approvals had been granted to commercially grow 8 transgenic crops and one flower crop of carnations with 8 different traits in 6 countries plus the EU till 1996 (Clive 1996 ). As of 2011, the USA leads a list of multiple countries in the production of GM crops. Currently, there are a number of food species in which a genetically modified version exists (Johnson 2008 ). Some of the foods that are available in the market include cotton, soybean, canola, potatoes, eggplant, strawberries, corn, tomatoes, lettuce, cantaloupe, carrots etc. GM products which are currently in the pipeline include medicines and vaccines, foods and food ingredients, feeds and fibres. Locating genes for important traits, such as those conferring insect resistance or desired nutrients-is one of the most limiting steps in the process.
Foods derived from GM crops
At present there are several GM crops used as food sources. As of now there are no GM animals approved for use as food, but a GM salmon has been proposed for FDA approval. In instances, the product is directly consumed as food, but in most of the cases, crops that have been genetically modified are sold as commodities, which are further processed into food ingredients.
Fruits and vegetables
Papaya has been developed by genetic engineering which is ring spot virus resistant and thus enhancing the productivity. This was very much in need as in the early 1990s the Hawaii’s papaya industry was facing disaster because of the deadly papaya ring spot virus. Its single-handed savior was a breed engineered to be resistant to the virus. Without it, the state’s papaya industry would have collapsed. Today 80 % of Hawaiian papaya is genetically engineered, and till now no conventional or organic method is available to control ring spot virus.
The NewLeaf™ potato, a GM food developed using naturally-occurring bacteria found in the soil known as Bacillus thuringiensis (Bt), was made to provide in-plant protection from the yield-robbing Colorado potato beetle. This was brought to market by Monsanto in the late 1990s, developed for the fast food market. This was forced to withdraw from the market in 2001as the fast food retailers did not pick it up and thereby the food processors ran into export problems. Reports say that currently no transgenic potatoes are marketed for the purpose of human consumption. However, BASF, one of the leading suppliers of plant biotechnology solutions for agriculture requested for the approval for cultivation and marketing as a food and feed for its ‘Fortuna potato’. This GM potato was made resistant to late blight by adding two resistance genes, blb1 and blb2, which was originated from the Mexican wild potato Solanum bulbocastanum . As of 2005, about 13 % of the zucchini grown in the USA is genetically modified to resist three viruses; the zucchini is also grown in Canada (Johnson 2008 ).
Vegetable oil
It is reported that there is no or a significantly small amount of protein or DNA remaining in vegetable oil extracted from the original GM crops in USA. Vegetable oil is sold to consumers as cooking oil, margarine and shortening, and is used in prepared foods. Vegetable oil is made of triglycerides extracted from plants or seeds and then refined, and may be further processed via hydrogenation to turn liquid oils into solids. The refining process removes nearly all non-triglyceride ingredients (Crevel et al. 2000 ). Cooking oil, margarine and shortening may also be made from several crops. A large percentage of Canola produced in USA is GM and is mainly used to produce vegetable oil. Canola oil is the third most widely consumed vegetable oil in the world. The genetic modifications are made for providing resistance to herbicides viz. glyphosate or glufosinate and also for improving the oil composition. After removing oil from canola seed, which is ∼43 %, the meal has been used as high quality animal feed. Canola oil is a key ingredient in many foods and is sold directly to consumers as margarine or cooking oil. The oil has many non-food uses, which includes making lipsticks.
Maize, also called corn in the USA and cornmeal, which is ground and dried maize constitute a staple food in many regions of the world. Grown since 1997 in the USA and Canada, 86 % of the USA maize crop was genetically modified in 2010 (Hamer and Scuse 2010 ) and 32 % of the worldwide maize crop was GM in 2011 (Clive 2011 ). A good amount of the total maize harvested go for livestock feed including the distillers grains. The remaining has been used for ethanol and high fructose corn syrup production, export, and also used for other sweeteners, cornstarch, alcohol, human food or drink. Corn oil is sold directly as cooking oil and to make shortening and margarine, in addition to make vitamin carriers, as a source of lecithin, as an ingredient in prepared foods like mayonnaise, sauces and soups, and also to fry potato chips and French fries. Cottonseed oil is used as a salad and cooking oil, both domestically and industrially. Nearly 93 % of the cotton crop in USA is GM.
The USA imports 10 % of its sugar from other countries, while the remaining 90 % is extracted from domestically grown sugar beet and sugarcane. Out of the domestically grown sugar crops, half of the extracted sugar is derived from sugar beet, and the other half is from sugarcane. After deregulation in 2005, glyphosate-resistant sugar beet was extensively adopted in the USA. In USA 95 % of sugar beet acres were planted with glyphosate-resistant seed (Clive 2011 ). Sugar beets that are herbicide-tolerant have been approved in Australia, Canada, Colombia, EU, Japan, Korea, Mexico, New Zealand, Philippines, Russian Federation, Singapore and USA. The food products of sugar beets are refined sugar and molasses. Pulp remaining from the refining process is used as animal feed. The sugar produced from GM sugar beets is highly refined and contains no DNA or protein—it is just sucrose, the same as sugar produced from non-GM sugar beets (Joana et al. 2010 ).
Quantification of genetically modified organisms (GMOs) in foods
Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73. The array based method combines multiplex PCR and array technology to screen samples for different potential GMO combining different approaches viz. screening elements, plant-specific markers, and event-specific markers. The qPCR is used to detect specific GMO events by usage of specific primers for screening elements or event specific markers. Controls are necessary to avoid false positive or false negative results. For example, a test for CaMV is used to avoid a false positive in the event of a virus contaminated sample.
Joana et al. ( 2010 ) reported the extraction and detection of DNA along with a complete industrial soybean oil processing chain to monitor the presence of Roundup Ready (RR) soybean. The amplification of soybean lectin gene by end-point polymerase chain reaction (PCR) was achieved in all the steps of extraction and refining processes. The amplification of RR soybean by PCR assays using event specific primers was also achieved for all the extraction and refining steps. This excluded the intermediate steps of refining viz. neutralization, washing and bleaching possibly due to sample instability. The real-time PCR assays using specific probes confirmed all the results and proved that it is possible to detect and quantify GMOs in the fully refined soybean oil.
Figure 1 gives the overall protocol for the testing of GMOs. This is based on a PCR detection system specific for 35S promoter region originating from cauliflower mosaic virus (Deisingh and Badrie 2005 ). The 35S-PCR technique permits detection of GMO contents of foods and raw materials in the range of 0.01–0.1 %. The development of quantitative detection systems such as quantitative competitive PCR (QC-PCR), real-time PCR and ELISA systems resulted in the advantage of survival of DNA in most manufacturing processes. Otherwise with ELISA, there can be protein denaturing during food processing. Inter-laboratory differences were found to be less with the QC-PCR than with quantitative PCR probably due to insufficient homogenisation of the sample. However, there are disadvantages, the major one being the amount of DNA, which could be amplified, is affected by food processing techniques and can vary up to 5-fold. Thus, results need to be normalised by using plant-specific QC-PCR system. Further, DNA, which cannot be amplified, will affect all quantitative PCR detection systems.

Protocol for the testing of genetically modified foods
In a recent work La Mura et al. ( 2011 ) applied QUIZ (quantization using informative zeros) to estimate the contents of RoundUp Ready™ soya and MON810 in processed food containing one or both GMs. They reported that the quantification of GM in samples can be performed without the need for certified reference materials using QUIZ. Results showed good agreement between derived values and known input of GM material and compare favourably with quantitative real-time PCR. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick has been reported recently (Xiumin et al. 2012 ).
