An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)


In Vitro Propagation and Acclimatization of Banana Plants: Antioxidant Enzymes, Chemical Assessments and Genetic Stability of Regenerates as a Response to Copper Sulphate
Doaa m. abou elyazid.
1 Horticulture Department, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh 33516, Egypt
2 Physiology and Breeding of Horticultural Crops Lab, Horticulture Department, Kafrelsheikh University, Kafr El-Sheikh 33516, Egypt
Abdel-Moety Salama
Abdel fattah m. el zanaty.
3 Genetics Department, Faculty of Agriculture, Menoufia University, Al Minufya 32514, Egypt; moc.liamg@6691ytanaz
Neama Abdalla
4 Plant Biotechnology Department, Genetic Engineering and Biotechnology Research Division, National Research Centre, 33 El Buhouth St., Dokki, Giza 12622, Egypt; moc.oohay@rcn_amaen
Developing a successful protocol for banana in vitro culture is a guarantee for the mass propagation of pathogen-free, high-quality, true-to-type planting materials with low production costs. The current work aimed to investigate the influence of increasing copper levels in an MS medium on endophytic bacterial contamination; shoot multiplication; rooting and the acclimatization of in vitro cultured banana; minerals and chlorophyll content; antioxidant enzymes activity; electrolyte leakage; and the genetic stability of banana regenerants. Four different concentrations of copper sulphate (0.025 as a control, and 30, 60, and 120 mg L −1 ) were examined. The growth of the endophytic bacteria was inhibited at 60 mg L −1 of copper sulphate which recorded zero contamination, without a significant difference at 120 mg L −1 . However, 0.025 mg L −1 of copper sulphate was optimal for the maximum shoot number and shoot length (10 shoots and 6 cm, respectively) without significant differences at 30 mg L −1 . The root length of banana plantlets was significantly enhanced at 30 mg L −1 of copper sulphate but without significant differences to the control, regarding the number of roots (9.92 cm and 3.80 roots, respectively). In vitro plants were acclimatized successfully at 30 mg L −1 of copper sulphate with 100% survival. The uptake of minerals, antioxidant enzyme activity and electrolyte leakage was improved because of the copper sulphate, but the chlorophyll level decreased. RAPD profiling showed polymorphism in only one plant treated with 60 mg L −1 of copper sulphate, with an average of 1.8%. The genome template stability percentage was almost 100% for all treated plants.
1. Introduction
Banana ( Musa sp.), which belongs to the family Musaceae , is considered one of the most popular fresh fruits worldwide and all cultivars of banana are nearly derived from Musa balbisiana and Musa acuminata [ 1 ]. Its name originates from the Arabic word “banan,” meaning the finger. Banana has a high nutritive value; a high content of pyridoxine: vitamin B6, potassium, carbohydrates and fiber. Banana plants are commercially propagated through the tissue culture technique [ 2 ], which can provide mass propagation, rejuvenation of older varieties, disease elimination, conservation of genetic resources, and the management of abiotic and biotic stresses [ 3 , 4 ]. Microbial contamination is one of the most important obstacles, as it prevents the successful micropropagation of in vitro plants [ 5 ]. Therefore, several methods have been used to obtain aseptic cultures of explants, such as ultraviolet, autoclaving of media [ 6 ] or the use of chemical antiseptic agents like sodium hypochlorite and mercuric chloride, whereas, antibiotics and copper sulphate can be used for eliminating the endogenous contamination [ 5 ].
As an essential micronutrient for plant growth, copper (Cu) is needed for several physiological functions, including photosynthesis, respiration, oxidative stress response, electron transport, plant hormone signaling, and cell wall biosynthesis and its lignification [ 7 ]. As a cofactor in many enzymes, Cu has a crucial role in many plant enzymes such as amino oxidase, laccase, superoxide dismutase (SOD), and polyphenol oxidase [ 5 ]. Copper and its compounds are important ingredients in several antibacterial and antifungal compounds, which have been applied against a wide range of pathogens including viruses, fungi and bacteria [ 8 , 9 ]. However, Cu is too toxic at high concentrations causing a reduced photosynthetic rate, increasing the formation of reactive oxygen species (ROS), causing rising leakage of potassium ions from the roots [ 10 ], and inducing the deficiency of iron [ 5 ]. Many reports have indicated the positive effects of higher Cu concentrations on in vitro culture of many plants, such as barley [ 11 ], tobacco [ 12 ], Lepidium sativum [ 13 ], rice [ 14 ], carrot [ 15 ] and Gymnema sylvestre [ 16 ], as reported by [ 5 , 6 ].
Few studies have reported the phytotoxicity of copper [ 17 , 18 ] on Musa acuminata and on Philodendron selloum [ 5 ] plants, which confirmed that the high activity of the antioxidant enzymes could cause ROS detoxification, hence, decreasing the oxidative damage of plants. Many studies have been published about the different stresses on in vitro Musa sp., such as water stress [ 19 ], osmotic stress [ 20 ], cold stress [ 21 ], stress of nanoparticles [ 22 , 23 , 24 ], and salinity stress [ 4 ], but very few on copper stress [ 18 , 25 ].
Optimizing the concentration of CuSO 4 in banana tissue culture media could be a more efficient way to improve the growth and development of micropropagated banana plants by enhancing shooting, rooting [ 25 ] and subsequent acclimatization. Therefore, the current study aims to evaluate the impact of increasing the concentration of Cu in the medium by the supplementation of the MS medium [ 26 ] with different high concentrations of copper sulphate; on endophytic bacterial contamination, shooting, rooting and acclimatization of in vitro cultured banana; chemical contents; antioxidant enzyme activity; electrolyte leakage; genetic fidelity and the stability of banana regenerants with the target of in vitro mass propagation of high-quality banana plants for commercial production.
2. Results and Discussion
2.1. copper sulphate and endophytic bacterial contamination percentage.
The primary experiment, in Figure 1 , showed that copper sulphate had a significant negative effect on the growth of isolated endophytic bacteria, which was completely inhibited at the higher concentration of copper sulphate (120 mg L −1 ), as it recorded zero contamination. Whereas, 0.025 mg L −1 of copper sulphate, which was found in the MS medium at this lower concentration, reported bacterial contamination. Copper exhibits antimicrobial activity, making it one of the most important components in most effective bactericides [ 5 , 8 , 27 , 28 , 29 ]. Accordingly, copper sulphate could be applied over other chemicals for controlling and eliminating the bacterial contamination. Depending on the primary experiment and according to the obtained information from the literature, four different concentrations of copper sulphate (0.025, 30, 60 and 120 mg L −1 ) were selected to limit the bacterial contamination percentage in banana in vitro cultures.

Effect of copper sulphate on endophytic bacteria contamination. Photo ( A ): Control (0.025 mg L −1 ) showed bacterial contamination. Photo ( B ): 120 mg L −1 was free from contamination.
The recorded results also clearly indicated that the increasing of copper sulphate concentration highly decreased the contamination percentage. The best result was observed with 60 mg L −1 of copper sulphate treatment. No significant differences were observed with 120 mg L −1 , where zero contamination was recorded ( Table 1 ).
Effect of different concentrations of copper sulphate on contamination % and shoot multiplication of banana plant after three subcultures, 3 weeks each in MS medium supplemented with 3 mg L −1 BA + 1 mg L −1 Kin.
Each treatment was the mean of five replicates (jars) and each replicate contained one explant, where explant was a cluster of three shoots, except for contamination % of each treatment, which was 20 jars. Values are the mean ± SE and values followed by the same letters in the same column were not significantly different, by 1 Kruskal–Wallis Test and 2 Duncan’s test at 0.05 level. * means Significant. ** means high significant. NS means not significant at p ≤ 0.05. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation). MS medium means Murashige and Skoog medium (1962) [ 26 ].
The recorded results agree with the findings of the study carried out by [ 5 ] on Philodendron selloum , in which the fortification of copper sulphate in the two concentrations (70 and 140 mg L −1 ) to the culture medium, completely inhibited the growth of endophytic bacteria compared to the control which recorded 100% contamination.
2.2. Copper Sulphate and Shoot Multiplication
In general, there were significant differences among copper sulphate treatments on all recorded measurements except in the number of leaves/shoot where the differences were insignificant. The highest value of shoots (11) was recorded with 30 mg L −1 of copper sulphate treatment followed by the control (10), without significant differences between them. Moreover, the number of shoots, as well as shoot length, decreased to almost half by increasing copper sulphate concentration. The control treatment (MS medium copper level) achieved the maximum shoot length (6 cm) without significant differences with 30 mg L −1 of copper sulphate ( Table 1 ).
The current study revealed that the fortification of the multiplication medium with 30 mg L −1 of copper sulphate had no significant effect on banana shooting compared to the control treatment. The obtained results differ from those recorded by [ 5 ] where, copper sulphate at 35 mg L −1 was the best treatment regarding the number of shoots in Philodendron selloum , with significant differences with the other higher concentrations in this study. The optimum concentration of copper in the culture medium mainly depended on the plant species [ 30 ]. In terms of shoot multiplication, some plants responded significantly to lower concentrations of Cu (0.01 to 20 mg L −1 ) [ 6 , 31 , 32 ]. Whereas, others, such as Arundo, can tolerate copper sulphate up to 300 mg L −1 without any adverse effects on its growth and production [ 5 ].
On the other hand, according to the current study, increasing the copper sulphate concentration to 60 and 120 mg L −1 yielded the opposite effect on the number of shoots. Similar results were observed by some previous researchers that reported a significant reduction in the number of shoots under high copper concentrations [ 5 , 33 ]. The higher copper levels reduced the uptake and transport of some essential metallic elements, such as iron and zinc, that resulted in a negative effect on shoot multiplication [ 34 ]. The toxic and useful copper content in the medium depended, to a great extent, on the plant species. Thus, the appropriate copper level should be accurately estimated [ 6 ].
2.3. Copper Sulphate and In Vitro Rooting
The results shown in Table 2 , clearly demonstrated that the (30 mg L −1 ) copper sulphate treatment registered a high efficiency for enhancing the in vitro rooting and growth development of banana plantlets. The highest significant values (14.26 cm, 2.47 g and 9.92 cm) were observed at 30 mg L −1 of copper sulphate for plantlet length, fresh weight, and root length, respectively, compared to the control, while there were no significant differences in the number of leaves or the number of roots. Previous investigations reported that the rooting of micropropagated banana plantlets was stimulated by supplementing 4 mg L −1 of copper sulphate into a rooting medium, while the rooting was reduced in a medium fortified by 8 mg L −1 of copper sulphate [ 25 ]. It could be concluded that the better responses of banana in vitro cultures for some growth parameters at higher concentrations of Cu, as compared to the control, were due to the fact that Cu could reduce the endogenous bacterial contamination of explants.
Effect of different concentrations of copper sulphate on in vitro rooting of banana plants after 4 weeks of culturing in MS medium fortified with 1 mg L −1 IBA.
Each treatment was the mean of five replicates (jars) and each replicate contained one shoot. Values are the mean ± SE and values followed by the same letters in the same column were not significantly different by 1 Kruskal–Wallis Test and 2 Duncan’s test at 0.05 level. * means Significant, ** means high significant. NS means not significant at p ≤ 0.05. Control = 0.025 mg L −1 represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
2.4. Copper Sulphate and Acclimatization
The obtained results in Table 3 and Figure 2 showed the effect of copper sulphate on the survival and growth parameters of acclimatized banana plants. The optimum treatment for plant length, root length, leaf area and fresh weight was 30 mg L −1 with significant differences observed with the other treatments. Despite this, Cu at 30 mg L −1 had no significant differences in survival percentage, number of leaves and number of roots compared to control treatment. The percentage of increase in plant length, number of leaves, number of roots, root length, and fresh weight from in vitro rooting to acclimatization, was 40, 11, 15.6, 22 and 56%, respectively. Moreover, copper sulphate at 30 mg L −1 recorded the highest significant values for plant length, root length, leaf area and fresh weight (23.83 cm, 12.67 cm, 59.23 cm 2 and 5.67 g, respectively) of acclimatized banana plants.

Effect of copper sulphate on in vitro propagation and the acclimatization of banana plants. ( A ) Shoot multiplication in MS medium supplemented with 3 mg L −1 BA + 1 mg L kin −1 . ( B ) Plantlets in elongation medium (MS +1 mg L −1 BA). ( C ) Plantlets in rooting medium (MS + 1 mg L −1 IBA). ( D ) In vitro rooted plants. ( E , F ) Acclimatized plants after 4 weeks (Control, 0.025 mg L −1 at Left and 120 mg L −1 copper sulphate at right).
Effect of different concentrations of copper sulphate added in multiplication and rooting stages on survival and growth parameters of banana plant after 4 weeks of acclimatization.
