

Class 9 Science Case Study Questions Chapter 2 Is Matter Around Us Pure
- Post author: studyrate
- Post published:
- Post category: class 9th
- Post comments: 0 Comments
Case study Questions in Class 9 Science Chapter 2 are very important to solve for your exam. Class 9 Science Chapter 2 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving Class 9 Science Case Study Questions Chapter 2 Is Matter Around Us Pure?
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Is Matter Around Us Pure? Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
Case Study/Passage-Based Questions
Case Study 1: Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper.

(i) Identify the technique used by the Akshita. (a) Sedimentation (b) Filtration (c) Chromatography (d) Distillation
Answer: (c) Chromatography.
(ii) What would you expect to see, if the ink contains three different coloured components? (a) We will not see any band on the filter paper. (b) We would see three bands on the filter paper at various lengths. (c) We would see infinite bands on the filter paper. (d) We would see the single band on the filter paper.
Answer: (b) The components of the ink will travel with water and we would see three bands on the filter paper at various lengths.
(iii) An application where you can use this technique is: (a) To separate salt from sand (b) To separate the wheat from the husk (c) To separate oil from water (d) To separate drugs from the blood.
Answer: (d) To separate drugs from blood.
(iv) The above process is used for the separation of : (a) insoluble substances (b) single solute that dissolves in the soluble solvent. (c) solutes that dissolve in the same solvent. (d) solutes that dissolve in the different solvents.
Answer: (c) For the separation of those solutes that dissolve in the same solvent.
(v) What is chromatography? (a) It is an agricultural method to separate grains (b) A method to separate magnetic impurities from non-magnetic impurities
(c) The process of separating the suspended particles of an insoluble substance (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Answer: (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Case Study 2: A homogeneous mixture of two or more substances is called a true solution. it consists of solute and solvent. The particle size of the true solution is less than 1 nanometer. A suspension is a heterogeneous mixture in which the solute particle does not dissolve but remains suspended throughout the bulk of the medium. A colloid is a mixture that is actually heterogeneous but appears to be homogeneous as the particles are uniformly spread throughout the solution.
(i) which one of the following is most stable?
A)True solution
B)Suspensions
D) both A and B
Answer: A)True solution
ii) which type of mixture can be separated by filtration?
D)All of these
Answer: B)Suspensions
iii) which statement is incorrect about the Tyndall effect. *
A)True solution shows Tyndall effect
B)Suspensions show the Tyndall effect
C)Colloid show Tyndall effect
D)Both B and C show the Tyndall effect
Answer: A)True solution shows Tyndall effect
iv) Which is the correct order of stability of solution *
A) True < Colloid<Suspension
B)Colloid<Suspension<True
C)Colloid<True<Suspension
D)Suspension<Colloid<True
Answer: D)Suspension Case Study 3: Matter can be classified into two categories: pure substances and mixtures. Pure substances are made up of a single type of particle and cannot be separated into other substances by physical methods. They have definite and constant properties. On the other hand, mixtures are made up of two or more substances that are physically combined and can be separated into their individual components. Mixtures can be further classified into homogeneous and heterogeneous mixtures. Homogeneous mixtures are uniform in composition, meaning the components are evenly distributed throughout the mixture. Heterogeneous mixtures, on the other hand, have non-uniform composition with visible different parts. It is important to understand the nature of matter around us and differentiate between pure substances and mixtures to comprehend their properties and behavior. What is the main characteristic of a pure substance? a) Made up of two or more substances b) Cannot be separated into other substances c) Has non-uniform composition d) Components are evenly distributed Answer: b) Cannot be separated into other substances Which of the following is an example of a pure substance? a) Air b) Saltwater c) Gold d) Soil Answer: c) Gold How are mixtures different from pure substances? a) Mixtures have definite and constant properties b) Mixtures are made up of a single type of particle c) Mixtures cannot be separated into other substances d) Mixtures are physically combined and can be separated Answer: d) Mixtures are physically combined and can be separated Which type of mixture has a non-uniform composition? a) Homogeneous mixture b) Heterogeneous mixture Answer: b) Heterogeneous mixture What is the primary reason for understanding the nature of matter around us? a) To separate mixtures into pure substances b) To comprehend the properties and behavior of matter c) To classify mixtures into homogeneous and heterogeneous d) To identify the components in pure substances Answer: b) To comprehend the properties and behavior of matter Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure? with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 9 Science Is Matter Around Us Pure? Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible By Team Study Rate Class 9 science mcq questions for chapter 12 sound with answers, class 9 science mcq questions for chapter 14 natural resources with answers, class 9 maths case study questions of chapter 10 circles pdf download, leave a reply cancel reply. Save my name, email, and website in this browser for the next time I comment.You Might Also Like

Class 9th Science - Is Matter Around Us Pure Case Study Questions and Answers 2022 - 2023
By QB365 on 09 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 9th Science Subject - Is Matter Around Us Pure, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Is matter around us pure case study questions with answer key.
9th Standard CBSE
Final Semester - June 2015
(ii) What would you expect to see, if the ink contains three different coloured components? (a) We will not see any band on the filter paper. (b) We would see three bands on the filter paper at various lengths. (c) We would see infinite bands on the filter paper. (d) We would see single band on the filter paper. iii) Give one application where you can use this technique. (a) To separate salt from sand (b) To separate wheat from husk (c) To separate oil from water (d) To separate drugs from blood. (iv) For the separation of what kind of substances is the above process used ? (a) For the separation of insoluble substances (b) For the separation of single solute that dissolves in single solvent. (c) For the separation of those solutes that dissolve in the same solvent. (d) For the separation of those solutes that dissolve in the different solvents. (v) What is chromatography ? (a) It is an agricultural method to separate grains (b) A method to separate magnetic impurities from non-magnetic impurities (c) The process of separating the suspended particles of an insoluble substance (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
(ii) Oil from water.
(iii) Sodium chloride from its solution in water.
(iv) Camphor from salt.
(v) Cream from milk
(ii) Which type of substance can be separated by this method ? (a) Any solvent from its non-volatile solute. (b) The volatile solvent from its non-volatile solute. (c) The non-volatile solvent from its non-volatile solute. (d) The volatile solvent from its volatile solute. (iii) What can we interpret about the nature of ink ?
(iv) Name the component which gets evaporated. ( a) Heating leads to the evaporation of water. (b) Heating leads to the evaporation of dyes. (c) Heating leads to the filtration of water. (d) Heating leads to the distillation of dyes. (v) Define the process shown in the diagram (a) It is the process of conversion of a liquid into its vapours. (b) It is a process of separating insoluble component by filtering the solution (c) It is a process that separates a pure solid in the form of its crystals (d) It is a technique to separate two miscible liquids
(ii) One of the following does not undergo sublimation. This one is :
(iii) The conversion of a solid into vapours without passing through the liquid state is called :
(iv) When heat is constantly supplied by a burner to boiling water, then the temperature of water during vaporisation :
(v) During summer days, water kept in an earthen pot (pitcher) becomes cool because of the phenomenon of :
*****************************************
Is matter around us pure case study questions with answer key answer keys.
(i) (c) Chromatography (ii) (b) We would see three bands on the filter paper at various lengths. (iii) (d) To separate drugs from blood. (iv) (c) For the separation of those solutes that dissolve in the same solvent. (v) (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
(i) (b) Sedimentation and Decantation. (ii) (c) Separating funnel (iii) (d) Evaporation (iv) (c) Sublimation (v) (d) Centrifugation.
(i) (a) Boiling (ii) (b) The volatile solvent from its non-volatile solute. (iii) (d) Ink is a mixture of dyes in water. (iv) (a) Heating leads to the evaporation of water. (v) (a) It is the process of conversion of a liquid into its vapours.
(i) (c) sublimation (ii) (b) sodium chloride (iii) (c) sublimation (iv) (d) does not rise at all (v) (d) evaporation.
Related 9th Standard CBSE Science Materials
9th standard cbse syllabus & materials, class 10th social science - pastoralists case study questions and answers 2022 - 2023, class 9th social science - forest society and colonialism case study questions and answers 2022 - 2023, class 9th social science - nazism and the rise of hitler case study questions and answers 2022 - 2023, class 9th social science - socialism in europe and the russian revolution case study questions and answers 2022 - 2023, class 9th social science - the french revolution case study questions and answers 2022 - 2023, class 9th maths - probability case study questions and answers 2022 - 2023, class 9th maths - statistics case study questions and answers 2022 - 2023, class 9th maths - surface case study questions and answers 2022 - 2023, class 9th maths - linear equations in two variables case study questions and answers 2022 - 2023, class 9th maths - coordinate geometry case study questions and answers 2022 - 2023, rs aggarwal 9th standard maths ncert solutions for probability, rs aggarwal 9th standard maths ncert solutions for statistics, rs aggarwal 9th standard maths ncert solutions for surface areas volumes, rs aggarwal 9th standard maths ncert solutions for heron's formula, rs aggarwal 9th standard maths ncert solutions for constructions.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

9th Standard CBSE Study Materials

9th Standard CBSE Subjects
CBSE Expert
Case Study Questions of Chapter 2 Is Matter Around Us Pure? PDF Download
Case study Questions on Class 9 Science Chapter 2 are very important to solve for your exam. Class 9 Science Chapter 2 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Is Matter Around Us Pure? Case Study Questions With answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
Case Study/Passage-Based Questions
Question 1:
Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper.