GM foods-merits and demerits
Before we think of having GM foods it is very important to know about is advantages and disadvantages especially with respect to its safety. These foods are made by inserting genes of other species into their DNA. Though this kind of genetic modification is used both in plants and animals, it is found more commonly in the former than in the latter. Experts are working on developing foods that have the ability to alleviate certain disorders and diseases. Though researchers and the manufacturers make sure that there are various advantages of consuming these foods, a fair bit of the population is entirely against them.
GM foods are useful in controlling the occurrence of certain diseases. By modifying the DNA system of these foods, the properties causing allergies are eliminated successfully. These foods grow faster than the foods that are grown traditionally. Probably because of this, the increased productivity provides the population with more food. Moreover these foods are a boon in places which experience frequent droughts, or where the soil is incompetent for agriculture. At times, genetically engineered food crops can be grown at places with unfavourable climatic conditions too. A normal crop can grow only in specific season or under some favourable climatic conditions. Though the seeds for such foods are quite expensive, their cost of production is reported to be less than that of the traditional crops due to the natural resistance towards pests and insects. This reduces the necessity of exposing GM crops to harmful pesticides and insecticides, making these foods free from chemicals and environment friendly as well. Genetically engineered foods are reported to be high in nutrients and contain more minerals and vitamins than those found in traditionally grown foods. Other than this, these foods are known to taste better. Another reason for people opting for genetically engineered foods is that they have an increased shelf life and hence there is less fear of foods getting spoiled quickly.
The biggest threat caused by GM foods is that they can have harmful effects on the human body. It is believed that consumption of these genetically engineered foods can cause the development of diseases which are immune to antibiotics. Besides, as these foods are new inventions, not much is known about their long term effects on human beings. As the health effects are unknown, many people prefer to stay away from these foods. Manufacturers do not mention on the label that foods are developed by genetic manipulation because they think that this would affect their business, which is not a good practice. Many religious and cultural communities are against such foods because they see it as an unnatural way of producing foods. Many people are also not comfortable with the idea of transferring animal genes into plants and vice versa. Also, this cross-pollination method can cause damage to other organisms that thrive in the environment. Experts are also of the opinion that with the increase of such foods, developing countries would start depending more on industrial countries because it is likely that the food production would be controlled by them in the time to come.
Safety tests on commercial GM crops
The GM tomatoes were produced by inserting kanr genes into a tomato by an ‘antisense’ GM method (IRDC 1998 ). The results show that there were no significant alterations in total protein, vitamins and mineral contents and in toxic glycoalkaloids (Redenbaugh et al. 1992 ). Therefore, the GM and parent tomatoes were deemed to be “substantially equivalent”. In acute toxicity studies with male/female rats, which were tube-fed with homogenized GM tomatoes, toxic effects were reported to be absent. A study with a GM tomato expressing B. thuringiensis toxin CRYIA (b) was underlined by the immunocytochemical demonstration of in vitro binding of Bt toxin to the caecum/colon from humans and rhesus monkeys (Noteborn et al. 1995 ).
Two lines of Chardon LL herbicide-resistant GM maize expressing the gene of phosphinothricin acetyltransferase before and after ensiling showed significant differences in fat and carbohydrate contents compared with non-GM maize and were therefore substantially different come. Toxicity tests were only performed with the maize even though with this the unpredictable effects of the gene transfer or the vector or gene insertion could not be demonstrated or excluded. The design of these experiments was also flawed because of poor digestibility and reduction in feed conversion efficiency of GM corn. One broiler chicken feeding study with rations containing transgenic Event 176 derived Bt corn (Novartis) has been published (Brake and Vlachos 1998 ). However, the results of this trial are more relevant to commercial than academic scientific studies.
GM soybeans
To make soybeans herbicide resistant, the gene of 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium was used. Safety tests claim the GM variety to be “substantially equivalent” to conventional soybeans (Padgette et al. 1996 ). The same was claimed for GTS (glyphosate-resistant soybeans) sprayed with this herbicide (Taylor et al. 1999 ). However, several significant differences between the GM and control lines were recorded (Padgette et al. 1996 ) and the study showed statistically significant changes in the contents of genistein (isoflavone) with significant importance for health (Lappe et al. 1999 ) and increased content in trypsin inhibitor.
Studies have been conducted on the feeding value (Hammond et al. 1996 ) and possible toxicity (Harrison et al. 1996 ) for rats, broiler chickens, catfish and dairy cows of two GM lines of glyphosate-resistant soybean (GTS). The growth, feed conversion efficiency, catfish fillet composition, broiler breast muscle and fat pad weights and milk production, rumen fermentation and digestibilities in cows were found to be similar for GTS and non-GTS. These studies had the following lacunae: (a) No individual feed intakes, body or organ weights were given and histology studies were qualitative microscopy on the pancreas, (b) The feeding value of the two GTS lines was not substantially equivalent either because the rats/catfish grew significantly better on one of the GTS lines than on the other, (c) The design of study with broiler chicken was not much convincing, (d) Milk production and performance of lactating cows also showed significant differences between cows fed GM and non-GM feeds and (e) Testing of the safety of 5-enolpyruvylshikimate-3-phosphate synthase, which renders soybeans glyphosate-resistant (Harrison et al. 1996 ), was irrelevant because in the gavage studies an E. coli recombinant and not the GTS product were used. In a separate study (Teshima et al. 2000 ), it was claimed that rats and mice which were fed 30 % toasted GTS or non-GTS in their diet had no significant differences in nutritional performance, organ weights, histopathology and production of IgE and IgG antibodies.
GM potatoes
There were no improvements in the protein content or amino acid profile of GM potatoes (Hashimoto et al. 1999a ). In a short feeding study to establish the safety of GM potatoes expressing the soybean glycinin gene, rats were daily force-fed with 2 g of GM or control potatoes/kg body weight (Hashimoto et al 1999b ). No differences in growth, feed intake, blood cell count and composition and organ weights between the groups were found. In this study, the intake of potato by animals was reported to be too low (Pusztai 2001 ).
Feeding mice with potatoes transformed with a Bacillus thuringiensis var. kurstaki Cry1 toxin gene or the toxin itself was shown to have caused villus epithelial cell hypertrophy and multinucleation, disrupted microvilli, mitochondrial degeneration, increased numbers of lysosomes and autophagic vacuoles and activation of crypt Paneth cells (Fares and El-Sayed 1998 ). The results showed CryI toxin which was stable in the mouse gut. Growing rats pair-fed on iso -proteinic and iso -caloric balanced diets containing raw or boiled non-GM potatoes and GM potatoes with the snowdrop ( Galanthus nivalis ) bulb lectin (GNA) gene (Ewen and Pusztai 1999 ) showed significant increase in the mucosal thickness of the stomach and the crypt length of the intestines of rats fed GM potatoes. Most of these effects were due to the insertion of the construct used for the transformation or the genetic transformation itself and not to GNA which had been pre-selected as a non-mitotic lectin unable to induce hyperplastic intestinal growth (Pusztai et al. 1990 ) and epithelial T lymphocyte infiltration.
The kind that expresses soybean glycinin gene (40–50 mg glycinin/g protein) was developed (Momma et al. 1999 ) and was claimed to contain 20 % more protein. However, the increased protein content was found probably due to a decrease in moisture rather than true increase in protein.