Each treatment was the mean of five replicates (pots) and each replicate contained one rooted plant, except survival percentage which was calculated the percentage of 10 plants (pots). Values are the mean ± SE and values followed by the same letters in the same column were not significantly different by 1 Kruskal–Wallis Test and 2 Duncan’s test at 0.05 level. * means Significant, ** means high significant, NS means not significant at p ≤ 0.05. Control = 0.025 mg L −1 represented the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
2.5. Copper Sulphate and Chemical Analyses
2.5.1. copper sulphate and mineral content of in vitro plantlets.
The mineral content of some nutrients was measured in two different growth stages of banana; in vitro rooted plantlets and acclimatized plants after 4 weeks ( Table 4 and Table 5 ). The applied copper to the rooting medium enhanced the uptake of the studied nutrients (Ca, K, Mg, Fe, Mn and Zn) but Cu uptake increased as copper concentration increased. The highest uptakes of K, Ca, Mg, Mn and Zn (0.548, 1.253, 0.249%, 398 and 103.3 mg kg −1 , respectively) were achieved in plants which had been treated with 60 mg L −1 . On the other hand, 120 mg L −1 of copper sulphate treatment recorded the highest significant content of Cu and Fe (44.69 and 394 mg kg −1 , respectively ( Table 6 ). This may explain the role of copper in the growth of plantlets during the rooting stage of banana as a main component/or as an activator of some enzymes in plants [ 35 ]. This also reflects the role of Cu in many plant biophysiological processes through the high uptake rate of Fe-nutrient (due to the participation in Fe-mobilization); nutrients of K, Ca, and Mg (for the photosynthetic process); protein trafficking; and the metabolism of the cell wall [ 36 , 37 , 38 ]. At the acclimatized plant stage, the uptake rates of the previously mentioned elements decreased with the increase in copper sulphate levels in the medium up to 120 mg L −1 except Cu ( Table 5 ). This decline in the nutrient uptake rate at this stage may reflect the need for plants to redistribute these nutrients in plant cells, based on the physiological activity. The previous result confirms that Cu has a dual impact (hermetic effect) in plants, including a positive impact at optimum or low levels and a toxic impact at high levels due to its high redox properties [ 35 ]. For in vitro rooted plantlets, the nutrients can be ordered as follows: Ca > K > P > Mg for macronutrients, and Mn > Fe > Zn > Cu for micronutrients. The previous order of nutrients for acclimatized plants was changed to K > Mg > Ca > P > Fe > Mn or Zn > Cu, due to the composition of the substrate used in acclimatization.
Effect of different concentrations of copper sulphate on mineral content of banana in vitro rooted plants after 4 weeks.
Values are the mean ± SE and values followed by the same letters in the same column were not significantly different by 1 Kruskal–Wallis Test and 2 Duncan’s test at 0.05 level Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
Effect of different concentrations of copper sulphate on mineral content of acclimatized banana plants after 4 weeks.
Values are the mean ± SE and values followed by the same letters in the same column were not significantly different by 1 Kruskal–Wallis Test and 2 Duncan’s test at 0.05 level. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
Sequences of the selected primers used in RAPD analysis and the number of generated RAPD markers and average polymorphism percentages.
2.5.2. Enzymatic Antioxidant Activities and Electrolyte Leakage
Significant increases due to copper treatments were shown in the activity of all the studied enzymes, including catalase (CAT), peroxidases (POX), and polyphenol oxidase (PPO). The 60 and 120 mg L −1 copper sulphate treatments produced the highest values of activity in the studied enzymes compared to the control ( Figure 3 a–c). The obtained results showed that the used levels of CuSO 4 5H 2 O up to 120 mg L −1 caused an increase in the activity of the studied antioxidant enzymes (CAT, POX and PPO) due to oxidative stress that resulted from high Cu doses, which were harmful to plant cells [ 5 ]. Under this stress, plants usually stimulated the antioxidant enzyme activities that occur as a consequence of elevated levels of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide [ 9 , 39 ]. These antioxidant enzymes, which mainly include CAT, superoxide dismutase (SOD) and POX, could alleviate the increased effect of ROS by scavenging them to harmless products [ 9 , 40 ].

Effect of copper sulphate at 0.025, 30, 60, 120 mg L −1 on the activity of antioxidant enzymes: [( A ) Catalase; ( B ) Peroxidases; ( C ) Polyphenol oxidase] and ( D ) Electrolyte leakage of banana shoots cultured in rooting medium (1 mg L −1 IBA) after 4 weeks of culture. Data represent the mean with SE. Letters above each bar shown the significant differences among the tested treatment, if letters were same corresponding treatments are statistically equal and vice versa according to Duncan’s multiple range tests at p < 0.05. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
The same trend of antioxidants was observed for electrolyte leakage (EL), for which the highest significant value of EL (~ 45 µS cm −2 ) was recorded for the highest dose of copper (120 mg L −1 ), as shown in Figure 3 d. Due to its importance as an indicator for stress, the measuring of EL was used to evaluate the injuries in the cell membranes as a response to different stresses. In the current study, an increase in EL was observed when the applied copper sulphate was increased to 120 mg L −1 ( Figure 3 d). This could be due to the increase of enzymatic antioxidants, which might correlate with the high levels of ROS. These ROS have the ability to damage the nucleic acids, cause the denaturation of proteins and lipids, and, ultimately, the death of the cells [ 5 ]. Many studies confirmed the increase in EL due to stresses on banana plants such as salinity, drought stress [ 41 ], and the stress of copper sulphate as presented in the current study, whereas other studies reported that EL decreased due to calcium nitrate (0.5 to 1.0 g L –1 ) in banana plantlets [ 42 ].
2.5.3. Copper Sulphate and Chlorophyll Content of In Vitro Plantlets
The chlorophyll content in plants is considered one of the most important components of photosynthetic pigments, which could be damaged under different stresses. The current results confirmed that the chlorophyll content (a and b) of banana plantlets decreased significantly with the increase in applied copper doses up to 120 mg L −1 , due to the phytotoxicity of the copper ( Figure 4 ). As the chlorophyll content was reduced due to its degradation, the photosynthesis of banana plantlets also reduced, as confirmed by many studies. Due to the stress resulting from nano silver, different concentrations led to a decrease in the chlorophyll content of banana plantlets, such as for 7 and 200 mg L −1 [ 22 , 23 ], respectively. The chlorophyll content of banana plants was significantly influenced by the liquid MS basal medium, containing excess salt (NaCl up to 800 mM) or suffering under drought stress (mannitol up to 600 mM), as reported by [ 41 ]. The stress of copper sulphate was also reported by some studies using different concentrations of up to 100 µM [ 17 ], and up to 8 mg L −1 [ 25 ]. The previous trend was not common for some other elements like calcium and silicon, which increased the chlorophyll content in banana plantlets when added at certain concentrations, such as with 0.5 g L −1 of calcium nitrate [ 42 ] and 1 g L −1 in the form of CaSiO 3 [ 43 ].

Effect of copper sulphate at 0.025, 30, 60, and 120 mg L −1 on chlorophyll content of in vitro banana plantlets. Data represent the mean with SE. Letters above each bar shown the significant differences among the tested treatment, if letters were same corresponding treatments are statistically equal and vice versa according to Duncan’s multiple range tests at p < 0.05. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
2.6. Impacts of CuSO 4 H 2 O Treatments on Genetic Fidelity and Genomic Template Stability
Seven -10 mer operon primers were used for screening the genome stability in response to CuSO 4 treatments. The primers yielded specific and stable banding patterns ( Table 6 and Table 7 , and Figure 5 ). RAPD patterns generated by the copper sulphate exposed plantlets that were not clearly different from those obtained using the control DNA for all copper sulphate concentrations. The number of total bands varied from 2 (OPE-11) to 7 (OPD-14). The tested primers produced only monomorphic bands, except primer OPD-12 which produced 1 polymorphic band in the plant treated with 60 mg L −1 of CuSO 4 . The polymorphism percentages were 0.0% for all primers, except primer OPD-12 which gave average of 1.8%. The differences in RAPD patterns refer to the loss of normal bands and/or the appearance of new bands as compared with the control. RAPD profiles of the randomly selected in vitro plants, in comparison to the mother plant, were almost identical, thus assuring a totally genetic fidelity-maintained protocol for in vitro propagated Musa plants. Additionally, the GTS% for all treated plants was calculated ( Figure 6 ). The GTS for control plants was fixed as 100%. There were non-significant ( p < 0.01) differences in the GTS % of all treated plants; the average genome stability was 100, 98.9, and 100 for CuSO4 concentrations of 30, 60 and 120 mg L −1 , respectively ( Figure 5 ). It can be concluded from the results that the CuSO 4 treatments did not significantly change the genome stability of in vitro Musa sp. plants. The RAPD method was sensitive and capable of detecting variations in plant genome profiles [ 44 , 45 , 46 ]. The RAPD technique was effectively utilized to detect genotoxic effects in several plants induced by various metals [ 5 , 47 , 48 ]. RAPD primers were used to study the genotoxic effects of CuSO 4 for both the control and treated in vitro plantlets [ 49 , 50 ].

RAPD profiles of banana plants treated with 30, 60 and 120 mg L −1 CuSO 4 5H 2 O using primers OPE-11 and OPD-12. M: Molecular weight marker (1000bpSizer DNA ladder), C: Control plant. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).

Genome template stability (GTS %) in in vitro banana plants treated with different concentrations of CuSO 4 5H 2 O. Control = 0.025 mg L −1 and represents the concentration of CuSO 4 5H 2 O in MS medium (without copper sulphate supplementation).
RAPD band patterns generated from genomic DNA of copper sulphate-treated and control banana in vitro plants.
3. Materials and Methods
3.1. plant materials.
In vitro plantlets of Musa sp. ‘Grand Nain’ were maintained in MS solid medium which contained 30 g L −1 sucrose + 2.0 g L −1 gelrite and was supplemented with 3 mg L −1 Benzyl adenine (BA) + 1 mg L −1 Kinetin (Kin). Cultures were kept at 25 ± 2 °C and 50 μmol m −2 s −1 photosynthetic photon flux (PPF: 16 h/d) for 3 weeks before using in this study.
3.2. Copper Sulphate and Bacterial Contamination (Primary Experiment)
Previously isolated endophytic bacteria as described in [ 5 ], were inoculated into MS medium containing different concentrations of copper sulphate (CuSO 4 5H 2 O: 0.025, 30, 60, and 120 mg L −1 ) to investigate their effect on the bacterial contamination. The medium was poured into sterilized 9 cm petri dishes and incubated for 4 days. After that they were inoculated by loop where each treatment had 4 replications. The appearance of clonal growth was used to detect the bacterial contamination of inoculated plates which were incubated at 28 °C for 3 days.
3.3. Copper Sulphate, Shoot Multiplication and In Vitro Rooting
Axillary shoots of about 3.0 cm were separated into clusters of three shoots each, which were used as explants. Twenty cylindrical culture jars of 375 mL capacity were used; each jar contained 60 mL MS medium, as described above, fortified with different concentrations of CuSO 4 5H 2 O as applied above in primary experiment (30, 60, and 120 mg L −1 ). The pH of media was adjusted to 5.8 by 0.1N KOH/HCl. The media were distributed in the culture jars and autoclaved for 20 min at 121 °C and 1.2 kg cm −2 . MS basal medium contained 0.025 mg L −1 CuSO 4 5H 2 O which was considered a control in the current study. Cultures were sub-cultured three times, 3 weeks each on the same medium, in the same conditions as mentioned above. Contamination percentage was recorded. Then, shoot clusters (three shoots, >5 cm long) of Musa sp. were cultured in MS rooting medium containing 1 mg L −1 BA and 1 mg L −1 indole-3-butyric acid (IBA) and fortified with copper sulphate treatments. After four weeks of culturing on rooting medium, five replicates (jars) for each treatment, each replicate contained three explants, were selected for determination of the number of shoots, number of leaves/shoots, shoot length(cm), number of roots, root length (cm) and plantlet fresh weight (g).
3.4. Copper Sulphate and Acclimatization
In vitro plantlets were transplanted at the stage of 4–6 leaves into 5 cm pots filled with a mixture of sterilized peat moss and perlite (1:1). The plantlets in pots were covered with clear plastic film during the first 20 days of transplanting in air-conditioned greenhouse. The air temperature in the greenhouse was adjusted to 25 ± 2 °C, the relative humidity was 60 to 70% and the PPF to 100 µmol·m –2 ·s –1 . After 4 weeks of acclimatization, survival percentage was calculated for the remaining plants (10 plants for each treatment at the beginning of acclimatization stage). Plant length (cm), number of leaves, number of roots, root length (cm), leaf area (cm 2 ) and plant fresh weight (g) were recorded for five plants for each treatment.
3.5. Chemical Analyses
3.5.1. enzymatic antioxidants and electrolyte leakage.