(i) Identify the technique used by the Akshita. (a) Sedimentation (b) Filtration (c) Chromatography (d) Distillation
Answer: (c) Chromatography.
(ii) What would you expect to see, if the ink contains three different coloured components? (a) We will not see any band on the filter paper. (b) We would see three bands on the filter paper at various lengths. (c) We would see infinite bands on the filter paper. (d) We would see the single band on the filter paper.
Answer: (b) The components of the ink will travel with water and we would see three bands on the filter paper at various lengths.
(iii) An application where you can use this technique is: (a) To separate salt from sand (b) To separate the wheat from the husk (c) To separate oil from water (d) To separate drugs from the blood.
Answer: (d) To separate drugs from blood.
(iv) The above process is used for the separation of : (a) insoluble substances (b) single solute that dissolves in the soluble solvent. (c) solutes that dissolve in the same solvent. (d) solutes that dissolve in the different solvents.
Answer: (c) For the separation of those solutes that dissolve in the same solvent.
(v) What is chromatography? (a) It is an agricultural method to separate grains (b) A method to separate magnetic impurities from non-magnetic impurities
(c) The process of separating the suspended particles of an insoluble substance (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Answer: (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Question 2:
A homogeneous mixture of two or more substances is called a true solution. it consists of solute and solvent. The particle size of the true solution is less than 1 nanometer. A suspension is a heterogeneous mixture in which the solute particle does not dissolve but remains suspended throughout the bulk of the medium. A colloid is a mixture that is actually heterogeneous but appears to be homogeneous as the particles are uniformly spread throughout the solution.
(i) which one of the following is most stable?
A)True solution
B)Suspensions
D) both A and B
Answer: A)True solution
ii) which type of mixture can be separated by filtration?
D)All of these
Answer: B)Suspensions
iii) which statement is incorrect about the Tyndall effect. *
A)True solution shows Tyndall effect
B)Suspensions show the Tyndall effect
C)Colloid show Tyndall effect
D)Both B and C show the Tyndall effect
Answer: A)True solution shows Tyndall effect
iv) Which is the correct order of stability of solution *
A) True < Colloid<Suspension
B)Colloid<Suspension<True
C)Colloid<True<Suspension
D)Suspension<Colloid<True
Answer: D)Suspension Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure? with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 9 Science Is Matter Around Us Pure? Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible Save my name, email, and website in this browser for the next time I comment. Key Features No thanks, I’m not interested! (I) Read the given passage and answer the questions that follow based on the passage and related studied concepts. A pure substance consist of single type of particles. Mixture consist of more than one kind of pure form of matter. Mixtures can be separated by physical methods but pure substances especially compounds cannot be separated into chemical constituents by physical methods. Pure substance has same composition throughout. Soil and soft drinks are mixtures. Mixtures can be separated by various methods depending upon nature of substance present in it. Solution is a homogeneous mixture. Q1: Name the process by which pure NaCl can be obtained from salt solution. Ans: Crystallisation Q2: What are alloys-compounds or mixtures? Ans: Alloys are homogeneous mixtures of two or more metals or a metal and a non-metal e.g. Brass is alloy of Cu and Zn Q3: What is size of particles in solution? Ans: The size of particles in solutions are less than 1nm (10 –9 m). Q4: What is solute and solvent in cold drinks? Ans: CO 2 gas, sugar, preservative are solute and water is solvent in cold drinks.Leave a Comment Cancel reply
Download India's best Exam Preparation App Now.
Class 9 Science Chapter 2 Case Based Questions - Is Matter Around Us Pure??
Q2: How can gases be separated that are present in air? Ans: By fractional distillation of liquid air.
Q3: If sample of urea has melting point 129°C, then: (a) It is impure (b) It is pure (c) It can not be predicted (d) The compound is not urea Ans: (a) It is impure
Q4: Which of the following will have boiling point 100°C? (a) Distilled water (b) Sea water (c) River water (d) Well water Ans: (a) Distilled water will have boiling point 100°C.
Top Courses for Class 9
Semester notes, objective type questions, study material, video lectures, extra questions, shortcuts and tricks, previous year questions with solutions, past year papers, sample paper, viva questions, important questions, practice quizzes, mock tests for examination.

Case Based Question Answer: Is Matter Around Us Pure? Free PDF Download
Importance of case based question answer: is matter around us pure, case based question answer: is matter around us pure notes, case based question answer: is matter around us pure class 9 questions, study case based question answer: is matter around us pure on the app, welcome back, create your account for free.

Forgot Password
Unattempted tests, change country.
- RS Aggarwal
- ML Aggarwal
- Merchant of Venice
- NCERT Books
- Questions and Answers
- NCERT Notes
- Important Questions
- Is Matter Around Us Pure
NCERT Revision Notes for Chapter 2 Is Matter Around Us Pure Class 9 Science
Anything which occupies space and has mass is called matter. Matter can be divided in two categories. They are:
(i) Pure Substance: It consists of single types of particles which are same in their chemical nature.
(ii) Mixtures: Mixture consists of two or more particles.
Mixture consists of more than one kind of pure substances which can be separated by physical method.
Mixtures are of two types, they are:
(i) Homogeneous mixture
(ii) Heterogeneous mixture
(i) Homogeneous mixture: A mixture is said to be homogeneous if all the components of the mixture are uniformly mixed and there are no boundaries of separation between them.
Ex: Sugar in water, etc.
(ii) Heterogeneous mixtures: A mixture is said to be heterogeneous if all the components of the mixture are not uniformly mixed and there are visible boundaries of separation between them.
Ex: Water and sand, Air etc.
Solution and its properties
A solution is a homogeneous mixture of two or more substances. Ex: Lemonade, soda water etc.
A solution has two components : (i) Solvent and (ii) Solute
(i) Solvent: The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
(ii) Solute: The component of the solution that is dissolved in the solvent (usually present in lesser quantity) is called the solute.
Properties of Solution:
1. A solution is a homogeneous mixture.
2. The particles of a solution are smaller than 1 nm (10 -9 ) in diameter which cannot be seen by naked eyes.
3. They do not scatter a beam of light passing through the solution that is they don’t show tyndall effect. So, the path of light is not visible in a solution.
4. The solute particles cannot be separated from the mixture by the process of filtration.
5. The solution is stable and solute particles do not settle down when left undisturbed.
Concentration of a solution
(i) Saturated solution: When no more amount of solute can be dissolved in a solution at a given temperature, it is called a saturated solution.
(ii) Unsaturated solution: When more amount of solute can be dissolved in a solution at a given temperature, it is called a saturated solution.
(ii) Solubility: The amount of the solute present in the saturated solution at the given temperature is
called its solubility.
The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution. Also, the amount of solute dissolved in a given mass or volume of solvent is called concentration of solution.
Concentration of solution = Amount of solute/Amount of solvent or Amount of solute/Amount of solution (Here, amount means mass or volume).
Two methods of finding concentration of solution:
(i) Mass by mass percentage of a solution = (Mass of solute/Mass of solution) ×100
(ii) Mass by volume percentage of a solution = (Mass of solute/Volume of solution) ×100
A suspension is a heterogeneous mixture in which the the solute particles do not dissolve but remain suspended throughout the bulk of the medium. Ex: Chalk in water, smoke in the air.
Properties of Suspension :
1. It is a heterogeneous mixture.
2. Particles of a suspension are visible to the naked eye.
3. Size of the particles is greater than 100 nm.
4. It is unstable mixture. Solute settles down at the bottom over period of time.
5. If the solution is passed through filter paper, solute and solvent gets separated.
6. It scatters light when light is passed through the solution i.e. it shows Tyndall effect.
Colloid solution is heterogeneous mixture in which the size of particles lies between the true solutions and suspensions.
• Colloidal particles can easily scatter a beam of visible light. This phenomenon is called Tyndall effect.
Properties of colloidal solution:
1. The particles of colloid can’t be seen by naked eyes individually.
2. It is a heterogeneous mixture and thus solute and solvent can’t be separated by filter paper.
3. Size of particles is smaller than suspensions but greater than solutions (1 nm to 100 nm).
4. It is a stable mixture. Particles do not settle down at the bottom over a period of time.
5. They do not settle down when left undisturbed which means colloid is quite stable.
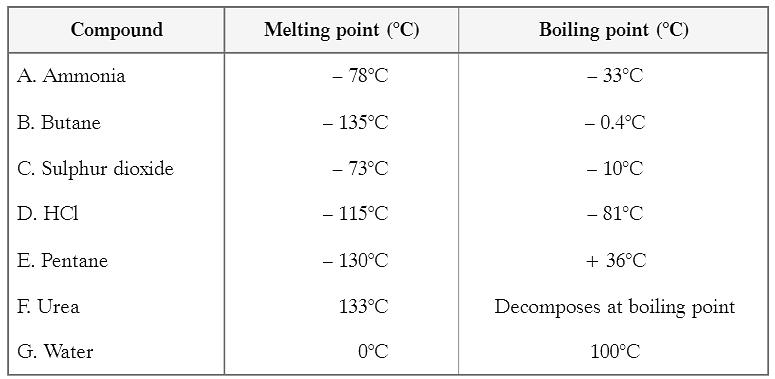
Some common examples of colloids (in the table)
Process of evaporation is used to obtain coloured components from blue/black ink. The process of evaporation is used to separate a substance which is dissolved in water.
• It is based on the fact that liquid vaporises easily than the solid.
• Helps in separating volatile substances from non-volatile substances.