Several lines of GM cotton plants have been developed using a gene from Bacillus thuringiensis subsp. kurstaki providing increased protection against major lepidopteran pests. The lines were claimed to be “substantially equivalent” to parent lines (Berberich et al. 1996 ) in levels of macronutrients and gossypol. Cyclopropenoid fatty acids and aflatoxin levels were less than those in conventional seeds. However, because of the use of inappropriate statistics it was questionable whether the GM and non-GM lines were equivalent, particularly as environmental stresses could have unpredictable effects on anti-nutrient/toxin levels (Novak and Haslberger 2000 ).
The nutritional value of diets containing GM peas expressing bean alpha-amylase inhibitor when fed to rats for 10 days at two different doses viz. 30 % and 65 % was shown to be similar to that of parent-line peas (Pusztai et al. 1999 ). At the same time in order to establish its safety for humans a more rigorous specific risk assessment will have to be carried out with several GM lines. Nutritional/toxicological testing on laboratory animals should follow the clinical, double-blind, placebo-type tests with human volunteers.
Allergenicity studies
When the gene is from a crop of known allergenicity, it is easy to establish whether the GM food is allergenic using in vitro tests, such as RAST or immunoblotting, with sera from individuals sensitised to the original crop. This was demonstrated in GM soybeans expressing the brasil nut 2S proteins (Nordlee et al. 1996 ) or in GM potatoes expressing cod protein genes (Noteborn et al. 1995 ). It is also relatively easy to assess whether genetic engineering affected the potency of endogenous allergens (Burks and Fuchs 1995 ). Farm workers exposed to B. thuringiensis pesticide were shown to have developed skin sensitization and IgE antibodies to the Bt spore extract. With their sera it may now therefore be possible to test for the allergenic potential of GM crops expressing Bt toxin (Bernstein et al. 1999 ). It is all the more important because Bt toxin Cry1Ac has been shown to be a potent oral/nasal antigen and adjuvant (Vazquez-Padron et al. 2000 ).
The decision-tree type of indirect approach based on factors such as size and stability of the transgenically expressed protein (O’Neil et al. 1998 ) is even more unsound, particularly as its stability to gut proteolysis is assessed by an in vitro (simulated) testing (Metcalf et al. 1996 ) instead of in vivo (human/animal) testing and this is fundamentally wrong. The concept that most allergens are abundant proteins may be misleading because, for example, Gad c 1, the major allergen in codfish, is not a predominant protein (Vazquez-Padron et al. 2000 ). However, when the gene responsible for the allergenicity is known, such as the gene of the alpha-amylase/trypsin inhibitors/allergens in rice, cloning and sequencing opens the way for reducing their level by antisense RNA strategy (Nakamura and Matsuda 1996 ).
It is known that the main concerns about adverse effects of GM foods on health are the transfer of antibiotic resistance, toxicity and allergenicity. There are two issues from an allergic standpoint. These are the transfer of a known allergen that may occur from a crop into a non-allergenic target crop and the creation of a neo-allergen where de novo sensitisation occurs in the population. Patients allergic to Brazil nuts and not to soy bean then showed an IgE mediated response towards GM soy bean. Lack ( 2002 ) argued that it is possible to prevent such occurrences by doing IgE-binding studies and taking into account physico-chemical characteristics of proteins and referring to known allergen databases. The second possible scenario of de novo sensitisation does not easily lend itself to risk assessment. He reports that evidence that the technology used for the production of GM foods poses an allergic threat per se is lacking very much compared to other methodologies widely accepted in the food industry.
Risks and controversy
There are controversies around GM food on several levels, including whether food produced with it is safe, whether it should be labelled and if so how, whether agricultural biotechnology and it is needed to address world hunger now or in the future, and more specifically with respect to intellectual property and market dynamics, environmental effects of GM crops and GM crops’ role in industrial agricultural more generally.
Many problems, viz. the risks of “tampering with Mother Nature”, the health concerns that consumers should be aware of and the benefits of recombinant technology, also arise with pest-resistant and herbicide-resistant plants. The evolution of resistant pests and weeds termed superbugs and super weeds is another problem. Resistance can evolve whenever selective pressure is strong enough. If these cultivars are planted on a commercial scale, there will be strong selective pressure in that habitat, which could cause the evolution of resistant insects in a few years and nullify the effects of the transgenic. Likewise, if spraying of herbicides becomes more regular due to new cultivars, surrounding weeds could develop a resistance to the herbicide tolerant by the crop. This would cause an increase in herbicide dose or change in herbicide, as well as an increase in the amount and types of herbicides on crop plants. Ironically, chemical companies that sell weed killers are a driving force behind this research (Steinbrecher 1996 ).
Another issue is the uncertainty in whether the pest-resistant characteristic of these crops can escape to their weedy relatives causing resistant and increased weeds (Louda 1999 ). It is also possible that if insect-resistant plants cause increased death in one particular pest, it may decrease competition and invite minor pests to become a major problem. In addition, it could cause the pest population to shift to another plant population that was once unthreatened. These effects can branch out much further. A study of Bt crops showed that “beneficial insects, so named because they prey on crop pests, were also exposed to harmful quantities of Bt.” It was stated that it is possible for the effects to reach further up the food web to effect plants and animals consumed by humans (Brian 1999 ). Also, from a toxicological standpoint, further investigation is required to determine if residues from herbicide or pest resistant plants could harm key groups of organisms found in surrounding soil, such as bacteria, fungi, nematodes, and other microorganisms (Allison and Palma 1997 ).
The potential risks accompanied by disease resistant plants deal mostly with viral resistance. It is possible that viral resistance can lead to the formation of new viruses and therefore new diseases. It has been reported that naturally occurring viruses can recombine with viral fragments that are introduced to create transgenic plants, forming new viruses. Additionally, there can be many variations of this newly formed virus (Steinbrecher 1996 ).
Health risks associated with GM foods are concerned with toxins, allergens, or genetic hazards. The mechanisms of food hazards fall into three main categories (Conner and Jacobs 1999 ). They are inserted genes and their expression products, secondary and pleiotropic effects of gene expression and the insertional mutagenesis resulting from gene integration. With regards to the first category, it is not the transferred gene itself that would pose a health risk. It should be the expression of the gene and the affects of the gene product that are considered. New proteins can be synthesized that can produce unpredictable allergenic effects. For example, bean plants that were genetically modified to increase cysteine and methionine content were discarded after the discovery that the expressed protein of the transgene was highly allergenic (Butler and Reichhardt 1999 ). Due attention should be taken for foods engineered with genes from foods that commonly cause allergies, such as milk, eggs, nuts, wheat, legumes, fish, molluscs and crustacean (Maryanski 1997 ). However, since the products of the transgenic are usually previously identified, the amount and effects of the product can be assessed before public consumption. Also, any potential risk, immunological, allergenic, toxic or genetically hazardous, could be recognized and evaluated if health concerns arise. The available allergen data bases with details are shown in Table 1 .
Allergen databases (Kleter and Peijnenburg 2002 )
More concern comes with secondary and pleiotropic effects. For example, many transgenes encode an enzyme that alters biochemical pathways. This could cause an increase or decrease in certain biochemicals. Also, the presence of a new enzyme could cause depletion in the enzymatic substrate and subsequent build up of the enzymatic product. In addition, newly expressed enzymes may cause metabolites to diverge from one secondary metabolic pathway to another (Conner and Jacobs 1999 ). These changes in metabolism can lead to an increase in toxin concentrations. Assessing toxins is a more difficult task due to limitations of animal models. Animals have high variation between experimental groups and it is challenging to attain relevant doses of transgenic foods in animals that would provide results comparable to humans (Butler and Reichhardt 1999 ). Consequently, biochemical and regulatory pathways in plants are poorly understood.