Full expanded leaves of in vitro plants were used to determine the activity of some enzymatic antioxidants, e.g., catalase (CAT; EC 1.11.1.6), peroxidase (POX; EC 1.11.1.7), and polyphenol oxidase (PPO; EC 1.10.3.1), according to [ 50 , 51 , 52 , 53 ], respectively. Electrolyte leakage percentage was measured as described by [ 54 , 55 ], with some modifications according to [ 56 ] using leaf discs of in vitro plantlets. More details about the measuring of enzymatic antioxidants and electrolyte leakage can be found in [ 5 ].
3.5.2. Measuring of Chlorophyll Contents
The amount of chlorophyll a (Chl a) and chlorophyll b (Chl b) in the fully expanded leaves of in vitro rooted plants was determined using spectrophotometric analysis (Double beam UV/Visible Spectrophotometer Libra S80PC, England) according to [ 57 ].
3.5.3. Chemical Composition of In Vitro Plantlets
The chemical composition was carried out for oven-dried plant samples at 70 °C for 24 h. According to [ 58 ], spectrophotometer (GT 80+, UK) was used for P and the atomic absorption spectrometry (Avanta E; GBC, Canton, USA) for K, Ca, Cu, Fe, Mg, Mn, and Zn.
3.6. Plant DNA Extraction and RAPD-PCR Conditions
DNA from leaf material was extracted using 3 plants for each treatment by acetyltrimethyl-ammonium bromide (CTAB) according to [ 59 ]. The DNA was resuspended in distilled water and quantified by Implen P330 nanophotometer (Implen GmbH, München, Germany). Seven 10-mer primers were used for PCR amplification (Operon Technologies, USA) as presented in Table 8 . PCR reactions were carried out in 20 μL volume containing: 2.0 μL of DNA (15 ng μL −1 ), 7.0 μL of dd.H2O, 10.0 μL of 10 × PCR master mix buffer, and 1.0 μL of single primer (10 pmol). The reaction mixture was subjected to the following conditions: initial denaturation at 94°C for 5 min then 35 cycles of amplification under the following parameters: template denaturation at 94 °C for 1min, primer annealing at 36 °C for 30 s, and extension at 72 °C for 3 min. By the end of the 35th cycle, final extension at 72 °C for 10 min was given, followed by storage at 4 °C. The amplification products were resolved by electrophoresis in 1.5% agarose gels in 0.5 Tris-borate-EDTA (TBE) buffer and documented on Gel Documentation system (Uvitec Cambridge Company, Cambridge, UK). The amplifications were repeated twice and only the reproducible bands were considered. Reproducible fragments were scored as ‘1’ or ‘0’ for presence or absence of the band on the gels, respectively.
The name of primers and their nucleotide sequences.
3.7. Genomic Template Stability and Its Estimation
The polymorphic pattern was generated by RAPD-PCR profiles by using the selected primers, allowing the calculation of Genomic Template Stability (GTS %) as follows:
where a is the average number of polymorphic bands detected in plants treated with different concentrations of copper sulphate and n is the number of total bands in the non-treated plants. Polymorphisms in RAPD profiles included appearance of a new band and disappearance of a band compared to the control profile. Changes in these values were calculated as a percentage of their control to compare the sensitivity of genomic template stability.
3.8. Statistical Analyses
All experiments under the current study were set up in a completely randomized design. Data were subjected to analysis of variance using SPSS software (version 20; IBM Corp., Armonk, NY, USA). The mean separations were performed using Duncan’s multiple range testing method and significance was determined at p ≤ 0.05. Deviation to the mean was calculated as Standard Error (SE). The normality of the data series was carried out by Kolmogorov–Smirnov test. When data or their transformations arcsine(x) functions) had a normal distribution, the differences in the parameter measurements due to Cu-Sulfate concentrations were analyzed by one-way analysis of variance (ANOVA). If data series did not have a normal distribution or homogeneity of variance after transformation, we evaluated response differences using a non-parametric Kruskal–Wallis H-test and a Mann–Whitney U post hoc test.
4. Conclusions
For the successful and efficient micropropagation protocol of banana plant, adding copper sulphate as external supplementations to culture media at the suitable concentration for each stage of in vitro propagation, could be recommended. Supplementing establishment media with 60 mg L −1 copper sulphate could be recommended to eliminate bacterial contamination in banana in vitro cultures with the aim of obtaining aseptic banana cultures. Banana shoots did not require the addition of external copper sulphate to multiplication media, whereas the standard content of Cu in the medium was optimal for shooting. However, copper sulphate at a level of 30 mg L −1 should be added to rooting media to enhance the rooting potential of micropropagated banana shoots and the acclimatization of in vitro plants. These current findings may help us to develop an applicable and cost-effective micropropagation protocol for banana plants. Further studies should be carried out to highlight the optimal concentration of Cu for in vitro cultures of uninfected explants. Moreover, future investigations should be conducted to examine the impact of the supplementation of culture media with micronutrients, rather than copper sulphate in different concentrations, on the efficiency of the micropropagation protocol of plants.

Acknowledgments
The authors greatly thank Mohammed El-Mahrouk, the head of Horticulture Department, Faculty of Agriculture, Kafrelsheikh University, Egypt, for his great help in the statistical analysis of data. The authors also thank the staff members of the Physiology and Breeding of Horticultural Crops Laboratory, Department of Horticulture, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt, for supporting the completion of this work, where all in vitro experiments were achieved. The authors extend their thanks to Yasser Hafez, Faculty of Agriculture, Agricultural Botany Department, EPCRS Excellence Center & Plant Pathology and Biotechnology Laboratory, Kafrelsheikh University, KafrEl-Sheikh 33516, Egypt, and Antar El-Banna, Faculty of Agriculture, Genetics Department, Kafrelsheikh University, Kafr El-Sheikh 33516, Egypt, for their great help in enzymatic analysis and plant DNA extraction and RAPD-PCR analysis. The authors also greatly thank Hany Sobhy, Faculty of Agriculture, Agronomy Department, Kafrelsheikh University, KafrEl-Sheikh 33516, Egypt, for his great help in statistical analysis.
Author Contributions
Conceptualization, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; methodology, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; software, D.M.A.E., A.-M.S. and N.A.; validation, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; formal analysis, D.M.A.E., A.-M.S. and N.A.; investigation, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; resources, D.M.A.E. and A.-M.S.; data curation, N.A. and A.F.M.E.Z.; writing—original draft preparation, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; writing—review and editing, D.M.A.E.; A.-M.S.; N.A. and A.F.M.E.Z.; visualization, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; supervision, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z.; project administration, D.M.A.E., A.-M.S., N.A. and A.F.M.E.Z. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
There is no conflict of interest among the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ORIGINAL RESEARCH article
Bio-priming of banana tissue culture plantlets with endophytic bacillus velezensis eb1 to improve fusarium wilt resistance.

- 1 Key Laboratory of South Subtropical Fruit Biology and Genetic Resource Utilization, Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of Tropical and Subtropical Fruit Tree Research, Institute of Fruit Tree Research, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2 College of Horticulture, Sichuan Agricultural University, Chengdu, China
Tissue culture techniques have been routinely used for banana propagation and offered rapid production of planting materials with favorable genotypes and free of pathogenic microorganisms in the banana industry. Meanwhile, extensive scientific work suggests that micropropagated plantlets are more susceptible to Fusarium oxysporum f. sp. cubense ( Foc ), the deadly strain that causes Fusarium wilt of bananas than conventional planting material due to the loss of indigenous endophytes. In this study, an endophytic bacterium Bacillus velezensis EB1 was isolated and characterized. EB1 shows remarkable in vitro antagonistic activity against Foc with an inhibition rate of 75.43% and induces significant morphological and ultrastructural changes and alterations in the hyphae of Foc . Colony-forming unit (c.f.u.) counting and scanning electron microscopy (SEM) revealed that EB1 could colonize both the surface and inner tissues of banana tissue culture plantlets. Banana tissue culture plantlets of late rooting stage bioprimed with EB1 could efficiently ward off the invasive of Foc . The bio-priming effect could maintain in the acclimatized banana plants and significantly decrease the disease severity of Fusarium wilt and induce strong disease resistance by manipulating plant defense signaling pathways in a pot experiment. Our results provide the adaptability and potential of native endophyte EB1 in protecting plants from pathogens and infer that banana tissue culture plantlets bio-priming with endophytic microbiota could be a promising biological solution in the fight against the Fusarium wilt of banana.
Introduction
As the most important fruit in the world and the major staple crop in more than 130 countries across the tropical belt, banana ( Musa spp.) production contributes significantly to income and food security ( Kema et al., 2021 ). However, the banana industry is under severe threat from Fusarium wilt, the most destructive disease of banana in history whose causal agent is Fusarium oxysporum f. sp. cubense ( Foc ). Foc is composed of different evolutionary lineages and at least 24 vegetative compatibility groups (VCGs). Foc race 1 wiped out the highly susceptible Gros Michel ( Musa AAA) variety in Central America in the mid-twentieth century ( O'Donnell et al., 2009 ; Staver et al., 2020 ). The plague caused by Foc race 1 was mitigated by gradually adopting a resistant cultivar Cavendish ( Musa AAA) as a replacement for Gros Michel ( Dita et al., 2018 ). The recent emergence of Foc tropical race 4 ( Foc TR4), the most destructive and uncontrollable pathogen of banana, to which Cavendish and many other cultivars are highly susceptible, has created havoc on banana production worldwide again ( Dita et al., 2018 ). Ever since it was first reported to destroy the Cavendish-based banana industry in the 1960s in Taiwan, Foc TR4 has expanded across Southeast Asia, the Middle East, Africa, and most recently has been reported in Colombia and is present in 27 countries where thousands of hectares have been affected in the past several years ( Ordonez et al., 2015 ; Galvis, 2019 ). A recent projection by the Food and Agriculture Organization of the United Nations (FAO) estimated that the inexorable spread of Foc TR4 would lead to a 2.0% drop in global output, 240,000 direct jobs loss, and induce a 9.2% rise in the global reference price for bananas by 2028 ( Altendorf, 2019 ).
The management of Fusarium wilt is particularly challenging due to several conspiring factors. First, as a soil-borne fungus, Foc can survive in the soil in the form of chlamydospore for up to 30 years even in the absence of host plants and be dispersed through diverse ways (i.e., infecting plant material, soil, water, and others) ( Cook et al., 2015 ; Dita et al., 2018 ). Second, the only effective measure to manage this disease is stated frequently as planting resistant cultivars, but resistant cultivars might not meet the current market demand and may be overcome by continually emerging pathogens ( Ploetz, 2015 ). The latter cultivated banana is almost exclusively of the Foc TR4 susceptible Cavendish for its export properties, which facilitates the dispersion of the disease worldwide. Third, as a typical vascular wilt disease, Foc can escape from contacting with non-contact fungicides, non-endophytic biological control agents (BCAs), and other control measures once it penetrates the host plant ( Bubici et al., 2019 ). Thus, it is almost impossible to eliminate the disease incidence once the field gets contaminated with Foc . Therefore, highly efficient and sustainable strategies should be implemented to alleviate the influences of Fusarium wilt on susceptible varieties and to improve the durability of available resistant varieties.
In recent years, the use of BCAs for the control of many plant diseases including the Fusarium wilt of banana has gained great interest as an alternative to chemical application. Among the BCAs, Among the BCAs, the pivotal role of endophyte in the health and fitness of their host plants has become evident only in recent years ( Papik et al., 2020 ; Matsumoto et al., 2021 ). Endophytes refer to microbes that colonize internally in different plant tissues and perform mutualistic symbiotic associations with their hosts ( Papik et al., 2020 ). Their unique ecological niches similar to that of vascular wilt pathogens make them better targets for biocontrol agents against wilt disease than their rhizospheric counterparts ( Strobel and Daisy, 2003 ; Eljounaidi et al., 2016 ). As the second microbiological layer of plant defense, endophytes can defend plants from biotic stresses either by showing direct antagonistic activity such as parasitism, antibiosis, and competition or by inducing indirect antagonism effects (induced systemic resistance, ISR) in host plants to an array of phytopathogens ( Dini-Andreote, 2020 ; Dubey et al., 2020 ). Several studies have shown that endophytic microbes may serve as environmentally safe measures to combat Fusarium wilt of banana ( Cao et al., 2005 ; Bubici et al., 2019 ; Gómez-Lama Cabanás et al., 2021 ; Savani et al., 2021 ; Zhang et al., 2022 ). Applications of endophytic Trichoderma asperellum Prr2 ( Thangavelu and Gopi, 2015 ), Pseudomonas aeruginosa ( Yu et al., 2010 ), Pseudomonas sp. UPMP3, and Burkholderia sp. UPMB3 ( Mohd Fishal et al., 2010 ) have reduced the disease incidence of Fusarium wilt in banana significantly under greenhouse and field conditions.