Steps of obtaining coloured components from blue/black ink:
• Fill half a beaker with water.
• Put a watch glass on the mouth of the beaker.
• Put few drops of ink on the watch glass.
• Now start heating the beaker. We do not want to heat the ink directly. You will see that evaporation is taking place from the watch glass.
• Continue heating as the evaporation goes on and stop heating when you do not see any further change on the watch glass.
• The process of centrifugation is used to separate the cream from milk. It is a method of separating the suspended particles of substance from a liquid.
• This process is carried out by the machine called centrifuge.
• Sometimes, the solid particles in a liquid are very small and pass through a filter paper. For such particles the filtration technique cannot be used.
• The mixture is rotated rapidly so that the heavier particles in the mixtures settle down to the bottom.
• The basic principle of centrifugation is that the denser particles are forced to the bottom and the liquid being lighter remains at the top.
Steps of separating cream from milk:
• Take some full-cream milk in a test tube.
• Centrifuge it by using a centrifuging machine for two minutes.
Application of centrifugation:
• Used in diagnostic laboratories for blood and urine tests.
• Used in dairies and home to separate butter from cream.
• Used in washing machines to squeeze out water from wet clothes.
• The separation of separating two immiscible liquid is carried out by the use of funnel.
• The basic principle involve is the difference between the densities of two liquids form two separate layers.
Steps of separating kerosene oil and water:
• Pour the mixture of kerosene oil and water in a separating funnel.
• Let it stand undisturbed for sometime so that separate layers of oil and water are formed.
• Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
• Close the stopcock of the separating funnel as the oil reaches the stop-cock.
Application of funnel:
• To separate mixture of oil and water.
• In the extraction of iron from its ore, the lighter slag is removed from the top by this method to leave the molten iron at the bottom in the furnace.
• This process is used to separate mixtures that contain a sublimable volatile component from a non-sublimable impurity.
• Sublimation is process where a substance directly changes from solid to gaseous state on heating.
• Ammonium chloride, camphor, naphthalene and anthracene are some examples which can sublime.
• Used for separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
• Mixture of acetone and water is separated by this method.
• Fractional distillation is used to separate a mixture of two or more miscible liquids for which the difference in boiling points is less than 25 K.
• Air is a homogeneous mixture and can be separated into its components by fractional distillation. Below is diagram which shows the steps of separation of air:
• The air is compressed by increasing the pressure and is then cooled by decreasing the temperature to get liquid air.
• The liquid air is warm-up slowly in a fractional distillation column, where gases get separated at different heights depending upon their boiling points.
• It used to separate a gas from the air.
• Used to remove impurities from solid and purify it.
• It separates a pure solid from mixture in the form of crystals.
• This process is used in purification of salt from sea water, separation of crystals of alum from impure samples.
• It is better method than evaporation because:
(i) Solids decompose or some, like sugar, may get charred on heating to dryness.
(ii) Some impurities may remain dissolved in the solution even after filtration. On evaporation these contaminate the solid.
• The process which brings about changes in physical properties and no new substances are formed are physical changes. The common physical changes are changes in colour, hardness, rigidity, fluidity, density, melting point, boiling point etc.
• The process in which new substances are formed and chemical properties of substances get changed are chemical changes. Some chemical properties are odour, inflammability etc.
The pure substance is divided in two types on the basis of their chemical composition: (i) Elements and (ii) Compounds
(i) Elements
• According to Antoine Laurent Lavoisier, element is a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
• It is divided in three types which are metals, non-metals and metalloids.
Properties of Metals
(i) They have a lustre (shine).
(ii) They have silvery-grey or golden-yellow colour.
(iii) They conduct heat and electricity.
(iv) They are ductile (can be drawn into wires).
(v) They are malleable (can be hammered into thin sheets).
(vi) They are sonorous (make a ringing sound when hit).
• Examples of metals are gold, silver, copper, iron, sodium, potassium etc.
• Mercury is the only metal that is liquid at room temperature.
Properties of non-metals
(i) They display a variety of colours.
(ii) They are poor conductors of heat and electricity.
(iii) They are not lustrous, sonorous or malleable.
• Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine etc.
Metalloids: Elements having intermediate properties between those of metals and non-metals are called metalloids. Examples are boron, silicon, germanium etc.
A compound is a substance composed of two or more elements, chemically combined with one another in a fixed proportion.
NCERT Solutions for Chapter 4 The Age of Industrialisation Class 10 History
Related chapters.
- Matter in Our Surroundings
- Atoms and Molecules
- Structure of the Atom
- The Fundamental Unit of Life
Related Questions
- NCERT Solutions for Chapter 2 Is Matter around us Pure? Class 9 Science
- VSAQ for Ch 2 Is Matter around us Pure Class 9 Science NCERT
Report a problem
- Question is incorrect
- Answer is Incorrect
- Spelling Mistakes
- Not explained in detail
NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure
NCERT Solutions for Class 9 Science (chemistry) Chapter 2 Is Matter Around Us Pure are given below. In these solutions, we have answered all the intext and exercise questions provided in NCERT class 9 science textbook. Class 9 NCERT Solutions Science Chapter 2 provided in this article are strictly based on the CBSE syllabus and curriculum. Students can easily download these solutions in PDF format for free from our app.
Class 9 Science Chapter 2 Textbook Questions and Answers
Intext Questions Page No. 15
Questions 1: what do mean by a pure substance?
Answer: A pure substance is the one that consists of a single type of particles, i.e., all constituent particles of the substance have the same chemical nature. Pure substances can be Classified as elements or compounds.
Question 2: List the points of differences between homogeneous and heterogeneous mixtures.
Page No. 18
Question 1: Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer: A homogeneous mixture is a mixture having a uniform composition throughout the mixture. For example, mixtures of salt in water, sugar in water, copper sulphate in water, iodine in alcohol, alloy, and air have uniform compositions throughout the mixtures.
On the other hand, a heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture. For example, composition of mixtures of sodium chloride and iron fillings, salt and sulphur, oil and water, chalk powder in water, wheat flour in water, milk and water are not uniform throughout the mixtures.
Question 2: How are sol, solution and suspension different from each other?
Answer: Sol is a heterogeneous mixture. In this mixture, the solute particles are so small that they cannot be seen with the naked eye. Also, they seem to be spread uniformly throughout the mixture. The Tyndall effect is observed in this mixture. For example: milk of magnesia, mud
Solution is a homogeneous mixture. In this mixture, the solute particles dissolve and spread uniformly throughout the mixture. The Tyndall effect is not observed in this mixture.
For example: salt in water, sugar in water, iodine in alcohol, alloy
Suspensions are heterogeneous mixtures. In this mixture, the solute particles are visible to the naked eye, and remain suspended throughout the bulk of the medium. The Tyndall effect is observed in this mixture.
For example: chalk powder and water, wheat flour and water
Question 3: To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
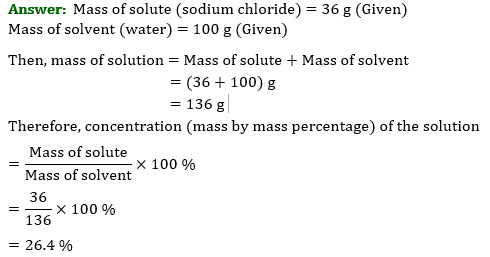
PAGE NO. 24 (I)
Question 1: How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer: A mixture of two miscible liquids having a difference in their boiling points more than 25°C can be separated by the method of distillation. Thus, kerosene and petrol can be separated by distillation.
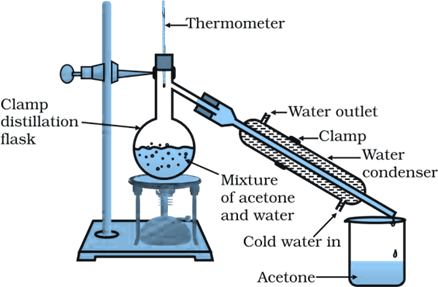
In this method, the mixture of kerosene and petrol is taken in a distillation flask with a thermometer fitted in it. We also need a beaker, a water condenser, and a Bunsen burner. The apparatus is arranged as shown in the above figure. Then, the mixture is heated slowly. The thermometer should be watched simultaneously. Kerosene will vaporize and condense in the water condenser. The condensed kerosene is collected from the condenser outlet, whereas petrol is left behind in the distillation flask.
Question 2: Name the technique to separate (i) butter from curd (ii) salt from sea-water (iii) camphor from salt
Answer: (i) Butter can be separated from curd by centrifugation.
(ii) Salt can be separated from sea-water by evaporation.
(iii) Camphor can be separated from salt by sublimation.
Question 3: What type of mixtures is separated by the technique of crystallization?
Answer: By the technique of crystallization, pure solids are separated from impurities. For example, salt obtained from sea is separated from impurities; crystals of alum (Phitkari) are separated from impure samples.
PAGE NO 24(II)
Question 1: Classify the following as chemical or physical changes:
- Cutting of trees
- Melting of butter in a pan
- Rusting of almirah
- Boiling of water to form steam
- Passing of electric current through water and water breaking into hydrogen and oxygen gases.
- Dissolving common salt in water
- Making a fruit salad with raw fruits, and
- Burning of paper and wood
Answer: Cutting of trees → Physical change
Melting of butter in a pan → Physical change
Rusting of almirah → Chemical change
Boiling of water to form steam → Physical change
Passing of electric current through water, and water breaking down into hydrogen and oxygen gas → Chemical change
Dissolving common salt in water → Physical change
Making a fruit salad with raw fruits → Physical change
Burning of paper and wood → Chemical change
Question 2: Try segregating the things around you as pure substances or mixtures.
Answer: Listed below are the classifications based on pure substances and mixtures:
Question 1: Which separation techniques will apply for the separation of the following? (a) Sodium chloride from its solution in water. (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride. (c) Small pieces of metal in the engine oil of a car. (d) Different pigments from an extract of flower petals. (e) Butter from curd. (f) Oil from water. (g) Tea leaves from tea. (h) Iron pins from sand. (i) Wheat grains from husk. (j) Fine mud particles suspended in water.
Answer: (a) Sodium chloride from its solution in water → Evaporation
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride → Sublimation
(c) Small pieces of metal in the engine oil of a car → Centrifugation or filtration or decantation
(d) Different pigments from an extract of flower petals → Chromatography
(e) Butter from curd → Centrifugation
(f) Oil from water → Using separating funnel
(g) Tea leaves from tea → Filtration
(h) Iron pins from sand → Magnetic separation
(i) Wheat grains from husk → Winnowing
(j) Fine mud particles suspended in water → Centrifugation
Question 2: Write the steps you would use for making tea. Use the words – solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer: 1. Take a cup of water in a container as solvent and heat it. 2. Add sugar in it which is solute. Heat it till all sugar dissolves. 3. You get a solution of water and sugar. 4. Sugar is soluble in water completely. 5. Add half a tea-spoon of tea-leaves, it is insoluble in water. 6. Boil the content, add milk which is also soluble in water, boil again. 7. Filter the tea with the help of strainer, the tea collected in cup is filtrate and the tea leaves collected on the strainer is residue.
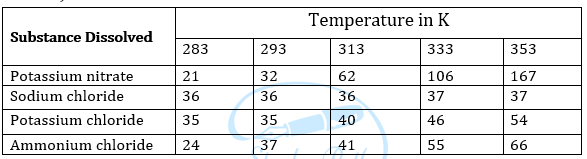
Question 3: Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer: (a) Mass of potassium nitrate required to produce a saturated solution in 100 g of water at 313 K = 62g
∴ Mass of potassium nitrate required to produce a saturated solution in 50 g of water = (62 × 50)/100 = 31 Hence 31 g of potassium nitrate is required.
(b) Some amount of dissolved Potassium Chloride will reappear as undissolved solid as solubility of solute decreases with the decrease of temperature.
(c) Solubility of each salt at 393 K are as follows:
- Potassium nitrate – 32 g
- Sodium chloride – 36 g
- Potassium chloride – 35 g
- Ammonium chloride – 37 g
- Ammonium chloride has the highest solubility at 293 K.
(d) Solubility of salt increases with the increase in temperature.
Question 4: Explain the following giving examples. (a) Saturated solution, (b) Pure substance, (c) Colloid, (d) Suspension.
Answer: (a) Saturated Solution: A solution in which no more of the solid (solute) can be dissolved at a given temperature is called a saturated solution. Suppose 50 gm of a solute is the maximum amount that can be dissolved in 100 gm water at 298 K. Then 150 gm of solution so obtained is the saturated solution at 298 K.
A saturated solution is a solution in which the maximum amount of solute has been dissolved at a given temperature. The solution cannot dissolve beyond that amount of solute at that temperature. Any more solute added will settle down at the bottom of the container as a precipitate. Suppose 500 g of a solvent can dissolve a maximum of 150 g of a particular solute at 40°C. Then, the solution obtained by dissolving 150 g of that solute in 500 g of that solvent at 300 K is said to be a saturated solution at 300 K.
Pure Substance: A pure substance consists of a single of matter or particles and cannot be separated into other kind of matter by any physical process. Pure substances always have the same colour, taste and texture at a given temperature and pressure. For example, pure water is always colourless, odorless and tasteless and boils at 373 K at normal atmospheric pressure.
Colloid: Colloid A colloid is a heterogeneous mixture. The size of the solutes in this mixture is so small that they cannot be seen individually with naked eyes, and seems to be distributed uniformly throughout the mixture. The solute particles do not settle down when the mixture is left undisturbed. This means that colloids are quite stable. Colloids cannot be separated by the process of filtration. They can be separated by centrifugation. Colloids show the Tyndall effect. For example, milk, butter, foam,fog, smoke, clouds.
Suspension: Suspension Suspensions are heterogeneous mixtures. The solute particles in this mixture remain suspended throughout the bulk of the medium. The particles can be seen with naked eyes. Suspension shows the Tyndall effect. The solute particles settle down when the mixture is left undisturbed. This means that suspensions are unstable. Suspensions can be separated by the method of filtration. For example, mixtures of chalk powder and water, wheat flour and water.
Question 5. Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air. soil, vinegar, filtered tea.
Answer: Homogeneous: Soda water, vinegar, filtered tea. Heterogeneous: Wood, air, soil.
Question 6. How would, you confirm that a colourless liquid given to you is pure water?
Answer: We can confirm if a colourless liquid is pure by setting it to boil. If it boils at 100°C it is said to be pure. But if there is a decrease or increase in the boiling point, we infer that water has added impurities hence not pure.
Question 7. Which of the following materials fall in the category of a “pure substance”? (a) Ice (b) Milk (c) Iron (d) Hydrochloric acid (e) Calcium oxide (f) Mercury (g) Back (h) Wood (i) Air.
Answer: Following substances from the above-mentioned list are pure substances:
- Hydrochloric acid
- Calcium oxide
Question 8. Identify the solutions among the following mixtures. (a) Soil (b) Sea water (c) Air (d) Coal (e) Soda water.
Answer: The following are the solutions from the above-mentioned list of mixture:
Question 9. Which of the following will show “Tyndall effect”? (a) Salt solution (b) Milk (c) Copper sulphate solution (d) Starch solution.
Answer: Milk and starch solution will show the “Tyndall effect”.
Question 10. Classify the following into elements, compounds and mixtures. (a) Sodium (b) Soil (c) Sugar solution (d) Silver (e) Calcium carbonate (f) Tin (g) Silicon (h) Coal (i) Air (j) Soap (k) Methane (l) Carbon dioxide (m) Blood