Insertional mutagenesis can disrupt or change the expression of existing genes in a host plant. Random insertion can cause inactivation of endogenous genes, producing mutant plants. Moreover, fusion proteins can be made from plant DNA and inserted DNA. Many of these genes create nonsense products or are eliminated in crop selection due to incorrect appearance. However, of most concern is the activation or up regulation of silent or low expressed genes. This is due to the fact that it is possible to activate “genes that encode enzymes in biochemical pathways toward the production of toxic secondary compounds” (Conner and Jacobs 1999 ). This becomes a greater issue when the new protein or toxic compound is expressed in the edible portion of the plant, so that the food is no longer substantially equal to its traditional counterpart.
There is a great deal of unknowns when it comes to the risks of GM foods. One critic declared “foreign proteins that have never been in the human food chain will soon be consumed in large amounts”. It took us many years to realize that DDT might have oestrogenic activities and affect humans, “but we are now being asked to believe that everything is OK with GM foods because we haven’t seen any dead bodies yet” (Butler and Reichhardt 1999 ). As a result of the growing public concerns over GM foods, national governments have been working to regulate production and trade of GM foods.
Reports say that GM crops are grown over 160 million hectares in 29 countries, and imported by countries (including European ones) that don’t grow them. Nearly 300 million Americans, 1350 million Chinese, 280 million Brazilians and millions elsewhere regularly eat GM foods, directly and indirectly. Though Europeans voice major fears about GM foods, they permit GM maize cultivation. It imports GM soy meal and maize as animal feed. Millions of Europeans visit the US and South America and eat GM food.
Around three million Indians have become US citizens, and millions more go to the US for tourism and business and they will be eating GM foods in the USA. Indian activists claim that GM foods are inherently dangerous and must not be cultivated in India. Activists strongly opposed Bt cotton in India, and published reports claiming that the crop had failed in the field. At the same time farmers soon learned from experience that Bt cotton was very profitable, and 30 million rushed to adopt it. In consequence, India’s cotton production doubled and exports zoomed, even while using much less pesticide. Punjab farmers lease land at Rs 30,000 per acre to grow Bt cotton.
Public concerns-global scenario
In the late 1980s, there was a major controversy associated with GM foods even when the GMOs were not in the market. But the industrial applications of gene technology were developed to the production and marketing status. After words, the European Commission harmonized the national regulations across Europe. Concerns from the community side on GMOs in particular about its authorization have taken place since 1990s and the regulatory frame work on the marketing aspects underwent refining. Issues specifically on the use of GMOs for human consumption were introduced in 1997, in the Regulation on Novel Foods Ingredients (258/97/EC of 27 January 1997). This Regulations deals with rules for authorization and labelling of novel foods including food products made from GMOs, recognizing for the first time the consumer’s right to information and labelling as a tool for making an informed choice. The labelling of GM maize varieties and GM soy varieties that did not fall under this Regulation are covered by Regulation (EC 1139/98). Further legislative initiatives concern the traceability and labelling of GMOs and the authorization of GMOs in food and feed.
The initial outcome of the implementation of the first European directive seemed to be a settlement of the conflicts over technologies related to gene applications. By 1996, the second international level controversy over gene technology came up and triggered the arrival of GM soybeans at European harbours (Lassen et al. 2002 ). The GM soy beans by Monsanto to resist the herbicide represented the first large scale marketing of GM foods in Europe. Events such as commercialisation of GM maize and other GM modified commodities focused the public attention on the emerging biosciences, as did other gene technology applications such as animal and human cloning. The public debate on the issues associated with the GM foods resulted in the formation of many non-governmental organizations with explicit interest. At the same time there is a great demand for public participation in the issues about regulation and scientific strategy who expresses acceptance or rejection of GM products through purchase decisions or consumer boycotts (Frewer and Salter 2002 ).
Most research effort has been devoted to assessing people’s attitudes towards GM foods as a technology. Numerous “opinion poll”—type surveys have been conducted on national and cross-national levels (Hamstra 1998 ). Ethical concerns are also important, that a particular technology is in some way “tampering with nature”, or that unintended effects are unpredictable and thus unknown to science (Miles and Frewer 2001 ).
Consumer’s attitude towards GM foods
Consumer acceptance is conditioned by the risk that they perceive from introducing food into their consumption habits processed through technology that they hardly understand. In a study conducted in Spain, the main conclusion was that the introduction of GM food into agro-food markets should be accompanied by adequate policies to guarantee consumer safety. These actions would allow a decrease in consumer-perceived risk by taking special care of the information provided, concretely relating to health. For, the most influential factor in consumer-perceived risk from these foods is concern about health (Martinez-Poveda et al. 2009 ).
Tsourgiannis et al. ( 2011 ) conducted a study aimed to identify the factors that affect consumers purchasing behaviour towards food products that are free from GMO (GM Free) in a European region and more precisely in the Prefecture of Drama-Kavala-Xanthi. Field interviews conducted in a random selected sample consisted of 337 consumers in the cities of Drama, Kavala, Xanthi in 2009. Principal components analysis (PCA) was conducted in order to identify the factors that affect people in preferring consuming products that are GM Free. The factors that influence people in the study area to buy GM Free products are: (a) products’ certification as GM Free or organic products, (b) interest about the protection of the environment and nutrition value, (c) marketing issues and (d) price and quality. Furthermore, cluster and discriminant analysis identified two groups of consumers: (a) those influenced by the product price, quality and marketing aspects and (b) those interested in product’s certification and environmental protection (Tsourgiannis et al. 2011 ).
Snell et al. ( 2012 ) examined 12 long-term studies (of more than 90 days, up to 2 years in duration) and 12 multigenerational studies (from 2 to 5 generations) on the effects of diets containing GM maize, potato, soybean, rice, or triticale on animal health. They referenced the 90-day studies on GM feed for which long-term or multigenerational study data were available. Many parameters have been examined using biochemical analyses, histological examination of specific organs, hematology and the detection of transgenic DNA. Results from all the 24 studies do not suggest any health hazards and, in general, there were no statistically significant differences within parameters observed. They observed some small differences, though these fell within the normal variation range of the considered parameter and thus had no biological or toxicological significance. The studies reviewed present evidence to show that GM plants are nutritionally equivalent to their non-GM counterparts and can be safely used in food and feed.
GM foods: issues with respect to India
In a major setback to the proponents of GM technology in farm crops, the Parliamentary Committee on Agriculture in 2012 asked Indian government to stop all field trials and sought a bar on GM food crops such as Bt. brinjal. Raising the “ethical dimensions” of transgenics in agricultural crops, as well as studies of a long-term environmental and chronic toxicology impact, the panel noted that there were no significant socio-economic benefits to farmers.
Countries like India have great security concerns at the same time specific problems exist for small and marginal farmers. India could use a toxin free variety of the Lathyrus sativus grown on marginal lands and consumed by the very poor. GM mustard is a variety using the barnase-barstar-bar gene complex, an unstable gene construct with possible undesirable effects, to achieve male sterile lines that are used to make hybrid mustard varieties. In India we have good non-GM alternatives for making male sterile lines for hybrid production so the Proagro variety is of little use. Being a food crop, GM mustard will have to be examined very carefully. Even if there were to be benefits, they have to be weighed against the risks posed to human health and the environment. Apart from this, mustard is a cross-pollinating crop and pollen with their foreign genes is bound to reach non-GM mustard and wild relatives. We do not know what impact this will have. If GM technology is to be used in India, it should be directed at the real needs of Indian farmers, on crops like legumes, oilseeds and fodder and traits like drought tolerance and salinity tolerance.