Nowadays, the most common application strategies of endophytes in agricultural systems are adding them directly into the soil and preparing them as seed-coating agents, which are rather inefficient in practice. Thus, it is imperative to explore alternative strategies for endophyte application ( Dubey et al., 2020 ). Even more significant is the fact that, unlike most other seed plants, the propagation of banana is mainly dependent on tissue culture with all microorganisms eliminated during the micropropagation process under strict aseptic conditions. The regenerated plants are, therefore, particularly vulnerable when transferred directly to natural conditions with multiple environmental stresses ( Lian et al., 2009 ; Soumare et al., 2021 ). In this sense, the establishment of beneficial interactions between explants and beneficial microbes to offer protection for young host plantlets against environmental stress in field conditions might represent a valuable approach to efficiently solve those restrictions ( Soumare et al., 2021 ). Unfortunately, only few studies have reported inoculation with endophytes in banana tissue culture plantlets during the rooting or acclimatization stages ( Guez-Romero et al., 2008 ; Lian et al., 2009 ; Kavino and Manoranjitham, 2018 ). As the key components for achieving sustainable agriculture, the interactions between plants, fungi, and endophytes in tissue culture plantlets have not been sufficiently studied. In the present study, a bacterial endophytic strain EB1 was isolated from a healthy banana plant in a wilt-diseased banana field in Dongguan, Guangdong Province, China (23.045315° N, 113.546177° E). We critically aimed to decipher (i) the phylogenetic, genomic, and antagonistic effect of EB1 against Foc by in vitro test and (ii) how EB1 modulates the resistance of banana plants against Foc by using a banana plant–EB1system created by inoculating banana tissue culture plantlets with EB1 at the end of rooting stage. Our study was designed to lend new insights into the sophisticated mechanisms of host plants–endophytes interactions for coping with environmental stresses and to provide potential strategies to control the Fusarium wilt of banana.
Materials and methods
Strain, media, and cultural conditions.
Wild-type Foc TR4 strain II5 (VCG01213) was cultivated on a potato dextrose agar (PDA) plate at 28°C and used in this study. Isolated endophytic bacteria were inoculated in Luria-Bertani (LB) agar (Sangong Co., Ltd., Shanghai, China) plates. Basal Murashige and Skoog (MS) medium was used for tissue culture experiments.
Isolation and selection of endophytic bacteria against Foc TR4 from healthy banana plant
The healthy banana plants used in this study were collected from a wilt-diseased banana field in Dongguan, Guangdong Province, China (23.045315° N, 113.546177° E). Banana plant samples were washed with tap water thoroughly to remove the airborne counterparts and soaked in 75% (v/v) ethanol for 1 min, 0.1% (v/v) NaClO for 15 min, followed by being rinsed 5 times with sterile water to deplete epiphytic microorganisms in aseptic conditions. Ten grams of plant tissue was weighed and ground with 20 ml sterilized distilled water premixed with sterilized quartz sand using a sterilized mortar and pestle for 5 min. Aliquots of 1 ml of the resulting suspension were diluted using a serial dilution method and spread evenly on an LB agar plate and incubated at 28°C for 5 days. All culturable bacterial colonies were purified and selected based on their morphological characteristics. The antagonistic efficacies of these endophytic bacterial isolates were evaluated against Foc TR4 by a dual-culture experiment ( Fan et al., 2019 ). One actively growing agar plug (5 mm diameter) of Foc TR4 was placed on the center of a fresh PDA plate. Then, 10 μL-drop of each isolate from an overnight culture (OD 600 = 1.0) was uniformly inoculated 2.0 cm away from the fungal inoculum. Plates inoculated only with Foc TR4 plug were served as control. Plates were incubated at 28°C for 5 days and recorded with a Canon EOS 77D camera (Canon, Tokyo, Japan) with the same parameters, and the surface area of the mycelia was measured using the Image J software (Image J, NIH, USA). The inhibitory effect was evaluated by calculating the percentage of area inhibition using the following formula: (Sc - St)/Sc × 100, where Sc and St represent the growth area of Foc TR4 in the control and treated plates, respectively. The experiment was repeated 3 times, with 4 replicates each time. Strain EB1 was isolated through the above screening and stored at −80°C with glycerol (50%, v/v). The antifungal efficiency of EB1 against Foc TR4 was further measured by observing the morphology and ultrastructure characteristics of Foc TR4 in the dual-culture experiment by applying a scanning electron microscope (SEM, Hitachi Model S-3400N, Hitachi, Tokyo, Japan) and a transmission electron microscope (TEM, Hitachi HT7700, Hitachi, Tokyo, Japan).
Whole-genome sequencing of EB1
Overnight bacterial cultures of EB1 in LB broth were collected, centrifuged at 3,000 rpm for 15 min, and washed two times with sterile PBS buffer (50 μM, pH = 7.4). Whole-Genome Sequencing of EB1 was performed using a combination of the Oxford Nanopore Technologies (ONT) GridION platform (Oxford Nanopore Technologies Ltd, Oxford, UK) and Illumina MiSeq platform (Illumina MiSeq PE300, Illumina, USA) by Gene Denovo Biotechnology Co. (Guangzhou, China). DNA was extracted from Qiagen's DNeasy UltraClean Microbial Kit (Qiagen GmbH, Hilden, Germany) and its quality and concentrations were determined using a Nanodrop spectrophotometer (NanoDrop, Wilmington, DE, USA) and Qubit Fluorometer (Thermo Fisher Scientific, MA, USA). For ONT sequencing, library preparation was conducted according to the manufacturer's protocol of the SQK-LSK109 sequencing kit (Oxford Nanopore Technologies Ltd., Oxford, UK). For Illumina sequencing, genomic DNA (gDNA) was fragmented and a paired-end library with an average DNA insert size of 300–400 bp was constructed using Illumina TruSeq Nano DNA Library Prep Kit (Illumina). The assembled sequences were deposited in the NCBI (BioProject ID: PRJNA807456). The components of coding genes, noncoding RNA (ncRNA), and functional annotation were analyzed using a range of databases including the non-redundant protein database (Nr), SwissProt, Cluster of Orthologous Groups (COGs), and Kyoto Encyclopedia of Genes and Genomes (KEGG). Gene clusters for the biosynthesis of secondary metabolites were identified by using antiSMASH.
Phylogenetic analysis of EB1
The 16S rDNA sequence of strain EB1 derived from the EB1 genome was aligned with an NCBI 16S ribosomal RNA sequences database by Nucleotide BLAST ( https://blast.ncbi.nlm.nih.gov/Blast.cgi ), and 16S rRNA gene sequences closest to the isolates (98% sequence homology) were recovered for further phylogenetic analysis. Strain EB1 was subjected to phylogenetic analysis using MEGA version 7 (University, Pennsylvania, PA, USA) based on a full-length 16S ribosomal RNA (16S rRNA) sequence, and a phylogenetic tree was constructed using the neighbor-joining method. The reliability of this resulting tree was evaluated by the bootstrap method with 1,000 replications.
Colonization capacity of EB1 on banana tissue culture plantlets
Uniformly grown banana tissue culture plantlets [“Cavendish” banana (AAA) cv. “Brazilian”] of rooting stage were surface sterilized in 75% (v/v) ethanol for 1 min and 0.1% (v/v) NaClO for 15 min, rinsed with sterile water for 5 times, air-dried, and then transferred and grown vertically in tissue culture flasks containing 100 ml of cooled-down MS. Four plantlets were transferred to each flask. For EB1 inoculation, overnight culture of EB1 in LB broth was harvested, centrifuged, and washed in liquid MS twice, and resuspended in MS to a final optical density (OD 600 ) = 0.2. Each flask was inoculated with 0.1 ml of the bacterial suspension (~10 6 colony-forming unit, c.f.u.) or 0.1 ml MS by pipetting to the rhizosphere of banana tissue culture plantlets and cultured at 22°C and 16/8 h light/dark cycle. The colonization and reproduction of EB1 on the plantlets were quantified each day over a period of 7 days. Total c.f.u. values of EB1 were quantified per the programs described previously ( de Zélicourt et al., 2018 ; Berlanga-Clavero et al., 2022 ). Briefly, 1.0 g root and pseudostem tissues of the banana tissue culture plantlets were sampled and gently washed by dipping in the distilled water to remove non-attached bacteria cells at the same time each day (±2 h). Each sample was transferred to a 2-ml microcentrifuge tube with 1 ml PBS buffer, sonicated on ice for 1 min, and vortexed for 10 min, and 100 μl of the resulting suspensions were spread on LB agar plates after a 10-time dilution. The c.f.u. was counted after overnight incubation at 28°C, and the total c.f.u. was normalized per gram of root or pseudostem. To explore the interactions between strain EB1 and Foc in planta , an additional experiment was conducted in banana tissue culture plantlets by pipetting 0.1 ml Foc spore suspension (1 × 10 8 spores/L) or 0.1 ml MS to the rhizosphere of banana tissue culture plantlets after prior inoculation with EB1 for 3 days and cultured at 22°C and 16:8 h light/dark cycle for another 4 days. The experiment was conducted in triplicate, with at least eight plants per treatment. After 7 days of successive culture, SEM was used to observe the distributions of EB1 and the interactions between EB1 and Foc on the banana tissue plantlets.
Biocontrol efficacy of EB1 on banana plantlets
The above banana tissue culture plantlets and symbionts (banana tissue culture plantlets colonized with EB1) after 7 days of successive culture were subjected to hardening for 10 days by transferring into pots with sterilized planting soil (40 × 19 × 15 cm pots, ca. 2.0 kg soil each). Then the biocontrol efficacy of EB1 on banana plantlets was investigated in greenhouse experiments with the acclimatized banana plants ( Supplementary Figure S1 ). Three treatments including EB1 only, TR4 only, EB1+ TR4, and control were applied in the pot. The banana plants were inoculated with or without Foc TR4 isolates at the concentration of 1,000 conidia/g soil, with a temperature ranging from 25 to 35°C. Three plantlets were grown in each pot and at least 20 plantlets were included in each treatment. In addition, due to the lethality of plantlets in the TR4 only, EB1 + TR4 groups, 60 plantlets were employed in each group to ensure sufficient plant material. Plant survival rates were recorded every 10 days, and observations on morphological characters such as plant height (cm) and fresh weight of shoot and root (g) were conducted after 60 days of planting. The disease index of each plantlet was assessed according to the rating scale of 0–4: 0 = no symptom; 1 = some brown spots in the inner rhizome; 2 = < 25% of the inner rhizome show browning; 3 = up to 75% of the inner rhizome show browning; and 4 = entire inner rhizome and pseudostem show dark brown, dead ( Liu S. et al., 2020 ). Harvested banana plant tissues were stored at −80°C pending for further analysis of defense-related enzymes and genes.
RNA extraction and gene expression analysis by RT-qPCR
Total RNA was extracted from the frozen banana plant using SteadyPure Plant RNA Extraction Kit (Accurate Biotechnology Co., Ltd., Hunan, China ) following the manufacturer's instructions. HiScript II One Step qRT-PCR SYBR Green kit (Vazyme Biotech, Nanjing, China) was employed for qRT-PCR assays according to the manufacturer's instructions. First-strand cDNA was prepared by reverse transcription from 1 μg of DNA-free total RNA in a final reaction volume of 20 μl. RT-qPCR was conducted using a QuantStudio 5 Real-Time PCR System (Applied Biosystems, CA, USA) in four replicates. The qTUB gene (banana) was used as a reference for data normalization, and the target genes were amplified using the primer sets listed in Supplementary Table S1 . The relative transcript abundance of each gene was estimated using the 2 −ΔΔ Ct method ( Livak and Schmittgen, 2001 ).
Statistical analyses
All statistical analyses were performed using the SPSS 20.0 statistical software package (SPSS, Chicago, IL, USA). Data were analyzed using Student's t -test and one-way ANOVA test after being verified for normality and homogeneity of variance with Kolmogorov-Smirnov and Levene's tests. Cases with p -values of < 0.05 were considered statistically significant.
EB1 shows strong inhibitory efficiency against Foc TR4
The morphological observation was preliminarily carried out for strain EB1. It was found that the colony of EB1 on LB medium was dry and round with irregular protrusions at the margin, showing the typical characteristics of Bacillus species ( Supplementary Figure S2A ). The cells were short rod-shaped structures and ~1.2–1.6 μm in length, 0.6–0.7 μm in width, as revealed by SEM ( Supplementary Figure S2B ). EB1 showed strong inhibitory activities with the inhibition rates of mycelium growth area 75.43% against Foc TR4 ( Figure 1A ) and other Fusarium pathogens ( Supplementary Figure S3 ) during co-cultivation compared to control. To confirm the antagonistic activity of EB1, the morphological and ultrastructural changes of Foc TR4 after a confrontation with EB1 were scrutinized by SEM ( Figure 1B ) and TEM ( Figure 1C ). The untreated hyphae of Foc TR4 appeared straight, uniform, and well-developed tube-like structure in shape under SEM. Conversely, phenotypes of abnormalities were noted in fungal hyphae co-culture with EB1. Severe forms of abnormalities, including highly deformed, irregular distorted, inflated were observed in fungal hyphae. TEM micrographs of untreated fungal hyphae had a well-defined cell wall (CW), intact plasma membrane (PM), and normal cytoplasm containing an intact nucleus and all organelles. In reverse, noticeably disruptions such as loss of cellular integrity, thickened CW, evident plasmolysis, serious vacuolation, invaginated PM, abnormal architecture of the nucleus and degenerated organelles were observed in EB1 treated hyphae. The results indicated that the morphology and structural integrity of the treated fungal were dramatically affected during co-culture with EB1.