Question 11. Which of the following are chemical changes? (a) Growth of a plant (b) Rusting of iron (c) Mixing of iron filings and sand (d) Cooking of food (e) Digestion of food (f) Freezing of water (g) Burning of a candle.
Answer: Chemical changes are: (a) Growth of a plant (b) Rusting of iron (d) Cooking of food (e) Digestion of food (g) Burning of candle
Class 9 Science NCERT Solutions Chapter 2 Is Matter Around Us Pure
CBSE Class 9 Science NCERT Solutions Chapter 2 helps students to clear their doubts and to score good marks in the board exam. All the questions are solved by experts with a detailed explanation that will help students complete their assignments & homework. Having a good grasp over CBSE NCERT Solutions for Class 9 Science will further help the students in their preparation for board exams and other competitive exams such as NTSE, Olympiad, etc.
NCERT Solutions for Class 9 Science Chapter 2 PDF
Below we have listed the topics discussed in NCERT Solutions for Class 9 Science Chapter 2. The list gives you a quick look at the different topics and subtopics of this chapter.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
The Site is down as we are performing important server maintenance, during which time the server will be unavailable for approximately 24 hours. Please hold off on any critical actions until we are finished. As always your feedback is appreciated.

- Study Packages
- NCERT Solutions
- Sample Papers
- Online Test

- Questions Bank
- Is Matter Around Us Pure
- Test Series
- Ncert Solutions
- Solved Papers
- Current Affairs
- JEE Main & Advanced
- Pre-Primary
- MP State Exams
- UP State Exams
- Rajasthan State Exams
- Jharkhand State Exams
- Chhattisgarh State Exams
- Bihar State Exams
- Haryana State Exams
- Gujarat State Exams
- MH State Exams
- Himachal State Exams
- Delhi State Exams
- Uttarakhand State Exams
- Punjab State Exams
- J&K State Exams
9th Class Science Is Matter Around Us Pure Question Bank
Done case based mcqs - is matter around us pure total questions - 30.
A) an element done clear
B) a compound done clear
C) a mixture done clear
D) a solution done clear
question_answer 2) Which of the following substances is a compound?
A) Oxygen done clear
B) Common salt done clear
C) Gold done clear
D) Air done clear
question_answer 3) The substance formed by mixing, crushing and heating iron filings sulphur powder is
A) an element done clear
C) a mixture done clear
question_answer 4) Which of the following property does not prove that water is a compound?
A) Water is made up of two different elements done clear
B) Water has fixed boiling point done clear
C) The constituents of water cannot be separated by simple physical methods done clear
D) Distilled water and tap water have same taste and constituents done clear
question_answer 5) Blood is considered as:
A) an element done clear
C) a mixture done clear
A) I done clear
B) III done clear
C) IV done clear
D) II done clear
question_answer 7) Which substance show maximum change in its solubility, when the temperature is raised from 313 K to 333 K.
B) II done clear
C) III done clear
D) IV done clear
question_answer 8) In the above question, if the amount of water taken is reduced by 20 per cent, what amount of ammonium chloride would be required to prepare its saturated solution at 353 K?
A) 86g done clear
B) 53g done clear
C) 50g done clear
D) 36g done clear
question_answer 9) What is the effect of temperature on the solubility of a salt?
A) It increases with decrease in temperature done clear
B) It decreases with increase in temperature done clear
C) It increases with increase in temperature done clear
D) It does not depend on temperature done clear
question_answer 10) To make lemonade, salts is added in water. The addition of salt in water is a.......
A) suspension done clear
B) colloid done clear
C) heterogeneous solution done clear
D) homogeneous solution done clear
A) In first case, the path is visible because of the presence of impurities done clear
B) In second case, the particles settle down done clear
C) In second case, the concentration increases done clear
D) In second case, the impurities dissolve in the solution done clear
question_answer 12) What is the nature of solution obtained, when X is added to water?
A) Colloid done clear
B) True solution done clear
C) Suspension done clear
D) Data insufficient done clear
question_answer 13) Which of the following show Tyndall effect?
A) Sugar solution done clear
B) Salt solution done clear
C) Starch solution done clear
D) Copper sulphate solution done clear
question_answer 14) Which of the following is an example of colloidal solution?
A) Milk done clear
B) Urea done clear
C) Sugar in water done clear
D) Common salt in water done clear
question_answer 15) When the light passes through the solution in this experiment, then
A) scattering of light is observed done clear
B) path of light becomes visible done clear
C) Tyndall effect is observed done clear
D) AII of the above done clear
A) maintain equal composition of substance done clear
B) obtain a pure sample of a substance done clear
C) make it homogeneous mixture done clear
question_answer 17) The mixture of ethyl alcohol and water can be separated by
A) distillation done clear
B) centrifugation done clear
C) filtration done clear
D) chromatography done clear
question_answer 18) Which of the following technique is used by farmers in village to purify food grains?
A) Sieving done clear
B) Hand picking done clear
C) Winnowing done clear
D) AII of these done clear
question_answer 19) The principle of separation based on
A) the difference in sizes of constituents done clear
B) the difference in physical or chemical properties of constituents done clear
C) the different techniques done clear
question_answer 20) By filtration, which of the following mixture can be separated?
A) Sugar in water done clear
B) Milk in water done clear
C) Sand in water done clear
D) Oil in water done clear
A) size done clear
B) temperature done clear
C) phase done clear
D) proportions done clear
question_answer 22) The interconversion of solid, liquid and gas state is a
A) physical change done clear
B) chemical change done clear
C) Both [a] and [b] done clear
D) no change done clear
question_answer 23) Burning is a chemical change and burning of candle is
question_answer 24) Colour, hardness, melting points, boiling points, odour, etc., are
A) chemical properties done clear
B) physical properties done clear
C) Both [a] and [b] done clear
D) example of chemical reactions done clear
question_answer 25) Chemical changes are
A) temporary and irreversible done clear
B) permanent and irreversible done clear
C) permanent and reversible done clear
D) temporary and reversible done clear
A) Chloroform and water done clear
B) Milk and water done clear
C) Acetone and ethanol done clear
D) Impurities in sea water done clear
question_answer 27) The distillation is the best technique to separate liquids having different.
A) Solubility done clear
B) Melting points done clear
C) Boiling point done clear
question_answer 28) The residue left in the round bottom flask in the distillation process is a liquid having
A) high boiling point done clear
B) low boiling point done clear
C) impurity done clear
D) high solubility done clear
question_answer 29) The observation made from distillation process is
A) acetone boils first done clear
B) water boils first done clear
C) impurity evaporates done clear
D) water boils at 363 K done clear
question_answer 30) The distillation process involves the
A) decomposition and condensation processes done clear
B) evaporation and condensation processes done clear
C) heating and sublimation processes done clear
D) only evaporation process done clear
Study Package

Case Based MCQs - Is Matter Around us Pure
Related question.

Reset Password.
OTP has been sent to your mobile number and is valid for one hour
Mobile Number Verified
Your mobile number is verified.