Basmati rice and Darjeeling tea are perhaps India’s most easily identifiable premium products in the area of food. Basmati is highly prized rice, its markets are growing and it is a high end, expensive product in the international market. Like Champagne wine and truffles from France, international consumers treat it as a special, luxury food. Since rice is nutritionally a poor cereal, it is thought that addition of iron and vitamin A by genetic modification would increase the nutritional quality. So does it make any sense at all to breed a GM Basmati, along the lines of Bt Cotton? However, premium wine makers have outright rejected the notion of GM doctored wines that were designed to cut out the hangover and were supposed to be ‘healthier’. Premium products like special wines, truffles and Basmati rice need to be handled in a special, premium way (Sahai 2003 ).
Traceability of GMOs in the food production chain
Traceability systems document the history of a product and may serve the purpose of both marketing and health protection. In this framework, segregation and identity preservation systems allow for the separation of GM and non-GM products from “farm to fork”. Implementation of these systems comes with specific technical requirements for each particular step of the food processing chain. In addition, the feasibility of traceability systems depends on a number of factors, including unique identifiers for each GM product, detection methods, permissible levels of contamination, and financial costs. Progress has been achieved in the field of sampling, detection, and traceability of GM products, while some issues remain to be solved. For success, much will depend on the threshold level for adventitious contamination set by legislation (Miraglia et al. 2004 ).
Issues related to detection and traceability of GMOs is gaining interest worldwide due to the global diffusion and the related socio-economical implications. The interest of the scientific community into traceability aspects has also been increased simultaneously. Crucial factors in sampling and detection methodologies are the number of the GMOs involved and international agreement on traceability. The availability of reliable traceability strategies is very important and this may increase public trust in transparency in GMO related issues.
Heat processing methods like autoclaving and microwave heating can damage the DNA and reduce the level to detectable DNA. The PCR based methods have been standardised to detect such DNA in GM soybean and maize (Vijayakumar et al. 2009 ). Molecular methods such as multiplex and real time PCR methods have been developed to detect even 20 pg of genomic DNA in genetically modified EE-1 brinjal (Ballari et al. 2012 ).
DNA and protein based methods have been adopted for the detection and identification of GMOs which is relatively a new area of diagnostics. New diagnostic methodologies are also being developed, viz. the microarray-based methods that allow for the simultaneous identification of the increasing number of GMOs on the global market in a single sample. Some of these techniques have also been discussed for the detection of unintended effects of genetic modification by Cellini et al. ( 2004 ). The implementation of adequate traceability systems requires more than technical tools alone and is strictly linked to labelling constraints. The more stringent the labelling requirements, the more expensive and difficult the associated traceability strategies are to meet these requirements.
Both labelling and traceability of GMOs are current issues that are considered in trade and regulation. Currently, labelling of GM foods containing detectable transgenic material is required by EU legislation. A proposed package of legislation would extend this labelling to foods without any traces of transgenics. These new legislations would also impose labelling and a traceability system based on documentation throughout the food and feed manufacture system. The regulatory issues of risk analysis and labelling are currently harmonised by Codex Alimentarius. The implementation and maintenance of the regulations necessitates sampling protocols and analytical methodologies that allow for accurate determination of the content of GM organisms within a food and feed sample. Current methodologies for the analysis of GMOs are focused on either one of two targets, the transgenic DNA inserted- or the novel protein(s) expressed- in a GM product. For most DNA-based detection methods, the polymerase chain reaction is employed. Items that need consideration in the use of DNA-based detection methods include the specificity, sensitivity, matrix effects, internal reference DNA, availability of external reference materials, hemizygosity versus homozygosity, extra chromosomal DNA and international harmonisation.
For most protein-based methods, enzyme-linked immunosorbent assays with antibodies binding the novel protein are employed. Consideration should be given to the selection of the antigen bound by the antibody, accuracy, validation and matrix effects. Currently, validation of detection methods for analysis of GMOs is taking place. New methodologies are developed, in addition to the use of microarrays, mass spectrometry and surface plasmon resonance. Challenges for GMO detection include the detection of transgenic material in materials with varying chromosome numbers. The existing and proposed regulatory EU requirements for traceability of GM products fit within a broader tendency towards traceability of foods in general and, commercially, towards products that can be distinguished from one another.
Gene transfer studies in human volunteers
As of January 2009, there has only been one human feeding study conducted on the effects of GM foods. The study involved seven human volunteers who previously had their large intestines removed for medical reasons. These volunteers were provided with GM soy to eat to see if the DNA of the GM soy transferred to the bacteria that naturally lives in the human gut. Researchers identified that three of the seven volunteers had transgenes from GM soya transferred into the bacteria living in their gut before the start of the feeding experiment. As this low-frequency transfer did not increase after the consumption of GM soy, the researchers concluded that gene transfer did not occur during the experiment. In volunteers with complete digestive tracts, the transgene did not survive passage through intact gastrointestinal tract (Netherwood 2004 ). Other studies have found DNA from M13 virus, GFP and even ribulose-1, 5-bisphosphate carboxylase (Rubisco) genes in the blood and tissue of ingesting animals (Guertler et al. 2009 ; Brigulla and Wackernagel 2010 ).
Two studies on the possible effects of giving GM feed to animals found that there were no significant differences in the safety and nutritional value of feedstuffs containing material derived from GM plants (Gerhard et al. 2005 ; Beagle et al. 2006 ). Specifically, the studies noted that no residues of recombinant DNA or novel proteins have been found in any organ or tissue samples obtained from animals fed with GM plants (Nordlee 1996 ; Streit 2001 ).
Future developments
The GM foods have the potential to solve many of the world’s hunger and malnutrition problems, and to help protect and preserve the environment by increasing yield and reducing reliance upon synthetic pesticides and herbicides. Challenges ahead lie in many areas viz. safety testing, regulation, policies and food labelling. Many people feel that genetic engineering is the inevitable wave of the future and that we cannot afford to ignore a technology that has such enormous potential benefits.
Future also envisages that applications of GMOs are diverse and include drugs in food, bananas that produce human vaccines against infectious diseases such as Hepatitis B (Kumar et al. 2005 ), metabolically engineered fish that mature more quickly, fruit and nut trees that yield years earlier, foods no longer containing properties associated with common intolerances, and plants that produce new biodegradable plastics with unique properties (van Beilen and Yves 2008 ). While their practicality or efficacy in commercial production has yet to be fully tested, the next decade may see exponential increases in GM product development as researchers gain increasing access to genomic resources that are applicable to organisms beyond the scope of individual projects.
One has to agree that there are many opinions (Domingo 2000 ) about scarce data on the potential health risks of GM food crops, even though these should have been tested for and eliminated before their introduction. Although it is argued that small differences between GM and non-GM crops have little biological meaning, it is opined that most GM and parental line crops fall short of the definition of substantial equivalence. In any case, we need novel methods and concepts to probe into the compositional, nutritional, toxicological and metabolic differences between GM and conventional crops and into the safety of the genetic techniques used in developing GM crops if we want to put this technology on a proper scientific foundation and allay the fears of the general public. Considerable effort need to be directed towards understanding people’s attitudes towards this gene technology. At the same time it is imperative to note the lack of trust in institutions and institutional activities regarding GMOs and the public perceive that institutions have failed to take account of the actual concerns of the public as part of their risk management activities.