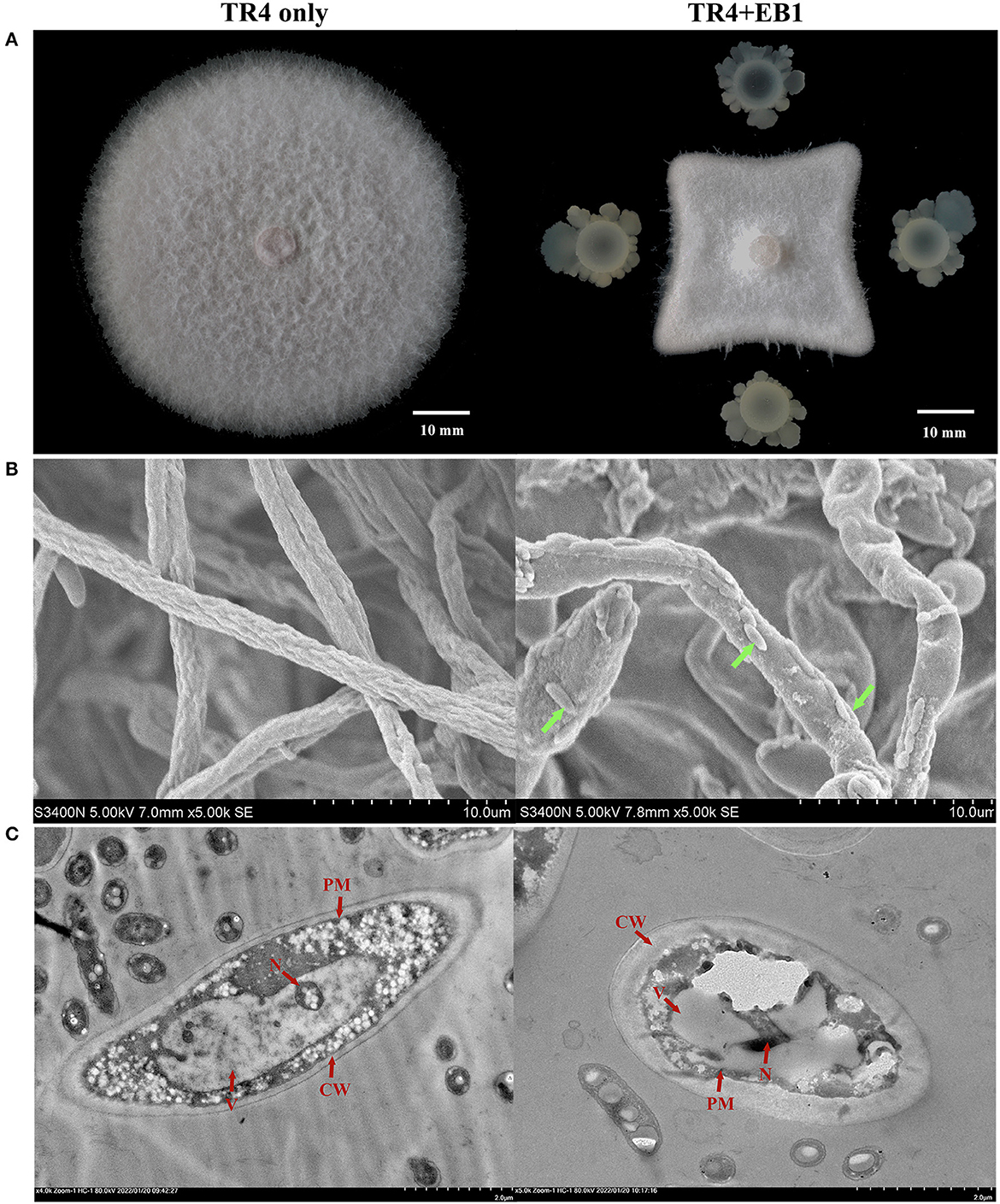
Figure 1 . The antagonistic potential of strain EB1 against Fusarium oxysporum f. sp. cubense tropical race 4 ( Foc TR4). (A) The antagonistic potential of EB1 against TR4 in vitro with a dual-culture experiment. Morphological (marked by green arrow heads) and ultrastructural changes (marked by red arrow heads) of TR4 after the confrontation with EB1 were scrutinized by scanning electron microscopy (SEM) (B) and transmission electron microscope (TEM) (C) , respectively.
Genome sequence assembly and general features of EB1
The whole genome of EB1 was sequenced and analyzed, through which the 16S rRNA region was extracted, and which was 1,404 bp in length. EB1 was identified as Bacillus velezensis based on a phylogenetic tree constructed from the 16S rRNA gene ( Figure 2A ). The complete genome sequence of EB1 was deposited in GenBank under accession number CP093218. Accordingly, the genome of EB1 consists of a single circular chromosome of 3,929,912 bp, with an average of 46.5% GC content and a clear GC skew transition ( Figure 2B ). All predicted 3,622 open reading frames (ORFs) with a maximum E -value of 1.0 E-5 were subjected to annotation analysis by comparing with Nr, SwissProt, COG, and KEGG databases, and a total of 3,606 candidate genes had annotation information. The overall functional annotation is depicted in Supplementary Figure S4 . A total of 2,756 genes were categorized into 21 functional groups using COG analysis ( Figure 2C ). Three main functional gene classes were revealed in the results: amino acid transport and metabolism (329 genes), transcription (267 genes), and carbohydrate transport and metabolism (240 genes), representing 30.33% of the predicted genes in the COG analysis. Other clusters of represented genes involved in inorganic ions transport and metabolism (200 genes), energy production and conversion (188 genes), cell wall/membrane/envelope biogenesis (182 genes), signal transduction (167 genes), and translation, ribosomal structure and biogenesis (161 genes) account for 32.58% of predicted genes. In addition, a high proportion of predicted genes (26.78%) involved in general function prediction only and function unknown is poorly characterized. A total of 2,250 genes were mapped to 5 KEGG branches, including metabolism, genetic information processing, environmental information processing, cellular processes, and organismal systems, and among which, a high proportion of the annotated genes were assigned to metabolism, especially the pathways belonging to global and overview maps (682 genes), carbohydrate metabolism (240 genes), and amino acid metabolism (201 genes) ( Figure 2D ). Twelve biosynthetic gene clusters (BGCs) involved in the biosynthesis of secondary metabolites including non-ribosomal peptides synthase (NRPS), bacteriocin-NRPS, trans-AT polyketide synthase (transatpks), type III polyketides synthase (t3pks), terpene, transatpks-nrps, lantipeptide, and other types of polyketide synthases (OtherKS) were identified in EB1 using AntiSMASH.
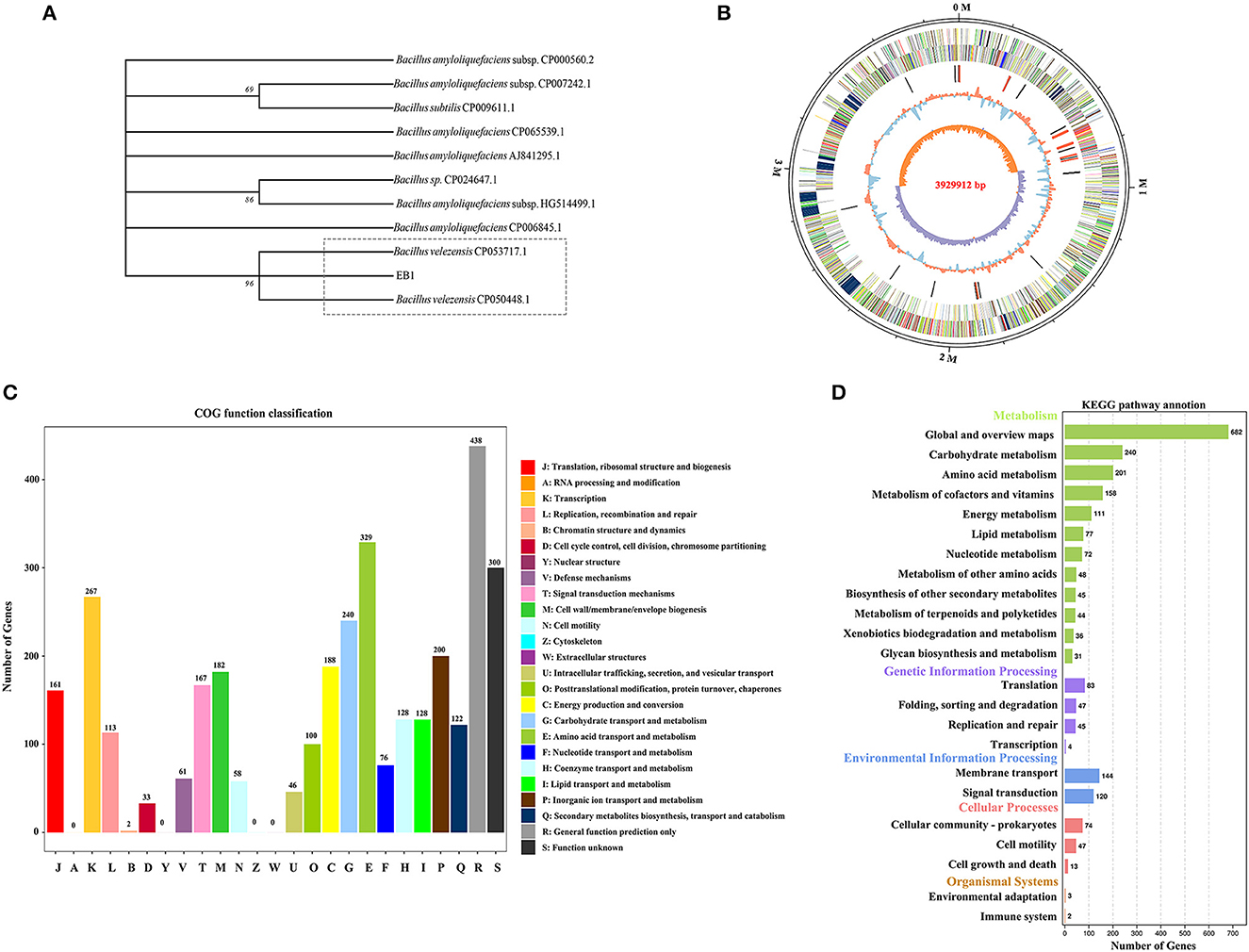
Figure 2 . Phylogenetic and genomic analyses of strain EB1. (A) Phylogenetic trees of strain EB1 based on 16S rRNA gene. The tree was constructed using the MEGA software. The level of bootstrap support (1,000 repetitions) was indicated at all nodes. (B) Graphical circular map of EB1 genome. The distribution of the circle from the outermost to the center is (i) scale marks of the genome; (ii) protein-coding genes on the forward strand; (iii) protein-coding genes on the reverse strand; (iv) tRNA (black) and rRNA (red); (v) GC content; (vi) GC skew. (C) The COG annotation of strain EB1 genome. (D) The KEGG pathway annotation of strainEB1 genome.
EB1 showed strong colonization ability on the banana plantlets
The growth dynamics of the EB1 population during the 7 days after banana tissue plantlets treatment in tissue culture flasks were investigated in the root and shoot by c.f.u. counts and SEM ( Figure 3 ). In the root, the colonization of EB1 numbered at 357 c.f.u./g on day 1 and increased to 1.20 × 10 8 c.f.u./g following an algorithm by day 5 and remained at 1.53 × 10 8 c.f.u./g by day 7. In contrast, in the case of shoot, no EB1 was detected on day 1, after which the colonization level slowly started to increase from 9.73 × 10 3 c.f.u./g on day 2 to 1.96 × 10 7 c.f.u./g by day 7 ( Figure 3A ). Using SEM, no colony could be observed in the root or shoot and the pant epidermal cells were integral and smooth in the control group ( Figure 3B ). In the EB1-inoculated group, small rod-shaped EB1 can be seen in grooves between epidermal cells and intercellular space in root cells ( Figure 3B ). To explore whether EB1 inhibits Foc in planta , an additional experiment was conducted in banana tissue culture plantlets by inoculating with Foc after prior inoculation with EB1 for 7 days. The SEM observation revealed that infection of Foc seriously destroyed the surface structures of the root of banana tissue culture plantlets ( Figure 3C ). However, inoculation of EB1 prior to Foc treatment led to serious morphological deformities of Foc and the damaging effects of pathogen infection were alleviated ( Figure 3C ).