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure
- Last modified on: 3 years ago
- Reading Time: 26 Minutes
Here we have given NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure.
INTEXT Questions
Question 1. What is meant by a pure substance? Solution: A pure substance is one which is made up of only one kind of atoms or molecules. Ex: water is made up of only one kind of particles. So water is a pure substance,
Question 2. List the points of differences between homogeneous and heterogeneous mixtures. Solution:
Question 3. Differentiate between homogeneous and heterogeneous mixtures with examples. Solution:
Question 4. How are sol, solution and suspension different from each other? Solution:
Question 6. How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other? Solution: The mixture of two miscible liquids such as kerosene and petrol whose boiling points differ by more than 25°C can be easily separated by the technique of simple distillation.
At the boiling point of more volatile (low boiling) liquid of the mixture, the vapours almost exclusively consist of the more volatile liquid. Likewise at the boiling point of the less volatile (high boiling) liquid, vapours almost entirely consist of the less volatile liquid since the more volatile liquid has already distilled over.
Question 7. Name the technique to separate (i) butter from curd (ii) salt from sea water (iii) camphor from salt. Solution: (i) Butter from curd can be separated by the technique of centrifugation. (ii) Salt from sea water can be separated by the technique of crystallisation or evaporation. (iii) Camphor is sublimable but salt is not. So, camphor can be separated from salt by sublimation.
Question 8. What type of mixtures are separated by the technique of crystallisation? Solution: Homogeneous mixtures such as common salt solution and copper sulphate solution are separated by the technique of crystallisation.
Question 9. Classify the following as chemical or physical changes:
- cutting of trees
- melting of butter in a pan
- rusting of almirah
- boiling of water to form steam
- passing of electric current through water and the water breaking down into hydrogen and oxygen
- dissolving common salt in water
- making a fruit salad with raw fruits
- burning of paper and wood
- Cutting of trees is a chemical change since all chemical reactions stop and we cannot get back the original tree from the wooden pieces.
- Melting of butter in a pan is a physical change since there is no change in the chemical composition of butter, only the physical state changes from solid to liquid.
- Rusting of almirah is a chemical change since during rusting, a new chemical compound called hydrated iron oxide (rust) is formed.
- Boiling of water to form steam is a physical change because during this change only change of state occurs from liquid water to steam (gaseous) without any change in its chemical composition.
- Passing of electric energy through water to form hydrogen and oxygen gases is a chemical change since the properties of hydrogen (combustible gas) and oxygen (supporter of combustion) are altogether different from those of water which is neither combustible nor a supporter of combustion but it actually extinguishes fire.
- Dissolution of common salt in water is a physical change since salt can be easily recovered by evaporating water.
- Making a fruit salad with raw fruits is a physical change since there is no change in the chemical properties of the fruits but only the physical appearance has changed.
- Burning of paper is a chemical change since carbon dioxide, water vapours, smoke and ash which are the products of combustion cannot be converted back into paper or wood by any physical method.
Question 10. Try segregating the things around you as pure substances or mixtures. Solution: Pure substances : Distilled water, diamond, graphite, gold, sulphur Mixtures : Curd, ice cream, kerosene oil, cooking oil, steel, vulcanised rubber, solder wire (alloy of lead and tin).
NCERT Exercises
Question 1. Which separation techniques will you apply for the separation of the following? (a) Sodium chloride from its solution in water. (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride. (c) Small pieces of metal in the engine oil of a car. (d) Different pigments from an extract of flower petals. (e) Butter from curd. (f) Oil from water. (g) Tea leaves from tea. (h) Iron pins,from sand. (i) Wheat grains from husk. (j) Fine mud particles suspended in water. Solution: (a) Evaporation : Water will evaporate leaving behind sodium chloride. (b) Sublimation : Ammonium chloride will be collected as sublimate. (c) Filtration : Pieces of metal can be separated by filtration. (d) Chromatography : Pigments (coloured components) from the extract of flower plants can be separated by chromatography. (e) Centrifugation: Butter will get separated upon centrifugation. (f) Separating funnel : Oil and water can be separated by the use of separating funnel. (g) Filtration: Upon filtration through a sieve, tea leaves will be collected on the sieve. (h) Magnetic separation : A magnet will attract iron pins and not sand particles. (i) Sieving : Wheat grains from husk can be separated with the help of sieves. (j) Sedimentation : As a result of sedimentation, mud particles will settle down and can be separated later on by filtration.
Question 2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue. Solution: Take 100 mL of water which acts as solvent. Boil water on a gas stove. Add one teaspoon of sugar which acts as solute. Sugar is soluble in water, so gets dissolved in water and forms a solution. Now add about half a teaspoon of tea leaves which are insoluble in water. Boil the contents for 4 to 5 minutes and add a half cup of milk and allow to boil again for 2-3 minutes. Filter the tea with the help of a sieve. Tea leaves will be left as residue while tea will be obtained as filtrate.
Question 3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to to from a saturated solution)
Question 4. Explain the following giving examples.
- Saturated solution
- Pure substance
- Saturated solution : A solution in whichno more solute canbe dissolved ina given amount of solvent at a particular temperature is called saturated solution. For example, if we dissolve 40 g sodium chloride in 100 g of water at 293 K, it will form a saturated solution because the solubility of sodium chloride at 293 K is 36 g per 100 g of water.
- Pure substance : A substance made up of only one kind of atoms or molecules is called a pure substance. A pure substance has the same colour, taste and texture at a given temperature and pressure. A pure substance also has a fixed melting and boiling point at a constant pressure. For example, hydrogen gas, sodium chloride, water, etc.
- Colloid : A substance is said to be a colloid if the particle size lies between 1 to 100 nm. A colloidal solution is heterogeneous and consists of two phases. i.e., dispersed phase (colloidal particles) and dispersion medium in which colloidal particles are suspended. For example, colloidal solution of sulphur or starch, milk, etc.
- Suspension : It is a heterogeneous mixture in which the particles of the solute do not dissolve but remain suspended throughout the bulk of the solvent. The size of the suspension particles is more than lCk7 m. For example, chalk powder in water is a suspension.
Question 5. Classify each of the following as a homogeneous or heterogeneous mixtures: Soda water, wood, air, soil, vinegar, filtered tea Solution: Homogeneous mixtures : Soda water, vinegar and filtered tea. Heterogeneous mixtures : Wood and soil. Air is a homogeneous mixture of different gases. However, if some dust or other particles are also present, then air becomes heterogeneous mixture.
Question 6. How would you confirm that a colourless liquid given to you is pure water? Solution: If the boiling point and freezing point of the given liquid comes out to be 100°C or 373 K and d°C or 273 K respectively under one atmospheric pressure, it confirms that the given liquid is pure water.
Question 7. Which of the following materials fall in the category of a “pure substance”? (a) Ice, (b) Milk, (c) Iron, (d) Hydroelectric acid, (e) Calcium oxide, (f) Mercury, (g) Brick, (h) Wood, (i) Air Solution: Ice, iron, calcium oxide and mercury are pure substances since they contain particles of only one kind of matter. In contrast, milk, hydroelectric acid (hydrogen chloride gas dissolved in water), brick and air cannot be called pure substances because they consist of particles of more than one kind of matter.
Question 8. Identify the solutions among the following mixtures. (a) Soil (b) Seawater (c) Air (d) Coal (e) Soda water Solution: A solution is a homogeneous mixture of two or more substances. In the light of this, the solutions among the given mixtures are (b) sea water, (c) air and (e) soda water.
Question 9. Which of the following will show Tyndall effect? (a) Salt solution (b) Milk (c) Copper Sulphate solution (d) Starch solution Solution: (b) milk and (d) starch solution show Tyndall effect because they are colloidal solutions, whereas (a) salt solution and (c) copper sulphate solution are true solutions. Their particle size is too small to scatter light, so they do not show Tyndall effect.
Question 10. Classify the following into elements, compounds and mixtures. (a) Sodium, (b) soil, (c) sugar solution, (d) silver, (e) calcium carbonate, (f) tin, (g) silicon, (h) coal, (i) air, (j) soap, (k) methane, (I) carbon dioxide, (m) blood Solution: Elements : The elements are regarded as the building blocks of the universe. So (a) sodium, (d) silver, (f) tin and (g) silicon are elements.
Compounds : It is a pure substance made up of two or more elements chemically combined in a fixed proportion by mass. So, (e) calcium carbonate, (k) methane and (1) carbon dioxide are compounds.
Mixtures : It is a substance containing two or more substances (elements or compounds) in any proportion. So (b) soil, (c) sugar solution, (h) coal, (i) air, (j) soap and (m) blood are mixtures.
Question 11. Which of the following are chemical changes? (a) Growth of a plant (b) Rusting of iron (c) Mixing of iron filings and sand (d) Cooking of food (e) Digestion of food (f) Freezing of water (g) Burning of a candle Solution: (a) Growth of a plant, (b) rusting of iron, (d) cooking of food (e) digestion of food and (g) burning of a candle, are chemical changes.
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
1 thought on “ NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure ”
- Pingback: NCERT Solutions for Class 9 Science – Gurukul For JEE & NEET
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Talk to our experts
1800-120-456-456
- Important Questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure

CBSE Class 9 Science Chapter-2 Important Questions - Free PDF Download
This chapter contains important questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure in the form of free to download review notes. These questions will undoubtedly aid students in improving their understanding of Chapter 2 of Class 9 Science. Both homogeneous and heterogeneous states of mixtures are covered in Class 9 Science Chapter 2. In this chapter, students will also learn about matter and consider whether the surrounding stuff is pure.
Vedantu is a platform that provides free CBSE Solutions (NCERT) and other study materials for students. You can download Class 9 Science and Class 9 Maths NCERT Solutions to help you to revise the complete syllabus and score more marks in your examinations.
Download CBSE Class 9 Science Important Questions 2023-24 PDF
Also, check CBSE Class 9 Science Important Questions for other chapters:

Study Important Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure
Very Short Answer Questions (1 Mark)
1. Try segregating the things around you as pure substances or mixtures.
Ans: Try mixing soil and water then separate them. Where soil is a mixture as it is the mix of more than one substance. And water is a pure substance because it’s made up of one kind of substance.
2. Classify each of the following as a homogeneous or heterogeneous mixture. soda water, wood, air, soil, vinegar, filtered tea.
Ans: Classification of the given into homogeneous or heterogeneous is enlisted below.
3. How would you confirm that a colorless liquid given to you is pure water?
Ans: Under the atmospheric pressure one, the boiling point of water is \[{{100}^{{}^\text{o}}}C\]. and freezing point is \[{{0}^{{}^\text{o}}}C\]. When we boil the given colorless liquid, if it boils at \[{{100}^{{}^\text{o}}}C\]then it is pure water. If not boiling at \[{{100}^{{}^\text{o}}}C\] temperature, then there will be impurities mixed with it, hence not pure water.
4. Which of the following materials fall in the category of a “pure substance”?
d) Hydrochloric acid
e) Calcium oxide
Ans: Pure substances: ice, iron, hydrochloric acid, calcium oxide, mercury.
5. Identify the solutions among the following mixtures.
b) Sea water
e) Soda water.
Ans: Solutions: seawater, air, soda water.
6. Which of the following will show the “Tyndall effect”?
a) Salt solution
c) Copper sulfate solution
d) Starch solution.
Ans: (b)Milk and (d)starch solution
7. Classify the following into elements, compounds, and mixtures.
c) Sugar solution
e) Calcium carbonate
l) Carbon dioxide
m) Blood
Ans: Classification of the given into elements, compounds, and mixtures are enlisted below.
8. Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Ans: Chemical changes: rusting of iron, cooking of food, digestion of food, burning of a candle.
9. Which of the following solutions scatter light?
(a) colloidal solution
(b) suspension
(d) none
Ans: (c) both
10. Which of the following methods would you use to separate cream from milk?
(a) fractional distillation
(b) distillation
(c) centrifugation
(d) filtration
Ans: (c) Centrifugation
11. Cooking of food and digestion of food:
(a) are both physical processes, (b) are both chemical processes, (c) cooking is physical whereas digestion is chemical.
(d) Cooking is chemical whereas digestion physical
Ans: (b) Are both chemical processes
12. Mercury and Bromine are both
(a) liquid at room temperature
(b) solid at room temperature
(c) gases at room temperature
(d) both (a) and (b)
Ans: (a) liquid at room temperature
13. Blood and seawater are:
(a) both mixtures
(b) both are compound
(c) blood is a mixture whereas seawater is a compound, (d) blood is a compound and seawater is a mixture , ans: (a) both mixtures.
14. Sol and Gel are examples of examples of
(a) Solid-solid colloids
(b) Sol is a solid-liquid colloid and Gel is a liquid-solid colloid
(c) Sol is a solid-solid colloid and Gel is a solid-liquid colloid
(d) Sol is a liquid-solid colloid and Gel is a solid-liquid colloid
Ans: (b) Sol is a solid-liquid colloid and Gel is a liquid-solid colloid
15. In a water-sugar solution:
(a) water is solute and sugar is solvent
(b) water is solvent and sugar is solute
(c) water is solute and water is also solute
(d) none of these
16. boron and carbon:.
(a) are metalloids
(b) boron is metalloid and carbon is non-metal
(c) boron is metallic and carbon is a metal
(d) boron is non-metal and carbon are a metalloid
Ans: (a) are metalloids
Short Answer Questions (2 Marks)
1. What is meant by a substance?
Ans: Substance will have similar chemical properties and can be defined as that kind of matter where constituent particles cannot be separated from each other by any physical process.
2. How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than \[{{25}^{{}^\text{o}}}C\] ), which are miscible with each other?
Ans: We can use the distillation technique to separate a mixture containing kerosene and petrol since the difference in their boiling points is more than \[{{25}^{{}^\text{o}}}C\].