Contributor Information
A. S. Bawa, Email: ni.oc.oohay@awabrednirama .
K. R. Anilakumar, Email: moc.liamg@rkramukalina .
- Allison S, Palma PM. Commercialization of transgenic plants: potential ecological risks. BioScience. 1997; 47 :86–96. doi: 10.2307/1313019. [ CrossRef ] [ Google Scholar ]
- Ballari VR, Martin A, Gowda LR (2012) Detection and identification of genetically modified EE-1 brinjal ( Solanum melongena ) by single, multiplex and SYBR® real-time PCR. J Sci Food Agric. doi:10.1002/jsfa.5764, Published online 22 June 2012 [ PubMed ]
- Beagle JM, Apgar GA, Jones KL, Griswold KE, Radcliffe JS, Qiu X, Lightfoot DA, Iqbal MJ. The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs. J Anim Sci. 2006; 84 (3):597–607. [ PubMed ] [ Google Scholar ]
- Berberich SA, Ream JE, Jackson TL, Wood R, Stipanovic R, Harvey P, Patzer S, Fuchs RL. The composition of insect-protected cottonseed is equivalent to that of conventional cottonseed. J Agric Food Chem. 1996; 44 :365–371. doi: 10.1021/jf950304i. [ CrossRef ] [ Google Scholar ]
- Bernstein IL, Bernstein JA, Miller M, Tierzieva S, Bernstein DI, Lummus Z, Selgrade MK, Doerfler DL, Seligy VL. Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides . Environ Health Perspect. 1999; 107 :575–582. doi: 10.1289/ehp.99107575. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brake J, Vlachos D. Evaluation of transgenic Event 176 “Bt” corn in broiler chicken. Poult Sci. 1998; 77 :648–653. [ PubMed ] [ Google Scholar ]
- Brian H. Unintended effects of Bt crops. World Watch. 1999; 12 :9–10. [ Google Scholar ]
- Brigulla M, Wackernagel W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl Microbiol Biotechnol. 2010; 86 (4):1027–1041. doi: 10.1007/s00253-010-2489-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burks AW, Fuchs RL. Assessment of the endogenous allergens in glyphosate-tolerant and commercial soybean varieties. J Allergy Clin Immunol. 1995; 96 :1008–1010. doi: 10.1016/S0091-6749(95)70243-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Butler T, Reichhardt T. Long-term effect of GM crops serves up food for thought. Nature. 1999; 398 (6729):651–653. doi: 10.1038/19348. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AMR, Karenlampi S, Kok EJ, Leguay JJ, Lehasranta S, Noteborn HPJM, Pedersen J, Smith M. Unintended effects and their detection in genetically modified crops. Food Chem Toxicol. 2004; 42 :1089–1125. doi: 10.1016/j.fct.2004.02.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chapman MD. Allergen nomenclature. In: Lockey RF, Dennis Ledford K, editors. Allergens and allergen immunotherapy. 4. New York: Informa Healthcare; 2008. pp. 47–58. [ Google Scholar ]
- Clive J (1996) Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. The International Service for the Acquisition of Agri-biotech Applications. http://www.isaaa.org/kc/Publications/pdfs/isaaabriefs/Briefs%201.pdf . Retrieved on 17 July 2010
- Clive J. Global status of commercialized Biotech/GM crops. ISAAA Briefs 43 . Ithaca: International Service for the Acquisition of Agri-biotech Applications; 2011. [ Google Scholar ]
- Conner AJ, Jacobs JME. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat Res Genet Toxicol Environ Mutagen. 1999; 443 :223–234. doi: 10.1016/S1383-5742(99)00020-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crevel RWR, Lerkhof MAT, Koning MMG. Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000; 38 (4):385–393. doi: 10.1016/S0278-6915(99)00158-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deisingh AK, Badrie N. Detection approaches for genetically modified organisms in foods. Food Res Int. 2005; 38 :639–649. doi: 10.1016/j.foodres.2005.01.003. [ CrossRef ] [ Google Scholar ]
- Domingo JL. Health risks of genetically modified foods: many opinions but few data. Science. 2000; 288 :1748–1749. doi: 10.1126/science.288.5472.1748. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ewen SWB, Pusztai A. Effects of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet. 1999; 354 :1353–1354. doi: 10.1016/S0140-6736(98)05860-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fares NH, El-Sayed AK. Fine structural changes in the ileum of mice fed on delta-endotoxin-treated potatoes and transgenic potatoes. Nat Toxins. 1998; 6 :219–233. doi: 10.1002/(SICI)1522-7189(199811/12)6:6<219::AID-NT30>3.0.CO;2-K. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frewer LI, Salter B. Public attitudes, scientific advice and the politics of regulatory policy the case of BSE. Sci Public Policy. 2002; 29 :137–145. doi: 10.3152/147154302781781092. [ CrossRef ] [ Google Scholar ]
- Gerhard F, Andrew C, Karen A. Animal nutrition with feeds from genetically modified plants. Arch Anim Nutr. 2005; 59 :1–40. doi: 10.1080/17450390512331342368. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guertler P, Paul V, Albrech C, Meyer HH. Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Anal Bioanal Chem. 2009; 393 :1629–1638. doi: 10.1007/s00216-009-2667-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamer H, Scuse T (2010) National Agricultural Statistics Service (NASS), Agricultural Statistics Board, US Department of Agriculture. Acreage report, NY
- Hammond BG, Vicini JL, Hartnell GF, Naylor MW, Knight CD, Robinson EH, Fuchs RL, Padgette SR. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. J Nutr. 1996; 126 :717–727. [ PubMed ] [ Google Scholar ]
- Hamstra A (1998) Public opinion about Biotechnology. A survey of surveys. European Federation of Biotechnology, The Hague
- Harrison LA, Bailey MR, Naylor MW, Ream JE, Hammond BG, Nida DL, Burnette BL, Nickson TE, Mitsky TA, Taylor ML, Fuchs RL, Padgette SR. The expressed protein in glyphosate-tolerant soybean, 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium sp. strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J Nutr. 1996; 126 :728–740. [ PubMed ] [ Google Scholar ]
- Hashimoto W, Momma K, Katsube T, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of genetically engineered potatoes with designed soybean glycinin: compositional analyses of the potato tubers and digestibility of the newly expressed protein in transgenic potatoes. J Sci Food Agric. 1999; 79 :1607–1612. doi: 10.1002/(SICI)1097-0010(199909)79:12<1607::AID-JSFA408>3.0.CO;2-T. [ CrossRef ] [ Google Scholar ]
- Hashimoto W, Momma K, Yoon HJ, Ozawa S, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of transgenic potatoes with soybean glycinin by feeding studies in rats. Biosci Biotechnol Biochem. 1999; 63 :1942–1946. doi: 10.1271/bbb.63.1942. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- IRDC (1998) Alliance for biointegrity. http://www.biointegrity.org including Calgene FLAVR SAVR™ tomato report, pp 1–604; International Research and Development Corp. first test report, pp 1736–1738; Conclusions of the expert panel regarding the safety of the FLAVR SAVR™ tomato, ENVIRON, Arlington VA, USA pp 2355–2382; Four week oral (intubation) toxicity study in rats by IRDC, pp 2895–3000
- Ivanciuc O, Schein CH, Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res. 2003; 31 :359–362. doi: 10.1093/nar/gkg010. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joana C, Isabel M, Joana SA, Oliveira MBPP. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Res Int. 2010; 43 :301–306. doi: 10.1016/j.foodres.2009.10.003. [ CrossRef ] [ Google Scholar ]
- Johnson SR. Quantification of the impacts on US Agriculture of Biotechnology-Derived Crops Planted in 2006. Washington DC: National Centre for Food and Agricultural Policy; 2008. [ Google Scholar ]
- Kleter GA, Peijnenburg AACM. Screening of transgenic proteins expressed in transgenic food crops for the presence of short amino acid sequences identical to potential, IgE-binding linear epitopes of allergens. BMC Struct Biol. 2002; 2 :8–19. doi: 10.1186/1472-6807-2-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar GBS, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005; 222 :484–493. doi: 10.1007/s00425-005-1556-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lack G. Clinical risk assessment of GM foods. Toxicol Lett. 2002; 127 :337–340. doi: 10.1016/S0378-4274(01)00517-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- La Mura M, Allnutt TR, Greenland A, Mackay LD. Application of QUIZ for GM quantification in food. Food Chem. 2011; 125 :1340–1344. doi: 10.1016/j.foodchem.2010.10.002. [ CrossRef ] [ Google Scholar ]