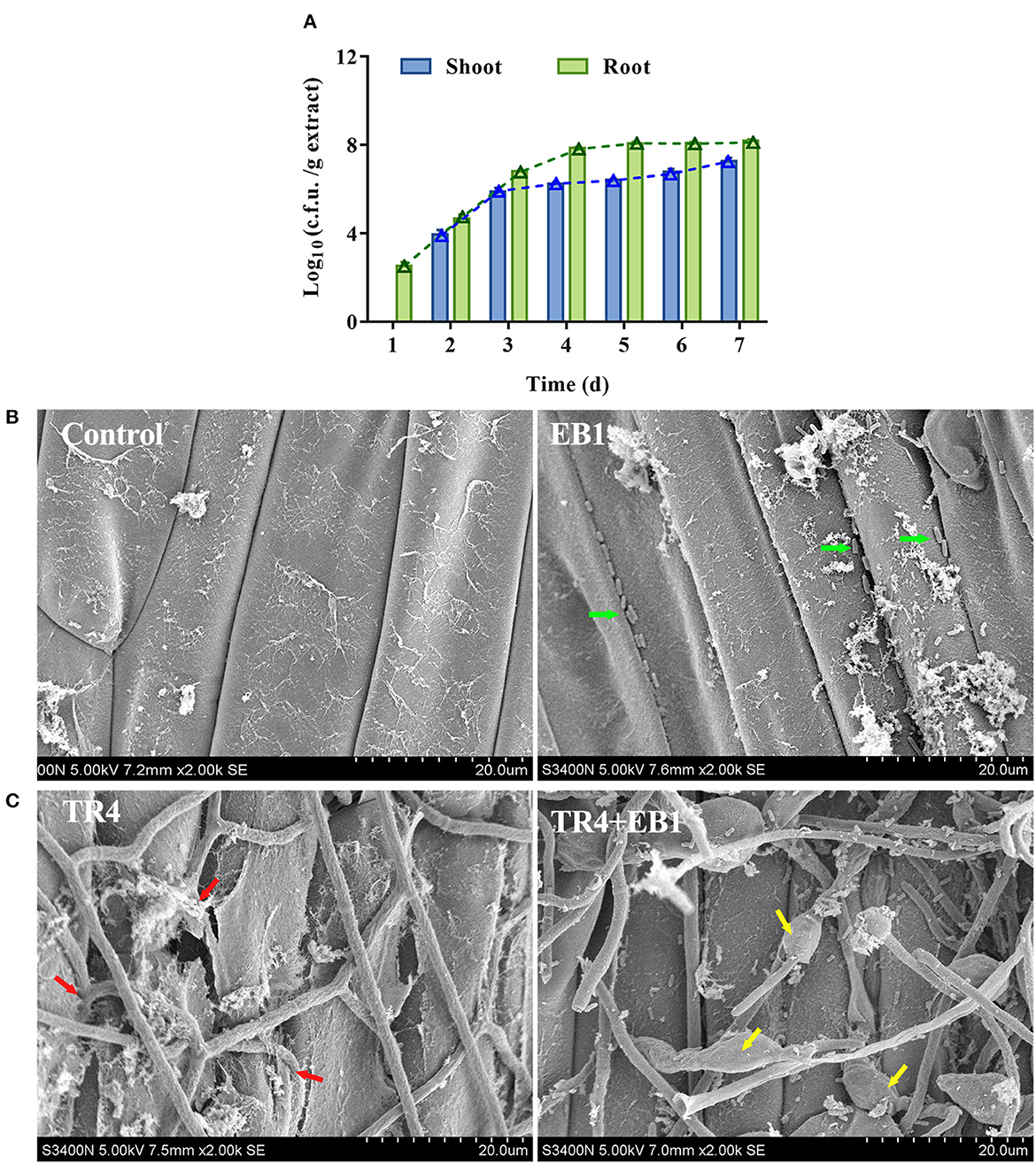
Figure 3 . Strain EB1 showed strong colonization ability on the banana plantlets and provided protection against the infection of TR4. (A) EB1 dynamics (c.f.u. counts) in shoot and root extracts during the first 7 days after banana tissue culture plantlets bacterized. Bars represent average values ± SD of total c.f.u. in shoot and root extracts. Green and blue lines represent c.f.u. corresponding to the number of spores in shoot and root extracts, respectively. (B) Root colonization of strain EB1 visualized by scanning electron microscopy (SEM). Strain EB1 colonies occur in grooves between epidermal cells and intercellular space in root cells (marked by green arrow heads). (C) Strain EB1 inhibits the infection of TR4 in planta . TR4 seriously destroyed the surface structures of the root of banana tissue culture plantlets (marked by red arrow heads) and inoculation of EB1 prior to Foc treatment led to serious morphological deformities of Foc and the damaging effects by pathogen infection were alleviated (marked by yellow arrow heads).
EB1 significantly promoted plant growth and conferred protection against Foc TR4
As EB1 was pre-inoculated on the culture plantlets at the rooting stage, to evaluate whether the acclimatized banana plants have been primed by EB1, the plant growth, survival, and disease severity were inspected in banana plants. Overall, pre-inoculation with EB1 at the rooting stage significantly promoted plant growth and conferred protection against Foc TR4 compared with the non-inoculation groups ( Figure 4 ). No differences were detected in the height of banana plants between the control and EB1 groups. By contrast, the heights of banana plants were significantly reduced when subjected to Foc TR4 infections ( p < 0.001), and this inhibitory effect was dramatically reversed upon co-inoculation with EB1 (TR4 + EB1) ( Figures 4A , B ). Compared with control, EB1 inoculation (EB1) significantly increased the plant biomass in both above-ground (shoot) and below-ground (roots) by 1.22- ( p = 0.002) and 1.49-fold ( p = 0.007), respectively. Plants in the EB1 + TR4 treatment group also had increased biomass compared with those treated by Foc TR4 only (shoot: 1.35-fold, p = 0.002 and root: 1.94-fold, p = 0.004). Moreover, EB1 did not cause any mortality or disease symptoms in banana plants when inoculated alone and greatly enhanced the survival rates and reduced the disease severity caused by Foc TR4 in banana plants who had been pre-inoculated with EB1 prior to inoculation with Foc TR4 (EB1 + TR4) compared with those inoculated only with Foc TR4 ( Figures 4D , E ; Supplementary Figure S5 ). Overall, these findings reveal that EB1 is a bacterial endophyte of banana plants that efficiently suppresses Fusarium wilt caused by Foc TR4.
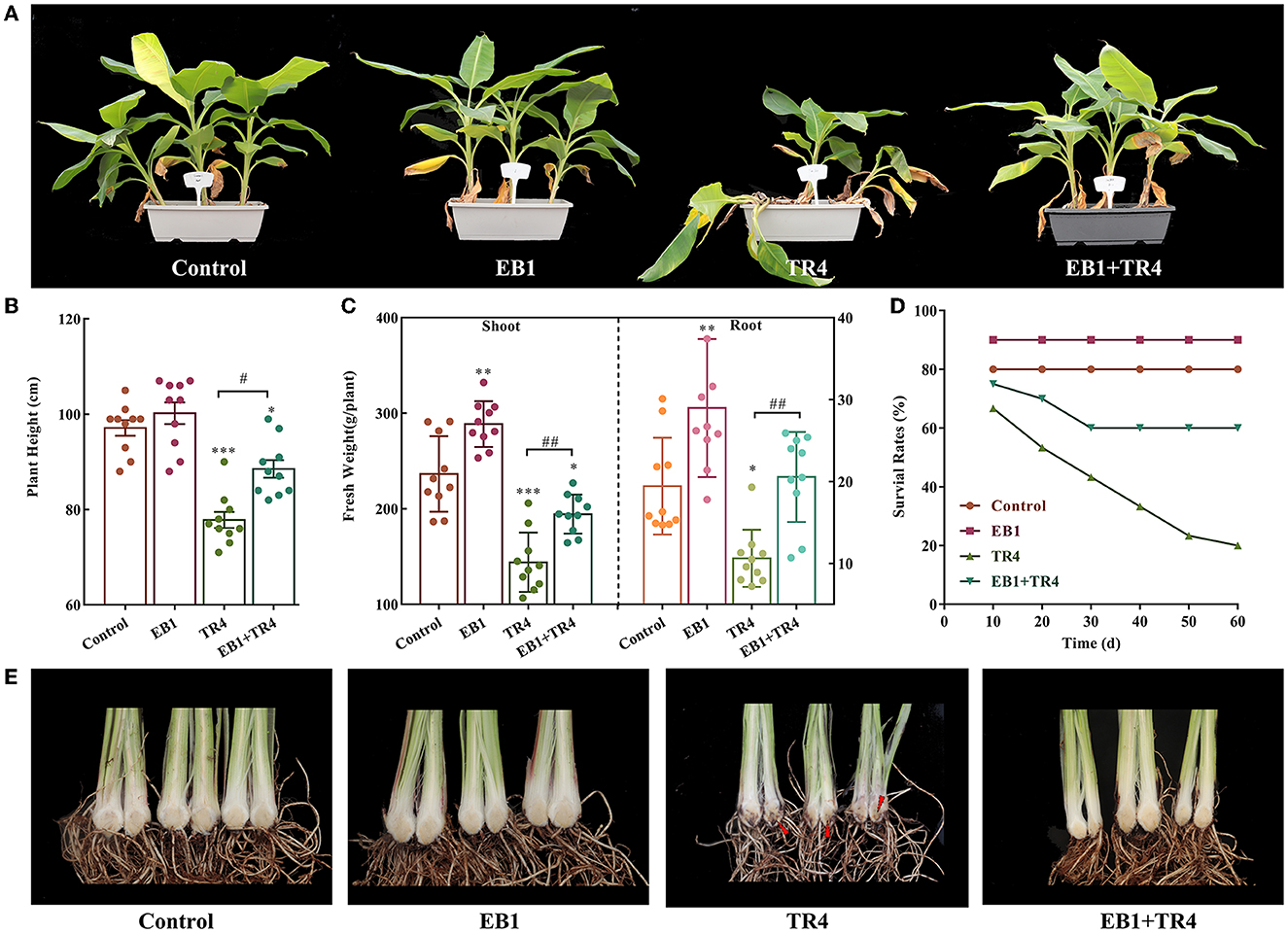
Figure 4 . Strain EB1 enhances Fusarium wilt resistance and promoted banana growth in the pot experiment. Control, no inoculation; EB1, inoculation with EB1 only, TR4, inoculation with TR4 only; EB1 + TR4, inoculation of EB1 prior to Foc treatment. (A) Phenotypes of acclimatized banana plants after being primed with EB1 at the rooting stage of tissue culture plantlets. (B–E) Plant height (B) , biomass of shoots and roots (C) , survival rates (D) , and severity of Fusarium wilt of acclimatized banana plants under different treatments (marked by red arrow heads) (E) . All data are expressed as the mean ± SD of at least 10 replicate samples. * p < 0.05, ** p < 0.01, and *** p < 0.001 indicate significant differences between the treatment groups and control group. # p < 0.05 and ## p < 0.01 indicate significant differences between treatment groups.
EB1 manipulated the SA and JA pathways in banana plants
To further examine whether the EB1 could activate defense signaling in the banana plant, the expression patterns of the defense-related marker genes involved in SA and JA pathways including NPR1, PR1, LOX2 , and MYC2 were analyzed ( Figure 5 ). Compared with the control, EB1 inoculation showed no induction in the expression of NPR1 and PR1 genes of the SA signaling pathway but showed an increase in the expression of the MYC2 gene of the JA signaling pathway by 1.67-fold ( p = 0.016). Whereas, the degree of expression changes in the Foc TR4 treatment group varied from gene to gene. Foc TR4 downregulated the expressions of NPR1 and LOX2 by 0.46- ( p = 0.0024) and 0.63-fold ( p = 0.016) and upregulated the expressions of PR1 and MYC2 by 16.62- ( p < 0.001) and 2.23-fold ( p < 0.001), respectively. Notably, both SA and JA signaling pathways were significantly upregulated by 1.40- ( p = 0.015), 35.80- ( p < 0.001), 1.50- ( p = 0.0028), and 2.44-fold ( p < 0.001) for NPR1, PR1, LOX2 , and MYC2 , respectively. The above results have indicated that EB1 primes the plants for enhanced immunity following a subsequent attack by Foc TR4.