3. Name the technique to separate
(i) Butter from curd
Ans: Centrifugation method.
(ii) Salt from sea-water
Ans: Evaporation method.
(iii) Camphor from salt
Ans: Sublimation method.
4. What type of mixtures are separated by the technique of crystallization?
Ans: From liquid solutions of impure samples, pure solid crystals can be separated. This method is known as crystallization.
Example: Pure sugar from impure sugar, salt from seawater.
5. What is a mixture? What are its various types?
Ans: A mixture is constituted by more than one element or compound or both mixed in any proportion. They are of two types:
(a) Homogenous mixture
(b) Heterogeneous mixture
6. Define solute, solvent, and solution?
Ans: Solute: It is the substance of the solution which is being added to the solvent.
Solvent: It dissolves the solute. The component of the solution to which the solute is added.
Solution: It is homogeneous, constituted by solute and solvent.
7. What is a solution? What are the properties of the solution?
Ans: A solution is a homogenous mixture of two or more substances. The various properties of the solution are: -
The particles of a solution cannot be seen by naked eyes because they are smaller than $1$ nm.
When the beam of light passes through a solution, it does not scatter.
Filtration cannot be used to separate the components of a solution from each other.
8. Differentiate between elements and compounds.
Ans: The difference between elements and compounds is enlisted below.
9. What is the Tyndall effect? Which kinds of solutions show it?
Ans: Tyndall effect is a process in which the scattering of beams of light takes place in particles of a colloid when that is directed towards them. Heterogeneous mixtures like Suspension solution and colloidal solution show the Tyndall effect.
10. Differentiate between homogeneous and heterogeneous mixture?
Ans: The difference between homogeneous and heterogeneous mixtures are listed below.
11. What is centrifugation? Where it is used?
Ans: Centrifugation is a technique used for the separation of suspended particles of a substance from liquid and is based upon the principle that denser particles stay at the bottom and lighter particles stay at the top when rotated at a high speed in a centrifuge application . It is used in separate butter from milk, also in washing machines for squeezing out water from clothes.
12. What is a suspension? What are the properties of suspension?
Ans: A suspension is a heterogeneous mixture in which the solute particles do
not dissolve in the solvent but remain suspended throughout the bulk of the medium. The suspension particle size is large enough to be visible from naked eyes.
Properties of suspension:
The particles are large so can be seen by naked eyes.
They scatter a beam of light passing through it.
When particles are left undisturbed, they settle down.
Short Answer Questions (3 Marks)
1. How are sol, solution, and suspension different from each other?
Ans: The difference between sol, solution, and suspension are enlisted below.
2. to make a saturated solution, $36$ g of sodium chloride is dissolved in \[100\] g of water at \[293\] k. find its concentration at this temperature..
Ans: In the problem, it is given that to make a saturated solution, $36$ g of sodium chloride is dissolved in \[100\] g of water at \[293\] K.
Mass of sodium chloride (solute) is $36$ g
Mass of water (solvent) is \[100\] g
Mass of solution is the sum of solute and solvent
\[\Rightarrow 36+100=136\]
Therefore, concentration percentage \[=\dfrac{mass\text{ }of\text{ }solute}{mass\text{ }of\text{ }solution}\times 100\]
$=\dfrac{36}{136}\times 100$
3. Classify the following as chemical or physical changes:
cutting of trees
melting of butter in a pan
rusting of almirah
boiling of water to form steam
the passing of electric current through water and the water breaking down into hydrogen and oxygen gases
dissolving common salt in water
Making a fruit salad with raw fruits burning of paper and wood.
Ans: When the chemical properties of a substance change then it’s called a chemical change.
Chemical change: rusting of almirah, passing of electric current, through water and the water breaking down into hydrogen and oxygen gases, burning of paper and wood.
Physical properties of a substance such as a shape, size, color, state change then it’s called a physical change.
Physical change: cutting of trees, melting of butter in a pan, boiling of water to form steam, dissolving common salt in water, making a fruit salad with raw fruits.
4. Which separation techniques will you apply for the separation of the following?
a) Sodium chloride from its solution in water.
Ans: Evaporation method
b) Ammonium chloride from a mixture containing sodium chloride and
Ammonium chloride., ans: sublimation method, c) small pieces of metal in the engine oil of a car..
Ans: Filtration method
d) Different pigments from an extract of flower petals.
Ans: chromatography.
e) Butter from curd.
Ans: Centrifugation method
f) Oil from water.
Ans: by using separating funnel.
g) Tea leaves from tea.
Ans: Filtration by using a strainer
h) Iron pins from sand.
Ans: magnetic separation.
i) Wheat grains from husk.
Ans: Winnowing method
j) Fine mud particles suspended in water.
5. write the steps you would use for making tea. use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate, and residue..
Ans: First, take the required amount of water as a solvent in a pan, and after boiling it add a little amount of sugar which is solute to the solvent. The solute will dissolve completely in the solvent forming the true solution, then add tea leaves that are insoluble along with another soluble liquid milk. After boiling the solution use the method of filtration with a sieve so that the filtrate obtained is tea while the residue has tea leaves that can be thrown away.
6. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below(results are given in the following table, as grams of substance dissolved in $100$ grams of water to form a saturated solution)
a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in \[50\] grams of water at $313$ K?
Ans: At temperature $313$ K the amount of potassium nitrate required was $62$ g in $100$ ml of water.
Therefore, in \[50\] g water we will need to dissolve $62\times \dfrac{50}{100}=31$ g potassium nitrate.
b) Pragya makes a saturated solution of potassium chloride in water at $353$ K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
Ans: at $353$ k saturated solution preparation needs \[54\] g potassium nitrate and at room temperature (\[293\]k) saturation solution formation occurs with \[35\] g potassium nitrate hence \[5435=19\] g potassium nitrate will precipitate out as undissolved salt..
c) Find the solubility of each salt at \[293\] K. Which salt has the highest solubility at this temperature?
Ans: Solubilities are (in \[100\] mg of water) \[32,36,35,37\] respectively for the mentioned salts and the highest solubility is of ammonium chloride at this temperature.
d) What is the effect of change of temperature on the solubility of a salt?
Ans: Solubility of salts is directly proportional to the temperature i.e., if temperature increases then solubility will also increase, and if the temperature decreases then solubility will also decrease.
7. Explain the following examples.
(a) Saturated solution
Ans: It is a solution in which no more solute particles can be dissolved at a particular temperature.
(b) Pure substance
Ans: It is a substance that has a fixed composition and is made up of only one type of particle.
(c) Colloid
Ans: It is a substance that has a fixed composition and is made up of only one type of particle. It is a kind of heterogeneous mixture/solution in which particle size is between $1$ nm and $1000$ nm that is intermediate between true solution and suspensions. Colloids have dispersion medium and dispersed phases.
(d) Suspension
Ans: It is a kind of heterogeneous mixture, in which insoluble solid particles remain suspended in the medium and dispersion particles are visible to the bare eyes.
8. Write a method to separate different gases from the air.
Ans: Air is a homogeneous mixture of various gases.
Fractional distillation can be used to separate its various components.
(a) First, compress air by increasing the pressure and cool the air by decreasing the temperature.
(b) The obtained air is liquid air; now allow the liquid air to warm up slowly in a fractional distillation column.
(c) The various gases separate from each other according to their boiling points at various heights of the fractionating column.
9. What is a colloid? What are its various properties?
Ans: The heterogeneous mixture of substances are colloids, in which the particle size is too small and cannot be seen by naked eyes.
(1) It is a heterogeneous mixture but appears homogeneous.
(2) The size of particles is too small so cannot be seen by naked eyes.
(3) They make its path visible by scattering the beam of light passing through it.
(4) When the colloid is left undisturbed, the particles of it do not settle down.
10. A solution contains $60$ g of $NaCl$ in $400$ g of water. Calculate the concentration in terms of mass-by-mass percentage of the solution.
Ans: In the problem, it is given that, A solution contains $60$ g of $NaCl$ in 400g of water.
Mass of solute ($NaCl$) is $60$ g
Mass of solvent (water) is $400$ g
Mass of solution $=$ Mass of solute $+$ Mass of solvent
$\Rightarrow 60+400=460$ g
Mass percentage of the solution is the percentage of the ratio of the mass of solute to the mass of solution.
\[\Rightarrow \dfrac{60}{460}\times 100=\dfrac{300}{23}~~\]
\[=13.4\%\]
11. Differentiate between metals and non-metal based upon the various properties that they show.
Ans : the difference between metals and non-metal based upon the various properties are enlisted below., 12. differentiate between mixtures and compounds by giving appropriate examples, ans : differences between mixtures and compounds are enlisted below., 13. write a method to separate a mixture of salt and ammonium chloride.
Ans: A mixture of salt and ammonium chloride can be separated by the process of sublimation. In this process, the solid substance is directly converted into a gaseous state. Since ammonium chloride changes directly from a solid into a gaseous state on heating and salt does not have that property, this principle can be used to the mixture of two.
The mixture of $N{{H}_{4}}Cl$ (ammonium chloride) and salt is taken in a china dish inside an inverted funnel.
The mixture is then heated using a burner and because $N{{H}_{4}}Cl$ sublimates thus it changes into vapors directly.
Salt settles into the inverted funnel as it is a non-sublimely substance.
Separation of $N{{H}_{4}}Cl$ salt by sublimation
14. What is crystallization? Where is it used? Why is this better than the simple evaporation technique?
Ans: Crystallization is the process of the transformation of solution into pure solid in the form of crystals. It is used to purify solids. For example, salt from seawater is purified using crystallization. It is a better technique than simple evaporation because:
Some solids may decompose or get charred on heating to dryness during evaporation.
Some of the impurities will remain dissolved in the solution.
15. What is chromatography? What are its various applications and underline the basic principle involved?
Ans: A technique used for the separation of those components whose solubility is different in the same solvent is chromatography. The basic principle in chromatography is that different solutes have different solubility in the same solvent.
Its various applications are:
It is used to separate different colors in dye .
It is used to separate pigments from natural colors.
It is used to separate drugs from the blood.
16. A solution of \[{{H}_{2}}S{{O}_{4}}\] acid is labeled is \[95\%\]. What is the mass of this that must be diluted with water to get \[5\]L of a solution containing \[10\] g of \[{{H}_{2}}S{{O}_{4}}\] per litre?
Ans: In the problem, it is given that, A solution of \[{{H}_{2}}S{{O}_{4}}\] acid is labeled is \[95\%\].
\[1\] L of the diluted solution must contain \[10\] g of \[{{H}_{2}}S{{O}_{4}}\]. Therefore, \[5\] L of the diluted solution must contain \[50\] g of \[{{H}_{2}}S{{O}_{4}}\].
The concentration of the acid in the bottle is \[95\%\] as per the problem.
This means that,
\[95\] g of \[{{H}_{2}}S{{O}_{4}}\] is present in \[100\] g of the acid solution .
\[50\] g of \[{{H}_{2}}S{{O}_{4}}\] will be present in \[\dfrac{\left( 50\times 100 \right)}{95}=52.64\] g of the solution.
Chemistry plays an important role in everyone's life, we might not know much about it but it is present in our everyday life, that is what this chapter tries to show where it indicates the different types of matters that are present everywhere around us. In this chapter, students will learn how matter is composed of and how it differs from various substances. Regular practice of Chapter 2 Class 9 Important Questions can help students improve, become through the concepts and topics, and be efficient during preparation or revision. Students must learn to utilize the material given to them to get more marks. In this article, we will also look at Class 9 Science Chapter 2 extra questions .
Important Questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure - Benefits of Class 9th Science Chapter 2 Important Questions
It is vital that students understand the importance of this subject and what it holds for students of Class 9. By utilising the important questions and with a rigorous practice regime, students will be able to score the most out of their exams. These exams can be a little difficult without the right guidance but by using Vedantu’s important questions on Chapter 2, students will be able to study in a more structured manner. Following is the list of benefits:
Students can use Vedantu to use their time wisely, it helps boost their confidence after consistent practice and students can plan their preparation accordingly.
It provides students with a structure with which they can study for their upcoming examinations.
This is a fundamental chapter for students and plays a crucial role in upcoming grades.
Students don’t have to worry about the relevance of these questions as they are all cross-checked and updated according to the latest CBSE guidelines and rules. So, the information in this article is genuine and reliable.
Topics Covered under CBSE Class 9 Chapter 2 ‘Is Matter Around Us Pure’
Following are the topics that are covered in CBSE Class 9 Chapter 2 ‘Is Matter Around Us Pure’:
Matter and its Types
Metals, Non-metals, and Metalloids
Mixture and its Types
Solution and its Types
Factors Affecting the Solubility
Concentration of Solution
Key Takeaways of CBSE Class 9 Science Chapter 2 Is Matter Around Us Pure
Students here will learn some of the basic elements of constituents of matters. This will help them in their future grades as it sets the groundwork. Constant practice of the essential questions should help students to tackle any difficult questions in their final examinations. Some of the topics that these chapter covers are as follows:
Chemistry
This subject is known as the central science subject that connects all the science subjects. This subject is very detailed and helps students understand the chemical constituents in different materials. it is connected to a lot of the physical subjects together such as Chemistry with Applied Science and Life Sciences such as Engineering and Medicine. Chemistry is defined as the study of the interaction, composition, and properties of matter.
Homogeneous and Heterogeneous Mixture
These are two very different mixtures as in a homogeneous mixture, it has a uniform composure of its constituents where heterogeneous is a nonuniform composure of its constituents.
Tyndall Effect
The scattering of a beam of light by particles of a solution when light is passed through it is known as the Tyndall effect. The solution where the size of the particle is very small.
Matter is defined as anything that possesses mass, occupies space, and the presence that can be felt by the five senses. Matter exists in three forms, namely, a solid, liquid, and gas. Solids are substances that possess a definite structure and a definite shape like sugar, iron, etc. Liquids are substances that have a definite volume but lack a definite form and take the shape of the vessel in which they are put — for example, mercury, milk, water, etc. Gases are substances that can neither possess a definite shape or definite volume like hydrogen, oxygen, nitrogen, etc.
The Difference Between Mixture and Compound
This chapter takes a detailed look into the difference between mixture and compounds. The mixture is basically the elements or compounds that are mixed together in a heterogeneous way. It has a variable composition and also shows us the properties of constituent elements and the various ways in which they can be mixed. The examples are air, blood, and water. In a compound when the elements react, they form new compounds. This new substance formed shows new properties and examples of this are sodium chloride.
Crystallisation
This is a very important process where we can separate the pure solid in the form of its crystals from its solutions. This is an important process when forming crystals. Unlike many processes where the solids may decompose because of the heat during the process of decomposition. In the process of evaporations, some solids stay intact.
Substance
In this situation, it is physically impossible to separate the constituent particles from one another on one's own. Chemical or electrochemical procedures can be used to separate them because their chemical properties are similar. A material possesses particular qualities or attributes. Physical properties and chemical properties are the two main categories into which properties of matter can be divided. Melting point, boiling point, colour, aroma, and other physical attributes can be observed or quantified without affecting the content or identification of the substance. Chemical characteristics, such as combustibility, basicity, or acidity, are the chemical transformations that result in a distinctive response.
Chromatography
This is a process where substances used for the separation of different substances have different solubility in the same solvent. It is used to separate different colours in the dye. It is used to separate different pigments from natural colours and separate drugs from the blood. There are various ways in which they can be separated and we will learn that in this chapter.
Colloid
These are heterogeneous mixtures of substances whose particles are too small for the naked eye and cannot be seen. It appears homogeneous but is actually a heterogeneous mixture. The particles are too small for the naked eye to see. They scatter a beam of light through it and make its path invisible. The particles of the colloid do not settle down when left undisturbed.
Important Questions on CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure
To get a better understanding of Class 9 Science Chapter 2 important questions , let's look at how the various essential questions are framed and how they can be beneficial to students. Using the following questions should help students in the long term.
1. What is chromatography? What are its various applications and underline the basic principles involved?
2. What is crystallisation? Why is crystallisation used?
3. Why is crystallisation a better technique than the evaporation process?
4. Write a method to separate salt from sodium chloride.
5. Differentiate between mixture and compound by giving appropriate examples.
6. Differentiate between metals and non-metals based on the various metal properties they show.
7. What is a colloid?
8. What are the various properties of a colloid?
9. Write a different method to separate gas from the air?
10. Explain and give the example of the following:
a. Saturated solution
b.Pure substance
c. Colloid
d.Suspension
Tips to Study Science Better
Following are some tips that will help the students to study science in a better way:
Follow the concepts and study them properly.
Try out the experiments under the guidance of someone elderly, which will help you to understand the topic better.
Practise solving the questions and answers, this will increase your chance of getting better results.
Practise with reliable notes for this chapter, you can refer to Is Matter Around Us Pure Class 9 Notes CBSE Science Chapter 2 (Free PDF Download) of Vedantu, this is quite reliable.
Conclusion
The Situation in the Matter Around Us Pure, students have found pure important questions to be incredibly helpful. As you can see from this article, this chapter is very important for students to begin their study of chemistry because it essentially lays the foundation for subsequent grades. This article can help students make the most of their time, build their confidence after constant practice, and manage their study sessions effectively. Students might aspire for higher grades by working more hard toward their goals. These significant questions ensure that students understand the chapter's numerous concepts, and with continued practice, they will develop the skills necessary to answer the challenging questions on exams.
Important Related Links for CBSE Class 9