- Lappe MA, Bailey EB, Childress C, Setchell KDR. Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. J Med Food. 1999; 1 :241–245. doi: 10.1089/jmf.1998.1.241. [ CrossRef ] [ Google Scholar ]
- Lassen J, Allansdottir A, Liakoupulos M, Olsson A, Mortensen AT. Testing times: the reception of round-up ready soya in Europe. In: Bauer M, Gaskell G, editors. Biotechnology—the making of a global controversy. Cambridge: Cambridge University Press; 2002. pp. 279–312. [ Google Scholar ]
- Louda SM (1999) Insect Limitation of weedy plants and its ecological implications. In: Traynor PL, Westwood J H (eds) Proceedings of a workshop on: ecological effects of pest resistance genes in managed ecosystems. Information Systems for Biotechnology. Blacksburg, Virginia, pp 43–48, http://www.isb.vt.edu
- Mari A, Riccioli D. The allergome web site—a database of allergenic molecules. Aim, structure, and data of a web-based resource. J Allergy Clin Immunol. 2004; 113 :S301. [ Google Scholar ]
- Martinez-Poveda A, Molla-Bauza MB, Gomis FJC, Martinez LMC. Consumer-perceived risk model for the introduction of genetically modified food in Spain. Food Policy. 2009; 34 :519–528. doi: 10.1016/j.foodpol.2009.08.001. [ CrossRef ] [ Google Scholar ]
- Maryanski JH. Bioengineered foods: will they cause allergic reactions? NY: U.S. Food and Drug Administration (FDA)/Centre for Food Safety and Applied Nutrition (CFSAN); 1997. [ Google Scholar ]
- Metcalf DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL (1996) Assessment of the allergenic potential of foods derived from genetically engineered crop plants. In: Crit Rev Food Sci Nutr 36(S):S165–S186. CRC, Boca Raton [ PubMed ]
- Miles S, Frewer LI. Investigating specific concerns about different food hazards—higher and lower order attributes. Food Qual Prefer. 2001; 12 :47–61. doi: 10.1016/S0950-3293(00)00029-X. [ CrossRef ] [ Google Scholar ]
- Miraglia M, Berdal K, Brera C, Corbisier P, Holst-jensen A, Kok E, Marvin H, Schimmel H, Rentsch J, van Rie J, Zagon J. Detection and traceability of genetically modified organisms in the food production chain. Food Chem Toxicol. 2004; 42 :1157–1180. doi: 10.1016/j.fct.2004.02.018. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Momma K, Hashimoto W, Ozawa S, Kawai S, Katsube T, Takaiwa F, Kito M, Utsumi S, Murata K. Quality and safety evaluation of genetically engineered rice with soybean glycinin: analyses of the grain composition and digestibility of glycinin in transgenic rice. Biosci Biotechnol Biochem. 1999; 63 :314–318. doi: 10.1271/bbb.63.314. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakamura R, Matsuda T. Rice allergenic protein and molecular-genetic approach for hypoallergenic rice. Biosci Biotechnol Biochem. 1996; 60 :1215–1221. doi: 10.1271/bbb.60.1215. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netherwood T. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 2004; 22 :204–209. doi: 10.1038/nbt934. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordlee JA. Identification of Brazil-Nut allergen in transgenic soybeans. New Engl J Med. 1996; 334 :688–692. doi: 10.1056/NEJM199603143341103. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordlee JA, Taylor SL, Townsend JA, Thomas LA. Identification of a Brazil nut allergen in transgenic soybean. New Engl J Med. 1996; 334 :688–692. doi: 10.1056/NEJM199603143341103. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Noteborn HPJM, Bienenmann-Ploum ME, van den Berg JHJ, Alink GM, Zolla L, Raynaerts A, Pensa M, Kuiper HA. Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in transgenic tomatoes. In: Engel KH, Takeoka GR, Teranishi R, editors. ACS Symp series 605 Genetically modified foods—safety issues. Washington, D.C: American Chemical Society; 1995. pp. 135–147. [ Google Scholar ]
- Novak WK, Haslberger AG. Substantial equivalence of antinutrients and inherent plant toxins in genetically modified novel foods. Food Chem Toxicol. 2000; 38 :473–483. doi: 10.1016/S0278-6915(00)00040-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Neil C, Reese G, Lehrer SB. Allergenic potential of recombinant food proteins. Allergy Clin Immunol Int. 1998; 10 :5–9. [ Google Scholar ]
- Padgette SR, Taylor NB, Nida DL, Bailey MR, MacDonald J, Holden LR, Fuchs RL. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. J Nutr. 1996; 126 :702–716. [ PubMed ] [ Google Scholar ]
- Pusztai A (2001) Safety tests on commercial crops. American Institute of Biological Sciences. actionbioscience.org, http://www.actionbioscience.org/biotech/pusztai.html viewed 2 March 2010
- Pusztai A, Ewen SWB, Grant G, Peumans WJ, van Damme EJM, Rubio L, Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990; 46 (suppl 2):308–316. doi: 10.1159/000200402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pusztai A, Grant G, Bardocz S, Alonso R, Chrispeels MJ, Schroeder HE, Tabe LM, Higgins TJV. Expression of the insecticidal bean alpha-amylase inhibitor transgene has minimal detrimental effect on the nutritional value of peas fed to rats at 30 % of the diet. J Nutr. 1999; 129 :1597–1603. [ PubMed ] [ Google Scholar ]
- Redenbaugh K, Hiatt W, Martineau B, Kramer M, Sheehy R, Sanders R, Houck C, Emlay D (1992) Safety assessment of genetically engineered fruits and vegetables: a case study of the Flavr Savr Tomato. CRC Press, Boca Raton
- Sahai S. Genetically modified crops: issues for India. Fin Agric. 2003; 35 :7–11. [ Google Scholar ]
- Snell C, Bernheim A, Bergé J-B, Kuntz M, Pascal G, Paris A, Agnès ER. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol. 2012; 50 :1134–1148. doi: 10.1016/j.fct.2011.11.048. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Steinbrecher RA. From green to gene evolution: the environmental risks of genetically engineered crops. Ecologist. 1996; 26 :273–281. [ Google Scholar ]
- Streit L. Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits. Crop Sci. 2001; 41 :1757–1760. doi: 10.2135/cropsci2001.1757. [ CrossRef ] [ Google Scholar ]
- Taylor NB, Fuchs RL, MacDonald J, Shariff AB, Padgette SR. Compositional analysis of glyphosate-tolerant soybeans treated with glyphosate. J Agric Food Chem. 1999; 47 :4469–4473. doi: 10.1021/jf990056g. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teshima R, Akiyama H, Okunuki H, Sakushima J-i, Goda Y, Onodera H, Sawada J-i, Toyoda M. Effect of GM and non-GM soybeans on the immune system of BN rats and B10A mice. J Food Hyg Soc Jpn. 2000; 41 :188–193. doi: 10.3358/shokueishi.41.188. [ CrossRef ] [ Google Scholar ]
- Tsourgiannis L, Karasavvoglou A, Florou G. Consumers’ attitudes towards GM free products in a European region. The case of the Prefecture of Drama-Kavala-Xanthi in Greece. Appetite. 2011; 57 :448–458. doi: 10.1016/j.appet.2011.06.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Beilen JB, Yves P. Harnessing plant biomass for biofuels and biomaterials: production of renewable polymers from crop plants. Plant J. 2008; 54 (4):684–701. doi: 10.1111/j.1365-313X.2008.03431.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vazquez-Padron RI, Moreno-Fierros L, Neri-Bazan L, Martinez-Gil AF, de la Riva GA, Lopez-Revilla R. Characterization of the mucosal and sytemic immune response induced by Cry1Ac protein from Bacillus thuringiensis HD 73 in mice. Braz J Med Biol Res. 2000; 33 :147–155. doi: 10.1590/S0100-879X2000000200002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vijayakumar KR, Martin A, Gowda LR, Prakash V. Detection of genetically modified soya and maize: impact of heat processing. Food Chem. 2009; 117 :514–521. doi: 10.1016/j.foodchem.2009.04.028. [ CrossRef ] [ Google Scholar ]
- Xiumin W, Da T, Qingfeng G, Fang T, Jianhua W. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Control. 2012; 29 :213–220. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Genetic engineering is the act of modifying the genetic makeup of an organism. Modifications can be generated by methods such as gene targeting, nuclear transplantation, transfection of synthetic ...