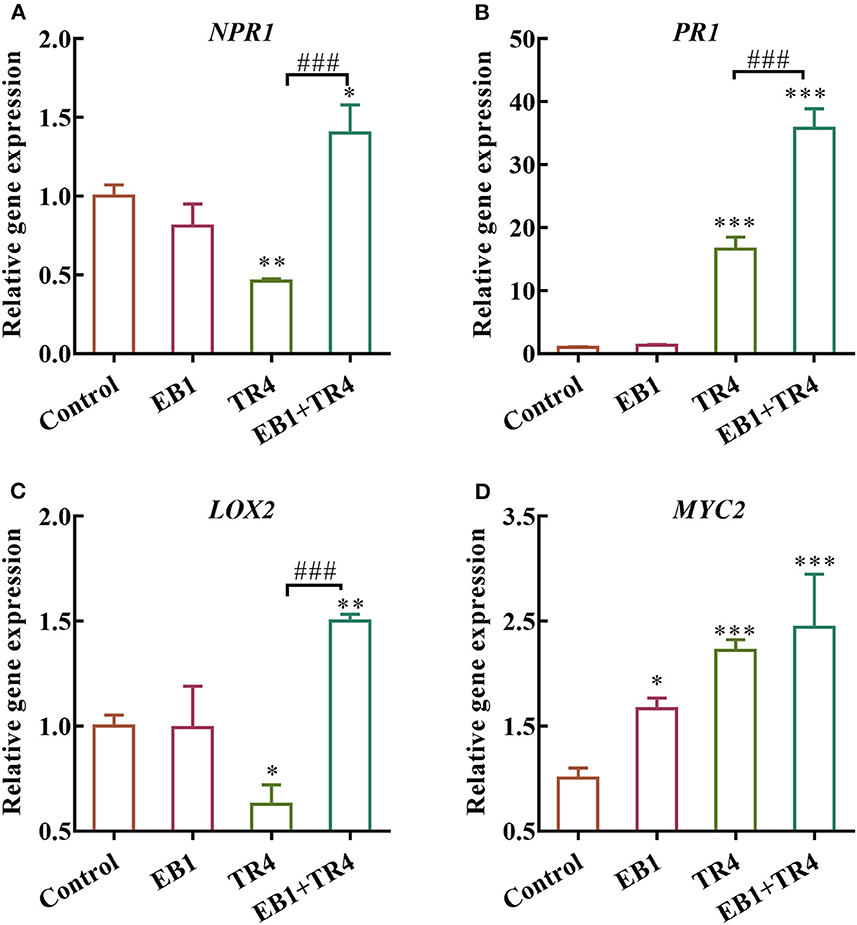
Figure 5 . Effects of strain EB1 and TR4 inoculations on the expression of defense genes. The expression patterns of (A) NPR1 , (B) PR1 , (C) LOX2 , and (D) MYC2 were analyzed by RT-qPCR. All data are expressed as the mean ± SD ( n = 4). * p < 0.05, ** p < 0.01, and *** p < 0.001 indicate significant differences between treatment groups and control group. ### p < 0.001 indicate significant differences between treatment groups.
Besides resistant cultivar breeding, BCA comprising endophytic bacteria has been considered another promising control strategy against Fusarium wilt that reached the field-testing stage ( Bubici et al., 2019 ). However, their control efficacy has been always unstable due to the varying environmental conditions, accentuating the need to develop a new efficient strategy for endophytes to harness their maximum benefits ( Dubey et al., 2020 ; Papik et al., 2020 ; Savani et al., 2021 ; Jana et al., 2022 ). Plant tissue culture is the main strategy for banana propagation with the advantages of high multiplication rates and the production of disease-free planting materials with high genetic fidelity and high standards of hygiene ( Pegg et al., 2019 ). More recently, tissue-cultured banana plantlets are questioned to be more susceptible to Fusarium wilt than vegetative planting materials because the first three stages (Stage I: establishment of explants, Stage II: elongation and multiplication, and Stage III: rooting) of the plant tissue culture process are taken place in aseptic conditions without the possibility of interaction with beneficial microbes ( Orlikowska et al., 2017 ). In this context, co-cultivation of the plant tissue culture plantlets with beneficial microorganisms may be indispensable to improve the adaptive ability of plantlets in the acclimation stage, which has accentuated the urgent to develop new technologies based on endophytes as microbial inoculants in tissue culture banana plantlets. Therefore, in the current study, the endophytic bacterial strain B. velezensis EB1 was isolated and selected for in-depth analysis based on its antifungal activity against Foc TR4 and strong colonization ability in banana plants to support a new approach using EB1 to control Fusarium wilt of banana by introducing into banana tissue culture plantlets at the end of rooting stage.
As one of the largest bacterial genera, Bacillus strains coexist with plants and are among the most studied microorganisms in the biological control of various plant diseases ( Shafi et al., 2017 ; Fira et al., 2018 ). For instance, endophytic bacteria B. mojavensis and B. cereus exhibited potent inhibition activities against various rice Fusarium pathogens such as F. proliferum, F. verticillioides , and F. fujikuroi ( Etesami and Alikhani, 2017 ). Consistent with these findings, the in vitro dual-culture experiment demonstrated that the growth of Foc TR4 could be significantly inhibited by EB1. Interestingly, considerable ultra-structural alterations such as wizened, flattened, thickened CW and plasmolysis that were observed in Foc TR4 cells at confronting with EB1 indicated that EB1 might be capable of producing antagonistic metabolites, penetrating into Foc TR4 cell, and leading to leakage of cytoplasm and disruption of internal organelles of Foc TR4. Accordingly, genes related to the biosynthesis of antifungal compounds such as lipopeptides and ketones, which were proven to inhibit hyphal extension and spore formation of phytopathogens, were identified in EB1 genome by the antiSMASH tool ( Arrebola et al., 2010 ; Li et al., 2015 ).
Plants can act as a filter of microbial communities and select the right endophytes to maintain their normal growth and development ( Dubey et al., 2020 ; Liu H. et al., 2020 ). Therefore, stable root colonization and persistence of BCAs in the plant is a key factor for their application in the biological management of microbial diseases ( Shafi et al., 2017 ). Detections of EB1 inside both roots and shoots of banana tissue culture plantlets in our study supporting EB1 is capable of entering through the root system and migrating upwards into the pseudostem. EB1 is an endophyte isolated from banana pseudostem; thus, it is conceivable that it has evolved strategies for efficient adaptation to this niche. To determine whether EB1 inhibits Fo c TR4 in planta , banana tissue culture plantlets were infected with Foc TR4 but only after a prior bacterization with an EB1. Using SEM, EB1 colonies were observed in grooves between root epidermal cells, indicating that the mechanism of entry of EB1 into roots occurs most probably via cracks, which also represent the major routes for phytopathogen to enter into plants ( Compant et al., 2010 ; de Zélicourt et al., 2018 ). Correspondingly, the deformed hyphae of Foc TR4 and alleviated host damage were observed in planta due to the inoculation of EB1, suggesting that penetration of Foc TR4 through cellophane membranes and invasion of banana tissue were impaired upon co-inoculation with EB1. Therefore, EB1 might occupy the ecological niches and nutrition rapidly and act as an extracellular barrier for the host plant for blocking the pathogen invasion ( Gao et al., 2010 ; Shafi et al., 2017 ; Dubey et al., 2020 ). It is noteworthy that the response of banana plants toward EB1 bacterization in the rooting stage was maintained and further amplified in the pot experiment. Plants whose roots had been pre-inoculated with EB1 at the rooting stage showed significantly higher survival rates and better growth states compared with those inoculated only with Foc TR4. Using antiSMASH, 12 BGCs responsible for the synthesis of 8 secondary metabolites including surfactin, bacilysin, bacillibactin, difficidin, fengycin, bacillaene, macrolactin, and butirosin have been identified in the genome of EB1. Surfactin and fengycin have been widely characterized to mediate biofilm formation and root colonization processes, which are suggested to have a role in plant development and growth promotion ( Aleti et al., 2016 ; Berlanga-Clavero et al., 2022 ). In addition, putative genes involved in the production of indole-3-acetic acid (IAA), spermidine, and polyamine, which are related to plant growth-prompting activity, have also been discovered in the genome of EB1 ( Xie et al., 2014 ; Zaid et al., 2022 ). Thus, our results have demonstrated the promising application of endophytic antifungal strains in agriculture to breed “microbe-optimized crops”.
Different from the fighting to the death in pathogen and host relationship, recent conceptual and experimental framework has indicated that beneficial endophytes usually can evade plant defense and reach a stable harmonious commensalism with the plant ( Sessitsch et al., 2012 ; Deng et al., 2019 ; Yu et al., 2019 ). To figure out the role EB1 plays in the three-way interactions with the host plant immunity and the fungal pathogen, expressions of genes known to be markers of plant defense signaling pathways including SA-mediated NPR1 and PR1 as well as JA-mediated LOX and MYC2 were analyzed ( Mhamdi, 2019 ). It is found that the expressions of these genes were stronger in plants with EB1 pretreatment and Foc TR4 infection than that in plants with pathogen infection only which is in accord with previous research ( Chandrasekaran and Chun, 2016 ; Nie et al., 2017 ). Similarly, inoculation of wheat with endophytic bacterium Stenotrophomonas rhizophila SR80 increased the expressions of a range of genes in SA and JA signaling pathways, but only when the F. pseudograminearum , the causal agent of Crown rot disease, was present ( Liu H. et al., 2020 ). Our findings suggested that EB1 plays a key role in the interactions with the host plant immunity and the fungal pathogen via a mechanism that enhances plant defense and growth ( Khare et al., 2018 ). A few studies mentioned that beneficial microbes can quench plant immune responses by downregulating the expression of the microbial-associated molecular patterns (MAMPs) ( Bardoel et al., 2011 ; Zamioudis and Pieterse, 2012 ), producing the MAMPs with a low-elicit ability ( Trda et al., 2014 ), or minimizing the stimulation of plant defensive response ( Liu et al., 2018 ; Deng et al., 2019 ). Accordingly, the whole-genome annotation data suggest that EB1 contains multiple genes that encode key components that function by these mechanisms. Combined with the localization and in vitro data, these observations suggest that EB1 forms a symbiotic relationship with banana plants and efficiently wards off the invasive of Foc TR4 in planta inferring the adaptability and potential of the banana tissue culture plantlets bio-primed with EB1 could be a promising biological solution for the management of Fusarium wilt of banana.
Our current study focused on providing a comprehensive understanding of the endophytic strain Bacillus velezensis EB1 isolated from a healthy banana plant in a wilt-diseased banana field and exploring its potential application in tissue culture plant of banana for the environmental sustainability management of Fusarium wilt based on its strong antagonistic effects against the devastating fungal pathogen Foc and mutualistic functional roles with banana plants. To realize large-scale implementation of microbial strains in agricultural practice, new strategies for successful delivery of BCAs into plant under field conditions are needed. Therefore, in the future, we intend to (1) understand the underlying molecular mechanisms of the beneficial effect of EB1 on the growth and stress tolerance of banana plants, (2) isolate more efficient, multifunctional, stress tolerant microbes and design an artificial disease suppressive synthetic community (SynCom) which comprised by multiple microbial strains rather than mono-strain inoculums to take advantage of functional complementarity to mimic a natural disease-suppressive community in plants, and (3) develop bioformulations for sustainable application of endophytic microbes in plant tissue culture. New strategies for the successful delivery of BCAs into the plant under field conditions are needed to realize the large-scale implementation of microbial strains in agricultural practice. The introduction of endophytic microbes, as a probiotic material that enhances plant growth as well as induces defense responses of plants to cope with stress, into tissue-cultured banana plantlets, could be a novel and stable biological control method to protect bananas from Foc infection.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary material .
Author contributions
DX and SL: conceptualization. DX, XY, BL, and YC: experimentation. DX, XY, and CL: review and drafting. SL and CL: validation and statistical analysis. All authors contributed to the article and approved the submitted version.
This study was supported by Grants from R&D Projects in Key Areas of Guangdong Province (Grant No. 2019B020216001), the Science and Technology Planning Project of Guangzhou Municipal Science and Technology Bureau, China (Grant No. 202102020567), and Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (Grant No. 2022KJ109).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1146331/full#supplementary-material
Aleti, G., Lehner, S., Bacher, M., Compant, S., Nikolic, B., Plesko, M., et al. (2016). Surfactin variants mediate species-specific biofilm formation and root colonization in Bacillus . Environ. Microbiol. 18, 2634–2645. doi: 10.1111/1462-2920.13405
PubMed Abstract | CrossRef Full Text | Google Scholar
Altendorf, S. (2019). Banana fusarium wilt tropical race 4: a mounting threat to global banana markets? Food outlook 11, 13–20.