FAQs on Important Questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure
1. What are the major differences between a mixture and a compound?
Mixture: Elements or compounds just mix together to form a mixture. It has a variable composition and shows the properties of constituent elements. These constituent particles can be separated by physical methods. For example, air, blood, etc.
Compound: When elements chemically react, they form new compounds. Compounds have a fixed composition. The new substance formed shows new properties. The constituent elements can only be separated by chemical methods. For example, Sodium Chloride, Calcium Sulphate, etc.
2. What is crystallisation? Why is it an important process?
Crystallisation is a process by which we can separate a pure solid in the form of crystals from its solutions. It is an important process which is used to purify solids. It is a better technique than many processes like simple evaporation as some solids may decompose on heating during evaporation. In evaporation, some impurities still remain dissolved in the solutions.
3. Where can I find Important Questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure?
Students can find a set of important questions for Class 9 Chapter 2 Is Matter Around Us Pure on Vedantu, a reliable online learning site. Vedantu caters to solve important questions chosen from the exam perspective. These questions are answered by subject matter experts who have immense subject knowledge and expertise. The material is available in the free PDF format and can be downloaded at students’ convenience for learning and during exam preparation.
4. What is meant by a substance?
Substance can be defined as the kind of matter where constituent particles cannot be separated from one another with the help of any physical process. Since they are similar in chemical properties, they can also be separated by chemical or electrochemical methods. Examples are diamond, water, sulphur, etc.
5. What is the saturated solution according to Chapter 2 of Class 9 Science?
A saturated solution is a solution in which no more solute can be added without increasing the temperature of a solution. It is stable at room temperature. Students study more about saturated solutions in their science books for Class 9. They will understand the concept of pure substances and matter too. All definitions related to different types of solutions and substances are given in detail on Vedantu.
6. What is a mixture and a compound according to Chapter 2 of Class 9 Science ?
A mixture and a compound are different from each other in many ways. A mixture is not a pure substance but a compound is a pure substance. A mixture has different constituents and the properties of the constituents do not change in a mixture but the properties of all constituents will change in a compound. In a mixture, two or more substances can be mixed together in any ratio whereas in a compound two or more substances are mixed together in a fixed ratio.
7. Write down the important properties of a colloidal solution?
Colloidal solutions are heterogeneous mixtures. The size of particles in a colloidal solution varies between 1nm and 100 nm. The particles are very small in size and cannot be seen easily. If the particles are left undisturbed, the solutions remain stable. Students of Class 9 can understand the concept of colloidal solution in Chapter 2. Students should read Chapter 2 of Class 9 carefully to understand the definitions of important terms given in the chapter.
8. Water acts as a compound and not a mixture. Give reasons.
Water acts as a compound because it is made of two components oxygen and hydrogen. The two constituents can be separated through the process of electrolysis. The two constituents are combined together in a fixed ratio of 1:2. The ratio of hydrogen and oxygen is fixed and does not change. It is not easy to separate the constituents of water easily. They can only be separated by a special process only called electrolysis.
9. What are the important features of important questions of Chapter 2 of Class 9 Science from Vedantu?
Important questions for Chapter 2 of Class 9 Science available on Vedantu include questions that can help students to score high marks in exams. These important questions are available at free of cost on Vedantu(vedantu.com) and mobile app. Important questions can also help students to understand the concepts and gain in-depth knowledge of all the concepts given in the chapter. Students can prepare for their exams by practising all-important questions given in Vedantu because they are prepared by expert and experienced teachers and professionals.
CBSE Class 9 Science Important Questions
Cbse study materials.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 2: Matter in Our Surroundings
Matter and particles that make up matter..
- Characteristics of particles of matter (Opens a modal)
- Characteristics of particles of matter Get 4 of 5 questions to level up!
States of matter
- States of matter (Opens a modal)
- Effect of temp and pressure on state change (Opens a modal)
- Evaporation and its cooling effect (Opens a modal)
- The states of matter. Get 3 of 4 questions to level up!
- Evaporation and its cooling effects Get 5 of 7 questions to level up!