Genetic engineering is the use of molecular biology technology to modify DNA sequence(s) in genomes, using a variety of approaches. For example, homologous recombination can be used to target specific sequences in mouse embryonic stem (ES) cell genomes or other cultured cells, but it is cumbersome, poorly efficient, and relies on drug positive/negative selection in cell culture for success.
Introduction. Gene therapy is a therapeutic strategy using genetic engineering techniques to treat various diseases. 1, 2) In the early 1960s, gene therapy first progressed with the development of recombinant DNA (rDNA) technology, 1) and was further developed using various genetic engineering tools, such as viral vectors. 3 - 5) More than ...
Manar A. Basheer, Khaled Abutaleb, Nermine N. Abed and Amal A. I. Mekawey. Journal of Genetic Engineering and Biotechnology 2023 21 :127. Research Published on: 21 November 2023. The Correction to this article has been published in Journal of Genetic Engineering and Biotechnology 2023 21 :164. Full Text.
In 1987, the New York Times Magazine characterized the Human Genome Project as the "biggest, costliest, most provocative biomedical research project in history." 2 But in the years between the ...
Journal of Genetic Engineering and Biotechnology was published by Springer between 2019-2023. Journal of Genetic Engineering and Biotechnology is now published by Elsevier, effective January 2024 Journal of Genetic Engineering and Biotechnology is devoted to rapid publication of full-length research papers that lead to significant contribution in advancing knowledge in genetic engineering and ...
K. K. Kang. Kambiz Kalhor. Lailiang Ou. Directed modification of the gene complement of a living organism by such techniques as altering the DNA, substituting genetic material by means ...
Genetic engineering can be used in a diverse range of contexts, including research (e.g., to build model organisms), pharmacology (e.g., for insulin production) and agriculture (e.g., to improve ...
Genetic engineering (GE) is often termed as gene manipulation or recombinant DNA technology with all three often used interchangeably--implying to the manipulation and alteration of the genetic ...
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context.
Abstract. Recent advances in genome engineering technologies based on the CRISPR-associated RNA-guided endonuclease Cas9 are enabling the systematic interrogation of mammalian genome function. Analogous to the search function in modern word processors, Cas9 can be guided to specific locations within complex genomes by a short RNA search string.
April 2001 · Nature Reviews Genetics. Michael Gasson. Derek Burke. Regulation is often seen as the dull end of science. The recent storm over the introduction of genetically modified foods and ...
7.21 Conclusion. Genetic engineering is a paradigmatic case for application of the PP to environmental and public health policy, due to the scientific uncertainty related to the consequences of genetic engineering and the moral uncertainty concerning those consequences. In this chapter I have applied the PP to genetic engineering of microbes ...
Abstract. CRISPR-Cas genetic engineering of plants holds tremendous potential for providing food security, battling biotic and abiotic crop stresses caused by climate change, and for ...
Journal of Genetic Engineering and Biotechnology is devoted to rapid publication of full-length research papers that leads to significant contribution in advancing knowledge in genetic engineering and biotechnology and provide novel perspectives in this research area. JGEB includes all major themes related to genetic engineering and recombinant ...
He stated that his research could help tamp down the HIV/AIDS epidemic; the most hard‐hit areas (such as Africa), however, would likely not gain much benefit from gene‐editing technologies. Per a December 2018 poll, 78 Americans draw the line at so‐called enhancement, but favor the use of genetic engineering to address disease and ...
Caption: Using a variant of CRISPR genome-editing known as prime editing, MIT researchers have developed a method to screen cancer-associated genetic mutations much more easily and quickly than any existing approach. This illustration, by Samuel Gould's brother Owen Gould, is an artistic interpretation of the research and the idea of "rewriting the genome," explains Samuel.
Abstract. Genetic engineering is the best technology that is promoting the world and this technology is applied to many plants, animals and microorganisms. It has wider applications in the field of Biology, Medicine, Industry, Research, Agriculture and many other fields of science. In this research paper I update the Roles of Genetic ...
Genomic engineering is the top-down, global approach to synthetic biology; to be distinguished from bottom-up, local genetic circuit engineering. Latest Research and Reviews Synthetic reversed ...
This sample DNA and genetic engineering research paper features: 10100 words (approx. 33 pages), an outline, and a bibliography with 23 sources. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help ...
Discussions and debates over some of these topics have been held numerous times in the last three decades, especially within the context of in vitro fertilization, transgenic animals, cloning, pre-implantation genetic diagnosis (PGD), research with stem cells and induced pluripotent stem cells, as well as related to the large scope of ...
Citation: Researchers use genetic engineering to create flood-tolerant plants (2024, March 13 ... New research on tungsten unlocks potential for improving fusion materials.
Genetic engineering is the best technology that is promoting the world and this technology is applied to many plants, animals ... Medicine, Industry, Research, Agriculture and many other fields of science. In this research paper I update the Roles of Genetic Engineering in Agriculture, Animals, Human enhancement and Evolution, Bacteriophage ...
Most research effort has been devoted to assessing people's attitudes towards GM foods as a technology. ... Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat Res Genet Toxicol Environ Mutagen. 1999; 443:223-234. doi: 10.1016/S1383-5742(99)00020-4. [Google Scholar] Crevel RWR, Lerkhof MAT, Koning MMG