Google Scholar
Arrebola, E., Sivakumar, D., and Korsten, L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 53, 122–128. doi: 10.1016/j.biocontrol.2009.11.010
CrossRef Full Text | Google Scholar
Bardoel, B. W., van der Ent, S., Pel, M. J., Tommassen, J., Pieterse, C. M., van Kessel, K. P., et al. (2011). Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathogens 7, e1002206. doi: 10.1371/journal.ppat.1002206
Berlanga-Clavero, M. V., Molina-Santiago, C., Caraballo-Rodríguez, A. M., Petras, D., Díaz-Martínez, L., Pérez-García, A., et al. (2022). Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 7, 1001–1005. doi: 10.1038/s41564-022-01134-8
Bubici, G., Kaushal, M., Prigigallo, M. I., Gómez-Lama Cabanás, C., and Mercado-Blanco, J. (2019). Biological control agents against Fusarium wilt of banana. Front. Microbiol. 10, 616. doi: 10.3389/fmicb.2019.00616
Cao, L., Qiu, Z., You, J., Tan, H., and Zhou, S. (2005). Isolation and characterization of endophytic streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. Fems Microbiol. Letters 247, 147–52. doi: 10.1016/j.femsle.2005.05.006
Chandrasekaran, M., and Chun, S. C. (2016). Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato ( Solanum lycopersicum ) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 80, 2277–2283. doi: 10.1080/09168451.2016.1206811
Compant, S., Clément, C., and Sessitsch, A. (2010). Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42, 669–678. doi: 10.1016/j.soilbio.2009.11.024
Cook, D. C., Taylor, A. S., Meldrum, R. A., and Drenth, A. (2015). Potential economic impact of Panama disease (tropical race 4) on the Australian banana industry. J. Plant Dis. Protect. 122, 229–237. doi: 10.1007/BF03356557
de Zélicourt, A., Synek, L., Saad, M. M., Alzubaidy, H., Jalal, R., Xie, Y., et al. (2018). Ethylene induced plant stress tolerance by Enterobacter sp. SA187 is mediated by 2-keto-4-methylthiobutyric acid production. PLoS Genetics , 14, e1007273. doi: 10.1371/journal.pgen.1007273
Deng, Y., Chen, H., Li, C., Xu, J., Qi, Q., Xu, Y., et al. (2019). Endophyte Bacillus subtilis evade plant defense by producing lantibiotic subtilomycin to mask self-produced flagellin. Commun. Biol. 2, 368. doi: 10.1038/s42003-019-0614-0
Dini-Andreote, F. (2020). Endophytes: the second layer of plant defense. Trends Plant Sci. 25, 319–322. doi: 10.1016/j.tplants.2020.01.007
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G., and Staver, C. P. (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 9, 1468. doi: 10.3389/fpls.2018.01468
Dubey, A., Malla, M. A., Kumar, A., Dayanandan, S., and Khan, M. L. (2020). Plants endophytes: unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 40, 1210–1321. doi: 10.1080/07388551.2020.1808584
Eljounaidi, K., Lee, S. K., and Bae, H. (2016). Bacterial endophytes as potential biocontrol agents of vascular wilt diseases-review and future prospects. Biol. Control 103, 62–68. doi: 10.1016/j.biocontrol.2016.07.013
Etesami, H., and Alikhani, H. A. (2017). Evaluation of gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice ( Oryzia sativa L.) pathogens. Eur. J. Plant Pathol. 147, 7–14. doi: 10.1007/s10658-016-0981-z
Fan, X., Matsumoto, H., Wang, Y., Hu, Y., Liu, Y., Fang, H., et al. (2019). Microenvironmental interplay predominated by beneficial Aspergillus abates fungal pathogen incidence in paddy environment. Environ. Sci. Technol. 53, 13042–13052. doi: 10.1021/acs.est.9b04616
Fira, D., Dimkić, I., Berić, T., Lozo, J., and Stanković, S. (2018). Biological control of plant pathogens by Bacillus species. J. Biotechnol. 285, 44–55. doi: 10.1016/j.jbiotec.2018.07.044
Galvis, S. (2019). Colombia confirms that dreaded fungus has hit its banana plantations. Science , 1–5. doi: 10.1126/science.aaz1033
CrossRef Full Text
Gao, F., Dai, C., and Liu, X. (2010). Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 4, 1346–1351. doi: 10.5897/AJMR.9000480
Gómez-Lama Cabanás, C., Fernández-González, A. J., Cardoni, M., Valverde-Corredor, A., López-Cepero, J., Fernández-López, M., et al. (2021). The Banana root endophytome: differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f. sp. cubense. J. Fungi 7, 194. doi: 10.3390/jof7030194
Guez-Romero, A. S. R., Badosa, E., Montesinos, E., and Jaizme-Vega, M. C. (2008). Growth promotion and biological control of root-knot nematodes in micropropagated banana during the nursery stage by treatment with specific bacterial strains. Ann. Appl. Biol. 152, 41–48. doi: 10.1111/j.1744-7348.2007.00189.x
Jana, S. K., Islam, M. M., and Mandal, S. (2022). Endophytic microbiota of rice and their collective impact on host fitness. Curr. Microbiol. 79, 37. doi: 10.1007/s00284-021-02737-w
Kavino, M., and Manoranjitham, S. K. (2018). In vitro bacterization of banana ( Musa spp.) with native endophytic and rhizospheric bacterial isolates: novel ways to combat Fusarium wilt. Eur. J. Plant Pathol. 151, 371–387. doi: 10.1007/s10658-017-1379-2
Kema, G. H. J., Drenth, A., Dita, M., Jansen, K., Vellema, S., and Stoorvogel, J. J. (2021). Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 11, 628888. doi: 10.3389/fpls.2020.628888
Khare, E., Mishra, J., and Arora, N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9, 2732. doi: 10.3389/fmicb.2018.02732
Li, X., Mao, Z., Wu, Y., Ho, H., and He, Y. (2015). Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci. Technol. 25, 132–143. doi: 10.1080/09583157.2014.960809
Lian, J., Wang, Z. F., Cao, L. X., Tan, H. M., Patrik, I., Jiang, Z., et al. (2009). Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biol. Control 51, 427–434. doi: 10.1016/j.biocontrol.2009.08.002
Liu, H., Li, J., Carvalhais, L. C., Percy, C. D., Prakash Verma, J., Schenk, P. M., et al. (2020). Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 229, 2873–2885. doi: 10.1111/nph.17057
Liu, S., Li, J., Zhang, Y., Liu, N., Viljoen, A., Mostert, D., et al. (2020). Fusaric acid instigates the invasion of banana by Fusarium oxysporum f. sp. cubense TR4. New Phytol . 225, 913–929. doi: 10.1111/nph.16193
Liu, Z., Beskrovnaya, P., Melnyk, R. A., Hossain, S. S., Khorasani, S., O'Sullivan, L. R., et al. (2018). A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. MBio , 9, 18. doi: 10.1128/mBio.00433-18
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ CT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Matsumoto, H., Fan, X., Wang, Y., Kusstatscher, P., Duan, J., Wu, S., et al. (2021). Bacterial seed endophyte shapes disease resistance in rice. Nature Plants 7, 60–72. doi: 10.1038/s41477-020-00826-5
Mhamdi, A. (2019). NPR1 has everything under control. Plant Physiol. 181, 6–7. doi: 10.1104/pp.19.00890
Mohd Fishal, E. M., Meon, S., and Yun, W. M. (2010). Induction of tolerance to Fusarium wilt and defense-related mechanisms in the plantlets of susceptible berangan banana pre-inoculated with Pseudomonas sp. (UPMP3) and Burkholderia sp. (UPMB3). Agric. Sci. China 9, 1140–1149. doi: 10.1016/S1671-2927(09)60201-7
Nie, P., Li, X., Wang, S., Guo, J., Zhao, H., and Niu, D. (2017). Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis . Front. Plant Sci. 8, 238. doi: 10.3389/fpls.2017.00238
O'Donnell, K., Gueidan, C., Sink, S., Johnston, P. R., Crous, P. W., et al. (2009). A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 46, 936–948. doi: 10.1016/j.fgb.2009.08.006
PubMed Abstract | CrossRef Full Text
Ordonez, N., Seidl, M. F., Waalwijk, C., Drenth, A., Kilian, A., Thomma, B. P. H. J., et al. (2015). Worse comes to worst: bananas and Panama disease-when plant and pathogen clones meet. PLoS Pathogens 11, e1005197. doi: 10.1371/journal.ppat.1005197
Orlikowska, T., Nowak, K., and Reed, B. (2017). Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Culture. 128, 487–508. doi: 10.1007/s11240-016-1144-9
Papik, J., Folkmanova, M., Polivkova-Majorova, M., Suman, J., and Uhlik, O. (2020). The invisible life inside plants: deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 44, 107614. doi: 10.1016/j.biotechadv.2020.107614
Pegg, K. G., Coates, L. M. O., Neill, W. T., and Turner, D. W. (2019). The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 10, 1395. doi: 10.3389/fpls.2019.01395
Ploetz, R. C. (2015). Management of Fusarium wilt of banana: a review with special reference to tropical race 4. Crop Prot. 73, 7–15. doi: 10.1016/j.cropro.2015.01.007
Savani, A. K., Bhattacharyya, A., Boro, R. C., Dinesh, K., and JC, N. S. (2021). Exemplifying endophytes of banana ( Musa paradisiaca ) for their potential role in growth stimulation and management of Fusarium oxysporum f. sp cubense causing panama wilt. Folia Microbiol. 66, 317–330. doi: 10.1007/s12223-021-00853-5
Sessitsch, A., Hardoim, P., Doring, J., Weilharter, A., Krause, A., Woyke, T., et al. (2012). Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant-Microbe Interact. 25, 28–36. doi: 10.1094/MPMI-08-11-0204
Shafi, J., Tian, H., and Ji, M. (2017). Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 31, 446–459. doi: 10.1080/13102818.2017.1286950
Soumare, A., Diédhiou, A. G., Arora, N. K., Tawfeeq Al-Ani, L. K., Ngom, M., Fall, S., et al. (2021). Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front. Microbiol. 12, 649878. doi: 10.3389/fmicb.2021.649878
Staver, C., Pemsl, D. E., Scheerer, L., Perez Vicente, L., and Dita, M. (2020). Ex ante assessment of returns on research investments to address the impact of Fusarium wilt tropical race 4 on global banana production. Front. Plant Sci. 11, 844. doi: 10.3389/fpls.2020.00844
Strobel, G., and Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67, 491–502. doi: 10.1128/MMBR.67.4.491-502.2003
Thangavelu, R., and Gopi, M. (2015). Combined application of native Trichoderma i solates possessing multiple functions for the control of Fusarium wilt disease in banana cv. Grand Naine. Biocontr. Sci. Technol. 25, 1147–1164. doi: 10.1080/09583157.2015.1036727
Trda, L., Fernandez, O., Boutrot, F., Heloir, M. C., Kelloniemi, J., Daire, X., et al. (2014). The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 201, 1371–1384. doi: 10.1111/nph.12592
Xie, S., Wu, H., Zang, H., Wu, L., Zhu, Q., and Gao, X. (2014). Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact . 27, 655–663. doi: 10.1094/MPMI-01-14-0010-R
Yu, C., Xiao, R., Liu, B., Lin, N., and Chen, L. (2010). Endophytic colonization of biocontrol bacterium FJAT-346-PA and its efficiency against Banana Fusarium Wilt. Acta Phytophylacica Sin. 37, 493–498. doi: 10.13802/j.cnki.zwbhxb.2010.06.020
Yu, K., Liu, Y., Tichelaar, R., Savant, N., Lagendijk, E., van Kuijk, S. J. L., et al. (2019). Rhizosphere-associated pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 29, 3913–3920. doi: 10.1016/j.cub.2019.09.015
Zaid, D. S., Cai, S., Hu, C., Li, Z., and Li, Y. (2022). Comparative genome analysis reveals phylogenetic identity of Bacillus velezensis HNA3 and genomic insights into its plant growth promotion and biocontrol effects. Microbiol. Spectr. 10, e216921. doi: 10.1128/spectrum.02169-21
Zamioudis, C., and Pieterse, C. M. J. (2012). Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 25, 139. doi: 10.1094/MPMI-06-11-0179
Zhang, L., Liu, Z., Wang, Y., Zhang, J., Wan, S., Huang, Y., et al. (2022). Biocontrol potential of endophytic Streptomyces malaysiensis 8ZJF-21 from medicinal plant against banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense tropical race 4. Front. Plant Sci. 13, 874819. doi: 10.3389/fpls.2022.874819
Keywords: endophyte, Bacillus , Fusarium wilt of banana, biological control, banana tissue culture plantlets
Citation: Xiang D, Yang X, Liu B, Chu Y, Liu S and Li C (2023) Bio-priming of banana tissue culture plantlets with endophytic Bacillus velezensis EB1 to improve Fusarium wilt resistance. Front. Microbiol. 14:1146331. doi: 10.3389/fmicb.2023.1146331
Received: 17 January 2023; Accepted: 20 February 2023; Published: 16 March 2023.
Reviewed by:
Copyright © 2023 Xiang, Yang, Liu, Chu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyu Li, lichunyu@gdaas.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Banana plants are commercially propagated through the tissue culture technique , which can provide mass propagation, rejuvenation of older varieties, disease elimination, conservation of genetic resources, and the management of abiotic and biotic stresses [3,4].
The tissue culture of banana is particularly important in virus indexing, production of clean planting material, and genetic transformation procedures [9]. However, contamination of in-vitro ...
The above banana tissue culture plantlets and symbionts (banana tissue culture plantlets colonized with EB1) after 7 days of successive culture were subjected to hardening for 10 days by transferring into pots with sterilized planting soil (40 × 19 × 15 cm pots, ca. 2.0 kg soil each).