Case Study Questions Class 9 Science Matter in our Surroundings
Case study questions class 9 science chapter 1 matter in our surroundings.
CBSE Class 9 Case Study Questions Science Matter in our Surroundings. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Matter in our Surroundings.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks.
CBSE Case Study Questions Class 9 Science – Matter in our Surroundings
Case study 1:.
1.) A matter is anything that has mass and occupies space. Pen, paper, clips, sand, air, ice, etc. are different forms of matter. Every matter is made up of small particles. These particles are so tiny that they can’t be seen with naked eyes. Let’s see about the different characteristics of particles of matter.
- All matter is made up of very small particles.
- .Particles of matter has spaces between them.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
Answer the following questions by referring above paragraph.
i.) Which of following is not matter?
c.) smell of perfume
d.) None of these
ii.) Thoughts coming in our mind are example of matter. True or false
c.) None of these
iii.) Which of the following is true about particles of matter?
a.) Particles of matter has spaces between them
b.) Particles of matter are continuously moving
c.) Particles of matter attract each other
d.) All of these
iv.) Give 5 examples of matter in our surroundings
v.) Enlist all properties of particles of matter
Answer key-1
iv.) pen, pencil, notebook, ice and water
v.) Different characteristics of particles of matter are
Case Study 2:
2.) There are three states of matter – solid, liquid and gas.
Solids have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
Liquids have no fixed shape but have a fixed volume. They take up the shape of the container in which they are kept. Liquids flow and change shape, so they are not rigid but can be called fluid.
Gas as has indefinite shape, no fixed volume. Gas gets the shape and volume of container.
Gas has very low density hence are light. Gas can flow easily and hence are called fluid.
i.) Which of the following state of matter takes shape of container in which it is filled?
d.) Both b and c
ii.) Distance between particles of matter least in
iii.) Compressibility is least in case of
iv.) Give properties of solids.
v.) Give properties of Gases.
Answer key-2
iv.) properties of solid are given below
- Solid has fixed volume.
- Solid has fixed shape.
- Solid has high density.
- Solids are heavy.
- Solid does not flow.
v.) Properties of gases are
- Gas has indefinite shape
- Gas has no fixed volume.
- Gas gets the shape and volume of container.
- Gas fills the container completely.
- Gas has very low density.
- Because of low density gas are light.
- Gas can flow easily and hence are called fluid.
Case Study 3:
3.) What happens inside the matter during change of state? On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the
Particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature of the system does not change after the melting point is reached, till all the ice melts. This happens even though we continue to heat the beaker, that is, we continue to supply heat. This heat gets used up in changing the state by overcoming the forces of attraction between the particles. The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0 0 C (273 K) have more energy as compared to particles in ice at the same temperature.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state. A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition.
i.) A change of state directly from solid to gas without changing into liquid state is called
a.) Sublimation
b.) Deposition
c.) Boiling point
ii.) The direct change of gas to solid without changing into liquid is called
iii.) The energy supplied by heat to solid is used to overcome the forces of attraction between the particles. True or false
iv.) Define melting point and boiling point
v.) Define latent heat of fusion
Answer key-3
iv.) The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
v.) The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Case Study 4:
4 .) Do we always need to heat or change pressure for changing the state of matter? Can you quote some examples from everyday life where change of state from liquid to vapour takes place without the liquid reaching the boiling point? In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
i.) Evaporation of liquid takes place at
a.) Boiling point
b.) Above boiling point
c.) Below boiling point
ii.) Evaporation takes place at surface of liquid because
a.) They are heavy as compare to other particles
b.) They have sufficient kinetic energy to break the force
c.) They are light weight as compare to other particles
iii.) During evaporation particles of liquid change into vapour
a.) From the surface
b.) From the bottom
c.) From all over the liquid
iv.) Define evaporation.
v.) Explain process of evaporation
Answer key-4
iv.) The phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
v.) In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
Case Study 5:
5.) You must have observed that the rate of evaporation increases with–
- an increase of surface area:
- We know that evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation increases. For example, while putting clothes for drying up we spread them out.
- an increase of temperature:
With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold. What happens when you pour some acetone (nail polish remover) on your palm? The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool. After a hot sunny day, people sprinkle water on the roof or open ground because the large latent heat of vaporization of water helps to cool the hot surface.
i.) Evaporation is surface phenomenon. True or false
ii.) As temperature increases the rate of evaporation is
a.) increases
b.) decreases
c.) remains constant
iii.) The rate of evaporation increases with
a.) Increase in wind speed
b.) Decrease in wind speed
c.) Does not have any effect from wind speed
iv.) What happens when you pour some acetone (nail polish remover) on your palm?
v.) We are able to sip hot tea from saucer than from cup. Why?
Answer key-5
iv.) The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool.
v.) We are able to sip hot tea from saucer than from cup. This is because saucer has large surface area, due to large surface area as compare to cut area tea evaporates at faster rate.
Thank you It helped me a lot
Why smell of Perfume is not a matter?
Because there is no particle
Because their are perfume particles suspended in air
These all case study questions are really helpful . Thanks
This is my first I was so nervous but these questions help me alot thank you
Smell of perfume is a matter because it have gas particles means perfume particles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- CBSE Study Material
- CBSE Important Questions
- Important Questions For Class 9 Science Chapter 2 Is Matter Around Us Pure
Important Questions for CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure
Important Questions for CBSE Class 9 Science Chapter 2 – Is Matter Around Us Pure covers topics such as mixtures, types of mixtures, etc. Students of Class 9 should go through these important questions to know what type of questions they can expect from this chapter. We have provided important questions of CBSE Class 9 Science Chapter 2, which are prepared by professional subject experts. It is considered as a valuable study material while preparing for exam. It helps students in quickly revising important topics of the chapter.
Students can download Important Questions for Class 9 Science Chapter 2 – Is Matter Around Us Pure by clicking the below-mentioned link.
Important Questions for Class 9 Science Chapter 2 – Is Matter Around Us Pure PDF

CBSE Class 9 Science Chapter 2 Extra Questions
- Which is the solute and the solvent in the ‘tincture of iodine’?
- ___________ is the mass percent of the solution.
- Give three properties of colloid. Differentiate between a true solution and a colloid.
- What do you observe when an aqueous sugar solution is heated to dryness?
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


IMAGES
VIDEO
COMMENTS
Case Study/Passage-Based Questions. Case Study 1: Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper.
Case Study. A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in figure. The filter paper was removed when the water moved near the top of the filter paper. (i) Identify the technique used by the child.
Question 1: Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper. (i) Identify the technique used by the ...
CASE STUDY QUESTIONS CLASS IX SCIENCE CHAPTER 2 - IS MATTER AROUND US PURE 1. A solution which can dissolve more of the solute at a given temperature is called unsaturated solution. However, a solution which cannot dissolve any more of the solute is called saturated solution. The amount of solute that can
Case Study Questions: Question 1: Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in figure. The filter paper was removed when the water moved near the top … Continue reading Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is ...
The Case Based Question Answer: Is Matter Around Us Pure? is an invaluable resource that delves deep into the core of the Class 9 exam. These study notes are curated by experts and cover all the essential topics and concepts, making your preparation more efficient and effective.
Class 9 Chemistry (India) 4 units · 15 skills. Unit 1 Is Matter Around Us Pure? Unit 2 Matter in Our Surroundings. Unit 3 Atoms and Molecules. Unit 4 Structure of the Atom. Course challenge. Test your knowledge of the skills in this course. Start Course challenge. Science.
Class 9 Chapter 2 - Is Matter Around Us Pure Important Questions with Answers Short Answer Type Questions. Q1. Suggest separation technique(s) one would need to employ to separate the following mixtures. (1) Mercury and water (2) Potassium chloride and ammonium chloride (3) Common salt, water and sand (4) Kerosene oil, water and salt. Answer:
Answer: (a) When the solid or liquid is dispersed in a gas it is called aerosol e.g. smoke. (b) When smoke mixes with fog it forms smog. (c) Rita's father is an aware citizen, environmentally concerned and dutiful. Extra questions for Class 9 Science Chapter 2 Is Matter Around Us Pure with answers is given below.
Class 9th Science - Is Matter Around Us Pure Case Study Questions and Answers 2022 - 2023 - Complete list of 9th Standard CBSE question papers, syllabus, exam tips, study material, previous year exam question papers, centum tips, formula, answer keys, solutions etc..
1. Introduction. Answer. Anything which occupies space and has mass is called matter. Matter can be divided in two categories. They are: (i) Pure Substance: It consists of single types of particles which are same in their chemical nature. (ii) Mixtures: Mixture consists of two or more particles. 2.
Course: Class 9 Chemistry (India) >. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
4. Sugar is soluble in water completely. 5. Add half a tea-spoon of tea-leaves, it is insoluble in water. 6. Boil the content, add milk which is also soluble in water, boil again. 7. Filter the tea with the help of strainer, the tea collected in cup is filtrate and the tea leaves collected on the strainer is residue.
Free Question Bank for 9th Class Science Is Matter Around Us Pure Case Based MCQs - Is Matter Around us Pure. Customer Care : 6267349244. Toggle navigation 0 . 0 . Railways; UPSC; CET; Banking; CUET; SSC; CLAT; JEE Main & Advanced ... Separation makes it possible to study and use the individual components of mixture. Heterogeneous mixtures can ...
Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue. Solution: Take 100 mL of water which acts as solvent. Boil water on a gas stove. Add one teaspoon of sugar which acts as solute. Sugar is soluble in water, so gets dissolved in water and forms a solution.
Free PDF download of NCERT Exemplar for Class 9 Science Chapter 2 - Is Matter Around Us Pure solved by expert Science teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 2 - Is Matter Around Us Pure exercise questions with solutions to help you to revise complete syllabus and score more marks in your examinations.
Study Important Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure. Very Short Answer Questions (1 Mark) 1. Try segregating the things around you as pure substances or mixtures. Ans: Try mixing soil and water then separate them. Where soil is a mixture as it is the mix of more than one substance.
2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate, and residue. Solution: (a) Into a vessel, add a cup of milk, which is the solvent, and supply it with heat. (b) Add tea powder or tea leaves to the boiling milk, which acts as a solute.
A pure substance that consists of a single type of element. For a human pure substance means having no mixed adulteration. According to the scientist, all these things like milk, salt, ghee, etc are a mixture of different substances therefore if it is not pure. Example: Milk is a mixture of water, fat, protein, etc. When a scientist says that ...
Test your Knowledge on Is matter around us pure! Put your understanding of this concept to test by answering a few MCQs. Click 'Start Quiz' to begin! Select the correct answer and click on the "Finish" button. Check your score and answers at the end of the quiz.
Class 9 Chemistry (India) 4 units · 15 skills. Unit 1 Is Matter Around Us Pure? Unit 2 Matter in Our Surroundings. Unit 3 Atoms and Molecules. Unit 4 Structure of the Atom. Course challenge. Test your knowledge of the skills in this course. Start Course challenge. Science.
Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Matter in our Surroundings. At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 ...
It is considered as a valuable study material while preparing for exam. It helps students in quickly revising important topics of the chapter. Students can download Important Questions for Class 9 Science Chapter 2 - Is Matter Around Us Pure by clicking the below-mentioned link.